Abstract
Objective
To evaluate the role of early-life exposure to airborne fine particulate matter (diameter, <2.5 µm [PM2.5]) pollution on metabolic parameters, inflammation, and adiposity; and to investigate the involvement of oxidative stress pathways in the development of metabolic abnormalities.
Methods and Results
PM2.5 inhalation exposure (6 h/d, 5 d/wk) was performed in C57BL/6 mice (wild type) and mice deficient in the cytosolic subunit of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase p47phox (p47phox−/−) beginning at the age of 3 weeks for a duration of 10 weeks. Both groups were simultaneously fed a normal diet or a high-fat diet for 10 weeks. PM2.5-exposed C57BL/6 mice fed a normal diet exhibited metabolic abnormalities after exposure to PM2.5 or FA for 10 weeks. Consistent with insulin resistance, these abnormalities included enlarged subcutaneous and visceral fat contents, increased macrophage infiltration in visceral adipose tissue, and vascular dysfunction. Ex vivo–labeled and infused monocytes demonstrated increased adherence in the microcirculation of normal diet– or high-fat diet–fed PM2.5-exposed mice. p47phox−/− mice exhibited an improvement in parameters of insulin resistance, vascular function, and visceral inflammation in response to PM2.5.
Conclusion
Early-life exposure to high levels of PM2.5 is a risk factor for subsequent development of insulin resistance, adiposity, and inflammation. Reactive oxygen species generation by NADPH oxidase appears to mediate this risk.
Keywords: PM2.5 air pollution, obesity, NADPH oxidase, inflammation, insulin resistance
Substantial epidemiological evidence implicates fine particulate matter (PM2.5) air pollution as a major adverse risk factor with serious consequences for human health in both developed and developing countries.1–4 Recent data from large population cohorts have provided compelling associations between ambient air PM2.5 pollution exposure and increased cardiovascular morbidity and mortality.5,6 Previously, important mechanistic links between inhaled PM2.5 exposure and exposure to high-fat diets (HFDs) in adult mice were demonstrated.7,8 Because propensity to insulin resistance (IR) develops via priming mechanisms early in childhood, we speculated that the appropriate time window for assessing the effects of exposure to pollutants in the eventual development of IR in adults is during infancy/childhood. Studying type 2 diabetes mellitus (T2DM)/IR development and its relation to environmental factors may help clarify the causative relations among IR, body fatness, and the development of cardiovascular risk.9,10 Inflammation and oxidative stress pathways appear to play critical roles in this process, as demonstrated by numerous investigations.11–14 Consistent with this hypothesis, targeted disruption of proinflammatory or pro-oxidant pathways have been effective in attenuating the development of IR.13,15–17 Furukawa et al18 have shown an important role of the NADPH oxidase in the development of obesity and IR in response to a HFD. We hypothesized that PM2.5 air pollution exerts important proinflammatory and pro-oxidant effects, mediated via NADPH oxidase, with early-life exposure eventually priming the development of IR.
Methods
Detailed methods are provided in the supplemental data (available online at http://atvb.ahajournals.org).
Animals
We used male 3-week-old C57BL/6 and p47phox−/− mice (both from Jackson Laboratories, Bar Harbor, Me). The protocols and the use of animals were approved by and in accordance with the Ohio State University Animal Care and Use Committee.
Diet and PM2.5 Exposure
The mice were fed with either a normal diet (ND) (Teklad 7012, 13% calories from fat; n = 16) or an HFD (Teklad TD 88137, 42% calories from fat; n = 16) beginning at the age of 3 weeks. The animals were exposed by inhalation to either filtered air (FA) or PM2.5 for 6 h/d, 5 d/wk, for 10 weeks in a mobile trailer exposure system (“Ohio Air Pollution Exposure System for Interrogation of Systemic Effects 1,” located at the Ohio State University Animal Facility in Columbus). Animal exposure and monitoring of the exposure environment and ambient aerosol were performed as previously described.7,8,19
Blood Glucose and Insulin Measurements, Magnetic Resonance Imaging, and Intravital Microscopy
Blood glucose measurement was conducted with a glucometer (Elite). Insulin levels were determined using a commercially available kit (Ultra Sensitive Mouse Insulin ELISA Kit).
An abdominal fat evaluation was performed by in vivo magnetic resonance imaging (MRI) with a T1-weighted gradient-echo sequence on all C57BL/6 and p47phox−/− mice, as previously described.8
Intravital microscopy was performed as described in the supplemental materials.
Blood Inflammatory Biomarkers
Blood inflammatory biomarkers, including interferon γ, monocyte chemoattractant protein-1, regulated on activation, normal T-cell expressed and secreted, and tumor necrosis factor (TNF) α, were measured by quantitative ELISA assays.
Statistical Analysis
Data are expressed as mean±SE unless otherwise indicated. For responses measured repeatedly at different points or dose levels, a series of 2-sample independent Student t tests was used to detect differences between the FA and PM2.5 treatment groups at every point and dose level, with Bonferroni correction for multiple-comparison adjustment. Comparisons of other continuous variables were conducted with an independent 2-sample Student t test, with P<0.05 considered significant.
Results
Exposure Characterization
The ambient mean daily PM2.5 concentration at the study site was 15.8 µg/m3 (SD, 6.1 µg/m3), whereas the mean concentration of PM2.5 in the exposure chamber was 111.0 µg/m3 (approximately a 7-fold greater concentration versus the ambient level). The elemental composition, as measured by energy-dispersive X-ray fluorescence (ED-XRF) analysis, is presented in supplemental Table I.
