Abstract
β1 integrins are ubiquitously expressed receptors that mediate cell–cell and cell–extracellular matrix interactions. To analyze the function of β1 integrin in skin we generated mice with a keratinocyte-restricted deletion of the β1 integrin gene using the cre–loxP system. Mutant mice developed severe hair loss due to a reduced proliferation of hair matrix cells and severe hair follicle abnormalities. Eventually, the malformed hair follicles were removed by infiltrating macrophages. The epidermis of the back skin became hyperthickened, the basal keratinocytes showed reduced expression of α6β4 integrin, and the number of hemidesmosomes decreased. Basement membrane components were atypically deposited and, at least in the case of laminin-5, improperly processed, leading to disruption of the basement membrane and blister formation at the dermal–epidermal junction. In contrast, the integrity of the basement membrane surrounding the β1-deficient hair follicle was not affected. Finally, the dermis became fibrotic. These results demonstrate an important role of β1 integrins in hair follicle morphogenesis, in the processing of basement membrane components, in the maintenance of some, but not all basement membranes, in keratinocyte differentiaton and proliferation, and in the formation and/or maintenance of hemidesmosomes.
Keywords: β1 integrin/epidermis/hair follicle/laminin-5/tissue-specific knockout
Introduction
The skin is composed of an epidermal and a dermal layer, which are separated by a basement membrane. The epidermis is made primarily of keratinocytes, while the dermis contains different cell types including fibroblasts, endothelial cells and macrophages as well as large amounts of extracellular matrix. Adhesion of the keratinocytes to the basement membrane and to each other is important for the development of skin and for the maintenance of skin integrity. During differentiation, basal keratinocytes detach from the basement membrane and migrate to suprabasal layers. This movement is accompanied by a complex change of cell–cell and cell–matrix interactions involving adhesion molecules such as cadherins and integrins (Hodivala and Watt, 1994; Hotchin et al., 1995; Zhu and Watt, 1996).
Integrins are heterodimeric transmembrane proteins consisting of an α and a β subunit, mediating the attachment of cells to the extracellular matrix and to other cells (Hynes, 1992). They are essential for cell adhesion and migration, but are also involved in proliferation, programmed cell death, tissue organization and differentiation (Brakebusch et al., 1997). In skin, several integrin receptors are expressed by the epidermal keratinocytes, including α2β1, α3β1 and α6β4 (Watt and Hertle, 1994). Basal keratinocytes attach to the underlying basement membrane via integrins. β1 integrin was found to be a marker for proliferation competent cells in vitro and in vivo (Jones and Watt, 1993; Jones et al., 1995). Upon initiation of terminal differentiation, keratinocytes downregulate integrin expression and migrate to the suprabasal layers where they keratinize and eventually die, forming the cornified layer of the skin. In normal conditions, therefore, integrin expression is confined mainly to the basal layer of the epidermis. However, during wound healing or in certain pathological conditions such as psoriasis, integrins are also expressed by suprabasal keratinocytes (Watt and Hertle, 1994). Ectopic expression of α2, α5 and β1 integrin in the suprabasal layers of transgenic mice resulted in hyperproliferation, perturbed keratinocyte differentiation and a psoriasis-like phenotype (Carroll et al., 1995). These reports, together with various in vitro findings, implicate β1 integrins in the regulation of differentiation and proliferation of keratinocytes (Zhu et al., 1999).
Integrins α3β1 and α6β4 bind to the basement membrane component laminin-5, while α2β1 mediates attachment to collagens, certain laminins and fibronectin, respectively (Hynes, 1992; Aumailley and Rousselle, 1999). Integrin α6β4 is an integral part of hemidesmosomes, which are specialized adherens junctions linking the basement membrane and the underlying dermis to the intracellular keratin filament network (Nievers et al., 1999). Genetic ablation of the α6 or β4 integrin gene in mice resulted in a complete absence of hemidesmosomes, creating large blisters between the dermis and the epidermis (Georges-Labouesse et al., 1996; van der Neut et al., 1996). Integrin α3β1 is expressed in between, but not within, mature hemidesmosomes. Mice carrying a deletion of the α3 integrin exhibited splits and disorganization of the epidermal basement membrane, but normal hemidesmosomes (DiPersio et al., 1997). Since these mice also suffer from a kidney defect that leads to perinatal lethality, it was impossible to study the role of α3β1 integrin in the development of hair and the maintenance of skin (Kreidberg et al., 1996).
Hair follicles develop by a sequence of epithelial–mesenchymal interactions, forming an initial hair germ during embryogenesis, which then grows down through the dermis and the subcutaneous fat layer reaching the muscle layer ∼9 days after birth in mice (Hardy, 1992; Paus and Cotsarelis, 1999). This developmental stage is called anagen. In the following catagen phase, starting ∼18 days after birth, hair proliferation stops and the hair involutes accompanied by apoptosis of hair matrix cells in the hair bulb. This process is halted when the dermal papilla reaches the stem cell-containing bulge region of the hair follicle in the dermis. After a resting or telogen phase, a new anagen phase is initiated by interactions between the dermal papilla and the overlying follicular epithelium. From the hair matrix at the proximal end of the follicle, several layers of different keratinocytes develop and form the outer root sheath (ORS), the companion layer (Mahony et al., 1999), the inner root sheath (IRS) and the hair itself (Paus and Cotsarelis, 1999). The hair follicle is surrounded by a basement membrane. The ORS cells interact with the basement membrane via α2β1, α3β1 and α6β4 integrins, which are expressed at different levels in different regions of the hair follicle (Commo and Bernard, 1997). In contrast to the epidermal–dermal junction, however, ORS cells do not form hemidesmosomes, which would inhibit the dynamic changes of the hair follicle during the hair cycles.
We have shown previously that β1-null embryonic stem (ES) cells can differentiate into keratinocytes in β1-null chimeric mice (Fässler and Meyer, 1995) and in teratomas, but not in embryoid bodies (Bagutti et al., 1996). In contrast to the blister formation in the α3-null skin, the small areas of β1-null skin were histologically normal in the β1-null chimeric mice (Bagutti et al., 1996). To study the function of β1 integrins in epidermis and hair follicles further, we generated mice with a keratinocyte-restricted deletion of the β1 integrin gene by mating mice carrying a floxed β1 integrin gene with transgenic mice expressing cre recombinase under the control of the keratin-5 promoter. This promoter has been shown to target the expression of transgenes to basal cells of stratified epithelia and to ORS cells of the hair follicles (Ramirez et al., 1994). Offspring with a keratinocyte-specific deletion of β1 showed an increasing loss of hair after birth, a hyperthickened epidermis of the back skin, blister formation and a fibrotic phenotype. We show that β1 integrins are important for the proliferation of the hair follicle keratinocytes and for the maintenance of the dermal–epidermal basement membrane. The integrity of the basement membrane surrounding the hair follicles was not obviously affected by the loss of β1 integrin, suggesting distinct functions of β1 integrins in different keratinocytes.
Results
Generation of mice with a keratinocyte-restricted deletion of the β1 integrin gene
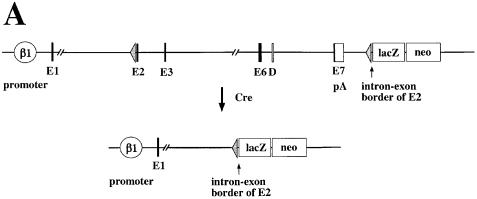
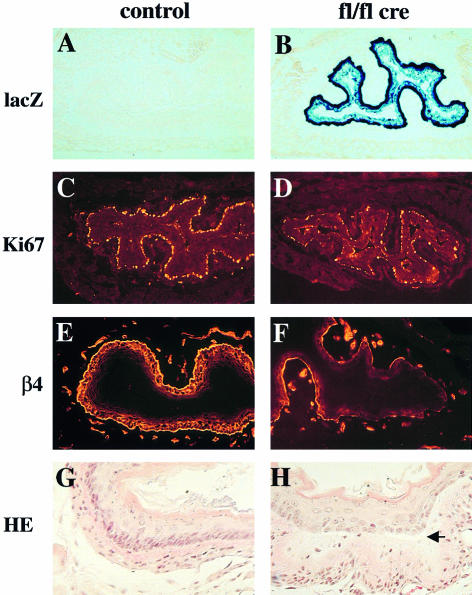
To study the role of β1 integrin in skin and hair development we generated mice with a keratinocyte-specific deletion of the β1 integrin gene (Figure 1A). First, we prepared mice in which the complete coding and 3′ non-coding region was flanked by loxP sites (floxed β1 integrin gene). In addition, a promoterless lacZ reporter gene was introduced after the downstream loxP site. After cre recombinase-mediated ablation of the β1 integrin gene, the lacZ reporter gene will be expressed under the control of the β1 integrin promoter, allowing simple monitoring of the gene deletion and of β1 integrin promoter activity in β1-deficient cells (Potocnik et al., 2000). Mice homozygous for the floxed β1 integrin gene were viable, fertile and phenotypically normal. We then mated these mice with transgenic mice expressing the cre recombinase under the control of the keratin-5 promoter (A.Ramirez and J.L.Jorcano, manuscript in preparation). In 2-day-old mice heterozygous for the floxed β1 gene carrying the keratin-5 cre transgene, strong lacZ staining was observed in the basal keratinocyte layer of back skin, ear, tail, foot pad, tongue, esophagus and fundus region of the stomach (data not shown). Strong lacZ activity was detected in all hair follicles. No lacZ activity was found in other mutant cell types where the keratin-5 promoter is not active or in mice lacking the keratin-5 cre transgene.
