Abstract
Renal injury is a common pathophysiological feature in women with preeclampsia as evidenced by increased protein leakage (proteinuria) and glomerular injury (glomerular endotheliosis). Recently, podocyturia was found in preeclampsia, suggesting podocyte shedding occurs in this pregnancy disorder. However, podocyte function in preeclampsia is poorly understood. In this study, the authors have examined podocyte-specific protein expressions for nephrin, glomerular epithelial protein 1 (GLEPP-1), and ezrin in kidney biopsy tissue sections from women with preeclampsia. Expressions for vascular endothelial growth factor (VEGF) and its receptor Flt-1 and oxidative stress marker nitrotyrosine and antioxidant CuZn-superoxide dismutase (CuZn-SOD) were also examined. Kidney tissue sections from nonhypertensive and chronic hypertensive participants were stained as controls. The findings were (1) nephrin and GLEPP-1 were mainly expressed in glomerular podocytes; (2) ezrin was expressed in both glomerular podocytes and tubular epithelial cells; (3) compared to tissue sections from nonhypertensive and chronic hypertensive participants, nephrin and GLEPP-1 expressions were much reduced in tissue sections from preeclampsia and ezrin expression was reduced in podocytes; (4) enhanced VEGF, Flt-1, and nitrotyrosine, but reduced CuZn-SOD, expressions were observed in both glomerular podocytes and endothelial cells in tissue sections from preeclampsia; and (5) the expression pattern for nephrin, GLEPP-1, ezrin, VEGF, Flt-1, and CuZn-SOD were similar between tissue sections from nonhypertensive and chronic hypertensive participants. Although the authors could not conclude from this biopsy study whether the podocyte injury is the cause or effect of the preeclampsia phenotype, the data provide compelling evidence that podocyte injury accompanied by altered angiogenesis process and increased oxidative stress occurs in kidney of patients with preeclampsia.
Keywords: podocyte, nephrin, VEGF, oxidative stress, kidney, preeclampsia
INTRODUCTION
Proteinuria is one of the clinical features of preeclampsia, the other being hypertension. The severity of preeclampsia is defined not only by the magnitude of the hypertension but also by the amount of protein present in the urine. The leakage of protein into the urine is a direct result of a disruption of the renal barrier structure in the kidney. The renal barrier structure has three components: glomerular endothelium, basement membrane, and podocytes. It is well known that in preeclampsia the glomerular endothelial cells present swelling, intracellular vacuoles, and certain degree of hypertrophy leading to loss of glomerular endothelial fenestrae known as glomerular endotheliosis.
Recent evidence suggests that podocyte injury might also play an important role in the pathophysiology of preeclampsia.1 By examining the renal autopsy tissues from women with preeclampsia, Garovic et al noticed reduced podocyte protein expressions for nephrin and synaptopodin.2 Nephrin is a marker for podocyte foot processes while synaptopodin is a marker for podocyte cytoskeleton. The same group also found that podocytes were present in the urine of women with preeclampsia but not in the urine of women with normal pregnancies, indicating podocytes detached from glomerular basement membranes in women with preeclampsia.3 Podocyte shedding has also been found in other renal and systemic diseases, such as diabetic nephropathy4 and lupus nephritis.5 Experimental nephropathy animal models suggested that podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria.6
Glomerular endothelial cells express vascular endothelial growth factor (VEGF) receptor-1 (also known as Flt-1) and glomerular podocytes express VEGF. Thus, it has been postulated that the counter urine flow diffusion of VEGF contributes to the maintenance of glomerular endothelial integrity.7 In mice, partial deletion and/or overexpression of podocyte-specific VEGF lead to proteinuria with glomerular endotheliosis and collapsing glomerulopathy,8 suggesting VEGF plays a key role in maintaining podocyte health. It is well known that in preeclampsia, there is an imbalance in angiogenic factors characterized by increased sFlt-1 levels in the maternal circulation.9 However, it remains unexplored whether this imbalance is associated with renal injury in preeclampsia.3 In this study, we examined podocyte-specific protein expressions for nephrin, glomerular epithelial protein 1 (GLEPP-1), and ezrin, as well as VEGF and its receptor Flt-1 expressions in kidney biopsy tissue sections from women with preeclampsia. Oxidative stress marker nitro-tyrosine and antioxidant CuZn-superoxide dismutase (CuZn-SOD) expressions were also examined. Kidney biopsy tissue sections from nonhypertensive patients and from patients with chronic hypertension were stained as controls.
