Abstract
Vascular endothelial growth factor (VEGF) has two highly homologous tyrosine kinase receptors: Flt-1 (VEGFR-1) and KDR (VEGFR-2). KDR is strongly phosphorylated on tyrosines and can transmit mitogenic and motogenic signals following VEGF binding, while Flt-1 is markedly less effective in mediating such functions. To dissect the regions that account for the differences between the two receptors, we generated a series of chimeric Flt-1–KDR molecules. We found that the juxtamembrane region of Flt-1 prevents key signaling functions. When the juxtamembrane region of Flt-1 is replaced by that of KDR, Flt-1 becomes competent to mediate endothelial cell migration and phosphatidylinositol 3′-kinase activation in response to VEGF. Further mutational analysis shows that a short divergent sequence is responsible for such repressor function. However, mutant Flt-1 receptors lacking this sequence do not transmit effective proliferative signals, suggesting that this receptor function is regulated separately. These results define a novel functional domain that serves to repress Flt-1 activity in endothelial cells.
Keywords: Flt-1/juxtamembrane region/KDR/phosphatidylinositol 3′-kinase/VEGF
Introduction
Vascular endothelial growth factor (VEGF), a major regulator of physiological and pathological angiogenesis (Leung et al., 1989), exerts its cellular functions on the vascular endothelium by binding to two different tyrosine kinase receptors: Flt-1 (VEGFR-1) and KDR (VEGFR-2) (Ferrara, 1999). Both receptors consist of seven immunoglobulin-like domains in their extracellular regions, followed by a transmembrane sequence and a short intracellular juxtamembrane region. The kinase domain is interrupted by a kinase-insert region and followed by a C-terminal sequence (Shibuya et al., 1990; Terman et al., 1991; de Vries et al., 1992). The structurally related tyrosine kinase Flt-4 (VEGFR-3) is not a receptor for VEGF, but instead binds VEGF-C and VEGF-D (Enholm et al., 1998). More recently, neuropilin-1 has been identified as an isoform-specific VEGF receptor that is thought to facilitate KDR-dependent signal transduction (Soker et al., 1998). Binding of the VEGF dimer to the second and third immunoglobin-like domains of Flt-1 or KDR is believed to result in receptor dimerization and initiates signal transduction (Davis-Smyth et al., 1996; Fuh et al., 1998). VEGF binding causes autophosphorylation of tyrosines in the cytoplasmic region of the KDR receptor, residues that may be recognized by various Src homology 2 (SH2) domain-binding proteins (Waltenberger et al., 1994; Guo et al., 1995).
Several different SH2 domain-containing proteins have been reported to interact with phosphotyrosines in the intracellular regions of KDR and Flt-1. Phosphopeptides based on the Flt-1 intracellular domain bind to phospholipase Cγ (PLCγ), Grb2 and the phosphatase SHP-2 in vitro (Ito et al., 1998). In vitro binding of PLCγ to full-length baculovirus-produced Flt-1 has also been reported. This interaction also occurs upon overexpression in NIH 3T3 fibroblasts (Sawano et al., 1997). In directed yeast two-hybrid assays, Flt-1 can interact with the phosphatidylinositol 3′-kinase (PI 3′-kinase) regulatory subunit, p85α, and with SHP-2 and Nck (Cunningham et al., 1995; Igarashi et al., 1998a). It is unclear whether any of these proteins can bind to Flt-1 in endothelial cells.
In a non-directed yeast screen, Sck was identified as a binding partner for KDR (Igarashi et al., 1998b). In 293 cells expressing KDR, PLCγ becomes phosphorylated after VEGF treatment (Dougher and Terman, 1999). PLCγ also interacts with Flk in VEGF-treated mouse mesentery (Mukhopadhyay et al., 1998).
While the biological functions of KDR activation are reasonably well understood, the function of Flt-1 in the adult endothelium is considerably less clear (Ferrara, 1999). We recently conducted a study using novel KDR- and Flt-selective VEGF mutants to elucidate the functions of the two receptors in primary endothelial cells. Intracellular signaling and cell migration in primary cells and in vivo angiogenesis could be obtained with the KDR-selective mutant alone, but not with the Flt- selective mutant (H.Gille, J.Kowalski, B.Li, B.Moffat, T.F.Zioncheck, N.Pelletier and N.Ferrara, submitted). Similar results have been obtained when comparing the effects of placenta-derived growth factor (PlGF), which binds to Flt-1 but not to KDR, with those of another VEGF-like molecule, VEGFOrf. The VEGFOrf gene was isolated from the Orf parapoxyvirus; the protein binds to KDR but not Flt-1 (Meyer et al., 1999; Wise et al., 1999).
Flt-1 contains a functional kinase domain, as autophosphorylation sites can be detected upon overexpression in heterologous systems (Sawano et al., 1997). The receptor can also be activated oncogenically by fusion to a dimerization motif followed by random mutagenesis (Maru et al., 1998). Flt-1-null mice die at embryonic day 9. They show an increase in endothelial progenitor cells leading to vascular disorganization (Fong et al., 1995, 1999). However, mice homozygous for a deletion of the Flt-1 cytoplasmic domain develop normally and are fertile. Their only defect is a loss of VEGF-induced macrophage migration in vitro (Hiratsuka et al., 1998). These observations have led to the hypothesis that Flt-1 may act as a decoy receptor in the vasculature (Park et al., 1994; Hiratsuka et al., 1998).
In order to understand the structural basis for the strikingly different properties of KDR and Flt-1, we constructed chimeric receptors by domain swapping. We found that the intracellular juxtamembrane (jx) region in Flt-1 suppresses activation of this receptor, as defined by VEGF-dependent chemotaxis. However, a chimeric Flt-1 receptor containing the KDR jx sequence can direct the phosphorylation of PI 3′-kinase and stimulate cell migration. PI 3′-kinase activity is required for endothelial migration. These results define a novel inhibitory domain in the VEGF receptor Flt-1.
Results
Generation of cell lines expressing chimeric receptors
We generated porcine aortic endothelial (PAE) cell lines stably expressing each VEGF receptor. PAE cells do not express endogenous KDR, Flt-1 or platelet-derived growth factor receptor (PDGFR) proteins (Miyazono et al., 1987; Waltenberger et al., 1994).
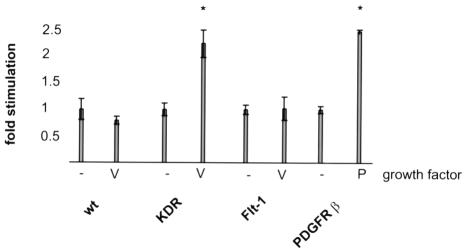
One of the central aspects of VEGF action on endothelial cells is its ability to act as a chemoattractant and stimulate the migration of endothelial cells. Figure 1 shows the results of a migration assay performed in a modified Boyden chamber with PAE cells expressing the various receptors. VEGF addition stimulates the migration of PAE cells expressing KDR, but not that of Flt-1-expressing cells, in agreement with a previous report (Waltenberger et al., 1994). Such KDR-dependent stimulation of migration is of the same order of magnitude as that observed in a well characterized PAE cell line expressing the PDGFβ receptor (Mori et al., 1993).