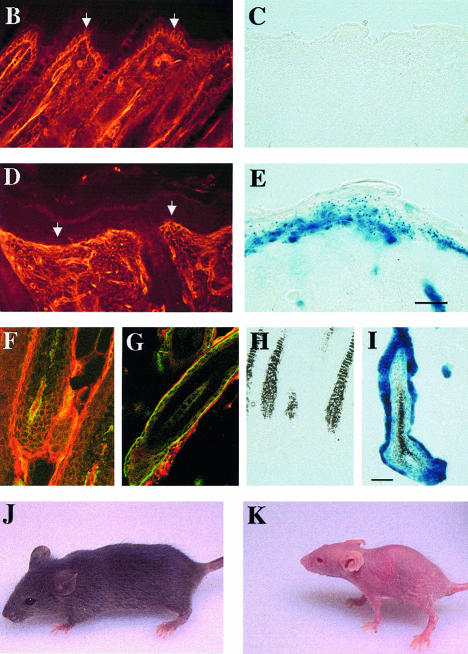
Fig. 1. Generation of mice with a keratinocyte-restricted ablation of the β1 integrin gene. (A) Schematic presentation of the floxed β1 integrin gene and the cre-mediated deletion of the gene. After deletion of the β1 integrin gene, the β1 integrin promoter will transcribe the lacZ cDNA. (E, exons in arbitrary numbering; D, splice variant; pA, polyadenylation sequence of β1 integrin gene). Epidermis of back skin of 9-day-old control (B and C) and mutant mice (D and E) stained for β1 integrin (B and D) and lacZ activity (C and E) (bar, 50 µm). Note the loss of β1 integrin expression in the mutant basal keratinocytes (arrows in B and D). Bulb region of hair follicles of back skin of 9-day-old control (F and H) and mutant mice (G and I) double-stained for β1 (red) and α6 integrin (green) (F and G) and for lacZ activity (H and I) (bar, 50 µm). Note the loss of α6 and β1 integrin in the hair matrix and the thin unstained region visible between the α6 integrin expressing ORS cells and β1 integrin expressing cells surrounding the hair follicle (G). Control (J) and mutant (K) mice at 4 weeks of age. Mutant mice were approximately half the weight of control littermates, lost nearly all hair, and had frequent wounds, especially in mechanically stressed regions of the skin.
Homozygously floxed mice expressing the cre recombinase (fl/fl cre) showed a similar, but more intense lacZ staining, due to the activation of two lacZ genes per cell (Figure 1E and I). However, staining of the basal keratinocyte layers was not as continuous as in the heterozygous mice, including cells with low (punctate) or little detectable lacZ. Staining for β1 integrin expression indicated a nearly complete loss of β1 integrin in the keratinocytes of the epidermis of 9-day-old animals (Figure 1D), while 3-day-old animals still expressed small amounts of β1 integrin (data not shown). Dermal cells were unaffected by cre and expressed normal amounts of β1 integrin. Confocal microscopy analysis of double stainings for α6 and β1 integrin showed that mutant hair follicles lost β1 integrin expression (Figure 1G). α6 integrin expression was lost in the hair matrix cells but was still present in the ORS cells, especially on their basal surface (Figure 1G). The mutant hair follicles were discontinuously surrounded by β1 integrin expressing cells, which in the immunostaining were clearly separated from the α6 integrin expressing ORS cells by a thin unstained space. These β1 integrin expressing cells were most probably dermal fibroblast and Schwann cells of nerves innervating the hair follicle.
Loss of β1 integrin in keratinocytes results in severe hair loss and skin abnormalities
Homozygously floxed mice carrying the keratin-5 cre transgene were born without an obvious phenotype. However, 2 days after birth, a reduced pigmentation of the back skin became visible in the midline and in several lines parallel to the ribs. Nine-day-old animals showed a clearly reduced number of hairs. No short hairs developed, indicating a defect in secondary, tertiary and quaternary hair follicle morphogenesis (Gibbs, 1941). At 14 days of age, the hair loss increased and after 4 weeks only a few hairs were left (Figure 1K). The skin turned reddish and became tight. Small skin wounds were observed, especially in areas of mechanical stress such as knees and elbows, and less frequently in other regions such as ears. Concomitantly, the mice developed a peculiar gait and walked with extended legs (Figure 1K). The ears acquired a crumpled appearance and were closely apposed to the head. The weight of the knockout mice was normal up to ∼9 days, but then fell behind their normal littermates, reaching a maximum of ∼10–11 g after 4 weeks compared with ∼20 g for littermate controls. Approximately 70% of the mice with a keratinocyte-specific deletion of the β1 integrin gene died within the first 6 weeks. Mice surviving >6 weeks showed β1 integrin expressing keratinocytes in some parts of the back skin, suggesting that β1 integrin was deleted less efficiently in these mice.
Integrin β1-null hair follicles show an aberrant morphology and a reduced proliferation
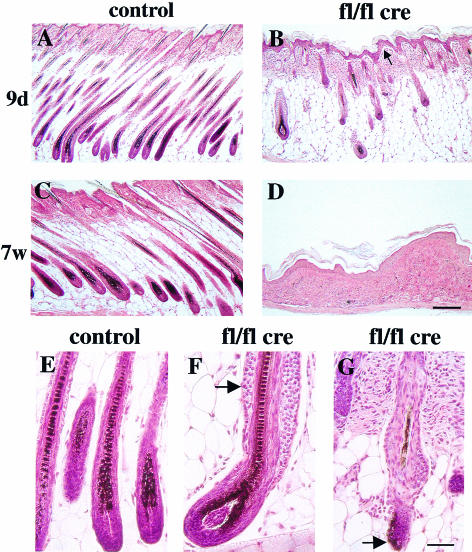
To find the reason for the hair loss, we assessed the morphology of the hair follicles at different time points (2, 3, 9 and 16 days, and 4, 6 and 7 weeks). At 9 days, many hair follicles showed gross abnormalities, ranging from a shortened hair bulb to an increased number of layers of ORS cells, foldings of the layers of the IRS cells, and amorphous, misclocated hairs (Figure 2B, F and G). At 16 days, only malformed hair follicles were detectable. These data suggest that β1 integrin is important for the organization of the hair follicles and/or for the proper differentiation of the cells forming them. At 7 weeks of age, no clearly identifiable remnants of hair follicles, hairs or sebaceous glands could be identified in mutant mice (Figure 2D).
Fig. 2. Aberrant hair follicle morphogenesis and loss of subcutaneous fat layer in keratinocyte-restricted β1-deficient mice. Hematoxylin–eosin stained sections of back skin of 9-day (A and B) and 7-week-old (C and D) control (A and C) and mutant mice (B and D). At 9 days of age the mutant hair follicles have grown less deeply into the subcutis than normal hair follicles (A, B). Seven-week-old mutant mice had lost all hair follicles and had no subcutaneous fat layer (C, D; bar, 200 µm). Hematoxylin–eosin staining of hair follicles derived from 9-day-old control (E) and mutant mice (F, G). Hair follicles in mutant mice showed gross abnormalities, ranging from multilayered ORS (F, arrow) to severely malformed follicles with peripheral hair deposition (G, arrow; bar, 100 µm).
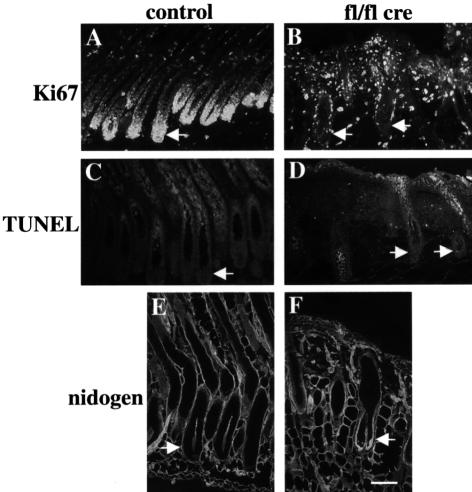
Whereas hair follicles in normal mice reach the muscle layer during the anagen growth (Figure 2A), β1-null hair follicles only reached the middle part of the subcutaneous fat layer (Figure 2B). Since the length of the hair follicle is directly proportional to the proliferative acitivity of its keratinocyte population (Pinkus, 1958), this finding suggested a reduced proliferation of hair follicle cells. This was confirmed by the expression of the proliferation marker Ki67 in a reduced number of mutant hair matrix cells at 2 days of age (data not shown) and by the absence of Ki67 staining at 16 days of age (Figure 3B). Bromodeoxyuridine (BrdU) incorporation studies similarly showed high mitotic activity in the matrix cells of normal hair follicles, and reduced or absent proliferation in mutant hair follicles at 9 and 16 days, respectively (data not shown).
Fig. 3. Reduced proliferation of hair matrix cells lacking β1 integrin. Back skin sections of 16-day-old control (A, C and E) and mutant mice (B, D and F) were stained for Ki67 (A and B), TUNEL (C and D) and nidogen (E and F). Hair matrix cells in the hair bulb (arrows) of the mutant mice showed no Ki67-positive proliferating cells (B), in contrast to normal mice (A). Ki67-positive cells between the mutant hair follicles were proliferating dermal fibroblasts. No increased apoptosis was observed in the matrix cells of mutants (D). Nidogen deposition around the β1-deficient hair follicles was thickened but continuous (F; bar, 100 µm).