Body Weight, Fat Content, Glucose Homeostasis, and Systemic Inflammation Changes
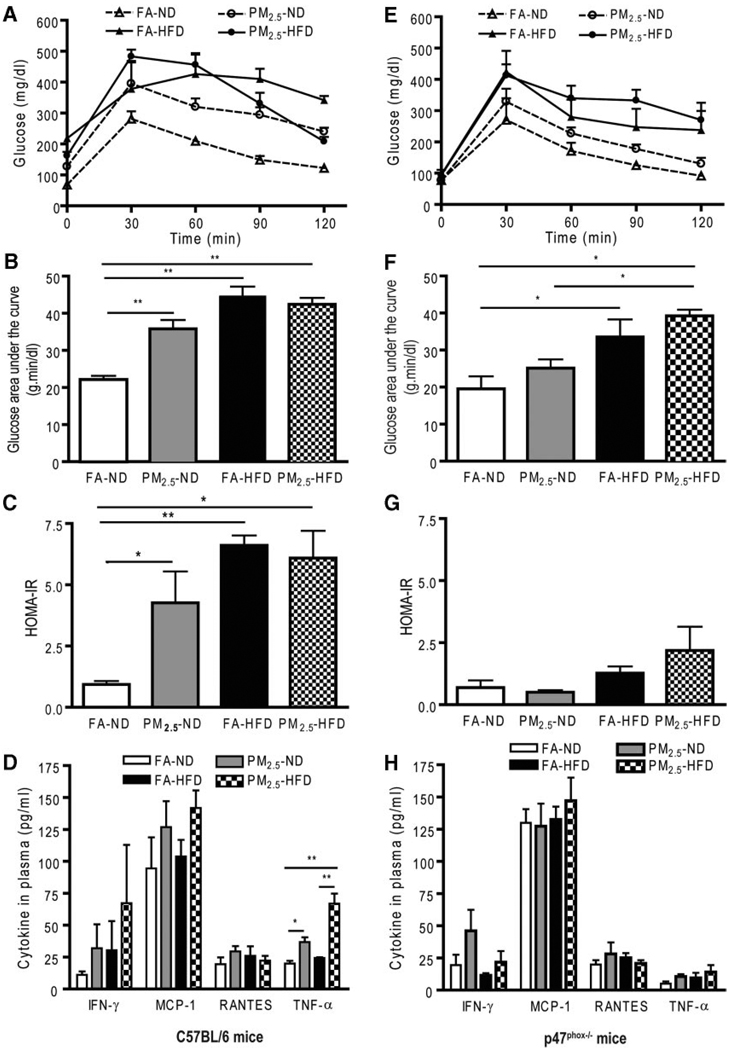
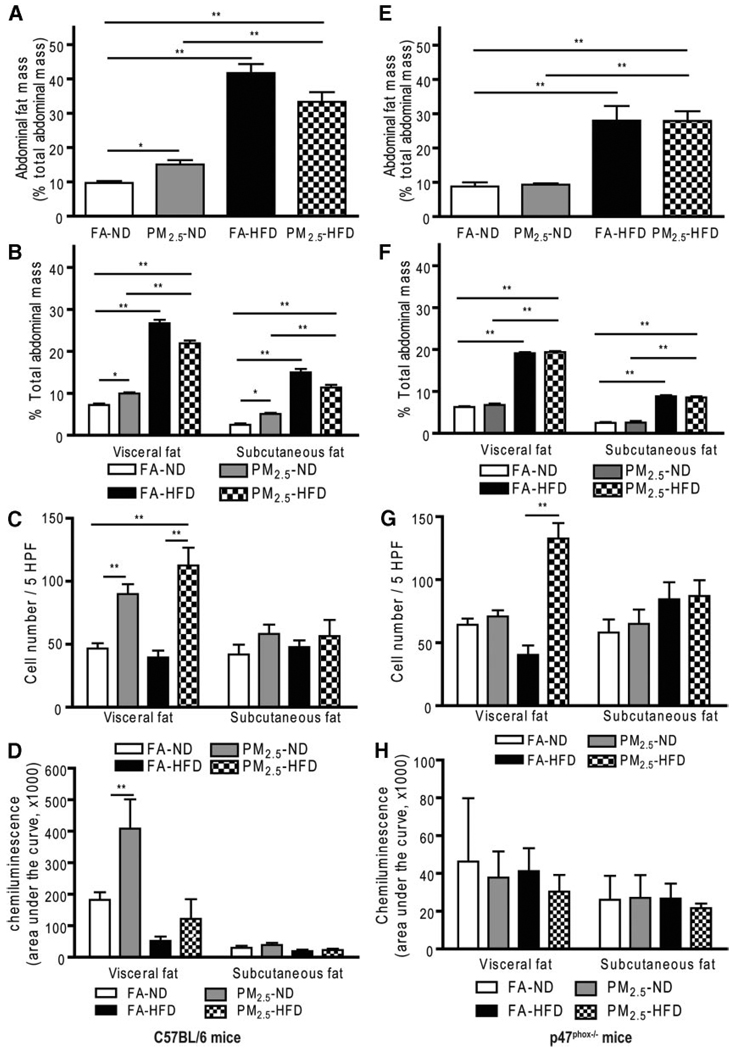
In wild-type C57BL/6 mice, there were no significant differences among the 4 groups in body weight (supplemental Table II) or glucose tolerance (supplemental Figure IA) at baseline before exposure. Mice on an HFD gained much more body weight compared with mice on an ND. Mice on an ND exposed to PM2.5 showed significant elevations in glucose levels by an intraperitoneal glucose tolerance test (Figure 1A and 1B). HFD (regardless of FA or PM2.5 exposure) and PM2.5 exposure in ND-fed mice led to IR, evaluated by a homeostasis model assessment index (Figure 1C). Figure 1D shows inflammatory biomarkers in blood from C57BL/6 mice exposed to either PM2.5 or FA. TNF-α was increased in the mice exposed to PM2.5 compared with the FA in either ND- or HFD-fed mice. To examine whether PM2.5 exposure altered adiposity, a T1-weighted MRI was performed before and after PM2.5 exposure. There was no significant difference in abdominal body fat among the experimental groups before exposure (supplemental Figure IB). However, PM2.5 exposure alone and HFD feeding significantly increased the total abdominal fat compared with FA-exposed mice fed an ND (Figure 2A and supplemental Figure IC), although the combination of PM2.5 with an HFD did not further increase abdominal fat. With respect to fat distribution, both visceral and subcutaneous fat contents were increased with PM2.5 exposure in the ND- or HFD-fed group (Figure 2B). Consistent with these results, adipocyte size was increased in the PM2.5-exposed mice fed an ND in both visceral fat (FA, 2137±45 µm2; PM2.5, 2698±80 µm2; P<0.01) and subcutaneous fat (FA, 1039±27 µm2; PM2.5, 1355±30 µm2; P<0.05). The increase in adipocyte size was extreme in the HFD groups, with no further changes due to PM2.5 exposure (supplemental Figure II). These data suggest that PM2.5 exposure alone, in the presence of ND, may potentiate adiposity and exert proinflammatory effects.
