Abstract
Neonectria is a cosmopolitan genus and it is, in part, defined by its link to the anamorph genus Cylindrocarpon. Neonectria has been divided into informal groups on the basis of combined morphology of anamorph and teleomorph. Previously, Cylindrocarpon was divided into four groups defined by presence or absence of microconidia and chlamydospores. Molecular phylogenetic analyses have indicated that Neonectria sensu stricto and Cylindrocarpon sensu stricto are phylogenetically congeneric. In addition, morphological and molecular data accumulated over several years have indicated that Neonectria sensu lato and Cylindrocarpon sensu lato do not form a monophyletic group and that the respective informal groups may represent distinct genera. In the present work, a multilocus analysis (act, ITS, LSU, rpb1, tef1, tub) was applied to representatives of the informal groups to determine their level of phylogenetic support as a first step towards taxonomic revision of Neonectria sensu lato. Results show five distinct highly supported clades that correspond to some extent with the informal Neonectria and Cylindrocarpon groups that are here recognised as genera: (1) N. coccinea-group and Cylindrocarpon groups 1 & 4 (Neonectria/Cylindrocarpon sensu stricto); (2) N. rugulosa-group (Rugonectria gen. nov.); (3) N. mammoidea/N. veuillotiana-groups and Cylindrocarpon group 2 (Thelonectria gen. nov.); (4) N. radicicola-group and Cylindrocarpon group 3 (Ilyonectria gen. nov.); and (5) anamorph genus Campylocarpon. Characteristics of the anamorphs and teleomorphs correlate with the five genera, three of which are newly described. New combinations are made for species where their classification is confirmed by phylogenetic data.
Keywords: Canker-causing fungi, molecular systematics, Nectria-like fungi, phylogeny, polyphasic taxonomy, root-rotting fungi, sequence analysis, systematics, taxonomy
INTRODUCTION
Species of Neonectria sensu lato and their anamorphs in Cylindrocarpon are common in tropical and temperate regions. They are generally found on bark of recently killed woody plants and sometimes on decaying herbaceous material (Samuels 1988, Samuels & Brayford 1990, Samuels et al. 1990, Samuels & Brayford 1993, 1994, Rossman et al. 1999, Castlebury et al. 2006). Some species of this genus are plant pathogens causing cankers, root rots, and other diseases on hardwood and coniferous trees, e.g. Abies and Acer cankers caused by Neonectria castaneicola; beech (Fagus) bark disease caused by N. coccinea, N. ditissima and N. faginata; black foot disease of grapevines (Vitis) caused by N. liriodendri; root rots caused by N. radicicola; and cankers caused by N. rugulosa, among others (Samuels & Brayford 1994, Hirooka et al. 2005, Kobayashi et al. 2005, Castlebury et al. 2006, Halleen et al. 2006). According to Index Fungorum (www.indexfungorum.org), 38 species have been placed in Neonectria and 143 in Cylindrocarpon. These numbers are underestimated because several species of Nectria-like fungi with Cylindrocarpon anamorphs have not been transferred to Neonectria (> 20 spp.). To date, the most comprehensive taxonomic works of Neonectria and species of Nectria having Cylindrocarpon anamorphs are those by Booth (1959, 1966) and Samuels, Brayford and collaborators (Samuels 1988, Brayford & Samuels 1993, Samuels & Brayford 1993, 1994, Brayford et al. 2004).
Species of Neonectria sensu lato are characterised by having perithecia that are subglobose to broadly obpyriform, smooth to roughened, red, becoming dark red in 3 % potassium hydroxide (KOH), and with an acute to constricted apex that is sometimes knobby; the perithecial wall is ca. 50 μm thick and generally composed of two regions, sometimes with an outer region that forms textura epidermoidea, that may or may not be covered with another region of cells; and the ascospores are hyaline, generally bicellular, rarely multi-cellular, and smooth or finely ornamented (Rossman et al. 1999). The anamorph of N. ramulariae (type of Neonectria) is Cylindrocarpon obtusiusculum and, consequently, species with the Neonectria-like morphology described above and Cylindrocarpon anamorphs have been classified as Neonectria. However, species that have been placed in Neonectria and species of Nectria having a Cylindrocarpon anamorph vary greatly in the morphology of their perithecia, some having perithecial walls < 50 μm or > 50 μm thick, others warted, and others with various degrees of ascospore ornamentation. Some species of Neonectria are morphologically similar in perithecial morphology with differences seen only in the anamorph (Samuels et al. 2006b).
The morphological variation in Neonectria resulted in the subdivision of species into five informal groups, mostly based on perithecial characteristics: (1) N. coccinea/galligena-group (Neonectria sensu stricto) (Booth 1959); (2) N. mammoidea-group (N. mammoidea = N. discophora (Booth 1959); (3) N. rugulosa-group (Samuels & Brayford 1994); (4) N. radicicola-group (Booth 1959); and (5) N. veuillotiana-group (Brayford & Samuels 1993). Species in the N. coccinea/galligena-group are characterised by having few to numerous perithecia clustered on wood; perithecial walls are ca. 50 μm thick, composed of relatively thick-walled, small cells; and ascospores are generally smooth (Booth 1959). Species in the N. mammoidea-group were originally defined as having a distinctive perithecial wall that comprises a layer of hyphae that have thickened walls and are typically arranged radially, giving the appearance of a palisade (Booth 1959, Brayford et al. 2004). This characteristic generally results in smooth, shiny perithecia. In addition to the perithecial anatomy, the N. mammoidea-group has spinulose, often yellow-brown ascospores, and a non-microconidial anamorph. The N. rugulosa-group includes species with warted perithecia, a perithecial wall > 50 μm thick, composed of large, thick-walled cells, and striate ascospores (Samuels & Brayford 1994). The N. radicicola-group includes species that have smooth to slightly warted, usually solitary perithecia, the outer region of the perithecial wall composed of large, thin-walled cells, and smooth ascospores (Samuels & Brayford 1990). Species in the N. veuillotiana-group have perithecia with a flattened or knobby apex, perithecial walls composed of thick-walled cells, and tuberculate ascospores (Brayford & Samuels 1993). Mantiri et al. (2001) revised the informal groupings of Neonectria based on phylogenetic analyses of DNA sequence data. Group or clade I was Neonectria sensu stricto or the N. coccinea/galligena-group; clade II included the N. mammoidea-, N. rugulosa-, and N. veuillotiana-groups; and clade III was the N. radicicola-group.
Booth (1966) subdivided Cylindrocarpon into four groups based on the presence or absence of microconidia and chlamydospores. The first three Cylindrocarpon groups in Booth (1966) correlate with the three groups/clades in Mantiri et al. 2001 (Castlebury et al. 2006). Anamorphs in the N. coccinea/galligena-group (clade I in Mantiri et al. 2001) belong to Cylindrocarpon group 1, which have micro- and macroconidia but lack chlamydospores, except N. ramulariae/C. obtusiusculum, which has chlamydospores and lacks microconidia. Cylindrocarpon obtusiusculum was originally placed in Cylindrocarpon group 4 by Booth (1966). The type species of Cylindrocarpon, C. cylindroides, belongs in Cylindrocarpon group 1 (Booth 1966, Mantiri et al. 2001, Brayford et al. 2004, Halleen et al. 2004, Castlebury et al. 2006). Anamorphs in the N. mammoidea/veuillotiana-group (clade II in Mantiri et al. 2001) belong to Cylindrocarpon group 2 and are characterised by the lack of microconidia and chlamydospores. Anamorphs in Cylindrocarpon group 3 belong to the N. radicicola-group (clade III in Mantiri et al. 2001) and are characterised by the presence of microconidia and chlamydospores.
The anamorphic genus Campylocarpon was described by Halleen et al. (2004) for species resembling Cylindrocarpon with 3–5-septate, curved macroconidia and lacking microconidia. Halleen et al. (2004) segregated Campylocarpon from Cylindrocarpon based on molecular phylogenetic data that placed it more closely to N. mammoidea-group than to Cylindrocarpon sensu stricto (N. coccinea-group). Halleen et al. (2004) noted the similarity of Campylocarpon to the Cylindrocarpon anamorphs of species in the N. mammoidea-group.
Even though morphological and phylogenetic studies suggest that Neonectria/Cylindrocarpon represents more than one genus (Samuels & Brayford 1994, Mantiri et al. 2001, Brayford et al. 2004, Halleen et al. 2004, Hirooka et al. 2005, Castlebury et al. 2006, Halleen et al. 2006), formal taxonomic segregation of these groups has not been proposed. The objectives of the present study are to: (1) define Neonectria sensu stricto; (2) determine if Neonectria/Cylindrocarpon should be divided into multiple genera using phylogenetic analyses of multiple loci; and (3) recognise these species in monophyletic genera as a first step toward their taxonomic revision.
MATERIALS AND METHODS
Morphological characterisation
Specimens were obtained from U.S. National Fungus Collections (BPI), Steere Herbarium, New York Botanical Garden (NY), and Manaaki Whenua Landcare Research, New Zealand (PDD), and collected in fresh conidition from the field. Some cultures were obtained from the Centraalbureau voor Schimmelcultures (CBS), Utrecht, Netherlands. For morphological characterisation of the teleomorph, the macromorphology of the perithecia was observed and described using the following characters: distribution of perithecia on the host; perithecial shape, colour, and reaction to 3 % w/v potassium hydroxide (KOH) and 100 % lactic acid; perithecial wall structure; and colour and appearance of the perithecial apex. Colour standards are from Kornerup & Wanscher (1978). To observe internal and microscopic characteristics, the perithecia were rehydrated briefly in KOH, then supported by Tissue-Tek O.C.T. Compound 4583 (Miles Inc., Elkhart, Indiana, USA), and sectioned at a thickness of ca. 15 μm with a freezing microtome. Characteristics of asci and ascospores were observed by rehydrating the perithecia in 3 % KOH, removing part of the centrum with a fine glass needle, and placing it on a glass slide. Microscopic observations were made using an Olympus BX51 microscope and DP71 digital camera. Cultures were obtained by isolating asci containing ascospores on cornmeal-dextrose agar (CMD; Difco™ cornmeal agar + 2 % w/v dextrose supplemented with antibiotics 0.2 % each neomycin and streptomycin). Morphological observations of the colonies and anamorph in culture were based on isolates grown on Difco™ potato-dextrose agar (PDA) and SNA (low nutrient agar, Nirenberg 1976) for 3 wk in an incubator at 25 °C with alternating 12 h/12 h fluorescent light/darkness. Measurements of continuous characters such as length and width for both anamorph and teleomorph were made using the beta 4.0.2 version of Scion Image software (Scion Corporation, Frederick, Maryland, USA). Continuous measurements are based on 10–30 measured units and are reported as the extremes (maximum and minimum) in brackets separated by the mean plus and minus one standard deviation.
DNA extraction, polymerase chain reaction (PCR), and sequencing
Strains listed in Table 1 were grown in Petri dishes (6 cm diam) containing Difco™ potato-dextrose broth. Plates were incubated at 25 °C for ca. 1 wk. DNA was extracted from the mycelial mat harvested from the surface of the broth. The PowerPlant™ DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, California, USA) was used to extract DNA from the samples. Other sequences used in the analyses were obtained from GenBank (Table 1).
Table 1.
Isolates used in the phylogenetic analyses with their corresponding GenBank accession numbers.