Fig. 1. Migration of PAE cells expressing VEGF and PDGFβ receptors. The ability of cell lines expressing the indicated receptors to mount a migratory response to 20 ng/ml growth factor was tested. Results are expressed relative to basal, factor-independent, migration. The bars represent the mean ± SEM. Experiments were performed in triplicate and significance was calculated to a 95% confidence level.
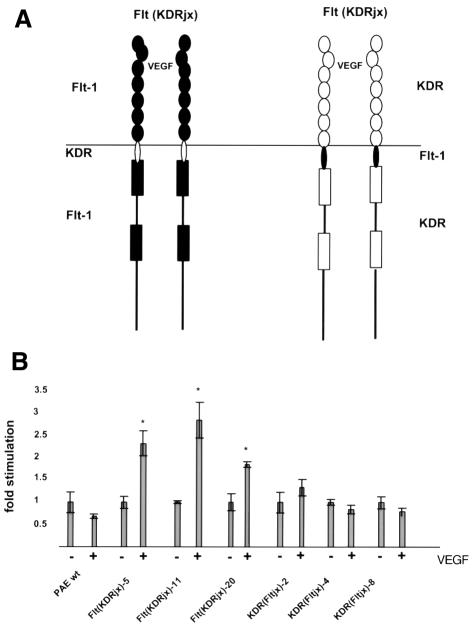
We wished to determine which domain(s) of Flt-1 is/are responsible for its failure to cause cell migration in endothelial cells. To this end, Flt–KDR chimeras were constructed and multiple clones were generated in PAE cells. In the initial chimeras, the kinase domain and the C-terminal sequences were exchanged. Surprisingly, PAE clones expressing chimeras of the Flt-1 kinase domain fused to the KDR intracellular jx and extracellular regions were able to migrate in response to VEGF stimulation to an extent similar to that of cells expressing wild-type KDR. Fusion of the Flt-1 jx and extracellular regions to the KDR kinase domain resulted in reduced migration of PAE cells in response to VEGF (data not shown). Therefore, we generated the chimeric receptors Flt(KDRjx) and KDR(Fltjx), outlined in Figure 2A, in which only the intracellular jx regions were exchanged. Several PAE cell lines expressing these chimeras were obtained and tested for their ability to migrate after VEGF treatment (Figure 2B). Comparison of the Flt(KDRjx) and KDR(Fltjx) cells demonstrates that the KDR jx region is sufficient to confer upon Flt-1 the ability to cause migration. On the other hand, when the Flt jx region is introduced into KDR, the mutant receptor appears unable to mediate chemotactic signaling in response to VEGF.
Fig. 2. Domain structure (A) and migration (B) of the KDR–Flt-1 chimeras. Schematic representations of the receptor chimeras are shown above. Flt-1 domains are in black. Migration results are presented as in Figure 1. At least five independent clones for each construct were analyzed and data shown reflect representative clones.
Mutational analysis of Flt-1 receptor juxtamembrane region
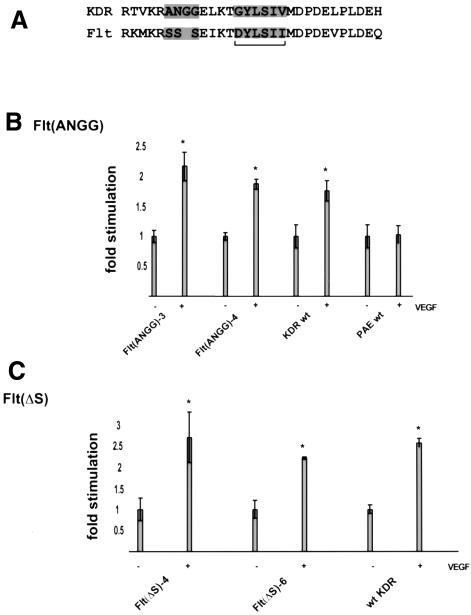
In order to characterize further the differences between the intracellular KDR and Flt jx regions, a sequence alignment was performed. Figure 3A shows that this region is highly conserved between the two receptors. One sequence feature of the jx regions is a potential SH2-binding site that is strongly conserved between the two receptors. At the +1 and +3 positions, which are major determinants of SH2 specificity, the sequence is identical in KDR and Flt-1, making it unlikely that this motif differs functionally in these two proteins. The major non-conservative divergence is a stretch of three serines in Flt-1. This sequence is replaced by the sequence ANGG in KDR. Therefore, the three serines in Flt-1 were replaced by the sequence ANGG by site-directed mutagenesis, generating Flt(ANGG). We also generated an Flt-1 deletion mutant, Flt(ΔS), lacking the three serines.
Fig. 3. Mutational analysis of the VEGF receptor jx regions and bioactivity. (A) Alignment of the KDR and Flt-1 jx regions. The potential SH2 domain-binding site (underlined) and the most divergent region are highlighted. Motility assay on VEGF receptor mutants. (B) Flt(ANGG) and (C) Flt(ΔS). Migration data are presented as in Figure 1B.
PAE cells expressing the receptor mutants were tested for their ability to migrate in response to VEGF. The migratory response of Flt(ANGG)-expressing cells to VEGF is indistinguishable from that of wild-type KDR-expressing cells (Figure 3B). Flt(ΔS) cells behaved in a similar fashion, migration being significantly stimulated by VEGF (Figure 3C). These results indicate that the inhibitory function that resides in the Flt-1 jx region is exerted by the divergent stretch of three serines in Flt.
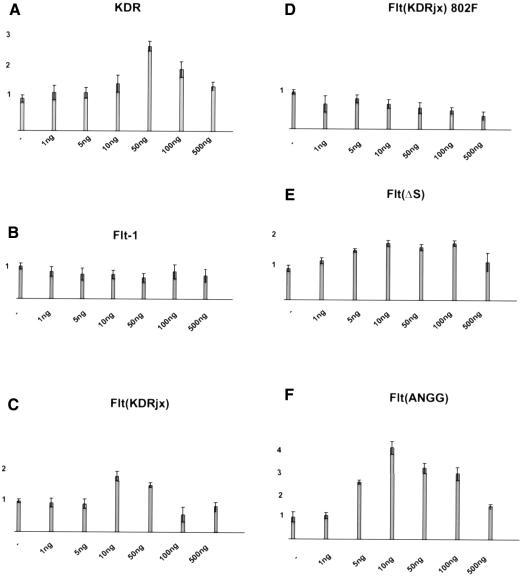
Some mutations in tyrosine kinase receptors shift the migration dose–response curve (Kundra et al., 1994), so we investigated whether any of our mutant receptors could do this. In addition to the mutants already described, we also analyzed a variant of Flt(KDR) in which the jx tyrosine had been mutated to phenylalanine, Flt(KDR)Y802F. Figure 4 shows that when motility was assessed at a VEGF concentration between 1 and 500 ng/ml, the maximal stimulation in cells expressing the receptors able to mediate migration was at 10–50 ng/ml. Small variations are likely to reflect the variability of the assay rather than any functional differences. No VEGF-dependent motility was detected in PAE cells expressing Flt(KDR)Y802F.
