Abstract
The Timed Up and Go (TUG) test has been used to assess balance and mobility in Parkinson’s Disease (PD). However, it is not known if this test is sensitive to subtle abnormalities present in early stages of the disease, when balance and gait problems are not clinically evident but may be detected with instrumented analysis of movement. We hypothesize that postural transitions and arm swing during gait will be the most sensitive characteristics of the TUG for early PD. In the present study, we instrumented the TUG test (iTUG) using portable inertial sensors, and extended the walking distance from 3 meters (traditional TUG) to 7 meters. Twelve subjects with early-to-moderate, untreated PD and 12 healthy individuals participated. Our findings show that although the stopwatch measure of TUG duration did not detect abnormalities in early-to-mid stage PD, the peak arm swing velocity on the more affected side, average turning velocity, cadence and peak trunk rotation velocity were significantly slower. These iTUG parameters were also correlated with the UPDRS Motor Scale. Thus, the iTUG test is sensitive to untreated PD and could potentially detect progression of PD and response to symptomatic and disease-modifying treatments.
Keywords: Parkinson’s Disease, Timed Up and Go, inertial sensors, gait, postural transitions, untreated
1. INTRODUCTION
The Timed Up and Go (TUG) test has been widely used as a clinical measure of balance and mobility in the elderly [1–3] and in neurological populations [4,5], including Parkinson’s Disease (PD) [6–9]. However, it is not known if this test is sensitive to early stages of PD. People in early stages of PD do not show significant signs of balance or gait problems on clinical examination. An instrumented gait and balance analysis might, however, detect such alterations [10–14]
The TUG has promise for evaluation of early PD because it consists of a sequence of sit-to-stand, walking, turning, and stand-to-sit tasks, each of which is eventually affected by PD, especially when performed as a sequence [15–17]. A shortcoming of the TUG test is that it relies on one measure, time, to evaluate the overall performance of a sequence of tasks. Therefore, it lacks specific information on components of each task that could reveal more specific mobility problems. For example, it is not known whether gait or postural transitions are affected first in early PD.
New technology has recently emerged that allows the recording of gait and postural transitions with portable devices. Aminian et al, 2002 [18] have successfully used miniature gyroscopes to measure spatio-temporal parameters of gait in young and elderly subjects. The algorithms were later improved to assess gait in PD [19]. Inertial sensors have also been used to assess postural transitions in elderly at risk for falling [20] as well as PD [21]. The present study applies this technology to instrument the TUG test and evaluate untreated subjects in early-to-mid stage of PD. Our objectives were to determine:
If the traditional TUG test, measured with a stopwatch, can differentiate between early-to-mid stage PDs and healthy individuals;
If the instrumented TUG (iTUG) test can better differentiate between untreated subjects with PD and healthy individuals than the traditional TUG; and if so, to identify the most sensitive components of the iTUG to detect mobility deficits; and
If iTUG measures are related to disease severity.
We hypothesize that the traditional stop-watch TUG test will not discriminate between early-to-mid stage PD subjects and healthy individuals, but specific components of the iTUG will be sensitive to and correlated with the severity of PD. If so, the iTUG would be a valuable tool to evaluate the progression of PD and effects of therapy.
2. METHODS
2.1. Subjects
Twelve subjects with idiopathic PD and 12 healthy subjects participated. PD subjects were diagnosed by a movement disorders neurologist. Only early-to-mid stage PD subjects who had never been treated with anti-parkinson medications were invited to participate. Healthy control subjects were either PD subject spouses or recruited from the community. Subjects were excluded if they presented any neurological disorders other than PD, orthopedic or other impairments that could interfere with gait, artificial joints or if they used orthotic devices. Participants signed informed consent forms approved by the Oregon Health & Science University Institutional Review Board.
Table 1 compares group characteristics. The groups were similar in age and weight. Height was significantly different; so we normalized our gait parameters to the subject height. The PD group was early-to-mid stage in the disease process as determined from the Unified Parkinson’s Disease Rating Scale, Motor Section (Motor UPDRS) and Hoehn and Yahr Scale (H&Y). Time since diagnosis was 13.7 ± 12.9 months (mean ± standard deviation (SD)).
Table 1.
Demographic characteristics of the untreated PD and control subjects. Values displayed as mean ± SE. Range of values are between parentheses.
| Untreated PDs (n = 12) | Controls (n = 12) | p-value | |
|---|---|---|---|
| Age | 60.4 ± 8.5 (48 – 77) | 60.2 ± 8.2 (56 – 76) | 0.96 |
| Gender | 7M,5F | 3M,9 F | ----- |
| Height (cm) | 174.4 ± 8.7 (154.9 – 185.4) | 166.1 ± 8.9 (152.4 – 182.9) | 0.03 |
| Weight (Kg) | 78.9 ± 12.8 (65.7 – 104.3) | 81.2 ± 22.6 (49.9 – 127.0) | 0.75 |
| Motor UPDRS | 20.0 ± 9.4 (7 – 35) | 0 (0 – 4) | ----- |
| Hoehn & Yahr | 1.6 ± 0.5 (1–2.5) | 0 |
2.2. Protocol
The PD subjects were rated by a trained examiner on the Motor UPDRS [22] and the H&Y [23]. Then all subjects performed the following tests 3 times:
Traditional 3 meter TUG: Subjects were asked to stand up from a chair, walk 3 meters to a horizontal line marked with red tape on the floor, turn around, walk back and sit down [24], at a comfortable pace.
