Abstract
A revision of Fusarium-like species associated with the plant genus Buxus led to a reconsideration of generic concepts in the Fusarium clade of the Nectriaceae. Phylogenetic analyses of the partial second largest subunit of the RNA polymerase II (rpb2) and the larger subunit of the ATP citrate lyase (acl1) gene exons confirm the existence of a clade, here called the terminal Fusarium clade, that includes genera such as Fusarium sensu stricto (including its Gibberella teleomorphs), Albonectria, Cyanonectria, “Haematonectria”, the newly described genus Geejayessia, and “Nectria” albida. Geejayessia accommodates five species. Four were previously classified in Nectria sensu lato, namely the black perithecial, KOH–species G. atrofusca and the orange or reddish, KOH+ G. cicatricum, G. desmazieri and G. zealandica. Geejayessia celtidicola is newly described. Following our phylogenetic analyses showing its close relationship with Cyanonectria cyanostoma, the former Gibbera buxi is recombined as the second species of Cyanonectria. A three gene phylogenetic analysis of multiple strains of each morphological species using translation elongation factor 1 α (tef-1), rpb2 and acl1 gene exons and introns confirms their status as distinct phylogenetic species. Internal transcribed spacer of the ribosomal RNA gene cluster and nuclear large ribosomal subunit sequences were generated as additional DNA barcodes for selected strains. The connection of Fusarium buxicola, often erroneously reported as the anamorph of G. desmazieri, with the bluish black and KOH+ perithecial species C. buxi is reinstated. Most Cyanonectria and Geejayessia species exhibit restricted host ranges on branches or twigs of Buxus species, Celtis occidentalis, or Staphylea trifolia. Their perithecia form caespitose clusters on well-developed, mostly erumpent stromata on the bark or outer cortex of the host and are relatively thin-walled, mostly smooth, and therefore reminiscent of the more or less astromatous, singly occurring perithecia of Cosmospora, Dialonectria, and Microcera. The cell walls in outer- and inner layers of the perithecial walls of Cyanonectria and Geejayessia have inconspicuous pore-like structures, as do representative species of Albonectria, Fusarium sensu stricto, “Haematonectria”, and “Nectria” albida. The taxonomic significance of these structures, which we call Samuels' pores, is discussed.
Keywords: Holomorph concept, nomenclature, peridial pores, taxonomy
INTRODUCTION
Species of Fusarium are of major agricultural, economic, and health importance because of their mycotoxin production and roles as crop and opportunistic human pathogens (Marasas et al. 1984, Summerbell 2003) or saprobes isolated from soil or decaying plant substrates (Domsch et al. 2007). Some Fusarium-like species inhabit lichens, other fungi, and insects, but many of these species are phylogenetically distantly related to F. sambucinum, the type species of Fusarium. Some of these were classified in Cosmospora by Rossman et al. (1999), and now placed in re-circumscribed genera such as Dialonectria, Fusicolla, Macroconia, Microcera, and Stylonectria in this volume, Gräfenhan et al. (2011).
