Abstract
LIM-Homeodomain genes encode a family of proteins defined by the cysteine-rich protein/protein interacting (Lin-11, Isl-1, and Mec-3) LIM domain and a highly conserved DNA-binding domain. Studies in several organisms have shown that these transcriptional regulators control multiple aspects of embryonic development and are responsible for the pathogenesis of several human diseases. Here we report the expression of Islet-1 (Isl-1) in the gastrointestinal epithelium in developing and adult mice. At embryonic day (E) 9.5–10.5, Isl-1 expression was first detected in the ventral gastric mesenchyme, and expression in the dorsal mesenchyme initiated a few days later. Isl-1 expression was first observed in the gastric epithelium at E13.5 and at E14.5 was restricted to the posterior half of the stomach. In the mature stomach, Isl-1 expression was detected only in subsets of enteroendocrine cells. Furthermore, Isl-1 expression in the intestinal epithelium was first detected at E15.5 and was restricted to subpopulations of enteroendocrine cells in adult mice. These expression analyses suggest that Isl-1 might have an early broad role in stomach and intestinal cells and a secondary role in terminal differentiation and/or maintenance of mature enteroendocrine subtypes in the gastrointestinal epithelium.
Keywords: Islet-1, stomach, intestine, gastrointestinal tract, development, transcriptional control, endocrine cell differentiation, somatostatin, gastrin, ghrelin
1. Results and Discussion
In mammals, the LIM-HD proteins form a family of 13 members of transcriptional regulators that control numerous developmental processes in the developing tissues and organs (Hunter and Rhodes, 2005). Some of these LIM-HD proteins have been associated with human diseases including leukemia, pituitary hormone deficiency, diabetes mellitus and nail-patella syndrome (Hunter and Rhodes, 2005), implicating this protein family in regulating key processes during embryonic and adult lives. Islet-1 (Isl-1), a LIM-HD family member, is required for cell specification and differentiation events in cardiac mesoderm, motor neuron and endocrine pancreas (Ahlgren et al., 1997; Cai et al., 2003; Du et al., 2009; Pfaff et al., 1996).
In the pancreas, Isl-1 is expressed in hormone expressing endocrine cells and has been shown to regulate their differentiation (Ahlgren et al., 1997; Du et al., 2009). Outside of the pancreas, endocrine cells are also found throughout the gastrointestinal (GI) mucosa (referred to as enteroendocrine cells). These cells play critical roles in regulating digestion, gut motility, appetite, and lipid absorption (May and Kaestner, 2010; Mellitzer et al., 2010). In contrast to endocrine cells of the pancreas, which cluster to form islets, enteroendocrine cells in the gastrointestinal tract are scattered as individual cells throughout the epithelium. Interestingly, while endocrine cells of the pancreas, stomach and intestine arise from different regions of the definite endoderm, they express a common set of genes and are also influenced by similar signaling pathways (May and Kaestner, 2010).
Differentiation of the mammalian gastric epithelium is a coordinated process involving morphogenesis, tissue interactions and cell differentiation. Initial specification of the gastric epithelium is dependent on the presence of the gastric mesenchyme (Koike and Yasugi, 1999); a similar dependence exists between the dorsal pancreatic bud and the pancreatic mesenchyme (Ahlgren et al., 1997). In mice, formation of the stomach begins as a bulge around E10.0, with subsequent extensive remodeling of the epithelium throughout development (Nyeng et al., 2007). While differentiation of cell lineages and invagination of the gastric epithelium occur at E15.5–E16.5, maturation of these cells continues into postnatal life (Nyeng et al., 2007). The mature stomach is divided into two main regions: the proximal stomach (also known as the forestomach) comprises keratinized squamous epithelium, while the posterior stomach (also known as glandular stomach) comprises the corpus and antrum and is made up of stratified epithelium. The glandular stomach is characterized by numerous tubular invaginations, also referred to as gastric units. Within each gastric unit, endocrine cells are found scattered at the bases surrounded by other cell types, including pepsinogen-secreting chief cells, gastric-acid producing parietal cells and mucus-producing pit cells (May and Kaestner, 2010).
Although formation of the gut tube occurs early during embryogenesis, remodeling of the pseudostratified intestinal epithelium to a monolayer does not begin until E14.0. Between E15.5 and E19.0, villus-like structures are present from duodenum to colon (Potten, 1995; Stappenbeck et al., 1998; Yang et al., 2001). The underlying mesenchyme differentiates into smooth muscle and connective stromal tissue, whereas the epithelium differentiates into enterocyte, goblet, paneth and endocrine cells (May and Kaestner, 2010). As in the stomach, maturation of these intestinal cell types continues from birth until weaning (Henning and Guerin, 1981).
In the adult, enteroendocrine cells are the least abundant cell types in the gastrointestinal epithelium and encompass at least 15 different subtypes classified based on their main hormonal products (i.e somatostatin, gastrin, serotonin, cholecystokinin, and ghrelin), ultrastructure of their secretory granules, and marker gene expression (May and Kaestner, 2010; Rindi et al., 2004). Most, but not all, enteroendocrine cells are positive for chromogranin A, a pan-endocrine cell marker, staining (Cetin et al., 1989). Although chromogranin A-positive enteroendocrine cells are detected at high levels around E16.5 (Nyeng et al., 2007), other markers of enteroendocrine cell differentiation (i.e. Pdx1, Ngn3 and Pax6) can be detected earlier (May and Kaestner, 2010). Due to the large number of enteroendocrine subtypes and the low number of each specific subpopulation, many studies have focused only on a subset of endocrine subpopulations (May and Kaestner, 2010).