Instrumented TUG (iTUG): Subjects performed the TUG test while wearing portable inertial sensors. The distance walked was increased to 7 meters to provide enough steps for gait analysis.
2.3. Apparatus
Subjects wore a portable data-logger (Physilog®) [18] with 5 inertial sensors attached to their body [21]. Two uni-axial gyroscopes (range 600°/s) were attached to the anterior shank, 4 cm above the ankle joint. Two 2-dimensional (2-D) gyroscopes (range ± 1200 °/s), that measured roll (axial rotation) and pitch (flexion extension) angular velocity, were attached to the dorsum of each wrist. One sensor, which contained a 2-D gyroscope (range ± 400 °/s, pitch and roll axis) and a 3-D accelerometer (range ± 2g), was attached to the chest on the sternum; 2 cm below the sternal notch. Data were recorded at a sampling rate of 200 Hz, with 16 bits/sample and stored in a flash memory card.
2.4. Data Analysis
A stopwatch was used to time the 3-meter TUG and the 7-meter iTUG. A Matlab program† automatically detected, separated and analyzed different components of gait and postural transition measures (sit-to-stand and turning) during the iTUG. The algorithms used have been discussed previously [19,21].
Motor UPDRS scores taken immediately prior to TUG and iTUG testing were also broken into 3 subscores:
Bradykinesia: sum of items 23 (finger tapping), 24 (hand open and closed), 25 (hand pronation/supination), and 26 (leg agility).
Rigidity: item 22 (neck, upper and lower body rigidity).
Gait/Posture: sum of items 27 (arising from chair), 28 (posture), 29 (gait), and 30 (postural stability).
During straight walk, individual gait cycles were detected and analyzed across 3 trials and the average values of the following gait parameters were investigated.
Upper Body
-
a.
Peak Arm Swing Velocity: The maximum angular velocity that achieved during the swing phase (deg/sec). The 2 axes of the forearm gyroscopes were combined.
-
b.
Arm Swing Range of Motion: Range of motion (i.e. max – min) of forearms in pitch axis of the body during arm swing (deg).
-
c.
Arm Swing Asymmetry: Difference in peak arm swing velocity between the faster and slower arm divided by the larger value (lv%).
Lower Body
-
d.
Temporal gait parameters including Cadence (steps/min), Gait Cycle Time (s), Double-Support (%)[25].
-
e.
Spatial gait parameters including Stride Velocity (%height/s) and Stride Length (%height)[25].
-
f.
Stride Time Variability: Coefficient of variability (CV = SD/mean) of Gait Cycle Time (%).
-
g.
Stride Length Variability: Coefficient of variability (CV = SD/mean) of stride length (%).
Trunk
-
h.
Peak Trunk Rotation Velocity: Peak angular velocity of the trunk rotation in yaw axis (deg/sec).
-
i.
Trunk Rotation Range of Motion: Range of rotation (i.e. max – min) of the trunk in yaw axis (deg).
In order to assess asymmetry in PD, we further classified selected Lower Body and Upper Body parameters into more affected and less affected sides (MAS and LAS). The MAS was determined based on a sum of bradykinesia sub-scores of the Motor UPDRS (items 23,24,25 and 26). For control subjects, the average of both sides was used for comparison.
The following postural transition parameters were investigated:
Turning
-
j.
Average Turning Velocity: Range of turning (180 degrees) divided by turning time in seconds (deg/sec).
-
k.
Peak Turning Velocity: The maximum achieved angular velocity of trunk rotation in the yaw axis during 180-degree turns (deg/sec).
Sit-to-stand
-
l.
Average Sit-to-Stand Velocity: average trunk angular velocity during Sit-to-Stand in pitch axis (i.e. flexion/extension; deg/sec).
-
m.
Peak Sit-to-Stand Velocity: maximum angular velocity of trunk reached during Sit-to-Stand in pitch axis (deg/sec).
2.5. Statistical Analysis
Statistical analysis was done with NCSS software‡. Skewness, kurtosis, and normality of the data were verified before specific analyses. A two-sample T-test was performed to investigate differences on the iTUG between the groups for each dependent variable. No correction was done for multiple comparisons because there were no multiple comparisons, each group was assessed only one time, on multiple variables. In addition, a receiver operating characteristics (ROC) analysis was used to evaluate the discriminative value of each variable. Finally, a Pearson product-moment correlation was performed to investigate the association between Motor UPDRS and selected variables (only those with high discriminative value and significant T-test). All hypotheses were non-directional and the critical α level was 0.05.
3. RESULTS
There were no significant differences between the number of gait cycles analyzed for PD (15.6 ± 3.2) and control (15.8 ± 3.0) groups (mean ± SD, p=0.83).
