Abstract
Energy transfer from antenna pigments to the reaction center is common in chlorophyll-based photosynthesis but never been observed in retinal-based ion pumps and photoreceptors. Here we describe xanthorhodopsin, a retinal protein/carotenoid complex in the eubacterium Salinibacter ruber, a proton pump. Difference absorption spectra measured under a variety of conditions and action spectra for pumping indicate that this protein contains two chromophores: retinal and the carotenoid, salinixanthin, in a molar ratio of about 1:1. The two chromophores strongly interact, and light energy absorbed by the carotenoid is efficiently transferred to the retinal and used for transmembrane proton transport.
The extreme halophile Salinibacter ruber isolated from salt crystallizer ponds (1, 2) can be grown in aerobic heterotrophic culture in 4 M NaCl. This eubacterium accumulates high concentrations of KCl to adapt to the high ionic strength (3), as the haloarchaea in the same environment. After several days of growth, Salinibacter ruber acquires a deep red color, from salinixanthin which constitutes nearly 100% of its carotenoid content and whose chemical structure was recently established (4). It was proposed to provide protection from photodamage and to stabilize the cell membrane because both the polyene and the fatty acid part of this carotenoid acyl glycoside will be immersed in the lipid bilayer (4). We report here that salinixanthin is not the only pigment in the Salinibacter ruber cell membrane, and heterotrophy is not the only source of energy for this organism. These cells contain an unusual retinal protein, which uses salinixanthin to assist harvesting light energy in a wider spectral range and utilizes it for transmembrane proton transport. Thus, it is a light-driven proton pump similar to bacteriorhodopsin (5) and the archaerhodopsins (6) of the archaea, the proteorhodopsins of planktobacteria (7), and leptosphaeria rhodopsin of an eukaryote (8), but with two chromophores. We term it here xanthorhodopsin. Its novel carotenoid antenna is a feature shared with chlorophyll-based light-harvesting complexes and reaction centers (9, 10).
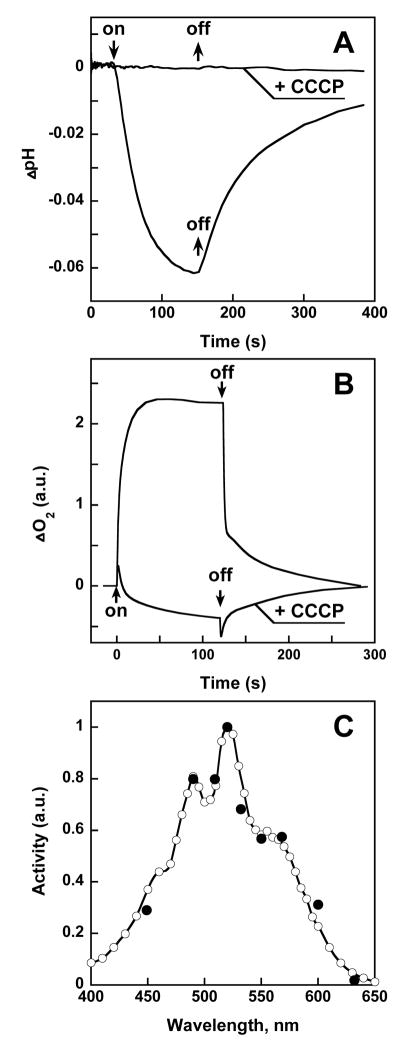
Illumination of cell membrane vesicles prepared from Salinibacter produces acidification of the medium, which is abolished by the protonophore carbonyl cyanide m-chlorophenyl-hydrazone (CCCP) (Fig. 1A). When assayed in 1 M Na2SO4, these light-dependent pH changes are unaffected by the presence of chloride ions (not shown). Thus, the vesicles contain an outward-directed light-driven proton pump like bacteriorhodopsin, and lack a chloride pump like halorhodopsin that would produce chloride-dependent light-dependent alkalinization in the presence of CCCP (11).
