Abstract
The genus Nectria is typified by N. cinnabarina, a wood-inhabiting fungus common in temperate regions of the Northern Hemisphere. To determine the diversity within N. cinnabarina, specimens and cultures from Asia, Europe, and North America were obtained and examined. Their phylogeny was determined using sequences of multiple loci, specifically act, ITS, LSU, rpb1, tef1, and tub. Based on these observations, four species are recognised within the N. cinnabarina complex. Each species is delimited based on DNA sequence analyses and described and illustrated from specimens and cultures. The basionym for N. cinnabarina, Sphaeria cinnabarina, is lectotypified based on an illustration that is part of the protologue, and an epitype specimen is designated. Nectria cinnabarina s. str. is recircumscribed as having 2-septate ascospores and long stipitate sporodochia. Nectria dematiosa, previously considered a synonym of N. cinnabarina, has up to 2-septate ascospores and sessile sporodochia or no anamorph on the natural substrate. A third species, Nectria nigrescens, has up to 3-septate ascospores and short to long stipitate sporodochia. One newly described species, Nectria asiatica with a distribution restricted to Asia, has (0–)1-septate ascospores and short stipitate sporodochia. Young and mature conidia developing on SNA were observed for each species. Mature conidia of N. asiatica, N. cinnabarina, and N. nigrescens but not N. dematiosa bud when the mature conidia are crowded. On PDA the optimal temperature for growth for N. dematiosa is 20 °C, while for the other three species it is 25 °C. Based on our phylogenetic analyses, three subclades are evident within N. dematiosa. Although subtle culture and geographical differences exist, these subclades are not recognised as distinct species because the number of samples is small and the few specimens are insufficient to determine if morphological differences exist in the natural environment.
Keywords: Ascomycota, Hypocreales, molecular systematics, Nectriaceae, plant pathogen, type species
INTRODUCTION
Nectria cinnabarina is the type species of the genus Nectria (Hypocreales, Nectriaceae). This species is characterised by red, globose, fleshy, warted perithecia that often become cupulate upon drying, 0–3-septate ascospores, and an anamorph referred to as Tubercularia vulgaris (Rossman et al. 1999). Nectria cinnabarina is a relatively common species that occurs on a range of hardwood trees and woody shrubs throughout the temperate regions of the Northern Hemisphere. It is occasionally considered to be a plant pathogen causing a disease on apple and other hardwood trees known as “coral spot” because of the pinkish sporodochia of its Tubercularia anamorph (Sinclair & Lyon 2005).
Nectria cinnabarina was originally described as Sphaeria cinnabarina by Tode (1791). When Fries (1849) sanctioned Sphaeria cinnabarina, he transferred this name to Nectria. Nectria cinnabarina was designated the lectotype species of the genus by Clements & Shear (1931). Nectria was conserved with this type species over Ephedrosphaera and Hydropisphaera (Cannon & Hawksworth 1983).
In studying the species of Nectria in the UK, Booth (1959) emphasised perithecial wall structure when he divided the large genus into groups. He included three species in what he referred to as the Nectria cinnabarina group: N. cinnabarina, N. aurantiaca, and N. ralfsii. When Rossman (1989) and Rossman et al. (1999) restricted Nectria s. str. to species congeneric with N. cinnabarina, they included N. aurantiaca and other species with a similar perithecial wall structure in Nectria s. str. Nectria ralfsii is now regarded a species of Bionectria, B. ralfsii (Schroers 2001).
Because of its morphological heterogeneity, 20 varieties and forms of Nectria cinnabarina exist as well as numerous synonyms. Wollenweber (1926, 1930) recognised three varieties of N. cinnabarina. Nectria cinnabarina var. minor was distinguished from the type variety by its smaller ascospores and conidia, while Nectria cinnabarina var. dendroidea has remarkably long, stipitate sporodochia. Nectria cinnabarina var. ribis (≡ N. ribis) was said to have larger ascospores and conidia than the other two varieties. Jøgensen (1952) published a monograph on N. cinnabarina and suggested that Nectria ribis was a “nomen confusum”, being a mixture of N. cinnabarina and N. berolinensis. Despite detailed observations, he did not find differences among specimens of N. cinnabarina; however, he noted differences between specimens on non-Ribes hosts and those on Ribes that he recognised as N. cinnabarina var. ribis.
Tubercularia (Tode 1790) includes anamorphs of several species in the Nectria cinnabarina group (Booth 1959, Rossman 1983). Tubercularia, conserved based on T. vulgaris, was segregated from fungi with black sporodochia by Fries (1832). Saccardo (1886) divided species of Tubercularia into four groups based on differences in substrate; however, his taxonomic concept was revised by Paoletti (1887) who emphasised the acropleurogenously developing phialides. Petch (1940) organised and revised the British records of Tubercularia. Seifert (1985) provided a thorough account of Tubercularia accepting eight species including T. vulgaris with many synonyms.
Although Tode (1790, 1791) described and illustrated both Sphaeria cinnabarina and Tubercularia vulgaris, he did not recognise their relationship as states of one species. Later, Fries (1828) determined that these were the sexual and asexual states of the same species. Modern authors have confirmed that N. cinnabarina and T. vulgaris are manifestations of the same species (Seifert 1985, Rossman 1989).
Nectria cinnabarina is commonly regarded as a saprobe; as mentioned above, it sometimes causes cankers on hardwood trees and woody shrubs. The parasitic occurrence of N. cinnabarina was first reported by Mayr (1883), who considered this species to be parasitic on Acer, Aesculus, Prunus, Robinia, Spiraea, Tilia, and Ulmus. Many hardwood trees and woody shrubs around the world have been reported as hosts for N. cinnabarina (Sinclair & Lyon 2005). Jøgensen (1952) demonstrated that N. cinnabarina was a facultative parasite and saprobe, but could not differentiate pathogenic races. He mentioned the following genera as the most common hosts of N. cinnabarina in Denmark: Acer, Aesculus, Carpinus, Fagus, Fraxinus, Malus, Prunus, Ribes, Tilia, and Ulmus. Similarly the anamorph has been commonly reported on woody substrates in many plant families (Seifert 1985).
Based on our hypothesis that Nectria cinnabarina is heterogeneous and might comprise several species, detailed morphological and molecular phylogenetic analyses of this species were undertaken. Many isolates of freshly collected and herbarium specimens from around the world were analysed to define phylogenetic species within the N. cinnabarina species complex (NCSC). Each species is described and illustrated and a key is provided.
MATERIALS AND METHODS
Source and deposition of specimens and isolates
Fresh specimens of the teleomorph and anamorph were collected from which single ascospores or conidia were isolated. Specimens are deposited in the US National Fungus Collections (BPI), Beltsville, Maryland, USA, or elsewhere as indicated in Table 1. Specimens were also obtained from other herbaria as listed in the specimens examined; herbaria are indicated using abbreviations according to Holmgren & Holmgren (1998). To obtain cultures from fresh material, a suspension in sterilised water was made from ascospores or conidia from a crushed fruiting body, streaked onto 2 % (w/v) water agar (WA) with streptomycin (streptomycin sulfate; Sigma Chemicals, St. Louis, Missouri, USA) or Difco™ cornmeal dextrose agar (CMD; Difco, Detroit, Michigan, USA, cornmeal agar + 2 % w/v dextrose) supplemented with antibiotics 0.2 % each neomycin (neomycin trisulfate salt hydrate; Sigma Chemicals, St. Louis, Missouri, USA), and incubated at 25 °C. After 24 h, a single germinating ascospore or conidium was transferred directly to slants or plates of Difco™ potato dextrose agar (PDA) with a tungsten needle (Nissin EM Co., Tokyo, Japan). Representative isolates are preserved at the CBS Fungal Biodiversity Centre (CBS, Utrecht, Netherlands), and/or Genebank, National Institute of Agrobiological Sciences (NIAS, Tsukuba, Ibaraki, Japan). Isolates were also obtained from other culture collections, including the CBS Fungal Biodiversity Center and the Global Bioresource Center (ATCC, Manassas, Virginia, USA).
Table 1.
Isolates and accession numbers used in the phylogenetic analyses.
| Species | Isolate No. | Herbarium No. | Substrate/Host | Country |
GenBank Accession No. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| act | ITS | LSU | rpb1 | tef1 | tub | |||||
| Cosmospora coccinea | A.R. 2741, CBS 114050 | BPI 802729 | Inonotus nodulosus | Germany | GQ505967a | HM484537 | GQ505990a | GQ506020a | HM484515 | HM484589 |
| Cyanonectria cyanostoma | G.J.S. 98-127, CBS 101734 | BPI 748307 | Buxaceae | France | GQ505961a | HM484558 | FJ474081a | GQ506017a | HM484535 | HM484611 |
| Nectria antarctica | A.R. 2767, CBS 115033, ATCC 204178 | BPI 746217 | Dead stem of Mahonia aquifolium | USA | HM484501 | HM484556 | HM484560 | HM484575 | HM484516 | HM484601 |
| Nectria aquifolii | A.R. 4108, CBS 125147 | BPI 880698 | Ilex aquifolium | UK | HM484506 | HM484538 | HM484565 | HM484579 | HM484522 | HM484590 |
| Nectria asiatica | MAFF 241408 | BPI 879980 | Dead wood | Japan | – | HM484703 | HM484744 | HM484790 | – | HM484815 |
| A.R. 4639, CBS 126568 | Dead wood | China | – | HM484713 | HM484727 | HM484787 | – | HM484811 | ||
| MAFF 241401 | BPI 879978 | Dead wood | Japan | HM484624 | HM484716 | HM484747 | HM484788 | – | HM484817 | |
| MAFF 241435 | BPI 879973 | Bark of dead wood | Japan | HM484625 | HM484709 | HM484749 | HM484794 | – | HM484816 | |
| MAFF 241399 | BPI 879976 | Prunus sp. | Japan | – | HM484715 | HM484751 | HM484791 | – | HM484813 | |
| MAFF 241448 | BPI 879974 | Dead twig | Japan | HM484626 | – | HM484728 | HM484793 | – | HM484809 | |
| MAFF 241398 | BPI 879975 | Dead wood of Zelkova serrata | Japan | HM484643 | HM484702 | HM484738 | HM484792 | – | HM484812 | |
| MAFF 241439 | BPI 879972 | Bark of dead wood | Japan | HM484505 | HM484701 | HM484563 | – | – | HM484604 | |
| MAFF 241405 | BPI 879979 | Dead twig of Prunus sp. | Japan | – | HM484708 | HM484748 | HM484789 | – | HM484814 | |
| MAFF 241400 | BPI 879977 | Dead stem of Sorbus commixta | Japan | HM484623 | HM484705 | HM484743 | HM484786 | – | HM484818 | |
| Nectria aurigera | A.R. 3717, CBS 109874 | BPI 841465 | Twigs dead, Fraxinus excelsior | France | HM484511 | HM484551 | HM484573 | HM484586 | HM484521 | HM484600 |
| Nectria austroamericana | A.R. 2808, CBS 126114 | BPI 746395 | Gleditsia triacanthos | USA | GQ505960a | HM484555 | GQ505988a | GQ506016a | HM484520 | HM484597 |
| Nectria balansae | A.R. 4446, CBS 123351 | BPI 878477 | Coronilla sp. | France | GQ505977a | HM484552 | GQ505996a | GQ506026a | HM484525 | HM484607 |
| Nectria balsamea | A.R. 4478, CBS 125166 | Pinus sylvestris | Germany | HM484508 | HM484540 | HM484567 | HM484580 | HM484528 | HM484591 | |
| Nectria berolinensis | A.R. 2776, CBS 126112 | BPI 746346 | Branches standing, Ribes rubrum | Austria | HM484510 | HM484543 | HM484568 | HM484583 | HM484517 | HM484594 |
| Nectria cinnabarina | A.R. 4327, CBS 125154 | Acer sp. | Canada | HM484642 | HM484688 | HM484733 | HM484778 | HM484666 | HM484824 | |
| G.J.S. 91-111, CBS 713.97 | BPI 1112880 | Acer sp. | USA | HM484629 | HM484693 | HM484724 | HM484777 | HM484665 | HM484825 | |
| A.R. 4340, CBS 125156 | BPI 878335 | Spiraea trilobata | Canada | HM484635 | HM484695 | HM484756 | HM484779 | HM484664 | HM484836 | |
| A.R. 4341, CBS 125157 | BPI 878311 | Acer saccharum | Canada | HM484636 | HM484687 | HM484741 | HM484780 | HM484667 | HM484822 | |
| G.J.S. 91-109 | BPI 1112878 | Fagus sp. | USA | HM484633 | HM484694 | HM484723 | HM484766 | HM484670 | HM484833 | |
| A.R. 4379, CBS 125158 | BPI 878313 | Twigs | Ireland | HM484640 | HM484696 | HM484739 | HM484772 | HM484668 | HM484830 | |
| A.R. 4337, CBS 127668 | BPI 878312 | Acer pseudoplatanus | Denmark | HM484631 | HM484690 | HM484726 | HM484775 | HM484659 | HM484826 | |
| A.R. 4477, CBS 125165 | BPI 879981 | Dead twigs of Aesculus sp. | France | HM484503 | HM484548 | HM484562 | HM484577 | HM484527 | HM484606 | |
| A.R. 4496 | BPI 878878 | Populus tremula | Ukraine | HM484641 | HM484712 | HM484731 | HM484768 | HM484658 | HM484831 | |
| A.R. 4302, CBS 125150 | BPI 878317 | Acer pseudoplatanus | Austria | HM484627 | HM484684 | HM484736 | HM484765 | HM484654 | HM484820 | |
| ATCC 11432, CBS 255.47 | Stem of Ulmus sp. | Netherlands | GQ505975a | HM484710 | GQ505997a | GQ506027a | HM484663 | HM484832 | ||
| CBS 256.47 | Twig of Ulmus sp. | Netherlands | HM484628 | HM484692 | HM484755 | HM484769 | HM484656 | HM484828 | ||
| A.R. 4303, CBS 125151 | BPI 878316 | Acer campestre | Austria | HM484630 | HM484686 | HM484740 | HM484776 | HM484669 | HM484821 | |
| CBS 189.87 | Sorbus aria | Germany | HM484644 | HM484699 | HM484746 | HM484796 | HM484671 | HM484835 | ||
| A.R. 4397, CBS 125163 | BPI 879983, C.L.L. 7027 | Acer sp. | France | HM484638 | HM484691 | HM484742 | HM484773 | HM484661 | HM484827 | |
| A.R. 4381, CBS 125160 | BPI 878310 | Root | UK | HM484632 | HM484685 | HM484752 | HM484774 | HM484657 | HM484837 | |
| A.R. 4304, CBS 125152 | BPI 879982 | Tilia sp. | Denmark | HM484637 | HM484698 | HM484734 | HM484767 | HM484655 | HM484829 | |
| A.R. 4388, CBS 125161 | BPI 878322 | Twigs of Acer pseudoplatanus | Poland | HM484639 | HM484689 | HM484735 | HM484771 | HM484662 | HM484823 | |