3.1. 3-meter TUG and iTUG times
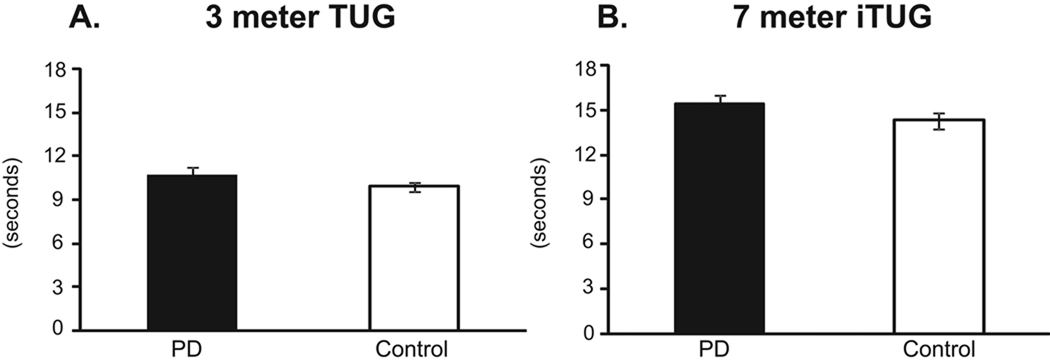
Performance duration of the 3-meter TUG and the 7-meter iTUG (Figure 1) was not significantly different between groups. On the 3-meter TUG, PDs required 10.8 ± 0.5 seconds and controls 9.9 ± 0.3 seconds to complete the task (p = 0.17). On the 7-meter iTUG, PDs required 15.4 ± 0.6 seconds and controls 14.3 ± 0.5 seconds (p = 0.18). Only 3 PD subjects performed the TUG or iTUG slower than the slowest control subject.
Figure 1.
Comparison in total performance time between untreated subjects with PD and control subjects on the traditional, 3-meter TUG test (A) and the 6-meter iTUG test (B). Differences are not significant. Vertical bars are standard errors.
3.2. Components of the iTUG
Ten of 22 gait and postural transition parameters were significantly different between PD and control subjects (Table 2). Arm swing showed the largest difference between groups. Identifying the MAS, based on the Motor UPDRS, increased the discriminative value of peak arm swing velocity and arm swing range of motion (Table 2).
Table 2.
Gait and postural transition parameters obtained with the iTUG test. Note, ht=height, MAS=more affected side, LAS=less affected side, gc=gait cycle, lv=largest value, Fl/Ext = flexion/extension, AUC = area under the curve.
| Untreated PD | Control | |||
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | p-value | AUC | |
| GAIT PARAMETERS | ||||
| Upper Body | ||||
| Peak Arm Velocity | 124.4 ± 9.2 | 187.5 ± 10.9 | 0.001 | 0.910 |
| Peak Arm Velocity ( MAS ) | 103.4 ± 10.6 | 187.5 ± 10.9 | 0.001 | 0.958 |
| Peak Arm Velocity ( LAS ) | 145.4 ± 13.7 | 187.5 ± 10.9 | 0.025 | 0.764 |
| Arm Swing Range of Motion (deg) | 29.2 ± 2.8 | 42.3 ± 3.2 | 0.005 | 0.826 |
| Arm Swing Range of Motion ( MAS) | 22.6 ± 2.9 | 42.3 ± 3.2 | 0.001 | 0.910 |
| Arm Swing Range of Motion( LAS ) | 35.7 ± 4.9 | 42.3 ± 3.2 | 0.266 | 0.660 |
| Arm Swing Asymmetry (% lv) | 35.5 ± 5.4 | 21.6 ± 2.7 | 0.031 | 0.708 |
| Lower Body | ||||
| Cadence (steps/min) | 111.7 ± 1.7 | 121.2 ± 2.1 | 0.001 | 0.854 |
| Stride Velocity (%ht/sec) | 71.0 ± 2.8 | 77.8 ± 2.0 | 0.065 | 0.729 |
| Stride Velocity (MAS) | 71.0 ± 2.9 | 77.8 ± 2.0 | 0.071 | 0.715 |
| Stride Velocity (LAS) | 71.0 ± 2.8 | 77.8 ± 2.0 | 0.063 | 0.729 |
| Stride Length (%ht) | 76.0 ± 2.2 | 76.9 ± 1.4 | 0.752 | 0.507 |
| Double Support Time (% gc) | 17.5 ± 0.9 | 18.9 ± 1.4 | 0.437 | 0.333 |
| Stride Time Variability (%) | 2.8 ± 0.3 | 2.9 ± 0.6 | 0.870 | 0.590 |
