Abstract
We have studied the effects of macromolecular crowding on protein folding kinetics by studying the oxidative refolding of hen lysozyme in the absence and presence of high concentrations of bovine serum albumin and Ficoll 70. The heterogeneity characteristic of the lysozyme refolding process is preserved under crowded conditions. This, together with the observation that the refolding intermediates that accumulate to significant levels are very similar in the absence and presence of Ficoll, suggests that crowding does not alter substantially the energetics of the protein folding reaction. However, the presence of high concentrations of macromolecules results in the acceleration of the fast track of the refolding process whereas the slow track is substantially retarded. The results can be explained by preferential excluded volume stabilization of compact states relative to more unfolded states, and suggest that, relative to dilute solutions, the rates of many protein folding processes are likely to be altered under conditions that more closely resemble the intracellular environment.
Keywords: excluded volume/Ficoll/hen lysozyme/macromolecular crowding/protein refolding kinetics
Introduction
Living cells are highly crowded due to the high total concentration of macromolecules they contain (20–30 volume %; Han and Herzfeld, 1993; Zimmerman and Minton, 1993; Record et al., 1998). Consequently, a substantial fraction of the intracellular space is occupied by macromolecules. By excluding volume, macromolecules reduce the configurational entropy and hence increase the free energy of a solution and the chemical potential of all macrosolutes present in that solution (Minton, 2000a,b). Crowding affects equilibria by preferentially destabilizing either reactants or products, such that the most favoured state excludes the least volume to the other macromolecular species present in solution. Association reactions are therefore highly favoured under crowded conditions, and it has been predicted that association constants under crowded conditions could be several orders of magnitude larger than those measured in dilute solutions (Zimmerman and Minton, 1993). This implies that aggregation of refolding protein molecules is a much greater problem under crowded conditions than it is in dilute solutions. This notion has been confirmed very recently for the refolding of reduced hen lysozyme in the presence of different crowding agents (van den Berg et al., 1999a; Minton, 2000c). Exclusion volume theory also predicts that compact (native) states are stabilized under crowded conditions relative to less compact partially folded or unfolded states of proteins (Minton, 1981, 2000a,b). This prediction has led to the suggestion that crowding could alter the free energy landscape of protein folding processes significantly (Minton, 2000b). The qualitative and quantitative effects of crowding on intrinsic features of refolding processes have, however, not been tested.
We have investigated the oxidative refolding of hen lysozyme in the absence and presence of high concentrations of bovine serum albumin (BSA) and Ficoll 70. The heterogeneity of the lysozyme refolding process is preserved in the presence of high concentrations of macromolecules. Moreover, the identities and distributions of the intermediates populated during refolding are very similar under crowded and non-crowded conditions. These observations suggest that crowding does not change dramatically the free energies (Baldwin, 1995; Dill and Chan, 1997; Dobson et al., 1998) of the various species involved in the refolding process in this particular case. However, excluded volume effects caused by crowding result in differential effects on the rates of the fast and slow tracks of lysozyme refolding relative to those in dilute solutions. This can be attributed to the higher stabilities of the fully native state and compact (native-like) intermediates relative to more unfolded states, resulting from macromolecular crowding.
Results and discussion
Kinetics of lysozyme refolding under crowded conditions
Reduced lysozyme is prone to aggregation during its refolding (Goldberg et al., 1991; van den Berg et al., 1999a). Under crowded conditions in the absence of denaturants, correct refolding is essentially abolished at higher lysozyme concentrations (≥5 µM), which is due to aggregation (van den Berg et al., 1999a). We therefore had to refold the reduced protein in buffer containing 2 M urea (van den Berg et al., 1999b,c) in order to obtain reasonable refolding yields in the presence of high concentrations of BSA and Ficoll 70. The refolding yields as measured by the extent of recovery of enzymatic activity are shown in Figure 1A for experiments carried out in the absence and presence of high concentrations of the crowding agents. In the presence of either BSA or Ficoll, the lysozyme refolding yields are decreased substantially relative to those obtained in the absence of crowding agents. As argued previously, this can be attributed to the greatly increased association constants under crowded conditions, which result in the increased aggregation of refolding lysozyme molecules and additional interactions between lysozyme intermediates and the crowding agents, both of which can contribute to the lower refolding yields (van den Berg et al., 1999a).
