Abstract
Extracellular matrices and degradable nanofibers are two very promising materials in the field of tissue engineering; however both of these structures face limitations as tissue engineering scaffolds. Extracellular matrices, such as collagen, gelatin, laminin, have excellent biocompatibility and allow cell in growth and survival, but structural weakness makes them difficult to handle and greatly limits their uses. Degradable nanofibers support cell attachment and can provide structural support and directional guidance, but individual degradable nanofibers are fragile and have a tendency to form dense fiber bundles which limited the cell penetration into the spaces between the nanofibers, especially in the case of aligned nanofibers. To overcome these difficulties, degradable loose nanofibers were embedded in protein matrix in an attempt to fabricate a hybrid scaffold with improved properties, such as improved strength, guidance, spacing among nanofibers, etc.. Polycaprolactone (PCL) was used as a model material for degradable nanofibers. Gelatin was employed as a model protein for matrix structure formation. Thin hybrid films (average thickness = 2.78 um) were fabricated by wetting the loose aligned undirectional nanofiber arrays or loose aligned bidirectional nanofiber grids with a gelatin aqueous solution, which also allows for live cell loading into the nanofiber-protein composite if cell are premixed with protein solution or on the surface of the films. Gelatin film alone without nanofiber reinforcement cannot be handled due to the weakness of the thin membrane. Gelatin films with a fiber density as low as 3% v/v were structurally robust enough for handling, and manipulation into complex shapes. Mechanical testing confirmed that the addition of nanofibers enhanced the strength of gelatin films, in both dry and hydrated state. In vitro testing confirmed that nanofiber reinforced films were biocompatible and provided cells with directional guidance. Results demonstrate the promise of gelatin/PCL nanofiber composites as a tissue scaffolding material.
Keywords: nanofiber, extracellular matrix, degradable polymer, gelatin, biaxial mechanical testing
1. Introduction
Many tissue engineering strategies involve the use of scaffolds that supports cell attachment, survival and proliferation, and formation of tissue-like structures. Natural extracellular matrices (ECMs), such as collagen, gelatin, laminin and fibronectin, promote cell attachment, survival, and growth. However, without extra-crosslinking, these materials are structurally very weak after in vitro processing, which greatly limits their potential use for guiding tissue regeneration 1. In addition, ECMs without appropriate alignment may not offer directional guidance to attached cells. On the other hand, degradable electrospun nanofibers support cell attachment, and provide excellent directional guidance to the attached cell 2–5. Like ECMs, individual degradable nanofibers are also very fragile and difficult to handle unless a relatively dense mat or dense bundle of nanofibers is formed. However, densely packed nanofibers provide a barrier for cell penetration inside the nanofiber bundles, especially in the case of aligned nanofibers, and thus preventing the use of 3D structures of aligned nanofibers as effective tissue engineering scaffolds. Interestingly, a combination of ECMs and loose degradable nanofiber arrays may help to address the weakness of each component as a scaffold. Since nanofibers can offer directional cues to the ECMs, and also provide extra structural integrity with nanofiber reinforcement. ECMs can act as a substrate to immobilize individual nanofibers and serve as spacers among nanofibers, thus creating nanofiber arrays with loose nanofiber packing that also provides adequate structural integrity. Since ECMs may degrade very fast in the body, the resulting spaces will allow cells to populate into the space among the nanofibers. To this end, degradable loose nanofibers were embedded in protein matrix in an attempt to fabricate a composite scaffold with improved properties; such as improved strength, guidance, spacing among nanofibers, etc.. The goal of this study was to design a method of fabricating hybrid protein structures by combining degradable nanofibers and protein matrices. The model system tested was the electospun PCL nanofibers embedded into gelatin thin films and the properties of these hybrid structures were evaluated with mechanical testing and in vitro cell culture.
