Abstract
Neuromodulation of synaptic plasticity by 17β-estradiol (E2) is thought to influence information processing and storage in the cortex and hippocampus. Because E2 rapidly affects cortical memory and synaptic plasticity, we examined its effects on phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII), extracellular signal-regulated kinase (ERK), and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) [AMPA-type glutamate receptor subunit 1 (GluR1 subunit)], all of which are important for the induction and maintenance of synaptic plasticity and memory. Acute E2 treatment resulted in an increased temporal and spatial phosphorylation pattern of CaMKII, ERK, and AMPAR (GluR1 subunit). By using inhibitors, we were able to attribute GluR1 phosphorylation to CaMKII at serine 831, and we also found that E2 treatment increased GluR1 insertion into the surface membrane. Because soluble amyloid-beta (Aβ) oligomers inhibit CaMKII and ERK activation, which is necessary for synaptic plasticity, we also tested E2’s ability to ameliorate Aβ-induced dysfunction of synaptic plasticity. We found that estrogen treatment in neuronal culture, slice culture, and in vivo, ameliorated Aβ oligomer-induced inhibition of CaMKII, ERK, and AMPAR phosphorylation, and also ameliorated the Aβ oligomer-induced reduction of dendritic spine density in a CaMKII-dependent manner. These phosphorylation events are correlated with the early stage of inhibitory avoidance learning, and our data show that E2 improved inhibitory avoidance memory deficits in animals treated with soluble Aβ oligomers. This study identifies E2-induced signaling that attenuates soluble Aβ peptide-mediated dysfunction of pathways in synaptic plasticity.
Keywords: 17β-estradiol, CaMKII, ERK, GluR1, synaptic plasticity
1. Introduction
Activity-dependent rapid structural and functional modulations of excitatory synapses contribute to synapse formation, experience-dependent plasticity, learning, and memory. In forebrain pyramidal neurons, induction of long-term potentiation (LTP) causes rapid enlargement of spine heads and simultaneous delivery of AMPAR (GluR1 subunits) into spines (Engert and Bonhoeffer, 1999; Kopec et al., 2006; Matsuzaki et al., 2004). Similar modifications have been observed in the cortex in response to LTP (Connor et al., 2006) and experience (Takahashi et al., 2003). These results provide the molecular mechanism for the structural basis of LTP in dendritic spines from both the hippocampus and cortex. Furthermore, it is known that NMDAR and L-type VGCC-dependent activation of CaMKII is necessary for both structural (increased spine density and enlargement of spine heads) and functional (induction and maintenance of LTP) synaptic plasticity [Matsuzaki et al., 2004; Lee et al., 2009].
Plasticity at synapses can be regulated at presynaptic sites by changing the release of neurotransmitters, or postsynaptically by changing the number or properties of neurotransmitter receptors. It has been shown that several activity-driven phosphorylation events at the C-terminus of GluR1 by protein kinase A (PKA) at serine 845 (Roche et al., 1996) and by CaMKII and protein kinase C (PKC) at serine 831 (Boehm et al., 2006) facilitate synaptic AMPAR delivery (Esteban et al., 2003; Song and Huganir, 2002). Therefore, monitoring phosphorylation and AMPAR trafficking provides an effective means to study cognitive function and dysfunction in animal models.
Modulation of synaptic plasticity is necessary for information processing and storage in hippocampal as well as cortical networks (Marder and Thirumalai, 2002). Recently, estrogen’s neuromodulatory role has been documented by showing that E2 treatment enhanced glutamate release via rapid nongenomic action of PI3K in hypothalamic presynaptic neurons and enhanced dendritic spine formation (Schwarz et al., 2008). Moreover, the neuromodulatory role of E2 is thought to occur through local estrogen formation in the pyramidal cells of the hippocampus and neocortex, thus affecting the functions of excitatory synapses (Yague et al., 2008). E2-induced signaling increases spine density, neuronal network connectivity, and synaptic transmission (Woolley, 2007; Spencer et al., 2008). Several mechanisms have been identified by which E2 may modulate synaptic plasticity. E2 has been shown to potentiate L-type VGCC (Sarkar et al., 2008) and enhance NMDAR-mediated synaptic activity and LTP (Smith et al., 2005). E2 has also been shown to activate CaMKII (Sawai et al., 2002). Because NMDAR and AMPAR transmission and LTP expression occur within minutes after E2 application, these effects of E2 are not believed to be mediated via estrogen receptor-dependent genomic actions.
Activation of synaptic plasticity related kinases and phosphorylation of synaptic proteins are the targets of Aβ. Soluble synthetic Aβ oligomers and dimers isolated from Alzheimer’s patients, decrease cell surface expression of NMDAR and AMPAR, inhibit LTP, inhibit phosphorylation of CaMKII, ERK, and GluR1, and decrease spine density (Snyder et al., 2005; Shrestha et al., 2006). Spine loss is prevented by Aβ specific antibody or a small molecule modulator of Aβ aggregation, scyllo-inositol (AZD 103) [Shankar et al., 2007]. In an effort to understand whether E2-induced signaling is mechanistically linked to synaptic plasticity, we have determined the phosphorylation patterns of CaMKII, ERK, and GluR1 subunit in cortical and hippocampal neurons following treatment with E2. Because the phosphorylation state of these proteins is down-regulated by soluble Aβ oligomers, which is linked to a decrease in spine density, we reasoned that E2 may ameliorate Aβ-induced dysfunction of synaptic plasticity. Our results show that estrogen treatment in neuronal culture, slice culture, and in vivo, ameliorates Aβ oligomer-induced inhibition of CaMKII, ERK, and AMPAR phosphorylation, and also ameliorates the Aβ oligomer-induced reduction of dendritic spine density in a CaMKII-dependent manner. These results suggest that estrogen has the potential to prevent Aβ oligomer-induced synaptic dysfunction.
