Abstract
INTRODUCTION
Traditional models of intestinal glucose absorption confine GLUT2 to the basolateral membrane. Evidence suggests that GLUT2 is translocated to the apical membrane when the enterocyte is exposed to high luminal glucose concentrations.
HYPOTHESIS
GLUT2 translocates to the apical membrane by a PKC signaling mechanism dependent on activity of SGLT1 and the cellular cytostructure.
METHODS
Transporter-mediated glucose uptake was studied in rat jejunum using everted sleeves under 7 conditions: Control, SGLT1 inhibition (phlorizin), GLUT2 inhibition (phloretin), both SGLT1 and GLUT2 inhibition, PKC inhibition (calphostin C or chelerythrine), and disruption of cellular cytostructure (nocodazole). Each condition was tested in iso-osmotic solutions of 1, 20, or 50 mM glucose for 1 or 5 min incubations (n=6 rats each).
RESULTS
Control rats exhibited a saturable pattern of uptake at both durations of incubation. Phlorizin (p≤0.006 each) inhibited markedly and phloretin (p≤0.01 each) inhibited partially glucose uptake in all concentrations and time. Phloretin and phlorizin together completely inhibited uptake (p=0.004 each). Calphostin C, chelerythrine, and nocodazole had little effect on glucose uptake at either 1 or 5 min.
SUMMARY
Inhibition of SGLT1 led to near complete cessation of transporter-mediated glucose uptake, while GLUT2 inhibition led to partial inhibition, suggesting some constitutive expression of GLUT2 in the apical membrane. Disruption of PKC signaling or cytoskeletal integrity partially inhibited transporter-mediated glucose uptake only in 1 mM glucose, suggesting a non-specific effect.
CONCLUSIONS
Under these conditions, it does not appear that GLUT2 is translocated to the apical membrane on the cellular cytostructure in response to PKC signaling.
Keywords: SGLT1, GLUT2, hexose transport, glucose uptake, intestinal absorption, protein kinase C
INTRODUCTION
The traditional model of glucose absorption from the brush border of the intestine has attributed the majority, if not all, of transporter-mediated glucose uptake, from the lumen into the enterocyte, to the secondary active glucose transporter, sodium glucose co-transporter 1 (SGLT1) (1–4). In this model, glucose transport into the enterocyte is driven by a basolateral, sodium-potassium ATPase pump which maintains a sodium gradient; this gradient allows SGLT1 to transport glucose actively along with sodium into the cell, after which glucose is either metabolized or exits the cell into the portal venous system via the facilitated transporter, glucose transporter 2 (GLUT2) which has been thought to be present only in the basolateral membrane of the enterocyte. In vivo rat studies, however, have demonstrated a very rapid, progressive, increase in glucose uptake at substrate concentrations far exceeding concentrations of glucose that fully saturate the SGLT1 transport system; thus, the idea that SGLT1 is responsible for all glucose uptake does not fit with experimental findings (5–8).
Two prominent theories have been proposed to explain this accessory pathway for glucose uptake (9–15). The first proposal (1983) to explain this phenomenon in intestinal glucose uptake has been termed “solvent drag” (9, 12–15). Investigators supporting this theory have demonstrated what appears to be opening of tight junctions between enterocytes mediated either by activation of SGLT1 or by the increases in luminal solute (glucose) concentrations, which, coupled with large water and solute shifts, leads to an augmented influx of glucose into the paracellular space where it is transported into the cell along a concentration gradient by a facilitated transporter, GLUT2 (14).