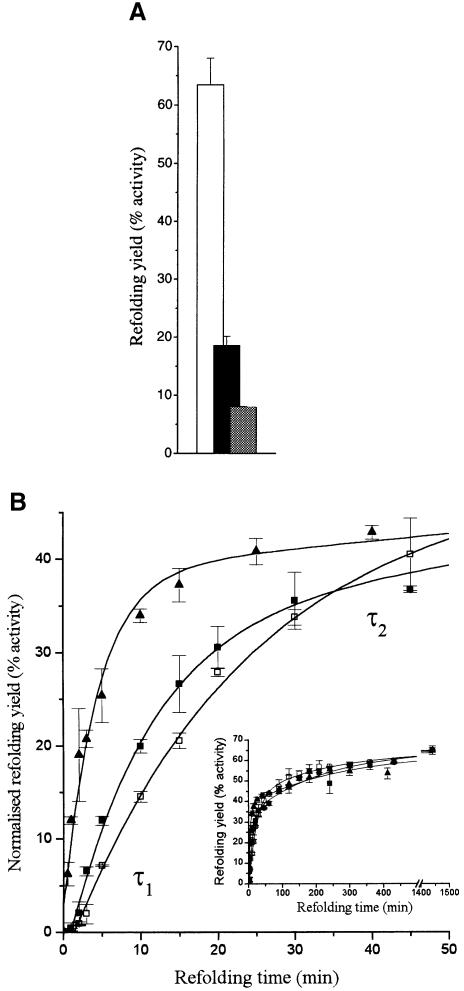
Fig. 1. (A) Refolding yields measured by the recovery of enzymatic activity during refolding of lysozyme in the absence of crowding agents (white bars) and in the presence of 200 g/l Ficoll 70 (black bars) or 100 g/l BSA (grey bars). (B) Initial refolding kinetics measured by the recovery of enzymatic activity during lysozyme refolding in the absence of crowding agents (open squares) and in the presence of 200 g/l Ficoll 70 (filled squares) or 100 g/l BSA (triangles). Refolding yields are normalized relative to those obtained in the absence of crowding agents to show the differences in the kinetics more clearly. The inset shows the complete kinetics.
During refolding of hen lysozyme, in both the absence and presence of its native disulfide bonds, there are distinct parallel fast and slow tracks via which the native state is reached (Radford et al., 1992; Dobson et al., 1994; Matagne et al., 1997; Kulkarni et al., 1999; van den Berg et al., 1999c). During oxidative refolding, this heterogeneity is evident from the two phases that are present in the recovery of enzymatic activity (van den Berg et al., 1999c). The initial phase with time constant τ1 represents the fast track, while the other with time constant τ2 represents the slow track (Figure 1B). Both phases are also present under crowded conditions, but analysis of the time constants shows that crowding significantly changes the rates of both tracks of the refolding process (Figure 1B; Table I). In the presence of high concentrations of BSA and Ficoll, the fast track of the refolding process is substantially faster (2- to 5-fold) compared with refolding in dilute solution. Interestingly, the slow track of the refolding process is nearly 50% slower in the presence of the crowding agents.