Fusarium species typically sporulate readily and grow moderately fast in culture. Perithecia are formed in vitro by a few species, often only after crossing of compatible mating types using special media and incubation conditions (Leslie 1991). Accordingly, the main Fusarium monographers of the 20th and 21st centuries were predominantly teleomorphically challenged and anamorph names are widely used (Wollenweber & Reinking 1935, Gerlach & Nirenberg 1982, Nelson et al. 1983, Gams et al. 1997, Leslie & Summerell 2006, Domsch et al. 2007). However, a parallel holomorphic system was initiated by other taxonomists, sometimes with less exposure to plant pathology and the Fusarium literature, and numerous Fusarium holomorphs were integrated taxonomically into the Nectriaceae, Hypocreales, under a variety of teleomorphic names, most notably Gibberella (Booth 1959, Samuels 1976, Samuels et al. 1990, 1991, Samuels & Brayford 1994, Rossman et al. 1999). The taxonomic segregation of species included in the broad concept of Nectria sensu Booth (1959) into distinct genera (Rossman et al. 1999), crystallised with the recognition or resurrection of holomorphic genera such as Albonectria, Cosmospora, Cyanonectria, Gibberella, Haematonectria, and Neocosmospora (Rossman et al. 1999, Samuels et al. 2009), all with the exception of the latter at least with some Fusarium-like anamorphs. This holomorphic system implied that the generic concept of Fusarium might not be monophyletic or that additional genera might be necessary to delimit monophyletic, morphologically homogenous, or natural species groups. Samuels et al. (2009) and the accompanying paper by Gräfenhan et al. (2011) provide evidence for a monophyletic Fusarium clade, once the species related to the revised concepts of Cosmospora, Dialonectria, Fusicolla, Macroconia, Microcera, and Stylonectria are removed; for convenience, we refer to this as the terminal Fusarium clade based on its position in the Nectriaceae in the phylogenetic analysis of Gräfenhan et al. (2011). In that study, this terminal Fusarium clade received low support in phylogenetic analyses and included several strongly supported phylogenetic lineages within it. Typically, the statistically supported phylogenetic clades corresponded in a nearly 1:1 fashion with taxonomic groupings earlier established on the basis of teleomorph (Samuels 1976, Samuels et al. 2001) and/or anamorph characters (Gerlach & Nirenberg 1982).
The taxonomic placements of some species formerly included in Nectria sensu Booth, including the black perithecial N. atrofusca, the orange N. desmazieri, and the red N. zealandica (the latter also included in Cosmospora sensu Rossman et al. 1999) are particularly puzzling. “Nectria” atrofusca, which has a macroconidial, Fusarium-like anamorph, cannot convincingly be placed phylogenetically among other species with darkly pigmented perithecia, in particular the large and well-known genus Gibberella (Samuels & Rogerson 1984, O'Donnell 1993, Samuels et al. 2009). Therefore, it remained classified in Booth's broadly delimited concept of “Nectria”, although its perithecia and macroconidial Fusarium anamorph are morphologically dissimilar to species of Nectria sensu stricto (Hirooka et al. 2011). The second species, “N.” desmazieri, was placed in the N. episphaeria species group by Booth (1959), but was not accepted as a species of Nectria subgenus Dialonectria by Samuels et al. (1991), nor was it transferred to Cosmospora by Rossman et al. (1999). Nirenberg & Samuels (2000) compared the third species, the plant-associated “N.” zealandica, with the scale insect pathogens now classified by Gräfenhan et al. (2011) as Microcera diploa and M. flammea. This might have suggested reclassification in Cosmospora sensu Rossman et al. (1999), but it has perithecia in caespitose clusters on well-developed stromata, atypical for Cosmospora.
The anamorphs of these three species are Fusarium-like and some anamorphic names have been proposed for them. Deviating descriptions and concepts exist for Fusarium buxicola, considered the anamorph of “N.” desmazieri by Wollenweber & Reinking (1935), Booth (1959, 1971), and Gerlach & Nirenberg (1982). Saccardo (1883) had proposed F. buxicola as the anamorph of an additional bluish-black perithecial species, `Gibbera' buxi, and the subsequent association with the orange or brownish orange perithecial species “N.”. desmazieri is mysterious. Wollenweber & Reinking (1935) classified F. buxicola and “N.” desmazieri in Nectria section Macroconia, which Wollenweber (1926) erected for “Nectria” stilbosporae, “N.” leptosphaeriae and “N.” aurantiicola, currently classified as “Fusarium” expansum, Macroconia leptosphaeriae and Microcera larvarum by Gräfenhan et al. (2011).
