Abstract
Cardiac dysfunction is a well-recognized complication of light chain amyloidosis (AL). Autologous stem cell transplant (auto-SCT) has emerged as a successful treatment modality for AL patients. In this study, we examined the effect of clonal immunoglobulin light chain genes (VL), which encodes the immunoglobulin light chain protein that ultimately forms amyloid, on cardiac function, in the context of auto-SCT and its impact on overall survival. Longitudinal Doppler myocardial imaging parameters along with cardiac biomarkers were used to assess for cardiac function pre and post auto-SCT. VL gene analysis revealed that Vλ genes, in particular VλVI, were associated with worse cardiac function parameters than Vκ genes. Clonal VL genes appeared to have an impact on left ventricular (LV) function post-transplant and also influenced mortality, with specific VL gene families associated with lower survival. Another key predictor of mortality in this report was change in tricuspid regurgitant flow velocity following auto-SCT. Correlations were also observed between systolic strain rate, systolic strain and VL genes associated with amyloid formation. In summary, clonal VL gene usage influences global cardiac function in AL, with patients having VλVI and VλII-III-associated amyloid more severely affected than those having Vκ or VλI amyloid. Pulsed wave tissue Doppler imaging along with immunoglobulin gene analysis offers novel insights into prediction of mortality and cardiac dysfunction in AL after auto-SCT.
INTRODUCTION
Light chain (AL) amyloidosis is the most common systemic amyloidosis with an age-adjusted incidence of 5–13 per million patients per year in the United States. The traditional low-dose oral melphalan treatment regimen is generally associated with a poor prognosis (median survival of 13 months).1 The recent introduction of autologous peripheral blood stem cell transplantation (auto-SCT) has dramatically improved median survival in a select group of AL patients.1,2 Both hematological response at Day 100 and cardiac function are key determinants of post auto-SCT survival.1–4
In earlier studies we reported the high sensitivity of longitudinal Doppler myocardial imaging (DMI) - myocardial velocity, strain and strain rate imaging in detecting left ventricular (LV) impairment in both early and advanced AL.5,6 Because an association among particular clonal VL genes, organ tropism of amyloid deposition and outcomes has been observed,7–10 this study had a twofold aim: 1) to evaluate whether clonal light chain VL gene usage influences changes in LV function post-SCT, and its impact on long-term mortality, and 2) to test whether serial measurement of cardiac biomarkers and longitudinal Doppler myocardial imaging (DMI) measures are useful for monitoring cardiac function, and for risk stratification.
METHODS
This study was approved by the Institutional Review Board of the Mayo Clinic. Fifty-three patients with AL systemic amyloidosis, consecutively selected from patients undergoing evaluation in the Divisions of Cardiovascular Diseases and Hematology at the Mayo Clinic, Rochester, Minnesota, from January 1st 2004 through April 30th 2007, and referred to auto-SCT, were enrolled in a prospective manner. Patients received either standard dose conditioning regimen (Melphalan) at 200 mg/m2 or reduced-dose (<200mg/m2) depending on patient age, renal function, presence of cardiomyopathy, or 3-organ involvement. Of the 53 patients who had immunoglobulin gene analysis performed, 28 received the standard dose, and 25 received the lower dose (see Supplementary Table 1). Follow-up ended June the 30th 2009. The diagnosis of AL amyloidosis was made by either subcutaneous fat biopsy or an involved organ biopsy study that demonstrated typical Congo red birefringence under polarized light. Endomyocardial biopsy was performed in 4 patients, for clinical purposes, and was positive for amyloid infiltration in all the cases. AL amyloidosis was further confirmed by the presence of a monoclonal protein in the serum or urine specimen and/or a monoclonal population of plasma cells in the bone marrow. Clinical, biomarkers and echocardiographic examinations were accomplished at 2 different time points (in a subset of 39 patients): the first assessment was performed within a month prior to auto-SCT while the second assessment was performed at Day 100 post-SCT.
Exclusion criteria were familial, secondary, or senile amyloidosis (n=2), all other causes of cardiomyopathy (including other forms of restrictive cardiomyopathy, hypertrophic cardiomyopathy, or any cause of LV hypertrophy, other than AL amyloidosis), diabetes mellitus (n=0), history of moderate or greater systemic or pulmonary hypertension (n=1), more than mild valvular heart disease (n=0), and coronary artery disease or previous myocardial infarction (n=0). Atrial fibrillation (n=3) was not an exclusion criterion.
Clinical classification of patients
Patients with AL were evaluated for the extent of amyloid-related organ involvement and for dominant organ involvement, integrating standard criteria described elsewhere11 along with information provided by the assessment of cardiac biomarkers and diastolic function. The patients were categorized according to clinical presentation as having renal, cardiac, hepatic, neurological or other dominant organ involvement. This clinical classification was performed independently blind to the clonal VL gene usage, Patients with more than one organ involved were categorized according to the most prominent and symptomatic affected organ. Since several patients had both cardiac and renal dysfunction due to amyloid deposition, information on dominant or co-dominant involvement was collected for these patients. Dominant gastrointestinal, pulmonary, or soft tissue AL (excluding cardiac, renal, hepatic, or neurological dominant involvement) were all classified in the category “Other”, to simplify analysis.
Dominant cardiac involvement was defined as having a positive endomyocardial biopsy, or abnormally elevated cardiac biomarkers, including brain natriuretic peptide (BNP), and cardiac Troponin T (cTnT), based on our previous observations.3,12 Additionally, echocardiographic parameters, such as mean left ventricular wall thickness (half the sum of the antero-septal and posterior wall thickness in the parasternal long-axis view) > 12 mm, and diastolic dysfunction with a grade of 3 or 4 (restrictive pattern) were also used to define dominant cardiac involvement.13,14 Dominant renal involvement was defined as having a positive kidney biopsy and a proteinuria more than 0.5 g/dl, dialysis dependence or creatinine clearance less than 10 mL/min.
Classification of enrolled patients followed a 3-step algorithm: firstly, patients were categorized based on clonal VL germline gene usage, and thus two groups were generated: Group Vκ, including patients using Vκ I family genes and Group Vλ, including patients using VλI, VλII, VλIII and Vλ VI families. Secondly, group Vλ patients were further subdivided based on cardiac function by standard echocardiography (left ventricular wall thickness, mass index, and ejection fraction) at baseline (pre-auto-SCT): Group Vλ I–II–III, and Group Vλ VI. Thirdly, patients of group Vλ I–II–III were sub-divided based on hematologic response;11 all Vλ I patients (n=7) had either partial or complete response at Day 100 and were therefore considered independently from Vλ II–III patients, who had heterogeneous hematological responses. In summary, 4 different groups were used in the analysis for comparison: Group Vκ; Group Vλ I; Group Vλ II–III; Group Vλ VI.
Specimen preparation and cloning of Immunoglobulin VL genes
Bone marrow samples from all 53 AL patients were processed to collect plasma cells and clonal immunoglobulin gene analysis was performed as per protocol (see Appendix for methodological details).
