Abstract
Background
Whether autonomic nerve activity is important in the development of pacing-induced sustained atrial fibrillation (AF) is unclear.
Objective
We tested the hypothesis that patterns of baseline autonomic nerve activities are important in the development of pacing-induced sustained AF.
Methods
We implanted radiotransmitters in 12 ambulatory dogs to record left stellate ganglion nerve activity (SGNA) and vagal nerve activity (VNA). Sustained (>48 hrs) AF was induced with intermittent rapid atrial pacing.
Results
At baseline (before pacing), the one-min integrated nerve activity between SGNA and VNA demonstrated either a single linear relationship with excellent correlation (Group 1, N=3, r=0.816±0.105) or non-linear relationships with poor correlation (Group 2, N=9, r=0.316±0.162, P<0.05 compared with Group 1). Group 1 dogs had higher VNA (97.0±11.5 mV-s) compared to Group 2 (33.4±21.7 mV-s, P<0.001). Group 1 dogs had more frequent sympathovagal coactivation episodes than Group 2 (50±19/d vs. 15±6/d, P<0.05) and more paroxysmal atrial tachycardia (PAT; 5±1/d vs. 2±1/d, P<0.05) at baseline. Sustained AF occurred after 16±4 d (range 13–20 d) of pacing in Group 1 and after 46±18 d (range 23–72 d) of pacing in Group 2 (P<0.05). In the week before the development of sustained AF, the VNA of Group 2 dogs had significantly (P<0.05) increased compared to baseline.
Conclusions
Ambulatory dogs with good linear sympathovagal correlation and higher vagal tone at baseline have more PAT episodes at baseline and faster induction of sustained AF by rapid pacing. Rapid atrial pacing increased the VNA of the remaining dogs before the induction of sustained AF.
Keywords: Autonomic nervous system, Nerve recording, Atrial fibrillation, Arrhythmia, Atrial pacing
Introduction
Atrial fibrillation (AF) can be either paroxysmal or sustained. In patients with paroxysmal AF at initial clinical evaluation, progression to more sustained forms is slow and/or unusual. It is estimated to be approximately 10% at 1 year and 25% to 30% at 5 years despite pharmacologic therapy.1 In patients with lone AF, only roughly half of the patients progressed to sustained AF after 25 years of follow up.2 Even if the progression occurs, the transition rate varies considerably among individuals.2 Reasons for such disparity in the susceptibility to AF progression remain poorly understood. It is known that intermittent rapid atrial pacing in large animals can lead to paroxysmal AF.3, 4 If the rapid pacing continues, sustained AF will develop.5 However, even with the same pacing protocol, sustained AF develops with variable durations of pacing among different animals.3, 5–7 The cause of such disparity of susceptibility to pacing-induced sustained AF is unclear. Wijffels et al5 proposed that AF begets AF through progressive pacing-induced electrical remodeling, such as shortening of the effective refractory periods. However, the authors also noted that the time course of changes in atrial refractoriness does not completely run parallel with the time course of development of sustained AF. These findings suggested to the authors that besides the shortening of refractoriness, other factors may play a role in the development of chronic fibrillation. A possible factor is the autonomic nerve activity because radiofrequency catheter ablation of vagal innervation could abolish pacing-induced sustained AF.8 However, no studies have compared the patterns of baseline nerve activity with the duration of rapid pacing needed to induce sustained AF. To determine if autonomic nerve activity is important in the development sustained AF, we developed methods to perform long-term recording of nerve activities in ambulatory dogs, and used rapid atrial protocol to determine the duration of pacing needed to induce sustained (> 48 hrs) of AF. The results were used to test the hypotheses that 1) differential patterns of interactions among cardiac autonomic structures naturally exist at baseline (before pacing) and that 2) certain distinct patterns of autonomic interactions are associated with faster development of sustained AF.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the Cedars-Sinai Medical Center,3 Los Angeles, California and Indiana University School of Medicine,4 and conforms to the guidelines of the American Heart Association.