Our study began with newly obtained collections of nectrioid fungi on species of Buxus and Celtis in Europe and North America. This led us to revise the taxonomy of the “N.” desmazieri species group, a monophyletic clade within the terminal Fusarium clade according to phylogenetic analyses (Samuels et al. 2009, Gräfenhan et al. 2011). We describe this clade as a new genus Geejayessia, with the former N. cicatricum as its type species, and including the former “N.” atrofusca, “N.” desmazieri, “N.” zealandica, and a new species collected on Celtis occidentalis, G. celtidicola; henceforth in this paper the Geejayessia names are used. The morphological and anatomical characters of the teleomorphs are compared with those of other teleomorphs in the terminal Fusarium clade. Specifically, perithecial wall layers and surface roughening are analysed, characters that were used, for example, when Booth (1959) placed G. desmazieri in the N. episphaeria species group.
Following the arguments of Gräfenhan et al. (2011) and similar opinions of others (Seifert & Samuels 2000, Cannon & Kirk 2000, Rossman & Samuels 2005), we have adopted a single name nomenclatural system in this paper. None of the species recognised here are solely anamorphic, and the oldest available species epithets are all teleomorphic. Therefore, all of the binomials adopted for species in this paper are valid, legitimate, and nomenclaturally correct according to the present International Code of Botanical Nomenclature (McNeill et al. 2006). We consider the available Fusarium binominals as synonyms of the names in Cyanonectria and the newly described genus Geejayessia and its anamorphs as Fusarium-like, and not part of our taxonomic concept of Fusarium sensu stricto.
MATERIALS AND METHODS
Specimens and strains
Dried reference specimens were obtained from the herbaria BPI, DAOM, G, K, M, and W. Herbarium abbreviations are from Holmgren et al. (1990). Cultures were obtained from the culure collections at the CBS Fungal Biodiversity Centre (CBS, Utrecht, the Netherlands), Eastern Cereal and Oilseed Research Centre (DAOM, Ottawa, Canada), and the Julius Kühn-Institute, Institute for Epidemiology and Pathogen Diagnostics (BBA, Berlin & Braunschweig, Germany).
Dead or decaying twigs attached to healthy Celtis occidentalis trees, Buxus sempervirens bushes, or detached twigs found below these trees, were examined for nectriaceous teleomorphs. Perithecia and supporting substrate were removed from specimens, rehydrated in water, embedded in Tissue Tek 4583 O.C.T.™, sectioned at –20 °C and 6–16 μm thickness using a Leica Cryotome CM 1850, and mounted in Shear's fluid (Gams et al. 1998). Microscopic structures such as conidia, phialides, asci, ascospores, details of stromata and walls of perithecia, etc. were studied with a Zeiss Imager microscope using differential interference contrast and luminance coded with 0.45 or, rarely, 1.0 gamma correction in the Zeiss AxioVision software v. 4.6, or an Olympus BX50 compound microscope. Anamorphic structures were studied in water, and teleomorphic structures in water, Shear's, 2 % KOH or 85–90 % lactic acid. Other methods for the study of micro- or macroscopical characters of strains including morphometrical analyses are described elsewhere (Schroers et al. 2009).
For ascospore isolates, single perithecia were squashed in a drop of sterile water. The resulting ascospore suspension was collected either with a 1 mL propipettor or a glass pasteur pipette with its tip sterilised and extended using an alcohol flame; the suspension was spread by moving the pipette over the surface of synthetic nutrient-poor agar (SNA; Nirenberg 1976) with penicillin and streptomycin (Gams et al. 1998). The next day isolated germinating ascospores were located under a compound microscope at low magnification or a dissecting microscope at high magnification and transferred to fresh media. For mycelial or conidial specimens, cultures were isolated by plating a piece of Buxus root surface sterilised with 70 % ethanol onto potato dextrose agar (PDA, Biolife, Italy) with penicillin and streptomycin or by streaking out macroconidia obtained from sporodochia on decaying Buxus branches.
