Abstract
Studies of early placental development in humans are difficult because of limitations on experimental material availability from the perimplantation period. We used a coculture system to determine the effects of various effector cell types on trophoblast differentiation. Enhanced green fluorescent protein (EGFP)-expressing H1 human embryonic stem cells were used in co-suspension with human term placental fibroblasts (TPFs) and dermal fibroblasts (CI2F) to form combination embryoid bodies (EBs), with the goal of recapitulating placental morphogenesis through incorporation of placental mesenchymal cells. Overall, the results demonstrated that when using mesenchymal cells for EB preparation from term placentas (TPF), combination EB-derived trophoblasts secrete higher levels of human chorionic gonadotropin (hCG) and progesterone compared to EBs made without effector cells, whereas there was no effect of dermal fibroblasts. This is due to the secretory activity of the EB-derived trophoblasts and not due to the number of differentiated trophoblasts per EB, demonstrating that nontrophoblast cells of the placenta can influence trophoblast endocrine activity.
Keywords: Embryonic stem cells, trophoblast, embryoid body, implantation
Introduction
Implantation in humans entails the embryo traversing the maternal uterine epithelium, resulting in intimate contact of the embryo with maternal tissue and the subsequent formation of the hemochorial placenta.1 During this development, an epithelial–mesenchymal transition takes place where fetally derived extravillous trophoblast cells will migrate and invade the maternal arteries.2 The trophoblasts, which are epithelial in origin, will acquire mesenchymal characteristics and express adhesion molecules representative of endothelial cells (ie, vascular cell adhesion molecule-1 (VCAM-1), platelet endothelial cell adhesion molecule-1 (PECAM-1)).3,4 During the following weeks, the endothelial cells within the fetal mesenchyme of the chorionic villi will initiate the formation of endothelial tubes and will undergo vasculogenesis as well as angiogenesis to form the fetal villous vasculature.5,6
A substantial number of pregnancies fail within the first weeks, at least in part, due to failure upon implantation of the embryo to properly form the placenta. Most models to study early pregnancy events use placental material from 6 to 12 weeks of gestation and thus do not represent the earliest stages of placental development. With the isolation of human embryonic stem cells (hESCs) and the recognition of their ability to differentiate into trophoblast cells spontaneously, following treatment with BMP4, or during the formation of embryoid bodies (EBs), methods are now readily available, which may more closely mimic early trophoblast differentiation and implantation events.7–9 Our laboratory has shown that when hESCs are allowed to differentiate via EB formation and are then placed into an extracellular matrix (Matrigel) environment, they will differentiate to trophoblasts and secrete high levels of placental hormones.9 However, the formation of chorionic villi does not take place within this 3-dimensional (3D) culture system, and it is therefore hypothesized that cell-cell interactions with nontrophoblast components of the fetal lineages, or their secreted factors, might guide further differentiation.
In the current study, we developed methods for the formation of combination EBs with several nontrophoblastic effector cell types. Growing hESCs on placental fibroblast feeder layers (ie, 2-dimensional [2D] environment) does not promote human chorionic gonadotropin (hCG) secretion and does not provide sufficient cues for trophoblast differentiation. However, combination EBs (ie, ESC-placental fibroblast combination cell aggregates) in suspension secreted higher levels of hCG than EBs without effector cells. In addition, effector cells isolated from nonplacental tissues did not enhance placental hormone secretion. Thus, placental fibroblast cues in the EB context, but not as a feeder monolayer, contribute to trophoblast differentiated function in EBs from hESCs.
Materials and Methods
Human Embryonic Stem Cell Culture
Undifferentiated hES cells (H1 cell line with National Institutes of Health (NIH) identifier WA01; Thompson, 1998) transfected to stably express enhanced green fluorescent protein (EGFP)10 were grown on growth factor-reduced Matrigel-coated plates (35 mm; BD-Biosciences, Franklin Lakes, New Jersey). Mouse embryonic fibroblast–conditioned medium (MEF-CM) was collected and used to maintain the undifferentiated hESCs. Conditioned medium consisting of 80% Dulbecco modified Eagle medium ([DMEM] Invitrogen, Carlsbad, California), 20% KnockOut Serum Replacement (Invitrogen), 1 mm l-glutamine (Invitrogen), 0.1-mm β-mercaptoethanol (Sigma, St Louis, Missouri), and 1% nonessential amino acid stock (Invitrogen) were harvested from irradiated MEFs after 24 hours in culture, and 4 ng/mL fibroblast growth factor-2 (FGF-2) was added to MEF-CM just before adding it to the hESCs.11
Human Term Placental Fibroblast Isolation
All studies with human tissues were approved by the UW-Madison Human Subjects Institutional Review Board. Informed consent was obtained from patients scheduled for cesarean section delivery at term. Term placentas were obtained immediately after surgery and were transferred on ice to the laboratory. The decidual layer of the placenta was removed to expose the villous tissue, and 30 to 40 g of villous tissue was rinsed of excess blood with Hank Buffered Salt Solution ([HBSS]; Invitrogen), transferred to a 150-mm dish, and finely minced. Obvious blood vessels were removed during mincing to decrease possible endothelial cell contamination. Minced tissue was then transferred to a cheesecloth-lined strainer and rinsed with HBSS several times before being transferred back to a 150-mm dish for weighing. When 20 to 30 g ofminced tissue was available, it was transferred to a 500-mL flask containing 120 mL HBSS. Trypsin (16 000 units/mL) and DNase (30 mg) were dissolved in 30 mL HBSS and added to the flask. The flask was then placed in an incubator shaker set on a slow shaking speed. After 20 minutes, the flask was removed from the shaker and an additional 10 mg of DNase dissolved in 5 mL HBSS was added to the flask. The flask was replaced on the shaker for an additional 10 minutes. The flask was then removed from the shaker, transferred to a sterile hood, and set at an angle to allow the tissue to settle to the bottom. As the tissue settled, 5 mL heat-inactivated fetal bovine serum ([FBS]; Atlanta Biologicals, Norcross, Georgia) was added to each of 3 50-mL conical tubes. A 100-µm cell strainer was placed over each tube, and the supernatant from the flask was transferred through the cell strainers to the 50-mL tubes. Fresh enzymes in HBSS were added to the tissue in the flask along with 120 mL HBSS for a second digestion as above, and 2 additional digests were completed, omitting the addition of extra DNase. During digestions, cells were harvested from the supernatant of the previous digest by centrifugation at 1200 rpm at 20°C for 7 minutes, and the cell pellets were resuspended in fibroblast media consisting of 90% DMEM (Invitrogen) + 10% FBS (Atlanta Biologicals). All cells were pooled and plated into 75 cm2 or 25 cm2 flasks with 10 mL or 5 mL, respectively, of fibroblast media. Flasks of plated cells were incubated under standard cell culture conditions for 1 hour, then the media and unattached cells were removed and fresh fibroblast media was replaced in the original flask. The short initial incubation was designed to selectively plate only fibroblasts, which attach earlier than other cell types. Following incubation under standard conditions for an additional 24 hours, flasks were washed 3 times with HBSS to remove unattached cells. Fresh fibroblast media was replaced and the flasks were returned to standard culture conditions.