Many transcription factors that are essential for endocrine pancreas differentiation also play important roles in the development of enteroendocrine cells (May and Kaestner, 2010; Oliver-Krasinski and Stoffers, 2008). Isl-1 has been shown to regulate the differentiation of glucagon, insulin, somatostatin and pancreatic polypeptide-expressing cells in the endocrine pancreas (Ahlgren et al., 1997; Du et al., 2009). In the stomach, Isl-1 expression has been characterized in rat, where it is first expressed in G/D-cell precursors and a subpopulation of somatostatin-producing D-cells, but not gastrin-producing G-cells (Larsson et al., 1995). In the intestine, Isl-1 expression is completely uncharacterized. To investigate the possibility that Isl-1 regulates enteroendocrine cell differentiation in the gastrointestinal epithelium, we characterized Isl-1 expression during various stages of development and in adult mice.
1.1. Isl-1 expression is detected in the entire gastrointestinal tract
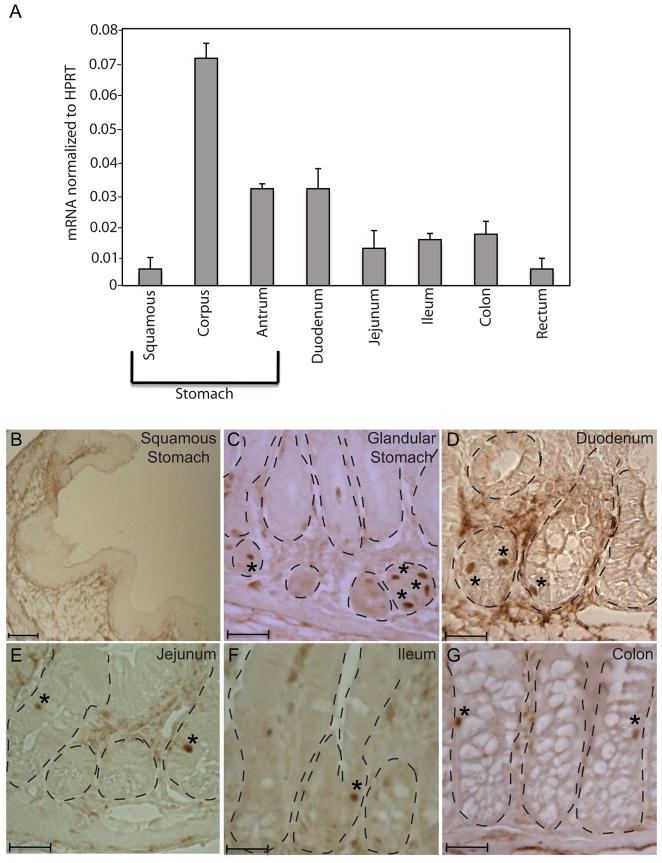
We first examined the presence of Isl-1 mRNA in different regions of the adult gastrointestinal tract by real-time PCR analysis. The expression of hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as an internal control. Highest levels of Isl-1 transcripts were observed in the corpus of the stomach, while squamous stomach and rectum had the least (Fig. 1A). The gastric antrum and the duodenum showed modest levels of Isl-1 transcripts, with lower amounts in the jejunum, ileum and colon (Fig. 1A).
Fig. 1.
Expression of Isl-1 in the adult gastrointestinal tract. (A) Relative expression of Isl-1 mRNAs in different regions of the gastrointestinal tract assessed by real-time PCR (n=4). All results were normalized to the level of hypoxanthine-guanine phosphoribosyl transferase (Hprt). (B–G) Immunohistochemical analyses of adult stomach and intestine detected Isl-1+ cells (*) in the epithelial compartment (dashed lines) of the digestive tract. Scale bars: 25μm.
To further characterize Isl-1 expression in the adult gastrointestinal tract, we performed immunohistochemistry (IHC) analysis using an Isl-1 antibody on different regions of the adult GI tract. Scattered Isl-1-positive cells were detected in the epithelium of the stomach, duodenum, jejunum, ileum and colon (Fig. 1C–G). Within the stomach, Isl-1 positive cells were more numerous in the corpus than the antrum region, and no Isl-1+ cells were observed in the squamous stomach, consistent with the mRNA quantitation (Fig. 1A–B).
