Abstract
Heme oxygenase-1 (HO-1) has been demonstrated to protect against tissue injury. Furthermore, HO-1 is also shown to be antioxidant. Our recent findings indicate that acute alcohol (EtOH) intoxication exacerbates postburn intestinal and lung tissue damage, and this was found to be neutrophil dependent. Because neutrophil-mediated tissue injury involves the release of superoxide anions , the present study examined the role of HO-1 in neutrophil production following EtOH and burn injury. Furthermore, we investigated whether HO-1 antioxidant properties are mediated via modulation of p47phox and/or p67phox proteins. Male rats (~250 g) were gavaged with EtOH to achieve a blood EtOH level of ~100 mg/dL before burn or sham injury (~12.5% total body surface area). Some rats were treated with HO-1 activator cobalt protoporphyrin IX chloride (Copp; 25 mg/kg body weight) at the time of injury. On day 1 after injury, we found that EtOH combined with burn injury significantly increased neutrophil production and p47phox and p67phox activation and decreased caspase-3 activity and apoptosis. This was accompanied with a decrease in neutrophil HO-1 levels. The treatment of animals with HO-1 activator Copp normalized neutrophil HO-1, , p47phox, and p67phox following EtOH and burn injury. The expression of caspase-3, however, was further decreased in Copp-treated sham and EtOH plus burn groups. Moreover, Copp treatment also prevented the increase in intestinal edema and permeability following EtOH and burn injury. Altogether, these findings provide a new insight into the mechanism by which HO-1 regulates neutrophil production and protect the intestine from damage following EtOH and burn injury.
Multiple organ dysfunction and failure (MOD/MOF)3 is the leading cause of death in trauma, burn, and intensive care unit patients (1–6). Many laboratory and clinical studies have indicated that intestine plays a critical role in the development of MOD/MOF (7–9). An estimated 1 million burn injuries are reported every year within the United States, and nearly half of them occur under the influence of alcohol (EtOH) (7, 10–14). Studies have shown that patients who are intoxicated at the time of injury are more susceptible to infection and have higher incidence of mortality compared with burn patients who have not consumed EtOH at time of injury (7, 10–15). Similarly, findings from experimental studies have also shown that EtOH intoxication before burn injury exacerbates the suppression of host immune defense, impairs intestinal barrier function, and increases bacterial translocation (7, 11–14, 16–21). Gut-derived bacteria are implicated in the development of MOD/MOF in injured patients.
Neutrophils are known as a major type of blood leukocytes and are among the first line of defense against invading pathogens. Under normal conditions, neutrophils rapidly migrate through the endothelium of blood vessels to extravascular inflammatory site to destroy pathogens by releasing toxic oxygen radical species and proteolytic enzymes. However, excess release of these agents may cause tissue damage in various inflammatory conditions, such as shock, trauma, and burn injury (2, 22–25). Such neutrophil accumulation and the release of superoxide anion and proteolytic enzymes may result in intestinal epithelial damage, capillary leak, alteration of intestine permeability, and increase in translocation of bacteria to extraintestinal sites (24, 26–28). Previous studies from our laboratory have shown that acute EtOH intoxication before burn injury increases neutrophil infiltration in intestine and lung, and thereby causes tissue damage in those organs (29 –31). Our studies also indicated that EtOH intoxication before burn injury delays neutrophil clearance (32). The treatment of animals with anti-neutrophil antiserum to deplete neutrophils prevented neutrophil-mediated intestinal injury (32). These findings strongly suggest that neutrophils play a critical role in organ damage following EtOH intoxication and burn injury.
A growing body of evidence indicates that heme oxygenase (HO)-1 is up-regulated following various pathophysiological conditions, including ischemia, oxidative stress, and endotoxemia, as well as following EtOH exposure (33, 34). HO-1, a microsomal enzyme, belongs to HO family. Three isoforms of HO have been identified (35) and are designated as HO-1, HO-2, and HO-3. HO-1, also known as heat shock protein 32, is the inducible isoform that catalyzes the degradation of heme to carbon monoxide, iron, and biliverdin. Biliverdin is rapidly converted to bilirubin, which is a potent endogenous antioxidant (36, 37). All three products of the HO reaction (biliverdin/bilirubin, Fe/ferritin, and carbon monoxide) participate in cellular defense. HO-1 has been reported to play a critical role in protection against oxidative tissue injury following ischemia, inflammation, and trauma-hemorrhage (37, 38); however, the mechanism is not clear. Because neutrophil-mediated tissue injury involves the release of , the present study examined the role of HO-1 in neutrophil regulation of using a two-hit rat model of EtOH and burn injury. The release of by neutrophils starts with the activation of NADPH oxidase, a multiprotein enzyme complex. NADPH oxidase is composed of cytosolic proteins (e.g., p40phox, p47phox, p67phox, and Rac2) and membrane proteins (e.g., p22phox and gp91phox) (39–43). During activation, cytosolic proteins translocate to membrane, associate with other components, and form the active oxidase that catalyzes the production of . Thus, although all six proteins contribute to the production of oxygen radical, studies have shown that p47phox and p67phox are up-regulated following major burn injury (44, 45). Therefore, we investigated whether the antioxidant properties of HO-1 could be mediated by modulation of p47phox and/or p67phox proteins of NADPH oxidase system. Furthermore, we determined the role of HO-1 in neutrophil-mediated intestinal damage following EtOH intoxication and burn injury. Although a role of HO-1 as antioxidant has been reported in many previous studies, most of these antioxidant properties were associated with the HO-1 product bilirubin. Our findings indicating that HO-1 modulates the activation of p47phox and p67phox provide a new insight into the mechanism to explain HO-1 antioxidant properties.
Materials and Methods
Animals and reagents
Male Sprague-Dawley rats (225–250 g) were obtained from Charles River Laboratories. Cobalt protoporphyrin IX chloride (Copp) was obtained from Frontier Scientific. Anti-HO-1 Ab was obtained from StressGen Biotechnologies. Anti-p47phox Ab and anti-p67phox Abs were obtained from Upstate Biotechnology. Anti-cleaved caspase-3 Ab was obtained from Cell Signaling Technology.
Rat model of acute EtOH and burn injury
As described previously (16, 30, 32), rats were randomly divided into four groups, as follows: saline plus sham, EtOH plus sham, saline plus burn, and EtOH plus burn. In EtOH-treated groups, the levels of blood EtOH equivalent to 90–100 mg/dL were achieved by gavage feeding of 5 ml of 20% EtOH in saline. In saline groups, animals were gavaged with 5 ml of saline. Four hours after gavage, all animals were anesthetized and transferred into a template, which was fabricated to expose ~12.5% of the total body surface area (TBSA). TBSA was calculated using Meeh’s formula (A = KW2/3), where K (constant factor) was equal to 10, as described in the manuscript of Walker and Mason (46). Animals were then immersed in a boiling water bath (95–97°C) for 10 –12 s. Sham-injured rats were subjected to identical anesthesia and immersed in lukewarm water. The animals were dried immediately and resuscitated i.p. with 10 ml of physiological saline. After recovery from anesthesia, the animals were returned to their cages and allowed food and water ad libitum. In some experiments, two groups of rats, one sham and other EtOH plus burn-injured rats, were treated i.p. with HO-1 activator Copp (25 mg/kg body weight (BW)) at the time of injury. Rats were sacrificed on day 1 after injury.
All of the experiments were conducted in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Neutrophil isolation
Rats were anesthetized and blood was drawn via cardiac puncture. As described previously (28), blood was slowly added to the top of Ficoll-Paque (GE Healthcare) and centrifuged at 300 × g for 30 min at 20°C. Supernatant was discarded, and the remaining RBC and neutrophils were resuspended in 3% dextran solution and stayed for 50 min. Neutrophil-rich supernatant was collected and centrifuged at 300 × g for 20 min at 10°C. The RBC were lysed by adding sterile-distilled H2O following 10 × HBSS and centrifuged at 300 × g for 30 min at 10°C. The purified neutrophils settled in the bottom were resuspended in HBSS and used for subsequent studies.
Neutrophil production
Neutrophil release was determined by the superoxide dismutase-inhibitable reduction of cytochrome c (26). Briefly, 0.1 ml of neutrophil (5 × 106 cells/ml in HBSS) was incubated with cytochrome c or cytochrome c plus superoxide dismutase for 5 min at 37°C in a 96-well plate. Neutrophil production was initiated by adding phorbol esters (PMA) at a dose of 500 ng/ml. Although we have used lower doses of PMA (50 and 100 ng/ml), a maximum response was obtained with a dose of 500 ng/ml. The absorbance of reduced cytochrome c was measured continuously at 550 nm for 60 min. The maximum rate of production was calculated from the slope of the response in nmol/min/105 cells using the specific absorbance of reduced cytochrome c of 21.1 mM/min. The peak concentration was achieved ~20–25 min after neutrophil stimulation with PMA. These peak values were recorded, pooled, and are expressed as mean ± SEM in Results.
