Abstract
Background
It has been suggested that the benefits of drug-eluting stents compared to bare metal stents (BMS) have been over-estimated in part because target lesion/vessel revascularization (TLR/TVR) rates in the BMS control group of these trials were spuriously high.
Methods
We used meta-analytic techniques to systematically compare clinical event rates among patients treated with BMS in trials where BMS were the experimental (BMSexperimental) rather than the control (BMScontrol) intervention. MEDLINE searches were performed to identify eligible randomized trials comparing either drug-eluting stents with BMScontrol, or BMSexperimental with balloon angioplasty in patients with non-acute coronary artery disease. Trial characteristics and 6 to 12 month rates for death, myocardial infarction, TLR/TVR and major adverse cardiac events (MACE) were extracted and assessed.
Results
Eligible trials yielded 50 BMS cohorts: 19 in the BMScontrol group (4 046 patients) and 31 in the BMSexperimental group (5 068 patients). Summary death and infarction rates did not differ between groups. The summary TLR/TVR rates were 16.2% (95% confidence interval, CI: 13.5, 19.3) versus 13.8% (95% CI: 12.0, 15.7) in BMScontrol versus BMSexperimental groups, respectively (p=0.15). Among 39 BMS cohorts with ≤250 patients, TLR/TVR rates were significantly higher in BMScontrol versus BMSexperimental groups (18.9% [95% CI: 16.0, 22.2] versus 13.7% [95% CI: 11.5, 16.3], p=0.01). There were no between-group differences among larger BMS cohorts (p=0.98).
Conclusions
While overall clinical event rates did not differ in the BMScontrol and the BMSexperimental groups, a higher rate of TVR/TLR was seen in the BMScontrol group among smaller trials.
Introduction
Over the last two decades, there have been several major technological innovations in the management of non-acute coronary artery disease. Over this period, percutaneous balloon angioplasty has been shown to improve outcomes compared to medical therapy alone;1 bare metal stents (BMS) have been shown to improve outcomes compared to balloon angioplasty;2 and, in turn, drug eluting stents (DES) have been shown to improve outcomes compared to BMS.3
However, the apparent progress has been largely based on non-uniform and composite outcomes that typically combine hard endpoints (such as death and myocardial infarction) with softer endpoints (such as need for revascularization). Indeed, meta-analyses have largely ruled out substantial improvements in myocardial infarction or mortality rates in the aforementioned succession of intervention comparisons.2,4,5
In the case of stents for coronary disease, technological innovations have been adopted mainly on the basis of softer endpoints: Benefits have been limited to a sequential decrease in the need for target lesion/vessel revascularization (TLR/TVR), an outcome much more common than death or infarctions. TLR/TVR is used in the context of protocol-driven angiography in clinical trials. It does not directly reflect patient response to clinical care, and is sensitive to physician judgment (and potentially to bias). A recent narrative review suggested that the TLR/TVR rate in the BMS control group of several DES trials may have been higher than expected compared to some other contemporary BMS trials.6
Such an observation might signal a more general, previously undescribed tendency toward “control rate inflation” with soft outcomes – something that might bias trial results in favor of the experimental intervention. We sought to systematically examine this possibility the rate of TLR/TVR in trials in which BMS were the experimental (BMSexperimental) intervention, compared to trials in which they were the control (BMScontrol) intervention.
Methods
Database formation and study eligibility
We utilized two comprehensive meta-analyses performed by the same team2,3 as a starting basis for our search. The meta-analyses evaluated randomized controlled trials comparing BMS with balloon angioplasty2, and DES with BMS3 in patients with coronary artery disease who were not treated for acute coronary syndromes. These two meta-analyses were updated from the end of their coverage period onwards, using similar MEDLINE search strategies (last search 08/21/2006) and the same eligibility criteria. Later-published meta-analyses from different teams were also searched for additional eligible randomized trials.5,7
Eligible trial reports randomized more than 10 patients per arm and evaluated the clinical outcomes of death, myocardial infarction, TLR/TVR, and major adverse cardiac events (MACE, defined as the first occurring of the aforementioned outcomes) at follow up times between 6 and 12 months. Outcomes from the latest available time point (within the window of 6 to 12 months) were collected.
Data extraction
From each trial, we extracted information describing patient characteristics (proportion of males, mean age and age range, and proportion with diabetes); trial characteristics and design (follow-up time; year that enrollment started; presence of protocol-driven co-treatments with antiplatelet agents; presence and timing of protocol-driven follow-up angiography; and type of bare metal stents); and the aforementioned clinical outcomes, including whether MACE was based on target lesion or target vessel revascularization.
