Abstract
This year marks the 50th anniversary of the discovery of the laser. The development of lasers for medical use, which became known as low-level laser therapy (LLLT) or photobiomodulation, followed in 1967. In recent years, LLLT has become an increasingly mainstream modality, especially in the areas of physical medicine and rehabilitation. At first used mainly for wound healing and pain relief, the medical applications of LLLT have broadened to include diseases such as stroke, myocardial infarction, and degenerative or traumatic brain disorders. This review will cover the mechanisms of LLLT that operate both on a cellular and a tissue level. Mitochondria are thought to be the principal photoreceptors, and increased adenosine triphosphate, reactive oxygen species, intracellular calcium, and release of nitric oxide are the initial events. Activation of transcription factors then leads to expression of many protective, anti-apoptotic, anti-oxidant, and pro-proliferation gene products. Animal studies and human clinical trials of LLLT for indications with relevance to neurology, such as stroke, traumatic brain injury, degenerative brain disease, spinal cord injury, and peripheral nerve regeneration, will be covered.
INTRODUCTION
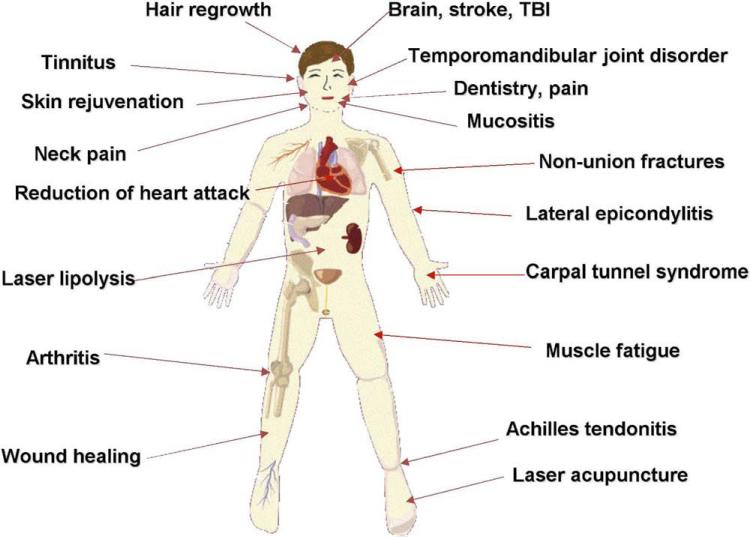
It was not long after the discovery of the first lasers (the ruby laser in 1960 and the helium-neon [HeNe] laser in 1961) that they began to be used in medical applications. In 1967, Endre Mester in Hungary noticed the ability of the HeNe laser to increase hair growth [1] and stimulate wound healing in mice [2], and, shortly afterward, he began to use lasers to treat patients with nonhealing skin ulcers [3]. Since those early days, the use of low-power lasers (as opposed to high-power lasers that can destroy tissue by a photothermal effect) has steadily increased in diverse areas of medical practice that require healing, prevention of tissue death, pain relief, reduction of inflammation, and regenerative medicine. Some of the different organ systems, diseases, and injuries that have been effectively treated with low-level laser therapy (LLLT) are schematically shown in Figure 1.
Figure 1.
Diagram of the various medical applications of low-level light therapy.
Nevertheless, this modality, which is variously known as LLLT or photobiomodulation, remains controversial. The reasons for this lack of general acceptance among both the medical community and the general public at large are 2-fold. First, widespread uncertainty and confusion exists about the mechanisms of action of LLLT at the molecular, cellular, and tissue levels. Second, a large number of parameters (eg, wavelength, fluence, irradiance, treatment timing and repetition, pulsing, and polarization) can be chosen in designing LLLT protocols. Furthermore, a biphasic dose response exists in laser therapy [4], which describes the observation that increasing the overall “dose” of the laser either by increasing the power density or by increasing the illumination time may have a counter-productive effect compared with the benefit obtained with lower doses. Taken together, these considerations may explain why a number of negative studies have been published; however, this should not be taken to imply that LLLT in general does not work but rather that the laser parameters used in those particular studies were ineffective.
In recent years, the development of light-emitting diodes (LEDs) as alternative light sources for LLLT has added to the confusion. These devices produce light with wavelengths similar to those of lasers, but they have broader output peaks (ie, they are less monochromatic) and lack the coherence that is a particular feature of laser light. LEDs have the advantage of being significantly less expensive than laser diodes (by a factor of approximately 100 on a milliwatt basis), and the LLLT community is engaged in a vigorous ongoing debate about their respective benefits.
This review covers the mechanisms that are thought to operate at molecular and cellular levels in LLLT. Many of the most compelling applications of LLLT are in the field of neurology (both central and peripheral). Many serious brain diseases and injuries can be successfully treated with noninvasive transcranial laser therapy. Furthermore, in the peripheral nervous system, LLLT can be used effectively for nerve regeneration and pain relief.
CELLULAR AND MOLECULAR MECHANISMS OF LLLT
LLLT uses low-powered laser light in the range of 1-1000 mW, at wavelengths from 632-1064 nm, to stimulate a biological response. These lasers emit no heat, sound, or vibration. Instead of generating a thermal effect, LLLT acts by inducing a photochemical reaction in the cell, a process referred to as biostimulation or photobiomodulation. Photo-biology works on the principle that, when light hits certain molecules called chromophores, the photon energy causes electrons to be excited and jump from low-energy orbits to higher-energy orbits. In nature, this stored energy can be used by the system to perform various cellular tasks, such as photosynthesis and photomorphogenesis. Numerous examples of chromophores exist in nature, such as chlorophyll in plants, bacteriochlorophyll in blue-green algae, flavoproteins, and hemoglobin found in red blood cells. The respective colors of chromophores are determined by the part of the spectrum of light they absorb: chlorophyll is green, flavoprotein is yellow, and hemoglobin is red [5].
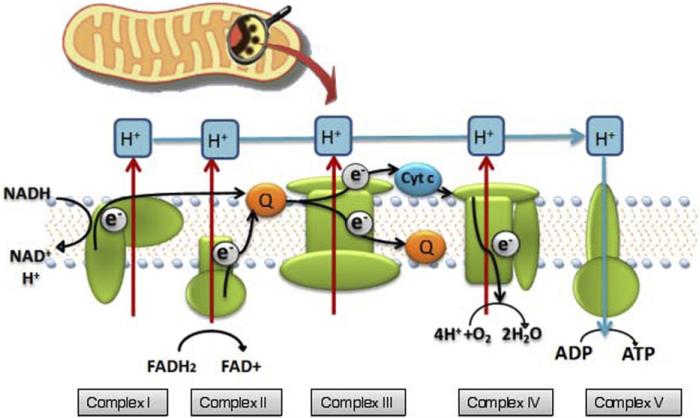
Mitochondria are considered the power generators of the eukaryotic cell, converting oxygen and nutrients through the oxidative phosphorylation process and electron transport chain into adenosine triphosphate (ATP), as shown in Figure 2. The basic idea behind cellular respiration is that high-energy electrons are passed from electron carriers, such as reduced nicotinamide adenine dinucleotide (NADH) and the reduced form of flavin adenine dinucleotide (FADH2), through a series of transmembrane complexes (including cytochrome c oxidase [CCO]) to the final electron acceptor, generating a proton gradient. The gradient is used by FOF1 ATP synthase to produce ATP. Various in vitro experiments, such as those that use rat liver isolates, found that cellular respiration was upregulated when mitochondria were exposed to an HeNe laser or other forms of illumination. Laser irradiation caused an increase in mitochondrial products (such as ATP [6], NADH, protein, ribonucleic acid [RNA] [7]) and a reciprocal augmentation in oxygen consumption. A similar effect is produced when tissue that contains mitochondria is exposed to low-level radiation. Visible and near-infrared (NIR) light is absorbed by the organelle, and an upregulation of cellular respiration is observed [8].
Figure 2.
Illustration of mitochondrion, as well as of the electron transport chain and oxidative metabolism.