2. Materials and Methods
2.1 Electrospinning
Polycaprolactone (PCL, Mn=80,000, Sigma, St Louis, MO) was dissolved in dichloromethane and dimethylformamide (3:1) (DCM:DMF, Sigma) at a concentration of 18% wt/v. A hydrophobic cyanine dye, 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen, Carlsbad, CA, USA), was added to the polymer solution at concentrations of 0.025 mg/ml to label the fabricated nanofibers. Polymer solution was feed by syringe pump (Medfusion 2010i; Smiths Medial Inc., Carlsbad, CA, USA) at a rate of 0.015 ml/min through a 23 G blunt tipped needle. A voltage of 8 KV was applied to the needle tip with a high voltage power supply (ES40P-10W, Gamma High Voltage Research, Ormond Beach, FL, USA). The needle tip was held at 10 cm above a custom built collecting device designed in our lab to collect large loose arrays of parallel nanofibers across a rectangular rack 6. This device utilized parallel mobile tracks that pulled aligned electrospun fiber arrays into a secondary collection area at a vertical speed of 16.9 mm/s.
2.2 Composite film fabrication
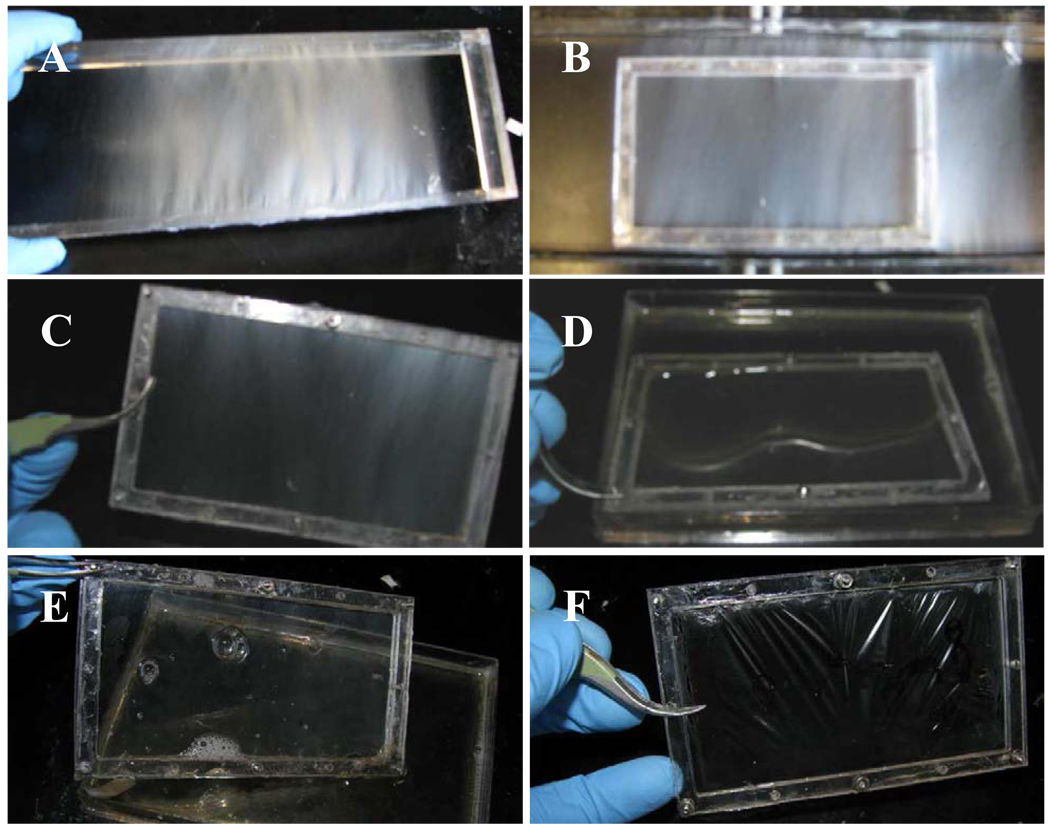
Parallel electrospun nanofibers were collected continuously across a rectangular rack (Figure 1A) for 3 or 6 minutes. This fiber array was then transferred to a two-piece polycarbonate frame that was held together with screws (Figure 1B and 1C). Composite films were fabricated with two different fiber orientations: unidirectional straight fibers (Figure 2A, 2C, and 2E) and bi-directional/criss-crossed fibers (Figure 2B, 2D, and 2F). During the fabrication of unidirectional composites, nanofibers were collected for 6 minutes first and transferred to a polycarbonate frame. For fabrication of bi-directional composites, nanofibers were collected twice for 3 minutes at two different directions and then transferred to the polycarbonate frame. The frames holding the nanofiber arrays were then coated in a 1 wt% gelatin aqueous solution (Bovine skin type B, Sigma, St Louis, MO, USA) and allowed drying. Some of the composite films were cross-linked with 1-Ethyl-3-(3-dimethylaminopropyl) carbodimide (EDC, TCI America). All the films were sterilized with 75% ethanol.