| Species (sexual/asexual state)** | Isolate |
Isolate |
|||||
|---|---|---|---|---|---|---|---|
| ITS | LSU | tef1 | tub | act | rpb1 | ||
| Campylocarpon fasciculare | CBS 112613 | HM364313 | HM352881 | HM364331 | |||
| Campylocarpon pseudofasciculare | CBS 112679 | HM364314 | HM352882 | HM364332 | |||
| Cosmospora coccinea / `Verticillium' olivaceaum | A.R. 2741 (= CBS 114050) | HM484537 | AY489734* | HM484515 | HM484589 | GQ505967* | AY489667* |
| Cosmospora vilior / Acremonium berkeleyanum | A.R. 4215 (= CBS 126111) | HM484854 | HM484869 | HM484846 | HM484875 | HM484838 | HM484872 |
| Cosmospora viliuscula | G.J.S. 96-6 (= CBS 455.96) | HM484855 | GQ506003* | HM484851 | HM484876 | GQ505966* | GQ506032* |
| Cosmospora sp. | G.J.S. 93-15 | HM484856 | GQ506006* | HM484849 | HM484878 | GQ505968* | GQ506035* |
| Cyanonectria cyanostoma / Fusarium sp. | G.J.S. 98-127 (= CBS 101734) | FJ474076* | FJ474081* | HM484611 | GQ505961* | GQ506017* | |
| Cylindrocarpon destructans var. crassum (I) | CBS 537.92 | EF607079* | |||||
| Cyl. destructans var. crassum (I) | CBS 605.92 | EF607078* | EF607065* | ||||
| Cyl. olidum (T) | CBS 215.67 | HM364317 | HM352884 | HM364334 | |||
| Emericellopsis glabra | A.R. 3614 (= CBS 125295) | HM484860 | GQ505993* | HM484843 | HM484879 | GQ505969* | GQ506023* |
| Gibberella fujikuroi / Fusarium moniliforme | FM 94 | FJ755697* | |||||
| Gibberella fujikuroi / Fusarium moniliforme | PMBMDF092 | FJ798606* | |||||
| Haematonectria haematococca / Fusarium solani | NRRL 22277 | AF178401* | AF178370* | ||||
| Haematonectria illudens / Fusarium illudens | NRRL 22090 | AF178393* | AF178362* | ||||
| Haematonectria sp. | G.J.S. 93-47 (= CBS 125113) | HM484862 | HM484870 | HM484850 | HM484880 | HM484839 | HM484873 |
| Hydropisphaera fungicola | A.R. 4170 (= CBS 122304) | HM484863 | GQ505995* | HM484845 | HM484877 | GQ505970* | GQ506025* |
| Lasionectria mantuana | A.R. 4029 (= CBS 114291) | HM484858 | HM484844 | ||||
| Leuconectria clusiae / Gliocephalotrichum bulbilium | ATCC 22228 | AY489732* | AY489664* | ||||
| Mycoarachis inversa | A.R. 2745 (= ATCC 22107) | HM484861 | GQ505991* | HM484840 | HM484882 | GQ505972* | GQ506021* |
| Nectria antarctica | A.R. 2767 (= CBS 115033) | HM484556 | HM484560 | HM484516 | HM484601 | HM484501 | HM484575 |
| Nectria aquifolii | A.R. 4108 (= CBS 125147) | HM484538 | HM484565 | HM484522 | HM484590 | ||
| Nectria aurigera | A.R. 3717 (= CBS 109874) | HM484551 | HM484573 | HM484521 | HM484600 | HM484511 | HM484586 |
| Nectria austroamericana / Gyrostroma austroamericanum | A.R. 2808 (= CBS 126114) | HM484555 | GQ505988 | HM484520 | HM484597 | ||
| Nectria balansae | G.J.S. 86-117 (= CBS 125119) | HM484857 | HM484868 | HM484848 | HM484874 | HM484871 | |
| Nectria balsamea | A.R. 4478 (= CBS 125166) | HM484540 | HM484567 | HM484528 | HM484591 | HM484508 | HM484580 |
| Nectria berolinensis / “Tubercularia” berolinensis | A.R. 2776 (= CBS 126112) | HM484543 | HM484568 | HM484517 | HM484594 | HM484510 | HM484583 |
| Nectria cinnabarina (dematiosa) / Tubercularia vulgaris | CBS 278.48 | HM484682 | HM484729 | HM484647 | HM484800 | HM484615 | HM484760 |
| Nectria coryli | Y.H. 0815 (= A.R. 4561) | HM484539 | HM484566 | HM484536 | HM484596 | HM484509 | |
| Nectria cucurbitula / Zythiostroma pinastri | CBS 259.58 | HM484541 | GQ505998 | HM484530 | HM484592 | GQ505974 | GQ506028 |
| Nectria lamyi | A.R. 2779 (= CBS 115034) | HM484544 | HM484569 | HM484518 | HM484593 | HM484507 | HM484582 |
| Nectria miltina | A.R. 4391 (= CBS 121121) | HM484547 | HM484609 | HM484514 | HM484587 | ||
| Nectria pseudotrichia / Tubercularia lateritia | CBS 551.84 | HM484554 | HM484532 | HM484602 | GQ505976 | GQ506030 | |
| Nectria pyrrhochlora | A.R. 2786 (= CBS 125131) | HM484545 | HM484570 | HM484519 | HM484598 | ||
| Nectria sinopica / Zythiostroma mougeotii | CBS 462.83 | HM484542 | GQ506001 | HM484531 | HM484595 | GQ505973 | GQ506031 |
| Nectria zanthoxyli | A.R. 4280 (= CBS 126113) | HM484546 | HM484571 | HM484523 | HM484599 | HM484513 | HM484585 |
| Nectriopsis exigua / Verticillium rexianum | G.J.S. 98-32 (= CBS 126110) | HM484865 | GQ505986* | HM484852 | HM484883 | GQ505979* | GQ506014* |
| Neo. castaneicola / Cyl. castaneicola (R) | TPPH 1 | AB233175* | |||||
| Neo. coprosmae / Cyl. coprosmae (I) | G.J.S. 85-39 (= CBS 119606) | HM364301 | |||||
| Neo. coronata / Cyl. coronatum (T) | A.R. 4505 (= CBS 125173) | HM364348 | HM352862 | HM352878 | HM364328 | ||
| Neo. discophora / Cyl. ianothele (T) | A.R. 4324 (= CBS 125153) | HM364294 | HM364307 | HM364345 | HM352860 | HM352875 | HM364326 |
| A.R. 4499 (= CBS 125172) | HM364296 | HM364309 | HM364347 | HM352877 | HM364327 | ||
| Neo. ditissima / Cyl. heteronemum | CBS 100316 | HM364298 | HM364311 | HM364350 | HM352864 | HM352880 | HM364330 |
| Neo. fuckeliana / Cyl. cylindroides var. tenue | A.R. 3103 (= CBS 125133) | HM364291 | HM446654 | HM364342 | HM352857 | HM352872 | |
| A.R. 4109 (= CBS 119723) | HM364292 | HM364305 | HM364343 | HM352858 | HM352873 | ||
| A.R. 4110 (= CBS 119200) | HM364293 | HM364306 | HM364344 | HM352859 | HM352874 | ||
| A.R. 4480 (= 126652) | HM364295 | HM364308 | HM364346 | HM352861 | HM352876 | ||
| G.J.S. 02-67 (= CBS 125109) | HM364300 | HM364320 | HM364354 | HM352867 | HM352886 | ||
| Neo. jungneri / Cyl. victoriae (T) | C.T.R. 71-244 | HM364299 | HM364319 | HM364353 | HM352866 | HM352885 | HM364336 |
| Neo. liriodendri / Cyl. liriodendri (I) | CBS 112602 | HM364302 | HM364323 | HM352853 | |||
| Neo. macrodidyma / Cyl. macrodydimum (I) | CBS 112615 | HM364315 | HM352883 | HM364333 | |||
| Neo. neobalansae / Cyl. sp. (R) | G.J.S. 85-219 (= CBS 125120) | HM364322 | HM352869 | ||||
| Neo. neomacrospora / Cyl. cylindroides var. cylindroides | CBS 198.62 | HM364316 | HM364351 | HM352865 | |||
| CBS 324.61 | HM364318 | HM364352 | HM352854 | HM364335 | |||
| Neo. radicicola / Cyl. destructans (I) | A.R. 2553 (= ATCC 208837) | HM364290 | HM364304 | HM364341 | HM352856 | HM352871 | HM364325 |
| Neo. ramulariae / Cyl. obtusiusculum | ATCC 16237 | HM364297 | HM364310 | HM364349 | HM352863 | HM352879 | HM364329 |
| CBS 151.29 | HM364303 | HM364324 | HM364340 | HM352855 | |||
| Neo. rugulosa / Cyl. rugulosum (R) | TPPH 32 | AB233176* | AB237526* | ||||
| Neo. trachosa / Cyl. sp. (T) | CBS 112467 | HM364312 | HM364356 | HM364339 | |||
| Neo. veuillotiana / Cyl. candidulum (T) | G.J.S. 90-48 (= CBS 125118) | HM364357 | HM352870 | HM352888 | HM364338 | ||
| Neo. westlandica / Cyl. sp. (T) | G.J.S. 83-156 (= CBS 112464) | HM364321 | HM364355 | HM352868 | HM352887 | HM364337 | |
| Neocosmospora vasinfecta / Acremonium-like | A.R. 3587 | HM484864 | HM484842 | HM484881 | |||
| Ophionectria trichospora / Antipodium spectabile | G.J.S. 01-206 | HM484867 | HM484847 | HM484886 | |||
| CBS 109876 | AF543790* | AY489669* | |||||
| Pseudonectria rousseliana / Volutella buxi | ATCC-MYA 627 | U17416* | AY489670* | ||||
| Rubrinectria olivacea / Nalanthamala sp. | CBS 102268 | AY554219* | AY554244* | AY554238* | |||
| Selinia pulchra / | A.R. 2812 | HM484859 | GQ505992* | HM484841 | HM484884 | GQ505982* | GQ506022* |
| Verrucostoma freycinetiae / Acremonium-like | MAFF240100/h523 | HM484866 | GQ506013* | HM484853 | HM484885 | GQ505984* | GQ506018* |
| Viridispora diparietispora / Penicillifer furcatus | CBS 114049 | AY489735* | AY489668* | ||||
Sequences obtained from GenBank.
Letters in parenthesis represent their classification in the newly segregated genera. I: Ilyonectria; R: Rugonectria; T: Thelonectria.
DNA sequences of partial large subunit (LSU, ca. 900 bp) and complete internal transcribed spacers 1 and 2 (ITS, ca. 600 bp), including 5.8S of the nuclear ribosomal DNA; partial β-tubulin (tub, ca. 500 bp); α-actin (act, ca. 600 bp); RNA polymerase II subunit 1 (rpb1, ca. 700 bp); and translation elongation factor 1α (tef1, ca. 700 bp) were used in the phylogenetic analyses (Table 2). The primers used and PCR protocols are listed in Table 2. Each 25 μL PCR reaction consisted of 12.5 μL Promega GreenTaq™ Master Mix 2× (Promega Corporation, Madison, Wisconsin, USA), 1.25 μL 10 mM forward primer, 1.25 μL 10 mM reverse primer, 1 μL of the DNA template, 1 μL of dimethyl sulfoxide (DMSO), and 8 μL of sterile RNAase-free water. PCR reactions were run in an Eppendorf Mastercycler ep using the parameters detailed in Table 2. PCR products were cleaned with ExoSAP-IT® (USB Corporation, Cleveland, Ohio, USA). Clean PCR products were sequenced at the DNA Sequencing Facility (Center for Agricultural Biotechnology, University of Maryland, College Park, Maryland, USA). Sequences were assembled and edited with Sequencher v. 4.9 (Gene Codes, Madison, Wisconsin, USA). Sequences were deposited in GenBank as listed in Table 1.
Table 2.
Genes/loci used in the phylogenetic analyses. Information on the primers, included bases pairs, PCR protocols, and models of nucleotide substitution are indicated.
| Locus | Primers used (reference) | PCR protocol: Annealing temp. & cycles | Nucleotide substitution models | Included sites (# of excluded sites) | Phylogenetically informative sites (%) | Uninformative polymorphic sites | Invariable sites |
|---|---|---|---|---|---|---|---|
| ITS | ITS5, ITS4 (White et al. 1990) | 53 °C, 1 min, 35′ | GTR+G | 670 (136) | 230 (34 %) | 95 | 345 |
| LSU | LR5, LROR (Vilgalys n.d.) | 53 °C, 1 min, 35′ | TIM+I+G | 915 (0) | 142 (16 %) | 44 | 729 |
| Tef1 | tef1-728, tef1-986 (Carbone & Kohn 1999) | 66 °C, 55 s, 9′ | GTR+I+G | 707 (524) | 200 (20 %) | 39 | 468 |
| 56 °C, 55 s, 35′ | |||||||
| Tub | Btub-T1, Btub-T2 (O'Donnell & Cigelnik 1997) | 55 °C, 30 s, 35′ | HKY+I+G | 535 (127) | 260 (26 %) | 49 | 226 |
| Act | Tact1, Tact2 (Samuels et al. 2006) | 65 °C, 30 s, 15′ | GTR+I+G | 635 (0) | 149 (15 %) | 37 | 4498 |
| 48 °C, 30 s, 30′ | |||||||
|
Rpb1 |
crpb1a, rpb1c (Castlebury et al. 2004)
|
50 °C, 2 min, 40′
|
GTR+I+G
|
722 (52)
|
378 (52 %)
|
61
|
283
|
| Total | 4184 | 1359 (33 %) | 325 | 2500 |
Phylogenetic analyses
Sixty-nine strains and their corresponding DNA sequences were analysed. Not all strains had all six loci sequenced and some sequences were obtained from GenBank; see Table 1. Seven species in the Bionectriaceae were selected as the outgroup: Emericellopsis glabra, Hydropisphaera fungicola, Lasionectria mantuana, Mycoarachis inversa, Nectriopsis exigua, Selinia pulchra, and Verrucostoma freycinetiae. The included sequences were aligned with MAFFT v. 5 (Katoh et al. 2005) using the E-INS-i strategy. The alignment was improved by hand with Seaview v. 2.4 (Galtier et al. 1996) and MESQUITE v. 2.5 (Maddison & Maddison 2009). Gaps (insertions/deletions) were treated as missing data. Maximum Likelihood (ML) and Bayesian (BI) analyses were performed with all sequences, first with each gene/locus separately, and then with the combined data sets. A reciprocal 70 % BP threshold (Mason-Gamer & Kellogg 1996, Reeb et al. 2004) was used to determine if partitions could be combined into a single phylogeny.
JMODELTEST (Rannala & Yang 1996, Posada & Buckley 2004, Posada 2008) was used to select the models of nucleotide substitution for the ML and BI analyses. The number of substitution schemes was set to 11, base frequencies +F, rate variation +I and +G, and the base tree for likelihood calculations was set to “ML optimised.” Once the likelihood scores were calculated, the models were selected according to the Akaike Information Criterion (AIC). After jMODELTEST was run, the parameters indicated in Table 2 were used for the ML and BI analyses.
GARLI v. 0.96 (Zwickl 2006) was used for the ML and bootstrap analyses through the Grid computing (Cummings & Huskamp 2005) and The Lattice Project (Bazinet & Cummings 2008), which includes clusters and desktops in one encompassing system (Myers et al. 2008). In GARLI, the starting tree was obtained by stepwise-addition and the number of runs or search replicates was set to 50. Bootstrap (BP) analyses were replicated 2000 times. BI analysis was done with MrBayes v. 3.1.2 (Rannala & Yang 1996, Mau et al. 1999, Huelsenbeck et al. 2001, Huelsenbeck et al. 2002). In MrBayes, data were partitioned by locus and the parameters of the nucleotide substitution models for each partition were set as described in Table 2. Two independent analyses of two parallel runs and four chains were carried out for 10 000 000 generations using MrBayes. Analyses were initiated from a random tree and trees were sampled every 100th generation. Convergence of the log likelihoods was analysed with TRACER v. 1.4.1 (beast.bio.ed.ac.uk/Tracer). The first 20 % of the resulting trees was eliminated (= “burn in”). A consensus tree (“sumt” option) and posterior probabilities (PP) were calculated in MrBayes. Phycas v. 1.1.2 (www.phycas.org) was used as another tree searching method and also to resolve possible polytomies (“Star Tree Paradox” problem), if any, as proposed by Lewis et al. (2005). Phycas uses reversible-jump MCMC to allow unresolved trees, i.e. with polytomies or very short and poorly supported branches, and fully resolved tree topologies to be sampled during a Bayesian analysis. Unresolved trees generally occur when the time between speciation events is so short or the substitution rate so low that no substitutions occurred along a particular internal edge in the true tree. The number of cycles in Phycas was set to 100 000, sampling every 100 cycles, and with a starting tree obtained randomly.
RESULTS
Molecular phylogenetic analyses
Multiple sequence alignment resulted in 4184 included base pairs, 1 359 (33 %) phylogenetically informative and 2 500 invariable sites; 325 sites presented unique non-informative polymorphic sites (Table 2). Ambiguously aligned regions were excluded from the analyses, especially in ITS, tef1, and tub loci, which possess highly variable regions, i.e. introns (Table 2). Phylogenetic analyses of six loci show high bootstrap (BP) and MrBayes posterior probabilities (PP) for most nodes in the combined cladogram, except for a few of the deeper nodes (Fig. 1). BI PPs were either 100 % (high support) or 50 % (low support). The negative log likelihoods (–Ln) for the ML, BI, and Phycas trees were 44603.27, 44959.23, and 44957.36, respectively. The reversible-jump MCMC run in Phycas resulted in a few improved posterior probabilities for some polytomies or poorly supported nodes in the ML or BI trees (Fig. 1). The reciprocal 70 % BP threshold used to determine topological conflicts between partitions resulted in complete congruence, that is, the topologies of each gene genealogy did not contradict each other (results not shown). This can be evidenced in the high BP and PP support found in most nodes (Fig. 1).
Fig. 1.
Multilocus phylogenetic tree (Bayesian Inference) with the best log likelihood (-44959.23). Support values indicated at nodes. Bayesian posterior probabilities ≥ 90 %, Maximum Likelihood bootstrap ≥ 70 % and Phycas posterior probabilities ≥ 90 % indicated by ***. If less than those values, then indicated by -. Cylindrocarpon-like anamorphs are in two paraphyletic clades: A and B.
Species with Cylindrocarpon-like anamorphs are contained in two paraphyletic clades (Fig. 1): Clade A with the N. rugulosa-group, N. mammoidea/veuillotiana-groups, and Campylocarpon (72 % BP, 100 % PP) and Clade B with the N. coccinea- and N. radicicola-groups (97 % BP, 100 % PP). These clades correspond generally to those reported by Mantiri et al. (2001). Figure 1 also shows that some of the groups defined by Booth (1959) and Samuels & Brayford (1994), i.e. N. mammoidea-, N. rugulosa-, N. coccinea-, and N. radicicola-groups, are supported by high or moderately high BP and PP values. Campylocarpon, an anamorph genus with morphology similar to Cylindrocarpon especially to those anamorphs in the N. mammoidea-group (Halleen et al. 2004), clusters with the N. mammoidea/veuillotiana-group supported by BI PP (100 %).