| CBS 125115, G.J.S. 91-121 | BPI 1112890 | Acer sp. | USA | HM484634 | HM484697 | HM484753 | HM484770 | HM484660 | HM484834 | |
| Nectria coryli | A.R. 4561, Y.H. 0815 | BPI 880697 | Twigs of Rhus copallinum | USA | HM484509 | HM484539 | HM484566 | HM484581 | HM484536 | HM484596 |
| Nectria cucurbitula | CBS 259.58 | Pinus sylvestris | Netherlands | GQ505974a | HM484541 | GQ505998a | GQ506028a | HM484530 | HM484592 | |
| Nectria dematiosa | CBS 126570, G.J.S. 94-37 | BPI 749337 | Bark | USA | HM484502 | HM484557 | HM484561 | HM484576 | HM484534 | HM484603 |
| A.R. 4328, CBS 125155 | Acer sp. | Canada | HM484616 | HM484680 | HM484725 | HM484761 | HM484648 | HM484799 | ||
| CBS 279.48 | Acer pseudoplatanus | – | HM484700 | HM484754 | HM484762 | HM484649 | HM484802 | |||
| CBS 278.48 | Ribes sp. | HM484615 | HM484682 | HM484729 | HM484760 | HM484647 | HM484800 | |||
| A.R. 4380, CBS 125159 | BPI 878308 | Twig | Poland | HM484614 | HM484681 | HM484722 | HM484759 | HM484650 | HM484801 | |
| A.R. 2699, CBS 125125 | BPI 802212 | Dead twig of Acer macrophyllum | Canada | HM484612 | HM484676 | HM484717 | HM484757 | HM484645 | HM484797 | |
| A.R. 2702, CBS 125127 | BPI 802215 | Dead twig of Rosa sp. | Canada | HM484613 | HM484677 | HM484719 | HM484758 | HM484646 | HM484798 | |
| MAFF 241430 | BPI 879985 | Branches standing | Japan | HM484617 | HM484704 | HM484750 | HM484795 | HM484653 | HM484803 | |
| A.R. 4638, CBS 127667 | Unknown | China | – | HM484706 | HM484718 | HM484763 | HM484651 | HM484805 | ||
| MAFF 241416 | BPI 879984 | Attached branches of Weigela coraeensis | Japan | – | HM484714 | HM484732 | HM484764 | HM484652 | HM484804 | |
| Nectria lamyi | A.R. 2779, CBS 115034 | BPI 746349 | Berberis vulgaris | Austria | HM484507 | HM484544 | HM484569 | HM484582 | HM484518 | HM484593 |
| Nectria miltina | A.R. 4391, CBS 121121 | BPI 878442 | Decaying leaves of Agave americana | Italy | HM484514 | HM484547 | HM484572 | HM484587 | HM484524 | HM484609 |
| Nectria nigrescens | A.R. 4282 | BPI 878455A | Dead twig of Acer sp. | France | HM484619 | HM484711 | HM484745 | HM484785 | HM484673 | HM484808 |
| A.R. 4211, CBS 125148 | BPI 871083 | Dead twig of dictyledonous tree | USA | HM484618 | HM484707 | HM484720 | HM484781 | HM484672 | HM484806 | |
| A.R. 4475, CBS 125164 | BPI 878457 | Twig of Fagus sylvatica | France | HM484504 | HM484550 | HM484564 | HM484578 | HM484526 | HM484605 | |
| AR 4565, CBS 127666 | BPI 879986 | Dead twig | USA | HM484620 | HM484683 | HM484730 | HM484784 | HM484674 | HM484810 | |
| A.R. 4213, CBS 125149 | BPI 871084 | Dead twig of Betula lutea | USA | HM484622 | HM484679 | HM484721 | HM484782 | HM484675 | HM484819 | |
| A.R. 4394, CBS 125162 | BPI 878449 | Twigs of Celtis occidentalis | Canada | HM484621 | HM484678 | HM484737 | HM484783 | – | HM484807 | |
| Nectria pseudocinnabarina | A.R. 4548 | C.L.L. 8299 | Unknown | French Guiana | – | HM484553 | HM484574 | HM484588 | HM484529 | HM484608 |
| Nectria pseudotrichia | CBS 551.84 | Unknown | Japan | GQ505976a | HM484554 | GQ506000a | GQ506030a | HM484532 | HM484602 | |
| Nectria pyrrhochlora | A.R. 2786, CBS 125131 | BPI 746398 | Acer campestre | Austria | HM484512 | HM484545 | HM484570 | HM484584 | HM484519 | HM484598 |
| Nectria sinopica | CBS 462.83 | CBS H-19479, CBS H-19485 | Hedera helix | Netherlands | GQ505973a | HM484542 | GQ506001a | GQ506031a | HM484531 | HM484595 |
| Nectria zanthoxyli | A.R. 4280, CBS 126113 | BPI 878445 | Crataegus sp. | France | HM484513 | HM484546 | HM484571 | HM484585 | HM484523 | HM484599 |
| Thelonectria westlandica | G.J.S. 83-156, CBS 112464 | Dacrydium cupressinum | New Zealand | GQ505959 | HM484559 | GQ505987a | GQ506015a | HM484533 | HM484610 | |
A.R.: Amy Y. Rossman, USDA-ARS MD USA; ATCC: American Type Culture collection, Manassas, VA, USA; BPI: U.S. National Fungus Collections USDA-ARS MD USA; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; C.L.L.: Christian Lechat, Ascofrance, Villiers en Bois, France; G.J.S.: Gary J. Samuels, USDA-ARS MD USA; MAFF: MAFF Genebank, National Institute of Agrobiological Sciences, Ibaraki, Japan; Y.H.: Yuuri Hirooka, USDA-ARS MD USA.
Sequences obtained from GenBank.
Morphological observations
For morphological characterisation of the teleomorph, the macromorphology of the perithecia and stroma was observed and described as follows: distribution of perithecia on the host; perithecium shape, colour and reaction to 3 % w/v potassium hydroxide (KOH) and 100 % lactic acid (LA) using a stereoscope (Zeiss, STEMI SV11, Jena, Germany). To observe internal and microscopic characteristics, the perithecia and stroma were sectioned by hand and rehydrated in water, KOH, and LA. Characteristics of asci and ascospores were observed by rehydrating the perithecia in water, removing part of the centrum with a fine glass needle, and placing it onto a glass slide. Microscopic observations were made using a compound microscope (Zeiss, Axioskop 2 Plus, Jena, Germany). To determine growth rates, colony colour, and odour, isolates were grown on PDA in 9-cm plastic dishes at 25 °C for 7 d in the dark. For observation of sporulating structures, the cultures were grown on a low nutrient agar (SNA; Nirenberg 1976). Cultures on SNA were incubated at 25 °C with alternating 12 h/12 h fluorescent light/darkness for 2–3 wk. Young conidia are those that develop after one or two d on SNA while mature conidia are 4–5 d old. To stimulate budding, mature conidia produced on SNA were suspended in distilled water and then streaked on SNA. After 24 h, budding mature conidia and germ tubes were produced. Images were captured with a Nikon DXM1200 digital camera. Some composite images were made with Helicon Focus v. 4.21.5 Pro (Helicon Soft, www.heliconfocus.com). All recognition of colour such as perithecia, ascospores, conidia, and top and reverse colony colour were described according to Kornerup & Wanscher (1978).
Statistical analysis
Measurements of continuous characters such as length and width were made using Scion Image software beta v. 4.0.2 (Scion Corporation, Frederick, Maryland, USA) and are based on up to 50 measurements for structures in each isolate. For morphological structures, descriptive statistics (minimum, mean, median, maximum, and standard deviation) were computed and variation of morphological characters displayed graphically using mean values and their corresponding 95 % confidence intervals. All computations were performed using Systat 10 (Systat Software, San José, California, USA). Only isolates for which all data were available were included in the analysis. Ranges are reported as mean values ± one standard deviation; the number of items measured is given in parentheses together with maximum and minimum.
Cardinal temperatures
Disks of 5 mm diam were cut from the edge of young colonies and placed in the centre of PDA plates, then incubated at temperatures from 15 to 35 °C at 5 °C intervals in complete darkness. Diameters of the colonies on three plates for each isolate at each temperature were measured daily for 1 wk.
DNA extraction, PCR, and sequencing
The forty-five cultures of N. cinnabarina used in the phylogenetic analyses (Table 1) and representatives of other species of Nectria s. str. were grown in Difco™ potato dextrose broth in 6 cm diam Petri plates for about 3 wk. Mycelial mats were harvested in a laminar flow hood and dried with clean, absorbent paper towels. DNA was extracted with Ultra Clean™ Plant DNA Isolation Kit (MO BIO Laboratories Inc., Solana Beach, California, USA).
Six loci were sequenced, namely a-actin (act) (Carbone & Kohn 1999), β-tubulin (tub) (O'Donnell & Cigelnik 1997), RNA polymerase II subunit one (rpb1) (Castlebury et al. 2004), the internal transcribed spacer (ITS) (White et al. 1990), large subunit nuclear ribosomal DNA (LSU) (Vilgalys n.d.), and translation elongation factor 1-a (tef1) (Carbone & Kohn 1999, Rehner 2001). The primers and PCR protocol information are listed in Tables 2 and 3. PCR products were cleaned with ExoSAP-IT® (USB Corporation, Cleveland, Ohio, USA) following the manufacturer's instructions. Clean PCR products were sequenced at the DNA Sequencing Facility (Center for Agricultural Biotechnology, University of Maryland, College Park, Maryland, USA) and at MCLAB (Molecular Cloning Laboratories, San Francisco, California, USA). Sequences were assembled and edited with Sequencher v. 4.9 (Gene Codes, Madison, Wisconsin, USA). Sequences are deposited in GenBank (Table 1).
Table 2.
Genes/loci used in the phylogenetic analyses for members of the genus Nectria. Information on the primers, base pairs, PCR protocols, and models of nucleotide substitution are indicated.
| Locus | Primers used (reference) | PCR protocol: Annealing temp. & cycles | Nucleotide substitution models | Included sites (# of excluded sites) | Phylogenetically informative sites (%) | Uninformative polymorphic sites | Invariable sites |
|---|---|---|---|---|---|---|---|
| Act | Tact1, Tact2 (Samuels et al. 2006) | 65 °C, 30 s, 15′ | GTR+G | 613 (127) | 111 (18 %) | 43 | 459 |
| 48 °C, 30 s, 30′ | |||||||
| ITS | ITS5, ITS4 (White et al. 1990) | 53 °C, 1 min, 35′ | TIM3+I+G | 539 (279) | 62 (12 %) | 52 | 425 |
| LSU | LR5, LROR (Vilgalys n.d.) | 53 °C, 1 min, 35′ | TIM3+I+G | 807 (150) | 67 (8.3 %) | 39 | 701 |
| Rpb1 | crpb1a, rpb1c (Castlebury et al. 2004) | 50 °C, 2 min, 40′ | TIM2+I+G | 590 (540) | 233 (40 %) | 65 | 292 |
| Tef1 | tef1-728, tef1-1567 (Carbone & Kohn 1999, Rehner 2001) | 66 °C, 55 s, 9′ | GTR+I+G | 645 (261) | 142 (22 %) | 43 | 460 |
| 56 °C, 55 s, 35′ | |||||||
|
Tub |
βtub-T1, βtub-T2
(O'Donnell & Cigelnik
1997)
|
55 °C, 30 s, 35′
|
TPM3uf+I+G
|
479 (408)
|
192 (40 %)
|
32
|
255
|
| Total | 3673 | 807 (22 %) | 274 | 2592 |
Table 3.
Genes/loci used in the phylogenetic analyses for members of Nectria cinnabarina species complex (NCSC). Information on the primers, base pairs, PCR protocols, and models of nucleotide substitution are indicated.
| Locus | Primers used (reference) | PCR protocol: Annealing temp. & cycles | Nucleotide substitution models | Included sites (# of excluded sites) | Phylogenetically informative sites (%) | Uninformative polymorphic sites | Invariable sites |
|---|---|---|---|---|---|---|---|
| Act | Tact1, Tact2 (Samuels et al. 2006) | 65 °C, 30 s, 15′ | TrN+G | 649 (91) | 47 (7 %) | 40 | 562 |
| 48 °C, 30 s, 30′ | |||||||
| ITS | ITS5, ITS4 (White et al. 1990) | 53 °C, 1 min, 35′ | TrNef+G | 475 (592) | 38 (8 %) | 19 | 418 |
| LSU | LR5, LROR (Vilgalys n.d.) | 53 °C, 1 min, 35′ | TIM1+I+G | 814 (260) | 18 (2 %) | 14 | 782 |
| Rpb1 | crpb1a, rpb1c (Castlebury et al. 2004) | 50 °C, 2 min, 40′ | TrN+G | 621 (123) | 111 (18 %) | 120 | 390 |
| Tef1 | tef1-728, tef1-1567 (Carbone & Kohn 1999, Rehner 2001) | 66 °C, 55 s, 9′ | TrN+G | 828 (186) | 158 (19 %) | 36 | 634 |
| 56 °C, 55 s, 35′ | |||||||
|
Tub |
βtub-T1, βtub-T2
(O'Donnell & Cigelnik
1997)
|
55 °C, 30 s, 35′
|
TPM3uf+G
|
527 (135)
|
88 (17 %)
|
138
|
301
|
| Total | 3914 | 460 (12 %) | 367 | 3087 |
Phylogenetic analyses
Sequences of the six genes were aligned with MAFFT v. 6 (Katoh 2008) and the alignment was visually improved with Mesquite v. 2.6 (Maddison & Maddison 2009). Maximum likelihood (ML) and Bayesian (BI) analyses were carried out with all sequences, first each locus separately, then with the combined/concatenated data sets. Representative members of the Nectriaceae, namely Cosmospora coccinea, Cyanonectria cyanostoma, and Thelonectria westlandica, were used as outgroups for inferring intrageneric relationships (Fig. 1). Nectria balansae, N. pseudocinnabarina, and N. pseudotrichia were used as outgroup taxa for the NCSC tree, including 45 isolates in the NCSC (Fig. 2). JMODELTEST (Posada 2008) was used to calculate the models of nucleotide substitutions of each gene/partition for the ML and BI analyses. The number of substitution schemes was set to 11, base frequencies +F, rate variation +I and +G, and the base tree for likelihood calculations was set to “ML optimised”. 88 models were compared. After the likelihood scores were calculated, the models were selected according to the Akaike information criterion (AIC) (Posada & Buckley 2004). Under the AIC settings, the AICc corrected for smaller samples was selected. After jMODELTEST was run, likelihood settings for trees of the Nectria tree and NCSC tree were set to each gene (Tables 2, 3). For the ML and bootstrap analyses (BP), GARLI version 0.96 (Zwickl 2006) was computed through the Grid computing (Cummings & Huskamp 2005) and The Lattice Project (Bazinet & Cummings 2008), which includes clusters and desk tops in one integrated network (Myers et al. 2008). In GARLI, the starting tree was made by stepwise-addition and the number of runs or search replicates was set to 50. 2000 ML BP replicates were done in GARLI with the starting tree chosen randomly. Bayesian analysis (BI) was done using MrBayes v. 3.1.2 (Huelsenbeck et al. 2001, 2002). In MrBayes, data were partitioned by locus and the parameters of the nucleotide substitution models for each partition were set as described (Tables 2, 3). For this analysis, two independent analyses of two parallel runs and four chains were carried out for 5 000 000 generations using MrBayes. Analyses were initiated from a random tree and trees sampled every 100th generation. The first 20 % of the resulting trees were eliminated (= “burn in”). A consensus tree (“sumt” option) and posterior probabilities (PP) were calculated in MrBayes, which combines the results from both parallel runs. A reciprocal 70 % BP threshold was used to detect topological incongruence among genes/partitions (Mason-Gamer & Kellogg 1996, Reeb et al. 2004).