Table I. Refolding time constants obtained from the recovery of enzymatic activity for the fast track (τ1) and the slow track (τ2) of lysozyme refolding in the absence of crowding agents (–) and in the presence of 200 g/l Ficoll 70 or 100 g/l BSA.
| Crowding agent | τ1 (min) | τ2 (min) |
|---|---|---|
| – | 21.2 ± 1.5 | 187 ± 37 |
| Ficoll | 11.6 ± 1.7 | 268 ± 40 |
| BSA | 4.3 ± 1.1 | 267 ± 11 |
It might be argued that the observed acceleration of the initial phase of the refolding process under crowded conditions is due to preferential aggregation of more slowly refolding populations of molecules, leading to an apparently faster refolding rate. The amplitudes of the fast phase during the recovery of enzymatic activity, however, are similar under crowded and non-crowded conditions (60 ± 7%). This observation indicates that the relative populations of refolding molecules that form the native state via the fast and the slow tracks are not greatly affected by crowding-induced aggregation, suggesting that such aggregation does not greatly change the apparent rate of the fast refolding track. Figure 1B and Table I show that the effect of 100 g/l BSA on the rate of acceleration of the fast track of the lysozyme refolding process is larger than that of 200 g/l Ficoll. In addition, the initial lag phase in the appearance of enzymatic activity (van den Berg et al., 1999b,c) has disappeared in the presence of BSA, but not in the presence of Ficoll (Figure 1B). The reason for the differential effect of BSA and Ficoll is not clear, although it could be related to the fact that Ficoll has a relatively open structure (Wenner and Bloomfield, 1999) and may be less effective in excluding volume to the refolding lysozyme molecules (see Materials and methods).
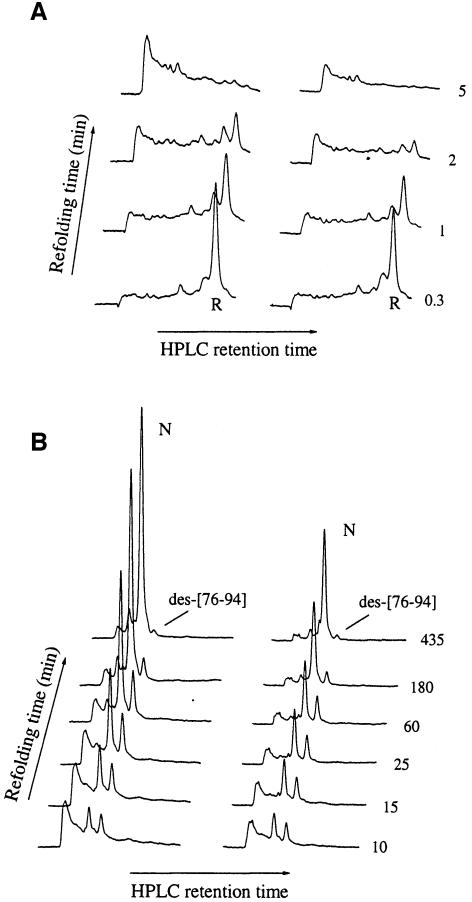
To determine whether similar refolding intermediates are populated under crowded and non-crowded conditions, we analysed aliquots of reduced lysozyme refolded in the absence and presence of Ficoll by reverse-phase HPLC at pH 2 (Weissman and Kim, 1991; van den Berg et al., 1999b,c). Although the presence of a large number of different refolding intermediates that accumulate to low levels during the early stages of the refolding process (Figure 2A; van den Berg et al., 1999b,c) limits their chromatographic separation, it is clear that the overall elution profiles are very similar in the absence and presence of Ficoll. During the later stages of the refolding process (Figure 2B), only two species accumulate to high levels in both the presence and absence of the crowding agent: the highly native-like three-disulfide intermediate des-[76-94] and the native protein (N) (van den Berg et al., 1999b,c).
Fig. 2. Reverse-phase HPLC elution profiles of intermediates observed in the absence (left panels) and presence (right panels) of 200 g/l Ficoll 70, during (A) the initial stages and (B) the later stages of the refolding process. R and N correspond to the fully reduced state and the native state, respectively.