Chronic Ambulatory Autonomic Nerve Recordings and Pacing-Induced AF
Twelve mongrel dogs of either sex (weighing 22 to 27kg, more than 2 years old) were studied over the past 5 years to determine the relationship between nerve activity and AF. The relationship between spontaneous paroxysmal AF and nerve activities in these dogs have been reported previously in two different manuscripts including 4 dogs in a report by Tan et al3 and 6 dogs in a report by Choi et al.4 (The study by Tan et al3 included a total of 7 dogs without cryoablation. Three dogs studied > 5 years ago were excluded because the data format used at that time was incompatible with the custom written analyses software used in the present study). We also include 2 dogs not used in these previous reports. All 12 dogs underwent the same surgical procedures that included a left thoracotomy through the 4th intercostal space under isoflurane general anesthesia. Data Sciences International (DSI) D70-EEE radiotransmitters were used in all 12 dogs to record left stellate ganglion nerve activity (SGNA) and vagal nerve activity (VNA). We also successfully recorded intrinsic cardiac nerve activity (ICNA) from the superior left ganglionated plexi (SLGP) in 8 of the 12 dogs. A pacing lead was implanted onto the left atrial appendage and connected to a Medtronic Itrel neurostimulator (N=4)3 or a modified Medtronic Kappa pacemaker (N=8; Medtronic Inc, Minneapolis, Minnesota) for intermittent high rate atrial pacing.4 All 12 dogs underwent the same pacing protocol that included alternating 6-d pacing with 1-d monitoring until sustained (>48 hrs) AF was documented. The dogs were then euthanized.
Data Analyses
We studied data of each dog to determine actual days of pacing needed to induce sustained AF (the net pacing days) and excluded the non-paced days in this analysis. We manually analyzed recordings from all channels. Nerve activities were considered present if there was a 3-fold increase in the amplitude over baseline noise. Co-activation was considered when the activation of two or more nerve structures overlaps for at least 5 sec. In addition, we also determined the occurrence of paroxysmal atrial tachycardia (PAT) at baseline. PAT was diagnosed when there was an abrupt (>50 bpm/s) increase in the atrial rate to >200 bpm that persisted for at least 10 seconds.9 Quantitative analyses were also preformed with the aid of custom-designed software to automatically import, filter and analyze the recordings. To optimize nerve signals, data from SGNA and VNA were high-passed filtered at 100 Hz.3, 10 To reduce the size of atrial electrograms from SLGPNA, wavelet transformation and spike-triggered (ECG-triggered) averaging of nerve activity were performed.4 An example of this method of ECG removal is provided in Online Supplement Figure 3. The filtered or transformed signals were then rectified, integrated with a 100-ms time constant, and summed to represent integrated nerve activity of 1-min segments over 24 hrs of baseline recordings.
Statistical Analysis
Data were presented as mean ± SD. Pearson’s correlation coefficients were used to assess correlations between integrated nerve activities. The correlation coefficients between integrated SGNA and integrated VNA were used to define groups. Group 1 dogs were defined as having a correlation coefficient of ≥0.7. The remaining dogs formed Group 2. The comparison of correlation coefficients among two groups was performed by the Mann-Whitney U test. Mann-Whitney U test was used to compare the differences of net pacing durations, baseline heart rates, the number of sympathovagal coactivations and PAT episodes between Groups 1 and 2. The slopes of the scatter plots were calculated by linear regression and compared with Mann-Whitney U test. Repeated measures analysis of variance (ANOVA) with Bonferroni post hoc test was performed to examine the circadian variation of the SGNA-VNA correlation. P<0.05 was considered statistically significant.