Meta-analyses
TLR (or, where not available, TVR) was the primary endpoint of this study. Trial arms that used BMS were considered as individual prospective cohorts, and were classified in two cohort groups, BMScontrol and BMSexperimental. Summary event rates per group were calculated with random effects meta-analyses using the DerSimonian and Laird method8 after a logit transformation, and were compared with a z-score, as previously described.9 We tested for heterogeneity with Cochran’s Q (considered significant at p<0.10), and quantified it’s extent with I2.10 I2 represents the proportion of between-study variability that is attributable to heterogeneity rather than chance.10
Subgroup and Sensitivity Analysis
Based on prior literature suggesting greater treatment effects in smaller versus larger trials,9,11,12 subgroup analysis was used to test the hypothesis that smaller trials might be more vulnerable to a control rate inflationary bias, should one exist. We arbitrarily defined as smaller BMS cohorts with ≤250 patients (i.e., we defined a trial of 500 people or less as being small, and assumed 1:1 patient allocation). We also assessed whether blinding of the patients or the outcome assessors differentiated the findings. In sensitivity analyses we excluded BMS cohorts from trials specifically targeting patients with difficult lesions, i.e. complex lesions or lesions in small arteries (≤3.0mm diameter), since these might have especially high revascularization rates.
Meta-regression
We used random-effects13 and fixed effects meta-regressions to adjust for the following trial-level characteristics: follow-up length, initial year of patient enrollment (as a proxy for evolution in patient care), whether the trial specifically targeted patients with difficult (i.e. complex, long or small vessel) lesions (yes/no) and trial size (square-root-transformed).
The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Characteristics of the treatment and control cohorts were compared with Mann-Whitney tests. All p-values are two-tailed and considered significant at the 0.05 level. Analyses were conducted in Intercooled Stata 8.2 (Stata Corporation, College Station, TX, US).
Results
Based on the selected meta-analyses and the updated searches 74 papers were retrieved in full text, and 50 were deemed eligible: Thirty-one describing BMSexperimental cohorts (published from 1994 to 2005, 5 068 patients) and 19 describing BMScontrol cohorts (published from 2001 to 2006, 4 046 patients) (see Supplementary Table). Cohort sample sizes, proportion of male enrollees and proportion of enrollees with diabetes did not differ beyond chance across the two groups (Table 1). On median, the average patient age was 62 years versus 60 years in the BMSexperimental and BMScontrol cohorts, respectively. BMScontrol cohorts had longer median follow-up times (median 9 versus 6 months), and belonged to trials that started enrolling patients in more recent years (median 2001 versus 1995) (Table 1). All trials had protocol-driven angiography.
Table 1.
Comparison of trial and arm characteristics of the two cohort groups
| Characteristic | BMScontrol | BMSexperimental | p |
|---|---|---|---|
| Number of cohorts (patients) | 19 (4 046) | 31 (5 068) | NA |
| Patients randomized to BMS | 140 (50, 263) | 125 (57, 204) | 0.69 |
| Publication year, median (IQR) | 2004 (2003, 2005) | 2000 (1998, 2000) | <0.01 |
| Enrollment initiation year, median (IQR) * | 2001 (2000, 2002) | 1995 (1992, 1996) | <0.01 |
| Follow up (months) | 9 (9, 12) | 6 (6, 12) | 0.01 |
| Proportion of males, (%) | 73 (70, 80) | 77 (72, 82) | 0.53 |
| Proportion of diabetics, (%)† | 21 (16, 25) | 17 (11, 21) | 0.10 |
| Mean participant age (years)‡ | 62 (62, 63) | 60 (58, 61) | <0.01 |
| Protocol driven angiography | 19/19 | 31/31 | 1.00 |
| Stent strut thickness ≤0.1mm|| | 1/11 | 17/27 | <0.01 |
five BMScontrol and six BMSexperimental cohorts did not provide this information
three BMSexperimental cohorts did not provide this information
one BMScontrol and two BMSexperimental cohorts did not provide this information
could not be ascertained in eight BMScontrol and four BMSexperimental cohorts
BMS: bare metal stents; IQR: interquartile range
We were able to determine strut thickness on 38 of the 50 trials. In the BMScontrol group 1 of 11 trials (9%) used only stents with strut thickness <0.1mm. The corresponding proportion in the BMSexperimental group was 17 out of 27 trials (63%) (p<0.01 for this comparison).
Twelve of the 50 trials presented data for both TLR and TVR rates (five in the BMSexperimental and seven in the BMScontrol group). Among them, the median difference (TVR minus TLR) in the outcome rate was 1.1% (IQR: 0.0 to 3.0%). TLR was not available in 18 of 31 BMSexperimental trials and 2 of 19 BMScontrol trials, and was substituted with TVR.