Fig. 1.
Light-induced proton transport in Salinibacter ruber, and its action spectrum. A. pH change in a membrane vesicle suspension produced by illumination at 550 nm at pH 6.5, in the absence and presence of 20 μM protonophore CCCP (carbonyl cyanide m-chlorophenyl-hydrazone), at a light intensity of 2 mW/cm2. B. Kinetics of light-induced changes in the rate of oxygen uptake caused by photo-inhibition of respiration in the cell suspension of Salinibacter ruber, in the absence and presence of 1 μM CCCP. C. Action spectra for photo-inhibition of the respiration by intact cells (open circles) and light-induced proton transport in membrane vesicles, latter measured as in Fig. 1 (closed circles). In both cases the optical density of the sample was below 0.2.
The observed light-driven proton transport suggests that Salinibacter might contain a bacterial rhodopsin. Action spectra for the transport were determined in two different ways. A temporary increase of oxygen concentration in a suspension of intact respiring Salinibacter ruber cells after the onset of continuous illumination will assay the electrochemical gradient produced by the light-driven proton pump, as indicated by its inhibition with the uncoupler CCCP (Fig. 1B). This method had been used for demonstrating the physiological function of bacteriorhodopsin in halobacterial cells (12, 13). The action spectrum exhibits a broad band with a maximum at 560 nm, as would be expected for a retinal chromophore, and additional sharp bands at 521, and 486 nm that correspond to the structured absorption band of salinixanthin (Fig. 1C). The action spectrum was determined also for light-induced pH changes in membrane vesicle suspensions, and is shown in Fig. 1C. These action spectra, and their virtually complete coincidence when determined by two independent methods, strongly suggest that this system depends on a complex of the retinal protein and a carotenoid, and that light absorbed by both carotenoid and retinal is effective in proton transport. The auxiliary carotenoid chromophore therefore serves as an antenna for the retinal chromophore.
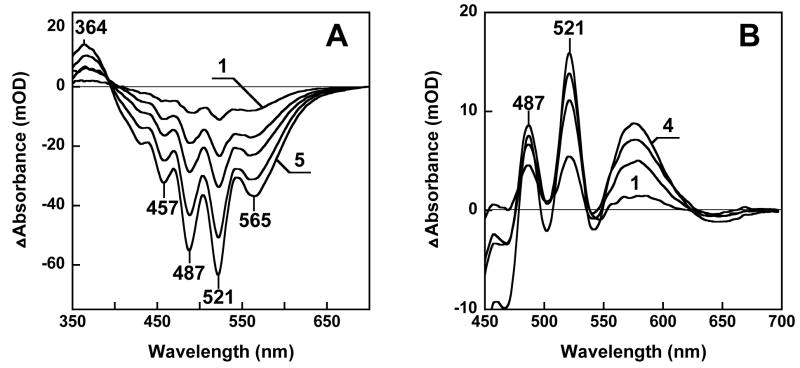
The presence of a retinal chromophore was confirmed by bleaching and reconstitution. Incubation of Salinibacter cell membranes with hydroxylamine produced a difference absorption spectrum which exhibited the broad minimum at 565 nm expected if retinal were released from a bacteriorhodopsin-like chromophore by this treatment (14), as well as the corresponding maximum at 364 nm from the retinal oxime produced (Fig. 2A). Additionally, the difference spectrum contains sharp depletion bands at 521, 487 and 457 nm. The latter three bands are close to the absorption maxima of salinixanthin, but show remarkably greater spectral resolution, like the action spectrum (Fig. 1C). The amplitude of the 487 nm difference band is 10–15% of the total carotenoid absorbance. Reconstitution of the membranes, washed free of hydroxylamine, with all-trans retinal resulted in the recovery of not only the broad band near 565 nm of the retinal chromophore but also sharp positive bands at 521, 487 and 456 nm (Fig. 2B) that correspond to the bleached negative peaks. These must originate from one or a few salinixanthin molecules that closely interact with the retinal protein and change their spectrum (mainly narrowing the vibronic bands) upon formation of the complex. Absorption changes similar to those produced by hydroxylamine were observed also upon shifting the pH above 12.5, which causes a shift of the absorption maximum of rhodopsins to 360 nm due to deprotonation of the retinal Schiff base and the subsequent destruction of the chromophore. As in the case of hydroxylamine treatment, deprotonation of the Schiff base at pH 12.5 was accompanied by sharp negative peaks at 521, 487 and 456 nm, confirming their dependence on retinal (not shown).