Results
Distinct Patterns of Nerve Activity at Baseline
We found some dogs had better SGNA-VNA correlations than others. A single linear correlation was observed in 3 dogs with excellent correlation (Group 1, r=0.816±0.105, P<0.001 for each dog; Figures 1A and 2A). The remaining dogs (Group 2) had poor SGNA-VNA correlations (N=9, r=0.316±0.162, P<0.001 for each dog; P<0.05 compared to Group 1; Figures 1B and 2B). Each dot in these figures represents an SGNA-VNA pair of 1-min integrated nerve activity. The entire plot has 1440 data points to cover a 24-hr period. As indicated by Figures 1A and 1B, the slope of Group 1’s scatter plot is similar to the slope of the lower arm of Group 2’s scatter plot. Overall, the slope of Group 1’s scatter plot (1.9±2.2) is not significantly different from that of the lower arm of Group 2’s scatter plot (1.5±0.8, P=NS). At baseline, the integrated VNA of Group 1 dogs (97.0±11.5 mV-s) was significantly higher than that of Group 2 (33.4±21.7 mV-s, P<0.001). The SGNA of Group 1 (90.7±17.7 mV-s) was lower than that of Group 2 (155.8±78.7 mV-s), but the difference was not significantly different (P=NS). Besides, Group 1 dogs had a significantly poorer VNA-SLGPNA correlation (r=0.452±0.11, P<0.001 for each dog) than Group 2 dogs (r=0.838±0.148, P<0.001 for each dog; P<0.05 compared to Group 1). Typical examples are shown in Figures 1C and 1D, respectively. Figure 1C shows that many dots deviated from the trend line (r=0.53, P<0.001) in a Group 1 dog, whereas Figure 1D shows an excellent linear relation (r=0.98, P<0.001) in a Group 2 dog. Poor but statistically significant correlations between SGNA-SLGPNA were observed in both groups of dogs (r=0.388±0.385, P<0.001 for Group 1 dog; r=0.465±0.261, P<0.001 for Group 2 dog; P=0.74 comparing Group 1 to Group 2). Overall, SLGPNA demonstrated a strong propensity to activate along with VNA rather than SGNA (P=0.03), particularly so in Group 2 dogs (P=0.01). However, in 13% of 210 randomly selected SLGPNA activations from Group 2 dogs, SLGPNA co-activated with SGNA but not VNA. The latter finding indicates that SLGPNA is not completely slaved to VNA in either group of dogs. Figure 1E shows typical nerve activation patterns from a Group 1 dog. Note that simultaneous SGNA and VNA (sympathovagal) co-activation (solid arrows) led to heart rate acceleration. Sporadic SLGPNA (arrowhead) occurred independently from VNA. Figure 1F shows typical nerve activity from a Group 2 dog. Note that SLGPNA co-activated with VNA (solid arrows) immediately after SGNA withdrawal (point a). There was heart rate deceleration (dashed arrow), consistent with vagal effects. Withdrawal of VNA and SLGPNA (point b) was followed by a short burst of sympathovagal co-activation and reactivation of the SGNA, leading to acceleration of heart rate.
Figure 1.
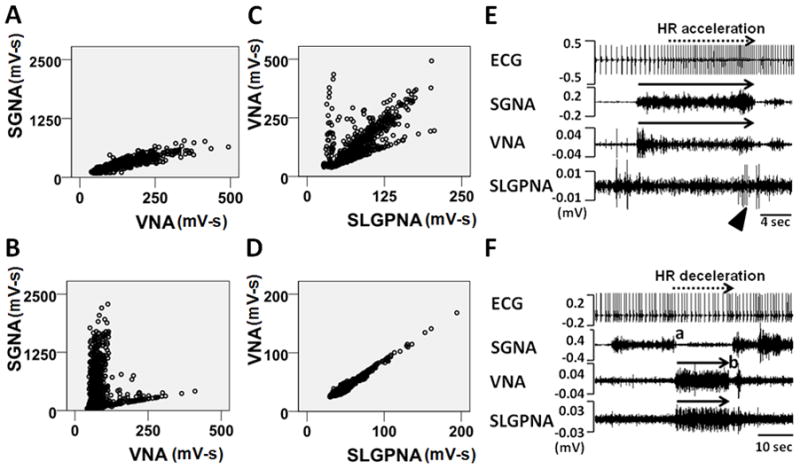
Patterns of autonomic interactions. A, Representative SGNA-VNA scatter plot of a Group 1 dog. Each dot represents an SGNA-VNA pair of nerve activity integrated over 1-min. The entire plot has 1440 data points to cover a 24-hr period. B, Representative SGNA-VNA scatter plot from a Group 2 dog. C, Representative VNA-SLGPNA scatter plot from a Group 1 dog. D, Representative VNA-SLGPNA scatter plot from a Group 2 dog. E, An example of simultaneous sympathovagal co-activation (black arrows) observed in a Group 1 dog that led to heart rate acceleration. Arrowhead shows independent SLGPNA. F, An example of recording from a Group 2 dog showing that simultaneously increased VNA and SLGPNA (black arrows) resulted in heart rate deceleration. ECG, electrocardiogram; SGNA, stellate ganglion nerve activity; VNA, vagal nerve activity; SLGPNA, superior left ganglionated plexi nerve activity.
Figure 2.