2. Results
2.1. Estrogen Rapidly Increased Phosphorylation of CaMKII, ERK, and GluR1 in Primary Neuronal Culture
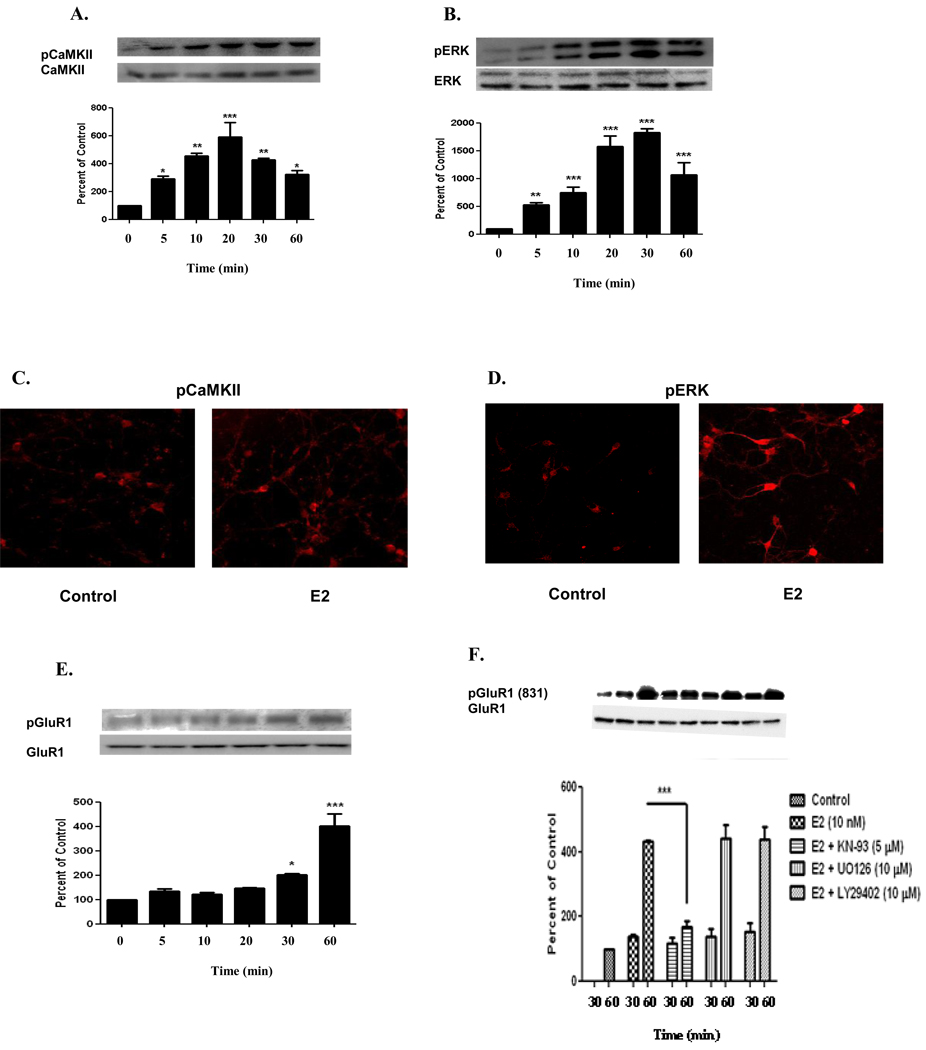
Estrogen has been shown to exert acute affects on synaptic physiology involving synaptic plasticity related kinases such as CaMKII (Sawai et al., 2002) and ERK in primary hippocampal neurons (Lee et al., 2004). Little is known about the temporal regulation of these kinases in cortical neurons. Therefore, we investigated the phosphorylation state of these kinases at different time points. E2 (10 nM) treatment in primary cortical neurons resulted in a steady increase of normalized pCaMKII immunoreactivity from basal, starting 5 min after E2 treatment and with its maximum at 20 min (592 ± 183%, compared to control, n=3, p<0.001) [Figure 1A], followed by a decline in phosphoryation at 30 min (427 ± 27%, compared to control, n=3, p<0.01) and 60 min (324 ± 50%, compared to control, n=3, p<0.05) [Figure 1A]. Next we studied ERK activity by measuring its phosphorylation state. E2 (10 nM) treatment also resulted in a steady increase of normalized pERK immunoreactivity from basal, starting 5 min after E2 treatment and with its maximum at 30 min (1827 ± 73%, compared to control, n=3, p<0.001) [Figure 1B], followed by a decline in phosphorylation at 60 min (1074 ± 204%, compared to control, n=3, p<0.001). To further investigate the phosphorylation state of CaMKII and ERK, we determined the spatial distribution of these kinases in primary cortical neurons. Immunoflorescent micrograph showed that E2 treatment for 20 and 30 min increased phosphorylation of CaMKII and ERK, respectively. This increased phosphorylation was visible in both the cell body and membrane of the dendrites (Figure 1C and 1D).
FIGURE 1. Estrogen Induces Phosphorylation of CaMKII, ERK, and GluR1 in Primary Neuronal Culture.
E2 (10 nM) temporally regulated the phosphorylation of CaMKII, ERK, and GluR1 in primary cortical neurons. A) Phosphorylation was seen within 5 min and was maximum at 20 min for CaMKII (592 ± 183%), while B) ERK phosphorylation was seen within 5 min but was maximum at 30 min (1827 ± 73%). E2 also induced phosphorylation of CaMKII and ERK in the cell body and dendritic extensions of primary cortical neurons. Cortical neurons untreated or treated with E2 (10 nM) for either 20 min or 30 min were labeled with C) phospho-CaMKII or D) phospho-ERK, respectively, and visualized by fluorescence microscopy. E) E2 (10 nM) temporally regulated the phosphorylation of GluR1 in primary cortical neurons. GluR1 was phosphorylated at 30 min but was maximal at 60 min (403 ± 88%). F) Primary cortical neurons were treated with E2 (10 nM) in the presence or absence of CaMKII inhibitor (KN-93, 5 µM), MEK inhibitor (UO126, 10 µM), and PI3K inhibitor (LY29402, 10 µM) for 30 and 60 min. Induction of phosphorylation of GluR1 at serine 831 was inhibited by KN-93 (169 ± 19%) but not U0126 (442 ± 40%) and LY29402 (401 ± 40%) compared to 60 min vehicle-treated (DMSO) control. Data are mean ± SD. *, p<0.05, **, p<0.01, ***, p<0.001, versus time 0 or 30 min or groups connected by bars as determined by one-way ANOVA followed by Newman-Keuls Multiple Comparison Test or two-way ANOVA followed by Bonferroni Test, n=3 (3 independent experiments).
We next sought to determine whether E2-induced activation of CaMKII can increase phosphorylation of one of its substrates, GluR1 (CaMKII site serine 831). Using serine 831 pGluR1 antibody we showed that E2 treatment in primary cortical neurons lead to the increase of GluR1 phosphorylation starting at 30 min (204 ± 20%, compared to control, n=3, p<0.05) and phosphorylation was maximal at 60 min (403 ± 88%, compared to control, n=3, p<0.001) [Figure 1E].
To determine which of the activated kinases (CaMKII, ERK, and/or PI3K) are necessary for the E2-induced increase in phosphorylation of GluR1, we used primary cortical neuronal culture and treated with a CaMKII inhibitor (KN-93), a MEK inhibitor (U0126), or a PI3K inhibitor (LY29402). Treatment with KN-93 for 30 min resulted in no inhibition of phosphorylation of GluR1 (E2, 140 ± 6%, compared to E2 and KN-93, 120 ± 16%), whereas, KN-93 treatment for 60 min severely inhibited phosphorylation of GluR1 at its CaMKII site (serine 831) [E2, 431 ± 24%, compared to E2 and KN-93, 169 ± 19%, n=3, p<0.001] (Figure 1F). The MEK inhibitor, U0126, had no inhibitory effect on the phosphorylation of GluR1 as shown in Figure 1F (E2, 431 ± 24%, compared to E2 and U0126, 442 ± 40%). The PI3K inhibitor, LY29402, was similar to that of U0126, in that it too had no inhibitory effect on the phosphorylation of GluR1 (Figure 1F) [E2, 431 ± 24%, compared to E2 and LY29402, 401 ± 40%].