Definition of clonal immunoglobulin gene usage
The term “immunoglobulin gene usage” refers to the concept that antibodies or immunoglobulin (Ig) molecules are generated in the immune system as a result of selection and molecular combining of 3–4 individual gene segments (3 for Ig light chain and 4 for Ig heavy chain; light and heavy chain being essential for formation of a complete antibody molecule). This process of selection and molecular recombination is a stochastic event where individual gene segments are apparently randomly “chosen” or “used” out of a larger array of such gene “families”. In a normal immune response, the diversity of antibody molecules produced by a terminally differentiated B cell (plasma cell) is large (polyclonal response), in contrast, to what is observed during a neoplastic process, such as in AL, where a single plasma cell undergoes expansion to produce a population of plasma cells (monoclonal or clonal) all secreting exactly the same antibody (immunoglobulin), from which the light chain forms amyloid fibrils and undergoes tissue deposition.
Biomarkers
BNP, NT-proBNP, cTnT, creatinine and glomerular filtration rate (GFR) were measured and collected at the time of the echocardiographic examination as previously described.6 Hematological assessment included percent of bone marrow clonal plasma cells, level of clonal serum free light chain (FLC),15 serum and urine protein electrophoresis (monoclonal protein quantitation) with immunofixation (monoclonal protein identification).
Standard Ultrasound Examination
All ultrasound examinations were performed with a commercially available echocardiographic instrument (Vivid 7 System, Vingmed, General electric 3135 Easton Turnpike Fairfield, CT 06828-0001). The thickness of the ventricular septum, and LV posterior wall, and end-systolic and end-diastolic LV diameters, and the left atrial antero-posterior diameter were determined from M-mode or two dimensional (2-D) imaging, while LV mass and ejection fraction were calculated. Left atrial volume measurement was carried out as previously described.16 Both LV mass and LA volume were indexed to body surface area. Pulsed wave Doppler of mitral inflow, pulmonary veins and LV outflow was performed as previously described.16 Pulsed wave tissue Doppler imaging was performed using a sample volume gate length of 0.17 cm. The sample volume was placed on the medial mitral annulus in the apical four chamber view, and the early (E’) and peak diastolic velocity were measured. Right index of myocardial performance (RIMP) was measured as previously described.17 RV systolic pressure (RVSP) was calculated by inserting the tricuspid regurgitation velocity obtained with continuous-wave Doppler, into the simplified Bernoulli equation.13,14
Off-line analysis of standard echocardiographic variables was performed with the use of dedicated software (ProSolv CV analyzer vers. 3.0 8021 Knue Road Indianapolis, IN 46250); three consecutive beats were measured and averaged for each measurement. All standard and DMI echocardiographic measures were collected with the two investigators obtaining the data (DB and GA) blind to the patients’ clinical status and the time point (pre-or post-SCT) that the images undergoing analysis were acquired.
Strain Imaging Data Acquisition and Analysis
For DMI, apical 4 chamber, long axis, and 2 chamber views were acquired. DMI was used to measure longitudinal left ventricular (LV) myocardial strain and strain rate in 2 separate echocardiographic examinations, as mentioned above. In addition, narrow sector views were acquired for each left ventricular wall from apical views. Color tissue recordings were collected with a frame rate ≥ 200 frames/sec during brief breath hold. Three consecutive cardiac cycles were recorded as cine loops and the acquired raw data were saved on magneto-optical disks for off-line analysis (Echopac BT06, GE Vingmed Ultrasound).
Sample volumes were placed on basal, middle and apical LV segments of the anterolateral, inferoseptal, posterior, anteroseptal, inferior and anterior walls to assess longitudinal DMI. Longitudinal systolic peak values were determined for systolic strain rate (sSR) and systolic strain (sS).18 Analysis was performed for all 16 LV segments individually (data not shown) and in clusters according to the LV level (basal, middle and apical). Specifically, the DMI values (sSR and sS) were averaged for the 6 basal segments (basal mean), the 6 middle segments (middle mean) and the 4 apical segments (apical mean).
Statistical analysis
Statistical analyses were performed with a commercially available software program (STATA 10, MP version, Statacorp. Ltd, College Station, Texas). Comparisons between groups were made using either the Kruskal-Wallis test or Fisher exact test to determine differences among groups at baseline (pre-auto-SCT). The Wilcoxon matched-pairs signed rank sum test was used to compare post-auto-SCT biomarkers and DMI measures to the pre-SCT values. The natural log of BNP and FLC were used in the analysis to meet the assumptions of a normal distribution.
Follow-up of the patients included a median follow-up time of 34 months (range: 0.9 – 64 months). Primary end-point was all-cause mortality and this was ascertained in all patients (N = 53), either while they were under medical management, or through the review of death certificates, or by querying the National Death index.
Because many of the patients in the study were first referred to Mayo Clinic several months after their initial diagnosis, left truncation was used for the Kaplan-Meier and Cox regression analyses; i.e. no subject was considered to be in the risk set until the time of echocardiography.19 Kaplan-Meier survival curves for the 4 groups were constructed following, which log-rank test of uniform survival distribution among groups was performed. By definition, TRM was excluded in the analysis of this subset. Univariate analysis of the time to events was performed with use of Cox proportional hazard models, considering the difference (Δ) between pre- and post-SCT of biomarkers, standard echocardiographic, and longitudinal DMI measurements as predictors of primary outcome. Delta (Δ) was computed for all collected variables as follows:
Data is presented as the mean value ± SD or median value ± inter-quartile range (biomarkers) or count (percentage). A difference was considered statistically significant when the p-value was <0.05.
Intra- Inter- observer variability
To examine intra-observer variability (repeatability), a sample of 10 echocardiographic examinations was randomly selected for masked review by the same investigator (DB). To examine inter-observer variability a co-investigator (GA), blinded to the clinical information and to the results of the first investigator, examined 10 randomly selected echocardiographic exams. Intraclass correlation coefficients (ICCs) for the same observer and different observers were calculated using previously described formulae20 for single segments and for the global mean of each DMI modality.
RESULTS
Immunoglobulin germ line gene usage and clinical characteristics
Baseline
The frequency distribution of clonal VL gene usage and dominant organ involvement are shown in Table 1. The Vλ VI gene −6a, was the most frequently used (n = 13; 25%) followed by the Vλ I genes (n=11; 21%), Vλ III genes (n=11, 21%), and Vκ genes (n=11; 21%); Vλ II genes were used the least frequently in this cohort (n =7; 13%). The time between diagnosis and enrollment in the study was not significantly different among the groups (p=0.51).
Table 1.
Distribution of dominant organ involvement as a function of immunoglobulin germ line variable gene (VL) use
| VL Gene Subtype |
Nb. | Dominant organ Involvement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nb Involved ≥ | ||||||||||
| Gene | Patients | Cardiac | Renal | PNS | Hepatic | Other | 3 | |||
| Primary | Secondary | Primary | Secondary | |||||||
| VλI | 1b | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 1c | 6 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | |
| 1e | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1g | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | |
| VλII | 2a2 | 4 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 2b2 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 2c | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| VλIII | 3h | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 3r | 10 | 5 | 1 | 3 | 3 | 1 | 0 | 0 | 1 | |
| Vλ VI | 6a | 13 | 6 | 0 | 4 | 1 | 1 | 0 | 1 | 5 |
| Vκ | L1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| L12 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| L2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| O12/O2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| O18/O8 | 6 | 0 | 2 | 3 | 0 | 0 | 0 | 1 | 0 | |
| Total | 53 | 17 | 5 | 21 | 9 | 3 | 1 | 3 | 7 | |
Frequency distribution of dominant and co-dominant organs affected by AL amyloid deposition, and number of organs involved, stratified by clonal immunoglobulin VL germline genes in 53 patients.
