Abstract
Nanos family members have been shown to act as translational repressors in the Drosophila and Caenorhabditis elegans germline, but direct evidence is missing for a similar function in vertebrates. Using a tethered function assay, we show that Xenopus Nanos1 is a translational repressor and that association with the RNA is required for this repression. We identified a 14 amino acid region within the N-terminal domain of Nanos1 that is conserved in organisms as diverse as sponge and Human. The region is found in all vertebrates but notably lacking in Drosophila and C. elegans. Deletion and substitution analysis revealed that this conserved region was required for Nanos1 repressive activity. Consistent with this observation, deletion of this region was sufficient to prevent abnormal development that results from ectopic expression of Nanos1 in oocytes. Although Nanos1 can repress capped and polyadenylated RNAs, Nanos1 mediated repression did not require the targeted RNA to have a cap or to be polyadenylated. These results suggest that Nanos1 is capable of repressing translation by several different mechanisms. We found that Nanos1, like Drosophila Nanos, associates with cyclin B1 RNA in vivo indicating that some Nanos targets may be evolutionarily conserved. Nanos1 protein was detected and thus available to repress mRNAs while PGCs were in the endoderm, but was not observed in PGCs after this stage.
Keywords: Xenopus, Nanos1/Xcat2, germline determination, translational repression
1. Introduction
Nanos homologues have been found in organisms as diverse as hydra (Mochizuki et al., 2000), leech (Kang et al., 2002), Caenorhabditis elegans (Subramaniam and Seydoux, 1999), Drosophila (Wang and Lehmann, 1991), zebrafish (Koprunner et al., 2001), frogs (Mosquera et al., 1993), mouse (Tsuda et al., 2003) and humans (Jaruzelska et al., 2003; Kusz et al., 2009). In each case, nanos members are expressed preferentially in the germline precursor cells (PGCs), germline stem cells or in multipotent stem cells (Mochizuki et al., 2000). Most importantly, the function of nanos in maintaining the germline by suppression of somatic cell fates has also largely been conserved (Hayashi et al., 2004; Tsuda et al., 2003; Wang and Lin, 2004; Koprunner et al., 2001). PGCs lacking Nanos activity inappropriately express somatic genes, such as Sex Lethal in flies (Asaoka et al., 1998; 1999; Deshpande et al., 1999, 2005), and enter apoptosis during migration (Sato et al., 2007;Hayashi et al., 2004; Koprunner et al., 2001; Maezawa et al., 2009; Tsuda et al., 2003; Subramaniam and Seydoux, 1999). In C. elegans and Drosophila, nanos mutants also fail to establish the germline specific histone modifications correlated with inactive chromatin (Schaner et al., 2003). These observations raise intriguing questions as to how Nanos may suppress somatic fates at multiple levels in the germline, i.e., preventing expression of somatic genes, repressing apoptotic pathways, and altering chromatin structure.
Insight into how nanos may affect these different molecular pathways has come from studies on Drosophila as well as C. elegans where Nanos was shown to function as a translational repressor (Wharton and Struhl, 1991; Wang and Lehmann, 1991; Kraemer et al., 1999). Although Nanos can bind RNA, it does so with little sequence specificity. Correct selection of the mRNA for repression requires binding of Pumilio (PUM) to the Nanos Response Element(s) (NRE) found in the 3'UTRs of targeted messages (Murata and Wharton, 1995; Sonoda and Wharton, 1999). Mutants in pumilio often mimic the nanos mutant phenotypes described above, arguing that these effects are co-regulated by Nanos/PUM repression (Parisi and Lin, 2000; Leatherman and Jongens, 2003).
Nanos repression of translation may depend on the target RNA itself (Kadyrova et al., 2007; Cheong and Hall, 2006; Wharton et al., 1998). Experiments on Drosophila hunchback (hb) and cyclin B1 RNAs reveal important differences although both are repressed by a PUM/Nanos interaction. Hb mRNA expression is repressed to allow correct abdominal formation while cyclin B1 repression must be restricted to PGCs for normal development. PUM binds the NRE in the hb 3'UTR, recruits Nanos, and subsequently Brat to this complex (Sonoda and Wharton, 2001). Brat can interact with 4E-HP preventing eIF-4E from cap binding, thus repressing translation (Cho et al., 2006). Nanos activity can accelerate hb RNA deadenylation followed by hb RNA degradation. Whether Nanos is directly responsible for these activities is not known (Wharton and Struhl, 1991; Sonoda and Wharton, 1999; Wreden et al., 1997). Interestingly, the presence of a poly(A) tail is not required for hb repression, suggesting this repression can occur upstream of polyadenylation. Taken together the data support both a poly(A) dependent and independent mechanism of repression (Chagnovich and Lehmann, 2001). Thus the PUM, NOS, BRAT complex can repress hb mRNA by interfering with translation at several different steps.
Cyclin B1 RNA is another important target of PUM/Nanos in Drosophila, but this interaction is restricted to PGCs (Deshpande et al., 1999; Wang and Lin, 2004). Brat is not recruited to this repressive complex, but another unknown co-factor(s) is required to restrict cyclin B1 repression to PGCs. If an interaction between Nanos and the RNA is forced by molecular tethering, PUM is not required for cyclin B1 repression as it is for hb repression, consistent with Pum simply acting to recruit Nanos to cyclin B1. In this case, Nanos interacts directly with the NOT4 subunit, part of the deadenylase complex, and could account for Nanos mediated repression (Kadyrova et al., 2007). Most recently, NANOS2 in the male mouse germline has also been found to interact with the deadenylase complex (Suzuki et al., 2010).
We identified Xcat2 as a nanos family member that is expressed in the Xenopus germline(Mosquera et al., 1993; Houston and King, 2000). In Xenopus oocytes, PUM directly binds cyclin B1 mRNA (Nakahata et al., 2001). Although Nakahata and co-workers provide evidence that Xcat2 can co-immunoprecipitate with PUM in vitro, there is no direct evidence that Xcat2 interacts with cyclin B1 in vivo. In oocytes, where Xcat2 is not present (MacArthur et al., 1999), PUM binds to the cytoplasmic polyadenylation element (CPE)-binding protein (CPEB) and this protein represses translation perhaps through Maskin. At maturation, CPEB is phosphorylated, causing the release of PUM and allowing translation events to occur (Nakahata et al., 2003). Thus, it is likely that cyclin B1 translation is regulated by different proteins in PGCs and not as part of the CPE-CPEB maturation translational pathway. Surprisingly, although the previous studies provide persuasive evidence that nanos activity is essential for maintaining the germline, there is no direct evidence that vertebrate Nanos functions to repress translation. Further, the identity of mRNA targeted for Nanos interaction in vertebrate PGCs remains largely unknown.
Here we identify the gene originally named Xcat2 (Mosquera et al., 1993) as homologous to human and mouse Nanos1 (Figs. S1, 2; Kurokawa et al., 2006) and therefore will use nanos1 throughout the rest of this report. nanos1 was previously found to be transcribed in pre-stage I oocytes and translationally sequestered during oogenesis (Zhou and King, 1996; MacArthur et al., 1999; Luo et al., 2011). Here we show directly that Xenopus Nanos1 can function as a translational repressor. Deletion analysis revealed the region required in vivo for Nanos1 repressive activity is highly conserved from sponge to humans, but that this region is missing in Drosophila and C. elegans. Although Nanos1 can repress capped and polyadenylated RNAs, Nanos1 mediated repression does not require the targeted RNA to have a cap or to be polyadenylated. Interestingly, the conserved region is not required for repression of uncapped RNA. These findings suggest that Nanos1 is capable of repressing translation by several different mechanisms. We identified cyclin B1 RNA as a target of Nanos1 in vivo, as previously found for Drosophila, suggesting that some nanos targets may be evolutionarily conserved. Nanos1 protein was detected in PGCs only while they were in the endoderm, thus restricting the developmental time period when Nanos1 is available to repress mRNAs.