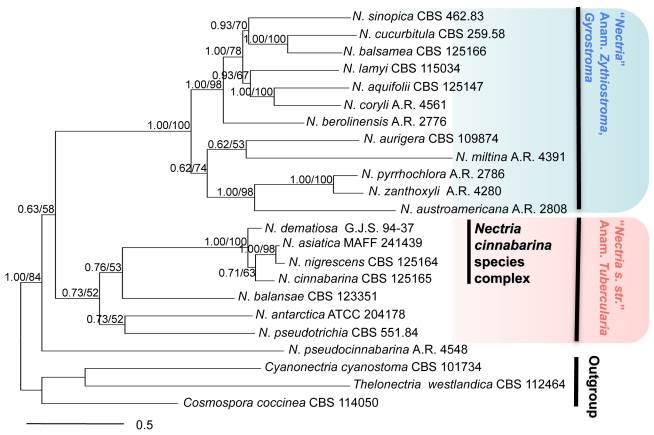
Fig. 1.
Members of the genus Nectria. Combined act, tub, rpb1, ITS, LSU, tef1 Bayesian cladogram (Ln –21514.704). BI posterior probabilities/ML bootstrap values indicated at branches.
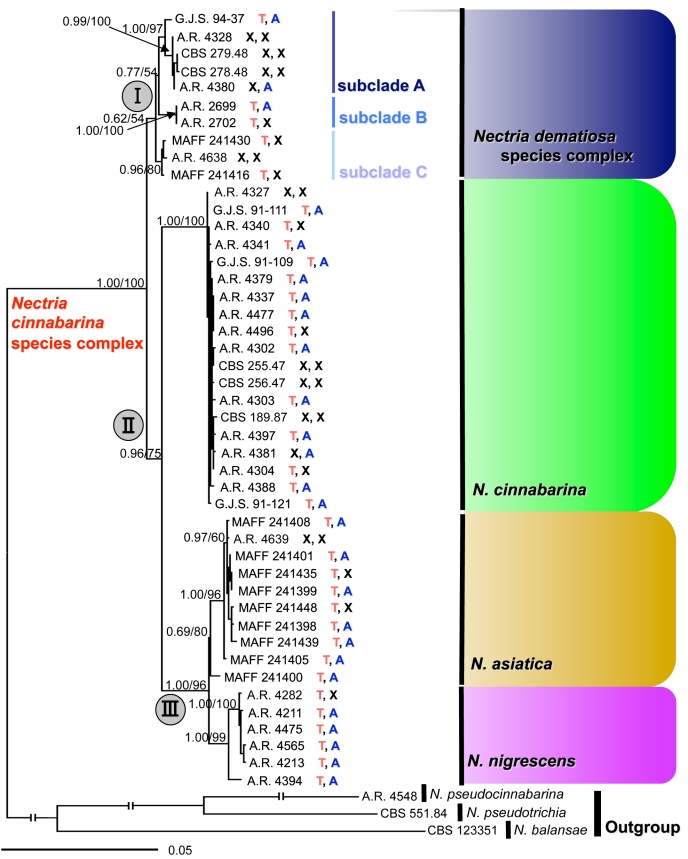
Fig. 2.
Members of the Nectria cinnabarina species complex (NCSC). Combined act, tub, rpb1, ITS, LSU, tef1 Bayesian cladogram (Ln –11408.155). BI posterior probabilities/ML bootstrap values indicated at branches. T: Teleomorph observed in the natural environment; A: Anamorph observed in the natural environment; X: no holomorph observed in the natural environment.
RESULTS
Phylogenetic analyses
Sequencing and alignment of the six loci for 23 taxa in Nectria resulted in 3 673 base pairs, 807 (22 %) phylogenetically informative, and 2 592 invariable sites; 325 sites presented unique non-informative polymorphic sites (Table 2). Sequencing and alignment of the six loci for 48 taxa for the NCSC tree included 3 914 base pairs, 460 (12 %) phylogenetically informative, and 3 087 invariable sites; 325 sites presented unique non-informative polymorphic sites (Table 3). Ambiguously aligned and poly-T/A regions were excluded from the analyses. For the species of Nectria, the ML and BI analyses of the combined six loci produced one tree with Ln likelihoods of –21393.478926 and –21514.704, respectively (Fig. 1). For the NCSC tree, ML and BI analyses produced one tree with Ln likelihoods of –11339.862470 and –11408.155, respectively (Fig. 2). The topologies of the ML and BI trees were congruent.
The topologies of each gene tree did not contradict each other, although the tef1 tree does not include N. asiatica (results not shown). All individual gene trees reveal three clades in N. dematiosa species complex. Among these trees, the act tree provides the best resolution with best BP support as evidenced in the high BP and PP support in most nodes.
The combined ML and BI analyses of six loci indicated that Nectria comprises two major clades: species with Tubercularia anamorphs (0.73 BI PP, 52 % ML BP) and species with pycnidial anamorphs (1.00 BI PP, 100 % ML BP) (Fig. 1). All isolates initially identified as N. cinnabarina formed a monophyletic Nectria-Tubercularia clade supported by high BI PP and ML BP value (1.00 BI PP, 100 % ML BP).
The combined ML and BI analyses of six loci using 45 isolates of the NCSC resolved four distinct species (Fig. 2). One major clade (clade II) included three species with high support (BI PP 0.96, ML BP 75 %). One of the species in clade II represents N. cinnabarina s. str. and includes the ex-epitype isolate from a hardwood tree in Europe with isolates on hardwoods in Europe and North America. Nectria cinnabarina s. str. is highly supported (BI PP 1.00, ML BP 100 %). A second segregate species occuring only in Asia is here described as a new species, N. asiatica. This species was supported by moderate values (BI PP 0.69, ML BP 80 %). A third species is recognised as N. nigrescens, previously considered a synonym of N. cinnabarina.
Nectria nigrescens also occurs on hardwoods in Europe and North America. This species is highly supported (BI PP 1.00, ML BP 99 %). A fourth segregate species, recognised as N. dematiosa (clade I), a previous synonym of N. cinnabarina, constitutes a sister clade to clade II. Within N. dematiosa, three subclades are highly supported (BI PP 1.00, ML BP 97 % for subclade A; BI PP 1.00, ML BP 100 % for subclade B; and BI PP 0.96, ML BP 80 % for subclade C). However, clades I was poorly supported (BI PP 0.62, ML BP 54 % for clade I) (Fig. 2). Nectria dematiosa subclade A is known from Europe and North America, N. dematiosa subclade B is representated by two isolates from Canada, while N. dematiosa subclade C is known only from Asia.
Morphological, colony growth, and temperature analyses
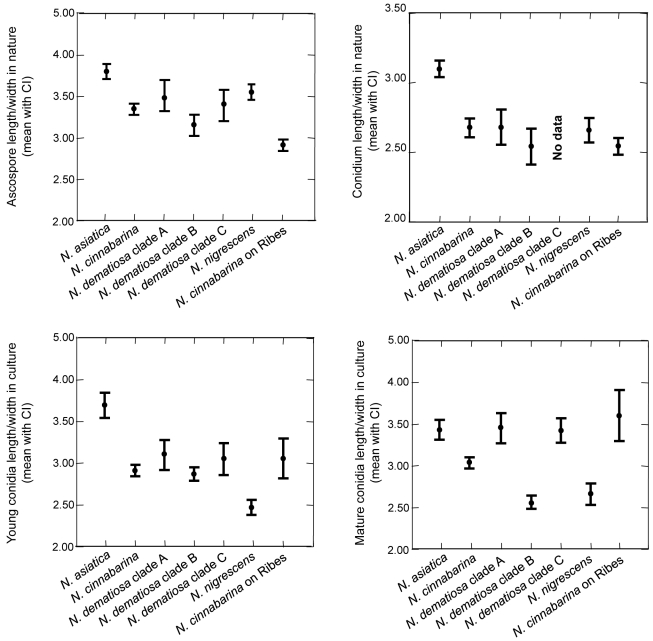
Morphological characters of the teleomorph and anamorph in the natural environment and cultural characteristics are useful in distinguishing species in the NCSC. Perithecial characters, such as colour, surface, and wall cell structure, are generally reliable for identifying the species complex, but not the segregate species. The perithecial wall surface of species in the NCSC is roughened, with conspicuous to small warts, 10–20 μm high, rarely smooth. In all species of the NCSC, the perithecial walls are about the same thickness and cell walls form similar textura globulosa or t. angularis; thus, perithecial wall structure is not useful in distinguishing species. Differences in ascospore septation correlate with phylogenetic species recognised in the NCSC. Nectria asiatica has up to 1-septate ascospores, N. cinnabarina and N. dematiosa have up to 2-septate ascospores, and N. nigrescens has up to 3-septate ascospores. The size ranges of ascospores in the four species overlap. However, in comparing 95 % confidence intervals of length/width ratios of ascospores on natural substrate, those of N. asiatica are greater than the other species while those of N. cinnabarina on Ribes are less than the other species (Fig. 3).
Fig. 3.
Graphs of 95 % confidence intervals of length to width ratios of ascospores and conidia.
Anamorph characters on natural substrate, especially presence or absence and length of the stipe of the sporodochia, are useful in distinguishing species. A distinction is made here between sporodochia that are astipitate i.e. lack any kind of stipe and sporodochia that are stipitate having a short stipe, less than 800 μm high, or a long stipe, 700–1600 μm high. The sporodochia of N. dematiosa are astipitate. In clade II, which includes N. cinnabarina, N. asiatica, and N. nigrescens, the sporodochia are short to long stipitate. Nectria asiatica has short stipitate sporodochia, N. cinnabarina has long stipitate sporodochia, and N. nigrescens has short to long stipitate sporodochia. The long stipitate sporodochia of N. cinnabarina and N. nigrescens have marginal cells arranged in a palisade, while the short stipitate sporodochia of N. asiatica and N. nigrescens lack these cells.
Additional morphological characteristics of the anamorph were also evaluated. These characteristics include the number of conidiophore branches and conidial size in the natural environment. No differences were found between species. The sizes of conidia among the four species overlap; however, in comparing 95 % confidence intervals of length/width ratios of conidia on natural substrate, those of N. asiatica are larger than other members of the NCSC (Fig. 3).
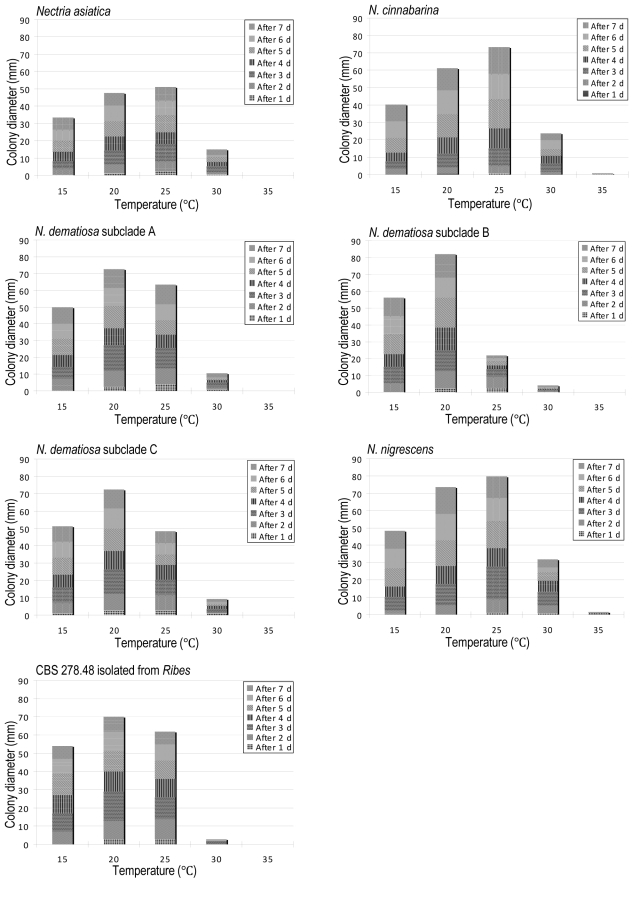
The optimal temperature for growth on PDA for N. dematiosa is 20 °C while that for N. asiatica, N. cinnabarina, and N. nigrescens is 25 °C (Fig. 4). In macroscopic appearance these colonies look similar.
Fig. 4.
Mycelial growth of NCSC at different temperatures on PDA.
Conidia produced in culture show differences that correlate with species. The size of conidia varies considerably when grown on different media (CMD, PDA, and SNA). On SNA conidia were classified into two types, namely young and mature conidia. Mature conidia appear after 3 to 4 d and are defined by extreme swelling to twice their original size, becoming 1-septate, often including vacuoles. The 95 % confidence interval of length/width ratios of young conidia in culture of N. asiastica was larger than that of other species of the NCSC (Fig. 3). By observing mature conidia on SNA, we could distinguish species in the NCSC. Mature conidia of N. cinnabarina budded abundantly while those of N. asiatica and N. nigrescens rarely budded. Mature conidia of N. dematiosa did not bud at all. In evaluating the 95 % confidence intervals of length/width ratios of mature conidia in culture, N. cinnabarina, N. dematiosa subclade B, and N. nigrescens were smaller than other members of the NCSC. Each subclade in N. dematiosa can be distinguished by the morphology of the anamorph in culture. Mature conidia of subclade A produced almost straight germ tubes that do not penetrate the agar immediately, while mature conidia of subclades B and C produced sinuous germ tubes that penetrate the agar after germination. The 95 % confidence interval of length/width ratio of mature conidia of subclade B was statistically different from subclades A and C (Fig. 3). On PDA at 25 °C for 7 d, subclade B grew more slowly than subclades A and C (Fig. 4).
