Abstract
Lay Abstract
Limited reciprocal social interactions are central to the diagnosis of autism. Behavioral intervention programs are effective in improving social and communication skills in children with autism. We are employing mouse models to understand the individual components of behavioral interventions which effectively improve social interactions. BTBR T+tf/J (BTBR) is an inbred strain of mice that exhibits multiple social deficits, unusual vocalizations, and high levels of repetitive behaviors, which are relevant to all three diagnostic symptom categories of autism. C57BL/6J (B6) is an inbred strain of mice that exhibits high sociability and low repetitive behaviors. We reasoned that these mouse strains with low versus high social interactions might be useful for evaluating social peer enrichment as a behavioral intervention during adolescence. Juvenile BTBR and B6 of the same sex were placed in a same home cage and lived together as cagemates until they reached young adulthood. The two control groups were juvenile B6 housed together, and juvenile BTBR housed together. B6 controls that lived with B6 cagemates showed their strain-typical high sociability. BTBR controls that lived with BTBR cagemates showed their strain-typical low sociability. Remarkably, BTBR that shared home cages with B6 showed high sociability as young adults. Peer rearing for either 20 days or 40 days were equally effective. These results from a robust mouse model of autism support the strategy of early behavioral intervention for treating the social domain in autism spectrum disorders, including beneficial interactions with social peers.
Scientific Abstract
Behavioral therapies are currently the most effective interventions for treating the diagnostic symptoms of autism. We employed a mouse model of autism to evaluate components of behavioral interventions that improve sociability in mice. BTBR T+tf/J (BTBR) is an inbred mouse strain that exhibits prominent behavioral phenotypes with face validity to all three diagnostic symptom categories of autism, including robust and well-replicated deficits in social approach and reciprocal social interactions. To investigate the role of peer interactions in the development of sociability, BTBR juvenile mice were reared in the same home cage with juvenile mice of a highly social inbred strain, C57BL/6J (B6). Subject mice were tested as young adults for sociability and repetitive behaviors. B6 controls reared with B6 showed their strain-typical high sociability. BTBR controls reared with BTBR cagemates showed their strain-typical lack of sociability. In contrast, BTBR reared with B6 as juveniles showed significant sociability as young adults. A 20-day intervention was as effective as a 40-day intervention for improving social approach behavior. High levels of repetitive self-grooming by BTBR were not rescued by peer-rearing with B6, indicating specificity of the intervention to the social domain. These results from a robust mouse model of autism support the interpretation that social enrichment with juvenile peers is a beneficial intervention for improving adult outcome in the social domain. This novel paradigm may prove useful for discovering factors that are essential for effective behavioral treatments, and biological mechanisms underlying effective behavioral interventions.
Keywords: Autism, BTBR inbred strain, mouse model, peer enrichment, social enrichment, behavioral intervention
Introduction
The first diagnostic symptom category of autism focuses on abnormalities in reciprocal social interactions (American Psychiatric Association, 2000). Prominent deficits in the social domain differentiate children with autism spectrum disorders from those with other developmental disorders (Klin et al., 2007; Reichow & Volkmar, 2009). Compared to typically developing peers, individuals with autism lack social skills that are necessary to interact with others and to form friendships (Causton-Theoharis, Ashby, & Cosier, 2009; Levy, Mandell, & Schultz, 2009). A lack of social experience during critical neurodevelopmental periods may amplify genetic and environmental risk factors, hampering normal development of social behaviors throughout life (Dawson, 2008). Although symptoms of autism tend to be lifelong (Billstedt, Gillberg, & Gillberg, 2005, 2007; Cederlund, Hagberg, Billstedt, Gillberg, & Gillberg, 2008; Nordin & Gillberg, 1998), early intensive behavioral interventions can result in substantial improvements in social and communication skills for a large proportion of children with autism (Dawson, 2008; Geschwind, 2009; Rogers, 2000; Sallows & Graupner, 2005). Behavioral intervention strategies have empirical support as effective treatment options for autism spectrum disorders (National Research Council, 2001; Vismara & Rogers, 2010). There are no effective pharmacological treatments for the social deficits in autism, and intensive research efforts are dedicated to finding drugs that could improve symptoms (Bartz & Hollander, 2008; Bear, Huber, & Warren, 2004; Blundell et al., 2010; Dolen et al., 2007; Ehninger et al., 2008; Hagerman et al., 2009; Silverman, Tolu, Barkan, & Crawley, 2009; West, Waldrop, & Brunssen, 2009; Wirojanan et al., 2009; Zhou et al., 2009)
Behavioral intervention programs based on principles of the Applied Behavioral Analysis (ABA) (Geschwind, 2009; Levy et al., 2009; Volkmar, Chawarska, & Klin, 2005) are generally adult directed (Reichow & Volkmar, 2009; Rogers & Vismara, 2008). Peer-mediated or child-directed intervention programs offer a complementary approach, in which typically developing peers with good social skills are instructed to actively engage in social interactions with children with autism by initiating, prompting, and responding (Goldstein, Kaczmarek, Pennington, & Shafer, 1992; Prendeville, Prelock, & Unwin, 2006; Trembath, Balandin, Togher, & Stancliffe, 2009). Sessions usually take place in natural settings such as school classrooms, playgrounds, or homes. Peer interventions programs such as the Integrated Play Group (IPG); Stay, Play, and Talk, Learning Experiences: An Alternate Program (LEAP); and Floor Time Play Dates have been reported to improve social-communicative behaviors including joint attention, reciprocal play behaviors, language development, and academic performance in many children with autism (Betz, Higbee, & Reagon, 2008; Eikeseth, Smith, Jahr, & Eldevik, 2002; Goldstein, English, Shafer, & Kaczmarek, 1997; Goldstein et al., 1992; Harper, Symon, & Frea, 2008; Hwang & Hughes, 2000; Kamps, Barbetta, Leonard, & Delquadri, 1994; Kohler et al., 1995; Landa, 2008; McGee, Almeida, Sulzer-Azaroff, & Feldman, 1992; Rogers, 2000; Rogers & Vismara, 2008; Strain & Kohler, 1998; Trembath et al., 2009; Wolfberg & Schuler, 1993; T. R. Yang, Wolfberg, Wu, & Hwu, 2003; Zercher, Hunt, Schuler, & Webster, 2001). Characteristics of successful programs are summarized in a number of reviews (Dawson, 2008; Koegel, Koegel, Frea, & Fredeen, 2001; Prendeville et al., 2006; Reichow & Volkmar, 2009; Rogers, 2000). These programs differ greatly in many ways, e.g. the characteristics of subjects, target behaviors, the characteristics of interventionists (e.g. age, sex, familiarity, amount of training received from an expert prior to the intervention sessions) intervention strategies, partner-to-subject ratio, and length of intervention. However, few rigorous randomized controlled studies of peer interventions have been reported (Dawson et al., 2009; Levy et al., 2009; Ospina et al., 2008). Current knowledge about the roles of these factors is based primarily on retrospective reviews which compare features of published studies (Hwang & Hughes, 2000; Koegel et al., 2001; Prendeville et al., 2006; Rogers, 2000). The question of which interventions and factors are most beneficial for improving social behaviors remains to be empirically tested.