2. Results
2.1 Nanos1 protein functions as a translational repressor
Maternal somatic determinants such as VegT RNA are present in primordial germ cells, but are prevented from functioning in the germline (Venkatarama et al., 2010). To test directly if Nanos1 protein can function as a translational repressor and thus contribute to preserving the germline, we used the tethered function assay (Gray et al., 2000; Coller and Wickens, 2007).
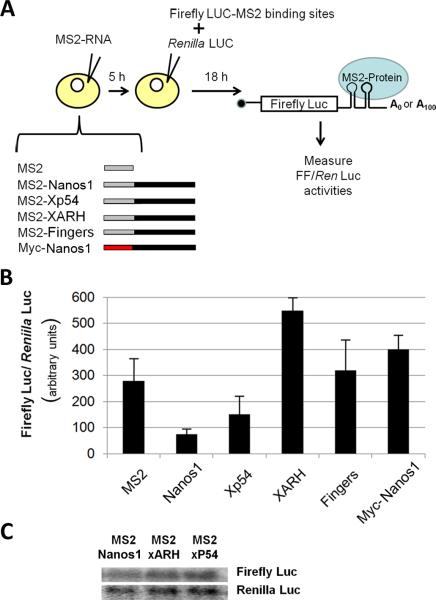
MS2 coat protein was fused upstream of Nanos1 allowing Nanos1 to bind to the 3'UTR of a Firefly (FF) luciferase reporter containing MS2 coat protein-binding sites. Both polyadenylated (A100) and non-adenylated (A0) reporters were tested. MS2 alone, MS2-Nanos1, MS2-Xp54, MS2-XARH or MS2-Fingers transcripts were injected into stage VI oocytes and the fusion proteins allowed to accumulate for five hours (Fig. 1A). MS2-Xp54, a potent repressor found in oocytes (Minshall and Standart, 2004) served as a positive control while MS2 alone should have no effect on translation. XARH is an adaptor protein involved in endocytosis and Fingers contains over 20 zinc fingers and served as a control for non-specific effects of these structures on translation (Zhou et al., 2004; King et al., 2005). After five hours, the FF luciferase reporter together with the control Renilla luciferase mRNA were injected and the oocytes cultured overnight. The effect of the tethered proteins on FF luciferase activity compared to the control Renilla luciferase was determined using a Dual-Luciferase assay system (Promega) (Fig. 1A).
Figure 1. Nanos1 functions as a translational repressor.
(A) Schematic of the tethered function assay used in these studies. Xenopus stage VI oocytes were first injected with mRNAs encoding MS2-protein fusions as indicated. Following 5hr incubation, all oocytes were co-injected with two luciferase mRNAs, encoding control Renilla luciferase that lacks 3'UTR regulatory sequences, and firefly luciferase mRNA containing MS2-binding sites in a nonadenylated or adenylated form. After 18hr incubation, both luciferase activities were assayed. (B) Firefly luciferase activity was normalized with Renilla luciferase activity. Three samples each containing three oocytes were assayed per experimental point from which mean values and standard deviations were determined. Experiments were repeated with three different female oocyte donors. xARH (Zhou et al., 2003) and xP54 (Minshall and Standart, 2004) served as the negative and positive controls respectively. (C) Nanos1 does not degrade the reporter RNAs as shown by blot analysis.
Tethered Nanos1 consistently repressed translation of the FF luciferase reporter RNA to 25% of the MS2 control levels, significantly more than Xp54 (n = 6) regardless of whether the reporter was adenylated (data not shown) or not (Fig. 1B). All fusion proteins were expressed at similar levels (data not shown). As expected, neither XARH nor Fingers negatively affected translation of the reporter indicating the repression observed was not due to the tethering process in general. To test whether MS2-Nanos1 somehow degraded the reporter RNAs, resulting in the loss of luciferase activity, levels of both FF and Renilla luciferase RNA were determined by blot analysis. Luciferase RNA levels were indistinguishable after injection of MS2-Nanos1, MS2-XARH, or MS2-Xp54 (Fig. 1C). Myc-Nanos1 did not repress FF reporter or control Renilla mRNA indicating that direct binding of Nanos1 to the RNA was required to repress translation. From these results we conclude that tethered Nanos1 acts to repress translation of either polyadenylated or non-adenylated RNAs.
2.2 Nanos1 contains a conserved region required for repression
The two zinc fingers located in the C terminal region are highly conserved among all nanos family members sharing 64.2% (34/53) identity with conserved substitutions (Fig 2C). The N terminal (N-ter) region is longer in human and mouse Nanos1 than it is in Xenopus and is less conserved overall (Fig. S1) with the exception of a small 14 amino acid motif that shows 57.1% conservation in all nanos family members (Fig. 2B). Interestingly, Drosophila and C. elegans do not contain this motif, suggesting that this region was independently lost in both species.
Figure 2. Comparison of Nanos family members identified new conserved N-terminal region.
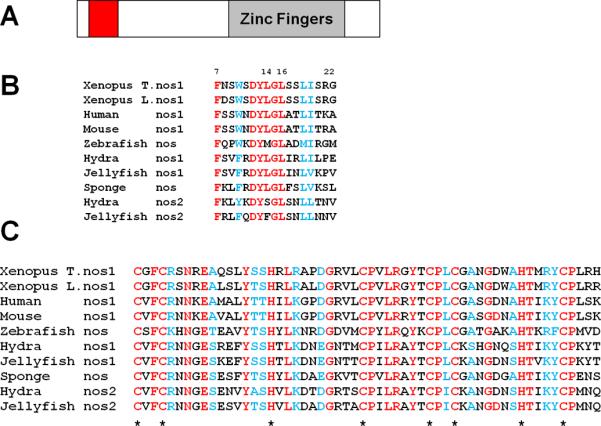
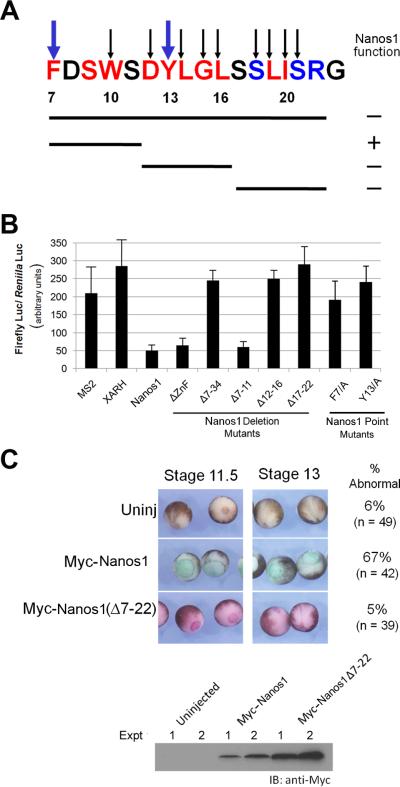
(A) Schematic of Nanos1 protein showing relative position of Nt-conserved region and Zinc Finger domains. (B) Nanos1 N-terminal region aa 7-22 and (C) zinc finger domain are conserved in divergent organisms. Identical residues (red), conserved residues (blue). * marks the location of the CCHC comprising the zinc fingers.