The second theory, proposed more recently by Kellett and others (7, 16–19), suggests that GLUT2, a facilitated transporter, is translocated rapidly to the apical membrane from pre-formed, intracellular stores of GLUT2 via a signaling mechanism initiated by activation of SGLT1 through protein kinase C (PKC); the increase in the brush border membrane of a transporter (GLUT2) with a lesser affinity but a greater transport capacity would explain this rapid increase in uptake of glucose at luminal concentrations of glucose much greater than the Km of SGLT1. Additional work suggests the presence of immunoreactive GLUT2 in the brush border membrane of enterocytes (20). In our own work, we have observed variations in Km along the intestinal tract; this observation suggests the presence of more than one transporter (21–23). Additional studies using phlorizin, a competitive inhibitor of SGLT1, exhibited a dramatic decrease in transporter-mediated glucose uptake but failed to achieve complete inhibition of transport, despite increasing doses of phlorizin (0.05–0.2 mM), which further suggests the presence of an additional glucose transporter in the brush border membrane (23). Additionally, in a cell culture model using several different intestinal epithelial cell lines, we have observed a rapid augmentation of glucose uptake, which is blunted by GLUT2 inhibition when the cells are exposed to high concentrations of glucose (24).
In this current study, we hypothesized that activation of SGLT1 leads to translocation of pre-formed GLUT2 to the apical membrane through a PKC-mediated signaling pathway via the cytoskeletal, microtubular architecture, where GLUT2 then functions to augment apical uptake of glucose.
METHODS
After approval by our Institutional Animal Care and Use Committee and in accordance with the guidelines of the National Institutes of Health for the humane use and care of laboratory animals, we studied transporter-mediated glucose uptake in jejunal segments of 250–300 g Lewis rats (Harlan, Indianapolis, IN) between 8:00 am and 10:00 am. Adherence to this schedule was undertaken to avoid the known, diurnal patterns in hexose transporter expression and function which would confound our results (3, 4, 25, 26). Transporter-mediated glucose uptake was measured using a modification of the everted sleeve technique as described previously (23, 27).
In brief, normal, nondiabetic Lewis rats were anesthetized using 2% inhaled isoflurane for induction followed by intraperitoneal injection of 50 mg/kg sodium pentobarbital. Rats were then subjected to a mid-ventral celiotomy, and intestinal segments from the proximal jejunum (5 cm from the ligament of Treitz) were flushed, everted, mounted, and secured rapidly to metal rods with 5-0 silk ties and kept in chilled (4°C), oxygenated (95% O2/5%CO2) mammalian Ringer’s solution (in mM: 128 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 20 NaHCO3; pH 7.3–7.4; 290 mOsm). When ready for study, the sleeves were preincubated in oxygenated, glucose-free, mammalian Ringer’s solution at 38°C for 5 min. Because our preliminary work in cell culture (24) suggested that transport of glucose was augmented by a GLUT2-dependent mechanism at glucose concentrations ≥ 20 mM and in vivo studies suggested that augmented jejunal absorption occurred only at greater concentrations of luminal glucose, transport studies were conducted by placing the sleeves in a flat-bottom test tube with 8 ml of mammalian Ringer’s solution which had iso-osmotic replacement of NaCl with 1, 20 or 50 mM d-glucose. Because our cell culture work (24) showed that the augmentation of glucose absorption took ≥ 2 min, sleeves were incubated for either a short duration (1 min) or a greater duration (5 min) at 38°C with a stir-bar at the bottom of the test tube spun at 1,200 rpm to promote mixing of the test solution and to ameliorate any effects of the “unstirred layer”. After incubation, sleeves were rinsed immediately with chilled (4°C), glucose-free Ringer’s solution for 20 s to stop further transporter-mediated uptake and placed in a glass, liquid scintillation, counting vials with 0.5 ml of tissue solubilizer (Solvable; Perkin-Elmer, Boston, MA) and incubated at 50°C overnight.
