Abstract
Background
Pentostatin, an adenosine deaminase (ADA) inhibitor, is a purine antimetabolite used for the treatment of leukemias. ADA inhibition blunts expansion of proliferating lymphocytes and increases adenosine release, a potent anti-inflammatory molecule. Human inflammatory bowel disease (IBD) is driven by expansion of effector T cells (Teff) that overwhelm reulatory T cells (Treg) and propagate innate immune reponses. Here we study the therapeutic benefits of ADA inhibition to impair Teff cell expansion and reduce inflammatory cytokine release in IL-10-deficient (IL-10−/−) mice.
Methods
Colitis was induced in IL-10−/− mice by administering piroxicam for two weeks. Mice were treated with daily pentostatin or phosphate-buffered saline for 1 week and effects on tissue inflammation, lymphocyte numbers and cytokine production examined.
Results
Pentostatin reduced inflammation by >50% and nearly normalized serum amyloid A levels. Lymphocyte expansions in the colon and mesenteric lymph node (MLN) (3.5-fold and >5-fold respectively) dropped by >50–90%. Pro-inflammatory factors in the colon and MLN (IL-1β, IFN-γ, IL-6, CXCL10, TNF) dropped whereas FoxP3 and TGF-β were unchanged. Reductions in cytokine production from equivalent numbers of T cells from pentostatin-treated mice after in vitro (36h) or in vivo (3h) activation suggested anti-inflammatory effects of pentostatin independent of lymphodepletion contributed to its therapeutic benefit. Analysis of mucosal lymphocyte subsets suggested pentostatin reduced numbers of effector CD4+ CD69+ T cells, while sparing CD4+ CD62L+ T cells.
Conclusions
Pentostatin dosages that avoid severe lymphocyte depletion effectively treat colitis by impairing Teff cell expansion and reducing pro-inflammatory cytokine production while preserving regulatory Treg populations and function.
Keywords: pentostatin, IL-10−/− colitis, pharmacotherapy, regulatory T cells, colitis, adenosine deaminase, animal models of IBD, inflammation
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is thought to result from a dysregulated immune response to commensal enteric bacterial antigens.1,2 The chronic nature of inflammation has been attributed to disturbed immune homeostasis due to polarized, dysregulated immune responses (Th1, Th17, and/or Th2) that potentiate inflammation.3–9 Because the underlying mechanism driving intestinal inflammation remains elusive, pharmacotherapy targeting downstream effects remains the mainstay of therapy. Traditional therapies result in nonspecific immune suppression (corticosteroids) or targeting of immune cell function (e.g., T and B lymphocytes, NK cells, etc.) by antimetabolites (e.g., azathioprine, methotrexate). Recent advances have focused on targeted biologic therapy (e.g., anti-TNF mAb) based on our evolving understanding of the immunopathogenesis of IBD.
Adenosine deaminase (ADA) is a ubiquitous enzyme involved in purine salvage and recycling. It is concentrated in lymphoid tissue with heightened activity in proliferating cells found in inflamed, injured, or ischemic remodeling tissue.10–13 The congenital absence in humans results in Severe Combined Immunodeficiency syndrome (SCID) with resultant lymphopenia. Accumulation of 2-deoxyadenosine appears to be instrumental in lymphopenia, likely through phosphorylation to dATP and subsequent allosteric inhibition of ribonucleotide reductase resulting in impaired DNA synthesis in dividing cells (e.g., effector sites within an active immune response).14 2-Deoxyadenosine also inhibits S-adenosyl homocysteine hydrolase, which modulates APO-1/FAS-mediated cell death.15 In addition, ADA deficiency/inhibition results in accumulation of adenosine. Adenosine is a nucleoside essential to DNA synthesis, but also functions as a signaling molecule through 4 described G-protein-coupled receptors, where it has been described to modulate immune responses.16,17 Receptor A2A signaling appears to impart an antiinflammatory effect. Its activation attenuates immune responses in models of tissue-specific immune activation such as colitis, asthma, diabetes, and others.18–20 A2A activation attenuates ischemia/reperfusion injury in the kidney, liver, heart, and lung.21–24 More important to IBD, selective A2A activation reduces murine intestinal inflammation through downregulation of lymphocyte function.25 In vitro data suggest that A2A activation downregulates proinflammatory cytokine secretion in both lymphocytes and macrophages.26–29 Additionally, adenosine receptor A1 null mice are more susceptible to kidney reperfusion injury, with the presence of A1 conferring a protective effect through modification of proinflammatory cytokine, in particular TNF.30 Adenosine receptor A3 agonism is protective in both dextran sulfate sodium-induced colitis and IL-10−/− colitis.31 Thus, signaling through adenosine receptors may diminish autoimmune inflammation in a wide range of tissues.
We hypothesized that the combination of focal lymphocyte toxicity within areas of active immune response (due to 2-deoxyadenosine accumulation) and downregulation of the proinflammatory response (due to adenosine accumulation) makes adenosine deaminase an attractive therapeutic target in IBD, where rapid induction of disease remission is desirable.
The aim of the present study was to examine whether inhibition of ADA attenuates experimental colitis by blunting expansion of disease-causing effector T cells while reducing proinflammatory cytokine responses. We postulated that these effects would combine to reestablish regulated mucosal immune environments. To test this hypothesis we used a specific commercially available ADA inhibitor, pentostatin (2-deoxycorformycin [Nipent]), currently indicated for use in hairy cell leukemia.32 To determine the relationship between ADA inhibition and colitis we examined the effects of pentostatin on colitis activity as well as mucosal lymphocyte subset numbers and immune cell function.
MATERIALS AND METHODS
Mice
The study used 5–7-week-old wildtype (WT) and IL-10−/− C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in the Northwestern University animal care facility under specific pathogen free (breeding) or conventional (colitis) conditions.
Colitis Induction and Pentostatin Treatment
All animal protocols were approved by the Animal Care and Use Committee of Northwestern University. The onset of spontaneous colitis was accelerated and synchronized by giving 5–6-week-old C57B6 IL-10−/− mice piroxicam (Sigma-Aldrich, St. Louis, MO) mixed into their feed (Harlan Teklad, Madison, WI, standard powdered rodent chow) for 2 weeks. They received 60 mg of piroxicam / 250 g of chow during week 1 and 80 mg piroxicam/250 g chow during week 2. Mice were then placed on normal rodent diet without piroxicam for the remainder of the protocol.33
Forty-eight hours after discontinuing piroxicam, mice were either given daily injections of intraperitoneal phosphate-buffered saline (PBS) or the ADA inhibitor pentostatin (Hospira, Lake Forrest, IL) at 0.75 mg/kg on days 16 to 22. Animals were sacrificed and colitis assessed on day 24.
