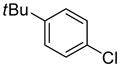
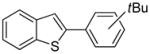
Abstract
A transition-metal-free method for arylation of heterocycle and arene carbon-hydrogen bonds by aryl chlorides and fluorides has been developed. The reactions proceed via aryne intermediates and are highly regioselective with respect to the C-H bond coupling component.
Regioselective formation of aryl-aryl bonds has attracted substantial interest due to prevalence of sp2-sp2 bonds in pharmaceuticals, natural products, and dyes.1 In recent years, classical methods for creating aryl-aryl bonds have been supplanted by direct arylation methodology where carbon-hydrogen bond is used as a functional group.2 Majority of the C-H bond arylation examples include palladium, rhodium, or ruthenium-catalyzed functionalization of five-membered ring heterocycles and directing-group containing arenes.3 Copper-catalyzed deprotonative heterocycle and electron-deficient arene arylation results in the functionalization of the most acidic sp2 C-H bonds.4 Several recent reports describe arene and electron-deficient heterocycle arylation by aryl halides that presumably proceed by radical-type mechanisms without transition metal involvement.5 The latter methods avoid heavy metal contamination of the products simplifying purification for pharmaceutical applications.6 Additionally, these methods are among the simplest synthetic routes to biaryls. However, arylation of simple arenes such as anisole afford isomer mixtures and the arene coupling component is often employed in large excess (up to 100 equivalents) as a solvent. We report here a method for transition-metal-free, intermolecular sp2 C-H bond arylation via benzyne intermediates that is highly regioselective with respect to arene coupling component and does not require large excess of any coupling components.
We have recently reported a transition-metal-free, base-mediated intramolecular C-arylation of phenols with aryl halides. In the presence of t-BuOK in dioxane at 140 °C, the cyclization of 3-(2-halobenzyloxy)phenols affords 6H-benzo[c]chromenes in high yields.7a The reaction proceeds by an initial formation of a benzyne intermediate followed by an aromatic sp2 C-H functionalization to form the carbon-carbon bond. To expand the synthetic utility of the reaction, we decided to investigate intermolecular carbon-carbon bond formation proceeding via benzyne intermediates. The reaction would occur by a simultaneous generation of benzyne and aryl anion8 followed by biaryl formation.9 Early examples of chloroarene phenylation by phenyllithium were reported by Huisgen more than 50 years ago.9a More recent examples of aryne arylations have been described by Hart, Schlosser, Meyers, Aubert, and Mamane.9 Bezyne can be generated from silyl aryl triflates under mild conditions.7b However, these starting materials are quite expensive and only a few of them are commercially available. Consequently, we decided to use readily accessible and cheap aryl chloride benzyne sources. Use of hindered 2,2,6,6-tetramethylpiperidides (TMP) should retard the reaction of benzyne with base.10 Relative reactivity of the base and aryl anion with benzyne can be modulated by employing a solvent where amide base is sparingly soluble. A brief optimization of reaction conditions showed that best results were obtained by employing a mixed pentane/THF solvent system. In pentane, the arylations are slow due to low base solubility. Competitive addition of TMPLi to benzyne decreases arylation yields in THF.