Figure 1.
Glucose homeostasis and systemic inflammation in wild-type C57BL/6 mice and p47phox−/− mice by ambient PM2.5 exposure compared with FA-exposed mice fed an ND (n = 16) or an HFD (n = 16). A and E, Effect of PM2.5 exposure on glucose tolerance by intra-peritoneal glucose tolerance test (IPGTT) in C57BL/6 mice and in p47phox−/− mice, respectively. B and F, The glucose area under the curve calculated from the glucose tolerance test from parts A and E, respectively. C and G, The homeostasis model assessment IR index in C57BL/6 mice and in p47phox−/− mice, respectively. D and H, Plasma cytokine measurement by ELISA in C57BL/6 mice and in p47phox−/− mice, respectively. n = 8 in each group. *P<0.05 and **P<0.001. IFN indicates interferon; MCP-1, monocyte chemoattractant protein-1; RANTES, regulated on activation, normal T cell expressed and secreted (or chemokine C-C motif ligand 5).
Figure 2.
Effect of PM2.5 exposure on abdominal fat and distribution, chemotactic migration, and superoxide production in wild-type C57BL/6 mice and p47phox−/− mice. A and E, Abdominal fat measured by MRI in C57BL/6 mice and p47phox−/− mice, respectively. B and F, Visceral and subcutaneuous fat (fat distribution) in the abdomen, measured by MRI, in C57BL/6 mice and p47phox−/− mice, respectively. C and G, Chemotactic migration of monocytes in C57BL/6 mice and p47phox−/− mice, respectively. D and H, Superoxide anion measurement by chemiluminescence in visceral and subcutaneous fat tissues in wild-type C57BL/6 and p47phox−/− mice, respectively. *P<0.05 and **P<0.001. 5HPF indicates 5 high-power fields.
Because the p47 subunit of the NADPH oxidase is homologous in both phagocytic and nonphagocytic cells, and is critical for a functional NADPH oxidase,20,21 we next investigated the importance of the NADPH oxidase in mediating the altered metabolic profile and IR in response to PM2.5 and diet. Age-matched male p47phox−/− mice were exposed to PM2.5 or FA using the same exposure protocol as the wild-type C57BL/6 mice. We found that there was no significant additional effect of PM2.5 on body weight in the different diet feeding groups (supplemental Table II). Glucose homeostasis in response to glucose loading from the PM2.5-exposed mice was comparable to that in the FA-exposed p47phox−/− mice (Figure 1E and 1F). Although an HFD led to a sharp increase in blood glucose levels, the increase was similar for PM2.5- and FA-exposed mice. Homeostasis model assessment IR indexes from the PM2.5-exposed p47phox−/− mice were significantly attenuated and comparable to those of the FA-exposed mice fed an ND (Figure 1G). Plasma inflammatory biomarkers in the p47phox−/− mice were similar to those in the wild-type C57BL/6 mice. Notably, the absence of a functional NADPH oxidase abrogated the previously noted difference in TNF-α in wild-type C57BL/6 with PM2.5 exposure (Figure 1H). Neither visceral nor subcutaneous fat in the abdomen, measured by MRI, was significantly different between the ND-fed groups exposed to PM2.5 or FA, which differed from the results in wild-type C57BL/6 mice (Figure 2E and 2F and supplemental Figure IF). In p47phox−/− mice, adipocyte size in the PM2.5-exposed mice fed an ND was similar to that in the FA-exposed mice on the same diet in either visceral fat (FA, 1221±50 µm2; PM2.5, 1184±38 µm2; P>0.05) or subcutaneous fat (FA, 769±44 µm2; PM2.5, 910±29 µm2; P>0.05) (supplemental Figure III). On the other hand, the adipocyte area of HFD-fed p47phox−/− mice was increased, albeit smaller than in the HFD-fed wild-type C57BL/6 mice, with considerable variation in size distribution.
Chemotaxis and Superoxide Generation
To investigate potential alteration in chemokine factors within visceral adipose tissue, we evaluated the chemotactic ability of conditioned media derived by culturing adipose samples from visceral and subcutaneous fat locations. The migratory capacity of monocytes was tested by quantifying the number of monocytes moving toward the chamber containing conditioned media. In wild-type C57BL/6 mice, more monocytes migrated toward conditioned media from visceral fat from mice exposed to PM2.5 fed an ND or an HFD when compared with those exposed to FA (Figure 2C). In contrast, there was no increase in cell migration with conditioned media from subcutaneous fat locations. The generation of an NADPH-derived anion by the adipose tissue was further assessed by a lucigenin-enhanced chemiluminescence technique. Interestingly, production was significantly increased in the epididymal fat (visceral fat) of the mice exposed to PM2.5 compared with the FA group, but not in the subcutaneous fat location (Figure 2D). However, in p47phox−/− mice, as shown in Figure 2G, differential chemotactic responses in response to PM2.5 exposure in the visceral fat of wild-type C57BL/6 mice were abolished in the p47phox−/− mice. Furthermore, there was no increase in generation in response to PM2.5 in the p47phox−/− mice exposed to PM2.5 (Figure 2H).