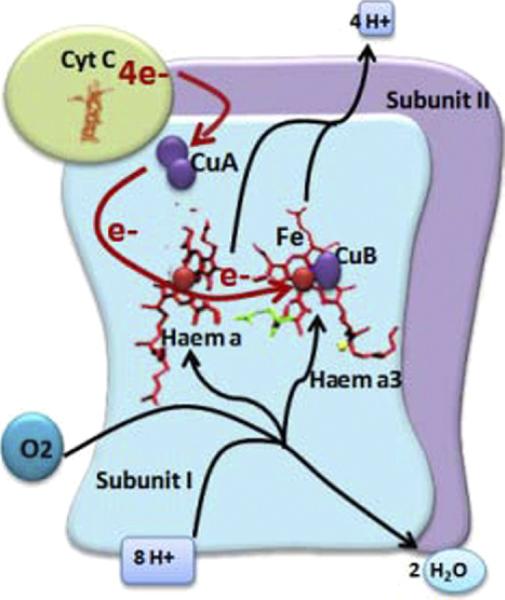
Once it was observed that LLLT's mechanism of action is at the level of the mitochondria, it remained to be determined what specific structure within the mitochondria acted as the chromophore. Four membrane-bound complexes have been identified in mitochondria, each constituting an extremely complex transmembrane structure embedded in the inner membrane. Complex IV, also known as CCO, is a large transmembrane protein complex found in mitochondria, which is a component of the respiratory electron transport chain (Figure 3). CCO appears to absorb the same spectrum of light as that observed for the action spectra for the biological response to light in the NIR range. Thus it is reasonable to assume that CCO acts as an important chromophore in LLLT [9]. CCO consists of 2 copper centers and 2 heme-iron centers that are capable of absorbing light over a wide range, including NIR.
Figure 3.
Complex IV (cytochrome c oxidase) is the principal chromophore involved in low-level light therapy. It has 2 copper centers and 2 heme prosthetic groups. Cytochrome c is oxidized and oxygen is reduced to water during respiration.
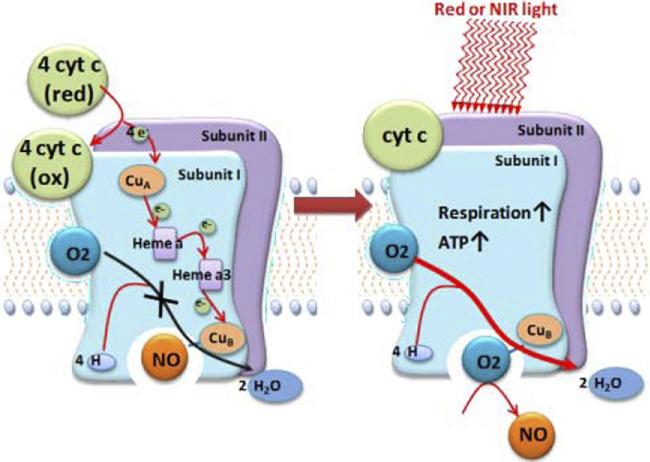
The next reasonable question to consider is: What action does CCO modulate once it absorbs the energy from light? On the cellular level, LLLT may cause photodissociation of nitric oxide (NO) from CCO. In a stressed cell, NO produced by mitochondrial NO synthase displaces oxygen from CCO, which results in a downregulation of cellular respiration and a subsequent decrease in the production of energy-storing compounds, such as ATP. By dissociating NO from CCO, LLLT prevents the displacement of oxygen from CCO and thereby promotes unhindered cellular respiration [10] (see Figure 4). Increased CCO enzyme activity can be measured [11]; increased ATP production [12] and increased electron transport [13] also have been reported. The basic idea behind cellular respiration is that high-energy electrons are passed from electron carriers, such as NADH and FADH2, through a series of transmembrane complexes (including CCO) to the final electron acceptor. Increased cellular ATP produced by LLLT may contribute to the positive effects, both by raising cellular energy levels and by upregulating the cyclic AMP molecule (biochemically formed from ATP) that is involved in many signaling pathways.
Figure 4.
Nitric oxide can bind to copper (or heme) centers in cytochrome c oxidase and inhibit respiration. The nitric oxide may be photodissociated by absorption of red or near infrared light, allowing oxygen to return and sharply increasing respiration and adenosine triphosphate formation.
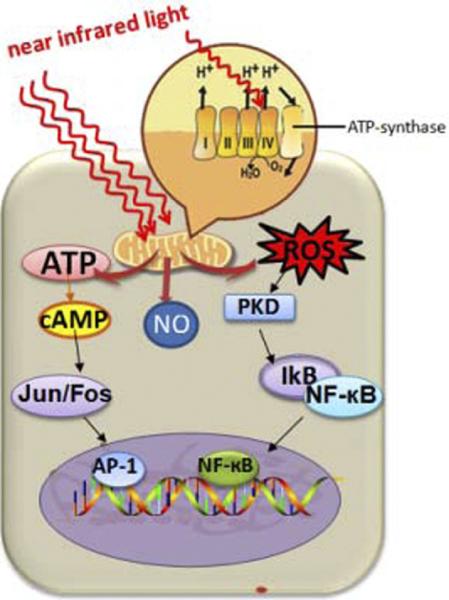
Oxygen acts as the final electron acceptor and is, in the process, converted to water. Part of the oxygen that is metabolized produces reactive oxygen species (ROS) as a natural by-product. ROS (eg, superoxide and hydrogen peroxide) are chemically active molecules that play an important role in cell signaling, regulation of cell cycle progression, enzyme activation, and nucleic acid and protein synthesis [14]. Because LLLT promotes the metabolism of oxygen, it also acts to increase ROS production. In turn, ROS activates certain redox-sensitive transcription factors such as nuclear factor-κB [NF-κB] and activator protein 1, which leads to the upregulation of various stimulatory and protective genes. The ultimate effect of LLLT is likely to be produced by transcription factor activation, which modulates the host's downstream cellular and tissue responses (see Figure 5).
Figure 5.
Diagram that illustrates the mechanism of low-level light therapy (LLLT) on the cellular and molecular level. Near infrared light, absorbed by the mitochondria, causes upregulation of the cellular respiratory chain. A host of downstream cellular responses involving nitric oxide, reactive oxygen species, and cyclic adenosine monophosphate ensues, which ultimately dictates LLLT effects.
Almost certainly, other mechanisms through which LLLT produces its effects are at play in addition to the one just described. For example, NO is a potent vasodilator via its effect on cyclic guanine monophosphate production. Cyclic guanine monophosphate is also involved in many other signaling pathways. LLLT may cause the photodissociation of NO from intracellular stores (ie, nitrosylated forms of both hemoglobin and myoglobin, in addition to CCO) [15]. LLLT promotes the synthesis of deoxyribonucleic acid (DNA) and RNA [16] and increases the production of proteins [17]. It also modulates enzymatic activity [18], affects intracellular and extracellular pH [17,18], and accelerates cell metabolism [18,19]. The expression of multiple genes related to cellular proliferation, migration, and the production of cytokines and growth factors also have been shown to be stimulated by low-level light [20].
Light is a powerful force and has a myriad of effects. The specific mechanisms of action may vary among various clinical applications of LLL and will be discussed in the respective sections below. Furthermore, in spite of a great number of studies that explored how LLLT works, the exact mechanism of action remains to be fully elucidated.
STROKE
Transcranial LLLT (808 nm) has significantly improved recovery after ischemic stroke in rats when they received one treatment 24 hours after sustaining a stroke [21,22]. Stroke was induced in rats by 2 different methods: (1) permanent occlusion of the middle cerebral artery through a craniotomy or (2) insertion of a filament. The laser was used transcranially on the exposed (shaved skin) skull by placing the tip of the 4-mm diameter fiber optic onto the skin at 2 locations on the head (3 mm dorsal to the eye and 2 mm anterior to the ear) on the contralateral hemisphere to the stroke. These locations had been determined from prior measurements to be sufficient to illuminate 1 brain hemisphere as a result of dispersion of the laser beam by the skin and the skull. Results of previous studies had shown that LLLT of the contralateral, or both hemispheres, demonstrated no difference in functional outcome [23]. An NIR gallium arsenic diode laser was used transcranially to illuminate the hemisphere contralateral to the stroke at a power density of 7.5 mW/cm2 to the brain tissue [22]. In both models of stroke, the neurologic deficits at 3 weeks after stroke were significantly reduced (by 32%) (P < .01) in the laser-treated rats compared with control subjects.