1.2 Isl-1 in enteroendocrine subpopulations in the adult stomach
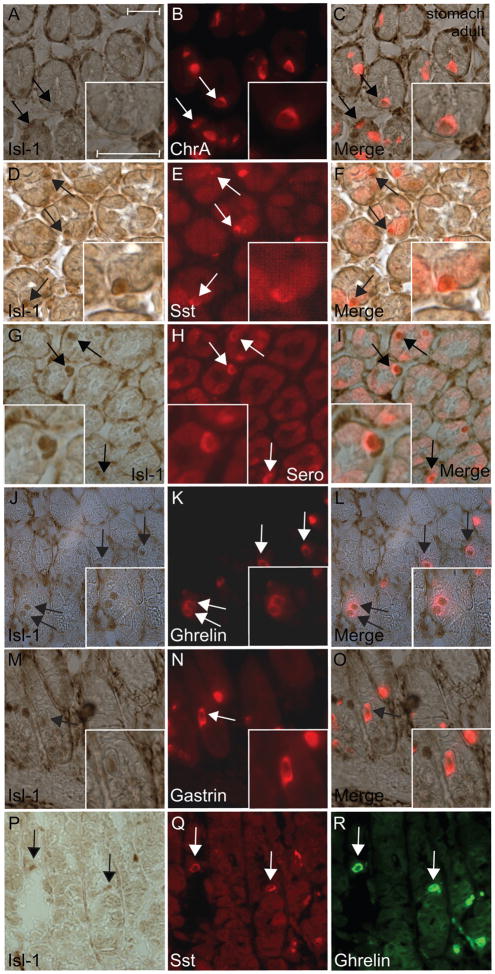
The distribution and frequency of Isl-1+ cells in the GI tract were suggestive of enteroendocrine cells. To confirm this, we carried out IHC analysis of Isl-1 with chromogranin A and various gastrointestinal hormones. Not all chromogranin A+ cells were positive for Isl-1, indicating that Isl-1 is differentially expressed in endocrine subtypes (Fig. 2A–C). Most of the somatostatin, serotonin and ghrelin producing cells were positive for Isl-1 expression (Fig. 2D–L), while there were fewer number of Isl-1+/gastrin+ cells (Fig. 2M–O). Isl-1+ cells co-expressing somatostatin and ghrelin were also detected in the adult stomach (Fig. 2P–R).
Fig. 2.
Immunohistochemical double staining for Isl-1 and endocrine hormones in the adult stomach. (A–C) Isl-1 with chromogranin A. (D–F) Isl-1 with somatostatin (Sst). (G–I) Isl-1 with serotonin (Sero). (J–L) Isl-1 with ghrelin. (M–O) Isl-1 with gastrin. (P–R) Isl-1 with somatostatin and ghrelin. Experiments were conducted on the same section for each hormone with Isl-1. Insets show co-localization of Isl-1 with the hormones in a magnified view. Arrows indicate co-expressing cells in all panels. Scale bars: 25μm.
Our observations are in agreement with Larsson and colleagues, who demonstrated that rat Isl-1 is first expressed in the gastrin and somatostatin coexpressing G/D precursors then later is restricted to mature somatostatin expressing D cells (Larsson et al., 1995). Based on these observations and our IHC analyses, Isl-1 is likely to play an important role in the asymmetric division of the G/D precursors and the differentiation/maturation of the D cells (Larsson et al., 1995). In addition, Isl-1 has been shown to regulate somatostatin expression in pancreatic cell lines (Leonard et al., 1992), and Isl-1 deficient mice lack somatostatin expressing cells in the endocrine pancreas (Ahlgren et al., 1997; Du et al., 2009). By analogy, Isl-1 may regulate the differentiation of D cells and/or somatostatin gene expression in the gastric epithelium.
Isl-1 was also detected in cells expressing serotonin or ghrelin, peptides that are responsible for feeding behavior and modulating gastric peristalsis, sensation, and secretion (Arvat et al., 2001; Englander et al., 2004; Gershon and Tack, 2007). Interestingly, Isl-1 was also detected in the ghrelin/somatostatin co-expressing cells, suggesting that these two enteroendorine subtypes may share a common precursor. Collectively, these observations suggest that Isl-1 alone or in combination with other transcriptional regulators may be involved in the differentiation of somatostatin, gastrin, ghrelin and serotonin producing cells in the gastric epithelium.
1.3 Isl-1 in somatostatin-expressing cells of the adult intestine
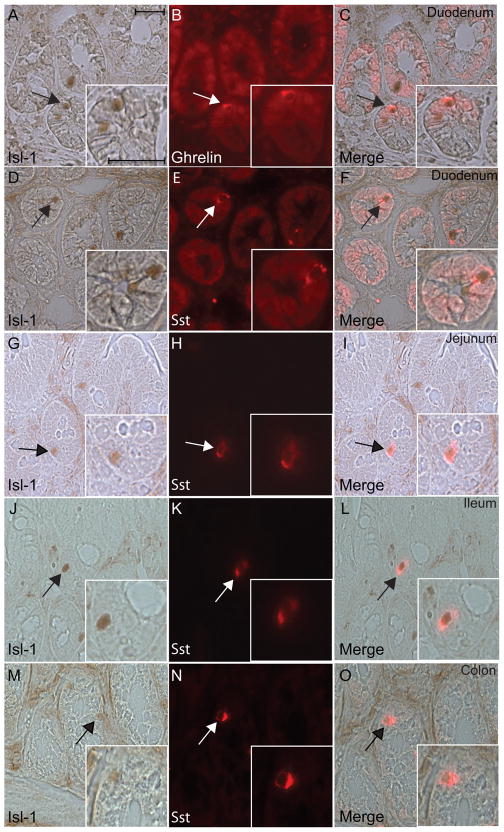
We next performed immunostaining to characterize Isl-1 expression with somatostatin and ghrelin in the adult intestine since these two enteroendocrine subtypes express Isl-1 abundantly in the stomach. IHC analyses demonstrated that while there were very few Isl-1+ cells detected in the adult intestinal epithelium from duodenum to colon, most of the Isl-1+ cells were positive for somatostatin or ghrelin expression (Fig. 3A–O). These results indicate that the function of Isl-1 in somatostatin- and ghrelin-expressing cells is likely to be conserved throughout the GI tract.
Fig. 3.
