Abstract
We investigated body mass index (BMI) and weight gain among pregnant women (ages 14 to 25) and assessed the relationship of BMI and weight gain on birth outcomes. We performed a secondary analysis of 841 women enrolled in a randomized controlled trial receiving prenatal care in two university-affiliated clinics. Almost half the patients were overweight or obese. An average of 32.3 ± 23.6 pounds was gained in pregnancy with only 25.3% gaining the recommended weight and over half overgaining. Weight gain had a significant relationship to birth weight. Multivariate analysis showed that prepregnancy BMI but not weight gain was a significant predictor of cesarean delivery (odds ratio [OR] 1.91, confidence interval [CI] 1.24 to 2.69, p < 0.0001). When large-for-gestational-age infants were removed from the analysis, there was still a significant effect of BMI on cesarean delivery (OR 1.76, CI 1.17 to 2.66, p = 0.007) but not of weight gain (OR 1.45, CI 0.94 to 2.17, p = 0.093). Prepregnancy BMI is a more significant predictor of cesarean delivery than pregnancy weight gain in young women.
Keywords: Obesity, pregnancy weight gain
Obesity rates are increasing. Recent National Health and Nutrition Examination Survey data from 2003 to 2004 show that the prevalence of overweight was 17.4% in women of reproductive age, which is almost triple the rate 30 years ago.1,2 Obstetric literature has recently focused on the rising incidence of complications with increases in both body mass index (BMI) and weight gain in pregnancy.3–7 Barau et al documented a linear association between maternal prepregnancy BMI and cesarean delivery in almost 17,000 term deliveries in France, while controlling for other risk factors for cesarean delivery.8
Rising cesarean delivery rates and other complications have significant short- and long-term implications for reproductive-age women. It is unclear whether obesity or excessive weight gain has more of an impact on complications, particularly cesarean delivery, as the effect of fetal size on the risk of cesarean delivery is often a confounding factor.9 The increased background risks of chronic illness, diabetes, and hypertension associated with obesity also elevate the estimated risks of pregnancy complications and cesarean delivery; therefore, examining the effects of obesity in young pregnant women prior to the development of chronic illnesses could help clarify the differential impact. A retrospective study of pregnant Caucasian adolescents focused specifically on maternal age less than 19 years and compared normal prepregnancy BMI with BMI greater than 25 through birth certificate records.10 Underweight women were excluded from the analysis. Results indicated that the risk of cesarean delivery, induction, gestational hypertension, and macrosomia were all elevated in the higher BMI category.
The aims of the present study are to: (1) describe BMI and weight gain among an ethnically diverse group of young (age 14 to 25) pregnant women; (2) assess the relationship of BMI and weight gain on pregnancy outcomes including birth weight and cesarean delivery.
MATERIALS AND METHODS
Study Participants
Data from this study come from a secondary analysis of 841 pregnant women enrolled in a prospective, randomized, controlled trial aimed at promoting improved general health and reproductive behaviors through group prenatal care.11 This is a prospective study following participants from early pregnancy through 1-year postpartum. Participants were recruited from two University-affiliated obstetrics clinics in New Haven, Connecticut and Atlanta, Georgia. Inclusion criteria were: (1) pregnant at less than 24 weeks’ gestation; (2) less than 25 years of age; (3) no severe medical problems necessitating individualized case management as a “high-risk” pregnancy (e.g., diabetes, hypertension, HIV); (4) English or Spanish speaking; and (5) willing to participate in a randomized clinical trial. All patients underwent written informed consent, and the study was approved by the Yale University and Emory University Human Investigations Committees. All patients had public (e.g., Medicaid) or hospital assistance for complete prenatal care insurance coverage. Of 1542 eligible women, 1047 enrolled in the study (68% participation rate). All participants received nutritional information and were informed of their target weight gain based on BMI at the first session. No further nutritional instruction was given unless they developed gestational diabetes during the pregnancy. Labor, delivery, and birth outcomes were obtained through medical chart review for 990 participants (95%). The current analysis was limited to singleton gestations. In addition, participants who were missing complete BMI or weight gain data were excluded resulting in 841 subjects. There were no differences between the 149 participants excluded and the 841 included on any demographics or measured study variables.