In this study, the number of newly formed neuronal cells, assessed by double immunoreactivity to bromodeoxyuridine and tubulin isotype III, as well as migrating cells (double Cortin immunoreactivity), was significantly elevated in the subventricular zone of the hemisphere ipsilateral to the induction of stroke when treated by LLLT [21,22]. No significant difference in the stroke lesion area was found between control and laser-irradiated rats. The researchers suggested that an underlying mechanism for the functional benefit after LLLT in this study was possible induction of neurogenesis. Results of other studies also suggested that, because improvement in neurologic outcome may not be evident for 2-4 weeks in the poststroke rat model, delayed benefits may in part be due to induction of neurogenesis and migration of neurons [24,25]. In addition, transcranial LLLT may prevent apoptosis and improve outcomes by exerting a neuroprotective effect, although these exact mechanisms are poorly understood [26].
Other studies in rat and rabbit models also have observed that transcranial LLLT improves functional outcome after stroke [25,27,28]. A recent rabbit study combined transcranial LLLT with thrombolytic therapy by using tissue plasminogen activator, with no increase in bleeding and good safety [29].
In the aforementioned studies, it has long been hypothesized that increased mitochondrial function (ie, increased ATP production) in brain cells irradiated with NIR LLLT was one of the major mechanisms involved with the beneficial behavioral effects observed after LLLT treatment. A recent animal study with rabbits has shown a direct relationship between the level of cortical fluence (energy density) delivered (in J/cm2) and cortical ATP content in embolized rabbits [30]. Five minutes after embolization (right carotid), the rabbits were exposed to 2 minutes of NIR transcranial LLLT with use of an 808-nm laser source (continuous wave [CW] or pulsed wave [PW] at 100 Hz or at 1000 Hz on the skin surface, posterior to bregma at midline). Three hours after embolization, the cerebral cortex was excised and processed for measurement of ATP content. Embolization decreased cortical ATP content in ischemic cortex by 45% compared with naive rabbits.A linear relationship up to 4.5 J/cm2 in fluence delivered, was observed for the relationship between cortical fluence (in J/cm2) verus percent increase in cortical ATP content (over sham-treated embolized rabbits). This linear relationship was observed with a power density of 7.5 mW/cm2 CW (0.9 J/cm2), where an increase of 41% in cortical ATP was observed; and with a power density of 37.5 mW/cm2 PW (100 Hz, 4.5 J/cm2), where an increase of 157% in cortical ATP was observed. An increase in cortical ATP of 221% was observed with fluence of 31.5 J/cm2, delivered with a power density of 262.5 mW/cm2 PW, 1000 Hz. This suggests that a near-plateau effect was present regarding the fluence level delivered above 4.5 J/cm2. It was surprising, however, that the increased cortical ATP levels of 157% and 221%, were higher than those measured in naive rabbits that had never suffered stroke. Because the authors observed that the PW modes (100 Hz and 1000 Hz) were more effective than the CW mode to increase cortical ATP, they hypothesized that in future stroke studies in animals and in humans, even greater improvement in clinical rating scores might be achieved by optimizing the method of NIR transcranial LLLT delivery, including the length of treatment and the mode of treatment (PW).
Transcranial LLLT has been shown to significantly improve outcome in acute human stroke patients when applied approximately 18 hours after the stroke occurs over the entire surface of the head (20 points in the 10/20 electroencephalographic system), regardless of the stroke location [31]. Only one LLLT treatment was administered, and, 5 days later, significantly greater improvement was found in the real-treated group but not in the sham-treated group (P < .05, National Institutes of Health Stroke Severity Scale). This significantly greater improvement was still present 90 days after –the stroke occurred, at which time 70% of the patients treated with real LLLT had a successful outcome compared with only 51% of control subjects. An NIR (808 nm) laser was used, which delivered a fluence of 0.9 J/cm2 over the entire surface (2 minutes per each of the 20 points; power density of 7.5 mW/cm2).
In a second, similar study with the same transcranial LLLT protocol, an additional 658 acute stroke patients were randomly assigned to receive real or sham treatments of transcranial LLLT. Similar significant beneficial results (P < .04) were observed for the patients who had a moderate or moderate to severe stroke (n = 434) and received the real laser protocol but not for the patients who had a severe stroke [32]. When all 656 cases were included in the data analysis (including the severe stroke cases), no significant real versus sham LLLT effect was seen. When data for both stroke studies were pooled (n = 778 [120 plus 658]) [31,32], a highly significant beneficial effect was seen for the real transcranial LLLT group (P = .003) compared with those who received the sham laser treatment [33].
Lampl et al [31] wrote that “Although the mechanism of action of infrared laser therapy for stroke is not completely understood . . . infrared laser therapy is a physical process that can produce biochemical changes at the tissue level. The putative mechanism . . . involves stimulation of ATP formation by mitochondria and may also involve prevention of apoptosis in the ischemic penumbra and enhancement of neurorecovery mechanisms.”
To date, no studies have been conducted to examine transcranial LLLT treatment of chronic stroke patients. Naeser et al [34] studied the application of LLLT-laser acupuncture (instead of needles) to stimulate acupuncture points on the body in chronic stroke patients with paralysis. Seven stroke patients (range, 48-71 years; 5 men) were treated, 5 of whom had single left hemisphere stroke, and 2 of whom had single right hemisphere stroke. Five patients were treated for hemiplegia, including severely reduced or no voluntary isolated finger movement, and 2 patients had hand paresis only. Six of the 7 patients received laser acupuncture during the chronic phase after the stroke had occurred (10 months to 6.5 years after stroke onset), clearly beyond the spontaneous recovery phase, which is considered to be up to 6 months after the stroke occurs [35,36]. The patients served as their own controls; no sham LLLT was administered. One patient (who had hand paresis) received LLLT during the acute phase after the stroke occurred (1 month after the stroke occurred). The patients did not receive any physical therapy or occupational therapy treatments while participating in this study.
A 20-mW gallium aluminum arsenide (780 nm) NIR CW laser with a 1-mm-diameter aperture was used (Unilaser, Copenhagen, Denmark). (At the time of this study, more powerful red or NIR lasers were not yet available.) Treatment consisted of stimulation of shallow acupuncture points (located on the hands and face) for 20 seconds per point (51 J/cm2). Deeper acupuncture points (located on the arms and legs) were treated for 40 seconds per point (103 J/cm2). Acupuncture points were treated on both the paralyzed side (arm, leg, and/or face) and on the nonparalyzed side by using primarily acupuncture meridians of the large intestine, triple warmer, gall bladder, liver, small intestine, and stomach [34]. The patients were treated 2-3 times per week for 3-4 months. They received a total of 20, 40, or 60 treatments (based on patient availability and transportation). Within a few days before the first treatment and a few days after the last treatment, physical therapy and/or occupational therapy testing was performed by therapists blinded to the acupuncture treatment program to which the patient had been assigned: LLLT, real or sham needle, or no acupuncture. Overall, 5 of 7 of the patients (71.4%) showed improvement.