To determine the region of Nanos1 required for repression, we deleted the N-terminal amino acids 7-34 which included the conserved region or the zinc finger domain and tested them in the tethered function assay (Fig. 3A, B). In all cases, IP with MS2 antibody confirmed that the deletion mutants were expressed in oocytes and at approximately equal levels. While the N-ter conserved region was essential for translational repression, the zinc finger domain was not required. We functionally tested a series of deletion and substitution mutants to pin down more precisely which residues were required. In summary, residues 7-22, the perfectly conserved D12YLGL16 region, and amino acids 17-22 were all required for Nanos1 function. Point mutation analysis showed that residues F7/A and Y13/A were also essential for function (Fig 3A). Although deletion of residues 17-22 abrogated function, point mutations (S18/A, L19/A, I20/A, S21/A) within this region had no effect on repression. We conclude from this analysis that the highly conserved N-ter region is essential for repressive function and that Nanos1, tethered to the RNA, does not require the zinc finger region to repress.
Figure 3. Nanos1 Nt-conserved region is essential for repressive function.
(A) Summary of results from tethered function assays testing deletion and point mutations in the Nanos1Nt-7-22 region. Identical residues between humans, mouse, and frogs are in red, conserved residues in blue with their respective numbers indicated. Lines below the sequence indicate region of deletions with loss (-) or retention (+) of repressive function shown. Arrows above sequence indicate those residues substituted for alanine and only the blue arrows indicate residues found to be required for translational repression. (B) Mutation analysis defining residues required for Nanos1 repressive ability using the tethered function assay (Fig. 1). (C) Host transfer with Myc-Nanos1 (green) or Myc-Nanos1 deletion mutant Δ7-22 (red). Note that expression of Myc-Nanos1 causes abnormal development while the mutant Δ7-22 does not. Blot shows that Myc-tagged proteins were expressed in donor oocytes used in host transfer.
To determine if the conserved region Nanos1 7-22 was required for in vivo function within the context of the embryo, we used the host transfer procedure to assess the deletion mutant covering these amino acids. We have previously observed that Nanos1 is translationally repressed if injected into oocytes, but this repression can be overcome by inserting any tag just after the 5'UTR. The Myc-Nanos1 injected embryos developed normally until gastrulation when Myc-Nanos1 injected embryos displayed large blastopores that failed to close, even by stage 13. Such embryos remained round with their endodermal mass exposed and most did not survive neurula stages (67% arrested gastrulation, n = 42). Presumably, Myc-Nanos1 together with endogenous PUM, inappropriately repress mRNAs required for normal somatic patterning. In contrast, embryos expressing Nanos1Δ7-22 developed normally (95% normal, n = 39) and could not be distinguished from uninjected controls (94% normal, n = 49) (Fig. 3C). From these results we conclude that the conserved N-ter region is required for Nanos1 repressive activity in vivo.
2.3 Nanos1 does not require a cap to repress translation
To determine if repression by Nanos1 functions through interference with initiation events, we used the tethered function assay with luciferase reporters that lacked a cap or contained a mutant GpppG-cap in front of a Cricket Paralysis Virus internal ribosome entry site (CrPV IRES) (Gorgoni et al., 2005). Repression by Nanos1 was compared between these and an m7G-capped luciferase reporter. CrPV IRES can recruit ribosomes directly in the absence of any initiation factors (Wilson et al., 2000). The RNA-binding protein U1A does not activate or repress translation and was expressed in oocytes along with the reporters as a control. MS2-U1A expression values for each reporter was normalized to 1 allowing for direct comparison of Nanos1-mediated translation repression with the CrPV IRES mRNAs as they translate less efficiently than capped reporters. As shown in Fig. 4, tethered Nanos1 effectively repressed translation of luciferase reporters regardless of whether the cap was present, mutant, or missing. Is the conserved N-ter region also required for repression of uncapped message? We tested the Nanos1 Δ7-22 mutant which failed to repress capped message and found that it retained the ability to repress uncapped RNA. Thus, Nanos1 can operate down-stream of initiation events to repress translation and this ability requires a different region of Nanos1 to function.
Figure 4. Nanos1 can repress IRES-driven translation.
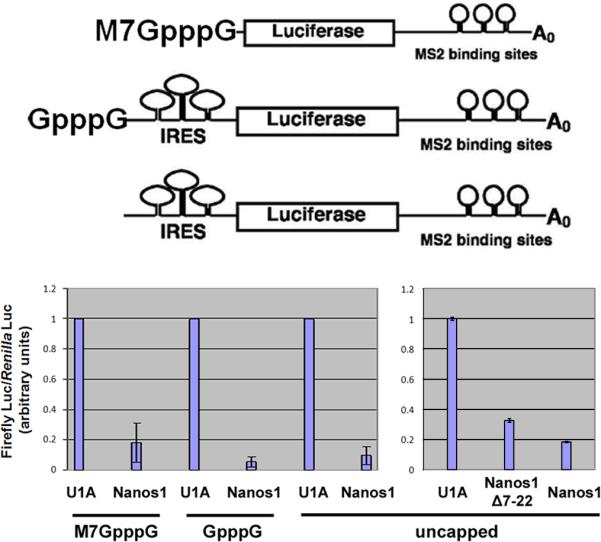
Capped, mutant capped, or IRES driven reporters were used in translational repression assays as described in Figure 1. All reporters were effectively repressed by Nanos1. Nanos1Δ7-22 repressed the translation of IRES driven reporter although it failed to repress capped reporter as shown in Fig 3. Splicing factor U1A was used as the positive control. The relative Luciferase ratio was normalized to the U1A value which was set at 1.
2.4 Nanos1 interacts with Cyclin B1 RNA in vivo
One target of Drosophila Nanos for translational repression is cyclin B1 RNA (Deshpande et al., 1999; Wang and Lin, 2004). This repression is critical for blocking the proliferation of PGCs. Indeed, previous reports have shown that Xenopus PUM interacts directly with cyclin B1 in frog oocytes and that injected GST-tagged Xcat2/Nanos1 can bind PUM after injection into oocytes (Nakahata et al., 2001). However, these studies did not show Xcat2/Nanos1 in a complex with cyclin B1 RNA. To determine if Nanos1 does indeed interact with cyclin B1 in vivo, myc-Nanos1 RNA was injected into stage VI oocytes. Myc-Nanos1 protein and the associated RNAs were then immunoprecipitated with anti-Myc antibody. The RNAs were analyzed by RT-PCR with specific primers. Cyclin B1 RNA, but not control ODC RNA, was pulled down by myc-Nanos1 protein (Fig 5). These results indicated that Nanos1 can associate with cyclin B1 RNA in vivo.
Figure 5. Nanos1 can pull down cyclin B1 RNA from oocyte extract.
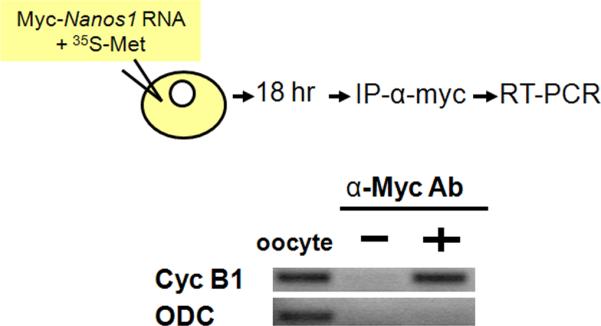
Myc-Nanos1 mRNA was injected into oocytes. After incubation, myc-Nanos1 protein was immunoprecipitated with anti-myc antibody. The associated mRNAs were analyzed by RT-PCR. Nanos1 can pull down cyclinB1 RNA, but not the negative control, ornithine decarboxylase RNA (ODC).