MATERIALS AND METHODS
Tissue Samples
The kidney biopsies were obtained retrospectively from the archives of kidney biopsy specimens evaluated in the department of pathology at our institution from 1999 through 2006. In nonhypertensive group (n = 5), the specimens were taken from the normal portion of the biopsies that were performed for excluding neoplasia; hypertension and chronic renal disease had been excluded based on clinical history, physical examination, and laboratory data. Biopsies for chronic hypertensive controls (n = 2) were from patients who had clinical history of hypertension, and the ranges of blood pressure were similar to that seen in preeclamptic patients. In preeclamptic patients (n = 3), the kidney biopsy was done for diagnostic purpose in all cases due to continued hypertension and proteinuria after clinical treatment. Institutional Review Board approval for using the remaining tissue sections was obtained. All biopsy specimens were processed for light microscopy and electron microscopy according to standard techniques.
Clinical information of the three preeclamptic cases was summarized in Table 1. The control cases were anonymous.
Table 1.
Clinical Information of Preeclamptic Cases
| Case 1 | A 24-year-old female, G3P2, was admitted to the hospital at 28 + 5 weeks of gestation with elevated blood pressure to180/100 mm Hg and proteinuria 14 g/d. She was diagnosed with preeclampsia and likely placental abruption and had an emergency C-section. Placental abruption was confirmed. The patient had 2 previous pregnancies, both were clinically diagnosed with preeclampsia first one was delivered at 37 weeks and the second one was delivered at 32 weeks. Kidney biopsy was done 2 weeks after the C-section due to persistent hypertension, proteinuria, and abnormal renal function. |
| Case 2 | A 25-year-old female, G2P1, was admitted to the hospital at 31 + 4 weeks of gestation with elevated blood pressure to 180/100 mm Hg and proteinuria 11 g/d. Her creatinine was within normal range. She had elective C-section delivery at 34 + 1 weeks. Ten days after C-section, she had kidney biopsy due to massive proteinuria. |
| Case 3 | A 25-year-old female, G1P0, was admitted to the hospital at 14 weeks of gestation with blurred vision, elevated blood pressure to 200/119 mm Hg and positive proteinuria (2+ in dip stick) and abnormal renal function. Initially, she was diagnosed severe preeclampsia. She had renal biopsy 2 days before elective termination of pregnancy due to multiple organ failure. After a serial clinical examination, she was diagnosed as preeclampsia complicated with Raynaud syndrome. |
Immunohistochemistry
Three groups of target proteins were examined by immunostaining of kidney tissue sections, including (1) podocyte markers: nephrin, GLEPP-1 and ezrin; (2) angiogenesis-related factors: CD31, VEGF, and Flt-1; and (3) oxidative stress marker nitrotyrosine and antioxidant enzyme CuZn-SOD. The sources of antibodies used in this study are given in Table 2. A standard immunohistochemistry staining procedure was performed as we previously described.10 Briefly, a serial of deparaffinization was done with xylene and ethanol alcohol. Antigen retrieval was performed by boiling tissue slides with 0.01 M citric buffer in microwave for 5 minutes. Hydrogen peroxide was used to quench the endogenous peroxidase activity. After blocking, the sections were incubated with primary antibodies overnight at 4°C. Corresponding biotinylate-conjugated secondary antibodies from ABC staining system (Santa Cruz Biotechnology, Santa Cruz, CA) were used. Slides stained with isotopic immunoglobulin g (IgG) or stained with secondary antibody only were used as negative control. The nuclei were counterstained by hematoxylin. Slides stained with the same antibody were processed altogether in the same day. Stained slides were examined by an Olympus microscope (Olympus IX71, Tokyo, Japan). Images were captured by a digital camera with PictureFrame computer software (Uptronics, Inc., Sunnyvale, CA) and recorded in a microscope-linked PC computer.
Table 2.
Antibodies Used for Immunohistochemistry in This Study
| Name | Origin | Catalog | Sources |
|---|---|---|---|
| Nephrin | Sheep-anti-human (polyclone) | AF4269 | R&D System (Minneapolis, MN) |
| GLEPP-1 | Mouse-anti-human (monoclone) | AM336-5M | BioGenex (San Ramon, CA) |
| Ezrin | Mouse-anti-human (monoclone) | Ab4069 | Abcam (Cambridge, MA) |
| VEGF-A | Rabbit-anti-human (polyclone) | SC-152 | Santa Cruz (Santa Cruz, CA) |
| Flt-1 | Rabbit-anti-human (polyclone) | SC-316 | Santa Cruz (Santa Cruz, CA) |
| CD31 | Mouse-anti-human (monoclone) | M0823 | Dako (Corpinteria, CA) |
| Nitrotyrosine | Rabbit-anti-human (polyclone) | 06-284 | Upstate (Temecula, CA) |
| CuZn-SOD | Rabbit-anti-human (polyclone) | Ab52950 | Abcam (Cambridge, MA) |
RESULTS
Clinical Information of Preeclamptic Patients
The clinical information of preeclamptic patients (n = 3) was summarized in Table 1. All preeclampsia patients presented with hypertension and proteinuria. The degree of proteinuria was variable, but at least 2 patients had proteinuria that was in nephrotic range. Elevated creatinine was noted in 2 of 3 patients.One patient (case 3) also had symptoms of pulmonary failure and abnormal liver function.