Amyloid deposition most frequently involved the heart and kidney, and in particular, the heart was the dominant organ affected in patients using Vλ VI, 6a gene (46%) with the Vλ III family genes coming close behind (45%). Dominant renal involvement was observed in 31% of the Vλ VI, 36% of the Vλ I, 57% of Vλ II, 27% of Vλ III, and 54% of the Vκ patients. The Vλ I, Vλ III and Vλ VI genes all had at least 1 patient with dominant peripheral nervous system involvement. Only one patient in the Vλ I group had hepatic involvement (Table 1).
There was no significant difference between the three V λ subgroups and the Vκ group patients with regard to age, gender and baseline clinical parameters (Table 2). At baseline, FLC levels were significantly lower in the Vλ VI group compared to the other patients. Furthermore, specific trends could be identified: patients using the Vλ VI gene appeared to have a higher prevalence of advanced NYHA functional class, and BNP and cTnT levels had a tendency to be lower in patients using the Vκ gene family and higher in patients using the Vλ VI family.
Table 2.
Biomarkers and Standard Echocardiography
| Variable (mean ± standard deviation) | Baseline* | Vκ (n = 11) | Vλ I (n = 11) | Vλ II–III (n = 18) | Vλ VI (n = 13) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| or median (Interquartile range) | p-value | Pre- PBSCT |
Post- PBSCT |
p- value |
Pre- PBSCT |
Post- PBSCT |
p- value |
Pre- PBSCT |
Post- PBSCT |
p- value |
Pre- PBSCT |
Post- PBSCT |
p- value |
| Females (n (%)) | 0.18 | 3 (28) | 8 (73) | 8 (45) | 5 (38) | ||||||||
| Age | 0.33 | 60 ± 5 | 55 ± 11 | 58 ± 11 | 59 ± 9 | ||||||||
| NYHA III/IV (n (%)) | 0.81 | 0 | 1 (12) | 0 | 3 (30) | ||||||||
| Body Mass Index | 0.64 | 26.21 ± 3.9 | 27.48 ± 4.6 | 25.8 ± 6 | 25 ± 3.6 | ||||||||
| Creatinine (mg/dl) | 0.69 | 1.20 (1.3) | 1.05 (1.1) | 1.0 (0.3) | 1.10 (0.4) | ||||||||
| GFR (ml/min/BSA) | 0.57 | 59 (56) | 65.5 (47) | 70 (18) | 72 (30) | ||||||||
| 24h Proteinuria (mg/24h) | 0.37 | 5,516 (7,469) | 4,859 (3,055) | 1,865 (4,178) | 3,538 (4,575) | ||||||||
| FLC κ | 0.003 | 14.1 (63) | 1.03 (4.8) | 0.04 | 1.24 (0.3) | 1.18 (1.5) | 0.70 | 0.88 (1.5) | 0.94 (2.4) | 0.33 | 1.41 (0.2) | 0.94 (1.4) | 0.78 |
| FLC λ | 0.004 | 1.64 (0.8) | 1.95 (1.9) | 0.90 | 13.55 (28.2) | 2.03 (5.2) | 0.02 | 20.95 (44.5) | 4.58 (12.6) | 0.01 | 5.68 (4.4) | 2.31 (3.9) | 0.71 |
| FLC κ/λ | 0.003 | 4.6 (6.1) | 1.94 (2.4) | 0.14 | 0.12 (0.2) | 0.2 (0.4) | 0.13 | 0.06 (0.1) | 0.48 (0.7) | 0.01 | 0.28 (0.2) | 0.36 (0.7) | 0.16 |
| BNP (pg/ml) | 0.59 | 79 (1158) | 80 (818) | 0.17 | 137 (290) | 198 (718) | 0.05 | 239 (262) | 154 (781) | 0.78 | 315 (480) | 293 (313) | 0.68 |
| Troponin T (ng/ml) | 0.95 | 0.01 (0.02) | 0.02 (0.02) | 0.67 | 0.01 (0.04) | 0.01 (0.19) | 0.42 | 0.01 (0.02) | 0.02 (0.06) | 0.12 | 0.02 (0.02) | 0.02 (0.02) | 0.23 |
| LV Thickness (mm) | 0.02 | 10.6 ± 2 | 11.9 ± 3 | 0.12 | 12.4 ± 2 | 14.3 ± 2 | 0.01 | 12.7 ± 3 | 13.5 ± 3 | 0.26 | 15.0 ± 4 | 15.7 ± 3 | 0.04 |
| LV Mass Index (gr/m2) | 0.04 | 95 ± 34 | 107 ± 49 | 0.26 | 80 ± 21 | 71 ± 22 | 0.01 | 92 ± 30 | 92 ± 33 | 0.38 | 157 ± 65 | 152 ± 35 | 0.62 |
| LVEDVolume (mm) | 0.65 | 86 ± 48 | 83 ± 34 | 0.23 | 37 ± 11 | 30 ± 9 | 0.34 | 41 ± 16 | 41 ± 19 | 0.95 | 73 ± 33 | 72 ± 35 | 0.04 |
| LVESVolume (mm) | 0.97 | 37.5 ± 17.4 | 33 ± 13.8 | 0.41 | 106 ± 18 | 132 ± 30 | 0.14 | 117 ± 46 | 122 ± 63 | 0.92 | 38.86 ± 19.8 | 33.25 ± 17 | 0.67 |
| LA Vol (cc/m2) | 0.68 | 47.7 ± 14.1 | 38.1 ± 10.9 | 0.02 | 37 ± 12 | 37 ± 9 | 0.25 | 40 ± 13 | 39 ± 19 | 0.86 | 41.8 ± 7.0 | 45.6 ± 9.6 | 0.31 |
| E (m/sec) | 0.48 | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.08 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.44 | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.69 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.27 |
| A (m/sec) | 0.87 | 0.65 ± 0.2 | 0.75 ± 0.2 | 0.15 | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.58 | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.78 | 0.65 ± 0.2 | 0.63 ± 0.3 | 0.72 |