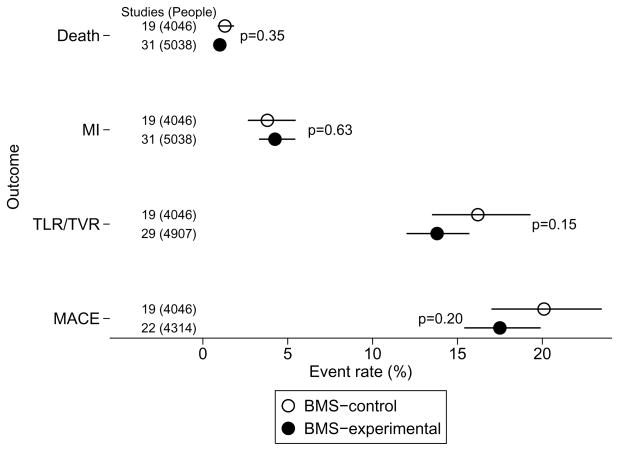
There was extensive between trial (within group) heterogeneity in the rate of TLR/TVR (See Supplementary Figure) (p<0.001, I2>67% for both cohort groups), indicating much greater between study variability than would be expected by chance alone. Despite a trend toward more frequent outcomes in BMScontrol cohorts, the summary rates did not differ significantly between the two groups: 16.2% (95% confidence interval, CI: 13.5, 19.3%) in the BMScontrol and 13.8% (95% CI: 12.0, 15.7%) in the BMSexperimental group (p = 0.15) (Figure 1). MACE rates, which are grossly determined by TLR/TVR rates, were 20.1% (95% CI: 17.0, 23.5%) in the BMScontrol cohorts and 17.5% (95% CI: 15.4, 19.9%) in the BMSexperimental cohorts (p=0.20) (Figure 1), again with extensive between study heterogeneity (p<0.001, I2>69% for both groups).
Figure 1.
Summary outcome rates in BMScontrol versus BMSexperimental cohorts
BMS: bare metal stents; MACE: major cardiac adverse events; MI: myocardial infarction; TLR/TVR: target lesion/vessel revascularization. The figure illustrates the summary event rates (random effects meta-analysis) in the two groups (BMScontrol versus BMSexperimental) for death, MI, TLR/TVR, and MACE. Dots stand for the point estimate of the summary rates and horizontal lines represent the 95% confidence intervals. P-values are for between group differences and are unadjusted for multiple comparisons.
Subgroup and sensitivity analysis
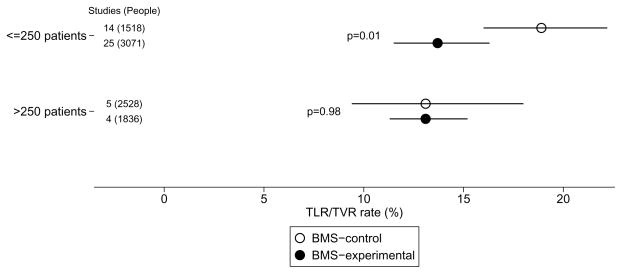
In the 9 larger cohorts (>250 patients in BMS arm), there was no difference in TLR/TVR rates in experimental compared to control treatment groups (p=0.98) (Table 2; Figure 2). However, in the 39 smaller cohorts a substantial and statistically significant effect was found, with almost 40% higher outcome rates in BMScontrol compared to BMSexperimental (18.9% versus 13.7%; p=0.01). At the same time, small BMScontrol cohorts had significantly higher rates that larger BMScontrol cohorts (18.9% vs 13.1%, p=0.04). Results for the overall analysis and the subgroup analysis remained consistent when we excluded BMS cohorts from trials targeting patients with small artery or complex lesions (Table 2).
Table 2.
Target Lesion/Vessel Revascularization: subgroup and sensitivity analyses
| Comparison or subgroup | BMScontrol | BMSExperimental | p | ||
|---|---|---|---|---|---|
| Studies (Pts) | TLR/TVR, (%) [95% CI] | Studies (Pts) | TLR/TVR, (%) [95% CI] | ||
| Overall | 19 (4 046) | 16.2 (13.5, 19.3) | 31 (5 068) | 13.8 (12.0, 15.7) | 0.15 |
| Excluding difficult lesions* | 15 (2 958) | 14.8 (11.8, 18.4) | 22 (3 903) | 13.0 (11.0, 15.3) | 0.35 |
| Cohort sample size | |||||
| N>250 | 5 (2 528) | 13.1 (9.4, 18.0) | 4 (1 836) | 13.1 (11.3, 15.2) | 0.98 |
| N≤250 | 14 (1 518) | 18.9 (16.0, 22.2) | 25 (3 071) | 13.7 (11.5, 16.3) | 0.01 |
| N≤250 and excluding difficult lesions* | 11 (1 006) | 17.4 (14.4, 20.9) | 18 (2 067) | 12.7 (10.0, 16.1) | 0.04 |
BMS: bare metal stents; CI: confidence interval; Pts: number of patients; TLR/TVR: Target lesion/vessel revascularization
Trials specifically enrolling patients with complex lesions or small artery lesions, as described in the supplementary Table.