2.2. Acute Estrogen Treatment Increased Phosphorylation of CaMKII, ERK, and GluR1 in vivo
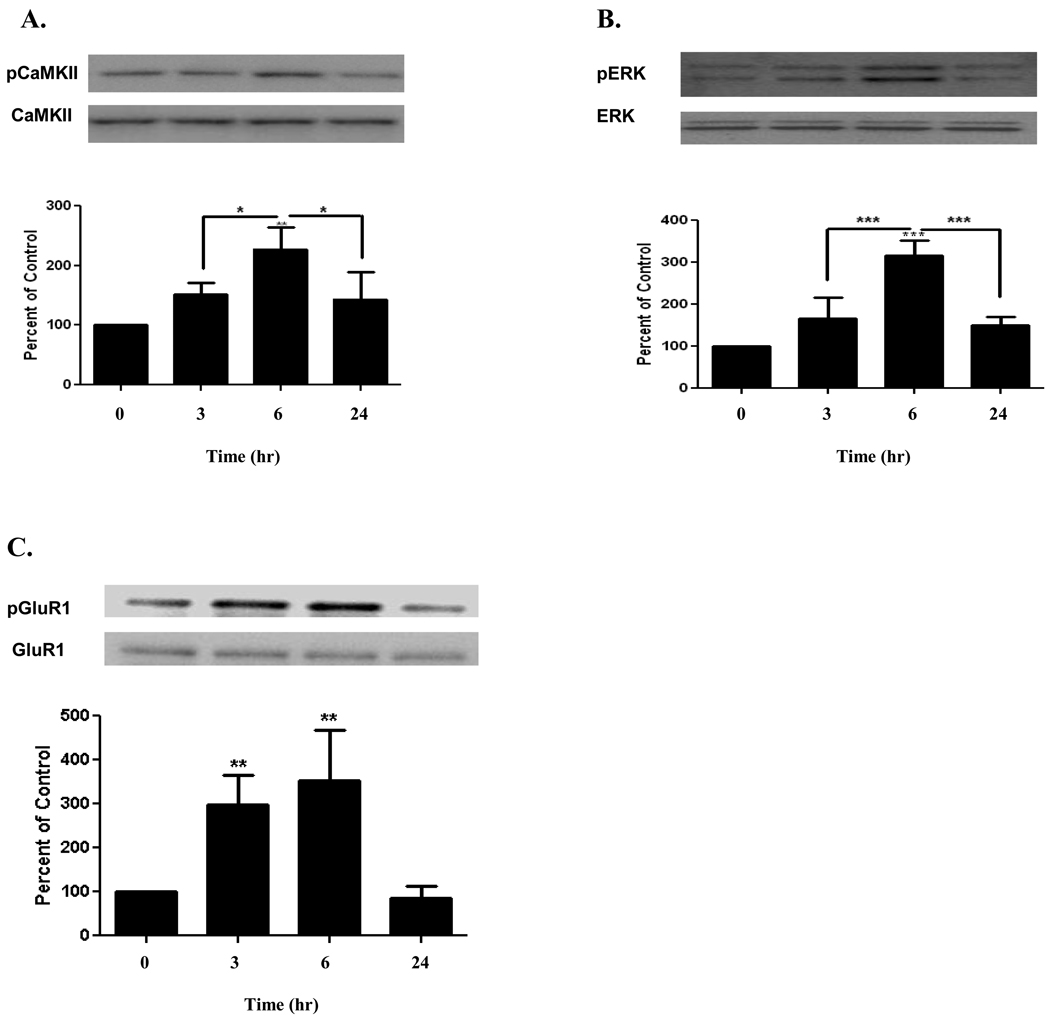
We next asked whether acute E2 treatment would have a similar effect on the phosphorylation state of CaMKII, ERK, and GluR1 in vivo as observed in vitro. For the in vivo studies, E2 was injected subcutaneously (320 µg/kg) in ovariectomized rats and the phosphorylation state of CaMKII, ERK, and GluR1 in the cortex was determined at 3, 6, and 24 hrs. The results for normalized pCaMKII identified an increase from basal level at 3 hrs (152 ± 19%), with its maximum at 6 hrs (227 ± 37%, compared to control, n=3, p<0.01), followed by return to basal level at 24 hrs (143 ± 46%) [Figure 2A]. For pERK, phosphorylation was highest at 6 hrs (316 ± 36%, compared to control, n=3, p<0.001), followed by a return to basal level at 24 hrs (150 ± 20%) [Figure 2B]. In the case of GluR1 in vivo, phosphorylation was maximum at 6 hrs (353 ± 116%, compared to control, n=3, p<0.01) and returned to basal level at 24 hrs (85 ± 28%) [Figure 2C].
FIGURE 2. Estrogen Induces Phosphorylation of CaMKII, ERK, and GluR1 In vivo.
Subcutaneous injection of E2 (320 µg/kg) in ovariectomized rats significantly increased phosphorylation of A) CaMKII, B) ERK, and C) GluR1, 6 hrs after injection (227 ± 37%, 316 ± 36%, and 353 ± 116%, respectively), and all returned to their respective basal levels at 24 hrs. Data are mean ± SD. *, p<0.05, **, p<0.01, ***, p<0.001, versus time 0 or groups connected by bars as determined by one-way ANOVA followed by Newman- Keuls Multiple Comparison Test, n=3 (3 independent experiments).
2.3. Soluble Aβ1–42 Oligomers Abrogated and E2 Ameliorated Phosphorylation of GluR1 at its CaMKII Site in Primary Cortical Neurons
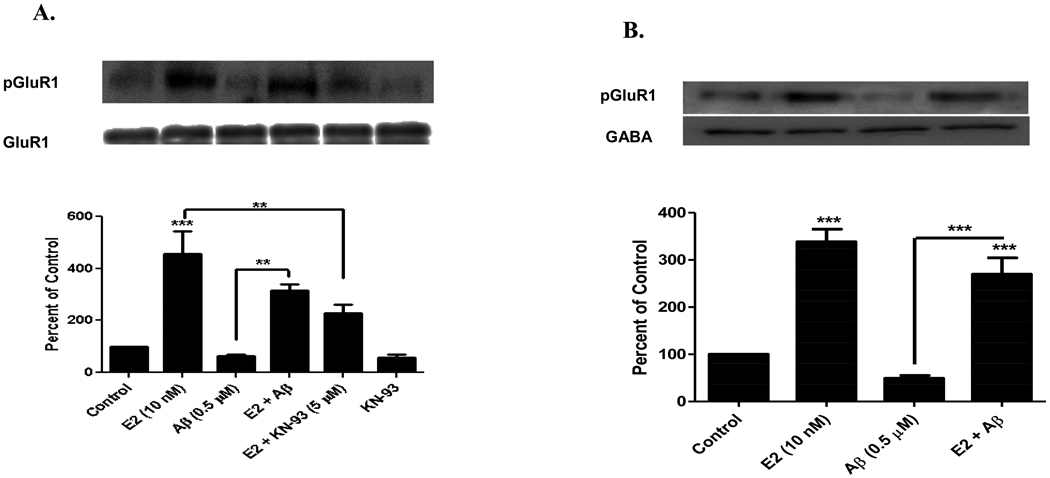
It has been reported that soluble Aβ1–42 treatment in hippocampal neurons, inhibits phosphorylation of both CaMKII and GluR1 (serine 831 site) [Zhao et al., 2004]. To determine the effect of Aβ in cortical neurons, we analyzed the phosphorylation state of GluR1 at its CaMKII site. As shown in Figure 3A, E2 (10 nM) treatment increased phosphorylation of GluR1 (455 ± 152%, compared to control, n=3, p<0.001). E2 (10 nM) exposure for 1 hr to Aβ (0.5 µM, 24 hrs.) pretreated primary cortical neurons and in the presence of Aβ, ameliorated Aβ-induced inhibition of phosphorylation of GluR1 (E2 and Aβ, 317 ± 38%, compared to Aβ, 63 ± 10%, n=3, p<0.01) [Figure 3A]. Figure 3A also shows that E2-induced phosphorylation of GluR1 was inhibited by CaMKII inhibitor (KN-93) [E2, 455 ± 152%, compared to E2 and KN-93, 218 ± 55%, n=3, p<0.01].
FIGURE 3. E2 Ameliorates Aβ-induced Inhibition of GluR1 Phosphorylation and Decreased GluR1 Membrane Insertion.
A) E2 ameliorates Aβ-induced inhibition of GluR1 phosphorylation in primary cortical neurons. Primary cortical neurons (E18) were grown 15 DIV and were pretreated with soluble Aβ1–42 oligomers (0.5 µM) for 24 hrs. Neurons were then treated with or without E2 (10 nM) for 1 hr in the presence or absence of KN-93. Aβ treatment decreased GluR1 phosphorylation (63 ± 10%), while E2 ameliorated Aβ’s effect (317 ± 38%). E2-induced phosphorylation was inhibited by KN-93 (218 ± 55%). B) GluR1 insertion into the membrane of primary cortical neurons. Primary cortical neurons (E18) were grown 15 DIV and were pretreated with soluble Aβ1–42 oligomers (0.5 µM) for 24 hrs. E2 (10 nM) treatment for 1 hr increased surface expression of pGluR1 (serine 831) and Aβ treatment inhibited this surface expression (338 ± 46% and 49 ± 10%, respectively). The surface expression of pGluR1 (serine 831) was normalized with surface expression of GABA receptor. E2 treatment ameliorated Aβ-induced inhibition of GluR1 insertion into the membrane of cortical neurons (270 ± 17%). Data are mean ± SD. **, p<0.01, ***, p<0.001, versus control or groups connected by bars as determined by one-way ANOVA followed by Newman-Keuls Multiple Comparison Test, n=3 (3 independent experiments).