| E/A | 0.42 | 1.4 ± 0.7 | 0.9 ± 0.5 | 0.03 | 1.4 ± 0.9 | 1.4 ± 0.7 | 0.14 | 1.3 ± 0.9 | 1.3 ± 0.6 | 0.37 | 1.5 ± 0.8 | 2.2 ± 1.9 | 0.41 |
| E Decelaration Time (msec) | 0.48 | 232 ± 52 | 248 ± 79 | 0.93 | 188 ± 36 | 203 ± 52 | 0.25 | 204 ± 48 | 195 ± 51 | 0.68 | 191 ± 43 | 194 ± 49 | 0.77 |
| E' (m/sec) | 0.51 | 0.06 ± 0.02 | 0.06 ± 0.03 | 0.22 | 0.06 ± 0.01 | 0.06 ± 0.03 | 0.67 | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.11 | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.04 |
| E/E' | 0.37 | 15.5 ± 8.9 | 13.9 ± 7.9 | 0.44 | 15.8 ± 6.7 | 13.3 ± 8.6 | 0.79 | 15.5 ± 11.6 | 19.0 ± 10.6 | 0.04 | 17.79 ± 7.5 | 22.79 ± 10.9 | 0.08 |
| Ejection Fraction (%) | 0.37 | 65 ± 9 | 62 ± 8.4 | 0.67 | 56 ± 9 | 56 ± 10 | 0.89 | 57 ± 7 | 57 ± 10 | 0.53 | 59 ± 12 | 60 ± 10 | 0.41 |
| Cardiac Index (l/min/m2) | 0.45 | 3.9 ± 0.7 | 3.2 ± 1.2 | 0.32 | 3.3 ± 1.1 | 3.1 ± 1 | 0.32 | 3.3 ± 0.9 | 2.9 ± 0.7 | 1.0 | 2.8 ± 1.2 | 3.0 ± 0.75 | 0.65 |
| RV tricuspid regurgitation vel. (m/s) | 0.57 | 2.6 ± 0.5 | 2.3 ± 0.3 | 0.05 | 2.7 ± 0.1 | 2.9 ± 0.3 | 0.14 | 2.5 ± 0.2 | 2.5 ± 0.4 | 0.80 | 2.6 ± 0.3 | 2.6 ± 0.5 | 0.91 |
| RV Systolic Pressure (mmHg) | 0.69 | 34.5 ± 11.6 | 25.67 ± 3.9 | 0.05 | 34.2 ± 3.5 | 42.4 ± 11 | 0.14 | 30.7 ± 7.3 | 36.4 ± 10.1 | 0.19 | 34.7 ± 8.4 | 35.2 ± 12 | 0.91 |
| RIMP | 0.54 | 0.36 ± 0.2 | 0.34 ± 0.2 | 0.67 | 0.32 ± 0.2 | 0.55 ± 0.3 | 0.15 | 0.26 ± 0.1 | 0.38 ± 0.1 | 0.03 | 0.38 ± 0.1 | 0.43 ± 0.2 | 0.41 |
Clinical, biomarkers, and standard echocardiographic variables for Vκ vs Vλ I vs Vλ II-III vs Vλ VI groups, pre- and post- peripheral blood stem cell transplant (auto-SCT). Descriptions are with mean ± SD, median ± inter-quartile range or count (percent). Comparisons of baseline values among groups (first column) are made using the Kruskal-Wallis test or Fisher exact test; comparison between pre- and post-auto-SCT value per variable are made using the Wilcoxon signed-rank paired sum test.
Post-SCT
The changes in BNP, cTnT and FLC κ levels following auto-SCT were not significant in any of the 4 groups, but, FLC λ was significantly reduced in both Vλ I and Vλ II–III groups, while the FLC k/ λ ratio was reduced in only the Vλ II–III group.
Standard echocardiography
Baseline
LV thickness and LV mass index at baseline was significantly different among the groups (Table 2) with the highest value being seen in Vλ VI patients. LV and left atrial volumes, ejection fraction and cardiac index, along with parameters of diastolic function (E and A wave velocities, E/A, pulsed wave tissue Doppler E’ velocity of the medial mitral annulus, and E/E’) were similar among all gene groups. Tricuspid regurgitant flow velocity, estimated right ventricular systolic pressure, and right index of myocardial performance (RIMP) were also similar among the groups.
Post-SCT
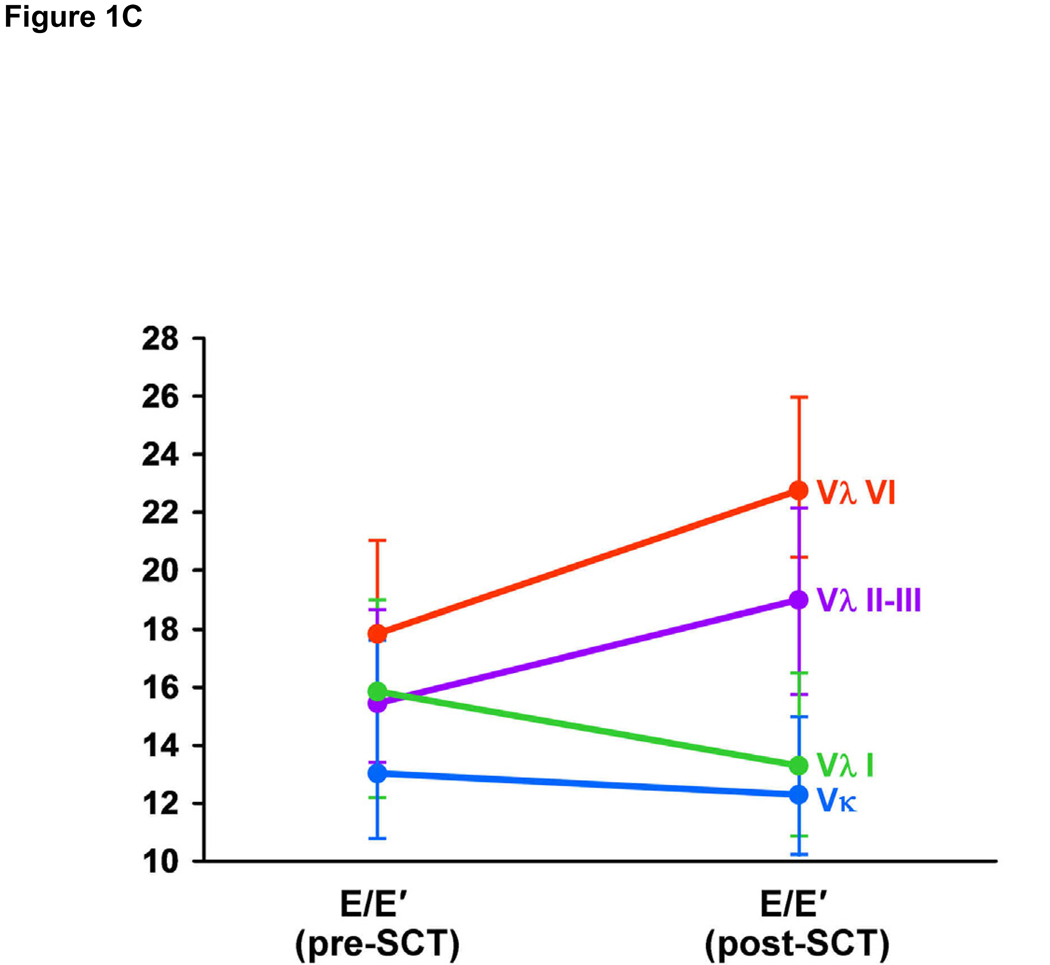
Post-SCT LV thickness was significantly increased in Vλ I and Vλ VI patients, while LV mass index was increased in Vλ I patients only (Figure 1A). LV end-diastolic and end-systolic volumes were not significantly changed following auto-SCT in any of the 4 groups. Left atrial volume was significantly reduced following auto-SCT in patients using the Vκ family (Figure 1B). Among diastolic measures, post-SCT trans-mitral E/A ratio was reduced in the Vκ group, while the difference after auto-SCT was not significant in patients using any of the Vλ families. E’ was reduced in Vλ VI patients and E/E’ ratio was increased in Vλ II–III patients. There were no significant variations in the other groups (Figure 1C), although post-auto-SCT E/E’ was marginally reduced in Vκ and in Vλ I patients. There was no variation in ejection fraction or cardiac index among groups. Both tricuspid regurgitant flow velocity and right ventricular systolic pressure showed a tendency to decrease only in patients using the Vκ family. At the same time, RIMP was unchanged in the Vκ group, while it increased in the Vλ II–III patients.