Histologic Assessment of Colitis
Entire colons were opened longitudinally and rolled up onto a syringe plunger. The tissue was fixed in 10% neutral buffered formalin overnight, removed from plungers without unrolling, and processed for sectioning. Then 4 µm longitudinal sections were cut and stained with hematoxylin and eosin (H&E) for histologic assessment. Inflammation was scored on a scale from 0–4 per the scoring system described by Berg et al.34
Cell Isolation and CD4+ T-Cell Enrichment
Spleen and mesenteric lymph node (MLN) were mechanically dissociated and red blood cells lysed with ACK lysis buffer (Cambrex Bio Science, Walkersville, MD). Cell suspensions were washed and stored in DMEM containing 5% FCS (Cellgro, Herndon, VA) on ice until used. Cells from colon lamina propria (LP) were isolated as described previously with modifications.35 In brief, colons were removed and flushed with ice-cold PBS to remove fecal contents, opened longitudinally, and cut into 1-cm pieces. The pieces were digested for 30 minutes in 5 mM EDTA and 0.014% dithiothreitol (DTT). Pieces were washed with cold PBS and supernatants discarded. The remaining tissue was minced and digested for 5 10-minute intervals in a buffer containing 1 mg/mL collagenase (Sigma-Aldrich), 4 mM CaCl2, and 2% FCS. After each 10-minute interval the cells released were centrifuged, washed, and stored in 5% DMEM on ice, and the mucosal pieces remaining were replaced in fresh digestion buffer. All 5 fractions were combined and viable cells were obtained by suspending the cells in 0.3 µg/mL DTT in DMEM and centrifuging them over 1-Step 1.077/265 (Accurate Chemical, Westbury, NY). For some experiments, populations of MLN lymphocytes were further purified utilizing a Mouse CD4 Subset Column Kit per the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
Flow Cytometry
Cells from the spleen, MLN, and colon were freshly isolated as described previously. For 4-color analysis, cells were stained with FITC-, PE-, and APC-conjugated or isotype matched mAbs (BD Biosciences, Franklin Lakes, NJ). Nonviable cells were excluded from analysis on the basis of propidium iodide staining. Data were collected and analyzed using a FACSCalibur flow cytometer and CELLQuest software (Becton Dickinson, San Jose, CA).
SAA ELISA
Blood was collected at the time of sacrifice. Mouse serum amyloid A (SAA) was measured on centrifuged serum using enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Camarillo, CA) following the manufacturer’s protocol.
RNA Extraction and Real-Time Polymerase Chain Reaction (PCR)
Total cellular RNA was extracted from freshly isolated MLNs or proximal colon by homogenization in Trizol reagent (Invitrogen, Carlsbad, CA) per the manufacturer’s protocol and quantified spectrophotometrically. One µg of RNA was reverse-transcribed with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) using RNase H+ MMLV reverse transcriptase and oligo(dT) primers. Four µL of cDNA was added to total volume of 50 µL of QuantiTect Sybr Green PCR kit (Qiagen, Valencia, CA). Primers were purchased from Integrated DNA Technologies (Coralville, IA). Applied Biosystems PCR 7500 Real Time cycler was used to run the PCR reaction, consisting of 40 cycles at 95° for 15 seconds, 60° for 1 minute. Data were analyzed utilizing 7500 system SDS software. GAPDH was utilized to standardize samples as the housekeeping gene to control for sample loading and to allow normalization between samples. Relative fold increase in mRNA was determined using ABI software.
Anti-CD3mAb Protocol
Hamster anti-mouse CD3 mAb (2C11) was purified from hybridoma supernatant as previously described.36 Mice were given 200 µg via intraperitoneal injection and sacrificed 3 hours later for assessment of cell yields and RNA isolation.
Cell Culture and ELISA
For IFN-γ analysis, MLN CD4+ T cells were cultured for 36 hours in 24-well culture plates (Falcon, Becton Dickinson) with 1 mL of media (1 × 106 cells/well) at 37°C. The culture media (DMEM) contained 10% FCS, 10 mM HEPES buffer 2 mM L-glutamine, 5 × 10−5 M 2-ME, 100 µg/mL penicillin, 100 mg/mL streptomycin, and 1× nonessential amino acids (Cellgro). Cells were cultured alone or in wells previously coated overnight with anti-CD3 mAb and anti-CD28 mAb (BD Pharmingen, San Diego, CA), each at 1 µg/mL. ELISA (Pierce, Rockford, IL) was used to measure IFN-γ in culture supernatants per the manufacturer’s protocol.
Statistical Analysis
Data are the mean ± standard error of the mean (SEM) of multiple determinations. Difference between 2 groups was compared using Student’s t-test. A value of p < 0.05 was considered significant.