While there is no substitute for randomized controlled human trials, a robust mouse model offers a translational tool to evaluate treatments for components of autism spectrum disorders. Mice are a social species which can be experimentally investigated to determine the early developmental factors that affect adult social behaviors (Crawley, 2004, 2007; Silverman, Yang, Lord, & Crawley, 2010; M. Yang, Scattoni, Chadman, Silverman, & Crawley, 2010). Inbred strains of mice are genetically homogeneous populations that exhibit large variations in social behaviors across strains (Bolivar, Walters, & Phoenix, 2007; Brodkin, 2007; Moy et al., 2008; Moy et al., 2007; Panksepp & Lahvis, 2007; Winslow, 2003). BTBR T+tf/J (BTBR) is an inbred strain that incorporates behavioral traits relevant to all three diagnostic symptoms of autism. As compared to the standard social C57BL/6J (B6) inbred strain, BTBR display low levels of reciprocal social interaction as juveniles, low social approach as adults, low social transmission of food preference, unusual patterns of vocalization as pups, fewer ultrasonic vocalizations in response to social cues as adults, and high repetitive self-grooming that persists throughout development (Bolivar et al., 2007; McFarlane et al., 2008; Moy et al., 2004; Moy et al., 2008; Scattoni, Gandhy, Ricceri, & Crawley, 2008; Silverman et al., 2009; Wöhr, Roullet, & Crawley, 2010; M. Yang, Clarke, & Crawley, 2009; M. Yang et al., 2007a; M. Yang, Zhodzishsky, & Crawley, 2007b). We previously discovered that cross-fostering of BTBR pups with B6 mothers did not improve sociability in BTBR, indicating that early maternal care was not a major factor in determining the social impairments of adult BTBR (M. Yang et al., 2007b). As a second hypothesis, we considered the possibility that the development of social behaviors in mice could be influenced by the interactions of cagemates living together in standard home cages. Specifically, we postulated that BTBR juveniles raised with B6 juveniles might benefit from peer interactions with social partners during adolescence. To directly test this hypothesis, home cages were assembled at the time of weaning to generate social groupings of a) juvenile B6, b) juvenile BTBR, and c) a combination of juvenile B6 + BTBR. Critical length of peer intervention was evaluated by comparing 20 and 40 days of peer rearing. Subject mice were tested as adults for social approach in our automated three-chambered apparatus, as well as an independent measure relevant to the third diagnostic symptom of autism, repetitive self-grooming. Both males and females were used as subjects (Newschaffer et al., 2007; Veenstra-Vanderweele, Christian, & Cook, 2004). Given the evidence that shows normal social and emotional adjustments in some siblings of children with autism (Kaminsky & Dewey, 2002; Macks & Reeve, 2007; Pilowsky, Yirmiya, Doppelt, Gross-Tsur, & Shalev, 2004; Verte, Roeyers, & Buysse, 2003), we did not test B6 cagemates of BTBR subjects.
Materials and Methods
Animals
C57BL/6J (B6) and BTBR T+tf/J (BTBR) breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at NIMH in Bethesda, Maryland. Breeding, housing, and behavioral testing were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. The colony room was maintained on a 12:12 light/dark cycle with lights on at 7:00 AM, at approximately 20°C and 55% humidity.
At postnatal day 20–21, juveniles were weaned and housed with same sex, age-matched cagemates in cages of 4 mice per cage. Cohort 1 consisted of a) 16 experimental cages, 8 male and 8 female, each containing 2 B6 littermates and 2 BTBR littermates; b) 8 control cages, 4 male and 4 female, each containing 4 B6 littermates; and c) 8 control cages, 4 male and 4 female, each containing 4 BTBR littermates. Cohort 2 consisted of a) 16 experimental cages, 8 male and 8 female, each containing 2 B6 littermates and 2 BTBR littermates; b) 8 control cages, 4 male and 4 female, each containing two sets of B6 littermates from two litters; and c) 8 control cages, 4 male and 4 female, each containing two sets of BTBR littermates from two litters.
Adult social approach task
Adult sociability testing was conducted between 10:00 AM and 4:00 PM. Mice used as the novel targets in the social approach task were 129Sv/ImJ mice of the same gender as the subject, aged 8–14 weeks old, purchased from The Jackson Laboratory. The novel mice were previously habituated to the apparatus but had no prior physical contact with subject mice, as previously described (Chadman et al., 2008; Silverman et al., 2009; M. Yang et al., 2009; M. Yang et al., 2007b).
Social approach behaviors were scored in an automated three-chambered apparatus using methods previously described (Chadman et al., 2008; Crawley et al., 2007; McFarlane et al., 2008; Moy et al., 2009; Moy et al., 2008; Silverman et al., 2009; M. Yang et al., 2009; M. Yang et al., 2007a; M. Yang et al., 2007b). In this test, sociability is defined by the subject mouse spending more time in the side chamber with a novel mouse than in the side chamber with a novel object, and more time sniffing the novel mouse than the novel object, during a ten minute test session. The apparatus was a rectangular, three-chambered box made of clear polycarbonate. Retractable doorways built into the two dividing walls controlled access to the side chambers. Number of entries and time spent in each chamber were automatically detected by photocells embedded in the doorways and tallied by the software. The test session began with a 10 min habituation session in the center chamber only, followed by a 10 min habituation to all three empty chambers. Lack of innate side preference was confirmed during the second 10 minute habituation. The subject was then briefly confined to the center chamber. A novel object (an inverted stainless steel wire pencil cup, Galaxy, Kitchen Plus, http://www.kitchen-plus.com) was placed in one of the side chambers. A novel mouse, previously habituated to the enclosure, was placed in an identical wire cup located in the other side chamber. A disposable plastic drinking cup containing a lead weight was placed on the top of each inverted wire pencil cup to prevent the subject from climbing on top. The side containing the novel object and the novel mouse alternated between the left and right chambers across subjects. After both stimuli were positioned, the two side doors were simultaneously lifted and the subject was allowed access to all three chambers for 10 min. In addition to the automatically tallied parameters (time spent in each chamber and entries into each chamber), an observer with two stopwatches scored cumulative time spent sniffing the novel object and the novel mouse. The apparatus was cleaned with 70% ethanol and water between subjects.
Repetitive self-grooming
To test for repetitive self-grooming, each mouse was individually placed in a clean empty cage without bedding and with lid on. After a 10 min habituation period in the empty cage, the subject mouse was scored for 10 min for cumulative time spent grooming. The investigator sat approximately 2 m from the test cage and recorded time spent in grooming with a stopwatch.
Cohort 1 was tested for adult social approach and self-grooming on postnatal day 61, i.e. after animals had been weaned from the mother and lived with cagemates for 40 days. Cohort 2 was tested for adult social approach on postnatal day 41, i.e. after animals had been weaned from the mother and lived with cagemates for 20 days. The control groups, B6 living with B6 cagemates and BTBR living with BTBR cagemates, were tested at the same age as BTBR subjects.
Statistical analysis
For the automated social approach test, time spent in the two side chambers was compared using within-group Repeated Measures ANOVAs, with the factor of chamber side (novel mouse side vs. novel object side). Time spent in the center chamber was included on the graphs for illustrative purposes, but was not included in the statistical analyses, for time in the three chambers adds to 100% and therefore the time spent in the left chamber, center chamber, and right chamber are not independent. Time spent sniffing the novel mouse versus the novel object was similarly analyzed using within-group Repeated Measures ANOVAs, with the factor of chamber side (novel mouse side vs. novel object side). One Way ANOVA was used to analyze total number of entries to the side chambers, and to analyze time spent self-grooming in an empty cage. Scheffe post hoc tests were used to compare individual groups following a significant ANOVA. Significance was defined as p < .05.