Fig. 4. Migration dose–response curves. The indicated cell lines were tested for migration towards a concentration range from 1 to 500 ng/ml VEGF.
Analysis of downstream signaling events
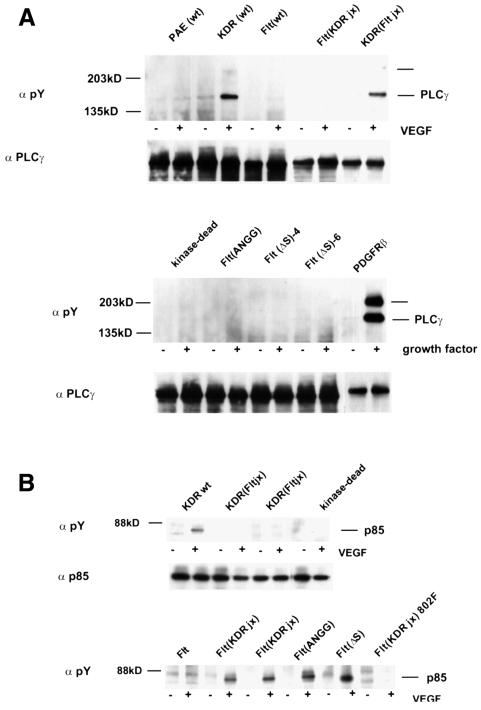
PLCγ can become tyrosine phosphorylated in response to VEGF treatment of endothelial cells (Guo et al., 1995). Phosphorylation of PLCγ occurs in PAE cells expressing KDR but not in Flt-1-expressing PAE cells, and its activation has been correlated with cell migration (Landgren et al., 1998). We therefore tested whether the Flt(KDRjx), KDR(Fltjx), Flt(ANGG) and FltΔS cells can cause PLCγ phosphorylation. Figure 5A shows that, as expected, PLCγ was phosphorylated after exposure to VEGF in cells expressing KDR, but not in those expressing Flt-1. PLCγ phosphorylation appeared to be weaker than in cells expressing PDGFβ receptors. Stimulation of KDR(Fltjx)-expressing cells causes PLCγ to become phosphorylated, but neither Flt(ANGG) nor Flt(ΔS) can trigger its phosphorylation. Since these cells can mount a migratory response after VEGF stimulation, PLCγ phosphorylation does not appear to be required for PAE cell migration.
Fig. 5. Analysis of downstream signaling. (A) PLCγ phosphorylation in response to VEGF stimulation. The migration of the faint bands at the top of the blot is consistent with the co-precipitated tyrosine-phosphorylated receptors. (B) PI 3′-kinase phosphorylation. Tyrosine phosphorylation of the endogenous p85 PI 3′-kinase regulatory subunit was assessed after immunoprecipitation.
PI 3′-kinase activity is required for the cytoskeletal changes involved in the migration of fibroblasts and epithelial cells (Toker and Cantley, 1997). It is also required to transmit signals that lead to membrane ruffling in PAE cells expressing PDGFβ receptors (Wennstrom et al., 1994). Therefore, the capacity of the mutant receptors to cause phosphorylation of the p85 subunit of the PI 3′-kinase was analyzed. Figure 5B shows that, as expected, p85 was not phosphorylated in Flt-1-expressing PAE cells. In contrast, p85 was strongly phosphorylated in PAE cells expressing KDR upon stimulation with VEGF. When the KDR jx region was introduced into Flt, the receptor gained the ability to induce p85 phosphorylation. Therefore, sequences in the Flt-1 jx region can repress this receptor function. In cells expressing Flt(ANGG) or Flt(ΔS), VEGF stimulation also results in p85 phosphorylation.
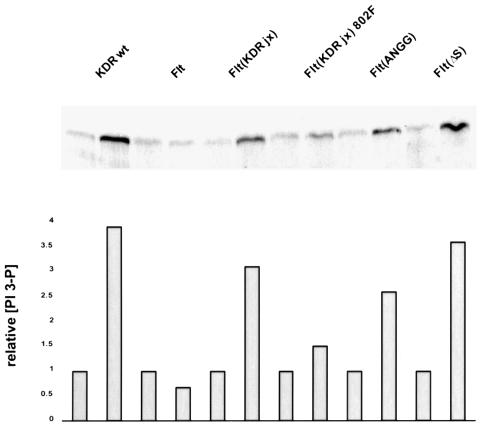
We then tested whether VEGF treatment of PAE cells expressing the mutant receptors resulted in an increase in PI 3′-kinase enzymatic activity. Initially, we attempted to use receptor antibodies to co-precipitate the lipid kinase. In agreement with Waltenberger et al. (1994), we did not detect KDR-associated PI 3′-kinase activity (data not shown). PI 3′-kinase activity was therefore measured in phosphotyrosine immunoprecipitates. VEGF stimulation resulted in an ∼4-fold increase in PI 3′-kinase activity in KDR-expressing PAE cells (Figure 6). The mutant receptors Flt(KDRjx), Flt(ΔS) and Flt(ANGG) mediated similar PI 3′-kinase activation. Also, cells expressing Flt(KDRjx)Y802F, in which p85 is not phosphorylated, showed only a marginal increase in PI 3′-kinase activity. Hence, the ability of the receptor mutants to trigger phosphorylation of the PI 3′-kinase regulatory subunit correlates tightly with their capacity to induce cell migration. The fact that activation of PI 3′-kinase was not detectable in receptor immunoprecipitates may indicate that activation proceeds through a transient interaction via an adaptor protein.
Fig. 6. Phosphotyrosine-associated PI 3′-kinase activity was measured before and after ligand stimulation of the indicated cell lines. Lower panel: phosphoimager analysis was performed and the activity in each clone was standardized against that observed in the absence of VEGF.
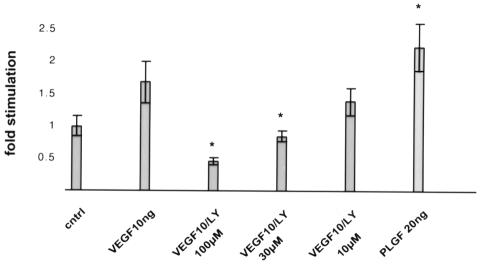
In order to determine whether PI 3′-kinase activity is required for endothelial cell migration in response to VEGF, we tested the effects of a specific PI 3′-kinase inhibitor, LY294002, on Flt(KDRjx) cells. PAE cell viability was not affected by exposure to LY294002 under our assay conditions. At 30 µM, LY294002 reduced migration to background levels (Figure 7), showing that PI 3′-kinase activity is required for endothelial cell migration.