Neutrophil apoptosis
Neutrophil apoptosis was measured by using cell death detection ELISA kit, according to the manufacturer’s instruction (Roche Applied Science).
Preparation of neutrophil membrane fraction
Neutrophils (2 × 106 cells/ml) were stimulated with or without PMA (500 ng/ml) for 10 min in PBS at 37°C and centrifuged at 300 × g for 20 min at 10°C. Neutrophils were suspended in the buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 100 mM NaF, 1 mM MgCl2, 10 mM Na4P2O7, 200 µM Na3VO4, and 10% glycerol and sonicated. The homogenate was centrifuged at 120,000 × g for 50 min at 4°C. The supernatant was collected for the measurement of cytosolic p47phox and p67phox. The pellet-containing membrane fraction was sonicated in lysis buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 100 mM NaF, 1 mM MgCl2, 10 mM Na4P2O7, 200 µM Na3 VO4, 10% glycerol, and 0.5% Triton X-100 and centrifuged at 9,000 × g for 10 min at 4°C. The supernatant was collected for the measurement of membrane p47phox and p67phox by Western blot using specific respective Abs.
Western blot
The equal amounts of protein from neutrophil total lysates, cytosolic fraction, and membrane fraction were analyzed on SDS-PAGE and transferred to immobilon membranes using a semidry Trans-Blot system (Bio-Rad) (47). The membranes were saturated with blocking buffer (10 mM Tris, 150 mM NaCl, 0.05% Tween 20, supplemented with 5% dry milk) for 2 h at room temperature and incubated with the desired primary Ab (1/1000 dilution) at 4°C overnight. The membranes were washed five times with TBS supplemented with 0.05% Tween 20 (TBST). The membranes were incubated with a secondary Ab conjugated with HRP for 1 h at room temperature. The membranes were washed five times with TBST and probed using ECL dye, and proteins were autoradiographed. Membranes were stripped by Western blot stripping buffer (Pierce) and reblotted with anti-β-actin Ab for equal protein loading in various lanes (47).
Intestinal tissue edema
Small intestine was removed, weighed, and dried for 48 h at 80°C. Water content (%) of intestinal tissue was calculated as (wet weight − dry weight)/wet weight × 100 and was used as a measure of tissue edema (29, 32).
Intestinal permeability
The intestinal permeability was measured using the procedure described previously (29, 32). In brief, the rat’s right femoral artery was cannulated under anesthesia, using PE-50 tubing filled with heparin saline (10 U/ml), and midline laparotomy was performed. Renal artery and vein in both kidneys were ligated. A 20-cm segment of the small intestine (ileum) was isolated without damaging intestinal and mesenteric structures, and PE-50 tubing was inserted into the isolated intestine from the proximal end. Solution (1 ml; 25 mg/ml 4 kDa FITC-conjugated dextran; Sigma-Aldrich) was injected into the isolated intestine. Blood samples were collected from femoral artery at 90 min after infusion of FITC-dextran. Plasma was separated by centrifuging at 4°C, 8000 rpm for 7 min, and was analyzed for FITC-dextran concentration using a fluorometer (FL500; Bio-Tek Instruments) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. Standard curves to calculate FITC-dextran concentration in the plasma samples were prepared from dilutions of FITC-dextran in PBS.
Statistical analysis
Results are presented as mean ± SEM and were analyzed using ANOVA. The significance between the groups was determined using Tukey’s test (Statistical Package for Social Sciences Software program, version 2.0, Sigma Stat). Value of p < 0.05 between two groups was considered statistically significant.
Results
Neutrophil production
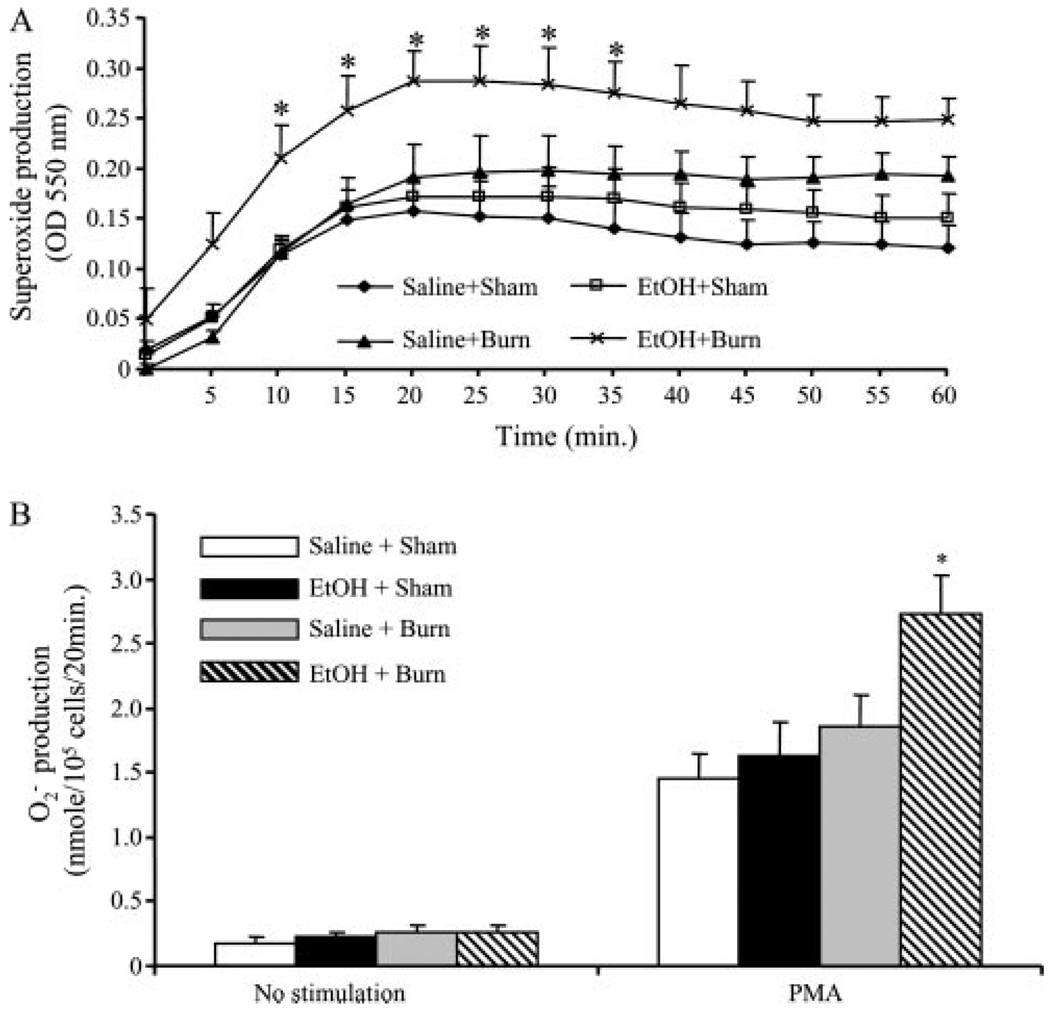
There was no significant difference in production of neutrophils without PMA stimulation in any group (Fig. 1). The addition of PMA resulted in an increase in neutrophil production in all four experimental groups. Neutrophil production was measured with or without their stimulation with PMA (500 ng/ml) by using cytochrome c reduction assay continuously at 550 nm for 60 min. The maximum rate of production was calculated as described in Materials and Methods. As shown in Fig. 1A, a peak elevation in was achieved within 20–25 min after the stimulation of neutrophils with PMA. The values obtained at 20-min time point were plotted in bar graph shown in Fig. 1B. Although neutrophils from rats receiving EtOH or burn injury alone also showed a tendency of an increase in their ability to produce , this increase was not found to be significantly different from sham animals. However, a significant increase in PMA-induced production was observed in rats receiving a combined insult of EtOH and burn injury compared with rats receiving either sham or burn injury alone (p < 0.05).
FIGURE 1.
Neutrophil production following EtOH intoxication and burn injury. One day after injury, blood was drawn via cardiac puncture and neutrophils were isolated. Neutrophil production was measured with or without their stimulation with PMA (500 ng/ml) by using cytochrome c reduction assay. Plates were read at an OD of 550 nm from 0 to 60 min (A). The maximum rate of production was calculated from the slope of the response in nmol/min/105 cells using the specific absorbance of reduced cytochrome c of 21.1 mM/min. The peak concentration was achieved ~20–25 min after neutrophil stimulation with PMA. These peak values were recorded and pooled, and are shown as mean ± SEM from at least six animals in each group (B). *, p < 0.05 vs other groups.