Results
Cohort 1. 40-day peer-enriched intervention improved sociability in BTBR male and female mice
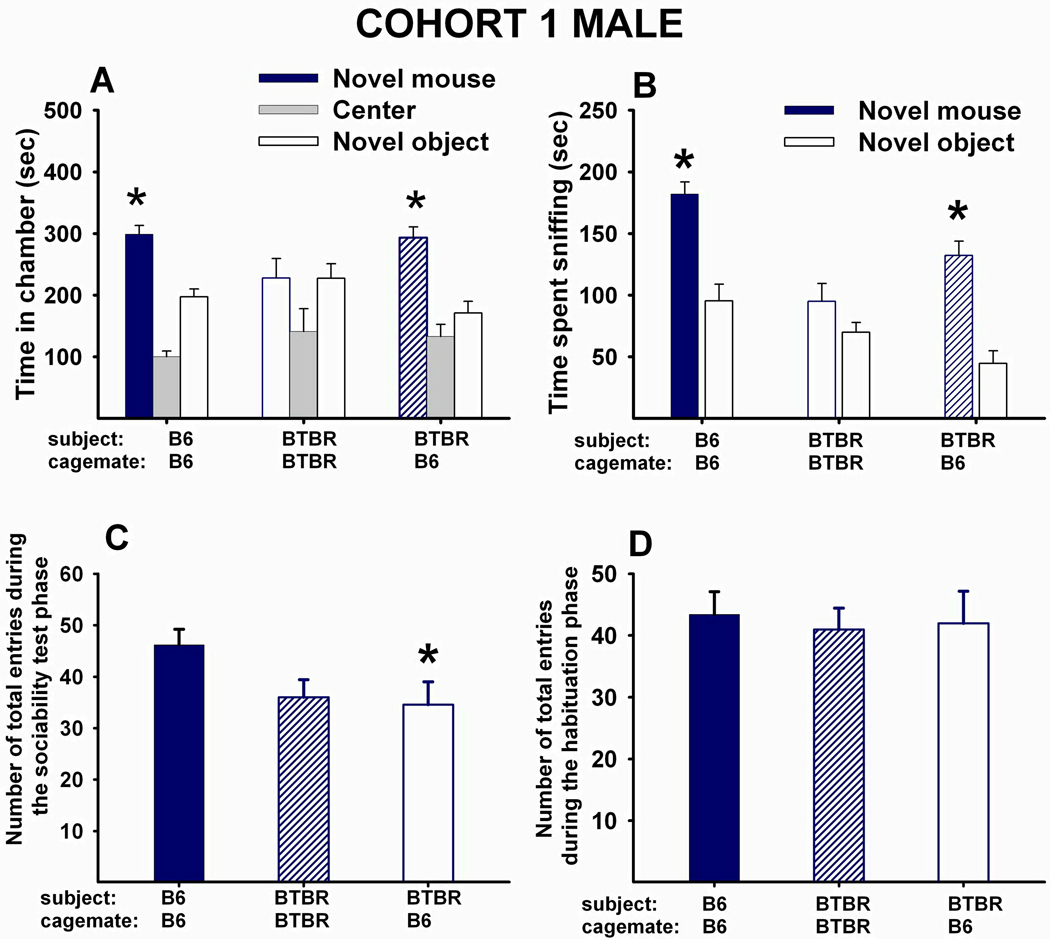
Fig 1 A–D shows social approach scores and number of entries of Cohort 1 males. (A) Adult male B6 that had been reared as juveniles with juvenile male B6 displayed typical sociability (N=16, F1,15=23.79, p<.001). Adult male BTBR reared as juveniles with juvenile male BTBR did not display sociability (N=16, F1,15=0.34, NS). Adult male BTBR reared as juveniles with juvenile male B6 displayed significant sociability (N=16, F1,15=16.02, p<.001). (B) Adult male B6 reared as juveniles with juvenile male B6 spent significantly more time sniffing the novel mouse than the novel object (F1,15=43.42, p<.001). Adult male BTBR reared as juveniles with juvenile male BTBR did not spend more time sniffing the novel mouse than the novel object (F1,15=2.12, NS). Adult male BTBR reared as juveniles with juvenile male B6 spent significantly more time sniffing the novel mouse than the novel object (F1,15=31.89, p<.001). (C) The main effect of social housing was significant for the number of total entries to the side chambers during the sociability test phase, (F2,45=3.43, p<.05). Posthoc Scheffe test showed that male BTBR reared with B6 made fewer entries into the side chambers as compared to male B6 controls (p<.05). (D) The main effect of social housing was not significant for the number of total entries to the side chambers during the habituation phase that preceded the social approach task (F2,45=1.14, NS), indicating no differences across groups in locomotor abilities or exploration of a novel non-social environment.
Figure 1. 40-day peer-enriched intervention improved sociability in BTBR male mice.
Sociability is defined as spending more time in the side chamber with a novel mouse than in the side chamber with a novel object, and more time sniffing the novel mouse than the novel object, during a ten minute test session in the automated three-chambered apparatus. (A) Time in chambers containing a novel object or novel mouse. Male B6 that were reared as juveniles with juvenile male B6 for 40 days displayed typical sociability as adults (N=16, F1,15=23.79, p<.001). Male BTBR reared as juveniles with juvenile male BTBR for 40 days did not display sociability as adults (N=16, F1,15=0.34, NS). Male BTBR reared as juveniles with juvenile male B6 for 40 days displayed significant sociability as adults (N=16, F1,15=16.02, p<.001). (B) Time spent sniffing the novel object and the novel mouse. Male B6 reared as juveniles with juvenile male B6 for 40 days displayed significant sociability as adults (N=16, F1,15=43.42, p<.001). Male BTBR reared as juveniles with juvenile male BTBR did not display significant sociability (N=16, F1,15=2.12, NS). Male BTBR reared as juveniles with juvenile male B6 spent significantly more time sniffing the novel mouse than the novel object as adults (N=16, F1,15=31.89, p<.001). (C) Total number of entries to the side chambers during the social approach task. A significant main effect of social housing was found (N=16 for each group, F2,45=3.43, p<.05), with male BTBR reared with B6 making fewer entries into the side chambers as compared to B6 control males (p<.05). (D) Total number of entries to the side chambers during the habituation phase that preceded the social approach task. No significant effect of social housing was found (N=16 for each group, F2,45=1.14, NS). (A and B) *p < .05 for comparison between novel mouse and novel object sides. Bars for the novel mouse are outlined in color, while bars for the novel object are outlined in black, in panels A and B of Figures 1–4. (C) *p < .05 as compared to B6 reared with B6.