Fig. 7. Migration of Flt(KDRjx) cells in the presence of the PI 3′-kinase activity inhibitor LY294002. Data were normalized against ligand-independent migration. Inhibition of migration was statistically significant at 30 and 100 µM LY294002.
PlGF is a VEGF-related protein (Maglione et al., 1991) that binds to Flt-1 but not to KDR (Park et al., 1994). Treatment of primary endothelial cells with PlGF (Cao et al., 1996) or an Flt-1 selective VEGF mutant (data not shown) does not trigger cell migration. Therefore, we were interested in determining whether the Flt(KDRjx) chimera containing the Flt-1 extracellular domain could promote migration in response to PlGF. Figure 7 demonstrates that exposure of Flt(KDRjx) cells to PlGF stimulates cell migration to an extent comparable to that observed in the presence of VEGF.
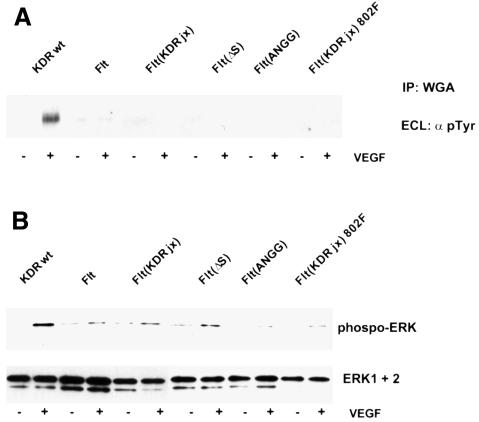
The results presented above suggest that the Flt-1 intracellular jx region suppresses the ability of the receptor to mediate cell migration. Since VEGF also transmits proliferative signals, we examined the ability of VEGF to cause tyrosine phosphorylation of the various receptor constructs. Figure 8A shows that while VEGF strongly stimulated tyrosine phosphorylation of KDR, phosphorylation of other receptor constructs was marginal or absent. Therefore, the overall tyrosine phosphorylation of the mutant receptors does not change appreciably upon VEGF treatment. However, these results do not rule out changes in phosphorylation at individual sites in the receptor.
Fig. 8. VEGF receptor and ERK phosphorylation. PAE cells were stimulated with VEGF for 5 min and receptors were precipitated with wheatgerm agglutinin and analyzed for tyrosine phosphorylation (A). In parallel, phosphorylation of ERK1 and ERK2 was assessed in total lysates with a phospho-specific antiserum (B).
We also examined the ability of VEGF to cause ERK phosphorylation. As shown in Figure 8B, induction of ERK phosphorylation is seen only in KDR-expressing cells. In several of the experiments performed, we occasionally detected minimal receptor phosphorylation or ERK activation in cells expressing the chimeric receptor constructs. However, this activation was always well below that seen in KDR-expressing cells.
These results indicate that while replacement or mutational inactivation of the inhibitory Flt-1 jx region confers the ability to transmit signals that lead to cell migration, these mutant receptors do not transmit effective proliferative signals.
Discussion
The expression of at least two VEGF receptor tyrosine kinases on endothelial cells has made it difficult to elucidate the individual contributions of each receptor to VEGF signaling. While PlGF binds selectively to Flt-1 (Park et al., 1994), its use has sometimes led to conflicting results, especially when heterologous cell types such as NIH 3T3 fibroblasts have been used (Seetharam et al., 1995; Landgren et al., 1998). Recently, we investigated the signaling properties of novel VEGF mutants that bind to either Flt-1 or KDR with high selectivity. KDR activation is sufficient to activate multiple signaling pathways and to stimulate migration and in vivo angiogenesis (H.Gille, J.Kowalski, B.Li, B.Moffat, T.F.Zioncheck, N.Pelletier and N.Ferrara, submitted). The properties of KDR-selective VEGF family members present in Orf parapoxyviruses (VEGFOrf) have also been analyzed. VEGFOrf mediates migration of KDR-expressing PAE cells and corneal angiogenesis to an extent comparable to VEGF (Ogawa et al., 1998; Meyer et al., 1999). The role of Flt-1 in the adult endothelium thus remains unclear. However, the Flt receptor contains a kinase domain that can be activated artificially (Sawano et al., 1997; Maru et al., 1998).
Despite their different signaling properties, the VEGF receptors KDR and Flt-1 are highly homologous. We generated receptor chimeras to investigate the structural basis for these differences. When the intracellular jx regions were exchanged between the two receptors, Flt-1 became able to activate PI 3′-kinase and trigger VEGF-dependent cell migration. In this chimera, mutation of the putative SH2 recognition site Y802 prevented PI 3′-kinase activation and cell migration. We also mutated Y802 in KDR to phenylalanine. However, in the context of KDR, this mutation did not have an appreciable effect on the ability of the receptor to mediate cell migration, indicating that phosphorylation of this site in KDR is not an essential step for chemotaxis (data not shown). These observations emphasize that the regulation of KDR may have a further level of complexity and differs in several respects from that of Flt-1, with or without the jx repressor.
The motility response in PAE cells is completely dependent on PI 3′-kinase activity, since LY294002 was able to abrogate migration at 30 µM. PI 3′-kinase activation has been associated with another important function of VEGF: the ability to act as a survival factor and inhibit endothelial cell apoptosis (Gerber et al., 1998). Therefore, a potential pro-survival function of Flt-1 may also be under constitutive repression. KDR-selective VEGF mutants have previously been shown to be capable of promoting mitogenesis, without involvement of Flt-1 (Keyt et al., 1996). However, the Flt-1 constructs described in this study, in which the intracellular jx region was mutated to allow PI 3′-kinase activation and cell migration, did not mediate ERK phosphorylation. In fact, KDR(Fltjx)-expressing cells still exhibited receptor tyrosine phosphorylation and phosphorylation of MAP kinase when exposed to VEGF, suggesting that the mitogenic pathway is regulated by a different region of the receptor (data not shown). Thus, elements outside the jx region of Flt-1, responsible for inhibition of tyrosine phosphorylation and mitogenesis, remain to be identified.
These results define a novel inhibitory domain in Flt-1 that serves to repress constitutively the ability of Flt-1 to mediate endothelial cell migration. These findings are consistent with the hypothesis that Flt-1 serves its function in angiogenesis primarily as a ligand-binding molecule rather than as a signaling receptor (Park et al., 1994; Hiratsuka et al., 1998). However, Flt-1 activation causes migration of macrophages (Barleon et al., 1996; Clauss et al., 1996). The only apparent defect observed in mice carrying a homozygous deletion of the Flt-1 kinase domain is a loss of VEGF-induced macrophage migration (Hiratsuka et al., 1998). These findings suggest that Flt-1-dependent migration in macrophages involves a pathway different from that repressed in endothelial cells. Alternatively, a repressor potentially present in endothelial cells may not be functional in macrophages.