Inflammation in Visceral Adipose Tissue and Microcirculatory Dysfunction
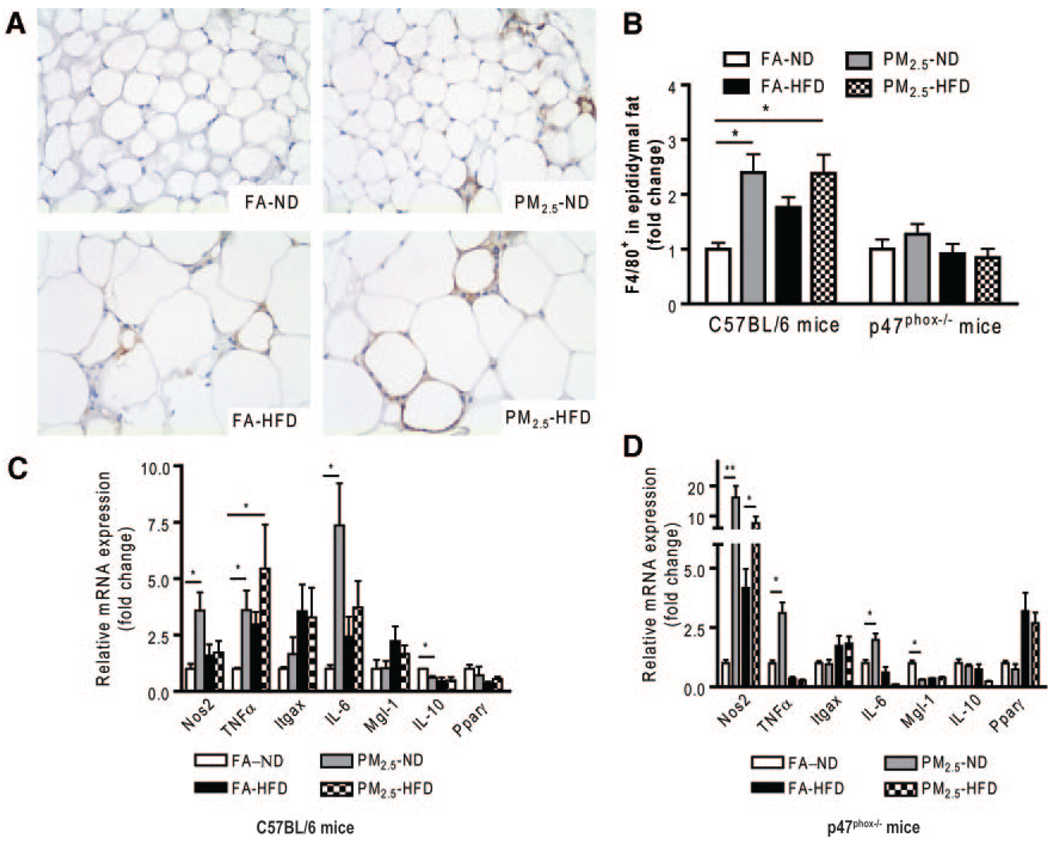
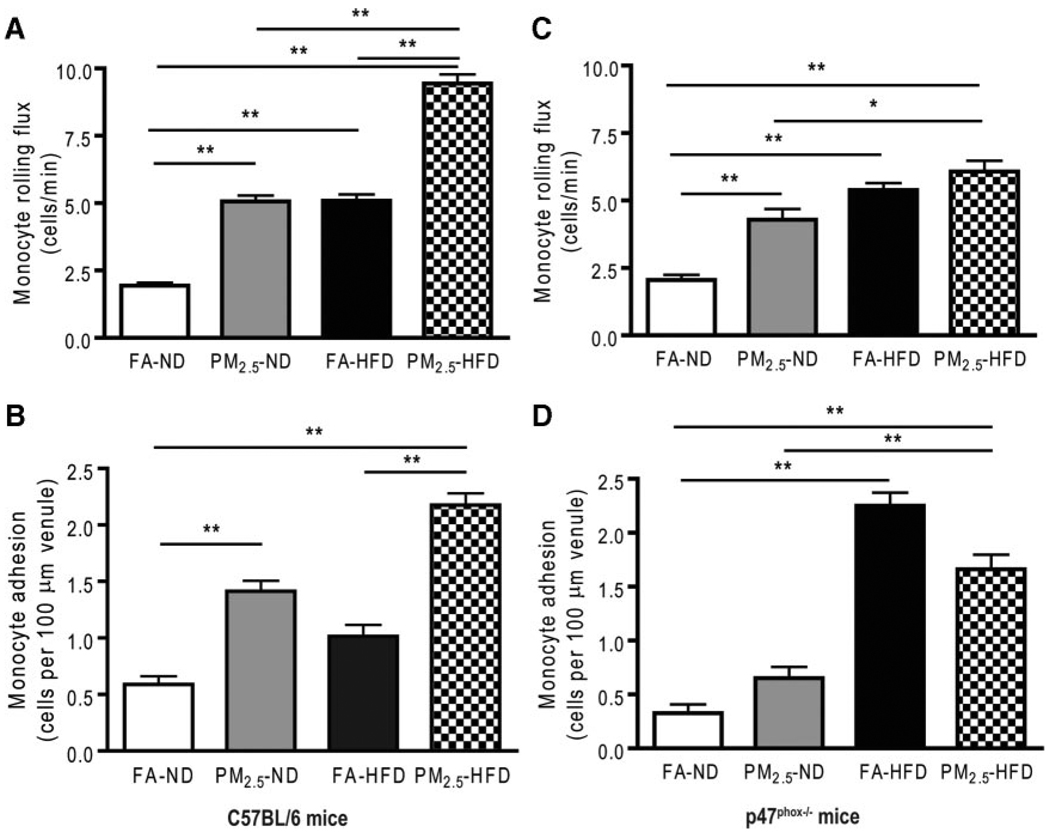
Adipose tissue macrophages (ATMs), which are thought to represent key cellular mediators of adipose tissue inflammatory response and IR development, were examined in C57BL/6 mice. PM2.5 exposure resulted in an increase in F4/80+ macrophages in epididymal (visceral) adipose tissue (Figure 3A and 3B). We then investigated the expression of ATM-specific genes in the macrophage-rich stromal vascular fraction of visceral adipose tissue (Figure 3C). PM2.5 exposure led to a significant increase in the expression of proinflammatory genes (M1, or “classically” activated) TNF-α, NO synthase 2, and interleukin (IL) 6, with no changes in integrin αX expression (Figure 2E). In contrast, IL-10, which is expressed at high levels in alternatively activated macrophages and adipocytes, was significantly downregulated by PM2.5 exposure. These results demonstrate that PM2.5 exposure downregulated genes associated with an anti-inflammatory M2 phenotype while inducing a proinflammatory phenotype. In view of the inflammatory macrophage phenotype in adipose tissue, we hypothesized that accelerated recruitment into adipose tissue may represent an important mechanism, as demonstrated by a prior study.22 At the end of the exposure to PM2.5 or FA, intravital microscopy with labeled monocytes/macrophages was performed to evaluate the number of rolling and adherent cells, as an index of recruitment into tissue depots. The results show that PM2.5 exposure resulted in an increase in adherent and rolling monocytes in the microcirculation when compared with the FA-exposed mice. There was an important synergistic effect of HFD in terms of exaggerating the PM2.5 effects (Figure 4A and 4B). Interestingly, there was no increase in ATM content in the p47phox−/− mice exposed to PM2.5 (Figure 3B), although gene expressions were similar to those in wild-type C57BL/6 mice (Figure 3D). Intravital microscopy showed that the increased adherence of monocytes in the cremaster microcirculation observed in the wild-type C57BL/6 mice that were exposed to PM2.5 was significantly decreased by the deletion of p47phox (Figure 4C and 4D). Although the number of rolling monocytes in response to an HFD was increased in the p47phox−/−, the extent was attenuated when compared with HFD-fed mice and either PM2.5 or FA exposure, suggesting that mechanisms other than NADPH oxidase may be involved.