In summary, clades I includes N. dematiosa with subclades A, B and C. This species is characterised by ascospores that are generally 1-septate, rarely 0- or 2-septate, sessile sporodochia or anamorph lacking, mature conidia that do not bud, and an optimum growth temperature of 20 °C on PDA. Clade II includes N. asiatica, N. cinnabarina and N. nigrescens, all of which have short to long stipitate sporodochia, mature conidia that bud, although sometimes only rarely, and an optimum growth temperature of 25 °C on PDA. Nectria cinnabarina has 1-septate, rarely 0- or 2-septate ascospores, long stipitate sporodochia, and mature conidia that bud abundantly. Nectria asiatica has 1-septate, rarely 0-septate ascospores, short stipitate sporodochia, and mature conidia that seldom bud. Nectria nigrescens has 1-, 2-, or occasionally 3-septate ascospores, short to long stipitate sporodochia, and mature conidia that bud infrequently.
TAXONOMY
Based on our morphological and molecular analyses, the N. cinnabarina species complex is recognised as four distinct species, each of which is described and illustrated below. A key to these four species is provided.
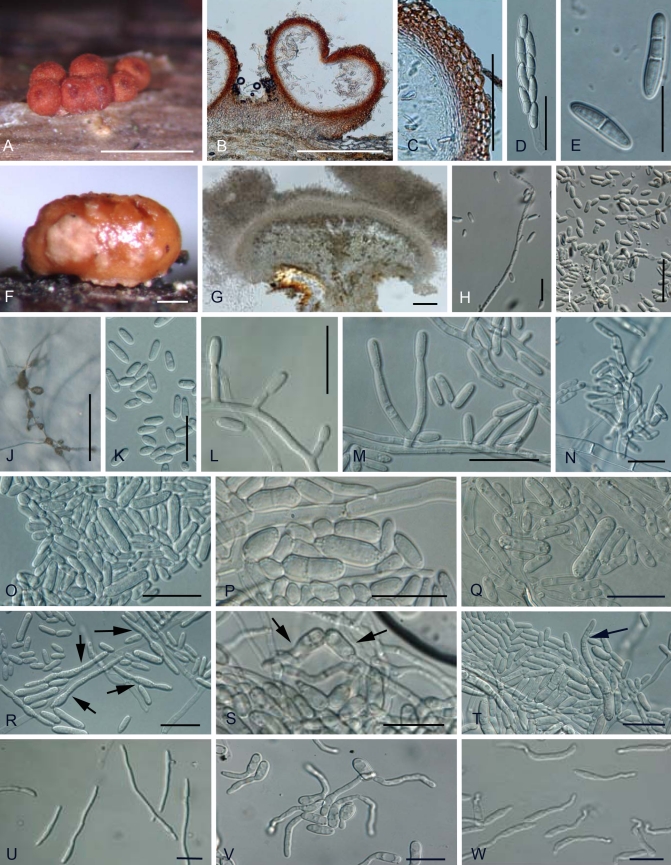
Nectria asiatica Hirooka, Rossman & P. Chaverri, sp. nov. MycoBank MB516721. Fig. 5.
Fig. 5.
A–R. Nectria asiatica. A. Perithecia and short stipitate sporodochia in the natural environment. B. Perithecia on nature. C. Median section of perithecium. D. Median section of perithecial wall. E. Ascus. F. 0–1 septates ascospores. G. Short stipitate sporodochium in the natural environment. H. Median section of short stipitate sporodochium. I. Edge of short stipitate sprodochium. J. Acropleurogenous conidiophores in the natural environment. K. Conidia in the natural environment. L. Aerial conidiophores and conidial mass on SNA. M. Lateral phialidic pegs and conidia on SNA. N. Short aerial conidiophores and conidia on SNA. O. Densely blanched aerial conidiophores and conidia on SNA. P. Mature conidia and young conidia on SNA. Q. Budding mature conidia on SNA. R. Budding and germinating mature conidia (arrow) that were streaked onto SNA. Scale bars: A, L = 1 mm; B, C, G, H = 300 μm; D, I = 100 μm; E, J, K, M, R = 30 μm; F, N, O, P, Q = 15 μm.
Anamorph: Tubercularia vulgaris-like.
Etymology: Asia + -tica - indicates the area from which this species is known.
Perithecia in cortice emortuo, solitaria vel gregaria, superficialia, subglobosa, 285–400 μm alta, 250–380 μm diam, rubella, KOH+, LA+. Asci unitunicati, clavate, apice simplici, 74–117 × 8.5–14.0 μm, octospori. Ascosporae ellipsoideae vel fusiformes, 10.5–19.0 × 3.0–6.0 μm, 0–1-septatae, hyalinae, laeves. Anamorphosis sporodochia discoida vel cylindrico-capitata, brevi-stipites, 250–800 mm alti, 300–2000 mm lati, atro-rubella vel raro niger, KOH+. Conidia oblonge ellipsoidea ad Cylindrica, 4.5–9.5 × 1.0–3.0 μm, hyalinae, leaves.
Holotype: Japan, Kanagawa Prefecture, Ashigarakami-gun, on dead wood, Oct. 2004, Y. Hirooka, holotype BPI 879972; ex-holotype culture MAFF 241439.
Teleomorph on natural substrata: Mycelium not visible around perithecia or on host. Stromata up to 1.0 mm high and 3 mm diam, erumpent through epidermis, whitish yellow to bay, sometimes darker red, KOH+ dark red, LA + yellow, pseudoparenchymatous; cells forming textura angularis to t. prismatica with cells oriented more or less vertically; cells 3–15 μm diam with walls 1–1.5 μm thick, intergrading with ascomatal wall. Perithecia superficial on well-developed stroma, solitary or caespitose, up to 20 on stroma, rarely clustered around base of stipitate sporodochia, subglobose to globose, 285–400 μm high × 250–380 μm diam (n = 39), red to reddish brown, sometimes cupulate upon drying, non-papillate, apical region darker, KOH+ dark red, LA+ yellow, surface with rough or concolourous warts, but sometimes smooth. Perithecial surface cells forming textura globulosa to t. angularis, with pigmented walls ca. 1.5 mm thick. Perithecial wall ca. 40–70 mm thick, of two distinct regions: outer region ca. 30–50 mm thick, intergrading with stroma, cells forming textura globulosa to t. angularis, walls pigmented, about 1.5 mm thick; inner region about 10–18 mm thick, of elongated, thin-walled, hyaline cells, forming textura prismatica. Asci unitunicate, (74–)89–101(–117) × (8.5–)10.0–12.5(–14.0) μm (n = 89), cylindrical to narrowly clavate, with an inconspicuous ring at apex, 8-spored, ascospores biseriate above, uniseriate below. Ascospores ellipsoidal to fusiform, straight, rarely slightly curved, hyaline, (0–)1-septate, (10.5–)14.5–17.5(–19.0) × (3.0–)3.5–5.0(–6.0) μm (n = 251), smooth-walled.
Anamorph on natural substrata: Stromata erumpent through epidermis, orange to red. Sporodochial conidiomata with stipe, superficial on well-developed stroma, smooth or cerebriform, scattered, solitary, or 2–4 gregarious, stipitate,, pustular, discoid or cylindrical-capitate, up to 250–800 mm high including stipe, 300–2000 mm diam, chestnut to black, sometimes whitish yellow to orange; stipe chestnut to black, sometimes dark green, up to 440–610 mm wide; stipe cells almost textura angularis, continuous with stroma, usually with wider cells in centre. Hymenium arising directly from textura prismatica, elongating from textura angularis, up to 110 μm long, of cells 2.0–7.0 μm wide, without curved margin. Conidiophores monoverticillate or rarely bi-verticillate, then developing acropleurogenously for 3–6 levels, strongly coiled, hyaline, rarely slightly pale green. Phialides intercalary, occurring below each septum, rarely terminal; intercalary phialides monophialidic, up to 3.5–7.5 μm long, 1.5–2.5 μm wide; terminal cells monophialidic, sometimes sterile, without collarettes. Conidia hyaline, narrowly long ellipsoidal to cylindrical, straight or slightly curved, non-septate, (4.5–)5.5–7.5(–9.5) × (1.0–)2.0–2.5(–3.0) μm (n = 258), smooth-walled.
Anamorph in culture: Optimum temperature for growth on PDA 25 °C, maximum temperature 30 °C; after 7 d at 25 °C colonies 40–75 mm diam (average 51 mm). Colony surface on PDA radiating sometimes wavy, slightly cottony with aerial mycelium, white to whitish saffron; aerial mycelium developing in a few isolates (CBS 125151, MAFF 241448); after 3 wk abundant white to whitish yellow sporodochial conidial masses produced; reverse white to slightly whitish yellow. Odour on PDA slightly fruity. Sporulation on SNA from lateral phialidic pegs on submerged or aerial hyphae, 3.0–5.0 μm long, 1.5–2.5 μm wide at base. Aerial conidiophores developing abundantly on aerial hyphae, unbranched, sometimes verticillate, 1–3 branched, becoming loosely to moderately densely branched, 6.0–25.5 μm long, 2.0–5.0 μm wide at base. Conidiogenous cells monophialidic, cylindrical, slightly tapering toward tip or narrowly flask-shaped with widest point in middle 7.5–22.5 μm long, 2.0–3.0 μm wide at base. Young conidia developing from monophialides on submerged or aerial hyphae, produced abundantly on slimy heads, non-septate, ellipsoidal, oblong to cylindrical, hyaline, smooth, straight or slightly curved, rounded at both ends, (4.0–) 6.0–12.0(–23.0) × (1.5–)2.0–3.0(–5.0) μm (n = 210). Mature conidia swollen, mostly 0-, rarely 1-septate, ellipsoidal, oblong or allantoid, rarely ellipsoidal with slightly constricted centre, smooth, straight or slightly curved, rounded at both ends, germinating or budding mature conidia (7.0–)11.5–17.5(–25.5) × (3.0–)3.5–4.5(–6.0) μm (n = 168). Chlamydospores and perithecia not produced in culture.
Distribution: Asia (China, Japan).
Habitat: On dead woody substrata, known in this study from Acer sp., Betula lutea, Prunus sp., Sorbus commixta, and Zelkova serrata.
Specimens and isolates examined: China, on dead wood, W.Y. Zhuang, culture CBS 126568 = A.R. 4639. Japan, Kanagawa Prefecture, Ashigarakami-gun, on bark of dead wood, Oct. 2004, Y. Hirooka, BPI 879973, culture MAFF 241435; Kanagawa Prefecture, Ashigarakami-gun, on dead twig, Apr. 2005, Y. Hirooka, BPI 879974, culture MAFF 241448; Kumamoto Prefecture, Kikuchi city, Kikuchi valley on dead wood of Zelkova serrata, Dec. 2000, Y. Hirooka, BPI 879975, culture MAFF 241398; Kumamoto Prefecture, Kikuchi city, Kikuchi valley, on twig of Prunus sp., Dec. 2000, Y. Hirooka, BPI 879976, culture MAFF 241399; Hokkaido, kamigawa-gun, mie-cho, on dead stem of Sorbus commixta, Sep. 1999, Y. Ono, BPI 879977, culture MAFF 241400; Nagano Prefecture, Ina city, on dead wood, Aug. 7, 1999, Y. Ono, BPI 879978, culture MAFF 241401; Saitama Prefecture, Kawaguchi city, Angyo, on dead twig of Prunus sp., Sep. 2002, Y. Hirooka, BPI 879979, culture MAFF 241405;Tokyo, Setagaya-ku, Tokyo University of Agriculture, on dead wood, Oct. 2002, Y. Hirooka, BPI 879980, culture MAFF 241408.
Notes: Nectria asiatica is known only from China and Japan, a range it shares with N. dematiosa subclade C. To differentiate these species, it is necessary to consider morphological characters of both the teleomorph and anamorph. Nectria asiatica has up to 1-septate ascospores (Fig. 5F) and budding mature conidia on SNA (Fig. 5Q, R) while N. dematiosa subclade C has up to 2-septate ascospores (Fig. 7E) and mature conidia that do not bud on SNA (Fig. 7R–W). In addition, N. asiatica has an optimal temperature for growth of 25 °C on PDA while N. dematiosa including subclade C has an optimal temperature for growth of 20 °C on PDA (Fig. 4). Although N. cinnabarina and N. nigrescens also produce budding mature conidia, N. asiatica forms up to 1-septate ascospores and stipitate sporodochia shorter than the former two species.
Fig. 7.
A–W. Nectria dematiosa species complex. A. Perithecia in the natural environment. B. Median section of perithecium. C. Median section of perithecial wall. D. Ascus. E. 1–2 septates ascospores. F. Astipitate sporodochium in the natural environment. G. Median section of stipitate sporodochium. H. Acropleurogenous conidiophore in the natural environment. I. Conidia in the natural environment. J. Aerial conidiophores and conidial mass on SNA. K. Young conidia on SNA. L. Lateral phialidic pegs and young conidia on SNA. M. Short aerial conidiophores and conidia on SNA. N. Densely blanched aerial conidiophores on SNA. O. Mature conidia and young conidia of N. dematiosa subclade A. P. Mature conidia and young conidia of N. dematiosa subclade B. Q. Mature conidia and young conidia of N. dematiosa subclade C. R. Germinating mature conidia (arrows) of N. dematiosa subclade A on SNA. S. Germinating mature conidia (arrows) of N. dematiosa subclade B on SNA. T. Germinating mature conidia (arrow) of N. dematiosa subclade C on SNA. U. Germinating mature conidia of N. dematiosa subclade A that were streaked onto SNA. V. Germinating mature conidia of N. dematiosa subclade B that were streaked onto SNA. W. Germinating mature conidia of N. dematiosa subclade C that were streaked onto SNA. Scale bars: A, J = 1 mm; B = 300 μm; C, F, G = 100 μm; D, H, I, R = 30 μm; E, K, L–W = 15 μm.
Hara (1918) described Nectria cinnabarina f. stromaticola on Dothichiza sp. (Dothioraceae, Dothideales) in Japan. He did not mention a type specimen and one could not be located. Based on his original description, this species had superficial, red, warted perithecia, asci with eight ascospores, and 1-septate ascospores. No anamorph was mentioned; however, it seems possible that the black stroma of the Dothichiza sp. listed as the substrate was actually the dark sporodochia of a Tubercularia anamorph. Most specimens of N. asiatica collected in Japan have chestnut to black sporodochial conidiomata. Because no type specimen could be located, we do not consider Nectria cinnabarina f. stromaticola to be a synonym of N. asiatica.
One isolate (MAFF 241400) is phylogenetically distinct from the other isolates of N. asiatica; however, the BI posterior probabilities and ML bootstrap values are not high enough to clearly segregate this strain from N. asiatica (0.69 BI PP, 80 % ML BP) (Fig. 2). In addition, the specimen of this isolate forms up to 1-septate ascospores, short stipitate sporodochia, and ellipsoidal, budding mature conidia with slightly constricted centres, morphological characteristics typical of N. asiatica. Based on these morphological and molecular phylogenetic analyses, we include MAFF 241400 in N. asiatica.