| Stride Length Variability (%) | 2.5 ±0.1 | 3.2 ± 0.7 | 0.363 | 0.535 |
| Trunk | ||||
| Peak Trunk Rotation Velocity (deg/sec) | 34.0 ± 2.6 | 44.6 ± 9.6 | 0.010 | 0.806 |
| Trunk Rotation Range of Motion (deg) | 7.3 ± 0.7 | 9.2 ± 0.5 | 0.041 | 0.764 |
| TURNING PARAMETERS | ||||
| Average Turning Velocity (deg/sec) | 76.2 ± 4.0 | 87.5 ± 3.2 | 0.037 | 0.764 |
| Peak Turning Velocity (deg/sec) | 163.3 ± 8.9 | 175.9 ± 8.3 | 0.312 | 0.617 |
| SIT-TO-STAND PARAMETERS | ||||
| Average Sit-to-Stand Velocity (deg/sec) | 30.9 ± 1.0 | 34.1 ± 2.1 | 0.199 | 0.618 |
| Peak Sit-to-Stand Velocity (deg/sec) | 89.2 ± 4.9 | 94.1 ± 5.0 | 0.421 | 0.562 |
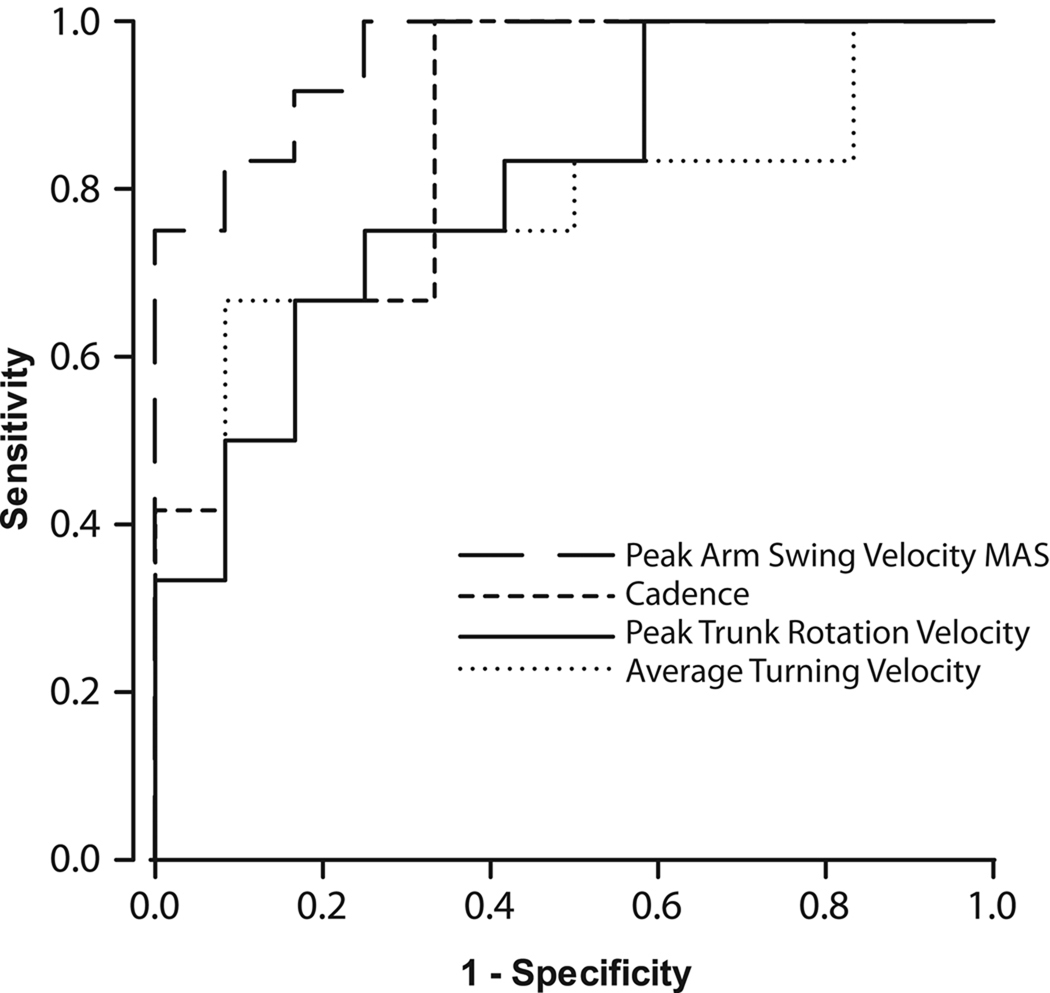
The most discriminative upper body, lower body and trunk parameters during gait and postural transitions are shown in Table 2. The PD group had a slower arm swing than the control group (p<0.001, Table 2), walked with slower cadence (p=0.01, Table 2), and rotated their trunks more slowly during gait (p<0.05, Table 2). Turning was also slower for PDs (p<0.01, Table 2). The ROC analysis revealed an area under the curve (AUC) of 0.95 (95% CI 0.821 – 1.041) for peak arm swing velocity of the MAS, which was the highest discriminative value of all variables (Table 2, Fig. 2). Among the other variables, cadence showed a discriminative value of 0.85 (95% CI 0.702 – 1.006), peak trunk rotation velocity was 0.80 (95% CI 0.632 – 0.979), and average turning velocity was 0.76 (95% CI 0.563 – 0.965) (Table 2, Fig. 2).
Figure 2.
The receiver operating characteristics (ROC) curve for peak arm swing velocity of the more affected side (MAS), cadence, peak trunk rotation velocity, and average turning velocity.
There were no significant differences between PD and control groups in stride velocity (although it approached significance), stride length, double support time, variability of stride time or stride length, peak turning velocity or sit-to-stand parameters. Those variables also showed lower AUC values (Table 2).
3.3. Correlations between iTUG and the Motor UPDRS
The Motor UPDRS total score and sub-scores were significantly correlated with the most discriminative parameters of the iTUG (Table 3). Higher UPDRS scores were associated with lower iTUG scores, which indicated more mobility deficits. The Motor UPDRS correlated significantly with arm swing, cadence and turning parameters. Turning velocity also correlated significantly with bradykinesia and Gait/Posture subscores. In contrast, peak trunk rotation velocity correlated significantly with rigidity.