Figure 3.
Effect of PM2.5 exposure on macrophage inflammation and gene expression in visceral adipose tissue in wild-type C57BL/6 and p47phox−/− mice. A, Representative images of immunohistochemical staining for macrophages (F4/80+) in visceral fat tissue. B, Statistical analysis of F4/80+ macrophage infiltration in visceral fat tissue in C57BL/6 and p47phox−/− mice. C and D, Real-time polymerase chain reaction analysis for macrophage M1/M2 phenotypic changes in C57BL/6 and p47phox−/− mice, respectively. Itgax indicates integrin αX (or CD11c); Mgl-1, macrophage galactose N-acetyl-galactosamine specific lectin-1; Nos2, NO synthase-2; Pparγ, peroxisome proliferator–activated receptor γ. *P<0.05 and **P<0.001.
Figure 4.
Effect of PM2.5 exposure on microvascular dysfunction in wild-type C57BL/6 and p47phox−/− mice. A and C, Monocyte rolling flux in cremasteric microcirculation via intravital microscopy in C57BL/6 and p47phox−/− mice, respectively. B and D, Monocyte adhesion in cremasteric microcirculation via intravital microscopy in C57BL/6 and p47phox−/− mice, respectively. *P<0.05 and **P<0.001.
Vasomotor Responses
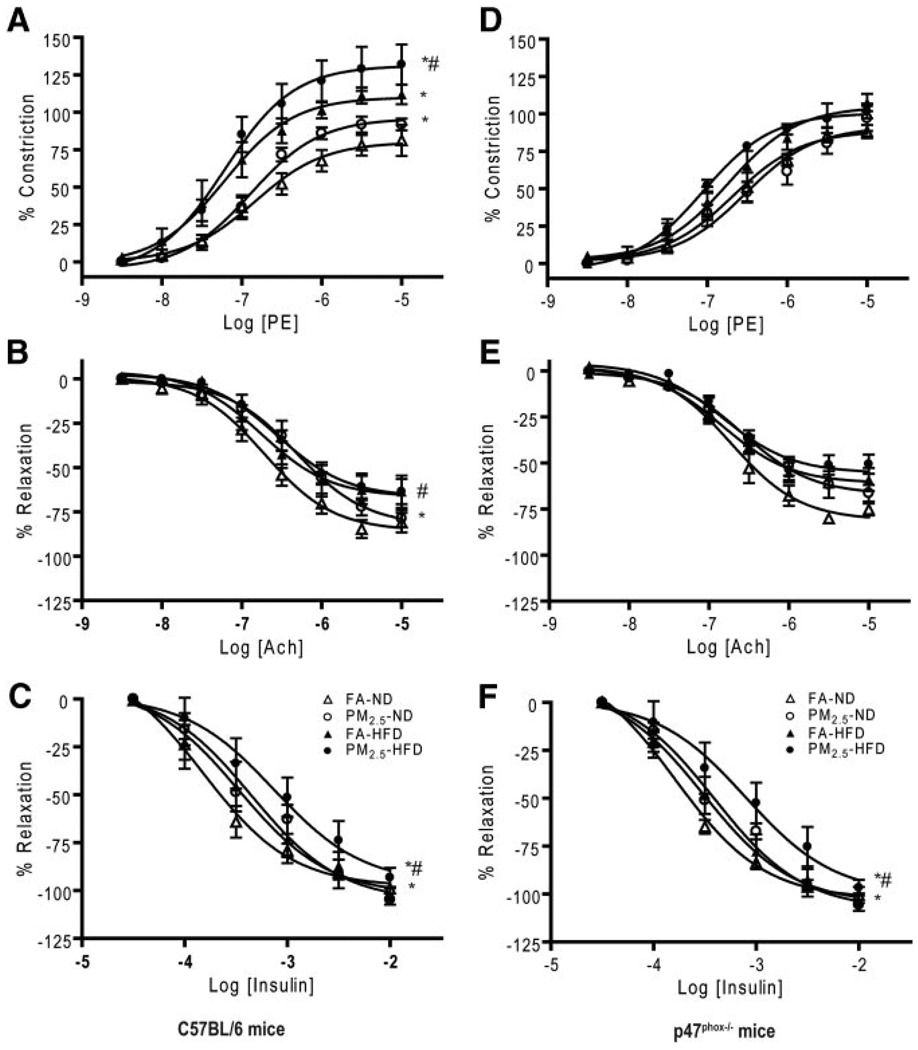
We then examined vascular responses to endothelium-dependent agonists, including insulin. Endothelial dysfunction and altered insulin sensitivity in blood vessels are characteristic of IR. As shown in Figure 5A through 5C and the Table, PM2.5-exposed wild-type C57BL/6 mice exhibited an enhanced constriction response to phenylephrine and a decreased relaxation response to endothelium-dependent vasodilator acetylcholine. The relaxation response to insulin was also reduced by PM2.5 exposure. PM2.5-exposed p47phox−/− mice fed an ND displayed a similar constriction response to phenylephrine and a relaxation response to acetylcholine when compared with the FA-exposed ND-fed mice (Figure 5D–5F and Table), although the response to insulin in p47phox−/− mice was comparable to that in the wild-type C57BL/6 mice. These data, collectively, indicate an important role for p47phox and NADPH oxidase in mediating responses to PM2.5.