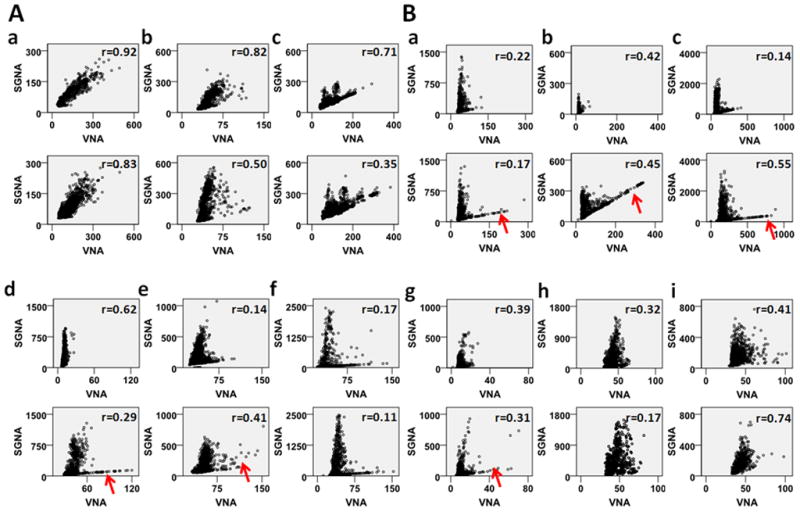
Sympathovagal correlations of all dogs studied. Panels A and B show dogs with excellent correlations (Group 1) and poor correlations (Group 2), respectively. The dogs of Group 2 show “L-shaped” correlation. However, because this designation is subjective, it is not used to determine the grouping of the dogs. The upper panels show the baseline correlation, while the lower panels show correlation in the week before the development of sustained AF. We used the same scales of the ordinate and abscissa within each dog to facilitate the comparison. The red arrows point to apparent increase of lower (vagal) arm of the correlation in six Group 2 dogs prior to the onset of sustained AF. All units are mV-s.
Patterns of Nerve Activity and Arrhythmia at Baseline
There is no significant difference of 24-hr average heart rate between two groups of dogs (Figure 3A). As suggested by Figure 1A and 1B, Group 1 dogs had more frequent sympathovagal coactivations than Group 2 dogs (Figure 3B). More episodes of PAT (Figure 3C) were observed in Group 1 dogs than in Group 2 dogs at baseline.
Figure 3.
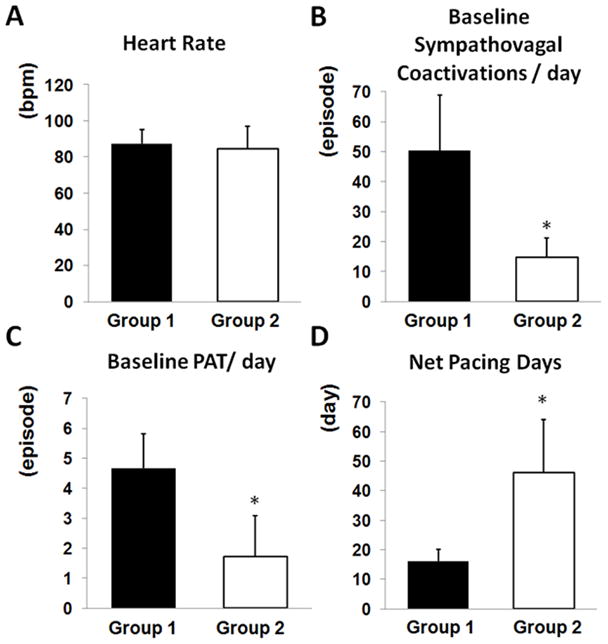
Comparing electrophysiologic characteristics between two groups of dogs. A, Baseline heart rate did not differ significantly between two groups. B, Group 1 dogs had more episodes of prolonged (≥5 sec) sympathovagal coactivations per day at baseline. C, Group 1 dogs had more episodes of PAT per day at baseline. D, Group 1 dogs required much fewer days of pacing than Group 2 dogs before developing sustained AF. *P<0.05.
The Two-Arm Sympathovagal Correlation
The baseline sympathovagal correlation in Group 2 resembles an “L” in most of the dogs (Figure 2). Typically, the upper arm (the red arm in Figure 4A) corresponded to a higher average heart rate (102±11 bpm) whereas the lower arm (the black arm in Figure 4A) corresponded to a significantly lower average heart rate (78±10 bpm, P<0.0001), suggesting that the upper and lower arms are sympathetic and parasympathetic dominant, respectively. Figure 4B is a sympathovagal plot from another dog from Group 2. As the color shows, most dots from the upper arm had an average heart rate over 100 bpm, suggesting that the upper arm is dominated by sympathetic activity. However, when lower arm is separately analyzed, it also shows that slight increase of SGNA is associated with increased heart rate (orange color). Figures 4C and 4D are recordings during the period representing a dot in the upper arm (red arrowhead) and a dot in the lower arm (black arrowhead), respectively, in Figure 4A. Abundant SGNA with scanty VNA led to heart rate acceleration (from 67 bpm to 122 bpm, Figure 4C). On the contrary, ample VNA with minimally increased SGNA gradually slowed the heart rate from 102 bpm to 71 bpm (Figure 4D). The shape of correlation in Group 2 dogs undergoes circadian changes (Figure 5A). The lower arm of the “L” is missing from 8 am to 4 pm, consistent with sympathetic dominance. The correlation coefficient (Figure 5B) increased from the midnight to reach its peak at 8 am–12 pm and then progressively decreased. There was no circadian variation in Group 1 dogs.
