Abstract
Twelve species of Hypomyces/Cladobotryum producing red pigments are reported growing in various tropical areas of the world. Ten of these are described as new, including teleomorphs for two previously known anamorphic species. In two species the teleomorph has been found in nature and in three others it was obtained in culture; only anamorphs are known for the rest. None of the studied tropical collections belongs to the common temperate species H. rosellus and H. odoratus to which the tropical teleomorphic collections had previously been assigned. Instead, taxa encountered in the tropics are genetically and morphologically distinct from the nine species of Hypomyces/Cladobotryum producing red pigments known from temperate regions. Besides observed host preferences, anamorphs of several species can spread fast on soft ephemeral agaricoid basidiomata but the slower developing teleomorphs are mostly found on polyporoid basidiomata or bark. While a majority of previous records from the tropics involve collections from Central America, this paper also reports the diversity of these fungi in the Paleotropics. Africa appears to hold a variety of taxa as five of the new species include material collected in scattered localities of this mostly unexplored continent. In examining distribution patterns, most of the taxa do not appear to be pantropical. Some species are known only from the Western Hemisphere, while others have a geographic range from southeastern Asia to Africa or Australia. The use of various morphological characters of anamorphs and teleomorphs as well as culture characteristics in species delimitation is evaluated. For detecting genetic segregation, partial sequences of the two largest subunits of the ribosomal polymerase perform the best in terms of providing informative sites and the number of well-supported groups recognised in the phylogenies. These are followed by the sequence data of the translation-elongation factor 1-alpha, while the ribosomal DNA ITS regions are of only limited use in distinguishing species and their phylogenetic relationships.
Keywords: aurofusarin, biogeography, fungiolous ascomycetes, Hypocreaceae, Hypocreales, ITS rDNA, RPB1, RPB2, systematics, TEF1
INTRODUCTION
The fungicolous habit is manifested in many lineages across the fungal kingdom. The diversity of this lifestyle, highest in ascomycetes, reaches its peak in the order Hypocreales. Here, the most numerous group of exclusively fungicolous species is the genus Hypomyces, members of which live in association with different asco- and basidiomycetes. Whereas the best studied regions in terms of these fungi include Europe and the eastern coast of the USA, the species richness appears to be highest in the tropics, as for the other groups in the Hypocreales (Samuels 1996). As in many groups of fungi, the level of documentation and classification of fungal diversity in temperate regions far exceeds that known for the tropics. Põldmaa & Samuels (2004) summarised the main literature on tropical Hypomyces and related taxa. The present study has largely been inspired by recent works in the sister genus Hypocrea/Trichoderma in the Hypocreaceae. Detecting genetic segregation combined with detailed morphological observations has furthered the understanding of species delimitation and geographic distribution in many taxa in this intricate group of ascomycetes.
The present paper deals with species of Hypomyces that grow on various basidiomycetes and are characterised by red-coloured perithecia and/or colonies in culture. The colouration is due to the chinonic pigment, aurofusarin, first described as occurring in Fusarium culmorum (Ashley et al. 1937). Helfer (1991) studied the chromatographic pattern of several species of the Hypomyces-group suggesting that the red-pigmented species were closely related and introduced the term aurofusarin-group for them. The subsequent phylogenetic analyses of Hypomyces and related taxa, based on LSU rDNA data, supported a monophyletic group of the few included species producing the red pigment (Põldmaa et al. 1999, Põldmaa 2000, Põldmaa & Samuels 2004). This group, like others distinguished among the diverse fungicolous genus, comprises species with and without a known teleomorph. Most of the anamorphs of Hypomyces species growing on basidiomycetes other than boletes are accepted in the anamorph genus Cladobotryum (Rogerson & Samuels 1993) that, in turn, is connected only to this holomorphic genus. Despite the evidence on the congeneric nature of all the red-pigmented taxa treated in this study, the tradition of using separate generic names for pleo- and anamorphic species is followed until the monophyletic groups within this diverse complex of fungicolous fungi will be distinguished and named.
To date, 13 aurofusarin-producing species are known, three of which have a teleomorph. Hypomyces rosellus is the only one in which the teleomorph often accompanies the common anamorph in the temperate regions. Only the type collection from New Zealand is known for H. dactylarioides. In H. odoratus, a ubiquitous anamorphic fungus in Europe, the teleomorph has been obtained by crossing sexually compatible strains in culture (Arnold 1964). The remaining species are represented by single collections without a known teleomorph, described in the anamorph genera Cladobotryum (= Sibirina) (Rogerson & Samuels 1993, Põldmaa 2000). Among the species known in tropical regions Sibirina coriolopsicola, C. cubitense and C. virescens have been described from Cuba (Castañeda-Ruiz 1987, Arnold 1987, 1988), while for C. semicirculare one collection was known also from Taiwan (Kirschner et al. 2007). Chen & Fu (1989) reported Sibirina asterophora and S. purpurea var. asterophora from China, while the type material of these species originates from Japan (Matsushima 1975, de Hoog 1978) or USA, Alabama (Gray & Morgan-Jones 1980), respectively.
Berkeley & Broome (1875) described H. paeonius as a roseous fungus from Sri Lanka. Although accepted by Petch (1912), the holotype, devoid of perithecia, does not confirm that it belongs to Hypomyces. Besides this doubtful taxon, no red-perithecial Hypomyces species have been described from the tropics. However, numerous teleomorphic specimens have been collected from the Americas for over a hundred years. A majority of these are preserved at The Mycological Herbarium of the New York Botanical Garden (NY) and lack cultures. These have been identified as H. rosellus, which was for a long time the only red-pigmented species of the genus with a described teleomorph, besides the neglected H. paeonius. Based on differences of the anamorph, a collection from Puerto Rico was published as H. odoratus (Rogerson & Samuels 1993). These authors state the absence of teleomorphic characters that would distinguish the two species, while admitting the possibility of error in identifying red-perithecial Hypomyces as H. rosellus in the absence of the anamorph. During recent decades, several new specimens of red-pigmented Hypomyces/Cladobotryum have been collected in various tropical areas of the world. Besides new localities in the well-sampled Central America, collecting has been carried out in Africa, Australia, Madagascar and southeastern Asia that all lacked records on the occurrence of these fungi. While some of the collections were easily distinguished as belonging to known species, many others presented difficulties in identification.
The present study aims to delimit species of Hypomyces/Cladobotryum that produce red pigments and occur in the tropics, describing their phylogenetic relationships, anamorph-teleomorph connections, host range, and geographic distribution. To complete this task morphological examination of specimens and all available cultures was undertaken. For a majority of the cultures partial sequences of four gene regions (ITS rDNA, RPB1, RPB2, TEF1) were obtained and analysed. The results reveal the occurrence of at least a dozen red-pigmented species in various tropical areas of the world. Eight of them are described here as new species, while teleomorphs are described for two previously known anamorphic species. These data demonstrate that none of the studied tropical collections belongs to H. odoratus or H. rosellus. Neither are the distinguished tropical taxa closely related to these two and other temperate aurofusarin-producing species of Hypomyces and Cladobotryum that are not considered in detail in this study.
MATERIALS AND METHODS
Characterisation of morphology and cultures
Twenty-five or more ascospores and conidia were measured from each specimen/culture. The given ranges represent the mean values of specimens (two innermost numbers) and the limits of the 90 % range of estimated normal distribution observed in the single available or most divergent specimens (the two outermost numbers) being rounded to the nearest 0.5 μm. For rest of the structures, the absolute ranges are presented. Ascospore size is presented as the total length and width as well as the size of the main part (body) of the spore, including or excluding the apiculi and ornamentation, respectively. In both cases also the length/width ratio (Q) was estimated.
Ascospores or conidia were isolated onto 1.5 % malt extract agar (MEA). The descriptions and illustrations of species were made of cultures grown on Bacto (Detroit, USA) or Oxoid (Cambridge, UK) MEA in darkness or alternating 12 h/12 h darkness and fluorescent light at 25 °C. Colony growth was measured from 9 cm-diam plastic Petri dishes into which a 4 × 4 mm plug taken from the edge of an actively growing colony was placed ca. 1 cm from the margin. Colony characters were evaluated also in cultures grown on cornmeal dextrose agar (CMD + 2 % dextrose), potato dextrose agar (PDA) and MEA from Merck (Darmstadt, Germany). Growth rates are presented as the colony radius on MEA in 4 d at 25 °C.
DNA extraction, PCR, and sequencing
Genomic DNA was extracted with High pure PCR template preparation kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions or with TES buffer (200 mM Tris-HCl, pH = 7.6, 0.1 % SDS, 10 mM EDTA), followed by treatment with chloroform, isopropanol and ethanol. In the latter case DNA purification followed with GeneClean®III kit (Qbiogene, California, USA) or UltraClean™15 kit (Mo Bio Laboratory, California, USA), according to the manufacturer's instructions. PCR was set up using the following primers for amplification of the different gene regions: ITS and 5' end of the LSU rDNA: ITS1 and ITS4 or LR5 (White et al. 1990); RPB1: cRPB1Af and RPB1Cr (Castlebury et al. 2004); domains 6 and 7 of RPB2: RPB2-5f and RPB2-7cR (Liu et al. 1999); TEF1, part of the largest exon: EF1-983f (Carbone & Kohn 1999) and EF1-2218r (Rehner 2001). PCR was performed using Illustra TM puReTaq Ready-To-Go PCR Beads (GE Healthcare Europe GmbH, Freiburg, Germany) with an Eppendorf Mastercycler or Techne Genius thermocycler. The following amplification conditions were used: an initial denaturation at 95 °C for 3 min, followed by 35 cycles at 95 °C for 30 s, at 55 °C for 30 s, at 72 °C for 1 min increasing time 2 s per cycle, and a final extension at 72 °C for 10 min. For amplifying the protein-coding genes annealing temperatures from 57 to 60 °C were applied; in both RPB regions each step of the 35 cycles was prolonged to 1 min, while 30 s denaturation and annealing steps were applied in the 40 cycles used for TEF1. In the presence of multiple bands in the PCR products of the protein-coding genes, a touchdown program and/or cutting out the correct bands, followed by treatment with gel extraction DNA purification kit by Fermentas UAB (Vilnius, Lithuania), was used. Routinely PCR products were purified with Exo-SAP (GE Healthcare GmbH) according to the manufacturer's instructions. Sequencing was performed by MWG-Biotech AG (Ebersberg, Germany) or Macrogen Inc. (Seoul, Korea).
Alignments and phylogenetic analyses
Sequences of the ITS and LSU rDNA, RPB1, RPB2 and TEF1 regions were obtained from 61 cultures and two specimens. The majority of the sequenced material represented red-coloured Hypomyces/Cladobotryum. Sequences were obtained also from morphologically similar species lacking red pigments and closely related species with orange perithecia (H. aurantius, H. lactifluorum and H. subiculosus), the latter selected as the outgroup.
Sequence fragments were assembled and corrected using Sequencer v. 4.9 (Gene Codes Corp.). DNA sequences were submitted to European Molecular Biology Laboratory (EMBL) database with accession numbers, including those used from previous studies, listed in Table 1.
Table 1.
Strains and specimens of Cladobotryum and Hypomyces included in the phylogenetic analyses, their origin and numbers in the International Sequence Databases.
Abbreviations of culture collections and collectors: CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; FSU – Pilz-Referenz-Zentrum Jena, Institute of Microbiology, Friedrich Schiller University Jena, Germany; IMI – International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; TFC – Tartu Fungal Culture Collection, University of Life Sciences and University of Tartu, Tartu, Estonia; C.T.R. = Clark T. Rogerson, G.A. – Günter Arnold, G.J.S – Gary J. Samuels.
Numbers of strains/specimens from which DNA was extracted and sequences obtained.
includes numbers of strains in personal collections, T ex-type cultures, * DNA isolated from specimen.
Alignments were performed using MAFFT v. 5.861 (Katoh et al. 2005), followed by manual adjustment in Genedoc v. 2.7 (Nicholas & et al. 1997). Maximum parsimony (MP) analyses were conducted in PAUP v. 4.0b10 (Swofford 2003) using 1000 heuristic searches with random taxon addition sequences, TBR branch swapping, and MulTrees on; the confidence of branching was assessed by 1000 bootstrap replicates applying 100 replicates with maxtrees set to 100. All characters were treated as unordered, equally weighted with gaps as missing data. MP trees were computed separately for each of the four gene regions as well as for the combined datasets of all four gene regions and the three protein-coding regions.
Bayesian inference of phylogeny was performed with MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003) using the combined datasets with partitions defined according to the four gene regions. The GTR+⌈+I model was applied separately for each of the four data partitions, conducting two runs of the Markov chain Monte Carlo (MCMC) with four chains for 5 mln generations. Every 500th generation was sampled, discarding the generations before the run reached stationarity for computing of the consensus trees and posterior probability (PP) scores.
RESULTS
Phylogenetic analyses
The RPB1 dataset included 56 sequences and 713 characters of which 222 were parsimony-informative. The MP analysis yielded 615 trees. The RPB2 dataset of 62 sequences and 1 069 characters (342 parsimony-informative) resulted in 24 trees in the MP analysis. The TEF1 dataset of 63 sequences and 921 characters (165 parsimony-informative) yielded 112 MP trees. The ITS regions were sequenced for all 63 isolates with the smallest number of total and parsimony-informative characters, i.e. 612 and 92, respectively. Due to ambiguous alignment of some regions, 70 characters of ITS rDNA were excluded. MP analyses of the remaining 542 (74 parsimony-informative) characters resulted in 972 trees. In general, the topologies of the four consensus trees obtained in the separate analyses of the different gene regions (not shown) were in agreement, i.e. none of the strongly supported monophyletic groups (bs > 90 %) were in conflict with these clades on other gene trees. In the bootstrap analyses of the protein-coding genes 14 (RPB1), 17 (RPB2) or 11 (TEF1) strongly supported groups were distinguished. The bootstrap consensus of the ITS regions recognised only four groups in more than 90 % of the trees.
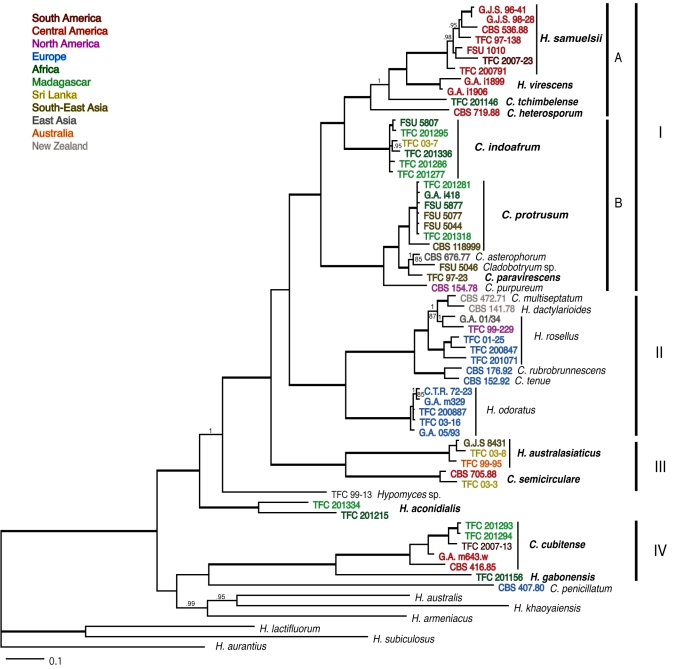
The combined dataset of four genes for 63 isolates included 3 246 characters of which 803 were parsimony-informative. MP analysis yielded 31 trees, the consensus of which is resolved in most parts. The topology generally concurs with the consensus tree obtained in Bayesian analysis of the combined dataset (Fig. 1). Therefore, support values for clades obtained in the bootstrap analysis are presented on the Bayesian tree. In several clades the support values were higher than observed in the bootstrap consensus trees in the analyses of individual genes. Exclusion of ITS rDNA from the combined dataset resulted in elevated support for some of the clades as well as revealed alternative relationships of two deeper branches discussed below.
Fig. 1.
Consensus tree obtained in partitioned Bayesian analysis of rDNA ITS regions and partial sequences of RPB1, RPB2 and TEF1 genes of Hypomyces/Cladobotryum producing red pigments. Names of tropical taxa are printed in bold. Font colours correspond to the geographic origin of the collection explained in the upper left hand side corner. Branches with posterior probability scores > 0.95 and bootstrap support > 95 % are in bold; in the case of individual values > 0.9 and > 85 %, these are given above and below the branches, respectively. Scale bar indicates subsitiutions per site.
Consensus trees of MP and Bayesian analyses of the four-gene combined dataset distinguish a well-supported clade of temperate taxa (clade II on Fig. 1), while all the tropical collections are included in its sister clade (clade I) or form basal lineages in regard to these two larger clades. The group comprising most of the species occurring in temperate areas (II) includes the best known red-coloured Hypomyces species, H. rosellus and H. odoratus together with other temperate species, each known only from type collection. These include C. rubrobrunnescens and C. tenue from Europe (Helfer 1991) as well as C. multiseptatum and H. dactylarioides from New Zealand (de Hoog 1978). The two European species form the sister-group of H. rosellus, which appears paraphyletic in regard to the two taxa from New Zealand.
The largest clade, comprising mostly tropical collections (Fig. 1 clade I), falls into two subclades (A, B) in the MP and Bayesian analyses. Among these, 10 of the well-supported groups or single-isolate lineages are considered to represent distinct species in accordance with morphological observations described below. Additional five isolates form third clade representing two tropical species. These form the moderately supported sister group of clade I in the consensus of MP trees obtained in the analyses of the combined four-gene and TEF1 datasets as well as in Bayesian and MP analyses based on the combined three coding genes. In the Bayesian phylogeny of the four-gene combined dataset this strongly supported clade (III in Fig. 1) is located in a more basal position. The remaining tropical, red-coloured Hypomyces/Cladobotrum isolates (clade IV) are included in the most basal clades of the ingroup. The ex-type isolate of C. penicillatum from The Netherlands forms the sister-group to two tropical taxa but this relationship is not supported in any of the analyses.
The outgroup species with orange-coloured teleomorphs, H. aurantius, H. lactifluorum, and H. subiculosus, form a well-supported group. These three produce the pigment skyrin (Helfer 1991), that, likewise aurofusarin turns purple in KOH solution. These taxa formed the sister-group of the clade including red-pigmented species in previous analyses based mostly on LSU rDNA data and broader taxon sampling (Põldmaa 2000, Põldmaa & Samuels 2004, Jaklitsch et al. 2008). These studies revealed the clade of red-pigmented taxa to comprise also some Hypomyces/Cladobotryum species that lack red pigmentation but show similarities in anamorph characters to those observed in the aurofusarin-producing species. The ubiquitous temperate parasite of Russulaceae, H. armeniacus as well as H. australis and H. khaoyaiensis occuring on various aphyllophoroid basidiomycetes in the tropics, were included in this study. In the consensus trees of the Bayesian and MP analyses these species represent a monophyletic group that is well-supported only in the Bayesian analysis. In both trees it forms the unsupported sister-group of clade IV and C. penicillatum. However, Bayesian and MP analyses of combined data of the three protein-coding gene regions (excluding ITS rDNA) resulted in consensus trees in which the three pallid KOH-negative species formed the sister clade of all the KOH-positive, mostly red-pigmented species. Adding similar pallid taxa and further gene regions to the analyses is expected to raise the support to this sister-group relationship.
Morphology
Teleomorphs
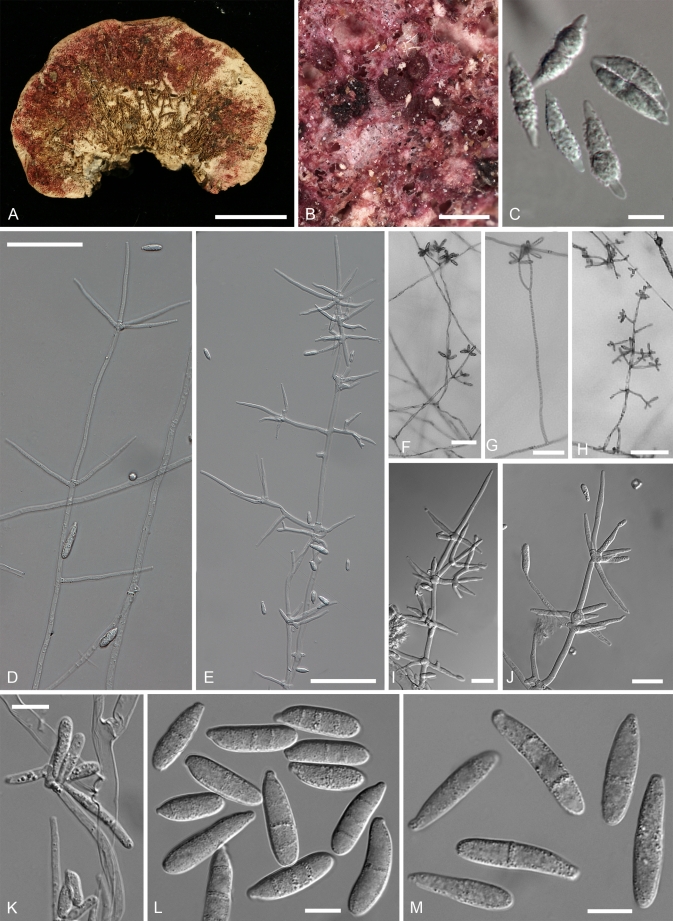
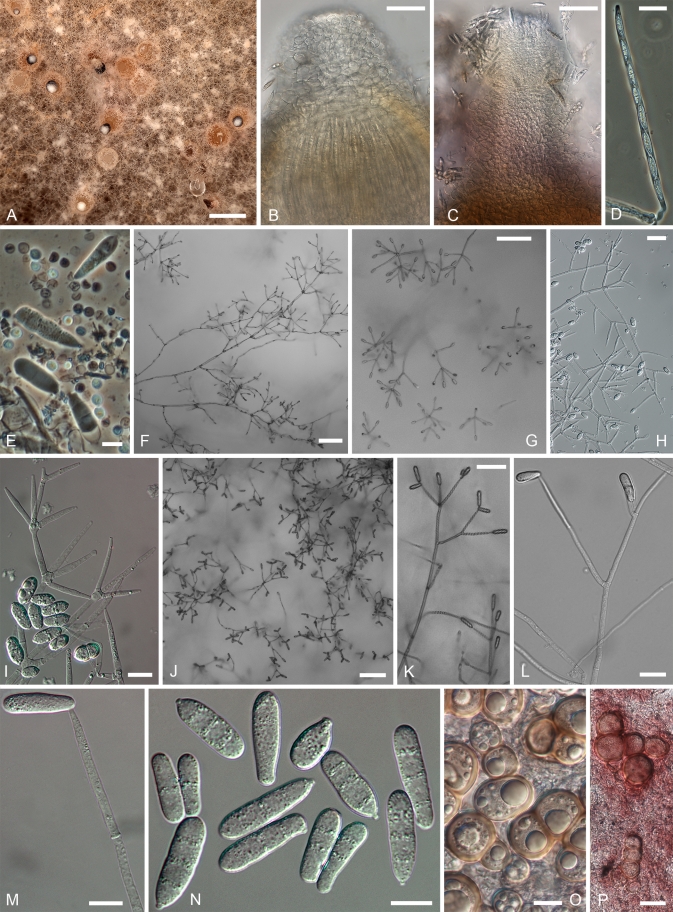
The sexual state is described here for five species, including those found in nature for H. australasiaticus and H. samuelsii. In H. aconidialis, H. gabonensis, and H. virescens the teleomorph has been obtained only in culture. In most species the subiculum develops as a scarce, arachnoid to profuse cottony, hyphal mat in which perithecia are formed. A restricted, pulvinate, stroma-like subiculum was observed only in cultures of H. aconidialis. In most species the perithecia are crimson to purplish red (11-12 C-D 6-8 according to Kornerup & Wanscher 1974). The subiculum is concolourous but usually much paler, appearing almost white in some collections. In the literature the colouration has also been described as (carmine-) red, pink, rosaceous lilac, and as “kirschrot” in German. The pigment reacts with aqueous KOH-solution, turning purple. Exceptional is the teleomorph of H. gabonensis, in which the subiculum and perithecia are buff-coloured with a faint colour change in KOH observed only in some parts of the subiculum. However, in cultures of this species, a KOH-positive, purplish red pigment develops. In all species the subiculum is composed of comparatively narrow, thin-walled hyphae, with only the cells surrounding the perithecia swollen. Perithecial anatomy is typical of most Hypomyces species growing on agaricoid and aphyllophoroid basidiomycetes (Rogerson & Samuels 1993, 1994, Põldmaa & Samuels 1999). The wall of the obpyriform perithecia is composed of a single region of thin-walled cells, compressed in the inner palisade and greatly swollen on the surface. The asci, containing eight, uniseriate, intact ascospores with ends overlapping, are released from the periphysate, ostiolar canal. The fusiform ascospores have a median septum, verrucose, warted or tuberculate wall and bear well-developed apiculi at their ends (Fig. 2).