The type species of Neonectria, N. ramulariae, and Cylindrocarpon, C. cylindroides, fall in the N. coccinea-group, i.e. Neonectria/Cylindrocarpon sensu stricto (94 % BP, 100 % PP) (Fig. 1), part of Clade B. This group also includes N. ditissima and N. fuckeliana. Morphological characteristics of the Neonectria/Cylindrocarpon sensu stricto clade include perithecia aggregated in an erumpent stroma, perithecial walls generally composed of two regions, somewhat ornamented ascospores, and macroconidia that are generally > 3-septate, cylindrical, and straight (Table 3). This clade is sister to the N. radicicola-group (Clade B). The monophyly of the N. radicicola-group is supported by 98 % BP and 100 % PP (Fig. 1). Characteristics of the teleomorph and anamorph clearly separate the N. radicicola-group from Neonectria/Cylindrocarpon sensu stricto (Table 3). Perithecia in the N. radicicola-group are superficial on the substrate and have a distinctive perithecial wall structure, smooth ascospores, and macroconidia that are straight, < 3-septate, with a prominent basal hilum.
Table 3.
Comparison of major diagnostic morphological characteristics between the newly segregated genera.
| Character | Campylocarpon (Clade A) | Rugonectria (Clade A) | Thelonectria (Clade A) | Ilyonectria (Clade B) | Neonectria (Clade B) |
|---|---|---|---|---|---|
| Teleomorph groups (Booth 1959, Brayford & Samuels 1993, Samuels & Brayford 1994) | – | N. rugulosa-group | N. mammoidea /veuillotiana-groups | N. radicicola-group | N. coccinea-group |
| Anamorph groups (Booth 1966) | – | Group 2 | Group 3 | Groups 1 & 4 | |
| Arrangement of perithecia on substrate | Teleomorph unknown | Perithecia, formed on, or sometimes partially immersed within a stroma | Perithecia solitary or in groups, superficial, sometimes seated on an immersed inconspicuous stroma | Generally solitary and loosely attached to substrate | Perithecia clustered on wood, generally seated on an erumpent stroma |
| Perithecial apex | – | Non-papillate | Most species with a prominent, areolate (darkened) papilla, if not, then at least with a darkly pigmented apex | Broadly conical papilla | Blunt or acute apex, rarely papillate |
| Perithecial wall | – | Warted, 50–150 μm thick; outer region, including warts, of thick-walled (3–4 μm), globose, 10–20 μm diam; perithecial wall merging with surrounding stroma | Smooth or sometimes warted, 20–50 (–100) μm thick; outer region of intertwined hyphae or cells lacking a definite outline, i.e. textura epidermoidea | Generally smooth to slightly roughened, 35–50 μm thick; outer region of thin-walled, globose, large cells | Generally smooth and shiny, sometimes scurfy, 35–50 μm thick; outer region of small, angular to globose, thick-walled cells (textura epidermoidea in one species) |
| Ascospores | – | 1-septate, striate | Generally 1-septate, smooth, rarely spinulose or striate | 1-septate, smooth | 1-septate, smooth or finely ornamented |
| Macroconidia shape | Fusiform, curved, often broadest at upper third, with rounded apical cells and flattened or rounded basal cells, inconspicuous hilum | Fusiform, curved, tapering towards ends (almost Fusarium-like), inconspicuous hilum | Fusiform, curved, often broadest at upper third, with rounded apical cells and flattened or rounded basal cells, inconspicuous hilum | Cylindrical, straight, rounded ends, prominent basal hilum | Cylindrical, generally straight, sometimes slightly curved toward ends, with rounded ends (except in one species, N. fuckeliana, which has fusiform straight conidia with pointed ends); inconspicuous hilum |
| Macroconidia septation | (1–) 3–5 (–6)-septate, average 4 septa | (3–) 5–7 (–9)-septate | (3–) 5–7 (–9)-septate, average 5 | 1–3-septate, rarely > 3-septate | 3–7 (–9)-septate, average 5-septate |
| Macroconidia size | (24–) 35–60 (–62) × 6.5–9 μm | (35–) 48–85 × 5–10 μm | (35–) 40–90 (–110) × 4–8 (–11) μm | 25–50 (–55) × 5–7.5 μm | 35–65 (–110) × 4–7 (–8) μm |
| Microconidia shape | Absent | Ovoid to cylindrical, hilum inconspicuous | Microconidia rare (seen only on natural substrate) | Ellipsoidal, prominent basal hilum | Ellipsoidal to oblong, inconspicuous hilum |
| Microconidia size | Absent | (3–) 5–15 (–20) × 2–5 μm | – | 3–15 × 2.5–5 (–6) μm | (2–) 6–10 (–15) × (1–) 2–5 (–6) μm |
| Chlamydospores | Uncommon | Absent | Uncommon (except in T. olida = C. olidum) | Abundant, generally intercalary, single or in chains, becoming brownish | Present in some species |
| Substrate | Pathogenic on roots and stems of grapevines | On bark of recently killed, dying or diseased trees, often causing cankers | On bark of recently killed, dying or diseased trees, often causing small cankers, sometimes on rotting roots | Generally a root pathogen. Anamorph common in the soil. Perithecia found mostly on decaying herbaceous material, sometimes branches or roots. | Generally on bark, sometimes causing cankers |
| Geographic distribution | South Africa, Uruguay | Widespread | Widespread, but more common in tropical regions | Widespread | Mostly in temperate regions |
The N. rugulosa group is sister to the N. mammoidea/veuillotiana-group and Campylocarpon (Clade A). The N. rugulosa-group is monophyletic (100 % BP, 100 % PP). It contains species with warted perithecia and a perithecial wall structure generally different that the N. radicicola-group and Neonectria/Cylindrocarpon sensu stricto, striate ascospores, microconidia, and no chlamydospores (Table 3). The clade that includes the N. mammoidea/veuillotiana-group is also supported by high BP and PP values (70 % BP and 100 % PP). Species in this clade have a perithecial wall comprised of thick-walled cells, a knobby or prominent apex, spinulose or tuberculate ascospores, and generally no microconidia or chlamydospores. Campylocarpon sequences form a distinct clade sister to the N. mammoidea-group and is supported by 100 % BP and 100 % PP. No teleomorph is known for Campylocarpon.
Morphological characterisation
Presence or absence of a stroma
In many cases perithecia are solitary, either seated directly on the substratum in the N. radicicola-group or on a minute basal stroma in the N. mammoidea/veuillotiana-group. In other cases, such as in N. discophora and N. lucida, perithecia are seated amidst erect hyphae that arise from a basal, almost inconspicuous stroma. A characteristic of N. coccinea, N. fuckeliana and other species of Neonectria sensu stricto and N. rugulosa-group is that they form in great numbers on a rather extensive, subcortical, basal stroma. An extensive stroma may also form in N. jungneri, but is not as conspicuous as in Neonectria sensu stricto and the N. rugulosa-group.
Perithecial wall
The perithecial wall of species of Neonectria sensu lato comprises at least two regions. The inner region is very thin, consisting of only a few layers of thin-walled, tangentially flattened cells lining the locule where the spores are formed. The outer regions vary. Essentially four distinctive types of outer perithecial walls are found among the groups studied here. In the N. radiciola-group the outer region of the perithecial wall is formed of one or two layers of large, round, thin-walled cells. This anatomy can be discerned even in whole mounts of perithecia. In species that have this anatomy, the surface of the perithecial wall, when seen in face view, is of large, round cells, mirroring what is seen in sections. The second perithecial wall anatomy consists of an outer region that is a palisade of short hyphae that are perpendicular to the locule, e.g. in the N. mammoidea-group. When seen in face view cells at the surface of the perithecium are small, < 5 μm diam. In some species, such as N. trachosa and N. westlandica, a superficial layer of large, angular cells that form warts obscure the palisade. When there is a palisade but no outer layer of large cells, the perithecial surface may be smooth and shiny. This wall anatomy typifies some members of the N. mammoidea-group, e.g. N. discophora, N. lucida, N. westlandica; and N. fuckeliana in Neonectria sensu stricto. The wall of species such as N. coronata or N. jungneri, both in the N. mammoidea/veuillotiana-group, which lacks any apparent cellular structure, is formed of intertwined hyphae having a seemingly random arrangement rather than a palisadal arrangement. The third perithecial wall type is characterised by the formation of thick-walled, round cells in the outer region that can be seen in section and in face view. This wall type characterises species of Neonectria sensu stricto. The fourth type of perithecial wall is that of the N. rugulosa-group. The perithecial wall is thick, 50–150 μm, with the outer region formed of several layers of cells, including warts, with small globose cells that are very thick-walled and merge with the surrounding stroma.
Ascospores
Although some species of Neonectria have been reported to have multiseptate ascospores (Rossman 1983, Samuels & Brayford 1993), the ascospores of the species included in the present study are bicellular. There is a tendency towards having spinulose ornamentation but there are exceptions. In species such as N. veuillotiana the ascospores may be nearly tuberculate. In N. jungneri the spores are coarsely striate. In N. coronata the spinules may be arranged in lines giving the appearance of striations. Ascospores of most species are hyaline, but, in N. discophora, N. lucida, and N. westlandica, the spores become pale yellow-brown. A species not included in the present study, Nectria viridispora, probably in the N. mammoidea-group, has green ascospores. Ascospores of species in Neonectria sensu stricto and the N. radicicola-group are smooth. Species in the N. rugulosa-group have striate ascospores, sometimes inconspicuous; cotton blue may be needed to observe these striations.
Paraphyses
The Nectria-type centrum (Luttrell 1951) is characterised by the formation of “apical paraphyses,” filaments that originate in a meristem situated at the top of the locule. Typically these filaments have dissolved by the time the ascospores form but often chains of saccate cells may persist among maturing asci. Most species of Neonectria sensu stricto have filaments that appear to be free at the apex and thus resemble paraphyses. These paraphyses are septate and constricted at each septum. The paraphyses are abundant especially in N. fuckeliana.
Conidiophores and phialides
Most conidiophores, especially those that give rise to the macroconidia, are formed laterally from hyphae; they are irregularly branched or form fascicles. In the case of Neonectria sensu stricto and the N. rugulosa-group, the macroconidia are produced from irregularly branched conidiophores or fascicles, and the microconidia from simple, generally unbranched, conidiophores. In the case of the N. radicicola-group, macro- and microconidia apparently originate from the same type of conidiophore. These are simple, unbranched or sparsely branched, irregularly or verticillately branched, or rarely densely branched. The N. mammoidea/veuillotiana-group and Campylocarpon produce only macroconidia that originate from irregularly branched conidiophores or fascicles. The morphology of the phialides is highly conserved. Phialides are generally long and cylindrical or somewhat flask-shaped, but mostly long.
Macro- and microconidia
Although the average size of the macroconidia varies among the groups, there is significant overlap. Campylocarpon, the N. radicicola-group, and Neonectria sensu stricto have macroconidia 25–65 × 4–9 μm, smaller than those of the N. mammoidea/veuillotiana- and N. rugulosa-groups that are 40–90 × 4–10 μm. With respect to shape, species in Clade A (Fig. 1) have curved macroconidia and species in Clade B have straight macroconidia. Within Clade A, macroconidia of the N. rugulosa-group can be easily distinguished from those in Campylocarpon and the N. mammoidea/veuillotiana-group. Species in N. rugulosa-group have curved, fusoid macroconidia with tapering ends that are almost Fusarium-like. Campylocarpon and the N. mammoidea/veuillotiana-group also have curved macroconidia but with rounded ends. Even though the macroconidia of Campylocarpon and the N. mammoidea/veuilotiana-group are similar, they can be distinguished on the basis of septation. Campylocarpon has 3–5-septate macroconidia while the N. mammoidea/veuilotiana-group has 5–7-septate macroconidia. Regarding septation of macroconidia, most species have on average five septa, with exceptions. On average species of Campylocarpon have four septa, the N. radicicola-group have up to three septa with exceptions, and N. jungneri (N. mammoidea-group) has generally > 5 septa. Microconidial morphology is highly conserved. They are generally ellipsoidal, 0–1-septate and measure 3–15 × 2–5 μm. Only the N. radicicola-group has microconidia with a prominent hilum or abscission scar. No microconidia are formed in the N. mammoidea/veuillotina-group and Campylocarpon. Some species in Neonectria sensu stricto may produce microconidia, but not as abundantly as in the N. radicicola- and N. rugulosa-groups. The only exception is N. fuckeliana in which microconidia are abundant and macroconidia are infrequently seen.
Chlamydospores
Chlamydospores are formed in the N. radicicola-group, in a few species in Neonectria sensu stricto, and are rarely formed in Campylocarpon. Species in the N. rugulosa-group rarely produce swollen and slightly pigmented hyphae that resemble chlamydospores. Most species in the N. mammoidea/veuillotiana-group do not produce chlamydospores, except in Cylindrocarpon olidum. The chlamydospores of the N. radicicola-group are generally intercalary, single or in chains, and yellow-brown. When produced in Campylocarpon, they are mostly terminal, single, or in chains of 2–3, and also yellow-brown.
Ecology
Species of Neonectria sensu lato and Cylindrocarpon sensu lato are either saprobes or plant pathogens. The only two known Campylocarpon species cause black foot disease of grapevines. Species in the N. mammoidea/veuillotiana-group are only known as saprobes growing on bark of recently killed woody trees. The only exception is Cylindrocarpon olidum, which has been reported as a root pathogen. Members of the N. rugulosa-group and Neonectria sensu stricto also grow on bark of recently killed trees and many species, e.g. N. castaneicola, N. ditissima, N. faginata, N. rugulosa among others, can cause cankers. In contrast, species in the N. radicicola-group are generally found in the soil and cause many root diseases. Based on the present study, the species that are commonly found in the soil causing root rots are the ones that produce chlamydospores. On the other hand, the species that grow on bark do not produce chlamydospores. Members of the N. rugulosa- and N. radicicola-groups are widespread, N. mammoidea/veuillotiana-group are mostly tropical and subtropical, Neonectria sensu stricto occur in temperate regions, and Campylocarpon is known only from South Africa and Uruguay (Abreo et al. 2010).
DISCUSSION
Genus concept
Several morphological characteristics of the teleomorphs and anamorphs have been used in defining informal groups in Nectria sensu lato. In the case of Neonectria and Cylindrocarpon groups (e.g. Booth 1959, 1966), they have been distinguished by the anatomy of the outer regions of the perithecial wall and presence or absence of microconidia and chlamydospores. Results from this study confirm previous suggestions that Neonectria/Cylindrocarpon is paraphyletic, comprising five independent lineages that may be interpreted as distinct genera. These segregate genera usually cannot be distinguished based on a single morphological or ecological character. However, the lineages or segregate genera correlate strongly with a combination of ecology and morphological characters of the perithecia and anamorphs (Table 3). Thus, the following genera are recognised: (1) Neonectria/Cylindrocarpon sensu stricto (N. coccinea-group); (2) N. rugulosa-group, hereafter Rugonectria gen. nov.; (3) N. mammoidea/veuillotiana-group, hereafter Thelonectria gen. nov.; (4) N. radicicola-group, hereafter Ilyonectria gen. nov.; and (5) Campylocarpon. The Neonectria and Cylindrocarpon groups defined by Booth (1959, 1966) based on morphological characters generally agree with the clades observed in the multilocus phylogeny (Fig. 1).