Figure 4.
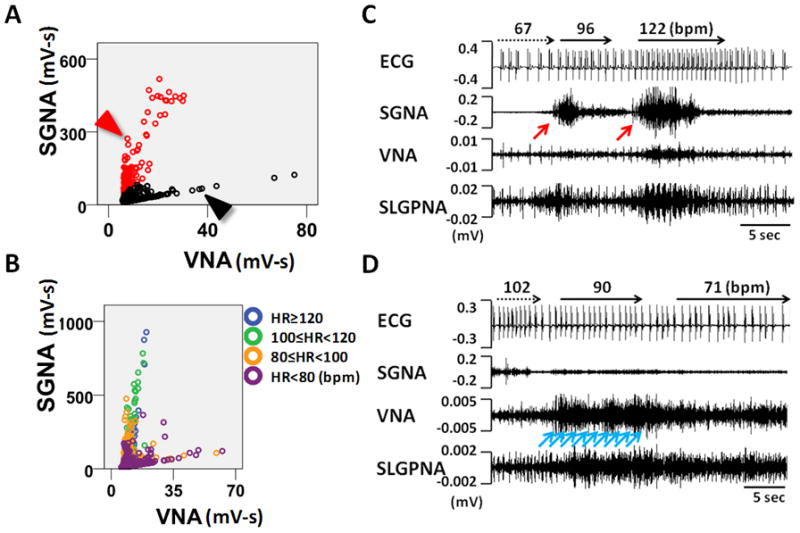
The physiology of L-shaped sympathovagal correlation. A, An example of a L-shaped sympathovagal plot. The upper and lower arms are coded with red and black circles, respectively. B, L-shaped sympathovagal plot from another dog. Each dot is labeled with different colors according to the average heart rate within the 1-min interval represented by each dot. The upper arm shows progressively increasing SGNA is associated with a progressively higher heart rate. The lower arm shows that increasing VNA is associated with increasing SGNA and heart rate. However, because all dots in the lower arm are associated with a heart rate of < 100 bpm, VNA has exerted a dominant effect on heart rate in these time points. C, Actual recordings at the red arrowhead in Panel A. Vigorous SGNA (pointed by a red arrow) caused heart rate acceleration. D, Actual recordings at the black arrowhead in Panel A. Gradually increasing VNA (pointed by blue arrows) led to progressive heart rate deceleration.
Figure 5.
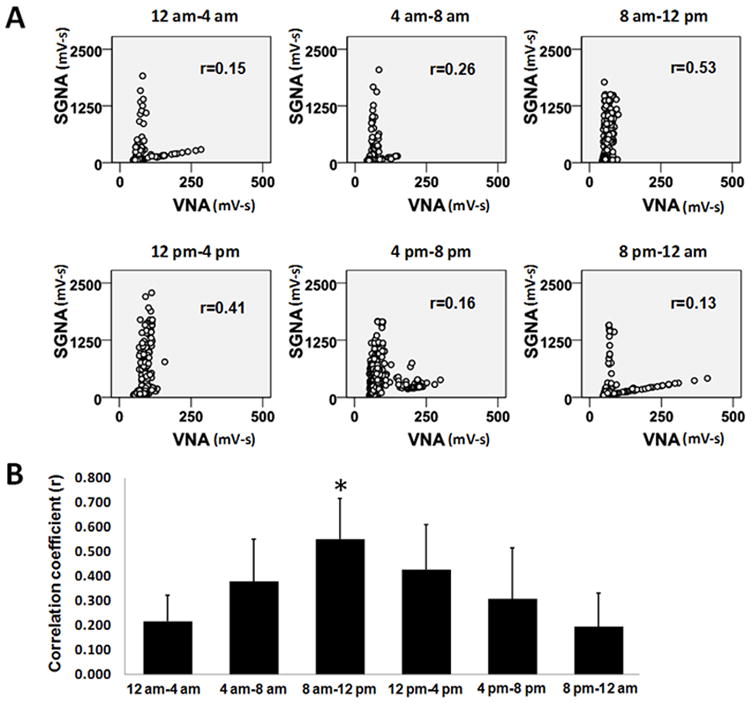
Circadian variations of the sympathovagal relationships. A shows the sympathovagal plots from one representative dog according to the time periods within 24 hrs. The Pearson’s correlation coefficients (r) are also shown on the corresponding plots. B, The circadian variations of the correlation coefficients of all dogs studied. *P<0.001 compared to 12 am–4 am.