Radiolabeled stereospecific glucose in the incubation solution was used to measure transporter-mediated and non-transporter-mediated glucose uptake. 14C-d-glucose (1.0 uCi/8ml) was used as a marker for transporter-mediated glucose uptake; 3H-l-glucose (a glucose enantiomer that is not transported by the stereospecific glucose transporters; 1.8 uCi/8ml) was used to account and correct for both passive glucose diffusion and glucose adherent to the mucosal surface. Once the tissues were solubilized, 15 ml of counting cocktail (Opti-Flour, Perkin-Elmer, Shelton, CT) was added to the glass vial, and counts were obtained using dual isotope, liquid scintillation counting on a Beckman LS6000SC counter (Fullerton, CA). Transporter mediated glucose uptake was calculated as total glucose uptake (total uptake [14C-glucose] minus adherence and passive uptake [3H-glucose]) for each sample and expressed in nmol·cm−1 per duration of incubation.
Transporter-mediated glucose uptake in normal rat jejunum was studied under six conditions: control, SGLT1 inhibition, GLUT2 inhibition, combined SGLT1 and GLUT2 inhibition, PKC inhibition, and disruption of intracellular microtubular structure (n=6 rats each); specific antagonists were added directly to the preincubation and incubation solutions. Using doses proven effective previously (28–34), phlorizin (0.2 mM) was used to inhibit SGLT1, phloretin (1 mM) was used to inhibit GLUT2, calphostin C (50 nM) was used to inhibit PKC activity, and nocodazole (10 µM) was used to inhibit intracellular translocation. Interim review of our results after jejunal sleeves had been tested in 1 and 20 mM glucose for 1 min incubation lead to the following changes in our experimental protocol: the concentration of calphostin C was increased to 500 nM and an additional PKC inhibitor (chelerythrine 50 µM) was tested separately (35, 36). These changes applied to all sleeves tested in 50 mM glucose for 1 min and for all glucose concentrations (1, 20, & 50 mM) in which everted sleeves were incubated for 5 min.
Phlorizin, phloretin, calphostin C, and chelerythrine were solubilized in ethanol with 100-µl aliquots used to obtain the above concentrations. Vehicle control studies with 100 µl of ethanol alone were conducted and did not show any difference in transporter-mediated glucose uptake (data not shown). Calphostin C was protected from light until it was added to the preincubation and incubation solutions. Nocodazole required solubilization in dimethyl sulfoxide (DMSO). Vehicle studies with 100 µl of DMSO in the incubation solution showed a significant decrease in transporter-mediated glucose uptake; however, 1 µl of DMSO had no effect on transporter-mediated glucose uptake (data not shown). Therefore, nocodazole was solubilized in DMSO and 1-µl aliquots were added to the preincubation and incubation solutions to obtain the above concentration. All agents were added to preincubation and incubation solutions at least 5 min before the sleeves were exposed to the solutions.
Nonlinear regression of transporter-mediated uptake at 1, 20, and 50 mM glucose was used to calculate Km values of the Michaelis-Menton kinetic equation (GraphPad Prism version 4.03).
Statistical Analysis
Statistical analysis was performed using the Kruskal-Wallis test to compare nonparametric data between groups, while the Wilcoxon rank sums test was used to compare directly the non-parametric datasets. P values were corrected according to the Bonferroni method, and a corrected p value of ≤0.05 was considered significant. All data are reported as the mean values ± standard error of the mean unless specified otherwise; n values are number of rats.
RESULTS
When the everted jejunal sleeves were exposed to increasing concentrations of luminal glucose for 1 minute, transporter-mediated uptake displayed a characteristic, saturable, single transporter pattern (figure 1A-control). Transporter-mediated uptake of glucose was increased from the uptake at 1 mM glucose in the 20 mM experiments, but there was no further increase at 50 mM glucose. The calculated Km was 4.0±2.4 which correlates with the Km reported previously for SGLT1.