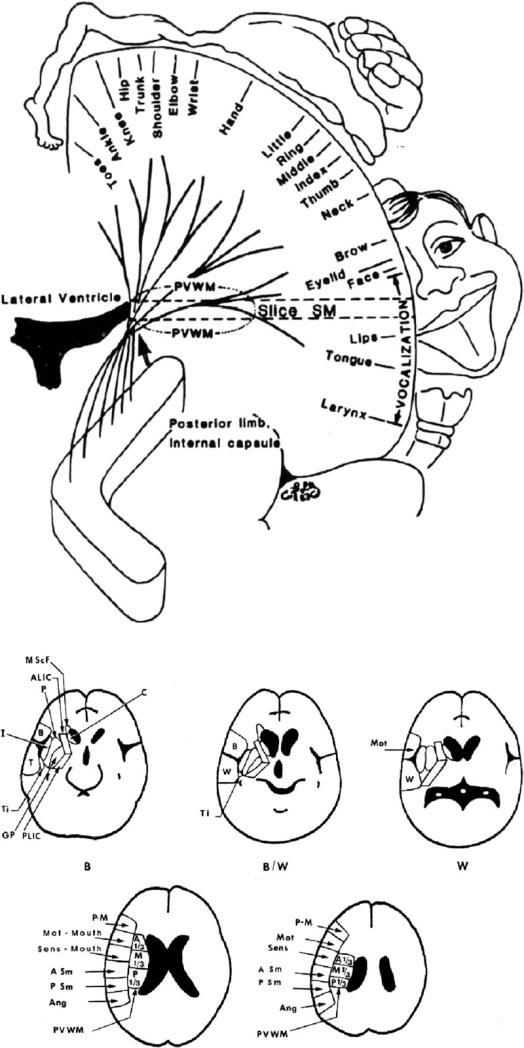
The 2 patients who showed no improvement had severe paralysis. We have observed that severity of paralysis and potential for improvement after LLLT-laser acupuncture (or needle acupuncture) is related to lesion location on chronic computed tomography (CT) scan acquired at least 3 months poststroke onset. Patients with lesion in more than half of the “periventricular white matter area” (PVWM) (adjacent to the body of the lateral ventricle, superior to the posterior limb, internal capsule), an area containing multiple efferent and afferent pathways (eg, thalamocortical, occipitofrontal, pathways from SMA/cingulate gyrus to the body of caudate, medial subcallosal fasciculus, and others), had severe paralysis which did not improve following LLLT-laser acupuncture (or needle) acupuncture treatments [34,37,38]. This area is diagrammed in Figure 6. The CT scan for a chronic stroke patient who had good response after LLLT-laser acupuncture treatments [34,37,38]. This area is diagrammed in Figure 7.
Figure 6.
Location of periventricular white matter (PVWM) area (black arrow), adjacent to the body of the lateral ventricle, located immediately superior to the posterior limb, internal capsule (computed tomography slice angulation, coronal and axial views). An extensive lesion in the PVWM was associated with severe paralysis and poor response following low-level light therapy (LLLT) or needle acupuncture treatments in chronic stroke patients with upper extremity, lower extremity, and hand paralysis. Patients with a lesion that was present in less than half of the PVWM area and who had a lesion that was not adjacent to the body of the lateral ventricle had less severe paralysis and good response after a series of LLLT or needle acupuncture treatments (34,37-39). Chronic stroke patients who had some preserved isolated finger flexion and extension before LLLT had the best potential for improvement after LLLT or needle acupuncture treatment. Other cases often had reduced spasticity after LLLT or needle acupuncture treatments.
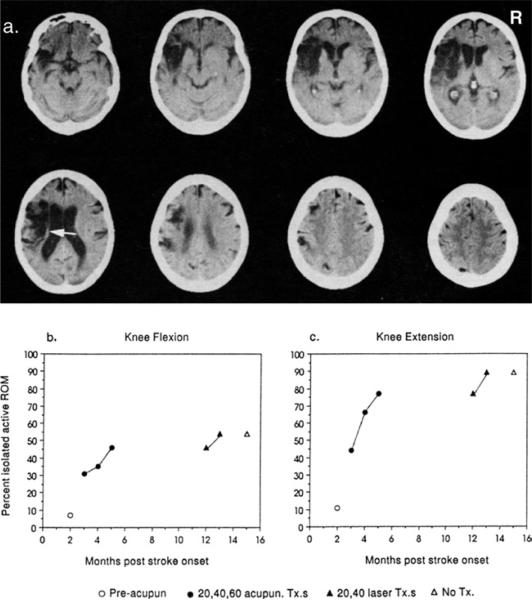
Figure 7.
(a.) Computed tomography (CT) scan of a 65-year-old woman obtained 5 months after stroke onset shows sparing of the most posterior portion of the periventricular white matter (PVWM) (white arrow), that is, likely sparing of some of the leg fibers. This patient showed improvement in knee flexion (b.) and knee extension (c.) after low-level light therapy (LLLT)-laser acupuncture treatments, which were initiated at 12 months after stroke onset. Knee extension increased from 77%-89% after 40 LLLT treatments, and her ability to climb up and down stairs improved. (She had shown some improvement on lower extremity tests after needle acupuncture treatments applied earlier after her stroke.) No improvement was seen in the upper extremity after LLLT or needle acupuncture, likely because of an extensive lesion in the more anterior portions of the PVWM. The arm paralysis was severe, scoring 0% isolated active range of motion for all arm tests at all times. The improvement in knee flexion and knee extension remained stable at 2 months after the last LLLT-laser acupuncture treatment (15 months after the stroke occurred). (Reprinted with author's permission, [34])
The 3 chronic stroke patients with hemiplegia who showed improvement after LLLT had an increase of 11%-28% in isolated, active range of motion for shoulder abduction, knee flexion, and/or knee extension (mean, 15.8%; SD, 7.1). This percentage increase after LLLT-laser acupuncture was similar to that observed after a series of 20 or 40 needle acupuncture treatments [37,38]. The person with hand paresis who was treated with LLLT at 33 months after stroke onset showed an increase of 2-6 lb in grip strength, 3-jaw chuck, tip pinch, and lateral pinch in the affected hand. These results are similar to those obtained with needle acupuncture [39]. These findings are intriguing and suggest that some recovery of motor function can occur with needle acupuncture or LLLT acupuncture applied to body acupuncture points in chronic stroke patients.
A reduction in hand spasticity also has been observed when chronic stroke patients are treated with a combination of red-beam laser applied to hand acupuncture points plus microamps transcutaneous electrical nerve stimulation (TENS). Figure 8 shows an immediate reduction in hand spasticity after the first hand treatment when LLLT-laser acupuncture and microamps TENS were used with 2 chronic stroke patients. This LLLT and microamps TENS hand treatment program also may be used with patients who have hand spasticity related to other etiologies, including, for example, traumatic brain injury (TBI), “stiff man syndrome,” and spinal cord injury (SCI) (personal observation, M.A.N., 2001). Similar to red and NIR LLLT, microamps TENS increases ATP levels when applied to the skin [40]. However, Cheng et al [40] observed that when stronger milliamps TENS was used (eg, similar to conventional TENS), the ATP levels were decreased. Hence when microamps TENS is used (as shown in Figure 8) [41], it is advisable to keep the sensation below threshold for the patient to increase ATP (not decrease ATP).
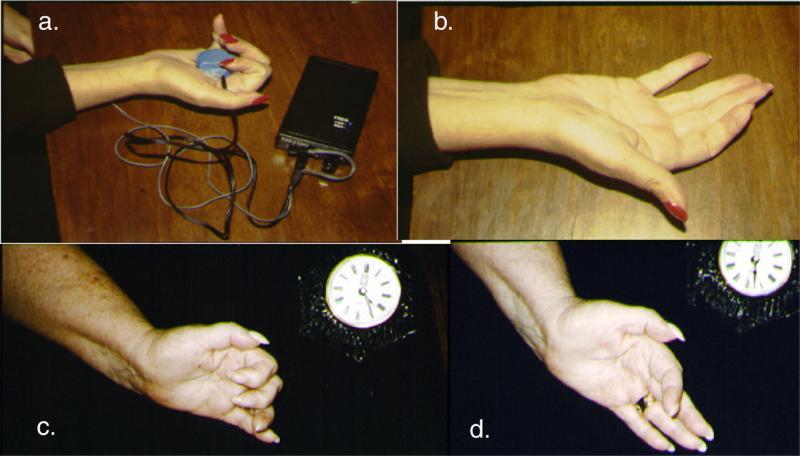
Figure 8.
(a.) Before the first low-level laser therapy (LLLT) and microamps transcutaneous electrical nerve stimulation (TENS) acupuncture treatment. It was 1.5 years after stroke onset and the patient still had right hand spasticity and was unable to extend her fingers into full extension. Microamps TENS was applied for 20 minutes to acupuncture point Heart 8 (in the palm of the hand) and Triple Warmer 5 (proximal to the dorsum of the wrist). Red-beam laser (670 nm, 5 mW, 4 J/cm2) was applied to the 6 Jing-Well points, located at base of fingernail beds on the hand, plus a few additional hand points. (b.) Immediately after the first 20-minute LLLT and microamps TENS acupuncture treatment, the patient had less hand spasticity and better control to open the fingers into full extension. More treatments are required for a longer-lasting effect. The patient can treat herself at home by using this LLLT and microamps TENS protocol, which is painless and noninvasive [41]. (c.) and (d.) A similar stroke case is shown.