Procedures
Trained study staff facilitated interviews on laptop computers and reviewed medical records. Structured interviews occurred after enrollment in the second trimester at an average gestational age of 18 weeks via audio computer-assisted self-interview. Participants were paid $25 for each interview.
Demographic and Medical History Measures
Patient demographics were obtained by questionnaires that assessed age, race, parity, and BMI prior to pregnancy. Participants were categorized into age groups (14 to 19 years old and 20 to 25 years old), racial groups (African-American, white, Latina, and other), parity groups (zero, one, two or more), and BMI groups (underweight [BMI < 19.8], normal [BMI 19.8 to 25.9], overweight [BMI 26 to 30], obese [BMI > 30]) based on the Institute of Medicine (IOM) classification. 12 Prepregnancy BMI and baseline BMI had a strong correlation (r = 0.95, p = 0.001) indicating the validity of the prepregnancy BMI report. Furthermore, medical record reviews obtained medical risk information including hypertension, diabetes, preeclampsia, multiple gestations, and fetal abnormalities as well as verification of prepregnancy BMI and weight gain in pregnancy.
The entire study population was subdivided according to prepregnancy BMI category as defined by the IOM as well as by categories of weight gain according to IOM guidelines for pregnancy.12 Weight gain was stratified as undergain, appropriate gain, and overgain in each BMI category based on the recommendations. Appropriate gain was 28 to 40 pounds for underweight participants, 25 to 35 pounds for normal-weight participants, 15 to 25 pounds for overweight participants, and 0 to 15 pounds for obese participants. Any gain below appropriate for that BMI class was classified as undergain and any gain above appropriate for that BMI class was classified as overgain.
Infants were categorized by gestational age and birth weight into small-for-gestational-age (SGA), appropriate-for-gestational-age (AGA), and large-for-gestational-age (LGA) categories using the Lubchenco curves.13 Type of delivery was coded as vaginal or cesarean delivery. Obstetric complications included vaginal lacerations, fetal distress, infant neonatal intensive care (NICU) admission, Apgar score at 5 minutes less < 7, hypertension, diabetes, oligohydramnios, and presence of infection during delivery or postpartum.
Analysis
To assess the first aim, we conducted descriptive statistics (frequencies, means, standard deviations) to analyze BMI and weight gain classes for our sample. Chi-square analyses were used to assess the relationship between BMI and weight gain. To assess the second aim, bivariate analyses were conducted between BMI and weight gain classes with birth outcomes using chi-square analyses. Multivariate analyses were conducted using a series of logistic regressions controlling for demographic and medical history variables known to relate to birth outcomes including cigarette use during pregnancy, alcohol use during pregnancy, age, race, and parity. We also controlled for the study intervention as well as gestational age at delivery. For birth weight, multinomial logistic regression modeling, an extension of logistic regression for outcomes with more than two levels, was used to assess the relationship between BMI class, weight gain class, and the three level birth weight variable (e.g., SGA, AGA, LGA) controlling for demographic and medical covariates. Multinomial logistic regression examines each level of the outcome with a specified comparison. For this analysis, we used AGA as the referent group. In addition, the BMI versus weight gain class interaction was assessed for all multivariate logistic and multinomial regression analyses to determine whether the influence of weight gain on birth outcomes differed across BMI classes. Significant interactions (p < 0.05) were included in the final model.
RESULTS
Almost half the patients were overweight or obese with 226 (26.9%) in the obese category (Table 1). Of the obese women, 20% had a BMI of 40 or greater (5.2% of the total sample). Women gained an average of 32.3 ± 23.6 pounds in pregnancy with only 25.3% gaining the amount of weight recommended by BMI class and more than half overgaining. There was a significant difference in weight gain between the groups, with obese women gaining less than the other groups (24.7 ± 27.5 pounds compared with 35.9 ± 18.8, 36.3 ± 20.3, and 31.2 ± 24.9 pounds in the underweight, normal, and overweight groups, respectively (F=13.53, p = 0.001). Fifty percent of the women with a BMI of 40 or greater overgained. There was no significant interaction of race or parity with weight gain patterns.
Table 1.