RESULTS
Pentostatin Treatment Results in a Dose-Dependent Lymphocyte Depletion
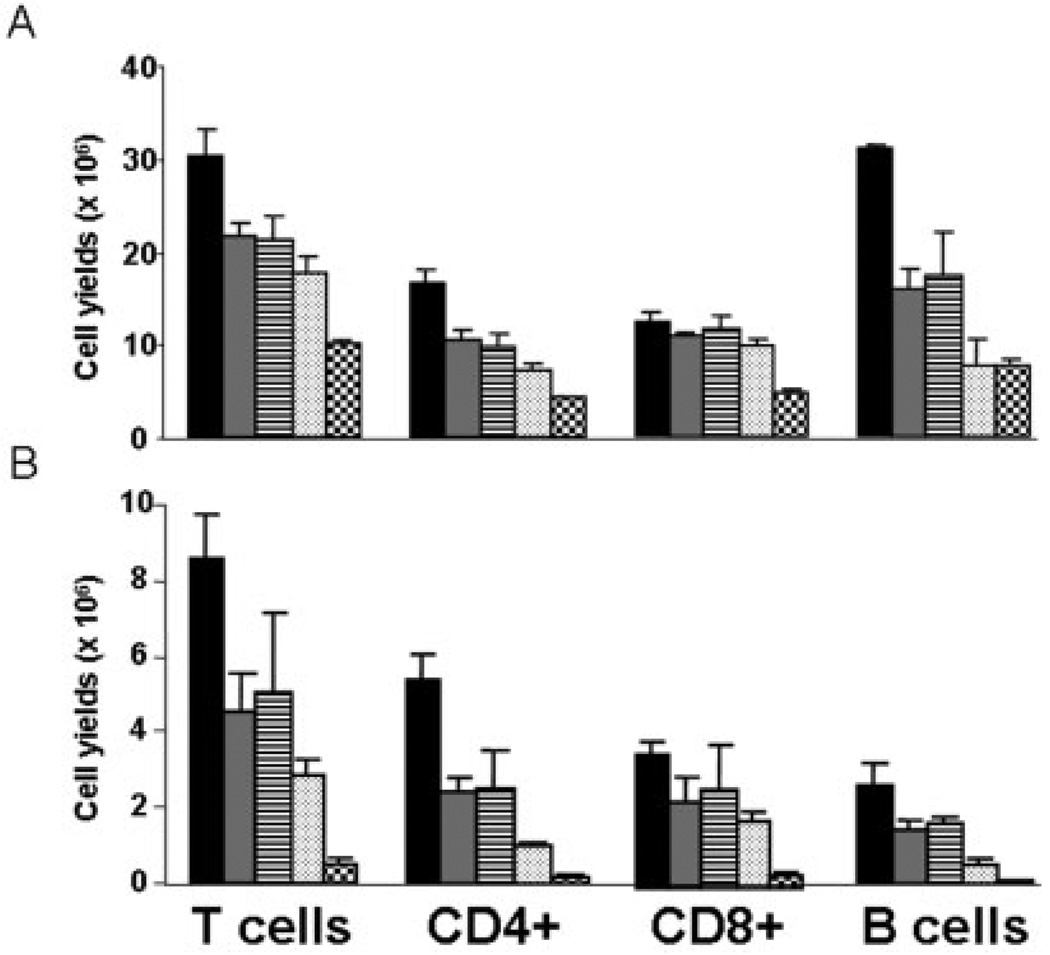
In IBD there is a robust lymphocyte expansion that occurs in draining lymph nodes as well as within mucosal tissues. To minimize systemic immunosuppression, we selected a dose of pentostatin that would limit lymphocyte expansion but not reduce levels below mean baseline numbers (i.e., pre-colitic). To identify a dose of pentostatin that would partially reduce the lymphocyte expansion induced by colitis without significant myelosuppression, a range of dosages were given to uninflamed WT B6 mice. Data in Figure 1 indicate that dosages between 0.5 and 1 mg/kg/day resulted in a 25%–30% reduction in splenic lymphocytes and a 50%–60% reduction in MLN lymphocytes, while at 5 mg/kg/day lymphocytes were reduced by 70% in the spleen and 95% in the MLN. A “sub-ablative” range of pentostatin dosages (0.5, 0.75, and 1 mg/kg/day) were then examined in established IL-10−/− colitis. We noted increased mortality and delayed recovery of weight loss (19% versus 5% below untreated) at 1 versus 0.75 mg/kg/day (data not shown). As histologic responses improved at 0.75 mg/kg/day without increased mortality, this dose was chosen for subsequent studies. At the 0.75 mg/kg/d dosage, lymphocyte counts in established colitis were “partially reduced” to levels above those detected in control mice. The effects of partial lymphodepletion were studied to test its usefulness while limiting the risk for immunocompromised infections and enhancing its potential for clinical usefulness in IBD.
FIGURE 1.
Dose-dependent lymphodepletion. WT B6 mice were treated with PBS control (black), 0.4 mg/kg/day (gray), 1 mg/kg/every other day (striped), 1 mg/kg/day (dotted), and 5 mg/kg/day (checkered) pentostatin over 7 days. Cells from spleens (A) and mesenteric lymph nodes (B) were isolated and enumerated, and lymphocyte subset yields were determined by flow cytometry. Data shown are means ± SEM (n = 3).
Pentostatin Administration Attenuates Established Colitis in IL-10−/− Mice
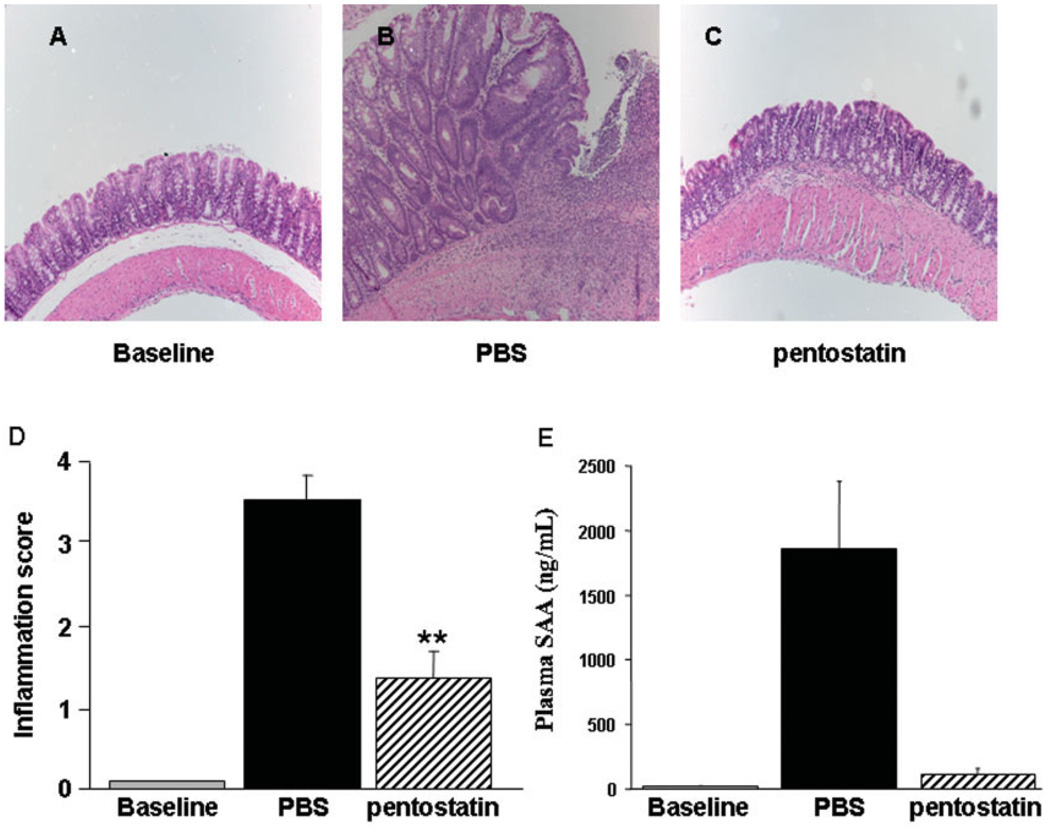
Administration of piroxicam for 2 weeks accelerates and synchronizes the onset of colitis in IL-10−/− mice with marked colonic crypt hyperplasia, muscle wall thickening, mononuclear cell infiltration of the LP, and transmural inflammation. By the end of piroxicam feeding, nearly all IL-10−/− mice (>90%) developed epithelial ulceration and significantly higher inflammatory scores compared to piroxicam-treated B6 controls.33 Treatment with pentostatin (initiated 2 days after cessation of piroxicam) reduced inflammatory scores (3.5 ± 0.5 versus 1.3 ± 0.3, P = 0.03) despite the aggressive nature of the transmural colitis (Fig. 2A,B). Mononuclear cell infiltration was diminished, with recovery of intact epithelium and normalization of muscularis mucosa (elimination of transmural lesions). Crypt hyperplasia improved and thickening of the muscle wall returned to near baseline (Fig. 2A). Furthermore, the dramatic induction of plasma serum amyloid A levels was nearly normalized by pentostatin therapy (Fig. 2C). Taken together, these results suggested that pentostatin has potent therapeutic effects in IBD, both locally (in the colon) and systemically (evidenced by SAA and data below).