For taxonomic studies, cultures were studied on agar media in 9 cm vented, plastic Petri dishes. Strains were grown on SNA with small pieces of sterile carnation leaves (CL) or Buxus leaves or twigs on the surface (SNA/CL or SNA/B). Growth rates were determined after 7 d on PDA (Difco, USA) incubated at 15, 20, 25, 30, and 35 °C. Colony colours were scored on the same medium after 14 d or later using Kornerup & Wanscher (1978). For the preparation of voucher material, cultures were first dried in a Christ LMC-2 lyophiliser and then killed with formalin as described by Gams et al. (1998).
The species included in the genus-level phylogenetic evaluation of the terminal Fusarium clade (Fig. 1) were Nectria cf. cinnabarina, USA, Pennsylvania, Salt Springs State Park, on Fagus grandifolia, T. Gräfenhan, May 2007, TG 2007-62, DAOM [HQ728160 (rpb2), HQ728179 (acl1)] (outgroup); Nalanthamala diospyri, USA, Tennessee, Readyville, wood of Diospyros virginiana, M.J. Wingfield, CBS 560.89 [HQ728156 (rpb2), HQ728175 (acl1)]; Fusarium avenaceum, Germany, north Germany, Solanum tuberosum, tuber, E. Langerfeld, August 1980, BBA 64151 [DNA barcodes: HQ728167 (rpb2), HQ728186 (acl1)]; Fusarium babinda, Spain, Morga, Pinus radiata/Hylurgops palliates, P. Romòn, CBS 122156 [HQ728168 (rpb2), HQ728187 (acl1)]; Fusarium compactum, Sudan, seed of Gossypium barbadense, G. Ibrahim, June 1989, BBA 65671 [HQ728165 (rpb2), HQ728184 (acl1)]; Fusarium graminum, Iran, Prov. Mazandaran, near Babol, Claviceps, on ear of Paspalum dilatatum, W. Gerlach & D. Ershad, October 1968, BBA 62226 [HQ728166 (rpb2), HQ728185 (acl1)]; Microcera larvarum, Iran, Prov. Guilan, near Rasht, Quadraspidiotus perniciosus, on living on branch of Prunus, W. Gerlach & D. Ershad, October 1968, BBA 62239 [HQ728163 (rpb2), HQ728182 (acl1)]; Microcera coccophila, New Zealand, Croesus Track, tree bark, H.I. Nirenberg, June 1991, BBA 65849 [HQ728158 (rpb2), HQ728177 (acl1)]; Macroconia leptosphaeriae, Netherlands, Tilburg, on Leptosphaeria sp./dead stem of Urtica dioica, L. Rommelaars, as “Fusarium sphaeriae`, CBS 100001 [HQ728164 (rpb2), HQ728183 (acl1)]; “Fusarium” melanochlorum, Austria, on branch canker of Fagus sylvatica, W. Gerlach, CBS 202.65 (= ATCC 16069, BBA 9831, DSM 62248) [HQ728162 (rpb2), HQ728181 (acl1)]; Cosmospora coccinea, Germany, Neubrandenburg, Kleppelshager Forst near Friedland, on Inonotus radiatus, P. Hübsch, 22 Oct 1978, CBS 704.79 [HQ728161 (rpb2), HQ728180 (acl1)]; Neonectria coccinea, Germany, Brandenburg, Stolpe, Fagus sylvatica, T. Gräfenhan, March 2007, TG 2007-24, DAOM [HQ728159 (rpb2), HQ728178 (acl1)]; Neonectria fuckeliana, Switzerland, KT. Graubunden, vic. Zuoz, along Ova d`Arpiglia, on branches of Picea sp., 6 Sep 1990, CBS 112466 (= IMI 342667) [HQ728157 (rpb2), HQ728176 (acl1)]; Volutella consors, India, Karnataka, Agumbe, on Agave americana, V. Rao, Oct 1985, CBS 130.86 [HQ728155 (rpb2), HQ728174 (acl1)]; Volutella consors, Brazil; Pará, 200 km SE from Belém, Capitão Poço, soil, L. Pfenning, CBS 549.89 [HQ728154 (rpb2), HQ728173 (acl1)]. Gräfenhan et al. (2011) list strain data and GenBank accession numbers for “Haematonectria” illudens (BBA 67606), Albonectria albosuccinea (BBA 64502), A. rigidiuscula (CBS 122570), Fusarium sambucinum (BBA 70569), F. sublunatum (BBA 62431), “Nectria” albida (BBA 67603), “Fusarium” ventricosum (CBS 748.79), Thelonectria discophora (CBS 125487), and Atractium crassum (CBS 180.31)].