Figure 2.
Summary outcome rates for TLR/TVR in BMScontrol versus BMSexperimental cohorts according to sample size. Layout is similar to Figure 1.
None of the 31 trials in the BMSexperimental group blinded outcome assessors. Summary TLR/TVR rates did not differ beyond chance between the 10 BMScontrol cohorts where outcome assessment was blinded and the remaining 9 (16.1% versus 16.4%, respectively; p=0.94). The same was true for the comparison of each subgroup against BMSexperimental cohorts. Among small BMS cohorts (≤250 patients), blinded BMScontrol cohorts still had higher summary TLR/TVR rates from BMSexperimental cohorts, but the difference was not formally significant (p=0.09).
Meta-regression
Meta-regression yielded a similar, statistically non-significant estimate of the overall BMScontrol versus BMSexperimental effect on TLR/TVR rates (p=0.16). Length of follow-up and cohort sample size did not have a significant effect on the rate of TLR/TVR in the meta-regressions (Table 3) using random effects analyses. TLR/TVR rates increased with later enrollment initiation and when patients with difficult lesions were studied. However, controlling for these trial-level characteristics did not reveal any differences in outcome rates between the BMScontrol and BMSexperimental groups overall (Table 3). The only significant effect in the random-effects meta-regression confirmed the aforementioned group-by-cohort size interaction (i.e. that different between group [BMScontrol versus BMSexperimental] outcome rates are observed between smaller, but not between larger cohorts).
Table 3.
Meta-regressions of target lesion/vessel revascularization on various covariates
| Effect of Group (BMScontrol/BMSExperimental) on TLR/TVR adjusting for | Covariate* effect direction | p for covariate* effect REM (FEM) | p between subgroups REM (FEM) |
|---|---|---|---|
| unadjusted | NA | NA | 0.16 (0.11) |
| Follow-up duration (continuous) | ↑ TLR/TVR | 0.72 (0.82) | 0.27 (0.25) |
| Initial year of enrollment† | ↑ TLR/TVR | 0.04 (0.05) | 0.45 (0.42) |
| Presence of difficult lesions‡ (yes/no) | ↑ TLR/TVR | 0.05 (0.04) | 0.15 (0.13) |
| Cohort sample size (square root transformation) | ↓ TLR/TVR | 0.16 (0.01) | 0.10 (0.03) |
| Cohort sample size, >250 patients (yes/no) | ↓ TLR/TVR | 0.18 (0.02) | 0.10 (0.04) |
| Cohort sample size, >250 patients (yes/no) and interaction of cohort size with Group | NS | NS | 0.05 (0.02) |
NA: Not applicable; NS: not shown; REM/FEM: Random/Fixed effects meta-regression; TLR/TVR: Target lesion/vessel revascularization.
Direction of effects in the meta-regression that incorporated the interaction terms are not shown because the interpretation is more complex.
Refers to independent variables other than subgroup participation
Missing values for year of initial enrollment were imputed using information from the year of publication
Trials specifically enrolling patients with complex lesions or small artery lesions, as described in the supplementary Table
Discussion
We did not find evidence for control rate inflation affecting the rates of TLR/TVR in our main analyses. Although a visual inspection of the summary estimates in Figure 1 across the BMS groups might imply higher TLR/TVR or MACE rates in the BMScontrol group of cohorts, this trend was relatively small compared to the substantial between trial outcome rate heterogeneity. However, a clinically substantial and statistically significant effect was seen among BMS cohorts from smaller trials, where TVR/TVL outcome rates were approximately 40% higher in the BMScontrol cohorts compared to the BMSexperimental cohorts. This could have a meaningful influence on the estimated treatment effect in these trials.
There are several potential explanations for this apparent inflation of BMScontrol rates among smaller trials, including chance. We used an arbitrary, non-data-driven cutoff in our subgroup analysis. However, we acknowledge that findings from subgroup analyses often result from the interplay of chance or confounding, and should be considered with due caution.14 Second, because patients were not randomized between the BMScontrol and BMSexperimental groups, the apparent control rate inflation may be ascribed to clinical heterogeneity across the different populations (e.g. higher risk lesions or patients in the smaller BMScontrol compared to BMSexperimental groups),15 or differences in design features of the trials. Most notably, DES trials (BMScontrol) had on average longer follow-up. However, on average, the magnitude of the effect of follow-up time on outcome rate was both negligible and not statistically significant. It is possible that the effect of follow up is masked by the ubiquity of protocol driven angiography, which actively detects lesions that may need revascularization. Moreover, other differences would have biased toward lower event rates in the BMScontrol versus the BMSexperimental group: In the former, the modal duration of dual-antiplatelet therapy was 6 months versus only 1 month; and there was more frequent use of the more restrictive endpoint of target lesion instead of target vessel revascularization (90% versus 42% of trials, in the two groups respectively).