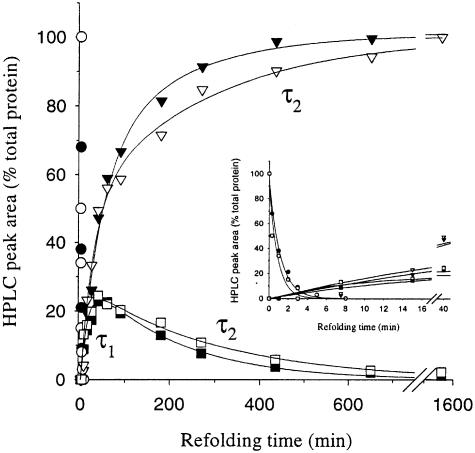
In order to obtain more detailed information about the refolding kinetics of individual species in the presence and absence of 200 g/l Ficoll 70, we analysed the levels of the fully reduced protein (R), the native-like three-disulfide intermediate des-[76-94] and the native protein (N) during refolding from the fully reduced state by reverse-phase HPLC (Figure 3; see Materials and methods). The time constants derived from the HPLC data are summarized in Table II. In accordance with the prediction that crowding is expected to affect in particular the conformations of unfolded states (Minton, 2000a,b), the disappearance of the fully reduced protein is faster by ∼25% in the presence of 200 g/l Ficoll (Figure 3; Table II).
Fig. 3. Analysis of the levels of the reduced protein (R; circles), des-[76-94] (squares) and the native state (N; triangles) during refolding of lysozyme from the fully reduced state. Amounts of protein were determined by measuring peak areas in reverse-phase HPLC chromatograms. The inset shows the early kinetics more clearly. Reduced lysozyme was refolded in the absence (filled symbols) or presence (open symbols) of 200 g/l Ficoll 70. For details see Materials and methods.
Table II. Refolding time constants determined by reverse-phase HPLC for the disappearance of the fully reduced protein (R), the formation (τ1) and disappearance (τ2) of des-[76-94] and the formation of the native state (N) during refolding of fully reduced lysozyme. In addition the time constants obtained for the conversion of purified des-[76-94] to the native state are shown.
| Protein species | τ1 (min) |
τ2 (min) |
||
|---|---|---|---|---|
| –Ficoll | +Ficoll | –Ficoll | +Ficoll | |
| Ra | 1.45 ± 0.04 | 1.09 ± 0.15 | – | – |
| des-[76-94]a | 16.8 ± 1.9 | 9.9 ± 1.6 | 209 ± 36 | 278 ± 45 |
| Na | 20.7 ± 2.5 | 15.7 ± 2.4 | 194 ± 50 | 265 ± 53 |
| des-[76-94]b | 264 ± 2 | 432 ± 14 | – | – |
Refolding was carried out in the absence and presence of 200 g/l Ficoll 70.
aRefolding kinetics determined during refolding from the fully reduced state.
bRefolding kinetics determined during refolding of purified des-[76-94].
The kinetic data for the formation of the native protein obtained by reverse-phase HPLC correspond well with those obtained from the measurements of recovery of enzymatic activity (Table I), which reflects formation not only of the fully native state but also of at least three highly native-like three-disulfide intermediates that have been identified during the oxidative refolding of lysozyme, the most abundant of which is des-[76-94] (van den Berg et al., 1999b). For the formation of the native state, the initial fast phase (τ1) is accelerated by crowding, while the subsequent slow phase (τ2) is retarded (Table II). Moreover, crowding results in the acceleration of the formation of des-[76-94] (τ1), whereas its disappearance (τ2) is retarded. The similar values of τ2 for the native protein and des-[76-94] support the previous conclusion (van den Berg et al., 1999c) that the conversion of des-[76-94] into the fully native state is largely responsible for the slow track of lysozyme refolding.