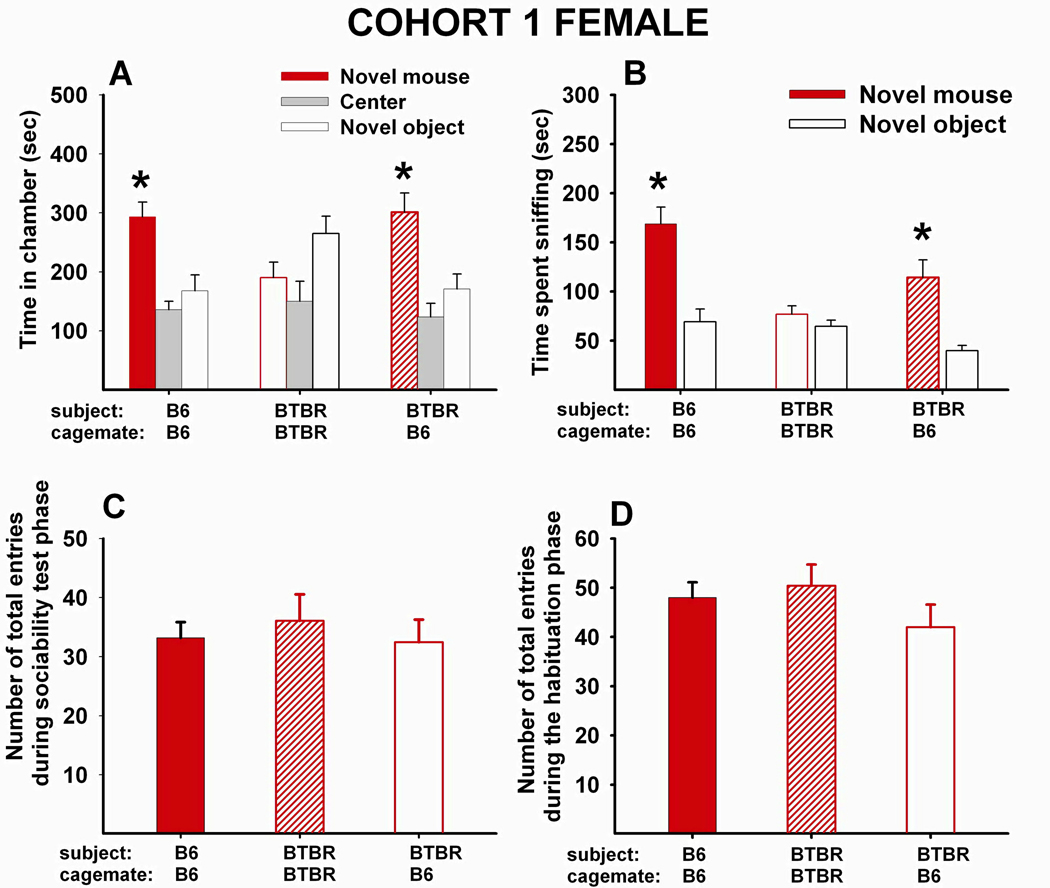
Fig 2 A–D shows social approach scores and number of entries for Cohort 1 females. (A) Adult female B6 that had been reared as juveniles with juvenile female B6 displayed typical sociability (N=16, F1,15=8.94, p<.01). Adult female BTBR reared as juveniles with juvenile female BTBR did not display sociability, spending more time in the chamber containing the novel object than in the chamber containing the novel mouse (N=16, F1,15=5.29, p<.05). Adult female BTBR reared as juveniles with juvenile female B6 displayed significant sociability (N=16, F1,15=12.7, p<.01). (B) Adult female B6 reared as juveniles with juvenile female B6 spent significantly more time sniffing the novel mouse than the novel object (F1,15=24.76, p<.001). Adult female BTBR reared as juveniles with juvenile female BTBR did not spend more time sniffing the novel mouse than the novel object (F1,15=0.18, NS). Adult female BTBR reared as juveniles with juvenile female B6 spent significantly more time sniffing the novel mouse than the novel object (F1,15=21.04, p<.001). (C) The main effect of social housing was not significant for the number of total entries to the side chambers during the sociability test phase, (F2,45=0.72, NS). (D) The main effect of social housing was not significant for the number of total entries to the side chambers during the habituation phase (F2,45=0.07, NS).
Figure 2. 40-day peer-enriched intervention improved sociability in BTBR female mice.
(A) Time in chambers containing a novel object or novel mouse. Female B6 that were reared as juveniles with juvenile female B6 for 40 days displayed typical sociability as adults (N=16, F1,15=8.94, p<.01). Female BTBR reared as juveniles with juvenile female BTBR for 40 days did not display sociability as adults (N=16, F1,15=5.29, p<.05). Female BTBR reared as juveniles with juvenile female B6 for 40 days displayed significant sociability as adults (N=16, F1,15=12.7, p<.01). (B) Time spent sniffing the novel object and the novel mouse. Female B6 reared as juveniles with juvenile female B6 for 40 days displayed significant sociability as adults (F1,15=24.76, p<.001). Female BTBR reared as juveniles with juvenile female BTBR did not display significant sociability (F1,15=0.18, NS). Female BTBR reared as juveniles with juvenile female B6 spent significantly more time sniffing the novel mouse than the novel object as adults (F1,15=21.04, p<.001). (C) Total number of entries to the side chambers during the social approach task. No significant effect of social housing was found (N=16 for each group, F2,45=0.72, NS). (D) Total number of entries to the side chambers during the habituation phase that preceded the social approach task. No significant effect of social housing was found (N=16 for each group, F2,45=0.73, NS). (A and B) *p < .05 for comparison between novel mouse and novel object sides.
Cohort 2. 20-day peer-enriched intervention improved sociability in BTBR male and female mice
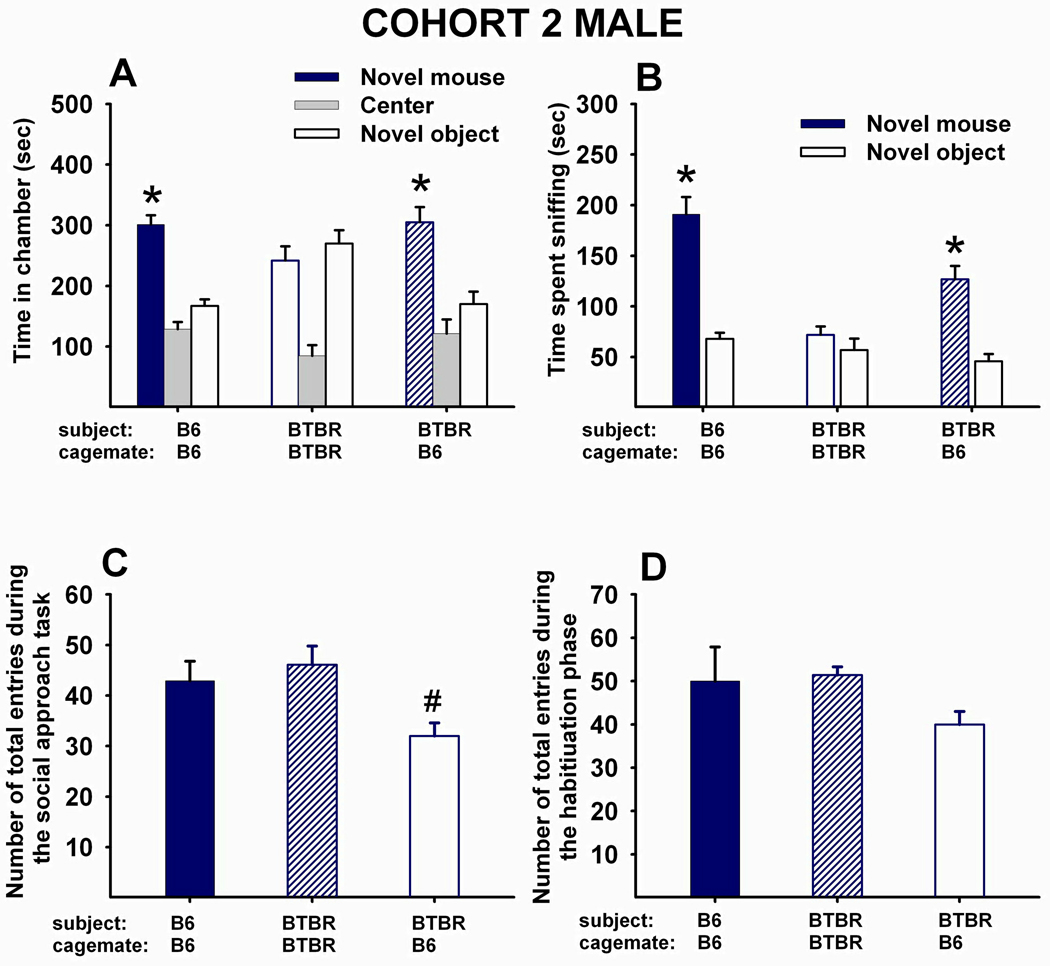
Fig 3 A–D shows social approach scores of Cohort 2 males. (A) Adult male B6 that had been reared as juveniles with juvenile male B6 displayed typical sociability (N=8, F1,7=93.77, p<.0001). Adult male BTBR reared as juveniles with juvenile male BTBR did not display sociability (N=8, F1,7=0.046, NS). Adult male BTBR reared as juveniles with juvenile male B6 displayed significant sociability (N=16, F1,15=12.24, p<.01). (B) Adult male B6 reared as juveniles with juvenile male B6 spent significantly more time sniffing the novel mouse than the novel object (F1,7=117.84, p<.001). Adult male BTBR reared as juveniles with juvenile male BTBR did not spend more time