No significant increase in apoptosis was observed in the hair matrix of β1-deficient hair follicles at 2, 9 and 16 days of age (Figure 3C and D and data not shown). Immunostainings of hair follicles for the expression of α6 and β4 integrin, and for basement membrane proteins including nidogen, laminin-5, collagen IV, perlecan and fibronectin were similar in mutant and control tissue of 9-day-old mice (data not shown). In 16-day-old mutant mice, staining for basement membrane components revealed a slightly thicker membrane than in hair follicles of control mice (Figure 3E and F). The presence of apparently normal basement membranes which were tightly associated with the mutant hair follicles was also confirmed by electron microscopy (data not shown). The expression patterns of cadherin as assessed by immunostaining with a pan-cadherin antibody, and of actin, were similar in β1-null and normal hair follicles (data not shown). In normal hair follicles, the expression of keratin-6 was confined to the companion layer between ORS and IRS. In contrast, all layers of the β1-null hair follicles expressed keratin-6 in 9-day-old mutant mice (data not shown).
Abnormalities in morphology, organization and differentiation of keratinocytes in the absence of β1 integrin
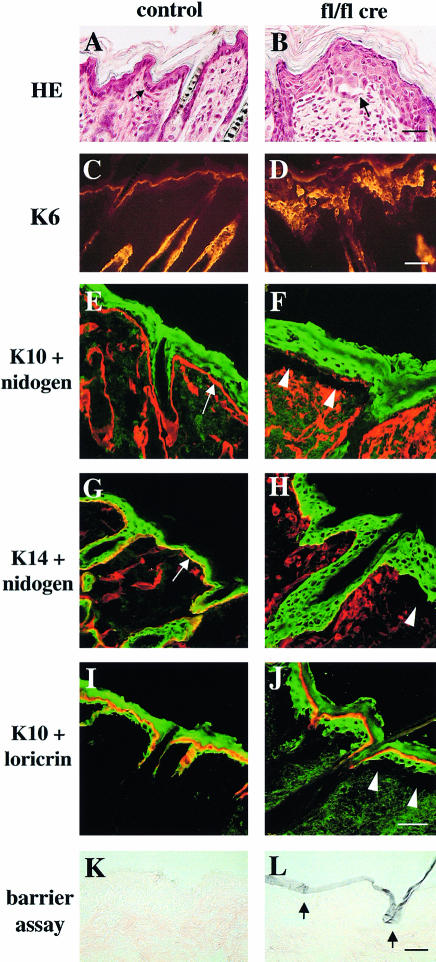
Deletion of the β1 integrin gene in keratinocytes caused abnormal morphology and disorganization of basal keratinocytes, and a hyperthickened epidermis with multiple layers of nucleated cells (Figure 4B). While in control mice basal keratinocytes of the back skin formed a monolayer of tightly packed cuboidal cells (Figure 4A), such cells were scarce in mice lacking β1 integrin expression. Instead, several layers of roundish or polygonal cells were found and often elongated cells were present in the basal layer of the mutant epidermis derived from 9-day-old mice (Figure 4B). Keratin-6, which is normally absent from keratinocytes in the interfollicular epidermis, was found throughout the mutant epidermis (Figure 4D), while the suprabasal marker keratin-10 was expressed in all suprabasal layers only (Figure 4F and J). Keratin-14 was detected in all layers of normal and mutant skin (Figure 4G and H). Expression of the terminal differentiation marker loricrin was confined to the cell layer just below the stratum corneum, both in wild-type and mutant mice (Figure 4I and J).
Fig. 4. Abnormalities in keratinocyte morphology and differentiation. Back skin sections of 9-day-old control (A, C, E, G and I) and mutant mice (B, D, F, H and J) were stained with hematoxylin–eosin (A and B; bar, 30 µm) and with antibodies for keratin-6 (K6; C and D; bar, 50 µm), keratin-10 (K10; green; E, F, I and J), keratin-14 (K14; green; G and H), nidogen (red; E–H) and loricrin (red; I and J). Basal keratinocytes from normal mice (A, arrow) attach to a nidogen-containing basement membrane (E and G, arrows). Mutant mice showed basal keratinocytes with an aberrant morphology, a thickened epidermis and blistering between epidermis and dermis (arrow and arrowheads in B, F, H and J). Keratin-6 is upregulated in mutant epidermis (D). Basal cells did not express keratin-10 either in normal or mutant skin (E, F, I and J). Suprabasal cells were positive for keratin-10 and keratin-14 (E–J). Most of the keratin-10 positive cells in the mutant skin showed no expression of loricrin (J), indicating a delayed terminal differentiation (E–J; bar, 40 µm). The barrier function of the skin was tested using a hematoxylin dye penetration assay (for details see Materials and methods). Sections of back skin of 4-week-old control (K) and mutant mice (L) were counterstained with eosin. Hematoxylin penetrated the superficial layers of the stratum corneum (violet), but neither the lower layers (L, arrows) nor any underlying tissue in the mutant mice, indicating an intact barrier function of the skin (bar, 50 µm).
To test the barrier function, normal and mutant skin was subjected to a dye penetration assay (Figure 4K and L). Hematoxylin penetrated the superficial, but not the lower layers (arrow in Figure 4L) of the stratum corneum of mutant mice. Skin of normal littermates and nude mice showed only occasional staining of the stratum corneum (Figure 4K and data not shown).
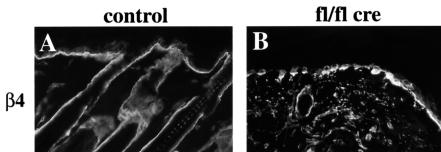
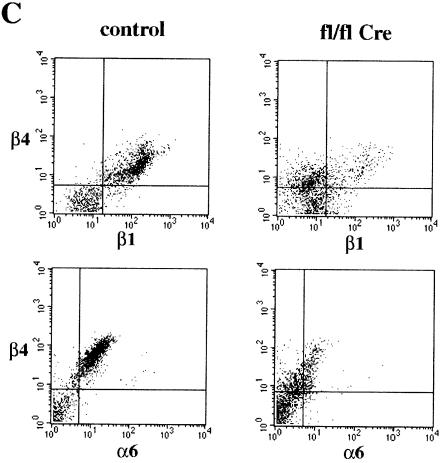
Immunostaining revealed that basal keratinocytes of mutant mice expressed reduced amounts of α6 and β4 integrin (Figure 5B and data not shown), indicating decreased expression of the laminin-5 receptor α6β4 integrin. This was confirmed by FACS analysis of keratinocytes isolated from 3-week-old normal and mutant mice (Figure 5C). Basal keratinocytes from normal mice expressed high amounts of β1, α6 and β4 integrin. In mutant keratinocyte preparations this population was very small. Instead, a new population lacking β1 integrin and expressing low amounts of α6 and β4 integrin was found. Cells lacking β1, α6 and β4 integrin are quite likely suprabasal keratinocytes. Their percentage increased from 20% in control to ∼40% in the mutant keratinocyte preparations (Figure 5C). Basal keratinocytes of mutant mice also showed a decreased expression of the laminin receptor dystroglycan (data not shown), which was recently found to be essential for the formation of basement membranes (Henry and Campbell, 1998).
Fig. 5. Reduced expression of α6β4 integrin in mutant epidermis. Back skin sections of 16-day-old control (A) and mutant (B) mice were stained for β4 integrin. In mutants, skin expression of β4 integrin by the basal keratinocytes was reduced and discontinuous (B) (bar, 50 µm). (C) FACS analysis of keratinocyte preparations from back skin of 3-week-old normal and mutant mice double stained for β4 and β1 integrin, and for β4 and α6 integrin, respectively. Basal keratinocytes expressing high amounts of β1, α6 and β4 integrin were found in normal, but hardly in mutant skin. Instead, mutant skin preparations contained a population of β1-null cells expressing low amounts of β4 and α6 integrin.
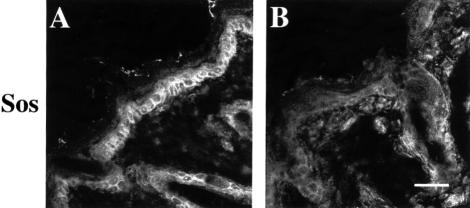
The number of cycling cells in the basal layer of the epidermis was reduced in mutant skin. Immunostaining for the Ki67 antigen revealed ∼30 positive cells out of 100 in 9-day-old control mice and 11.7 positive cells out of 100 in mutant mice. Similarly, BrdU incorporating cells diminished from 16.7 per 100 in controls to 8.0 per 100 in mutants. This defect in proliferation might be connected to a reduced expression of the Ras guanine nucleotide exchange factor Sos in basal keratinocytes (Figure 6). While normal skin showed strong Sos staining in basal keratinocytes, mutant skin had a significantly reduced signal. Antibodies against the phosphorylated active form of the MAPK, a possible downstream target of Sos, showed only very weak cytoplasmic staining in basal keratinocytes of normal and mutant mice (data not shown). We performed TUNEL stainings to test whether β1-deficient basal keratinocytes were lost by apoptosis. Mutant epidermis revealed no apoptotic cells in the basal layer and few in the suprabasal layer, similar to normal epidermis, indicating that β1 integrin expression is apparently not crucial for the survival of the basal keratinocytes. These data indicate that the formation of multiple nucleated cell layers in mutant skin is possibly caused by a delay in terminal differentiation of the suprabasal cell layers in the mutant skin. This is supported by the lack of loricrin expression in the additional keratin-10-positive suprabasal cells of the mutant mice (Figure 4J).
Fig. 6. Reduced Sos expression of basal keratinocytes in mutant skin. Back skin sections of 9-day-old control (A) and mutant (B) mice were stained for Sos. Sos expression was significantly reduced in basal keratinocytes of mutant skin compared with normal (bar, 50 µm).