Third, there was heterogeneity in the type of BMS stents used. For example, we found a higher frequency of stents with thick struts among the BMScontrol cohorts. This observation has been made before, when it was argued that the DES were compared against a “straw man”6 (namely the thick strut BMS) rather than thin strut BMS (which might be more effective16). However, strut thickness alone seems unlikely to account for the difference in TLR/TVR rates among smaller BMScontrol cohorts, since all larger BMScontrol cohorts used thick strut stents and had very similar TLR/TVR rates to the BMSexperimental cohorts.
Finally, it is possible that biases (systematic errors) may have influenced outcomes rates. TLR/TVR is a physician-determined outcome, and it is not impossible that it might be differentially ascertained by assessors with strong prior beliefs on the effectiveness of different types of stents. In our case, blinding did not appear to influence outcome rates in the BMScontrol cohorts, suggesting that systematic errors are not the most plausible explanation. Nevertheless, the potential for biases should always be considered when evaluating clinical research.
Whatever its cause, control rate inflation may provide a much-needed opportunity to demonstrate yet another advancement, another step in a sequence of ever successful interventions. Small trials were found to provide the wider margin of opportunity in our evaluation. Small trials are more susceptible to the interplay of chance and systematic errors, as several lines of empirical evidence suggests. Estimates from small trials differ beyond chance from those of larger trials 10–25% of the time,11,12 and usually claim stronger and more impressive effects.9,11 Inflated control rates in some of the small DES trials may have biased toward larger, more positive effect sizes, as the dependency of effect sizes on control rates is well appreciated in the methodological literature.17
Continual innovation leads to progressively diminishing control rates and diminishing control rates leads to diminishing benefits for new therapeutic advances. Indeed, sustained continual innovation theoretically requires an inexhaustible control rate; one could hypothesize that this might exert subtle pressure for higher event rates in control interventions that might manifest in trial design or trial conduct, resulting in an exaggeration of the degree of true progress. The existence and importance of a general tendency toward control rate inflation deserves systematic (not just anecdotal) study. Our evaluation did not find evidence that the control rate was inflated when all DES trials were assessed. However, the accumulated evidence suggests a higher than expected TLR/TVR control rate in smaller trials. While these results do not challenge the overall superiority of DES for reducing TLR/TVR compared to BMS, they add to others that caution against early enthusiasm for new technologies based on small trials, especially when this is based on soft rather than hard endpoints.
Supplementary Material
Acknowledgments
This study was supported by grant No. K23 NS044929 from the National Institute of Neurological Disorders and Stroke (NINDS).
Appendix A: References of studies included in meta-analysis
- 1.Versaci F, Gaspardone A, Tomai F, et al. A comparison of coronary-artery stenting with angioplasty for isolated stenosis of the proximal left anterior descending coronary artery. N Engl J Med. 1997;336:817–822. doi: 10.1056/NEJM199703203361201. [DOI] [PubMed] [Google Scholar]
- 2.Weaver WD, Reisman MA, Griffin JJ, et al. Optimum percutaneous transluminal coronary angioplasty compared with routine stent strategy trial (OPUS-1): a randomised trial. Lancet. 2000;355:2199–2203. doi: 10.1016/s0140-6736(00)02403-x. [DOI] [PubMed] [Google Scholar]
- 3.Witkowski A, Ruzyllo W, Gil R, et al. A randomized comparison of elective high-pressure stenting with balloon angioplasty: six-month angiographic and two-year clinical follow-up. On behalf of AS (Angioplasty or Stent) trial investigators. Am Heart J. 2000;140:264–271. doi: 10.1067/mhj.2000.107555. [DOI] [PubMed] [Google Scholar]
- 4.Tamai H, Berger PB, Tsuchikane E, et al. Frequency and time course of reocclusion and restenosis in coronary artery occlusions after balloon angioplasty versus Wiktor stent implantation: results from the Mayo-Japan Investigation for Chronic Total Occlusion (MAJIC) trial. Am Heart J. 2004;147:E9. doi: 10.1016/j.ahj.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, de Jong P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 6.Sirnes PA, Golf S, Myreng Y, et al. Stenting in Chronic Coronary Occlusion (SICCO): a randomized, controlled trial of adding stent implantation after successful angioplasty. J Am Coll Cardiol. 1996;28:1444–1451. doi: 10.1016/s0735-1097(96)00349-x. [DOI] [PubMed] [Google Scholar]