Neutrophil apoptosis
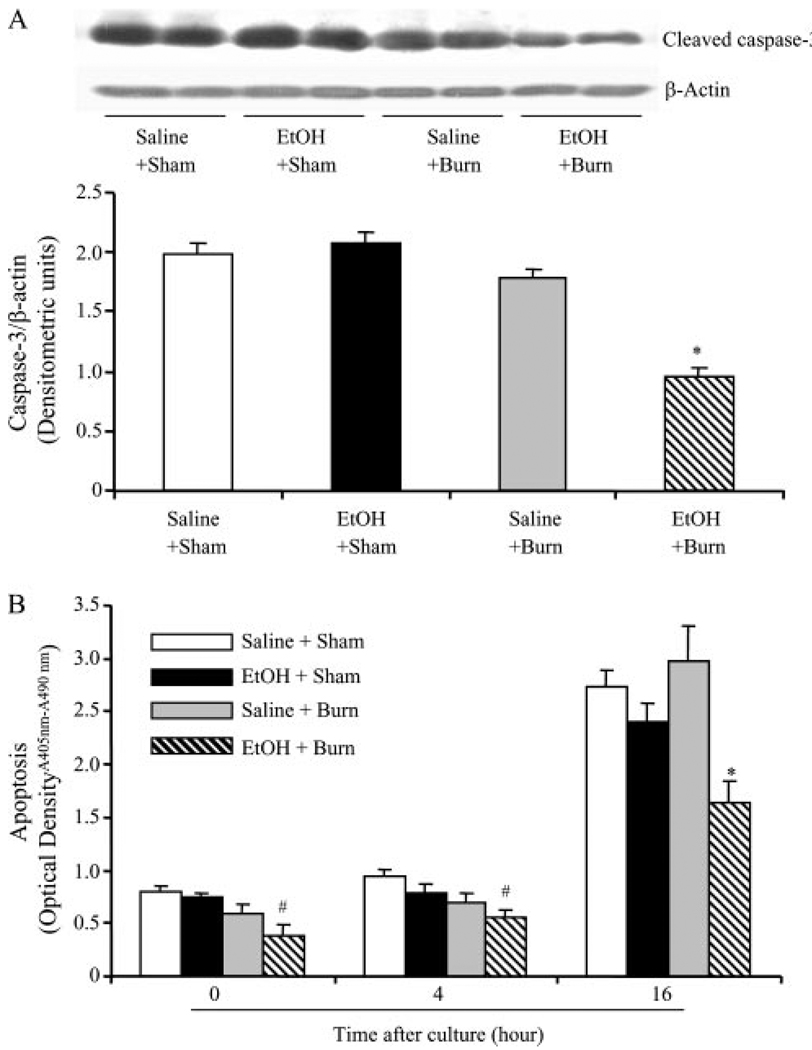
Neutrophil apoptosis was examined by determining caspase-3 expression. As shown in Fig. 2A, the expression of cleaved caspase-3 was not found to be significantly different in neutrophils from sham rats gavaged with saline or EtOH. There was a trend toward a decrease in caspase-3 in neutrophils after burn injury alone in the absence of EtOH exposure. However, this decrease was not found to be significantly different from shams. A significant decrease in caspase-3 expression was observed in neutrophils from rats receiving a combined insult of EtOH intoxication and burn injury compared with rats receiving either sham injury regardless of their EtOH exposure or burn injury alone.
FIGURE 2.
Neutrophil caspase-3 activity and apoptosis following EtOH intoxication and burn injury. One day after injury, neutrophils were isolated from blood and the neutrophil caspase-3 activity was measured by Western blot (A). For loading control in various lanes, membranes were erased and reblotted for β-actin. Blots were analyzed using densitometry. The densitometric values were normalized to the β-actin and are shown in bar graph as mean ± SEM. Values are mean ± SEM from at least five animals in each group. *, p < 0.05 vs other groups. In addition to caspase-3 expression, apoptosis was also measured by cell death detection ELISA kit in freshly isolated (0-h) neutrophils and after their culture for 4 and 16 h (B). Values are mean ± SEM from at least six animals in each group. *, p < 0.05 vs other groups. #, p < 0.05 vs sham + saline.
In addition to caspase-3 expression, neutrophil apoptosis was further confirmed by measuring cytoplasmic histone-associated DNA fragments using freshly isolated neutrophil, as well as after their culture for 4 and 16 h, and the results are shown in Fig. 2B. Because neutrophils have a short lifetime, apoptosis was significantly increased in cells cultured for 16 h compared with cells from 0- and 4-h cultures in all groups. Results shown in Fig. 2B indicate no significant difference in the neutrophil apoptosis at any time point between rats receiving sham injury regardless of EtOH intoxication and burn injury alone. A significant decrease in neutrophil apoptosis was observed at 0-, 4-, and 16-h culture in rats receiving a combined insult of EtOH intoxication and burn injury compared with rats receiving either sham injury or burn injury alone.
Neutrophil HO-1
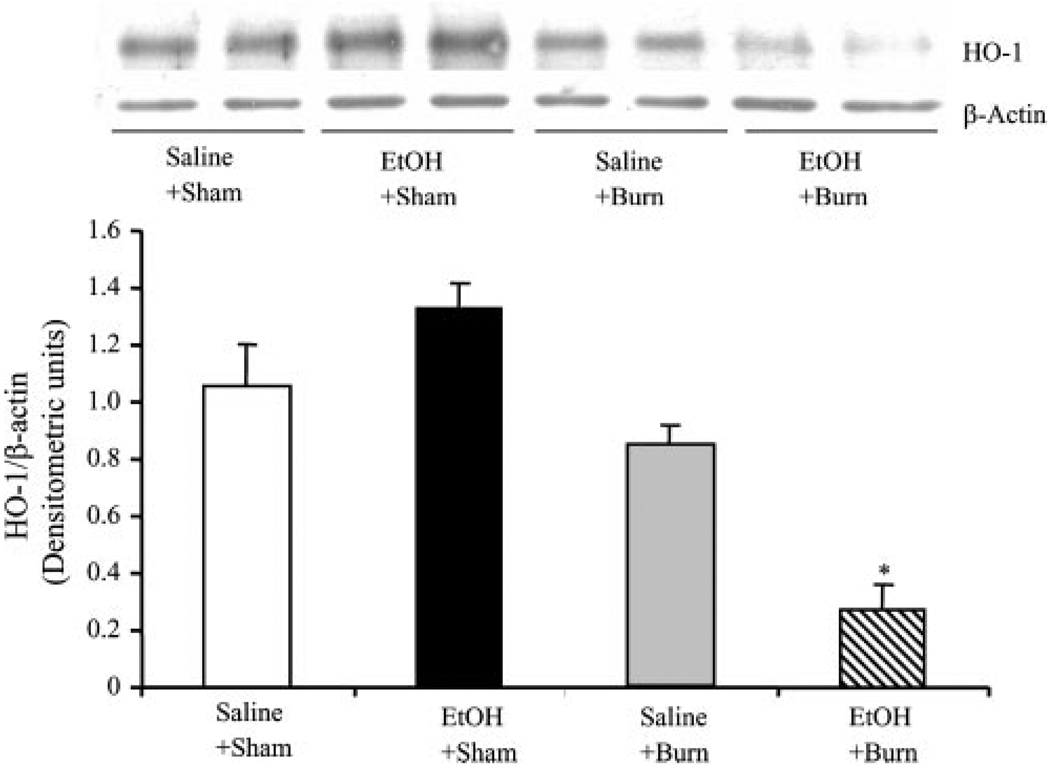
As compared with shams, there was a tendency toward an increase in neutrophil HO-1 expression in sham rats gavaged with EtOH, but this increase was not found to be significantly different (Fig. 3). Similarly, neutrophil HO-1 expression was not significantly different in rats receiving burn injury alone compared with shams gavaged with saline. However, as compared with rats receiving EtOH intoxication, neutrophil HO-1 expression in burn rats was significantly decreased. Furthermore, a significant decrease in neutrophil HO-1 expression was observed in rats receiving a combined insult of EtOH intoxication and burn injury compared with rats receiving either sham or burn injury alone.
FIGURE 3.
Neutrophil HO-1 expression following EtOH intoxication and burn injury. One day after injury, neutrophils were isolated from the blood. Neutrophil HO-1 expression was measured by Western blot. For loading control in various lanes, membranes were erased and reblotted for β-actin. Blots were analyzed using densitometry. The densitometric values were normalized to β-actin and are shown in bar graph as mean ± SEM. Values are mean ± SEM from four to six animals in each group. *, p < 0.05 vs other groups.