In order to verify the effect of high macromolecule concentrations on the rate of the slow track for lysozyme refolding, purified des-[76-94] was refolded in the absence and presence of 200 g/l Ficoll 70 (Table II). The data confirm that the conversion of des-[76-94] to the native state is slower (by ∼60%) under crowded conditions than in dilute solutions. Since the effect of a macromolecular crowding agent such as Ficoll on viscosity is macroscopic, the lower rate of conversion is not likely to result from the high viscosity of the Ficoll solutions.
Implications for protein folding
The present study demonstrates that high concentrations of two crowding agents with very different physico-chemical properties do not significantly affect the partitioning of the refolding process between molecules that form the native state efficiently and those that form the native state much more slowly. Together with the observation that the distributions of intermediates during lysozyme refolding are very similar in the absence or presence of high concentrations of macromolecules, these results suggest that the qualitative effects of crowding on the overall energetics of the lysozyme refolding process are small.
We have shown that crowding increases somewhat the rate of the initial formation of disulfide bonds during lysozyme refolding (Table II; Figures 3 and 4). There are, however, more dramatic differences in the rates of both tracks of the later stages of lysozyme refolding under crowded conditions relative to dilute solutions. The kinetic data obtained from the recovery of enzymatic activity in the presence of high concentrations of both BSA and Ficoll 70 suggest that the fast track of lysozyme refolding is accelerated substantially under crowded conditions, whereas the slow track is retarded (Figure 4). A similar conclusion can be drawn from an analysis of the populations of individual species during refolding in the presence and absence of Ficoll 70, as derived from reverse-phase HPLC. The HPLC data suggest that the acceleration of the fast track under crowded conditions is not likely to be caused by the faster disappearance of the fully reduced state under crowded conditions, since this process is already fast in the absence of Ficoll (τ ∼1 min; Table II).
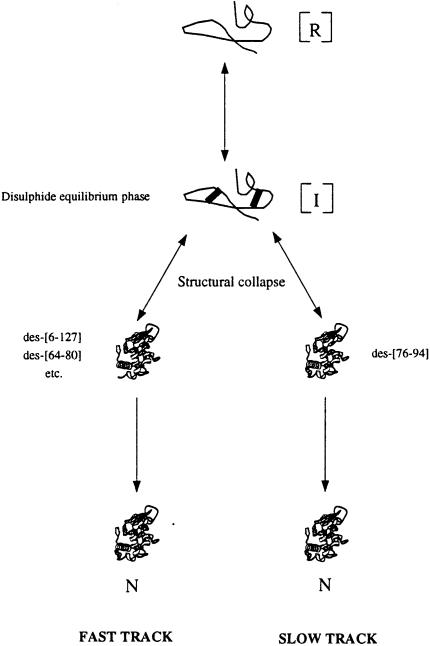
Fig. 4. Schematic diagram summarizing the oxidative refolding of lysozyme in the absence and presence of high concentrations of macromolecules. The changes in the rate of the various stages in the refolding process are discussed in the text. Both the reduced protein (R) and the early intermediates (I) are present in solution as an ensemble of species that are relatively unstructured, which facilitates rapid interconversion between them during the disulfide equilibrium phase (Roux et al., 1999; van den Berg et al., 1999c). Subsequent structural collapse from a subset of these early intermediates results in the formation of a limited number of highly native-like three-disulfide intermediates, which appear more rapidly under crowded conditions. Of these, des-[6-127] and des-[64-80] do not accumulate to high levels during refolding since they are converted rapidly to the fully native state. This gives rise to the fast track in lysozyme refolding, the rate of which is increased under crowded conditions. The slow track of lysozyme refolding results from the need for unfolding of the dominant intermediate des-[76-94], and is slower in the presence of crowding. Details of the oxidative refolding of lysozyme have been published (van den Berg et al., 1999c).