2.5 Germline Nanos1 RNA is maternally derived
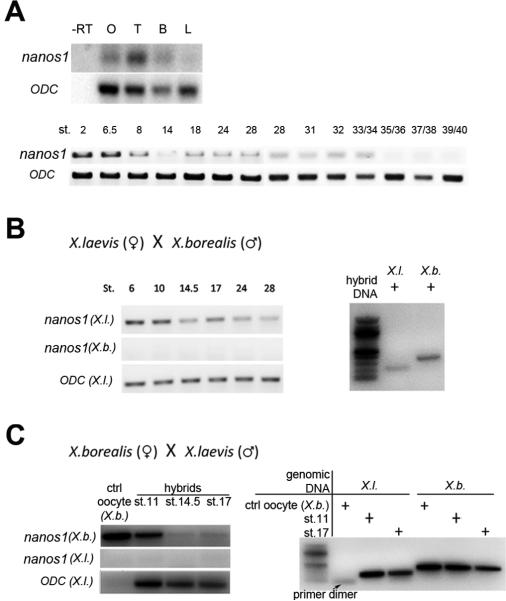
As multiple nanos family members have been described in mouse (Haraguchi et al., 2003; Tsuda et al., 2003), we searched the Xenopus tropicalis genome for possible nanos members in addition to Nanos1. Three candidates were identified and tested for expression in germ cells, embryos or in adult X. tropicalis tissues using primers specific to each candidate. We found no evidence for expression for any of these putative nanos family members. However, we detected Nanos1 in adult testis and brain in addition to the previously described expression sites: oocytes and PGCs (Fig. 6A; Mosquera et al., 1993). Using the more sensitive method of RT-PCR, we now show that maternal nanos1 RNA persists until tailbud stage 33/34 (Fig. 6A).
Figure 6. Expression of laevis nanos1 during development.
(A) nanos1 RNA was detected by RT-PCR in adult ovary (O), testes (T), brain(B), but not in liver (L). In embryogenesis it was detected until stage 33/34 (tailbud). ODC served as a loading control. (B and C) nanos1 expression is strictly maternal. (B) Zygotic transcription of nanos1 RNA was examined using Xenopus laevis (female) and borealis (male) hybrid embryos. RT-PCR analysis using species specific primers for laevis nanos1 (X.l.) or borealis nanos1 (X.b.) at the indicated stages. Note that while laevis Nanos1 was present as expected, borealis nanos1 RNA was not detected in the hybrid embryos at any stage. (C) laevis nanos1 RNA was not detected in Xenopus laevis (male) and borealis (female) hybrid embryos at any stage tested. In each case, hybrid embryos were confirmed to be authentic hybrids by PCR amplification of species specific genomic DNA.
The extended persistence of nanos1 raised the question of whether it was strictly of maternal origin or whether it was zygotically transcribed at stage 14 when PGCs first become transcriptionally active (Venkatarama et al., 2010). In mouse, all three Nanos members are zygotically expressed although Nanos1 is maternally expressed as well (Haraguchi et al., 2003; Tsuda et al., 2003). To address this question we created laevis/ borealis hybrids. In the X. laevis (female) and X. borealis (male) hybrid embryos, borealis nanos1 was not detected by RT-PCR, indicating that zygotic transcription of nanos1 does not occur (Fig. 6B). This result was confirmed by the reverse hybrid experiment, using laevis (male) and borealis (female) hybrids (Brun and Kobel, 1977). Analysis of genomic DNA confirmed authentic hybrids had been generated. The mid-blastula transition occurred normally in these hybrids as demonstrated by testing known zygotic genes including, ornithine decarboxylase (ODC) and Bix4 (data not shown) (Fig. 6C). In either hybrid cross, the paternal nanos1 contribution was not detected, suggesting that nanos1 is not zygotically transcribed. However, we cannot rule out parental imprinting and embryonic transcription of nanos1 from the maternal genome. It is worth noting that no evidence for imprinting has ever been reported in frogs (de la Casa-Esperon and Sapienza, 2003). The data presented above show that nanos1 RNA is strictly of maternal origin in the early embryo and that it likely persists not only for months during oogenesis but three days into embryogenesis. nanos1 RNA is zygotically transcribed later in the brain and testis.
2.6 Expression of Nanos1 protein
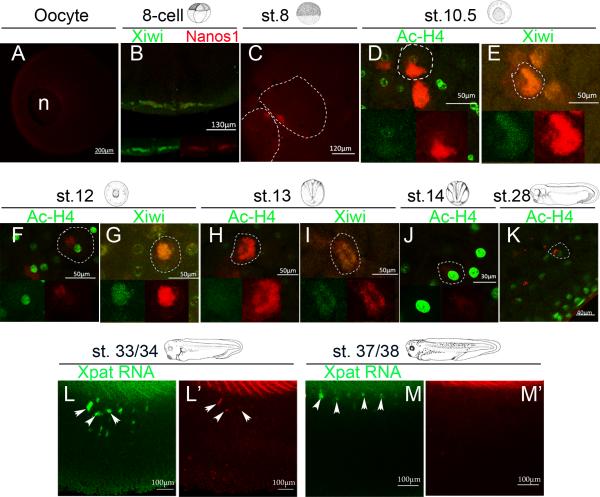
To determine the developmental time period when Nanos1 protein is available to repress RNAs, we generated a polyclonal antibody against Nanos1 recombinant protein. nanos1 RNA is translationally silenced during oogenesis (MacArthur et al., 1999), therefore stage VI oocytes served as a negative control (Fig. 7A). During early cleavage and blastula stages, Nanos1 was found close to the cell periphery in a germ plasm-like pattern (Fig 7C) where it co-localized with Xiwi (Fig. 7 B, E, G, I; Lau et al., 2009; Wilczynska et al., 2009). Xiwi has only been detected in PGC germ plasm where it persists until late neurula stage (Wilczynska et al., 2009). From gastrula stage on, Nanos1 was detected predominantly in a perinuclear location (Fig. 7 D, F, H, J, K). Xpat RNA served as a germ plasm indicator for tailbud stages (from stage 28 to stage 37/38) (Hudson and Woodland, 1998). Nanos1 protein co-localized with Xpat RNA until stage 34 after which Nanos1 was no longer detected (Fig. 7L, L’,M, M’). Therefore, Nanos1 is potentially available to repress translation of germ plasm RNAs while PGCs are in the endoderm.
Figure 7. Nanos1 protein expression during X. laevis development.
Endogenous Nanos1 was detected by confocal immunofluorescence with anti-Nanos1 antibody (red in all images). (A) Nanos1 was not expressed in stage VI oocytes. (C) Nanos1 protein was concentrated in a sub-cellular cytoplasmic domain close to the cell periphery in stage 8 embryos. (B, E, G, I) Nanos1 protein co-localizes with the germ plasm at stages 4 (8 cell), 10.5, 12, and 13. Xiwi, a germ plasm specific protein, was detected by anti-Xiwi antibody (green). (D, F, H, J, K) Hyperacetylated histone H4 (Penta), a nuclear marker, was detected by immunostaining (Green). Nanos1 protein (red) was found only in perinuclear locations in stages 10.5, 12, 13, 14, and 28. (L and M) Stages 33/34 and 37/38. Xpat RNA was detected by whole mount in situ hybridization using fluorescein tyramide (green). L’ and M’, same sample as in L and M. Nanos1 protein could no longer be detected in PGCs after stage 34. C-K, images were from the endoderm mass. L and M, lateral view, with anterior to the left. Scale bars are as indicated.