Immunohistochemical double staining for Isl-1 and ghrelin or somatostatin in the adult small and large intestines. (A–C) Isl-1 with ghrelin in the duodenum. (D–O) Isl-1 with somatostatin in the duodenum (D–F), jejunum (G–I), ileum (J–L) and colon (M–O). Experiments were conducted on the same section for each hormone with Isl-1. Insets show co-localization of Isl-1 with the hormones in a magnified view. Arrows indicate co-expressing cells in all panels. Scale bars: 25μm.
1.4. Isl-1 in the stomach and intestine during embryogenesis
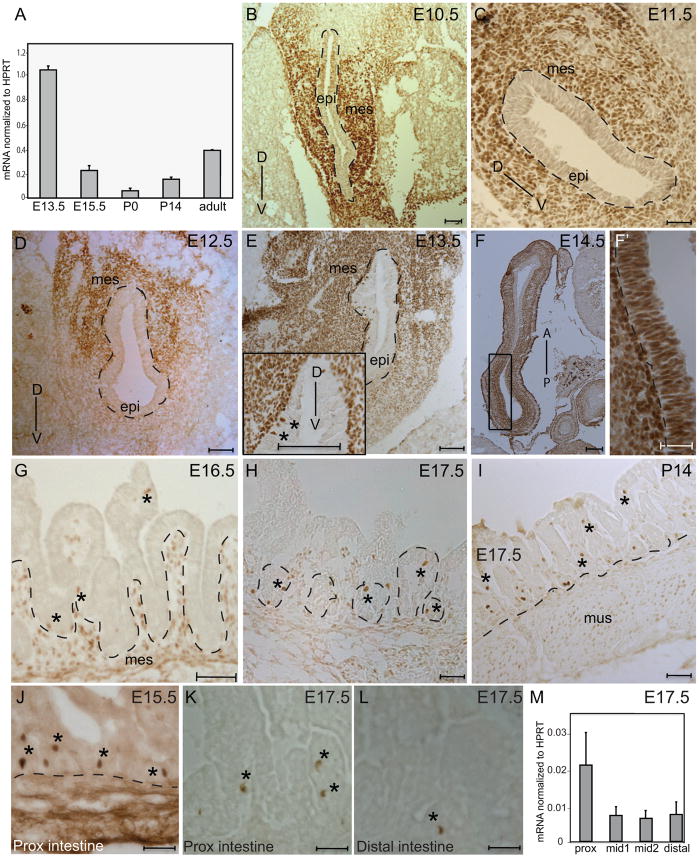
Since the differentiation of enteroendocrine cells occurs during embryogenesis and Isl-1 showed highest levels of gene expression in the glandular stomach, we focused our embryonic/perinatal expression analysis on stomach at various developmental stages by real-time PCR (Fig. 4A). Expression levels at these stages were compared to that of the adult glandular stomach. Isl-1 expression was high at the onset of gastric epithelial differentiation and decreased as development progressed. At birth, Isl-1 expression reached a nadir and then began to increase (Fig. 4A).
Fig. 4.
Isl-1 expression in the developing stomach and intestine. (A) Relative expression of Isl-1 mRNAs in different stages of stomach development from E13.5 to adult measured by real-time PCR (n=4). (B–I) Immunohistochemical staining for Isl-1 in the stomach at E10.5 (B), E11.5 (C), E12.5 (D), E13.5 (E), E14.5 (F–F′), E16.5 (G), E17.5 (H) and P14 (I). Dashed lines outline epithelial compartments. Isl-1+ cells in the epithelium are denoted by “*” in E, G, H, and I. D: Dorsal; V: Ventral; A: Anterior; P: Posterior; mes: mesenchyme and epi: epithelium. (J–L) Immunohistochemical staining for Isl-1 in the intestine at E15.5 (J) and proximal (K) and distal (L) regions of the E17.5 intestine. Isl-1+ cells were denoted by *. (M) Relative expression of Isl-1 mRNAs in different regions of the E17.5 intestine assessed by real-time PCR (n=3). Prox: proximal region; mid1: mid-region 1; mid2: mid-region 2; distal: distal region. Scale bars: 25μm.
To localize the developmental expression of Isl-1, IHC analyses were performed on transverse sections prepared from mouse embryos (E9.5–E17.5) and tissue sections from postnatal day (P)14. Isl-1 was first observed at E9.5 in the ventral gastric mesenchyme (data not shown). At E10.5 as the stomach begins to develop as a tubular structure, Isl-1 expression was detected in the ventral and lateral mesenchyme (Fig. 4B). By E11.5, Isl-1 expression was distributed uniformly in the mesenchyme surrounding the entire gastric epithelium (Fig. 4C). At E12.5, there was greater expression of Isl-1 in the dorsal mesenchyme than in the ventral mesenchyme (Fig. 4D). Isl-1 was restricted to the mesenchyme until E13.5 when the protein was first detected in the gastric epithelium (asterisks; Fig. 4E). Interestingly, Isl-1 expression in the gastric epithelium coincided with the onset of gut rotation, which takes place between E13.5 and E14.5 (Kluth et al., 1995). The Isl-1 expression domain expanded to the entire posterior region of the stomach at E14.5; this region gives rise to the glandular stomach (Fig. 4F-F′). It is not until E15.5–E17.5 that rudimentary epithelial invaginations are formed in the gastric epithelium, where few scattered Isl-1+ cells were found in the base and middle of each gastric unit (Fig. 4G–I). As Isl-1 is abundantly expressed in the mesenchyme and scarcely in the gastric epithelium during early embryonic stages (E12.5– E13.5), the high level of Isl-1 expression detected in our PCR analysis at E13.5 (Fig. 4A) is a reflection of Isl-1 expression in the mesenchyme and not epithelium (Fig. 4E).