Light Microscopy and Electron Microscopy
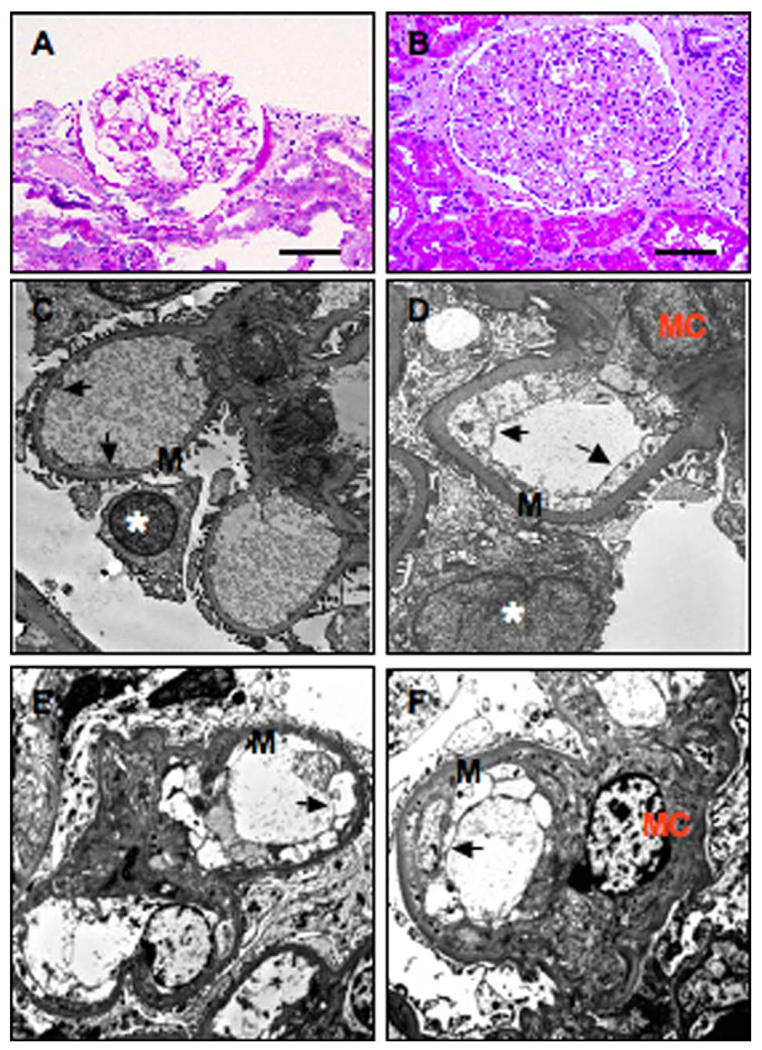
Representative micrographs of glomeruli from a nonhypertensive control and from a patient with preeclampsia are shown in Figure 1. In nonhypertensive controls, the glomeruli reveal Bowman’s capsule and normal glomerular structures. The capillaries show open loops, smooth, and thin capillary membranes (Figure 1A). In hypertensive patients, the glomeruli appear slightly enlarged. Segmental wrinkling and thickening of capillary loops, features associated with hypertension, are present (not shown). In the glomeruli from patients with preeclampsia, there are diffuse thickening of capillary membranes, obliteration of capillary lumina, and accentuated lobulation of capillary tufts (Figure 1B). Ultrastructurally, the glomeruli from nonhypertensive controls revealed open capillary loops and uniform basement membranes that showed fenestrations in the inner sides and regularly distributed foot processes in the peripheral of the capillary loops (Figure 1C). In patients with preeclampsia, the glomeruli revealed diffuse effacement of foot process and widening of subendothelial spaces. The number of endothelial cells per capillary loop was increased. The endothelial cells revealed slightly enlarged nuclei and prominent edema of cytoplasm with diminishing of fenestration (Figure 1D–F) that has been described as endotheliosis, a well-known kidney injury in patients with preeclampsia.
Figure 1.
Representative micrographs of HE staining and EM examination in kidney tissue sections from nonhypertensive and preeclamptic patients. A and C, Nonhypertensive. B and D–F, Preeclampsia. A and B, HE staining and C and D–F, Electron macroscopic micrographs. Arrow indicates glomerular endothelial cells; EM = electron microscopic; HE = hematoxylin and eosin; M = basement membrance; Asterisk = podocytes; MC = mesenchymal cells. Bar = 50 µm for A and B. Magnification: C: 5200×; D: 8900×; E: 8000×; and F: 8000×, respectively.