Effect of HO-1 inducer Copp on neutrophil HO-1 expression, production, and caspase-3 expression
In the subsequent experiments, we measured whether HO-1 influences neutrophil production and caspase-3 expression following a combined insult of EtOH intoxication and burn injury. In these experiments, rats were treated with Copp (25 mg/kg BW) at the time of burn injury. We did not include EtOH-alone and burn-alone groups because the above parameters (i.e., production, apoptosis, and HO-1) were not significantly different in those groups compared with sham animals.
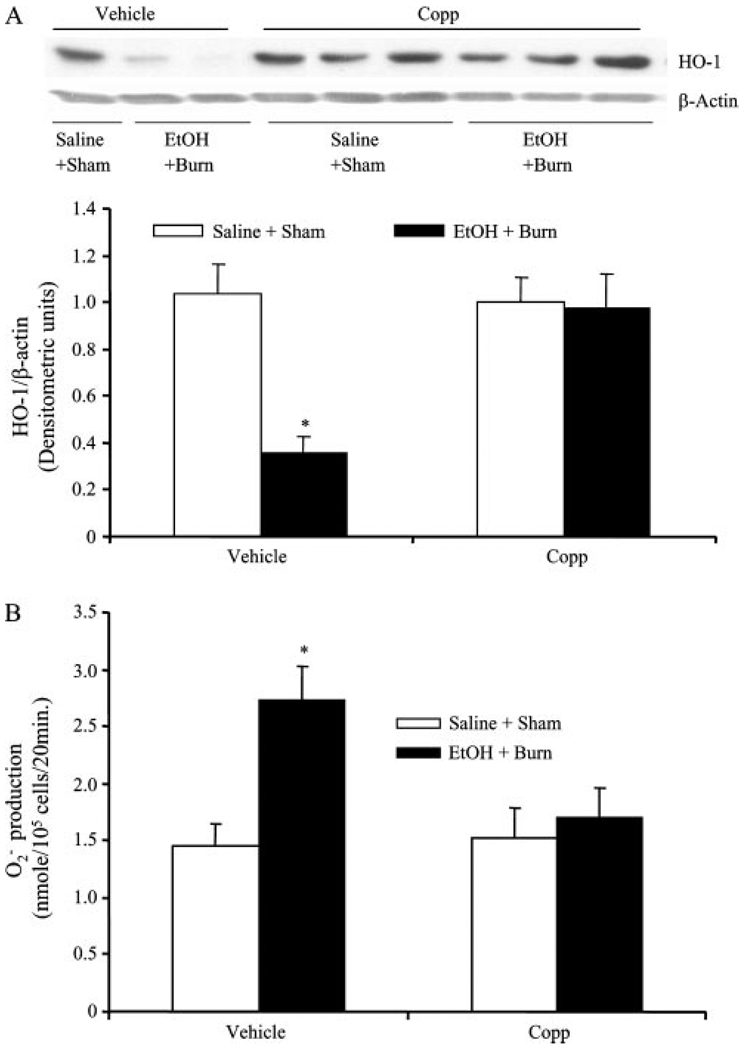
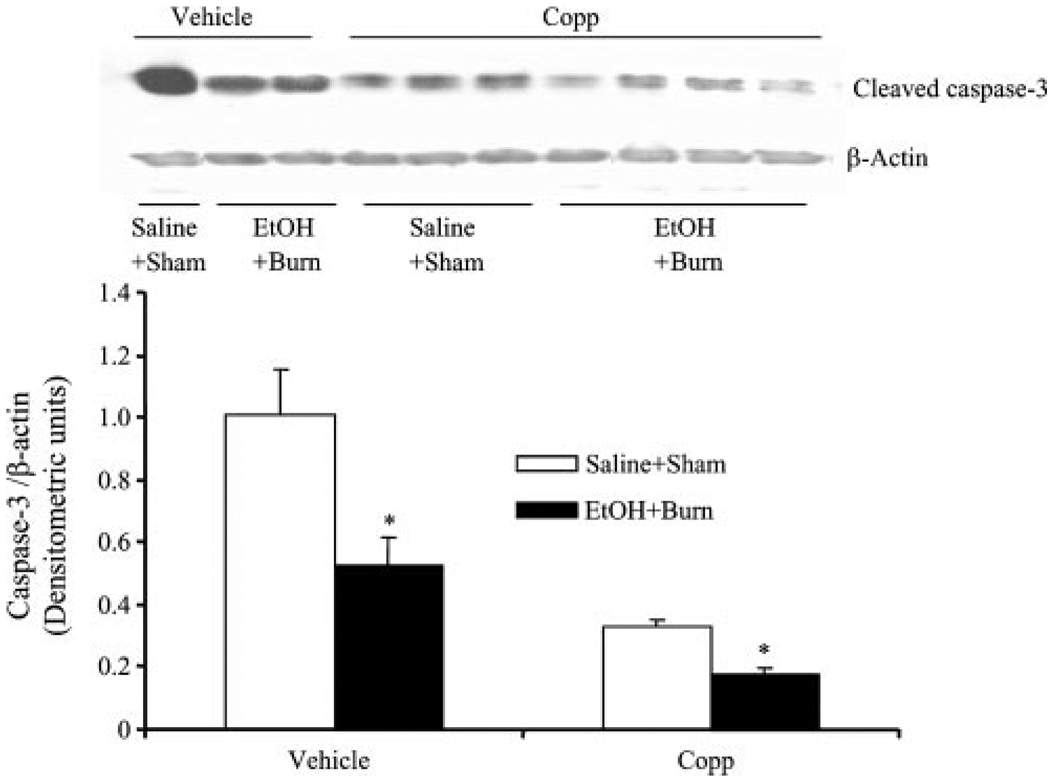
The results as shown in Fig. 4 indicate that treatment of rats with Copp normalized HO-1 expression following EtOH and burn injury to sham levels (Fig. 4A). Furthermore, treatment of rats with Copp prevented the increase in neutrophil production compared with rats treated with vehicle following EtOH intoxication in burn injury (Fig. 4B). However, there was no difference in neutrophil HO-1 expression and production between sham rats treated with Copp or vehicle (Fig. 4). The caspase-3 expression was significantly decreased in sham rats treated with Copp compared with vehicle-treated shams (Fig. 5). Administration of Copp in EtOH plus burn rats resulted in a further significant decrease in neutrophil caspase-3 expression compared with vehicle-treated sham and EtOH plus burn-injured rats (Fig. 5).
FIGURE 4.
The effect of Copp on neutrophil HO-1 expression and production following EtOH intoxication and burn injury. Rats were treated with Copp (25 mg/kg BW) or vehicle at the time of injury. One day after injury, neutrophils were isolated from the blood; HO-1 expression (A) and production (B) were measured. For HO-1 loading control in various lanes, membranes were erased and reblotted for β-actin. Blots were analyzed using densitometry. The densitometric values were normalized to β-actin and are shown in bar graph as mean ± SEM from four to six animals in each group. For neutrophil production, we recorded the peak concentration, which was achieved ~20–25 min after neutrophil stimulation with PMA. These peak values were pooled and are shown as mean ± SEM from at least six animals in each group (B). *, p < 0.05 vs other groups in respective panels.
FIGURE 5.
The effect of Copp on neutrophil caspase-3 activity following EtOH intoxication and burn injury. Rats were treated with Copp (25 mg/kg BW) or vehicle at the time of injury. One day after injury, blood was drawn via cardiac puncture, neutrophils were isolated, and neutrophil caspase-3 activity was measured by Western blot. For loading control in various lanes, membranes were erased and reblotted for β-actin. Blots were analyzed using densitometry. The densitometric values were normalized to β-actin and are shown in bar graph as mean ± SEM from at least five animals in each group. *, p < 0.05 vs respective shams.