- 7.Sievert H, Rohde S, Utech A, et al. Stent or angioplasty after recanalization of chronic coronary occlusions? (The SARECCO Trial) Am J Cardiol. 1999;84:386–390. doi: 10.1016/s0002-9149(99)00320-3. [DOI] [PubMed] [Google Scholar]
- 8.Serruys PW, de BB, Carlier S, et al. Randomized comparison of primary stenting and provisional balloon angioplasty guided by flow velocity measurement. Doppler Endpoints Balloon Angioplasty Trial Europe (DEBATE) II Study Group. Circulation. 2000;102:2930–2937. doi: 10.1161/01.cir.102.24.2930. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Foley DP, Suttorp MJ, et al. A randomized comparison of the value of additional stenting after optimal balloon angioplasty for long coronary lesions: final results of the additional value of NIR stents for treatment of long coronary lesions (ADVANCE) study. J Am Coll Cardiol. 2002;39:393–399. doi: 10.1016/s0735-1097(01)01760-0. [DOI] [PubMed] [Google Scholar]
- 10.Rubartelli P, Niccoli L, Verna E, et al. Stent implantation versus balloon angioplasty in chronic coronary occlusions: results from the GISSOC trial. Gruppo Italiano di Studio sullo Stent nelle Occlusioni Coronariche. J Am Coll Cardiol. 1998;32:90–96. doi: 10.1016/s0735-1097(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 11.Serruys PW, van HB, Bonnier H, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II) Lancet. 1998;352:673–681. doi: 10.1016/s0140-6736(97)11128-x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez A, Ayala F, Bernardi V, et al. Optimal coronary balloon angioplasty with provisional stenting versus primary stent (OCBAS): immediate and long-term follow-up results. J Am Coll Cardiol. 1998;32:1351–1357. doi: 10.1016/s0735-1097(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 13.Moer R, Myreng Y, Molstad P, et al. Stenting in small coronary arteries (SISCA) trial. A randomized comparison between balloon angioplasty and the heparin-coated beStent. J Am Coll Cardiol. 2001;38:1598–1603. doi: 10.1016/s0735-1097(01)01602-3. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez AE, Rodriguez AM, Fernandez PC, et al. Latin American randomized trial of balloon angioplasty versus coronary stenting in diabetic patients with small vessel reference size (Latin American Small Vessel [LASMAL II] Trial): immediate and long-term results. Am Heart J. 2005;150:188. doi: 10.1016/j.ahj.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Lincoff AM, Califf RM, Moliterno DJ, et al. Complementary clinical benefits of coronary-artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of Platelet IIb/IIIa Inhibition in Stenting Investigators. N Engl J Med. 1999;341:319–327. doi: 10.1056/NEJM199907293410503. [DOI] [PubMed] [Google Scholar]
- 16.Lafont A, Dubois-Rande JL, Steg PG, et al. The French Randomized Optimal Stenting Trial: a prospective evaluation of provisional stenting guided by coronary velocity reserve and quantitative coronary angiography. F.R.O.S.T. Study Group. J Am Coll Cardiol. 2000;36:404–409. doi: 10.1016/s0735-1097(00)00747-6. [DOI] [PubMed] [Google Scholar]
- 17.Lotan C, Rozenman Y, Hendler A, et al. Stents in total occlusion for restenosis prevention. The multicentre randomized STOP study. The Israeli Working Group for Interventional Cardiology. Eur Heart J. 2000;21:1960–1966. doi: 10.1053/euhj.2000.2295. [DOI] [PubMed] [Google Scholar]
- 18.Kastrati A, Schomig A, Dirschinger J, et al. A randomized trial comparing stenting with balloon angioplasty in small vessels in patients with symptomatic coronary artery disease. ISAR-SMART Study Investigators. Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries. Circulation. 2000;102:2593–2598. doi: 10.1161/01.cir.102.21.2593. [DOI] [PubMed] [Google Scholar]
- 19.Koning R, Eltchaninoff H, Commeau P, et al. Stent placement compared with balloon angioplasty for small coronary arteries: in-hospital and 6-month clinical and angiographic results. Circulation. 2001;104:1604–1608. doi: 10.1161/hc3901.096695. [DOI] [PubMed] [Google Scholar]
- 20.Kinsara AJ, Niazi K, Patel I, et al. Effectiveness of stents in small coronary arteries. Am J Cardiol. 2003;92:584–587. doi: 10.1016/s0002-9149(03)00727-6. [DOI] [PubMed] [Google Scholar]