2.4. E2 Prevented and Soluble Aβ1–42 Inhibited GluR1 Trafficking in Primary Cortical Neurons
It has been previously documented that activated CaMKII can modulate synaptic plasticity by enhancing AMPAR channel conductance via GluR1 phosphorylation at its CaMKII site (Barria et al., 1997) and by delivering AMPAR to the synapse (Poncer et al., 2002). Thus we sought to determine whether E2 treatment prevents Aβ1–42 inhibition of GluR1 insertion into the surface membrane. We used the surface labeling technique of biotinylation in primary cortical neurons to quantify GluR1 surface expression. As shown in Figure 3B, E2 (10 nM, 1 hr) treatment increased surface expression of GluR1 (338 ± 46%, compared to control, n=3, p<0.001). Aβ-induced inhibition of GluR1 insertion was ameliorated by E2 treatment (E2 and Aβ, 270 ± 17%, compared to Aβ, 49 ± 10%, n=3, p<0.001) [Figure 3B].
2.5. Aβ Oligomers Inhibited and Acute E2 Ameliorated Phosphorylation of CaMKII, ERK, and GluR1 in Hippocampal Neurons
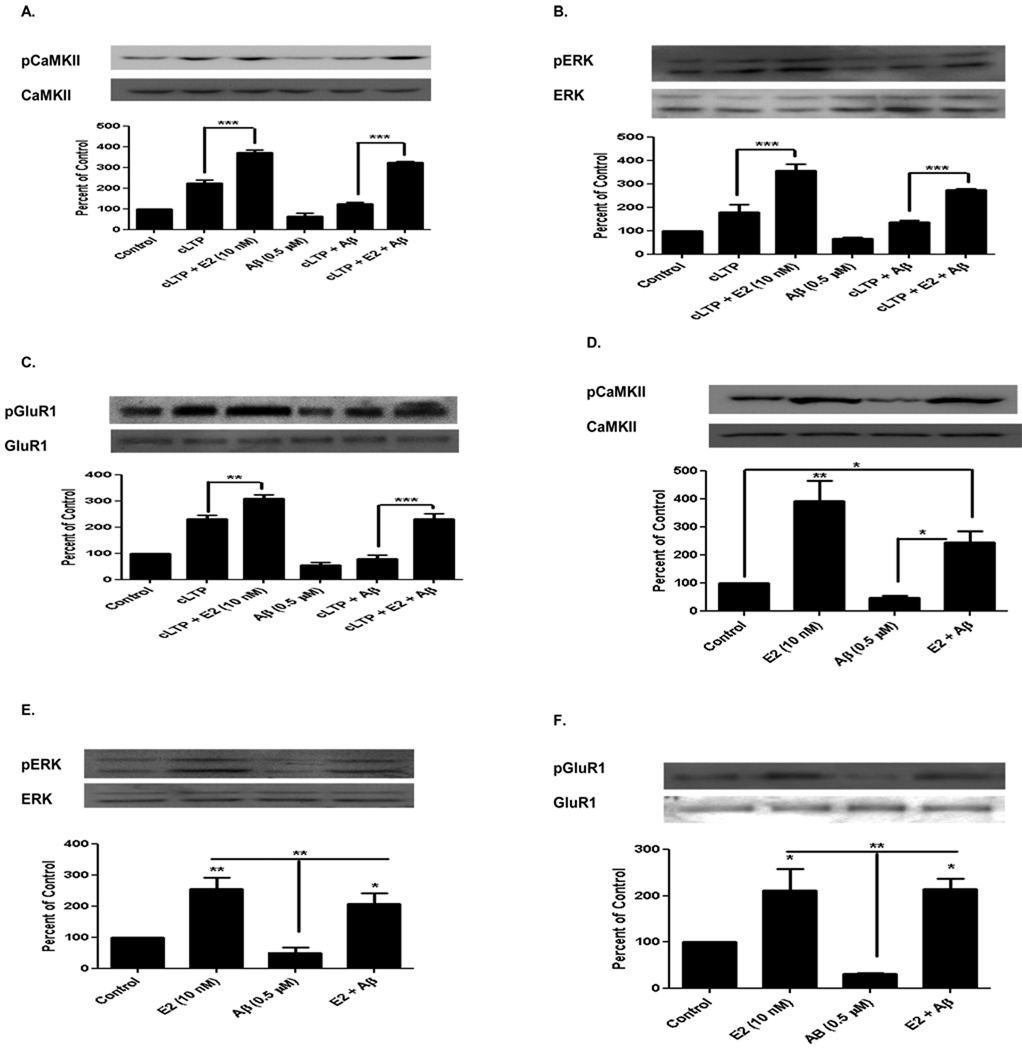
It has been shown that soluble Aβ treatment disrupts activation of CaMKII, ERK, and Akt/PKB in chemically-induced LTP (cLTP) in hippocampal neurons (Townsend et al., 2007). We report here that acute hippocampal slices prepared from normal mice showed Aβ-induced inhibition of cLTP-induced phosphorylation of CaMKII (cLTP, 223 ± 25%, compared to cLTP and Aβ, 124 ± 14, n=3, p<0.001, Figure 4A). E2 treatment increased phosphorylation of CaMKII (cLTP and E2, 373 ± 30%, compared to cLTP, 223 ± 25%, n=3, p<0.001), and E2 treatment ameliorated Aβ mediated cLTP-induced inhibition of phosphorylation of CaMKII (cLTP, E2, and Aβ, 325 ± 50%, compared to cLTP and Aβ, 64 ± 24%, n=3, p<0.001). Similar inactivation patterns were seen with ERK when compared to CaMKII, as shown in Figure 4B (cLTP, E2, and Aβ, 274 ± 20%, compared to cLTP and Aβ, 68 ± 8%, n=3, p<0.001). AMPAR (GluR1 subunit) at the CaMKII phosphorylation site (serine 831) but not the PKA phosphorylation site (serine 845), is known to be required for LTP induction and memory formation (Lee et al., 2000 and 2003; Whitlock et al., 2006). cLTP increased phosphorylation of GluR1 at the CaMKII site (Figure 4C, 231 ± 25%, compared to control 100%, n=3, p<0.001). Aβ inhibited cLTP-induced phosphorylation (cLTP and Aβ, 78 ± 25%, compared to cLTP, 231 ± 25%, n=3, p<0.001), and E2 ameliorated Aβ-mediated cLTP-induced inhibition of GluR1 phosphorylation (Figure 4C, cLTP, E2, and Aβ, 232 ± 34%, compared to cLTP and Aβ, 78 ± 25%, n=3, p<0.001).
FIGURE 4. Aβ1–42 Oligomers Inhibit and Acute E2 Ameliorates Phosphorylation of CaMKII, ERK, and GluR1 in Hippocampal Neurons.
E2 (10 nM) showed a significant increase in phosphorylation of CaMKII (Figure 4A), ERK (Figure 4B), and GluR1 (Figure 4C), but Aβ inhibited the induction of their phosphorylation (Figure 4A–4C). E2 ameliorated Aβ-mediated cLTP-induced inhibition of phosphorylation of these proteins. In vivo Aβ oligomer (0.5 µM) exposure to the hippocampus by icv injection to ovariectomized rats for 24 hrs inhibited the phosphorylation of CaMKII (Figure 4D), ERK (Figure 4E), and GluR1 (Figure 4F). Subcutaneous E2 treatment (320 µg/kg) 24 hrs later for 3 hrs, ameliorated Aβ-mediated inhibition of phosphorylation of these proteins. Data are mean ± SD. * p<0.05, ** p<0.01, *** p<0.001, versus control or groups connected by bars as determined by one-way ANOVA followed by Newman-Keuls Multiple Comparison Test, n=3 (3 independent experiments).
In vivo studies revealed that like in hippocampal slices, E2 increased the phosphorylation of CaMKII (366 ± 60%, compared to control 100%, n=3, p<0.01), ERK (256 ± 62%, compared to control 100%, n=3, p<0.01), and GluR1 (211 ± 49%, compared to control 100%, n=3, p<0.01). E2 was also able to ameliorate the inhibitory effects of Aβ1–42 oligomers (CaMKII, 244 ± 69%, ERK, 207 ± 45%, GluR1, 200 ± 37%, compared to Aβ, n=3, p< 0.5 and p<0.01) [Figure 4D, 4E, and 4F].