FIGURE 2.
Pentostatin reduces histologic inflammation and systemic inflammatory responses in established colitis. (A) Representative histology of an uninflamed IL-10−/− mouse maintained in pathogen-free housing prior to the initiation of piroxicam protocol. IL-10−/− mice were transferred to conventional housing for 1 week, fed piroxicam for 14 days, then treated with PBS (B) or 0.75 mg/kg/day pentostatin (C) from day 16–23. Note epithelial ulceration, transmural inflammation, and crypt hyperplasia in colitic (PBS-treated) mice. H&E staining, 10×. (D) Histologic assessment of inflammation scores. (E) Serum Amyloid A (SAA) protein was measured by ELISA prior to piroxicam (baseline) and at the time of sacrifice (day 24) in colitic (PBS-treated) and pentostatin-treated mice. Data represented as mean ± SEM.
Pentostatin Administration has a Lymphocyte-Specific Depleting Effect and Reduces the Production of Th1 Proinflammatory Cytokines
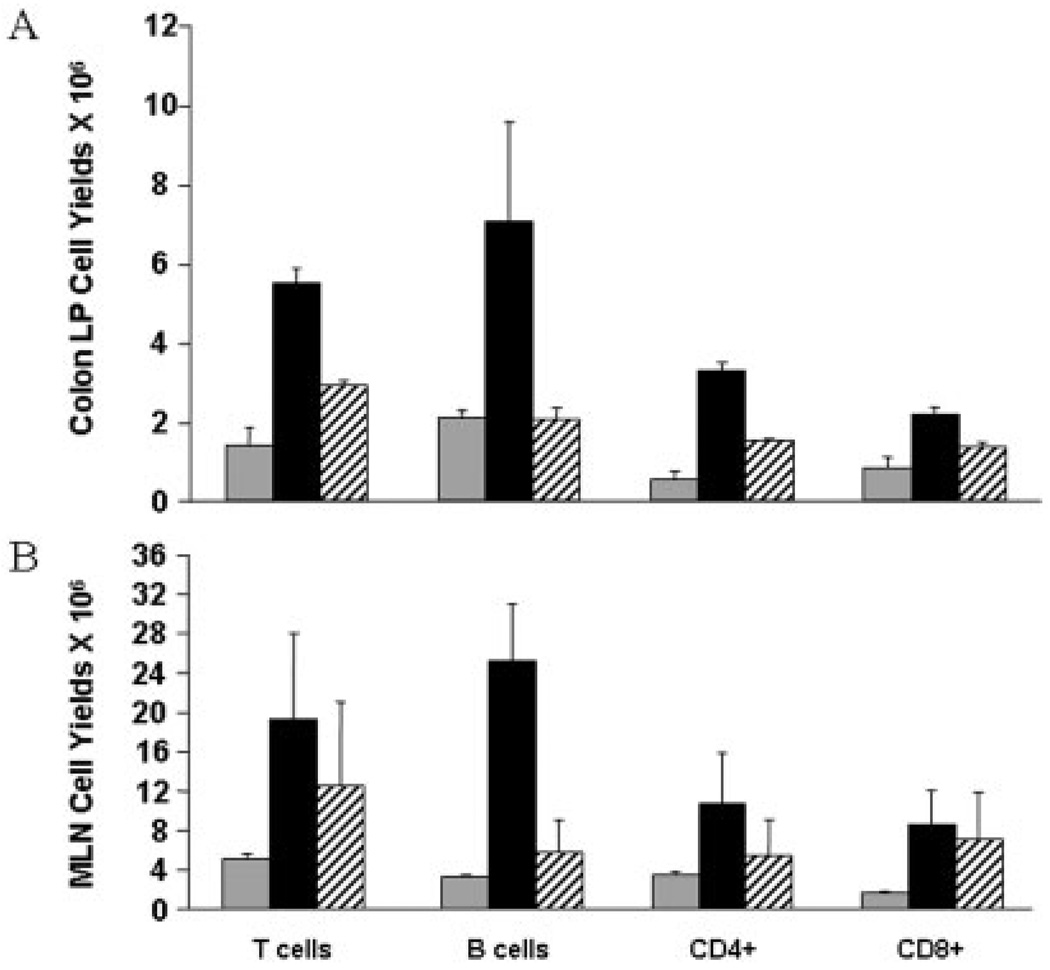
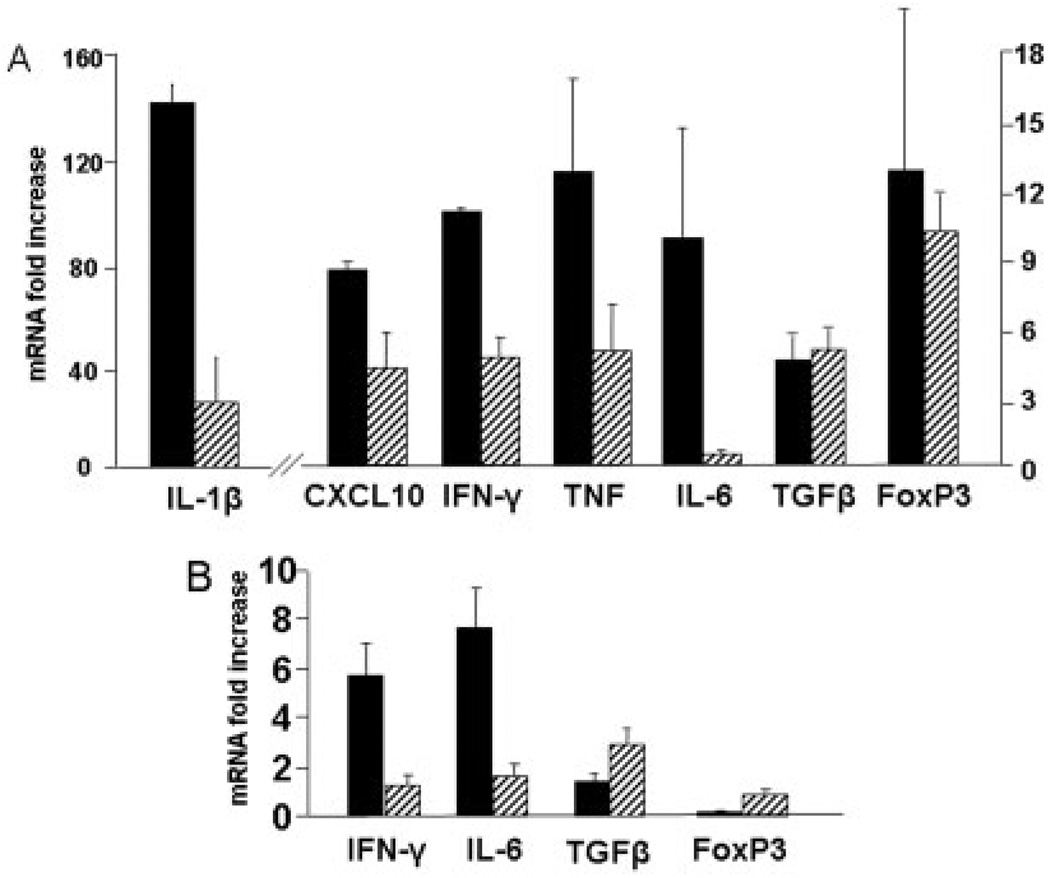
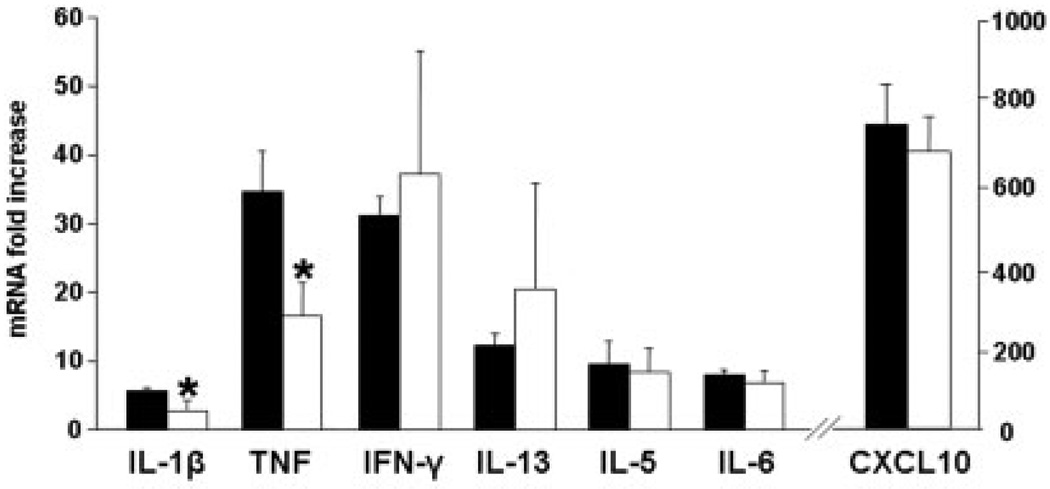
Inflammation in IL-10−/− colitis results from dysregulated CD4-driven innate immune responses. We hypothesized that inhibition of ADA would result in lymphocyte-specific depletion. Our results show a significant reduction in both T and B cells within the effector (colon) and inductive (draining MLN) sites (Fig. 3A,B). The data show 0.75 mg/kg/d of pentostatin reduced T and B cell expansion. However, in no case were numbers reduced to levels below those detected in control (uninflamed) mice. IL-10−/− colitis is mediated through secretion of Th1 cytokines (e.g., IL-1β, IFN-γ, TNF, IL-6) and chemokine (e.g., CXCL10).34 Data in Figure 4 show that pentostatin treatment reduced mRNA levels of Th1 cytokine and chemokine levels in colon (Fig. 4A) and MLN (Fig. 4B), while preserving induction of mRNA elevated in regulatory responses (TGF-β, FoxP3). Reductions were especially pronounced for cytokines made by M1 macrophages (>80% reduction in IL-6 and IL-1β). These findings were consistent with the notion that pentostatin imparted antiinflammatory effects independent of blunted lymphocyte expansion.