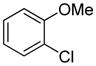
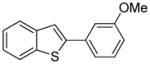
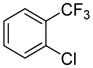
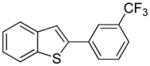
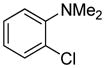
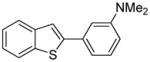
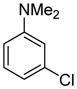
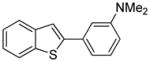
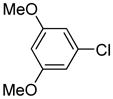
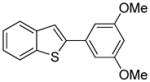
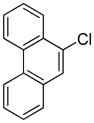
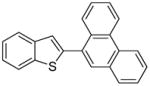
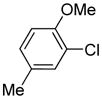
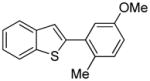
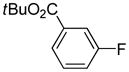
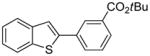
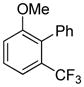
Arylation scope with respect to aryl halides is presented in Table 1. Benzothiophene is arylated by aryl halides in the most acidic position. Arylation by 2-chloroanisole and 2-chlorobenzotrifluoride (entries 1 and 2) affords the meta-substituted products in good yields. If benzothiophene is arylated by either 2- or 3-chloro-N, N-dimethylaniline, the m-substitution product is obtained (entries 3 and 4). This regioselectivity pattern may be advantageous if o-chloroarene is more available than the corresponding m-isomer. Substitutions by a benzyne mechanism often produce the same isomer if 2- and 3-haloarene starting materials are used.11 Regioselectivity is explained by energy required to distort aryne into two possible transition states and by ground-state polarization of aryne by electron-withdrawing substituents.11c 3,5-Dimethoxychlorobenzene is reactive and arylation product is obtained in good yield (entry 5). 2,3,4,5-Tetrasubstituted chloroarenes can be employed and benzothiophene is arylated by 9-chlorophenanthrene (entry 6). If 3-chloro-4-methoxytoluene is used, the arylation occurs meta to the methoxy substituent (entry 7). Fluoroarene starting materials can be employed and the reaction tolerates an ester group (entry 8). If 4-substituted chloroarenes are used, nearly 1/1 isomer mixture is obtained as expected (entry 9).11
Table 1.
Arylation scope with respect to aryl halidesa
Aryl halide (1.6–2.5 equiv), benzothiophene (1 equiv), 0.5 mmol scale. Yields are isolated yields. See Supporting Information for details.
tert-Butyl-3-bromobenzoate used.
Isomer mixture; m/p ratio 1/1.2.
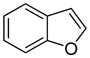
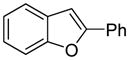
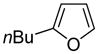
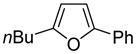
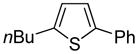
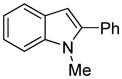
The arylation scope with respect to heterocycles and arenes is shown in Table 2. In most cases, TMPLi base affords slightly higher yields than either lithium dicyclohexylamide or diisopropylamide (entries 1, 2, 3, 4, 6). Fluorobenzene can be used instead of chlorobenzene and nearly identical yields were obtained in the reactions with benzothiophene (entry 1). Furan derivatives, such as benzofuran and 2-butylfuran, are arylated in good yields (entries 2 and 3). N-Methylbenzimidazole and benzothiazole are reactive (entries 4 and 5). Thiophene, indole, and pyrrole derivatives afford the arylation products in excellent yields (entries 6, 7, 8). The arylation is not limited to five-membered ring heterocycles. Pyridine and pyridazine derivatives are arylated in reasonable yields (entries 9 and 10). Arenes such as 1,3-dimethoxybenzene and 3-methoxybenzotrifluoride are reactive (entries 11 and 12).
Table 2.
Arylation scope with respect to heterocycles and arenesa
 | |||
|---|---|---|---|
| entry | arene | product | yield, % |
| 1 |
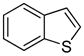
|
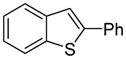
|
86 72b 85c |
| 2 |
|
|
81 75d |
| 3 |
|
|
80 68b |
| 4 |
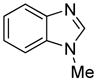
|
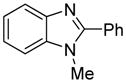
|
91 81b |
| 5 |
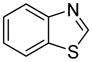
|
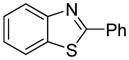
|
72 |
| 6 |
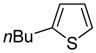
|
|
80 74b |
| 7 |
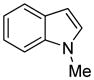
|
|
90 |
| 8e |
|
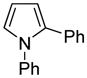
|
78 |
| 9 |
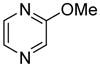
|
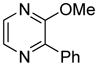
|
55 |
| 10 |
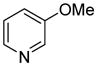
|
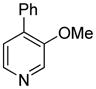
|
71 |
| 11 |
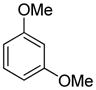
|
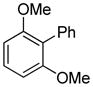
|
95 |
| 12 |
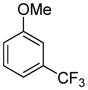
|
|
81 |
Chlorobenzene (1.3–2 equiv), arene or heterocycle (1 equiv), 0.5 mmol scale. Yields are isolated yields. See Supporting Information for details.