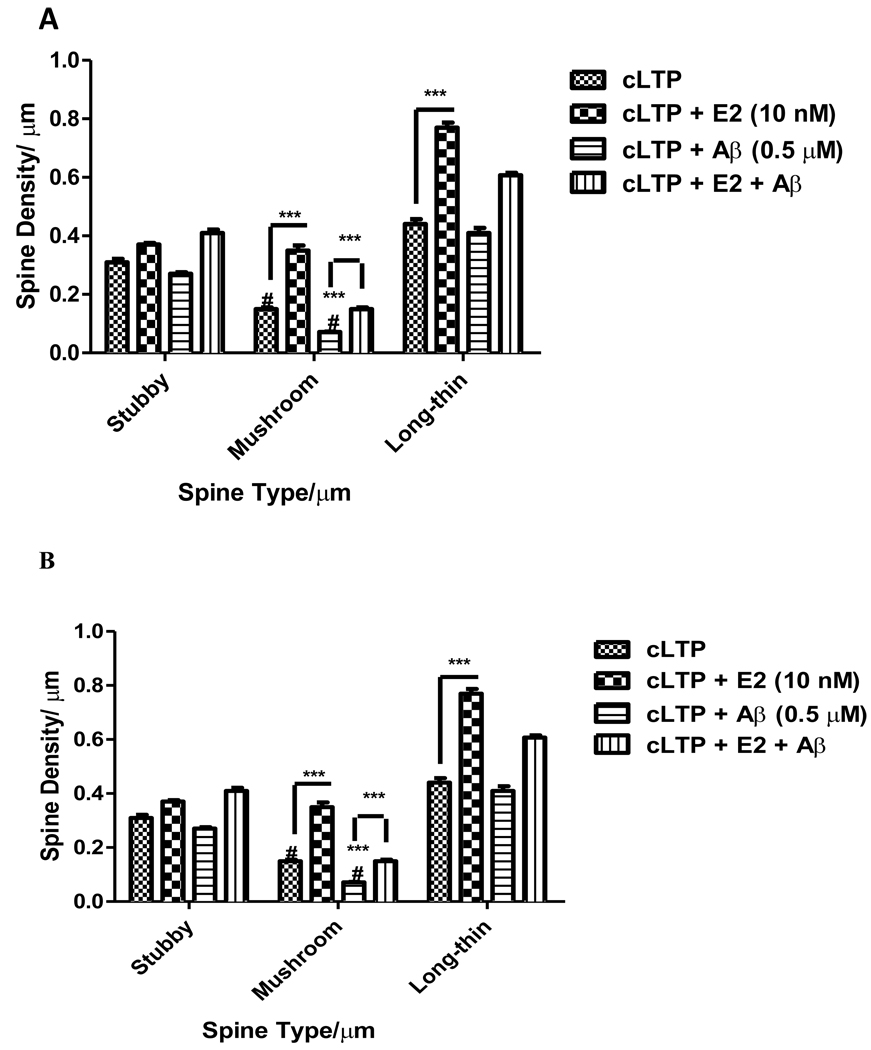
2.6. Aβ Oligomers Decreased and E2 Ameliorated cLTP-induced Mushroom-type Spine Density
To examine whether E2-mediated activation of CaMKII and/or increased phosphorylation of GluR1 correlates with the changes in Aβ-induced synaptic architecture and/or synaptic loss, we analyzed spine morphology and spine density in adeno-associated virus containing green florescent protein (AAV2-GFP)-infected pyramidal neurons from organotypic mouse hippocampal slices (Figure 5A). Aβ1–42 exposure reduced by 50% number of mushroom-type spines without affecting total spine density in cLTP activated pyramidal neurons. E2 treatment increased both mushroom-type and long-thin spines compared to cLTP. Aβ-mediated cLTP-induced inhibition of mushroom-type spine number was ameliorated by E2 treatment. Taken together, our results show that E2 ameliorates Aβ-induced decreased phosphorylation of CaMKII, ERK, and GluR1, and spine density.
FIGURE 5. Effects of Aβ1–42 Oligomers and E2 on Hippocampal Spine Density and Spine Type.
A) E2 (10 nM) increased mushroom- and long-thin-type spine density [0.35 ± 0.03 spines/µm and 0.77 ± 0.035 spines/µm, respectively]. Soluble Aβ1–42 oligomer (0.5 µM) treatment reduced mushroom-type spine density (0.07 ± 0.005 spines/µm). E2 (10 nM) treatment ameliorates Aβ-induced mushroom-type spine density loss (0.15 ± 0.01 spines/µm). B) KN-93, but not U0126, inhibits an E2-induced increase in mushroom-type spine density (E2, 0.59 ± 0.02 spines/µm, E2 and KN-93, 0.20 ± 0.02 spines/µm). Data are mean ± SD. ***, p<0.001, versus control or groups connected by bars as determined by two-way ANOVA followed by Bonferroni Test, n=6 (3 independent experiments).
Next, by using CaMKII and ERK inhibitors, we studied the mechanism by which E2 ameliorated Aβ-induced decreases in spine density. In the presence of E2, both mushroom-type and long-thin spine density is increased, while total number of spines remains unaffected (Figure 5B). Aβ reduced mushroom-type spines and E2 ameliorated this decline (Figure 5B). KN-93, a potent CaMKII inhibitor, but not MEK inhibitor U0126, inhibited the E2-induced increase in mushroom-type spine density. Induced CaMKII activation appears to be the primary mechanism by which E2 ameliorates Aβ-induced decrease in spine density. Previously we have shown that E2 directly potentiates L-type VGCC in hippocampal neurons (Sarkar et al., 2008). Thus, our results raise the possibility that E2 by potentiating L-type VGCC activates CaMKII and ERK at the synapse, which has the potential to ameliorate Aβ-induced synaptic dysfunction.