- 21.Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 22.Hancock J, Thomas MR, Holmberg S, et al. Randomised trial of elective stenting after successful percutaneous transluminal coronary angioplasty of occluded coronary arteries. Heart. 1998;79:18–23. doi: 10.1136/hrt.79.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbel R, Haude M, Hopp HW, et al. Coronary-artery stenting compared with balloon angioplasty for restenosis after initial balloon angioplasty. Restenosis Stent Study Group. N Engl J Med. 1998;339:1672–1678. doi: 10.1056/NEJM199812033392304. [DOI] [PubMed] [Google Scholar]
- 24.Hoher M, Wohrle J, Grebe OC, et al. A randomized trial of elective stenting after balloon recanalization of chronic total occlusions. J Am Coll Cardiol. 1999;34:722–729. doi: 10.1016/s0735-1097(99)00254-5. [DOI] [PubMed] [Google Scholar]
- 25.Fluck DS, Chenu P, Mills P, et al. Is provisional stenting the effective option? The WIDEST study (Wiktor stent in de novo stenosis). Widest Trial Investigators’ Group. Heart. 2000;84:522–528. doi: 10.1136/heart.84.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eeckhout E, Stauffer JC, Vogt P, et al. Comparison of elective Wiktor stent placement with conventional balloon angioplasty for new-onset lesions of the right coronary artery. Am Heart J. 1996;132:263–268. doi: 10.1016/s0002-8703(96)90420-2. [DOI] [PubMed] [Google Scholar]
- 27.Buller CE, Dzavik V, Carere RG, et al. Primary stenting versus balloon angioplasty in occluded coronary arteries: the Total Occlusion Study of Canada (TOSCA) Circulation. 1999;100:236–242. doi: 10.1161/01.cir.100.3.236. [DOI] [PubMed] [Google Scholar]
- 28.Di Mario C, Moses JW, Anderson TJ, et al. Randomized comparison of elective stent implantation and coronary balloon angioplasty guided by online quantitative angiography and intracoronary Doppler. DESTINI Study Group (Doppler Endpoint STenting INternational Investigation) Circulation. 2000;102:2938–2944. doi: 10.1161/01.cir.102.24.2938. [DOI] [PubMed] [Google Scholar]
- 29.Dangas G, Ambrose JA, Rehmann D, et al. Balloon optimization versus stent study (BOSS): provisional stenting and early recoil after balloon angioplasty. Am J Cardiol. 2000;85:957–961. doi: 10.1016/s0002-9149(99)00909-1. [DOI] [PubMed] [Google Scholar]
- 30.Doucet S, Schalij MJ, Vrolix MC, et al. Stent placement to prevent restenosis after angioplasty in small coronary arteries. Circulation. 2001;104:2029–2033. [PubMed] [Google Scholar]
- 31.Betriu A, Masotti M, Serra A, et al. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START): a four-year follow-up. J Am Coll Cardiol. 1999;34:1498–1506. doi: 10.1016/s0735-1097(99)00366-6. [DOI] [PubMed] [Google Scholar]
- 32.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 33.Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS) J Am Coll Cardiol. 2004;43:1110–1115. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Pache J, Dibra A, Mehilli J, et al. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur Heart J. 2005;26:1262–1268. doi: 10.1093/eurheartj/ehi098. [DOI] [PubMed] [Google Scholar]
- 35.Kelbaek H, Thuesen L, Helqvist S, et al. The Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) trial. J Am Coll Cardiol. 2006;47:449–455. doi: 10.1016/j.jacc.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 36.Heldman AW, Farhat N, Fry E, et al. Paclitaxel-eluting stent for cytostatic prevention of restenosis: PATENCY study follow-up. Transcatheter Cardiovascular Therapeutics. 2002 [Google Scholar]
- 37.Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 38.Holmes DR, Jr, Leon MB, Moses JW, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109:634–640. doi: 10.1161/01.CIR.0000112572.57794.22. [DOI] [PubMed] [Google Scholar]
- 39.Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–794. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 40.Park SJ, Shim WH, Ho DS, et al. A paclitaxel-eluting stent for the prevention of coronary restenosis. N Engl J Med. 2003;348:1537–1545. doi: 10.1056/NEJMoa021007. [DOI] [PubMed] [Google Scholar]
- 41.Gershlick A, De SI, Chevalier B, et al. Inhibition of restenosis with a paclitaxel-eluting, polymer-free coronary stent: the European evaLUation of pacliTaxel Eluting Stent (ELUTES) trial. Circulation. 2004;109:487–493. doi: 10.1161/01.CIR.0000109694.58299.A0. [DOI] [PubMed] [Google Scholar]
- 42.Lansky AJ, Costa RA, Mintz GS, et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow-up of the DELIVER clinical trial. Circulation. 2004;109:1948–1954. doi: 10.1161/01.CIR.0000127129.94129.6F. [DOI] [PubMed] [Google Scholar]
- 43.Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–1947. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 44.Grube E, Sonoda S, Ikeno F, et al. Six- and twelve-month results from first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation. 2004;109:2168–2171. doi: 10.1161/01.CIR.0000128850.84227.FD. [DOI] [PubMed] [Google Scholar]