Based on the morphological similarity between Campylocarpon and Thelonectria, it could be argued that these two are congeneric. However, phylogenetic analyses do not support the monophyly of these two genera (short branch length, and low BP and PP supports, Fig. 1). Therefore, Campylocarpon and Thelonectria are recognised as separate. Several morphological and ecological traits aid in distinguishing these two genera (Table 3).
Although Clades A and B (Fig. 1) could be recognised as two genera, the multiple morphological and ecological traits of each of the five segregate genera are distinctive enough to justify their taxonomic subdivision. There are other similar cases in the Ascomycota. For example, although genera with fast-growing Fusarium anamorphs form a monophyletic group, they are still recognised as separate genera and have morphologically different teleomorphs (e.g. Albonectria, Cyanonectria, Gibberella, and Haematonectria) (O'Donnell 1996, Samuels et al. 2009, Luo & Zhuang 2010a). Another example is Calonectria/Cylindrocladium and related genera. Glionectria/Gliocladiopsis, Nectricladiella/Cylindrocladiella, and Xenocalonectria/Xenocylindrocladium all have similar anamorph and teleomorph morphology and were previously classified in Calonectria. Later they were segregated from Calonectria/Cylindrocladium based mostly on anamorph characteristics even though they form a monophyletic group (Rossman 1983, 1993, Schoch et al. 2000, Crous 2002, Samuels et al. 2009, Luo & Zhuang 2010a). A third example is Botryosphaeria sensu lato. Many recognised monophyletic anamorphic genera, e.g. Fusicoccum, Lasiodiplodia, and Neofusicoccum among others, are associated with Botryosphaeria teleomorphs, yet, Botryosphaeria s. l. forms a monophyletic group (Crous et al. 2006).
Results from thepresent study show that Neonectria fuckeliana clusters with Neonectria/Cylindrocarpon sensu stricto, and T. jungneri with Thelonectria. The branch lengths (substitutions/site) that separate these species from Neonectria/Cylindrocarpon sensu stricto and Thelonectria, respectively, are similar to the branch lengths between Thelonectria and Rugonectria (Fig. 1). This could be interpreted as evidence that N. fuckeliana or T. jungneri should be recognised as distinct genera. However, these species are not separated due to the lack of additional morphologically similar species and to avoid monotypic genera with further splitting of genera. It is possible that the addition of morphologically similar species will support the establishment of new genera.
Neonectria/Cylindrocarpon sensu stricto
Neonectria/Cylindrocarpon sensu stricto is characterised by having few to numerous perithecia clustered on wood and seated on an erumpent stroma; perithecial walls are generally composed of two regions with the outer region comprising small, thick-walled cells; generally septate paraphyses; smooth or finely ornamented ascospores; generally straight, typically 5-septate macroconidia with rounded ends; either microconidia or chlamydospores formed, generally not both; and, if microconidia are present, they are produced from simple, generally unbranched, conidiophores and lack a prominent abscission scar. Anamorphs of Neonectria/Cylindrocarpon belong in Booth's groups 1 and 4 (Booth 1966).
Neonectria/Cylindrocarpon sensu stricto species are mostly found in temperate regions on woody substrata, e.g. bark, often causing cankers, and rarely found in soil. This genus includes species such as N. coccinea/C. candidum, N. ditissima/C. heteronemum, N. faginata/C. faginatum, N. fuckeliana/C. cylindroides var. tenue, N. hederae/C. hederae, N. major/Cylindrocarpon sp., N. neomacrospora/C. cylindroides, N. punicea/C. album, and N. ramulariae/C. obtusiusculum (Castlebury et al. 2006). The monophyly of the N. coccinea-group was shown in Castlebury et al. (2006). Although some authors suggested that N. fuckeliana belongs in the N. mammoidea-group based on the morphology of the perithecia (Booth 1959, Brayford et al. 2004), this study supports more recent accounts that place this species close to Neonectria sensu stricto (Halleen et al. 2004, Castlebury et al. 2006, Luo & Zhuang 2010b).
The teleomorph of the type species of Neonectria, N. ramulariae, apparently has not been collected again since it was described by Wollenweber (1917) (Rossman et al. 1999). Rossman et al. (1999) examined the type specimen and noted that it had only immature perithecia along with its anamorph, C. obtusiusculum (= C. magnusianum Wollenw. 1928 non Wollenw. 1926). Domsch et al. (1980) followed Wollenweber (1928) in recognising N. ramulariae to be the teleomorph of C. obtusiusculum (then known as C. magnusianum), based on the anamorph present in the type specimen of N. ramulariae. Although Rossman et al. (1999) designated an iconotype for N. ramulariae, new collections of the anamorph and teleomorph are needed to better describe N. ramulariae/C. obtusiusculum. The morphology of C. obtusiusculum is similar to the anamorphs in Ilyonectria. However, the lignicolous habit, straight macroconidia, absence of microconidia, absence of a prominent basal abscission scar or hilum, and molecular phylogenetic analyses place C. obtusiusculum in Neonectria/Cylindrocarpon sensu stricto.
The segregate genera: Campylocarpon, Ilyonectria, Rugonectria, and Thelonectria
A sister clade to Neonectria/Cylindrocarpon, Ilyonectria (N. radicicola-group), is described here based on Ilyonectria radicicola comb. nov. (anamorph C. destructans). Anamorphs in Ilyonectria belong in Booth's group 3 (Booth 1966). Contrary to Neonectria/Cylindrocarpon, Ilyonectria and its anamorphs are common in the soil and rhizosphere or as agents causing root rots. Chlamydospores are generally present in species of Ilyonectria, possibly as an adaptation for survival in soil. Chlamydospores are generally absent in species that are associated with bark or cankers, e.g. Neonectria, Rugonectria and Thelonectria. Perithecia in Ilyonectria are not as commonly encountered as the anamorphs, and, if found, they are mostly on herbaceous substrata. The species of this genus are cosmopolitan and are found on a wide range of hosts.
Neonectria-like species included here in Ilyonectria are: I. coprosmae/C. coprosmae, I. liriodendri/C. liriodendri, I. macrodydima/C. macrodydimum, and I. radicicola/C. destructans (Samuels & Brayford 1990, Seifert et al. 2003, Halleen et al. 2004, 2006). The monophyly of species in Ilyonectria, viz. the N. radicicola-group, has also been shown in previous studies (Seifert et al. 2003, Halleen et al. 2004, 2006). These studies suggest that C. destructans is a species complex. Thus, defining C. destructans sensu stricto through the examination of many cultures derived from ascospores as well as cultures isolated directly from diverse substrata is a necessary future endeavour. Many other species have been described that may fit in Ilyonectria.
Rugonectria gen. nov. (N. rugulosa-group) is described here based on Rugonectria rugulosa comb. nov. (anamorph C. rugulosum). Members of the genus occur on recently killed or dying woody substrata, mostly bark, and are sometimes found causing cankers. Some species of Neonectria now included in Rugonectria are: R. castaneicola/C. castaneicola, R. neobalansae, and R. rugulosa/C. rugulosum. Another species that may fit in Rugonectria is Nectria pulcherrima (Samuels & Brayford 1994). This species has multiseptate, curved macroconidia with tapering ends, microconidia, and warted perithecia that are caespitose, somewhat immersed in an erumpent stroma, all characteristics of Rugonectria. This species is morphologically similar to R. neobalansae. A new combination has not been made due to the lack of DNA data to confirm its phylogenetic placement.
The new genus Thelonectria is established here to accommodate species in the N. mammoidea- and N. veuillotiana-groups. Species of Thelonectria are mostly tropical and subtropical, and are found on bark of recently killed or dying trees, often causing small cankers, rarely in soil except in one species, C. olidum. Some species included in this genus are: T. coronata/C. coronatum, T. discophora/C. ianothele, T. jungneri/C. victoriae, T. lucida/C. lucidum, T. olida, T. trachosa, T. veuillotiana/C. candidulum, T. viridispora, and T. westlandica (Mantiri et al. 2001, Brayford et al. 2004). Anamorphs in Thelonectria belong in Booth's group 2 (Booth 1966).
Although Thelonectria can generally be recognised by perithecia with prominent or darkened papilla, macroconidia that are curved with rounded ends, > 3-septate (average 5-septate), and absence of microconidia, some species deviate from this trend. For example, 3-septate macroconidia have been reported for T. lucida and T. trachosa (Booth 1966, Brayford et al. 2004). Thelonectria trachosa mostly forms 3-septate macroconidia, but > 3-septate macroconidia can be found in the same culture (Brayford et al. 2004). Brayford et al. (2004) reported that the majority of the T. lucida cultures formed > 3-septate macroconidia. Brayford et al. (2004) also suggested that T. lucida might comprise a species complex, thus, further taxonomic studies are needed to explain the morphological variation within this species. Thelonectria lucida and T. trachosa can be easily classified in Thelonectria based on the anatomy of the perithecia and curved macroconidia with rounded ends and absence of microconidia and chlamydospores. A similar case is T. olida, which produces 3–5-septate macroconidia and chlamydospores although Booth (1966) reports many > 3-septate macroconidia. This species is classified in Thelonectria based on the curved macroconidia with rounded ends and absence of microconidia. However, T. olida is difficult to distinguish from Campylocarpon based on morphology and ecology.
Conidia in Campylocarpon are similar to those in Thelonectria, as also reported by Halleen et al. (2004). The only morphological difference is the average number of septa in the macroconidia: four in Campylocarpon and five in Thelonectria. Despite the morphological similarity of the conidia, phylogenetic analysis distinguishes the two genera. Campylocarpon species were collected from diseased roots and stems of grapevines in South Africa. This is in contrast to most species of Thelonectria, which are found on above ground parts of woody plants. Thelonectria olida, associated with roots, is the exception.
Previous molecular phylogenetic studies (Mantiri et al. 2001, Brayford et al. 2004) did not show that the N. rugulosa-group was distinct from N. mammoidea-group, as suggested by Samuels & Brayford (1994). This was probably due to the few phylogenetically informative loci and few taxa that were used in those studies. The monophyly of the N. rugulosa-group (= Rugonectria) and its close relationship to the N. mammoidea/veuillotiana-group (= Thelonectria) are shown here (Fig. 1). Rugonectria is distinguished from Thelonectria by perithecial anatomy, presence of microconidia in Rugonectria, and morphology of the macroconidia (Table 3).
As has been the case with several groups of fungi (Chaverri et al. 2003, Frisvad & Samson 2004, Schmidt et al. 2004, Samuels et al. 2006a, Chaverri et al. 2008, Degenkolb et al. 2008, Andersen et al. 2009), a multiphasic approach, i.e. using a combination of independently derived characters such as morphological, ecological, and molecular phylogenetic, is necessary to identify monophyletic groups with Neonectria/Cylindrocarpon-like morphology. For example, the presence of microconidia alone is not useful to identify groups with Cylindrocarpon-like morphology, because microconidia are always present in Ilyonectria and Rugonectria, sometimes present in Neonectria, and absent in Thelonectria, and their morphology is highly conserved. However, if characters are combined such as the presence of 3-septate, straight macroconidia with a prominent abscission scar, presence of chlamydospores, and perithecia with a particular wall anatomy, they can be used to classify a particular specimen as Ilyonectria. Thus, in this study our genus concept is based on a multilocus phylogenetic analyses correlated with a combination of multiple morphological and ecological characters. Each of the proposed genera is further described in the Taxonomy section.
Species of Neonectria/Cylindrocarpon of uncertain classification
In this study we present a general overview of genera with Neonectria/Cylindrocarpon-like morphology. There are still species classified in Neonectria and Cylindrocarpon that have teleomorph and anamorph morphology different than those presented here and also quite distinct from Neonectria/Cylindrocarpon sensu stricto. Additional specimens, cultures, and DNA sequences are needed to infer their phylogenetic position within the Nectriaceae. For example, Neonectria macroconidialis has morphological characteristics of both Neonectria sensu stricto and Ilyonectria. This species is not formally included in Ilyonectria because phylogenetic studies including this species in the ITS tree (Seifert et al. 2003, Halleen et al. 2004) show low bootstrap support for the clade with N. macroconidialis and other species in the N. radicicola-group. In contrast, the β-tubulin tree places N. macroconidialis basal and outside the N. radicicola complex. Therefore, the phylogenetic position of this species is uncertain. The straight macroconidia, prominent basal hilum, and anatomy of the perithecia suggest that N. macroconidialis belongs in Ilyonectria. However, the > 4-septate macroconidia, a characteristic of Neonectria sensu stricto, would be an exception if this species were included in Ilyonectria. This species and others previously placed in the N. radicicola-group (Samuels & Brayford 1990) are morphologically atypical of this group, specifically N. austroradicicola/C. austrodestructans and N. radicicola variant ex Gahnia.
Brayford & Samuels (1993) described three species of Nectria with Cylindrocarpon-like anamorphs and mentioned that they could not be classified in any of the then recognised groups of Nectria. Nectria neblinensis and N. verrucospora are distinct because they have macroconidia that are torpedo-like, viz. straight, wider near the middle or towards the base, and tapering and truncated at the ends. The perithecial wall anatomy somewhat resembles Rugonectria, but the ascospores in these two species are warted and not striate as in Rugonectria. Other species that have been placed in Cylindrocarpon that have torpedo-like macroconidia are C. fusiforme, C. supersimplex, and N. laetidiscoides; however, these are straight in the middle and the terminal cells taper almost to a point (Matsushima 1975, Samuels & Brayford 1993).
Several species previously classified in Neonectria/Cylindrocarpon are distinct from those treated here because they have phragmosporous ascospores, e.g. N. fusispora, N. laetidisca, N. laetidiscoides, N. phaeodisca, N. philodendri, N. septospora and N. vermispora among others (Rossman 1983, Samuels & Brayford 1993). Most of the above appear to belong in Thelonectria, or at least they are closely related, except N. laetidiscoides and N. septospora, which have distinct macroconidia.
Another species with uncertain affinity is N. cinnamomea. The perithecia do not change colour in 3 % KOH, a typical characteristic of members of the Nectriaceae (Brayford & Samuels 1993). In addition, the perithecial wall is completely different from the genera treated in this study or any other genus in Nectriaceae, and the ascospores have a conspicuous wrinkled sheath. The macroconidia are also distinct; they are curved, fusiform, and 3-septate.
Luo & Zhuang (2010b) described Neonectria shennongjiana based mostly on the distinctive macroconidia that are cylindrical-clavate to clove-shaped. The phylogenetic analysis in Luo & Zhuang (2010b) shows that N. shennongjiana may be closely related to Neonectria sensu stricto. Their parsimony cladogram reveals that N. shennongjiana clusters within Neonectria sensu stricto (BP 72 % if N. fuckeliana is included). However, in their phylogenetic tree based on parsimony analysis of two loci (ITS nrDNA and tub), the position of N. shennongjiana is not clear. The bootstrap value supporting the clade of N. shennongjiana and C. obtusisporum is low (62 %). Additional phylogenetic and taxonomic studies are needed to confirm if N. shennongjiana and other species with odd-shaped macroconidia belong in Neonectria s.str. Another species with clove-shaped macroconidia is described in the literature, i.e. Nectria lugdunensis (Webster 1959), the teleomorph of Heliscus lugdunensis.