The HPLC data obtained for des-[76-94] and the native state suggest that the rate of acceleration of the fast track is more likely to arise from the preferential stabilization through excluded volume effects of the compact native-like three-disulfide intermediates and the fully native state relative to the more unstructured early intermediates (Minton, 2000b). This stabilization effect would result in an increased population of more compact conformers, leading to a more rapid appearance of native-like structure (Figure 4). The recovery of enzymatic activity in the presence of BSA supports such a conclusion, since the initial lag phase in the appearance of enzymatic activity (van den Berg et al., 1999b,c) is essentially abolished in the presence of high concentrations of the crowding agent (Figure 1B). In contrast, the conversion of the highly native-like three-disulfide intermediate des-[76-94] into the native state, which gives rise to the slow track in lysozyme refolding, involves the unfolding of at least part of the structure of the intermediate prior to the formation of the last disulfide bond (van den Berg et al., 1999b,c). Excluded volume effects would make this less favourable, and therefore provide an explanation for the slower rate of this process under crowded conditions compared with dilute solutions (Minton, 2000b).
In terms of the new view of protein folding (Baldwin, 1995; Dill and Chan, 1997; Dobson et al., 1998), the data obtained for the oxidative refolding of lysozyme suggest that crowding does not alter the main features of the free energy surface of the refolding process dramatically, at least not in this particular case. However, kinetic changes occur for both tracks of the lysozyme refolding process. We propose, on the basis of this observation, that excluded volume stabilization of compact states relative to more unstructured ones caused by macromolecular crowding generally could result in acceleration of folding steps that involve structural collapse, and deceleration of steps that involve (local) unfolding of pre-formed structure. If this model is correct, the free energy surface of folding processes under crowded conditions would differ from those in dilute solution by steeper drops along the folding trajectories and deeper local energy minima (Dobson et al., 1998).
Effects of macromolecular crowding on protein folding kinetics of a magnitude similar to those observed in this study might well be very important in vivo, since the ultimate fate of a refolding protein inside the cell is very subtly dependent on the balance between a number of competing processes (Dobson and Karplus, 1999). Aggregation, for example, is generally thought to be relatively slow (Dobson and Karplus, 1999), and a more rapid folding process as a result of crowding could therefore decrease competition with aggregation. It is clear, therefore, that the effects of macromolecular crowding could influence many aspects of protein folding inside the cell, including not only intermolecular interactions that result in increased aggregation and enhanced chaperone–substrate interactions (Martin and Hartl, 1997; van den Berg et al., 1999a), but also intrinsic properties of folding polypeptide chains. The extension of these studies to other proteins, and the exploration of effects of protein folding catalysts and chaperones, should lead to a better understanding of how proteins fold in their intracellular environment.
Materials and methods
Crowding agents
There is considerable evidence in the literature that the thermodynamic and hydrodynamic properties of Ficoll 70 approximate much more closely a sphere than those of linear polysaccharides such as dextran (Luby-Phelps et al., 1987). The radius of Ficoll 70, which has an average mol. wt. of ∼74 kDa, has been determined recently by light scattering to be 55 Å. Together with the observation that at higher Ficoll concentrations (>100 g/l) interpenetration and/or compression occurs (Wenner and Bloomfield, 1999), this suggests that Ficoll has a relatively open structure and is not a hard, tightly packed sphere. However, the advantages of using Ficoll as a crowding agent are that it is inert, polar and does not interfere with the separation of intermediates on HPLC columns. Protein crowding agents such as BSA are more relevant from a physiological point of view, but interfere with the HPLC separations (data not shown) and may have interactions with refolding proteins in addition to the effects caused by excluded volume (van den Berg et al., 1999a; Minton, 2000a,b). The concentrations of Ficoll 70 and BSA used in this study were chosen as 200 and 100 g/l, respectively, because these concentrations were demonstrated previously to have comparable effects on the refolding yields of reduced lysozyme (van den Berg et al., 1999a). In addition, these concentrations are likely to be within the range of total macromolecular concentrations present inside cells (Swaminathan et al., 1997; Elowitz et al., 1999).