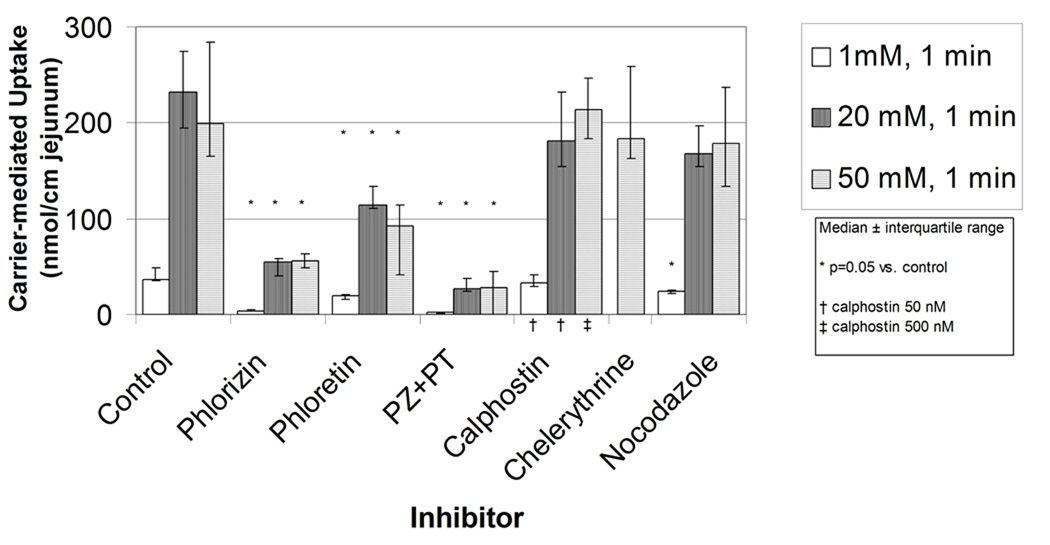
Figure 1.
A) Effects on transporter mediated glucose uptake of inhibition of SGLT1 (phlorizin 0.2 mM), GLUT2 (phloretin 1 mM), both SGLT1 and GLUT2 (phlorizin 0.2 mM and phloretin 1 mM), PKC activity (calphostin C 50 or 500 nM or chelerythrine 50 µM), and disruption of the microtubular system (nocodazole 10 µM) in jejunum using everted sleeves incubated for 1 min. B) same as (A) except everted sleeves incubated for 5 minutes and calphostin C 500 nM.
We then measured transporter-mediated uptake of glucose at these same concentrations of glucose (1, 20, and 50 mM) but for 5 minutes exposure. We were expecting to see an augmentation of transporter-mediated glucose uptake at the 50 mM glucose exposure that would be much greater than the uptake at 20 mM glucose suggesting recruitment of another transporter different from SGLT1. We found that the pattern of transporter-mediated uptake, however, again resembled that of a saturable, single transporter system without a marked increase in transporter-mediated uptake at 50 mM glucose as we had expected, and the Km was 8.9±7.6, again consistent with SGLT1 as the transporter and not different statistically to the Km for transporter-mediated transport at the 1 minute duration of exposure.
Inhibition of SGLT1 and/or GLUT2
When the jejunal everted sleeves were exposed to glucose concentrations of 1, 20, and 50 mM for the short, 1 min duration, addition of the SGLT1 inhibitor, phlorizin resulted in marked inhibition of transporter-mediated glucose uptake at all glucose concentrations (figure 1A). Treatment with phloretin, the GLUT2 inhibitor, also decreased transporter-mediated glucose uptake for the 1 minute duration by approximately 50%. The combination of both phlorizin and phloretin further decreased glucose uptake and inhibited transporter-mediated uptake almost completely.
When the duration of incubation was increased to 5 minutes (figure 1B), very similar effects were seen. Phlorizin and phloretin alone each inhibited glucose uptake at all concentrations of glucose, and the combination of both inhibitors lead to a further decrease in glucose uptake.
Inhibition of mechanisms of intracellular GLUT2 translocation
Two inhibitors of PKC activity, calphostin C and chelerythrine, were used to inhibit PKC activity in attempt to disrupt potential intracellular translocation of GLUT2 to the apical membrane of the enterocytes. When evaluated at either 1 min (figure 1A) or 5 min (figure 1B) of exposure to glucose at the lesser concentrations of glucose (1 and 20 mM), as well as at the greatest concentration (50 mM), no consistent effect on suppressing glucose uptake was seen with either concentration of calphostin C or with chelerythrine. Similarly, when nocodazole was used to disrupt the cellular cytostructure within the enterocyte by depolymerizing the microtubules, no consistent effect was noted on glucose uptake at either the lesser or greater durations of exposure to either the lesser or greater concentrations of glucose.