Table 3.
Correlation between instrumented Timed Up and Go components and Unified Parkinson’s Disease Rating Scale (UPDRS) motor section scores
| UPDRS motor score | UPDRS bradykinesia | UPDRS gait/posture | UPDRS rigidity | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | |
| GAIT | ||||||||
| Upper Body parameters | ||||||||
| Arm Swing Range of Motion (°) | −0.58 | 0.04 | −0.49 | 0.09 | −0.40 | 0.18 | −0.08 | 0.79 |
| Peak Arm Swing Velocity (°/sec) | −0.62 | 0.02 | −0.49 | 0.10 | −0.50 | 0.09 | −0.18 | 0.57 |
| Arm Swing Asymmetry (% lv) | −0.34 | 0.27 | −0.53 | 0.07 | 0.23 | 0.45 | 0.05 | 0.86 |
| Trunk parameters | ||||||||
| Trunk Rotation Range of Motion (°) | −0.02 | 0.94 | 0.06 | 0.84 | 0.01 | 0.97 | −0.50 | 0.09 |
| Peak Trunk Rotation Velocity (°/sec) | −0.22 | 0.49 | −0.14 | 0.65 | −0.17 | 0.58 | −0.68 | 0.01 |
| Lower Body parameters | ||||||||
| Cadence (steps/min) | −0.58 | 0.04 | −0.49 | 0.10 | −0.26 | 0.40 | −0.52 | 0.08 |
| TURNING | ||||||||
| Average Turning Velocity (°/sec) | −0.73 | 0.006 | −0.65 | 0.02 | −0.71 | 0.009 | −0.39 | 0.20 |
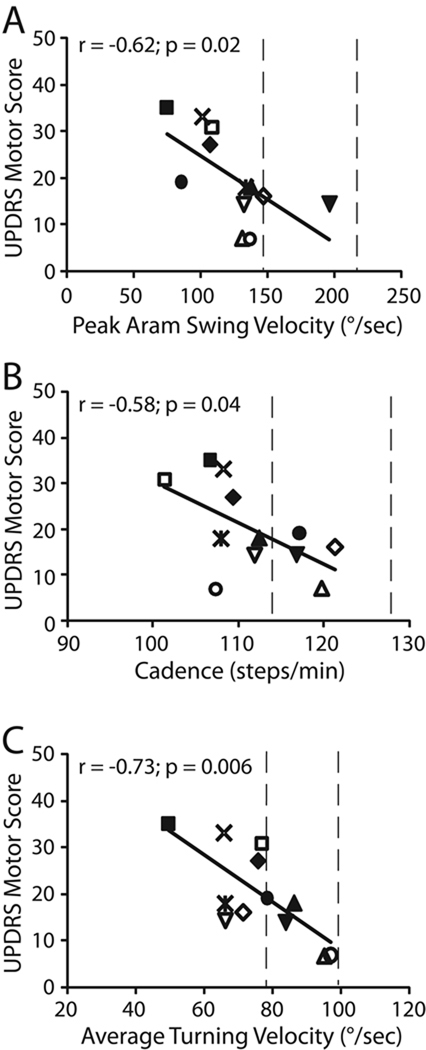
Figure 3 shows the largest correlations between the Motor UPDRS and iTUG components for the PD subjects: peak arm swing velocity (Fig 3A), cadence (Fig 3B), and average turning velocity (Fig. 3C). Each subject is represented by a different symbol. It can be seen that only one subject fell within the normal range of peak arm swing velocity, and 4 subjects were within the normal range of gait cadence and turning velocity (vertical dashed lines). Also evident, is that individual PD subjects had different mobility problems. For example, subject “open zero” had abnormal cadence and arm swing, but normal turning velocity, whereas subject “solid zero” had abnormal arm swing but normal cadence and near normal turning. Every PD subject, except for the subject represented by “solid inverse triangle”, had abnormal performance in at least one parameter.
Figure 3.
The relationship between UPDRS Motor Scores and iTUG parameters in the PD group. (A) correlation between UPDRS scores and Peak Arm Swing Velocity, (B) UPDRS scores and Cadence, (C) UPDRS scores and Average Turning Velocity. Each PD subject is represented with a different symbol. Vertical dashed lines delimit the range of values for the control group, 1 SD above and below the average. Patients with different levels of mobility deficits = 
4. DISCUSSION
Our key findings were: 1) The traditional TUG, measured with a stopwatch, was not a sensitive tool to detect gait and posture abnormalities in early-to-mid stage PD; 2) The iTUG can reveal specific gait and turning deficits in early-to-mid stage PD; and 3) Components of the iTUG correlated with PD severity.
The traditional TUG is not a sensitive tool to differentiate between untreated PD and controls
The lack of sensitivity of the traditional 3-meter TUG or 7-meter iTUG time to differentiate between early, untreated PD and control subjects was not surprising. Although previous studies have validated the use of the 3-meter TUG test in PD [8,9], they examined subjects with more advanced disease compared to our group. Our PD subjects had average H&Y 1.6, were 60 yrs. old, and approximately 1 yr. disease duration whereas the other studies included subjects with H&Y 2.6 [9] or ranging from 1–4 [7], mean age between 67 [7] and 69 yrs. [9], and 9 yrs. disease duration [8]. Our findings confirmed that a stopwatch measure is not sensitive to detect problems in people with PD, who do not show obvious clinical signs of impaired gait and balance.