Distortion of the basement membrane at the dermal–epidermal junction after deletion of the β1 integrin gene in keratinocytes
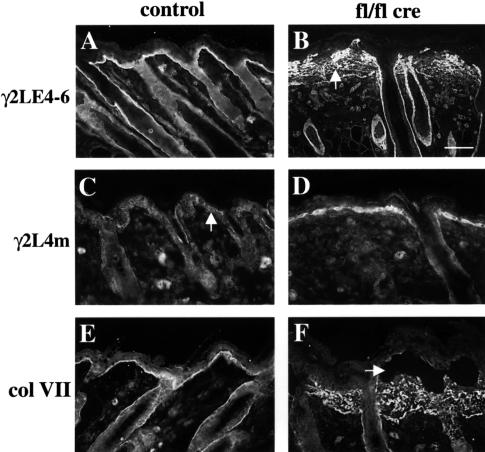
In contrast to the normal appearance of the basement membrane around hair follicles, the basement membrane at the dermal–epidermal junction was severely abnormal. Laminin-5 (chain composition α3β3γ2) is a major component of anchoring filaments at this junction, and its proper function depends on proteolytic processing of the α3 and γ2 chain (Burgeson, 1996). Two specific antisera were generated against recombinant fragment γ2LE4-6, which is retained in mature laminin-5, and fragment γ2L4m, which is cleaved and lost. The antibodies to γ2LE4-6 stained the normal epidermal basement membrane in a linear fashion (Figure 7A). This staining is largely lost in 9-day-old mutants and replaced by diffuse laminin-5 deposition in the dermis (Figure 7B). These antibodies also already demonstrated the splitting of the basement membrane 2 days after birth (data not shown). Antibodies to γ2L4m showed a weak and scattered staining in the basement membrane of normal mice consistent with the loss of this domain in the majority of the laminin-5 molecules present in the basement membrane (Figure 7C). In mutant mice, strong staining was observed around the basal keratinocytes, which did not extend into the dermis (Figure 7D). Also, other basement membrane proteins like nidogen-1 and collagen IV and the anchoring fibril protein collagen VII were diffusely and irregularly deposited in the dermis (Figures 4F and H and 7F and data not shown).
Fig. 7. Aberrant processing and deposition of laminin-5 and collagen VII in mutant skin. Back skin sections of 16-day-old control (A, C and E) and mutant mice (B, D and F) were stained for the γ2 chain of laminin-5 using antibodies recognizing both the unprocessed and the processed form (γ2LE4-6; A and B), and antibodies specific for a fragment present only in the unprocessed form (γ2L4m; C and D). γ2LE4-6 showed diffuse staining in the dermis of mutant skin (B, arrow). Significantly increased amounts of unprocessed laminin γ2 were detected with the γ2L4m specific antibody around the basal keratinocytes of mutant mice, while dermally deposited laminin-5 was processed normally (D). Note the virtual absence of unprocessed laminin γ2 in the basement membrane of normal epidermis (C) and the normal γ2LE4-6 staining around the hair follicle in mutant skin (B; bar, 50 µm). Staining for the anchoring fibril component collagen VII (E, F) revealed a diffuse, dermal staining in mutant mice (F). In blisters (F, arrow), small amounts of collagen VII could be detected at the epidermal side.
Electron microscopy of normal skin revealed a regular and well developed lamina lucida and densa separating the epidermis from the dermal connective tissue (Figure 8A). Mutant skin showed an aberrant basement membrane with absent or reduced lamina densa between the hemidesmosomes, similar to that described for the α3-null neonates (Figure 8B and C). Some regions lacked any detectable basement membrane formation and showed basal keratinocytes with protrusions into the underlying matrix (Figure 8C and data not shown). The number of their hemidesmosomes was reduced. Anchoring filaments and anchoring fibrils were found both in normal and mutant skin (data not shown). In blisters, very few hemidesmosomes with patches of basement membrane were found at the epidermal side. The frequency and morphological appearance of desmosomes at the lateral cell membrane of the basal keratinocytes were similar in normal and mutant skin (data not shown).
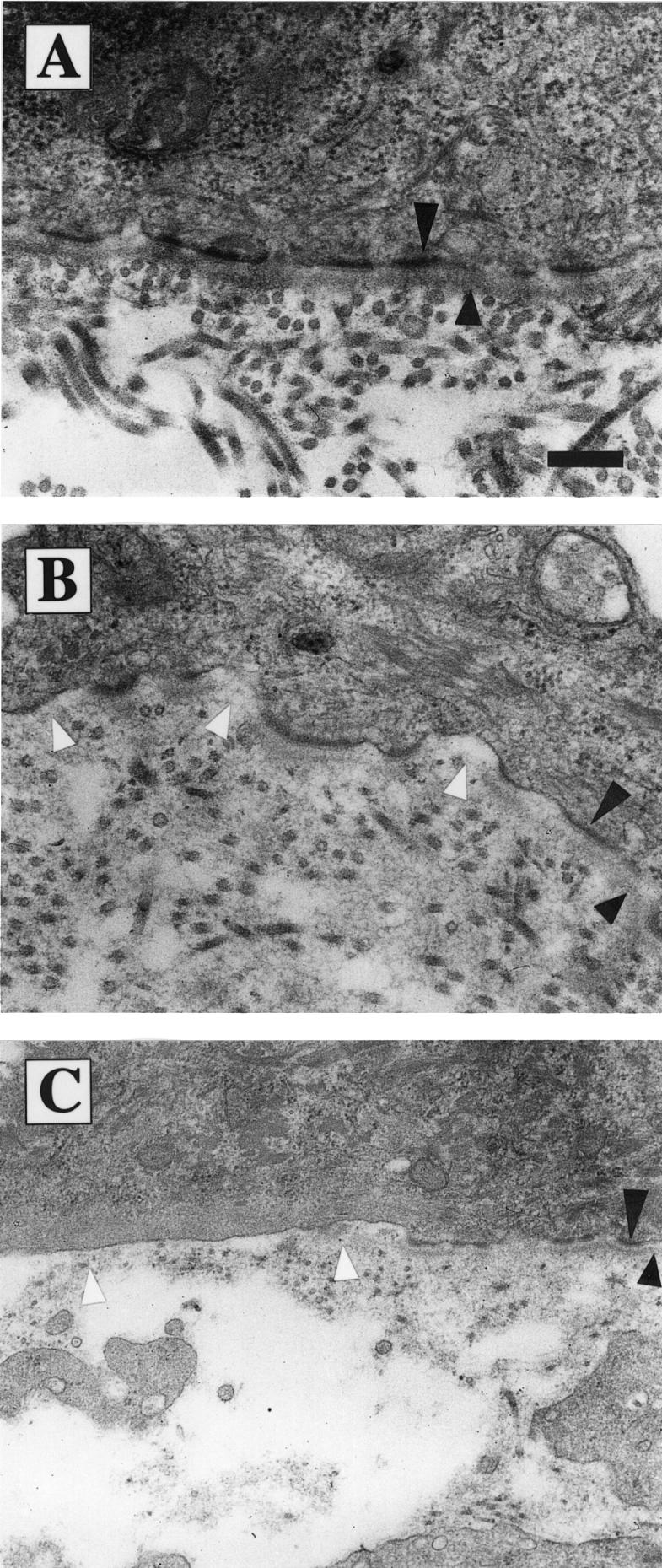
Fig. 8. Distortion of the basement membrane at the dermal–epidermal junction. Electron microscopy of back skin sections of 4-week-old control (A) and mutant (B and C) mice. The lamina densa is marked by short and hemidesmosomes by long arrowheads. Loss of β1 integrin expression on keratinocytes resulted in a discontinuous lamina densa [gaps marked by white arrowheads in (B)] and in a reduction of hemidesmosomes [region without hemidesmosomes and basement membrane marked by white arrowheads in (C)]. (A and B) Bar, 0.25 µm; (C) bar, 0.42 µm.
Development of a dermal fibrosis and inflammation
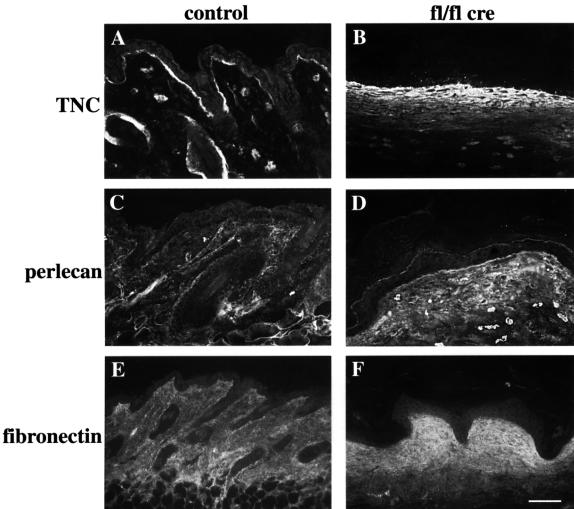
Concomitant with the loss of hair, the mutant mice showed increased deposition of extracellular matrix proteins in the dermis and lost all fat cells in their subcutis. Immunostaining revealed elevated deposition of tenascinC, perlecan, fibronectin and collagen I (Figure 9 and data not shown).
Fig. 9. Increased deposition of extracellular matrix components in mutant skin. Back skin sections of 6-week-old control (A, C and E) and mutant mice (B, D and F) were stained for tenascinC (TNC) (A, B), perlecan (C, D), and fibronectin (E, F). All three proteins showed increased staining in the mutant dermis (bar, 100 µm).