TRAUMATIC BRAIN INJURY
Each year in the United States, more than 1.4 million new cases of TBI occur, and more than 80,000 persons are left with permanent disability [42]. Mild TBI (mTBI) from single and multiple events is the most frequent type of head injury experienced by military personnel deployed to Iraq and Afghanistan [43]. TBI is known to cause damage that ranges from observable to microscopic throughout the gray and white matter of the brain. Diffuse axonal injury [44] is often observed in the anterior corona radiata and frontotemporal regions [45]. Two regions highly susceptible to damage within the frontal lobes are the prefrontal cortex and the anterior cingulate gyrus. Cognitive processing problems result from tissue damage and inefficient cellular function in these brain regions. The prefrontal cortex is involved with maintaining, monitoring, and manipulating information in working memory [46] and particularly in sustained attention [47,48].
In the first reported study of the use of transcranial LLLT to treat traumatic brain injury, an animal model was used [49]. Mice were subjected to closed-head injury (CHI) by using a weight-drop procedure, and 4 hours after CHI, either sham or real NIR LLLT (808 nm) was administered transcranially. The control group received no laser therapy (n = 8); the laser-treated group (n = 16) received 1 transcranial LLLT treatment by using a 200-mW, 808-nm NIR laser with a 3-mm-diameter probe tip (Photothera Inc, Carlsbad, CA). Either 10 or 20 mW/cm2 was administered. A single point was treated on the skull (a skin incision was made) that was located 4 mm caudal to the coronal suture line on the midline. The point was treated for 2 minutes (1.2-2.4 J/cm2). At 24 and 48 hours after CHI, no significant difference in motor behavior was seen between mice in the laser-treated and control groups. After 5 days, the motor behavior was significantly better (P < .05) in the laser-treated group; in addition, the neurobehavioral scores were 26%-27% better (lower scores indicated better motor behavior). At 28 days after CHI, the brain-tissue volume was examined for mice in each group. The mean lesion size of 1.4% in the laser-treated group (SD 0.1) was significantly smaller (P < .001) than in the control group (12.1%, SD 1.3). No difference in lesion size or behavior was observed in the mice treated with 10 mW/cm2 and those treated with 20 mW/cm2. The researchers suggested various possible mechanisms, including an increase in ATP, total antioxidants, angiogenesis, neurogenesis, heat shock proteins content, and an antiapoptotic effect, similar to observations reported after LLLT treatment of ischemic heart skeletal muscles [50-54].
Moreira et al [55] conducted a study in 2009 using phototherapy with low-intensity lasers and observed the effect on local and systemic immunomodulation after cryogenic brain injury in rats. Brain and blood samples were analyzed by enzyme-linked immunosorbent assay for the production of cytokines interleukin (IL)-6 , IL-10, IL-1b, and tumor necrosis factor (TNF)-α. The study concluded that laser phototherapy could positively affect the balance of IL-1b, TNF-α, and IL-6 in rats and thereby prevent cell death after TBI.
Wu et al [56] reported another mouse study of LLLT mediated by transcranial laser therapy. A nonfocal (diffuse) TBI was produced by a CHI caused by a calibrated weight-drop device. A neurologic severity score for each mouse was determined based on 10 standardized performance tests (involving beam balancing and maze exiting) administered at specified times. Mice with a neurologic severity score of 7-8 (moderately severe brain injury) were used in the study. Mice were given a single treatment to the top of the head with 36 J/cm2 of a 665-nm, 810-nm, or 980-nm laser 4 hours after the closed head TBI. Both 665-nm and 810-nm lasers were highly effective in improving the neurologic performance of the mice during the succeeding 4 weeks. The 980-nm wavelength was ineffective (negative control). We believe that this difference in results can be explained by the absorption spectrum of the different chromophores; CCO has peaks at 660 nm and 810 nm, whereas water has a peak at 980 nm.
In humans, 2 persons with chronic mTBI recently have been reported to have improved cognition after a series of treatments with transcranial, red, and NIR LEDs [57,58]. The LED cluster heads were applied to the forehead and scalp areas (the hair was not shaved off but was parted underneath each 2-inch-diameter LED cluster head). Each cluster head had 61 diodes (9 red 633-nm diodes and 52 NIR 870-nm diodes). Each diode was 12-15 mW, and the total power output was 500 mW. The LED cluster heads were applied to bilateral frontal, parietal, and temporal areas and to the mid-sagittal suture line.
Each LED cluster head was applied for 10 minutes per placement. With the device used here (parameters described above), 1 joule per cm2 (J/cm2) energy density was produced during every 45 seconds of exposure time. The energy density dose at the forehead-scalp was 13.3 J/ cm2; the power density was 22.2 mW/cm2 (±20%). The power density refers to the mW of power applied per cm2. The ± refers to the range of fluctuation (plus or minus 20%) on the power density per cm2. This power density is well below that used in other transcranial laser or LED studies to treat acute stroke cases or severe depression cases (225 mW/cm2) [59]. It is estimated that only approximately 3% of the photons delivered to the forehead-scalp surface will reach 1 cm, to the cortex [60]. The dose of 13.3 J/cm2 per placement area was estimated to deliver only 0.4 J/cm2 to the brain cortex. No sensation of heat or pain was reported during the LED application to the skin or scalp. These LED cluster heads (MedX Health Corp, Mississauga, Ontario, Canada) are approved by the U.S. Food and Drug Administration for treatment of musculoskeletal pain; they were used off-label for treatment of cognition in the mTBI cases. No potential existed for ocular damage because the LEDs produce noncoherent light. These LED cluster heads also have been approved by the Food and Drug Administration for home treatment.
A 66-year-old woman (case 1) began transcranial LED treatments 7 years after a motor vehicle–related TBI. Before LED treatment, she could focus on her computer for only 20 minutes. After 8 weekly LED treatments, her focused computer time increased to 3 hours. She has treated herself nightly at home for 5.5 years, with transcranial LED. She maintains her improved cognition at age 72 years.
Case 2 involved a 52-year-old retired, high-ranking female military officer who had a history of multiple TBIs. Her brain MRI showed frontoparietal atrophy. She was medically disabled for 5 months before beginning nightly transcranial LED treatments at home (see Figure 9, A and B). After 4 months of nightly LED treatments, she returned to work full time as an executive consultant for an international technology consulting firm and discontinued medical disability. Neuropsychological tests performed after 9 months of transcranial LED showed significant improvement in cognition (see Figure 9, C). After LED treatments, she improved on tests of executive function (inhibition and inhibition accuracy, +2 SD) and on memory (immediate and delayed recall +1, +2 SD). The improvement of +1 or +2 standard deviations on her scores refers to the degree of improvement on her scores after 9 months of LED treatments (versus before LED treatments). The SDs are provided with the test materials, and they are based on the published norms for each test.
Figure 9.
(a.) Red and near-infrared (NIR) light-emitting diode (LED) cluster head (2-inch diameter) for transcranial LED treatments. (b.) Sample placement location on right forehead for one of the LED cluster heads during transcranial LED treatment. (c.) Graph that shows significant improvement in cognition on tests of Executive Function (inhibition, and inhibition accuracy, +2 SD) after LED treatments in the second patient with chronic, mild traumatic brain injury. The patient returned to full-time employment after 4 months of nightly transcranial LED treatments. (c reprinted with permission, (58).)
Both patients with TBI reported that they needed to continue with home treatments. If they stop treatment for 1 or 2 weeks, then their cognitive problems started to return. Both patients with TBI reported improved sleep. The second patient with TBI reported a decrease in her posttraumatic stress disorder symptoms after a few months of using the transcranial LEDs, and Schiffer et al [59] also reported a reduction in posttraumatic stress disorder symptoms in 3 of 10 patients with major depression who were treated with transcranial LED.
Several possible mechanisms may be associated with the improved cognition in the mTBI cases treated with transcranial LEDs [58]. Mitochondria display a significant amount of dysfunction after TBI [61-63]. The primary mechanism for improvement posited in one study with human acute stroke patients was an increase in ATP, with photons being used by CCO in the mitochondria to increase ATP, especially in the cortex [64].