Reduced Nephrin, GLEPP-1, and Ezrin Expressions in Kidney Tissue Sections From Preeclampsia
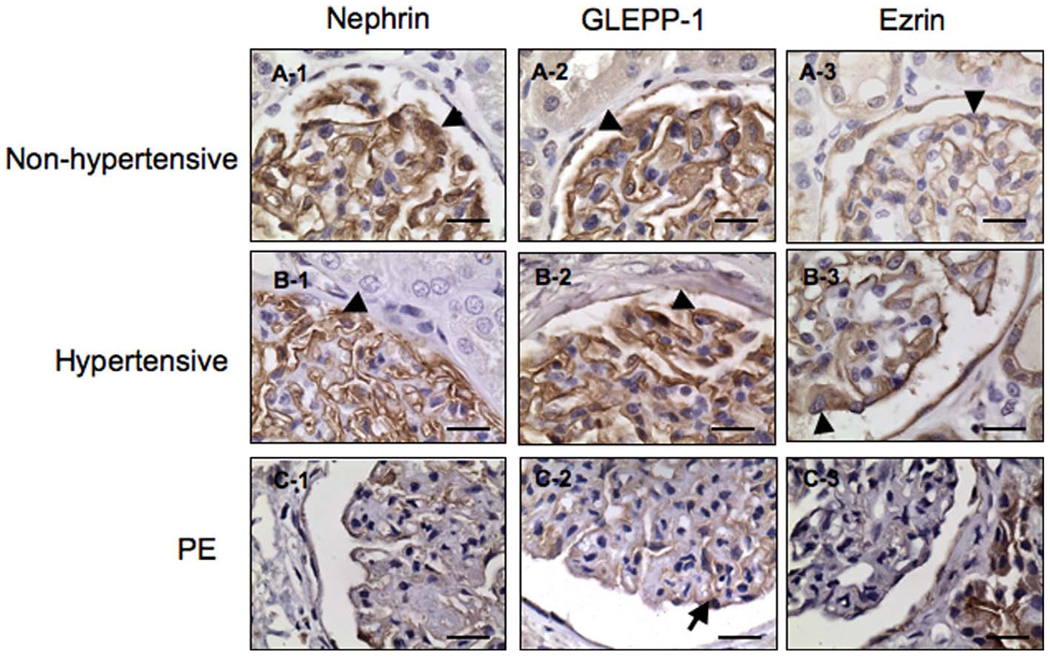
Nephrin is a specific protein localized in slit diaphragm of glomerular podocytes, and GLEPP-1 is an apical marker of podocytes. In nonhypertensive kidney tissue sections, immunoreactions for nephrin and GLEPP-1 are noted along the capillary epithelial side. Both endothelial cells and mesangial cells appear negative for nephrin (Figure 2, A-1) and GLEPP-1 (Figure 2, A-2). In hypertensive patients, the pattern for nephrin and GLEPP-1 (Figure 2, B-1 and B-2) is similar to that identified in nonhypertensive participants. Again the immunoreaction is noted along the capillary epithelial side. In glomeruli from preeclamptic patients, immunoreactions for nephrin and GLEPP-1 are much reduced along the capillary epithelial side (Figure 2, C-1 and C-2). Ezrin is expressed on both the glomerular and tubular epithelial cells. For both non-hypertensive and hypertensive participants, the ezrin immunoreaction is noted along the capillary epithelial side. In patients with preeclampsia, the immunoreaction for ezrin is greatly reduced from the glomerular epithelial cells whereas the staining signal looks relatively enhanced in tubular compartment compared to that found in non-hypertensive and hypertensive participants.
Figure 2.
Immunostaining of podocyte markers, nephrin, GLEPP-1, and ezrin, in kidney tissue sections from nonhypertensive, hypertensive, and preeclamptic patients. Expressions for nephrin, GLEPP-1, and ezrin expression are much reduced in podocytes (C-1, C-2, and C-3) from preeclamptic tissue sections compared to those from nonhypertensive (A-1, A-2, and A-3) and hypertensive controls (B-1, B-2, and B-3), respectively. Arrowhead indicates podocytes; GLEPP-1 = glomerular epithelial protein 1. Bar = 20 µm.