Effect of HO-1 inducer Copp on neutrophil p47phox and p67phox activity
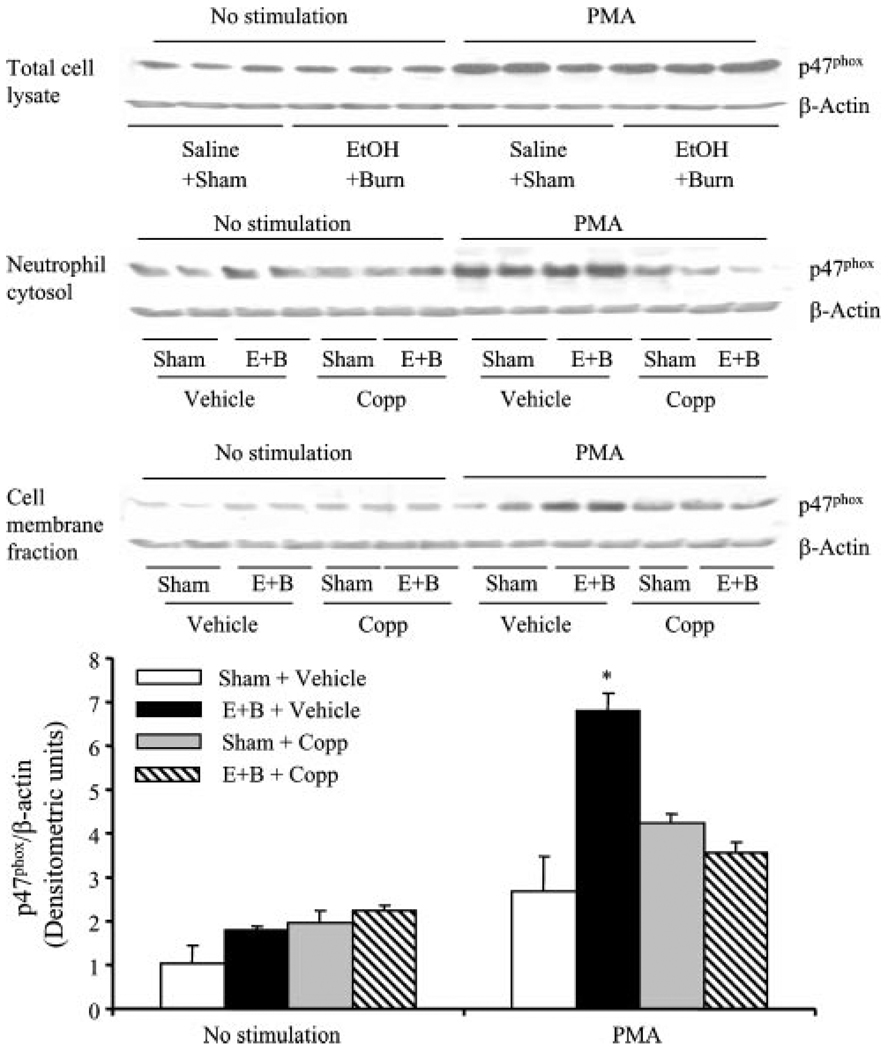
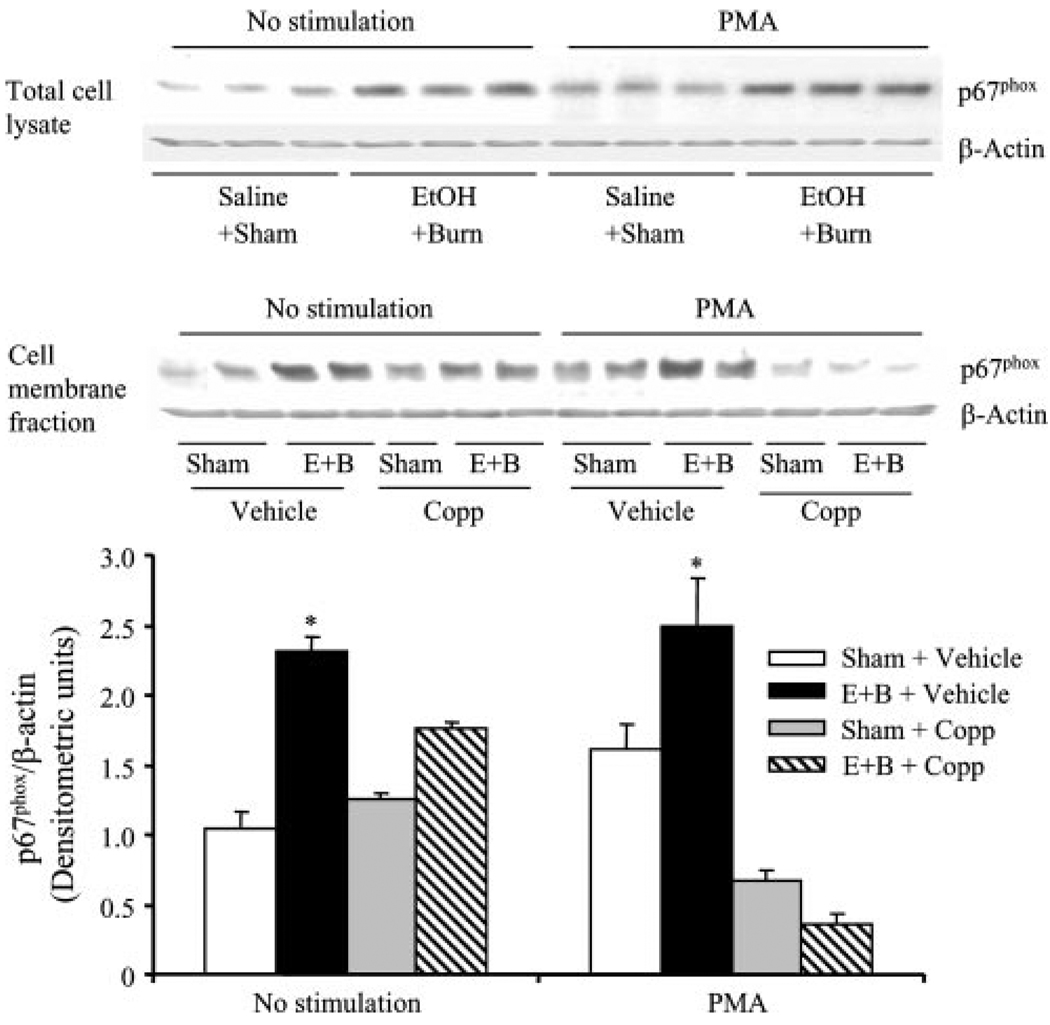
p47phox and p67phox proteins are the components of NADPH oxidase, which are involved in the formation of . We determined whether HO-1 regulates neutrophil production via modulating the activation of p47phox and p67phox following a combined insult of EtOH intoxication and burn injury. Activation of p47phox and p67phox was assessed by determining their association with the cell membrane fraction. The results indicate that there was no significant difference in p47phox expression in neutrophil total cell lysates with or without PMA stimulation between sham and EtOH plus burn-injured rats (Fig. 6). However, a significant increase in p67phox expression was observed in the neutrophil total cell lysates regardless of their stimulation with PMA in rats receiving a combined insult of EtOH and burn injury compared with shams (Fig. 7). p47phox and p67phox are cytosolic proteins, but upon stimulation with an agonist such as PMA, they translocate to the membrane and associate with other NADPH components to form the active oxidase system. We isolated neutrophil membranes to determine p47phox and p67phox levels (Figs. 6 and 7). No significant difference was observed in p47phox levels in membrane fractions of unstimulated neutrophils between sham and EtOH plus burn rats (Fig. 6). Treatment with Copp also did not influence p47phox levels in unstimulated neutrophil membrane fractions. However, there was a significant increase in p47phox levels of neutrophil membrane stimulated with PMA in rats receiving a combined insult of EtOH intoxication and burn injury compared with sham rats. Treatment with Copp prevented the increase in p47phox levels in neutrophil membrane fractions following EtOH and burn injury (Fig. 6). The levels of p67phox in contrast were significantly increased in both unstimulated and PMA-stimulated neutrophil membrane fractions following a combined insult of EtOH intoxication and burn injury compared with sham rats (Fig. 7). This increase in membrane p67phox levels was prevented in neutrophils harvested from rats treated with Copp following EtOH and burn injury (Fig. 7). In addition to the total and membrane fractions, we also measured p47phox and p67phox levels in cytosolic fractions. There was no significant difference in p47phox in cytosolic fractions from unstimulated cells (Fig. 6). However, following PMA stimulation, there was a tendency of an increase in p47phox levels in neutrophil cytosolic fractions following EtOH and burn injury compared with shams, but this increase was not found to be significantly different. The treatment of animals with Copp normalized cytosolic p47phox levels similar to those observed in unstimulated cells. p67phox in contrast was not detectable in cytosolic fractions.
FIGURE 6.
The effect of Copp on neutrophil p47phox activity following EtOH intoxication and burn injury. Rats were treated with Copp (25 mg/kg BW) or vehicle at the time of injury. One day after injury, blood was drawn via cardiac puncture; neutrophils were isolated and either remained unstimulated or stimulated with PMA (500 ng/ml) and lysed. p47phox expression was measured in neutrophil total cell lysates, as well as in cytosolic and membrane fractions by Western blot. For loading control in various lanes, membranes were erased and reblotted for β-actin. Blots obtained from membrane fractions were analyzed using densitometry. The densitometric values were normalized to β-actin and are shown in bar graph as mean ± SEM from five animals in each group. *, p < 0.05 vs other groups. E+B: EtOH + burn.
FIGURE 7.