Figure 5.
Effect of PM2.5 exposure on macrovascular dysfunction in wild-type C57BL/6 and p47phox−/− mice. A through F, Vasomotor tone change in aortic rings via myograph in response to phenylephrine (A and D), acetylcholine (B and E), or insulin (C and F) in C57BL/6 and p47phox−/− mice, respectively. *P<0.05 vs FA-ND, and #P<0.05 vs FA-HFD.
Table 1.
Vascular Responses to Phenylephrine, Acetylcholine, and Insulin From Aortic Rings in Wild-Type C57BL/6 Mice and in p47phox−/− Mice*
| Agent | FA-ND | PM2.5-ND | FA-HFD | PM2.5-HFD |
|---|---|---|---|---|
| C57BL/6 Mice | ||||
| Phenylephrine | ||||
| Peak constriction, % | 80.1± −4.6 | 96.0± −2.8† | 110.1± −5.5† | 131.2± −6.4†‡ |
| EC50, mol/L | 1.4×10−7±0.1×10−7 | 1.3×10−7±0.1×10−7 | 0.6×10−7±0.2×10−7 | 0.6×10−7±0.1×10−7 |
| Acetylcholine | ||||
| Peak relaxation, % | −85.7± −3.5 | −82.6± −4.7 | −66.5± −4.6 | −66.5± −3.8 |
| ED50, mol/L | 1.9×10−7±0.1×10−7 | 4.9×10−7±0.1×10−7† | 1.8×10−7±0.2×10−7 | 2.8×10−7±0.1×10−7‡ |
| Insulin | ||||
| Peak relaxation, % | −98± −4.6 | −105.7± −6.0 | −102.1± −7.4 | −96.3± −10.5 |
| ED50, mol/L | 1.5×10−4± −0.1×10−4 | 4.5×10−4± −0.1×10−4† | 3.4×10−4± −0.2×10−4 | 7.4×10−4± −0.2×10−4†‡ |
| p47phox−/− Mice | ||||
| Phenylephrine | ||||
| Peak constriction, % | 88.8±4.5 | 91.4±5.2 | 104.9±5.3 | 100.6±1.9 |
| EC50, mol/L | 2.1×10−7±0.1×10−7 | 3.0×10−7±0.1×10−7 | 1.8×10−7±0.1×10−7 | 0.9×10−7±0.1×10−7 |
| Acetylcholine | ||||
| Peak relaxation, % | −81.1±3.0 | −67.0±3.2† | −61.0±2.7 | −55.5±2.9 |
| ED50, mol/L | 1.9×10−7±0.1×10−7 | 2.6×10−7±0.1×10−7 | 1.5×10−7±0.1×10−7 | 1.5×10−7±0.1×10−7 |
| Insulin | ||||
| Peak relaxation, % | −102.6±2.6 | −108.1±3.4 | −105.0±5.6 | −100.1±10.9 |
| ED50, mol/L | 1.6×10−4± −0.1×10−4 | 3.9×10−4± −0.1×10−4† | 3.1×10−4± −0.1×10−4 | 7.9×10−4± −0.2×10−4†‡ |
EC50 indicates the half-maximal dose for constriction; ED50, half-maximal dose for dilation.
Data are given as mean±SE.
P<0.05 vs FA-ND.
P<0.05 vs FA-HFD.
PM2.5 Exposure Induces p47phox Phosphorylation
On activation, a p47phox cytosolic subunit phosphorylates, translocates to the membranes, and associates with the membrane-bound components. Then, the newly assembled enzyme complex actively catalyzes the production of . Therefore, we next wanted to know if exposure to PM2.5, regardless of diet impact, may have effects on the p47phox subunit. As shown in supplemental Figure IVA and IVB, PM2.5 exposure significantly increased the phosphorylation of the p47phox subunit of NADPH oxidase in epididymal adipose tissue compared with the FA-exposed control, indicating the effect of PM2.5 exposure on NADPH oxidase.
Discussion
In this study, we evaluated the role of early-life inhalation exposure to concentrated airborne PM2.5 on systemic and adipose tissue inflammation and susceptibility to IR development in adulthood. There are several important findings in this study. First, early-life PM2.5 exposure, even in the absence of dietary indiscretion, is sufficient to induce metabolic dysfunction and inflammation (“metaflammation”). Second, PM2.5 exposure for only 10 weeks is sufficient to induce visceral adiposity and vascular dysfunction, consistent with IR. Finally, NADPH oxidases may play a critical role in PM2.5-induced development of T2DM/IR.
Risk factors, such as inappropriate diet and inactivity, play fundamental roles in the propensity for T2DM.14,23,24 Underlying genetics also provide an explanation, particularly among certain defined populations.25,26 On the other hand, the link between exposure to environmental factors in the air/water and propensity for cardiometabolic disorders has only recently gained attention.7,8,27,28 This issue is of special importance given the extraordinary confluence of changing levels of airborne and water pollutants and shifts in diet/exercise in urban populations. There appear to be additional interactions between the effects of inhaled PM2.5 and metabolic disorders, such as T2DM, as shown by the study of O’Neill et al,29 in which diabetic individuals seemed more vulnerable to PM-associated impairment in endothelial function.