Demographic and Medical History by BMI and Weight Gain Groups
| Variable | Underweight | Normal | Overweight | Obese | χ2 | Undergain | Appropriate Gain |
Overgain | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| n | 110 | 361 | 144 | 226 | 168 | 213 | 460 | ||
| % | 13.1% | 42.9% | 20% | 25.5% | 54.7% | ||||
| Weight gain (lbs) | 35.9 ± 18.8 | 36.3 ± 20.3 | 31.2 ± 24.9 | 24.7 ± 27.5 | 13.74 | ||||
| p = 0.001 | |||||||||
| Undergain | 33% | 29% | 19% | 0% | 100.56 | ||||
| Appropriate | 31% | 22% | 16% | 33% | p < 0.001 | ||||
| Overgain | 36% | 49% | 65% | 67% | |||||
| Age (y) | |||||||||
| 14–19 | 52.6% | 56.3% | 41.0% | 38.7% | 22.03 | 52.5% | 47.8% | 48.0% | 0.20 |
| 20–25 | 47.4% | 43.7% | 59.0% | 61.3% | p = 0.001* | 47.5% | 52.2% | 52.0% | p = 0.55 |
| Race | |||||||||
| Black | 74.6% | 79.6% | 79.2% | 76.1% | 3.83 | 85.9% | 75.4% | 77.0% | 11.57 |
| Latina | 16.7% | 12.6% | 13.9% | 14.2% | p = 0.70 | 16.7% | 16.5% | 13.1% | p = 0.07 |
| White/other | 8.7% | 7.8% | 6.9% | 9.7% | 8.7% | 8.1% | 9.9% | ||
| Parity | |||||||||
| Nulliparous | 71.9% | 69.4% | 56.9% | 56.5% | 17.54 | 61.6% | 58.5% | 66.6% | 4.71 |
| p = 0.001* | p = 0.095 | ||||||||
| Smoking | 20.2% | 19.1% | 18.8% | 21.2% | 0.69 | 17.1% | 15.6% | 22.8% | 6.13 |
| during | p = 0.88 | p = 0.04* | |||||||
| Pregnancy | |||||||||
| Alcohol use | 5.3% | 8.6% | 7.6% | 8.0% | 1.16 | 6.8% | 8.5% | 8.1% | 0.43 |
| during | p = 0.70 | p = 0.81 | |||||||
| pregnancy |
p < 0.05.
BMI, body mass index.
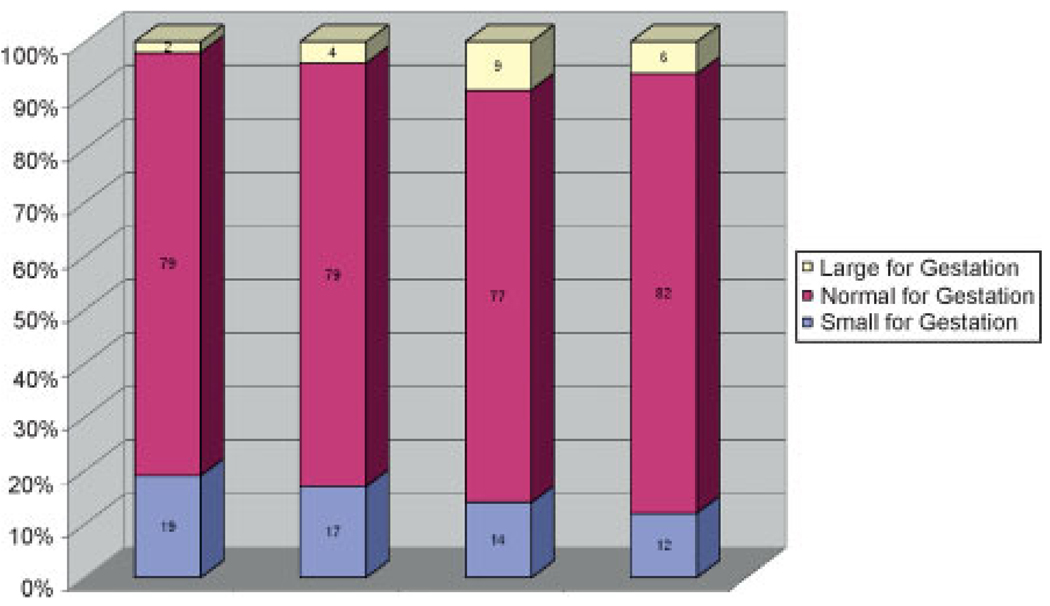
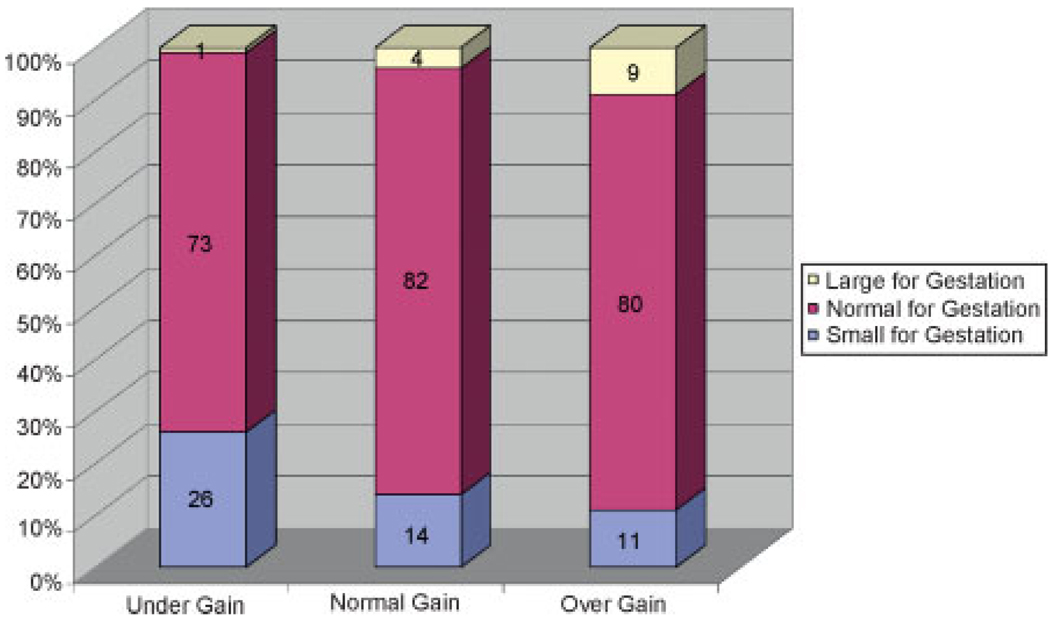