3. Discussion
The function of nanos in maintaining the germline by suppression of somatic cell fates and cell death appears to be conserved (Hayashi et al., 2004; Tsuda et al., 2003; Wang and Lin, 2004; Koprunner et al., 2001; Sato et al., 2007; Hayashi et al., 2004; Koprunner et al., 2001; Maezawa et al., 2009; Tsuda et al., 2003; Subramaniam et al., 1999). Here we show directly that Xenopus Nanos1 represses the translation of reporter RNAs to which it is bound and that it associates with cyclin B1 RNA in vivo. Repression by Nanos1 does not require a cap or polyadenylation, indicating that Nanos1 can operate down-stream of cap binding events. We identified an N-terminal conserved sequence of nanos in widely divergent species and showed that this region is required for repression of capped but not uncapped reporter RNAs.
Current thinking suggests that Nanos translationally represses specific mRNAs that are recognized by PUM (Sonoda and Wharton, 1999). In Drosophila, Nanos binds preformed PUM/RNA complexes (Sonoda and Wharton, 1999) while in Xenopus, Nanos1 can bind PUM directly without a requirement for RNA (Nakahata et al., 2001). The assay used in this study directly tethered Nanos1 to the RNA, bypassing the known function of PUM in RNA recognition and Nanos recruitment. After being tethered, Nanos1 was competent to form a potent repressive “complex” as reporter RNAs were strongly inhibited. Whether tethered Nanos1 can recruit PUM to this complex is not known.
Deletion analysis revealed the region required for Nanos1 repressive activity is found in sponges as well as humans, but missing in Drosophila and C. elegans. It is striking that the only phenylalanine within this region is absolutely conserved in all species examined. Substitution of this residue with alanine completely abrogated function, while deletion of five residues including the phenylalanine had no effect. Thus the deletion was tolerated but substituting the chemical structure was not. On the other hand, the deletion 17-22 lost repressive activity while four point mutations in this area had no effect. The simplest interpretation is that point mutations do not disturb any protein/protein interactions that may occur at this site while a complete deletion does. The only other substitution that was not tolerated was that of tyrosine in the conserved DYLGL sequence. Tyrosine is very close in chemical structure to phenylalanine. The conserved DYLGL sequence is essential for repressive activity but individual substitutions in this region revealed only tyrosine as being required. Our studies begin to indicate which parts of the conserved N-terminus are important for Nanos1 activity but additional studies will be required to fully understand the interactions involved.
We found that Nanos1 ectopically expressed in oocytes can interact with cyclin B1 RNA. Nakahata and coworkers showed that in fully grown oocytes PUM can directly bind cyclin B1 RNA and CPEB, repressing cyclin B1 through the involvement of the eIF4E-binding protein Maskin (Cao and Richter 2002). CPEB is phosphorylated at maturation, triggering the release of PUM and subsequent translation of cyclin B1, critical for somatic cell cycle progression (Nakahata et al., 2003). The endogenous PUM complex that forms in oocytes to repress cyclin B1 cannot involve Nanos1 however, as Nanos1 is not translated until after fertilization (MacArthur et al., 1999; Fig. 7A,B). Whether the repressive mechanism for cyclin B1 in PGCs is different from that described for oocytes remains to be determined. Based on the observation that Xenopus PGCs divide more infrequently than somatic cells (Dziadek and Dixon, 1977) and that PUM and Nanos1 can bind cyclin B1 RNA, it is very likely that cyclin B1 is negatively regulated in Xenopus PGCs as has been reported for Drosophila (Cao and Richter, 2002; Wang and Lin, 2004). However, we have no direct evidence for Nanos1 and PUM both binding cyclin B1 RNA in the germline.
The efficient repression of capped RNA by Nanos1 raised the possibility that Nanos1 binds cap. Drosophila Nanos is known to associate with cap, but indirectly through Cup binding (Verrotti and Wharton, 2000). We found that Nanos1 added to oocyte extract was not able to specifically bind to cap in a cap affinity binding assay (data not shown). However, Xenopus Nanos1 may also associate indirectly with cap through PUM as PUM was recently shown to have 7-methyl guanosine cap-binding activity (Cao et al., 2010). Future experiments will have to tease out the repressive complex that includes Nanos1 and PUM.
Drosophila nanos is able to repress a reporter driven by the Ubx IRES, although the possible requirement for initiation factors with this IRES is unclear (Wharton et al., 1998). We found that tethered Nanos1 was an effective repressor of IRES-driven translation in oocytes. The CrPV IRES we used does not require initiation factors to recruit ribosomes, effectively bypassing cap binding events (Wilson et al., 2000). Repression in this case interferes with events downstream which could include assembly of the ribosome, elongation and termination events. Interestingly, a different region of the Nanos1 sequence was required for repressing IRES-driven translation, suggesting that Nanos1 can form different repressive complexes. Several factors can function in both mechanisms including eIF4G, 4A, 3, and 2-PABP and may serve as one point of commonality. Lipoxygenase mRNA is another message that has been found to be regulated through both mechanisms, but the nature of the transacting factors remains unknown (Ostareck et al., 1997).
Our data suggest that Nanos1 can mediate repression affecting different steps in the translation process: cap-driven, IRES-driven, polyadenylated RNAs or nonpolyadenylated RNAs. In contrast, tethered Xp54 only represses non-adenylated RNA (Minshall and Standart, 2004). Previous work examining how Nanos/Pum complex represses translation in Drosophila revealed that depending on the RNA, PUM recruited different proteins to the complex. In the case of cyclin B1, Nanos itself recruited the deadenylase NOT (Kadyrova et al., 2007). It appears that the targeted RNA dictates, at least to some extent, the repressive complex that forms. Opperman and coworkers (2005) have provided a model for how this may work. Their model suggests that minor variations in the NRE sequence cause small alterations in how PUM binds, which in turn promotes different PUM-protein associations (Bernstein et al., 2005). Understanding what mechanism of translational repression may be operating would thus depend on the particular variant NRE and the PUM-complex formed.
To date, very few RNA targets of Nanos/PUM have been identified in any organism. Nanos1 protein was detected in germ plasm until PGCs exited the endoderm, suggesting that Nanos1 function may be primarily to repress endoderm fates. Future experiments will be directed at identifying the RNA targets of Nanos1/PUM in PGCs to better understand how translational repression maintains the germline.
4. Experimental Procedures
4.1 Tethered function assay
Xenopus VI oocytes were obtained after collagenase treatment as previously described (MacArthur et al., 1999). 25ng of MS2-Nanos1 RNA (2µg/µL) was injected into each st VI oocyte. After 5hrs, 10nl of the reporter RNAs (Firefly luciferase-MS2 (a generous gift from Dr. Nancy Standart (T7/Bgl II) and Renilla luciferase (pRL-CMV) from Promega (T7/BamH I) were injected into the oocytes at 5ng/µL. Luc-MS2 reporter constructs with a poly(A) tail were synthesized using the poly(A) tailing kit (Ambion) as per the manufacturer's instructions.
The oocytes were collected after an additional 18hr incubation and the luciferase activities were assayed with the Dual-Glo kit from Promega. Three oocytes were collected into one tube, 10µL of water and 10µL of Dual-Glo substrate/buffer were added. After centrifugation at 16,000 g for 2 minutes at room temperature, the supernatant was transferred to a Microliter 1 plate. The firefly luciferase activity was measured with a microplate luminometer. 10µL of Stop-Glo substrate/buffer was added to the microplate and mixed well. After 10 minutes at room temperature, the Renilla luciferase activity was measured.