Fig. 1.
Hybrid film fabrication: (a) PCL nanofibers on collecting rack (b) nanofiber array is transferred to smaller frame (c) nanofiber array inside of frame (d) nanofiber array is wetted in gelatin solution (e) nanofiber array is removed from gelatin solution and (f) dried hybrid film.
Fig. 2.
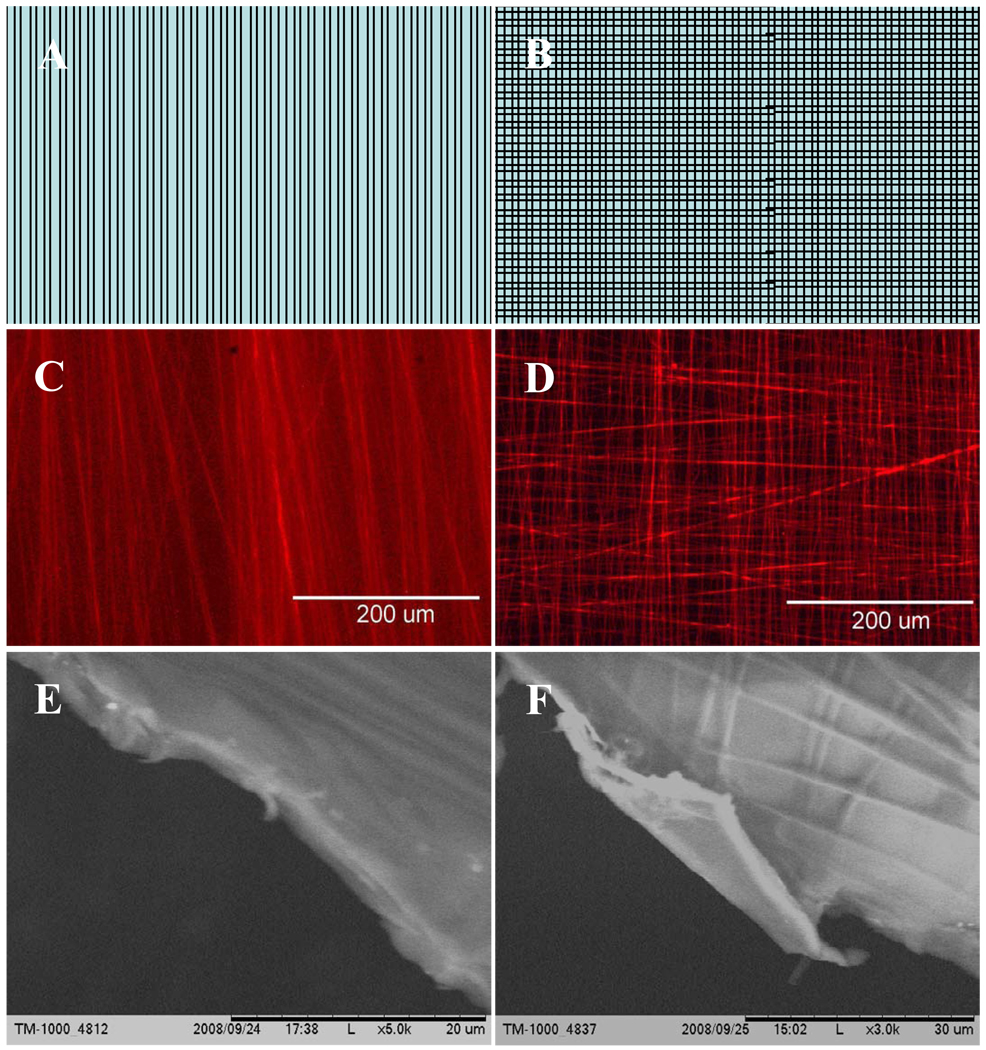
Hybrid gelatin/PCL nanofiber membranes: (A) Schematic of unidirectional aligned nanofiber reinforced protein membrane. (B) Schematic of criss-cross nanofiber reinforced protein membrane. (C) Fluorescent image of gelatin/PCL membrane. PCL fibers dyed with DiI in a unidirectional pattern. (D) Fluorescent image of gelatin/PCL membrane. PCL fibers dyed with DiI in a bi-directional pattern. (E) SEM image of gelatin/PCL membrane. PCL fibers are in a unidirectional pattern and (F) PCL fibers are in a bi-directional pattern.
2.3 Visualization
All SEM images were taken at 1000 to 5,000 times magnification using a scanning electron microscope (SEM, Hitachi TM-1000). Samples were not sputter coated. In order to visualize the embedded fibers, the composite film edges were selected for taking pictures. All fluorescent and light microscope images were taken using a Nikon Eclipse TE2000-S microscope with an EXFO X-cite 120 