In the developing intestine, Isl-1+ cells were first detected in the intestinal epithelium at E15.5 (Fig. 4J and data not shown). The scattered expression pattern of Isl-1 continued throughout development, with more Isl-1+ cells located in the proximal region of the E17.5 intestine (Fig. 4K–L). As in the adult (Fig 1A), the highest levels of Isl-1 transcripts during development were in the proximal intestine (Fig. 4M).
1.5. Isl-1 in gastric enteroendocrine cells at E17.5 and postnatal day 10
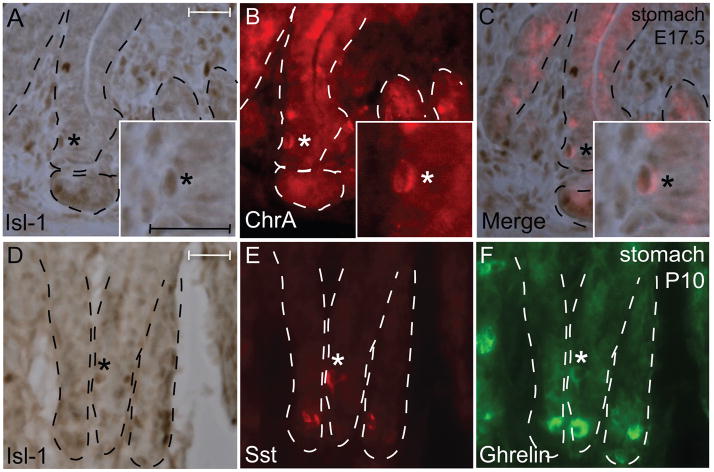
As chromogranin A-positive cells are found during late gestation (~E16.5) in the gastric epithelium (Nyeng et al., 2007), we performed IHC analysis of Isl-1 with chromogranin A at E17.5 and found only a few Isl-1/chromogranin A double positive cells (Fig. 5A–C). This is likely due to the low number of endocrine cells at this stage. To determine if Isl-1 is co-expressed with somatostatin and/or ghrelin as in the adult stomach (Fig. 2P–R), we performed IHC in P10 stomach and detected some Isl-1+/somatostatin+/ghrelin+ cells (Fig. 5D–F).
Fig. 5.
Isl-1 expression with endocrine hormones in E17.5 and P10 stomach. (A–F) Immunohistochemical staining for Isl-1 and chromogranin A at E17.5 (A–C) and somatostatin and ghrelin at P10 (D–F). Experiments were conducted on the same section for each hormone with Isl-1. Insets show co-localization of Isl-1 with the hormones in a magnified view. Co-expressing cells are indicated by *. Dashed lines outline epithelial compartments. Scale bars: 25 μm.
1.6. Dynamic expression of Isl-1 in comparison with Pdx1, Ngn3 and Pax6 in the gastrointestinal epithelium
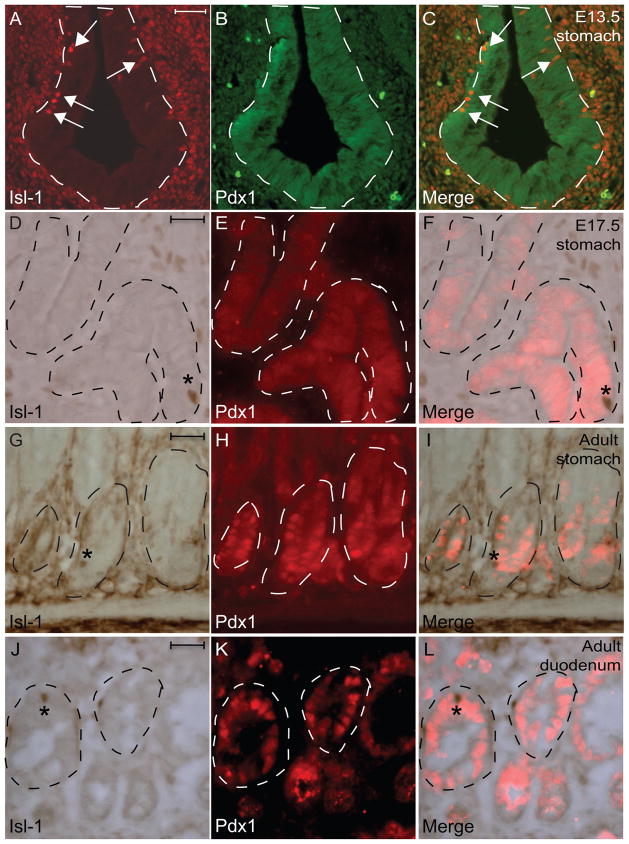
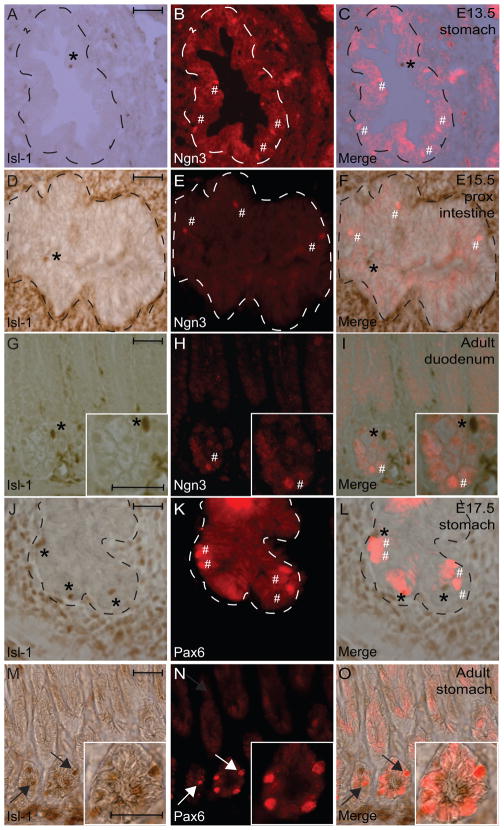
Several transcription factors, including Pancreatic duodenal homeobox gene (Pdx1), Neurogenin3 (Ngn3) and Paired homeobox gene (Pax6), have been demonstrated to play critical roles in regulating differentiation of the gastrin-, somatostatin-, or serotonin-expressing cells in the GI tract (Jenny et al., 2002; Larsson et al., 1998; Lee et al., 2002; Mellitzer et al., 2010; Offield et al., 1996). To investigate whether Isl-1 expression correlates with any of these factors, IHC analyses were performed using sections from embryonic and adult tissues. While Pdx1 expression was detected in the entire gastric epithelium with few scattered Isl-1+ cells at E13.5 (Fig. 6A–C), we did not detect Isl-1+ cells in the Pdx1+ gastric epithelium at E17.5 (Fig. 6D–F). Furthermore, we also did not detect any Isl-1+/Pdx1+ co-expressing cells in adult stomach