Human Term Placental Fibroblast Culture
Human term placental fibroblasts (TPFs) were grown under standard culture conditions with medium(90%DMEM; Invitrogen) + 10% FBS (Atlanta Biologicals) changes 2 to 3 times per week until 100% confluence was reached. Confluent flasks were split by washing twice with PBS and treating with trypsin for approximately 5 minutes at 37°C. Fibroblast medium was then added to flasks to inactivate the trypsin, and the cell suspension was transferred to 15 mL conical tubes. Tubes were spun at 2000 rpm for 3 to 5 minutes, the supernatant was removed and the cell pellet was resuspended in fibroblast medium. The cell suspension was then spun as described above, the supernatant aspirated off, and the cell pellet resuspended in fibroblast medium. Cells were then plated into new 75 cm2 flasks at a density of approximately 5 × 105 cells per flask.
Dermal Fibroblast Cell Culture
Dermal fibroblasts (CI2F) were provided by Dr Lynn Allen-Hoffman, Department of Pathology and Laboratory Medicine, University of Wisconsin–Madison. Stocks were frozen back at 3 to 6 passages and thawed and expanded for preparation of combination EBs as needed. Human effector cells (TPF or CI2F) were maintained in DMEM (Invitrogen) + 10% heat-inactivated FBS (Atlanta Biologicals) or F12 (Invitrogen) + 10% FBS (Atlanta Biologicals), respectively.
Cell Tracker Staining of Effector Fibroblasts
Cell Tracker Red (Invitrogen) was prepared by adding 7.3 µL dimethyl sulfoxide (Sigma) to 50 µg cell tracker powder, yielding a 10-mmol/L stock solution. Appropriate volumes of the stock solution were added to fibroblast culture media to a 25-µmol/L final concentration. Three milliliters of 25 µmol/L dye were added to each 75 cm2 flask of effector cells, and the flasks were allowed to incubate under standard culture conditions for 45 minutes. After incubation, the flasks were washed 2 to 3 times with PBS (Invitrogen) before replacement with fresh media. The flasks were returned to standard culture conditions until experimental use, approximately 1 to 2 days.
Combination EB Preparation
For each combination EB mixture, effector cells were prepared as a single cell suspension by trypsin treatment and approximately 2 to 12 × 106 cells were used for each experiment. Approximately 107 cells or the equivalent of a near-confluent 6-well (35 mm) dish of H1EGFP hESC colonies10 were enzymatically released from the culture dish and passed through a 100-µm cell strainer (BD Falcon, Franklin Lakes, New Jersey). The hESC colonies that remained on the strainer were resuspended in EB media. Effector and hESC suspensions were combined into a total of 15 mL EB media in a 100-mm Optilux non-TC culture dish (BD) and placed on a thermolyne rocker (speed setting 25) at 37°C overnight. After 24 to 48 hours of suspension culture, combination EBs were passed through a 100-µm cell strainer and subsequently through a 70-µm strainer. All size-selected EBs were transferred to 35-mm dishes (Corning, Corning, New York) and then manually separated on the basis of green and red fluorescence with visualization in a Leica DMIRB microscope (Leica Microsystems, Bannockburn, Illinois). Only EBs that expressed both green and red fluorescence were selected and transferred to a new 35-mm dish with a Hamilton syringe and unipet. Within 24 hours, combination EBs were counted and seeded at 25 to 50 EBs per 35-mm dish. The same procedure was followed for control EBs using H1EGFP hESCs but without adding effector cells. All cultures were kept in suspension on a rocker for up to 30 days and 1 mL of EB medium was collected daily from each 35-mm dish and replaced with fresh EB medium. All collected samples were stored at −20°C.
Confocal Imaging
Images were acquired using a Leica TCS SP2 AOBS laser scanning confocal microscope (Leica Microsystems) with a 20× objective and 2.3× magnification, giving a pixel dimension of 320 nm. Green channel images were acquired using a 488 excitation wavelength and fluorescence emission was collected from 500 to 535 nm. Red channel images were acquired using a 543 nm excitation wavelength and fluorescence emission was collected from 573 to 637 nm. For each channel, confocal images were acquired at 1.67 µm interval thickness along the Z-axis through the entire EB.
Hormone Assays
All stored samples were allowed to thaw and hCG and progesterone levels were determined in duplicate by radioimmunoassay and enzyme immune assay, respectively, by Assay Services at the National Primate Research Center, University of Wisconsin–Madison, as previously described.9
Immunostaining
Embryoid bodies were fixed with 2% paraformaldehyde for 1 to 2 hours and then washed twice with PBS (Invitrogen). Embryoid bodies were then placed in a warmed (48°C), 1% agarose solution, allowed to gel for 20 minutes, and subsequently dehydrated and embedded in paraffin. Paraffin-embedded specimens were then sectioned at 6 to 7 µm and used for staining. Cell cultures were fixed in wells with 2% paraformaldehyde for 1 to 2 hours and then washed twice with PBS (Invitrogen). All tissues were boiled either in 10 mmol/L sodium citrate in a microwave (800 W, Samsung, model MW5536) for 6 minutes, level 10, and then for another 3 to 6 minutes, level 6, or were pretreated with proteinase K (10 µg/µL) made in Tris buffered saline (TBS) for 4 minutes at 37°C. For immunostaining, mouse monoclonal anti-human fibroblast surface protein clone 1B10 (Sigma) was used at 0.4 µg/mL, mouse monoclonal anti-hCG (Neomarkers, Fremont, California) was used at 0.83 µg/mL, mouse monoclonal anti-human cytokeratin (BD) was used at 0.125 µg/mL, mouse monoclonal anti-human vimentin (Sigma) was used at 1.275 µg/mL, and mouse monoclonal anti-human Ki67 (Vector Laboratories, Burlingame, California) was used at 0.37 µg/mL. Human ES cell cultures were stained with mouse monoclonal anti-human Oct-3/4 (Santa-Cruz Biotechnology, Santa Cruz, California) at 10 µg/mL. In each experiment, a nonspecific isotype-matched antibody was used as a control. All slides were counterstained with hematoxylin (Richard-Allan Scientific, Thermo Scientific, Waltham, Massachusetts) with the exception of the Oct-3/4 stained cell culture tissue where a 7-amino-4-methylcoumarin-3-acetic acid (AMCA)-conjugated sheep anti-mouse antibody was used to visualize stained cells (Vector Laboratories).