Fig. 2.
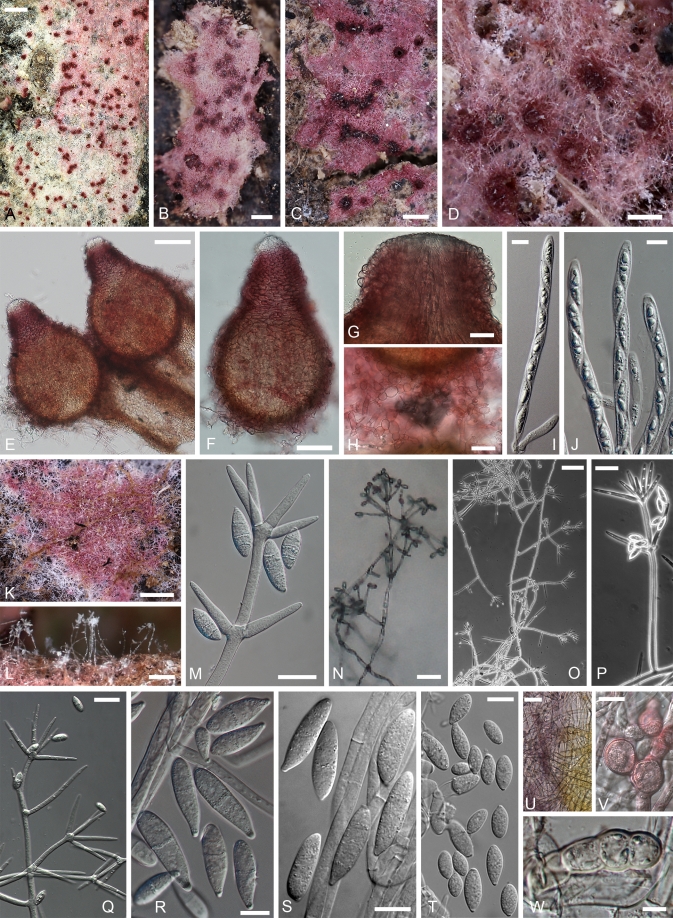
Ascospores of red-coloured Hypomyces; all except H. rosellus from type collections. A. H. samuelsii. B. H. virescens. C. H. australasiaticus. D. H. gabonensis. E. H. odoratus. F. H. rosellus. (A. BPI 748258; B. TU 112905; C. TAAM 170757; D. TU 112024; E. Type JE; F. TAAM 161043). Scale bar on E = 10 μm applies to all figures.
The main differences observed among the teleomorphs were in the size of perithecia and ascospores. The largest perithecia, up to 600 μm high, occur in H. gabonensis. In this species the papilla is very prominent, reaching 250 μm in length. In H. virescens the perithecia, obtained only in culture, are 380–460 μm high and 280–350 μm wide. In the rest of the species the perithecia remain < 400 μm high and < 300 μm diam with the cylindrical to conical papillae not exceeding 140 μm in height.
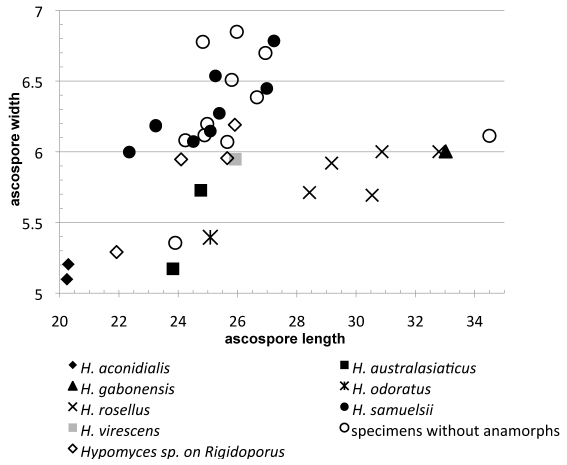
Only in H. samuelsii are a fair number of teleomorphic specimens known. Ascospore size measured in seven specimens verified by cultures and sequence data revealed remarkable variation both in length and width (Fig. 3). Ranges of ascospore size in the single specimen of H. virescens and the two of H. australasiaticus overlap with that observed for H. samuelsii. Among the temperate species, the ascospore size of H. odoratus overlaps with that of these three tropical species, while H. rosellus differs from these by its considerably larger ascospores (Fig. 2). The mean values of spore size differ only slightly among the three tropical species as well as H. odoratus (Fig. 3) but the sample size is limited. The longest ascospores in H. gabonensis and smallest in H. aconidialis clearly distinguish these species from all the rest. Because the teleomorphs of three species of the group have been obtained only in culture and single specimens of species other than H. samuelsii are available, evaluating the statistical significance of spore size differences is premature. For the same reason, specimens from tropical America that share similar red-perithecia but lack anamorphs, can only tentatively be identified as belonging to H. samuelsii as discussed below.
Fig. 3.
Scatterplots of ascospore measurements of six red-coloured Hypomyces. The points represent mean values of specimens, units in μm.
Another feature distinguishing the treated species is the length/width ratio (Q) of ascospores. The ascospores of H. gabonensis are narrower than in other Hypomyces species discussed, with Q > 5. In the type specimens of H. australasiaticus and H. virescens, the mean value of Q ranges from 4.3 to 4.6, remaining less than 4.3 in H. aconidialis, H. samuelsii and most of the old collections lacking anamorph data. In H. odoratus and H. rosellus Q is 4.7 or 5.0–5.5, respectively.
All the treated tropical species are characterised by well-developed apiculi at the ends of ascospores (Fig. 2). These may vary from simple conical to hat-shaped with their tips mostly obtuse, rarely bent. Often these different forms are present in one specimen. Variation in size follows the pattern described for ascospores. The ranges and their mean values, mostly falling between 3 and 4 μm, vary considerably among the specimens of H. samuelsii, overlapping with those from all other species. At the same time, the temperate H. odoratus and H. rosellus are distinguished by smaller or larger apiculi with mean values < 2.7 or > 5.0 μm, respectively. The apiculi of H. rosellus are the most prominent, attenuating from a broad base to the very narrow tip. The apiculi of H. gabonensis are similar, yet narrower and shorter, with mean length of 4 μm.
So far, the ascospore measurements of Hypomyces have always been presented including the apiculi and ornamentation. However, these represent separate structures that may exhibit independent patterns of variation. Therefore, it would be correct to measure spore bodies separately from the apiculi and ornamentation. By comparing the mean values of length and width of the main part of the ascospores, differences similar to those described above for the inclusive measurements were observed among species. In most cases the size of the apiculi appears to be correlated with that of the main part of the ascospore. Yet in specimens of H. samuelsii and H. rosellus in which the longest apiculi cause the highest value of total length of ascospores, the mean value of the main part falls within the range observed for most other specimens. In H. aconidialis and H. gabonensis mean values of both measurements are among the smallest or largest detected in the group.
Anamorphs
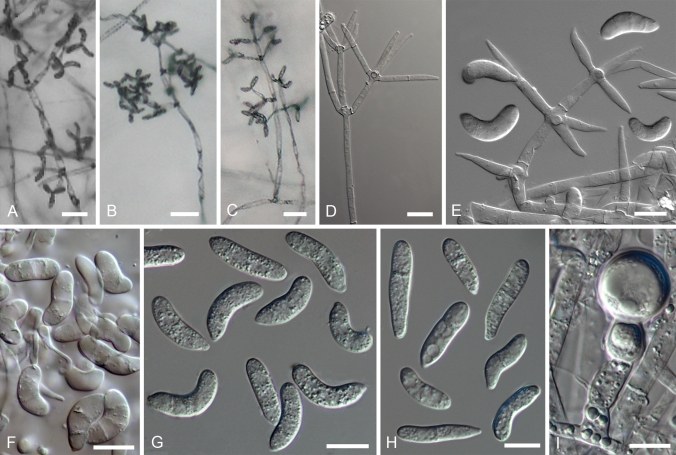
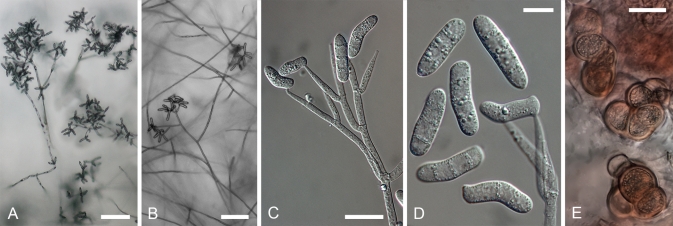
On the natural substratum, structures associated with asexual reproduction are mostly formed on a delicate, whitish to buff mycelium bearing scattered, suberect to erect conidiophores. A well-developed, easily observable white mat, characteristic of the anamorphs of the temperate H. odoratus and H. rosellus, has been recorded only in the case of C. semicirculare infecting cultivated Ganoderma in Taiwan (Kirschner et al. 2007). Despite this, fast-growing, profusely conidiating colonies develop upon germination of ascospores or conidia in culture. On MEA the aerial mycelium appears whitish, buff or yellowish, the colouration being affected by the underlying strong pigmentation of the medium. The aerial mycelium is either scarce arachnoid or profuse and cottony, often without clear demarcation between aerial hyphae and conidiophores. The submerged hyphae mostly extend in a verticillate manner with branches regularly arising in opposite position in the agar, with an additional branch usually growing upwards. Once reaching above the agar, hyphae form alternating or opposite extensions that usually produce further branches. The whole branching system or only its uppermost parts should be called conidiophores. Their stipes, which arise from aerial hyphae at right angles, are often slightly wider and yellowish ochraceous, turning purple in KOH solution except for the uppermost part. The conidiophores branch verticillately or irregularly, sometimes dichotomously; branching is more or less symmetrical or repeatedly one sided (drepanoid). The branching, either uniformly distributed or confined to the top of the conidiophores giving these a tree-like aspect, is quite characteristic of each taxon. There can be up to four levels of side branches, the ultimate ones giving rise to conidiogenous cells. The conidiogenous cells are held in comparatively dense verticils in most species, being less numerous in lower than uppermost position. Cylindrical or elongated-ampulliform conidiogenous cells deviate from the prevailing subulate form in which the cell is widest just above its base and attenuates gradually towards the apex. In contrast to the monoblastic, conidiogenous cells observed in most species, in C. protrusum their apices are slightly inflated or bear narrow elongations with several conidiogenous loci on irregular protrusions. Additional loci are observed also in the primary anamorph of H. gabonensis and occasionally in C. heterosporum and C. paravirescens.
Conidia observed in the anamorphs of tropical Hypomyces producing red pigments are often species-specific, while exhibiting high infraspecific variation and similarity among some of the taxa. Even though in a majority of species one to three septa are formed, single septate conidia can prevail in cultures of most of the species. Their shape varies from ellipsoidal to cylindrical, clavate, fusiform, obovoid or ovoid; the conidia are either straight or often curved in different ways with a part of almost circular conidia found in C. semicirculare. In H. samuelsii as well as in some other species, all forms are present either in a single or different strains. There is a tendency to form greenish conidia. Although prevailing in the Trichoderma anamorphs of the sister genus Hypocrea, in the Cladobotryum anamorphs of Hypomyces green conidia have been observed only in the anamorph of H. viridigriseus. The colouration is often faint and cannot always be observed even in conidial masses. However, the four species with greenish conidia do not constitute a monophyletic group indicating the homoplasious nature of this characteristic in the treated group. The conidial length and width as well as their ratio appear to be species-specific, with considerable range overlap observed among some taxa. The high variability of conidial size in C. heterosporum can be expressed by its coefficient of variation that exceeds 0.2, while it ranges from 0.01–0.15 in all other species (data not shown).
The differences in the width of the apex of the conidiogenous cell and that of the corresponding conidial hilum refer to retrogressive proliferation of the conidiogenous locus in most of the treated anamorphs. Specifically, width of these structures can vary to a considerable extent, reflecting their age and, in the case of hila, also formation order of successive conidia. Typically, the conidia are formed in an oblique position and held through their bases in imbricate chains. A single terminal conidiogenous locus usually produces two to five but never more than 10 conidia. The tips of conidia are pointed in different directions, giving the impression of star-like heads formed at the apices of conidiogenous cells. Distinct conidial columns composed of dozens of conidia held in one vertical plane are characteristic of the anamorph of H. odoratus, but are not found in the treated group. Neither are changes in the length of the conidiogenous cell and the width of its tip and conidial hila, also remarkable in H. odoratus. Likewise, annellidic tips of conidiogenous cells or those with a short rachis, both found in the anamorph of H. rosellus, are lacking in the tropical species. In C. protrusum each locus, formed at the tip of a small protrusion, presumably produces one conidium, with up to 12 conidia observed at the apex of each conidiogenous cell.
The anamorph of H. gabonensis provides an unusual phenomenon that illustrates the plasticity of the anamorphic state. The colonies on various media start growing by producing profusely branched conidiophores and comparatively small, 1-septate conidia from the uppermost and intercalary loci. Subsequently, a large-conidial anamorph, almost indistinguishable from C. cubitense, forms in most of the cultures at different times and location. Equally unique is H. aconidialis, representing the only species of the genus not found conidiating on the host or in the fresh isolations on different culture media.
Chlamydospores or thick-walled structures
Most of the species treated herein produce thick-walled, subglobose cells, referred to as chlamydospores, in nature as well as in culture. In nature they are found among the mycelium on which the conidiophores develop or near perithecia. In these fungal parasites chlamydospores obviously serve as survival structures to overcome periods between the availability of host fruiting bodies as well as unfavourable conditions like drought. Although seemingly more important for parasites of soft, ephermeral fruiting bodies of agarics, they are found also in cultures of species isolated from the more persistent basidiomata of wood-rotting aphyllophores. On natural substrata, the chlamydospores occur as single cells or are held in short simple chains. In cultures these can be followed by the formation of more complex aggregations. Generally, the chains of swollen and thick-walled cells grow out from a similar or simple intercalary cell on submerged or aerial hyphae. In some species the chains form branches and can develop into an irregular to globose mass of cells visible under the stereomicroscope. These are often light, almost colourless to pale ochraceous, soft, and lack inner structure characteristic of true sclerotia. The dark, tough, purplish brown sclerotia-like aggregations, common in temperate red Hypomyces species, were found only in C. paravirescens and C. protrusum.
Collections from tropical America lacking anamorph data
Over 20 specimens of red Hypomyces collected from tropical Central, North and South America in the 20th century are preserved at NY as H. rosellus. The US National Fungus Collection (BPI) holds fewer such specimens, some of which are accessioned as H. odoratus. Most of the specimens comprise purplish red perithecia developed in paler subiculum as typical of the members of the aurofusarin group of Hypomyces. The perithecia measure 300–430 μm in height and 200–340 μm in length, with papilla 50–150 μm high. Despite the similarity in perithecia, the morphology of ascospores clearly distinguishes all the studied mature collections from H. rosellus. The fusiform ascospores, 21.0–29.0 × (5.0–)5.5–7.5 μm, and their apiculi, 2.0–4.5(–5.5) μm, are shorter than in H. rosellus. Ascospore measurements, including the more diagnostic mean values of length and width, fall in the range described for the cultured specimens of H. samuelsii. Moreover, the grossly warted to tuberculate ornamentation is similar to that observed in H. samuelsii, while in H. rosellus the ascospores are covered with fine low warts (Fig. 2). In addition, ascospore length covers the range observed in the type specimens of H. odoratus and H. virescens, teleomorphs of which have been observed only in culture. However, these two species differ from the described specimens at NY and BPI in smaller mean width of ascospores (Fig. 3), less prominent ascospore ornamentation and larger perithecia.
Four specimens at NY differ from the remaining collections in having ivory to buff, dense cottony subiculum with contrasting deep purplish red perithecia. These have been collected in the West Indies (Dominica), Guyana, and Puerto Rico, all growing on Rigidoporus sp. Their ascospore morphology and measurements, (19.0)–21.9–25.6(–29.0) × (5.0)–5.3–6.0(–7.0) μm, Q = (2.8–)3.4–4.4(–5.0), provide no distinction from H. samuelsii. However, the conidia (seen only in Setliff 1249), remind those of C. cubitense. In contrast, another specimen collected on Datronia mollis in Panama (Dumont-PA 2018) comprises ascospores that deviate from all other red perithecial Hypomyces. These resemble ascospores of H. rosellus but are even larger, measuring (31.0–)34.5(–38.0) × (5.5–)6.1–6.5 μm. Whether these collections represent two undescribed species or teleomorphs of known anamorphic species has to await furher collecting along with isolation of pure cultures.
None of the old specimens have been inoculated into pure culture but anamorph structures were sometimes observed in close proximity to the teleomorphs. Besides the collection on Rigidoporus sp., described above, the fusiform 3-septate conidia allowed their identification as H. samuelsii. Cylindrical-ellipsoidal 3-septate conidia and conidiogenous cells with a sympodial rachis at their apex, characteristic of H. rosellus, were not observed in any of the collections. Neither could the long chains of 1–3-septate cylindrical conidia produced from retrogressively proliferating conidiogenous cells be found, known only in H. odoratus.
In conclusion, the collections without and those with cultures provide no evidence on the occurrence of H. odoratus or H. rosellus in the tropics. Among the five teleomorphs described in this paper, those of H. samuelsii and H. virescens originate from tropical America. In addition to these two very similar teleomorphs, anamorphic Cladobotryum cubitense, C. heterosporum and C. semicirculare, have been found in Cuba. An immature teleomorph of C. cubitense was found accompanying the anamorph in a collection from Louisiana, USA, and it is likely that teleomorphs of the other two also grow in this region. As in other groups of fungi with limited variation in teleomorphs, old collections lacking anamorph data cannot always be unambiguously identified to species. However, considering the frequency of the recent samples of morphologically similar H. samuelsii and the fact that the teleomorphs of H. virescens and the three Cladoboryum species have never been found in nature, it is most likely that large part of the historical collections from tropical America represent H. samuelsii.
Culture characteristics
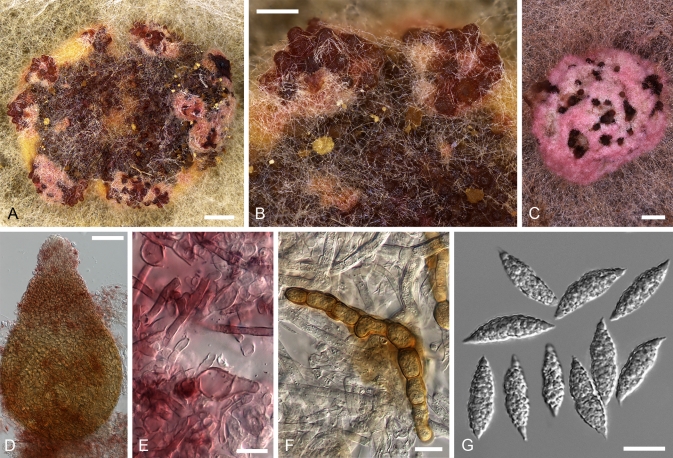
Most of the tropical red-coloured Hypomyces share the characters of fast growing, intensely coloured colonies on different media (Figs 4, 5). Colours and their succession are more or less identical in the strains studied, except for some species described below. On MEA whitish to buff mycelium develops after inoculation, with the colony reverse turning yellow in a few days. Usually in 2–4 wk, depending on the medium/brand and conditions, the colonies turn intensely red. The pigment, presumably aurofusarin in all these species, is most abundantly formed in submerged hyphae. Under the microscope, the colouration appears crimson to reddish or yellowish ochraceous, always turning purple in 3 % aquaeous KOH solution in which the pigment is partially dissolved from hyphae. In the isolates of H. australasiaticus and C. semicirculare, the pigmentation can be very pale, while C. cubitense, C. heterosporum and H. gabonensis differ in having a colony reverse that remains ochraceous for a long period. There is clearly a detectable KOH-reaction in all these species. In several isolates the ability to produce red pigments diminishes with age.