An increase in ATP after red and/or NIR LED treatments in patients with chronic TBI would have beneficial effects, including an increase in cellular respiration and oxygenation. Oxidative stress plays a role in the damage present after TBI [65]. One hypothesis is that LLLT produces low levels of ROS in mitochondria of illuminated cells and that these ROS cause NF-κB activation via the redox sensitive sensor enzyme protein kinase D1, which results in upregulation of the mitochondrial superoxide dismutase [66]. A single exposure of LLLT-LED in vitro with fibroblasts has been observed to increase NF-κB in the short term [67]. In stimulated dendritic cells in the longer term, however, NF-κB and pro-inflammatory cytokines were reduced [68]. Thus, in the long term, repeated LED treatments are hypothesized to decrease inflammation (less NF-κB) and upregulate gene products that are cytoprotective, such as superoxide dismutase, glutathione peroxidase, and heat shock protein 70 [54,69]. It is hypothesized that an overall protective response occurs with repeated LED treatments and that major ROS-mediated damage and chronic inflammation that occur in the brain after TBI may actually be reduced.
Acupuncture points located on the scalp were treated with the red-NIR LEDs [57]. This includes points along the Governing Vessel (GV) acupuncture meridian, located on the midline of the skull (including, in part, the mid-sagittal suture line). Some acupuncture points located on the GV meridian have been used historically to help treat patients in coma [70] and stroke [71], for example, GV 16 (inferior to occipital protuberance), GV 20 (vertex), and GV 24 (near center-front hairline); these points were treated in both patients with TBI reported in this study.
Transcranial red-NIR LED may have irradiated the blood via the valveless, emissary veins located on the scalp surface but interconnecting with veins in the superior sagittal sinus (M. Dyson, oral personal communication, June 2009). If red-NIR photons penetrate deeply enough to reach the cortex, then it also is possible they are entering small vessels located between the arachnoid and the pia mater, including those that supply arterial blood to superficial areas of the cortex. Direct in vitro blood irradiation with a red-beam laser has been observed to improve erythrocyte deformability (flexibility) and rheology [72,73]. A beneficial effect from direct-laser blood irradiation in vivo has been observed during stenting procedures where a low-level, red-beam laser (10 mW, 650 nm) was used, with the beam placed directly into a coronary artery [74]. The restenosis rate was reduced and no adverse effects or complications were noted. Thus blood irradiation at the scalp may have affected local intracerebral blood and circulation; however; whether this effect occurred is unknown and would require further study.
An increase in regional cerebral blood flow may have occurred, specifically to the frontal lobes. The second TBI case showed significant improvement on objective, neuro-psychological testing for executive function (inhibition) after administration of LED. These results suggest improved function in the prefrontal cortex and anterior cingulate gyrus regions. Significant improvement on “inhibition” on the Stroop test particularly suggests improved function of the medial prefrontal cortex, anterior cingulate gyrus area [75]. It is possible that this medial prefrontal cortex area could have been treated with NIR photons, especially when the LED cluster head was placed over the midline, front hairline area. The dorsolateral prefrontal cortex also was likely irradiated when the LEDs were placed on the left and right high-frontal areas of the scalp. Increased regional cerebral blood flow also could have occurred in frontal pole areas with the TBI cases, as was observed in the recent transcranial LED study to treat major depression [59]. Additional controlled studies with real and sham transcranial LLLT and LED are recommended to investigate whether these methods can be applied to improve cognition and reduce symptom severity in persons with acute and chronic TBI. The LED technology is not expensive ($1400 for a single LED cluster head and approximately $4000 to $5000 for a unit with 3 LED cluster heads). The transcranial LED treatment protocol can be used in the home.
DEGENERATIVE CENTRAL NERVOUS SYSTEM DISEASE
The positive effects of transcranial laser therapy on stroke and TBI have led to early investigations into whether LLLT may have benefits for persons with degenerative brain disorders, which are a rapidly growing affliction of the world's aging population. Moges et al [76] tested whether LLLT had a role to play in treating familial amyotrophic lateral sclerosis (FALS), which is a neurodegenerative disease characterized by progressive loss of motor neurons and death. Mitochondrial dysfunction and oxidative stress play an important role in motor neuron loss in ALS. The study combined LLLT (with use of an 810-nm diode laser with 140-mW output power targeting a 1.4-cm2 spot area for 120 seconds using 12 J/cm2 energy density) and riboflavin to test the survival of motor neurons in a mouse model of FALS. Motor function (determined with use of the Rota rod test) was significantly improved in the LLLT group in the early stage of the disease. Immunohistochemical expression of the astrocyte marker glial fibrillary acidic protein was significantly reduced in the cervical and lumbar enlargements of the spinal cord as a result of LLLT.
Trimmer et al [77] carried out preliminary studies that may have relevance to Parkinson disease (PD). Mitochondria supply the ATP needed to support axonal transport, which contributes to many other cellular functions essential for the survival of neuronal cells. Furthermore, mitochondria in PD tissues are metabolically and functionally compromised. The researchers measured the velocity of mitochondrial movement in human transmitochondrial cybrid “cytoplasmic hybrid” neuronal cells with mitochondrial DNA from patients with sporadic PD and disease-free age-matched volunteer control subjects (CNT). PD and CNT cybrid neuronal cells were exposed to NIR laser light (an 810-nm diode laser using 50 mW/cm2 for 40 seconds), and axonal transport of labeled mitochondria was measured. The velocity of mitochondrial movement in PD cybrid neuronal cells was significantly reduced compared with mitochondrial movement in disease-free CNT cybrid neuronal cells, and 2 hours after LLLT, the average velocity of mitochondrial movement in PD cybrid neurites was significantly increased and restored to levels comparable with those of CNT. Mitochondrial movement in CNT hybrids was unaltered by LLLT. PD cybrid neuronal cell lines with the most dysfunctional mtETC assembly and oxygen utilization profiles were least responsive to LLLT.
Zhang et al [78] likewise did preliminary experiments with relevance to Alzheimer disease. They showed that LLLT (0.156 -0.624 J/cm2 from a 5-mW HeNe laser) could protect rat pheochromocytoma PC12 cells (a model of cortical neurons) from apoptosis caused by amyloid β peptide (Aβ25-35). This protection was mediated by protein kinase C activation caused by an increase in the cell survival protein bcl-xl and a decrease in cell death protein bax. Although no peer-reviewed publications have been published to date, it is known that transcranial LLLT has been applied to patients with moderate Alzheimer disease.
Michalikova et al [79] treated middle-aged (12–month-old) female CD-1 mice with a daily 6-minute exposure to 1072-nm LED light for 10 days and found that LLLT yielded a number of significant behavioral effects upon testing in a 3-dimensional maze. Middle-aged mice showed significant deficits in a working memory test, and LLLT reversed this deficit. LLLT-treated middle-aged mice were more considerate in their decision making, which resulted in an overall improved cognitive performance comparable with that of young (3-month-old) CD-1 mice. These results suggest that LLLT could be applied in cases of general cognitive impairment in elderly persons.
SPINAL CORD INJURY
SCI is a severe central nervous system trauma with no effective restorative therapies. Light therapy has biomodulatory effects on central and peripheral nervous tissue. Several groups investigated the effectiveness of LLLT on SCI. Roch-kind et al [80] demonstrated that LLLT applied simultaneously to the injured sciatic nerve and the corresponding segment of the spinal cord accelerates the process of regeneration of the injured peripheral nerve.
Light therapy (810 nm, 150 mW) significantly increased the axonal number and distance of regrowth in 2 SCI models: a contusion model and a dorsal hemisection model [81,82]. In addition, LLLT returned aspects of function to baseline levels and significantly suppressed immune cell activation and cytokine-chemokine expression [81].
Moreover, light therapy significantly improved the average length of axonal regrowth and increased the total axon number for both injury models. A statistically significant lower angle of rotation of the feet was observed during a walking test in the hemisection model and a statistically significant overall functional recovery in contusion model was seen in the LLLT groups. These results suggest that light may be a promising therapy for human SCI [82].