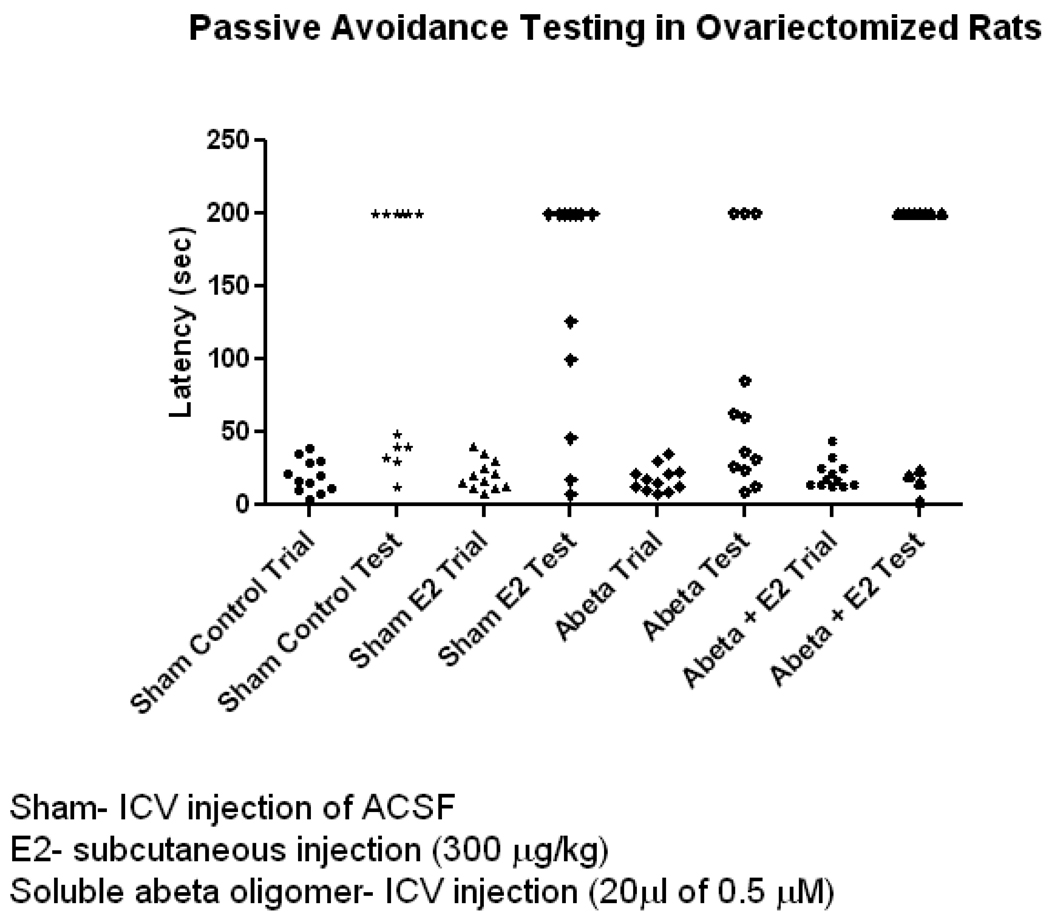
2.7. E2 Treatment Prevented Soluble Aβ Oligomer-induced Impairment of Inhibitory Avoidance Memory in Ovariectomized Rats
Increased phosphorylation of CaMKII and GluR1 in the hippocampus correlates with the early stage of IA learning memory formation (Cammarota et al., 1998; Whitlock et al., 2006). Additionally, recent findings demonstrate that icv injection of soluble Aβ oligomers isolated from AD patients impair IA memory (Townsend et al., 2007). In an effort to demonstrate estrogen’s ameliorating effect on Aβ-induced inhibition of phosphorylation of CaMKII and GluR1 and how this correlates with improvement of memory deficits caused by Aβ, we measured the IA memory performance of ovariectomized rats. Aβ treatment showed impairment of IA memory and this impairment was reduced as a result of E2 treatment. The latency differences between trial and test groups were all statistically significant as analyzed by one way ANOVA with non parametric Kruskal-wallis test and Dunns post test (n=12, p<0.05) but not the pair wise tests.(n=12, p>0.05). However unpaired t-test with 95% confidence interval analysis revealed that Aβ treatment in the presence of E2 showed increased latency and was statistically significant (79 ± 22% trial compared to 138 ± 26% test, n=12, one-tailed p value p<0.05) [Figure 6]. This is evident by counting the no of animals in the test with 200 sec latency as is shown in the figure and described here. The numbers are control:6, E2 :7, Aβ: 3, Aβ+E2: 8. Because increased latency signifies better IA memory formation, these results can be interpreted as E2 improved IA memory deficits in animals treated with soluble Aβ oligomers.
FIGURE 6. Effects of E2 and Soluble Aβ Oligomers Administered Alone or in Combination on Inhibitory Avoidance Memory in Ovariectomized Rats.
Columns depict latency taken to enter the dark chamber before and after foot shock (trial and test, respectively). 24 hrs after icv injection of ACSF (sham treatment) animals were trained and IA memory task was tested 3 hrs after foot shock (column 1 trial, column 2 test). E2 was subcutaneously injected (300 µg/kg) 1 hr before the trail and animals were tested 3 hrs after the trial [column 3 and 4]. 24 hrs after icv injection of Aβ1–42 oligomers (0.5 µM) memory task was tested as described above (column 5 trial, column 6 test). For treatment with Aβ and E2 the above protocol was again followed (column 7 trial, column 8 test). Data are mean ± SEM. *, p<0.05, versus groups connected by bars as determined by unpaired t test, n=12 (12 animals per group).
3. Discussion
E2-induced increases in structural and functional plasticity are associated with learning and memory, whereas, decreases are associated with cognitive dysfunction (Sinopoli et al., 2006; Spencer et al., 2008). Structural and functional synaptic plasticity dysfunction have been implicated as the neuropathological correlates of severity of cognitive dysfunction in AD (Terry et al., 1991; Shankar et al., 2008). The signaling mechanism by which E2 modulates synaptic plasticity at the postsynaptic cortical neuron has not been well studied. We therefore investigated E2-induced signaling pathways and asked which signaling mechanism is responsible for linking structural and functional synaptic plasticity. We also asked whether soluble Aβ -induced dysfunction of signaling pathways involved in synaptic plasticity can be attenuated by E2 treatment in cortical neurons.
Here we used a cortical and hippocampal neuronal system to elucidate the molecular components involved in E2-induced rapid signaling pathways which link structural and functional plasticity. After first determining that E2 was able to induce the activation of both CaMKII and ERK, we assessed the possibility that either or both could be involved in linking structural and functional plasticity. We next identified that activation of CaMKII is the signaling mechanism for the E2-mediated increase in GluR1 phosphorylation. Reduction in CaMKII activity by CaMKII inhibitor, KN-93, resulted in a decrease in the phosphorylation of GluR1.
Previous studies have shown that E2 modulates LTP (Cordoba Montoya and Carrer, 1997; Foy et al., 1999) in the hippocampal region and increases CaMKII activation (Sawai et al., 2002). It is also well known that NMDAR and L-type VGCC activation of CaMKII is necessary for both structural (spine growth and spine number) and functional (induction and/or expression of LTP) plasticity in hippocampal neurons (Lee et al., 2009). Previously we have shown that estrogen directly potentiates L-type VGCC (Sarkar et al., 2008). Therefore, E2 by potentiating L-type VGCC in cortical neurons may activate CaMKII which in turn phosphorylates GluR1 and modulates LTP induction and/or expression. LTP-inducing stimuli cause the formation of new spines and enlargement of existing spines (Matsuzaki et al., 2004; Nagerl et al., 2004; Okamoto et al., 2004). Insertion of more AMPAR (containing GluR1 subunits) to activated synapses is known to be critical for LTP induction and/or maintenance (Malinow and Malenka, 2002). Phosphorylation of GluR1 by CaMKII also modulates synaptic plasticity by delivering AMPAR to the synapse (Poncer et al., 2002). Our surface biotinylation studies revealed that E2 increased surface levels of GluR1 phosphorylated at serine 831. These findings are consistent with studies showing that serine 831 phosphorylation is critical for regulating subcellular trafficking of GluR1 (Liao et al., 2001). Also, our results raise the intriguing possibility that AMPAR can be modified and recruited rapidly to silent synapses via the E2-induced signaling mechanism in spontaneously activated primary cortical neurons.
Acute application of soluble Aβ oligomers impaired CaMKII, ERK, and Akt/ protein kinase B (PKB) activation in mature hippocampal culture (Townsend et al., 2007). Furthermore, soluble Aβ dimers isolated directly from the cerebral cortex of patients with AD, potently inhibited LTP and reduced dendritic spine density (Shankar et al., 2008). As these activated kinases are key players for induction and/or maintanience of LTP as well as spine growth, inhibition of these processes by soluble Aβ oligomers could lead to dysfunction of structural and functional synaptic plasticity. Because soluble Aβ oligomers inhibited CaMKII activation, blocked onset of LTP, and inhibited phosphorylation of GluR1 at its CaMKII, we tested the possibility that E2 can ameliorate Aβ-induced inhibition of CaMKII-dependent phosphorylation of GluR1. Our data substantiate this possibility, providing direct evidence that E2 has the potential for ameliorating Aβ-induced inhibition of CaMKII mediated phosphorylation and loss of GluR1 surface expression. Electronmicroscopic studies show a positive correlation between spine size, synapse number, and synaptic AMPAR number (Takumi et al., 1999). Thus E2-induced increments of surface expression of GluR1 may be mechanistically linked to new spine formation. Recent findings show that increased phosphorylation of CaMKII and GluR1, in addition to rapid stabilization, accumulation, and enlargement of dendritic spines (Roberts et al., 2010), occur at the onset of behavioral learning. Soluble Aβ inhibits many of these synaptic plasticity related events (Townsend et al., 2007). Mechanistically, we show that E2 by ameliorating Aβ-induced inhibition of phosphorylation of CaMKII and GluR1, as well as inhibition of accumulation of mushroom-type dendritic spines, improves IA memory deficits in ovariectomized rats treated with soluble Aβ oligomers. The particular mechanism (s) by which E2 attenuates soluble Aβ-mediated dysfunction of synaptic plasticity is unknown. We and others have shown that primary hippocampal neurons express both ER-α and ER-β (Jelks KB et.al, 2007. Yang S-H et al. 2009). Also it has been reported that non genomic and very rapid activation of CaMKII is ER-dependent (Sawai T et al., 2002.). Moreover, in hippocampal neurons phosphorylation of GluR1 is induced by ER-β specific agonist (Liu et al., 2008). The identity of the ER involved in potentiation of synaptic plasticity and memory has not yet been fully confirmed. For example, in one report, ER-α but not ER-β (Ikeda M et al., 2008), yet in another report ER-β but not ER-α (Liu et al 2008.) regulates hippocampal synaptic plasticity and enhances cognitive performances in rodents. Previously we and other laboratory have shown that estrogen directly potentiates L-type VGCC (Sarkar et al., 2008 ; Wu et al., 2005). We hypothesize E2 by potentiating L-type VGCC in cortical as well as hippocampal neurons activate CaMKII which in turn phosphorylates GluR1 and modulates LTP induction and/or expression. LTP-inducing stimuli cause the formation of new spines.