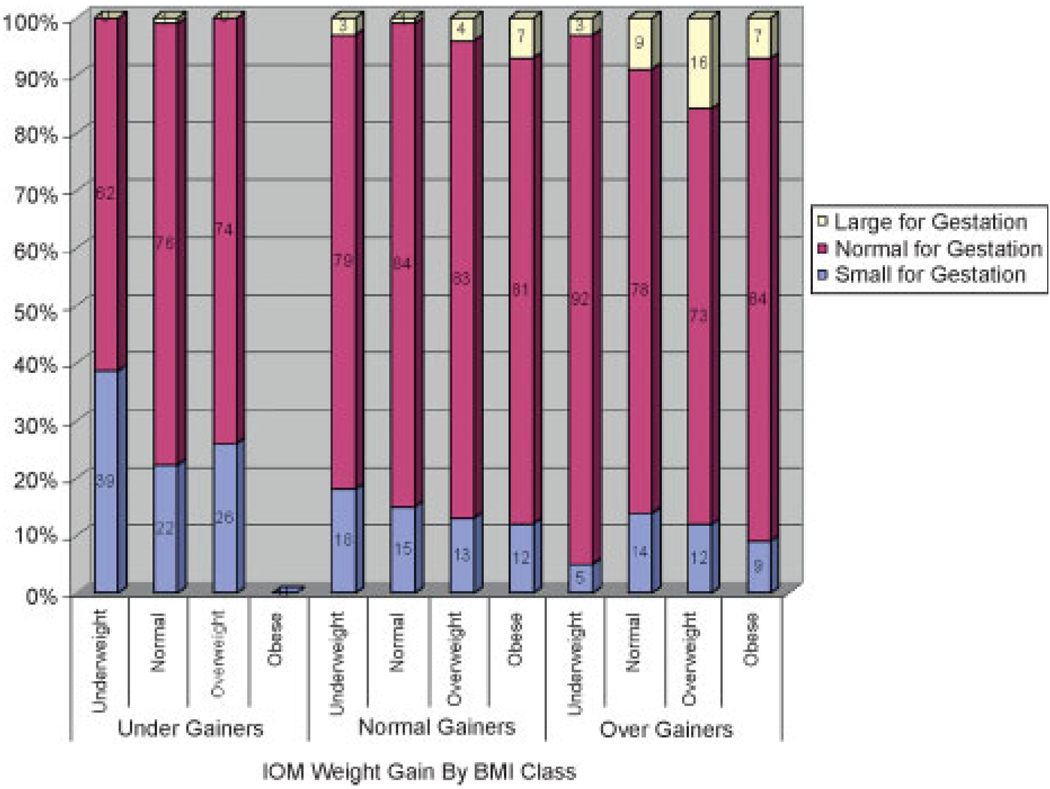
There was a significant relationship between infant size and BMI class with decreasing rates of SGA and increasing rates of LGA with increasing BMI category (χ2 = 17.31, p = 0.008; Fig. 1). Weight gain also had a significant effect on infant size with undergainers having an increased risk of SGA infants and overgainers more likely to have LGA infants (χ2 = 37.68, p = 0.001; Fig. 2). When weight gain was further analyzed by BMI class, a relationship of weight gain to infant weight was found for the underweight (χ2 = 14.41, p = 0.006) and normal weight groups (χ2 = 14.71, p = 0.005), but only marginally for the overweight group (χ2 = 9.15, p = 0.057) and not significant for the obese group (χ2 = 0.41, p = 0.81). For underweight women, overgaining gave the best infant weight outcome with 92% of the infants being AGA with 5% SGA and 3% LGA as compared with 15% SGA with appropriate weight gain and 39% SGA with inadequate weight gain. Overgaining in the normal weight group had no significant effect on the percentage of SGA (14% versus 15%) but accounted for an eightfold increase in LGA (8% versus 1%). In overweight women, overgaining also had a large effect, increasing LGA by fourfold (16% versus 4%). Undergaining for overweight women had the lowest rate of LGA (0%) compared with normal gainers and overgainers (4% and 16%) but it also had the highest rates of SGA (26% versus 13% and 12%; Fig. 3). In obese women, normal gainers did not differ substantively from overgainers on LGA (7% versus 7%) or SGA (12% versus 9%).