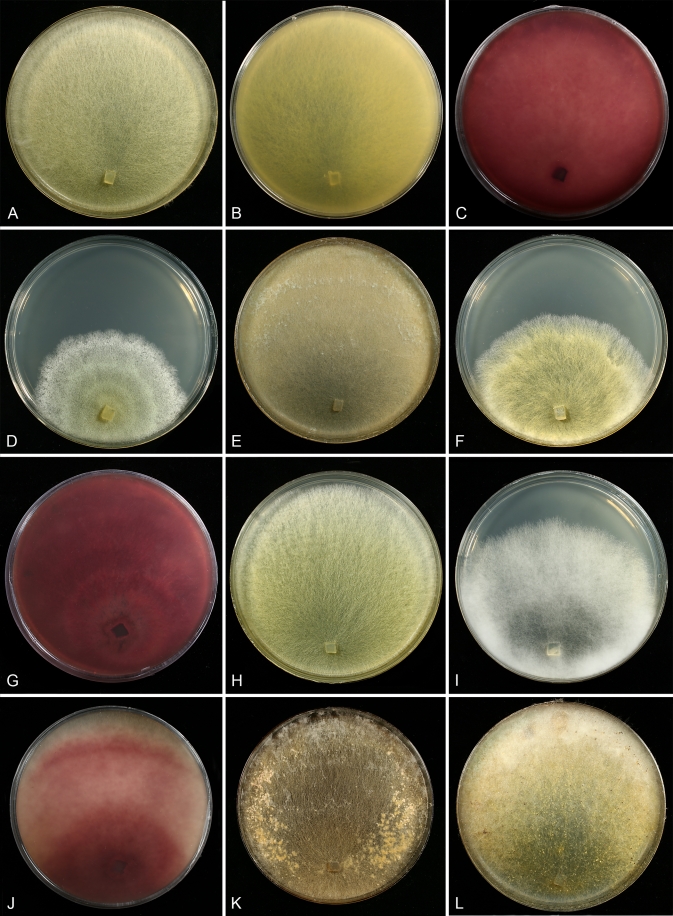
Fig. 4.
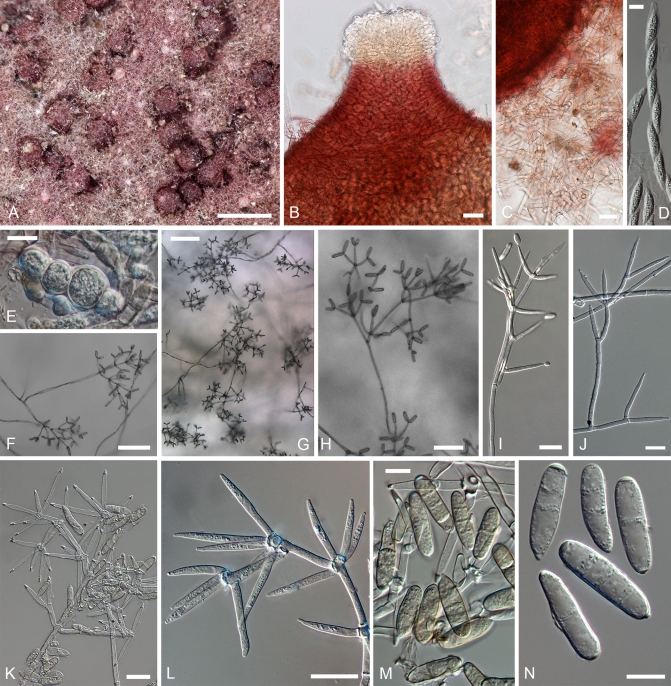
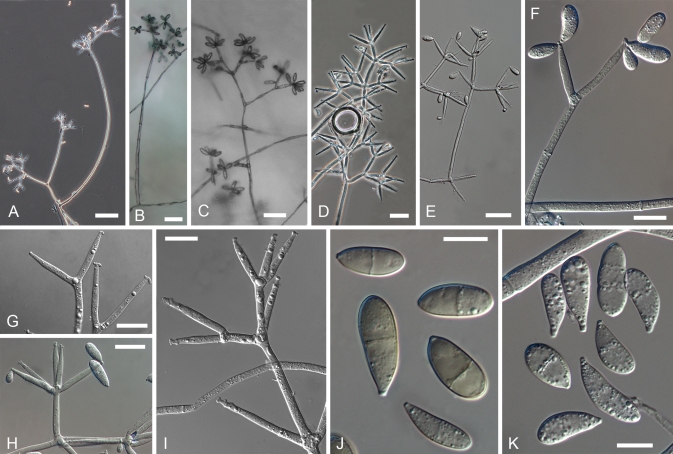
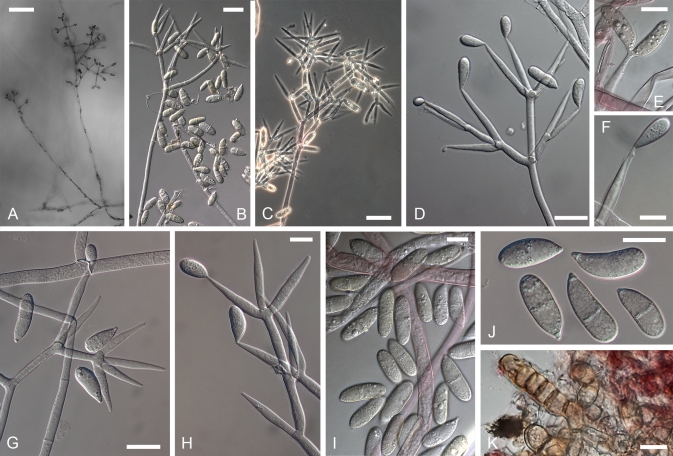
Cultures of seven species of Hypomyces/Cladobotryum grown at 25 °C in 12/12 h alternating darkness and fluorescent light. A–C. H. samuelsii. D, E. H. virescens. F, G. C. heterosporum. H. C. indoafrum. I, J. C. semicirculare. K. C. protrusum. L. C. paravirescens. (A–C. G.J.S. 98-28; D. G.A. i1899; E. G.A. i1906; F, G. CBS 719.88; H. TFC 03-7; I, J. CBS 705.88; K. FSU 5077; L. TFC 97-23; C, J on PDA, rest on MEA. A, B, D, F, H, I after 4 d; C, G, J. 2 mo; E, K, L. 1 mo).
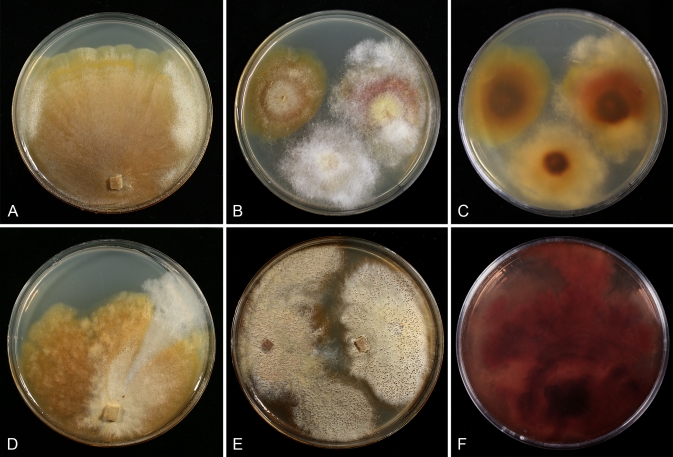
Fig. 5.
Cultures of C. cubitense and H. gabonensis on MEA after 25 °C grown in 12/12 h darkness and fluorescent light. A. C. cubitense G.A. i1361. B–F. H. gabonensis TFC 201156. B–D. Ochraceous colonies with the primary anamorph, white colonies/sectors with reddish reverse representing the secondary anamorph. (A, D grown for 1 mo; B, C, 2 wk; E, F 2 mo).
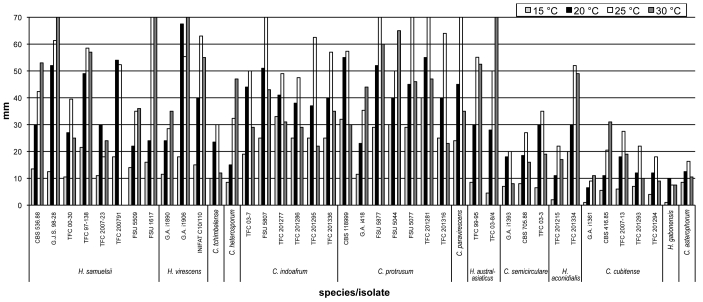
A majority of the observed strains and species grow fast on different media with obvious differences among the growth rates as well as optimum temperatures among the studied species (Fig. 6). All strains grow slowest at 15 °C with no growth observed at 35 °C. In several species the fastest growth is observed at 25 °C (C. asterophorum, C. indoafrum, C. paravirescens, C. semicirculare, C. tchimbelense). Many isolates of H. australasiaticus, H. samuelsii, H. virescens, C. heterosporum, and C. protrusum grow equally fast or even faster at 30 °C. Four of these species excluding C. heterosporum as well as C. indoafrum grow fastest at the four observed temperatures. Unlike other species the colony radius of C. protrusum exceeds 25 mm at 15 °C. On the contrary, C. asterophorum, C. cubitense, and H. gabonensis have slowest growth at all four temperatures. Because considerable infraspecific variation in growth, the values based on single strains should be interpreted with caution. For identification purposes three categories of growth rates can be distinguished, given here as the colony radius on MEA after 4 d at 25 °C. The fast growing species C. indoafrum, C. paravirescens, C. protrusum, H. samuelsii, and H. virescens exceed 50 mm, the slow growing C. asterophorum, C. cubitense, and H. gabonensis remain less than 20 mm, with intermediate values observed for the rest of the species.
Fig. 6.
Colony radius of 40 isolates of 12 tropical Hypomyces/Cladobotryum species and ex-type culture of C. asterophorum grown for 4 d on MEA at four different temperatures. Values represent means of 2–3 experiments.
Most species produce cottony, scarce to profuse, homogenous aerial mycelium on different media, while in others, profusely branching aerial hyphae or conidiophores form tufts throughout the colony (Fig. 4). Exceptional members of the group, C. cubitense and H. gabonensis, are characterised by slowly growing colonies, differing also in the pattern of colour change characteristic of the remaining taxa (Fig. 5). In these two species the colony reverse is intensely ochraceous, sometimes with an olivaceous tinge, turning purplish red in a few to several weeks. In both of these sister-species transfers resulted in subcolonies lacking the red pigment.
Several of the species produce odours detectable upon lifting the lid of the Petri dish. Often the smell is bitterish sweet, sometimes reminescent of camphour as described in the protologue of H. odoratus (Arnold 1964). The production and intensity of the smell depends on the medium and age of the strain. Because only some of the tropical strains were observed after their initial isolation, the data on strain odours is not extrapolated to species.
MEA was used as the standard medium for studying microscopic structures and colony characters because this medium guarantees optimal conidiation compared to CMD and PDA as well as the production of characteristic pigments. As observed in Hypocrea/Trichoderma strains (Jaklitsch 2009), the colony characters can vary depending on the brand of the extract used. MEA prepared from extract from Oxoid enhanced perithecial production, while extract from Bacto and Merck improved pigment production. For some strains, the anamorph structures were characterised on both media with no obvious differences observed.
On PDA the colony appearance is similar to that on MEA, with more intense colouration, turning from paler or darker egg-yolk yellow to crimson. The cottony aerial mycelium is generally more abundant, often reaching the lid of the Petri dish throughout the colony. On CMD all strains produce colonies with scarce aerial mycelium and the reverse turns bright yellow early. Generally the mycelium is homogenous with less conidiation than observed on other media. Only in C. tchimbelense, C. heterosporum, and one strain of C. protrusum growth is fasciculate.
Substrata
Species of the aurofusarin-group of Hypomyces/Cladobotryum grow on fruiting bodies of basidiomycetes belonging to specific taxonomic groups. The documented hosts represent saprotrophic, wood-decaying homobasidiomycetes, including species with soft, annual, or tough, perennial basidiomata either with poroid or gilled hymenophores. The host species belong to the families Agaricaceae, Crepidotaceae, Pleurotaceae, Schizophyllaceae, and Tricholomataceae in the Agaricales or to the Coriolaceae, Cyphellaceae, Ganodermataceae, Lentinaceae, Polyporaceae, and Pterulaceae in the Polyporales. Only H. samuelsii has also been collected on members of Auriculariales and Hymenochaetales.
While in temperate regions various ectomycorrhizal (EcM) taxa are frequently recorded as hosts of red-pigmented Hypomyces/Cladobotryum, these have never been observed to parasitise EcM fungi in the tropics. Such differences may be due to the scarcity and patchy distribution of ectomycorrhizal trees in the tropical forests. The red species have been found also on bark, sometimes in association with black ascomata. In such cases observation on the actual host remains obscure because wood can always contains fungal hyphae.
Host preference apprears to characterise several taxa even if their host ranges are mutually not exclusive. Hypomyces samuelsii, with the most numerous available collections, grows on different kinds of fruiting bodies of members of various basidiomycete taxa. It is the only species of the group that has repeatedly been found on Auricularia spp., that are otherwise only infrequently parasitised (Põldmaa & Samuels 2004). Cladobotryum semicirculare appears to grow often on members of the Polyporales, while H. australasiaticus has yet been reported only on polypores including the not closely related Antrodiella, Earliella, and Microporus. The few collections of C. tchimbelense and H. aconidialis are on saprotrophic Tricholomataceae. Members of this family appear as preferred hosts also for C. indoafrum and C. protrusum. These differences may partially be explained by the state in which the parasite was found. The tropical red-pigmented Hypomyces follow the substrate pattern of Hypomyces species with Cladobotryum anamorphs, in which the anamorphs and teleomorphs can differ in their host range. While the anamorphs of several species can spread fast on soft ephemeral agaricoid basidiomata, the slower developing teleomorphs are only formed on more durable substrata. These include polyporoid basidiomata, wood or other substrata of the fungal host that were observed in all the studied teleomorphic collections except for one specimen of H. samuelsii on Crepidotus sp.
The anamorphs of temperate, red perithecial Hypomyces are causal agents of the cobweb disease responsible for epidemics in mushroom farms (McKay et al. 1999). In Taiwan C. semicirculare has been isolated growing on basidiomata of Ganoderma distributed as G. tsugae (Kirschner et al. 2007). Besides this record, we are not aware of similar cases in tropical regions.
Geographic distribution
The sparse data resulting from sporadic collecting activities of Hypomyces in the tropics support Samuels (1996) who stated that most species of the Hypocreales are either temperate or tropical and subtropical. From the phylogenies presented herein, it seems obvious that the species growing in various (sub)tropical areas of the world are distinct from the well-known temperate species to which many of the previous tropical collections had been attributed. This conforms to the pattern detected in some taxa of the sister genus Hypocrea/Trichoderma in which detailed studies have revealed more refined geographic distribution for many of the species (e.g. Jaklitsch et al. 2006, Samuels 2006). In red Hypomyces/Cladobotryum a number of closely related tropical species form the sister group of temperate taxa (Fig. 1, clades I and II, respectively). The rest of the tropical taxa represent earliest diverged lineages in the whole group that has also been observed in other hypocrealean fungi (e.g. O'Donnell et al. 2000).
The data presented here, as well as unpublished observations, reveal that none of the red-pigmented Hypomyces/Cladobotryum species crosses the line between holarctic and paleo- and/or neotropical distribution. Moreover, these results challenge the idea of pantropical distribution in most of the studied fungi. With two exceptions, the species occurring in tropical America have not been collected on other continents. The numerous collections of H. samuelsii suggest that this species is common in Central America. Thus far, H. virescens and C. heterosporum have been found only from Cuba but for C. cubitense records are added from Peru and Madagascar. In C. semicirculare, the genetic segregation between isolates from Central America and southeastern Asia suggests that morphological comparison coupled with analysing more variable gene regions may warrant the distinction of two species.
The remaining species in the treated group have not been found in the Western Hemisphere. Hypomyces australasiaticus has been collected in Australia, Sri Lanka and Thailand, while C. paravirescens is known only from its type specimen in Thailand. For the rest of the species at least some of the specimens originate from Africa. However, the scattered sites sampled on that continent give a mere hint of the great diversity of Hypomyces in the vast, unexplored areas. Namely, the few collections from Gabon, Republic of South Africa, Uganda and Zimbabwe belong to five new species that do not appear as closest relatives to each other. A dozen specimens collected from close localities in southeastern Madagascar belong to three of these taxa. Whereas C. tchimbelense and H. gabonensis are described from Gabon, H. aconidialis was also found in Madagascar. Cladobotryum indoafrum, common in Madagascar but collected also in southern Africa and Sri Lanka, is presumed to represent a species with an African-Indian distribution pattern. Even wider distribution is documented for C. protrusum, extending from southern Africa and Madagascar to southeastern China and Taiwan.
Despite the scarcity of data it is obvious from the phylogeny of the red-pigmented Hypomyces that different distribution events have resulted in the geographic pattern of extant taxa. The species occurring in temperate North America, H. odoratus, H. rosellus and C. purpureum do not show affinities to the several species found in tropical America. On the other hand, the clade comprising C. asterophorum, C. protrusum and C. paravirescens suggests extensive dispersal events related to speciation taking place along the tropical and temperate regions of eastern Asia. Disjunct distribution, described in saprotrophic and ectomycorrhizal fungi (e.g. Matheny et al. 2009) is observed in C. cubitense and C. semicirculare. Also the sister taxa of C. tchimbelense from Africa, H. samuelsii and H. virescens grow in America. Similar African-South American disjunctions have been attributed to transoceanic dispersals in the Fusarium graminearum-group (O'Donnell et al. 2000). Estimating the divergence dates of lineages is required to understand whether also vicariance events have contributed to the observed distribution pattern as has been suggested for other groups of fungi (e.g. Hosaka et al. 2008, Matheny et al. 2009).
Species delimitation and phylogenetic relationships
The present study combines morphology, culture characteristics, and phylogenetic analyses of four gene regions for determining species and phylogenetic relationships among the red-pigmented Hypomyces/Cladobotryum. The analyses include pleomorphic taxa as well as those for which no teleomorph has been found. Tropical collections appear distinct from the temperate species, most of which form one clade (Fig. 1, clade II) comprising the common and well-known H. odoratus and H. rosellus. All the specimens from tropical areas of the world are distributed among other lineages. Most of them fall in the large clade l that appears as the sister-group to the temperate taxa in clade II. Members of this tropical clade share characters typical of the temperate taxa in producing fast-growing colonies that turn from yellow to purplish red in culture. Although all the isolates with greenish conidia are included in this clade, these do not form a monophyletic subclade. Moreover, none the four species forming green conidia reveal close affinities to another taxon sharing this feature. Neither do the studied green-conidial non-American isolates belong to C. virescens described from Cuba, the only previously known red-pigmented species producing green conidia. Therefore three new species, C. indoafrum, C. paravirescens and C. protrusum, are described based on material collected in Africa, Madagascar and southeastern Asia.
Clade I, including mostly tropical red Hypomyces/Cladobotryum, is composed of two subclades (Fig. 1). One of these, subclade A, includes five distinct lineages, each characterised by a unique combination of morphology. Members of three of the lineages are described below as new anamorphic species C. heterosporum, C. indoafrum, and C. tchimbelense. For the other two species, earlier known only from their anamorphic type material, teleomorphs are described herein. In H. samuelsii, previously known as Sibirina coriolopsicola, recently isolated and sequenced material provides evidence for the connection with teleomorphic specimens collected for over a hundred years. In H. virescens, the teleomorph has been obtained only in culture in a pairing of the only two known strains.
The sister-group, subclade B (Fig. 1), is well-supported but poses problems for species delimitation. Besides C. purpureum, described from North America, members of this subclade have been isolated outside the Western Hemisphere, mostly from tropical areas. The only other previously described species is C. asterophorum, known from the ex-type strain isolated from Japan. Characteristic of this strain is the production of polyblastic conidiogenous cells, a feature that is shared by most of the strains in subclade B. However, isolates forming several loci at the swollen apex of the conidiogenous cell do not form a monophyletic group. Rather, the ex-type isolate of C. asterophorum forms a strongly supported group with two strains characterised by monoblastic conidiogenous cells. The isolate TFC 97-23 from Thailand was previously reported as belonging to C. virescens (Põldmaa & Samuels 2004), while that from China (FSU 5046) was published as Sibirina purpurea var. purpurea (Chen & Fu 1989). Species delimitation is based on the correlation between genetic segregation and unique combinations of characters. The new species C. paravirescens and C. protrusum produce green conidia from poly- or monoblastic conidiogenous cells, respectively. Cladobotryum asterophorum differs in forming hyaline conidia from polyblastic cells.
As the well-supported sister-group of clades I and II, clade III (Fig. 1) is composed of tropical isolates that are often weakly pigmented and produce indistinct conidiophores. Molecular data support the distinction of H. australasiaticus with the longest conidia in the group from C. semicirculare with strongly curved conidia. A conidial isolate from Azerbaijan (TFC 99-13), forming an individual lineage, represents an undescribed species lacking a voucher specimen. A distinct lineage is formed of two isolates described as H. aconidialis; these are unique in lacking anamorph structures on natural substrate and culture media, while forming a discrete pulvinate subiculum with abundant perithecia reaching maturity in culture.
The most basal clade of the ingroup includes two tropical taxa (Fig. 1, clade IV) with limited production of red pigments. Hypomyces gabonensis, described here, forms the sister group to C. cubitense. These species differ in several aspects from other red-pigmented Hypomyces/Cladobotryum. Their colonies grow slowly on different media with intensive ochraceous colouration in H. gabonensis. The red pigments are absent or develop only in older cultures. While an immature teleomorph has been found for C. cubitense in nature, abundant buff-coloured perithecia with mature ascospores are produced in polysporic isolates of H. gabonensis.