The effect of Copp on neutrophil p67phox activity following EtOH intoxication and burn injury. Rats were treated with Copp (25 mg/kg BW) or vehicle at the time of injury. One day after injury, blood was drawn via cardiac puncture; neutrophils were isolated and either remained unstimulated or stimulated with PMA (500 ng/ml) and lysed. p67phox expression was measured in neutrophil total cell lysates, as well as in neutrophil membrane fractions by Western blot. For loading control in various lanes, membranes were erased and reblotted for β-actin. Blots obtained from membrane fractions were analyzed using densitometry. The densitometric values were normalized to β-actin and are shown in bar graph as mean ± SEM from five animals in each group. *, p < 0.05 vs other groups. E+B: EtOH + burn.
Effect of HO-1 inducer Copp on intestinal tissue damage
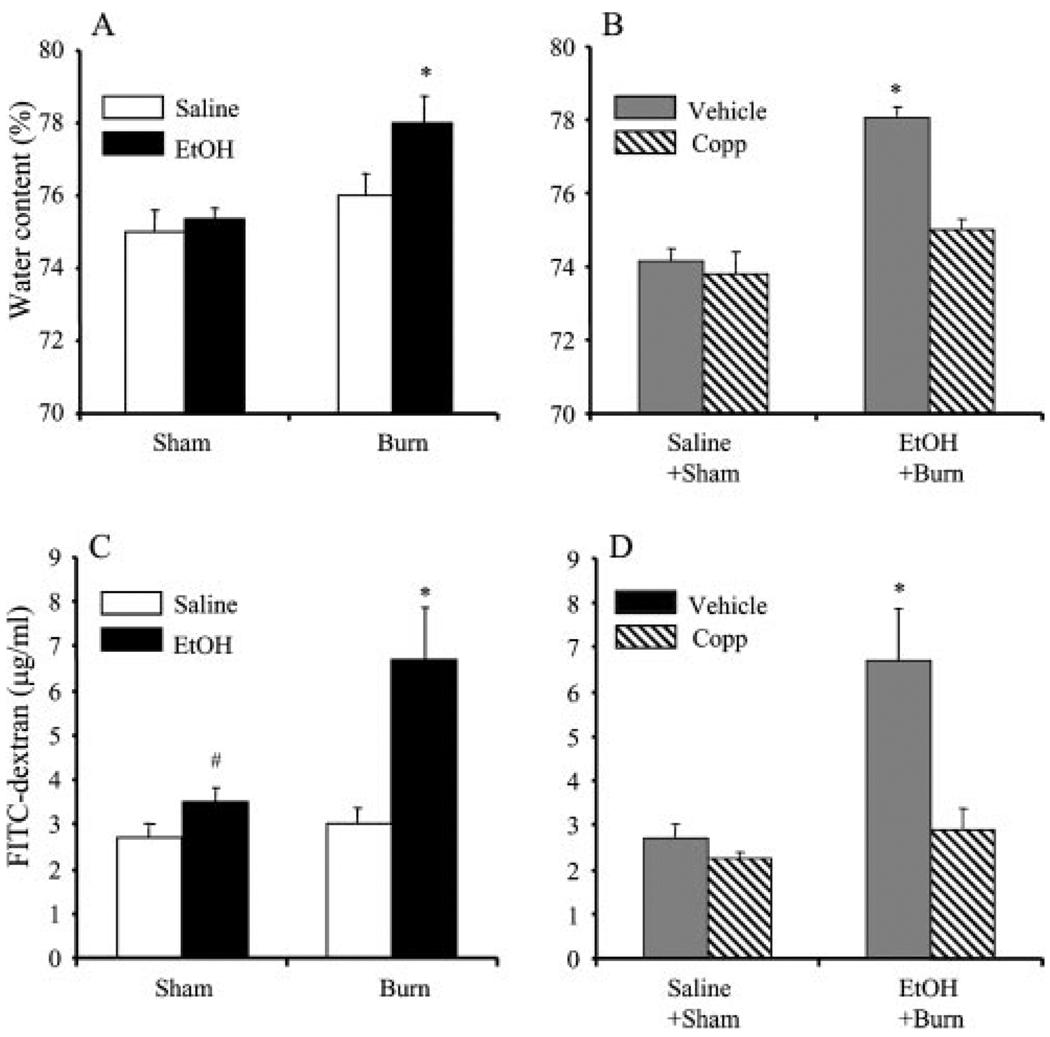
The intestinal tissue edema is a marker of tissue damage and is determined by intestinal tissue water content. As shown in Fig. 8, there was a significant increase in water content in intestinal tissue of rats receiving a combined insult of EtOH intoxication and burn injury compared with rats receiving sham injury regardless of their EtOH intoxication or burn injury without EtOH intoxication (Fig. 8A). The treatment of rats with Copp prevented the increase in intestinal edema following EtOH intoxication prior to injury (Fig. 8B). To further confirm the protective role of Copp in intestinal tissue damage, we determined intestinal permeability, which is another marker of intestinal tissue damage. The results as shown in Fig. 8C indicate a significant increase in plasma FITC-dextran accumulation in femoral artery at 90 min after infusion of FITC-dextran in the intestines of rats receiving a combined insult of EtOH intoxication and burn injury compared with sham rats regardless of EtOH intoxication and burn injury alone. Treatment of rats with Copp prevented the increase in plasma FITC-dextran accumulation in femoral artery at 90 min after infusion of FITC-dextran following EtOH intoxication and burn injury (Fig. 8D).
FIGURE 8.
The effect of Copp on intestinal tissue edema formation (A and B) and intestinal permeability (C and D) following EtOH intoxication and burn injury. Rats were subjected to sham or burn injury with or without EtOH intoxication. Some animals in sham and EtOH + burn groups were treated with Copp (25 mg/kg BW). One day after injury, rats were sacrificed and intestine edema formation and permeability were determined, as described in Materials and Methods. Values are mean ± SEM from six animals in each group. *, p < 0.05 vs other groups in respective panel; #, p < 0.05 vs saline + sham.
Discussion
Our findings indicate that EtOH intoxication before burn injury significantly increases neutrophil production, decreases caspase-3 activity, and delays neutrophil apoptosis. We also found an increase in neutrophil p47phox and p67phox activation following a combined insult of EtOH and burn injury. These changes in neutrophil , p47phox, p67phox, and apoptosis are accompanied with a decrease in HO-1. The treatment of animals with HO-1 activator Copp normalized neutrophil , p47phox, and p67phox following EtOH and burn injury. The expression of caspase-3, however, was further decreased in Copp-treated sham and EtOH plus burn groups. Moreover, Copp treatment also prevented the increase in intestinal edema and permeability following EtOH and burn injury. These findings indicate that acute EtOH intoxication combined with burn injury potentiates the alterations in neutrophil release and apoptosis, and that HO-1 appears to play a role in this process.
Neutrophils have a short lifetime (t1/2 of ~7 h) and are normally present in the circulatory system (48). Under inflammatory conditions, their lifetime is increased and large numbers of neutrophils migrate to the inflammatory site to destroy the invading pathogens by releasing proteases (e.g., elastase) and reactive oxygen species (e.g., ) (24). Many studies indicated that neutrophils are key components in early stage of the inflammatory process against microbial pathogens. However, the excess of neutrophil-derived proteases and is shown to inflict damage to surrounding tissue. Neutrophil recruitment to the inflammatory sites is induced by several factors, including cytokines (e.g., TNF-α, IL-6, and IL-18), chemokines (e.g., MIPs, keratinocyte-induced chemokine, cytokine-induced neutrophil chemoattractant (CINC)-1, and CINC-3), and adhesion molecules (e.g., ICAM-1). Once the job is done, neutrophils are cleared by apoptosis and subsequent phagocytosis by macrophages (49). In a recent study, we observed an increase in neutrophil infiltration and the levels of IL-18, ICAM-1, and CINC-1 in both intestine and lung tissue (29, 30). Another significant observation in that study was that following burn injury alone, neutrophils were cleared within the first 24 h after injury; however, when it was combined with prior EtOH exposure, neutrophil infiltration and other inflammatory markers sustained up to 24 h (32). We also found that the depletion of neutrophils prevented the intestine and lung tissue damage following EtOH and burn injury (30, 32). Altogether, these findings indicated that acute EtOH intoxication potentiates the neutrophil infiltration and tissue damage in intestine and lung following burn injury, and neutrophils play a pivotal role in intestine and lung tissue damage under those conditions.