Figure 1.
Birth weight by gestation by body mass index (BMI) class (χ2 = 17.31, p = 0.008).
Figure 2.
Birth weight by gestation by weight gain (χ2 = 37.68, p = 0.001).
Figure 3.
Institute of Medicine (IOM) weight gain by body mass index (BMI) class.
Next, we conducted multinomial regression analyses to predict birth weight. Results showed that weight gain was a significant predictor of infant size with undergainers more likely to have SGA infants (odds ratio [OR] 1.99, confidence interval [CI] 1.11 to 3.55, p = 0.001) and overgainers more likely to have LGA infants (OR 2.52, CI 1.08 to 5.89, p = 0.008; Table 2). BMI did not relate to infant size in the multivariate analysis. Furthermore, there was no significant BMI ± weight gain interaction.
Table 2.
Multinomial Regression Predicting Birth Weight by Gestation
| SGA versus Normal for Gestational Age |
LGA versus Normal for Gestational Age |
|
|---|---|---|
| BMI | p = 0.89 | p = 0.09 |
| Underweight | 1.21 (0.69–2.12) | 0.46 (0.10–2.10) |
| Normal | 1.00 | 1.00 |
| Overweight | 1.19 (0.65–2.18) | 2.16 (0.98–4.75) |
| Obese | 1.00 (0.55–1.82) | 1.00 (0.45–2.20) |
| Weight gain | p = 0.001* | p = 0.008* |
| Undergain | 1.99 (1.11–3.55)* | 0.21 (0.02–1.67) |
| Appropriate gain | 1.00 | 1.00 |
| Overgain | 0.75 (0.44–1.28) | 2.52 (1.08–5.89)* |
p < 0.05.
Note: Controlling for cigarette use, alcohol use, age, parity, race, gestational age at delivery, and study intervention condition. BMI, body mass index; SGA, small for gestational age; LGA, large for gestational age.