Enhanced VEGF and Flt-1, But Reduced CD31, Expressions in Kidney Tissue Section From Preeclampsia
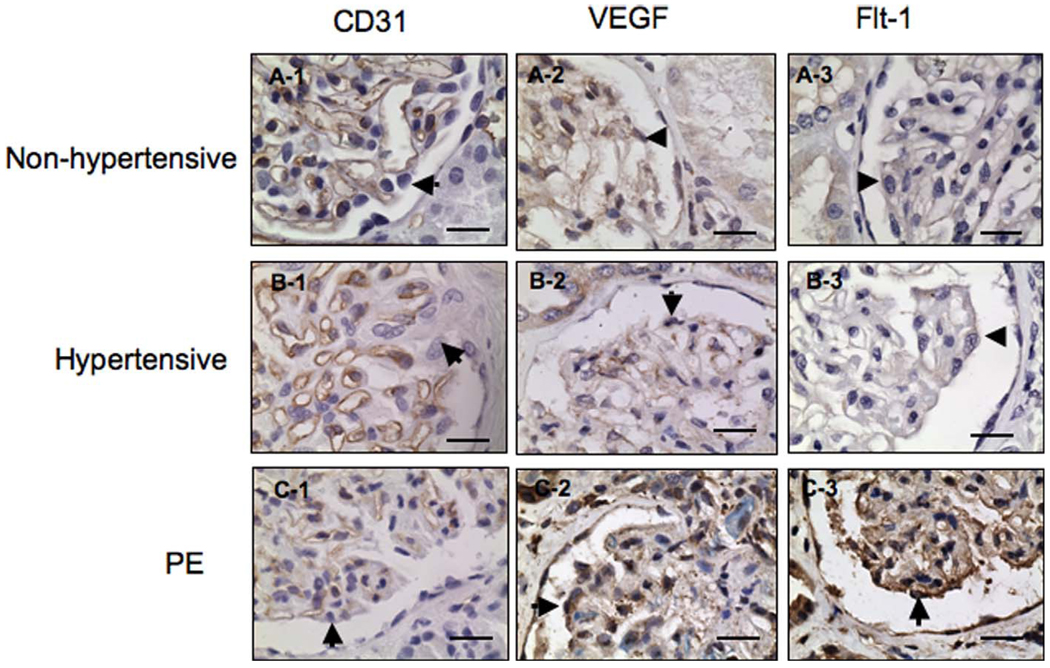
CD31 is a marker of vascular endothelial cells. CD31 is positively expressed and highlights the glomerular endothelial cells from both nonhypertensive and hypertensive tissue sections, Figure 3, A-1 and B-1, respectively. CD31 expression is much reduced in kidney biopsies from patients with preeclampsia (Figure 3, C-1). CD31 is negatively expressed in glomerular podocytes, mesangial cells, and tubular epithelial cells in sections from nonhypertensive, hypertensive as well as preeclampsia patients. In contrast to CD31, immunoreactions for both VEGF and Flt-1 are strongly positive expressed in tissue sections from preeclampsia patients (Figure 3, C-2 and C-3), while VEGF and Flt-1 expressions are weakly or barely visible in either glomerular or tubular epithelial cells from both nonhypertensive and hypertensive participants (Figure 3, A-2, A-3, B-2 and B-3), respectively.
Figure 3.
Immunostaining of CD31, VEGF, and Flt-1 in kidney tissue sections from nonhypertensive, hypertensive, and preeclamptic patients. In both nonhypertensive and hypertensive participants, immunostaining of CD31 highlights the glomerular endothelial cells, A-1 and B-1, respectively. In preeclampsia, the intensity of CD31 immunostaining is markedly reduced. C-1, VEGF and Flt-1 are weakly or negatively expressed in tissue sections from nonhypertensive samples, A-2 and A-3. Similar patterns of VEGF and Flt-1 expression are observed in tissue sections from hypertensive participants, B-2 and B-3. In contrast, intensive expressions for VEGF and Flt-1 are seen in tissue section from preeclamptic patients, C-2 and C-3, respectively. Arrowhead indicates podocytes; PE = preeclampsia; VEGF = vascular endothelial growth factor. Bar = 20 µm.