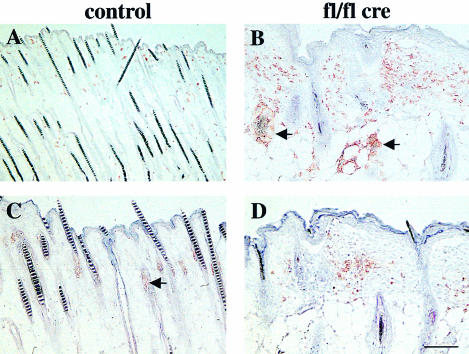
Infiltration of inflammatory cells is a key event in the pathogenesis of many skin disorders. To test for the presence of macrophages, granulocytes, mast cells and T cells, tissue sections from normal and mutant mice were stained with several cell type-specific antibodies. Infiltration of macrophages was significantly increased in mutant skin samples. At 9 days, and more so at 16 days of age, increased numbers of macrophages accumulated around some but not all deformed hair follicles (Figure 10B). Furthermore, foreign-body giant cells, a subset of skin macrophages, were frequently found close to some hair bulbs. In some areas, granuloma formation was visible (data not shown). These data suggest that macrophages might play a role in the removal of the deteriorating β1-null hair follicles. At 16 days, granulocytes, which are rarely found in normal skin (Figure 10C), were detected in the dermis of mutant skin (Figure 10D). Immunostaining for CD3 and the γδ T-cell receptor revealed a slight increase in the number of T cells in the mutant dermis at 16 days of age. The number of mast cells, which are suggested to play a role in hair follicle remodeling (Maurer et al., 1995), increased slightly from 16 days of age onwards (data not shown).
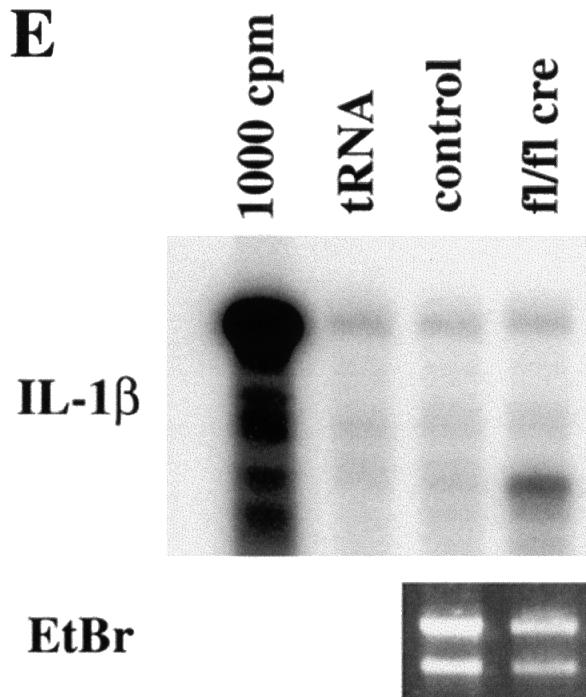
Fig. 10. Infiltration of inflammatory cells in the mutant skin and RNase protection assay. Back skin sections of 16-day-old control (A and C) and mutant (B and D) mice were stained for F4/80 (macrophage/monocyte specific antigen; A, B) and Gr-1 (granulocyte specific antigen; C, D). In mutant skin, an infiltration of macrophages and granulocytes was observed. Some deformed hair follicles were surrounded by macrophages (arrows in B). (Staining of the sebaceous glands in (C) (arrow) and (D) is background staining; bar, 100 µm.) (E) RNA isolated from skin of 4-week-old mutant and normal mice was analyzed by RNase protection (for details see Materials and methods). Mutant skin (fl/fl cre) showed a strong upregulation of IL-1β mRNA compared with littermate controls (control) (1000 c.p.m.: radiolabeled RNA probe; tRNA: negative control). Gel separated RNA samples were stained with ethidium bromide (EtBr) to control RNA amounts used in the assay.
To test whether the accumulation of macrophages was associated with increased production of cytokines, RNase protection assays were carried out using RNA from skin samples derived from 2- and 4-week-old control and mutant mice. Figure 10E shows that increased mRNA levels of the pro-inflammatory cytokine interleukin-1β (IL-1β) were expressed in the skin of mutant mice. Furthermore, we observed upregulation of transforming growth factor-β1 (TGF-β), connective tissue growth factor (CTGF) and activin mRNA expression in the skin of mutant mice (data not shown).
Defects in internal stratified epithelia
Keratin-5 promoter-controlled cre expression also resulted in a loss of β1 integrin in internal stratified epithelia. Strong lacZ activity was detected in tongue, esophagus, the fundus region of the stomach, and anus, and weaker activity in trachea, salivary gland and bladder (Figure 11B and data not shown). No lacZ staining was found in the intestine. Several of these organs, including the esophagus, displayed similar changes to those observed in the skin: different basal cell morphology, reduced proliferation as assessed by Ki67 staining, reduced expression of β4 integrin and Sos, and altered laminin-5 deposition (Figure 11D and F and data not shown). While small blisters were observed in tongue and esophagus of 6-week-old mutant mice, no obvious bleedings were detected in these tissues (Figure 11H). In contrast, blisters, bleedings and ulcerations were frequently found in the anus region of the mutant mice (data not shown).
Fig. 11. Defects in the esophagus of mutant mice. Esophagus sections of 2- (A–F) and 6-week-old (G and H) control (A, C, E and G) and mutant mice (B, D, F and H) were stained for lacZ activity (A and B), Ki67 (C and D), β4 integrin (E and F), and with hematoxylin–eosin (G and H). Basal cells in the mutant esophagus showed strong lacZ activity (B), reduced Ki67-positive proliferating cells (D) and a reduced staining for β4 integrin (F). In 6-week-old mice, mutant basal cells (H) displayed a different morphology than in normal mice (G). In addition, blisters were observed (H, arrow).
Discussion
In this paper we describe the skin phenotype of mice with a keratinocyte-restricted deletion of the β1 integrin gene. These mice mice show a progressive loss of hair, malformations of the hair follicles, a hyperthickened epidermis, a reduction of hemidesmosomes, a defective basement membrane at the dermal–epidermal junction, blister formation, inflammation and dermal fibrosis. These data demonstrate that β1 integrin is important for the proliferation of the matrix cells of the hair follicle, and for the proper organization of the different layers of the hair follicle. Furthermore, we show that β1 integrin is crucial for the maintenance of the basement membrane at the dermal–epidermal junction, but not around the hair follicle. We suggest that reduced expression of α6β4 integrin, defective processing of laminin-5, and impaired arrangement of basement membrane (laminin-5, nidogen-1, collagen IV) and anchoring fibril (collagen VII) components are crucial for the blister formation in mutant mice.
β1 integrin is important for proliferation, but not for survival of hair matrix cells
β1 integrin is strongly expressed in the ORS and the matrix cells of the hair follicle. Rapid proliferation of the matrix cells leads to the extension of the hair follicle into the subcutis during anagen. In the absence of β1, mutant hair follicles have a reduced length, suggesting a reduced proliferative activity of the matrix cells. Furthermore, β1-null matrix cells showed a dramatically decreased expression of Ki67 and incorporation of BrdU, which identify cycling cells. During normal hair follicle morphogenesis, the proliferation of matrix cells halts at the beginning of catagen. This stop in proliferation is accompanied by a burst in apoptosis in the hair matrix (Lindner et al., 1997), which was not observed in mutant hair follicles. Apparently, loss of β1 integrin selectively reduces proliferation and is not simply accelerating the hair cycle, leading to a premature catagen. No new hair cycle is initiated in the β1-null hair follicles after the growth arrest. Moreover, in mutant mice the hair follicles are completely lost after 4–6 weeks. This finding suggests that β1 integrin might be involved in the maintenance of an epithelial stem cell population, or alternatively, play an essential role in the activation of these stem cells at the beginning of anagen. Potential ligands for the integrins of the matrix cells eliciting the proliferative response could be components of the basement membrane that separate the matrix cells from the dermal papilla, such as collagen IV, laminin-5, perlecan and nidogen. The downstream targets of β1 integrin are less clear. Several transcription factors, such as hairless, as well as the cateninβ–LEF-1–TCF-1 complex or Sonic hedgehog (Shh) were found to be involved in the proliferation of certain subsets of mouse hairs (Oro and Scott, 1998; Panteleyev et al., 1999). Loss-of-function models of these proteins, however, did not show a similar phenotype to that observed in the β1-null hair follicle. Hairless mutants show premature apoptosis of hair matrix cells at ∼15 days after birth, together with a high rate of proliferation (Panteleyev et al., 1999). Lef-1–TCF-1 mutants have only mild defects in the body hair, but lack whiskers, which are present in the keratinocyte-specific β1-null mice (van Genderen et al., 1994). Shh–/– skin transplanted onto nude mice failed to differentiate properly and formed hyperproliferative follicle-like structures that did not produce mature hair shafts (St Jacques et al., 1998; Chiang et al., 1999). It would be interesting to know whether keratinocyte-specific gain-of-function mutations of these molecules can at least partially rescue the phenotype of the β1-null hair follicles.