PERIPHERAL NERVE
The use of new therapeutic instruments such as electric stimulation, ultrasound, and LLLT for peripheral nervous system regeneration is currently being investigated in an attempt to achieve early functional recovery. LLLT has been used in several clinical and experimental research studies on peripheral nerves injuries.
In a pilot double-blind randomized study, Rochkind et al showed that postoperative 780-nm laser phototherapy enhances the regenerative process of the peripheral nerve after reconnection of the nerve defect by using a PGA neurotube. Morphologically, the laser-treated group showed an increased total number of myelinated axons [83]. These researchers also reported that, in patients with long-term peripheral nerve injury, 780-nm laser therapy (250 mW) can progressively improve nerve motor function, which leads to significant functional recovery [84].
Barbosa et al [85] observed that, compared with the 830-nm laser group and the sham group, rats in the 660-nm laser group had the best sciatic functional index scores on average, which indicates that the use of these parameters was more efficient. Differences in sciatic functional index were found among the 660-nm laser group and the other ones at the 14th day [85]. However, Gigo-Benato et al [86] found that pulsed (905 nm) continuous (808 nm) combined laser biostimulation showed the best effectiveness in promoting peripheral nerve regeneration.
CONCLUSION
LLLT is steadily moving into mainstream medical practice. As the Western populations continue to age, the incidence of the degenerative diseases of old age will only continue to increase and produce an evermore severe financial and societal burden. Moreover, despite the best efforts of “big pharma,” distrust of pharmaceuticals is growing in general because of uncertain efficacy and troublesome adverse effects. LLLT has no reported adverse effects, and no reports of adverse events can be directly attributed to laser or light therapy. We believe that the high benefit:risk ratio of LLLT should be better appreciated by medical professionals in the rehabilitation and physical medicine specialties. The introduction of affordable LED devices powered by rechargeable batteries will lead to many home-use applications of LLLT. The concept of “wearable” light sources is not far off. Moreover, the particular benefits of LLLT to both the central and peripheral nervous systems suggest that much wider use of LLLT could or should be made in cases of both brain diseases and injuries.
Acknowledgments
Disclosure: research in the Hamblin laboratory supported by NIH grant R01AI050875, Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), CDMRP Program in TBI (W81XWH-09-1-0514), and Air Force Office of Scientific Research (FA9950-04-1-0079)
Footnotes
Disclosure key can be found on the Table of contents and at www.pmrjournal.org
Contributor Information
Javad T. Hashmi, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA; Department of Dermatology, Harvard Medical School, Boston, MA Disclosure: nothing to disclose.
Ying-Ying Huang, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA; Department of Dermatology, Harvard Medical School, Boston, MA; Aesthetic and Plastic Center, Guangxi Medical University, Nanning, PR China Disclosure: nothing to disclose.
Bushra Z. Osmani, Aga Khan Medical College, Karachi, Pakistan Disclosure: nothing to disclose.
Sulbha K. Sharma, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA Disclosure: nothing to disclose.
Margaret A. Naeser, VA Boston Healthcare System, Boston, MA; Department of Neurology, Boston University School of Medicine, Boston, MA Disclosure: nothing to disclose.
Michael R. Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA; Department of Dermatology, Harvard Medical School, 40 Blossom St BAR414, Boston, MA 02114 ; Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA. Address correspondence to: M.R.H.; hamblin@helix.mgh.harvard.edu.
REFERENCES
- 1.Mester E, Szende B, Tota JG. Effect of laser on hair growth of mice. Kiserl Orvostud. 1967;19:628–631. [Google Scholar]
- 2.Mester E, Spiry T, Szende B, et al. Effect of laser rays on wound healing. Am J Surg. 1971;122:532–535. doi: 10.1016/0002-9610(71)90482-x. [DOI] [PubMed] [Google Scholar]
- 3.Mester E, Szende B, Spiry T, et al. Stimulation of wound healing by laser rays. Acta Chir Acad Sci Hung. 1972;13:315–324. [PubMed] [Google Scholar]
- 4.Huang YY, Chen AC, Carroll JD, et al. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aimbire F, Albertini R, Pacheco MT, et al. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24:33–37. doi: 10.1089/pho.2006.24.33. [DOI] [PubMed] [Google Scholar]
- 6.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 7.Passarella S, Casamassima E, Molinari S, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175:95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 8.Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428–1434. doi: 10.1016/0006-291x(89)91138-8. [DOI] [PubMed] [Google Scholar]
- 9.Karu T. Photobiology of low-power laser effects. Health Phys. 1989;56:691–704. doi: 10.1097/00004032-198905000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Karu T. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol B. 1989;3:638–640. doi: 10.1016/1011-1344(89)80088-0. [DOI] [PubMed] [Google Scholar]
- 11.Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 12.Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B. 1995;27:219–223. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 13.Pastore D, Greco M, Petragallo VA, et al. Increase in ≤H+/e– ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser. Biochem Mol Biol Int. 1994;34:817–826. [PubMed] [Google Scholar]
- 14.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 15.Lohr NL, Keszler A, Pratt P, et al. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol. 2009;47:256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kam T, Kalendo G, Lethokov V. Lobko. Biostimulation of HeLa cells by low-intensity visible light II. Stimulation of DNA and RNA synthesis in a wide spectral range. Nuevo Cimento. 1984:309–318. [Google Scholar]
- 17.Baxter GD. Therapeutic Lasers: Theory and Practice. Churchill Livingstone; London, England: 1994. pp. 89–138. [Google Scholar]
- 18.Longo L. Terapia Laser. USES; Firenze, Italy: 1986. p. 9^+0. [Google Scholar]
- 19.Cruanes CJ. Laser Therapy Today. Laser Documentation Centre; Barcelona, Spain: 1984. pp. 95–98. [Google Scholar]
- 20.Zhang Y, Song S, Fong CC, et al. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 2003;120:849–857. doi: 10.1046/j.1523-1747.2003.12133.x. [DOI] [PubMed] [Google Scholar]
- 21.Lampl Y. Laser treatment for stroke. Expert Rev Neurother. 2007;7:961–965. doi: 10.1586/14737175.7.8.961. [DOI] [PubMed] [Google Scholar]
- 22.Oron A, Oron U, Chen J, et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 23.Zhang RL, Chopp M, Zhang ZG, et al. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 24.Shen J, Xie L, Mao X, et al. Neurogenesis after primary intracerebral hemorrhage in adult human brain. J Cereb Blood Flow Metab. 2008;28:1460–1468. doi: 10.1038/jcbfm.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Detaboada L, Ilic S, Leichliter-Martha S, et al. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg Med. 2006;38:70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 26.Carnevalli CM, Soares CP, Zangaro RA, et al. Laser light prevents apoptosis in Cho K-1 cell line. J Clin Laser Med Surg. 2003;21:193–196. doi: 10.1089/104454703768247756. [DOI] [PubMed] [Google Scholar]
- 27.Lapchak PA, Salgado KF, Chao CH, et al. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 29.Lapchak PA, Han MK, Salgado KF, et al. Safety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbits. Stroke. 2008;39:3073–3078. doi: 10.1161/STROKEAHA.108.516393. [DOI] [PubMed] [Google Scholar]
- 30.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2009;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 32.Zivin JA, Albers GW, Bornstein N, et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 33.Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2. Curr Cardiol Rep. 2010;12:29–33. doi: 10.1007/s11886-009-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naeser MA, Stiassny-Eder D, Galler V, Hobbs J, Bachman D, Lannin L. Laser acupuncture in the treatment of paralysis in stroke patients: a CT scan lesion site study. [November 30, 2010];Am J Acupuncture. 1995 23:13–28. Available at: http://www.bu.edu/naeser/acupuncture.
- 35.Bard G, Hirschberg GG. Recovery of voluntary motion in upper extremity following hemiplegia. Arch Phys Med Rehabil. 1965;46:567–572. [PubMed] [Google Scholar]
- 36.Sunderland A, Tinson D, Bradley L, et al. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989;52:1267–1272. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naeser MA, Alexander MP, Stiassny-Eder D, et al. Acupuncture in the treatment of paralysis in chronic and acute stroke patients—improvement correlated with specific CT scan lesion sites. Acupunct Electrother Res. 1994;19:227–249. doi: 10.3727/036012994816357231. [DOI] [PubMed] [Google Scholar]
- 38.Naeser MA AM, Stiassny-Eder D, Galler V, Hobbs J, Bachman D. Real versus sham acupuncture in the treatment of paralysis in acute stroke patients: a CT scan lesion site study. [November 30, 2010];J Neurologic Rehab. 1992 6:163–173. Available at: http://www.bu.edu/naeser/acupuncture.