Patterns of Nerve Activity After Rapid Atrial Pacing
We analyzed the 24-hr recording in the week before the development of sustained AF and the sympathovagal correlation plots of all dogs are shown in the lower panels of Figure 2. The SGNA-VNA correlation coefficient in this period was 0.560±0.246 (P<0.001 for each dog) for Group 1, still significantly (P<0.05) higher than that of Group 2 (0.356±0.204, P<0.001 for each dog), suggesting that no dogs switched groups after pacing. However, after atrial pacing, there was an obvious increase of the lower (vagal) arms in 6 of the 9 dogs, as indicated by red arrows in Figure 2B. The VNA in Group 2 dogs increased from 33.4±21.7 mV-s at baseline to 72.0±46.8 mV-s before onset of sustained AF (P<0.01). Meanwhile, the slopes of the upper arm of Group 2 animals significantly decreased from 42.2±19.8 to 23.9±8.8 (P<0.05, Online Supplement Table 1). There were no significant changes in the slopes of either Group 1 or the lower arm of Group 2 dogs.
Patterns of Nerve Activity and the Development of Sustained AF
Sustained AF developed significantly earlier in Group 1 dogs (16±4 d, range 13–20 d) than in Group 2 dogs (46±18 d, range 23–72 d, P<0.05) (Figure 3D). In Figure 6, Group 1 dogs are identified by either filled or unfilled circles, while Group 2 dogs are identified as filled or unfilled triangles depending on the types of pacemaker used. Figures 6A and 6B show that better SGNA-VNA correlation and poorer SLGPNA-VNA correlation at baseline are associated with shorter periods of atrial pacing needed to induce sustained AF (r=−0.458, P=0.134 in Figure 6A and r=0.823, P<0.05 in Figure 6B; dashed regression line is shown in the scatter plots).
Figure 6.
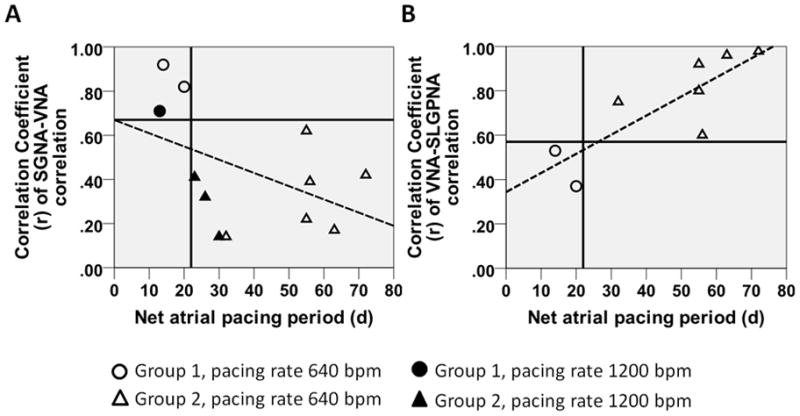
The relationship between autonomic interactions at baseline and the susceptibility to pacing induced sustained AF. A, Better SGNA-VNA correlation is associated with shorter periods of atrial tachypacing needed for induction of sustained AF. The Group 1 dogs (circles) are exclusively in the left upper quadrant of the scatter plot whereas the Group 2 dogs (triangles) are entirely in the right lower one. Dogs paced at 1200 bpm are labeled with filled circles/triangles while those paced at 640 bpm are labeled with unfilled circles/triangles. B, Poorer VNA-SLGPNA correlation is associated with shorter periods of atrial rapid pacing.
Discussion
Major findings include 1) Differential patterns of interactions among cardiac autonomic structures naturally exist at baseline. 2) Ambulatory dogs with good linear sympathovagal correlation and higher vagal tone at baseline have more PAT episodes at baseline and more rapid induction of sustained AF by rapid pacing. Rapid atrial pacing increases the VNA of the remaining dogs prior to the induction of sustained AF.