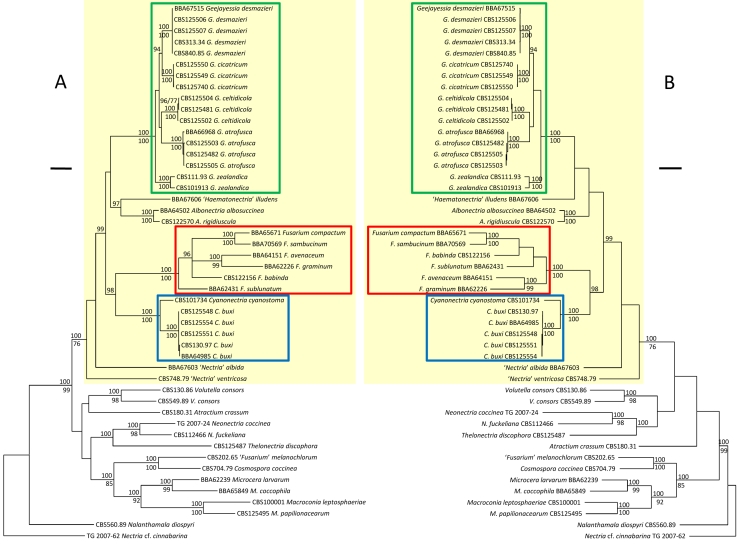
Fig. 1.
Phylogrammes showing generic relationships in the terminal Fusarium clade inferred from partial sequences of the second largest subunit of the RNA polymerase II and the larger subunit of ATP citrate lyase gene exons using Nectria cf. cinnabarina as outgroup. A. Majority rule consensus tree of a Bayesian Markov chain Monte Carlo sampling. B. One of 145 equally parsimonious trees. Numbers above branches are Bayesian posterior probabilities multiplied by 100 (p.p. > 90 are shown); those below lines are parsimony bootstrap proportions (> 70 % are shown). Fusarium sensu stricto is demarcated by a red frame, Geejayessia by green, Cyanonectria by blue, and the terminal Fusarium clade by yellow. Scale bars: A 0.05 substitutions per site, B 50 steps.
The sequences HM068357 (rpb2, NRRL 36148, O'Donnell et al., unpubl. data, as “Nectria desmazieri”), EU329502 (rpb2, NRRL 22316, O`Donnell et al. 2008), and AF178361 (tef-1α, NRRL 22316, O'Donnell et al., unpubl. data) were included in the three gene analysis for testing phylogenetic species boundaries (Fig. 2B, C).