sniffing the novel mouse than the novel object (F1,7=1.78, NS). Adult male BTBR reared as juveniles with juvenile male B6 spent significantly more time sniffing the novel mouse than the novel object (F1,15=20.46, p<.001). (C) A significant main effect of social housing was found for the number of total entries to the side chambers in cohort2 males (F2,29=5.83, p<.01). Posthoc Scheffe test indicated that male BTBR reared with B6 made significantly fewer entries than BTBR reared with BTBR (p<.05). (D) The main effect of social housing was not significant for the number of total entries to the side chambers during the habituation phase (F2,29=2.11, NS), indicating no differences across groups in locomotor abilities or exploration of a novel non-social environment.
Figure 3. 20-day peer-enriched intervention improved sociability in BTBR male mice.
(A) Time in chambers containing a novel object or novel mouse. Male B6 that were reared as juveniles with juvenile male B6 for 20 days displayed typical sociability as adults (N=8, F1,7=93.77, p<.0001). Male BTBR reared as juveniles with juvenile male BTBR for 20 days did not display sociability as adults (N=8, F1,7=0.046, NS). Male BTBR reared as juveniles with juvenile male B6 for 20 days displayed significant sociability as adults (N=16, F1,15=12.24, p<.01). (B) Time spent sniffing the novel object and the novel mouse. Male B6 reared as juveniles with juvenile male B6 for 20 days displayed significant sociability as adults (N=8, F1,7=117.84, p<.001). Male BTBR reared as juveniles with juvenile male BTBR did not display significant sociability (N=8, F1,7=1.78, NS). Male BTBR reared as juveniles with juvenile male B6 displayed sociability as adults (N=16, F1,15=20.46, p<.001). (C) Total number of entries to the side chambers during the social approach task. A significant main effect of social housing was found (F2,29=5.83, p<.01), with male BTBR reared with B6 showing fewer entries into the side chambers as compared to BTBR reared with BTBR (p<.05). (D) Total number of entries to the side chambers during the habituation phase that preceded the social approach task. No significant effect of social housing was found (F2,29=2.11, NS). (A and B) *p < .05 for comparison between novel mouse and novel object sides. (C) #p < .05 as compared to BTBR reared with BTBR.
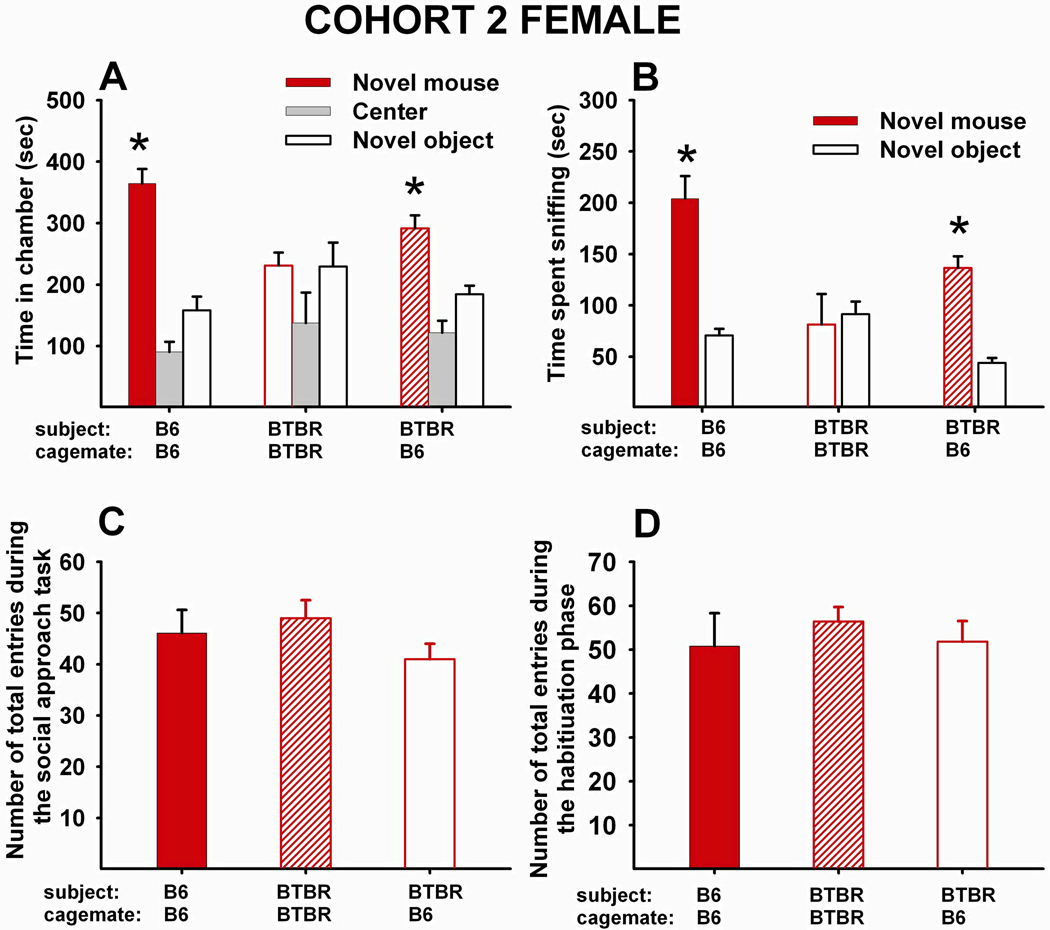
Fig 4 A–D shows social approach scores of Cohort 2 females. (A) Adult female B6 that had been reared as juveniles with juvenile female B6 displayed typical sociability (N=8, F1,7=52.20, p<.001). Adult female BTBR reared as juveniles with juvenile female BTBR did not display sociability, spending more time in the chamber containing the novel object than in the chamber containing the novel mouse (N=8, F1,7=0.07, NS). Adult female BTBR reared as juveniles with juvenile female B6 displayed significant sociability (N=16, F1,15=12.18, p<.01). (B) Adult female B6 reared as juveniles with juvenile female B6 spent significantly more time sniffing the novel mouse than the novel object (F1,7=42.73, p<.001). Adult female BTBR reared as juveniles with juvenile female BTBR did not spend significantly more time sniffing the novel mouse than the novel object (F1,15=0.06, NS). Adult female BTBR reared as juvenile with juvenile female B6 spent significantly more time sniffing the novel mouse than the novel object (F1,15=48.28, p<.001). (C) The main effect of social housing was not significant for total number of entries during the social approach task (F2,29=1.51, NS). (D) The main effect of social housing was not significant for the number of total entries to the side chambers during the habituation phase (F2,29=0.24, NS).