Enhanced Nitrotyrosine, But Reduced CuZn-SOD, Expression in Kidney Tissue Sections From Preeclampsia
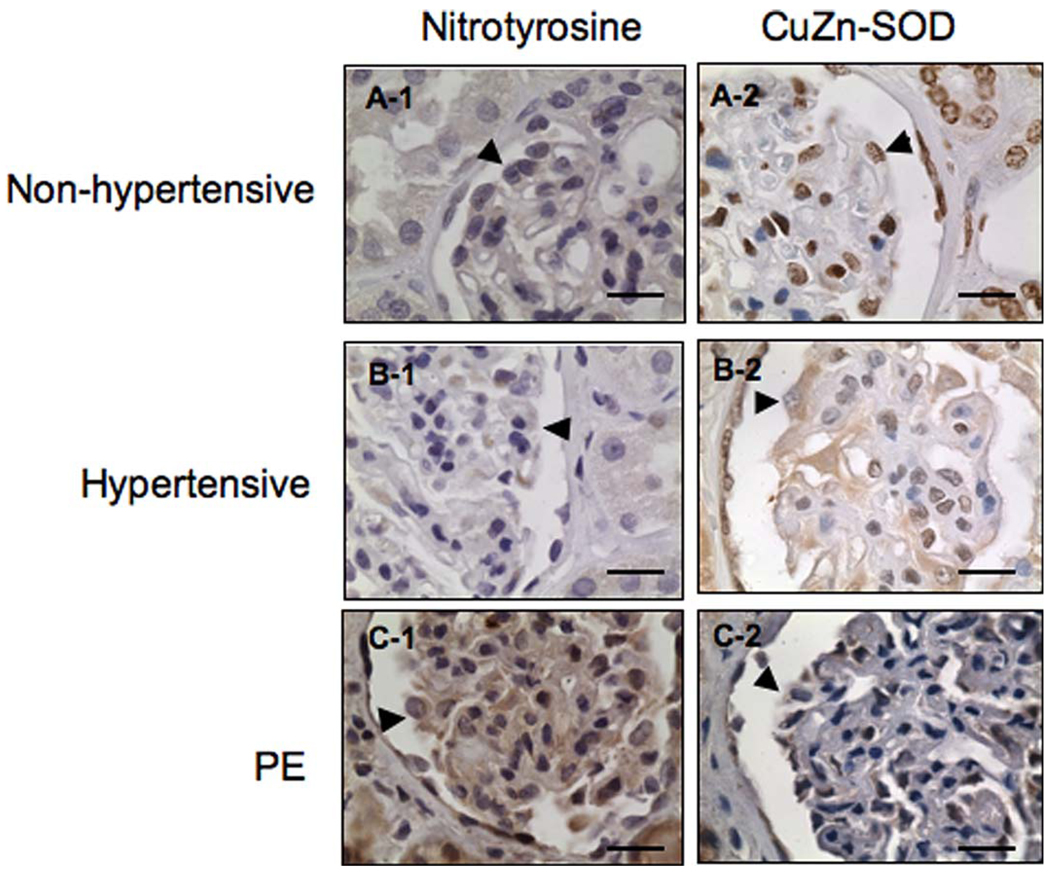
Nitrotyrosine is a marker for increased oxidative stress, while CuZn-SOD is an antioxidant enzyme to dismutate superoxide radicals generated in living cells. To investigate whether increased oxidative stress also occurs in the kidney tissue in women with preeclampsia, nitrotyrosine and CuZn-SOD expressions were examined. Compared to nonhypertensive and hypertensive participants, our results showed that nitrotyrosine immunostain is much enhanced in tissue sections from preeclamptic participants (Figure 4, C-1). There is no immunoreactivity for nitro-tyrosine on either glomerular or tubular epithelial cells from both nonhypertensive and hypertensive participants (Figure 4, A-1 and B-1), respectively. In contrast, reduced CuZn-SOD expression is observed in tissue sections from patients with preeclampsia (Figure 4, C-2) compared to nonhypertensive and hypertensive controls (Figure 4, A-2 and B-2), respectively.
Figure 4.
Immunostaining of nitrotyrosine and CuZn-SOD in kidney tissue sections from nonhypertensive, hypertensive, and preeclamptic patients. Enhanced nitrotyrosine staining is seen in tissue sections from preeclampsia, C-1, compared to those of controls, A-1 and B-1, respectively. The pattern for CuZn-SOD expression is opposite. CuZn-SOD immunostaining is much reduced in preeclamptic tissue sections, C-2, compared to those from nonhypertensive and hypertensive controls, A-2 and B-2, respectively. Arrowhead indicates podocytes; CuZn-SOD = CuZn-superoxide dismutase; PE = preeclampsia. Bar = 20 µm.