DISCUSSION
Formerly, the presence of GLUT2 in the apical membrane was associated with diabetes and was presumed to be a pathologic event in response to increasing blood concentrations of glucose (37). Currently, some groups still dismiss the theory of a rapid, reversible translocation of GLUT2 to the apical membrane, citing previous work that demonstrated GLUT2 only in the basolateral membrane using immunohistochemical techniques targeting the C-terminal of GLUT2 (38–40). More recent data, however, using immunohistochemical assays targeting the extracellular loop of GLUT2, have demonstrated its presence at both the basolateral and brush border apical membranes of the enterocyte (16, 20). Additional studies have shown that activities of the membrane-bound transporter SGLT1 and intracellular enzyme PKC appear to be integral, inter-related initiators of an intracellular signaling pathway that appears to regulate the translocation of GLUT2 into the apical membrane; this pathway establishes the rapid increase in transporter-mediated uptake of glucose when the intraluminal concentration of glucose is high, such as early after a meal. Indeed, in the presence of high concentrations of intraluminal glucose (≥ 50 mM) that far exceed the Km of SGLT1 (~3–5 mM) and that saturate all SGLT1-mediated uptake of glucose, the combined activities of SGLT1 and PKC appear to be necessary to initiate translocation of pre-formed, cytoplasmic stores of GLUT2 rapidly to the apical membrane. This translocation of GLUT2, which has a lesser affinity but a much greater Vmax and thus a greater capacity for glucose absorption, allows for the marked increase in absorption of luminal glucose at concentrations that are far greater than the Km of SGLT1. Once the intraluminal concentration of glucose decreases, GLUT2 appears to be either degraded rapidly or internalized rapidly such that it does not maintain a constant presence in large amounts in the apical membrane (17, 18, 20).
Our initial experiments were designed to reproduce with an in vitro, full-thickness, tissue-based model the augmented, transporter-mediated uptake of glucose at concentrations > 20 mM in the lumen when the everted sleeves were exposed for short (1 min) and greater (5 min) durations. Our ultimate goal is to have an in vivo, in vitro, and cell culture-based methodology to study the phenomenon of GLUT2 translocation. To our surprise however, we were unable to show augmentation in transporter-mediated uptake, and the Km of the transport measured at 1 minute and 5 minutes of exposure were similar to one another and similar to that reported by others for SGLT1. Therefore, under the constraints of our experimental preparation and conditions, we were unable to provide evidence for recruitment of another glucose transporter with a greater Km (as expected for GLUT2) within the 5 minutes of exposure, an effect that differs from our preliminary observations in cell culture in several intestinal epithelial cell lines (24, preliminary data).