and intestine (Fig. 6G–L). In addition, we did not detect any Isl-1+/Ngn3+ co-expressing cells in the gastrointestinal epithelium during development or in adult mice (Fig. 7A–I and data not shown). Lastly, while Isl-1 and Pax6 expression did not overlap in the gastric epithelium at E17.5 and P7 (Fig. 7J-L and data not shown), we did detect some Isl-1+/Pax6+ co-expressing cells in adult mice (Fig. M–O).
Fig. 6.
Immunohistochemical double-staining for Isl-1 and Pdx1 in the gastrointestinal epithelium. (A–I) Isl-1 with Pdx1 at E13.5 (A–C), E17.5 (D–F) and adult (G–L). Experiments were conducted on the same section. Dashed lines outline epithelial compartments. Arrows in A and C indicate Isl-1+ cells. * in D–L indicates Isl-1+/Pdx1− cells. Scale bars: 25μm.
Fig. 7.
Immunohistochemical double staining for Isl-1 and Ngn3 or Pdx1. (A–I) Isl-1 with Ngn3 in E13.5 stomach (A–C), in E15.5 proximal intestine (D–F), in adult duodenum (G-I). (J–O) Isl-1 with Pax6 in E17.5 stomach (J–L) and adult stomach (M–O). Dashed lines outline epithelial compartments. Experiments were conducted on the same section. * indicates Isl-1+/Ngn3− or Pax6− cells and # denotes Isl-1−/Ngn3+ or Pax6+ cells in A–L. Arrows indicate Isl-1+/Pax6+ cells in M–O. Scale bars: 25μm.
Collectively, these results suggest that Isl-1 is likely to play important regulatory roles in the formation enteroendocrine subtypes after the initial specification of Ngn3+ endocrine progenitors. Since we did not observe any overlaps between Isl-1 and Pdx1 expression in adult stomach and duodenum, we hypothesize that Isl-1 and Pdx1 may be involved in the differentiation of distinct enteroendocrine lineages. In fact, Pdx1 has been shown to be necessary for the development of antropyloric gastrin-secreting G cells (Larsson et al., 1996) and the majority of gastrin+ cells in the adult stomach are Isl-1 negative (Fig. 2M–O) highlighting the importance of Pdx1 and not Isl-1 during later stages of G cell differentiation. However, this does not preclude the possibility that Isl-1 may still be involved in the early differentiation of gastrin cells. Furthermore, we did detect some Isl-1+/Pax6+ co-expressing cells in the adult stomach suggesting a potential overlapping role between Isl-1 and Pax6. In fact, it has been shown that Pax6 is required for the development of gastrin and somatostatin producing cells in the stomach (Larsson et al., 1998), prompting us to hypothesize that Isl-1 and Pax6 work in parallel or in a hierarchical manner during the development of gastrin and somatostatin producing cells.