Our study provides important insights into potential mechanisms by which PM2.5 may induce obesity/IR. The mechanisms seem broadly similar to other dietary mediators, such as an HFD, in which an important role for innate immune mechanisms has been highlighted.30 Interestingly, our studies clearly show a phenotypic shift in response to PM2.5 exposure, which is typified by enhanced ATM infiltration, proinflammatory gene expression, and an altered chemokine profile that may play important roles in stimulating infiltration of these tissues by monocytes. Although this study was not designed to address the relative importance of phagocyte-versus non–phagocyte-derived NADPH oxidases, several findings suggest that phagocyte derived NADPH oxidases may be playing an important role. Phagocytic NADPH oxidases are well-known to produce much at levels that are several orders of magnitude higher than those of nonphagocytic systems.31,32 In addition, there were further indirect observations in this study that support the concept that activation of monocytes/macrophages in visceral adipose tissue may contribute to the excess levels noted in the study. These findings include the following: (1) normalization of ATM content in the p47phox−/− and generation, (2) reduction in the recruitment of monocytes/macrophages into the microcirculation in labeled monocyte experiments, (3) reduction in chemotactic responses to monocytes with deletion of p47phox, and (4) attenuation of classically activated M1 gene expression in visceral adipose tissue of TNF-α and IL-6 in p47phox−/− mice. However, there were additional findings that support the involvement of nonphagocytic NADPH oxidase pathways or distinct pathways unrelated to oxidant systems. For instance, there was continued upregulation of TNF-α and IL-6 (albeit attenuated) gene expression in the p47phox−/−, suggesting that reactive oxygen species and/or NADPH oxidase independent pathways may still be involved, especially in response to HFD and potentially in response to PM2.5. Interestingly, NO synthase 2 gene expression was markedly increased in response to PM2.5 in the p47phox−/−. The reasons for this are unclear at this point. In addition, adipocyte remodeling seen with HFD feeding continued in the p47phox−/− mice, suggesting reactive oxygen species– and/or NADPH oxidase–independent pathways in the pathogenesis of IR. Thus, our data suggest an important interaction of PM2.5 in activating NADPH oxidases in both phagocytic and potentially nonphagocytic cells and are consistent with an important role for the p47phox cytosolic subunit. Further studies in tissue-specific and conditional knockout models will be needed to distinguish relative contributions of the phagocyte NADPH oxidase from nonphagocyte systems. Taken together, our findings are consistent with the studies by Furukawa et al18 and Schroder et al33; they also support an important role for early priming by PM2.5 exposure. The increase in ATM was paralleled by an increase in adipose tissue size and visceral adipose tissue content in the PM2.5-exposed group. Adipose tissue synthesizes and secretes proinflammatory substances, which are upregulated in obesity and play important roles in mediating obesity-linked IR.12,14 Accumulating evidence indicates that visceral fat is the most predictive of postprandial glucose levels, indexes of IR, and cardiovascular disease.34–37 Thus, adipose tissue and adipose tissue– derived signals may be key mediators and may play a pivotal role in PM2.5-induced adiposity and IR.
Many studies38,39 have shown that exposure to PM2.5 is associated with a systemic proinflammatory response (eg, increased TNF-α and IL-6 levels) in humans and animals. Brook et al40 previously showed that long-term exposure to nitrogen dioxide (NO2) (a marker of traffic-related PM exposure) was associated with the prevalence of diabetes among women. Across the interquartile range (approximately 4 parts per billion NO2) in 2 cities in Canada, there was an approximate 17% increase in the odds of diabetes. This association was likely because of NO2-associated vehicle-related PM exposure, not a biological effect of NO2 itself. In a recent set of key mechanistic experiments, in which 12 weeks of HFD feeding of adult male C57BL/6 mice that were subsequently exposed to PM2.5 demonstrated overt glucose intolerance and diabetes.8 MRI measurements in these mice revealed an increase in visceral fat with abnormalities in phosphatidylinositol 3-kinase/Akt/endothelial NO synthase signaling and adipocytokines. In contrast, findings from an earlier study8 demonstrated an important synergistic effect of diet with PM2.5 exposure, with the current study revealing an effect of PM2.5 alone in the absence of HFD. In this study, exposure began at the age of 3 weeks and was continued for 10 weeks; the previous study focused on the adult mice (aged > 18 weeks) that were already considered obese (HFD feeding for 10 weeks before PM2.5 exposure). The finding that PM2.5 exposure alone, in the absence of dietary influences, over a shorter time than in a previously reported experiment was sufficient to induce changes characteristic of IR and obesity is particularly noteworthy. This suggests that early-life exposure may play an important priming influence in the eventual development of obesity.
The p47phox cytosolic subunit plays a pivotal role in NADPH oxidase activation in inflammatory cells by providing physical binding domains to cytochrome b558 and p67phox, and genetic mutations of p47phox lead to loss of production during cell activation.41,42 Several studies43–45 in animals and humans have shown an important role for this subunit in inflammatory disorders, such as IR, and complications of diabetes. p47phox has been regulated via phosphorylation by IL-1 receptor–associated kinase 4 in response to Toll-like receptor (TLR) 4 ligation.46,47 Diet-induced obesity and IR are well-known to elevate multiple mediators of TLR4 activation, including via endogenous fatty acids.48,49 Chen and colleagues50 showed that diet-induced obesity activates NADPH oxidase and mediates the expression of TLR in the vascular tissues, whereas deficiency of the NADPH oxidase subunit p47phox attenuates inflammatory response and neointimal formation in mice with diet-induced obesity. In line with these findings, we recently demonstrated that oxidized phospholipids generated in response to PM2.5 exposure in the lung and perhaps in the systemic circulation lead to activation of NADPH oxidase via TLR4/IL-1 receptor–associated kinase-dependent pathways (T.K., A.M., Z.Y., Z.S., J.A.D, X.X., N.K., R.D.B., K.M.R., N.P.P., S.P., L.C.C., S.M-B, Q.S., H.M., S.R., unpublished data, 2010). From our current data, the increase in inflammation and oxidative stress seemed to be associated with p47phox phosphorylation in response to PM2.5 exposure.