fluorescence illumination system, and a Q-Imaging Micropublisher 3.3 RTV camera with Q-Capture software. ImagePro Plus 4.0 (Media Cybernetics Inc., Bethesda, MD, USA) was used to measure the average thickness of composite film samples using SEM images of the cross sections.
2.4 Mechanical testing
Mechanical Properties of the hybrid PCL nanofiber reinforce gelatin films were characterized using both biaxial tensile testing and uniaxial tensile testing.
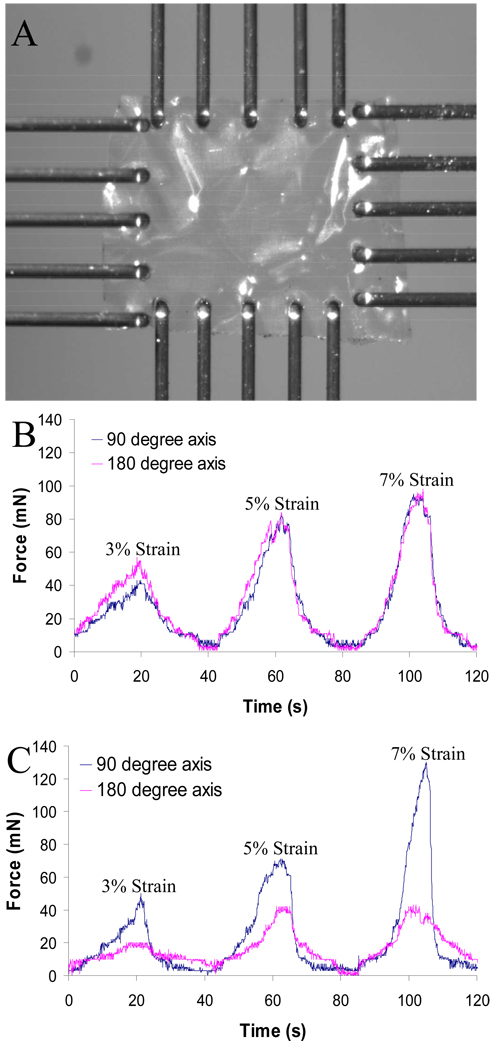
Biaxial tensile testing was performed using a BioTester5000 Biaxial Test System (CellScale division of Waterloo Instruments Inc., Waterloo, Ontario, Canada) to examine the mechanical properties of the membrane samples in two directions. In brief, gelatin-PCL nanofiber based thin film (5 mm × 5 mm) was attached with 20 tungsten finger-grips (300µm diameter) five on each side and stretched in the nanofiber direction or perpendicular to the nanofiber direction in dry condition (Figure 3A). Each set of five finger grips was attached to an actuator, and load cells measured the force along the two pulling axes. The load cell used was 2500 mN. The samples were sequentially tested with 3% strain, 5% stain and 7% strain and each stain rate was done in 20 seconds.