1.7 Isl-1+ cells are post-mitotic and do not express proliferating markers
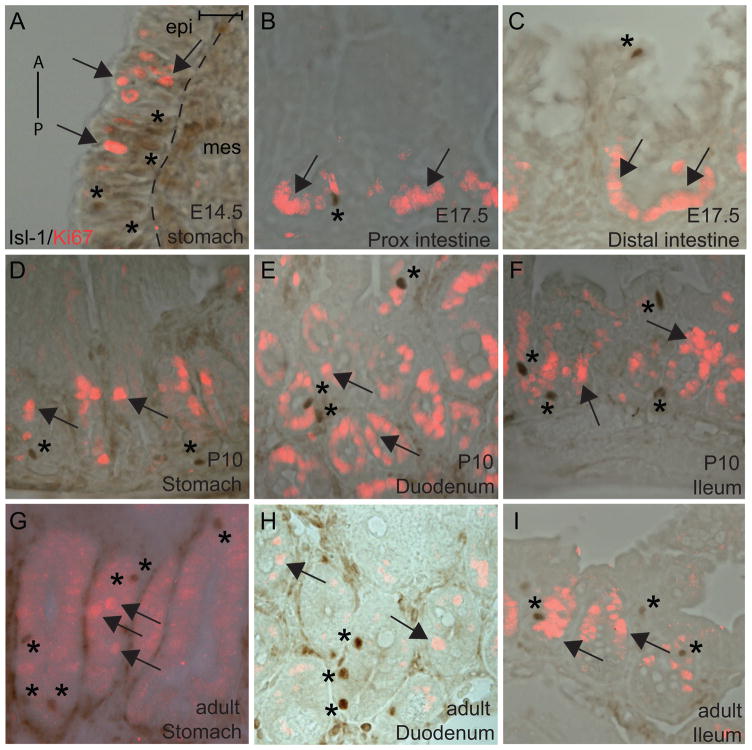
Most Isl-1+ cells are postmitotic in the motor neuron and pancreas (Ahlgren et al., 1997; Ericson et al., 1992). We performed IHC analysis for Isl-1 and Ki67, a proliferation marker, in the developing and postnatal stomach and intestine. Similar to what has been shown in other tissues, we did not detect Isl-1+/Ki67+ cells at any of the stages examined, indicating that Isl-1+ cells in the gastrointestinal epithelium are also postmitotic (Fig. 8A–I). These results suggest that Isl-1 is not likely to be involved in promoting cell proliferation in the gastrointestinal epithelium.
Fig. 8.
Immunohistochemical analyses of Isl-1 expression with Ki67. (A–I) Immunostaining of Isl-1 and Ki67 in E14.5 stomach (A), E17.5 proximal intestine (B), E17.5 distal intestine, P10 stomach (D), P10 duodenum (E), P10 ileum (F), adult stomach (G), adult duodenum (H) and adult ileum (I). A: anterior, P: posterior. Arrows denote Ki67+ cells and * denotes Isl-1+ cells. Isl-1 and Ki67 staining were done on the same section and images were merged. Scale bars: 25μm.
1.8 Conclusions
We have documented the expression patterns of Isl-1 in the developing and adult gastrointestinal epithelium. The implication of these patterns remains to be determined using tissue-specific Isl-1 deletion mouse models. Our expression data analysis implicates the involvement of Isl-1 in the differentiation of somatostatin, serotonin, ghrelin and gastrin-producing cells. As there are many endocrine subtypes in the GI tract, transcriptional regulators are likely to work in concert and/or have redundant roles to drive the differentiation and function of these cells. Isl-1 is first expressed in a few gastric epithelial cells at E13.5 and is then expressed abundantly in glandular epithelium by E14.5, prior to the secondary transition at E15.5–E16.5. Once the stomach matures, Isl-1 expression is restricted to subsets of enteroendocrine cells. These observations prompt us to hypothesize that Isl-1 plays a role in general enteroendocrine differentiation prior to the activation of specific hormone genes, and may then have a secondary function in further fine-tuning enteroendocrine subtypes. The importance of enteroendocrine cells has been demonstrated in mice (May and Kaestner, 2010; Mellitzer et al., 2010) and humans; mutations in the Ngn3 gene cause enteroendocrine cell dysgenesis and severe congenital chronic diarrhea (Wang et al., 2006). The characterization of Isl-1 expression in the present study is expected to open new avenues in the molecular analysis of enteroendocrine cell differentiation and function in the GI tract. Results from our study can also be used to define and isolate endocrine subtypes during in vitro differentiation of embryonic stem cells towards enteroendocrine cell lineages.
2. Experimental Procedures
2.1. Animals and tissue preparation
CD1 wild type mice were used for this study. Embryonic age was determined by the appearance of the vaginal plug as E0.5. All tissues were fixed in 4% paraformaldehyde, washed in phosphate buffered saline (PBS), cryoprotected in 30% sucrose, embedded in OCT and sectioned at 10μm. All animal procedures were done following the guidelines of the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
2.2. Immunohistochemistry and immunofluorescence
Tissue sections were subjected to microwave antigen retrieval by boiling for 14 min in 10mM citric acid buffer (pH 6.0) and allowing to cool for 15 min at room temperature. Sections were quenched in 30% hydrogen peroxide at room temperature for 20 min, followed by blocking with Avidin D and Biotin blocking reagent (Vector), for 15 min each at room temperature with a quick rinse of PBS in between. All slides were blocked with CAS-Block (Invitrogen, Cat No. 008120) for 30 min at room temperature. Isl-1 antibody was diluted in PBT (10%BSA and 10% Triton-X100 in PBS) and incubated overnight at 4°C. Slides were washed in PBS and incubated with goat anti-mouse (1:100; Vector Laboratories) for 30 min at 37°C. Slides were rinsed with PBS and incubated with HRP-conjugated ABC reagent (Vector Elite Kit) for 30 min at 37°C, followed by developing with DAB substrate kit (Vector), washing with water, dehydrating in a graded series of alcohol concentrations, clearing in xylene, and mounting in Permount.