KEY TO ANAMORPHS OF HYPOMYCES/CLADOBOTRYUM SPECIES PRODUCING RED PIGMENTS
1. Conidia observed on natural substratum and on standard culture media................................................................................................. 2 1. Conidia not observed on natural substratum or on standard culture media.................................................................... 10. H. aconidialis
2. At least part of conidia greenish................................................................................................................................................................ 3 2. All conidia hyaline...................................................................................................................................................................................... 6
3. Conidiogenous cells polyblastic, tips with protrusions........................................................................................................ 6. C. protrusum 3. Conidiogenous cells monoblastic, tips simple........................................................................................................................................... 4
4. Conidia mostly uniformly cylindrical, mean l/w ratio > 3.0................................................................................................... 2. H. virescens 4. Conidia mostly ellipsoidal or irregular in shape, often curved at base or both ends, mean l/w ratio < 3.0................................................ 5
5. Conidia mostly 1-septate, 2–3-septate conidia rare, conidial bases acuminate........................................................... 7. C. paravirescens 5. Conidia 1–3-septate, 2–3-septate conidia common, conidial bases rounded.................................................................... 5. C. indoafrum
6. Aerial mycelium of long unbranched hyphae that form short lateral branches supporting verticils of conidiogenous cells; conidia slender, with mean l/w ratio > 4............................................................................................................................................... 8. H. australasiaticus 6. Aerial mycelium of moderately to profusely branched hyphae, usually forming long lateral branches that function as conidiophores with further branching, often confined to the apex; conidia with mean l/w ratio < 4..................................................................................................... 7
7. Conidia mostly 1-, rarely 2-septate............................................................................................................................................................ 8 7. Conidia 1–3-septate, 3-septate conidia always present.......................................................................................................................... 10
8. Conidia > 20 μm long, > 7 μm wide............................................................................................................................... 3. C. tchimbelense 8. Conidia < 20 μm long, < 7 μm wide........................................................................................................................................................... 9
9. Conidial shape homogenous, mostly one conidium at apex of conidiogenous cell........................................................ 12. H. gabonensis 9. Conidia variable in shape, 1–4 conidia at apex of conidiogenous cell......................................................................... 4. C. heterosporum
10. Conidia ellipsoidal to fusiform, straight, slightly curved or twisted with ends curved in different direction.......................... 1. H. samuelsii 10. Conidia ellipsoidal to clavate, often curved............................................................................................................................................. 11
11. Conidia ellipsoidal, often strongly curved to semicircular, held in radiating heads at the single locus at apex of conidiogenous cell, mean length < 19.5 μm, l/w ratio < 3.5............................................................................................................................................... 9. C. semicirculare 11. Conidia mostly cylindrical to clavate, less prominently curved, not appearing semicircular, held horizontally in imbricate chains at uppermost locus, occasionally also singly at intercalary loci, mean length > 19.5 μm, l/w ratio > 3.5...................................................................... 12
12. One type of conidiophore and conidia formed in culture................................................................................................... 11. C. cubitense 12. Cultures initially produce profusely branched conidiophores with 1-septate conidia on terminal and intercalary conidiogenous loci, followed by formation of moderately branched conidiophores producing larger 3-septate conidia from a single locus.................... 12. H. gabonensis
DESCRIPTIONS OF SPECIES
1. Hypomyces samuelsii K. Põldmaa, sp. nov. MycoBank MB518517. Figs 2A, 4A–C, 7.
Fig. 7.
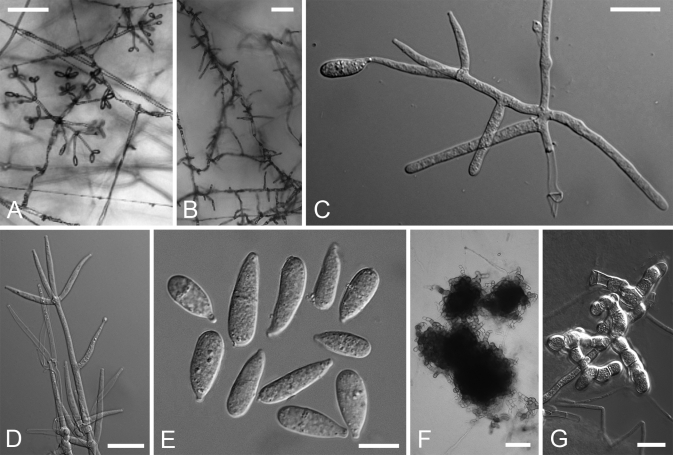
Hypomyces samuelsii. A–D. Perithecia embedded in subiculum effused over the substratum. E. Two perithecia seated on host's pores. F. Perithecium. G. Perithecial papilla with ostiolar canal in the center and swollen cells on the surface. H. Swollen cells surrounding perithecia. I, J. Asci. K–M. Anamorph on the host. N–V. Anamorph in culture. N–Q. Conidiophores with verticillately placed conidiogenous cells bearing conidia at their tips. R–T. Conidia. U. Hyphae turning from initial yellow to purple in KOH. V, W. Chlamydospores. (A, H, I. TU 112902; B, G, J. BPI 749247; C, K. TFC 97-138; D, E. Holotype, BPI 748258; F. TU 112903; L, M. TU 112901; N, S, V. TFC 00-30; O–Q. TFC 200789; R, U. Ex-type culture, G.J.S. 98-28; T, W. G.J.S. 96-41). Scale bars: A = 1 cm; B, C = 500 μm; D, K, L = 250 μm; E, O = 100 μm; F, H = 50 μm; G, M, N, P, Q, U = 20 μm; I, J, R–T, V, W = 10 μm.
Anamorph: Cladobotryum coriolopsicola (R.F. Castañeda) K. Põldmaa, comb. nov. MycoBank MB519537. Basionym: Sibirina coriolopsicola R.F. Castañeda, Fungi Cubenses II, 10–11. 1987.
Etymology: Named to honour Gary J. Samuels whose long and extremely productive mycological career is mostly dedicated to the taxonomy of the Hypocreales with passion for Hypomyces among many others.
Perithecia in effuso subiculo dispersa, semiimmersa, coccinea purpurescentia, obpyriformia, (250–)270–370 × (160–)200–260 μm; papilla late conica, 65–120 μm alta, basi (60–)80–105 μm lata. Asci cylindrici, 130–160 × 7–9 μm. Ascosporae fusiformes, 21.0–23.2–27.6–29.0 × 5.0–6.1–6.8–8.0 μm, septo mediano, dense verrucatae, apiculo 2.5–3.3–4.4–5.5 μm longo. Conidiophora 100–400 μm longa, 7–12 μm lata. Cellulae conidiogenae cylindraceae vel subulatae, 25–45 μm longae, propre basin 4–6 μm latae, uno loco. Conidia ellipsoidea vel cylindracea, (late-) fusiformia, recta vel extremo extremibusque flexa, 15–30 × 6–8 μm, hyalina, 1–3(–4)-septata. Chlamydosporae 12–14 μm diametro, ochroleucae.
Subiculum with embedded perithecia widely effused over host or in small, < 1 cm diam patches, forming dense, cottony or sometimes scarce, arachnoid mat, whitish to pale crimson, buff to yellowish; hyphae hyaline to pale purplish red, 3–6 wide, with cells partially swollen to 17 μm diam, especially near the perithecia, thin-walled. Perithecia scattered in subiculum, semi-immersed to almost superficial, crimson to purplish red, turning purple in KOH with tip of papilla remaining hyaline and occasionally lower part of venter reddish brown; flask-shaped, (250–)270–370 × (160–)200–260 μm; wall 12–20 μm wide, composed of a single region of flattened thin-walled cells, cells greatly swollen, 12–20 μm diam, at surface; papilla prominent, broadly conical, 65–120 μm high, (60–)80–105 μm wide at base, with cells at surface 11–17 μm diam, attenuating to 30–60 μm at tip, tip obtuse with oblong-clavate cells, 6–14 × 3–4.5 μm reaching surface; ostiolar canal periphysate. Asci cylindrical, 130–160 × 7–9 μm, apex thickened, 0.5–1.5(–2.0) μm; ascospores uniseriate with ends overlapping. Ascospores fusiform, often inequilateral, (21.0–)23.2–27.6(–29.0) × (5.0–)6.1–6.8(–8.0) μm, Q = (3.2–)3.8–4.2(–4.9), main part of ascospore (14.5–)16.6–19.7(–22.5) × (4.5–)5.2–5.6(–6.0) μm, Q = (2.5–)3.2–3.5(–4.1); 1-septate, septum median; densely warted, warts to 1 μm high; apiculate, apiculi (2.5–)3.3–4.4(–5.5) μm long and (1.0–)1.6–2.4 (–3.0) μm wide at base, tips obtuse or sometimes acute.
Anamorph effused on host, also on subiculum. Conidiophores borne on scarce mycelium, erect, 100–400 μm long, 7–10 (–12) μm wide at base, tapering to 5–6 μm below uppermost verticil of conidiogenous cells, frequently septate, especially near base, thin-walled, hyaline, forming 1–2 verticils of conidiogenous cells. Conidiogenous cells held by 2–4, cylindrical to subulate, sometimes widest in middle, often constricted in upper part, 25–45 μm long, 4–6 μm wide near base, attenuating to 1–2 μm at apex, with one uppermost locus sometimes bearing a collarette. Conidiaellipsoidal to cylindrical, fusiform to broadly fusiform, occasionally long obovoid, equi- or inequilateral, straight or curved at one or both ends; 15–30 × 6–8(–10) μm; hyaline, apex sometimes refractive; 1–3(–4) septate; basal hilum small, central or slightly shifted to side. Chlamydospores of 2–4 cells, in lateral position on intercalary cells, subglobose, 12–14 μm diam, pale ochraceous, wall 1–1.5 μm thick, smooth.
Colonies on MEA spreading fast, reaching 45–50 mm in 4 d; margin even or slightly fasciculate; reverse initially yellow, turning purplish red; yellowish brown, round or fan-shaped crystals and/or pigment patches with needle-like margins, turning deep purple in KOH, abundant in agar. Odour sweet or bitter-sweet, strong in recently isolated cultures, disappearing in old cultures. Aerial mycelium scanty to abundant, cottony, to 7 mm high or < 2mm in cultures producing teleomorph; mostly homogenous, occasionally with tufts; yellowish white, amber or buff, partially turning violet in KOH. Submerged hyphae often turning violet in KOH, cells infrequently swollen. Conidiation abundant in fresh isolates, becoming moderate to scarce in older strains. Conidiophores arising from aerial hyphae at right angles, not differentiated from these or distinct with main axis yellowish ochraceous, KOH+ and wall slightly thickened; ascending to suberect, 200–400(–1000) μm long, main axis near base 4–10 μm wide; branching profuse or sometimes sparse, verticillate or irregular, occasionally drepanoid, widely distributed, sometimes confined to uppermost parts, conidiophores then appearing irregularly tree-like in aspect; lateral branches formed at 1–2 levels, 1–4 developing from one point, 30–60 × 3.5–4.5 μm. Conidiogenous cells formed directly on conidiophores or from lateral branches that are often integrated in a previous verticil of conidiogenous cells, developing singly or (2–)3–6(–8) in a verticil, sometimes singly below verticil; subulate, 25–40 μm long, 2.5–4.5 μm wide near base, attenuating gradually to 0.8–2.0 μm at apex; aseptate; forming one conidiogenous locus at apex. Conidia ellipsoidal to fusiform, long obovoid i.e. droplet-shaped or sometimes widest in lower half (oblong-ovoid); equi- or inequilateral, straight but sometimes with basal or both ends curved; attenuated at base to a narrow but prominent central hilum, often attenuated also at apex; (9.5–)11.7–22.2(–26.5) × (4.0–)5.4–7.2 (–9.0) μm, Q = (1.6–)2.2–3.8(–4.6); 1–3-septate, in 1-septate conidia septum median or in upper 1/3 or 2/3; hyaline or occasionally with tinge of green when old, with refractive thickening at base or sometimes also at apex; formed obliquely from uppermost locus, held by (1–)2–3(–8) in imbricate chains appearing as radiating heads. Chlamydosporesformed among aerial or submerged mycelium, hyaline; cells subglobose, 13–23 μm diam, wall 1–2 μm thick, smooth; 2–5 cells in intercalary chains or in lateral, irregular chains or sclerotia-like aggregations formed from an intercalary cell. Perithecia produced in abundance in recent cultures isolated from ascospores.
Substrata: Basidiomata of various wood-decaying members of Agaricales, Hymenochaetales and Polyporales, also on Auriculariales; in some collections host fungus not detected and then observed growing on bark, wood or associated with other ascomycetes.
Distribution: Tropical America.
Holotype: Puerto Rico, Luquillo, Chicken Farm, on Phellinus cf. chryseus, 10 June 1998, G.J. Samuels, BPI 748258, ex-type culture G.J.S. 98-28 = CBS 127157.
Specimens with living cultures examined: Costa Rica, Guanacaste Conservation Area, Santa Rosa National Park, on Crepidotus sp. and wood, 9 Oct. 1997, P. Chaverri & S. Salas, InBio 3-233, culture TFC 97-138 = CBS 127159; Guanacaste Conservation Area, Rincón de la Vieja Nat. Park, Pailas, on Hexagonia glabra, 1 July 1998, P. Chaverri & S. Salas InBio 5-183, culture IB8029 = TFC 00-30. Cuba, Guantanamo Prov., Imías, on Coriolopsis sp., 29 Apr. 1986, M. Camino, C 86/138, Holotype of Sibirina coriolopsicola, INIFAT, ex-type culture CBS 536.88; Sierra del Rosario, El Salon, on Crepidotus sp., 14 July 1984, G. Arnold A 84/790, culture FSU 1617 = m659; Soroa, on an agaric, 10 Nov. 1985, G. Arnold A 85/318, culture FSU 5509 = i1931; locality unknown, on Pleurotus sp., 1988, INIFAT Castañeda 87/261, culture G.A. i1716 = FSU 1010. Peru, Junin Dept., Chanchamayo Distr., Kimo, on fruitbodies of an agaricoid basidiomycete on a stem of a palm, 2 Mar. 2007, K. Põldmaa, TU 107212 (anamorph), conidial isolate TFC 2007-23 = CBS 127160). Puerto Rico, Caribbean National forest, Luquillo Mts, Trail to El Toro from Rte. 186, on wood, 24 Feb. 1996, G. J. Samuels & H. J. Schroers, BPI 749247, culture G.J.S. 96-41 = CBS 127158; Luquillo Mts., La Coca trail, on black mycelium on palm, 10 June 1998, P. Chaverri, BPI 748259, culture G.J.S. 98-29. West Indies, Martinique, Pointe La Philippe, on Auricularia cf. polytricha on bark of Cyathea, associated with Gliocladium sp., 19 Aug. 2007, C. L. Lechat 7259, TU 112901, conidial isolate TFC 200791 = CBS 127155; Anse Noire, on bark, 22. Aug. 2007, C. L. Lechat 7265, TU 112902, culture TFC 200793 = CBS 127156; same collecting data, JF07018, TU 112904, culture TFC 200790; Anse Noire, Les Anses d'Arlet, on bark of a dead standing stem and on black effused stromata incl. Camillea sp., 22 Aug. 2007, J. Fournier, JF07016, TU 112903, culture TFC 200789.
Specimens without living cultures but accompanied by dried cultures or anamorph on the host: Cuba, Santa Clara Prov., Santa Clara, on Auricularia sp., 17 Mar. 1905, F. S. Earle & W. Murrill 424, NY. Jamaica, Tray, on wood, 19 June 1909, A.E. Wight 473, NY, perithecia immature, anamorph present. Puerto Rico, Bosque Estatal de Guajataca Trail 1 to cave, on leaf litter, 24 Nov. 1992, S. M. Huhndorf 239, CTR 92-87, NY, dried culture BPI 747860. USA, Florida, Highlands Hammock, on a resupinate polypore, 1 Feb. 1937, C. L. Shear #288, BPI 630895; Rock Spa, on Daedaleopsis confragosa, 11 Jan. 1942, C. L. Shear, BPI 630911; Alachua Co., Univ. of Florida Horticultural Farm, 6 miles NW of Gainesville, on Auricularia sp., 26 Aug 1977, C. T. Rogerson 77-121, NY.
Notes: Most of the tropical collections of red perithecial Hypomyces at BPI were preserved as H. odoratus. Anamorphs studied in pure cultures, available for three of these, clearly differed from the anamorph of H. odoratus that is frequently found on mostly agaricoid basidiomycetes in Europe. Another specimen at NY (Huhndorf 239), accompanied by a dried culture and drawings representing similar morphology, had been published as one of first collections of H. odoratus in nature (Rogerson & Samuels 1994). Teleomorphs and anamorphs of these four specimens from Puerto Rico were similar to those collected in Costa Rica and the West Indies. Analyses of sequence data confirmed conspecificity of all the ascosporic isolates but revealed these not to be related to H. odoratus. The strongly supported monophyletic group comprised also three conidial isolates from Cuba, including the ex-type strain of Sibirina coriolopsicola, and one isolate from Peru. Based on these data, a new pleomorphic species, Hypomyces samuelsii is described.
Besides these collections of H. samuelsii, numerous specimens, including similar teleomorphs but lacking cultures, have been collected mostly from the the Caribbean region since the end of the nineteenth century. Several originate from Puerto Rico, with the oldest collection at NY dating back to 1899 (collected by G. P. Goll in Bairoa, Caguas). In 1930 a specimen has been sampled in the Luquillo mountains, as is a more recent collection with a living culture that was selected as the holotype of H. samuelsii. Rest of the specimens at NY originate from Cuba, Guatemala, Jamaica, USA (Florida, Louisiana) and the West Indies. While most of the specimens have been growing on various polypores, several were collected on Auricularia spp. as was a recent isolate from the West Indies. In most of these the morphology of the teleomorph and anamorph (if present) matches that of the cultured collections of H. samuelsii. The measurements of the conspicuously warted ascospores are described and compared to those of similar species in the section of “Collections from tropical America lacking anamorph data”. It was concluded that large part of the old collections apparently belong to H. samuelsii which can be considered a common species at least in the tropical forests surrounding the Caribbean Sea.
Until now, Sibirina coriolopsicola was known from the type collection containing only the anamorph. In the original description only the anamorph on natural substratum was described. Despite scarce conidation in the ex-type culture, it produced the characteristic fusiform 1(–3)-septate conidia, slightly smaller than reported in the protologue, 13–26 × 4.5–8 μm. The main differences between the studied isolates and the protologue are the rarity of 2–3-septate conidia in culture and much smaller conidia in some of the strains, e.g. G.J.S. 96-41. The fusiform, sometimes twisted form of conidia is usually not as pronounced on culture media as it is on natural substratum. The moon-shaped conidia described in the protologue were not observed in culture nor on natural substrata. In several strains, including the ex-type culture of the anamorph and that of the holomorph of H. samuelsii designated here, 1-septate conidia were prevalent. The conidial size differs considerably among the studied strains, with minimal overlap in length of the short- and long-conidial isolates. Conidiation appears retrogressive; in the older cultures conidiogenous cells become shorter and their tips wider. The anamorph was originally described in Sibirina, presumably because of verticillately placed conidiogenous cells, but fits the expanded concept of Cladobotryum proposed by Rogerson & Samuels (1993). The recognition of Sibirina is not justified based on the molecular and morphological data provided here as well as in previous studies (Põldmaa 2003).
2. Hypomyces virescens G.R.W. Arnold & K. Põldmaa, sp. nov. MycoBank MB518518. Figs 2B, 4D, E, 8.
Fig. 8.
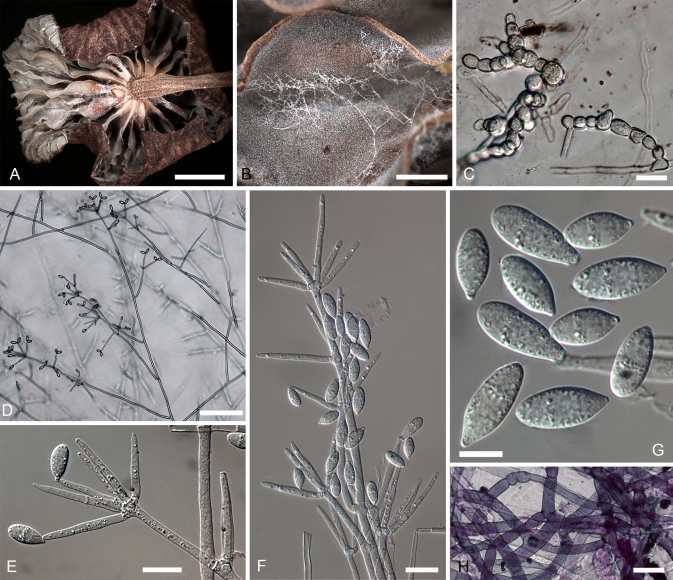
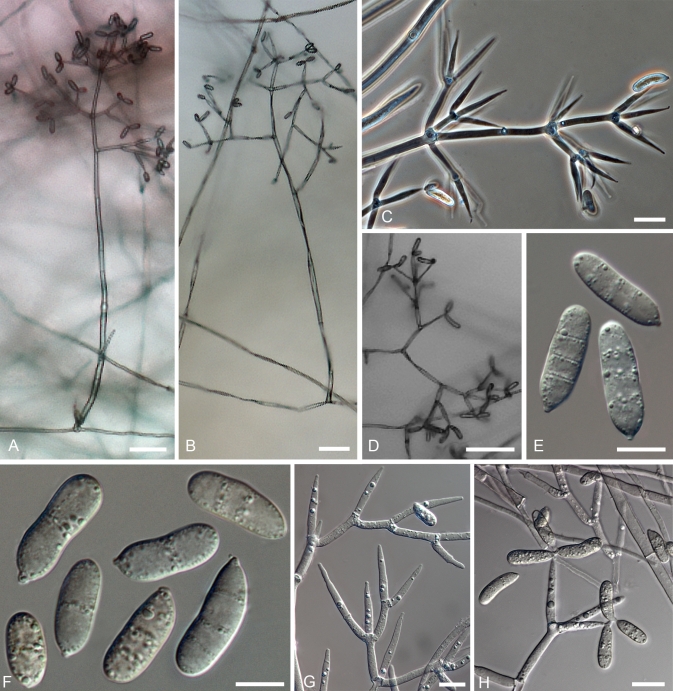
Hypomyces virescens. A–D. Teleomorph from a dried culture on MEA. E–N. Anamorph on MEA. A. Perithecia embedded in the subiculum. B. Upper part of a perithecium. C. Base of a perithecium and subicular hyphae. F. Asci and ascospores. E. Chlamydospores among subiculum. F–J. Conidiophores with conidiogenous cells and conidia. K, L. Upper parts of conidiophores. M, N. Conidia. (A–E. Isotype, TU 112905; F–I, K–M. G.A. i1906; J, N INIFAT C10/110). Scale bars: A = 500 μm; F, G = 100 μm; H = 50 μm; B, C, I–L = 20 μm; D, E, M, N = 10 μm.