Figure 4. 20-day peer-enriched intervention improved sociability in BTBR female mice.
(A) Time in chambers containing a novel object or novel mouse. Female B6 that were reared as juveniles with juvenile female B6 for 20 days displayed typical sociability as adults (N=8, F1,7=52.20, p<.001). Female BTBR reared as juveniles with juvenile female BTBR for 20 days did not display sociability as adults (N=8, F1,7=0.07, NS). Female BTBR reared as juveniles with juvenile female B6 for 20 days displayed significant sociability as adults (N=16, F1,15=12.18, p<.01). (B) Time spent sniffing the novel object and the novel mouse. Female B6 reared as juveniles with juvenile female B6 for 20 days displayed significant sociability as adults (F1,7=42.73, p<.001). Female BTBR reared as juveniles with juvenile female BTBR did not display significant sociability (F1,7=0.06, NS). Female BTBR reared as juveniles with juvenile female B6 displayed significant sociability as adults (F1,15=48.28, p<.001). (C) Total number of entries to the side chambers during the social approach task. No significant effect of social housing was found (F2,29=1.51, NS). (D) Total number of entries to the side chambers during the habituation phase that preceded the social approach task. No significant effect of social housing was found (F2,29=0.24, NS). (A and B) *p < .05 for comparison between novel mouse and novel object sides.
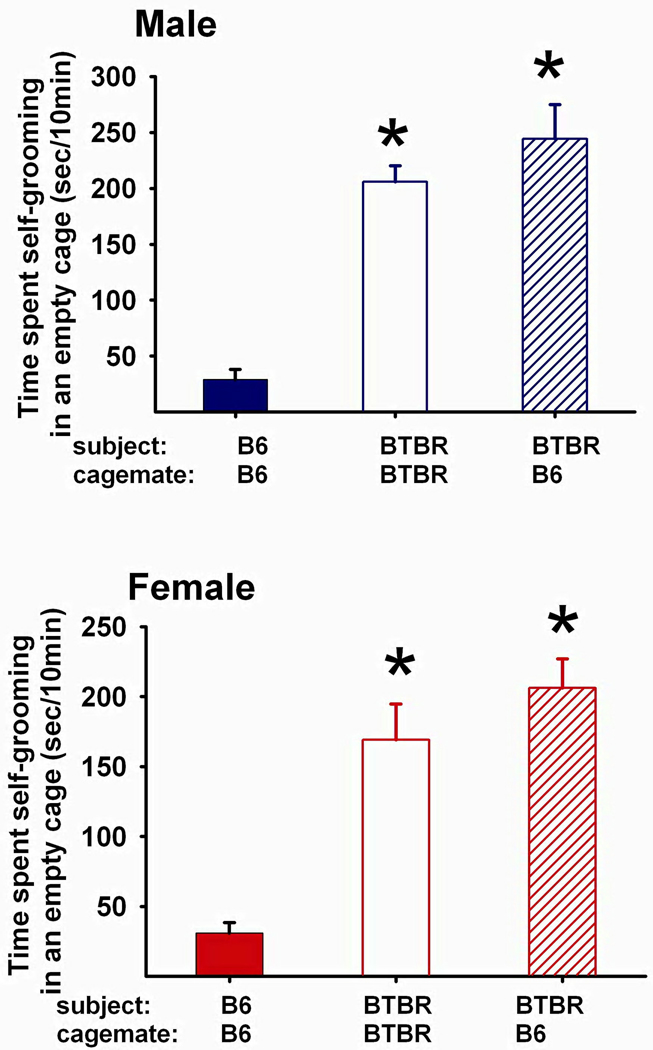
Social peer enrichment did not affect the high levels of repetitive self-grooming in male and female BTBR
Figure 5 shows time spent in self-grooming in an empty cage over 10 min for cohort 1. The main effect of social housing was significant for adult cohort 1 males (F2,45=53.81, p<.001). Adult male BTBR that had been reared with either B6 or BTBR as juveniles showed higher levels of self-grooming as compared to B6 control males (p<.01 for each posthoc comparison). Similarly, the main effect of social housing was significant for adult cohort 1 females (F2,45=32.70, p<.001). Female BTBR reared with either B6 or BTBR as juveniles showed higher levels of self-grooming as compared to B6 control females (p<.01 for each posthoc comparison). Since the 40 day intervention had no effect on repetitive self-grooming in cohort 1 BTBR, self-grooming was not tested after the 20 day intervention in cohort 2 mice.
Figure 5. Social peer enrichment did not affect high levels of repetitive self-grooming in male and female BTBR.
Time spent in repetitive self-grooming was significantly higher in adult male BTBR that were reared with either B6 (N=16) or BTBR (N=16) as juveniles, as compared to B6 controls (N=16) (Main effect of social housing, F2,45=53.81, p<.001; posthoc Scheffe test, p<.01 for each comparison). Similarly, adult female BTBR reared with either B6 (N=16) or BTBR (N=16) as juveniles showed higher levels of self-grooming as compared to B6 control females (N=16) (Main effect of social housing, F2,45=32.70, p<.001; Posthoc Scheffe test, p<.01 for each comparison). *p < .05 as compared to B6 reared with B6.
Discussion
A large body of literature demonstrates that early social environment, including social isolation in monkeys (Harlow and Suomi, 1971) and communal nesting in mice (Branchi, 2009), exerts a profound influence on the development of adult social behaviors. Animal models of autism provide preclinical tools to evaluate treatment interventions, including behavioral and pharmacological therapeutics (Blundell et al., 2010; Chadman, Yang, & Crawley, 2009; Dolen et al., 2007; Ehninger et al., 2008; Silverman et al., 2009; Silverman et al., 2010; M. Yang et al., 2010; M. Yang et al., 2007b; Zhou et al., 2009). In the current study we report the development of a new mouse model of early social enrichment and the novel finding that social deficits in adult BTBR are prevented by social peer enrichment during the period between weaning and sexual maturity. Robust social deficits in adult BTBR were prevented by rearing 21 day old juvenile BTBR with juvenile males of a social strain, B6. Time spent in social approach and social sniffing were both significantly higher in BTBR reared with B6 than in BTBR reared with BTBR, as tested at adult ages. The same beneficial effect of rearing with social cagemates was seen in female BTBR reared with female B6 cagemates. These findings indicate that the social milieu in the home cage during early development has a direct influence on emerging social behaviors in adult mice.