Fig. 2.
Spectroscopic detection of the retinal protein of Salinibacter. A. Curves 1 through 5, difference spectra upon illumination of a cell membrane suspension at >550 nm in the presence of 0.2 M hydroxylamine for 4, 12, 20, 36, and 60 min at 20°C, respectively. B. Curves 1 through 4, difference spectra measured 5, 15, 25, and 60 min after addition of all-trans retinal to the Salinibacter ruber cell membranes which had been bleached with 0.2 M hydroxylamine.
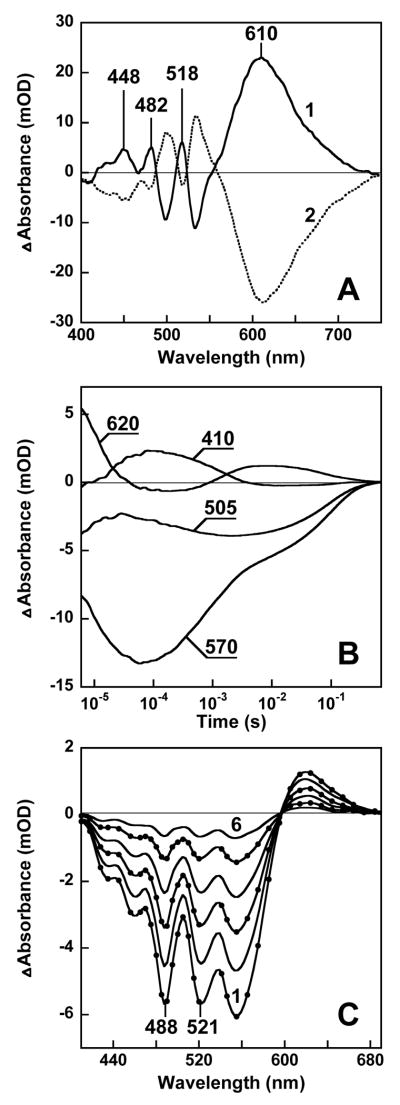
The interaction of the two chromophores is modulated in the photocycle. Illumination of the Salinibacter membranes with green light at 175K causes formation of a photoproduct with a red-shifted retinal band, which is stable at this temperature but reconverts upon red light illumination (Fig. 3A). This is behavior characteristic of the K photointermediate of bacteriorhodopsin (15–17). The peculiarity of the difference spectrum of Salinibacter retinal-protein is that besides the increase of absorbance near 610 nm from the red-shifted retinal chromophore, a set of three additional sharp bands appear at 448, 482 and 518 nm. These peaks originate from a small (ca. 2 nm) blue shift of the three main carotenoid bands. Perturbation of the carotenoid in the K-like photoproduct suggests that it closely interacts with the retinal and responds to its electrostatic or conformational changes. Reversal of the changes of the carotenoid bands with red light (Fig. 3A), which is absorbed by the retinal but not the carotenoid, is further evidence for interaction between the carotenoid and the retinal in the complex.
Fig. 3.