Decades of research in both humans and other animals has provided evidence that E2 enhances synaptic plasticity and memory. Investigating E2-induced signaling molecules that target synaptic plasticity machinery will not only provide the molecular mechanisms of synaptic plasticity that correlate with memory, but will also provide a therapeutic strategy that may ameliorate age and AD-related dementia.
4. Experimental Procedures
4.1. Primary Neuronal Culture
At embryonic day 18 (E18), pregnant rats were anesthetized and cervically dislocated. The brains of pups were removed and placed into magnesium (Mg2+) free Hank’s balance salt solution (HBSS). Cortices and hippocampi were removed under a dissecting microscope, washed, and placed into neurobasal culture media (without phenol red) supplemented with B27 and pen-strep (all from Gibco, Carlsbad, CA). The cortices and hippocampi were triturated using a graded series of fine polished Pasteur pipettes, and then filtered through a 40 µm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). The neurons were plated on poly-L-lysine coated 100 mm dishes and glass coverslips, and cultured in vitro in 95% humidity and 5% CO2 atmosphere for 15 days. At day 2 cells were treated with 5 µM 1-beta-d-arabinofuranosylcytosine (AraC) to inhibit glial cell growth.
4.2. Immunocytochemistry
Cells were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 minutes. After a 1 hour block with 5% bovine serum albumin (BSA), coverslips were incubated with the appropriate primary antibody (anti-phospho-CaMKII; anti-phospho-ERK1/2) over night at 4°C, then washed with PBS (three 5 minute washes) and incubated with the appropriate secondary antibody (goat anti-mouse or goat anti-rabbit Alexa Fluor 633, Invitrogen, Carlsbad, California) for 1 hour, followed by 3 PBS washes. Samples were visualized using laser scanning confocal microscopy.
4.3. Surface Labeling
Cortical neurons, while still in the petry dish, were incubated with PBS containing 1.5 mg/ml EZ-link sulfo-NAS-LC-biotin (Pierce Biotechnology, Inc., Rockford, IL) for 20– 30 minutes at 4°C. Cells were removed from the dish, washed with PBS, and then centrifuged. Washed cells were lysed and the supernatant was incubated with immobilized neutravidin protein (Pierce Biotechnology, Inc., Rockford, IL) for 1 hour at room temperature. Protein beads were obtained by centrifugation and washed 3 times with PBS. Protein beads were dissolved in sodium dodecyl sulfate (SDS) sample buffer.
4.4. Slice Preparation
The hippocampus encompassing the CA3-CA1 region was dissected out and 360 µm thick slices were prepared from postnatal day 5 (P5) male C57BL/6 mice in oxygenated artificial cerebral spinal fluid (ACSF) solution using a vibrotone. Immediately after cross-sectioning, organotypic hippocampal slices were maintained as roller-tube cultures as described by the published method (Gahwiler, 1981). Cultures were grown in steroid deficient and phenol-red free neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen, Carlsbad, CA). 3–4 days after culturing, these slices were used for the experiments described.
4.5. cLTP Induction
Hippocampal slices were infected with AAV2-GFP viruses in neurobasal medium and incubated for four days. Infected slices were then first washed with ACSF [mM: Sucrose 206, KCl 2.8, CaCl 1, NaH2PO4 1.25, NaHCO3 26, D-glucose 10, Sodium Ascorbate 0.4, pH 7.4], and then cLTP was induced by incubating the slices in ACSF containing 10 µM picrotoxin and 200 µM glycine for 3min according to the protocol described by Lu et al., 2001. Immediately after 3min. ACSF solution was replaced and the slices were incubated in neurobasal medium in the presence or absence of soluble Aβ (0.5µM) and E2 (10nM) for 2hr. Immediately after the treatment two-photon confocal images of live slices were acquired.
4.6. Soluble Aβ Oligomer Preparation
Synthetic Aβ1–42 (Tocris, Ellisville, Missouri) was prepared without the fibrillar component according to the published method (Lambert et al., 2001). In brief, Aβ1–42 was dissolved in anhydrous dimethyl sulfoxide (DMSO) to 5 mM, which was then added to ice cold ACSF to 100 µM. This solution was incubated at 4°C for 24 hours, and then centrifuged at 14,000g for 10 minutes. The supernatant comprised of fibrillar-free oligomers, as well as monomers, was used for the experiments performed in this study. Protein concentration was determined from the supernatant and its molarity was calculated (0.5 µM).
4.7. Western Blot Analysis
After the respective treatments, primary neurons, neuronal tissue, or individual slices were homogenized in 100 µL of ice cold buffer containing 50 mM Tris, 10 mM Mg2+, 1 mM EDTA, 1 mM EGTA, 10 mM benzamide, 100 ng/ml leupeptin, 100 ng/ml aproteinin, 0.08 mM sodium molybdate, 0.01% tritonX-100, 10 µM okadoic acid, and 2 mM sodium pyrophosphate, pH 7.4. Aliquots of the lysed and sonicated homogenate were taken to determine protein concentration using protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Samples containing 30 µg protein were electrophoresed on a SDS/PAGE gel. The protein was transferred onto PVDF membrane (Millipore, Billerica, MA), blocked for 1 hour with PBS containing 4% non-fat dried milk, and probed overnight at 4°C with primary antibody. A polyclonal antibody against the alpha subunit of CaMKII phosphorylated at threonine 286 (Cell Signaling Technology, Danvers, MA) was used at a dilution of 1:1000 to detect activation of the kinase (autophosphorylation). A monoclonal antibody against ERK phosphorylated at tyrosine 204 (Santa Cruz, CA) was used at a dilution of 1:1000 to detect activation. A polyclonal antibody was used at a dilution of 1:1000 to recognize GluR1 phosphorylation at the CaMKII site (serine 831, Upstate, Temecula, CA). After washing 3 times with PBS, the membranes were further incubated at room temperature with horseradish peroxidase conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA) at a dilution of 1:1000. The proteins were visualized with supersignal chemiluminesence (Pierce Biotechnology, Rockford, IL) using UVP software (Upland, CA). For the loading control, membranes were stripped and reprobed with total antibody. Antibodies for total CaMKII, total ERK, and total GluR1 were used at a dilution of 1:1000.
4.8. Icv Injection
A well established icv injection protocol was used (Lu et al., 2002). In brief, the anesthetized ovariectomized female rats were fixed in the stereotaxic frame, and the subcutaneous tissue over the bregma was anesthetized with Xylocaine. The scalp was incised and a bar hole was drilled in the skull near the right coronal suture, 0.9 mm posterior to the bregma and 1.5 mm lateral to the midline. A needle (30-gage) connected to a 10 µl Hamilton syringe, fixed in the stereotaxic frame 4 mm ventral to the skull surface, was then slowly inserted and 3 µl of either vehicle (ACSF) or Aβ1–42 oligomer solution was injected slowly at a rate of 1 µl per minute. The needle was kept in place for 15 minutes to prevent any leakage from the ventricle, before being removed.