TAXONOMY
Many of the species of Neonectria sensu lato, including those considered here, are known in both their teleomorph and anamorph states. Although Article 59 of the International Code of Botanical Nomenclature (ICBN) allows the use of two scientific names for some groups of pleomorphic fungi including ascomycetes, a trend exists toward the use of just one scientific name for each species regardless of the state manifested (Rossman & Samuels 2005, Rossman 2009). Additionally, generic names of asexual fungi are now being used in a narrower, phylogenetic sense rather than as broad form-genera that encompass unrelated fungi. For example, the genus Verticillium sensu lato, which traditionally included many species with verticillate branching, has been segregated into distinct phylogenetic genera in spite of morphological similarities. Recently, Verticillium sensu stricto was conserved with a different type so that it represents the plant pathogenic species such as V. alboatrum and V. dahliae (Zare et al. 2004). Moreover, other genera separated from Verticillium sensu stricto are now recognised based on distinctive morphological and ecological characteristics, e.g. Lecanicillium and Pochonia (Gams & Van Zaayen 1982, Zare et al. 2000, Gams & Zare 2001, Zare & Gams 2001a, b, Zare et al. 2001).
The anamorphs of Neonectria sensu lato have been classified in the genus Cylindrocarpon. Just as Neonectria is now conceived in a narrow sense, the genus Cylindrocarpon is herein defined phylogenetically and restricted to only anamorphs of Neonectria sensu stricto. Thus, the anamorph name in Cylindrocarpon is listed for only those species that belong in Neonectria sensu stricto. However, for species in genera segregated from Neonectria sensu lato with an anamorph name in Cylindrocarpon, the scientific name of the anamorph is listed in quotes, e.g. “Cylindrocarpon” destructans, or as Cylindrocarpon-like, if no epithet exists, to indicate that it does not belong in Cylindrocarpon sensu stricto.
In this paper, some species described in Cylindrocarpon have no known teleomorph, but, phylogenetically, they fall into a recognised genus (e.g. “C.” olidum = Thelonectria olida comb. nov). As permitted by the ICBN this scientific name is recombined in the new genus. Recent examples in the literature include Lombard et al. (2009), in which species are described in Calonectria despite the lack of known teleomorphs. Although it would be possible and correct according to ICBN Art. 59 to place these taxa into newly described or existing anamorph genera, this has not been done to avoid separating anamorph names from holomorph genera, which is redundant, confusing, and unnecessary. If and when a teleomorph were discovered for this species and a new name were proposed for it, at present, priority would be given to that teleomorph name rather than the anamorph name. Alternatively, the anamorph name could be epitypified with an element that represents the teleomorph in accordance with ICBN Art. 59.7. Given the confusion that has arisen because of the dual nomenclature associated with pleomorphic fungi and the usefulness of molecular systematics in determining the accurate taxonomic placement of asexually reproducing fungi, it would seem expedient to move toward the use of only one scientific name for all fungi.
KEY TO SEGREGATE GENERA OF NEONECTRIA/CYLINDROCARPON
1. Perithecia generally on herbaceous material, rarely on bark or woody parts; perithecia superficial, loosely attached to substratum; perithecial wall of two regions, outer region of thin-walled (ca. 1 μm), globose, large cells; ascospores smooth; anamorph in soil, generally associated with diseased roots; microconidia generally with a prominent abscission scar; chlamydospores present; macroconidia straight, generally < 3-septate, generally with a prominent abscission scar............................................................................................ Ilyonectria 1. Perithecia and macroconidia not as above............................................................................................................................................... 2
2. Perithecia smooth to slightly roughened, generally red, with a prominent papilla or non-papillate; ascospores generally smooth or slightly ornamented; microconidia present or absent; chlamydospores present or absent; macroconidia curved or almost straight, with rounded ends, generally 3–5-septate; on bark or roots........................................................................................................................................... 3 2. Perithecia conspicuously warted, orange-red, generally aggregated, with an inconspicuous papilla, perithecial wall 50–150 μm thick; ascospores striate; microconidia present; chlamydospores absent; macroconidia fusiform with tapering ends; generally on bark of recently killed trees or causing small cankers......................................................................................................................... Rugonectria
3. Perithecia clustered on wood, generally seated on an erumpent stroma, generally smooth and shiny, sometimes scurfy with a blunt or acute apex, rarely papillate; perithecial walls of 2–3 regions, outer region of small, angular to globose, thick-walled cells, rarely of textura epidermoidea; many species with septate paraphyses; ascospores ellipsoidal, smooth or finely ornamented; either microconidia or chlamydospores present; macroconidia generally straight or slightly curved toward ends, rarely clove-shaped, with rounded ends, rarely tapering, 5–7-septate; chlamydospores rare; on bark of recently killed trees or forming cankers.................................. Neonectria 3. Perithecia mostly aggregated, generally smooth and shiny, with a prominent papilla; ascospores generally ornamented; microconidia and chlamydospores absent; macroconidia curved, often broadest at upper third, with rounded apical cells and flattened or rounded basal cells, 3–7-septate; on bark of recently killed trees, on small cankers, or diseased roots.......................................................................... 4
4. Teleomorph unknown; macroconidia on average 4-septate; on diseased roots and stems of grapevines; generally pathogenic; macroconidia generally 3–5-septate (average 4); known from South Africa and Uruguay................................................ Campylocarpon 4. Teleomorph common, on bark of recently killed trees or causing small cankers; perithecia superficial, most species with a prominent, darkened papilla, if not, then at least with a darkly pigmented apex; perithecial walls of 2–3 regions; outer region of intertwined hyphae or cells lacking a definite outline i.e. textura epidermoidea, with thickened and pigmented walls; ascospores mostly ornamented, becoming brownish at maturity; anamorphs rarely encountered apart from their teleomorph; macroconidia (4–)5–7(–9)-septate (average 5) (except T. olida; see section on Description of Genera)....................................................................................................................... Thelonectria
DESCRIPTION OF GENERA
In this paper five genera are described that have neonectria- and Cylindrocarpon-like morphology: Campylocarpon (teleomorph unknown); Ilyonectria gen. nov. (anam. Cylindrocarpon-like); Neonectria sensu stricto (anam. Cylindrocarpon sensu stricto); Rugonectria gen. nov. (anam. Cylindrocarpon-like); and Thelonectria (anam. Cylindrocarpon-like). New combinations are made only for those species that are confirmed to belong to the new genera based on molecular phylogenetic data presented here or in previous studies (Seifert et al. 2003, Brayford et al. 2004, Halleen et al. 2004, 2006, Castlebury et al. 2006).
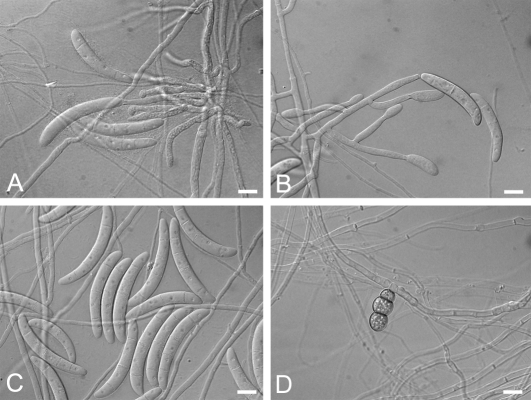
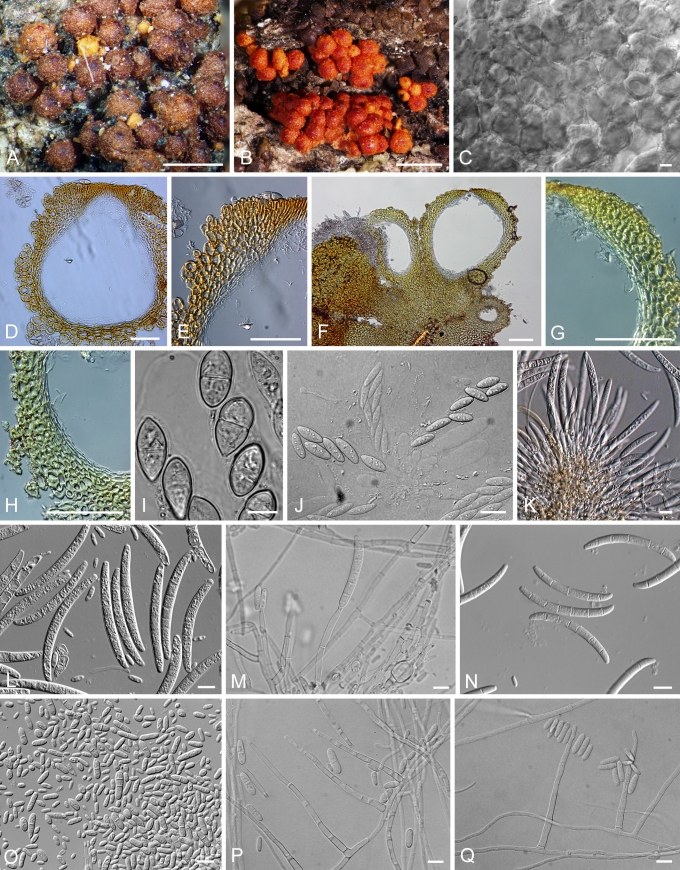
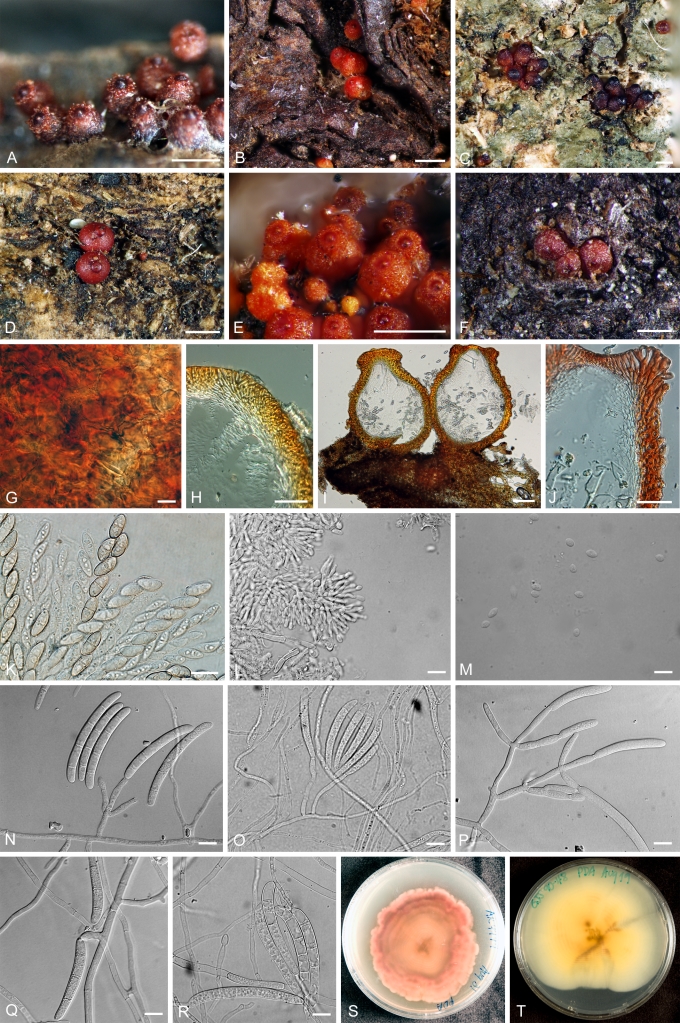
CAMPYLOCARPON Halleen, Schroers & Crous, Stud. Mycol. 50: 449. 2004. Fig. 2.
Fig. 2.
A–D.Campylocarpon. A–C. C. fasciculare conidiophores and macroconidia (CBS 112613). D. C. pseudofasciculare chlamydospores (CBS 112679). Bars: 10 μm.
Type: Campylocarpon fasciculare Schroers, Halleen & Crous, Stud. Mycol. 50: 449. 2004.
Teleomorph: Unknown.
Anamorph: Cylindrocarpon-like; microconidia not observed; chlamydospores rarely observed; conidiophores arising laterally from hyphae, irregularly branched conidiophores or forming fascicles; phialides cylindrical, (13–)15–20(–25) × (2–)3.5–4 μm; macroconidia curved, often broadest at upper third, with rounded apical cells and flattened or rounded basal cells, (1–)3–5(–6)-septate (average 4), with inconspicuous hilum, (24–)35–60(–62) × 6.5–9 μm.
Habitat: On roots and stems of grapevines; generally pathogenic.
Distribution: Known from South Africa and Uruguay (Abreo et al. 2010).
Campylocarpon fasciculare Schroers, Halleen & Crous, Stud. Mycol. 50: 449. 2004.
Teleomorph: Unknown.
Habitat: On diseased roots, rootstock and stems of grapevines.
Distribution: South Africa.
Description and illustrations: Halleen et al. (2004).
Campylocarpon pseudofasciculare Halleen, Schroers & Crous, Stud. Mycol. 50: 451. 2004.
Teleomorph: Unknown.
Habitat: On asymptomatic grapevine roots.
Distribution: South Africa.
Description and illustrations: Halleen et al. (2004).
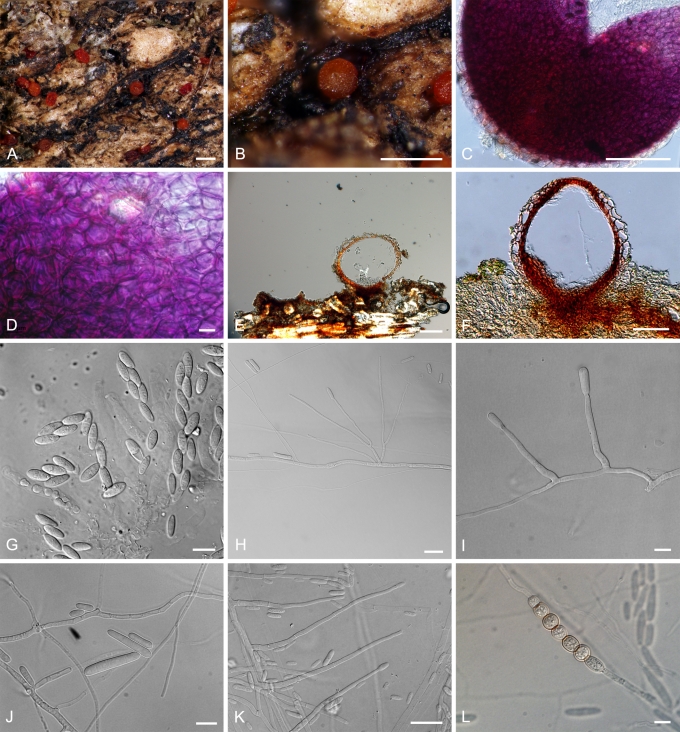
ILYONECTRIA P. Chaverri & C. Salgado, gen. nov. MycoBank MB518558. Fig. 3.
Fig. 3.
Ilyonectria. A, B. I. radicicola perithecia (A.R. 2553). C, D. Crushed perithecium of I. radicicola showing perithecium wall surface (A.R. 2553). E, F. Longitudinal section of perithecium (TFM FPH-7807) of I. radicicola. G. Asci and ascospores of I. radicicola (A.R. 2553). H–J. Conidiophores and conidia of I. macrodydima (CBS 112615). K. Conidiophores and conidia of I. radicicola (C.T.R. 71-76). L. Chlamydospores of I. radicicola (A.R. 2553). Bars: A, B = 500 μm; C, E, F = 100 μm; D, G, J, L = 10 μm; H, I = 20 μm; K = 50 μm.