FIGURE 3.
Pentostatin treatment reduces lymphocyte subset yields. (A) Colon lamina propria and (B) MLN cells were isolated and numerated at day 24. Lymphocyte subset yields were determined by flow cytometry at baseline (gray bars), day 24 colitic (PBS-treated) IL-10−/− mice (black bars), and day 24 pentostatin treated colitic mice (striped bar). Data represented as mean ± SEM.
FIGURE 4.
Effect of pentostatin on expression of proinflammatory and regulatory mRNA in IL-10−/− mice. Real-time PCR analysis was done on colon and MLN. Tissues were obtained from colitic IL-10−/− mice treated for 7 days as control (PBS) versus pentostatin. Data represented as mean ± SEM.
To directly examine whether antiinflammatory effects of ADA inhibition were evident prior to lymphodepletion, mucosal T cell functional responses were assessed prior to lymphocyte expansion. We previously observed that in WT B6 mice the earliest sign of mucosal T cell expansion (BrDU incorporation) after anti-CD3 mAb injection was detected at 18 hours.35 Thus, cytokine mRNA was measured 3 hours after anti-CD3 mAb to permit measurement of function prior to changes in T cell numbers. Administration of pentostatin 1 hour prior to anti-CD3 mAb failed to alter splenic, MLN, or colonic lymphocyte yields 3 hours after anti-CD3 mAb injection (data not shown). By comparison, pentostatin significantly reduced both TNF and IL-1β mRNA inductions (p < 0.05) without affecting significant changes in other cytokine or chemokine mRNA noted (Fig. 5). Taken together, these results support the hypothesis that pentostatin inhibited cytokine synthesis in colitis.