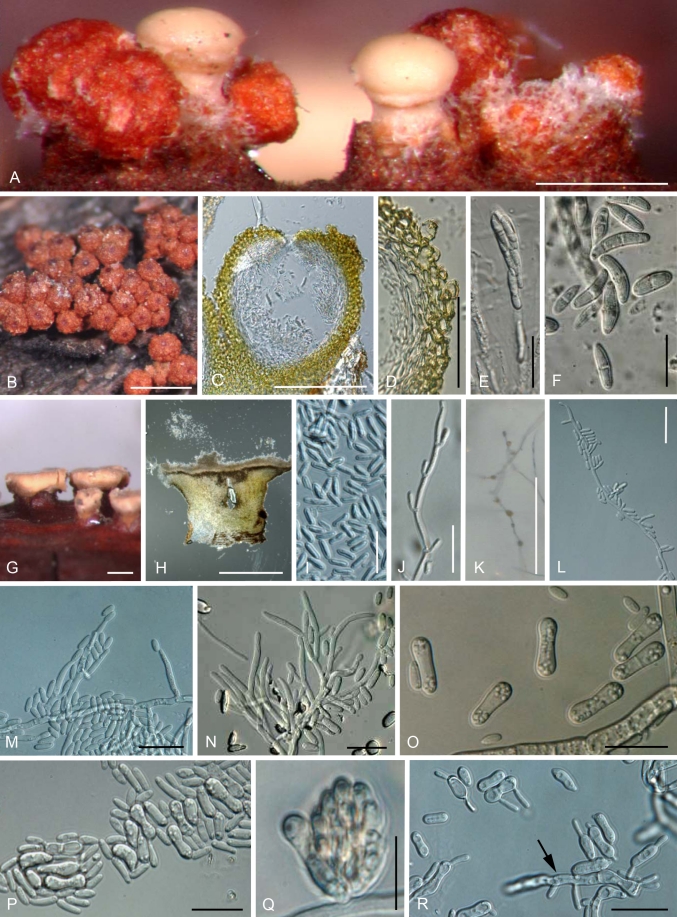
Nectria cinnabarina (Tode: Fr.) Fr., Summa Veg. Scand. 2: 388. 1849. Fig. 6. Basionym: Sphaeria cinnabarina Tode: Fr., Tode, Fungi Mecklenb. sel. 2: 9, 1791: Fries, Syst. Mycol. 2: 412. 1823.
Fig. 6.
A–R. Nectria cinnabarina. A. Perithecia and long stipitate sporodochia in the natural environment. B. Peritheica in the natural environment. C. Median section of perithecium. D. Median section of perithecial wall. E. Ascus. F. 0–2 septates ascospores. G. Long stipitate sporodochia in the natural environment. H. Median section of long stipitate sporodochia. I. Conidia in the natural environment. J. Acropleurogenous conidiophore in the natural environment. K. Aerial conidiophores and conidial mass on SNA. L. Lateral phialidic pegs on SNA. M. Aerial conidiophores and young conidia. N. Densely blanched aerial conidiophores and young conidia. O. Mature conidia on SNA. P. Budding mature conidia and secondly conidia on SNA. Q. Slimy head of young and mature conidia on lateral phialidic peg on SNA. R. Budding and germinating mature conidia (arrow) that were streaked onto SNA. Scale bars: A = 500 μm; C = 300 μm; D, = 100 μm; E, J, L, M, N, P, R = 30 μm; F, I, O, Q = 15 μm; B, G, H, K = 1 mm.
≡ Cucurbitaria cinnabarina (Tode: Fr.) Grev., Scot. Crypt. Fl. 3: 135. 1825.
= Sphaeria tremelloides Weigel, Obs. Bot. p. 46, 1772.
= Sphaeria decolorans Pers.: Fr., Persoon, Neues Magazin für Botanik, Rőmer 1: 83. 1794: Fries, Syst. Mycol. 2: 412, 1823.
= Sphaeria celastri Fr., Elenchus Fungorum 2: 81. 1827.
= Nectria russellii Berk. & M.A. Curtis in Berkeley, Grevillea 4: 45. 1875.
= Nectria offuscata Berk. & M.A. Curtis in Berkeley, Grevillea 4: 45. 1875.
Anamorph: Tubercularia vulgaris Tode: Fr., Tode, Fungi Mecklenb. sel. 1: 18, 1790: Fries, Syst. Mycol. 3: 464. 1832.
Teleomorph from natural substrata: Mycelium rarely visible around perithecia and on host. Stromata up to 2.0 mm high and 5 mm diam, erumpent through epidermis, whitish yellow to bay, KOH+ dark red, LA+ yellow, pseudoparenchymatous, cells forming textura angularis to t. prismatica with cells oriented more or less vertically; cells 5–20 μm diam, with 1–2 μm thick walls, intergrading with ascomatal wall. Perithecia superficial on well-developed stroma, solitary or caespitose, up to 25 on stroma, sometimes clustered around base of stipitate sporodochia, subglobose to globose, 275–400 μm high × 250–370 μm diam (n = 55), red to reddish brown, sometimes cupulate upon drying, non-papillate, apical region darker, KOH+ dark red, LA+ yellow, surface roughened with concolourous warts, but sometimes smooth. Perithecial surface cells forming textura globulosa or t. angularis, with walls pigmented ca. 1.5 mm thick. Perithecial wall ca. 40–60 mm thick, of two distinct regions: outer region ca. 35–55 mm thick, intergrading with stroma, cells forming textura globulosa or t. angularis, walls pigmented, ca. 1.5 mm thick; inner region ca. 15–20 mm thick, of elongated, thin-walled, hyaline cells, forming textura prismatica. Asci unitunicate, (81–)85–96(–105) × (7.5–)8.0–9.5(–11.0) μm (n = 129), cylindrical to narrowly clavate, with inconspicuous ring at apex, 8-spored, ascospores biseriate above, uniseriate below. Ascospores ellipsoidal to fusiform, straight, sometimes slightly curved, hyaline, (0–)1(–2)-septate, (11.5–)14.0–17.5(–21.5) × (3.0–)4.0–5.5(–7.0) μm (n = 558), smooth-walled.
Anamorph on natural substrata: Stromata erumpent through epidermis, pale yellow to orange, rarely reddish brown. Sporodochial conidiomata with stipe, superficial on well-developed stroma, smooth, cerebriform, or tubercularoid, scattered, solitary or 2–4 gregarious, stipitate, pustulate, discoid, or cylindrical-capitate, up to 700–1600 mm high including stipe, 300–2500 mm wide, white, whitish yellow to orange, sometimes darker red. Stipe white to whitish red, rarely darker red, up to 250–600 mm wide, solitary or 2–6 gregarious; stipe cells almost textura angularis, continuous with stroma, usually with wider cells in centre. Hymenium arising directly from textura prismatica, elongating from textura angularis, up to 150 μm long, of cells 2.5–5 μm wide; in stipitate forms marginal cells arranged in a palisade as described above for surface of stroma; curved margin, up to 100 μm long, of parallel hyphae 1.5–2.5 μm wide. Conidiophores monoverticillate or rarely bi-verticillate, then developing acropleurogenously for 3–10 levels, straight, curved. Phialides intercalary, occurring below each septum, or rarely terminal; intercalary phialides monophialidic, up to 3–9 μm long, 1.5–2 μm wide; terminal cells monophialidic, sometimes sterile, no collarettes. Conidia hyaline, narrowly long ellipsoidal to cylindrical, straight or slightly curved, non-septate, (4.0–)5.2–7.0(–8.5) × (1.3–) 1.9–2.7(–3.4) μm (n = 355), smooth-walled.
Anamorph in culture: Optimum temperature for growth on PDA 25 °C, maximum temperature 30 °C. After 7 d at 25 °C, colonies 60–85 mm (average 73 mm) diam. Colony surface radial, sometimes wavy, slightly cottony with aerial mycelium, white to whitish saffron; aerial mycelium developed, in some isolates (A.R. 4338, CBS 127668, CBS 125154, CBS 125157, CBS 125165) abundant, white to whitish yellow sporodochial conidial masses produced after 2 wk; reverse white to slightly whitish yellow. Odour on PDA slightly fruity. Sporulation on SNA from lateral phialidic pegs common, 1.5–4.5 μm long, 1.0–1.5 μm wide near aperture. Aerial conidiophores abundantly formed, unbranched, sometimes verticillate, 1–3 branched, becoming loosely to moderately densely branched, 5.5–38.0 μm long, 2.0–3.5 μm wide at base. Conidiogenous cells monophialidic, cylindrical and slightly tapering toward tip or narrowly flask-shaped with widest point in middle, 5–22 μm long, 2.0–3.2 μm wide at base. Young conidia formed from monophialides on submerged or aerial hyphae, formed abundantly on slimy heads or sporodochia, ellipsoidal, oblong to cylindrical, hyaline, smooth, straight or slightly curved with round at both end, non-septate, (3.0–) 5.5–9.0(–15.0) × (1.5–)2.0–3.0(–3.5) μm (n = 764), smooth-walled. Mature conidia swollen, mostly 0-, rarely 1-septate, allantoid, oblong, ellipsoidal, or ellipsoidal with strongly constricted centre, hyaline, smooth, straight or slightly curved, rounded at both ends, germinating and budding on media, (5.5–)10.5–17.0(–27.0) × (3.0–)4.0–5.0(–7.0) μm (n = 668). Chlamydospores rarely present, globose, subglobose, broadly ellipsoidal, 0(–1)-septate, solitary or chains, 8.5–12 μm diam. Perithecia not produced in culture.
Distribution: Europe (Austria, Denmark, France, Germany, Ireland, Netherlands, Poland, Sweden, Ukraine, UK) and North America (Canada, USA).
Habitat: On dead woody substrata including Acer campestre, A. platanoides, A. pseudoplatanus, A. saccharum, Acer sp., Aesculus sp., Celastris scandens, Fagus sp., Gleditsia sp., Populus tremula, Sorbus aria, Spiraea trilobata, Tilia sp., and Ulmus × hollandica.
Lectotype of Sphaeria cinnabarina designated here: figs 68a–e in the copy of Tode HJ (1791). Fungi Mecklenburgenses selecti. 2: 9 associated with BPI.
Epitype of Sphaeria cinnabarina designated here. France: Villiers en Bois, on dead twigs of Aesculus sp., Feb. 13, 2008, C. Lechat, epitype BPI 879981 = C.L.L. 7152, ex-epitype culture CBS 125165 = A.R. 4477.
Additional type specimens examined: The type specimen of Sphaeria tremelloides exists at K but these specimens are no longer sent for examination. This name is retained as a synonym of N. cinnabarina. A lectotype for Sphaeria decolorans is designated here: Country unknown: on branch of Acer platanoides, ex Herb. Persoon, BPI 799523). Additional Persoon material examined: Country unknown: on bark of Ribes rubrum, Mougeot, ex Herb. Persoon, BPI 799524). The lectotype and additional specimens of Sphaeria decolorans were examined, but these lacked the anamorphic structures needed to identify species within the NCSC. This name is retained as a synonym of N. cinnabarina. Type specimen of Sphaeria celastri: USA, Philadelphia, on dead branch of Celastrus scandens L., coll. possibly L.D. Schweinitz, holotype Schweinitz Syn. PH 1421. Type of Nectria russellii: USA Massachusetts, Jan. 1856, J.L. Russell, holotype FH 284394. Lectotype of Nectria offuscata designated here: USA, South Carolina, on Hibiscus syriacus L., lectotype BPI, Michener Collection 32, Sheet 12.
Additional specimens and isolates examined: Austria, Vienna, 19th district, base of the mountain Kahlenberg, MTB 7763/2, on Acer campestre L., 25 May 2006, W. Jaklitsch, BPI 878316, culture CBS 125151 = A.R. 4303; Vienna, on Acer pseudoplatanus L., 25 May 2006, coll W. Jaklitsch, BPI 878317, culture CBS 125150 = A.R. 4302. Canada, Ontario, Ottawa, on Acer sp., 26 Sep. 2006, K.A. Seifert 961, culture CBS 125154 = A.R. 4327; Quebec, Gatineau Park, Lac Philippe sector, ca. 45°35'24”N 75°59'25”W, on Acer saccharum Marsh., 15 Sep. 2006, K.A. Seifert, W. Gams, T. Gräfenhan, BPI 878311, culture CBS 125157 = A.R. 4341; Quebec, Quebec City. Lake St. Charles, on Spiraea trilobata L., 18 Aug. 2006, G. Laflamme, BPI 878335, culture CBS 125156 = A.R. 4340. Denmark, on bark of Tilia sp., 21 May 2006, T. Laessoe, BPI 879982, culture CBS 125152 = A.R. 4304; Sjaelland, Gadevang, on Acer pseudoplatanus L., 25 Aug. 2006, W. Jaklitsch, BPI 878312, culture CBS 127668 = A.R. 4337. France, Chize, on Acer sp., Jan. 18, 2007, C. Lechat 7027, BPI 879983, culture CBS 125163 = A.R. 4397. Germany, on Sorbus aria (L.) Crantz, Oct. 1986, H. Reinartz, anamorph only, culture CBS 189.87. Ireland, Dublin, Phoenix Park 53°20'59.91”N 6°17'56.87”W, on twigs, 21 Sep. 2006, K. Seifert, BPI 878313, culture CBS 125158 = A.R. 4379. Netherlands, on stem of Ulmus sp., (culture CBS 255.47, ATCC 11432; on twig of Ulmus sp., culture CBS 256.47. Poland, Sudetes, Zlote Mts., Zloty Stok, on twigs of Acer pseudoplatanus L., 6 Jun. 2006, A. Chlebicki, BPI 878322, culture A.R. 4388. Sweden, Fries, Scleromyceti Sueciae no. 184 as Sphaeria cinnabarina, BPI 799329, BPI 799330, BPI 799331, UPS. Ukraine, Kharkov-city, University botanic garden, on fallen twigs of Populus tremula L., 3 Mar. 2007, A. Akulov, BPI 878878, culture A.R. 4496. U.K, Wales, Hafod, logged area, ca. 52°22'N 3°51'W, on root, 1 Oct. 2006, K. Seifert, BPI 878310, culture CBS 125160 = A.R. 4381. USA, Virginia, Giles Co., Cascades Recreation Site, 4 Mi N of Pembroke, Little Stony Creek, 37d2d'n, 80d35'w. alt. 840 meters, on Acer sp., 18 Sep. 1991, G.J. Samuels,C.T. Rogerson, S. Huhndorf, S. Rehner, BPI 1112890, culture CBS 125115 = G.J.S. 91-121; Virginia, Giles Co., Mountain Lake, alt. 1160 meters, 37d22'n,80d31'w, near hotel pond drain, on Fagus sp., 17 Sep. 1991, G.J. Samuels, BPI 1112878, culture G.J.S. 91-109; Virginia, Giles Co., Mountain Lake, alt. 1160 meters, 37d22'n, 80d31'w, near hotel, Pond Drain, on Acer sp., 17 Sep. 1991, G.J. Samuels, BPI 1112880, culture CBS 713.97 = G.J.S. 91-111.