Type: Ilyonectria radicicola (Gerlach & L. Nilsson) Chaverri & C. Salgado.
Etymology: “ilyo” = Greek for “mud” or “dirt”. The name is given because most species are found as soil inhabitants.
Ascomata superficialia, globosa vel sublobosa, verrucata vel squamosa, rubra, KOH+ phaeorubra, papilla conica vel subconica. Ascosporae ellipsoidea, 1-septatae, hyalinae, glabra. Anamorphosis cylindrocarpon-similis. Microconidia et chlamydosporae abundans. Phialide cylindrici. Macroconidia cylindrici, recte, hyaline, 1–3-septatae, hilum conspicue. Microconidia ellipsoidea vel oblonga, hyaline, 0–1-septatae, hilum conspicue. Typus: Ilyonectria radicicola.
Teleomorph: Perithecia superficial, loosely attached to substrate, red, KOH+, globose to subglobose, 175–350 μm diam, with a broadly conical papilla, scaly or slightly warted; perithecial wall of two regions, 35–50 μm thick: outer region 25–30 μm thick, of thin-walled, ca. 1 μm, globose, large cells; inner region of compressed, flattened cells. Ascospores ellipsoidal, 1-septate, smooth, hyaline.
Anamorph: Cylindrocarpon-like; microconidia and chlamydospores abundant; macro- and microconidia apparently originating from same conidiophores. Conidiophores 40–160 um long, generally simple, unbranched or sparsely branched, irregularly or verticillately branched, rarely densely branched. Phialides cylindrical, 15–40 (–50) × 1.5–3 μm. Macroconidia straight, hyaline, 1–3-septate, rarely > 3-septate, 25–50(–55) × 5–7.5 μm, generally with a prominent basal or lateral abscission scar or hilum. Microconidia ellipsoidal to ovoid, hyaline, 0–1-septate, with a lateral or basal hilum, 3–15 × 2.5–5(–6) μm. Chlamydospores abundant, generally intercalary, globose, single or in chains, becoming brownish.
Habitat: On roots, soil, woody and herbaceous plants, often pathogenic.
Notes: One potential existing generic name for this group is Coleomyces Moreau & M. Moreau that Booth (1966) listed as a synonym of Cylindrocarpon. The illustration in the original description of Coleomyces, based on C. rufus (Moreau & Moreau 1937), suggests that it belongs in the N. radicicola-group. However, in the original description the authors refer to this name as “ad interim.” Ad interim means it is a provisional name and, according to the ICBN (Art. 34.1, Ex. 6), it is not validly published. The authors of the present study were not able to find a later publication validating this name. Therefore, Coleomyces cannot be used for species in the N. radicicola-group.
Ilyonectria coprosmae (Dingley) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518559. Basionym: Nectria coprosmae Dingley, Trans. Roy. Soc. New Zealand 79: 200. 1951.
≡ Nectria radicicola var. coprosmae (Dingley) Samuels & Brayford, Mycol. Res. 94: 438. 1990.
≡ Neonectria coprosmae (Dingley) Seifert, Phytopathology 93: 1541. 2003.
Anamorph: “Cylindrocarpon” coprosmae C. Booth, Mycol. Pap. 104: 16. 1966. Basionym: Cylindrocarpon destructans var. coprosmae (C. Booth) Brayford & Samuels, Mycol. Res. 94: 438. 1990.
Habitat: On various decaying woody and herbaceous plants.
Distribution: New Zealand.
Descriptions and illustrations: Booth (1966) and Samuels & Brayford (1990).
Notes: Brayford & Samuels (1990) accepted this species as a variety of Cylindrocarpon destructans. However, Seifert et al. (2003) recognised it as a separate species. To better elucidate the taxonomic and phylogenetic relationship of I. coprosmae/'C.' coprosmae to I. radicicola/'C.' destructans sensu stricto, further detailed taxonomic studies are needed.
Ilyonectria radicicola (Gerlach & L. Nilsson) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518560. Basionym: Nectria radicicola Gerlach & L. Nilsson, Phytopath. Z. 48: 225. 1963.
≡ Neonectria radicicola (Gerlach & L. Nilsson) Mantiri & Samuels, Canad. J. Bot. 79: 339. 2001.
Anamorph: “Cylindrocarpon” destructans (Zinssm.) Scholten var. destructans, Netherl. J. Plant Path. 70 suppl. (2): 9. 1964. Basionym: Ramularia destructans Zinssm., Phytopathology 8: 570. 1918.
= Cylindrocarpon radicicola Wollenw., Fus. Autogr. Delin. 2: 651. 1924.
[= Ramularia macrospora Wollenw. Phytopathology 3: 222. 1913 non Fresen., Beitr. Mykol. 3: 88. 1863. hom. illeg.]
[= Fusarium polymorphum Marchal, Bull. Soc. Roy. Bot. Belgique 34: 145-148. 1895 non Matruchot, Rech. Dével. Mucéd. 84: 1892. hom. illeg.]
Habitat: On soil, roots, wood, and herbaceous debris.
Distribution: Cosmopolitan.
Descriptions and illustrations: Booth (1966, 1967), Samuels & Brayford (1990).
Ilyonectria liriodendri (Halleen et al.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518561. Basionym: Neonectria liriodendri Halleen, Rego & Crous, Stud. Mycol. 55: 232. 2006.
Anamorph: “Cylindrocarpon” liriodendri J.D. MacDon. & E.E. Butler, Pl. Dis. 65: 156. 1981.
Habitat: On diseased roots and rootstocks.
Distribution: France, Portugal, New Zealand, South Africa, USA.
Description and illustrations: Halleen et al. (2006).
Ilyonectria macrodidyma (Halleen, Schroers & Crous) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518562. Basionym: Neonectria macrodidyma Halleen, Schroers & Crous, Stud. Mycol. 50: 446. 2004.
Anamorph: “Cylindrocarpon” macrodidymum Schroers, Halleen & Crous, Stud. Mycol. 50: 447. 2004.
Habitat: On diseased roots and rootstocks.
Distribution: Australia, Canada, New Zealand, South Africa.
Description and illustrations: Halleen et al. (2004).
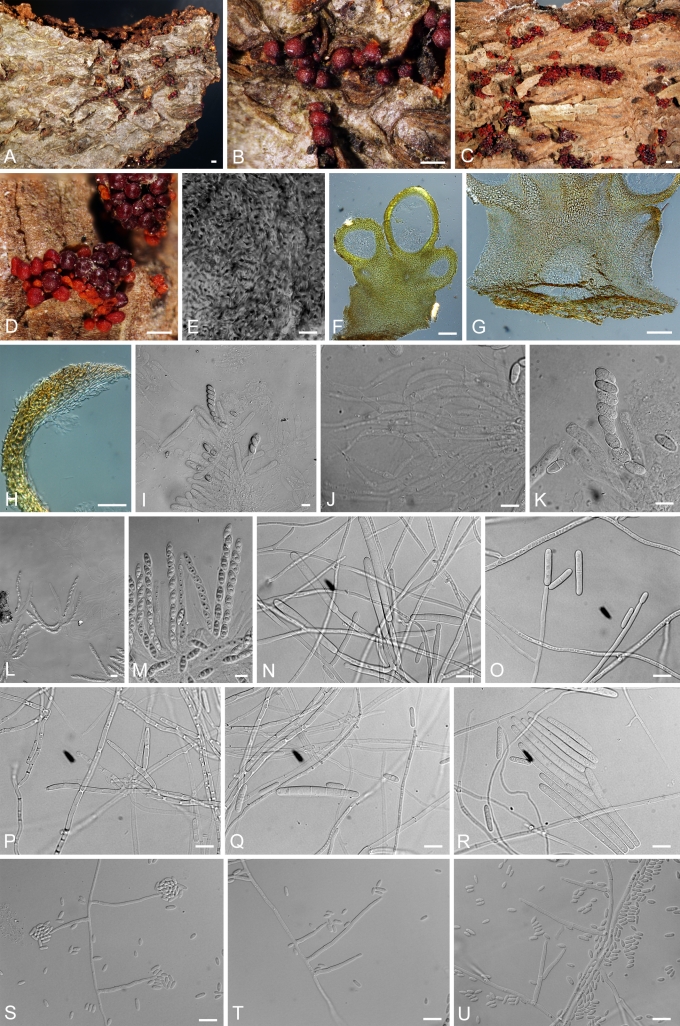
NEONECTRIA Wollenw., Ann. Mycol. 15: 52. 1917. Fig. 4.
Fig. 4.
Neonectria. A, B. N. ditissima perithecia (A.R. 3690 = BPI 870951). C, D.N. fuckeliana perithecia (A.R. 3103 = BPI 842140). E. Top view of surface of N. fuckeliana perithecium (A.R. 3103 = BPI 842140). F–H. Longitudinal section of N. ditissima perithecia (A.R. 3690 = BPI 870951). I. Asci and ascospores of N. ditissima (A.R. 3703 = BPI 871120). J. Paraphyses of N. ditissima (A.R. = BPI 871120). K. Asci and ascospores of N. ditissima (A.R. 3703 = BPI 871120). L, M. Asci and ascospores of N. fuckeliana (A.R. 3103 = BPI 842140). N–R. Conidiophores and macroconidia of N. ditissima (A.R. 3692 = CBS 119521 = BPI 871119). S–U. Conidiophores and microconidia of N. fuckeliana (G.J.S. 02-67 = CBS 125109 = BPI 842434). Bars: A, C = 1 mm; B, D = 500 μm; E, I–U = 10 μm; F, G = 100 μm; H = 50 μm.
Type: Neonectria ramulariae Wollenw.
= Chitinonectria Morelet, Bull. Soc. Sci. Nat. Archéol. Toulon Var 178: 6. 1969. Type: Ch. coccinea (Pers.: Fr.) Morelet (≡ Sphaeria coccinea Pers.: Fr., ≡ Neonectria coccinea (Pers.: Fr.) Rossman & Samuels).
Anamorph: Cylindrocarpon Wollenw., Phytopathology 3: 225. 1913. Type species Cylindrocarpon cylindroides Wollenw.
[= Fusidium Link: Fr., Syst. Mycol. 1: x1. 1821: 3(2): 480. 1832 nomen rejiciendum]
Teleomorph: Perithecia clustered on wood, generally seated on an erumpent stroma, red, KOH+ dark red, yellow in lactic acid, generally smooth and shiny, sometimes scurfy, subglobose to broadly obpyriform, 200–400 μm diam, generally not collapsing when dry, with a blunt or acute apex, rarely papillate. Perithecial walls of 2–3 regions, generally 35–50 μm thick: outer region of small, angular to globose, thick-walled cells, rarely of textura epidermoidea; inner region of flattened thin-walled cells. Paraphyses when present, septate, slightly constricted at each septum. Ascospores ellipsoidal, smooth or finely ornamented, 1-septate, hyaline, sometimes becoming pale brown at maturity.
Anamorph: Either microconidia or chlamydospores present. Macroconidia produced from irregularly branched conidiophores or fascicles. Phialides cylindrical, typically 10–20(–30) μm long. Macroconidia hyaline, smooth, generally straight, sometimes slightly curved toward ends, with rounded ends except in one species, N. fuckeliana, which has fusiform conidia with pointed ends, 3–7(–9)-septate, mostly 5-septate, lacking a prominent scar or basal hilum, 35–65(–110) × 4–7(–8) μm. Microconidia produced from simple, generally unbranched conidiophores, short or long; microconidia hyaline, smooth, ellipsoidal to oblong, 0–1-septate, mostly unicellular, (2–)6–10(–15) × (1–)2–5(–6) μm. When present, chlamydospores globose to subglobose, hyaline.
Habitat: Generally on bark, sometimes causing cankers. Mostly in temperate regions.
Representative species: N. coccinea/C. candidum, N. ditissima/C. heteronemum, N. faginata/C. faginatum, N. fuckeliana/C. cylindroides var. tenue, N. hederae/C. hederae, N. major/Cylindrocarpon sp., N. neomacrospora/C. cylindroides, N. punicea/C. album, and N. ramulariae/C. obtusiusculum.
Notes: Three names exist that could be considered synonyms of Cylindrocarpon, i.e. Allantospora, Cylindrodendrum, and Heliscus (Booth, 1966). The protologue and illustrations of Allantospora suggest that it is probably not congeneric with Cylindrocarpon (Wakker 1895). Wakker (1895) illustrated this genus based on the type species, A. radicicola, as having Verticillium-like conidiophores and small, allantoid conidia. Therefore, this synonymy is doubtful. Cylindrodendrum could also be considered a synonym of Cylindrocarpon, based on the cylindrical conidia although many other genera in the Hypocreales have cylindrical conidia. The original description and illustration of this genus based on the type species, Cylindrodendrum album, shows that most characteristics are quite distinct from Cylindrocarpon (Bonorden 1851). In Cylindrodendrum, the conidiophore branches have sterile, terminal elongations that are generally hooked, phialides with a swollen base and narrow neck, and relatively small conidia that are not septate. Regarding Heliscus as a possible synonym of Cylindrocarpon, Luo & Zhuang (2010b) placed the morphologically similar species, Neonectria shennongjiana, close to Neonectria/Cylindrocarpon sensu stricto. However, more studies are needed to confirm if Heliscus is congeneric with Cylindrocarpon. If it is congeneric, then, Heliscus is an older name (1880) and thus would have priority over Cylindrocarpon. Due to the extensive use of the name Cylindrocarpon, its economic importance, and some doubts about the phylogenetic placement of Heliscus, the authors of the present study would argue for conservation of Cylindrocarpon over Heliscus.
RUGONECTRIA P. Chaverri & Samuels, gen. nov. MycoBank MB518563. Fig. 5.
Fig. 5.
Rugonectria. A. Perithecia of R. neobalansae (G.J.S. 85-219, NY). B. Perithecia of R. rugulosa (G.J.S. 90-238 = BPI 1107399). C. Top view of surface of R. rugulosa perithecium (G.J.S. 90-238 = BPI 1107399). D, E. Longitudinal section of R. neobalansae perithecium (G.J.S. 85-219, NY). F–H. Longitudinal section of R. rugulosa (G.J.S. 90-238 = BPI 1107399). I. Ascospores of R. neobalansae (G.J.S. 85-219, NY). J. Asci and ascospores of R. rugulosa (G.J.S. 90-238 = BPI 1107399). K, L. Conidiophores and macroconidia of R. castaneicola (MAFF 237284). M. Conidiophores and macroconidia of R. rugulosa (G.J.S. 09-1337). N. Macroconidia of R. rugulosa (MAFF 241491). O. Microconidia of R. castaneicola (MAFF 237284). P, Q. Conidiophores and microconidia of R. rugulosa (09-1337). Bars: A, B = 1 mm; C, I–Q = 10 μm; D–H = 100 μm.
Etymology: “rugo” = Latin for “wrinkled”. The perithecial wall surface for species of this genus is warted or rugose.
Type: Rugonectria rugulosa(Pat. & Gaill.) Chaverri, C. Salgado & Samuels.