Flow Cytometry
Cells were fixed and permeabilized first using the Cytofix/Cytoperm Kit (BD Pharmingen, Franklin Lakes, New Jersey) according to the manufacturer’s protocol, and then subjected to monoclonal antibody (mAb). Perm/Wash buffer (BD Pharmingen) was used as the washing and blocking buffer and as the antibody diluent. Cells at 0.3 × 106 per sample were incubated with the anti-vimentin mAb (Sigma) or an isotype control for 30 minutes at 4°C. After 2 washes cells were incubated with 10 µg/mL of rat immunoglobulin G ([IgG]; Sigma) for 10 minutes at room temperature (RT) to block nonspecific binding of secondary mAb. R-phycoerythrin (PE)-conjugated rat anti-mouse IgG1 mAb (BD Pharmingen) was added to all samples for 30 minutes at 4°C. Samples were washed twice and mouse IgG (Sigma) at 10 µg/mL was used as the next blocking agent for 10 minutes at RT. Fluorescein isothiocyanate (FITC)-labeled anti-pan cytokeratin (clone C-11, Sigma) or FITC-conjugated isotype control was then added and cells were incubated for 30 minutes at 4°C. After 2 washes, the cells were resuspended in Perm/Wash buffer for the analysis. For each sample, 20,000 cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, California) and CellQuest Software. Data analysis was done using FlowJo software 8.7.1 (Tree Star Inc, Ashland, Oregon).
Morphometric Analysis
Morphometric analysis was performed as previously described by Bondarenko et al.12 Briefly, digital images of EBs from each treatment group were captured using a Leica DM IRB microscope equipped with an Optronics MagnaFire digital camera and software. From each EB, 2 to 5 selected microscopic fields were acquired. Morphometric analyses were performed using NIH ImageJ 1.32j software for measuring photomicrograph image features of EB sections. Embryoid body sections were traced manually on the computer monitor with a mouse-controlled cursor at on-screen magnification to calculate the areas of the entire section in addition to the area with positive staining for immunohistochemical stains: cytokeratin, hCG, and Ki67. The results from each EB were averaged to give one value for each EB type. Sections from 3 (CI2F) or 4 (TPF) independent experiments, each representing 1 to 2 EBs were analyzed for each treatment group.
Statistical Analyses
Mean ± SEM were calculated for each combination and control EB group for hCG and progesterone secretion. The statistical significance of the differences between groups was assessed using a 2-way analysis of variance (ANOVA) with repeated measures (SAS, Cary, North Carolina) followed by Tukey Honestly Significant Difference test. Mean ± SEM were calculated for each immunohistochemical stain and the statistical significance of the differences between EB groups was assessed using a 2-way ANOVA (GraphPad Prism 5.0a, GraphPad Software Inc, San Diego, http://www.graphpad.com) followed by the Bonferroni correction. Values were considered significant if P < .05.
Results
Embryoid Bodies on TPF Feeders
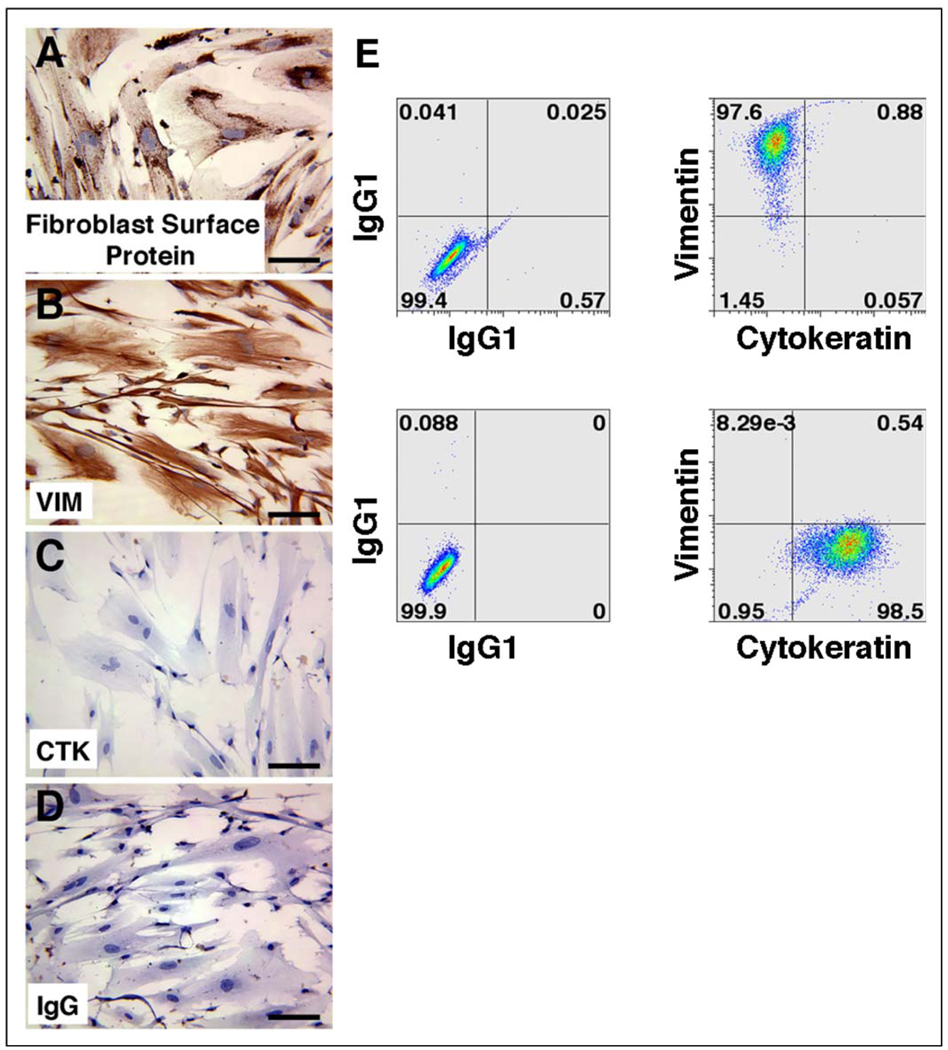
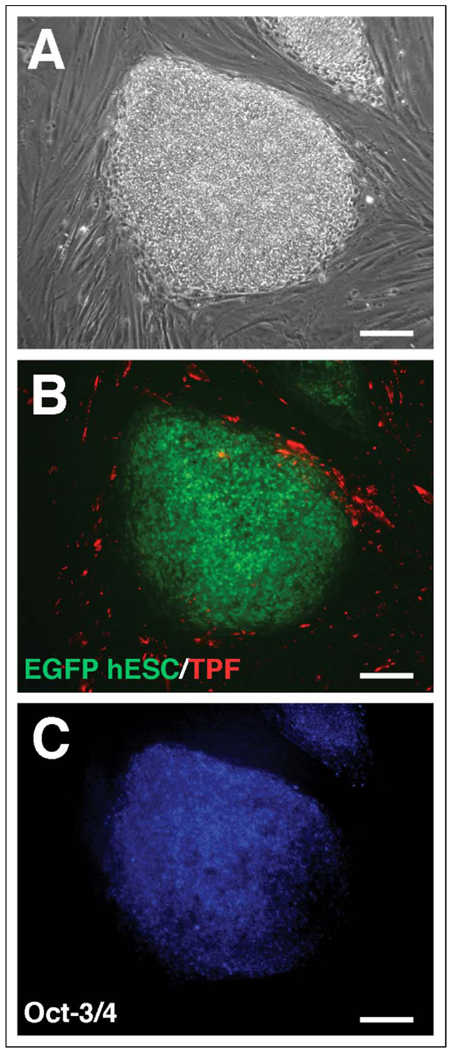