Notes: Nectria cinnabarina is the type species of the genus Nectria. Tode (1791) described and illustrated the superficial, red, warted perithecia and 1-septate ascospores, but did not mention any detailed morphology of perithecial wall structure or stroma. Because the type specimen was lost, the name Sphaeria cinnabarina is lectotypified by the original illustration in the copy of Tode (1791) associated with BPI. A stipitate sporodochium with perithecia at the base is clearly illustrated by Tode (1791), thus assuring the identity of N. cinnabarina. Based on Article 7.8 of the ICBN (McNeill et al. 2006), an illustration from the protologue may serve as a lectotype, thus this lectotypification supersedes the neotypification by Rossman et al. (1999). We here epitypify N. cinnabarina with BPI 879981, a specimen collected in France with abundant mature perithecial and anamorph structures as well as a living culture.
Nectria cinnabarina can be identified by morphological characteristics of the teleomorph and anamorph in the natural environment and in culture. On natural substrate, N. cinnabarina has up to 2-septate ascospores and long stipitate sporodochia (Fig. 6A, F–H). Among species in the NCSC, N. cinnabarina is similar to N. nigrescens in having long stipitate sporodochia; however, N. nigrescens is distinct in having up to 3-septate ascospores. Unlike N. asiatica, N. dematiosa, and N. nigrescens, N. cinnabarina is distinguished in culture by abundant budding mature conidia that are ellipsoidal and strongly constricted in the centre (Fig. 6O, P).
Nectria dematiosa (Schwein.) Berk., Grevillea, 4: 16. 1875. Fig. 7. Basionym: Sphaeria dematiosa Schwein., Trans. Amer. Philos. Soc. II, 4: 205. 1832.
≡ Cucurbitaria dematiosa (Schwein.) Kuntze, Revisio Generum Plantarum 3: 461. 1898.
= Nectria sambuci Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 1890: 246. 1891.
= Nectria cinnabarina subsp. amygdalina P. Karst., Rev. Mycol. 37: 205. 1889.
≡ Nectria amygdalina (P. Karst.) Mussat in Saccardo, Syll. Fung. 15: 225. 1901.
Anamorph: Tubercularia vulgaris-like.
Teleomorph on natural substrata: Mycelium not visible around perithecia and on host. Stromata up to 0.3 mm high and 2 mm diam, erumpent through epidermis, orange to bay, sometimes darker red, KOH+ dark red, LA+ yellow, pseudoparenchymatous, cells forming textura angularis to t. prismatica with cells oriented more or less vertically; cells 3–10 μm diam, with 1–1.5 μm thick walls, intergrading with the ascomatal wall. Perithecia superficial on well-developed, erumpent stroma, solitary or caespitose, up to 20 on a stroma, rarely clustered around sessile sporodochia, subglobose to globose, 260–380 μm high × 220–380 μm diam (n = 40), red to reddish brown, sometimes cupulate upon drying, non-papillate, apical region darker, KOH+ dark red, LA+ yellow, surface with rough or concolourous warts, but sometimes smooth. Perithecial surface cells forming textura globulosa or t. angularis, with walls pigmented, ca. 1.5 mm thick. Perithecial wall ca. 35–60 mm thick, of two distinct regions: outer region ca. 25–40 mm thick, intergrading with stroma, cells forming textura globulosa or t. angularis, walls pigmented, ca. 1.5 mm thick; inner region ca. 10–20 mm thick, of elongated, thin-walled, hyaline cells, forming textura prismatica. Asci unitunicate, (64–)77–91(–108) × (6.3–) 9.4–11.0(–12.0) μm (n = 68), cylindrical to narrowly clavate, with an inconspicuous ring at apex, 8-spored, ascospores biseriate above, uniseriate below. Ascospores ellipsoidal to fusiform, sometimes long fusiform, straight or slightly curved, hyaline, smooth-walled, (0–)1(–2)-septate, (12.6–)15.2–17.2(–22.2) × (3.2–)4.3–5.7(–6.4) μm (n = 150); subclade A: (12.6–)13.9–16.9(–18.5) × (3.4–)3.9–4.9(–5.3) μm (n = 30); subclade B: (13.6–)14.7–17.9(–20.5) × (3.8–)4.7–5.7(–6.4) μm (n = 60); subclade C: (12.6–)14.3–18.9(–22.2) × (3.2–)4.3–5.7(–6.2) μm (n = 60).
Anamorph on natural substrata: Stromata erumpent through epidermis, orange to red. Sporodochial conidiomata without stipe, superficial on well-developed stroma, smooth, cerebriform or tubercularoid, scattered, solitary, rarely caespitose, astipitate, sessile, pustular, discoid or cylindrical-capitate, up to 200–700 mm high, 250–1000 mm wide, white, whitish yellow to orange, sometimes brown. Hymenium arising directly from textura prismatica elongating from textura angularis, up to 90 μm long, of cells 2.0–7.5 μm wide, not curved at margin. Conidiophores monoverticillate or sometimes bi-verticillate, then developing acropleurogenously for 3–6 levels, straight, curved hyaline. Phialides intercalary occurring below each septum, or rarely terminal; intercalary phialides monophialidic, 2.5–8.5 μm long, 1.3–2.4 μm wide at base; terminal cells monophialidic, sometimes sterile, no collarettes, 10.5–15 μm long, 2.3–2.8 μm wide at base. Conidia hyaline, narrowly long ellipsoidal to cylindrical, straight or slightly curved, non-septate, (4.5–)5.7–7.1(–8.8) × (1.7–)2.2–2.8(–3.1) μm (n = 60). Subclade A: (4.5–)5.5–7.1(–8.8) × (2.0–)2.2–2.6(–2.9) μm (n = 30), subclade B: (5.2–)5.8–7.0(–7.8) × (1.7–)2.3–2.9(–3.1) μm (n = 30), subclade C: none present.
Anamorph in culture: Optimum temperature for growth on PDA 20 °C, colonies 65–85 mm (average 70 mm) diam at 20 °C after 7 d, maximum temperature 30 °C. Colony surface on PDA, radial, sometimes wavy, slightly cottony with aerial mycelium, white to whitish saffron; aerial mycelium developing in a few isolates (CBS 125127, CBS 126570), white to whitish yellow sporodochial conidial masses produced after 2 wk; reverse white to slightly whitish yellow. Odour slightly fruity. Sporulation on SNA from lateral phialidic pegs on submerged or aerial hyphae common, 2.5–4.5 μm long, 1.5–3.0 μm wide at base. Aerial conidiophores occasionally developing on aerial hyphae, unbranched, sometimes verticillate, 1–2-branched, becoming loosely to moderately densely branched, 6.0–34 μm long, 2.1–4.5 μm wide at base. Conidiogenous cells monophialidic, cylindrical, slightly tapering toward tip or narrowly flask-shaped with widest point in middle, 8–26 μm long, 2.5–3.5 μm wide at base. Young conidia formed by monophialides on submerged or aerial hyphae, formed abundantly on slimy heads, non-septate, ellipsoidal, oblong to cylindrical, hyaline, smooth, straight or slightly curved with round at both ends, (4.1–)6.0–10.6 (–17.3) × (1.6–)2.4–3.4(–5.1) μm (n = 496); subclade A: (4.6–) 5.9–10.1(–14.0) × (1.6–)2.3–3.1(–4.0) μm (n = 200); subclade B: (4.1–) 6.0–10.6(–16.8) × (1.6–)2.4–3.6(–5.1) μm (n = 213); subclade C: (5.0–)6.5–11.5(–17.3) × (2.2–)2.6–3.4(–4.0) μm (n = 83). Mature conidia swollen, mostly 0-, rarely 1-septate, ellipsoidal, oblong or allantoid, rarely ellipsoidal, straight or slightly curved, rounded at both ends, germinating, never budding secondary conidia on media, (7.1–)10.0–17.4(–29.3) × (2.8–)3.8–5.6(–7.9) μm (n = 429); subclade A: (8.2–)10.7–19.1(–27.8) × (2.9–)3.6–5.0(–6.1) μm (n = 136); subclade B: (7.1–)9.7–16.7(–29.3) × (3.5–)4.3–6.1(–7.9) μm (n = 211); subclade C: (8.0–)10.7–15.9(–23.2) × (2.8–)3.3–4.7 (–5.6) μm (n = 82). Chlamydospores and perithecia not produced in culture.
Distribution: Asia (China, Japan), Europe (Finland, Poland), New Zealand, North America (Canada, USA).
Habitat: On dead woody substrata including Acer macrophyllum, A. pseudoplatanus, Acer sp., Morus sp., Prunus tenella, Ribes sp., Rosa sp., Sambucus nigra ssp. canadensis, and Weigela coraeensis.
Lectotype of Nectria dematiosa designated here: USA, Pennsylvania, on Morus sp., Bethlehem, Schweinitz, lectotype BPI 799536, isolectotype BPI 799535 anamorph only. The two isotype specimens of S. dematiosa have sessile sporodochia; on BPI 799536 ascospores up to 2-septate were observed. This specimen has only 4 or 5 perithecia and a few sessile sporodochia.
Epitype of Nectria dematiosa designated here: USA, North Carolina, Highlands, Macon Co. Highlands Biological Station, Lake Ravenel, on bark, 31 Aug. 1994, G.J. Samuels & H.-J. Schroers, epitype BPI 749337, ex-epitype culture CBS 126570 = G.J.S. 94-37.
Additional type specimens examined. Holotype of Nectria sambuci: USA, Nebraska, Lincoln, on Sambucus nigra ssp. canadensis, Aug. 1888, H.J. Webber, holotype NY 00927949. Holotype of Nectria cinnabarina subsp. amygdalina: Finland, Mustiala, on dead branch of Amygdalus nana, now considered to be Prunus tenella, 28 May 1889, P.A. Karsten. Holotype H 6009374.
Specimens and isolate examined. Canada, British Columbia, Sidney, Dogwood, on dead twig of Acer macrophyllum, 2 May 1992, M.E. Barr, BPI 802212, culture CBS 125125 = A.R. 2699; British Columbia, Sidney, on dead twig of Rosa sp., 5 Feb. 1992, M.E. Barr, BPI 802215, culture CBS 125127 = A.R. 2702; Ontario, Ottawa, on Acer sp., K. Seifert 1450, culture CBS 125155 = A.R. 4328. China, Jun. 2009, W.Y. Zhuang, culture CBS 127667 = A.R. 4638. Japan, Gunma Prefecture, Seta-gun, Fujimi-son, on twig of Weigela coraeensis Thunb., May 2003, Y. Hirooka, BPI 879984, culture MAFF 241416; Tokyo, Okutama-gun, on twig, Nov. 2003, Y. Hirooka, BPI 879985, culture MAFF 241430. New Zealand, Otago, on dead twig of Ribes sativum, 1 Feb. 1948, BPI 880708. Poland, Bialowieza forest, NW part of the forest near Lipiny reserve, section 271c, alt. 170 m. 52°45'13”N 23°37'59”E, on twig, 21 May 2006, D. Karasinski and D. Ronikier, BPI 878308, culture CBS 125159 = A.R. 4380. Unknown: on Acer pseudoplatanus, culture CBS 279.48; on Ribes sp., culture CBS 278.48.
Notes: Nectria dematiosa is distinguished from other species of the NCSC by sessile sporodochia and ascospores that are up to 2-septate. Care must be taken in observing these characters, because the short stipitate sporodochia of N. asiatica and N. nigrescens are often covered by a mass of conidia, thus appearing sessile. In addition, the 2-septate ascospores of N. dematiosa occur relatively infrequently (Fig. 7E). Additional differences include mature conidia of N. dematiosa that never bud on SNA (Fig. 7R–W). Finally, the optimum temperature for growth of N. dematiosa on PDA is 20 °C, while the optimum temperature for growth of N. asiatica, N. cinnabarina, and N. nigrescens is 25 °C (Fig. 4).
Our molecular phylogenetic analyses suggest that three subclades can be distinguished within N. dematiosa (Fig. 2). Some subtle differences among subclades were observed specifically differences in the shape and behavior of germ tubes, mycelial growth at 25 °C on PDA, and geographic range. Mature conidia of subclade A produce almost straight germ tubes that did not grow into the agar immediately, while mature conidia of subclades B and C produced sinuate germ tubes that grew into the agar after germination (Fig. 7U–W). The 95 % confidence intervals of mature conidial length/width ratio of subclade B were statistically different from subclades A and C (Fig. 3). According to mycelial growth at 25 °C for 7 d on PDA, subclade B showed slower growth than subclades A and C (20–30 mm vs. 40–70 mm) (Fig. 4).
For several reasons, we do not recognise these N. dematiosa subclades as distinct species. First of all, in subclade A the five collections from Canada, Poland and the USA contain only one specimen with the teleomorph (BPI 749337), while anamorphs on natural substrate were observed on only two specimens (BPI 749337, BPI 878308). In subclade B, there are only two specimens both collected in Canada (BPI 802212, BPI 802215). In addition, the anamorph of BPI 802215 was not found on natural substrate. Subclade C is known only from Asia and no anamorph was observed on natural substrate (Fig. 2). The number of samples available is relatively small and the few specimens were insufficient to determine if morphological differences exist and are constant on natural substrate.
Jøgensen (1952) found morphological differences between typical N. cinnabarina and N. cinnabarina on Ribes. Jøgensen (1952) also mentioned that the fungus grew faster than N. cinnabarina from other hosts. One isolate was obtained of N. `cinnabarina' on Ribes sp. (CBS 278.48). In growth trials this isolate showed growth similar to that of N. dematiosa subclade A (Fig. 4). Based on our phylogenetic analysis, this isolate falls in N. dematiosa subclade A with isolates collected on Acer pseudoplatanus and Acer sp.
Nectria nigrescens Cooke, Grevillea 7: 50. 1878. Fig. 8.
Fig. 8.
A–S. Nectria nigrescens. A. Perithecia and short stipitate sporodochia in the natural environment. B. Peritheica in the natural environment. C. Median section of perithecia. D. Median section of perithecial wall. E. Ascus. F. One and three septatas ascospores. G. Long stipitate sporodochia in the natural environment. H. Median section of long stipitate sporodochium. I. Edge of long stipitate sporodochium. J. Acropleurogenous conidiophore in the natural environment. K. Conidia in the natural environment. L. Aerial conidiophores and conidial mass on SNA. M. Young conidia on SNA. N. Lateral phialidic pegs on SNA. O. Short and densely blanched aerial conidiophores, and conidia on SNA. P. Mature conidia and young conidia on SNA. Q, R. Budding mature conidia on SNA. S. Germinating mature conidia that were streaked onto SNA. Scale bars: A, G, H, L = 1 mm; B, C = 300 μm; D, I = 100 μm; E, J, K, O, R = 30 μm; F, M, N, P, Q, S = 15 μm.
= Nectria cinnabarina f. dendroidea Fuckel, Fungi rhenani 2657. 1874.