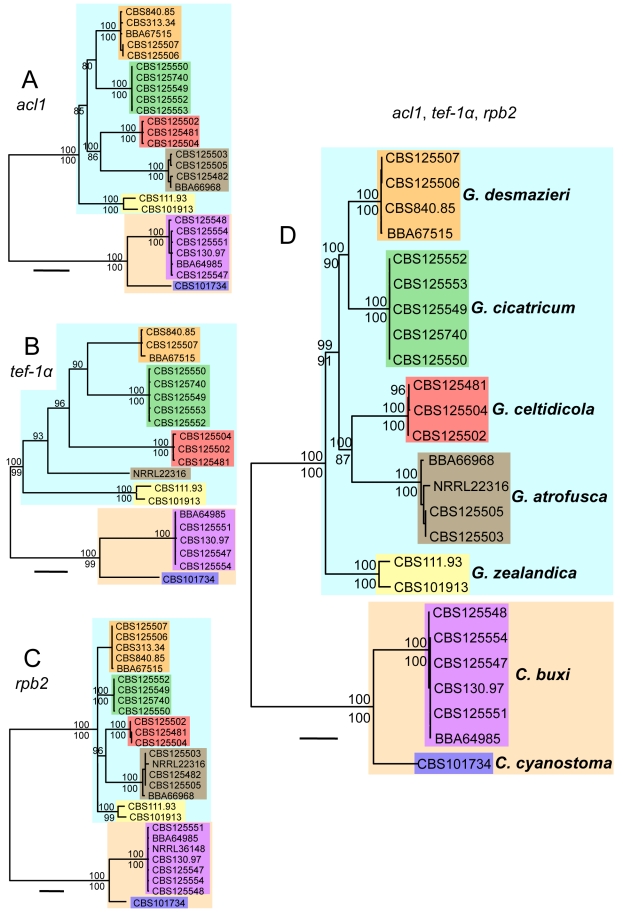
Fig. 2.
Phylogrammes showing individual gene phylogenies and combined phylogeny of species of Geejayessia and Cyanonectria. Majority rule consensus trees of Bayesian Markov chain Monte Carlo sampling inferred from introns and exons of the ATP citrate lyase (acl1, A), translation elongation factor 1 alpha (tef-1α, B), and the second largest subunit of the RNA polymerase II (rpb2, C). D. Phylogramme based on the combined data sets of the three genes. Numbers above branches are Bayesian posterior probabilities multiplied by 100 (p.p. > 90 are shown). Numbers below the branches are parsimony bootstrap proportions (> 70 % are shown). Scale bars: 0.04 substitutions per site.
DNA sequencing
Following the methods of Gräfenhan et al. (2011), partial sequences of the second largest subunit of the RNA polymerase II (rpb2) flanked by the primers 5F2/7cR (O'Donnell et al. 2007) and the larger subunit of ATP citrate lyase (acl1) were generated for strains not included in that study. In addition, sequences of the internal transcribed spacer regions 1 and 2 and the 5.8S nuclear ribosomal DNA (ITS rDNA), partial nuclear ribosomal large subunit DNA (LSU rDNA), and the partial nuclear translation elongation factor 1-alpha (tef-1α), were sequenced following published protocols (Schroers et al. 2005, 2009). For tef-1α, we used an initial denaturation step at 94 °C for 3 min, 35 cycles of 94 °C for 60 s, 54 °C for 60 s, 72 °C for 90 s and a final extension at 72 °C for 6 min. Sequencing reactions were performed at the Macrogen sequencing facility (Seoul, Korea). Newly generated sequences were deposited at GenBank under accession numbers HM626622–HM626690 and HQ728144–HQ728187.
Phylogenetic analyses
Two data sets were assembled. The first data set combined sequences of rpb2 (948 bp alignment) and the exon regions of the acl1 (477 bp alignment) to address the generic relationships of the terminal Fusarium clade (Fig. 1A, B). The second analysis included rpb2, acl1, and tef-1α gene exons and introns of multiple strains of each species to evalute species boundaries by geneological concordance.