4.2 RNA IP
2ng of Myc-Nanos1 RNA was injected into stage VI oocytes. After overnight culture, 5 oocytes were homogenized in 1ml YSS buffer (50mM Tris pH8.0, 50mM NaCl, 0.1% NP40, 0.02U/µL superRNAsin, 1X protease inhibitors from Roche, 0.5mM DTT, 100mM sucrose) and centrifuged at 16,000g for 2 minutes to prepare extract. 2ug anti-myc antibody (9E10 from Calbiochem) and 10µL protein G beads were added and incubated at 4°C over night. The beads were then washed four times with 1ml YSS buffer. The RNAs were released from the beads by protease K treatment and analyzed by RT-PCR. The primers used for cyclinB1 were L: ttgaggatgcacaagcagtc and R: aagcggtcaattatgccaac.
4.3 DNA Constructs and primer sequences
MS2 coat protein was PCR amplified from MS2-xP54 (from Nancy Standart) with agcact GGATCC gccacT ATGGCTTCTAACTTTACTCAG and agcact CCATGG t GTAGATGCCGGAGTTTGC. The PCR product was digested with BamHI+NcoI and cloned into pCS2 or pCS2+MT-xARH (Zhou et al., 2004) to generate pCS2+MS2 and pCS2+MS2-xARH respectively.
Nanos1 coding region was PCR amplified with agttcacc ATGGATGGCGGTCTCTGC and atcgac ctcgag TCAGTGTCTCAGCTTTGG. The PCR product was digested with NcoI+XhoI and cloned into pCS2+MS2.
Nanos1 residues 7-34 were deleted using primers Atgatg GGCCatgatGGCC GCAGAGACCGCCATCCAT and Atgatg GGCCatcatGGCC tt TGGGACGTTTTATCTCCG. The PCR product was digested with SfiI and self-ligated (see Quickchange protocol). Nanos1 residues 35-56 were deleted with primers Atgatg GGCCatgatGGCCTCTTGGCCTTTCGCCTTC and Atgatg GGCCatcatGGCC tt TGCGGGTTCTGCAGGAGC. Nanos1 residues 57-111 were deleted with primers Atgatg GGCCatgatGGCC GCCTTTGTGTCCCACAGA and Atgatg GGCCatcatGGCC tt CGGCTCCTCAGAGATCCC.
MS2-Nanos1 point mutations are made with Quickchange kit (Stratagene). The mutations were confirmed by sequencing.
Nanos1 coding region was amplified with primers cagctt gaattc a ATGGATGGCGGTCTCTGC and actagt ctcgag TCAGTGTCTCAGCTTTGG. Nanos1 N terminal region was amplified with cagctt gaattc a ATGGATGGCGGTCTCTGC and actagt ctcgag GCCTTTGTGTCCCACAGA. Nanos1 C terminal region was amplified with cagctt gaattc a TGCGGGTTCTGCAGGAGC and actagt ctcgag TCAGTGTCTCAGCTTTGG. The PCR product was digested with EcoRI+XhoI and cloned into the pCS2+MT vector.
4.4 RT-PCR
Staged embryos were collected and total RNA was extracted using TRIzol® Reagent (Invitrogen #15596-026) and reverse transcribed to cDNA using SuperScript® III First-Strand Synthesis System (Invitrogen #18080-051). PCR was done for 32 cycles and the products were analyzed by electrophoresis through 1% agarose gel. RT-PCR primers: ODC 53°C (Xanthos et al., 2001); Xenopus laevis nanos1: 5’-CTGCAGCCTCAGAGAGAAGG-3’ (forward), 5’-CCACACAAAGGGCAAGTGTA-3’ (reverse), 58°C; Xenopus borealis nanos1: 5’-AGCCCAGTAGGGAAGTTGTG-3’ (forward), 5’-GCAGGTGTAGCCCCTCAGTA-3’ (reverse), 55°C.
4.5 Host transfer
Performed as described by Mir and Heasman (2008).
4.6 X. laevis X. borealis Hybrids
Adult Xenopus borealis frogs were purchased from Xenopus Express. Borealis female eggs have been shown to strongly block cross fertilization due to the thick jelly coat. An egg-transfer technique designed by Brun and Kobel (1977) was used: Xenopus borealis female (donor) was induced to ovulate with 600U hCG; the donor was sacrificed at the appearance of eggs at the cloaca; and the eggs from the body cavity were collected (Brun and Kobel, 1977). Xenopus laevis ovulating female (previously induced by 900U hCG) was immobilized with 900mg/L MS222 and borealis eggs were transferred into her coelom by a small incision in the ventral body wall. After recovery, ovulation continued and the donor eggs would appear about two hours later (distinguished by the smaller size of borealis eggs). Laevis female was gently stripped and borealis eggs were collected and fertilized by laevis sperm in 1X MMR for 10min. Embryos were cultured in 0.1X MMR and collected at specific stages.
4.7 Immunofluorescence microscopy
Staged embryos were fixed in Dent's fixative (80% Methanol, 20% DMSO) at -20°C overnight and then bleached in 0.5X Standard Saline Citrate (SSC) solution with 5% formamide and 1% H2O2 for 1~2 hours under fluorescent light. Whole-mount immunofluorescence was performed as detailed by Venkatarama et al. (2010). Samples were analyzed in Murray's Clear solution (2:1 benzylbenzoate:benzylalcohol), after dehydration with ethanol, using an inverted Zeiss LSM-510 Confocal Laser Scanning Microscope equipped with Argon ion, Helium-Neon and Green-Neon lasers. Primary antibodies used were: affinity purified anti-Nanos1 antibody made in goat against total recombinant expressed Nanos1 protein (Invitrogen), 1:50; rabbit anti-Xiwi1 (a gift from Dr. Nelson Lau), 1:1000. Secondary antibodies were from Invitrogen: AlexaFluor 488 donkey anti- rabbit IgG (H+L), 1:500; AlexaFluor 555 donkey anti-goat IgG (H+L), 1:500.
4.8 Whole-mount fluorescent in situ hybridization
Whole-mount in situ hybridization was performed as detailed by Houston et al. (1998), and the signal was detected by Tyramide Signal Amplification system (TSA, from Perkin Elmer). Staged embryos were fixed in MEMFA at 4°C overnight, bleached, and then stored in methanol at -20°C. After rehydration into PBST, embryos were then permeabilized in 10μg/mL Proteinase K and digestion was terminated by washing in PBST. Embryos were re-fixed in 4% paraformaldehyde (PFA) in PBST, followed by washing in PBST. After incubation in prehybidization buffer for 4h at 60°C, embryos were transferred into hybridization buffer containing 1μg/mL DIG-labeled probe and incubated overnight at 60°C. Embryos were washed and then blocked in Blocking solution (2% blocking reagent (Roche) in MABT) for 1h at room temperature, and then incubated in Blocking solution containing anti-DIG-POD (Roche) (1:1000) overnight at 4°C. Embryos were washed four times in MABT for 1h and two times in PBS for 10min at room temperature, then incubated in 1/50 volume of fluorescein tyramide in 1×Plus Amplification Diluent (Perkin Elmer) for 30 min. The reaction was terminated by washing two times in PBST for 10min. Embryos were re-fixed in 4% PFA/PBST for 20min and stored in PBST at 4°C. Analysis was carried out in Murray's Clear solution using an inverted Zeiss LSM-510 Confocal Laser Scanning Microscope equipped with Argon ion, Helium-Neon and Green-Neon lasers.
Full length Xpat containing construct pBluescript SK-Xpat was linearized by restrictive digestion enzyme HindIII. Digoxigenin (DIG)-labeled Xpat probe was transcribed using T7 RNA polymerase (Ambion # 2082) and purified using mini Quick Spin RNA Columns (Roche, #11814427001).