For combined immunohistochemistry and immunofluorescence, slides were first processed for IHC as described above, then rinsed with PBS and blocked again for 30 min at room temperature with CAS-Block. Slides were incubated with the primary antibodies diluted in antibody diluent (Invitrogen, Cat No. 00-3118) overnight at 4°C. The following day, slides were washed with PBS, incubated with appropriate Cy3- or Cy2-conjugated secondary antibodies (1:600, Jackson Laboratories) for two hours at room temperature, washed, and then mounted with an aqueous mounting medium (KPL, Cat No. 71-00-16).
| Antibody | Vendor | Dilution | Species |
|---|---|---|---|
| Isl1 (39.4 D5) or (40.2 D6) | DSHB | 1:50 | Mouse |
| Chromogranin A | DiaSorin | 1:3000 | Rabbit |
| Gastrin | Santa Cruz | 1:2000 | Goat |
| Ghrelin | Santa Cruz | 1: 200 | Goat |
| Somatostatin | Santa Cruz | 1:5000 | Goat |
| Somatostatin | Zymed | 1:50 | Rabbit |
| Serotonin (5-HT) | Immunostar | 1: 30,000 | Rabbit |
| Pax6 | Covance | 1:300 | Rabbit |
| Pdx1 | Santa Cruz | 1: 100 | Goat |
| Ngn3 | Gift from Maike Sander | 1:1000 | Guinea pig |
| Ki67 | Vector Labs | 1:200 | Rabbit |
2.3. Imaging and Microscopy
All images were taken on Leica Microscope using Leica AF software. IHC and IF images were merged using the Leica AF software.
2.4. Quantitative RT-PCR
RNA was extracted from dissected tissues at different stages in Trizol (Invitrogen). After a reverse transcription reaction using oligo dT, cDNA was amplified using an Isl-1 specific primer and HPRT as an internal standard. A Stratagene Mx3005 Real-Time PCR machine was used for the quantitative PCR analysis. Conditions and primer concentrations suggested by the SYBR Green Assay protocol was followed. The following forward and reverse primers were used for amplification:
Hprt: 5′GGCCAGACTTTGTTGGATTTG 3′/5′ TGCGCTCATCTTAGGCTTTGT 3′
Isl-1: 5′CGGAGAGACATGATGGTGGTT 3′/5′ GGCTGATCTATGTCGCTTTGC 3′
2.5. Statistical Analyses
All values are depicted as mean ± standard error of mean from at least 4 animals. All data were statistically analyzed with Student’s two-tailed t-test.
Acknowledgments
We thank Dr. Gary Swain, and members of the Morphology Core in the Center for Molecular Studies in Digestive and Liver Disease (P30-DK050306). We are also grateful to Dr. Joshua Friedman for critical reading of the manuscript. CLM is supported by NIH-DK078606, NIH-DK019525 and JDRF 2-2007-703.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–1174. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin Y, Muller-Koppel L, Aunis D, Bader MF, Grube D. Chromogranin A (CgA) in the gastro-entero-pancreatic (GEP) endocrine system. II. CgA in mammalian entero-endocrine cells. Histochemistry. 1989;92:265–275. doi: 10.1007/BF00500540. [DOI] [PubMed] [Google Scholar]
- Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander EW, Gomez GA, Greeley GH., Jr Alterations in stomach ghrelin production and in ghrelin-induced growth hormone secretion in the aged rat. Mech Ageing Dev. 2004;125:871–875. doi: 10.1016/j.mad.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Henning SJ, Guerin DM. Role of diet in the determination of jejunal sucrase activity in the weanling rat. Pediatr Res. 1981;15:1068–1072. doi: 10.1203/00006450-198107000-00019. [DOI] [PubMed] [Google Scholar]
- Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32:67–77. doi: 10.1007/s11033-004-7657-z. [DOI] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. Embo J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth D, Kaestner M, Tibboel D, Lambrecht W. Rotation of the gut: fact or fantasy? J Pediatr Surg. 1995;30:448–453. doi: 10.1016/0022-3468(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Koike T, Yasugi S. In vitro analysis of mesenchymal influences on the differentiation of stomach epithelial cells of the chicken embryo. Differentiation. 1999;65:13–25. doi: 10.1046/j.1432-0436.1999.6510013.x. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Madsen OD, Serup P, Jonsson J, Edlund H. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev. 1996;60:175–184. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- Larsson LI, St-Onge L, Hougaard DM, Sosa-Pineda B, Gruss P. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–159. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Tingstedt JE, Madsen OD, Serup P, Hougaard DM. The LIM-homeodomain protein Isl-1 segregates with somatostatin but not with gastrin expression during differentiation of somatostatin/gastrin precursor cells. Endocrine. 1995;3:519–524. doi: 10.1007/BF02738827. [DOI] [PubMed] [Google Scholar]
- Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J, Serup P, Gonzalez G, Edlund T, Montminy M. The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc Natl Acad Sci U S A. 1992;89:6247–6251. doi: 10.1073/pnas.89.14.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120:1708–1721. doi: 10.1172/JCI40794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 signaling controls stomach morphogenesis. Dev Biol. 2007;303:295–310. doi: 10.1016/j.ydbio.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Potten CS. Radiation and Gut. Elsevier Science B.V; Amsterdam: 1995. Structure, function, and proliferative organisation of the mammalian gut. [Google Scholar]
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Wong MH, Saam JR, Mysorekar IU, Gordon JI. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr Opin Cell Biol. 1998;10:702–709. doi: 10.1016/s0955-0674(98)80110-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martin MG. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for Secretory Cell Lineage Commitment in the Mouse Intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]