BMI groups significantly differed on cesarean delivery rates with obese women at significantly greater risk for cesarean delivery by bivariate analysis (29.6% compared with 14.7% in the underweight, 15.6% in the normal weight, and 21.0% in the overweight groups, χ2 = 19.23, p = 0.001). Weight gain groups also significantly differed on cesarean delivery rates, with overgainers more likely to have cesarean delivery (24.3% compared with 16.9% in the appropriate gain and 13.3% in the undergain groups, p = 0.004). Multivariate analysis using logistic regression showed that BMI and not weight gain was a significant predictor of cesarean delivery. The odds of cesarean delivery for obese women were 2.3 times more likely than for normal weight women (OR 2.30, CI 1.48 to 3.58, p < 0.0001; Table 3). Only 6% of the women developed diabetes or hypertension/preeclampsia during the pregnancy. Exclusion of these patients did not significantly alter the analyses. We conducted post hoc analyses of the individual negative birth outcomes with large enough frequencies. Results showed no relationship with BMI and any of the individual negative birth outcomes (e.g., vaginal laceration, fetal distress, NICU admission, Apgar below 7). However, weight gain was significantly related to laceration and fetal distress. Undergainers were less likely to have a laceration (OR 0.39, CI 0.22 to 0.69, p < 0.05) and fetal distress (OR 0.28, CI 0.09 to 0.86, p < 0.05) than appropriate gainers.
Table 3.
Multivariate Results and Odds Ratios Using Logistic Regression for BMI and Weight Gain on Cesarean Delivery
| Cesarean Delivery | |
|---|---|
| BMI | p = 0.000* |
| Underweight | 0.99 (0.54–1.83) |
| Normal | 1.00 |
| Overweight | 1.44 (0.86–2.39) |
| Obese | 2.30 (1.48–3.58)* |
| Weight gain | p = 0.084 |
| Undergain | 0.97 (0.53–1.79) |
| Appropriate gain | 1.00 |
| Overgain | 1.51 (0.98–2.33) |
p < 0.05.
Note: Controlling for smoking, alcohol and drug use, age, parity, race, gestational age at delivery and intervention condition. BMI, body mass index.
Post hoc analysis demonstrated that even when LGA infants were removed from the analysis, there was still a significant effect of obese women on cesarean delivery (OR 2.09, CI 1.32 to 3.32, p = 0.007) but there was no effect of weight gain (OR 1.45, CI 0.94 to 2.17, p = 0.093).
DISCUSSION
This represents a prospective study examining both weight gain patterns and obstetric outcomes in young women. Previous studies in adolescents have predominantly focused on the relationship of weight gain to birth weight.14,15 There has been debate on whether adolescents should have different guidelines for weight gain than adult women secondary to their still developing nutritional requirements, leading some authors advocating for increases in weight gain.16 Our results demonstrate that it is only the underweight group that significantly decreases SGA risks by overgaining. They represent a minority of our population and a minority of the reproductive age women in the United States today. The predominance of adolescent women should follow guidelines similar to adult women. Our results showed that women who were outside the guidelines of appropriate gain (e.g., undergainers and overgainers) were more likely to have adverse birth weight outcomes. Undergainers were almost 2 times more likely to have SGA babies and overgainers were almost 2.5 times more likely to have LGA babies. Weight gain had more of a significant impact on birth weight than BMI.
However, BMI had a more significant impact on the occurrence of cesarean delivery than weight gain. Sukalich et al first reported on the increased risks of cesarean delivery and BMI of obesity in adolescents in a retrospective study of birth certificate data of predominantly Caucasian teenagers.10 They excluded underweight women from their control group and found increasing risk of cesarean delivery with increasing BMI class and increasing weight gain. Multivariate analysis in their study showed that both BMI and weight gain were independent risk factors. Our study confirms these findings but demonstrates that BMI seems to be a more important predictor of cesarean delivery than pregnancy weight gain. When controlling for the causative effect of LGA on cesarean delivery, obese women were still at significant risk for cesarean delivery regardless of whether they had a macrosomic infant.
This study is limited by a sample that represents a relatively restricted group of young, ethnic minority women of low socioeconomic status who registered for prenatal care prior to the second trimester and agreed to participate in a randomized clinical trial. Their weight and weight gain patterns may not be generalizable to other populations. They do reflect the patient population seen in many urban settings. The frequency of negative birth outcomes other than cesarean delivery was relatively low because of the sample size and the lack of chronic disease in this population. The lack of chronic disease in the population does mitigate its effect on the cesarean delivery rate.