Anamorph: Cladobotryum virescens G.R.W. Arnold, Feddes Repertorium 98: 351. 1987.
Etymology: The epithet of the previously described anamorph referring to the greenish conidia.
Teleomorphosis crescens in MEA substrato; colonia crescens in subiculum. Perithecia dispersa, immersa, obpyriformia, 380–460 × 280–350 μm, coccinea purpurescentia; papilla brevi, cylindracea, 70–100 μm alta, basi 70–100 μm lata. Asci cylindrici, 160–180 × 7.0–8.5 μm. Ascosporae fusiformes, (22.0–)26.0(–30.0) × (5.0–)5.9(–7.0) μm, septo mediano, habentes densas breves verrucas, apiculo 2.5–4.5 μm longo.
Teleomorph produced in culture on MEA; colony becoming subiculum with embedded perithecia. Subiculum dense cottony mat, roseous with scattered buff patches; hyphae hyaline to pale crimson, KOH + purple, 2.5–4 μm wide, with cells surrounding perithecia often swollen to 15 μm in diam, thin-walled. Perithecia scattered in subiculum, immersed; flask-shaped, 380–460 × 280–350 μm; purplish red, in KOH base of papilla, upper part of venter turning purple with lower part of venter reddish brown; wall of a single region of flattened, thin-walled cells, at surface cells broadly ellipsoidal, 20–30 × 10–16 μm; papilla short, cylindrical, 70–100 μm high, 70–100 μm wide, apex obtuse with oblong-clavate cells, 5.5–8.0 μm diam at surface. Asci cylindrical, 160–180 × 7.0–8.5 μm, ascospores uniseriate with ends overlapping. Ascospores fusiform, equi- or inequilateral, (22.0–)26.0(–30.0) × (5.0–)5.9 (–7.0) μm, Q = (3.6–)4.4(–5.1); ascospore body (16.5–)19.5(–22.5) × (4.5–)5.2(–6.0) μm, Q = (3.0–)3.7(–4.5); 1-septate, septum median; densely covered with low warts to 0.5 μm high; apiculi 2.5–4.5 μm long, 2–3 μm wide at base, straight or sometimes hooked, simple or hat shaped, occasionally branched, tips obtuse or acute.
Colonies on MEA spreading fast to very fast, reaching (30–) 50–70 mm in 4 d, reverse first yellowish ochraceous or bright yellow, turning slowly into yellowish or reddish brown; margin even. Odour absent or sweetish. Aerial mycelium scanty to moderate, cottony, to 3 mm high or reaching the lid in some parts; homogenous or with small tufts; pale whitish buff or yellowish, becoming greenish with formation of conidia, hyphae partially turning purple in KOH. Submerged hyphae often turning purple in KOH, cells not swollen. Conidiation abundant, not diminishing with age. Conidiophores arising from aerial hyphae at right angles, not differentiated; ascending to suberect or erect, main axis 6–10 μm wide, brownish yellow, except tips, pigmented parts turning purple in KOH; branching profuse, verticillate, dichotomous, drepanoid or irregular, confined to upper half giving conidiophores tree-like aspect, forming tufts in colonies; often conidiophores borne as side branches from a verticil of conidiogenous cells, branching further at top; lateral branches, formed by 1–3 from one point, 20–30 × 3–4 μm. Conidiogenous cells developing mostly on short lateral branches, 3–6 in a verticil; subulate, aseptate, (25–)30.3–34.2 (–40) μm long, (2.5–)3.1–3.5(–4.0) μm wide near base, attenuating gradually to 0.6–1.2 μm at apex, straight or slightly curved at apex; producing one conidiogenous locus at apex. Conidia cylindrical or long ellipsoidal, sometimes narrowly clavate, equi- or inequilateral, straight or curved in lower half, often also attenuated towards base, (15.0–)20.6–23.6(–29.0) × (5.0–)6.1–6.5(–8.0) μm, Q = (2.5–) 3.4–4.0(–5.0); 1–3-septate, hyaline to pale green, pigmentation sometimes visible only in conidial masses, may be refractive at apex and hilum; hilum prominent, central or slightly off center; conidia produced obliquely from uppermost locus, (1–)2–3(–5) in radiating heads. Chlamydospores absent or formed in short lateral chains among aerial mycelium, cells globose, 9–16 μm diam, wall 1.0–1.5 μm thick; sclerotia-like aggregations absent.
Substrata: Wood-decaying basidiomycetes, wood.
Distribution: Central America.
Holotype: Cuba, G. Arnold, dried cultures with perithecia obtained from pairing isolates G.A. i1906 and G.A. i1899, data listed below, JE, isotypes BPI, TU 112905.
Cultures examined: Cuba, Matanzas Prov., Coliseo, on old decaying wood on the ground, 21 Feb. 1983, G. Arnold, A 83/244, holotype of the anamorph, permanent slide at JE, isotype HAJB, ex-type culture G.A. i1906 = TFC 98-37 = FSU 5526 = ATCC 66118 = CBS 676.92; Camaguey Prov., Moron, on Schizophyllum commune, 10 Oct. 1985, G. Arnold A 85/616-3, G.A. i 1899 = FSU 5525 = CBS 127161; Santiago de Las Vegas, on Lentinellus sp., 10 June 2010, R.F. Castañeda-Ruiz, INIFAT C10/110, culture TFC 201449.
Notes: Cladobotryum virescens was described based on a single collection from Cuba. Crossing the ex-type strain with another strain of this species from a different locality in Cuba by the author of the species in 1992 resulted in the production of perithecia in culture. This dried culture, deposited at JE (part of it as the isotype at TU), serves as the holotype of the teleomorph described herein. Another dried culture obtained from pairing the same two cultures is preserved at BPI. The ascospores formed in the perithecia of the two dried cultures differ to some extent. In the material at BPI ascospores are shorter and bear very low and broad apiculi, whereas in the holotype material, ascospores and apiculi are more slender with their tips acute. Formation of the teleomorph could not be repeated even when including the recently isolated strain in the pairing experiments.
The protologue describes the conidiogenous cells as producing one, seldom two conidia that are narrower (4.5–5.5 μm) than in current observations. In the isolates grown on MEA usually two to three, sometimes also four or five conidia are held at the tip of the conidiogenous cell. While on MEA 1-septate conidia prevail, a few 4–6-septate conidia were seen among the usual 3-septate ones on PDA. Although reported as lacking in the protologue, chlamydospores were found among the mycelium in the dried culture designated as the holotype.
In contrast to other red-pigmented Hypomyces, the isolates of H. virescens produce brownish rather than yellow pigments on different brands of MEA media. The final brownish red colouration develops quite late. Only on PDA the medium is initially yellow and starts to turn deep red after one wk. While G.A. i1906 is one of the fastest growing isolates among the red-pigmented Hypomyces, G.A. i1899 is characterised by considerably slower growth (Fig. 6).
Analyses of the four genes reveal H. virescens to be the sister-species of H. samuelsii (Fig. 1). The larger perithecia of H. virescens and ascospores with less pronounced ornamentation are the only differences observed between the two species (Figs 2, 3). Finding the teleomorph of H. virescens in nature would allow more precise comparison. The anamorphs of these two species, developing in culture, differ mainly in the colour and shape of conidia, being hyaline and often distinctively fusiform, sometimes also curved at both ends in H. samuelsii. The anamorph of H. virescens is distinguished by the green colouration of conidia easily observed in cultures due to profuse conidiation. It differs from other geen-conidial species by slender, comparatively regular, cylindrical, mostly straight, 1–3-septate conidia (Fig. 8M, N) formed from a single locus at the tip of the conidiogenous cell. Only the last formed conidium at the tip of each conidiogenous cell developing from a laterally displaced hilum is slightly curved at the base.
3. Cladobotryum tchimbelenseK. Põldmaa, sp. nov. MycoBank MB518515. Fig. 9.
Fig. 9.
Cladobotryum tchimbelense. A, B. Delicate mycelium on host gills. C. Chlamydospores. D–F. Conidiophores with conidiogenous cells and conidia. G. Conidia. H. Submerged hyphae turning purple in KOH. (A, B. Holotype, TU 112007; C–H. Ex-type culture TFC 201146 on MEA). Scale bars: A = 1 cm; B = 250 μm; C, E = 20 μm; D = 100 μm; F = 25 μm; G, H = 10 μm.
Etymology: Refers to the type locality in Gabon, Africa.
Mycelium tenue, lactescens, in hospitis lamellas; hyphae parce ramosae, septatae, 3–6 μm latae, hyalinae. Conidiophora et conidia n.v. In MEA substratum, conidiophora 200–1500 μm longa, 8–10 μm lata prope basin; conidiogenae cellulae subulatae vel fere cylindraceae, 25–50 μm longae, 3.5–5.0 μm latae prope basin, fascientes unum conidiogenum locum. Conidia ellipsoidea, fusiformes, clavata, obovoidea vel ovoidea, recta, basi attenuata, (16.0–)20.1(–24.0) × (7.5–)8.4(–9.5) μm, 1(–2)-septata, hyalina, (1–)2–3(–8) catenatae. Chlamydosporae subglobosae, 7–17 μm diametro, hyalinae vel ochrol.
Delicate whitish mycelium on lamellae of host; hyphae sparingly branched, septate, 3–6 μm wide, hyaline. Conidiophores and conidia not observed in nature. Colonies on MEA growing fast, reaching 40–65 mm in 4 d; reverse first yellow turning yellowish ochraceous or purple; margin even to fasciculate. Odour absent. Aerial mycelium scanty, arachnoid, 1–2 mm high; homogenous or forming mycelial tufts of variable size, to 1 cm diam; buff, turning ochraceous or salmon in compacted areas of 1–1.5 cm diam, turning purple in KOH. Submerged hyphae often turning purple in KOH. Conidiation abundant. Conidiophores arising from submerged and aerial hyphae, not differentiated or slightly wider at base, ascending to suberect, 200–1500 μm long, near base 8–10 μm wide with wall to 1.3 μm thick; branching sparse to moderate, mostly forming single side branches that function as conidiophores or shorter supporting branches of conidiogenous cells; supporting branches arising singly or by 2–3 from one point, 25–40 × 4–5 μm. Conidiogenous cells formed singly or by 2–3 directly on conidiophores, or 4–7(–12) in verticil at top of conidiophore and on lateral branches that can be integrated in verticil of previously formed conidiogenous cells; subulate to almost cylindrical, 25–50 μm long, 3.5–5.0 μm wide near base, attenuating gradually to 1–2.0 μm at the tip; aseptate or rarely with one septum in middle; forming one conidiogenous locus at tip. Conidia ellipsoidal to fusiform, clavate, obovoid, or ovoid, straight, equilateral, occasionally inequilateral, slightly curved at top, attenuated at base to a narrow, prominent or wider, indistinct central refractive hilum; (16.0–)20.1(–24.0) × (7.5–) 8.4(–9.5) μm, Q = (2.0–)2.4(–2.8), 1(–2)-septate, septum median or in upper 2/3, hyaline; formed obliquely from uppermost locus, (1–) 2–3(–8) in short imbricate chains that appear as radiating heads or columns in case of longer chains. Chlamydospores formed among aerial or submerged mycelium, hyaline to pale ochraceous, cells subglobose, 7–17 μm diam, wall 1–1.5 μm thick, intercalary on submerged hyphae or in long branching chains, sometimes forming soft pale orange-brown sclerotia-like aggregations in old cultures.
Substrata: Agaricoid basidiomata of Tricholomataceae growing on wood.
Distribution: Central Africa, known only from the type locality.
Holotype: Gabon, Crystal Mountains National Park, Tchimbele, on Gymnopus sp. on wood, 30 Apr. 2009, K. Põldmaa, TU 112007, ex-type culture TFC 201146 = CBS 127166.
Notes: Cladobotryum tchimbelense is distinguished by the monoblastic conidiogenous cells arranged in dense verticils as well as the ellipsoidal to fusiform, straight, mostly 1-septate conidia. It is similar to the anamorph of H. samuelsii in which especially the 3-septate conidia tend to be curved at one or both ends. Their conidial measurements overlap, with only the mean width in the single collection of C. tchimbelense falling outside the range of H. samuelsii. On MEA colonies of C. tchimbelense differ in having an uneven margin that is even more fasciculate on CMD, distinguishing the single known strain from others examined in this study. In old cultures on MEA main branches of the submerged hyphae contain conglomerations of brick-ochraceous pigment that partly diffuses into the medium resulting in patchy pigmentation of the agar.
4. Cladobotryum heterosporum K. Põldmaa, sp. nov. MycoBank MB518511. Figs 4F, G; 10.
Fig. 10.
Cladobotryum heterosporum, ex-type culture CBS 719.88 grown on MEA for 5 d. A. Conidiophores with verticillately arranged conidiogenous cells bearing radiating clusters of conidia. B. Hyphae with many short sterile lateral branches. C. Part of a conidiophore with two sterile branches. D. Top of a conidiophore. E. Conidia. F. Sclerotia-like aggregations above agar. G. Aggregations of thick-walled cells. Scale bars: A, B, F = 50 μm; C, D, G = 20 μm; E = 10 μm.
Etymology: refers to the variable shape and size of conidia.
In MEA substratum, hyphae aeriae cum pluribus erectis libris extrematibus; conidiophora 7–8 μm lata. Cellulae conidiogenae subulatae vel fere cylindraceae, 25–40 μm longae, 2.5–3.5 μm latae prope basin, apice fascientes unum vel paucos conidiogenos locos, interdum ferentes parvam irregularem protuberationem. Conidia forma et amplitudine irregulares, ellipsoidea vel (angusta) clavata vel (ob)ovoidea, basi rotundata vel saepe attenuata ad hilum angustatum, vulgo aequilateralia et basi leviter curva, (12.0–)16.2(–20.5) x (4.5–)5.8(–7.0) μm, 1(–2)-septata, 2–5 aggregata in radiantibus capitulis. Chlamydosporae sublgobosae, 9–13 × 7–9 μm, hyalinae, catenatae vel sclerotiis aggregatae.
Colonies on MEA spreading moderately fast reaching 30–40 mm in 4 d; reverse initially yellowish ochraceous turning roseous or brownish red in 7–10 d, finally brownish red or crimson; margin even; pigment allocated in irregular patches in medium. Odour sweetish bitter or sweet, strong. Aerial mycelium scarce to moderate, homogenous or sometimes with small patches, 1–2(–5) mm high, buff; branching profusely with many erect free ends remeniscent of conidiophores, reaching lid of Petri dish, frequently also forming short, sterile lateral branches, 30–90 × 4–5 μm, divided by a few septa; hyphae hyaline to pale ochraceous, turning partially purplish in KOH. Submerged hyphae 4–6 μm diam, turning pinkish in KOH, cells not or irregularly swollen to 12 μm diam. Conidiation moderate. Conidiophores not differentiated from aerial hyphae, sometimes arranged in tufts, suberect to erect, main axis 7–8 μm wide, thin-walled, hyaline; branching profuse, irregular. Conidiogenous cells 3–5(–8) in a verticil or occasionally 1–2 just below it; subulate to almost cylindrical, occasionally ampulliform, 25–40 μm long, 2.5–3.5 μm wide near base, attenuating gradually to 0.7–1.5 μm at tip, straight or occasionally curved at apex, aseptate, producing 1 or a few conidiogenous loci, mostly arranged at apex that sometimes bears small irregular protrusions. Conidiairregular in shape and size,ellipsoidal, clavate to narrowly clavate, ovoid or obovoid, base rounded or often attenuating to a narrow hilum, mostly equi-, seldom inequilateral, slightly curved at base, (12.0–)16.2(–20.5) x (4.5–)5.8(–7.0) μm; Q = (2.0–)2.9(–3.7), 1(–2)-septate, septum median or supramedian; hilum prominent, 1–1.5 × 1.3–1.7 μm, central or slightly off-center; held by 2–5 in radiating heads at apex of conidiogenous cell. Chlamydospores sublgobose, 9–13 × 7–9 μm, wall 0.7–1.2 μm, hyaline, forming chains of 2–6 cells in terminal position on lateral branches of submerged hyphae; often many chains formed from closely placed cells further developing into soft, almost hyaline sclerotia like-aggregations, held singly or 2–4 together in irregular clusters just above agar surface.
Holotype: Cuba, Soroa, on an agaric, 10 Oct. 1982, G. Arnold A 82/633, dried culture, TU 112906, ex-type culture G.A. i1898 = FSU 5514 = CBS 719.88.
Notes: Cladobotryum heterosporum is unique in the group due to the process of conidiation, small irregular conidia, and conidiophore system that forms abundant, short sterile side branches. The species can be differentiated through its mostly ellispoidal to clavate, 1-septate conidia that are variable in shape and size. Together with the primary anamorph described below for H. gabonensis in which the conidia are less than 15 μm, they share the shortest conidia in the group, being the only ones in which the conidial length does not exeed 20 μm.
Most of the conidiogenous cells in C. heterosporum bear 2–3 conidia at the apex. Often it is obvious that the conidia are held a short distance from each other, presumably as the result of each being formed from a different locus. This cannot be unequivocally stated as there are no clear denticles or scars demarcating the loci on the conidiogenous cells. Thus, it can only be presumed that each locus produces a single conidium, with the irregular protrusions, sometimes visible at the tips of conidiogenous cells, incorporating several loci. This is in agreement with the centrally based hilum, evident at the base of each conidium. Occasionally single conidia are seen also attached to the middle part of the conidiogenous cell that may be the result of the detached conidium sliding downwards after its release. Similar conidiogenesis has been observed in the anamorph of H. orthosporus (Põldmaa 1996, Põldmaa & Samuels 1999).
On CMD growth is fasciculate as in C. tchimbelense. On PDA C. heterosporum differs from most strains of the group in forming low, compact whitish, ochraceous to cocoa-brown aerial mycelium with reverse turning partially red or reddish brown in 1 wk. The sweet odour is also characteristic of C. heterosporum.
5. Cladobotryum indoafrumK. Põldmaa, sp. nov. MycoBank MB518510. Figs 4H, 11.
Fig. 11.
Cladobotryum indoafrum on MEA. A, B. Conidiophores. C, D, G. Conidiogenous cells on short lateral branches at the apex of conidiophores. E, F. Conidia. H. Radiating heads of conidia at tips of two conidiogenous cells. A, F. TFC 03-7. B, D. Ex-type culture, TFC 201286. C, E. Holotype, TU 112289. G, H. FSU 5807. Scale bars: A, B, D = 50 μm; E–G = 10 μm; C, H = 25 μm.
Etymology: refers to the geographic range of the species.
In MEA substratum, conidiophorae ascendentes vel (sub)erectae, 300–1500 μm longae, 8–10 μm latae. Cellulae conidiogenae subulatae, 20–45 μm longae et prope basin 3–5 μm latae, apice fascientes unum conidiogenum locum. Conidia forma irregulares, ellipsoidea vel cylindracea, aliquando clavata, recta vel in dimidio inferiore sparse curva, basi rotundata, (15.5–)18.9–24.5(–28.5) × (5.5–)5.9–7.5(–9.0) μm, 1–3(–4)-septata, primo hyalina, partim diluta viridescentes; conidia fascientes oblique in summo loco, (1–)2–4(–6) aggregata in radiantibus capitulis. Chlamydosporae fascientes in submersis hyphis.
Colonies on MEA spreading fast, reaching 50–70 mm in 4 d; reverse first yellow turning yellowish ochraceous to crimson; margin even; pigment patchy in agar. Odour absent. Aerial mycelium moderate, cottony, 1–5 mm high, homogenous; whitish yellowish buff, hyphae turning pinkish in KOH. Submerged hyphae turning violet in KOH, cells 5–9 diam, not swollen. Conidiation moderate. Conidiophores not differentiated from aerial hyphae or with distinguishable stipe, ascending to erect or suberect, 300–1500 μm long, main axis 8–10 μm wide near base, thin-walled, hyaline, not reacting in KOH; branching evenly distributed or confined to tip of conidiophore, giving it a tree-like aspect, verticillate, occasionally dichotomous, irregular or drepanoid; side branches sometimes incorporated in verticils of conidiogenous cells at lateral position, 1–4 arising from one point, 20–35 × 3.5–5 μm, aseptate or with 1 septum, branching further once or twice, uppermost branches 15–25 × 3.5–4.5 μm. Conidiogenous cells formed on lateral branches, 3–4(–6) in a verticil; subulate, 20–45 μm long and 3–5 μm wide near base, occasionally widest in middle, attenuating gradually or sometimes abruptly in upper quarter to 0.6–1.5 μm at tip; aseptate or occasionally longer ones with one septum in the middle; forming one, sometimes refractive conidiogenous locus at tip. Conidia of variable shape,ellipsoidal or cylindrical, occasionally suballantoid, clavate or with lower and/or upper half swollen, equi- or inequilateral, straight or curved in lower half, base rounded; (15.5–)18.9–24.5(–28.5) × (5.5–)5.9–7.5(–9.0) μm, Q = (2.1–)2.8–3.5(–4.1); 1–3(–4)-septate; hilum minute, narrow, 0.5–1.5 μm high and wide, central or slightly off center; first hyaline, partially turning pale green, white to pale green in mass; formed obliquely from uppermost locus, held by (1–)2–4(–6) in radiating heads. Chlamydosporesformed on comparatively long, lateral branches of submerged hyphae, held by 2–10 in unbranched chains in terminal position; sclerotia-like aggregations absent.