We first characterized the TPF cultures to confirm the absence of trophoblast contamination. Term placental fibroblasts maintained appropriate fibroblast surface markers, as they stained positively for both fibroblast surface protein (Figure 1A) and vimentin (Figure 1B) but did not stain for the epithelial marker cytokeratin (Figure 1C). In addition, hCG secretion was below the detectable limits of the assay in TPF cultures (data not shown). Double staining for vimentin and cytokeratin by flow cytometry showed that TPFs stained for vimentin but did not stain for cytokeratin, whereas the converse was true for positive control JEG-3 choriocarcinoma cells (Figure 1E). To determine whether the term fibroblast cells would stimulate hESC differentiation toward the trophoblast lineage, EGFP hESCs were next grown on cell tracker stained TPF feeder layers both with and without FGF-2 supplementation. After 1 week in culture, the stem cell colonies had increased in size and individual cell borders were not distinguishable, characteristic of undifferentiated cell proliferation, so the colonies were split onto new TPF feeder layers, this time in the absence of FGF-2. After passage, the hESC colonies continued to proliferate in an undifferentiated state. Surviving wells were maintained for 6 passages (approximately 6 weeks), and immunocytochemical staining for Oct-3/4, a marker of pluripotency, was strongly localized to the hESC colonies, revealing that colonies remained undifferentiated (Figure 2C). Cells that were stained with an IgG negative control did not display any positively stained colonies (data not shown). When compared with control wells of hES colonies grown on traditional MEF feeder layers, hES colonies on TPFs were morphologically indistinguishable from controls: compact colonies composed of many small cells with individual cell borders not easily identified, giving the colonies a large, round shape, and well-defined edges (data not shown). In addition, hCG secretion was always below the limit of detection in the culture medium (data not shown). Differentiated hESCs, identified by an increase in cell size, and distinct borders from surrounding cells and a lack of Oct-3/4 staining, were observed at a very low frequency in both MEF and TPF feeder layer cultures and were generally found around the periphery of the colonies. As with MEF feeder layers, TPFs were never observed growing over the top of hES colonies, as observed by distinct green (hESC) and red (TPF) areas (Figure 2B).
Figure 1.
Immunohistochemical staining of term placental fibroblasts (TPFs) for fibroblast surface protein (A), vimentin (B), and cytokeratin (C). Panel D depicts a nonspecific isotype-matched IgG negative control. TPF (top panels) and choriocarcinoma cell line, JEG-3, (lower panels) were analyzed by flow cytometry with cytokeratin and vimentin mAbs. The presence of vimentin staining and the lack of cytokeratin staining of permeabilized TPF cells demonstrate the purity of fibroblast cultures without contaminating trophoblasts. JEG-3 cells were used as a positive control to demonstrate cytokeratin staining in trophoblasts (E). IgG indicates immunoglobulin G; mAbs, monoclonal antibodies; VIM, vimentin; CTK, cytokeratin.
Figure 2.
Oct-3/4 staining of EGFP hESCs grown on TPF. Representative undifferentiated hESC colonies are presented as phase contrast (A); Fluorescent EGFP (hESCs) and Texas Red fluorescence (TPF monolayer) (B); or Oct-3/4 immunostaining (hESCs) (C). EGFP indicates enhanced green fluorescent protein; hESCs, human embryonic stem cells; TPF, term placental fibroblast.