Refolding experiments
Reduced lysozyme (prepared as described in van den Berg et al., 1999b) was dissolved in buffer A containing 8 M urea, 100 mM Tris–HCl, 100 mM NaCl, 1 mM EDTA pH 8.5. Refolding was initiated by rapid 4-fold dilution into buffer B: 100 mM Tris, 100 mM NaCl, 1 mM EDTA pH 8.5 to give a final protein concentration of 25 µM, in the presence of 1.0/0.2 mM of reduced/oxidized glutathione (freshly made before use). For experiments with crowding agents, buffer B contained an additional 130 g/l BSA or 260 g/l Ficoll 70, resulting in a BSA concentration of 100 g/l or a Ficoll concentration of 200 g/l during refolding. The pH in the refolding experiments was 8.50 ± 0.05. Refolding experiments were carried out at 20°C. Details of the refolding procedure and assay of enzymatic activity have been published elsewhere (van den Berg et al., 1999b,c). The three-disulfide lysozyme refolding intermediate des-[76-94] was purified as described earlier (van den Berg et al., 1999b), dissolved in a small volume of acetic acid pH 3, and refolded at 25 µM in buffer B with or without 200 g/l Ficoll in the absence of urea (1/0.2 mM GSH/GSSG, 20°C).
Reverse-phase HPLC
After various times of refolding in the absence and presence of Ficoll, aliquots were taken and refolding was halted by acidification to pH 2 by adding a 1/20 vol. of 2.5 M HCl as described previously (Weissman and Kim, 1991; van den Berg et al., 1999b,c). Native and reduced lysozyme and the various refolding intermediates were separated by reverse-phase HPLC at pH 2 using a semi-preparative Phenomenex Jupiter 10 µ C5 300 Å column (00G-4054-N0; 250 × 10 mm), with linear gradients consisting of solvent A [H2O/0.1% trifluoroacetic acid (TFA)] and solvent B (CH3CN/0.1% TFA): 0–3 min, 30% B; 3–25 min, 37–43% B; 25–28 min, 90% B, with a flow rate of 4 ml/min. Refolding kinetics were determined for the fully reduced state (R), des-[76-94] and the native state (N) by measuring their peak areas in the HPLC chromatograms using the Gilson Unipoint analysis software program (Figure 3). The data were subsequently fitted to single (for R and refolding of purified des-[76-94]) or double exponential models using ORIGIN 5.0 (Microcal software). The reported time constants, τ, are averages from three to five separate experiments and correspond to the half-times of the reaction (van den Berg et al., 1999c).
References
- Baldwin R.L. (1995) The nature of protein folding pathways: the classical versus the new view. J. Biomol. NMR, 5, 103–109. [DOI] [PubMed] [Google Scholar]
- Dill K.A. and Chan,H.S. (1997) From Levinthal to pathways to funnels. Nature Struct. Biol., 4, 10–19. [DOI] [PubMed] [Google Scholar]
- Dobson C.M. and Karplus,M. (1999) The fundamentals of protein folding: bringing together theory and experiment. Curr. Opin. Struct. Biol., 9, 92–101. [DOI] [PubMed] [Google Scholar]
- Dobson C.M., Evans,P.A. and Radford,S.E. (1994) Understanding how proteins fold: the lysozyme story so far. Trends Biochem. Sci., 19, 31–37. [DOI] [PubMed] [Google Scholar]
- Dobson C.M., Sali,A. and Karplus,M. (1998) Protein folding: a perspective from theory and experiment. Angew. Chem. Int. Ed., 37, 868–893. [DOI] [PubMed] [Google Scholar]
- Elowitz M.B., Surette,M.G., Wolf,P.-E., Stock,J.B. and Leibler,S. (1999) Protein mobility in the cytoplasm of Escherichia coli. J. Bacteriol., 181, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M.E., Rudolph,R. and Jaenicke,R.A. (1991) Kinetic study of the competition between renaturation and aggregation during the refolding of denatured–reduced egg-white lysozyme. Biochemistry, 30, 2790–2797. [DOI] [PubMed] [Google Scholar]