Substrata: Basidiomata of Agaricales and Polyporales.
Distribution: Africa, Madagascar, South Asia.
Holotype: Madagascar, Anosy region, Tolagnaro distr., Manantantely, on Neonothopanus sp., 13 Mar. 2010, K. Põldmaa, TU 112289, ex-type culture TFC 201286 = CBS 127529.
Other specimens/cultures examined: Madagascar, Anosy region, Tolagnaro distr., Mandena Conservation Zone, littoral forest with Uapaca, Intsia, Sarcolaena, on a small agaricoid basidiomycete on a hanging branchlet, 11 Mar. 2010, K. Põldmaa, TU 112251, culture TFC 201277; same locality, on Neonothopanus sp., 18 Mar. 2010, K. Põldmaa, TU 112391, culture TFC 201319; same locality, on a decayed brown agaricoid basidiomycete, 18 Mar. 2010, E. Randrianjohany, TU 112487, culture TFC 201335; Petriky, littoral forest, on an agaricoid basidiomycete laterally attached to a living? trunk, 14 Mar. 2010, K. Põldmaa, TU 112338, culture TFC 201295. Republic of South Africa, Kwazulu-Natal Prov., Albert Falls Nature Reserve, on old aphyllophoralean basidiomycete, 15 Mar. 1995, G. Arnold A 95/12, culture G.A. i3463 = FSU 5807 = CBS 127163. Sri Lanka, Wagumba Prov., near Kurunegala, Aranyakele, 07°38'N, 80°25'E, on small agaricoid basidiomycetes, 12 Dec. 2002, K. Põldmaa, TAAM 170750, culture TFC 03-7 = CBS 127162. Uganda, Kibale National Park, on an aphyllophoralean basidiomycete, Mar. 2010, T. Tammaru, TU 112490, culture TFC 201336 = CBS 127530.
Notes: Cladobotryum indoafrum is characterised by tree-like conidiophores, monoblastic conidiogenous cells, and greenish, 1–3-septate conidia of variable shapes. In most of these features it resembles the anamorphs of H. virescens and H. paravirescens. In H. virescens the conidia are much narrower, slightly longer, and very uniform in shape, being cylindrical and always straight. The conidial measurements of H. paravirescens overlap with those of C. indoafrum but in the former conidia are mostly 1-septate with their bases acuminate. The frequently curved, 3-septate conidia of C. indoafrum resemble those of C. semicirculare, which differs in producing much wider conidiogenous cells and conidia that remain hyaline. This newly descibed species was frequent in localities near Fort Dauphin in southeastern Madagascar suggesting that it might be a common species in other parts of its geographic range.
6. Cladobotryum protrusumK. Põldmaa, sp. nov. MycoBank MB518513. Figs 4K, 12.
Fig. 12.
Cladobotryum protrusum. A–E. Conidiophores with clusters of conidia. F. Conidia at tips of conidiogenous cells. G–I. Tips of conidiogenous cells bearing irregular protrusions. J, K. Conidia. (A, I. FSU 5044. B, D. Ex-type culture, TFC 201318. C. CBS 118999. E, H. FSU 5877. F, K. FSU 5077. G. IMI 165503. J. Holotype, TU 112389. A–I, K on MEA, J on natural substratum). Scale bars: A, B = 50 μm; C–E, H = 25 μm; F, G, I = 20 μm; J, K = 10 μm.
= Sibirina purpurea var. asterophora J. D. Chen, Acta Mycologica Sinica 8: 129. 1989.
Etymology: refers to the morphology of the apex of conidiogenous cells that bears small protrusions from which conidia are formed.
In MEA substratum, conidiophora 200–700(–2000) μm longa, prope basin 7–9 μm lata. Cellulae conidiogenae subulatae, apice tumido et cum protubertionibus, ferentes aliquot locos, 25–45 μm longae, prope basin 3.5–5.0 μm latae. Conidia ellipsoidea vel cylindracea, interdum longo-clavatae vel fusiformes, (16.0–)20.0–23.0(–27.0) × (5.5–)6.2–7.9(–9.0) μm, basi attenuata, saepe curva in dimidio inferiore, (0–)1(–3)-septata, hyalina vel diluta flavovirentes; < 12 conidia ad apicem cellulae conidiogenae. Chlamydosporae non visae; structurae aggregatae similes sclerotiis carentes vel fuliginosae, pseudoparenchymatae.
Colonies on MEA spreading fast to very fast, reaching 40–70 mm in 4 d; margin even; reverse first yellowish ochraceous, turning brick, brick-brown or purple. Odour absent or faint sweetish. Aerial mycelium scarce, cottony, 1–3 mm, to 4 mm high at margin, in some strains forming pinkish tufts, whitish yellowish buff, in some strains with olivaceous tinge, turning partially purple in KOH; submerged hyphae not or slightly swollen, (5–)7–10(–12) μm diam, yellowish ochraceous, becoming roseous to pale purple in KOH. Conidiation scarce to abundant. Conidiophores with a differentiated stipe, yellowish, becoming purplish in KOH; 200–700(–2000) μm long, 7–10 μm wide near base, branching moderately, confined to top of conidiophore, verticillate or occasionally drepanoid, forming one to two levels of branches; 2–4 branches producing conidiogenous cells formed from one point, sometimes in lateral position in a verticil of conidiogenous cells, 15–50 × 3–5 μm. Conidiogenous cells 2–5 in a verticil; subulate, attenuating slightly in upper part before formation of an irregular, often transversely placed or sometimes sublgobose conidiogenous elongation that bears several protrusions, protrusions formed also in subterminal position; 25–45 μm long, 3.5–5.0 μm wide near base, attenuating to 1.0–2.0 μm below protrusion where occasionally collarette is observed; terminal elongation with protrusions 1–2.5 μm high, 2–4(–7) μm wide. Conidia ellipsoidal, sometimes cylindrical, long clavate or fusiform, (16.0)–20.0–23.0(–27.0) × (5.5–)6.2–7.9(–9.0) μm, Q = (2.0–)2.6–3.3(–4.2), attenuating at base to a narrow but prominent central to laterally placed, sometimes refractive hilum, 1–2 μm long and 0.6–1.5 μm wide; inequi- or equilateral, often curved in lower half; (0–)1(–3)-septate, septum median or rarely sub- or supramedian; hyaline to pale yellowish green, white to pale green in mass; up to 12 conidia held at top of a conidiogenous cell. Chlamydospores not observed; in some isolates dark brown sclerotia-like aggregations scattered above agar, to 1 mm in diam, forming aggregations up to 7 mm in diam; context homogenous, pseudoparenchymatous, cells ellipsoidal to subglobose 13–19 × 12–14 μm, with wall 1.0–1.3 thick, yellowish ochraceous.
Substrata: Basidiomata of Agaricales and Polyporales, wood, and bark.
Distribution: Madagascar, southern Africa, southeastern Asia.
Holotype: Madagascar, Anosy region, Tolagnaro distr., Mandena Conservation Zone, eucalypt forest, 24.952 S, 47.002 E, on Mycena sp. on decorticated wood of a burnt dead trunk, 16 Mar. 2010, K. Põldmaa, TU 112389, ex-type culture TFC 201318 = CBS 12753.
Other specimens/cultures examined: China, Fujian Province, Sanmin, on Agaricus bisporus, Chen 68, culture HMAS 54138 = FSU 5044; Guangdong, on Pleurotus ostreatus), Chen 584, culture FSU 5077. Madagascar, Anosy region, Tolagnaro distr., Mandena Conservation Zone, littoral forest with Uapaca, Intsia, Sarcolaena, on Neonothopanus sp., 11 Mar. 2010, K. Põldmaa, TU 112269, culture TFC 201281; Mandena Conservation Zone, Eucalyptus forest, 24.952 S, 47.002 E, on Neonothopanus sp. on a stump, 16 Mar. 2010, K. Põldmaa, TU 112384, culture TFC 201316. Zimbabwe, Melfort, on cultivated Agaricus sp., 13. Mar. 1972, A. Rothwell, culture G.A. i418 = IMI 165503 = CBS 127164. Republic of South Africa, Kwazulu-Natal Prov., Kwambonambi State Forest Office, Arboretum, on bark of a lying trunk of Eucalyptus sp., 17 Mar. 1995, G. Arnold A 95/32.4, culture G.A. i3542 = FSU 5877 = CBS 127165. Taiwan, Taitung, Zhiben, on polypore and adjacent bark on dead wood, 15 Aug. 2002, R. Kirschner & H.-C. Kuo 1422, TNM, culture CBS 118999.
Notes: Isolates of this new species are similar in forming mostly pale yellowish green 1-septate conidia, often acuminate and curved at base. The conidia are formed on small protrusions at the apex of the conidiogenous cell. Although species having these characters occur in the group of red-coloured Hypomyces/Cladobotryum, their unique combination clearly distinguishes C. protrusum. Two of the studied isolates, including that from Taiwan (Kirschner et al. 2007), had earlier been published as belonging to C. asterophorum, a species producing hyaline conidia from swollen apices of conidogenous cells. When reporting this species from China, Chen & Fu (1989) transferred it to Sibirina, based on the isolate Chen 584. For another strain (Chen 68), he described S. purpurea var. asterophora. This new variety was differentiated from the type by sympodial proliferation of the tips of conidiogenous cells and the formation of sclerotia. The morphological and molecular data reported herein strongly support the conspecificity of the strains from China with those from Madagascar and Zimbabwe as well as C. asterophorum and C. purpureum as distinct species (Fig. 1). These two taxa have not been found in the tropics and will be treated elsewhere together with other temperate red-coloured Hypomyces/Cladobotryum.
Most of the reported anamorphic isolates of C. protrusum differ from C. asterophorum by the tree-like conidiophores, profusely branched at the apex, protrusions formed at conidiogenous elongations that are often transversely placed at the apices of conidiogenous cells, and formation of greenish conidia. The conidia of C. protrusum are also slightly longer and much wider than in C. asterophorum, with 2–3-septate ones occurring among the prevailing conidia with one septum. A distinguishing feature is also the formation of up to 5 or 12 conidia at the apex of each conidiogenous cell in C. asterophorum and C. protrusum, respectively, as well as colony characters.
All the strains from Africa, China, and Madagascar exhibit only two bp difference among all the four gene regions studied. Despite the genetic homogeneity, considerable morphological variation was observed among the strains of C. protrusum from these distant regions. Namely, G.A. i3542 differs by having much less branched conidiophores that do not appear tree-like. In the isolates Chen 584 and TFC 201281 3–5 conidiophores are formed from one point on the aerial hyphae, with conidia remaining hyaline in the former. Both isolates from China are similar to the ex-type strain in the olive tinge of the aerial mycelium and in changing the agar reddish brown to dark-brown. In these strains, except for Chen 584, pinkish tufts are formed among aerial mycelium that develops into dark, tough sclerotia-like aggregations, common in C. purpureum (pers. obs.) and identical to those described in detail for C. paravirescens. In Chen 68 the terminal swellings or elongations on the conidiogenous cells are lacking or are much less developed than in other strains in which there are only a few subterminal protrusions. In this strain and TFC 201281 protrusions are scattered at the top of the conidiogenous cell with up to four denticles forming a subterminal ring in addition to the ones below. The strain from Taiwan isolated from a polypore and wood (CBS 118999) forms the sister-group to other strains, isolated from agaricoid basidiomycetes. Except for the somewhat larger mean conidial length (23 μm), it agrees morphologically with the other strains. While the greenish colouration of conidia is most obvious in the strain from IMI and Chen 68, they vary from hyaline to green in the other isolates. The colour is less intense than in H. paravirescens and H. virescens, with conidial masses often not appearing greenish when observed with the naked eye.
7. Cladobotryum paravirescens K. Põldmaa, sp. nov. MycoBank MB518512. Figs 4L, 13.
Fig. 13.
Cladobotryum paravirescens, ex-type culture TFC 97-23 on MEA. A. Conidiophore. B–D. Topmost parts of conidiophores with conidiogenous cells and conidia. E, F. Conidiogenous cells with conidia at their tips. G, H. Conidiogenous cells formed in verticils. I, J. Conidia. K. Chlamydospores. Scale bars: A = 100 μm; B, C = 30 μm; D, G = 20 μm; E, F, H–K = 10 μm.
Etymology: refers to the similarity to the anamorph of H. virescens although phylogenetically not closely related.
In MEA substratum, conidiophora 300–600 μm longa, prope basin 6–9 μm lata. Cellulae conidiogenae subulatae, cum unum vel paucos conidiogenos locos, 25–35 μm longae, prope basin 4.0–5.0 μm latae. Conidia ellipsoidea, interdum clavatae, (18.0–)22.5(–27.5) × (6.5–)8.3(–10.0) μm, basi attenuata, saepe curva in dimidio inferiore, 1(–3)-septata, hyalina vel diluta flavovirentes; 2–3(–4) conidia ad apicem cellulae conidiogenae. Chlamydosporae sublgobosae, 14–17 × 12–14 μm, hyalinae vel ochraceae, catenatae; structurae aggregatae similes sclerotiis fuliginosae, pseudoparenchymatae.
Colonies on MEA spreading fast, reaching 70 mm in 4 d; reverse first ochraceous or yellow turning crimson; margin even. Odour absent. Aerial mycelium moderate, cottony,up to 4 mm high, reaching lid of Petri dish at margin; buff, obtaining greenish tinge with formation of conidia, hyphae turning purple in KOH. Submerged hyphae often turning purple in KOH, cells infrequently becoming swollen. Conidiation abundant, often in patches. Conidiophores not differentiated from aerial hyphae; ascending to suberect or erect, main axis 300–600 μm long, 6–9 μm wide, thin-walled, brownish yellow, except at apex, pigmented parts turning purple in KOH; branching moderate to profuse, verticillate or irregular, often drepanoid, mostly in uppermost part; conidiophores often borne as side branches from a verticil of conidiogenous cells, further branching at apex; lateral branches supporting 1–3 conidiogenous cells, 25–35 μm long and 4.0–5.0 μm wide, often integrated into a previously formed verticil. Conidiogenous cells 3–4(–6) in a verticil; subulate, 30–40 μm long, 3–4.5 μm wide near base, attenuating to 0.7–1.3 μm at apex;aseptate, forming one conidiogenous locus at tip or occasionally an additional locus on a small protrusion in the middle of cell or ca. 3–5 μm from tip. Conidia ellipsoidal, some slightly clavate, mostly inequi–, some equilateral, straight or curved in lower half, mostly attenuated towards base; (18.0–)22.5(–27.5) × (6.5–)8.3(–10.0) μm, Q = (2.2–)2.7(–3.3); 1(–3)-septate, septum median or sometimes sub- or supramedian; hyaline or pale yellowish green, pale green in conidial masses in culture; hilum prominent, narrow, refractive, central or laterally placed; conidia formed obliquely from uppermost locus, held by 2–3(–4) in radiating heads. Chlamydospores formed among submerged mycelium, cells subglobose, 14–17 × 12–14.5 μm, wall 0.7–1 thick, hyaline to pale ochraceous, in intercalary or lateral chains; dark brown sclerotia-like aggregations scattered above agar, to 1 mm diam, homogenous, pseudoparenchymatous, cells 13–19 × 12–14 μm, with wall 1.0–1.3 thick, yellowish ochraceous.
Substrata: Aphyllophoralean basidiomycete.
Distribution: Southeastern Asia, known only from the type locality.
Holotype: Thailand, Khao Yai National Park, Nature trail km 33 to Nong Pak Chi, on an aphyllophoralean basidiomycete, 1 Aug. 1997, K. Põldmaa, TAAM169726, ex-type culture TFC 97-23 = CBS 100366.
Notes: Cladobotryum paravirescens is distinct in producing yellowish green, mainly 1-septate conidia with acuminate bases that are often curved. The colouration of conidia, led to the original identification of these two Thai collections as C. virescens (Põldmaa & Samuels 2004), previously the only known species producing red pigment and green conidia. Furthemore, the branching system of conidiophores and the mode of conidiation in the Thai specimen resembles those observed in H. virescens. The main distinguishing features include much broader, mostly ellipsoidal, 1-septate conida with curved acuminate bases in C. paravirescens compared to the narrow cylindrical, 1–3-septate, straight conidia of H. virescens. Cladobotryum paravirescens forms dark tough sclerotia-like aggregations, common in cultures of temperate species. Among other red-pigmented tropical Hypomyces/Cladobotryum these have been observed only in C. protrusum.
The molecular data presented herein clearly support the distinctness of C. paravirescens from H. virescens, revealing its affinities with an isolate from China (Chen 339-2A = FSU 5046) and the single known isolate of C. asterophorum, both of which produce hyaline conidia. The clade joining these three isolates forms the sister-group of C. protrusum, characterised by green conidia and prominent protrusions at the apices of conidiogenous cells. Among this group of species C. paravirescens is distinguished by having green conidia and conidiogenous cells with simple tips. Occasionally single inconspicuous outgrowths were observed in the middle or upper part of the conidiogenous cell. The frequently drepanoid branching of conidiophores resembles that described for C. asterophorum (de Hoog 1978). In contrast to this species, the conidia of C. paravirescens are green and wider, with a few 2-septate conidia usually present. In these features as well as the conidial shape and size, C. paravirescens is similar to C. protrusum. Although appearing most closely related to C. paravirescens (Fig. 1), the isolate Chen 339-2A differs in having hyaline, 0–1-septate, straight, ellipsoidal conidia that are smaller, (11.5–)15.7(–20.0) × (5.5–)6.6(–7.7), Q = (1.8–)2.4(–3.0). The conidiogenous cells attenuate into simple apices with one locus that forms 2–3 conidia. The isolate Chen 339-2A is similar to C. paravirescens in the abundant production of sclerotia-like aggregations which, however, are more light-coloured. This isolate was originally identified as Sibirina purpurea var. purpurea (Chen & Fu 1989). This species, now regarded as C. purpureum, was described from Alabama, USA. According to the morphology and phylogenetic analyses of molecular data, it is a distinct species. The Chinese strain Chen 339-2A probably represents an undescribed species, with additional strains reported by Chen & Fu (1989).
8. Hypomyces australasiaticusK. Põldmaa, sp. nov. MycoBank MB518515. Figs 2C, 14.
Fig. 14.
Hypomyces australasiaticus. A. Teleomorph effused over the host's hymenophore. B. Perithecia scattered in the subiculum. C. Ascospores. D–J. Conidiophores with conidiogenous cells and conidia. K. Apex of a conidiogenous cell with a cluster of conidia. L, M. Conidia. (A, B. Holotype, TAAM 170757; C. BPI 745759; D, E, G–K, M. Ex-type culture, TFC 03-8; F, L. TFC 99-95; A, B on Earliella scabrosa, D–M on 7 d MEA). Scale bars: A = 1 cm; B = 0.5 cm; D–H = 50 μm; I, J = 20 μm. C, K–M = 10 μm.
Etymology: refers to the presumable geographic range of the species.
Subiculum effusum super hospitis hymenophorum; perithecia dispersa, semidimmersa vel fere superficialia, obpyriformia, 330–400 × 260–300 μm, coccinea purpurescentia; papilla (55–)100–120 μm alta, basi (80–)100–130 μm lata. Asci cylindrici, 140–160 × 7–8. Ascosporae fusiformes, (20.5–)23.4–23.8(–26.0)× (4.5–)5.2–5.9(–6.5) μm, septo mediano, parietibus verrucosis, apiculo (2.0–)3.5–3.9(–4.6) μm longo. Conidiophora 3.5–5.5 μm lata; cellulae conidiogenae subulatae vel fere cylindraceae, 25–50 μm longae, basi 2.5–4.0 μm latae. Conidia cylindracea vel (oblonga) clavata, recta, (10.0–)15.8(–21.0) × (3.5–)5.2(–7.0), 1–3-septata, hyalina.
Subiculum effused over most of the host's hymenophore; thin, cottony, whitish to pink, crimson or purplish, turning purplish in KOH; hyphae 3–4 μm diam. Perithecia scattered among subiculum, singly or in groups of two to three, semi-immersed to almost superficial, obpyriform, 330–400 × 260–300 μm, crimson, turning purple in KOH, wall ca. 20 μm thick, with inner cells flattened, those on surface swollen, 17–27 × 15–15 μm, walls 1–2 μm thick; papilla conical, (55–)100–120 μm high, (80–)100–130 μm wide at base, tapering to 20–40 μm at apex, cells on surface of base swollen, 18–24 × 15–19 μm, apex obtuse. Asci cylindrical, 140–160 × 7–8 μm, apex slightly thickened, 1.0–1.5 μm; ascospores uniseriate with ends overlapping. Ascospores fusiform, inequilateral, (21.5–) 23.8–24.7(–27.0) × (4.5–)5.2–5.7(–6.5) μm, Q = (4.0–)4.3–4.6(–5.3), ascospore body (14.0–)16.1–17.1(–18.5) × (4.0–)4.4–4.6(–5.0) μm, Q = (3.0–)3.7–3.8(–4.3); septum median; wall verrucose to warted, warts discrete or confluent, 0.3–0.7 μm high; apiculi conical, occasionally hat-shaped or hooked, (2.5–)3.5–3.9(–4.5) μm long, (1.5–)2.0(–2.5) μm wide at base, apices obtuse.
Mycelium delicate, scarce, whitish, effused over host, apart from subiculum, bearing erect conidiophores with stipes 3.5–5.5 μm wide. Conidiogenous cells held by 1–3, subulate to almost cylindrical, 25–50 μm long, attenuating from 2.5–4.0 at base to 1–2.3 μm at apex. Conidia cylindrical or clavate to oblong clavate, (10.0–)15.8(–21.0) × (3.5–)5.2(–7.0), Q = (2.1–)3.1(–4.1), 1–3-septate, equilateral, straight, hilum mostly wide and laterally displaced or narrow and central.