Figure 1.
Figure 1A: Pre- and post- peripheral blood stem cell transplant (auto-SCT) values for left ventricular (LV) thickness, by clonal VL germ line gene families (Vκ vs Vλ I vs Vλ II–III vs Vλ VI). * = p-value < 0.05.
Figure 1B: Pre- and post-auto-SCT values for left atrial volume, by clonal VL germ line gene families (Vκ vs Vλ I vs Vλ II–III vs Vλ VI). * = p-value < 0.05
Figure 1C: Pre- and post-auto-SCT values for E/E’, by clonal VL germ line gene families (Vκ vs Vλ I vs Vλ II–III vs Vλ VI). * = p-value < 0.05
Doppler myocardial imaging
Baseline
Longitudinal sSR mean of the 6 middle segments and the mean of the 4 apical segments, as well as the mean of 16 LV segments were significantly different among all groups, with the highest values in patients using the Vκ gene family, intermediate in Vλ II–III groups and least in patients using the Vλ VI and Vλ I gene families (Table 3).
Table 3.
Systolic Doppler Myocardial Imaging
| Variable (mean ± standard deviation) |
Baseline* | Vκ (n = 11) | Vλ I (n = 11) | Vλ II III (n = 18) |
Vλ VI (n = 13) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-value | Pre-PBSCT | Post- PBSCT |
p-value | Pre-PBSCT | Post- PBSCT |
p- value |
Pre-PBSCT | Post- PBSCT |
p- value |
Pre-PBSCT | Post- PBSCT |
p- value |
|
| Strain Rate Imaging (1/s) | Strain Rate Imaging (1/s) | ||||||||||||
| Basal Mean | 0.22 | −0.9 ± 0.5 | −0.9 ± 0.3 | 0.4 | −0.8 ± 0.3 | −0.7 ± 0.3 | 0.88 | −0.8 ± 0.3 | −0.7 ± 0.3 | 0.01 | −0.6 ± 0.4 | −0.5 ± 0.2 | 0.37 |
| Middle Mean | 0.009 | −1.2 ± 0.3 | −1.0 ± 0.2 | 0.26 | −0.9 ± 0.2 | −0.8 ± 0.3 | 0.4 | −1.1 ± 0.3 | −0.8 ± 0.3 | 0.001 | −0.7 ± 0.3 | −0.7 ± 0.3 | 0.53 |
| Apex Mean | 0.02 | −1.2 ± 0.2 | −1.1 ± 0.3 | 0.16 | −0.9 ± 0.3 | −0.8 ± 0.2 | 0.12 | −1.2 ± 0.4 | −0.9 ± 0.3 | 0.001 | −0.8 ± 0.2 | −0.7 ± 0.2 | 0.03 |
| Global Average | 0.02 | −1.1 ± 0.3 | −0.1 ± 0.3 | 0.26 | −0.9 ± 0.2 | −0.8 ± 0.3 | 0.78 | −1.0 ± 0.3 | −0.8 ± 0.2 | 0.001 | −0.7 ± 0.3 | −0.6 ± 0.2 | 0.11 |
| Strain Imaging (%) | Strain Imaging (%) | ||||||||||||
| Basal Mean | 0.06 | −15.6 ± 5.7 | −12.63 ± 5.9 | 0.09 | −10.2 ± 3.2 | −9.3 ± 6.4 | 0.78 | −11.3 ± 4.3 | −9.9 ± 4.8 | 0.48 | −8.7 ± 6.9 | −8.2 ± 5.3 | 0.72 |
| Middle Mean | 0.08 | −17.1 ± 4.6 | −16.11 ± 3 | 0.33 | −13.0 ± 4.7 | −12.5 ± 5.5 | 1 | −15.7 ± 3.3 | −14.1 ± 5.7 | 0.18 | −11.5 ± 5.4 | −9.8 ± 4.3 | 0.04 |
| Apex Mean | 0.009 | −17.1 ± 2.9 | −14.51 ± 3.6 | 0.09 | −12.5 ± 3.7 | −12.1 ± 3.9 | 0.41 | −17.3 ± 4.7 | −13.8 ± 4.3 | 0.009 | −12.2 ± 3.9 | −9.6 ± 3.3 | 0.04 |
| Global Average | 0.02 | −16.6 ± 4.2 | −14.41 ± 4.1 | 0.09 | −11.8 ± 2.6 | −11.2 ± 4.9 | 0.78 | −14.5 ± 3.3 | −12.5 ± 4.3 | 0.02 | −10.6 ± 5.2 | −9.2 ± 4.1 | 0.06 |
Longitudinal systolic Doppler myocardial imaging (DMI) variables, including systolic strain rate (sSR) and strain (sS), for Vκ vs Vλ I vs Vλ II–III vs Vλ VI groups, pre- and post- peripheral blood stem cell transplant (auto-SCT). Descriptions are with mean ± SD, median ± inter-quartile range or count (percent). Comparisons of baseline values among groups (first column) are made using the Kruskal-Wallis test or Fisher exact test; comparison between pre- and post-auto-SCT value per variable are made using the Wilcoxon signed-rank paired sum test
Longitudinal sS mean of the 4 apical segments, and the global mean of 16 LV segments were also different among the groups, again with highest values in patients using the Vκ gene family and least in patients using the Vλ gene families
Post-SCT
Longitudinal sSR was consistently decreased for all 4 groups and clusters of segments, including the global mean of 16 LV segments. In particular, the reduction was not significant in patients using the Vκ gene family and in patients using the Vλ I gene family. Patients using the Vλ II–III gene families had a significant reduction post-auto-SCT in the longitudinal sSR of the mean of the basal, middle and apical segments, as well as the sSR global average of 16 LV segments. On the other hand, in patients using the Vλ VI gene family, sSR appeared to be reduced when considering the mean of the 6 basal, mean of the 6 middle, and mean of the 4 apical segments, as well as the global average of all 16 segments. However, this trend reached statistical significance only for the sSR mean of the apical segments.
Longitudinal sS had a tendency to be low in all groups (Figure 2), although statistical significance was reached only in the Vλ II–III and Vλ VI groups. Patients using Vλ II–III gene families had a reduction post-SCT of the sS mean of the 4 apical segments and global average of 16 LV segments. Patients using the Vλ VI family had a reduction of longitudinal sS measured as mean of the 6 middle and 4 apical segments, as well as global average of 16 LV segments.
Figure 2.
Pre- and post-auto-SCT values for longitudinal sS global average of the 16 LV segments, by clonal VL germ line gene families (Vκ vs Vλ I vs Vλ II–III vs Vλ VI). * = p-value < 0.05
To further demonstrate the differences in longitudinal systolic strain (sS) between patients using the Vκ and Vλ gene families, representative images of the inferoseptal wall are shown for a Vκ and Vλ-II patient pre and post-auto-SCT (Day 100) (Figure 3A–D).