Because the production of has been implicated in neutrophil-mediated tissue damage, this study examined the effect of EtOH intoxication and burn injury on neutrophil production. We found that EtOH intoxication before burn injury significantly increases neutrophil production. Moreover, neutrophils from EtOH plus burn-injured rats exhibited less apoptosis than sham animals. Because neutrophil apoptosis is critical to their clearance, the finding of a delay in neutrophil apoptosis may further their ability to produce and proteases in excess, and thereby accelerates the tissue injury. The generation of by neutrophils results from the activation of multiprotein enzyme complex known as NADPH oxidase. NADPH oxidase is present in many cells, including neutrophils, eosinophils, and mononuclear phagocytes (42). NADPH oxidase is composed of membrane proteins (gp91phox, p22phox, and the small G protein Rap1A) and cytosolic proteins (p47phox, p67phox, p40phox, the small G protein Rac2, and Cdc42) (42). In resting cells, these components exist in free form; upon activation, the membrane-bound subunits gp91phox and p22phox together form the heterodimeric cytochrome b558 located in the plasma membrane. The cytosolic proteins, p47phox and p67phox, translocate to the plasma membrane and combine with cytochrome b558 to form a complex enzyme system, which transfers electrons from NADPH to oxygen to generate . In the membrane-associated complex, p47phox stabilizes the interaction of p67phox with the cytochrome b558 complex. p67phox contains high-affinity binding site for NADPH and can bind gp91phox to form an active enzyme (42). Dusi et al. (41) demonstrated that the activation of NADPH oxidase involves the phosphorylation of p47phox and p67phox. The phosphorylation results in the conformational changes of p47phox and p67phox and neutralization of the cationic domain, which in turn allows interaction with other membrane proteins (48). The phosphorylation of p47phox is associated with production during neutrophil activation. The stimuli such as PMA and fMLP lead to the phosphorylation of p47phox and p67phox (48, 50). In this study, we determined the activation of neutrophil p47phox and p67phox following EtOH intoxication and burn injury. We observed that neutrophils stimulated with PMA resulted in a significant increase in neutrophil membrane levels of p47phox following a combined EtOH and burn injury compared with shams. However, p47phox expression was not significantly increased in total cell lysates following EtOH and burn injury compared with sham. In contrast, p67phox levels were significantly elevated in both stimulated and unstimulated neutrophil total cell lysates and membrane fractions. We also measured p47phox and p67phox levels in cytosolic fractions and found that p47phox similar to the membrane fractions was significantly increased following EtOH plus burn injury compared with shams. However, p67phox in contrast to total cell lysates was not detectable in cytosolic fractions. Thus, it appears that p67phox protein is translocated to the membrane following EtOH and burn injury, and PMA has no further effect on this. PMA, an agonist of protein kinase C (PKC), has been used in many previous studies for determining neutrophil production. The activation of PKC results in the phosphorylation of p47phox and p67phox, and thus in the activation of NADPH oxidase (51, 52). Although the activation of PKC may be involved in the regulation of p47phox and p67phox, whether the up-regulation of p47phox and p67phox is a direct effect of EtOH plus burn injury or is indirectly mediated via up-regulation of upstream molecules such as PKC remains to be established. Furthermore, because PMA has no further influence on neutrophil membrane p67phox in our study, it appears that p47phox play a predominant role in PMA-induced neutrophil production following EtOH and burn injury. However, more studies are needed to establish this fact.
An imbalance between release and antioxidant capability results in oxidative stress and oxidative damage. Many studies have shown that HO represents an important mechanism of protection against oxidative stress by its anti-inflammatory, antiapoptotic, and cytoprotective properties (34, 38, 53, 54). Furthermore, a role of HO-1 is also implicated in neutrophil rolling, adhesion, and migration to the inflammatory sites (38, 53–56). The treatment of animal with chromium-mesoporphyrin (a specific inhibitor of HO-1) enhanced the neutrophil infiltration (38). In the present study, we found a decrease in neutrophil HO-1 expression and an increase in production following EtOH and burn injury. The treatment of animals with Copp, a known HO-1 activator, prevented the decrease in neutrophil HO-1 expression and increase in production following EtOH and burn injury. Furthermore, we found the treatment of animals with Copp also prevented the increase in p47phox and p67phox activity following EtOH and burn injury. This finding indicates that in addition to the classical scavenging role, HO-1 also plays a role in the formation of NADPH assembly by regulating the activation of p47phox and p67phox. Although a similar role of HO-1 in regulation of gp91phox in a macrophage cell line was suggested in a previous study (57), our findings provide new additional evidence that it also regulates the activation of p47phox and p67phox. Thus, multiple mechanisms may exist by which HO-1 may exhibit its antioxidant properties.
Because administration of Copp further decreased neutrophil caspase-3 expression following EtOH plus burn injury, it appears that HO-1 up-regulation decreases neutrophil apoptosis. A precise mechanism of HO-1 antiapoptotic effect following EtOH and burn injury remains to be determined; a previous study indicated that antiapoptotic effect of HO-1 in endothelial cells involves the degradation of p38 α MAPK isoforms (54). HO-1 overexpression has been demonstrated to down-regulate neutrophil-mediated tissue damage (38). Similarly, we observed that treatment of rats with Copp prevented the increase in intestinal edema formation and intestinal permeability (the markers of intestinal injury) following combined insult of EtOH intoxication and burn injury. Although the mechanism by which HO-1 regulates neutrophil infiltration following EtOH and burn injury remains to be established, studies have indicated that HO-1 overexpression down-regulates adhesion molecule expression and neutrophil chemokines and reduces neutrophil infiltration (35, 38, 53). Thus, an increase in HO-1 expression may not only down-regulate neutrophil production ability, but also prevents their recruitment to the intestine and protects intestine from injury following EtOH and burn injury.
Recent findings indicate that IL-18 down-regulates HO-1 expression in endothelial cells (58). Because we also observed an increase in IL-18 levels following EtOH and burn injury (29, 32), it is plausible that IL-18 may regulate HO-1 under the present conditions; however, this remains to be established. We recognize that the present study has used a ~12.5% TBSA burn injury, which by itself did not produce any adverse effects on the intestine at 24 h after injury. There is evidence that burn injury size is a critical factor in postburn complications (4). However, other factors such as age, gender, and preclinical manifestation can also influence the outcome of burn patients, especially the patients with small burn injury. Similarly, EtOH exposure at the time of burn injury has been shown to further confound postburn pathogenesis (7, 14, 15). Thus, whereas a smaller burn by itself may not have an adverse effect on host defense, when combined with existing conditions such as EtOH intoxication, it may become detrimental.
In summary, our findings indicate that EtOH intoxication combined with burn injury suppresses neutrophil HO-1 expression, decreases neutrophil apoptosis, and up-regulates neutrophil p47phox and p67phox activity and production. Treatment of rats with HO-1 inducer Copp at the time of injury normalizes all the above parameter except the apoptosis, which was further decreased. Furthermore, HO-1 induction also protected intestine from damage following EtOH and burn injury. These results suggest that HO-1 plays a critical role in protection from neutrophil-mediated intestinal tissue damage following a combined insult of EtOH intoxication and burn injury. These findings provide new insight into the mechanism by which HO-1 may regulate neutrophil production, and thus may help in developing the new therapeutic strategies for patients suffering from a combined insult of EtOH intoxication and burn injury.
Footnotes
This study was supported by National Institutes of Health Grants R01AA015731-01A2 and R21AA015979-01A1.