Term Placental Fibroblast Combination EBs
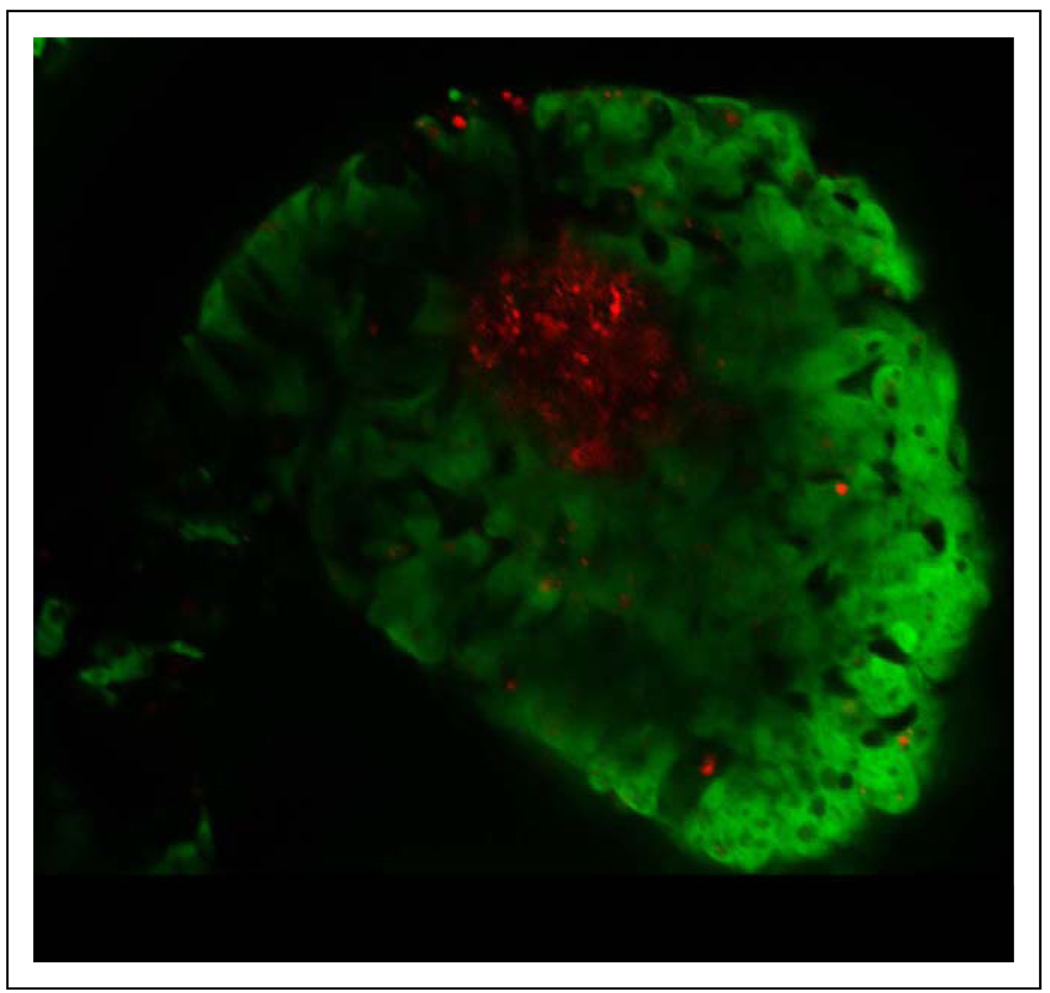
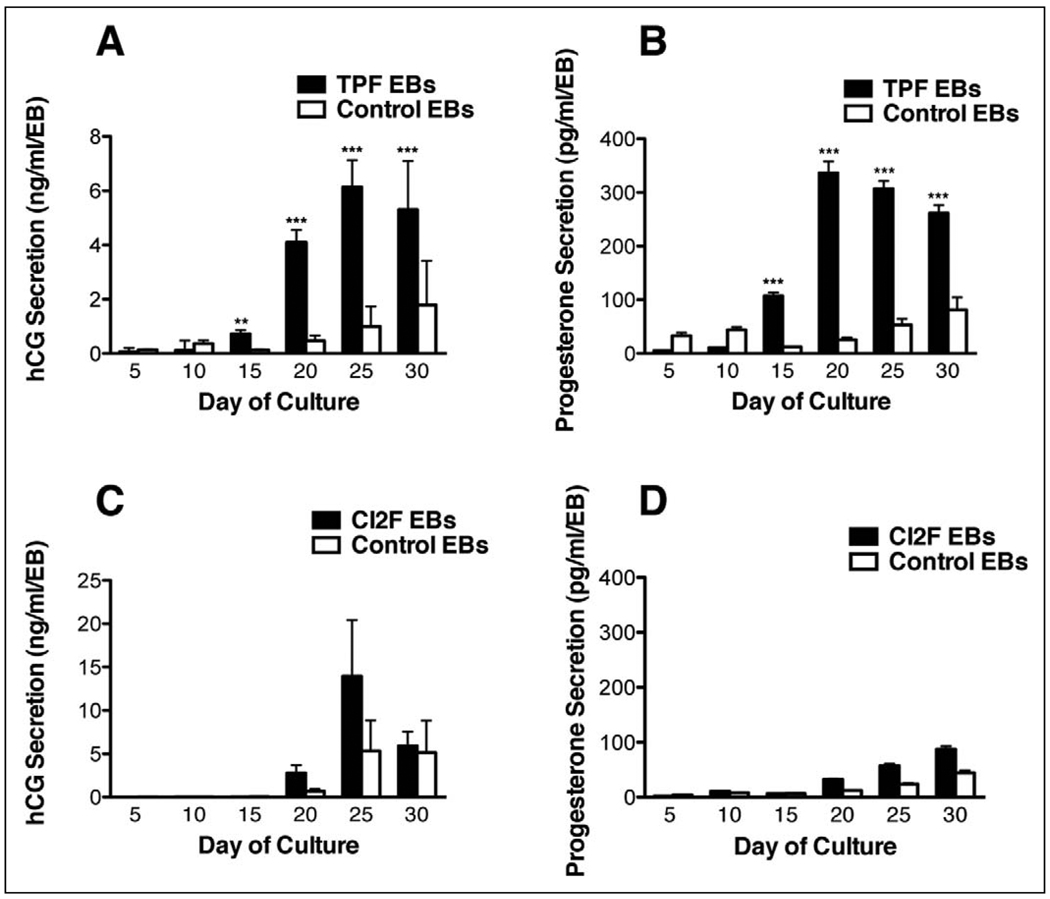
The EB as a model for trophoblast differentiation has been previously established.9 To identify the effects of epithelial–mesenchymal interactions on trophoblast differentiation and placental development, we cocultured cell tracker red-labeled fibroblasts with GFP-labeled hESCs in an EB system (Figure 3). Combination TPF-EBs were cultured in suspension for 30 days and half the media was collected from each well daily. Human chorionic gonadotropin secretion from each well was evaluated on days 5, 10, 15, 20, 25, and 30 of culture. An increase in hCG secretion was observed in the TPF-EB combination from days 15 to 30 of culture, with a peak secretion level of approximately 6 ng/mL per EB on day 25 (Figure 4A). Also, in comparison with the control EBs (ie, EBs made without effector fibroblasts), the combination EBs secreted significantly higher levels of hCG on days 15, 20, 25, and 30 of culture (Figure 4A; P < .01 day 15; P < .0001 days 20–30). Progesterone secretion was also significantly higher on the same days (Figure 4B; P < .0001 days 15 and 20; P < .01 days 25 and 30). These results indicate that by day 15 in suspension culture, EBs prepared with the effector TPF cells had increased secretion of the pregnancy hormones hCG and progesterone, suggesting that the TPF-hESC interactions were enhancing differentiated trophoblast function.
Figure 3.
Confocal image of a TPF combination EB in suspension. Fluorescent EGFP (hESCs) and Texas Red fluorescence (TPF inner core). EB indicates embryoid body; TPF, term placental fibroblast; EGFP indicates enhanced green fluorescent protein; hESCs, human embryonic stem cells.