The iTUG revealed specific deficits in gait and turning in untreated PD
This is the first study to use an instrumented TUG test to identify specific mobility deficits in early-to-mid stage, untreated PD. A recent study used inertial sensors to separate the TUG into 4 subtasks (sit-to-stand, walk, turn, and stand-to-sit) and showed that the duration of these subtasks were slower in hemiplegics than in controls, although the total TUG duration was also slower in hemiplegics [26]. By assessing a wide variety of spatial and temporal components of the modified TUG with twice the gait length, we uncovered mobility deficits that were not evident with the stopwatch measure in PDs.
The most sensitive deficits in early PD were arm swing, cadence and trunk rotation during gait, and turning velocity. These findings suggest a generalized bradykinesia, rather than bradykinesia specific to body part or to posture versus gait, early in the disease. Since these deficits were apparent in subjects who had never taken antiparkinsonian medication, this slowed performance was likely due to reduced dopaminergic activity and not secondary to medication effects. The individual differences in relative slowing of arms, legs and trunk during gait and turning among the PD subjects suggests that different parts of the basal ganglia may be responsible for the control of different body parts and are differently affected in PD [27].
Other investigations on gait and postural transitions in early PD have been limited to only the lower body and/or trunk parameters, and limited to one task; either gait, turning or sit-to-stand [10,12,28–30]. In our study, the tasks were performed in a remembered sequence. The iTUG task may be more sensitive to early PD than single tasks because PD is known to affect the ability to sequence motor tasks [15–17,31].
Our findings are consistent with studies reporting slower walking for PD subjects than control subjects [10,12,28,32]; cadence was significantly reduced and stride velocity was reduced, approaching significance. However, contrary to the literature [10,12], we observed no deficits in double support time or stride length in early-to-mid stage PD. Differences in results may be due to different disease severities as well as differing sensitivities of the measuring techniques. Also, contrary to some reports of increased variability of stride time [10,11], and stride length [33], our PD subjects did not show deficits in temporal or spatial gait variability. We don’t think the lack of increased gait variability in our PD subjects was due to the relatively short distance subjects walked, because we evaluated variability of parameters across 7 meters × 3 trials = 21 meters, which is similar to previous studies [10,11], [33]. Our findings are consistent with the lack of increased gait variability seen by Ebersbach et al [28] and Carpinella et al [32].
The iTUG revealed deficits in trunk mobility in untreated PDs, with reduced peak trunk rotation velocity during walking and reduced average angular velocity during turning, both variables with high discriminative values. These results are consistent with ‘enbloc’ trunk motion in PD and partially in line with the findings by Visser et al [30], who also used portable technology to measure trunk motion during walking and turning in PD. Visser et al [30] reported a high AUC for peak turn velocity (0.83), however their reported AUC value for peak trunk rotation during walking was low (0.56). One reason for the difference might be the sensor placement, which was on the lower back in Visser’s study [30] and on the upper chest (sternum) in our study. Placing the sensor on the sternum may be more sensitive to reduced trunk rotation associated with reduced arm swing during gait.
Surprisingly, we did not observe any differences between groups in angular trunk velocity during the sit-to-stand task. This result is in contrast with a previous study by Mak et al [29], who reported reduced peak horizontal and vertical trunk velocities during sit-to-stand in PD. The difference is likely due to subjects in Mak’s study [29] being more severe (H&Y 2.58), older (65 yrs.) and mean disease duration five years longer. These results suggest that sit-to-stand deficits appear in later stages of the disease than turning and gait deficits, possibly related to weakness secondary to lack of mobility.
Components of the iTUG correlate with disease severity
Our findings showed that deficits in arm swing, gait and turning increase with the severity of PD as measured by the Motor UPDRS. However, these findings should be interpreted with caution given that our sample size was small for correlation analyses. Additional investigation involving larger groups are necessary to confirm our findings. We only found one study correlating clinical scales with gait or turning measurements in early PD; and they reported a negative, moderate correlation (ρ=−0.76) between maximum gait velocity and the Webster rating scale [34]. Our results agree with their findings; cadence decreased with an increase in disease severity. Curiously, cadence is faster than normal later in PD [35], possibly as a consequence of shorter stride length or because of propulsion associated with freezing and equilibrium problems.
The mobility deficits presented by our PD subjects were not uniform. Although the majority of the subjects (8 out of 12) showed impairments on gait or turning, a PD subject who had reduced cadence during gait did not necessarily also have reduced arm swing or turning deficits and vice versa. In fact, several PD subjects had normal values in some parameters and severe impairments in others. These results indicate that in early-to-mid stages of PD, the spectrum of mobility abnormalities vary from person to person. Thus, a test that includes a variety of postural transitions and gait tasks will be more sensitive in detecting early mobility deficits in PD. The iTUG seems to be an effective tool for this purpose.