β1 integrin is necessary for the normal morphology of the hair follicle
Hair follicles are composed of multiple layers of keratinocytes. In the absence of β1 integrin these distinct layers can no longer be identified. Various morphological aberrations were observed. These ranged from relatively mild defects like multilayered ORS cells or additional foldings of the IRS cell layers, to completely distorted hair follicles, in which no layer organization was recognizable and amorphous hair material was deposited in the periphery of the hair bulb instead of the center. This phenotype could be caused by a defective differentiation of the hair matrix cells. Our data, however, indicate only a very non-specific change in the expression profile of β1-null keratinocytes in the hair follicle, such as ubiquitous expression of keratin-6. Keratin-6 was shown to be upregulated in wound healing and in several pathological conditions such as psoriasis and skin tumors, and in several mouse models inducing hyperproliferation as well as growth suppression of keratinocytes (Stoler et al., 1988; Greenhalgh and Roop, 1993; Sellheyer et al., 1993). However, expression of keratin-14 in the ORS cells and staining for cadherins and actin were similar in both mutant and wild-type hair follicles.
Alternatively, uncoordinated growth rates or defective cell–cell interactions of correctly differentiated keratinocytes in the hair follicle could cause the morphological aberrations. Structural abnormalities in the hair follicles were observed in several other mouse mutants (Yamanishi, 1998). For example, expression of a dominant-negative form of the epidermal growth factor receptor (EGF-R) in the epidermis prevents the hair follicles from entering the catagen phase and results in thinning and loss of the IRS and ORS (Murillas et al., 1995). Knockout of TGF-α (Luetteke et al., 1993) or EGF-R (Sibilia and Wagner, 1995) results in mice with wavy whiskers and fur, and disoriented and misaligned hair follicles. However, no mutants have been described with a phenotype resembling β1-deficient hair follicles.
Loss of β1 integrin results in abnormal basal keratinocytes and a hyperthickened epidermis
Keratinocyte-restricted deletion of the β1 integrin gene changed the morphology and the expression profile of the basal keratinocytes in the back skin. The normally cuboidal basal keratinocytes became roundish, polygonal or flattened, had a reduced expression of α6β4 integrin, and showed a decreased proliferative potential. Although these findings suggested a premature differentiation of β1-deficient basal keratinocytes, we could not detect expression of the suprabasal marker protein keratin-10 in the basal keratinocyte layer. Nevertheless, it is possible that other differentiation markers, which we have not used in this study, are expressed in the basal cell layer of mutant mice. Since the additional keratin-10-positive suprabasal layers do not express loricrin, terminal differentiation seems to be delayed in mutant keratinocytes (Mehrel et al., 1990; Hohl et al., 1991). Also, the ubiquitous expression of keratin-6 in the epidermis indicates a disturbed differentiation of the keratinocytes. Such a delay could cause the hyperthickened back skin epidermis of the mutant mice, since no increased proliferation or decreased apoptosis was detected.
β1 integrin is important but not essential for the proliferation of basal keratinocytes in vivo
It has been shown previously that high expression of β1 integrin is a marker for keratinocyte stem cells within the basal keratinocyte layer and that keratinocyte differentiation is associated with a downregulation of β1 integrin (Jones and Watt, 1993; Jones et al., 1995). Furthermore, it is well known that integrin-mediated adhesion to the extracellular matrix and signals provided by growth factors are jointly required to stimulate cyclin-dependent kinases resulting in cell-cycle progression through the G1 phase (Bottazzi and Assoian, 1997). Using our in vivo model we show that loss of β1 integrins in basal cells of the epidermis reduces proliferation and/or facilitates differentiation-inducing signaling pathways. However, ablation of β1 integrin-mediated signaling did not lead to a complete block of proliferation in the keratinocytes of the epidermis. β1 integrin therefore seems to be important, but not essential for cell-cycle progression in basal epithelial cells.
Since loss of β1 integrin resulted in a significant reduction of α6β4 integrin expression by the basal keratinocytes, a reduced signaling through this integrin receptor could also contribute to the cell-cycle defects observed in mutant epidermis. It was shown recently that mice carrying a targeted deletion of the integrin β4 cytoplasmic domain have a significantly reduced number of Ki67-positive cells in the epidermis at E18.5 (Murgia et al., 1998).
The reduced number of cycling cells in the basal keratinocytes corresponded to a decreased expression of the signal transduction molecule Sos, which is involved in the integrin and tyrosine kinase controlled activation of MAPK (Giancotti and Ruoslahti, 1999). β1 integrin signaling to MAPK was shown recently to be essential in maintaining the keratinocyte stem-cell compartment in vitro (Zhu et al., 1999). However, using antibodies against the phosphorylated active form of MAPK, we could not detect any nuclear staining either in normal or in mutant basal keratinocytes. Although this could be a problem of the detection limit, it is also possible that proliferation of keratinocytes in vivo is controlled by other mechanisms.
β1 integrin is important for the maintenance of the basement membrane at the dermal–epidermal junction, but not around the hair follicles
Keratinocyte-specific loss of β1 integrin led to basement membrane defects and blister formation similar to those described for the complete knockout of α3 integrin (DiPersio et al., 1997). In β1-null teratomas, basement membranes were detected but were partially or completely detached from the cells and biochemically altered with a reduced content of both laminin-1 and nidogen (Bloch et al., 1997; Sasaki et al., 1998).
While α3-null mice constitutively lack α3β1 integrin, cre-mediated deletion of β1 in keratinocytes led to a loss of α2β1 and α3β1 during development. β1 mutant mice showed a similar, but more severe phenotype than the α3-null mice, with some areas lacking hemidesmosomes as well as basement membrane. This could be due to the additional loss of α2β1 or to the longer lifespan of the β1-mutant mice, allowing further deterioration than in neonatal α3-null mice. As in the α3-deficient neonates, we observed blister formation due to disruption of the basement membrane itself. This was confirmed by the presence of tiny patches of basement membrane that attached to remaining hemidesmosomes at the epidermal side of the blister, and by the absence of cells expressing keratin-14, -10 or -6 at the dermal side of the blister.
Loss of β1 integrin in the basal keratinocytes is promoting blister formation by various mechanisms. First, it decreases the adhesion of basal keratinocytes to the underlying basement membrane by loss or decreased expression of the laminin receptors α3β1 integrin, α6β4 integrin and dystroglycan. The reduced expression of α6β4 integrin corresponds to a reduction of hemidesmosomal adhesion junctions. Complete absence of functional hemidesmosomes due to mutations in the genes for α6 or β4 integrin leads to severe skin detachment both in man and mice (Georges-Labouesse et al., 1996; van der Neut et al., 1996). Secondly, it results in incomplete processing of basement membrane components and irregular deposition of basement membrane components and anchoring fibril forming collagen VII. Laminin-5 is a major component of anchoring filaments which bridge the surface of basal keratinocytes to the lamina densa, presumably through covalent connections to laminins-6 and -7 (Burgeson, 1996). It also binds tightly to α3β1 integrins (Aumailley and Rousselle, 1999). The absence of β1 integrin does not interfere with the production and secretion of laminin-5, which, however, in the absence of a strong integrin anchor, becomes dislocated from the dermis. Laminin-5 binding to basement membrane components also requires the proteolytic processing of its α3 and γ2 chains including the loss of the γ2L4m module (Burgeson et al., 1996). The increased staining for the γ2L4m module around basal keratinocytes in mutant compared with normal skin indicates that this processing is impaired. This would be an additional cause of the lack of basement membrane association. Deposition of processed laminin-5, nidogen-1 and collagen VII in the mutant dermis where β1 integrin is still highly expressed on fibroblasts points to a crucial role of β1 integrins in organizing the formation of the epidermal basement membrane and also of the anchoring fibrils in vivo.
Interestingly, no such changes were observed at the basement membrane around the hair follicle. Expression of laminin-5, nidogen and collagen IV, as well as of α6 and β4 integrin was similar around hair follicles of mutant and control mice. Also, ultrastructural analysis did not reveal significant changes after loss of β1 integrin expression. The most striking structural difference between the basement membrane around the hair follicles and at the dermal–epidermal junction is the complete lack of hemidesmosomes in the hair follicle, while anchoring fibrils are present (Nutbrown and Randall, 1995). In addition, mechanical stress might be higher in the epidermis than in the tissue surrounding the hair follicles. Obviously, lack of β1 integrin reduces the attachment of the basal keratinocytes to the basement membrane to such a degree that even the remaining hemidesmosomes cannot prevent blistering and ruptures of the basement membrane. Perhaps it is the lack of rigid hemidesmosomal connections that enables the hair follicle basement membrane to survive mechanical stress without permanent damage, enabling ‘gliding’ or transient blisters. Alternatively, the maintenance of the basement membrane around the hair follicle is less dependent on β1 integrins than the epidermal–dermal junction.
Keratinocyte-specific deletion of β1 integrin causes inflammation and dermal fibrosis
Deletion of β1 integrin in the epidermal keratinocytes induced an influx of inflammatory cells, mainly macrophages and to a lesser extent granulocytes and T cells, into the dermis. In parallel, or more likely caused by the inflammation, a fibrosis characterized by upregulation of fibronectin, perlecan and collagen I expression was observed in the dermis of mutant mice. The infiltration of macrophages could have been triggered by the deteriorating hair follicles, which in some cases led to the exposure of the ‘foreign’ hair antigen to the immune system. In line with this explanation, we could find multinucleated foreign-body giant cells surrounding hair remnants and accumulations of macrophages around deformed hair follicles. Phagocytotic removal of damaged hair follicles could explain the complete lack of hairs in older mutant mice. Inflammation and phagocytic hair removal were also observed in mice expressing a dominant-negative form of the EGF-R in keratinocytes (Murillas et al., 1995). A second cause for the inflammation could be the secretion of inflammatory cytokines or chemokines by β1-null keratinocytes. We could find no evidence for bacterial or fungal infection or for a strong acute-phase response, suggesting that the inflammation is not caused by an infection.