- 39.Naeser MA, Alexander MP, Stiassny-Eder D, Nobles L, Bachman D. Acupuncture in the treatment of hand paresis in chronic and acute stroke patients: Improvement observed in all cases. Clin Rehab. 1994;8:127–141. [Google Scholar]
- 40.Cheng N, Van Hoof H, Bockx E, et al. The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin Orthop Relat Res. 1982;(171):264–272. [PubMed] [Google Scholar]
- 41.Naeser MA, Wei XB. Laser acupuncture, an introductory textbook for treatment of pain, paralysis, spasticity and other disorders. clinical, research uses of laser acupuncture from around the world. Boston Chinese Medicine. 1994:40. [Google Scholar]
- 42.Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 44.Taber KH, Warden DL, Hurley RA. Blast-related traumatic brain injury: what is known? J Neuropsychiatry Clin Neurosci. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- 45.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am J Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- 47.Lewin JS, Friedman L, Wu D, et al. Cortical localization of human sustained attention: detection with functional MR using a visual vigilance paradigm. J Comput Assist Tomogr. 1996;20:695–701. doi: 10.1097/00004728-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 49.Oron A, Oron U, Streeter J, et al. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 50.Oron U, Yaakobi T, Oron A, et al. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg Med. 2001;28:204–211. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- 51.Oron U, Yaakobi T, Oron A, et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103:296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 52.Yaakobi T, Shoshany Y, Levkovitz S, et al. Long-term effect of low energy laser irradiation on infarction and reperfusion injury in the rat heart. J Appl Physiol. 2001;90:2411–2419. doi: 10.1152/jappl.2001.90.6.2411. [DOI] [PubMed] [Google Scholar]
- 53.Shefer G, Partridge TA, Heslop L, et al. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115:1461–1469. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- 54.Avni D, Levkovitz S, Maltz L, et al. Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg. 2005;23:273–277. doi: 10.1089/pho.2005.23.273. [DOI] [PubMed] [Google Scholar]
- 55.Moreira MS, Velasco IT, Ferreira LS, et al. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B. 2009;97:145–151. doi: 10.1016/j.jphotobiol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Wu Q, Huang Y-Y, Dhital S, et al. Hamblin MR, Anders JJ, Waynant RW, editors. Low level laser therapy for traumatic brain injury. Mechanisms for Low-Light Therapy V. Proc SPIE. 2010;7552 Article No. 755206. [Google Scholar]
- 57.Naeser MA, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function post-transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2010 doi: 10.1089/pho.2010.2814. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naeser MA, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function post-transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2010 doi: 10.1089/pho.2010.2814. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiffer F, Johnston AL, Ravichandran C, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan S, Parrish JA, Anderson RR, et al. Transmittance of nonionizing radiation in human tissues. Photochem Photobiol. 1981;34:679–681. doi: 10.1111/j.1751-1097.1981.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 61.Verweij BH, Muizelaar JP, Vinas FC, et al. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- 62.Gilmer LK, Roberts KN, Joy K, et al. Early mitochondrial dysfunction after cortical contusion injury. J Neurotrauma. 2009;26:1271–1280. doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lifshitz J, Sullivan PG, Hovda DA, et al. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 64.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 65.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sompol P, Xu Y, Ittarat W, et al. NF-kappaB-associated MnSOD induction protects against beta-amyloid-induced neuronal apoptosis. J Mol Neurosci. 2006;29:279–288. [PubMed] [Google Scholar]
- 67.Chen AC-H, Arany PR, Huang Y-Y, et al. Hamblin MR, Anders JJ, Waynant RW, editors. Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. Mechanisms for Low-Light Therapy IV. Proc SPIE. 2009;7165 doi: 10.1371/journal.pone.0022453. Article No. 71650B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen AC-H, Huang Y-Y, Sharma SK, Hamblin MR. Effects of 810-nm laser on murine bone marrow derived dendritic cells. Photomed Laser Surg. 2010 doi: 10.1089/pho.2010.2837. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang YH, Takahashi K, Jiang GZ, et al. In vivo production of heat shock protein in mouse peritoneal macrophages by administration of lipopolysaccharide. Infect Immun. 1994;62:4140–4144. doi: 10.1128/iai.62.10.4140-4144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frost E. Acupuncture for the comatose patient. Am J Acupuncture. 1976;4:45–48. [Google Scholar]
- 71.Deadman P, Al-Khafaji M. A Manual of Acupuncture. Hove, East Essex, England: Journal of Chinese Medicine Publications. 1998;256:548–560. [Google Scholar]
- 72.Mi XQ, Chen JY, Liang ZJ, et al. In vitro effects of helium-neon laser irradiation on human blood: blood viscosity and deformability of erythrocytes. Photomed Laser Surg. 2004;22:477–482. doi: 10.1089/pho.2004.22.477. [DOI] [PubMed] [Google Scholar]
- 73.Mi XQ, Chen JY, Cen Y, et al. A comparative study of 632.8 and 532 nm laser irradiation on some rheological factors in human blood in vitro. J Photochem Photobiol B. 2004;74:7–12. doi: 10.1016/j.jphotobiol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 74.De Scheerder IK, Wang K, Kaul U, et al. Intravascular low-power laser irradiation after coronary stenting: long-term follow-up. Lasers Surg Med. 2001;28:212–215. doi: 10.1002/lsm.1040. [DOI] [PubMed] [Google Scholar]
- 75.Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- 76.Moges H, Vasconcelos OM, Campbell WW, et al. Light therapy and supplementary riboflavin in the SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis (FALS). Lasers Surg Med. 2009;41:52–59. doi: 10.1002/lsm.20732. [DOI] [PubMed] [Google Scholar]
- 77.Trimmer PA, Schwartz KM, Borland MK, et al. Reduced axonal transport in Parkinson's disease cybrid neurites is restored by light therapy. Mol Neurodegener. 2009;4:26. doi: 10.1186/1750-1326-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Xing D, Zhu D, et al. Low-power laser irradiation inhibiting Abeta25-35-induced PC12 cell apoptosis via PKC activation. Cell Physiol Biochem. 2008;22:215–222. doi: 10.1159/000149799. [DOI] [PubMed] [Google Scholar]
- 79.Michalikova S, Ennaceur A, van Rensburg R, et al. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol Learn Mem. 2008;89:480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Rochkind S, Barr-Nea L, Bartal A, et al. New methods of treatment of severely injured sciatic nerve and spinal cord. An experimental study. Acta Neurochir Suppl (Wien) 1988;43:91–93. doi: 10.1007/978-3-7091-8978-8_20. [DOI] [PubMed] [Google Scholar]
- 81.Byrnes KR, Waynant RW, Ilev IK, et al. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 82.Wu X, Dmitriev AE, Cardoso MJ, et al. 810 nm Wavelength light: an effective therapy for transected or contused rat spinal cord. Lasers Surg Med. 2009;41:36–41. doi: 10.1002/lsm.20729. [DOI] [PubMed] [Google Scholar]
- 83.Rochkind S, Leider-Trejo L, Nissan M, et al. Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed Laser Surg. 2007;25:137–143. doi: 10.1089/pho.2007.2076. [DOI] [PubMed] [Google Scholar]
- 84.Rochkind S, Drory V, Alon M, et al. Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg. 2007;25:436–442. doi: 10.1089/pho.2007.2093. [DOI] [PubMed] [Google Scholar]
- 85.Barbosa RI, Marcolino AM, de Jesus Guirro RR, et al. Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci. 2010;25:423–430. doi: 10.1007/s10103-009-0750-8. [DOI] [PubMed] [Google Scholar]
- 86.Gigo-Benato D, Geuna S, de Castro Rodrigues A, et al. Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: a double-blind randomized study in the rat median nerve model. Lasers Med Sci. 2004;19:57–65. doi: 10.1007/s10103-004-0300-3. [DOI] [PubMed] [Google Scholar]