Several lines of evidence suggest that a negative regulatory function may reside in the intracellular jx domain of other kinase-insert domain-containing receptors. Missense mutations in the c-Kit jx region are found in canine mastocytomas and human gastrointestinal tumors and have been suggested to be markers for poor prognosis (Ma et al., 1999; Taniguchi et al., 1999). When the c-Met jx sequence is reintroduced into TPR–Met, the oncogenic activity of the fusion is lost despite the presence of the constitutive dimerization motif in TPR (Vigna et al., 1999). Also, in-frame duplications in the Flt-3 jx region correlate with poor prognosis in childhood acute myeloid leukemia (Iwai et al., 1999). However, to our knowledge, this is the first example of a receptor in which some key signaling functions are constantly repressed and short amino acid substitutions are able to restore ligand-dependent activation. Mutational analysis suggests that a divergent stretch of three serines in Flt-1 mediates repression. So far, we have not been able to detect serine phosphorylation of Flt-1, so the precise mechanism by which this region regulates receptor activity will require further study.
A single amino acid substitution in the PDGFR jx domain causes ligand-independent activation (Irusta and DiMaio, 1998). The authors of the study suggested that the PDGFR jx region may fold as a WW domain. In order to understand the role of these activating mutations, a three-dimensional model of the Flt-1 jx region was generated based on its homology with the PDGFR and the yap and Pin WW domains (Koepf et al., 1999). The alignment in Figure 9A shows that the residues forming the core of the WW domain are present in both KDR and Flt-1. Although the first tryptophan residue of the WW domain is replaced by a tyrosine in KDR and Flt-1, this residue could play a similar role in forming the core of the protein. In fact, this sequence replacement can be found in other reported WW domains. This tyrosine is part of a potential SH2 recognition sequence and would not be available for protein binding as it forms the hydrophobic core in our model. The second tryptophan of the WW domain, which is involved in ligand recognition (Macias et al., 1996), is conserved in both KDR and Flt-1.
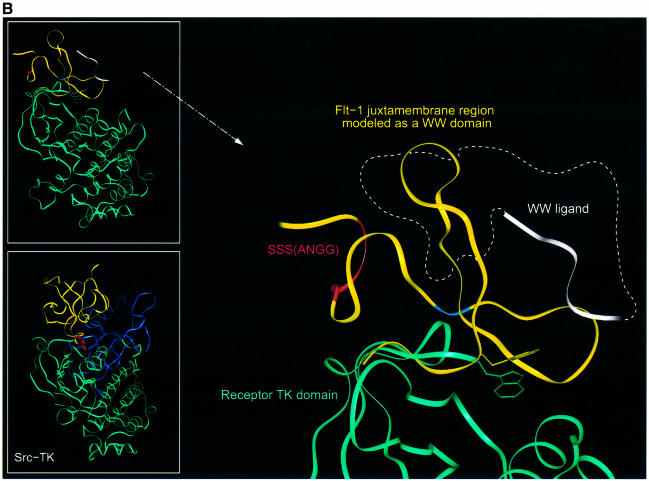
Fig. 9. (A) Sequence alignment of pin and yap WW domains with the jx regions of PDGFR, Flt-1 and KDR. Residues forming the protein core are boxed in green. The tryptophan residues defining the WW motif are indicated by an asterisk at the top of the alignment. The residues responsible for the different signaling properties of KDR and Flt-1 are shown in red. The constitutively activating mutation in the PDGFR (Irusta and DiMaio, 1998) is shown in blue. Residues included in the IR-TK (PDB:1rk) and KDR-TK (PDB: 1vrz) structures, which were used for superimposition on our model, are indicated at the bottom of the alignment as a dashed and solid black line, respectively. (B) Proposed model for the regulation of receptor activation by the Flt-1 jx region. The Flt-1 jx region modeled by homology as a WW domain is shown as a yellow ribbon. The three serines responsible of Flt-1 repressor function are indicated in red, the position corresponding to the activating mutation in PDGFR (Irusta and DiMaio, 1998) is shown in blue and the side chain of the tryptophan important for ligand binding to the WW domain is displayed in yellow. The C-terminus of the modeled WW is superimposed on the N-terminus of the receptor kinase domain shown in green. The side chain of the tryptophan corresponding to the one responsible for activity regulation in Src-TK is displayed in green (see text for details). The white ribbon indicates where peptide ligands have been shown to bind WW domains (Macias et al., 1996) and the dashed white line illustrates the possibility of an inhibitor or regulator binding to the same region of Flt-1 and/or KDR and controlling receptor activity. (Top inset) Full view of the Flt-1 jx model (in yellow) superimposed on the receptor tyrosine kinase domain (in green). (Bottom inset) Full view of the Src-TK structure (PDB:1fmk). The SH3 domain and linker are shown in yellow, the SH2 in blue and the kinase domain in green. The side chain of the tryptophan responsible for Src-TK activity regulation is displayed in red.
The X-ray structures of other receptor tyrosine kinases, such as that of the KDR kinase domain (KDR-TK) and insulin receptor (IR-TK) (Hubbard et al., 1994; McTigue et al., 1999), include in their N-terminus the last residues of the corresponding jx region. Using these common residues, IR-TK and KDR-TK can be superimposed on our Flt-1 jx model without steric clashes (Figure 9B). In this model, the three serines in the Flt-1 jx region could be near the activating mutation described in PDGFR and on the same face as the WW peptide recognition region (Macias et al., 1996). Thus, the three serines could be involved in aiding the recognition of a WW-binding partner, which may modulate receptor function. Interestingly, comparison of this model with the Src tyrosine kinase X-ray structure (Moarefi et al., 1997) shows that the potential WW domain and its second tryptophan are at an analogous position to the SH3 domain, reponsible for regulating Src activity via intramolecular interactions with the kinase domain (Hubbard, 1999) (Figure 9B, inset).
We have defined a novel functional domain in the intracellular jx region of the Flt-1 receptor tyrosine kinase. A short peptide sequence in this region, which is divergent between KDR and Flt-1, represses the ability of Flt-1 to activate PI 3′-kinase and stimulate VEGF-dependent cell migration. It is not clear how the Flt-1 jx domain suppresses activation of the receptor. The region may either prevent receptor activation per se or bind to a cellular inhibitor. Finally, we anticipate the existence of additional repressor elements in Flt-1, responsible for the inhibition of tyrosine kinase activity and mitogenesis. Efforts are currently under way to identify and characterize such sequences and further elucidate the structure and function of this unusual receptor.