Lithium dicyclohexylamide base.
PhF used instead of PhCl.
LiNiPr2 base.
Phenylpyrrole (2 equiv), PhCl (1 equiv).
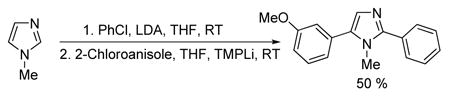
We performed sequential, one-pot diarylation of N-methylimidazole. The heterocycle mixture with chlorobenzene was treated with LDA in THF at RT, followed by quench with MeOH and evaporation. Following addition of 2-chloroanisole and TMPLi in THF, a single isomer of diarylation product was obtained in 50% yield (eq 1).
 |
(1) |
In conclusion, we have developed a transition-metal free method for base-promoted arylation of heterocycles and arenes by aryl chlorides and fluorides. The reactions proceed via aryne intermediates at mild temperatures and allow for highly regioselective arylation of the arene and heterocycle C-H bonds. Functionalization occurs at the most acidic carbon-hydrogen bond.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Sciences (Grant No. R01GM077635), A. P. Sloan Foundation, and Camille and Henry Dreyfus Foundation for supporting this research.
Footnotes
SUPPORTING INFORMATION AVAILABE: Experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem Rev. 2002;102:1359. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 2.Selected reviews: Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n.Ackermann L, Vicente R, Kapdi AR. Angew Chem, Int Ed. 2009;48:9792. doi: 10.1002/anie.200902996.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem, Int Ed. 2009;48:5094. doi: 10.1002/anie.200806273.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173. doi: 10.1039/b606984n.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174. doi: 10.1021/cr0509760.
- 3.Selected examples: Akita Y, Inoue A, Yamamoto K, Ohta A, Kurihara T, Shimizu M. Heterocycles. 1985;23:2327.Catellani M, Chiusoli GP, Ricotti S. J Organomet Chem. 1985;296:C11.Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Bull Chem Soc Jpn. 1998;71:467.Rieth RD, Mankad NP, Calimano E, Sadighi JP. Org Lett. 2004;6:3981. doi: 10.1021/ol048367m.Chiong HA, Daugulis O. Org Lett. 2007;9:1449. doi: 10.1021/ol0702324.Liégault B, Petrov I, Gorelsky SI, Fagnou K. J Org Chem. 2010;75:1047. doi: 10.1021/jo902515z.Deprez NR, Kalyani D, Krause A, Sanford MS. J Am Chem Soc. 2006;128:4972. doi: 10.1021/ja060809x.Kakiuchi F, Kan S, Igi K, Chatani N, Murai S. J Am Chem Soc. 2003;125:1698. doi: 10.1021/ja029273f.Oi S, Fukita S, Inoue Y. Chem Commun. 1998:2439.Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. Angew Chem, Int Ed. 2003;42:112. doi: 10.1002/anie.200390037.Caron L, Campeau LC, Fagnou K. Org Lett. 2008;10:4533. doi: 10.1021/ol801839w.Cho SH, Hwang SJ, Chang S. J Am Chem Soc. 2008;130:9254. doi: 10.1021/ja8026295.Chen X, Li JJ, Hao XS, Goodhue CE, Yu JQ. J Am Chem Soc. 2006;128:78. doi: 10.1021/ja0570943.Chiong HA, Pham QN, Daugulis O. J Am Chem Soc. 2007;129:9879. doi: 10.1021/ja071845e.Desai LV, Stowers KJ, Sanford MS. J Am Chem Soc. 2008;130:13285. doi: 10.1021/ja8045519.Shi Z, Li B, Wan X, Cheng J, Fang Z, Cao B, Qin C, Wang Y. Angew Chem, Int Ed. 2007;46:5554. doi: 10.1002/anie.200700590.