Absorption changes during the photocycle. A. Light-induced absorption changes in water-glycerol suspension of cell membranes at 175 K. Spectrum 1, illumination of membranes with 520 nm light, spectrum 2, subsequent illumination at > 650 nm. B. Kinetics of laser flash induced absorption changes at selected characteristic wavelengths in membranes of Salinibacter solubilized with 0.15% dodecyl maltoside, in 100 mM NaCl, pH 8.8, 20°C. The global fit indicates that kinetics include at least six components, with time-constants of 7.5 μs, 35 μs, 280 μs, 1.3 ms, 11 ms, and 100 ms. C. Transient difference spectra during the photocycle, measured at 10, 30, 60, 100, 160, and 250 ms (curves 1 through 6) after a 532 nm laser flash. Conditions and sample are as in B.
The absorption changes during the photocycle at ambient temperature (Figs. 3B and 3C), at characteristic wavelengths indicate formation of intermediates similar to the K, L, M, N, O photoproducts of bacteriorhodopsin (15) and proteorhodopsin (18). The absorption changes at 410 nm reflect mainly the formation of the M intermediate with deprotonated retinal Schiff base, as expected in a proton pump. The difference spectra associated with all photointermediates exhibit changed absorption bands of salinixanthin also (Fig. 3C). The changes in the early intermediates (not shown) appear to be band-shifts similar to what is observed after illumination at low temperature (Fig. 3A), but those of the late states involve band-broadening similar to the spectra after bleaching (Fig. 2A), suggesting that the carotenoid senses both electrostatic changes and conformational shifts of the retinal or the protein during the photocycle.
Substantial purification of the complex was achieved by repeated incubation of Salinibacter membranes with 0.015% dodecyl maltoside, followed by centrifugation to recover the membranes. This extracts most of the excess bulk carotenoids as well as nearly all other proteins (19), and the xanthorhodopsin can be then solubilized with 0.15% dodecyl maltoside. The solubilized xanthorhodopsin exhibits a slight blue shift of the retinal chromophore band but retains all the characteristic absorption bands of the membranes, and because of less light-scattering reveals more spectroscopic details, particularly at shorter wavelengths.
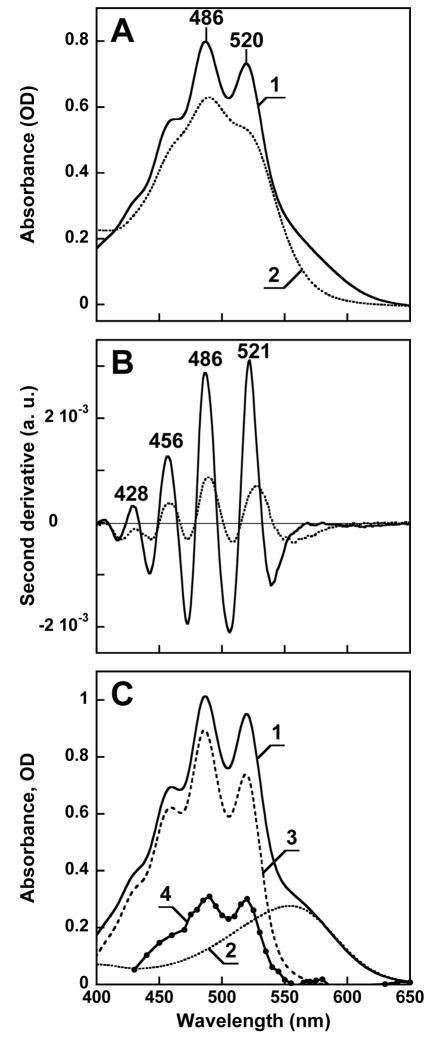
The broadening of the carotenoid absorption bands (Fig. 4A) upon hydrolysis of the Schiff base is revealed by second-derivative spectra which better resolve overlapping bands. In second-derivate spectra the narrow carotenoid bands are more pronounced than the wider band of the retinal chromophore (Fig. 4B), because the amplitude of the second derivative is inversely proportional to the square of the half-width. The second-derivative spectra indicate that the overall shape of the vibronic bands after bleaching resembles those before bleaching, but the bands shift slightly to the red and become wider (e.g., the width of the 486 nm band increases from ca. 24 to 36 nm). Further, the shapes and the amplitude ratios of the difference bands after bleaching the fraction enriched in xanthorhodopsin are the same as in a sample enriched in bulk salinixanthin. This strongly suggests that salinixanthin is a stoichiometric component of the retinal protein complex. It appears that upon hydrolysis of the Schiff base, salinixanthin loses its rigid environment and acquires a broader, less structured spectrum, similar to that of the bulk carotenoid in the membrane.