Fig. 3.
Biaxial tensile testing on hybrid gelatin membrane embedded with PCL degradable nanofiber arrays. (A) Example of a gelatin-PCL membrane mounted in the biaxial tester. (B) Gelatin membrane embedded with criss-crossed PCL fibers were tested at 3% strain, 5% strain and 7% strain condition. (C) Gelatin membrane embedded with unidirectional aligned PCL fibers were tested at 3% strain, 5% strain and 7% strain condition.
Uniaxial tensile testing was performed in a Shimadzu EZ Graph tensile tester (Nakagyo-ku, Kyoto, Japan) with Trapezium 2.32 software for data acquisition to examine the strength and stiffness of the thin films. SEM tape was adhered to both ends of 20 mm × 5 mm rectangular strips of composite film and these were attached to the tensile tester’s grips leaving an initial length of 10 mm of sample between the grips prior to elongation. The purpose of the tape was to limit stress concentrations on the thin films and to prevent slippage from the grips. A total of 20 samples were fabricated for mechanical testing, with 5 samples for each of 4 groups: (1) Unidirectional (2) Bi-directional (3) Unidirectional + EDC and (4) Bi-directional + EDC. Strips were cut from the samples in the direction of the aligned fibers (90°) and perpendicular to the direction of the aligned fibers (180°). Strips cut from each sample were tensile tested in both directions under both wet and dry conditions. Under wet conditions the samples were secured in the grips of the tensile tester and then hydrated. The elastic modulus, maximum stress, and elongation were measured from the stress strain curves. Statistical analysis was done using SPSS 15.0 software (SPSS Inc. Chicago, Illinois, USA). All p-values (p) were calculated using the Mann-Whitney test.
2.6 In vitro cell culture study
Circular pieces of unidirectional and bi-directional EDC crosslinked hybrid film with a diameter of 19 mm were cut and placed at the bottom of 12 well cell culture plates. Half-inch sections of polycarbonate rod (Outer Diameter: 19 mm, Inner Diameter: 13 mm) were place on top of the films to hold them down. Sample wells were sterilized with 75% ethanol and 80,000 3T3 fibroblast cells were seeded on each sample and allowed to incubate for 3 days. Cells were fixed in 4% paraformaldehyde and stained with AlexaFluor 488 Phalloidin (Invitrogen) for the actin filament inside the cells and 4′-6-Diamidino-2-phenylindole (DAPI, Invitrogen) for the cell nuclei.
3. Results
3.1 Hybrid Film Fabrication
Thin gelatin/PCL degradable nanofiber hybrid films were fabricated using the techniques described above. The surface tension of the gelatin solution caused the formation of a thin film over both unidirectional and bi-directional nanofiber arrays. A thin hybrid film remained intact after drying. Fluorescent and SEM microscopy confirmed that the nanofibers remained embedded in the gelatin films in their original configurations (Figure 2 C–F). The average thickness of the films was 2.73 um with a standard deviation of 0.28. It was estimated from previously collected data that PCL fibers were approximately 585 nm in diameter and collected at a rate of approximately 0.05 fibers/um/min (data not shown), therefore the estimated volume fraction of PCL nanofibers embedded in the composite films was around 3% v/v. The volume fraction and the size of the nanofibers can be readily adjusted by controlling the nanofiber fabrication conditions and collecting time. Fabricated films demonstrated the strength and structural integrity for manipulation into complex shapes.