DISCUSSION
Previous studies have indicated that injury of glomerular endothelial cells is pathognomonic in preeclampsia.11 In our study, glomerular endothelial cell injury and basement membrane alterations were confirmed by electron microscopy in all three preeclamptic cases. Because podocyte shedding contributes to the development of proteinuria, and podocyturia is believed to be a specific marker for preeclampsia,3 we specifically examined immunoreactivities of 3 target podocyte proteins in kidney tissue sections: nephrin, GLEPP-1, and ezrin. Our results showed that immunostains for nephrin, GLEPP-1, and ezrin were all reduced in glomerular podocytes in kidney biopsies from women with preeclampsia compared to those from nonhypertensive and hypertensive participants. These results are consistent with the findings reported by Garovic et al in which reduced nephrin expression and downregulation of synaptopodin expression, a cytoskeleton/actin associated protein, were noticed on glomerular podocytes of kidney tissues from women with preeclampsia.2
These podocyte expressing proteins are thought to play an important role in maintaining the integrity of kidney podocyte function. Nephrin is a specific marker for glomerular podocyte foot process. It is localized in the slit diaphragm.12 Nephrin is critical in regulation of protein filtration in the kidney. Nephrin knock-out mice were unable to develop foot process and present massive proteinuria,13 demonstrating the importance of nephrin in renal barrier functionality. In humans, decreased nephrin expression has been found in patients with diabetic nephropathy.14 Studies also demonstrated that mutation of nephrin coding gene NPSH1 causes congenital nephrotic syndrome of Finnish type, an autosomal recessive disease characterized by fetal massive proteinuria in utero and nephrotic syndrome at birth.15,16 GLEPP-1 is a negatively charged podocyte-specific receptor phosphatase localized on the apical surface of podocytes.17 It is responsible for the maintenance of the apical domain sialyzation.18 The negative charge of the foot process apical domain is not only indispensable for the prevention of negatively charged plasma protein that pass through the renal ultrafiltration barrier but also necessary for the slit diaphragm stability. Ezrin is a plasma membrane-actin linking protein and also considered a marker for glomerular podocytes.19 The network of foot process is connected to the actin cytoskeleton through a complex including ezrin and PDZ protein NHERV-1 by phosphorylation process possibly.20 Ezrin expression is altered in podocytes undergoing injury and/or proliferation and is believed to be associated with glumerulogenesis.19
In the preeclamptic tissue sections, we noticed loss of immunoreaction for nephrin in the epithelial side of glomerular basement membranes. This observation is in line with increased podocyte detachment from basement membrane or increased podocyte shedding into the urine of patients with preeclampsia.3 Although currently no information is available regarding the viability of the detached podocytes in urine of preeclamptic women, in other renal pathology such as focal segmental glomerulosclerosis (FSGS) and lupus nephritis the detached podocytes retrieved from patient urine showed stronger viability than control specimen when cultured in vitro.21 Thus, it is tempting to speculate that in preeclampsia the detachment of podocytes from the basement membrane might be partially attributable to the altered local environment in the glomerulus per se, including imbalanced angiogenesis and oxidative stress.
It has been well accepted that imbalanced angiogenesis factor VEGF and its soluble receptor sFlt-1 productions or increased sFlt-1 levels in the maternal circulation play an important role in the pathophysiology in preeclampsia.9,22 Although the mechanism of downregulation of podocyte specific protein expression in preeclampsia is not known, an animal study conduced by Sugimoto et al provided convincing evidence of the harmful effects of sFlt-1 on kidney glomerular podocytes.23 Their study showed that wild-type CD1 mice administrated with anti-VEGF antibody or sFlt-1/Fc chimera produced proteinuria and showed downregulation of glomerular nephrin expression in kidney tissues.23 To study whether altered angiogenic factor expressions are also involved in renal injury in preeclampsia, we examined VEGF and Flt-1 expressions in kidney biopsies. Interestingly, in contrast to nephrin, GLEPP-1, and ezrin, both VEGF and Flt-1 expressions were markedly increased in tissue sections from preeclampsia particularly compared to those from nonhypertensive participants and chronic hypertension patients. Expressing the endothelial marker protein CD31 is much reduced in glomerular endothelium in samples from preeclampsia compared to those of controls. These observations support the concept that altered glomerular angiogenesis might take place in preeclampsia. It is not known whether increased VEGF expression is a compensatory mechanism of increased glomerular angiogenesis to offset increased sFlt-1 production in preeclampsia. However, our observation of increased glomerular VEGF expression in kidney tissue sections from preeclamptic patients supports the hypothesis that endogenous production of VEGF from renal tissue might contribute to the elevated urine excretion of VEGF in preeclamptic patients.24
Another important finding of the current study is increased oxidative stress in the kidney of women with preeclampsia as evidenced by increased nitrotyrosine expression and decreased CuZn-SOD expression in tissue sections from preeclampsia compared to those from controls. Nitrotyrosine is a maker of increased oxidative stress and it is formed when a protein molecule is nitrated by peroxynitrite. Superoxide dismutase is the only antioxidant enzyme to dismutate superoxide radicals generated by living cells. In our samples, enhanced nitrotyrosine expression and reduced CuZn-SOD expression are seen in glomeruli in preeclamptic tissues. It is well established that increased oxidative stress occurs in the placenta during preeclampsia.25,26 Although there is no direct evidence that oxidative stress induces renal injury in preeclampsia, increased oxidative stress has been implicated in several renal disease models, such as membrane nephropathy and diabetic nephropathy.27 The mechanism and the association of reduced antioxidant activity and podocyte injury in preeclampsia has not yet been established. Nevertheless, data from animal studies did show that podocyte-specific overexpression of the antioxidant metallothionein could reduce diabetic nephropathy by improving podocyte function and reducing albumin excretion,28,29 indicating that increased oxidative stress could produce podocyte injury. This underlying mechanism may also apply to the podocyte injury in preeclampsia.