Colonies on MEA growing fast, reaching 50–60 mm in 4 d; reverse first uncoloured, turning slowly reddish purple; margin even. Odour absent or sweet. Aerial mycelium abundant, cottony, to 7 mm high, homogenous, white, hyphae not changing colour in KOH. Submerged hyphae turning purple in KOH, cells not swollen. Conidiation moderate or abundant. Conidiophores not differentiated from aerial hyphae; ascending to suberect, to 1700 μm long, main axis 2.5–3 μm wide, thin-walled, unbranched but forming numerous short lateral branches, 19–24 × 2–3 μm, bearing verticils of conidiogenous cells or an additional level of supporting branches. Conidiogenous cells formed on supporting branches, sometimes directly on conidiophore, 3–6 in verticils, sometimes sinlgy or in pseudoverticils just below true verticil; subulate to almost cylindrical, 20–50 μm long and 1.5–3 μm wide near base, attenuated to 0.8–1.9 μm at apex; aseptate or occasionally with one septum in the middle, forming one conidiogenous locus at apex. Conidia oblong clavate, clavate, ellipsoidal or cylindrical, occasionally long fusiform, mostly equilateral, straight or curved in lower half; (15.0–)20.7–24.4(–35) × (3.5–)4.8–5.6(–6.5) μm, Q = (2.8–)3.7–5.1(–7.0), 1–3-septate, hyaline; hilum low, 0.5–1.3 μm high, narrow to very broad, 0.8–2.2 μm wide, central or slightly off-center; conidia formed obliquely from uppermost locus, 4–8(–10) in imbricate chains that appear as radiating heads. Chlamydospores and sclerotia-like aggregations absent.
Substrata: Basidiomata of Polyporales.
Distribution: Australia, south and southeastern Asia.
Holotype: Sri Lanka, Wagumba Prov., near Kurunegala, Aranyakele, 07°38'N, 80°25'E, on Earliella scabrosa, 12 Dec. 2002, K. Põldmaa, TAAM 170757, ex-type culture TFC 03-8 = CBS 127152.
Other specimens examined: Australia, Queensland, Daintree Nat. Park, Daintree Rainforest Environmental Centre, near Oliver Creek, on Microporus affinis, 27 Aug. 1999, K. Põldmaa, TAAM 170182, anamorph only, culture TFC 99-95 = CBS 127153. Thailand, Saraburi Province, Khao Yai National Park, Haew Narok, on Antrodiella sp., 13. Aug. 1996, G. J. Samuels 8431 & P. Chaverri, BPI 745759, accompanied by a dried culture; TU 112950; no living culture preserved.
Notes: Among the three collections of H. australasiaticus, only the one from Sri Lanka contains the teleomorph accompanied by a living culture. Therefore it was selected as the holotype despite the fact that the abundant perithecia are mostly overmature, asci have disintegrated, and many of the ascospores are swollen. In the Thai collection, ascospores are more slender, apiculi longer, and the ornamentation composed of larger confluent warts compared to the wider ascospores with more fine verrucose pattern observed in the specimen from Sri Lanka. The observed size variation may be the result of age differences when the specimens were dried. The material from Thailand also contains an anamorph that is absent in the specimen from Sri Lanka.
The teleomorph of this new species shares the typical features of the basidiomyceticolous members of Hypomyces with Cladobotryum anamorphs. It differs from the red tropical species with most similar teleomorphs, H. samuelsii and H. virescens, only by slightly smaller ascospores (Figs 2, 3). The characteristics of cultures and the anamorph provide further delimitors from related taxa. The cultures are distinct due to the abundant, white aerial mycelium from which arise very long, mostly unbranched conidiophore systems. These bear only very short lateral branches that form verticils of conidiogenous cells either directly or on a further level of supporting branches. Conidiogenous cells are narrow, tapering only slightly towards the apex. The conidiogenous locus produces numerous conidia that are held obliquely to almost horizontally with their bases connected, reminescent of the pattern observed in C. cubitense and the secondary anamorph type of H. gabonensis. Hypomyces australasiaticus is also distinct in the often clavate-shaped conidia. In these characteristic features the anamorph is reminiscent that of H. polyporinus, H. pseudopolyporinus (Rogerson & Samuels 1993, Põldmaa & Samuels 1999) and H. puertoricensis (Põldmaa et al. 1997). However, these species all have pallid, KOH-negative perithecia and cultures producing KOH-negative cocoa-brownish pigments. Cladobotryum curvatum (de Hoog 1978), known only from the type collection from Java, is similar to the anamorph of H. australasiaticus as well as to C. cubitense and C. semicirculare. However, conspecificity cannot be tested due to the lack of a living culture.
In the cultures obtained from the specimens from Australia and Sri Lanka, dimorphic conidia were observed. In addition to the comparatively long (up to 47 μm in older cultures), 1–3-septate conidia, small, 0–1-septate conidia measuring 10–17 × 3.5–6.0 μm, Q = 2.2–3.6 were found. These were abundant in one of the monosporic isolates from the Sri Lankan specimen.
Based on the few available collections H. australasiaticus is presumed to be distributed in southeastern Asia and Australasia. It is the sister species of C. semicirculare, some populations of which are apparently sympatric with H. australasiaticus in Asia. Their morphological similarities include indistinct conidiophores.
9. Cladobotryum semicirculare G.R.W. Arnold, R. Kirschner, Chee J. Chen, Sydowia 59: 118. 2007. Figs 4I–J, 15.
Fig. 15.
Cladobotryum semicirculare on MEA. A–C. Upper parts of conidiophores bearing conidiogenous cells with conidia held in radiating heads. D, E. Verticillately placed conidiogenous cells. F–H. Conidia. I. Chlamydospore. (A, B, D, G, I. Ex-type culture, CBS 705.88; C, H. TFC 03-3; E. Isotype, IMI 394236; F. Holotype of H. paeonius, K(M) 168029). Scale bars: A–C = 30 μm; D = 15 μm; E–I = 10 μm.
Mycelium whitish, effused on host; conidiogenous cells subulate, 12–24 μm long, 2–4 μm wide at base, 0.5–1.5 μm at tip, with tips sometimes curved; conidia (11.5–)15.0–18.5(–25.0) × (4.5–) 5.6–6.2(–8.0) μm, Q = (2.1–)2.7–3.0(–3.6); chlamydospores subglobose to globose, 13–15 μm diam, wall 1.0–1.5 μm thick, smooth.
Colonies on MEA spreading moderately fast, reaching 25–35 mm in 4 d; reverse ivory or uncoloured initially, turning yellowish buff or ochraceous, finally reddish brown; margin even. Odour absent or faint, reminescent of agarics. Aerial mycelium scanty or abundant, cottony, 2–7 mm high, homogenous, primary axes of hyphae wider than secondary branches, sometimes becoming moniliform or with single cells inflated, white, partially turning purple in KOH. Submerged hyphae with cells occasionally swollen, partially turning (pale) purplish red in KOH. Conidiation very abundant in fresh isolates, becoming moderate to scarce in old isolates. Conidiophores arising from aerial hyphae at right angles, not differentiated or wider from these, ascending to suberect, 70–450 μm long, main axis 3–5 μm wide; branching moderate, irregular or verticillate, mostly evenly distributed; lateral branches arising mostly singly from one point, 15–35 × 3–4 μm. Conidiogenous cells formed on conidiophores or supporting branches, 2–4 in a verticil, occasionally also formed singly just below verticil, verticils not always symmetrical, not all conidiogenous cells formed at same level; subulate, 20–40 μm long, 2–3 μm wide at base, attenuating gradually to 0.6–1.5 μm at apex, aseptate or sometimes with one septum, often slightly curved at apex, forming one conidiogenous locus at apex. Conidia ellipsoidal, clavate, sigmoid or semicircular, occasionally with two shallow branches at tip; inequilateral, often curved or straight, base rounded or acuminate, sometimes slightly attenuated at ends, then subfusiform; (12.0–)16.6–19(–25.5) × (4.5–)5.2–5.8(–7.2) μm, Q = (2.4–)3.2–3.4(–4.4), 1–3-septate, hyaline; with minute hilum of variable width, central or slightly off-center; formed obliquely from uppermost locus, held by 3–6(–8) in imbricate chains that appear as radiating heads. Chlamydosporesformed among aerial mycelium, cells subglobose, 11–18 μm diam, wall 0.6–1.0 μm thick, ochraceous, in short chains on supporting cells or forming irregular clusters.
Substrata: Basidiomata of Agaricales and Polyporales.
Distribution: Central America, south and southeastern Asia.
Specimens/cultures examined: Cuba, Prov. Habana, Santiago de las Vegas, on old polypore, 9 Jan. 1985, G. Arnold A 85/185, isotype IMI 394236, ex-type culture CBS 705.88; same locality, on old agaric, 29 July 1985, G.A. A85/380, culture i1393; same locality, on Lentinus scleropus, 12 Aug. 1987, R. Castañeda, G. Arnold INIFAT C87/249, i 1715, CBS 533.88. Sri Lanka, on a resupinate polypore, Nov. 1867, G. H. K. Thwaites 127, K(M) 168028, ex herb. Berkeley, holotype of H. paeonius; K(M) 168029, ex herb. Broome, isotype of H. paeonius; Sabaragumuwa Prov., Sinharaja Man and Biosphere Reserve, Morning site, near bungalow in a forested slope, 06°24'N, 80°36'E, elev. 1035 m, 10 Dec. 2002, K. Põldmaa, TAAM 170728, culture TFC 03-2 = CBS 112421; Wagumba Prov., near Kurunegala, Aranyakele, 07°38'N, 80°25'E, on Pterula sp. cf. P. typhuloides on a decaying log, 12 Dec. 2002, K. Põldmaa, TAAM 170744, culture TFC 03-3.
Notes: Observations on the examined strains of Cladobotryum semicirculare generally match the protologue, except that the conidiophores and conidiogenous cells are much narrower and the conidia are wider. Kirschner et al. (2007) stated that strains from Cuba have larger conidia than those from Taiwan. According to my observations, conidia in the studied isotype measured (12.0–) 18.5(–25.0) × (4.5–)6.2(–8.0) μm, Q = (2.3–)3.0(–3.6) and those in the ex-type strain (13.5–)18.6(–25.5) × (4.5–)5.8(–7.0) μm. In the strains from Sri Lanka conidia were slightly shorter and narrower, (11.5–)16.6(–21.0) × (4.5–)5.2(–7.0) μm. The latter agree with the measurements given by Kirschner et al. (2007) for Taiwanese material for which no living strain is available. These morphological and genetic differences (data not shown) between the strains from Cuba and those from Asia indicate that these may represent two species sharing the unusual shape of conidia.
In C. semicirculare the branching of the conidiophorous system is much less pronounced than in most species of the group. The conidiogenous cells are usually held in the uppermost and one, seldom two, lower verticils that are formed on conidiophore-like branches of the aerial mycelium. Additional short branches supporting the verticils may also be involved. In this regard, C. semicirculare resembles the anamorph of its sister species, H. australasiaticus with even more poorly developed conidiophores. Conidiogenous cells of C. semicirculare with tips that are often curved are unique in the group. Although lower parts of the conidia are curved in several species, most often in C. cubitense and C. indoafrum, they are strongly curved, with some appearing almost circular in C. semicirculare.
Berkeley and Broome (1875) described Hypomyces paeonius based on material collected by Thwaites in Ceylon (= Sri Lanka) in 1867. The protologue describes a widely effused roseous fungus having subcymbiform spores with obtuse tips. In addition, conidia, resembling those of H. rosellus are mentioned. Upon examination of the holo- and isotype from Kew I could not find any perithecia or their initials among the purplish red mycelium covering a resupinate polypore. The same had been stated by Clark T. Rogerson and G. Arnold, whose notes are accompanying the type materials. These include also drawings (presumably by Berkeley) showing 1-septate curved ellipsoidal ascospores with obtuse ends, irregularly arranged in upper half of a clavate ascus. Although not suggestive of a species of Hypomyces, these could represent an immature collection of a member of this genus. Both the holo- and isotype material contain a well developed anamorph. The 1–3-septate ellipsoidal, often strongly curved hyaline conidia, measuring (11.5–)15.0(–18.5) × (4.5–)5.6(–7.0) μm with Q = (2.1–)2.7(–3.3), are formed on subulate conidiogenous cells, 10–22 μm long and attenuating from 2.0–4.5 μm at base to 0.5–1.5 μm at tip. With a proportion of conidia being semicircular, the anamorphs in these specimens clearly match the recently described C. semicirculare. Yet, because the holo- and isotype of H. paeonius do not contain teleomorph structures and the protologue with accompanying figures cannot unequivocally be considered as representing a species of Hypomyces, this material has to be regarded as a collection of the anamorphic C. semicirculare Petch (1912) accepted H. paeonius identifying two other specimens, collected from Sri Lanka at the beginning of 20th century, as belonging to this species. The strongly verrucose ascospores 25–30 × 5–7 μm and narrow-oval or clavate, 1–2-septate hyaline conidia, 15–28 × 5–6 μm, described by Petch (1912) match the characteristics of H. australasiaticus. However, the examination of one of these specimens [Petch 2345 = K(M) 168030] revealed ochraceous perithecia with faint purplish colour observed in KOH only at the base of the papilla and upper part of the venter. The ascospores inside asci appeared lanceolate or narrow-oval and non-apiculate as noted by Petch but the detached ascospores were grossly warted, bearing prominent, (2.0–)4.1(–6.0) μm long apiculi. These, as well as the ascospores, measuring (21.0–)26.5(–32.0) × (4.5–)5.9(–7.0) μm, Q = (3.6–)4.6(–5.5), were larger than in H. australasiaticus. Whereas no anamorph could be seen, the identity of this material remains unclear.
10. Hypomyces aconidialis K. Põldmaa, sp. nov. MycoBank MB518608. Fig. 16.
Fig. 16.
Hypomyces aconidialis. A–C. Pulvinate stromatic subiculum with perithecia. D. Perithecium. E. Chains of chlamydospores. F. Cells at the surface of perithecium. G. Ascospores. (A, B, E–G. Ex-type culture, TFC 201334; C, D. TFC 201215). Scale bars: A, C = 1 mm; B = 500 μm; D = 50 μm; E–G = 10 μm.
Etymology: indicates the absence of conidia, found neither on the host nor in culture.
Teleomorphosis crescens in MEA substrato; perithecia inclusa in pulvinato simile stromate subiculo. Perithecia dense aggregata, immersa, obpyriformia, 320–410 × 200–300 μm, rubra flavescentia; papilla conica, 90–140 μm alta, basi 90–130 μm lata. Ascosporae fusiformes, (13.0–)14.3–15.3(–16.5) × (3.5–)4.0–4.2(–5.0) μm, septo mediano, habentes densum breve tuberculare ornamentum; apiculo 2.0–3.0(–4.0) μm longo.
Teleomorph produced in culture on MEA; restricted patches turning into pulvinate stroma-like subiculum with embedded perithecia. Subiculum dense cottony mat, buff to roseous, of tightly interwoven hyphae; hyphae hyaline to pale crimson, turning purple in KOH, 2.5–4 μm wide, cells not swollen. Perithecia caespitose, immersed, flask-shaped, 320–410 × 200–300 μm; yellowish red, with whole perithecium or only the base of papilla turning purple in KOH; wall of a single region of flattened, thin-walled cells, at surface cells broadly ellipsoidal, 15–30 × 13–21 μm; papilla conical, 90–140 μm high, 90–130 μm wide at base, attenuating to 40–60 μm at apex, apex obtuse, with oblong-clavate cells, 5.0–8.0 μm diam at surface. Ascospores fusiform, equilateral, (18.0–)20.2–20.3(–22.5) × (4.5–)5.1–5.2(–5.5) μm, Q = (3.3–)3.9–4.0(–4.6); ascospore body (13.0–)14.3–15.3(–16.5) × (3.5–)4.0–4.2(–5.0) μm, Q = (2.9–)3.4–3.9(–4.5); 1-septate, septum median; densely tuberculate, ornamentation < 0.5 μm high; apiculi 2.0–3.0(–4.0) μm long, 1–2 μm wide at base, straight, simple or hat shaped, tips obtuse.
Colonies on MEA growing moderately fast to fast, reaching 25–50 mm in 4 d, reverse first yellowish ochraceous, turning slowly into yellowish or purplish red; margin even or fasciculate. Odour absent. Aerial mycelium moderate to profuse, cottony, to 3 mm high, homogenous or partly fasciculate, pale whitish or yellowish buff. Submerged hyphae partly turning purple in KOH, cells not swollen. Conidiation absent. Some parts of aerial hyphae becoming moniliform, with cells turning into chlamydospores, cells swollen, 9–15 μm diam, wall 1–1.5 μm thick, brownish yellow.
Substrata: Basidiomata of Tricholomataceae (Agaricales).
Distribution: Central Africa, Madagascar.
Holotype: Madagascar, Anosy region, Tolagnaro district, Mandena Conservation Zone, littoral forest with Uapaca, Intsia, Sarcolaena, on Mycena sp. on wood, 18 Mar. 2010, E. Randrianjohany, TU 112486, dried culture containing the teleomorph deposited together with anamorph material on natural host; ex-type culture TFC 201334 = CBS 127527.
Other specimen examined: Gabon, Crystal Mountains National Park, Tchimbele, on an agaricoid basidiomycete (Tricholomataceae, cf. Gerronema) on wood, 6 May 2009, K. Põldmaa, TU 112133, culture TFC 201215 = CBS 127526.
Notes: Both collections of Hypomyces aconidialis contain delicate whitish mycelium without any structures associated with conidiation. In the material from Gabon, there is scanty mycelium loosely attached to the gills of the host. In the holotype, more profuse mycelium covers most parts of the decayed host. In addition, there are small patches of subiculum with perithecial initials on a piece of adjacent wood.
This species appears unique because isolations from both collections do not form any conidiophores or conidia on any of the three inoculated media. However, on MEA both strains produce pulvinate stroma-like subiculum with immersed perithecia. This feature distinguishes H. aconidialis from other red-perithecial species that all have effused, comparatively thin subiculum. The abundantly produced, mature ascospores with tuberculate ornamentation are smallest among the five tropical species for which the teleomorph has been observed (Fig. 3).
Cultures isolated from the holotype differ from the those isolated from the other specimen in having faster growing colonies, production of crystals in agar, and absence of fasciculate growth.
11. Cladobotryum cubitense R.F. Castañeda & G.R.W. Arnold, Feddes Repertorium 98: 414. 1987. Figs 5A, 17.
Fig. 17.
Cladobotryum cubitense. A–C. Upper parts of conidiophores bearing conidiogenous cells with transversely placed conidia at their tips. D. Conidia. E. Chlamydospores. (A–D. TFC 07-13, 9 d on MEA; E. Ex-type culture, G.A. i1361, 3 mo on MEA). Scale bars: A = 100 μm; B = 50 μm; C, E = 20 μm; D = 10 μm.
Mycelium on host cottony, buff, producing erect conidiophores, ca. 8 μm wide near base, branching at top; conidiogenous cells subulate, (15–)20–30 long, 2.5–4.0 μm wide in widest place, gradually attenuating to 0.8–1.8 μm at apex bearing one locus, held by 2–3 on short lateral branches. Conidia mostly cylindrical, some slightly curved, rarely sigmoid, (20.0–)23.7–25.0(–30.0) × (6.0–) 6.6–7.0(–8.0) μm, Q = (2.9–)3.6(–4.3), hyaline, 3-septate; hilum laterally displaced, held transversely at the apex of conidiogenous cell.
Colonies on MEA spreading comparatively slowly, 15–25 mm in 4 d; margin even to slightly fasciculate; reverse first pale yellowish ivory or ochraceous, becoming paler purplish in some isolates. Odour absent or faint sweet. Conidiation abundant in fresh isolates. Aerial mycelium moderate, cottony, becoming compacted near inoculum, whitish to buff, 1–3 mm high. Submerged hyphae not swollen, turning purple in KOH. Aerial hyphae arising from agar, extending several millimeters, producing single branches at irregular intervals that function as conidiophores or branch further in irregular manner. Conidiophores not differentiated from aerial hyphae or with a well-defined stipe, ascending to suberect, 300–900 μm long, 7.5–9.0 μm wide near base, branched at top with one to three levels of branches ultimately bearing verticils of conidiogenous cells, hyaline or yellowish ochraceous, then turning purplish red in KOH; 2–4 branches supporting conidiogenous cells formed from one point, 18–35 × 2.5–4.0 μm. Conidiogenous cells held by 2–3, subulate or long ampulliform, occasionally attenuating slightly towards base, (15–)20–30(–50) μm long and 2–3.5 μm wide in widest place, gradually attenuating to 0.6–1.5 μm at apex bearing one locus. Conidia cylindrical, rarely ellipsoidal, often irregularly shaped, curved at base, lower half or middle, some sigmoid, (15.0–)19.5–25.0(–28.5) × (4.5–)5.5–7.5(–8.5) μm, Q = (2.1–)2.9–3.7(–4.6), hyaline, (1–)3-septate, hilum narrow to wide, central or at side of conidium, sometimes with a scar of attachment at base of conidium in opposite position to hilum; formed transversely from conidiogenous locus, held by 3–5(–12) in imbricate chains at apex of conidiogenous cell, rarely singly at intercalary loci on conidiophores. Chlamydospores abundant in old cultures, in and on agar, cells subglobose to globose, 10–30 μm diam, pale yellowish to ochraceous, wall 1–1.5 μm thick, smooth, held by a few to a dozen in chains or forming irregular clusters often held on thin-walled, hyaline supporting cell arising from an intercalary cell of aerial or submerged hyphae.
Substrata: Agaricoid, corticioid and cyphelloid basidiomata, wood.
Distribution: Tropical North, Central and South America, Madagascar.
Specimens and/or cultures examined: Cuba, Camagüey Prov., Sierra de Cubitas, Hoyo de Bonet, on dead fruiting bodies of an agaricoid basidiomycete, 6 Mar. 1985, R. Castañeda C85/29, holotype: INIFAT permanent slide, ex-type culture G.A. i1361 = CBS 416.85; Guantanamo Prov., Sierra de Imias, Las Cabezas del Arroyo Los Cacaos, on fruitbody of an old agaricoid basidiomycete, 9 Apr. 1984, G. Arnold, culture m643.w, GA 84/688-w white form, TFC 98-35. Madagascar, Anosy region, Tolagnaro distr., Petriky, littoral forest, on Cyphellaceae, 14 Mar. 2010, K. Põldmaa, TU 112334, culture TFC 201293; Mandena Conservation Zone, Eucalyptus forest, 24.952 S, 47.002 E, on wood next to Rigidoporus sp., 16 Mar. 2010, K. Põldmaa, TU 112379b, culture TFC 201294; same collecting data, on a corticioid basidiomycete on a living trunk of Eucalyptus sp., TU 112380, TFC 201315 = CBS 127528. Peru, Junin Dept., Chanchamayo Distr., Kimiri, on fruiting bodies of an agaricoid basidiomycete cf. Lentinus sp., 2 Mar. 2007, K. Põldmaa, TU 107195, cultures TFC 2007-13 = CBS 121646. USA, Louisiana, near Walker, Livingston Parish, on a log, 23 Aug. 1960, C. T. Rogerson 60-189, NY, anamorph and immature teleomorph.