To begin to evaluate the essential components of social peer intervention, we compared 20 days of BTBR+B6 peer rearing with 40 days of BTBR+B6 peer rearing. BTBR males that had spent only 20 days after weaning with B6 cagemates showed significant sociability at age 41 days. BTBR males that had spent 40 days after weaning with B6 cagemates similarly showed significant sociability at age 61 days. The magnitude of sociability rescue was similar after the 20 day and the 40 day intervention periods. This finding indicates that constant interactions with social peers from a juvenile age to a late adolescent/young adult age prevented social deficits in BTBR as effectively as a longer length of home cage interactions from a juvenile age to an adult age. Further experiments will investigate even shorter intervention periods, and will explore whether a critical window exists during a specific age within early development when peer intervention is likely to produce maximal effects. We speculate that it is critical for intervention to begin at an early age. A related interesting question is whether home cage interactions between BTBR and BTBR are strikingly deficient, which could represent a social deprivation environment during early development. Specific qualities of home cage interactions that contribute to the improved sociability in BTBR reared with B6 will require intensive observations of a wide range of home cage behaviors in identified combinations of juvenile mice. Subsequently, it will be important to investigate long-term outcomes of peer intervention in adult mice that are no longer living with social peers. To further understand which aspects of social behaviors are changed by social peer intervention, it will be important to test reciprocal social interactions between the subject mouse and a freely moving novel partner mouse in various settings, including male-female and male-male pairings.
Unusually low or unusually high levels of general exploratory activity in the three-chambered apparatus would introduce artifacts due to sedation or hyperactivity, that could represent an experimental confound and lead to an overinterpretation of the social approach data. Entries between compartments affords a built-in measure of general exploratory activity in our automated three-chambered apparatus. Number of entries was significantly lower in male BTBR reared with male B6 in both cohorts, but not in female BTBR reared with female B6 in either cohort. To further determine exploratory locomotion in BTBR males reared with B6 males, we analyzed number of entries during the habituation phase that preceded the social approach task. In the novel but non-social test environment, BTBR that had been reared with B6 made similar numbers of entries as compared to B6 reared with B6 and BTBR reared with BTBR. This finding rules out differences in general exploratory tendencies as an explanation for entry differences during the social approach task.
Repetitive self-grooming, one of several other well-replicated phenotypes in BTBR, is relevant to the third diagnostic symptom of autism. High levels of self-grooming in BTBR were present in both BTBR reared with BTBR cagemates and in BTBR reared with B6 cagemates. Thus, the home cage interactions between BTBR and B6 reared together since a juvenile age were beneficial in the social domain but not in the repetitive domain. This finding is consistent with the reported need for different types of behavioral interventions to improve social behaviors and repetitive behaviors in autistic children. A recent randomized control study found that the Early Start Denver Model improved IQ and adaptive behaviors in children with autism but was without effect on repetitive behaviors scores (Dawson et al., 2009). Genetic studies indicate that the symptoms of social-communication deficits and symptoms of repetitive behaviors are regulated by different genetic factors (Happe & Ronald, 2008) and thus may respond differentially to treatments. Previous studies have shown that environmental enrichment was effective in reducing repetitive jumping stereotypies in deer mice (Tanimura, Yang, & Lewis, 2008), that a serotonin reuptake inhibitor was effective in preventing injurious self-grooming in Sapap3 mutant mice (Welch et al., 2007), and that an mGluR5 receptor antagonist MPEP was effective in reducing repetitive self-grooming, but not social deficits, in BTBR mice (Silverman et al., 2009), indicating a separation of therapeutic responses for the social and repetitive domains.
An important question relevant to the translational value of this mouse model is how the ages of these mice at the beginning and the end of peer intervention translate to human ages. Large species differences in developmental trajectories prohibit direct comparisons between mice and humans. However, reasoning that mice are weaned at 3–4 weeks of age and reach sexual maturity 6–8 weeks of age, it is interesting to speculate that the current intervention period in mice, from approximately 3 to 8 weeks, is roughly equivalent to human ages from approximately 2 to 18 years.
Conclusions
Structured behavioral interventions through special education programs are currently the most effective treatment available for autistic individuals (Dawson, 2008; Rogers, 2000; Vismara & Rogers). Our findings demonstrate that the profound social deficits in BTBR are significantly improved when BTBR adolescents share home cages with highly social B6 adolescent cagemates. Detailed investigation into the nature of the beneficial peer interactions between B6 and BTBR may reveal the specific ameliorative components of the juvenile intervention. It will be interesting to compare the nature of social interactions between B6 and BTBR juveniles in the home cage to the types of interactions that occur between children with autism and their siblings at home, and to the types of interactions between children with autism and classmates in a mainstreamed school environment. Our results from a robust mouse model of autism may support the strategy of targeted behavioral intervention with peers for improving social interactions in children with autism spectrum disorders.
Acknowledgments
We extend our gratitude to Dr. Sally Ozonoff, University of California Davis M.I.N.D. Institute, for insightful comments on this manuscript. Supported by the National Institute of Mental Health Intramural Research Program.
References
- American Psychiatric Association, W., DC. Diagnostic and Statistical Manual of Mental Disorders, 4th edn text rev (DSM-IV-TR) Washington, DC: American Psychological Association; 2000. [Google Scholar]
- Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Betz A, Higbee TS, Reagon KA. Using joint activity schedules to promote peer engagement in preschoolers with autism. J Appl Behav Anal. 2008;41(2):237–241. doi: 10.1901/jaba.2008.41-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C. Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord. 2005;35(3):351–360. doi: 10.1007/s10803-005-3302-5. [DOI] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry. 2007;48(11):1102–1110. doi: 10.1111/j.1469-7610.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30(6):2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176(1):21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009 Apr;33(4):551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176(1):53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Causton-Theoharis J, Ashby C, Cosier M. Islands of loneliness: exploring social interaction through the autobiographies of individuals with autism. Intellect Dev Disabil. 2009;47(2):84–96. doi: 10.1352/1934-9556-47.2.84. [DOI] [PubMed] [Google Scholar]
- Cederlund M, Hagberg B, Billstedt E, Gillberg IC, Gillberg C. Asperger syndrome and autism: a comparative longitudinal follow-up study more than 5 years after original diagnosis. J Autism Dev Disord. 2008;38(1):72–85. doi: 10.1007/s10803-007-0364-6. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1(3):147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Medicine. Testing hypotheses about autism. Science. 2007;318(5847):56–57. doi: 10.1126/science.1149801. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41(3):145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20(3):775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2009;125(1) doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14(8):843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikeseth S, Smith T, Jahr E, Eldevik S. Intensive behavioral treatment at school for 4- to 7-year-old children with autism. A 1-year comparison controlled study. Behav Modif. 2002;26(1):49–68. doi: 10.1177/0145445502026001004. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H, English K, Shafer K, Kaczmarek L. Interaction among preschoolers with and without disabilities: effects of across-the-day peer intervention. J Speech Lang Hear Res. 1997;40(1):33–48. doi: 10.1044/jslhr.4001.33. [DOI] [PubMed] [Google Scholar]