Our subsequent experiments were designed to examine this model of intestinal uptake of glucose for evidence of GLUT2-mediated uptake of glucose under basal conditions and the role of SGLT1, PKC, and the intracellular, cytoskeletal microtubular structure on GLUT2-mediated uptake of glucose. Prior work in vivo has suggested that SGLT1 activity is necessary for GLUT2 activation and translocation (41, 42); inhibition of SGLT1 by phlorizin should, therefore, disrupt uptake of glucose by SGLT1 and GLUT2 and prevent the translocation of GLUT2 to the apical membrane. Our studies with phlorizin in this everted sleeve model inhibited markedly the transporter-mediated uptake of glucose in the jejunum. The small amount of persistent glucose uptake in the jejunum could be due to either uninhibited SGLT1 or to GLUT2 (or another transporter) expressed constitutively in the apical membrane. While most groups comment on the rapid translocation of GLUT2 to and from the apical membrane in vivo in response to glucose, few studies have commented on the presence of GLUT2 in the apical membrane in “unstimulated,” nondiabetic enterocytes; the presence of GLUT2 in the apical membrane of the enterocyte in the absence of a high concentration of luminal glucose has been demonstrated in several studies using western blots and could be the explanation for the phlorizin-insensitive absorption in our experiment. (16, 18–20, 28, 37, 42). Another potential explanation for the phlorizin-insensitive glucose transport observed in our experiment is that GLUT2 was present in the apical membrane as a result of taste receptor-induced GLUT2 translocation, another recently described pathway for GLUT2 translocation (43, 44). Treatment of everted sleeves with phloretin, which inhibits GLUT2 selectively, should not affect SGLT1-mediated glucose uptake but should inhibit glucose uptake by GLUT2, if GLUT2 was present in the apical membrane. Our findings of partial inhibition of transporter-mediated glucose uptake appear to confirm this concept in these experiments with the short duration of exposure to glucose (1 min). Although we would expect a greater Km (possibly > 30 mM based on others work (7)) and therefore an increase in transporter-mediated uptake at concentrations of glucose greater than 20 mM, we could not demonstrate greater transporter-mediated uptake at 50 mM glucose solutions. We can only attempt to explain these findings by suggesting that constitutive GLUT2 in the apical membrane may have a lesser Km similar to that of SGLT1; if true, this hypothesis would explain our findings.
We were also interested in studying the intracellular pathways regulating GLUT2 activity, as well as GLUT2 translocation in response to high luminal concentrations of glucose. When we targeted the role of PKC with the PKC inhibitors calphostin C and chelerythrine, we anticipated that uptake of glucose would be similar to that of the phloretin-treated sleeves if SGLT1 signaling through PKC was, in fact, necessary for GLUT2 activation and translocation to the apical membrane. We found no inhibition of transporter-mediated uptake during the short duration of exposure (1 min) to glucose concentrations greater than 1 mM and were somewhat surprised that inhibition of PKC activity did not decrease glucose absorption at the greater duration of incubation (5 min).
We further complemented these experiments by exploring the role of the integrity of the cytoskeleton in the apical translocation of GLUT2 by using nocodazole which depolymerizes intracellular microtubules. We hypothesized that if GLUT2 is translocated to the apical membrane, then disruption of the microtubule structure should prevent this translocation and blunt transporter-mediated glucose uptake similar to that expected with inhibition of PKC by calphostin C, chelerythrine, and by inhibition of GLUT2 by phloretin. Our results with nocodazole were similar to those seen with PKC inhibition, and no effect was noted in transporter-mediated uptake.
One other goal of our study was to use this in vitro technique of full thickness everted sleeves to study this presumed dynamic process of rapid (within minutes) apical translocation of glucose transporters. Unfortunately, our study shows that this model is not a useful preparation to study rapid translocation of glucose transport for several reasons. One reason is that the time necessary to harvest and prepare the everted sleeves and to equilibrate the tissue in the solution may be associated with translocation (or degradation) of GLUT2 out of the apical membrane. Indeed, this phenomenon has been blamed as the cause by which the role of GLUT2 in the apical membrane has been overlooked (7). Second, a 5 minute exposure to high concentrations of glucose, although satisfactory to see translocation in cell culture, may be too short a duration in everted sleeves; indeed, in vivo, this phenomenon appears to take 10 to 15 minutes. Longer exposures in the everted sleeve technique have not proved reliable in our extensive preliminary studies, because glucose uptake decreases markedly over time.