≡ Nectria cinnabarina var. dendroidea (Fuckel) Wollenw., Angew. Bot. 8: 186. 1926.
= Nectria cinnabarina var. minor Wollenw., Angew. Bot. 8: 185. 1926.
= Nectria meliae Earle, Bull. Torrey Bot. Club 25: 364. 1898.
= Nectria fuscopurpurea Wakef., Kew Bull., p. 232. 1918.
Anamorph: Tubercularia vulgaris-like.
Teleomorph on natural substrata: Mycelium rarely visible around perithecia and on host. Stromata up to 2.0 mm high and 4 mm diam, erumpent through epidermis, whitish yellow to bay, sometimes darker red, KOH+ dark red, LA+ yellow, pseudoparenchymatous, cells forming textura angularis to t. prismatica with cells oriented more or less vertically; cells 4–17 μm diam, with 1–1.5 μm thick walls, intergrading with the ascomatal wall. Perithecia superficial on well-developed stroma, solitary or caespitose, up to 20 on an erumpent stroma, rarely clustered around base of stipitate sporodochia, subglobose to globose, 265–420 μm high × 236–410 μm diam (n = 38), red to reddish brown, sometimes cupulate upon drying, non-papillate, apical region darker, KOH+ dark red, LA+ yellow, surface with rough or concolourous warts, but sometimes smooth. Perithecial surface cells forming textura globulosa or t. angularis, with walls pigmented ca. 1.5 mm thick. Perithecial wall ca. 40–65 mm thick, of two distinct regions: outer region about 25–45 mm thick, intergrading with stroma, cells forming textura globulosa or t. angularis, walls pigmented, ca. 1.5 mm thick; inner region ca. 7–18 mm thick, of elongated, thin-walled, hyaline cells, forming textura prismatica. Asci unitunicate, (62–)70–98(–113) × (6.5–)7.5–10.0(–11.5) μm (n = 63), cylindrical to narrowly clavate, with an inconspicuous ring at apex, 8-spored, ascospores biseriate above, uniseriate below. Ascospores ellipsoidal to fusiform, straight, sometimes slightly curved, hyaline, (0–)1(–3)-septate, (10.5–)13.5–18.0(–22.0) × (2.5–)3.5–5.5(–8.0) μm (n = 320), smooth-walled.
Anamorph on natural substrata: Stromata erumpent through epidermis, pale yellow to orange, rarely reddish brown. Sporodochial conidiomata with stipe, superficial on well-developed stroma, smooth, cerebriform or tubercularoid, scattered, solitary, or 2–4 gregarious, stipitate, pustular, discoid or cylindrical-capitate, up to 250–1700 mm high, 300–1700 mm wide, white, whitish yellow to orange, sometimes brown, red or dark red; stipe white to whitish red, rarely dark red, up to 340–640 mm wide; stipe cells almost textura angularis, continuous with stroma, usually with wider cells in centre. Hymenium arising directly from textura prismatica elongating from textura angularis, up to 120 μm long, of cells 2.5–6.0 μm wide, curved margin, up to 150 μm long, of parallel hyphae 1.5–2.5 μm wide. Conidiophores monoverticillate or rarely bi-verticillate, then developing acropleurogenously for 3–7 levels, straight to curved, sometimes coiled. Phialides intercalary, occurring below each septum, or rarely terminal; intercalary phialides monophialidic, up to 3.0–5.0 μm long, 1.0–2.0 μm wide; terminal cells monophialidic, sometimes sterile, no collarettes. Conidia hyaline, narrowly long ellipsoidal to cylindrical, straight or slightly curved, (4.7–)5.5–6.9(–8.4) × (1.6–)2.1–2.7(–3.0) μm (n = 343), non-septate.
Anamorph in culture: Optimum temperature for growth on PDA 25 °C, maximum temperature 35 °C, after 7 d colonies 70–85 mm (av. 80 mm) diam. Colony surface on PDA, radial, sometimes wavy, slightly cottony with aerial mycelium, white to whitish saffron; aerial mycelium developing only in CBS 125148, white to whitish yellow, sporodochial conidial masses produced after 2 wk; reverse white to slightly whitish yellow. Odour on PDA slightly fruity. Sporulation on SNA from lateral phialidic pegs on submerged or aerial hyphae common, 2.4–5.3 μm long, 1–1.9 μm wide near aperture. Aerial conidiophores abundantly developed on aerial hyphae, unbranched, sometimes verticillate, 1–2-branched, becoming loosely to moderately densely branched, 5.5–21.5 μm long, 2.0–3.0 μm wide at base. Conidiogenous cells monophialidic, cylindrical, slightly tapering toward tip or narrowly flask-shaped with widest point in middle, 9.5–17.0 μm long, 1.5–2.0 μm wide at base. Young conidia formed by monophialides on submerged or aerial hyphae, formed abundantly on slimy heads, non-septate, ellipsoidal, oblong to cylindrical, hyaline, smooth, straight or slightly curved with rounded ends, (3.0–)4.0–7.0(–14.5) × (1.5–)2.0–2.5(–3.5) μm (n = 250). Mature conidia swollen, mostly 0-, rarely 1-septate, ellipsoidal, oblong, or allantoid, rarely ellipsoidal with slightly constricted centre, hyaline, smooth, straight or slightly curved, rounded at both ends, germinating or budding secondary conidia on media, (5.0–)7.6–14.6(–24.3) × (2.3–)3.5–4.9(–6.6) μm (n = 180). Chlamydospores rare, globose, subglobose, broadly ellipsoidal, 0(–1)-septate, solitary or chains, 8.0–13.0 μm wide. Perithecia not produced in culture.
Distribution: Europe (France, Germany, UK), North America (Canada, USA).
Habitat: On dead woody substrata including Acer sp., Betula lutea, Celtis occidentalis, and Fagus sylvatica.
Holotype of Nectria nigrescens: USA, South Carolina, on Gleditsia sp., S.C. Aiken, K165219, Ravenel, American Fungi 2380a.
Epitype of Nectria nigrescens designated here. USA, North Carolina, Haywood Co., Great Smoky Mountains National Park, Purchase Knob. Cataloochees Divide Trail, alt. 5000 ft. 35°35'9.9”N 83°4'25.5”W, on dead twig of dictyledonous tree, 7 Sep. 2005, A.Y. Rossman, epitype BPI 871083, ex-epitype culture CBS 125148 = A.R. 4211.
Additional type specimens examined. Holotype of Nectria cinnabarina f. dendroidea: Germany, Fungi Rehnani 2657, FH. Holotype of Nectria fuscopurpurea: UK, Wisbech, on dead branch of Prunus domestica L., 1917, J.C.F. Fryer or A.D. Cotton, K98615. Neotype of Nectria meliae designated here: USA, Alabama, on Melia sp., 1 Dec. 1896, C.F. Baker, BPI 552588.
Specimens and isolates examined: Canada, Ontario, Carleton Place, near the Mississippi River, on twigs of Celtis occidentalis, 31 Jun. 2007, T. Gräfenhan, BPI 878449, culture CBS 125162 = A.R. 4394. France, Foret le Chize, Les Essarts, on twig of Fagus sylvatica, 27 Nov. 2007, C. Lechat, BPI 878457, culture CBS 125164 = A.R. 4475; Foret le Chize, Puymardier, on dead twig of Acer sp., 18 May 2006, C. Lechat, BPI 878455A = C.L.L. 684, culture A.R. 4282. USA, Tennessee, Sevier Co., Great Smoky Mountains National Park. Alum Cave Bluff Trail, alt. 3900 ft. 35°37'43.3”N 83°27'32”W, on dead twig of Betula lutea, 8 Sep. 2005, A.Y. Rossman, BPI 871084, culture CBS 125149 = A.R. 4213; Vermont, Windham County, Putney, Fort Hill Road, along a stream in a wet site, on dead twig, 17 Oct. 2008, G.J. Samuels, BPI 879986, culture CBS 127668 = A.R. 4565.
Notes: Nectria nigrescens resembles N. asiatica and N. cinnabarina in producing short to long stipitate sporodochia and mature conidia that bud (Fig. 8A, G, H, Q, R). Nectria nigrescens has up to 3-septate ascospores, short or long stipitate sporodochia, and length/width ratios of young and mature conidia that are somewhat smaller than the other species of the NCSC (Figs 3, 8A, F, G, H). Budding mature conidia of N. nigrescens on SNA (Fig. 8Q, R) are less commonly observed than in N. asiatica and N. cinnabarina.
The name N. cinnabarina f. dendroidea was published on the label of Fuckel's Fungi Rhenani 2657, issued in 1874 (Pfister 1985). Fuckel (1874) provided a name on this label that referred to a previously published description of the specimen (Fuckel 1873). We examined photographs and a microscope slide of the exsiccati (Fuckel, Fungi Rhen. 2657 from FH) and determined this name to be a synonym of N. nigrescens. Wollenweber (1926) attributed his name Nectria cinnabarina var. dendroidea (Fuckel) Wollenw. to Fuckel (1873). Wollenweber (1926) noted the presence of long, stipitate sporodochia on the type specimen and was the first to regard this as an important characteristic. He described and illustrated both N. cinnabarina var. dendroidea and N. cinnabarina var. minor as having 1-septate ascospores. His later illustration of N. cinnabarina var. minor showed this variety with up to 3-septate ascospores (Wollenweber 1930, no. 778). Although Wollenwever (1926) did not document stipitate sporodochia of N. cinnabarina var. minor, his illustration showed well developed stroma (Wollenweber 1926, table 3, 21f). From these reasons, we include N. cinnabarina f. dendroidea and N. cinnabarina var. minor as synonyms of N. nigrescens.
The holotype specimen of N. meliae is lost, therefore, a specimen collected in the same year, on the same genus of host, and at the same place i.e. a topotype, specifically BPI 552588, is designated the neotype of N. meliae.
Our phylogenetic analyses suggest a sister-group relationship between N. nigrescens and CBS 125162, supported by high BI posterior probabilities and ML bootstrap (1.00 BI PP, 99 % ML BP) (Fig. 2). However, based on morphological characters in the natural environment and culture, CBS 125162 completely matches N. nigrescens and is regarded as N. nigrescens.
SPECIES EXCLUDED OR OF UNCERTAIN STATUS
Nectria cinnabarina var. ribis(Tode) Wollenw., Fusaria autographica delineata, Edn 1: no. 787. 1930. Basionym: Sphaeria ribis Tode, Fungi Mecklenb. sel. 2: 31. 1791.
≡ Hypoxylon ribis (Tode) J. Kickx f., Fl. Crypt. Louvain p. 113. 1835.
≡ Nectria ribis (Tode) Nießl, Verh. Naturf. Vereins Brünn 3: 171. 1865.
≡ Nectria ribis (Tode) Rabenh. in Sacc., Syll. Fung. 2: 480. 1883.
Notes: Nectria cinnabarina var. ribis was originally described as Sphaeria ribis by Tode (1791). Because Tode's specimens were destroyed (Kirk et al. 2008), his illustrations are regarded as lectotype (tabula XII, fig. 103a–f). Tode (1791) described and illustrated smooth, pyriform perithecia immersed at the base of a well-developed stroma, possibly as a parasite, and thus do not belong in the N. cinnabarina species complex. Rather it appears to be related to Cosmospora.
Tremella purpurea L., Spec. Plant. 2: 1158. 1753. Basionym: Nectria purpurea (L.) G.W. Wilson & Seaver, J. Mycol. 13: 51. 1907.
≡ Cucurbitaria purpurea (L.) Seaver, Mycologia 1: 184. 1909.
Notes: The name Tremella purpurea was listed as a synonym of N. cinnabarina (Rossman et al. 1999). However, according to Spencer et al. (2009), this name is invalid because the genus was not validly published by Linnaeus (1753). Names based on this invalidly published name are either invalid or illegitimate.
KEY TO THE SPECIES IN THE NECTRIA CINNABARINA SPECIES COMPLEX
On natural substrate
1. Ascospores up to 3-septate, 1-septate (91 %), 2-septate (5 %), 3-septate (4 %); sporodochia short (65 %) to long stipitate (35 %), 250–1700 μm high; Europe or North America..................................................................................................................... N. nigrescens 1. Ascospores up to 1-, rarely 2-septate; sporodochia sessile or stipitate; Asia, Europe or North America.................................................. 2
2. Ascospores up to 1-septate; sporodochia less than 800 μm high, short stipitate; Asia............................................................. N. asiatica 2. Ascospores up to 1- or rarely 2-septate (3 %); sporodochia sessile or long stipitate; Asia, Europe or North America............................. 3
3. Sporodochia 700–1600 μm high, long stipitate (70 %); Europe or North America............................................................. N. cinnabarina 3. Sporodochia sessile or anamorph lacking; Asia, Europe or North America......................................................................... N. dematiosa
In pure culture
1. Mature conidia not budding on SNA after 7 d; optimum temperature for growth 20 °C on PDA...................................................................................................................................................... N. dematiosa, subclades A–C, go to 4 1. Mature conidia budding on SNA after 7 d; optimum temperature for growth 25 °C on PDA..................................................................... 2
2. Mature conidia ellipsoidal, strongly constricted, budding; Europe or North America.......................................................... N. cinnabarina 2. Mature conidia ellipsoidal, straight, or slightly curved, rarely slightly constricted, rarely budding; Asia, Europe or North America........... 3
3. Young conidia averaging 10 μm long; mature conidia averaging 15 μm long; Asia.................................................................. N. asiatica 3. Young conidia averaging 5 μm long; mature conidia averaging 10 μm long; Europe or North America.............................. N. nigrescens
4. Germ tubes more or less straight, not penetrating agar immediately; Canada, Poland, USA.......................... N. dematiosa subclade A 4. Germ tube sinuate, penetrating agar immediately after germination; Canada, China, Japan................................................................... 5
5. Mean of 95 % confidence intervals of mature conidial length/width ratio 2.5; mycelial growth 20–30 mm after 7 d at 25 °C; Canada.......................................................................................................................................................................... N. dematiosa subclade B 5. Mean of 95 % confidence intervals of mature conidial length/width ratio 3.5; mycelial growth 40–50 mm after 7 d at 25 °C; China, Japan................................................................................................................................................................. N. dematiosa subclade C
DISCUSSION
Nectria cinnabarina and other species in the NCSC form a monophyletic group within Nectria, all having Tubercularia anamorphs (Fig. 1). The molecular analyses of the NCSC resolve four phylogenetically distinct species (Fig. 2), each of which is described and illustrated above.
The anamorph of N. cinnabarina sensu lato has been referred to as Tubercularia vulgaris. Many synonyms are known for T. vulgaris (Jøgensen 1952, Booth 1959, Seifert 1985) for which authentic and type specimens were examined by Seifert (1985). Although differences exist in stipe length in the natural environment and in size and shape of conidia in culture, it is not possible to determine which synonym of T. vulgaris represents each species in the NCSC. Thus, the anamorph of N. cinnabarina is referred to as T. vulgaris, while the anamorph of other species in the NCSC is referred to as Tubercularia vulgaris-like.