Fig. 4.
Absorption spectrum of xanthorhodopsin: its carotenoid and retinal chromophore components, and estimation of the efficiency of energy transfer from the carotenoid. A. Absorption spectrum of solubilized and purified xanthorhodopsin, before (spectrum 1) and after (spectrum 2) bleaching with hydroxylamine. B. Second derivatives of the two spectra in A. C. Estimated absorption spectrum of the complex and the contribution of carotenoid to it and to the action spectrum. Spectrum 1: calculated spectrum of xanthorhodopsin; spectrum 2: bacteriorhodopsin, 10 nm shifted to the blue and appropriately scaled to model the retinal component of xanthorhodopsin; spectra 3 and 4: carotenoid component of the absorption spectrum and the action spectrum, respectively, obtained by subtracting the contribution of the retinal.
The spectrum of the xanthorhodopsin complex in Fig. 4C was calculated from the spectrum of the purified sample (Fig. 4A) by satisfying the condition that the resolution of the vibronic bands should be the same as in the action spectrum (19). From the published extinction coefficients of the two chromophores, 250,000 M−1 cm−1 for salinixanthin (4) and 63,000 M−1 cm−1 for the retinal chromophore (20) we estimate that the ratio of salinixanthin to retinal is 0.75:1. However, the extinction coefficient for most carotenoids with the same number of conjugated bonds as salinixanthin is lower, ca. 180,000 M−1 cm−1 (19). With this number, the retinal to carotenoid ratio in the complex will be 1:1.
The action spectrum will differ from the absorption spectrum, and consist of the retinal band plus the carotenoid band multiplied by the efficiency of energy transfer to the retinal. Because the contribution of the carotenoid bands to the action spectrum is about 40% of their contribution to the absorption spectrum (Fig. 4C), we conclude that the quantum efficiency of energy transfer from the salinixanthin to retinal in the complex is ca. 40%. In photosynthetic organisms the efficiency of energy transfer between carotenoids and chlorophyll is from 15 to 100% (9, 21, 22).
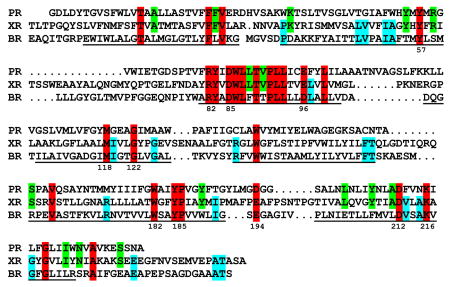
Mass spectrometry of the more intense of the two bands on a SDS-polyacrylamide gel from the purified sample (19) identified the gene product from a proteorhodopsin/bacteriorhodopsin-like open reading frame in the Salinibacter genome (NC_006812). No other protein that could bind retinal, such as halorhodopsin identified in the genome (23) was found. The sequence below indicates that xanthorhodopsin contains all of the functionally important residues known for retinal binding and proton transport, including homologues of tyr57, arg82, asp85, trp86, asp96, trp182, glu194, tyr185, asp212, and lys216 (numbering for bacteriorhodopsin, helical segments underlined). There are 27 residues (marked red) common to xanthorhodopsin (XR), bacteriorhodopsin (BR) and proteorhodopsin (PR). The number of residues in xanthorhodopsin with counterparts in bacteriorhodopsin (red plus cyan) is about the same (45 vs. 41) as those with proteorhodopsin (red plus green). As in proteorhodopsin, the internal proton donor homologous to asp96 is glu. A set of four phenylalanine residues, located on what should be the side of helix E that faces toward the lipid bilayer, might be involved in binding the carotenoid.