FIGURE 5.
Pentostatin impairs proinflammatory cytokine transcription after T-cell activation. Wildtype B6 mice were treated with either PBS (black bars) or 1 mg/kg pentostatin (open bars) 1 hour prior to systemic activation of T cells with 200 µg intra-peritoneal anti-CD3 mAb. Colons were removed 3 hours after anti-CD3 mAb administration. Real-time PCR analysis was done on colonic RNA to determine proinflammatory cytokine mRNA.
ADA Inhibition Results in Reduced Lymphocyte Activity In Vitro
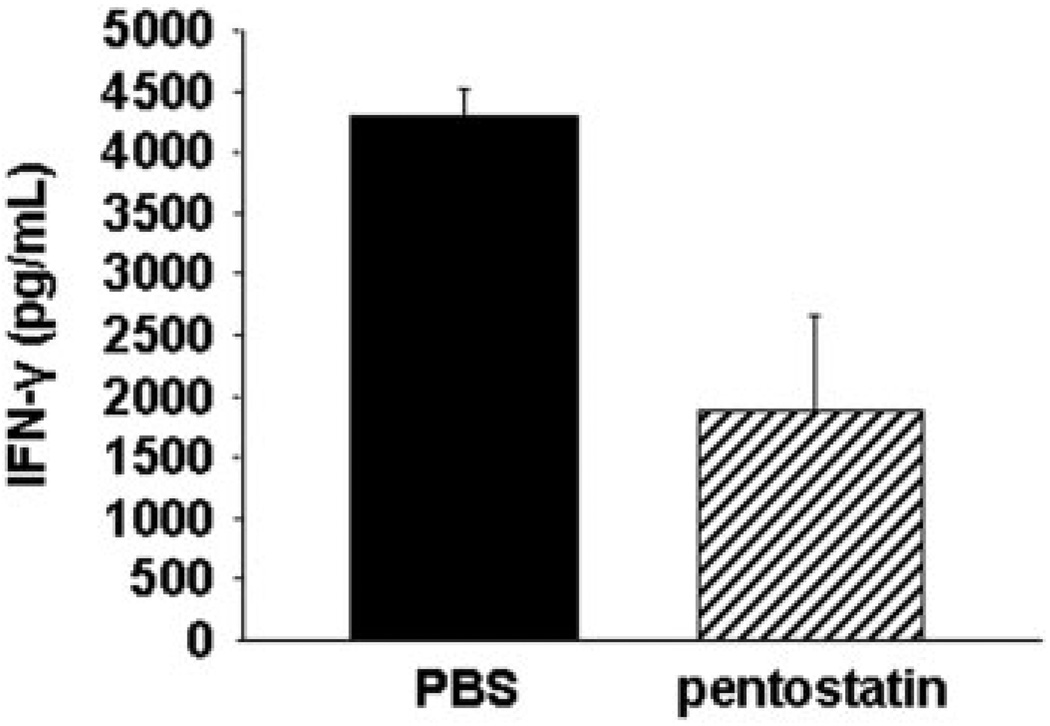
To further examine the independent effect of ADA inhibition on T cell function, CD4+ T cells were purified (>98% purity) from MLN of control and pentostatin-treated colitic mice. Equivalent numbers of CD4+ T cells were activated with anti-CD3 and anti-CD28 mAbs. IFN-γ levels were measured in the supernatant after 36 hours. The results (Fig. 6) show that activated CD4+ T cells from pentostatin-treated IL-10−/− mice produced >50% less cytokine compared to untreated colitic IL-10−/− mice. Thus, pentostatin impaired functional responses of Teff populations in colitis.
FIGURE 6.
Effect of pentostatin on expression of effector cytokine in cells stimulated in vitro with anti-CD3 mAb and anti-CD28 mAb. MLNs were isolated from colitic IL-10−/− mice after 7 days of treatment with PBS (control) or pentostatin. Equal amounts of freshly isolated CD4+ T cells (1 × 106 cells) were stimulated in vitro. After 36 hours, supernatants were collected and expression of IFN-γ protein analyzed by ELISA. Data represented as mean ± SEM.
ADA Inhibition Reduces Effector Cell Responses While Sparing Regulatory Responses: A Possible Mechanism for Reestablishing Immune Homeostasis
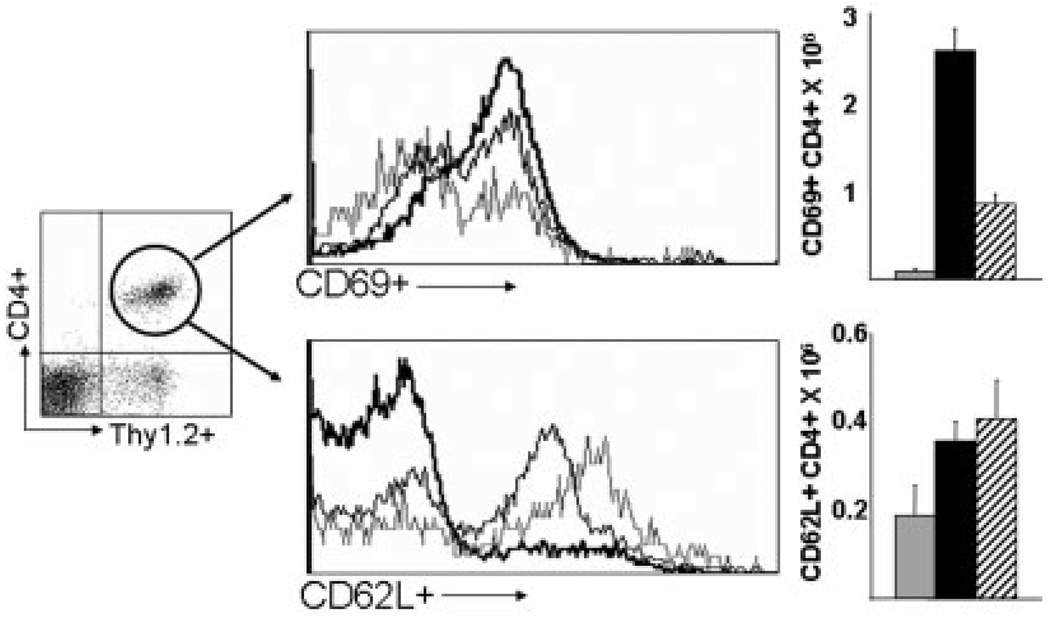
To examine whether pentostatin differentially affected Teff versus Treg lymphocyte populations, colonic surface phenotype and cell numbers were assessed in colonic lamina propria. Data in Figure 7A,B show that pentostatin reduced increases in numbers of activated CD4+ T cells (CD69+, CD4+) by >60%. By comparison, numbers of naïve and/or regulatory CD4+ T-cell populations bearing CD62L were unaffected by pentostatin (Fig. 7C,D). No consistent changes in intracellular FoxP3-stained cells were detected in multiple experiments (data not shown). As CD62L has recently been shown to be expressed on peripheral Treg populations,37–39 we consider these data to be consistent with the notion that Treg populations are relatively spared by pentostatin treatment in colitis. Further studies will need to be performed as reliable reagents in the intestine are made available. These results were consistent with other supportive data in Figure 4 indicating that despite a drop in nearly all effector cytokines, TGF-β and FoxP3 mRNA levels were either unaffected or increased. Taken together, the data are consistent with the notion that pentostatin targets rapidly proliferating Teff populations while sparing Treg cells that proliferate at lower rates. Thus, by default, pentostatin may enhance the capacity of Treg cells to reestablish mucosal dominance over highly proliferative activated Teff cells that have greater dependence on ADA for accelerated DNA synthesis.