Ascomata superficialia vel gregaria in stromatae, ascomata globosa vel sublobosa, verrucata vel tuberculata, rubra, KOH+ phaeorubra, non papillata. Ascosporae ellipsoidea vel oblongata, 1-septatae, hyalinae vel pallide brunneae, striatae. Anamorphosis Cylindrocarpon-similis. Phialide cylindrici. Macroconidia fusiformes, hyaline, (3–)5–7(–9)-septatae, hilum inconspicue. Microconidia ellipsoidea vel cylindrici, hyaline, 0–1-septatae, hilum inconspicue. Chlamydosporae absens. Typus: R. rugulosa.
Teleomorph: Perithecia solitary or in groups, formed on or sometimes partially immersed within a stroma. Perithecia globose to subglobose, warted, non-papillate, orange to red, dark red in KOH+, yellow in lactic acid. Perithecial wall 50–150 μm thick, generally of two indistinct regions: outer region including warts with cells circular, 10–20 μm diam, cell walls 3–4 μm thick, merging with surrounding stroma; inner region with cells becoming progressively flattened, thinner, and less pigmented toward locule. Ascospores ellipsoidal to oblong, striate, hyaline, or sometimes yellowish, bicellular.
Anamorph: Cylindrocarpon-like; microconidia present; chlamydospores lacking. Macroconidia arising laterally from hyphae, irregularly branched conidiophores or in fascicles, generally with a short base. Phialides from macroconidiophores cylindrical, 15–25 × 3–5 μm. Macroconidia curved, fusiform, tapering towards ends, (3–)5–7(–9)-septate, with inconspicuous hilum, (35–)48–85 × 5–10 μm. Microconidia produced from simple monophialidic or sparsely branched conidiophores, scattered, ca. 20–100 μm long. Phialides from microconidiophores cylindrical, 20–40 × 3–4 μm. Microconidia ovoid to cylindrical, with rounded ends, generally blunt, 0–1-septate, hyaline, (3–)5–15(–20) × 2–5 μm, lacking a prominent basal hilum.
Habitat: On bark of recently killed, dying or diseased trees, often causing cankers.
Rugonectria castaneicola(W. Yamam. & Oyasu) Hirooka & P. Chaverri, comb. nov. MycoBank MB518564. Basionym: Nectria castaneicola W. Yamam. & Oyasu, Sci. Rep. Hyogo Univ. Agric. Biol. 3: 17. 1957.
≡ Neonectria castaneicola (W. Yamam. & Oyasu) Tak. Kobay. & Hirooka, J. Gen. Plant Pathol. 71: 126. 2005.
Anamorph: “Cylindrocarpon” castaneicola Tak. Kobay. & Hirooka, J. Gen. Plant Pathol. 71: 126. 2005.
Habitat: On bark of conifers, generally causing cankers.
Distribution: Japan.
Description and illustrations: Kobayashi et al. (2005).
Rugonectria neobalansae (Samuels) P. Chaverri & Samuels, comb. nov. MycoBank MB518565. Basionym: Nectria neobalansae Samuels, Mem. N. Y. Bot. Gard. 59: 60. 1990.
Anamorph: Cylindrocarpon-like.
Habitat: On bark of living and recently killed trees.
Distribution: Indonesia, known only from the type locality.
Description and illustrations: Samuels et al. (1990).
Notes: Rugonectria neobalansae is distinct in being almost completely immersed in an orange-red stroma and having, large striate ascospores.
Rugonectria rugulosa (Pat. & Gaill.) Samuels, P. Chaverri & C. Salgado, comb. nov. MycoBank MB518566. Basionym: Nectria rugulosa Pat. & Gaill., Bull. Soc. Mycol. France 4: 115. 1888 [1889].
≡ Neonectria rugulosa (Pat. & Gaill.) Mantiri & Samuels, Canad. J. Bot. 79: 339. 2001.
= Nectria congoensis Sydow in Hennings in Wildeman Mycetes. Ann. Mus. Congo. Bot. V. Études Syst. Geog. Bot. Flore du Baset du Moyen Congo 14. 1909.
Anamorph: “Cylindrocarpon” rugulosum Brayford & Samuels, Sydowia 46: 146. 1994.
Habitat: On bark of living and recently killed trees, sometimes causing cankers.
Distribution: Pantropical.
Descriptions and illustrations: Samuels et al. (1990), Samuels & Brayford (1994).
THELONECTRIA P. Chaverri & C. Salgado, gen. nov. MycoBank MB518567. Fig. 6.
Fig. 6.
Thelonectria. A. T. veuillotiana perithecia (A.R. 4505 = BPI 878946). B. T. discophora perithecia (A.R. 4499 = BPI 878945). C. T. jungneri perithecia (C.T.R. 71-244, NY). D. T. lucida perithecia (C.T.R. 72-180, NY). E. T. veuillotiana perithecia (G.J.S. 90-48 = BPI 1107127). F. T. westlandica perithecia (G.J.S. 83-156, PDD). G. Top view of surface of T. veuillotiana perithecium (A.R. 4505 = BPI 878946). H. Longitudinal section of T. discophora perithecium (A.R. 4499 = BPI 878945). I, J. Longitudinal section of T. veuillotiana perithecium (G.J.S. 90-48 = BPI 1107127). K. Asci and ascospores of T. lucida (C.T.R. 72-180, NY). L, M. Conidiophores and conidia of T. veuillotiana on natural substrate (G.J.S. 90-48 = BPI 1107127). N. Conidiophores and macroconidia of T. discophora (A.R. 4499 = BPI 878945). O. Conidiophores and macroconidia of T. olida (CBS 215.67). P. Conidiophores and macroconidia of T. veuillotiana (G.J.S. 90-48 = BPI 1107127). Q. Conidia of T. trachosa (CBS 112467). R. Macroconidia of T. westlandica (G.J.S. 83-156, PDD). S. Reverse colony of T. discophora on PDA (A.R. 4499 = BPI 878945). T. Reverse colony of T. veuillotiana on PDA (G.J.S. 90-48 = BPI 1107127). Bars: A–F = 500 μm; G, K–R = 10 μm; H, J = 50 μm; I = 100 μm.
Etymology: “thelo” –Greek for “nipple”. Many species in this genus have a raised, papilla that is sometimes darkened, and thus resembles a nipple.
Type species: Thelonectria discophora (Mont.) P. Chaverri & C. Salgado (new combination made below).
Ascomata superficialia vel gregaria, ascomata globosa vel sublobosa, glabra, rubra, KOH+ phaeorubra, atropapillata. Ascosporae ellipsoidea vel oblongata, 1-septatae, hyalinae, glabra. Anamorphosis Cylindrocarpon-similis. Phialide cylindrici. Macroconidia fusiformes, curva, saepe triente apicali latiore, cellulis apicalibus rotundatis et cellulis basalibus rotundatis vel complanatis, hyaline, (3–)5–7(–9)-septatae, hilum inconspicue. Microconidia absens. Chlamydosporae absens. Typus: T. discophora.
Teleomorph: Perithecia superficial, sometimes seated on an immersed inconspicuous stroma, smooth or sometimes warted, sometimes shiny, globose, subglobose, or pyriform to elongated, 300–600 μm diam, most species with a prominent, areolate (darkened) papilla, if not, then at least with a darkly pigmented apex; perithecial walls of 2 or 3 regions, 20–50(–100) μm thick: outer region of intertwined hyphae or cells lacking a definite outline i.e. textura epidermoidea, with thickened, pigmented walls; inner region of thin-walled, non-pigmented, flattened cells. Ascospores mostly smooth, rarely spinulose or striate, hyaline, becoming brownish at maturity, generally 1-septate.
Anamorph: Cylindrocarpon-like; microconidia rare, sometimes seen on natural substrata; chlamydospores rare, abundant in one species; conidiophores arising laterally from hyphae, irregularly branched conidiophores or forming fascicles; phialides cylindrical, 10–25 × 3–6 μm; macroconidia curved, often broadest at upper third, with rounded apical cells and flattened or rounded basal cells, (3–)5–7(–9)-septate, with inconspicuous hilum, (35–)40–90(–110) × 4–8(–11) μm.
Habitat: On bark of recently killed, dying or diseased trees, often causing small cankers, sometimes on rotting roots.
Thelonectria coronata (Penz. & Sacc.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518568. Basionym: Nectria coronata Penz. & Sacc., Malpighia 11: 510. 1897.
≡ Neonectria coronata(Penz. & Sacc.) Mantiri & Samuels,Canad. J. Bot. 79: 339. 2001.
Anamorph: “Cylindrocarpon” coronatum Brayford & Samuels, Sydowia 46: 91. 1993.
Habitat: On bark, often associated with small cankers.
Distribution: Probably pantropical.
Descriptions and illustrations: Brayford & Samuels (1993); Samuels & Brayford (1994)
Thelonectria discophora (Mont.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518569. Basionym: Sphaeria discophora Mont., Ann. Sci. Nat. Bot. II 3: 353. 1835.
≡ Neonectria discophora (Mont.) var. discophora Mantiri & Samuels,
= Nectria tasmanica Berk. in Hooker, Flora Tasmaniae 2: 279. 1860.
= Nectria mammoidea W. Phillips & Plowr. Grevillea 3: 126. 1875.
≡ Creonectria mammoidea (W. Phillips & Plowr.) Seaver, Mycologia 1: 188. 1909 (as Creonectria mammoides).
= Nectria nelumbicola Henn., Verh. Bot. Vereins. Prov. Brandenburg 40: 151. 1898.
= Nectria umbilicata Henn., Hedwigia 41: 3. 1902.
= Nectria mammoidea var. rugulosa Weese, Akad. Wiss. Wien Math.-Naturw. Kl., Abt. 1, 125: 552. 1916.
= Nectria mammoidea var. minor Reinking, Zentralbl. Bakteriol., Abt. 2, 94: 135. 1936.
= Creonectria discostiolata Chardón, Bol. Soc. Venez. Ci. Nat. 5: 341. 1939.
= Nectria pinea Dingley, Trans. Roy. Soc. New Zealand 79: 198. 1951.
Anamorph: “Cylindrocarpon” ianothele var. majus Wollenw., Z. Parasitenk. (Berlin) 1: 161. 1928.
= Cylindrocarpon ianthothele var. minus Reinking, Zentralbl. Bakteriol., Abt. 2, 94: 135. 1936.
= Cylindrocarpon ianthothele var. rugulosum C. Booth, Mycol. Pap. 104: 25. 1966.
= Cylindrocarpon pineum C. Booth, Mycol. Pap. 104: 26. 1966.
Habitat: On bark and twigs of recently killed trees, rarely on palm trunks.
Distribution: Cosmopolitan.
Description and illustrations: Brayford et al. (2004).
Thelonectria jungneri (Henn.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518570. Basionym: Nectria jungneri Henn., Bot. Jahrb. Syst. 22: 75. 1897.
= Nectria eustoma Penz. & Sacc., Malpighia 11: 509. 1898 [1897]
= Nectria leucoloma Starbäck, Bih. Kongl. Svenska Vetensk.-Akad. Handl. 25: 28. 1899.
= Nectria cinereopapillata Henn. & Nyman in Warburg, Monsunia 1: 161. 1900 [1899]
= Nectria striatospora Zimm., Centralbl. Bakteriol. II, 7: 105. 1901.
= Nectria azureostiolata Doi, Mem. Nat. Sci. Mus. Tokyo 10: 23. 1977.
Anamorph: “Cylindrocarpon” victoriae Wollenw., Z. Parasitenk. (Berlin) 1: 161. 1928.
Habitat: On bark of recently killed or dying trees.
Distribution: Pantropical.
Description and illustrations: Samuels et al. (1990).
Thelonectria lucida (Höhn.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518571. Basionym: Nectria lucida Höhn., Akad. Wiss. Wien math. Naturw. Kl., Abt. 1, 118: 289. 1909.
≡ Neonectria lucida (Höhn.) Samuels & Brayford, Mycologia 96: 590. 2004.
Anamorph: “Cylindrocarpon” lucidum Booth, Mycol. Pap. 104: 21. 1966.
Habitat: On bark of recently killed or dying trees, rarely on vines.
Distribution: Asia, New Zealand, South America, North America, probably cosmopolitan.
Description and illustrations: Brayford et al. (2004).
Thelonectria olida (Wollenw.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518572. Basionym: Ramularia olida Wollenw., Phytopathology 3: 223. 1913.
≡ Cylindrocarpon olidum var. olidum (Wollenw.) Wollenw., Fus. Autogr. Del., ed. 1: 471. 1916.
= Cylindrocarpon curvatum Hochapfel in Wollenw., Z. Parasitenk. 3: 495. 1931.
Teleomorph: Unknown.
Habitat: On rotting roots of various plants.
Distribution: Probably widespread.
Descriptions and illustrations: Booth (1966), Brayford (1987).
Notes: This species is somewhat different from the rest of Thelonectria in having shorter macroconidia, fewer septa, and abundant chlamydospores. However, it also has similarities with Thelonectria. Thelonectria olida has short conidiophores, lacks microconidia, and has curved macroconidia with rounded ends. Molecular phylogenetic data presented here also places this species in Thelonectria.
Thelonectria trachosa(Samuels & Brayford) Samuels, P. Chaverri & C. Salgado, comb. nov. MycoBank MB518573. Basionym: Neonectria trachosa Samuels & Brayford, Mycologia 96: 592. 2004.
Anamorph:Cylindrocarpon-like
Habitat: On bark of unknown conifer.
Distribution: Scotland, only known from the type locality.
Description and illustrations: Brayford et al. (2004).
Thelonectria veuillotiana (Sacc. & Roum.) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518574. Basionym: Nectria veuillotiana Sacc. & Roum. in Désmazières, Rev. Mycol. (Toulouse) 2: 189. 1880.
≡ Dialonectria veuillotiana (Sacc. & Roum.) Cooke, Grevillea 12: 110. 1884.
≡ Cucurbitaria veuillotiana (Sacc. & Roum.) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 462. 1898.
≡ Neonectria veuillotiana (Sacc. & Roum.) Mantiri & Samuels, Canad. J. Bot. 79: 339. 2001.
= Sphaerostilbe sanguinea Fuckel, Symb. Myc. App. 3: 22. 1877.
Anamorph: “Cylindrocarpon” candidulum (Sacc.) Wollenw., Z. Parasitenk. 1: 160. 1928.
≡ Atractium candiduli Sacc., Syll. Fung. (Abellini) 2: 512.1883.
Habitat: On bark of recently killed trees, rarely on wood or leaves.
Distribution: Probably widespread.
Description and illustrations: Brayford & Samuels (1993).
Thelonectria viridispora (Samuels & Brayford) P. Chaverri, C. Salgado, & Samuels, comb. nov. MycoBank MB518575. Basionym: Neonectria viridispora Samuels & Brayford, Mycologia 96: 592. 2004.
Anamorph: Cylindrocarpon-like.
Habitat: On bark of Ochroma.
Distribution: Ecuador, only known from the type locality.
Description and illustrations: Brayford et al. (2004).
Thelonectria westlandica (Dingley) P. Chaverri & C. Salgado, comb. nov. MycoBank MB518576. Basionym: Nectria westlandica Dingley, Trans. Roy. Soc. New Zealand 79: 201. 1951.
≡Neonectria westlandica (Dingley) Samuels & Brayford, Mycologia 96: 595. 2004.
Anamorph: Cylindrocarpon-like.
Habitat: On bark of dicotyledonous trees, sometimes gymnosperms.
Distribution: New Zealand.