Abbreviations used in this paper: MOD/MOF, multiple organ dysfunction and failure; CINC, cytokine-induced neutrophil chemoattractant; Copp, cobalt protoporphyrin IX chloride; EtOH, alcohol; HO, heme oxygenase; , superoxide anion; PKC, protein kinase C; TBSA, total body surface area; BW, body weight.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Arturson G. Forty years in burns research: the postburn inflammatory response. Burns. 2000;26:599–604. doi: 10.1016/s0305-4179(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima R, Alexander JW, Gianotti L, Pyles T, Ogle CK. Bacterial translocation-related mortality may be associated with neutrophil-mediated organ damage. Shock. 1995;3:323–328. [PubMed] [Google Scholar]
- 3.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J. Leukocyte Biol. 2004;75:400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 4.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int. J. Mol. Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 5.Smith JW, Gamelli RL, Jones SB, Shankar R. Immunologic responses to critical injury and sepsis. J. Intensive Care Med. 2006;21:160–172. doi: 10.1177/0885066605284330. [DOI] [PubMed] [Google Scholar]
- 6.Carrico CJ, Holcomb JB, Chaudry IH. Scientific priorities and strategic planning for resuscitation research and life saving therapy following traumatic injury: report of the PULSE Trauma Work Group: Post Resuscitative and Initial Utility of Life Saving Efforts. Shock. 2002;17:165–168. doi: 10.1097/00024382-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA. Role of the gut lymphatic system in multiple organ failure. Curr. Opin. Crit. Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Moore EE. Mesenteric lymph: the critical bridge between dysfunctional gut and multiple organ failure. Shock. 1998;10:415–416. [PubMed] [Google Scholar]
- 10.Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J. Burn Care Rehabil. 1996;17:532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Kelley D, Lynch JB. Burns in alcohol and drug users result in longer treatment times with more complications. J. Burn Care Rehabil. 1992;13:218–220. doi: 10.1097/00004630-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 12.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J. Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 13.McGwin G, Jr, Chapman V, Rousculp M, Robison J, Fine P. The epidemiology of fire-related deaths in Alabama, 1992–1997. J. Burn Care Rehabil. 2000;21:75–83. doi: 10.1097/00004630-200021010-00016. [DOI] [PubMed] [Google Scholar]
- 14.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 15.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. J. Burn Care Rehabil. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am. J. Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 17.Maier RV. Ethanol abuse and the trauma patient. Surg. Infect. 2001;2:133–141. doi: 10.1089/109629601750469456. [DOI] [PubMed] [Google Scholar]
- 18.Napolitano LM, Koruda MJ, Zimmerman K, McCowan K, Chang J, Meyer AA. Chronic ethanol intake and burn injury: evidence for synergistic alteration in gut and immune integrity. J. Trauma. 1995;38:198–207. doi: 10.1097/00005373-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Szabo G, Mandrekar P, Verma B, Isaac A, Catalano D. Acute ethanol consumption synergizes with trauma to increase monocyte tumor necrosis factor α production late postinjury. J. Clin. Immunol. 1994;14:340–352. doi: 10.1007/BF01546318. [DOI] [PubMed] [Google Scholar]
- 20.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J. Leukocyte Biol. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 21.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am. J. Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, Chaudry IH. Are the protective effects of 17β-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-α or ER-β? J. Leukocyte Biol. 2006;79:1173–1180. doi: 10.1189/jlb.0106029. [DOI] [PubMed] [Google Scholar]
- 23.Partrick DA, Moore EE, Offner PJ, Meldrum DR, Tamura DY, Johnson JL, Silliman CC. Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch. Surg. 2000;135:219–225. doi: 10.1001/archsurg.135.2.219. [DOI] [PubMed] [Google Scholar]
- 24.Sayeed MM. Exuberant Ca2+ signaling in neutrophils: a cause for concern. News Physiol. Sci. 2000;15:130–136. [PubMed] [Google Scholar]
- 25.Sir O, Fazal N, Choudhry MA, Goris RJ, Gamelli RL, Sayeed MM. Role of neutrophils in burn-induced microvascular injury in the intestine. Shock. 2000;14:113–117. doi: 10.1097/00024382-200014020-00006. [DOI] [PubMed] [Google Scholar]
- 26.Sabeh F, Hockberger P, Sayeed MM. Signaling mechanisms of elevated neutrophil generation after burn injury. Am. J. Physiol. 1998;274:R476–R485. doi: 10.1152/ajpregu.1998.274.2.R476. [DOI] [PubMed] [Google Scholar]
- 27.Fazal N, Shamim M, Zagorski J, Choudhry MA, Ravindranath T, Sayeed MM. CINC blockade prevents neutrophil Ca2+ signaling up-regulation and gut bacterial translocation in thermal injury. Biochim. Biophys. Acta. 2000;1535:50–59. doi: 10.1016/s0925-4439(00)00082-x. [DOI] [PubMed] [Google Scholar]
- 28.Fazal N, Al Ghoul WM, Schmidt MJ, Choudhry MA, Sayeed MM. Lyn- and ERK-mediated vs. Ca2+-mediated neutrophil O responses with thermal injury. Am. J. Physiol. 2002;283:C1469–C1479. doi: 10.1152/ajpcell.00114.2002. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Rana SN, Schwacha MG, Chaudry IH, Choudhry MA. A novel role for IL-18 in corticosterone-mediated intestinal damage in a two-hit rodent model of alcohol intoxication and injury. J. Leukocyte Biol. 2006;80:367–375. doi: 10.1189/jlb.1205745. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin 18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am. J. Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- 31.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J. Leukocyte Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication potentiates neutrophil-mediated intestine tissue damage following burn injury. Shock. 2008;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer I, Rensing H, Florax A, Ulrich C, Pistorius G, Redl H, Bauer M. Expression pattern and regulation of heme oxygenase-1/heat shock protein 32 in human liver cells. Shock. 2003;20:116–122. doi: 10.1097/01.shk.0000075568.93053.fa. [DOI] [PubMed] [Google Scholar]
- 34.Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, Bach FH, Mandrekar P, Szabo G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J. Immunol. 2006;177:2592–2600. doi: 10.4049/jimmunol.177.4.2592. [DOI] [PubMed] [Google Scholar]
- 35.Wagener FA, van Beurden HE, von den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system: a molecular switch in wound healing. Blood. 2003;102:521–528. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 36.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 37.Terry CM, Clikeman JA, Hoidal JR, Callahan KS. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am. J. Physiol. 1998;274:H883–H891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- 38.Yu HP, Choudhry MA, Shimizu T, Hsieh YC, Schwacha MG, Yang S, Chaudry IH. Mechanism of the salutary effects of flutamide on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of estrogen receptor-β-dependent HO-1. J. Leukocyte Biol. 2006;79:277–284. doi: 10.1189/jlb.0705363. [DOI] [PubMed] [Google Scholar]
- 39.Azim AC, Cao H, Gao X, Joo M, Malik AB, van Breemen RB, Sadikot RT, Park G, Christman JW. Regulation of cyclooxygenase-2 expression by small GTPase Rac2 in bone marrow macrophages. Am. J. Physiol. 2007;293:L668–L673. doi: 10.1152/ajplung.00043.2007. [DOI] [PubMed] [Google Scholar]
- 40.Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- 41.Dusi S, Della VB, Grzeskowiak M, Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca2+-depleted human neutrophils. Biochem. J. 1993;290:173–178. doi: 10.1042/bj2900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J. Leukocyte Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 43.Gao XP, Xu N, Sekosan M, Mehta D, Ma SY, Rahman A, Malik AB. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J. Immunol. 2001;167:2895–2901. doi: 10.4049/jimmunol.167.5.2895. [DOI] [PubMed] [Google Scholar]
- 44.Fazal N, Sabeh F, Gamelli RL, Sayeed MM. Elevated expression of p47phox and p67phox proteins in neutrophils from burned rats. Shock. 1997;8:256–260. doi: 10.1097/00024382-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Fazal N, Knaus UG, Sabeh F, Gamelli RL, McNulty JA, Sayeed MM. Enhanced expression of neutrophil NADPH oxidase components in intestine of rats after burn injury. Shock. 1999;12:438–442. doi: 10.1097/00024382-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Walker HL, Mason AD., Jr A standard animal burn. J. Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J. Leukocyte Biol. 2006;79:453–462. doi: 10.1189/jlb.0705369. [DOI] [PubMed] [Google Scholar]
- 48.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukocyte Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 49.Mayadas TN, Cullere X. Neutrophil β2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Babior BM. Phagocytes and oxidative stress. Am. J. Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 51.Benna JE, Dang PM, Gaudry M, Fay M, Morel F, Hakim J, Gougerot-Pocidalo MA. Phosphorylation of the respiratory burst oxidase subunit p67phox during human neutrophil activation: regulation by protein kinase C-dependent and independent pathways. J. Biol. Chem. 1997;272:17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 52.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase Cδ is required for p47phox phosphorylation and translocation in activated human monocytes. J. Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 53.Seldon MP, Silva G, Pejanovic N, Larsen R, Gregoire IP, Filipe J, Anrather J, Soares MP. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-κB RelA phosphorylation at serine 276. J. Immunol. 2007;179:7840–7851. doi: 10.4049/jimmunol.179.11.7840. [DOI] [PubMed] [Google Scholar]
- 54.Silva G, Cunha A, Gregoire IP, Seldon MP, Soares MP. The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 α MAPK isoform. J. Immunol. 2006;177:1894–1903. doi: 10.4049/jimmunol.177.3.1894. [DOI] [PubMed] [Google Scholar]
- 55.Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, Cunha FQ. Heme oxygenase/carbon monoxide-biliverdin pathway down-regulates neutrophil rolling, adhesion and migration in acute inflammation. Br. J. Pharmacol. 2006;149:345–354. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 57.Taille C, El Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J. Biol. Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- 58.Tranter M, Jones WK. Anti-inflammatory effects of HO-1 activity in vascular endothelial cells, commentary on “Carbon monoxide donors or heme oxygenase (HO-1) overexpression blocks interleukin-18-mediated NF-κB-PTEN-dependent human cardiac endothelial cell death.”. Free Radical Biol. Med. 2008;44:261–263. doi: 10.1016/j.freeradbiomed.2007.10.051. [DOI] [PubMed] [Google Scholar]