Figure 3.
A: - Longitudinal systolic strain (sS) curve of the inferoseptal wall pre-auto-SCT (first echocardiographic examination). sS of the basal LV segment (yellow), sS of the middle LV segment (magenta), sS of the apical LV segment (red) in an AL patient using the Vκ gene family. B: Longitudinal systolic strain (sS) curve of the inferoseptal wall at Day 100 post-SCT (second echocardiographic examination). C: - Longitudinal systolic strain (sS) curve of the inferoseptal wall pre-auto-SCT (first echocardiographic examination). sS of the basal LV segment (yellow), sS of the middle LV segment (magenta), sS of the apical LV segment (red) in an AL patient using the Vλ-II gene family. D:- Longitudinal systolic strain (sS) curve of the inferoseptal wall at Day 100 post-SCT (second echocardiographic examination).
Survival Analysis
During a median follow-up time of 34 months following pre-SCT evaluation (range: 0.9 – 64 months) there were 17 (32%) deaths in the entire cohort studied (n = 53) Survival curves and event rates per group are shown in Figure 4A and Figure 4B. Four patients had transplant-related mortality (TRM) (2 -Vλ II-III and 2 - Vλ VI patients); the cause of death was acute heart failure in 2 patients (where both had multiple episodes of sustained ventricular tachycardia followed by asystole), the 3rd patient developed a massive pericardial effusion with subsequent cardiac tamponade, while the last patient died of acute renal complications and failure of engraftment.
Figure 4.
Figure 4A: Cumulative survival in an analysis of death according VL gene usage groups classified as Vκ, Vλ-I, Vλ-II–III, and Vλ-VI. Analysis time expressed in months median = 34 months). Log-rank test for equality of survivor functions p-value = 0.04. N = 53.
Figure 4B: Cumulative survival in an analysis of death according VL gene usage groups classified as Vκ and Vλ-I (considered together) vs Vλ-II–III and Vλ-VI (considered together). Analysis time expressed in months, log-rank test p-value = 0.005. N = 53.
Survival distribution was significantly different among patients using the different gene families (log-rank test, p = 0.04). When the Vλ II–II and Vλ VI gene families were grouped together and compared to a group including the Vκ and Vλ I gene families, the difference in mortality had greater significance relative to the overall comparison, (n = 53, log-rank test p = 0.005, Figure 4B).
By Cox proportional models, AL patients using either the Vλ II–III or the Vλ VI gene family had a significantly higher risk of death due to all causes compared to patients using the Vκ or Vλ-I families for the follow-up period of this study (HR=6.5 p= 0.002).
By univariate survival analysis, Δ of cardiac biomarkers at 3 months was not a significant predictor of death (p = 0.58 and 0.70 for BNP and cTnT, respectively).
Using standard echocardiography, only the Δ of tricuspid regurgitation flow velocity as detected by standard Doppler was significant (HR = 21.1, p = 0.01). Among longitudinal systolic DMI measurements, Δ sSR mean of basal segments (HR=0.1 p=0.007), Δ sSR mean of apical segments (HR=5.8, p=0.04), Δ sS mean of basal segments (HR=0.1, p=0.009) and Δ sS mean of apical segments (HR=1.3 p=0.008) were all significant predictors of mortality.
Inter- and Intra- observer variability
Considering longitudinal systolic global average of the 16 LV segments, ICC for intra-reader reproducibility was similar for all the modalities - (0.99 [95% CI 0.99, 0.99] for sMVI, 0.99 [0.99, 0.99] for sS, and 0.98 [0.93, 0.99] for sSR). The ICC for inter-reader reproducibility was similarly high for sMVI, sS and sSR (0.99 [95% CI 0.98, 0.99], 0.99, [0.99, 0.99], and 0.99 [0.97, 0.99], respectively).
DISCUSSION
This is the first report, to the best of our knowledge, which evaluates clonal immunoglobulin VL gene usage, changes in cardiac biomarkers and longitudinal DMI of the LV following auto-SCT to predict all-cause mortality in patients with AL amyloidosis. The salient observations of this study are as follows: 1) Patients with AL have changes in LV function post-SCT, which can be detected by echocardiography at Day 100, and appears to be substantially influenced by clonal immunoglobulin (Ig) VL germline gene usage. Clonal VL gene usage also influences mortality, with patients using the Vκ or Vλ-I families having a significant lower risk compared to patients using the Vλ II-III or Vλ VI families. 2) By standard echocardiography, left atrial (LA) volume, parameters of diastolic function (E/A), and estimation of LV filling pressures (E/E’) are the ones most significantly altered after auto-SCT, while change in tricuspid regurgitant flow velocity following auto-SCT is the most significant predictor of all causes mortality 3) Clonal immunoglobulin VL gene usage influences longitudinal systolic DMI values both at baseline (prior to auto-SCT) and post-transplant. 4) The difference (Δ) between pre- and post-SCT DMI measures is a significant prognostic factor of mortality with sSR and sS mean of the 6 LV basal segments being particularly useful in this regard.
Several studies have shown that patients with AL preferentially utilize a restricted repertoire of Ig VL genes compared to the polyclonal Ig VL repertoire seen in healthy subjects.7–10 Patients using the Vλ-I, II or III gene families demonstrated less severe (yet significant) cardiac dysfunction by standard echocardiography or cardiac biomarkers. Patients using the Vκ-genes had the least amount of cardiac involvement.
Not surprisingly, the serum FLC (κ FLC and λ FLC respectively) was reduced significantly after auto-SCT in all VL gene groups,15 and λ FLC Δ (pre and post-transplant) was useful in stratifying risk in our study population.
One of the hallmarks of light chain (AL) amyloidosis is the remarkable heterogeneity in organ involvement and the contributing amyloidogenic immunoglobulin light chain protein. There is significant diversity in the immunoglobulin light chain genes as part of the natural mechanism of the immune response. For example, there are approximately 31–35 functional Vκ genes organized into 5 subgroups and 29–33 functional Vλ genes organized into 10 subgroups. A first level of diversity is achieved by somatic recombination between various Vκ or Vλ gene segments (variable (V) and constant (C), but a further layer of diversity is added through the process of somatic hypermutation where single base-pair mutations occur randomly at a relatively high frequency in the variable region of the immunoglobulin light chain gene. In AL clonal light chains (i.e. the secreted immunoglobulin light chains derived from the neoplastic expansion (monoclonal) of an antibody-producing plasma cell), the extent of somatic hypermutation in the V region of the light chain protein is very high and likely contributes to its propensity to structural dysregulation and amyloid formation. In general, somatic mutations in the immunoglobulin genes target specific sections of the V region called complementarity determining regions (CDRs) leaving the scaffolding regions, the framework (FR) segments, relatively untouched. However, in AL, it has been shown that mutations in the FR region of the light chain genes facilitate abnormal protein folding and subsequent fibril formation. Therefore, the location and the number of somatic mutations in the V regions of the light chain genes play a key role in modifying the tertiary and quaternary structure leading to destabilization of the protein.21
Also, the nature of the individual somatic mutations may dictate the extent and type of post-translational modifications, such as glycosylation, which can further alter protein structure and stability leading to amyloid formation. It is well known that there is an over-representation of λ light chains in AL (3:1,λ:κ) compared to normal immunoglobulin light chain use (1:2,λ:κ), and in particular the Vλ VI gene family, substantiating the hypothesis that the sequence of the underlying gene may dictate the type and possibly, the location of somatic mutations acquired, which in turn predisposes to protein structural changes favoring amyloidogenesis. The alterations in light chain protein structure may also influence the kinetics of amyloid formation, and λ light chains may be inherently more amyloidogenic because their germline (i.e. native) sequence and secondary somatic mutations supporting more rapid fibril formation.22–24 In fact, the presence of low levels of circulating free light chain protein may not necessarily mean a lower level of production of the specific immunoglobulin light chain by the clonal plasma cell, but rather a right shift in the kinetics of amyloidogenesis facilitating enhanced tissue deposition. We have observed lower levels of free light chain in the Vλ VI patients, despite the severity of their clinical phenotype, lending credence to this hypothesis.