Description and illustrations: Brayford et al. (2004)
Acknowledgments
We appreciate the nomenclatural advice given by Drew Minnis (USDA). We also greatly appreciate the assistance of Adam Bazinet (UMD) in the use of the GRID-computing system. This study was funded by a grant from United States National Science Foundation (PEET program) DEB-0925696: “Monographic Studies in the Nectriaceae, Hypocreales: Nectria, Cosmospora, and Neonectria” to University of Maryland (P. Chaverri, G.J. Samuels & A.Y. Rossman).
Taxonomic novelties: Ilyonectria P. Chaverri & C. Salgado, gen. nov.; Ilyonectria coprosmae (Dingley) P. Chaverri & C. Salgado, comb. nov.; Ilyonectria liriodendri (Halleen et al.) P. Chaverri & C. Salgado, comb. nov.; Ilyonectria macrodydima (Halleen, Schroers & Crous) P. Chaverri & C. Salgado, comb. nov.; Ilyonectria radicicola (Gerlach & L. Nilsoon) P. Chaverri & C. Salgado, comb. nov.; Rugonectria P. Chaverri & Samuels, gen. nov.; Rugonectria castaneicola (W. Yamam. & Oyasu) Hirooka & P. Chaverri, comb. nov; Rugonectria neobalansae (Samuels) P. Chaverri & Samuels, comb. nov.; Rugonectria rugulosa (Pat. & Gaill.) Samuels, P. Chaverri & C. Salgado, comb. nov.; Thelonectria P. Chaverri & C. Salgado, gen. nov.; Thelonectria coronata (Penz. & Sacc.) P. Chaverri & C. Salgado, comb. nov.; Thelonectria discophora (Mont.) P. Chaverri & C. Salgado, comb. nov.; Thelonectria jungneri (Henn.) P. Chaverri & C. Salgado, comb. nov.; Thelonectria lucida (Höhnel) P. Chaverri & C. Salgado, comb. nov.; Thelonectria olida (Wollenw.) P. Chaverri & C. Salgado, comb. nov; Thelonectria trachosa (Samuels & Brayford) Samuels, P. Chaverri & C. Salgado, comb. nov.; Thelonectria veuillotiana (Sacc. & Roum.) P. Chaverri & C. Salgado, comb. nov.; Thelonectria viridispora (Samuels & Brayford) P. Chaverri, C. Salgado, & Samuels, comb. nov.; Thelonectria westlandica (Dingley) P. Chaverri & C. Salgado, comb. nov.
References
- Abreo E, Martinez S, Bettucci L, Lupo S (2010). Morphological and molecular characterisation of Campylocarpon and Cylindrocarpon spp. associated with black foot disease of grapevines in Uruguay. Australasian Plant Pathology 39: 446–452. [Google Scholar]
- Andersen B, Sorensen JL, Nielsen KF, van den Ende BG, de Hoog S (2009). A polyphasic approach to the taxonomy of the Alternaria infectoria species-group. Fungal Genetics and Biology 46: 642–656. [DOI] [PubMed] [Google Scholar]
- Bazinet AL, Cummings MP (2008). The Lattice Project: a grid research and production environment combining multiple grid computing models. In: Distributed & Grid Computing - Science Made Transparent for Everyone. Weber MHW, ed. Rechenkraft.net:
- Bonorden HF (1851). Handbuch der Allgemeinen Mykologie (Stuttgart). E. Schweiserbart'sche Verlagshandlung und Druckerel, Stuttgart.
- Booth C (1959). Studies of pyrenomycetes. IV. Nectria (part 1). Mycological Papers 73: 1–115. [Google Scholar]
- Booth C (1966). The genus Cylindrocarpon. Mycological Papers 104: 1–56. [Google Scholar]
- Booth C (1967). Nectria radicicola. C.M.I. Descriptions of Pathogenic Fungi and Bacteria 148: 1–2. [Google Scholar]
- Brayford D (1987). Cylindrocarpon olidum var. olidum. CMI Descriptions of Pathogenic Fungi and Bacteria 929: 131–132. [DOI] [PubMed] [Google Scholar]
- Brayford D, Honda BM, Mantiri FR, Samuels GJ (2004). Neonectria and Cylindrocarpon: the Nectria mammoidea group and species lacking microconidia. Mycologia 96: 572–597. [PubMed] [Google Scholar]
- Brayford D, Samuels GJ (1993). Some didymosporous species of Nectria with non-microconidial Cylindrocarpon anamorphs. Mycologia 85: 612–637. [Google Scholar]
- Castlebury LA, Rossman AY, Hyten AS (2006). Phylogenetic relationships of Neonectria/Cylindrocarpon on Fagus in North America. Canadian Journal of Botany 84: 1417–1433. [Google Scholar]
- Chaverri P, Castlebury LA, Overton BE, Samuels GJ (2003). Hypocrea/Trichoderma: species with conidiophore elongations and green conidia. Mycologia 95: 1100–1140. [PubMed] [Google Scholar]
- Chaverri P, Liu M, Hodge KT (2008). Neotropical Hypocrella (anamorph Aschersonia)., Moelleriella, and Samuelsia. Studies in Mycology 60: 1–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW (2002). Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, St. Paul, MN.
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings MP, Huskamp JC (2005). Grid computing. Educause Review 40: 116–117. [Google Scholar]
- Degenkolb T, Ralf D, Nielsen KF, Grafenhan T, Theis C, et al. (2008). The Trichoderma brevicompactum clade: a new lineage with new species, new peptaibiotics, and mycotoxins. Mycological Progress 7: 177–209. [Google Scholar]
- Domsch KH, Gams W, Anderson T-H (1980). Compendium of Soil Fungi. Academic Press, London.
- Frisvad JC, Samson RA (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium - A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49: 1–173. [Google Scholar]
- Galtier N, Gouy M, Gautier C (1996). SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Computational Applied Biosciences 12: 543–548. [DOI] [PubMed] [Google Scholar]
- Gams W, Van Zaayen A (1982). Contribution to the taxonomy and pathogenicity of fungicolous Verticillium species. I. Taxonomy. Netherlands Journal of Plant Pathology 88: 57–78. [Google Scholar]
- Gams W, Zare R (2001). A revision of Verticillium sect. Prostrata. III. Generic classification. Nova Hedwigia 72: 329–337. [Google Scholar]
- Halleen F, Schroers H-J, Groenewald JZ, Crous PW (2004). Novel species of Cylindrocarpon (Neonectria). and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.). Studies in Mycology 50: 431–455. [Google Scholar]
- Halleen F, Schroers H-J, Groenewald JZ, Rego C, Oliveira H, Crous PW (2006). Neonectria liriodendri sp. nov., the main causal agent of black foot disease of grapevines. Studies in Mycology 55: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Kobayashi T, Natsuaki KT (2005). Neonectria castaneicola and Neo. rugulosa in Japan. Mycologia 97: 1058–1066. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Larget B, Miller RE, Ronquist F (2002). Potential applications and pitfalls of Bayesian inference of phylogeny. Systematic Biology 51: 673–688. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F, Nielsen ES, Bollback JP (2001). Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294: 2310–2314. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K-i, Toh H, Miyata T (2005). MAFFT version 5: improvement in accuracy of multiple sequence alignment Nucleic Acids Research 33: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Hirooka Y, Natsuaki KT, Kawashima Y, Ushiyama K (2005). New canker diseases of Abies veitchii and Acer crataegifolium caused by Neonectria castaneicola. Journal of General Plant Pathology 71: 124–126. [Google Scholar]
- Kornerup A, Wanscher JH (1978). Methuen Colour Handbook. 3rd edition. Methuen London Ltd., London.
- Lewis PO, Holder MT, Holsinger KE (2005). Polytomies and Bayesian phylogenetic inference. Systematic Biology 54: 241–253. [DOI] [PubMed] [Google Scholar]
- Lombard L, Rodas CA, Crous PW, Wingfield BD, Wingfield MJ (2009). Calonectria (Cylindrocladium). species associated with dying Pinus cuttings. Persoonia 23: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Zhuang W-Y (2010a). Chaetopsinectria (Nectriaceae, Hypocreales), a new genus with Chaetopsina anamorphs. Mycologia 102: 976–984. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhuang W-Y (2010b). Three new species of Neonectria (Nectriaceae, Hypocreales) with notes on their phylogenetic positions. Mycologia 102: 142–152. [DOI] [PubMed] [Google Scholar]
- Luttrell ES (1951). Taxonomy of the Pyrenomycetes. University of Missouri, Columbia, MO.
- Maddison WP, Maddison DR. 2009. Mesquite: a modular system for evolutionary analysis. Version 2.5. http://mesquiteproject.org.
- Mantiri FR, Samuels GJ, Rahe JE, Honda BM (2001). Phylogenetic relationships in Neonectria species having Cylindrocarpon anamorphs inferred from mitochondrial ribosomal DNA sequences. Canadian Journal of Botany 79: 334–340. [Google Scholar]
- Mason-Gamer RJ, Kellogg EA (1996). Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545. [Google Scholar]
- Matsushima T (1975). Icones Microfungorum: A Matsushima Lectorum. (published by the author), Kobe, Japan.
- Mau B, Newton M, Larget B (1999). Bayesian phylogenetic inference via Markov chain Monte Carlo methods. Biometrics 55: 1–12. [DOI] [PubMed] [Google Scholar]
- Moreau F, Moreau M (1937). Sur un nouveau champignon a collarette, Coleomyces rufus, nov. gen., nov. sp., ad interim. Bulletin Trimestriel de la Societe Mycologique de France 53: 33–38. [Google Scholar]
- Myers DS, Bazinet AL, Cummings MP (2008). Expanding the reach of Grid computing: combining Globus- and BOINC-based systems. In: Grids for Bioinformatics and Computational Biology. Talbi E-G, Zomaya A, ed. John Wiley & Sons, New York: 71–85.
- Nirenberg HI (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt Biol Bundesanst Land-Forstw Berlin-Dahlem 169: 1–117. [Google Scholar]
- O'Donnell K (1996). Progress towards a phylogenetic classification of Fusarium. Sydowia 48: 57–70. [Google Scholar]
- Posada D (2008). jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Posada D, Buckley TR (2004). Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- Rannala B, Yang ZH (1996). Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311. [DOI] [PubMed] [Google Scholar]
- Reeb V, Lutzoni F, Roux C (2004). Contribution of RPB2 to multilocus phylogenetic studies of the Euascomycetes (Pezizomycotina, Fungi). with special emphasis on lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution 32: 1036–1060. [DOI] [PubMed] [Google Scholar]
- Rossman AY (1983). The phragmosporous species of Nectria and related genera. Mycological Papers 150: 1–164. [Google Scholar]
- Rossman AY (1993). Holomorphic hypocrealean fungi: Nectria sensu stricto and teleomorphs of Fusarium. In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. Reynolds DR, Taylor JW, ed. CAB International: Wallingford, UK: 149–160.
- Rossman AY (2009). One scientific name for fungi: how and when can this happen? Inoculum 60: 38. [Google Scholar]
- Rossman AY, Samuels GJ (2005). Towards a single scientific name for species of fungi. Inoculum 56: 3–6. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of the Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Samuels GJ (1988). Species of Nectria (Ascomycetes, Hypocreales). having orange perithecia and colorless, striate ascospores. Brittonia 40: 306–331. [Google Scholar]
- Samuels GJ, Brayford D (1990). Variation in Nectria radicicola and its anamorph, Cylindrocarpon destructans. Mycologia 94: 433–442. [Google Scholar]
- Samuels GJ, Brayford D (1993). Phragmosporous Nectria species with Cylindrocarpon anamorphs. Sydowia 45: 55–80. [Google Scholar]
- Samuels GJ, Brayford D (1994). Species of Nectria (sensu lato). with red perithecia and striate ascospores. Sydowia 46: 75–161. [Google Scholar]
- Samuels GJ, Dodd S, Lu B-S, Petrini O, Schroers H-J, Druzhinina I-S (2006a). The Trichoderma koningii aggregate species. Studies in Mycology 56: 67–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ, Doi Y, Rogerson CT (1990). Hypocreales. In: Contributions toward a mycobiota of Indonesia: Hypocreales, synnematous Hyphomycetes, Aphyllophorales, Phragmobasidiomycetes, and Myxomycetes. Samuels GJ, ed. New York Botanical Garden, New York: 6–108.
- Samuels GJ, Lu B-S, Chaverri P, Candoussau F, Fournier J, Rossman AY (2009). Cyanonectria, a new genus for Nectria cyanostoma and its Fusarium anamorph. Mycological Progress 8: 49–58. [Google Scholar]
- Samuels GJ, Rossman AY, Chaverri P, Overton BE, Põldmaa K (2006b). Hypocreales of the southeastern United States: An identification guide. CBS Biodiversity Series No. 4. CBS, Utrecht.
- Schmidt H, Adler A, Holst-Jensen A, Klemsdal SS, Logrieco A, Mach RL, Nirenberg HI, Thrane U, Torp M, Vogel RF, Yli-Mattila T, Niessen L (2004). An integrated taxonomic study of Fusarium langsethiae, Fusarium poae and Fusarium sporotrichioides based on the use of composite datasets. International Journal of Food Microbiology 95: 341–349. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Wingfield MJ, Wingfield BD (2000). Phylogeny of Calonectria and selected hypocrealean genera with cylindrical macroconidia. Studies in Mycology 45: 45–62. [Google Scholar]
- Seifert KA, McMullen CR, Yee D, Reeleder RD, Dobinson KF (2003). Molecular differentiation and detection of ginseng-adapted isolates of the root rot fungus Cylindrocarpon destructans. Phytopathology 93: 1533–1542. [DOI] [PubMed] [Google Scholar]
- Wakker JH (1895). De Schimmeis in de Wortels van het Suikerriet. Mededeelingen van het Proefstation Oost-Java, Series 2 28: 1–9. [Google Scholar]
- Webster J (1959). Nectria lugdunensis sp. nov., the perfect state of Heliscus lugdunensis. Transactions of the British Mycological Society 42: 322–327. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications. Innis MA, Gelfand DH, Sninsky JJ, White. TJ, ed. Academic Press, Inc., New York: 315–322.
- Wollenweber HW (1917). Fusaria autographice delineata. Published by the author, Berlin.
- Wollenweber HW (1928). Über Fructformen der krebserregenden Nectriaceen. Z. Parasitenk. (Berlin). 1: 138–173. [Google Scholar]
- Zare R, Gams W (2001a). A revision of Verticillium sect. Prostrata IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 73: 1–50. [Google Scholar]
- Zare R, Gams W (2001b). A revision of Verticillium section Prostrata. VI. The genus Haptocillium. Nova Hedwigia 73: 271–292. [Google Scholar]
- Zare R, Gams W, Culham A (2000). A revision of Verticillium sect. Prostrata I. Phylogenetic studies using ITS sequences. Nova Hedwigia 71: 465–480. [Google Scholar]
- Zare R, Gams W, Evans HC (2001). A revision of Verticillium sect. Prostrata V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwigia 73: 51–86. [Google Scholar]
- Zare R, Gams W, Schroers HJ (2004). The type species of Verticillium is not congeneric with the plant-pathogenic species placed in Verticillium and it is not the anamorph of “Nectria” inventa. Mycological Research 108: 576–582. [DOI] [PubMed] [Google Scholar]
- Zwickl DJ (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion (Ph.D. dissertation). The University of Texas at Austin, Austin,TX.