- Goldstein H, Kaczmarek L, Pennington R, Shafer K. Peer-mediated intervention: attending to, commenting on, and acknowledging the behavior of preschoolers with autism. J Appl Behav Anal. 1992;25(2):289–305. doi: 10.1901/jaba.1992.25-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Ronald A. The 'fractionable autism triad': a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18(4):287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Suomi SJ. Social recovery by isolation-reared monkeys. Proc NAtl Acad Sci USA. 1971;68(7):1534–1538. doi: 10.1073/pnas.68.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CB, Symon JB, Frea WD. Recess is time-in: using peers to improve social skills of children with autism. J Autism Dev Disord. 2008;38(5):815–826. doi: 10.1007/s10803-007-0449-2. [DOI] [PubMed] [Google Scholar]
- Hwang B, Hughes C. The effects of social interactive training on early social communicative skills of children with autism. J Autism Dev Disord. 2000;30(4):331–343. doi: 10.1023/a:1005579317085. [DOI] [PubMed] [Google Scholar]
- Kaminsky L, Dewey D. Psychosocial adjustment in siblings of children with autism. J Child Psychol Psychiatry. 2002;43(2):225–232. doi: 10.1111/1469-7610.00015. [DOI] [PubMed] [Google Scholar]
- Kamps DM, Barbetta PM, Leonard BR, Delquadri J. Classwide peer tutoring: an integration strategy to improve reading skills and promote peer interactions among students with autism and general education peers. J Appl Behav Anal. 1994;27(1):49–61. doi: 10.1901/jaba.1994.27-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: the Vineland and the ADOS. J Autism Dev Disord. 2007;37(4):748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL, Frea WD, Fredeen RM. Identifying early intervention targets for children with autism in inclusive school settings. Behav Modif. 2001;25(5):745–761. doi: 10.1177/0145445501255005. [DOI] [PubMed] [Google Scholar]
- Kohler FW, Strain PS, Hoyson M, Davis L, Donina WM, Rapp N. Using a group-oriented contingency to increase social interactions between children with autism and their peers. A preliminary analysis of corollary supportive behaviors. Behav Modif. 1995;19(1):10–32. doi: 10.1177/01454455950191002. [DOI] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nat Clin Pract Neurol. 2008;4(3):138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374(9701):1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macks RJ, Reeve RE. The adjustment of non-disabled siblings of children with autism. J Autism Dev Disord. 2007;37(6):1060–1067. doi: 10.1007/s10803-006-0249-0. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McGee GG, Almeida MC, Sulzer-Azaroff B, Feldman RS. Promoting reciprocal interactions via peer incidental teaching. J Appl Behav Anal. 1992;25(1):117–126. doi: 10.1901/jaba.1992.25-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8(2):129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191(1):118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Educating Young Children with Autism. Washington, DC: Natl. Acad. Press; 2001. [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J. The Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health. 2007 doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Nordin V, Gillberg C. The long-term course of autistic disorders: update on follow-up studies. Acta Psychiatr Scand. 1998;97(2):99–108. doi: 10.1111/j.1600-0447.1998.tb09970.x. [DOI] [PubMed] [Google Scholar]
- Ospina MB, Krebs Seida J, Clark B, Karkhaneh M, Hartling L, Tjosvold L, Vandermeer B, Smith V. Behavioural and developmental interventions for autism spectrum disorder: a clinical systematic review. PLoS One. 2008;3(11):e3755. doi: 10.1371/journal.pone.0003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6(7):661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Doppelt O, Gross-Tsur V, Shalev RS. Social and emotional adjustment of siblings of children with autism. J Child Psychol Psychiatry. 2004;45(4):855–865. doi: 10.1111/j.1469-7610.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Prendeville JA, Prelock PA, Unwin G. Peer play interventions to support the social competence of children with autism spectrum disorders. Semin Speech Lang. 2006;27(1):32–46. doi: 10.1055/s-2006-932437. [DOI] [PubMed] [Google Scholar]
- Reichow B, Volkmar FR. Social skills interventions for individuals with autism: evaluation for evidence-based practices within a best evidence synthesis framework. J Autism Dev Disord. 40(2):149–166. doi: 10.1007/s10803-009-0842-0. [DOI] [PubMed] [Google Scholar]
- Reichow B, Volkmar FR. Social skills interventions for individuals with autism: evaluation for evidence-based practices within a best evidence synthesis framework. J Autism Dev Disord. 2009;40(2):149–166. doi: 10.1007/s10803-009-0842-0. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. Interventions that facilitate socialization in children with autism. J Autism Dev Disord. 2000;30(5):399–409. doi: 10.1023/a:1005543321840. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37(1):8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four-year outcome and predictors. Am J Ment Retard. 2005;110(6):417–438. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J, Tolu SS, Barkan CL, Crawley JN. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J, Yang M, Lord C, Crawley JN. Behavioural Phenotyping Assays for Mouse Models of Autism. Nat. Rev. Neuroscience. 2010 doi: 10.1038/nrn2851. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain PS, Kohler F. Peer-mediated social intervention for young children with autism. Semin Speech Lang. 1998;19(4):391–404. doi: 10.1055/s-2008-1064056. quiz 404–395; 424. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008;189(2):250–256. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Trembath D, Balandin S, Togher L, Stancliffe RJ. Peer-mediated teaching and augmentative and alternative communication for preschool-aged children with autism. J Intellect Dev Disabil. 2009;34(2):173–186. doi: 10.1080/13668250902845210. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Verte S, Roeyers H, Buysse A. Behavioural problems, social competence and self-concept in siblings of children with autism. Child Care Health Dev. 2003;29(3):193–205. doi: 10.1046/j.1365-2214.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- Vismara LA, Rogers SJ. Behavioral Treatments in Autism Spectrum Disorder: What Do We Know? Annu Rev Clin Psychol. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- Vismara LA, Rogers SJ. Behavioral Treatments in Autism Spectrum Disorder: What Do We Know? Annu Rev Clin Psychol. 2010;6:447–468. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- Volkmar F, Chawarska K, Klin A. Autism in infancy and early childhood. Annu Rev Psychol. 2005;56:315–336. doi: 10.1146/annurev.psych.56.091103.070159. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West L, Waldrop J, Brunssen S. Pharmacologic treatment for the core deficits and associated symptoms of autism in children. J Pediatr Health Care. 2009;23(2):75–89. doi: 10.1016/j.pedhc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Winslow JT. Mouse social recognition and preference. Chapter 8. Curr Protoc Neurosci. 2003 doi: 10.1002/0471142301.ns0816s22. Unit 8 16. [DOI] [PubMed] [Google Scholar]
- Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, Goodlin-Jones BL. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009;5(2):145–150. [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced Scent Marking and Ultrasonic Vocalizations in the BTBR T+tf/J Mouse Model of Autism. Genes, Brain and Behavior. 2010 doi: 10.1111/j.1601-183X.2010.00582.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfberg PJ, Schuler AL. Integrated play groups: a model for promoting the social and cognitive dimensions of play in children with autism. J Autism Dev Disord. 1993;23(3):467–489. doi: 10.1007/BF01046051. [DOI] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(8):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Chadman CC, Silverman JL, Crawley JN. Behavioral Evaluation of Genetic Mouse Models of Autism. In Autism Spectrum Disorders. Oxford Press; 2010. in press. [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25(8):515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TR, Wolfberg PJ, Wu SC, Hwu PY. Supporting children on the autism spectrum in peer play at home and school: piloting the integrated play groups model in Taiwan. Autism. 2003;7(4):437–453. doi: 10.1177/1362361303007004009. [DOI] [PubMed] [Google Scholar]
- Zercher C, Hunt P, Schuler A, Webster J. Increasing joint attention, play and language through peer supported play. Autism. 2001;5(4):374–398. doi: 10.1177/1362361301005004004. [DOI] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29(6):1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]