Of all parameters, turning velocity was best related to progression of PD. Turning was also the only parameter correlated with bradykinesia and gait/posture subscores. These relationships may be a reflection of early balance problems, since PD subjects may slow down as their balance deteriorates with disease progression. Previous studies on more severe PD have shown that turning becomes a major problem later in the disease [36–39] and is related to falls [40]. Our findings, along with the literature, show that decreased turning velocity seems to be a good measure of disease progression and it may be specific to PD. Our research group is currently conducting a longitudinal study to further investigate turning parameters as a potential marker of disease progression.
Future research and clinical use of the iTUG
Further research should investigate the potential of the iTUG to monitor the progression of PD over time. The iTUG may also be useful in studies of potential disease-modifying treatments or in rehabilitation studies aimed to improve gait and balance. In addition, the iTUG could be a quick and easy test to monitor PD course in the clinical setting, indicating when therapies are efficacious and when new interventions are needed. For better clinical applicability of the iTUG, studies should focus on exploring methods to combine the most important variables into a single score.
As mobility is a problem with aging and many other neurological disorders, the usefulness of the iTUG may extend well beyond PD. Detection of specific mobility problems in the elderly, or other populations with gait and balance deficits, would allow rehabilitation professionals to focus on each individual’s mobility deficits.
In summary, this study showed that unlike the traditional TUG, components of the iTUG can reveal specific deficits of gait and turning parameters in early-to-mid stage PD. People with untreated PD showed reduced cadence, range and velocity of trunk rotation and arm swing, and they also turned around (with a 180 degree-turn) slower. Such deficits increased with the severity of the disease from mild to moderate, however they are not uniformly present, varying from person to person. Further research is necessary to investigate the potential of the iTUG test to detect changes in PD progression, sensitivity to treatment, and test its application to other disorders.
ACKNOWLEDGEMENTS
We thank our research subjects, Triana Nagel-Nelson for assisting with subject scheduling and data collection, and Edward King for technical assistance. This research was supported by the Kinetics Foundation, the National Institutes on Aging (006457) and the Oregon Center for Aging and Technology (AG024978). Dr. Horak was a consultant for the Kinetics Foundation.
Footnotes
This potential conflict of interest has been reviewed and managed by OHSU.
REFERENCES
- 1.Berg KO, Maki BE, Williams KI. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73:1073–1080. [PubMed] [Google Scholar]
- 2.Lin M, Hwang H, Hu M, et al. Psychometric Comparisons of the Timed Up and Go, One-Leg Stand, Functional Reach, and Tinetti Balance Measures in Community-Dwelling Older People. JAGS. 2004;52:1343–1348. doi: 10.1111/j.1532-5415.2004.52366.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson M, Medley A. Performance of community dwelling elderly on the Timed Up and Go test. Phys Occup Ther Geriatr. 1995;13:17–29. [Google Scholar]
- 4.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86:1641–1647. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Nilsagard Y, Lundholm C, Gunnarsson LG, et al. Clinical relevance using timed walk tests and 'timed up and go' testing in persons with multiple sclerosis. Physiother Res Int. 2007;12:105–114. doi: 10.1002/pri.358. [DOI] [PubMed] [Google Scholar]
- 6.Brusse KJ, Zimdars S, Zalewski KR, et al. Testing functional performance in people with Parkinson disease. Phys Ther. 2005;85:134–141. [PubMed] [Google Scholar]
- 7.Martínez-Martín P, Urra DG, Quijano TS, et al. A New Clinical Tool for Gait Evaluation in Parkinson's Disease. Clin Neuropharmacol. 1997;20:183–194. doi: 10.1097/00002826-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Morris S, Morris ME, Iansek R. Reliability of Measurements Obtained With the Timed “Up & Go” Test in People With Parkinson Disease. Phys Ther. 2001;81:810–819. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 9.Dibble LE, Lange M. Predicting Falls In Individuals with Parkinson Disease: A Reconsideration of Clinical Balance Measures. J Neurol Phys Ther. 2006;30:60–67. doi: 10.1097/01.npt.0000282569.70920.dc. [DOI] [PubMed] [Google Scholar]
- 10.Baltadjieva R, Giladi N, Gruendlinger L, et al. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson's disease. Eur J Neurosci. 2006;24:1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Cudkowicz ME, Firtion R, et al. Gait Variability and Basal Ganglia Disorders: Stride-to-stride Variations of Gait Cycle Timing in Parkinson's Disease and Huntington's Disease. Mov Dis. 1998;13:428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 12.Nelson AJ, Zwick D, Brody S, et al. The validity of the GaitRite and the Functional Ambulation Performance scoring system in the analysis of Parkinson gait. NeuroRehabil. 2002;17:255–262. [PubMed] [Google Scholar]