Another component to the concept of tissue tropism or differential specificity in organ involvement between the κ and λ light chains could be organ or tissue-specific factors, including dynamics of blood flow, acting in concert with the structure of the protein, favoring post-translational modifications in situ and/or allowing increased retention of the structurally modified light chains within the organ ultimately leading to greater amyloid burden. Therefore, the underlying etio-pathogenesis of greater cardiac involvement in λ AL as opposed to κ AL is multifactorial and likely related to the intrinsic sequence and somatic mutations in the immunoglobulin light chain gene, and its interaction with the tissue microenvironment.
Interestingly, cardiac biomarkers, including BNP and cardiac troponin T did not show any change post-transplant and furthermore, no association between post-transplant cardiac biomarkers and FLC levels was observed as previously reported.25 This observation is also inconsistent with previous reports where a significant correlation between BNP levels and longitudinal strain measures has been noted,26 and this discrepancy is likely due to a combination of the large variability in the BNP levels and the relatively small size of this patient cohort. Also, in contrast to the observations by Palladini et al, we did not observe a significant reduction in LV thickness or LV mass index following auto-SCT in this cohort of patients. However, it is possible that the 3 month time point may be too early to assess cardiac biomarkers in amyloid patients who have recently undergone the rigors of SCT. In contrast to the FLC levels, the LV thickness or LV mass index and parameters of LV filling pressures (E/E’) increased in all groups with the most significant change observed in patients using the Vλ-I and Vλ-II–III genes.
Despite the failure to reduce LV thickness, several parameters of diastolic function improved in patients using the VλI and Vκ genes (specifically, LA volume, E/A and E/E’ values), and these were associated with a lower mortality. It is also important to highlight the role of change in tricuspid regurgitant flow velocity following auto-SCT for prognostic aims. This novel observation certainly needs to be validated through a multivariate analysis on larger samples of patients, but it is potentially an immediate and effective measure for risk stratification of patients with AL amyloidosis.
The increased diagnostic sensitivity of longitudinal sSR and sS, as well as their prognostic utility, particularly for early detection of cardiac involvement and prediction of all-causes mortality in patients with AL has been previously reported.6,18,27–29 With the present data, we have confirmed and expanded our previous observations and demonstrated that even prior to auto-SCT, there are differences in sSR and sS which correlate with specific clonal Ig VL gene usage. The primary advantage of DMI analyses in defining risk of mortality in patients with AL amyloidosis when compared to FLC levels is that longitudinal DMI measures provide a prognostic assessment at baseline (before auto-SCT), while FLC levels depict responsiveness to specific treatment (either melphalan/prednisone alone or in combination with auto-SCT). This permits identification of patients who are likely to tolerate, and therefore, benefit the most from the peripheral blood stem cell transplant procedure.
The post-transplant DMI measures indicate that there is a decrease in sSR and sS for all the patients in this cohort. The decrease in these parameters post-SCT could simply be due to the fact that the time of re-evaluation (Day 100) is not sufficient to detect the therapeutic effects of auto-SCT on LV performance. On the other hand, this finding would be consistent with the overall, long-term unfavorable prognosis of this disease, and more specifically in this cohort, which did not demonstrate an improvement in LV thickness or LV mass at Day 100.
Though there is a consistent and general trend of impairment in LV systolic function at Day 100 post-transplant, the degree of dysfunction appears to correlate with clonal Ig VL gene use. The decrease in longitudinal systolic DMI values was minimal for patients using Vκ and Vλ I genes (patients demonstrating the least mortality), while it was significant for patients using the Vλ-II–III and Vλ VI genes (patients with the highest mortality).
Although patients using VλI genes had a somewhat more abnormal baseline cardiac function (biomarkers, standard and strain echocardiography) relative to patients using the VλII–III genes with a significant increase in LV thickness and mass following transplant, these patients (Vλ I) demonstrated a smaller decline in LV longitudinal sSR, and sS post-transplant compared to the latter group, suggesting a slower progression of disease in the VλI patients post-transplant (Figures 1 and 2).
It is important to note that the observations obtained by longitudinal systolic DMI, specifically, sSR and sS do not completely correlate with the longitudinal diastolic DMI measures: none of the diastolic DMI measures were significantly different at Day 100 post-transplant and no trend was discernable at this time point. These results are consistent with our previous observations,30 which demonstrated that LV longitudinal diastolic DMI modalities, either dMV or dSR, have a diagnostic sensitivity for detecting cardiac dysfunction in AL comparable to pulsed wave tissue Doppler of the medial mitral annulus, but lower than the sensitivity obtained with longitudinal systolic DMI.
Despite the intriguing findings presented herein, there are certain limitations to the study that need to be considered thoughtfully. The main limitation of the present study is the small number of patients enrolled in the different groups, a common issue in longitudinal studies involving serial assessments of patients. The small sample size precluded multivariate analysis, thereby making it difficult to prove that specific (clonal) immunoglobulin gene usage is an independent predictor of mortality in patients with AL amyloidosis. For this reason as well, the associations between specific immunoglobulin gene family usage and echocardiographic or biochemical markers should be considered preliminary and hypothesis generating data, but not conclusive. Therefore, additional studies involving larger study populations with longer follow-up are needed to confirm these initial results.
Though the post-auto-SCT echocardiographic analysis was performed on Day 100, a typical assessment time-point for hematopoietic stem cell transplantation, an interval of 3 months is likely to be insufficient to detect significant changes in LV thickness and cardiac performance by standard echocardiography. To obviate this shortcoming, we additionally used highly sensitive techniques to assess LV function post-auto-SCT, such as cardiac biomarkers and DMI, and the results showed significant changes, in spite of the small sample size of this cohort.