FIGURE 7.
Lymphodepletion from pentostatin targets rapidly proliferating effector T cells in IL-10−/− colitis. Colon LP cells were isolated from baseline, uninflamed mice (light line, gray bar), colitic IL-10−/− mice at day 24 (dark line, black box), and colitic IL-10−/− mice treated with 7 days of pentostatin (medium line, striped box) and stained with anti-Thy1.2 mAb-APC and anti-CD4 mAb-PE (for gating), and anti-CD69 mAb-FITC to identify the distribution of proliferating effector CD4+ T cells or stained with anti-Thy 1.2 mAb-APC and anti-CD4 mAb-FITC (for gating), and anti-CD62L mAb-PE to identify naive/resting/regulatory CD4+ T cells. Yields of CD4+ T cells are indicated for each group. Data represented as mean ± SEM.
DISCUSSION
The goal of treatment in severe acute IBD is to induce remission before embarking on maintenance programs. In clinically severe patients, regimens include agents that impart profound systemic immunosuppression (e.g., steroids, methotrexate). Agents proposed in UC that specifically target T-cell populations with anti-CD3 mAb induce transient severe lymphopenia,40,41 which may enhance risks for PTLD-like responses or infection. Although newer immunomodulatory agents target key cytokine pathways (e.g., TNF), their clinical use is compromised by anti-idiotype responses to mAbs, thus frequently necessitating concomitant use of a second immunosuppressant.42 Newer approaches aim to alter the balance of T-cell functional subsets by decreasing effector cells and enhancing regulatory cell numbers and responses (e.g., through use of probiotic or helminthic therapies).43–45 In the current study, we took advantage of differences in Teff and Treg proliferative rates. As Teff cells expand at higher rates compared to Treg subsets, we hypothesized that treatment with pentostatin would preferentially reduce Teff cell numbers in colitic mice while sparing Treg cells. In support of this notion, pentostatin imparted potent antiinflammatory effects that paralleled reductions in colitis-induced T-cell expansion. The argument that this effect was related to changes in Teff populations was supported by data in Figure 7, where numbers of colitis-induced CD69+ T cells were reduced by >60%. The reduction in CD69+ cells occurred in mice where CD62L+ cell numbers increased slightly. The preferential reduction in Teff cell responses was supported by decreases in IFN-γ and TNF with concomitant reduction in macrophage-related IL-1β and IL-6 (Fig. 4). Reductions in effector cell cytokine mRNA occurred in mice where mRNA levels for FoxP3 and TGF-β were either unchanged or slightly increased. Thus, the data presented are consistent with the notion that targeting rapidly expanding populations of Teff cells while sparing Treg cells provides a novel strategy for inducing remission in patients with severe colitis. The striking reversal of severe transmural colitis with pentostatin suggests that it will have utility in more severe patients, where clinical trials tend to be more successful due to lower placebo rates.
Compared to other immunomodulators that induce lymphodepletion or biologic therapies that target individual pathways, ADA inhibition presents a bimodal therapy. In addition to its lymphodepleting effect on rapidly proliferating cells, ADA inhibition results in the accumulation of adenosine, which has potent anti-inflammatory properties. This second therapeutic arm could facilitate using a lower, less myeloablative dose of pentostatin. In the current study we proposed that pentostatin would have an independent anti-inflammatory effect on IL-10−/− colitis in addition to its lymphodepleting effect. When colonic tissue mRNA were analyzed, a disproportionately exaggerated reduction in macrophage versus T-cell-derived cytokine mRNA was observed. Specifically, IL-1β, IL-6, and TNF were significantly reduced. This is consistent with adenosine’s described suppressive effect on IL-1β and TNF in a septic model of inflammation,46 as well as adenosine-induced reduction of LPS-induced TNF secretion from human monocytes and mouse macrophages.29,47 Adenosine receptor agonism has also been demonstrated to impair secretion of IL-6 and TNF in DSS-induced colitis31 and attenuate experimental diabetic nephropathy partly though reducing TNF secretion.20 To further explore the effect of pentostatin independent of its lymphodepletion, we analyzed cytokine mRNA prior to lymphodepletion. The goal of this study was to assess the functional effect pentostatin had on anti-CD3 mAb-induced T-cell-mediated immune cell activation. Even without lymphodepletion, we noted a significant reduction in IL-1β and TNF mRNA, consistent with the notion that pentostatin has an independent anti-inflammatory effect on T cell-mediated inflammation. Finally, we assessed equal numbers of in vitro stimulated CD4+ T cells isolated from colitic IL-10−/− mice previously treated with PBS (control) or pentostatin. Again, a significant reduction in proinflammatory cytokine (IFN-γ) production was seen, with equal numbers of cells stimulated. Thus, by focusing on functional responses that occur prior to lymphodepletion, we tested the notion that pentostatin has a more broad anti-inflammatory effect in autoimmune disease. Although this may be related to adenosine (a topic beyond the scope of the current study), we suspect that the combined impact of limiting effector cell expansion and impairing inflammatory cytokine production (from adaptive and innate immune cells) may explain the pronounced and rapid effects observed in severely inflamed IL-10−/− mice.