For mechanistic reasons that have been thoroughly explored in bacteriorhodopsin (24), retinal must be the site of proton translocation in xanthorhodopsin also. The obvious rationale for the carotenoid in the dual light-harvesting system is that it increases the cross-section for light absorption and, through energy transfer, extends it to the spectral region where the absorption of the retinal chromophore is low. Another function might be protection of the retinal protein from photo-oxidation with singlet oxygen since carotenoids are known to be efficient deactivators of singlet states. Such functions of carotenoids are common in chlorophyll-based photosynthetic systems where they harvest light energy and transfer it to chlorophyll (21), but they have never been described for retinal based pumps or receptors (25, 26). The formation of such a complex seems to be specific for Salinibacter. Action spectra for bacteriorhodopsin ruled out an antenna function for bacterioruberin, the main carotenoid in halobacteria (13, 27). No energy transfer was observed for archaerhodopsin either (28). We found that incubation of bacteriorhodopsin and proteorhodopsin with dodecyl maltoside-solubilized salinixanthin yielded no evidence for a complex (spectra not shown).
The discovery of a carotenoid antenna in a retinal protein is relevant to current ideas about how carotenoids function in energy transfer. The intense absorption bands of carotenoids are caused mainly by singlet-singlet S0–S2 transition to the vibrational levels of the strongly allowed S2 state (21). The S2 state is very short-lived (50–200 fsec) and populates internal lower lying excited states including the S1 state with a lifetime in the psec domain. S1 and S2 are both involved in energy transfer to bacteriochlorophyll. However, the energy of S1 in spirilloxanthin, a carotenoid with 13 conjugated bonds as salinixanthin, is far below the 560 nm absorption band of the retinal chromophore. If the same is true for salinixanthin, energy transfer should be very fast, as it must involve the higher excited state. Energy transfer from an excited state located between S2 and S1 was suggested for carotenoid-chlorophyll complexes (29). High rate of energy transfer implies close proximity of donor and acceptor. Our observation that the carotenoid undergoes substantial spectral changes upon its retinal-dependent binding to the opsin and during the photocycle indicates that the binding site is specially “crafted” to accommodate the carotenoid molecule in a position optimal for energy transfer to the retinal. Xanthorhodopsin represents the simplest electrogenic pump with an accessory antenna pigment, and might be an early evolutionary development in utilizing energy transfer for energy capture.
Supplementary Material
References and notes
- 1.Antón J, Rosselló-Mora R, Rodríguez-Valera F, Amann R. Appl Environ Microbiol. 2000;66:3052. doi: 10.1128/aem.66.7.3052-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antón J, et al. Int J Syst Evol Micrbiol. 2002;52:485. doi: 10.1099/00207713-52-2-485. [DOI] [PubMed] [Google Scholar]
- 3.Oren A, Heldal M, Norland S, Galinski EA. Extremophiles. 2002;6:491. doi: 10.1007/s00792-002-0286-3. [DOI] [PubMed] [Google Scholar]
- 4.Lutnaes BF, Oren A, Liaaen-Jensen S. J Nat Prod. 2002;65:1340. doi: 10.1021/np020125c. [DOI] [PubMed] [Google Scholar]