Supplementary Material
Figure S1. Sequence alignment of nanos family members. Human and mouse Nanos1, Xenopus Nanos1 and zebrafish Nanos are aligned using ClustalX program. The highly conserved residues at the N terminal region are in red. The zinc fingers at the C terminal region are underlined.
Acknowledgements
We thank Nicola Gray for her generosity in providing the luciferase reporters containing the CrPV IRES and for advice during the course of these studies. We also thank Nelson Lau for the generous gift of Xiwi antibody, Hugh Woodland for the Xpat plasmid, and Amar Singh for doing the nanos1 expression analysis in tissues. This work was supported by NIH grant GM33932 to MLK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asaoka-Taguchi M, Sano H, Obara Y, Kobayashi S. Maternal Nanos regulates zygotic gene expression in germline progenitors of Drosophila melanogaster. Mech Dev. 1998;78(1-2):153–158. doi: 10.1016/s0925-4773(98)00164-6. [DOI] [PubMed] [Google Scholar]
- Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nature Cell Biology. 1999;1:431–437. doi: 10.1038/15666. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Hook B, Hajarnavis A, Opperman L, Wickens M. Binding specificity and mRNA targets of a C. elegans PUF protein, FBF-1. RNA. 2005;11:447–458. doi: 10.1261/rna.7255805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R, Kobel HR. Observations on the Fertilization Block between Xenopus borealis and Xenopus laevis laevis. J. Exp. Zool. 1977;201:135–138. [Google Scholar]
- Cao Q, Richter JD. Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 2002;21(14):3852–3862. doi: 10.1093/emboj/cdf353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Padmanabhan K, Richter JD. Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition. RNA. 2010;16:221–227. doi: 10.1261/rna.1884610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong CG, Hall TM. Engineering RNA sequence specificity of Pumilio repeats. Proc Natl Acad Sci U S A. 2006;103(37):13635–13639. doi: 10.1073/pnas.0606294103. Epub 2006 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho PF, Gamberi C, Cho-Park YA, Cho-Park IB, Lasko P, Sonenberg N. Cap-dependent translational inhibition establishes two opposing morphogen gradients in Drosophila embryos. Curr. Biol. 2006;16:2035–2041. doi: 10.1016/j.cub.2006.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnovich D, Lehmann R. Poly(A)-independent regulation of maternal hunchback translation in the Drosophila embryo. Proc.Natl.Acad.Sci. USA. 2001;98(20):11359–11364. doi: 10.1073/pnas.201284398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J, Wickens M. Tethered function assays: an adaptable approach to study RNA regulatory proteins. Methods Enzymol. 2007;429:299–321. doi: 10.1016/S0076-6879(07)29014-7. [DOI] [PubMed] [Google Scholar]
- de la Casa-Esperon E, Sapienza C. Natural selection and the evolution of genome imprinting. Annu Rev Genet. 2003;37:349–370. doi: 10.1146/annurev.genet.37.110801.143741. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Yanowitz J, Schedl P. Novel functions of nanos in downregulating mitosis and transcription during the development of the Drosophila germline. Cell. 1999;99:271–281. doi: 10.1016/s0092-8674(00)81658-x. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Jinks TM, Polydorides AD, Schedl P. Nanos downregulates transcription and modulates CTD phosphorylation in the soma of early Drosophila embryos. Mech. Dev. 2005;122(5):645–657. doi: 10.1016/j.mod.2004.12.009. Epub 2005 Jan 26. [DOI] [PubMed] [Google Scholar]
- Dziadek M, Dixon KE. An autoradiographic analysis of nucleic acid synthesis inthe presumptive primordial germ cells of Xenopus laevis. J. Embryol. Exp. Morphol. 1977;37:13–31. [PubMed] [Google Scholar]
- Gorgoni B, Andrews S, Schaller A, Schümperli D, Gray NK, Müller B. The stem-loop binding protein stimulates histone translation at an early step in the initiation pathway. RNA. 2005;11(7):1030–1042. doi: 10.1261/rna.7281305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NK, Coller JM, Dickson KS, Wickens M. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 2000;19(17):4723–4733. doi: 10.1093/emboj/19.17.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi S, Tsuda M, Kitajima S, Sasaoka Y, Nomura-Kitabayashid A, Kurokawa K, Saga Y. nanos1: a mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mech. Dev. 2003;120(6):721–731. doi: 10.1016/s0925-4773(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Hayashi M, Kobayashi S. Nanos suppresses somatic cell fate in Drosophila germ line. Proc. Natl. Acad. Sci. USA. 2004;101:10338–10342. doi: 10.1073/pnas.0401647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DW, King ML. Germ plasm and molecular determinants of germ cell fate. Curr. Top. Dev. Biol. 2000;50:155–81. doi: 10.1016/s0070-2153(00)50008-8. [DOI] [PubMed] [Google Scholar]
- Houston DW, Zhang J, Maines JZ, Wasserman SA, King ML. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development. 1998;125(2):171–180. doi: 10.1242/dev.125.2.171. [DOI] [PubMed] [Google Scholar]
- Hudson C, Woodland R. Xpat, a gene expressed specifically in germ plasm and primordial germ cells of Xenopus laevis. Mech. Dev. 1998;73:159–168. doi: 10.1016/s0925-4773(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Jaruzelska J, Kotecki M, Kusz K, Spik A, Firpo M, Reijo Pera RA. Conservation of a Pumilio-Nanos complex from Drosophila germ plasm to human germ cells. Dev. Genes Evol. 2003;213:120–126. doi: 10.1007/s00427-003-0303-2. [DOI] [PubMed] [Google Scholar]
- Kadyrova LY, Habara Y, Lee TH, Wharton RP. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development. 2007;134(8):1519–1527. doi: 10.1242/dev.002212. [DOI] [PubMed] [Google Scholar]
- Kang D, Pilon M, Weisblat DA. Maternal and zygotic expression of a nanos-class gene in the leech Helobdella robusta: primordial germ cells arise from segmental mesoderm. Dev. Biol. 2002;245(1):28–41. doi: 10.1006/dbio.2002.0615. [DOI] [PubMed] [Google Scholar]
- King ML, Messitt TJ, Mowry KL. Putting RNAs in the right place at the right time: RNA localization in the frog oocyte. Biol. Cell. 2005;97(1):19–33. doi: 10.1042/BC20040067. [DOI] [PubMed] [Google Scholar]
- Koprunner M, Thisse C, Thisse B, Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 2001;15:2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M. NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr. Biol. 1999;9(18):1009–1018. doi: 10.1016/s0960-9822(99)80449-7. [DOI] [PubMed] [Google Scholar]
- Kusz KM, Tomczyk L, Sajek M, Spik A, Latos-Bielenska A, Jedrzejczak P, Pawelczyk L, Jaruzelska J. The highly conserved NANOS2 protein: testis-specific expression and significance for the human male reproduction. Mol. Hum. Reprod. 2009;15(3):165–171. doi: 10.1093/molehr/gap003. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Aoki Y, Nakamura S, Ebe Y, Kobayashi D, Tanaka M. Time-lapse analysis reveals different modes of primordial germ cell migration in the medaka Oryzias latipes. Develop. Growth Differ. 2006;48:209–221. doi: 10.1111/j.1440-169X.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- Lau NC, Ohsumi T, Borowsky M, Kingston RE, Blower MD. Systematic and single cell analysis of Xenopus Piwi-interacting RNAs and Xiwi. EMBO J. 2009;28(19):2945–2958. doi: 10.1038/emboj.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Jongens TA. Transcriptional silencing and translational control: key features of early germline development. Bioessays. 2003;25(4):326–335. doi: 10.1002/bies.10247. [DOI] [PubMed] [Google Scholar]