- 45.Grube E, Lansky A, Hauptmann KE, et al. High-dose 7-hexanoyltaxol-eluting stent with polymer sleeves for coronary revascularization: one-year results from the SCORE randomized trial. J Am Coll Cardiol. 2004;44:1368–1372. doi: 10.1016/j.jacc.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Ardissino D, Cavallini C, Bramucci E, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA. 2004;292:2727–2734. doi: 10.1001/jama.292.22.2727. [DOI] [PubMed] [Google Scholar]
- 47.Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 48.Dawkins KD, Grube E, Guagliumi G, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005;112:3306–3313. doi: 10.1161/CIRCULATIONAHA.105.552190. [DOI] [PubMed] [Google Scholar]
- 49.Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362:1093–1099. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 50.Serruys PW, Ormiston JA, Sianos G, et al. Actinomycin-eluting stent for coronary revascularization: a randomized feasibility and safety study: the ACTION trial. J Am Coll Cardiol. 2004;44:1363–1367. doi: 10.1016/j.jacc.2004.03.084. [DOI] [PubMed] [Google Scholar]
Footnotes
Author Contributions
Dr Kent and Dr Trikalinos were involved in all aspects of this research project including development of the research plan, literature search, data extraction, manuscript drafting and approval of the final manuscript. DK had the initial idea for the study, while TT performed the statistical analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bucher H, Hengstler P, Schindler C, et al. Percutaneous transluminal coronary angioplasty versus medical treatment for non-acute coronary heart disease: meta-analysis of randomised controlled trials. BMJ. 2000;321:73–77. doi: 10.1136/bmj.321.7253.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 2003;138:777–786. doi: 10.7326/0003-4819-138-10-200305200-00005. [DOI] [PubMed] [Google Scholar]
- 3.Babapulle MN, Joseph L, Belisle P, et al. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364:583–591. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- 4.Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease. Circulation. 2005;111:2906–2912. doi: 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- 5.Katritsis DG, Karvouni E, Ioannidis JP. Meta-analysis comparing drug-eluting stents with bare metal stents. Am J Cardiol. 2005;95:640–643. doi: 10.1016/j.amjcard.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Tung R, Kaul S, Diamond GA, Shah PK. Narrative review: drug-eluting stents for the management of restenosis: a critical appraisal of the evidence. Ann Intern Med. 2006;144(12):913–919. doi: 10.7326/0003-4819-144-12-200606200-00009. [DOI] [PubMed] [Google Scholar]
- 7.Shafiq N, Malhotra S, Pandhi P, et al. A meta-analysis of clinical trials of paclitaxel- and sirolimus-eluting stents in patients with obstructive coronary artery disease. Br J Clin Pharmacol. 2005;59:94–101. doi: 10.1111/j.1365-2125.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Cappelleri JC, Ioannidis JP, Schmid CH, et al. Large trials vs meta-analysis of smaller trials: how do their results compare? JAMA. 1996;276:1332–1338. [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Contopoulos-Ioannidis DG, Gilbody SM, Trikalinos TA, et al. Comparison of large versus smaller randomized trials for mental health-related interventions. Am J Psychiatry. 2005;162:578–584. doi: 10.1176/appi.ajp.162.3.578. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis JP, Cappelleri JC, Lau J. Issues in comparisons between meta-analyses and large trials. JAMA. 1998;279:1089–1093. doi: 10.1001/jama.279.14.1089. [DOI] [PubMed] [Google Scholar]
- 13.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 14.Oxman AD, Guyatt GH. A consumer’s guide to subgroup analyses. Ann Intern Med. 1992;116:78–84. doi: 10.7326/0003-4819-116-1-78. [DOI] [PubMed] [Google Scholar]
- 15.Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet. 1998;351:123–127. doi: 10.1016/S0140-6736(97)08468-7. [DOI] [PubMed] [Google Scholar]
- 16.Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283–1288. doi: 10.1016/s0735-1097(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 17.Schmid CH, Lau J, McIntosh MW, et al. An empirical study of the effect of the control rate as a predictor of treatment efficacy in meta-analysis of clinical trials. Stat Med. 1998;17:1923–1942. doi: 10.1002/(sici)1097-0258(19980915)17:17<1923::aid-sim874>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.