Therefore, we propose that ADA inhibition may provide a novel target for therapy in IBD. The effects take advantage of the differential proliferative rates of Teff and Treg cells, as noted by several other investigators.48,49 As IBD tends to have a rapid onset characterized by clinically severe (occasionally lethal) complications, the need for a rapidly acting, yet safe agent is real. Although anti-TNF mAb has revolutionized the treatment of IBD, it has limited usefulness in clinically severe patients where needed results are measured in days and not weeks. We propose that pentostatin will offer clinicians another tool in their arsenal of agents to use in patients facing the prospect of life-altering surgery. Safety concerns will need to be explored further but the history of this agent has created a number of experienced clinicians with vast experience and the ability to guide those of us in search for another choice for patients facing decisions with severe implications on quality of life.
Acknowledgments
Supported by Hospira, Inc. (Lake Forrest, IL) (to T.A.B.), NIH RO1 AI61702-01A1 (to T.A.B.), NIH R01 DK054778-06 (to T.A.B), NIH KO8DK066161-01A1 (to J.B.B.), Crohn’s and Colitis Foundation of America Career Development Award (to J.B.B.), the Thomas Russell Charitable Foundation, Inc. (to T.A.B.).
REFERENCES
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 4.West GA, Matsuura T, Levine AD, et al. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–1695. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- 5.Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan JL, Osborne WR, Wedgewood RJ. Adenosine deaminase activity in lymphocytes. Br J Haematol. 1977;37:157–158. [PubMed] [Google Scholar]
- 11.Kose K, Yazici C, Assioglu O. The evaluation of lipid peroxidation and adenosine deaminase activity in patients with Behcet’s disease. Clin Biochem. 2001;34:125–129. doi: 10.1016/s0009-9120(01)00190-4. [DOI] [PubMed] [Google Scholar]
- 12.Hitoglou S, Hatzistilianou M, Gougoustamou D, et al. Adenosine deaminase activity and its isoenzyme pattern in patients with juvenile rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2001;20:411–416. doi: 10.1007/s100670170005. [DOI] [PubMed] [Google Scholar]
- 13.Law WR, Valli VE, Conlon BA. Therapeutic potential for transient inhibition of adenosine deaminase in systemic inflammatory response syndrome. Crit Care Med. 2003;31:1475–1481. doi: 10.1097/01.CCM.0000063259.09580.D8. [DOI] [PubMed] [Google Scholar]
- 14.Hirschhorn R. Adenosine deaminase deficiency: molecular basis and recent developments. Clin Immunol Immunopathol. 1995;76:S219–S227. doi: 10.1016/s0090-1229(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 15.Resta R, Thompson LF. SCID: the role of adenosine deaminase deficiency. Immunol Today. 1997;18:371–374. doi: 10.1016/s0167-5699(97)01047-5. [DOI] [PubMed] [Google Scholar]
- 16.Kuno M, Seki N, Tsujimoto S, et al. Anti-inflammatory activity of non-nucleoside adenosine deaminase inhibitor FR234938. Eur J Pharmacol. 2006;534:241–249. doi: 10.1016/j.ejphar.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Siegmund B, Rieder F, Albrich S, et al. Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther. 2001;296:99–105. [PubMed] [Google Scholar]
- 18.Naganuma M, Wiznerowicz EB, Lappas CM, et al. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 19.Bonneau O, Wyss D, Ferretti S, et al. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- 20.Awad AS, Huang L, Ye H, et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 21.Okusa MD, Linden J, Huang L, et al. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 22.Jordan JE, Zhao ZQ, Sato H, et al. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther. 1997;280:301–309. [PubMed] [Google Scholar]
- 23.Harada N, Okajima K, Murakami K, et al. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J Pharmacol Exp Ther. 2000;294:1034–1042. [PubMed] [Google Scholar]
- 24.Ross SD, Tribble CG, Linden J, et al. Selective adenosine-A2A activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant. 1999;18:994–1002. doi: 10.1016/s1053-2498(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 25.Odashima M, Bamias G, Rivera-Nieves J, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Link AA, Kino T, Worth JA, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 27.Hasko G, Kuhel DG, Chen JF, et al. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 28.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 29.Eigler A, Greten TF, Sinha B, et al. Endogenous adenosine curtails lipopolysaccharide-stimulated tumour necrosis factor synthesis. Scand J Immunol. 1997;45:132–139. doi: 10.1046/j.1365-3083.1997.d01-377.x. [DOI] [PubMed] [Google Scholar]
- 30.Gallos G, Ruyle TD, Emala CW, et al. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 31.Mabley J, Soriano F, Pacher P, et al. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5’-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 32.Saven A, Piro L. Newer purine analogues for the treatment of hairy-cell leukemia. N Engl J Med. 1994;330:691–697. doi: 10.1056/NEJM199403103301007. [DOI] [PubMed] [Google Scholar]
- 33.Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 34.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst SD, Cooper CJ, Sitterding SM, et al. The differentiated state of intestinal lamina propria CD4+ T cells results in altered cytokine production, activation threshold, and costimulatory requirements. J Immunol. 1999;163:5937–5945. [PubMed] [Google Scholar]
- 36.Musch MW, Clarke LL, Mamah D, et al. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez I, Zeiser R, Karsunky H, et al. CD101 surface expression discriminates potency among murine FoxP3+ regulatory T cells. J Immunol. 2007;179:2808–2814. doi: 10.4049/jimmunol.179.5.2808. [DOI] [PubMed] [Google Scholar]
- 38.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 39.Fu S, Yopp AC, Mao X, et al. CD4+ CD25+ CD62+ T-regulatory cell subset has optimal suppressive and proliferative potential. Am J Transplant. 2004;4:65–78. doi: 10.1046/j.1600-6143.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 40.Plevy S, Salzberg B, Van Assche G, et al. A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis. Gastroenterology. 2007;133:1414–1422. doi: 10.1053/j.gastro.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 41.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 42.Hanauer SB. Immunogenicity of infliximab in Crohn’s disease. N Engl J Med. 2003;348:2155–2156. doi: 10.1056/NEJM200305223482121. author reply 2156. [DOI] [PubMed] [Google Scholar]
- 43.Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol. 2005;21:44–50. [PubMed] [Google Scholar]
- 44.Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 45.Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adanin S, Yalovetskiy IV, Nardulli BA, et al. Inhibiting adenosine deaminase modulates the systemic inflammatory response syndrome in endotoxemia and sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1324–R1332. doi: 10.1152/ajpregu.00373.2001. [DOI] [PubMed] [Google Scholar]
- 47.Sajjadi FG, Takabayashi K, Foster AC, et al. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 48.Yamazaki S, Tomonori I, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-producing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]