Notes: Characteristic of Cladobotryum cubitense are the comparatively long, narrow, clavate, mostly 3-septate conidia that are held horizontally at the tip of the conidiogenous cell. Indicative of the formation of conidia in long imbricate chains are the hila, often observed at the side of the conidia, close to the base. The more conidia are produced from one locus, the lower, wider and more laterally placed become the hila on the successive conidia. In most isolates part of the conidia are curved. Dinstinctive of the species are also the pale ochraceous, irregular aggregations of thick-walled swollen cells, often held on a thin-walled, hyaline supporting cell as in anamorphs of Mycogone. These are formed abundantly in old cultures. The more recently isolated strains from Madagascar and Peru differ from the two isolates from Cuba by the profuse branching of conidiophores, abundant conidiation, and much smaller conidiogenous cells.
All examined living cultures of C. cubitense have been isolated from the anamorph found in nature. In the collection from Louisiana, USA, the typical anamorph structures are accompanied by the teleomorph. It comprises buff subiculum with irregular pinkish patches effused over a resupinate polypore. The colour pattern of perithecia follows that of the subiculum, with the roseous perithecia turning pale purplish in KOH but the buff perithecia not changing colour. These characters fit the morphology expected for the sister taxon of H. gabonensis, described below. The red pigment is absent or less developed in both of these species that form the sister group to the rest of the red-coloured Hypomyces/Cladobotryum. In the specimen from Louisiana, the scattered perithecia are immersed except for the papilla that is mostly covered with a waxy cap of agglutinated extruded asci. The ascal content, however, has only started to differentiate, for what reason mature ascospores could not be observed. The oblong-clavate 1–4-septate conidia measure 20.0–40.0 × 6.5–9.0 μm and have a distinctive wide, often laterally placed flat base. The 0–1-septate and 30–50 μm long conidiogenous cells, attenuate from 3–5.5 μm at base to 1.5–2.5 μm at tip. While this collection is believed to represent C. cubitense, describing the teleomorph has to await for a collection with mature ascospores. Whether the four collections with pale subiculum but purplish red perithecia, described in the section describing uncultured collections, also belong to C. cubitense needs isolation of cultures from similar fresh collections on Rigidoporus spp.
Regarding colony characteristics, C. cubitense resembles H. gabonensis in which colonies grow more slowly, aerial mycelium is intensively buff, and the reverse remains ochraceous or turns pinkish. The type strain of C. cubitense was obtained consisting of a red and white form with the latter remaining uncoloured on different media. The conidial apparatus of C. cubitense is similar to that of the secondary anamorph of H. gabonensis, descibed below, and to the anamorph of H. khaoyaiensis. However, the latter has pallid, KOH-negative perithecia and does not produce red pigment in culture (Põldmaa & Samuels, 2004).
12. Hypomyces gabonensis K. Põldmaa, sp. nov. MycoBank MB518516. Figs 2D, 5B–F, 18.
Fig. 18.
Hypomyces gabonensis. A–D. Teleomorph formed in culture. A. Perithecia embedded in the subiculum. B–C. Upper parts of perithecia. D. Ascus. E. Conidia and basidiospores of the host on natural substratum. F–I. Conidiophores with verticillately placed conidiogenous cells and conidia formed first in culture. J–N. Conidiophores with sparingly placed conidiogenous branches and larger conidia produced after the first formed anamorph structures in culture. O, P. Chlamydospores among subiculum. (E. Holotype, TU 112024 on host; A–D, F–P. Ex-type culture, TFC 201156 on MEA; F–N. 12 d; A–D, O. 3 mo; P. 6 mo). Scale bars: A = 500 μm; F, G, J = 100 μm; B, C = 50 μm; H = 25 μm; D, K, L, P = 20 μm; E, I, M–O = 10 μm.
Etymology: refers to the country of the type collection.
Mycelium tenue bubalinum, dependens sub hospitis hymenophoro; hyphae hyalinae, 7–9 μm diam. Conidia longo-clavata, cylindracea vel ellipsoidea, 15–30 × 5–7 μm, recta, hyalina, 1–3-septata. In MEA substratum, subiculum factum in partibus coloniae, tenue, hyphis 2.5–4.5 μm latis; perithecia fere superficiales, dispersa, obpyriformes, 400–600 × 300–400 μm, papilla cylindracea, 100–250 μm longa et basi 140–190 μm lata. Ascosporae fusiformes, (28.0–)30.7(–33.0) × (5.0–)5.7(–6.5) μm, septo mediano, subtiliter verrucosae, verrucis < 0.5 μm, apiculo (2.5–)4.0(–5.5) μm, angustatissimo, aciculari.
Mycelium delicate buff, hanging under host hymenophore, attached only from edge, of hyaline, frequently septate hyphae 7–9 μm diam, one simple, septate conidiogenous branch observed. Conidia long clavate, cylindrical or ellipsoidal, 15–30 × 5–7 μm, straight, hyaline, 1–3 septate, hilum narrow, central or wider, laterally displaced.
Teleomorph on MEA. Subiculum formed in parts of colony, thin, delicate; hyphae 2–4.5 μm wide, cells not swollen, buff, partially turning purple in KOH; perithecia semi-immersed to almost superficial on subiculum, scattered, obpyriform, 400–600 × 300–400 μm, buff to yellowish ochraceous, not reacting in KOH; papilla prominent, cylindrical, 100–250 μm long, 140–190 μm wide at base; cells at surface greatly swollen, 15–30 μm diam with wall 1.5–2.5 μm thick; surface cells at base of papilla 17–27 × 11–19 μm. Asci cylindrical, 180–210 × 6.5–7.5 μm, apex thickened, 1.5–2.5 μm, ascospores uniseriate with ends overlapping. Ascospores fusiform, (28.0–)33.0(–38) × (5.0–)6.0(–7.0) μm, Q = (4.6–)5.5(–6.4), main part (20.0–)23.0(–26.0) × (4.0–)5.0(–6.0) μm, Q = (4.0–)4.6(–5.3); (0–)1-septate, septum median; hyaline, becoming olivaceous-brown when old; finely verrucose, verrucae < 0.5 μm, not confluent; apiculi very narrow, needle-like, rarely with base wider, then hat-shaped, (3.5–)4.8(–6.3) μm long, (1.0–)1.5(–2.0) μm wide at base, ends acute, straight or sometimes curved.
Colonies on MEA spreading slowly, reaching 6–10 mm in 4 d, margin fasciculate. Odour absent. Aerial mycelium scarce, low, < 1 mm, compact, buff, hyphae turning purple in KOH; reverse yellowish ochraceous, turning darker, yellowish brown with age. Conidiation abundant. Conidiophores arising from submerged or aerial hyphae, ascending to suberect or erect, 300–750 μm long, branching profuse, mostly verticillate, often drepanoid, many verticils of conidiogenous cells producing one branch at lateral position, ending with a verticil or branching further several times; 1–4 lateral branches formed from one point, branches 23–30 × 2–3 μm. Conidiogenous cells borne on supporting branches, held in verticils by 2–7; subulate, 18–30 μm long, attenuating from 2–3 at base to 0.7–1.5 at apex, aseptate or sometimse with one septum in the middle; with one terminal, often with an additional intercalary conidiogenous locus formed on short denticle, often delimited below by a septum, denticles 1.0–2.5 μm high, 1.0–1.5 μm wide; intercalary loci forming also on stipes of conidiophores. Conidia ellipsoidal, ovoid or cylindrical, straight, (9.5–)12.5(–15.5) × (4.5–) 5.3(–6.0), Q = (1.8–)2.4(–3.0), (0–)1(–2)-septate, septum supramedian or median; hilum narrow, central; held by 1(–3) at uppermost or singly at intercalary loci.
Most colonies produce a secondary anamorph, developing in a section starting from inoculum or margin of existing colony. Mycelium whitish, growing faster than primary anamorph. Odour sweetish. Aerial mycelium moderate, sparse cottony, 1–3 mm high; reverse initially white, turning yellowish brown in a few days, finally brownish red to purplish brown (10–11 C–E 6–7). Conidiation abundant. Conidiophores arising from aerial hyphae, ascending, 350–1200 μm long, 4–5 μm wide near base, branching moderate, irregular or partly dichotomous. Conidiogenous cells or branches borne on lateral branches of conidiophore, rarely on conidiophore, 1–3 formed from one point, almost cylindrical, 35–55 μm long, attenuating from 2–3 at base to 0.7–1.7 at apex, with one terminal conidiogenous locus that produces first upright conidium, subsequent ones formed horizontally. Conidia cylindrical, long-clavate, seldom ellipsoidal, straight, (17.5–)22.9(–28.0) × (5.5–) 6.4(–7.0), Q = (2.7–)3.6(–4.5), (1–)3(–4)-septate; hilum narrow to wide, ca. 1 μm high and 1.5 μm wide, central, laterally displaced or shifted to side of conidium; held by 3–7 at apex of conidiogenous cells. Chlamydospores abundant in old cultures in and on the agar, cells subglobose to globose, 10–20 μm diam, pale brown, wall 1.0–2.5 μm thick, smooth, formed by a few to dozen in chains or irregular clusters often held on a smaller, hyaline thin-walled supporting cell.
Substrata: Resupinate basidiomata of Polyporales.
Distribution: Central Africa, known only from the type locality.
Holotype: Gabon, Crystal Mountains National Park, Tchimbele, on Rigidoporus lineatus, 1 May 2009, K. Põldmaa, TU 112024, dried culture containing the teleomorph deposited together with anamorph material on natural host; ex-type culture TFC 201156 = CBS 127154.
Notes: Hypomyces gabonensis was found as mycelium, loosely attached to the host, with relatively long, narrow 3-septate conidia (Fig. 18E). Although lacking teleomorph structures on the host, several conidial isolates produced perithecia in abundance. Therefore, H. gabonensis is described as a pleomorphic species with a dried culture containing both forms designated as the holotype.
The large, pallid perithecia (Fig. 18A–C) with long, slender ascospores clearly distinguish H. gabonensis from the other three teleomorphs described in this study. The perithecia lack any red colouration as well as the KOH reaction. However, red pigments are produced in the subicular hyphae and submerged mycelium, resulting in purplish colouration of the agar medium. The ascospores of H. gabonensis are remarkably long and narrow bearing long, very narrow needle-like apiculi at their ends (Figs 2D, 3). The ascospores resemble those of H. rosellus as well as H. tegillum. In the latter species, the ascospores and the apiculi are even longer than in H. gabonensis.
The cultures obtained from the germination of conidia are remarkable because of the formation of two types of anamorphs. To exclude the possibility of contamination, several isolations were made from single conidia as well as 16 ascospores. All the isolates started with the growth of profusely branching conidiophores bearing dense verticils of conidiogenous cells (Fig. 18F–H). The conidiogenous cells produce one, less frequently 2–3, ellipsoidal to ovate, 1-rarely 2–3-septate, conidia (Fig. 18I). These are formed either only at the single apical conidiogenous locus or also from a few additional loci on a short sympodium. While in C. protrusum the several conidiogenous loci are mostly arranged at the transversely placed elongation at the tip of the conidiogenous cell, a few closely formed terminal loci are seen in C. heterosporum. The widely distributed, single loci distinguish the primary anamorph of H. gabonensis from all other taxa treated in this study. An even more elongated denticulate rachis has been described in Pseudohansfordia irregularis (Arnold 1969) and Cladobotryum stereicola (Põldmaa & Samuels 1999). Occasionally the intercalary loci are scattered on the conidiophores, likewise observed in one isolate of C. cubitense.
Approximately 1 wk after inoculation several of the monosporic isolates start to form whitish mycelium that proceeds more quickly from one place of the primary yellowish ochraceous colony (Fig. 5D–F). Such mycelium bears long, loosely, yet profusely branched conidiophores with conidiogenous cells held singly or by 2–3 and forming one apical conidiogenous locus (Fig. 18J–M). These produce relatively long, cylindrical to long-clavate, 3-septate conidia (Fig. 18N). Such conidiophores are sometimes formed above the primary, profusely branched ones or are seen at the margins of colonies. This secondary anamorph is clearly distinct from the primary type. All the structures and mode of conidiation of the secondary anamorph are similar to those observed in the sister species C. cubitense. In both species conidia grow horizontally from the conidiogenous locus, while in all other species they develop obliquely. Consequently, narrow to wide hila are observed either centrally or laterally at base or often at the side of conidia. In C. cubitense the shape of conidia is often irregular due to variously curved conidia. In H. gabonensis, the straight conidia are uniform in shape. The irregular clusters of chlamydospores associated with the secondary anamorph (Fig. 18O, P) are identical to those found in old cultures of C. cubitense. In H. gabonensis the yellowish ochraceous, later yellowish brown colouration of the reverse of colonies is much more intense and growth slower compared to C. cubitense. The anamorph of H. gabonensis presents a unique feature in the genus, producing two distinct anamorphs in culture. In the isolate of C. cubitense from Peru conidia appeared dimorphic, without differences observed in the conidiophorous system. Formation of secondary conidiophores and conidia has also been observed in H. khaoyaiensis and C. dimorphicum. In these species the smaller structures regularly precede or follow the characteristic primary anamorph. In H. gabonensis the two morphs represent distinct forms, which, on their own, could be described as different species of Cladobotryum. Isolates from single ascospores and conidia differ substantially in the timing and abundance of secondary anamorph production with the stimulation and mechanisms for their production remaining unknown.
Acknowledgments
I would like to thank the keen hypocreologists Gary J. Samuels and Günter Arnold for the many valuable discussions on these fungi and for sharing their collections of Hypomyces and Cladobotryum. Priscila Chaverri, Jacques Fournier, Christian Lechat and Rafael Castañeda Ruiz provided further specimens. I am indebted to Amy Y. Rossman for reviewing the manuscript, Christian Feuillet for preparing the Latin diagnoses, as well as to Leif Ryvarden and Erast Parmasto for identifying the polypores as hosts. Curators of the following herbaria and fungal culture collections are acknowledged for sending specimens and cultures: Ellen D. Bloch (NY), Erin McCray, Shannon Dominick (BPI), Begoña Aguirre-Hudson (K), Kerstin Voigt (FSU) and Walter Gams, previously at CBS. Several lab assistants helped with PCR. The study was supported by the Estonian Science Foundation grants (4994 and 6939), the Estonian Ministry of Education and Science (project SF0180012s09), and the European Union through the European Regional Development Fund (Centre of Excellence FIBIR).
Taxonomic novelties: Cladobotryum coriolopsicola (R.F. Castañeda) K. Põldmaa, comb. nov., Hypomyces aconidialis K. Põldmaa, sp. nov., Hypomyces australasiaticus K. Põldmaa, sp. nov., Hypomyces gabonensis K. Põldmaa, sp. nov., Hypomyces samuelsii K. Põldmaa, sp. nov., Hypomyces virescens G.R.W. Arnold & K. Põldmaa, sp. nov., Cladobotryum heterosporum K. Põldmaa, sp. nov., Cladobotryum indoafrum K. Põldmaa, sp. nov., Cladobotryum paravirescens K. Põldmaa, sp. nov., Cladobotryum protrusum K. Põldmaa, sp. nov., Cladobotryum tchimbelense K. Põldmaa, sp. nov.
References
- Arnold GRW (1964). Über eine neue Hypomycetazee, Hypomyces odoratus Arnold sp. nov. Ceska Mykologie 18: 144–146. [Google Scholar]
- Arnold GRW (1969). Über Zwei Neue Imperfekten-Gattungen, Eurasina gen. nov. und Pseudohansfordia gen. nov. Zeitschrift für Pilzkunde 35: 305–310. [Google Scholar]
- Arnold GRW (1987). Beitrag zur Kenntnis der Pilzflora Cubas. III. Feddes Repertorium 98: 351–355. [Google Scholar]
- Arnold GRW (1988). Beitrag zur Kenntnis der Pilzflora Cubas. IV Feddes Repertorium 99: 27–31. [Google Scholar]
- Ashley JN, Hobbs BC, Raistrick (1937). Studies in the biochemistry of micro-organisms. LIII. The crystalline colouring matters of Fusarium culmorum (W. G. Smith) Sacc. and related forms. Biochemical Journal 31: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkeley MJ, Broome CE (1875). Enumeration of the fungi of Ceylon. Part II. Journal of the Linnean Society, Botany 14: 29–141. [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castañeda-Ruiz R (1987). Fungi Cubense 2. Insituto de Investigaciones Fundamentales en Agricultura Tropical, La Habana, Cuba.
- Castlebury LA, Rossman AY, Sung G-H, Hyten AS, Spatafora JW (2004). Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 1–9. [DOI] [PubMed] [Google Scholar]
- Chen J-D, Fu X-H (1989). The Verticillium-like genera parasitic on mushrooms. Acta Mycologica Sinica 8: 123–132. [Google Scholar]
- Gray DJ, Morgan-Jones G (1980). Notes on Hyphomycetes. XXXIV. Some mycoparasitic species. Mycotaxon 10: 375–404. [Google Scholar]
- Helfer W (1991). Pilze auf Pilzfruchtkorpern. Untersuchungen zur Ökologie, Systematik und Chemie. Libri Botanici 1: 1–157. [Google Scholar]
- Hoog GS de (1978). Notes on some fungicolous hyphomycetes and their relatives. Persoonia 10: 33–81. [Google Scholar]
- Hosaka K, Castellano MA, Spatafora JW (2008). Biogeography of Hysterangiales (Phallomycetidae, Basidiomycota). Mycological Research 112: 448–462. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W (2009). European species of Hypocrea Part I. The green-spored species. Studies in Mycology 63: 1–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W, Põldmaa K, Samuels GJ (2008). Reconsideration of Protocrea (Hypocreales, Hypocreaceae). Mycologia 100: 962–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W, Samuels GJ, Dodd SL, Lu B-S, Druzhinina IS (2006). Hypocrea rufa/ Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Studies in Mycology 56: 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T (2005). MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R, Arnold GRW, Chen CJ (2007). Cladobotryum semicirculare sp. nov. (Hyphomycetes) from commercially grown Ganoderma tsugae in Taiwan and other Basidiomycota in Cuba. Sydowia 59: 114–124. [Google Scholar]
- Kornerup A, Wanscher JH (1974). Farver i farver. Copenhagen.
- Liu YJ, Whelen S, Hall BD (1999). Phylogenetic relationships among ascomycetes, as inferred from RNA polymerase II phylogeny. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Aime MC, Bougher NL, Buyck B, Desjardin DE, Horak E, Kropp BR, Lodge DJ, Trappe JM, Hibbett DS (2009). Out of the palaeotropics? Historical biogeography and diversification of the cosmopolitan mushroom family Inocybaceae. Journal of Biogeography 36: 577–592. [Google Scholar]
- Matsushima T (1975). Icones Microfungorum a Matsushima Lectorum. Kobe, Japan.
- McKay GJ, Egan D, Morris E, Scott C, Brown AE (1999). Genetic and morphological characterization of Cladobotryum species causing cobweb disease of mushrooms. Applied and Environmental Microbiology 65: 606–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB, Deerfield DW (1997). GeneDoc: Analysis and Visualization of Genetic Variation. Embnew News 4: 14. [Google Scholar]
- O'Donnell K, Kistler HC, Tacke BK, Casper HH (2000). Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proceedings of the National Academy of Sciences of the United States of America 97: 7905–7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch T (1912). Revisions of Ceylon fungi. (Part III). Annals of the Royal BotanicGardens, Peradeniya 5: 265–301. [Google Scholar]
- Põldmaa K (1996). A new species of Hypomyces and three of Cladobotryum from Estonia. Mycotaxon 59: 389–405. [Google Scholar]
- Põldmaa K (2000). Generic delimitation of the fungicolous Hypocreaceae. Studies in Mycology 45: 83–94. [Google Scholar]
- Põldmaa K (2003). Three species of Hypomyces growing on basidiomata of Stereaceae. Mycologia 95: 921–933. [DOI] [PubMed] [Google Scholar]
- Põldmaa K, Larsson E, Kõjalg U (1999). Phylogenetic relationships in Hypomyces and allied genera, with emphasis on species growing on wood-decaying homobasidiomycetes. Canadian Journal of Botany 88: 1756–1768. [Google Scholar]
- Põldmaa K, Lodge DJ, Samuels GJ (1997). Three new polyporicolous species of Hypomyces and their Cladobotryum anamorphs. Sydowia 49: 80–93. [Google Scholar]
- Põldmaa K, Samuels GJ (1999). Aphyllophoricolous species of Hypomyces with KOH negative perithecia. Mycologia 91: 177–199. [Google Scholar]
- Põldmaa K, Samuels GJ (2004). Fungicolous hypocreaceae (Ascomycota: Hypocreales) from Khao Yai National Park, Thailand. Sydowia 56: 79–130. [Google Scholar]
- Rehner SA (2001). Primers for Elongation Factor 1-alpha (EF1-alpha). http://ocid.nacse.org/research/deephyphae/EF1primer.pdf.
- Rogerson CT, Samuels GJ (1993). Polyporicolous species of Hypomyces. Mycologia 85: 231–272. [Google Scholar]
- Rogerson CT, Samuels GJ (1994). Agaricolous species of Hypomyces. Mycologia 86: 839–866. [Google Scholar]
- Ronquist F & Huelsenbeck JP (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Samuels GJ (1996). Tropical Hypocreales. In: Diversity of tropical microfungi (Hyde K, ed). University of Hong Kong Press: 297–325.
- Samuels GJ (2006). Trichoderma: systematics, the sexual state, and ecology. Phytopathology 96: 195–206. [DOI] [PubMed] [Google Scholar]
- Swofford DL (2003). PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods), Version 4. Sinauer Associates, Sunderland, Massachusetts.
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: A guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego: 315–322.