Materials and methods
Reagents
LY294002 was purchased from Biomol. Anti-phospho-ERK was purchased from Promega. p85 was imunoprecipitated with antibodies from Transduction Labs (P13020) and Neomarkers (MS 424-P). Escherichia coli-expressed rhVEGF165 was produced at Genentech. PlGF was purchased from R&D Systems. The phosphotyrosine antibodies used were PY20 or E120H (both from Transduction Labs). Wheatgerm agglutinin–Sepharose was from Pharmacia. Anti-PLCγ antiserum was obtained from Dr G.Carpenter (Vanderbilt University). PAE cells were a gift from Dr C.-H.Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden). Transfected PAE cells expressing PDGFβ receptor were obtained from Dr L.Claesson-Welsh (Uppsala University Biomedical Center, Sweden).
Generation of chimeric VEGF receptors
Following an alignment of the nucleotide sequence of the VEGF receptors, the various domains within the intracellular domain were defined along with the transmembrane and jx regions. Oligonucleotides were designed that would introduce unique silent restriction sites at or near each domain. Because the single-stranded DNA templates generated were of the coding strand, the oligonucleotides used for the initial mutagenesis (Kunkel, 1985) were of the non-coding strand. Identical sites were placed in the analogous location of each VEGF receptor. We started with cDNA clones encoding full-length VEGF receptors, Flt-1/pRK and KDR/pRK (Davis-Smyth et al., 1996).
The locations of the restriction sites are underlined in the following oligonucleotide sequences. To place a BsiWI site in the coding sequence near the beginning of kinase domain 1, 5′-CTCCCACTTGCTGGCATCGTACGGGAGCCGCTCAC-3′ was used for Flt-1 and 5′-GAATTCCCATTTGCTGGCATCGTACGGCAGTCGTTCACAATG-3′ for KDR.
The Quick Change kit (Stratagene) was used to introduce further silent restriction sites. First, an AccIII site, located in the intracellular domain of KDR, was removed. The coding strand sequences of the complementary oligonucleotide pairs used were: Flt.tm/jx.accIII: 5′-CTTCTGGCTCCTATTACGTTACTTATCCGGAAAATGAAAAGGTCTTCTTC; and KDR.tm/jx.accIII: 5′-GCCATGTTCTTCTGGCTACTACTAGTCATCATCCTCCGGACCGTTAAGCGGGCC. Flt(KDRjx) and KDR(Fltjx) were then generated by standard subcloning.
Further point mutations were introduced using FltΔS (5′-CGCTCTTCATCCGAAAAATGAAAAGGGAAATAAAGACTGACTACCTATC), Flt(ANGG) (5′-GGTAGTCAGTCTTTATTTCCCCTCCATTGGCGCGCTTCATTTTTCGGATAAAGAGGG) and Flt(KDRjx)Y802F (5′-GAACTGAAGACAGGCTTCTTATCGATCGTCATGGATCCAGATG). GAll constructs were verified by sequence analysis.
Cell culture
PAE cells were maintained in Ham’s F12 medium containing 10% fetal bovine serum (FBS). For the generation of stable clones, pRK plasmid vectors (Leung et al., 1989) harboring the receptor constructs were co-transfected with pNeo in six-well dishes. After 48 h, the cells were replated on to 10 cm dishes in the presence of 0.25 µg/ml G418. Colonies were isolated after 10–14 days and screened in triplicate for functional VEGF receptor expression by binding to [125I]VEGF, as previously reported (Davis-Smyth et al., 1996). Parental PAE cells did not show any displaceable VEGF binding, in agreement with a published report that these cells do not express significant levels of endogenous VEGF receptors (Waltenberger et al., 1994). Clones exhibiting comparable amounts of displaceable c.p.m. bound were selected for further analysis. The various clones were plated on 10 cm dishes and allowed to reach near confluence. Cells were made quiescent by 14 h starvation in Ham’s F12 with 0.05% bovine serum albumin (BSA) and 0.05% FBS followed by an additional 2 h incubation in Ham’s F12 with 0.05% BSA. Cells were pre-incubated for 5 min in 0.1 µM sodium orthovanadate and stimulated or left untreated. After the appropriate treatment, cells were washed once in ice-cold phosphate-buffered saline (PBS) containing 0.1 mM sodium orthovanadate. Cells were lysed in 0.5–1 ml lysis buffer, consisting of RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 50 mM Tris pH 8.0) plus 0.1 mM sodium orthovanadate, 5 mM p-nitrophenylphosphate, 10 mM sodium fluoride, 0.5 µM okadaic acid and a protease inhibitor cocktail (Roche MB 1836145).
PAE migration assays
Falcon 8.0 mm filter inserts (Falcon 3097) were coated with type 1 collagen (Cohesion; Vitrogen). Cells were trypsinized and transferred to endothelial basal medium (EBM; Clonetics) with 0.1% BSA for the assay. Cells were plated at 5 × 104 into the upper chamber. Growth factors were placed in the lower chamber and inhibitors in the upper chamber. The assay was routinely an 18 h assay at 37°C. Viability in the presence of LY294002 was confirmed by trypan blue exlusion. Cells were removed from the upper side of the membrane by scraping with a polyurethane keyboard swab, then the remaining cells on the bottom side of the membrane were fixed with methanol. Cells were stained with Yo-Pro Iodide nuclear stain (Molecular Probes) and counted under low power fluorescence using an Image-Pro cell recognition program. At least five independent clones for each construct were analyzed; the data shown reflect representative clones. Error bars represent the SEM. Significance was calculated to a confidence level of 95% by analysis of variance.
Immunoprecipitation and western analysis
Protein A/G beads (Pierce) were blocked for non-specific protein binding in 50 mM HEPES pH 7.2, 0.1% Triton X-100, 150 mM NaCl, 1 mg/ml ovalbumin for 30 min. Antibodies were pre-coupled in the same buffer for 1 h at 4°C with head-over-end rotation and beads were washed three times in lysis buffer as defined above. Beads were added to the lysates and rotated overnight. For the precipitation of VEGF receptors, wheatgerm agglutinin beads (Pharmacia) were employed instead of protein A/G. Beads were washed sequentially in (i) 50 mM Tris pH 7.6, 150 mM NaCl, 1% Triton X-100, 1 mM CaCl2; (ii) 50 mM Tris pH 7.6, 500 mM NaCl, 0.1% Triton X-100, 1 mM CaCl2; and (iii) 50 mM Tris pH 7.6, 150 mM NaCl, 0.05% Triton X-100, 1 mM CaCl2. Beads were resuspended in 2× sample buffer (100 mM Tris–HCl pH 6.8, 20 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) and applied directly to 4–12% Tris–glycine gradient gels (Novex).
Proteins were transferred on to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 3% BSA in PBS/0.1% Tween for 1 h and probed with the appropiate antibodies for at least 2 h. Immunoblots were washed and incubated with the corresponding horseradish peroxidase-conjugated Pierce secondary antibodies at 1:30 000 dilution. Pierce ECL reagents were used for signal detection.