The current guidelines of appropriate weight gain in pregnancy are currently under review. These data support the importance of appropriate weight and weight gain and the potential for decreasing the weight gain parameters in overweight women and increasing the weight gain parameters in underweight women. These changes must be balanced with the increased risk of SGA children seen with undergaining.
There are many theories to account for the increased risk of cesarean delivery in obese women in the absence of macrosomia. The increased risk may be due to the increase in soft tissue in the pelvis narrowing the pelvic outlet, the negative effect of poor pelvic and abdominal tone on fetal position, the relatively higher dosages of oxytocin required for induction and/or maintenance of adequate contractile forces, the technical difficulty in fetal monitoring, as well as the concerns by the physician about macrosomia and shoulder dystocia and therefore the more liberal use of cesarean delivery for failure to progress. There have been no prospective studies on the effect of obesity on fetal heart rate patterns and whether maternal habitus or excessive weight gain causes a redistribution of blood in addition to the normal physiological changes in pregnancy and therefore adversely affect placental perfusion and increase abnormal fetal heart rate patterns. There is no doubt that with the increasing rates of obesity demonstrated worldwide, there should be a concerted effort to prepregnancy weight reduction and strict adherence to weight gain guidelines. Future research will need to focus on whether antepartum or postpartum interventions will be successful in reducing these risks.
ACKNOWLEDGMENTS
This research was funded by National Institute of Mental Health grant R01 MH/HD61175 to Jeannette R. Ickovics, Ph.D.
REFERENCES
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among U.S. children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Hall LF, Neubert AG. Obesity and pregnancy. Obstet Gynecol Surv. 2005;60:253–260. doi: 10.1097/01.ogx.0000158509.04154.9e. [DOI] [PubMed] [Google Scholar]
- 4.ACOG Committee Opinion on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2005;106:671–675. doi: 10.1097/00006250-200509000-00054. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complication and cesarean delivery rate: a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 6.Pathi A, Esen U, Hildreth A. A comparison of complications of pregnancy and delivery in morbidly obese and non-obese women. J Obstet Gynaecol. 2006;26:527–530. doi: 10.1080/01443610600810914. [DOI] [PubMed] [Google Scholar]
- 7.Kiel DW, Dodson EA, Artal R, Boehmer T, Leet TL. Gestational weight gain and pregnancy outcomes in obese women. How much is enough? Obstet Gynecol. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 8.Barau G, Robillard PY, Hulsey TC, et al. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG. 2006;113:1173–1177. doi: 10.1111/j.1471-0528.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 9.Stotland NE, Hopkins LM, Caughey AB. Gestational weight gain, macrosomia and risk of Cesarean birth in nondiabetic nulliparas. Obstet Gynecol. 2004;104:671–677. doi: 10.1097/01.AOG.0000139515.97799.f6. [DOI] [PubMed] [Google Scholar]
- 10.Sukalich S, Mingione MJ, Glantz JC. Obstetrical outcomes in overweight and obese adolescents. Am J Obstet Gynecol. 2006;195:851–855. doi: 10.1016/j.ajog.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 11.Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110:330–339. doi: 10.1097/01.AOG.0000275284.24298.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nutrition during Pregnancy. Washington DC: National Academy Press; 2000. Subcommittee on Nutritional Status and Weight Gain in Pregnancy, Institute of Medicine. [Google Scholar]
- 13.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–163. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 14.Hediger ML, Scholl TO, Ances IG, Belsky DH, Salmon RW. Rate and amount of weight gain during adolescent pregnancy: associations with maternal weight-for-height and birth weight. Am J Clin Nutr. 1990;52:793–799. doi: 10.1093/ajcn/52.5.793. [DOI] [PubMed] [Google Scholar]
- 15.Stevens-Simon C, McAnarney ER, Roghmann KJ. Adolescent gestational weight gain and birth weight. Pediatrics. 1993;92:805–809. [PubMed] [Google Scholar]
- 16.Johnston CS, Christopher FS, Kandell LA. Pregnancy weight gain in adolescents and young adults. J Am Coll Nutr. 1991;10:185–189. doi: 10.1080/07315724.1991.10718142. [DOI] [PubMed] [Google Scholar]