Figure 4.
Hormone secretion of combination EBs compared to control EBs made without effector cells. A, C present hCG secretion by TPF and CI2F EBs, respectively, on days 5, 10, 15, 20, 25, 30 of culture. B, D present progesterone secretion by TPF and CI2F EBs, respectively, on days 5, 10, 15, 20, 25, 30 of culture. **P < .01 and ***P < .001, comparing a combination EB value with its control on a given day. Experiments with each combination cell type were not conducted contemporaneously and thus should not be compared between effector cells. EBs indicates embryoid bodies; TPF, term placental fibroblast; hCG, human chorionic gonadotropin.
Dermal Fibroblast (CI2F) Combination EBs
To explore whether there were tissue-specific effects of TPF effector cells, we prepared combination EBs from the human dermal fibroblast cell line C12F and H1EGFP hESCs. Embryoid bodies were maintained in suspension for 30 days, as in the preceding experiments. CI2F combination EBs secreted similar levels of hCG and progesterone compared to control EBs, with no statistical difference found between the 2 groups (Figure 4C, D). Detectable hCG and progesterone secretion indicate that trophoblast differentiation was not suppressed in the presence of effector cells from a nonplacental source.
Immunohistochemical Evaluation of Combination EBs in Suspension Culture
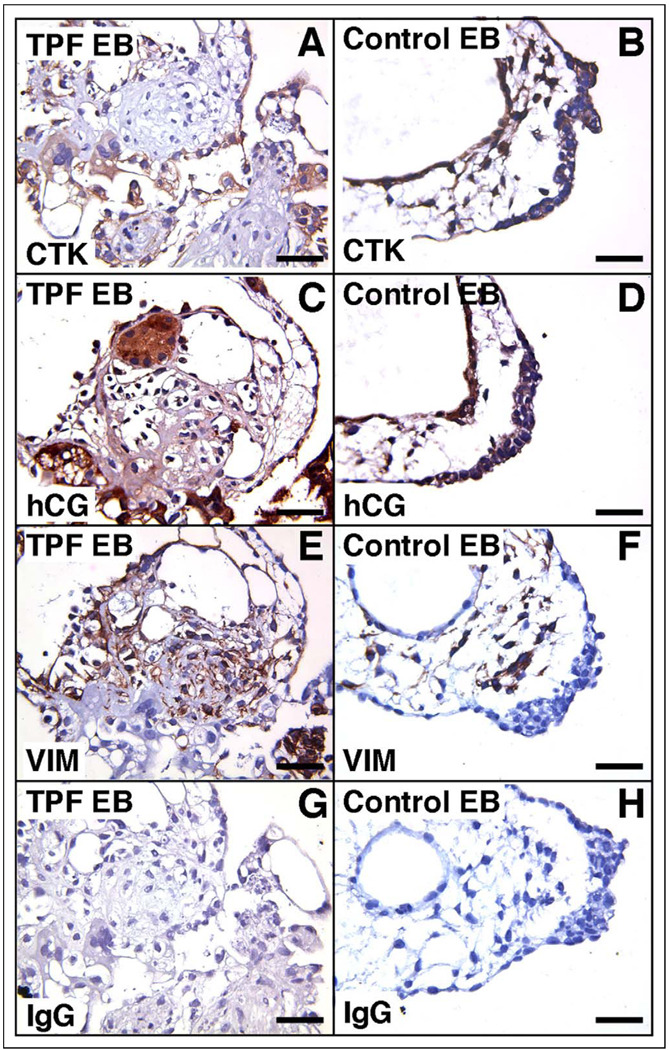
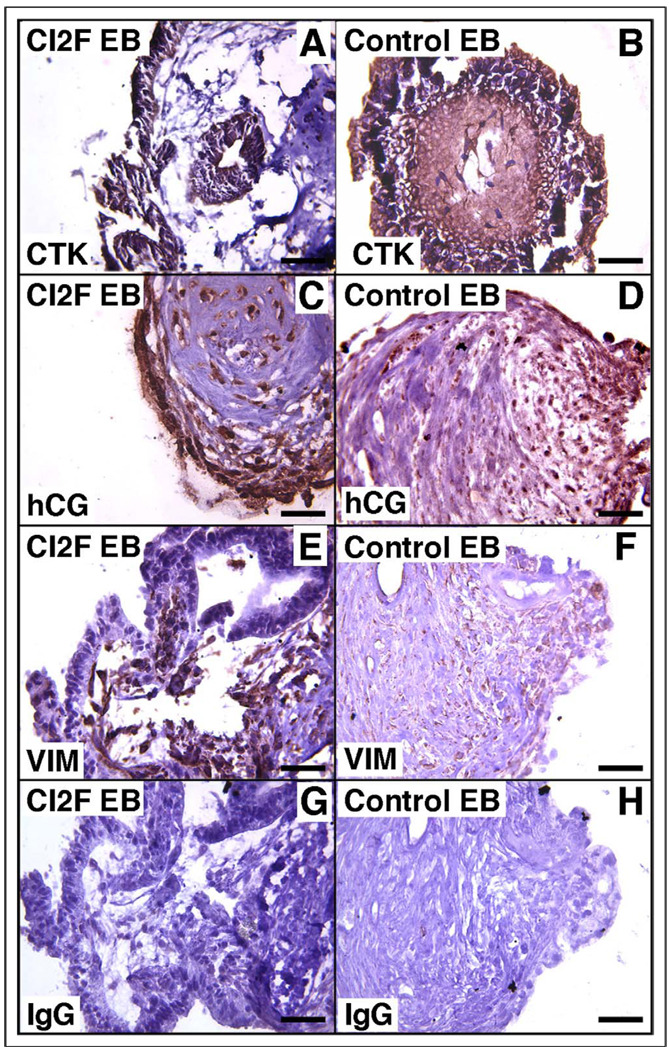
We sought to define trophoblast differentiation by immunohistochemistry within combination EBs (Figures 5, 6). The expression of hCG was generally restricted to a cytokeratin-positive population at the periphery of the combination EBs, similar to the staining pattern seen in control EBs without effector cells (Figures 5, 6 A–D). Vimentin-positive cells were typically localized to the core of most EBs (Figures 5, 6 E–F). Similar staining patterns were found for all combination (TPF and CI2F) EBs.
Figure 5.