Chronic inflammation is known to be the cause of fibrosis in various tissues and organs. Similarly, in our mutant mice, the onset of inflammation was followed by a progressive fibrosis in the dermis, possibly as a result of upregulation of TGF-β1, CTGF and/or activin expression. These cytokines have been shown to play an important role in the induction of extracellular matrix molecule expression by fibroblasts and thus in the pathogenesis of fibrotic disease (reviewed by Lawrence et al., 1996; Brigstock, 1999; Munz et al., 1999). The accumulation of extracellular matrix material led to a stiffening of the skin, an increased number of skin lesions, reduced flexibility of the skin and to the peculiar gait of the knockout mice. These features are also found in patients suffering from scleroderma, a complex connective issue disorder of unknown etiology, which is characterized by excessive extracellular matrix deposition in the dermis (Jimenez et al., 1996). It will be of interest to determine to what degree keratinocytes of the epidermis or the hair follicle are involved in the etiology of scleroderma.
Internal defects could contribute to the reduced lifespan of the mutant mice
The skin of mutant mice still functions as a fluid barrier preventing dehydration, but shows blisters and wounds, especially in mechanically stressed areas. The loss of hairs and subcutaneous fat results in a hypothermic condition and increased energy consumption by the mutant mice. Blisters in the tongue and esophagus, perhaps resulting in trickle bleedings, and bleeding and ulcers in the anus region further deteriorate their condition. Finally, the sclerodermic stiffening of the skin due to excessive deposition of extracellular matrix proteins will restrict the ability of the mice to move normally. While we could not determine a single cause, it is likely that a variety of pathological conditions resulting in reduced food uptake and grossly disturbed development combine to cause the premature death of the mutant mice.
Materials and methods
Generation of conditional knockout mice
The generation of mice carrying a floxed β1 integrin gene was described elsewhere (Potocnik et al., 2000). Briefly, the complete coding and 3′ non-coding region of the β1 integrin gene was flanked by loxP sites. The 3′ loxP site is followed by the intron–exon border of exon 2 and a promoterless lacZ gene. This design enabled activation of the lacZ reporter gene upon cre-mediated deletion of the β1 integrin gene. Transgenic mice expressing the cre recombinase under the control of the keratin-5 promoter (A.Ramirez and J.L.Jorcano, manuscript in preparation) were mated with the floxed β1 integrin mice. Offspring were genotyped and analyzed as described. The mice were kept in a barrier animal facility according to the Swedish rules of animal welfare.
Histology and immunofluorescence
Mouse tissues were dissected, fixed in 4% paraformaldehyde in 0.15 mM phosphate-buffered saline (PBS; pH 7.4) and embedded in paraffin or frozen unfixed in optimal cutting temperature compound (OCT). Paraffin sections of 7 µm thickness and cryo sections of 10 µm thickness were cut and used for histochemistry and immunofluorescence. Hematoxylin–eosin stainings were carried out according to standard procedures. Immunohistochemistry was performed as described previously (Fässler and Meyer, 1995). Antibodies against the following proteins were used: α6 integrin, β4 integrin, CD3, γδ TCR, Gr-1 (all from PharMingen, San Diego, CA), β1 integrin (obtained from S.Johansson, Uppsala, Sweden), β-dystroglycan (obtained from K.Campbell, Iowa City, IA), collagen VII (obtained from L.Bruckner-Tuderman, Münster, Germany), Sos, pan-cadherin (both from Santa Cruz, CA), keratin-10 (DAKO, Denmark), keratin-6, loricrin (both from Babco, Richmond, CA), keratin-14 (Serotec, Germany), F4/80 (Biomedicals, Germany), phospho-p44/42 MAP kinase (NEB, USA) and Ki67 (Dianova, Hamburg, Germany). Affinity-purified antibodies against various extracellular matrix proteins were those used previously (Sasaki et al., 1998). Similar antibodies were also produced against mouse laminin-5 γ2 chain using recombinant fragments γ2L4m (position 185–461) and γ2LE4-6 (position 460–606) following procedures previously used for γ1 chain fragments (Mayer et al., 1993). Cy3- or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were purchased from Sigma (Germany) or Jackson Immunoresearch (West Grove, CA). Actin was detected by Cy3-conjugated phalloidin and BrdU and TUNEL staining was carried out according to the manufacturer’s instructions (Roche Diagnostics, Germany).
Barrier dye penetration assay
The barrier assay was carried out according to Hardman et al. (1998). Four-week-old mutant and control mice, including a nude mouse, were killed with carbon dioxide, fixed for 5 min with methanol, and then incubated for 4 h in 0.5% hematoxylin. After brief washing with water, skin pieces were dissected and snap frozen with OCT. Cryosections of 10 µm thickness were counterstained with eosin.
Flow cytometry
Keratinocytes were isolated from the back skin of 2- to 3-week-old mice as described earlier (Dlugosz et al., 1995), and then stained with monoclonal antibodies as indicated in the figure legends. Dead cells were excluded from analysis on a FACSort (Becton-Dickinson) by propidium iodide (1 µg/ml; Sigma) counterstaining. The following antibodies were used for flow cytometry: FITC anti-β1 integrin (Ha2/5; PharMingen); FITC anti-α6 (GoH3; PharMingen); and anti-β4 integrin (346-11A; PharMingen), visualized with anti-rat-PE (PharMingen).
Electron microscopy
After the mice were killed, small fragments of back skin were fixed overnight in a solution containing 3% paraformaldehyde, 3% glutaraldehyde in 0.2 M Sörensen’s buffer pH 7.4. Tissue fragments were washed for 20 min in 0.2 M Sörensen’s buffer and subsequently treated with 1% osmium tetroxide in PBS for 2 h. Tissue fragments were washed for 20 min in PBS and subsequently treated with 1% osmium tetroxide in PBS for 2 h. After washing, specimens were dehydrated in a graded ethanol series and embedded in epon. For orientation purposes, semithin sections (1 µm) were cut and stained with toluidine blue. Ultrathin sections (70–80 nm) were then cut with a Reichert–Jung ultramicrotome, collected on formvar-coated nickel grids, stained with uranyl acetate for 10 min and with lead citrate for 7 min. Samples were examined with a Zeiss EM 109 electron microscope (Zeiss, Germany).
RNase protection assay
RNA isolation was carried out as described by Chomczynski and Sacchi (1987). RNase protection analysis was performed as described by Werner et al. (1993). As a loading control, 1 µg of all RNA samples was loaded on a 1% agarose gel before hybridization and stained with ethidium bromide. All protection assays were carried out at least in duplicate with RNAs from different skin samples. The same RNA preparations were used for all protection assays. The IL-1β and mouse activin βA cDNA templates used for the RNase protection assay were described by Hübner et al. (1996), the CTGF probe was described by Dammeier et al. (1998), and the TGF-β1 probe was described by Frank et al. (1996).
Acknowledgments
Acknowledgements
We thank Drs Kevin Campbell, Leena Bruckner-Tuderman and Staffan Johansson for antibodies, Dr Wiliam Agace for help with the T-cell stainings, Mrs Keiko Sakai for technical assistance, Sebastian Barg for help with the confocal microscopy, Dr Carolyn Byrne for advice with the barrier assay, and Drs Mike Dictor and Ray Boot-Handford for discussions and critical reading of the manuscript. The work was supported by grants from the Swedish National Science Foundation, the SSF-Inflammations programme, Österlund Foundation, Deutscher Akademischer Austauschdienst (to R.F. and S.W.) and the Human Frontier Science Program (to S.W.).