- Han J. and Herzfeld,J. (1993) Macromolecular diffusion in crowded solutions. Biophys. J., 65, 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S.K., Ashcroft,A.E., Carey,M., Masselos,D., Robinson,C.V. and Radford,S.E. (1999) A near-native intermediate on the slow refolding pathway of hen lysozyme. Protein Sci., 8, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Castle,P.E., Lansing Taylor,D. and Lanni,F. (1987) Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Natl Acad. Sci. USA, 84, 4910–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. and Hartl,F.-U. (1997) The effect of macromolecular crowding on chaperonin-mediated protein folding. Proc. Natl Acad. Sci. USA, 94, 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A., Radford,S.E. and Dobson,C.M. (1997) Fast and slow tracks in lysozyme folding: insight into the roles of domains in the folding process. J. Mol. Biol., 267, 1068–1074. [DOI] [PubMed] [Google Scholar]
- Minton A.P. (1981) Excluded volume as a determinant of macro- molecular structure and reactivity. Biopolymers, 20, 2093–2120. [Google Scholar]
- Minton A.P. (2000a) Effect of a concentrated ‘inert’ macromolecule cosolute on the stability of a globular protein with respect to denaturation by heat and by chaotropes: a statistical-thermodynamic model. Biophys. J., 78, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A.P. (2000b) Implications of macromolecular crowding on protein assembly. Curr. Opin. Struct. Biol., 10, 34–39. [DOI] [PubMed] [Google Scholar]
- Minton A.P. (2000c) Protein folding: thickening the broth. Curr. Biol., 10, R97–R99. [DOI] [PubMed] [Google Scholar]
- Radford S.E., Dobson,C.M. and Evans,P.A. (1992) The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature, 358, 302–307. [DOI] [PubMed] [Google Scholar]
- Record M.T., Courtenay,D.S., Cayley,D.S. and Guttman,H.J. (1998) Biophysical compensation mechanisms buffering E.coli protein–nucleic acid interactions against changing environments. Trends Biochem. Sci., 23, 190–194. [DOI] [PubMed] [Google Scholar]
- Roux P., Ruoppolo,M., Chaffotte,A.-F. and Goldberg,M.E. (1999) Comparison of the kinetics of S–S bond, secondary structure and active site formation during refolding of reduced denatured hen egg white lysozyme. Protein Sci., 8, 2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan R., Hwang,C.P. and Verkman,A.S. (1997) Photobleaching recovery and anisotropy decay of green fluorescent protein GFP–S65T in solution and cells: cytoplasmic viscosity probed by green fluorescent protein translational and rotational diffusion. Biophys. J., 72, 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B., Ellis,R.J. and Dobson,C.M. (1999a) Effects of macromolecular crowding on protein folding and aggregation. EMBO J., 18, 6927–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B., Chung,E.W., Robinson,C.V. and Dobson,C.M. (1999b) Characterisation of the dominant oxidative folding intermediate of hen lysozyme. J. Mol. Biol., 290, 781–796. [DOI] [PubMed] [Google Scholar]
- van den Berg B., Chung,E.W., Robinson,C.V., Mateo,P.L. and Dobson,C.M (1999c) The oxidative refolding of hen lysozyme and its catalysis by protein disulfide isomerase. EMBO J., 18, 4794–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J.S. and Kim,P.S. (1991) Re-examination of the folding of BPTI: predominance of native intermediates. Science, 253, 1386–1393. [DOI] [PubMed] [Google Scholar]
- Wenner J.R. and Bloomfield,V.A. (1999) Crowding effects on EcoRV kinetics and binding. Biophys. J., 77, 3234–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S.B. and Minton,A.P. (1993) Macromolecular crowding: biochemical, biophysical and physiological consequences. Annu. Rev. Biophys. Biomol. Struct., 22, 27–75. [DOI] [PubMed] [Google Scholar]