In previous studies,51,52 long-term effects of concentrated ambient particle exposure on heart rates and heart rate variability in C57 and apolipoprotein E–knockout mice were observed. Although the experimental procedure of placing the mice in the chamber itself increased physical activity by 0.9 counts, there were no relationships with concentrated ambient particle concentration. Moreover, heart rate change during the exposure period was marginally associated with the concentrated ambient particle level. A potential limitation of the present study is that the exposure procedure might alter activity in home cages, although we deliberately exposed the mice during the daytime, while the mice were sleeping as they normally do, as part of their sleep/wake cycle. The mice of all groups were under the same experimental conditions (ie, light-dark cycle) except that a high-efficiency particulate air filter was positioned in the inlet valve to the exposure system to remove all of the PM2.5 from that air stream. Furthermore, the mice are not able to move around ad libitum during the exposure because the space/cell for each mouse is limited or restricted (approximately 6×7 cm).
In summary, our data suggest an important impact of early-life exposure to PM2.5 exposure on T2DM/IR development. The investigation of environmental factors, such as PM2.5 air pollution, on the increased susceptibility to T2DM/IR development is significant; these findings suggest an important public health impact on the health of both children and adults. Further understanding of the factors regulating the activation and accumulation of macrophages in the adipose tissue may provide novel prevention and therapeutic strategies for better control and treatment of obesity, T2DM, and IR.
Supplementary Material
Acknowledgments
We thank Silis Y. Jiang for superb technical assistance.
Sources of Funding
This study was supported by the Diabetes Action Research and Education Foundation (#268); and grants RO1 ES015146 (Dr Rajagopalan) and R21 ES017412 and K01 ES016588 (Dr Sun) from the National Institutes of Health.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Schwartz J. Air pollution and hospital admissions for heart disease in eight U.S. counties. Epidemiology. 1999;10:17–22. [PubMed] [Google Scholar]
- 2.Pope C, III, Thun M, Namboodiri M, Dockery D, Evans J, Speizer F, Heath C., Jr Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 3.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 4.Kan H, Chen B, Hong C. Health impact of outdoor air pollution in China: current knowledge and future research needs. Environ Health Perspect. 2009;117:A187. doi: 10.1289/ehp.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 6.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 7.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattsson N, Ronnemaa T, Juonala M, Viikari JS, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood: the Cardiovascular Risk in Young Finns Study. Ann Med. 2008;40:542–552. doi: 10.1080/07853890802307709. [DOI] [PubMed] [Google Scholar]
- 10.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 13.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 14.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 16.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 17.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LC, Nadziejko C. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice, V: CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhal Toxicol. 2005;17:217–224. doi: 10.1080/08958370590912815. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 21.Lasségue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunstan DW, Barr EL, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121:384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 24.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baier LJ, Hanson RL. Genetic studies of the etiology of type 2 diabetes in Pima Indians: hunting for pieces to a complicated puzzle. Diabetes. 2004;53:1181–1186. doi: 10.2337/diabetes.53.5.1181. [DOI] [PubMed] [Google Scholar]
- 26.Wolf G. Insulin resistance associated with leptin deficiency in mice: a possible model for noninsulin-dependent diabetes mellitus. Nutr Rev. 2001;59:177–179. doi: 10.1111/j.1753-4887.2001.tb07009.x. [DOI] [PubMed] [Google Scholar]
- 27.Bhatnagar A. Could dirty air cause diabetes? Circulation. 2009;119:492–494. doi: 10.1161/CIRCULATIONAHA.108.831404. [DOI] [PubMed] [Google Scholar]
- 28.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A. 2009;106:10740–10745. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal. 2006;8:1443–1445. doi: 10.1089/ars.2006.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T. Vascular effects following homozygous disruption of p47(phox): an essential component of NADPH oxidase. Circulation. 2000;101:1234–1236. doi: 10.1161/01.cir.101.11.1234. [DOI] [PubMed] [Google Scholar]
- 33.Schroder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 34.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 35.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120:S3–S8. doi: 10.1016/j.amjmed.2006.11.012. discussion, S29–S32. [DOI] [PubMed] [Google Scholar]
- 36.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 37.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 38.Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calderon-Garciduenas L, Villarreal-Calderon R, Valencia-Salazar G, Henriquez-Roldan C, Gutierrez-Castrellon P, Torres-Jardon R, Osnaya-Brizuela N, Romero L, Torres-Jardon R, Solt A, Reed W. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- 40.Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50:32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- 41.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 42.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- 43.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 44.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 45.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 46.Pacquelet S, Johnson JL, Ellis BA, Brzezinska AA, Lane WS, Munafo DB, Catz SD. Cross-talk between IRAK-4 and the NADPH oxidase. Biochem J. 2007;403:451–461. doi: 10.1042/BJ20061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 49.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen JX, Stinnett A. Critical role of the NADPH oxidase subunit p47phox on vascular TLR expression and neointimal lesion formation in high-fat diet-induced obesity. Lab Invest. 2008;88:1316–1328. doi: 10.1038/labinvest.2008.92. [DOI] [PubMed] [Google Scholar]
- 51.Hwang JS, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice, III: acute and chronic effects of CAPs on heart rate, heart-rate fluctuation, and body temperature. Inhal Toxicol. 2005;17:199–207. doi: 10.1080/08958370590912761. [DOI] [PubMed] [Google Scholar]
- 52.Chen LC, Hwang JS. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice, IV: characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart-rate variability. Inhal Toxicol. 2005;17:209–216. doi: 10.1080/08958370590912789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.