Sympathovagal Coactivations at Baseline and the Development of Sustained AF
Simultaneous sympathovagal discharges are important triggers of spontaneous atrial tachyarrhythmias after intermittent rapid atrial pacing.3 Heart rate variability analyses showed that sympathovagal activation precedes the onset of paroxysmal AF episodes in humans.11 However, sympathovagal coactivations at baseline have not been implicated in the subsequent development of sustained AF. A major finding of the present study is that an excellent sympathovagal correlation at baseline is a risk factor for pacing-induced sustained AF. Sympathetic activation increases cardiac calcium entry and spontaneous sarcoplasmic reticulum calcium release.12 Vagal activation may reduce atrial effective refractory period,5 augment spatial electrophysiological heterogeneity13 and promote late phase 3 early afterdepolarizations14 when there is intracellular calcium overload. Therefore, more frequent sympathovagal coactivations at baseline may work synergistically with rapid atrial pacing to facilitate the development of sustained AF.
VNA and the Development of Sustained AF
In addition to sympathovagal coactivation, we also observed that Group 1 dogs apparently had VNA dominance, and noted the increased vagal tone prior to the development of sustained AF. Increased vagal tone has been associated with the early recurrence of AF after electrical cardioversion.15 In the present study, the slope of the Group 1 scatter plot matches with that of the slope of the lower arm of Group 2, suggesting that Group 1 has parasympathetic dominance. After atrial pacing, the VNA rose more drastically compared to SGNA before the induction of sustained AF, as shown in the Online Supplement Figure 3 of the paper by Choi et al.4 In the meantime, the slope of the upper arm of Group 2 animals significantly decreased, indicating the sympathovagal balance had been shifted toward vagal dominance. These findings suggest that increased vagal dominance may facilitate the development of pacing-induced sustained AF. Group 1 dogs had a natural vagal predominance at baseline, hence the greater susceptibility to pacing-induced sustained AF.
VNA-ICNA Relationship in Ambulatory Dogs
The SLGP, located within an epicardial fat pad at the junction of left superior pulmonary vein and the left atrium,16, 17 is believed to modulate the effects of the VNA on cardiac physiology and inducibility of AF18 and is close to where most ectopic trigger beats arise in paroxysmal AF.19 High-frequency stimulation20 or radiofrequency application21 to the neural elements around pulmonary veins, inclusive of the SLGP, can evoke vagal reflexes. A potentially important finding of the present study is that SLGPNA is highly correlated with VNA in a majority of dogs studied. Together with the finding in anesthetized dogs that SLGP ablation significantly attenuated the bradycardiac responses induced by right or left vagosympathetic trunk stimulation,18 our data suggest that SLGP is responsible for relaying left VNA to the heart. However, this relationship is not mandatory in all dogs studied because occasionally SLGPNA may co-activate with SGNA but not VNA. Another important discovery in this study is that unusually poor correlation between VNA and SLGPNA is associated with high susceptibility to sustained AF. Since intrinsic cardiac nervous system forms a complex neural network composed of GP that provide innervation to discrete cardiac tissues,16, 22 it is possible that in some dogs the VNA may have bypassed the SLGP and gone through other GP en route to innervate the atria. A more extensive vagal innervation pattern may have resulted in a poor VNA-SLGPNA correlation and a higher propensity for the development of sustained AF. Alternatively, since ICNA can function independently of extrinsic cardiac nerve inputs,23 and can be independently arrhythmogenic,4 a more independent SLGPNA at baseline may have predisposed dogs to sustained AF through atrial rapid pacing.
Clinical Implications
While progression from paroxysmal to sustained AF within a year is an unusual event,24 it is associated with increased incidence of hospital admissions and other major adverse cardiovascular events as compared with patients demonstrating no AF progression. It is possible that preventing AF progression from paroxysmal to sustained forms can prevent these adverse cardiovascular outcomes. The present study provided new insights into the mechanisms of AF progression. Interventions that effectively alter the nerve discharge patterns may be useful in preventing the development of sustained AF and other adverse cardiovascular events.