- 13.Yang Y, Lee Y, Cheng S, et al. Relationships between gait and dynamic balance in early Parkinson’s disease. Gait Posture. 2008;27:611–615. doi: 10.1016/j.gaitpost.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Chastan N, Debono B, Maltête D, et al. Discordance Between Measured Postural Instability and Absence of Clinical Symptoms in Parkinson’s Disease Patients in the Early Stages of the Disease. Mov Dis. 2008;23:366–372. doi: 10.1002/mds.21840. [DOI] [PubMed] [Google Scholar]
- 15.Benecke R, Rothwell JC, Dick JPR, et al. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 16.Bloem BR, Valkenburg VV, Slabbekoorn M, et al. The multiple tasks test. Strategies in Parkinson's disease. Exp Brain Res. 2001;137:478–486. doi: 10.1007/s002210000672. [DOI] [PubMed] [Google Scholar]
- 17.Rogers MA, Phillips JG, Bradshaw JL, et al. Provision of external cues and movement sequencing in Parkinson's disease. Motor Control. 1998;2:125–132. doi: 10.1123/mcj.2.2.125. [DOI] [PubMed] [Google Scholar]
- 18.Aminian K, Najafi B, Bula C, et al. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35:689–699. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Salarian A, Russmann H, Vingerhoets FJ, et al. Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. IEEE Trans Biom Eng. 2004;51:1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 20.Najafi B, Aminian K, Loew F, et al. Measurement of Stand–Sit and Sit–Stand Transitions Using a Miniature Gyroscope and Its Application in Fall Risk Evaluation in the Elderly. IEEE Trans Biom Eng. 2002;49:843–851. doi: 10.1109/TBME.2002.800763. [DOI] [PubMed] [Google Scholar]
- 21.Salarian A, Russmann H, Vingerhoets FJ, et al. Ambulatory monitoring of physical activities in patients with Parkinson's disease. IEEE Trans Biom Eng. 2007;54:2296–2299. doi: 10.1109/tbme.2007.896591. [DOI] [PubMed] [Google Scholar]
- 22.Fahn S, Elton RL. Unified parkinson's disease rating scale. Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, prognosis and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The Timed "Up & Go": a test of basic functional mobility for frail elderly persons. JAGS. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Salarian A, Horak FB, Zampieri C, et al. The iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009 doi: 10.1109/TNSRE.2010.2047606. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashi Y, Yamakoshi K, Fujimoto T, et al. Quantitative Evaluation of Movement Using the Timed Up-and-Go Test. IEEE Eng Med Biol Mag. 2008:38–46. [Google Scholar]
- 27.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev of Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 28.Ebersbach G, Heijmenberg M, Kindermann L, et al. Interference of Rhythmic Constraint on Gait in Healthy Subjects and Patients With Early Parkinson’s Disease: Evidence for Impaired Locomotor Pattern Generation in Early Parkinson’s Disease. Mov Dis. 1999;14:619–625. doi: 10.1002/1531-8257(199907)14:4<619::aid-mds1011>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Mak MKY, Hui-Chan CWY. Switching of Movement Direction Is Central to Parkinsonian Bradykinesia in Sit-to-Stand. Mov Dis. 2002;17:1188–1195. doi: 10.1002/mds.10257. [DOI] [PubMed] [Google Scholar]
- 30.Visser JE, Voermans NC, Nijhuis LBO, et al. Quantification of trunk rotations during turning and walking in Parkinson’s disease. Clin Neurophysiol. 2007;118:1602–1606. doi: 10.1016/j.clinph.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Fama R, Sullivan EV. Motor sequencing in Parkinson's disease: relationship to executive function and motor rigidity. Cortex. 2002;38:753–767. doi: 10.1016/s0010-9452(08)70042-x. [DOI] [PubMed] [Google Scholar]
- 32.Carpinella I, Crenna P, Calabrese E, et al. Locomotor function in the early stage of Parkinson's disease. IEEE Trans Neur Sys Rehabil Eng. 2007;15:543–551. doi: 10.1109/TNSRE.2007.908933. [DOI] [PubMed] [Google Scholar]
- 33.Blin O, Ferrandez AM, Serratrice G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J Neurol Sci. 1990;98:91–97. doi: 10.1016/0022-510x(90)90184-o. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen SW, Eriksson T, Oberg B. Effects of withdrawal of antiparkinson medication on gait and clinical score in the Parkinson patient. Acta Neurol Scand. 1991;84:7–13. doi: 10.1111/j.1600-0404.1991.tb04894.x. [DOI] [PubMed] [Google Scholar]
- 35.Morris ME, Iansek R, Matyas TA, et al. Ability to modulate walking cadence remains intact in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:1532–1534. doi: 10.1136/jnnp.57.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crenna P, Carpinella I, Rabuffetti M, et al. The association between impaired turning and normal straight walking in Parkinson's disease. Gait Posture. 2007;26:172–178. doi: 10.1016/j.gaitpost.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Huxham F, Baker R, Morris ME, et al. Footstep adjustments used to turn during walking in Parkinson's disease. Mov Dis. 2008;23:817–823. doi: 10.1002/mds.21932. [DOI] [PubMed] [Google Scholar]
- 38.Huxham F, Baker R, Morris ME, et al. Head and trunk rotation during walking turns in Parkinson's disease. Mov Dis. 2008;23:1391–1397. doi: 10.1002/mds.21943. [DOI] [PubMed] [Google Scholar]
- 39.Stack E, Ashburn A. Dysfunctional turning in Parkinson's disease. Disabil Rehabil. 2008:1–8. doi: 10.1080/09638280701829938. [DOI] [PubMed] [Google Scholar]
- 40.Stack E, Ashburn A. Fall events described by people with Parkinson's disease: implications for clinical interviewing and the research agenda. Physiother Res Int. 1999;4:190–200. doi: 10.1002/pri.165. [DOI] [PubMed] [Google Scholar]