- Luo X, Nerlick S, An W, King ML. Xenopus germline nanos1 is translationally repressed by a novel structure based mechanism. Development. 2011;138:589–598. doi: 10.1242/dev.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur H, Bubunenko M, Houston DW, King ML. Xcat2 RNA is a translationally sequestered germ plasm component in Xenopus. Mech. Dev. 1999;84:75–88. doi: 10.1016/s0925-4773(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Maezawa T, Arita K, Shigenobu S, Kobayashi S. Expression of the apoptosis inducer gene head involution defective in primordial germ cells of the Drosophila embryo requires eiger, p53, and loki function. Dev. Growth Differ. 2009;51(4):453–461. doi: 10.1111/j.1440-169X.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Minshall N, Standart N. The active form of Xp54 RNA helicase in translational repression is an RNA-mediated oligomer. Nucleic Acids Res. 2004;32(4):1325–1334. doi: 10.1093/nar/gkh303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir A, Heasman J. How the mother can help: studying maternal Wnt signaling by anti-sense-mediated depletion of maternal mRNAs and the host transfer technique. Methods Mol. Biol. 2008;469:417–429. doi: 10.1007/978-1-60327-469-2_26. [DOI] [PubMed] [Google Scholar]
- Mochizuki K, Sano H, Kobayashi S, Nishimiya-Fujisawa C, Fujisawa T. Expression and evolutionary conservation of nanos-related genes in Hydra. Dev. Genes Evol. 2000;210:591–602. doi: 10.1007/s004270000105. [DOI] [PubMed] [Google Scholar]
- Mosquera L, Forristall C, Zhou Y, King ML. A mRNA localized to the vegetal cortex of Xenopus oocytes encodes a protein with a nanos-like zinc finger domain. Development. 1993;117:377–386. doi: 10.1242/dev.117.1.377. [DOI] [PubMed] [Google Scholar]
- Murata Y, Wharton RP. Binding of Pumilio to Maternal hunchback mRNA Is Required for Posterior Patterning in Drosophila Embryos. Cell. 1995;80:747–756. doi: 10.1016/0092-8674(95)90353-4. [DOI] [PubMed] [Google Scholar]
- Nakahata S, Katsu Y, Mita K, Inoue K, Nagahama Y, Yamashita M. Biochemical identification of Xenopus pumilio as a sequencespecific cyclin B1 mRNA-binding protein that physically interacts with a nanos homolog, Xcat-2, and a cytoplasmic polyadenylation elementbinding protein. J. Biol. Chem. 2001a;276:20945–20953. doi: 10.1074/jbc.M010528200. [DOI] [PubMed] [Google Scholar]
- Nakahata S, Kotani T, Mita K, Kawasaki T, Katsu Y, Nagahama Y, Yamashita M. Involvement of Xenopus Pumilio in the translational regulation that is specific to cyclin B1 mRNA during oocyte maturation. Mech. Dev. 2003;120(8):865–880. doi: 10.1016/s0925-4773(03)00160-6. [DOI] [PubMed] [Google Scholar]
- Opperman L, Hook B, DeFino M, Bernstein D, Wickens M. A single spacer nucleotide determines the specificities of two mRNA regulatory proteins. Nat. Struct. Mol. Biol. 2005;12:945–951. doi: 10.1038/nsmb1010. [DOI] [PubMed] [Google Scholar]
- Ostareck DH, Ostareck-Lederer A, Wilm M, Thiele BJ, Mann M, Hentze MW. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3' end. Cell. 1997;89(4):597–606. doi: 10.1016/s0092-8674(00)80241-x. [DOI] [PubMed] [Google Scholar]
- Parisi M, Lin H. Translational repression: a duet of Nanos and Pumilio. Curr. Biol. 2000;10(2):R81–3. doi: 10.1016/s0960-9822(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Sato K, Hayashi Y, Ninomiya Y, Shigenobu S, Arita K, Mukai M, Kobayashi S. Maternal Nanos represses hid/skl-dependent apoptosis to maintain the germ line in Drosophila embryos. Proc.Natl.Acad.Sci. USA. 2007;104:7455–7460. doi: 10.1073/pnas.0610052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaner CE, Deshpande G, Schedl PD, Kelly WG. A conserved chromatin architecture marks and maintains the restricted germ cell lineage in worms and flies. Dev. Cell. 2003;5(5):747–757. doi: 10.1016/s1534-5807(03)00327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev. 1999;13(20):2704–2712. doi: 10.1101/gad.13.20.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Wharton RP. Drosophila Brain Tumor is a translational repressor. Genes Dev. 2001;15(6):762–773. doi: 10.1101/gad.870801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development. 1999;126(21):4861–4871. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc. Natl. Acad. Sci. USA. 2010;107(8):3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science. 2003;301(5637):1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- Venkatarama T, Lai F, Luo X, Zhou Y, Newman K, King ML. Repression of zygotic gene expression in the Xenopus germline. Development. 2010;137(4):651–660. doi: 10.1242/dev.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti AC, Wharton RP. Nanos interacts with cup in the female germline of Drosophila. Development. 2000;127(23):5225–32. doi: 10.1242/dev.127.23.5225. [DOI] [PubMed] [Google Scholar]
- Wang C, Lehmann R. nanos is the localized posterior determinant in Drosophila. Cell. 1991;66:637–647. doi: 10.1016/0092-8674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- Wharton RP, Struhl G. RNA Regulatory Elements Mediate Control of Drosophila Body Pattern by the Posterior Morphogen nanos. Cell. 1991;67:955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- Wharton RP, Sonoda J, Lee T, Patterson M, Murata Y. The Pumilio RNA-Binding Domain Is Also a Translational Regulator. Molecular Cell. 1998:863–872. doi: 10.1016/s1097-2765(00)80085-4. [DOI] [PubMed] [Google Scholar]
- Wilczynska A, Minshall N, Armisen J, Miska EA, Standart N. Two Piwi proteins, Xiwi and Xili, are expressed in the Xenopus female germline. RNA. 2009;15(2):337–345. doi: 10.1261/rna.1422509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE, Powell MJ, Hoover SE, Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol. Cell Biol. 2000;20(14):4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreden C, Verrotti AC, Schisa JA, Lieberfarb ME, Strickland S. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development. 1997;124(15):3015–3023. doi: 10.1242/dev.124.15.3015. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Wylie C, Heasman J. Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development. 2001;128:167–180. doi: 10.1242/dev.128.2.167. [DOI] [PubMed] [Google Scholar]
- Zhou Y, King ML. Localization of Xcat2 RNA, a putative germ plasm component, to the mitochondrial cloud in Xenopus stage 1 oocytes. Development. 1996;122:2947–2953. doi: 10.1242/dev.122.9.2947. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang J, King ML. Xenopus autosomal recessive hypercholesterolemia protein couples lipoprotein receptors with the AP-2 complex in oocytes and embryos and is required for vitellogenesis. J. Biol. Chem. 2003;278(45):44584–44592. doi: 10.1074/jbc.M308870200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang J, King ML. Polarized distribution of mRNAs encoding a putative LDL receptor adaptor protein, xARH (autosomal recessive hypercholesterolemia) in Xenopus oocytes. Mech. Dev. 2004;121(10):1249–1258. doi: 10.1016/j.mod.2004.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sequence alignment of nanos family members. Human and mouse Nanos1, Xenopus Nanos1 and zebrafish Nanos are aligned using ClustalX program. The highly conserved residues at the N terminal region are in red. The zinc fingers at the C terminal region are underlined.