3.2 Mechanical Testing
Results for biaxial testing in dry conditions can be observed in Figure 3A. Hybrid membranes reinforced with PCL nanofibers in criss-cross pattern possessed similar mechanical properties in both 90° and 180° directions (Figure 3B). In contrast, membranes with PCL nanofibers in a unidirectional pattern had significant differences in strength in different directions (Figure 3C).
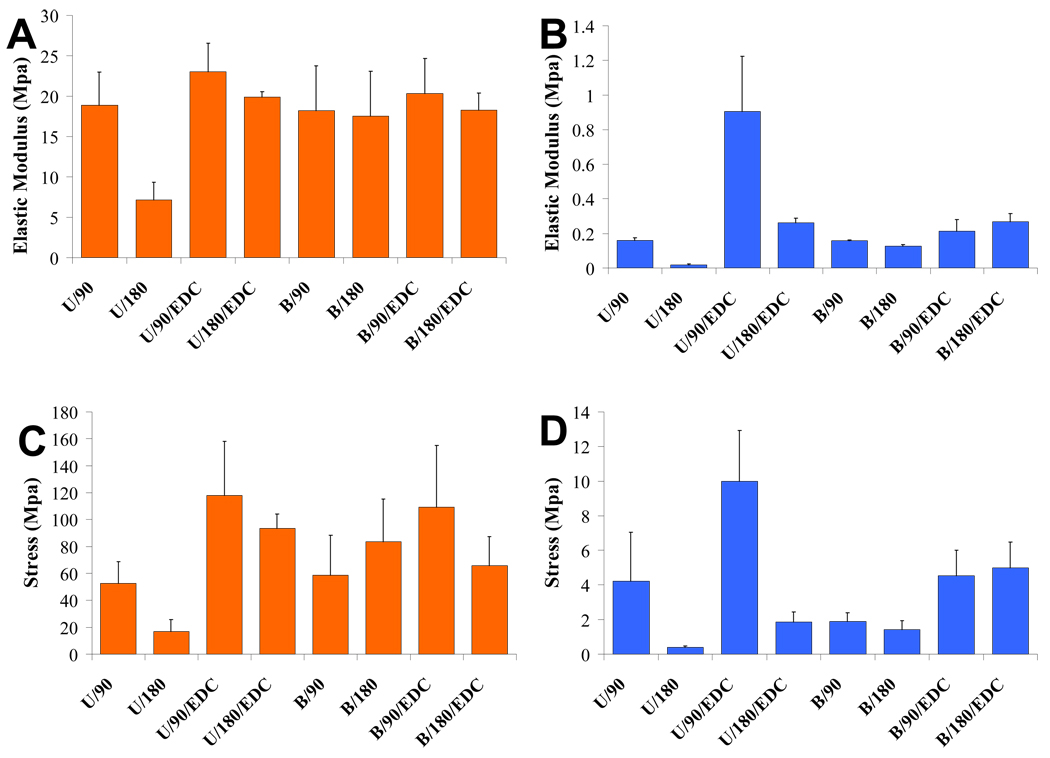
Unaxial tensile testing was done in the 90 and 180° planes under both wet and dry conditions for samples that were uncrosslinked and crosslinked with EDC. The elastic modulus and failure strength for each set of conditions is displayed in Figure 4. Fiber orientation had significant effects on the strength of composite films for unidirectional samples with and without EDC in the dry and hydrated state. The elastic modulus of unidirectional hybrid films were 392% (p=0.05), 231% (p=0.02), and 164%(p=0.03) higher in the direction of the aligned fibers for hydrated, hydrated+ EDC, and dry films respectively. The failure strength of unidirectional hybrid films were 1265% (p=0.05), 437% (p=0.03), and 212% (p=0.02) higher in the direction of the aligned fibers for hydrated, hydrated+ EDC, and dry films respectively. Composite films cross-linked with EDC were stronger than films without EDC cross-linking over all fiber orientations (p<0.05). The elastic modulus of cross-linked films were 36% (p=0.02) higher in the dehydrated state, and 306% (p<0.01) higher when hydrated. The failure strength of cross-linked films were 86% (p<0.01) higher in the dehydrated state, and 139% (p=0.01) higher when hydrated.