PI 3′-kinase assays
VEGF-treated or unstimulated PAE cells were lysed in 1% Nonidet P-40, 20 mM Tris pH 7.5, 150 mM NaCl2, 5 mM EDTA, 50 mM NaF, 1 mM sodium vanadate and protease inhibitor cocktail (Roche MB 1836145). PI 3′-kinase assays were performed as described (Ruderman et al., 1990) with the following modifications. Lysates were incubated with 2 µg of PY20 antibody on ice for 90 min and immunocomplexes were collected on protein G–Sepharose beads with head-over-end rotation for 45 min. Beads were washed three times with lysis buffer, once with PBS, once with 0.5 M LiCl, 100 mM Tris pH 7.5, once with distilled water, once with 20 mM Tris pH 7.5, 100 mM NaCl, 0.5 mM EDTA and once with 20 mM Tris pH 7.5, 100 mM NaCl, 0.5 mM EGTA. Phosphatidylinositol (Sigma) and phosphatidylserine (Sigma) were disolved in chloroform and mixed at a 1:1 (w/w) ratio. After drying, the glass vials were stored at –20°C until use. Lipids were suspended in 20 mM Tris pH 7.5, 100 mM NaCl, 0.5 mM EGTA at 1 mg/ml and placed in a sonicating water bath for 4 min. Twenty microliters of the lipid mixture were added to the immunoprecipitates. Reactions were initiated by addition of another 25 µl of 20 mM Tris pH 7.5, 100 mM NaCl, 0.5 mM EGTA, 20 mM MgCl2, 100 µM ATP, 10 µCi of [32P]ATP and allowed to proceed for 8 min at 22°C. Reactions were terminated by addition of 400 µl of chloroform–methanol (1:2) containing 1% HCl. One hundred and fifty microliters of chloroform and 150 µl of 10 mM HCl were added. After phase separation, the lower phase was transferred to a new tube and the chloroform was evaporated. Lipids were resuspended in 20 µl of chloroform and spotted on to Whatman silica gel 60 plates that had been pre-treated with 1% oxalate and dried. Chromatography was performed in 65 ml n-propanol, 33 ml H2O and 2 ml glacial acetic acid. Dried plates were exposed to film.
Molecular modeling
The three-dimensional models of the jx region of KDR and Flt-1 were built using homology modeling techniques with the Modeler program INSIGHT II 97.0 (Molecular Simulations Inc., San Diego, CA), based on the 1.35 Å resolution X-ray structure of the WW domain of the human peptidyl-prolyl cis–trans isomerase (PDB: 1PIN).
Acknowledgments
Acknowledgements
We thank Graham Carpenter, Carl-Henrik Heldin and Lena Claesson-Welsh for providing reagents and Pablo Rodriguez-Viciana for advice on PI 3′-kinase assays.
References
- Barleon B., Sozzani,S., Zhou,D., Weich,H.A., Mantovani,A. and Marme,D. (1996) Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood, 87, 3336–3343. [PubMed] [Google Scholar]
- Cao Y. et al. (1996) Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression and high affinity binding to Flk-1/KDR. J. Biol. Chem., 271, 3154–3162. [DOI] [PubMed] [Google Scholar]
- Clauss M., Weich,H., Breier,G., Knies,U., Rockl,W., Waltenberger,J. and Risau,W. (1996) The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem., 271, 17629–17634. [DOI] [PubMed] [Google Scholar]
- Cunningham S.A., Waxham,M.N., Arrate,P.M. and Brock,T.A. (1995) Interaction of the Flt-1 tyrosine kinase receptor with the p85 subunit of phosphatidylinositol 3-kinase. Mapping of a novel site involved in binding. J. Biol. Chem., 270, 20254–20257. [DOI] [PubMed] [Google Scholar]
- Davis-Smyth T., Chen,H., Park,J., Presta,L.G. and Ferrara,N. (1996) The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J., 15, 4919–4927. [PMC free article] [PubMed] [Google Scholar]
- de Vries C., Escobedo,J.A., Ueno,H., Houck,K., Ferrara,N. and Williams,L.T. (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science, 255, 989–991. [DOI] [PubMed] [Google Scholar]
- Dougher M. and Terman,B.I. (1999) Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene, 18, 1619–1627. [DOI] [PubMed] [Google Scholar]
- Enholm B., Jussila,L., Karkkainen,M. and Alitalo,K. (1998) Vascular endothelial growth factor-C: a growth factor for lymphatic and blood vessel endothelial cells. Trends Cardiovasc. Med., 8, 292–296. [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int., 56, 794–814. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant,J., Gertsenstein,M. and Breitman,M.L. (1995) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature, 376, 66–70. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Zhang,L., Bryce,D.M. and Peng,J. (1999) Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development, 126, 3015–3025. [DOI] [PubMed] [Google Scholar]
- Fuh G., Li,B., Crowley,C., Cunningham,B. and Wells,J.A. (1998) Requirements for binding and signaling of the kinase domain receptor for vascular endothelial growth factor. J. Biol. Chem., 273, 11197–11204. [DOI] [PubMed] [Google Scholar]
- Gerber H.P., McMurtrey,A., Kowalski,J., Yan,M., Keyt,B.A., Dixit,V. and Ferrara,N. (1998) VEGF regulates endothelial cell survival by the PI3-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem., 273, 30366–30343. [DOI] [PubMed] [Google Scholar]
- Guo D., Jia,Q., Song,H.Y., Warren,R.S. and Donner,D.B. (1995) Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J. Biol. Chem., 270, 6729–6733. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S., Minowa,O., Kuno,J., Noda,T. and Shibuya,M. (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl Acad. Sci. USA, 95, 9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S.R. (1999) Src autoinhibition: let us count the ways. Nature Struct. Biol., 6, 711–714. [DOI] [PubMed] [Google Scholar]
- Hubbard S.R., Wei,L., Ellis,L. and Hendrickson,W.A. (1994) Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature, 372, 746–754. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Isohara,T., Kato,T., Shigeta,K., Yamano,T. and Uno,I. (1998a) Tyrosine 1213 of Flt-1 is a major binding site of Nck and SHP-2. Biochem. Biophys. Res. Commun., 246, 95–99. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Shigeta,K., Isohara,T., Yamano,T. and Uno,I. (1998b) Sck interacts with KDR and Flt-1 via its SH2 domain. Biochem. Biophys. Res. Commun., 251, 77–82. [DOI] [PubMed] [Google Scholar]
- Irusta P.M. and DiMaio,D. (1998) A single amino acid substitution in a WW-like domain of diverse members of the PDGF receptor subfamily of tyrosine kinases causes constitutive receptor activation. EMBO J., 17, 6912–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N., Wernstedt,C., Engstrom,U. and Claesson-Welsh,L. (1998) Identification of vascular endothelial growth factor receptor-1 tyrosine phosphorylation sites and binding of SH2 domain-containing molecules. J. Biol. Chem., 273, 23410–23418. [DOI] [PubMed] [Google Scholar]