Representative immunohistochemical staining of TPF EBs for cytokeratin (A), human chorionic gonadotrophin (C), and vimentin (E) and of control EBs for cytokeratin (B), human chorionic gonadotrophin (D), and vimentin (F). Panels G and H depict nonspecific isotype-matched IgG negative controls. IgG indicates immunoglobulin G; VIM, vimentin; CTK, cytokeratin; EB, embryoid body.
Figure 6.
Representative immunohistochemical staining CI2F EBs for cytokeratin (A), human chorionic gonadotrophin (C), and vimentin (E) and of control EBs for cytokeratin (B), human chorionic gonadotrophin (D), and vimentin (F). Panels G and H depict nonspecific isotype-matched IgG negative controls. IgG indicates immunoglobulin G; hCG, human chorionic gonadotropin; CTK, cytokeratin; EB, embryoid body.
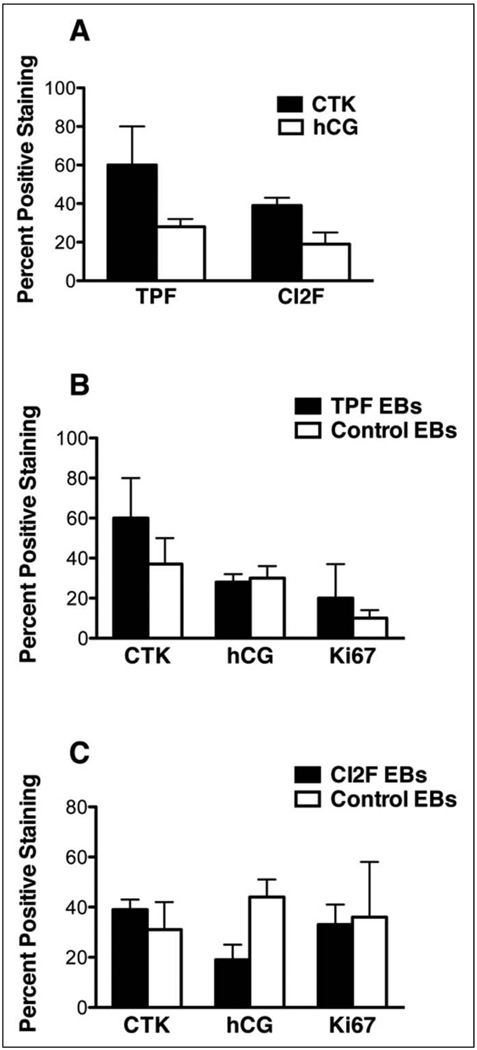
Because double immunostaining for cytokeratin and hCG proved to be technically challenging, separate analysis of each trophoblast marker was carried out to calculate the percentage of cytokeratin-positive cells and hCG-positive cells found in combination and control EBs. The same morphometric analysis was performed on sections immunostained for the proliferation maker Ki67 to determine the proliferative capacity of each EB type, assess the number of trophoblasts within combination EBs and control EBs, and assess proliferation in EB trophoblasts. Ki67 expression also demonstrated maintenance of proliferation and overall viability of EBs in all treatment groups. When comparing the percentage of cells in each of the combination EB groups that were cytokeratin and hCG positive, there were no statistically significant differences between the TPF and CI2F EBs (Figure 7A), nor were they significantly different from their respective control EBs (Figure 7 B,C). In addition, the size of the combination and control EBs was similar as determined by the area of each EB type.
Figure 7.
Morphometric analysis of immunohistochemical staining. Panel (A) presents the percentage of cells positively stained in 2 to 5 random fields for cytokeratin and human chorionic gonadotrophin for all (TPF and CI2F) combination EB groups. Panel (B) presents the percentage of positively stained cells of TPF EBs compared to control EBs for cytokeratin, human chorionic gonadotrophin, and Ki67. Panel (C) presents the percentage of positively stained cells of CI2F EBs compared to control EBs for cytokeratin, human chorionic gonadotrophin, and Ki67. hCG indicates human chorionic gonadotropin; CTK, cytokeratin; EBs, embryoid bodies; TPF, term placental fibroblast.
Discussion
Although placental explant studies provide a great deal of information about early pregnancy, the study of early implantation events and trophoblast differentiation would significantly benefit from a model system that does not rely on fresh tissue in which the trophectoderm has already undergone differentiation into the various trophoblasts of mature chorionic villi. The model of EB formation from hESCs for trophoblast differentiation provides an appropriate in vitro alternative for investigating these earliest time points in development. The aim of the current study was to determine the effects of coculture of placental fibroblast cells with hESCs on trophoblast differentiation by combining these effector cells with hESCs to form a combination EB, reasoning that this may recapitulate cell-cell interactions that are found in vivo. The key findings from these experiments were that EBs made with fibroblasts from term placentae were able to secrete higher levels of hCG and progesterone compared to EBs made without effector cells, when placed in suspension culture. Morphometric analyses confirmed that this was due to the secretory activity of the differentiated trophoblasts and not due to the percentage of trophoblasts present in the TPF EBs. However, fibroblasts from human dermal fibroblasts did not have a similar enhancement of hCG and progesterone secretion.
Similar to nonhuman primate ESC, hESCs can differentiate spontaneously into trophoblast cells of the placenta, therefore providing the platform for understanding early implantation events.7,13 It has also been shown that trophoblast differentiation from hESCs can be promoted by exposure to bone morphogenetic protein (BMP) or by the formation of EBs.8,9,14–17 Although these methods provide consistent trophoblast differentiation, further placental development and morphogenesis of appropriate villous structures fails to occur. The first nontrophoblastic, fetally derived cells that are in intimate contact with the trophoblasts of the developing placenta in vivo are fetal mesenchymal cells that will give rise to the villous stroma. As a first approximation of these cells, placental fibroblasts were used for coculture with hESCs. When placed in 2D culture on top of the placental fibroblasts, the hESCs were maintained undifferentiated and continued to express Oct-3/4, a marker of pluripotency. We demonstrated that hESCs grown on TPFs proliferate in an undifferentiated state. This data is consistent with recent reports that first trimester placental fibroblast feeder layers are capable of maintaining hESCs in an undifferentiated state.18,19 Other recent data suggest that FGF-2 can support undifferentiated hESC growth in the absence of feeder layers or conditioned medium, and the mechanism responsible for this effect may involve the inhibition of BMP signaling.20 In addition to FGF-2, noggin or other BMP antagonists may also be involved in the maintenance of undifferentiated stem cell growth.20 Given these data, it is conceivable that the TPFs produce FGF-2, among other factors, as hESC growth was maintained without the growth factor supplement that is typically required.