Fig. 4.
Elastic modulus of samples tested under (A) dry and (B) wet conditions and failure stress of samples tested under (C) dry and (D) wet conditions: U=unidirectional sample, B=bi-directional sample, 90 = force applied in the direction of aligned fibers, 180 = force applied perpendicular to aligned fibers, EDC=cross-linked sample.
3.3 In vitro cell culture study
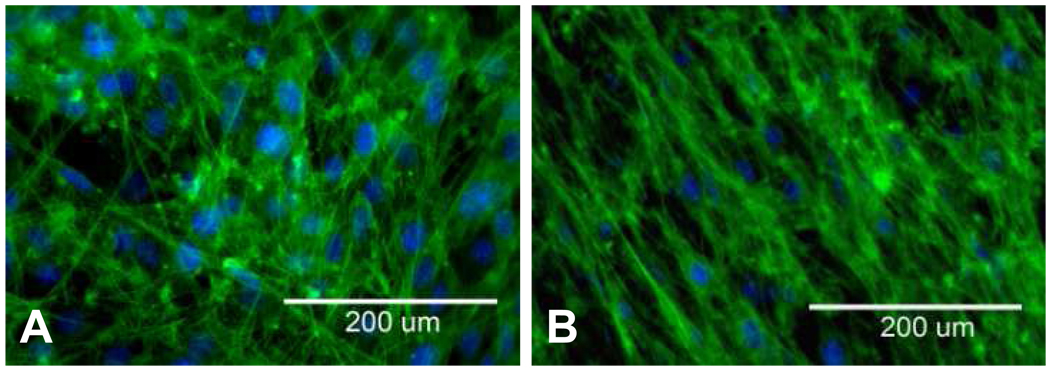
PCL nanofiber/gelatin hybrid thin films maintained their integrity under cell culture conditions and promoted the attachment of 3T3 fibroblast cells. Since the membrane is very thin, cells were able to sense the embedded PCL nanofibers. Fibroblast cells on composite films containing bidirectional nanofibers adopted a somewhat random orientation, although it appeared that some cells may have oriented in the crisscrossed pattern of the fibers (Figure 5A). Fibroblast cells on composite films containing unidirectional nanofibers however, clearly elongated in the direction of the aligned nanofibers (Figure 5B).
Fig. 5.
3T3 fibroblasts grown on gelatin/PCL nanofiber composites with (A) bi-directional and (B) unidirectional fiber orientations
4. Discussion
Extracellular matrix proteins, such as collagen, gelatin, laminin, fibronectin, ect. have favorable properties as tissue engineering scaffolds. Because most of these materials are naturally found in the body, they promote cell attachment, survival and growth and are fully biodegradable. The concern about the immunogenicity can be overcome by using human recombinant ECM molecules. Unfortunately natural ECMs are mechanically weak after in vitro manipulation, which limits their practicality as tissue engineering scaffolds. Structural weakness not only limits these proteins as coating materials or applications in non-load bearing environment, but it also makes them very difficult to handle and manipulate in the fabrication, processing, and implantation steps.
Degradable polymer nanofibers exhibit many properties that make it an excellent substrate to add to protein matrices to construct hybrid structures with improved structural properties. Nanofibers mimic the size and structure of the natural guidance cues and therefore provide physical guidance for tissue regeneration, which is critical for regenerating aligned tissues such as nerve, tendon/ligament, or muscle tissue. Nanofibers can provide mechanical strength due to their high surface area to volume ratio and act as a barrier to crack propagation because of their long continuous length.