4.9. Live Animal Drug Administration and Tissue Procurement
Female Charles River Sprague-Dawley rats were maintained in laboratory acclimatization for 3 days before ovariectomy. All animal procedures were reviewed and approved by the University of North Texas Health Science Center Institutional Animal Care and Use Community. Bilateral ovariectomy was performed 3 weeks prior to subcutaneous drug administration. E2 was dissolved in absolute ethanol and then corn oil (Penta Manufacturing, Airfield, NJ) at a concentration of 320 µg/ml. Ethanol was evaporated by incubation at 50°C overnight. A single subcutaneous injection of E2 (320 µg/kg, 3hrs.) or vehicle was administered, as described by our laboratory (Yang et al., 2003).
4.10. Production of Recombinant Adeno-Associated Virus 2 and Infection of Hippocampal Slice Culture
The helper plasmid pDG, which contains both the AAV2 genes (rep and cap) and helper genes (Grimm et al., 1998), was used to generate recombinant AAV2. Recombinant AAV2 vectors were packaged, purified, concentrated, and tittered as described by the published method (Zolotukhin et al., 1999). Recombinant GFP containing AAV2 particles were used at a multiplicity of infection (MOI) of 100. For spine structure analysis the hippocampal slices were incubated with AAV2-GFP containing viruses and grown for 3 days for the expression of GFP in hippocampal neurons.
4.11. Inhibitory Avoidance Test
Female Charles River Sprague-Dawley rats (2 months old) were ovariectomized according to the protocol previously mentioned, and 3 weeks later were given an icv injection of Aβ1–42 oligomers or ACSF (0.5 µM), and/or a subcutaneous injection of E2 (300 µg/kg). Animals that were icv injected were trained for inhibitory avoidance memory using a GEMINI inhibitory avoidance (IA) system (San Diego Instruments San Diego, CA), according to the suppliers protocol 24 hrs after injection. Training consisted of allowing the rat to cross from an illuminated chamber into a dark chamber where a foot shock was delivered (scrambled shock was delivered at 2.0 mA for 2 sec). All animals underwent 1 training session (50 sec maximum allowed time), and were immediately re-caged for 3 hrs and then were tested (200 sec maximum allowed time). Memory of this experience was assessed by measuring latency to enter the dark chamber. Animals subcutaneously injected with E2 were trained 1 hr. after injection. Again, animals underwent 1 training session (50 sec maximum allowed time), and were immediately re-caged for 3 hrs and then were tested (200 sec maximum allowed time).
4.12. Statistical Analysis
Densitometric analysis of western blots was conducted using LabWorks Image Acquisition and Analysis software (UVP, Inc., Upland, CA). Densitometric data from at least three independent experiments was subjected to ANOVA, followed by Newman- Keuls Multiple Comparison Test or Bonferroni Test, for the assessment of group differences, and was presented as a bar graph depicting the average ± SD, using GraphPad Prism software (La Jolla, CA). *, p<0.05, **, p<0.01, ***, p<0.001. For the spine structure and spine density analysis the hippocampal slices expressing GFP were used for live cell imaging while submerged in ACSF. Whole pyramidal neurons were imaged by two-photon laser scanning microscopy using Zeiss LSM510 with wide tunable, mode locked, TiSapphire Laser (1.5W Chamelon-XR, Coherent, Inc., CA). 910nm excitation was used for imaging GFP. Images of individual CA1 green (GFP) pyramidal neurons were used to make filament objects of spines and dendrites using Imaris XT software (Bitplane, Saint paul, MN). From the traces stubby, mushroom, and thin type spines were sorted using MatLab XT software (Bitplane, Saint paul, MN). Finally, spines were classified by using “classify spines” program from the MatLab software and this was displayed as color coded spine heads, for example red, green, and blue as stubby, mushroom, and long-thin, respectively. Spine data from at least three independent experiments was subjected to two-way ANOVA, followed by Bonferroni Test, for the assessment of group differences, and was presented as a bar graph depicting the average ± SD, using GraphPad Prism software (La Jolla, CA). ***, p<0.001. For behavioral data all the groups were analyzed both by one way ANOVA with non parametric Kruskal-wallis test and Dunns post test and by unpaired t-test with 95% confidence interval using GraphPad Prism software.
Acknowledgements
This study was supported by NIH Grants P01 AG10485, P01 AG22550, and P01 AG27956, and a Minority Supplement P01 AG22550-06S1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations
AAV2
AD
Aβ
ACSF
AMPAR
AraC
BSA
CaMKII
cLTP
DMSO
E2
E18
ERK
GFP
GluR1
HBSS
IA
LTP
L-type VGCC
MEK
Mg2+
MOI
NMDA
P5
PBS
PKA
PKB
PKC
PI3K
SDS
References
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bernabeu R, Levi De Stein M, Izquierdo I, Medina JH. Learning-specific, time-dependent increases in hippocampal Ca2+/calmodulin-dependent protein kinase II activity and AMPA GluR1 subunit immunoreactivity. Eur J Neurosci. 1998;10:2669–2676. doi: 10.1046/j.1460-9568.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–2611. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor S, Williams PT, Armstrong B, Petit TL, Ivanco TL, Weeks AC. Long-term potentiation is associated with changes in synaptic ultrastructure in the rat neocortex. Synapse. 2006;59:378–382. doi: 10.1002/syn.20248. [DOI] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, Lacombe D, Kong X, Aman A, Laurin J, Szarek WA, Tremblay P. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28:537–547. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Chen CM, Silva A, Fox K. Requirement for alpha-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–423. doi: 10.1126/science.272.5260.421. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Giese KP, Silva A, Fox K. The role of alpha-CaMKII autophosphorylation in neocortical experience-dependent plasticity. Nat Neurosci. 2000;3:911–918. doi: 10.1038/78820. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Nobuaki T, Hideo M, Yasushi H, Gen M, Tomokazu T, Norio T, Tetsuya K, Suguru K. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Research Reviews. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, Kimberly McAllister A, Phyllis Wise Estradiol Targets Synaptic Proteins to Induce Glutamatergic Synapse Formation in Cultured Hippocampal Neurons: Critical Role of Estrogen Receptor-α. J Neurosci. 2007;27:6903–6913. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, Venton DL, Krafft GA, Finch CE, Klein WL. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz L, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, pangalos MN, Brandon NJ. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto T, Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2008;105:15148–15153. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T, Bernier F, Fukushima T, Hashimoto T, Ogura H, Nishizawa Y. Estrogen induces a rapid increase of calcium-calmodulin-dependent protein kinase II activity in the hippocampus. Brain Res. 2002;950:308–311. doi: 10.1016/s0006-8993(02)03186-4. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha BR, Vitolo OV, Joshi P, Lordkipanidze T, Shelanski M, Dunaevsky A. Amyloid beta peptide adversely affects spine number and motility in hippocampal neurons. Mol Cell Neurosci. 2006;33:274–282. doi: 10.1016/j.mcn.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Sinopoli KJ, Floresco SB, Galea LA. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem. 2006;86:293–304. doi: 10.1016/j.nlm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Mielke ML, Rozkalne A, Meyer-Luehmann M, de Calignon A, Bacskai BJ, Schenk D, Hyman BT. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol Dis. 2009;33:213–220. doi: 10.1016/j.nbd.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T-W, Wang JM, Chen S, Brinton RD. 17β-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–127. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- Yang S-H, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW. Estrogen Receptor β as a Mitochondrial Vulnerability Factor. J.Biol.Chem. 2009;284:9540–9548. doi: 10.1074/jbc.M808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Watson JB, Xie CW. Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol. 2004;92:2853–2858. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]