References
- Aumailley M. and Rousselle,P. (1999) Laminins of the dermo-epidermal junction. Matrix Biol., 18, 19–28. [DOI] [PubMed] [Google Scholar]
- Bagutti C., Wobus,A.M., Fässler,R. and Watt,F.M. (1996) Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and β1 integrin-deficient cells. Dev. Biol., 179, 184–196. [DOI] [PubMed] [Google Scholar]
- Bloch W. et al. (1997) β1 integrin is essential for teratoma growth and angiogenesis. J. Cell Biol., 139, 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi M.E. and Assoian,R.K. (1997) The extracellular matrix and mitogenic growth factors control G1 phase cyclins and cyclin-dependent kinase inhibitors. Trends Cell Biol., 7, 348–352. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Hirsch,E., Potocnik,A. and Fässler,R. (1997) Genetic analysis of β1 integrin function: confirmed, new and revised roles for a crucial family of cell adhesion molecules. J. Cell Sci., 110, 2895–2904. [DOI] [PubMed] [Google Scholar]
- Brigstock D.R. (1999) The connective tissue growth factor/cysteine-rich 61/nephroblastome overexpressed (CCN) family. Endocrine Rev., 20, 189–206. [DOI] [PubMed] [Google Scholar]
- Burgeson R.E. (1996) Laminins in epidermal structures. In Ekblom,P. and Timpl,R. (eds), The Laminins. Harwood Academic, Reading, UK, pp. 65–96. [Google Scholar]
- Carroll J.M., Rosario Romero,M. and Watt,F.M. (1995) Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell, 83, 957–968. [DOI] [PubMed] [Google Scholar]
- Chiang C. et al. (1999) Essential role for sonic hedgehog during hair follicle morphogenesis. Dev. Biol., 205, 1–9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. and Sacchi,N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem., 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Commo S. and Bernard,B.A. (1997) The distribution of α2β1, α3β1 and α6β4 integrins identifies distinct subpopulations of basal keratinocytes in the outer root sheath of the human anagen hair follicle. Cell. Mol. Life Sci., 53, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammeier J., Beer,H.-D., Brauchle,M. and Werner,S. (1998) Dexamethasone is a novel potent inducer of connective tissue growth factor expression. J. Biol. Chem., 273, 18185–18190. [DOI] [PubMed] [Google Scholar]
- DiPersio C.M., Hodivala-Dilke,K.M., Jaenisch,R., Kreidberg,J.A. and Hynes,R.O. (1997) α3β1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol., 137, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A.A., Glick,A.B., Tennebaum,T., Weinberg,W.C. and Yuspa,S.H. (1995) Isolation and utilisation of epidermal keratinocytes for oncogene research. Methods Enzymol., 254, 3–20. [DOI] [PubMed] [Google Scholar]
- Fässler R. and Meyer,M. (1995) Consequences of lack of β1 integrin gene expression in mice. Genes Dev., 9, 1896–1908. [DOI] [PubMed] [Google Scholar]
- Frank S., Madlener,M. and Werner,S. (1996) Transforming growth factors β1, β2, β3 and their receptors are differentially regulated during normal and impaired wound healing. J. Biol. Chem., 271, 10188–10193. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E., Messaddeq,N., Yehia,G., Cadalbert,L., Dierich,A. and Le Meur,M. (1996) Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nature Genet., 13, 370–373. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti,E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Gibbs H.F. (1941) A study of the post-natal development of the skin and hair of the mouse. Anat. Rec., 80, 61–81. [Google Scholar]
- Greenhalgh D.A. and Roop,D.R. (1993) Targeted overexpression of TGFα in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis and spontaneous, squamous papillomas. Cell Growth Differ., 4, 1071–1082. [PubMed] [Google Scholar]
- Hardman M.J., Sisi,P., Banbury,D.N. and Byrne,C. (1998) Patterned acquisition of skin barrier function during development. Development, 125, 1541–1552. [DOI] [PubMed] [Google Scholar]
- Hardy M.H. (1992) The secret life of the hair follicle. Trends Genet., 8, 55–61. [DOI] [PubMed] [Google Scholar]
- Henry M.D. and Campbell,K.P. (1998) A role for dystroglycan in basement membrane assembly. Cell, 95, 859–870. [DOI] [PubMed] [Google Scholar]
- Hodivala K.J. and Watt,F.M. (1994) Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol., 124, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl D., Mehrel,T., Lichti,M., Turner,L., Roop,D.R. and Steinert,P.M. (1991) Characterisation of human loricrin. Structure and function of a new class of epidermal envelope proteins. J. Biol. Chem., 266, 6626–6636. [PubMed] [Google Scholar]
- Hotchin N.A., Gandarillas,A. and Watt,F.M. (1995) Regulation of cell surface β 1 integrin levels during keratinocyte terminal differentiation. J. Cell Biol., 128, 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner G., Brauchle,M., Smola,H., Madlener,M., Fässler,R. and Werner,S. (1996) Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine, 8, 548–556. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Jimenez S.A., Hitraya,E. and Varga,J. (1996) Pathogenesis of scleroderma. Collagen. Rheum. Dis. Clin. North Am., 22, 647–674. [DOI] [PubMed] [Google Scholar]
- Jones P.H. and Watt,F.M. (1993) Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell, 73, 713–724. [DOI] [PubMed] [Google Scholar]
- Jones P.H., Harper,S. and Watt,F.M. (1995) Stem cell patterning and fate in human epidermis. Cell, 80, 83–93. [DOI] [PubMed] [Google Scholar]
- Kreidberg J.A., Donovan,M.J., Goldstein,S.L., Rennke,H., Shepherd,K., Jones,R.C. and Jaenisch,R. (1996) α3β1 integrin has a crucial role in kidney and lung organogenesis. Development, 122, 3537–3547. [DOI] [PubMed] [Google Scholar]
- Lawrence D.A. (1996) Transforming growth factor-β: a general review. Eur. Cytokine Netw., 7, 363–374. [PubMed] [Google Scholar]
- Lindner G., Botchkarev,V.A., Botchkareva,N.V., Ling,G., van der Veen,C. and Paus,R. (1997) Analysis of apoptosis during hair follicle regression. Am. J. Pathol., 151, 1601–1617. [PMC free article] [PubMed] [Google Scholar]
- Luetteke N.C., Qiu,T.H., Peiffer,R.L., Oliver,P., Smithies,O. and Lee,D.C. (1993) TGFα deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell, 73, 263–278. [DOI] [PubMed] [Google Scholar]
- Mahony D., Karunaratne,S. and Rothnagel,J.A. (1999) The companion layer and outer root sheath of the anagen hair follicle. Exp. Dermatol., 8, 329–331. [PubMed] [Google Scholar]
- Maurer M., Paus,R. and Czarnetzki,B.M. (1995) Mast cells as modulators of hair follicle cycling. Exp. Dermatol., 4, 266–271. [DOI] [PubMed] [Google Scholar]
- Mayer U., Nischt,R., Pöschl,E., Mann,K., Fukuda,K., Gerl,M., Yamada,Y. and Timpl,R. (1993) A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J., 12, 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrel T. et al. (1990) Identification of a major keratinocyte cell envelope protein, loricrin. Cell, 61, 1103–1112. [DOI] [PubMed] [Google Scholar]
- Munz B. et al. (1999) Overexpression of activin in the epidermis of transgenic mice reveals new activities of activin in keratinocyte differentiation, cutaneous fibrosis and wound repair. EMBO J., 18, 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia C., Blaikie,P., Kim,N., Dans,M., Petrie,H.T. and Giancotti,F.G. (1998) Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J., 17, 3940–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillas R., Larcher,F., Conti,C.J., Santos,M., Ullrich,A. and Jorcano,J.L. (1995) Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J., 14, 5216–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievers M.G., Schaapveld,R.Q. and Sonnenberg,A. (1999) Biology and function of hemidesmosomes. Matrix Biol., 18, 5–17. [DOI] [PubMed] [Google Scholar]
- Nutbrown M. and Randall,V.A. (1995) Differences between connective tissue–epithelial junctions in human skin and the anagen hair follicle. J. Invest. Dermatol., 104, 90–94. [DOI] [PubMed] [Google Scholar]
- Oro A.E. and Scott,M.P. (1998) Splitting hairs: Dissecting roles of signaling in epidermal development. Cell, 95, 575–578. [DOI] [PubMed] [Google Scholar]
- Panteleyev A.A., Botchkareva,N.V., Sundberg,J.P., Christiano,A.M. and Paus,R. (1999) The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am. J. Pathol., 155, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R. and Cotsarelis,G. (1999) The biology of hair follicles. N. Engl. J. Med., 341, 491–497. [DOI] [PubMed] [Google Scholar]
- Pinkus H. (1958) Embryology of hair. In Montagna,W. and Ellis,R.A. (eds), The Biology of Hair Growth. Academic Press, New York, NY. pp. 1–32. [Google Scholar]
- Potocnik A.J., Brakebusch,C. and Fässler,R. (2000) Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen and bone marrow. Immunity, 12, 653–663. [DOI] [PubMed] [Google Scholar]
- Ramirez A., Bravo,A., Jorcano,J.L. and Vidal,M. (1994) Sequences 5′ of the bovine keratin-5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation, 58, 53–64. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Forsberg,E., Bloch,W., Addicks,K., Fässler,R. and Timpl,R. (1998) Deficiency of β1 integrins in teratoma interferes with basement membrane assembly and laminin-1 expression. Exp. Cell Res., 238, 70–81. [DOI] [PubMed] [Google Scholar]
- Sellheyer K. et al. (1993) Inhibition of skin development by overexpression of TGFβ 1 in the epidermis of transgenic mice. Proc. Natl Acad. Sci. USA, 90, 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M. and Wagner,E.F. (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science, 269, 234–238. [DOI] [PubMed] [Google Scholar]
- St Jacques B. et al. (1998) Sonic hedgehog signalling is essential for hair development. Curr. Biol., 8, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Stoler A., Kopan,R., Duvic,M. and Fuchs,E. (1988) Use of monospecific antisera and cRNA probes to localise major changes in keratin expression during normal and abnormal epithelial differentiation. J. Cell Biol., 107, 427–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R., Krimpenfort,P., Calafat,J., Niessen,C.M. and Sonnenberg,A. (1996) Epithelial detachment due to the absence of hemidesmosomes in integrin β4 null mice. Nature Genet., 13, 366–369. [DOI] [PubMed] [Google Scholar]
- van Genderen C., Okamura,R.M., Farinas,I., Quo,R.-G., Parslow,T.G., Bruhn,L. and Grosschedl,R. (1994) Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in Lef-1-deficient mice. Genes Dev., 8, 2691–2703. [DOI] [PubMed] [Google Scholar]
- Watt F.M. and Hertle,M.D. (1994) Keratinocyte integrins. In Leigh,I.M., Lane,E.B. and Watt,F.M. (eds), The Keratinocyte Handbook. Cambridge University Press, Cambridge, UK, pp. 153–164. [Google Scholar]
- Werner S., Weinberg,W., Liao,X., Peters,K.G., Blessing,M., Yuspa,S.H., Weiner,R.I. and Williams,L.T. (1993) Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. EMBO J., 12, 2635–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K. (1998) Gene-knockout mice with abnormal epidermal and hair follicular development. J. Dermatol. Sci., 18, 75–89. [DOI] [PubMed] [Google Scholar]
- Zhu A.J. and Watt,F.M. (1996) Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J. Cell Sci., 109, 3013–3023. [DOI] [PubMed] [Google Scholar]
- Zhu A.J., Haase,I. and Watt F. (1999) Signaling via β1 integrins and MAPK determines human epidermal stem cell fate in vitro. Proc. Natl Acad. Sci. USA, 96, 6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]