- Iwai T. et al. (1999) Internal tandem duplication of the FLT3 gene and clinical evaluation in childhood acute myeloid leukemia. The Children’s Cancer and Leukemia Study Group, Japan. Leukemia, 13, 38–43. [DOI] [PubMed] [Google Scholar]
- Keyt B.A., Nguyen,H.V., Berleau,L.T., Duarte,C.M., Park,J., Chen,H. and Ferrara,N. (1996) Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J. Biol. Chem., 271, 5638–5646. [DOI] [PubMed] [Google Scholar]
- Koepf E.K., Petrassi,H.M., Ratnaswamy,G., Huff,M.E., Sudol,M. and Kelly,J.W. (1999) Characterization of the structure and function of W→F WW domain variants: identification of a natively unfolded protein that folds upon ligand binding. Biochemistry, 38, 14338–14351. [DOI] [PubMed] [Google Scholar]
- Kundra V., Escobedo,J.A., Kazlauskas,A., Kim,H.K., Rhee,S.G., Williams,L.T. and Zetter,B.R. (1994) Regulation of chemotaxis by the platelet-derived growth factor receptor-β. Nature, 367, 474–476. [DOI] [PubMed] [Google Scholar]
- Kunkel T.A. (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl Acad. Sci. USA, 82, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren E., Schiller,P., Cao,Y. and Claesson-Welsh,L. (1998) Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-γ and migration of endothelial cells expressing Flt 1. Oncogene, 16, 359–367. [DOI] [PubMed] [Google Scholar]
- Leung D.W., Cachianes,G., Kuang,W.J., Goeddel,D.V. and Ferrara,N. (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science, 246, 1306–1309. [DOI] [PubMed] [Google Scholar]
- Ma Y., Longley,B.J., Wang,X., Blount,J.L., Langley,K. and Caughey,G.H. (1999) Clustering of activating mutations in c-Kit’s juxtamembrane coding region in canine mast cell neoplasms. J. Invest. Dermatol., 112, 165–170. [DOI] [PubMed] [Google Scholar]
- Macias M.J., Hyvonen,M., Baraldi,E., Schultz,J., Sudol,M., Saraste,M. and Oschkinat,H. (1996) Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature, 382, 646–649. [DOI] [PubMed] [Google Scholar]
- Maglione D., Guerriero,V., Viglietto,G., Delli-Bovi,P. and Persico,M.G. (1991) Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl Acad. Sci. USA, 88, 9267–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y., Yamaguchi,S. and Shibuya,M. (1998) Flt-1, a receptor for vascular endothelial growth factor, has transforming and morphogenic potentials. Oncogene, 16, 2585–2595. [DOI] [PubMed] [Google Scholar]
- McTigue M.A. et al. (1999) Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure Fold Des., 7, 319–330. [DOI] [PubMed] [Google Scholar]
- Meyer M. et al. (1999) A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J., 18, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., Okabe,T., Urabe,A., Takaku,F. and Heldin,C.H. (1987) Purification and properties of an endothelial cell growth factor from human platelets. J. Biol. Chem., 262, 4098–4103. [PubMed] [Google Scholar]
- Moarefi I., LaFevre-Bernt,M., Sicheri,F., Huse,M., Lee,C.H., Kuriyan,J. and Miller,W.T. (1997) Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature, 385, 650–653. [DOI] [PubMed] [Google Scholar]
- Mori S., Ronnstrand,L., Yokote,K., Engstrom,A., Courtneidge,S.A., Claesson-Welsh,L. and Heldin,C.H. (1993) Identification of two juxtamembrane autophosphorylation sites in the PDGFβ-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J., 12, 2257–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D., Nagy,J.A., Manseau,E.J. and Dvorak,H.F. (1998) Vascular permeability factor/vascular endothelial growth factor-mediated signaling in mouse mesentery vascular endothelium. Cancer Res., 58, 1278–1284. [PubMed] [Google Scholar]
- Ogawa S., Oku,A., Sawano,A., Yamaguchi,S., Yazaki,Y. and Shibuya,M. (1998) A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem., 273, 31273–31282. [DOI] [PubMed] [Google Scholar]
- Park J.E., Chen,H.H., Winer,J., Houck,K.A. and Ferrara,N. (1994) Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem., 269, 25646–25654. [PubMed] [Google Scholar]
- Ruderman N.B., Kapeller,R., White,M.F. and Cantley,L.C. (1990) Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl Acad. Sci. USA, 87, 1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawano A., Takahashi,T., Yamaguchi,S. and Shibuya,M. (1997) The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCγ. Biochem. Biophys. Res. Commun., 238, 487–491. [DOI] [PubMed] [Google Scholar]
- Seetharam L., Gotoh,N., Maru,Y., Neufeld,G., Yamaguchi,S. and Shibuya,M. (1995) A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene, 10, 135–147. [PubMed] [Google Scholar]
- Shibuya M., Yamaguchi,S., Yamane,A., Ikeda,T., Tojo,A., Matsushime,H. and Sato,M. (1990) Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase (flt) closely related to the fms family. Oncogene, 8, 519–527. [PubMed] [Google Scholar]
- Soker S., Takashima,S., Miao,H.Q., Neufeld,G. and Klagsbrun,M. (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell, 92, 735–745. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Nishida,T., Hirota,S., Isozaki,K., Ito,T., Nomura,T., Matsuda,H. and Kitamura,Y. (1999) Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res., 59, 4297–4300. [PubMed] [Google Scholar]
- Terman B.I., Carrion,M.E., Kovacs,E., Rasmussen,B.A., Eddy,R.L. and Shows,T.B. (1991) Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene, 6, 1677–1683. [PubMed] [Google Scholar]
- Toker A. and Cantley,L.C. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature, 387, 673–676. [DOI] [PubMed] [Google Scholar]
- Vigna E., Gramaglia,D., Longati,P., Bardelli,A. and Comoglio,P.M. (1999) Loss of the exon encoding the juxtamembrane domain is essential for the oncogenic activation of TPR-MET. Oncogene, 18, 4275–4281. [DOI] [PubMed] [Google Scholar]
- Waltenberger J., Claesson Welsh,L., Siegbahn,A., Shibuya,M. and Heldin,C.H. (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem., 269, 26988–26995. [PubMed] [Google Scholar]
- Wennstrom S., Hawkins,P., Cooke,F., Hara,K., Yonezawa,K., Kasuga,M., Jackson,T., Claesson-Welsh,L. and Stephens,L. (1994) Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr. Biol., 4, 385–393. [DOI] [PubMed] [Google Scholar]
- Wise L.M. et al. (1999) Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc. Natl Acad. Sci. USA, 96, 3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]