Study limitations
Atrial cardiac ganglionated plexi are reported to contain four types of neurons - efferent sympathetic, efferent parasympathetic, afferent neurons and local circuit neurons (interneurons).22 The latter neurons may be the dominant population recorded in this study. A second limitation is that we only recorded from the SLGP. Other potentially important GP such as the GP between the superior vena cava and aortic root25 and the GP near the ligament of Marshall26 were not studied due to the limited number of recording channels. Moreover, we only recorded from the left stellate ganglion and the left vagus nerve. The autonomic interactions from the right side remain to be determined. However, because the conclusions were based primarily on the patterns of correlation between left SGNA and VNA, the inability to selectively record sensory nerve activity or from multiple other GPs does not invalidate the conclusions of the study. Most (but not all) Group 2 dogs showed typical “L-shaped” sympathovagal correlation at baseline or before onset of sustained AF. Other dogs did not have typical L-shaped correlation: One dog in Group 2 (Figure 2B, d) had only the sympathetic (upper) arm of the sympathovagal correlation graph, and two dogs in Group 2 (Figure 2B, h and i) had poor correlation but not a typical L-shaped pattern either before or after rapid pacing. We do not have enough number of dogs to determine if these 3 dogs are significantly different from the ones with typical L-shaped correlation in terms of outcomes of pacing. Finally, the stellate ganglia also innervate the skeletal muscle, and the vagal nerves innervate the gut. Therefore, we cannot be sure that all recordings are cardiac specific.
Supplementary Material
Acknowledgments
Supported in part by NIH Grants P01 HL78931, R01 HL78932, 71140, a Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology (M.J.S.), a Korea Research Foundation Grant (KRF-2008-357-E00028) funded by the Korean Government (E.-K.C.), an AHA Established Investigator Award (S.-F.L.) and a Medtronic-Zipes Endowment (P.-S.C.). Medtronic Inc and St Jude Inc donated research equipment used in our lab.
We thank Lei Lin and Jian Tan for their assistance; Dr Xiaohong Zhou of Medtronic Inc and Mr Michael Bova of St Jude Medical Inc for donating the pacemaker equipment used in the study; and Amy Chang for her help in the preparation of the manuscript.
Abbreviations
- AF
atrial fibrillation
- DSI
Data Sciences International
- ECG
electrocardiography
- GP
ganglionated plexi
- ICNA
intrinsic cardiac nerve activity
- PAT
paroxysmal atrial tachycardia
- SGNA
stellate ganglion nerve activity
- SLGP
superior left ganglionated plexi
- SLGPNA
superior left ganglionated plexi nerve activity
- VNA
vagal nerve activity
Footnotes
This manuscript was processed by a Guest Editor
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 3.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi E-K, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 6.Wu TJ, Ong JJC, Chang CM, et al. Pulmonary veins and ligament of Marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–1163. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 7.Chang CM, Wu TJ, Zhou SM, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 8.Elvan A, Huang XD, Pressler ML, Zipes DP. Radiofrequency catheter ablation of the atria eliminates pacing-induced sustained atrial fibrillation and reduces connexin 43 in dogs. Circulation. 1997;96:1675–1685. doi: 10.1161/01.cir.96.5.1675. [DOI] [PubMed] [Google Scholar]
- 9.Swissa M, Zhou S, Paz O, Fishbein MC, Chen LS, Chen PS. A Canine Model of Paroxysmal Atrial Fibrillation and Paroxysmal Atrial Tachycardia. Am J Physiol Heart Circ Physiol. 2005;289:H1851–H1857. doi: 10.1152/ajpheart.00083.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. Journal of the American College of Cardiology. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 12.ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation. 1998;98:2202–2209. doi: 10.1161/01.cir.98.20.2202. [DOI] [PubMed] [Google Scholar]
- 14.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 15.Bertaglia E, Zoppo F, Bonanno C, Pellizzari N, Frigato N, Pascotto P. Autonomic modulation of the sinus node following electrical cardioversion of persistent atrial fibrillation: relation with early recurrence. Int J Cardiol. 2005;102:219–223. doi: 10.1016/j.ijcard.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- 17.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y, Scherlag BJ, Lin J, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 20.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 (Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh MH, Chiou CW, Wen ZC, et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation. 1999;100:2237–2243. doi: 10.1161/01.cir.100.22.2237. [DOI] [PubMed] [Google Scholar]
- 22.Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm. 2010;7:994–6. doi: 10.1016/j.hrthm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. American Journal of Physiology. 1991;260:H713–H721. doi: 10.1152/ajpheart.1991.260.3.H713. [DOI] [PubMed] [Google Scholar]
- 24.de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes--the third fat pad. Circulation. 1997;95:2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 26.Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. Journal of the American College of Cardiology. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