However, when an aggregate coculture system was developed, the TPF combination EBs secreted significantly more hCG and progesterone than control EBs, marking the importance of the dimensional effect on trophoblast differentiation. These results reinforce the evidence that not only does the source of fibroblast determine EB-derived trophoblast endocrine-differentiated function, but that the cell-cell interactions found in the aggregate combination EB seem to play a role as well. Traditional cell culture techniques fail to mimic the spatial dynamics that are seen in vivo. In vivo, cells grow by contacting other cells and are surrounded by different cell types and their secreted matrix proteins, in 3D, thus our combination EB model appears to provide some spatial cues that enhance trophoblast-differentiated function.
To distinguish whether the source of fibroblast must be placental in origin, combination EBs were also prepared with dermal fibroblasts isolated from fetal foreskin (CI2F). There was no statistically significant difference in hCG or progesterone secretion between CI2F EBs and control EBs, indicating that dermal fibroblasts do not modify hormone secretion in combination EBs. An additional point is that there seems to be qualitative differences among the control EBs between experimental groups. This may be due to the variation in the size of control EBs and the methods used to make them. Alternative methods to produce EBs of uniform cell number, size, and shape would allow significant refinement of the approaches available in this field.
Immunohistochemical staining and subsequent morphometric analysis of EBs has also provided evidence that the percentage of trophoblasts that are found in all combination EB types (TPF and CI2F) are similar. The pattern of cytokeratin and hCG staining is restricted to the outermost layer of cells in all combination and control EBs. In addition, the percentage of positive stained cytokeratin and hCG cells are not different between combination groups, indicating that the actual numbers of trophoblasts that are found in each combination EB are similar. Because the area of combination EBs and control EBs is similar, these results indicate that increased hCG secretion by TPF EBs compared to controls is due to the ability of the EB-derived trophoblast cells to secrete more hormone and not due to the percentage of trophoblast cells that were differentiated in the EB. Moreover, there were no differences in the percentage of Ki67-positive cells in combination and control EBs and between Ki67, cytokeratin, and hCG staining in any EB type, therefore we can conclude that the balance of proliferation and differentiation is similar among both experimental groups.
Overall, these findings provide evidence that cell-cell interactions in an aggregate coculture system influence trophoblast differentiation in the in vitro EB model. It has been well established that the creation of a stem cell niche is necessary to maintain an undifferentiated population of cells. Some of the basic components of a stem cell niche included stromal cells, cell-cell adhesions, soluble factors, and the extracellular matrix (ECM).21 Current and future directions for this EB model include the addition of other components such as ECM interactions to create a 3D system to induce differentiation.9 These studies would help elucidate the necessary inductive signals that EB-derived trophoblasts need to further differentiate into the extravillous trophoblast phenotype, therefore allowing this model to be used to study endovascular remodeling of vessels that is often impaired in preeclamptic pregnancies. Thus, this in vitro model provides a novel platform for continuing research to better understand the initial developmental events that occur in early implantation.
Acknowledgments
We thank Fritz Wegner and Dan Wittwer of the Wisconsin National Primate Research Center Hormone Assay Laboratory for assistance with hCG and progesterone assays; Nicholas Keuler of the Department of Computing and Biometry at the University of Wisconsin–Madison for statistical assistance; and Judith Peterson for help with manuscript preparation. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Funding
Research was conducted at a facility with support from Research Facilities Improvement Program grant no: RR15459-01 and RR020141-01, by NIH Clinical and Translational Science Award UL1 RR025011 to the UW Institute for Clinical and Translational Research, and by P51 RR000167 to the WNPRC. Research was supported by NIH grants HD046919 and HD038843 to TGG, HD T32 041921 Predoctoral Fellowship to MG.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Benirschke K, Kaufman P, Baergen RN. Pathology of the Human Placenta. 5th ed. New York, NY: Springer-Verlag; 1999. [Google Scholar]
- 2.Vicovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 1996;156(3):202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 3.Damsky CH, Fisher SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10(5):660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Red-Horse K, Zhou Y, Genbacev O, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114(6):744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25(2–3):114–126. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Golos TG, Pollastrinin LM, Gerami-Naini B. Human embryonic stem cells as a model for trophoblast differentiation. Semin Reprod Med. 2006;24(5):314–321. doi: 10.1055/s-2006-952154. [DOI] [PubMed] [Google Scholar]
- 7.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20(12):1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 9.Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells as a model for trophoblast differentiation. Endocrinology. 2004;145(4):1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- 10.Liu YP, Dovzhenko OV, Garthwaite MA, et al. Maintenance of pluripotency in human embryonic stem cells stably overexpressing enhanced green fluorescent protein. Stem Cells Dev. 2004;13(6):636–645. doi: 10.1089/scd.2004.13.636. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 12.Bondarenko GI, Burleigh DW, Durning M, Breburda EE, Grendell RL, Golos TG. Passive immunization against the MHC class I molecule Mamu-AG disrupts rhesus placental development and endometrial responses. J Immunol. 2007;179(12):8042–8050. doi: 10.4049/jimmunol.179.12.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci. U S A. 1995;92(17):7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peiffer I, Belhomme D, Barbet R, et al. Simultaneous differentiation of endothelial and trophoblastic cells derived from human embryonic stem cells. Stem Cells Dev. 2007;16(3):393–402. doi: 10.1089/scd.2006.0013. [DOI] [PubMed] [Google Scholar]
- 15.Soares MJ, Wolfe MW. Human embryonic stem cells assemble and fulfill their developmental destiny. Endocrinology. 2004;145(4):1514–1516. doi: 10.1210/en.2003-1737. [DOI] [PubMed] [Google Scholar]
- 16.Schulz LC, Ezashi T, Das P, Westfall SD, Livingston KA, Roberts RM. Human embryonic stem cells as models for trophoblast differentiation. Placenta. 2008;29 suppl A:S10–S16. doi: 10.1016/j.placenta.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harun R, Ruban L, Matin M, et al. Cytotrophoblast stem cell lines derived from human embryonic stem cells and their capacity to mimic invasive implantation events. Hum Reprod. 2006;21(6):1349–1358. doi: 10.1093/humrep/del017. [DOI] [PubMed] [Google Scholar]
- 18.Genbacev O, Krtolica A, Zdravkovic T, et al. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil Steril. 2005;83(5):1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 19.Simón C, Escobedo C, Valbuena D, et al. First derivation in Spain of human embryonic stem cell lines: use of long-term cryopreserved embryos and animal-free conditions. Fertil Steril. 2005;83(1):246–249. doi: 10.1016/j.fertnstert.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2(3):185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 21.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9(1):11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]