CONCLUSION
While these data, under the experimental conditions used, do not support conclusively the presence of an intracellular signaling pathway mediated by PKC that promotes translocation of additional glucose transporters (GLUT2) to the apical membrane, it is clear that inhibition of SGLT1 activity with phlorizin and/or GLUT2 with phloretin has an inhibitory effect on transporter-mediated glucose uptake. The partial inhibition of transporter-mediated glucose uptake in the presence of GLUT2 inhibition implicates GLUT2 as being involved in apical glucose transport at the “luminal” concentrations tested, while the inhibition of GLUT2 activity by SGLT1 inhibitor phlorizin suggests that SGLT1 activity is required for GLUT2 uptake of glucose. These data show that GLUT2 be constitutively active at the apical membrane of nondiabetic rats under basal conditions with a Km not significantly different from that of SGLT1.
ACKOWLEDGEMENTS
The authors wish to thank Judith Duenes for her expert assistance in the performance of the experiments and Deborah Frank for her expertise in the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Corpe CP, Burant CF. Hexose transporter expression in rat small intestine: effect of diet on diurnal variations. Am J Physiol. 1996;271:G211–G216. doi: 10.1152/ajpgi.1996.271.1.G211. [DOI] [PubMed] [Google Scholar]
- 2.Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265–276. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavakkolizadeh A, Berger UV, Shen KR, Levitsky LL, Zinner MJ, Hediger MA, Ashley SW, Whang EE, Rhoads DB. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G209–G215. doi: 10.1152/ajpgi.2001.280.2.G209. [DOI] [PubMed] [Google Scholar]
- 4.Houghton SG, Zarroug AE, Duenes JA, Fernandez-Zapico ME, Sarr MG. The diurnal periodicity of hexose transporter mRNA and protein levels in the rat jejunum: role of vagal innervation. Surgery. 2006;139:542–549. doi: 10.1016/j.surg.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Fullerton PM, Parsons DS. The absorption of sugars and water from rat intestine in vivo. Q J Exp Physiol Cogn Med Sci. 1956;41:387–397. [Google Scholar]
- 6.Holdsworth CD, Dawson AM. The Absorption of Monosaccharides in Man. Clin Sci. 1964;27:371–379. [PubMed] [Google Scholar]
- 7.Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons DS, Prichard JS. Properties of some model systems for transcellular active transport. Biochim Biophys Acta. 1966;126:471–491. doi: 10.1016/0926-6585(66)90006-9. [DOI] [PubMed] [Google Scholar]
- 9.Drozdowski LA, Thomson AB. Intestinal sugar transport. World J Gastroenterol. 2006;12:1657–1670. doi: 10.3748/wjg.v12.i11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev. 1997;77:257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- 11.Ferraris RP, Diamond JM. Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu Rev Physiol. 1989;51:125–141. doi: 10.1146/annurev.ph.51.030189.001013. [DOI] [PubMed] [Google Scholar]
- 12.Mullen TL, Muller M, Van Bruggen JT. Role of solute drag in intestinal transport. J Gen Physiol. 1985;85:347–363. doi: 10.1085/jgp.85.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappenheimer JR. Scaling of dimensions of small intestines in non-ruminant eutherian mammals and its significance for absorptive mechanisms. Comp Biochem Physiol A Mol Integr Physiol. 1998;121:45–58. doi: 10.1016/s1095-6433(98)10100-9. [DOI] [PubMed] [Google Scholar]
- 14.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 15.Pohl P, Saparov SM. Solvent drag across gramicidin channels demonstrated by microelectrodes. Biophys J. 2000;78:2426–2434. doi: 10.1016/S0006-3495(00)76786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000;350(Pt 1):163–169. [PMC free article] [PubMed] [Google Scholar]
- 18.Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000;350(Pt 1):149–154. [PMC free article] [PubMed] [Google Scholar]
- 19.Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C betaII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem. 2003;278:28644–28650. doi: 10.1074/jbc.M301479200. [DOI] [PubMed] [Google Scholar]