There are limitations of the current study. First, because this was a retrospective study, we were unable to conduct a simultaneous study to collect maternal urine or blood samples from these patients. Therefore, we were unable to determine whether increased podocyte shedding could be found in the patient urine or whethr corresponding renal injury was associated with angiogenesis factor or oxidative stress marker levels in the maternal circulation. Second, among the three preeclamptic cases, kidney biopsy was done almost 2 weeks after cesarean section delivery in 2 cases due to uncontrollable hypertension. The third one was done at 14 weeks of gestation due to multiple organ failure. In addition, there is lacking of follow-up information because all preeclampsia patients were only refereed to our institution for managing pregnancy-associated complications. We were unable to trace the updated information for these patients and unable to provide current renal function or long-term maternal health information. Despite these limitations, we believe that these results provide compelling evidence that podocyte injury is associated with altered angiogenesis factor expression and increased oxidative stress in kidney tissues in women with preeclampsia. At the present time, we could not find whether podocyte injury is the consequence of adverse effects of elevated sFlt-1 levels on glomerular endothelial cells or downstream of increased oxidative stress in the systemic circulation, and we could not conclude from this biopsy study whether the podocyte injury is the cause or effect of the preeclampsia phenotype. However, we believe that podocyte injury contributes to the renal barrier lesion in patients with preeclampsia. Further studies are needed to dissect the cellular and molecular details that underlie preeclamptic nephropathy to better understand the mechanisms of renal injury in this pregnancy disorder.
ACKNOWLEDGMENTS
This study was supported in part by grants from National Institute of Health, NHLBI (HL65997) and NICHD (HD36822).
Footnotes
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
This study was presented at the 16th World Congress of the International Society for the Study of Hypertension in Pregnancy (ISSHP), Washington DC, September 21–24, 2008.
REFERENCES
- 1.Karumanchi SA, Lindheimer MD. Preeclampsia and the kidney: footprints in the urine. Am J Obstet Gynecol. 2007;196(4):287–288. doi: 10.1016/j.ajog.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garovic VD, Wagner SJ, Petrovic LM, et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant. 2007;22(4):1136–1143. doi: 10.1093/ndt/gfl711. [DOI] [PubMed] [Google Scholar]
- 3.Garovic VD, Wagner SJ, Turner ST, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196(4):320.e1–320.e7. doi: 10.1016/j.ajog.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Ushiyama C, Suzuki S, et al. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15(9):1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Ushiyama C, Suzuki S, et al. Urinary podocytes for the assessment of disease activity in lupus nephritis. Am J Med Sci. 2000;320(2):112–116. doi: 10.1097/00000441-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16(6):1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- 7.Foster RR, Hole R, Anderson K, et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol. 2003;284(6):F1263–F1273. doi: 10.1152/ajprenal.00276.2002. [DOI] [PubMed] [Google Scholar]
- 8.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S, Gu Y, Lewis DF, Wang Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29(1):81–88. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–8884. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 12.Ruotsalainen V, Ljungberg P, Wartiovaara J, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96(14):7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10(1):1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Doublier S, Salvidio G, Lupia E, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52(4):1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 15.Kestilä M, Lenkkeri U, Männikkö M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 16.de Zoysa JR, Topham PS. Podocyte biology in human disease. Nephrology. 2005;10(4):362–367. doi: 10.1111/j.1440-1797.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC. GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J Biol Chem. 1994;269(31):19953–19962. [PubMed] [Google Scholar]
- 18.Wharram BL, Goyal M, Gillespie PJ, et al. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest. 2000;106(10):1281–1290. doi: 10.1172/JCI7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugo C, Nangaku M, Shankland SJ, et al. The plasma membrane-actin linking protein, ezrin, is a glomerular epithelial cell marker in glomerulogenesis, in the adult kidney and in glomerular injury. Kidney Int. 1998;54(6):1934–1944. doi: 10.1046/j.1523-1755.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 20.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108(11):1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285(1):F40–F48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. New Eng J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto H, Hamano Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278(15):12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 24.Buhimschi CS, Magloire L, Funai E, et al. Fractional excretion of angiogenic factors in women with severe preeclampsia. Obstet Gynecol. 2006;107(5):1103–1113. doi: 10.1097/01.AOG.0000207698.74104.4f. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in preeclampsia. Placenta. 2001;22(2–3):206–212. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- 26.Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension. 1996;28(3):488–493. doi: 10.1161/01.hyp.28.3.488. [DOI] [PubMed] [Google Scholar]
- 27.Neale TJ, Ojha PP, Exner M, et al. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest. 1994;94(4):1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng S, Carlson EC, Yang L, Kralik PM, Huang Y, Epstein PN. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol. 2008;19(11):2077–2085. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spurney RF, Coffman TM. Stressed-out podocytes in diabetes? J Am Soc Nephrol. 2008;19(11):2035–2037. doi: 10.1681/ASN.2008090955. [DOI] [PubMed] [Google Scholar]