The combination of polymer nanofibers with gelatin in a hybrid membrane expands the versatility of both of these promising materials or structures. The addition of as low as 3% v/v PCL nanofibers improved the strength of gelatin film in the direction of unidirectional aligned fibers when compared to the strength in the direction perpendicular to the fibers, especially for hydrated samples. It could be hypothesized that addition of a greater percentage of nanofibers could strengthen protein matrices much further. And furthermore, chemical modification of the nanofiber surface to promote the covalent bonding between nanofibers and protein matrix will further improve the mechanical properties of the composite structures. These hybrid structures also address one of the greatest limitations of nanofibers as a scaffolding material. Nanofibers are very difficult to handle, process and implant into the body, and in addition, the tendency of forming dense nanofiber mats greatly limits cell population inside the nanofiber structure. The protein matrix in hybrid structures allows the fabrication of scaffolds containing loose nanofiber arrays with sufficient space for cell growth inside the nanofiber bundles, which can provide directional guidance without obstructing cell penetration.
The fabrication techniques used to manufacture these composite films is readily adaptable for scale up and use in a Good Manufacturing Practices (GMP) fabrication facility for human tissue regeneration applications. Because the films were fabricated in atmospheric conditions there is no need to remove them from any substrate and they can be easily handled and manipulated as shown in Figure 4. Complex two and three-dimensional structures assembled from dry composite films become gelatinous scaffolds once again upon rehydration, and thus this material may be a versatile tool in the fabrication of tissue engineering scaffolds.
5. Conclusions
A method for fabricating nanofiber and protein hybrid thin membranes was developed. Hybrid membranes could be fabricated with a relatively consistent thickness between 2 and 4 um. The hybrid films were structurally robust enough for handling and manipulation into complex structures. Hybrid membranes could be fabricated with unidirectional and bi-directional embedded fibers, and cross-linking with EDC strengthened the films significantly. Increased strength of films in the direction of unidirectional nanofibers suggests that an embedded volume fraction of PCL nanofibers as low as 3% can improve the mechanical properties of gelatin significantly. In vitro culture of fibroblasts confirmed that these composite films supported cell attachment, survival, and growth, and that embedded nanofibers could provide directional guidance to cells growing on the hybrid membranes. It was shown that nanofibers and protein matrices can be easily combined into a composite structure that has improved properties as a tissue engineering scaffold when compared to either component alone. This versatile technique can be used to develop tissue scaffolds that are biodegradable and biocompatible; promote cell attachment, survival, and growth; provide directional guidance; and possess the structural stability required for practical handling. Studies in progress are evaluating direct cell loading into the hybrid membrane and the use of nanofiber reinforced protein structures for engineering blood vessel, skeletal muscle, tendon/ligament and nervous tissue.
Acknowledgments
This work was made possible by the NIH/NINDS USA (R01 NS050243) and Early Career Translational Research Awards in Biomedical Engineering from Wallace H. Coulter Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang X, Li X, Yost MJ. Microtensile testing of collagen fibril for cardiovascular tissue engineering. J Biomed Mater Res A. 2005;74(2):263–268. doi: 10.1002/jbm.a.30387. [DOI] [PubMed] [Google Scholar]
- 2.Chua KN, Chai C, Lee PC, Tang YN, Ramakrishna S, Leong KW, Mao HQ. Surface-aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials. 2006;27(36):6043–6051. doi: 10.1016/j.biomaterials.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24(2):426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 4.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 6.Beachley V, Wen X. Three Dimensional Aligned Individual Nano-fibers For Neural Tissue Engineering. Chicago, IL, USA: 2007. [Google Scholar]
- 7.Huang Z, Kotaki, Ramakrishna A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology. 2003;63:2223–2253. [Google Scholar]
- 8.Li D, Wang Yuliang, Younan Xia. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Letters. 2003;3(8):1167–1171. [Google Scholar]