- 20.Affleck JA, Helliwell PA, Kellett GL. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J Histochem Cytochem. 2003;51:1567–1574. doi: 10.1177/002215540305101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatima J, Iqbal CW, Houghton SG, Kasparek MS, Duenes JA, Zheng Y, Sarr MG. Hexose transporter expression and function in mouse small intestine: role of diurnal rhythm. J Gastrointest Surg. 2009;13:634–641. doi: 10.1007/s11605-008-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houghton SG, Iqbal CW, Duenes JA, Fatima J, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93. doi: 10.1016/j.surg.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal CW, Fatima J, Duenes J, Houghton SG, Kasparek MS, Sarr MG. Expression and function of intestinal hexose transporters after small intestinal denervation. Surgery. 2009;146:100–112. doi: 10.1016/j.surg.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Duenes JA, Qandeel HG, Sarr MG. Translocation of Transfected GLUT2 to the Apical Membrane in Rat Intestinal IEC-6 Cells. Gastroenterology. 2009;136:A95. doi: 10.1007/s10620-011-1984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X, Terada T, Okuda M, Inui K. The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 is regulated by the feeding conditions in rats. J Nutr. 2004;134:2211–2215. doi: 10.1093/jn/134.9.2211. [DOI] [PubMed] [Google Scholar]
- 26.Tavakkolizadeh A, Ramsanahie A, Levitsky LL, Zinner MJ, Whang EE, Ashley SW, Rhoads DB. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. 2005;129:73–78. doi: 10.1016/j.jss.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Karasov WH, Diamond JM. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983;1983:105–116. [Google Scholar]
- 28.Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol. 2007;292:R862–R867. doi: 10.1152/ajpregu.00655.2006. [DOI] [PubMed] [Google Scholar]
- 29.Loike JD, Hickman S, Kuang K, Xu M, Cao L, Vera JC, Silverstein SC, Fischbarg J. Sodium-glucose cotransporters display sodium- and phlorizin-dependent water permeability. Am J Physiol. 1996;271:C1774–C1779. doi: 10.1152/ajpcell.1996.271.5.C1774. [DOI] [PubMed] [Google Scholar]
- 30.Manome S, Kuriaki K. Effect of insulin, phlorizin and some metabolic inhibitors on the glucose absorption from the intestine. Arch Int Pharmacodyn Ther. 1961;130:187–194. [PubMed] [Google Scholar]
- 31.Oulianova N, Falk S, Berteloot A. Two-step mechanism of phlorizin binding to the SGLT1 protein in the kidney. J Membr Biol. 2001;179:223–242. doi: 10.1007/s002320010049. [DOI] [PubMed] [Google Scholar]
- 32.Rotenberg SA, Huang MH, Zhu J, Su L, Riedel H. Deletion analysis of protein kinase C inactivation by calphostin C. Mol Carcinog. 1995;12:42–49. doi: 10.1002/mc.2940120107. [DOI] [PubMed] [Google Scholar]
- 33.Forsling ML, Widdas WF. The effect of temperature on the competitive inhibition of glucose transfer in human erythrocytes by phenolphthalein, phloretin and stilboestrol. J Physiol. 1968;194:545–554. doi: 10.1113/jphysiol.1968.sp008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao MD, Chen IS, Cheng JT. Inhibition of protein kinase C translocation from cytosol to membrane by chelerythrine. Planta Med. 1998;64:662–663. doi: 10.1055/s-2006-957545. [DOI] [PubMed] [Google Scholar]
- 36.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 37.Corpe CP, Basaleh MM, Affleck J, Gould G, Jess TJ, Kellett GL. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996;432:192–201. doi: 10.1007/s004240050124. [DOI] [PubMed] [Google Scholar]
- 38.Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990;259:C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- 39.Thorens B, Lodish HF, Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990;259:C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- 40.Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 41.Mace OJ, Morgan EL, Affleck JA, Lister N, Kellett GL. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol. 2007;580:605–616. doi: 10.1113/jphysiol.2006.124784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350(Pt 1):155–162. [PMC free article] [PubMed] [Google Scholar]
- 43.Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol. 2007;213:834–843. doi: 10.1002/jcp.21245. [DOI] [PubMed] [Google Scholar]
- 44.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]