In this study, we did not use speckle myocardial imaging, a novel semi-automatic method to assess cardiac mechanics, which shows less variability than DMI. However, it has been previously shown that there is a close correlation between these two techniques in AL patients.31 Moreover, we have demonstrated in this report that the reproducibility of our measurements is acceptable for either systolic or diastolic DMI modalities. We did not measure the radial or circumferential DMI in this cohort due to the previously established lack of sensitivity of these measurements in detecting cardiac amyloidosis.6,30
CONCLUSIONS
Clonal immunoglobulin light chain variable gene (VL) usage impacts cardiac function prior to auto-SCT, influences changes in cardiac performance post-transplant and long-term mortality. Longitudinal DMI measures suggest strongly that 3 months after auto-SCT there is progression of cardiac dysfunction in all AL patients regardless of their clonal Ig VL gene usage. But, the grade of baseline dysfunction and the rate of LV systolic function impairment is directly correlated with the Ig VL gene used; patients with Vκ, and Vλ-I genes had lower progression compared to patients using the Vλ-II–III and Vλ VI genes. Further, the Vκ and Vλ I patients had lower mortality. Additional studies with longer follow-up period, involving new and complementary imaging techniques (i.e. cardiac magnetic resonance delayed gadolinium enhancement) are required to confirm the observations reported herein and to distinguish whether the changes observed 100 days post-SCT are sub-acute related to toxicity of the SCT or truly reflect continuous progressive damage related to the underlying amyloid-related pathogenesis. Thus far, longitudinal sS and sSR are the most sensitive measures, not only to detect impairment of LV function in patients with AL amyloidosis pre-transplant but also to assess variation in cardiac performance over time and stratify risk. Therefore, we would recommend assessment of longitudinal DMI in AL patients at each follow-up. In particular, sS mean of the 6 LV basal segments would provide diagnostic and prognostic information at the same time.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by National Institute of Health (R21 HL 76513) and American Society of Echocardiography (ASE Research Award 2008–2009).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar S. Transplantation for amyloidosis. Curr Opin Oncol. 2007;19:136–141. doi: 10.1097/CCO.0b013e32801494c6. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Palladini G, Campana C, Klersy C, Balduini A, Vadacca G, Perfetti V, et al. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107:2440–2445. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- 5.Bellavia D, Abraham TP, Pellikka PA, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20:1194–1202. doi: 10.1016/j.echo.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Bellavia D, Pellikka PA, Abraham TP, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Cardiol. 2008;101:1039–1045. doi: 10.1016/j.amjcard.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Abraham RS, Geyer SM, Price-Troska TL, Allmer C, Kyle RA, Gertz MA, et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL) Blood. 2003;101:3801–3808. doi: 10.1182/blood-2002-09-2707. [DOI] [PubMed] [Google Scholar]
- 8.Comenzo RL, Wally J, Kica G, Murray J, Ericsson T, Skinner M, et al. Clonal immunoglobulin light chain variable region germline gene use in AL amyloidosis: association with dominant amyloid-related organ involvement and survival after stem cell transplantation. Br J Haematol. 1999;106:744–751. doi: 10.1046/j.1365-2141.1999.01591.x. [DOI] [PubMed] [Google Scholar]
- 9.Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–720. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 10.Perfetti V, Casarini S, Palladini G, Vignarelli MC, Klersy C, Diegoli M, et al. Analysis of V(lambda)-J(lambda) expression in plasma cells from primary (AL) amyloidosis and normal bone marrow identifies 3r (lambdaIII) as a new amyloid-associated germline gene segment. Blood. 2002;100:948–953. doi: 10.1182/blood-2002-01-0114. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Kyle RA, Gertz MA, Therneau TM, Miller WL, Chandrasekaran K, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361:1787–1789. doi: 10.1016/S0140-6736(03)13396-X. [DOI] [PubMed] [Google Scholar]
- 13.Klein AL, Hatle LK, Burstow DJ, Taliercio CP, Seward JB, Kyle RA, et al. Comprehensive Doppler assessment of right ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;15:99–108. doi: 10.1016/0735-1097(90)90183-p. [DOI] [PubMed] [Google Scholar]
- 14.Klein AL, Hatle LK, Taliercio CP, Taylor CL, Kyle RA, Bailey KR, et al. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;16:1135–1141. doi: 10.1016/0735-1097(90)90545-z. [DOI] [PubMed] [Google Scholar]
- 15.Dispenzieri A, Lacy MQ, Katzmann JA, Rajkumar SV, Abraham RS, Hayman SR, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107:3378–3383. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–664. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 18.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 19.Andersen PKGR. Cox's regression model for counting processes: a large sample study. Annals of Statistics. 1982;10:1100–1120. [Google Scholar]
- 20.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 21.Poshusta TL, Sikkink LA, Leung N, Clark RJ, Dispenzieri A, Ramirez-Alvarado M. Mutations in specific structural regions of immunoglobulin light chains are associated with free light chain levels in patients with AL amyloidosis. PLoS One. 2009;4:e5169. doi: 10.1371/journal.pone.0005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellotti V, Mangione P, Merlini G. Review: immunoglobulin light chain amyloidosis--the archetype of structural and pathogenic variability. J Struct Biol. 2000;130:280–289. doi: 10.1006/jsbi.2000.4248. [DOI] [PubMed] [Google Scholar]
- 23.Enqvist S, Sletten K, Stevens FJ, Hellman U, Westermark P. Germ line origin and somatic mutations determine the target tissues in systemic AL-amyloidosis. PLoS One. 2007;2:e981. doi: 10.1371/journal.pone.0000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randles EG, Thompson JR, Martin DJ, Ramirez-Alvarado M. Structural alterations within native amyloidogenic immunoglobulin light chains. J Mol Biol. 2009;389:199–210. doi: 10.1016/j.jmb.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107:3854–3858. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 26.Yoneyama A, Koyama J, Tomita T, Kumazaki S, Tsutsui H, Watanabe N, et al. Relationship of plasma brain-type natriuretic peptide levels to left ventricular longitudinal function in patients with congestive heart failure assessed by strain Doppler imaging. Int J Cardiol. 2008;130:56–63. doi: 10.1016/j.ijcard.2007.07.171. [DOI] [PubMed] [Google Scholar]
- 27.Bellavia D, Pellikka PA, Al-Zahrani GB, Abraham TP, Dispenzieri A, Miyazaki C, et al. Independent predictors of survival in primary systemic (Al) amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr. 2010;23:643–652. doi: 10.1016/j.echo.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3:333–342. doi: 10.1016/j.jcmg.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Koyama J, Ray-Sequin PA, Falk RH. Prognostic significance of ultrasound myocardial tissue characterization in patients with cardiac amyloidosis. Circulation. 2002;106:556–561. doi: 10.1161/01.cir.0000023530.86718.b0. [DOI] [PubMed] [Google Scholar]
- 30.Al-Zahrani GB, Bellavia D, Pellikka PA, Dispenzieri A, Hayman SR, Oh JK, et al. Doppler myocardial imaging compared to standard two-dimensional and Doppler echocardiography for assessment of diastolic function in patients with systemic amyloidosis. J Am Soc Echocardiogr. 2009;22:290–298. doi: 10.1016/j.echo.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Modesto KM, Cauduro S, Dispenzieri A, Khandheria B, Belohlavek M, Lysyansky P, et al. Two-dimensional acoustic pattern derived strain parameters closely correlate with one-dimensional tissue Doppler derived strain measurements. Eur J Echocardiogr. 2006;7:315–321. doi: 10.1016/j.euje.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Retter I, Althaus HH, Munch R, Muller W. VBASE2, an integrative V gene database. Nucleic Acids Res. 2005;33:D671–D674. doi: 10.1093/nar/gki088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.