- 5.Oesterhelt D, Stoeckenius W. Nature. 1971;233:149. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 6.Mukohata Y, Ihara K, Tamura T, Sugiyama Y. J Biochem. 1999;125:649. doi: 10.1093/oxfordjournals.jbchem.a022332. [DOI] [PubMed] [Google Scholar]
- 7.Béjà O, Spudich EN, Spudich JL, Leclerc M, DeLong EF. Nature. 2001;411:786. doi: 10.1038/35081051. [DOI] [PubMed] [Google Scholar]
- 8.Waschuk SA, Bezerra J, Shi AGL, Brown LS. Proc Natl Acad Sci USA. 2005;102:6879. doi: 10.1073/pnas.0409659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert B, Cogdell RJ, van Grondelle R. In: Light-Harvesting Antennas in Photosynthesis. Green BR, Parson WW, editors. Vol. 13. Kluwer Academic Publishers; Dordrecht/Boston/London: 2003. pp. 169–194. [Google Scholar]
- 10.Green BR, Parson WW, editors. Light-Harvesting Antennas in Photosynythesis. Vol. 13. Kluwer Academic Publishers; Dordrecht/Boston/London: 2003. [Google Scholar]
- 11.Schobert B, Lanyi JK. J Biol Chem. 1982;257:10306. [PubMed] [Google Scholar]
- 12.Oesterhelt D, Stoeckenius W. Proc Natl Acad Sci USA. 1973;70:2853. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litvin FF, Boichenko VA, Balashov SP, Dubrovskii VT. Biofizika. 1977;22:1062. [PubMed] [Google Scholar]
- 14.Oesterhelt D, Schuhman L, Gruber H. FEBS Lett. 1974;44:257. doi: 10.1016/0014-5793(74)81152-x. [DOI] [PubMed] [Google Scholar]
- 15.Lozier RH, Bogomolni RA, Stoeckenius W. Biophys J. 1975;15:955. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litvin FF, Balashov SP, Sineshchekov VA. Bioorgan Khim. 1975;1:1767. [Google Scholar]
- 17.Balashov SP, Ebrey TG. Photochem Photobiol. 2001;73:453. doi: 10.1562/0031-8655(2001)073<0453:tasiot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Dioumaev AK, et al. Biochemistry. 2002;41:5348. doi: 10.1021/bi025563x. [DOI] [PubMed] [Google Scholar]
- 19.Materials and methods are available as supporting material on Science Online.
- 20.Oesterhelt D, Meentzen M, Schuhmann L. Eur J Biochem. 1973;40:453. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 21.Polivka T, Sundström V. Chem Rev. 2004;104:2021. doi: 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]
- 22.Montano GA, Xin YY, Lin S, Blankenship RE. J Phys Chem B. 2004;108:10607. [Google Scholar]
- 23.Peña A, et al. Extremophiles. 2005;9:151. doi: 10.1007/s00792-005-0430-y. [DOI] [PubMed] [Google Scholar]
- 24.Lanyi JK, Schobert B. J Mol Biol. 2003;328:439. doi: 10.1016/s0022-2836(03)00263-8. [DOI] [PubMed] [Google Scholar]
- 25.Spudich JL, Yang CS, Jung KH, Spudich EN. Annu Rev Cell Dev Biol. 2000;16:365. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- 26.Kumauchi M, Ebrey T. In: Handbook of Photosensory Receptors. Briggs WR, Spudich JL, editors. Wiley-VCH; Darmstadt: 2005. pp. 43–76. [Google Scholar]
- 27.Danon A, Stoeckenius W. Proc Natl Acad Sci USA. 1974;71:1234. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukohata Y, Ihara K, Uegaki K, Miyashita Y, Sugiyama Y. Photochem Photobiol. 1991;54:1039. doi: 10.1111/j.1751-1097.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 29.Cerullo G, et al. Science. 2002;298:2395. doi: 10.1126/science.1074685. [DOI] [PubMed] [Google Scholar]
- 30.The authors are grateful to Dennis T. Ta (UCI) for sharing his expertise in SDS gel preparation and Dr. Deliang Chen for help to optimize culture growth conditions. This work was supported by NIH grant GM29498 and DOE grant DEFG03-86ER13525 (both to J. K. L.). V.A.B. thanks the “Molecular and Cellular Biology” program of the Russian Academy of Sciences for support.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.