Seifert (1985) recognised that T. vulgaris in the natural environment had two types of sporodochia, i.e. sessile and stipitate sporodochia with marginal cells arranged in a palisade. These differences correlate with the species recognised here. Specifically, N. dematiosa has sessile sporodochia while N. asiastica, N. cinnabarina, and N. nigrescens have short to long stipitate sporodochia. Except for conidia in culture, no differences were found in other morphological characteristics of the anamorph including the number of conidiophore branches and conidial size in the natural environment. Morphological heterogeneity of conidia in culture was noted for many years (Mayr 1883, Brefeld 1891, Beck 1902, Jøgensen 1952). Beck (1902) observed conidia that were much larger than normal conidia and suggested that their size depended on the nutritional content of the media. To standardise cultural conditions, Jøgensen (1952) used a detached branch instead of artificial media. He determined that the range of conidial size was variable but not useful in distinguishing taxa within specimens identified as N. cinnabarina. By observing mature conidia on SNA, we could distinguish species in the NCSC including the subclades within N. dematiosa. Budding of mature conidia in culture was observed for N. asiatica, N. cinnabarina, and N. nigrescens, a characteristic not noted for other Nectria-like fungi. Differences in the size of mature conidia and the shape of its germ tube can be used to distinguish the subclades in the N. dematiosa clade.
Nectria cinnabarina sensu lato has been considered a cosmopolitan species (Farr & Rossman 2010). This study shows that N. cinnabarina, N. nigrescens, and N. dematiosa subclades A and B are widespread on hardwood trees and woody shrubs in Europe and North America, while Nectria asiatica and N. dematiosa subclade C are known only in Asia. Nectria cinnabarina has been reported in tropical regions and the Southern Hemisphere (Cunningham 1922, Tunstall 1923, Booth 1977, Debons et al. 1993), however, none of these reports could be confirmed because of the lack of specimens and cultures.
Species of the NCSC occur on a wide range of woody shrubs and trees in many families including the Arecaceae and Pinaceae; it is occasionally reported on herbaceous hosts (Farr & Rossman 2010). Most specimens used in this study were collected on newly killed branches suggesting that these fungi may exist as endophytes that then sporulate when the substrate dies (Wang et al. 2000). Nectria cinnabarina sensu lato causes a disease referred to as “coral spot Nectria canker” because of the conspicuous erumpent pink sporodochia of the anamorph (Sinclair & Lyon 2005). Trees and woody plants growing in plantations and nurseries or those damaged by frost or other causes appear to be especially susceptible. The pathogenicity of this fungus has been proven by host inoculation studies (Bedker & Blanchette 1984, Yasuda & Izawa 2007). Jøgensen (1952) demonstrated that N. cinnabarina was a facultative parasite and saprobe of mainly deciduous trees but was unable to correlate his results with specific hosts. Although N. cinnabarina and N. nigrescens produce chlamydospores, they are rarely found in soil.
Chaverri and Samuels (2003) used a morphological species concept (John & Maggs 1997, Kirk et al. 2008) and genealogical concordance phylogenetic species recognition (Taylor et al. 2000) to delimit Hypocrea/Trichoderma species with green ascospores. According to their species concept, each of the three subclades (A, B, and C) in N. dematiosa would be a distinct species. Even though we found that the subclades of N. dematiosa could be distinguished by subtle anamorph characters in culture and by biogeography, we prefer not to give them names because of the small number of available specimens.
Our study clearly indicates that to define and characterise species in the N. cinnabarina species complex, an integrated approach should be used. The use of phylogenetic analyses of DNA sequences from six loci, observations and analyses of morphological characters of teleomorph and anamorph, mycelial growth, and geographical data indicates the existence of four species within the NCSC. This study will pave the way for understanding the evolutionary diversification and taxonomic implications of morphology using robust phylogenetic analyses and comprehensive character sampling.
Acknowledgments
The authors thank Dr Gary J. Samuels (USDA-ARS, Beltsville, Maryland, USA) for his contributions to the systematics of the hypocrealean fungi including discussions about the NCSC and other Nectria-like fungi. It is a distinct honour to publish our paper here. We thank the many mycologists who shared their fresh specimens and/or cultures of these fungi with us, specifically Alex Akulov, Margaret Barr, Alex Chlebicki, Walter Gams, Tom Gräfenhan, Walter Jaklitsch, Thomas Laessoe, Gaston Laflamme, Christian Lechat, Yasunori Ono, Gary Samuels, Hans-Josef Schroers, Keith Seifert, and Wenying Zhuang. We are indebted to Sato Toyozo, Takayuki Aoki, and Keisuke Tomioka (NIAS Genebank, National Institute of Agrobiological Sciences, Tukuba, Ibaraki, Japan), and Keiko T. Natuaki (Tokyo University of Agriculture) for sending and quickly depositing Japanese cultures. We also thank Orlando Petrini (Repubblica e Cantone Ticino, Bellinzona, Switzerland) for giving many ideas and advice about statistical analyses. We express sincere thanks to Andrew Minnis for providing nomenclatural advice and to our excellent technicians, Sasha Allen, Aimee Hyten and Tunesha Phillips. We also gratefully acknowledge the assistance of the curators and staff of the BPI, FH, H, TNS, K, NY, PH, SPA, and UPS. This study was supported in part by the United States National Science Foundation (NSF) PEET grant DEB-0731510 “Monographic Studies in the Nectriaceae, Hypocreales: Nectria, Cosmospora, and Neonectria” to University of Maryland (PIs: P. Chaverri, A.Y. Rossman, G.J. Samuels).
Taxonomic novelty: Nectria asiatica Hirooka, Rossman & P. Chaverri, sp. nov.
References
- Bazinet AL, Cummings MP (2008). The Lattice Project: a grid research and production environment combining multiple grid computing models. In: Distributed & grid computing - science made transparent for everyone. Weber MHW, ed. Rechenkraft.net.
- Beck R (1902). Beiträge zur Morphologie und Biologie der forstlich wichtigen Nectria-Arten, insbes. Der Nectria cinnabarina (Tode) Fr. Tharander forstliches Jahrbuch. 52: 161–206. [Google Scholar]
- Bedker PJ, Blanchette RA (1984). Identification and control of cankers caused by Nectria cinnabarina of honey locust. Journal of Arboriculture 10: 33–39. [Google Scholar]
- Booth C (1959). Studies of Pyrenomycetes: IV. Nectria (Part I). Mycological Papers 73: 1–115. [Google Scholar]
- Booth C (1977). Nectria cinnabarina. CMI Descriptions of Pathogenic Fungi and Bacteria. No. 531.
- Brefeld O (1891). Untersuchungen aus dem Gesammtgebiete der Mykologie. Heft 10: Ascomyceten II. Münster.
- Cannon PF, Hawksworth DL (1983) (701) Proposal to conserve Nectria over Ephedrosphaera and Hydropisphaera (Fungi). Taxon 32: 476–477. [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Sung GH, Hyten AS, Spatafora JW (2004). Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 864–872. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ (2003). Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with green ascospores. Studies in Mycology 48: 1–116. [Google Scholar]
- Clements RE, Shear CL (1931). The Genera of Fungi, 2nd Edition. H.W. Wilson, New York.
- Cummings MP, Huskamp JC (2005). Grid computing. Educause Review 40: 116–117. [Google Scholar]
- Cunningham GH (1922). Coral-spot, Nectria cinnabarina (Tode) Fries. A wound parasite of fruit-trees. New Zealand Journal of Agriculture 25: 354–359. [Google Scholar]
- Debons J, Chilembwe E, Braunwork Jr. WS, Mainjeni CED (1993). First report of Nectria twig blight on apple trees in Malawi, Africa. Plant Disease 77: 428. [Google Scholar]
- Farr DF, Rossman AY (2010). Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. Retrieved January 21, 2010, from http://nt.ars-grin.gov/fungaldatabases/
- Fries EM (1828). Elenchus Fungorum Vol. 1, E. Mauritius, Greifswald.
- Fries EM (1832). Systema mycologicum vol. 3, part 2. Greifswald.
- Fries EM (1849). Summa vegetabilium Scandinaviae, Sectio posterior p. 259–572.
- Fuckel L (1873). Symbolae mycologicae. Beitrage zur Kenntniss der rheinischen Pilze. Zweiter Nachtrag. Jahrbücher des Nassauischen Vereins für Naturkunde 27–28: 1–99. [Google Scholar]
- Fuckel L (1874). Fungi rhenani exsicacati a Leopoldo Fuckel collecti. No. 2657.
- Hara K (1918). Plant pathogenic fungi on mulberry tree. Journal of the Sericultural Association of Japan 27: 223–229. [Google Scholar]
- Holmgren PK, Holmgren NH (1998). Index Herbariorum. New York Botanical Garden. < http://sciweb.nybg.org/science2/IndexHerbariorum.asp>.
- Huelsenbeck JP, Ronquist F, Nielsen ES, Bollback JP, (2001). Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294: 2310–2314. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Larget B, Miller RE, Ronquist F (2002). Potential applications and pitfalls of Bayesian inference of phylogeny. Systematic Biology 51: 673–688. [DOI] [PubMed] [Google Scholar]
- Jøgensen HA (1952). Studies on Nectria cinnabarina hosts and variation. Den Konglige Veterinaerog Landbohøjskoles Årsskrift Copenhagen 35: 57–120. [Google Scholar]
- John DM, Maggs CA (1997). Species problems in eukaryotic algae: a modern perspective. In: Species: The units of biodiversity. (Claridge MF, Dawah HA, Wilson MR, eds). Chapman & Hall, London: vol 54, 83–107. [Google Scholar]
- Katoh T (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286–298. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Dictionary of the Fungi 10th Edition, CAB International: Wallingford, UK.
- Kornerup A, Wanscher JH (1978). Methuen Handbook of Colour. 3rd edn. Methuen, London.
- Linnaeus C (1753). Species Plantarum. Laurentii Salvii, Holmiae.
- Maddison WP, Maddison DR (2009). Mesquite: a modular system for evolutionary analysis. Version 2.6 http://mesquiteproject.org.
- Mason-Gamer RJ, Kellogg EA (1996). Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545. [Google Scholar]
- Mayr H (1883). Ueber den Parasitismus von Nectria cinnabarina Fr. Untersuchungen aus dem forstbotanischen Institute zu München. 3: 1–16. [Google Scholar]
- McNeill JF, Barrie F, Burdet HM, Demoulin V, Hawksworth DL, Marhold K, Nicolson DH, Prado J, Silva PC, Skog JE, Wiersema J, Turland NJ (eds) (2006). International Code of Botanical Nomenclature (Vienna Code). Regnum Vegetabile Vol. 146. A.R.G. Ganter Verlag, Ruggell.
- Myers DS, Bazinet AL, Cummings MP (2008). Expanding the reach of Grid computing: combining Globus- and BOINC-based systems. In: Grids for bioinformatics and computational biology. (Talbi E-G, Zomaya A, eds) John Wiley & Sons, New York: 71–85.
- Nirenberg HI (1976). Untersuchungen über die Morphologische und biologische differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land und Forstwirtschaft, Berlin-Dahlem 169: 1–117. [Google Scholar]
- O'Donnell K, Cigelnik E (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Paoletti G (1887). Revisione del genere Tubercularia. Atti della Societa veneto-trentina di scienze naturali 11: 52–66. [Google Scholar]
- Petch T (1940). Tubercularia. Transactions of the British Mycological Society 24: 33–58. [Google Scholar]
- Pfister DH (1985). A bibliographic account of exsiccatae containing fungi. Mycotaxon 23: 1–139. [Google Scholar]
- Posada D (2008). jModelTest:Phylogenetic Model Averaging Averaging. http://darwin.uvigo.es/ [DOI] [PubMed]
- Posada D, Buckley TR (2004). Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- Reeb V, Lutzoni F, Roux C (2004). Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution 32: 1036–1060. [DOI] [PubMed] [Google Scholar]
- Rehner SA (2001). Primers for elongation factor 1-a (EF1-a). www.nacse.org/yfaaberg/aftol/EF1primer.pdf.
- Rossman AY (1983). The phragmosporous species of Nectria and related genera. Mycological Papers 150: 1–164. [Google Scholar]
- Rossman AY (1989). A synopsis of the Nectria cinnabarina group. Memoirs of the New York Botanical Garden 49: 253–255. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Saccardo PA (1886). Sylloge fungorum omnium hucusque cognitorum vol. IV. Padova.
- Schroers HJ (2001). A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Studies in Mycology 46: 1–214. [Google Scholar]
- Seifert KA (1985). A monograph of Stilbella and some allied Hyphomycetes. Studies in Mycology 27: 1–235. [Google Scholar]
- Sinclair WA, Lyon H (2005). Diseases of Trees and Shrubs, Second Edition, Cornell University Press, Ithaca.
- Spencer JA, Irvine LM, Jarvis CE (2009). Typification of Linnaean names relevant to algal nomenclature. Taxon 58: 237–260. [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000). Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Tode HJ (1790). Fungi mecklenburgenses selecti. Vol. 1: 1–47. Lüneburg. [Google Scholar]
- Tode HJ (1791). Fungi mecklenburgenses selecti. Vol. 2: 1–64. Lüneburg. [Google Scholar]
- Tunstall AC (1923). Some observations on stem end root diseases. Quartity Journal of Indian Tea Association pp. 86–91.
- Vilgalys R. n.d. Conserved primer sequences for PCR amplification and sequencing from nuclear ribosomal RNA. http://www.biology.duke.edu/fungi/mycolab/primers.htm Vilgalys Lab, Durham, NC.
- Wang J, Li G, Lu, H, Zheng Z, Huang Y, Su W (2000). Taxol from Tubercularia sp. Strain TF5, an endophytic fungus of Taxus mairei. Federation of European Microbiological Societies Microbiology Letters 193: 249–253. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ. eds). San Diego, California, USA: Academic Press: 315–322.
- Wollenweber HW (1926). Pyrenomyceten-studien. II. Angewandte Botanik Bd. VII: 169–212. [Google Scholar]
- Wollenweber HW (1930). Fusaria Autographice Delineata. Collectio Specierum ex Herbariis Variis Selectarum et ab Auctore Lectarum Cultarumque Synonymis et Excludendis Additis quas Determinavit, in Sectiones Digessit, Comparavit cum Hypocreaceis Analogis Praemissis ad Methodi Naturalis Normas et Culturae Purae Experientiam H.W. Wollenweber 3: i, 660–1100. Germany, Berlin. [Google Scholar]
- Yasuda F, Izawa H (2007). The occurrence of coral spot of Japanese persimmon caused by Nectria cinnabarina (Tode:Fries) Fries. Journal of General Plant Pathology 73: 405–407. [Google Scholar]
- Zwickl DJ (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion (Ph.D. dissertation). The University of Texas at Austin, Austin, TX.