- 4.(a) Do HQ, Daugulis O. J Am Chem Soc. 2007;129:12404. doi: 10.1021/ja075802+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Do HQ, Khan RMK, Daugulis O. J Am Chem Soc. 2008;130:15185. doi: 10.1021/ja805688p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yotphan S, Bergman RG, Ellman JA. Org Lett. 2009;11:1511. doi: 10.1021/ol900103a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagisawa S, Ueda K, Taniguchi T, Itami K. Org Lett. 2008;10:4673. doi: 10.1021/ol8019764.Liu W, Cao H, Zhang H, Zhang H, Chung KH, He C, Wang H, Kwong FY, Lei A. J Am Chem Soc. 2010;132:16737. doi: 10.1021/ja103050x.Shirakawa E, Itoh KI, Higashino T, Hayashi T. J Am Chem Soc. 2010;132:15537. doi: 10.1021/ja1080822.Sun CL, Li H, Yu DG, Yu M, Zhou X, Lu XY, Huang K, Zheng SF, Li BJ, Shi ZJ. Nature Chem. 2010;2:1044. doi: 10.1038/nchem.862.Five-membered ring heterocycle arylation by iodonium salts: Kita Y, Morimoto K, Ito M, Ogawa C, Goto A, Dohi T. J Am Chem Soc. 2009;131:1668. doi: 10.1021/ja808940n.Photochemical arylation: Fagnoni M, Albini A. Acc Chem Rev. 2005;38:713. doi: 10.1021/ar0402356.
- 6.Prasad K, Repic O, Blacklock TJ. Org Process Res Dev. 2003;7:733. [Google Scholar]
- 7.(a) Bajracharya GB, Daugulis O. Org Lett. 2008;10:4625. doi: 10.1021/ol801897m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Himeshima Y, Sonoda T, Kobayashi H. Chem Lett. 1983;8:1211. [Google Scholar]
- 8.(a) Shen K, Fu Y, Li JN, Liu L, Guo QX. Tetrahedron. 2007;63:1568. [Google Scholar]; (b) Bordwell FG. Acc Chem Res. 1988;21:456. [Google Scholar]
- 9.Addition of ArM to benzynes: Huisgen R, Sauer J, Hauser A. Chem Ber. 1958;91:2366.Meyers AI. J Chem Soc, Chem Commun. 1985:690.Hart H, Harada K, Du CJF. J Org Chem. 1985;50:3104.Leroux F, Schlosser M. Angew Chem, Int Ed. 2002;41:4272. doi: 10.1002/1521-3773(20021115)41:22<4272::AID-ANIE4272>3.0.CO;2-B.Addition of ArPd to benzynes: Henderson JL, Edwards AS, Greaney MF. Org Lett. 2007;9:5589. doi: 10.1021/ol702584t.Liu Z, Larock RC. Angew Chem, Int Ed. 2007;46:2535. doi: 10.1002/anie.200604969.Becht MJ, Gissot A, Wagner A, Mioskowski C. Chem-Eur J. 2003;9:3209. doi: 10.1002/chem.200204373.Abboud M, Mamane V, Aubert E, Lecomte C, Fort Y. J Org Chem. 2010;75:3224. doi: 10.1021/jo100152e.
- 10.Dougherty CM, Olofson RA. J Am Chem Soc. 1973;95:582. [Google Scholar]
- 11.(a) Huisgen R, Sauer J. Angew Chem. 1960;72:91. [Google Scholar]; (b) Tadross PM, Gilmore CD, Bugga P, Virgil SC, Stoltz BM. Org Lett. 2010;12:1224. doi: 10.1021/ol1000796. [DOI] [PubMed] [Google Scholar]; (c) Im GYJ, Bronner SM, Goetz AE, Paton RS, Cheong PHY, Houk KN, Garg NK. J Am Chem Soc. 2010;132:17933. doi: 10.1021/ja1086485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.