Bayesian phylogeny (BP) inferences with MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003) implemented substitution models selected according to the akaike information criterion calculated with the software jmodeltest based on 24 models (Guindon & Gascuel 2003, Posada 2008). The software MrBayes v. 3.1.2 was run for 10 M generations with four Markov chains sampled every 100 generations starting from a randomly selected tree. A 50 % majority rule consensus tree and posterior probabilities for each split was calculated after excluding the first 25000 sampled trees. In analyses of combined data (Figs 1A, 2D), stationary nucleotide frequencies, relative rates of substitution, alpha shape parameter of the gamma distribution, and the proportion of invariable sites was estimated in MrBayes independently for each of the partitions, and the site specific rates were set variable. Trees illustrating the relationships of the species (Fig. 2A–D) were rooted at the longest split using the tools provided in the software MEGA (Tamura et al. 2007) after they were inspected unrooted using the software TreeView (Page 1996).
Heuristic searches for shortest trees in parsimony analyses (PA) generated with PAUP v. 4.0b10 (Swofford 2003) were based on parsimony informative, unordered, and equally weighted characters; gaps were treated as missing data. Starting trees were obtained with 1 000 (Fig. 1B analyses) or 1 0000 (Fig. 2 analyses) stepwise, random addition sequences. Other settings included the treebisection-reconnection branch-swapping algorithm and the MULTREES option. Branch robustness was assessed by 1000 heuristic bootstrap replicates using the same settings, but with 10 stepwise, random addition sequences. A MAXTREE setting of 1 000 was effective for bootstrap analyses of the combined data sets summarised in Fig. 1B and Fig. 2D and the analyses of the rpb2 gene (Fig. 2C). Either fewer than 1000 trees were collected or an automatically increased MAXTREE setting was adopted for the other parsimony analyses.
For the second set of analyses, the phylogenetic relationships of strains identified as Cyanonectria cyanostoma, `Fusarium buxicola', F. staphyleae, G. atrofusca, G. desmazieri, G. zealandica, and others isolated from Buxus sempervirens or Celtis occidentalis were estimated from the aligned DNA sequences of the individual genes (Fig. 2A–C). The combined data sets (Fig. 2D) comprised the acl1 (915 bp alignment) and tef-1α (751 bp) gene exons and introns and the rpb2 gene fragment (1033 bp alignment). Strain data and sequence accession numbers are listed in the Taxonomy section below. Sequences of the ITS and LSU rDNA were generated only for representatives of the ingroup taxa and are cited in the taxonomic part below as DNA barcodes. Phylogenetic analyses based on these sequences (not shown) were consistent with the inferences summarised in Fig. 2. They also included AF178423 and AF178392 (ITS and LSU, NRRL 22316; O'Donnell et al., unpublished), U88116 (LSU, NRRL 20428; O'Donnell 1993) and U88125 (LSU, NRRL 20474; O'Donnell 1993).
RESULTS
A General Time Reversible plus Gamma model and gamma distribution of rate variation with a proportion of invariable sites (GTR+G+I) was selected for each of the individual data sets of the 48 taxon analyses (Fig. 1A). The proportion of invariable sites was 0.4170 in the rpb2 gene (acl1: 0.5510). The shape parameter of the gamma distribution was 0.9300 (rpb2) and 1.4020 (acl1) across sites. In modeltest analyses, basefrequencies were calculated as 0.2456, 0.2658, 0.2599, 0.2288 for A, C, G, T, respectively (rpb2) and 0.2098, 0.3162, 0.2601, 0.2139 (acl1); substitution rates were AC = 1.3301, AG = 3.6324, AT = 1.2458, CG = 0.6151, CT = 7.6227, GT = 1.000 (rpb2) and 1.1272, 2.6353, 0.4595, 1.1210, 10.9535, 1.0000 (acl1). The most negative likelihood (–lnL) score was –15.758.620 for the combined analysis. The overall topologies of the 48 equally most parsimonious trees did not differ significantly from each other. Based on 590 parsimony-informative characters (PIC), they were 3 330 steps in length and had a consistency index (CI) of 0.305 and a retention index (RI) of 0.620.
Based on the partial rpb2 and acl1 loci, phylogenetic analyses identified a statistically moderately or strongly supported clade [Bayesian posterior probability (B-PP), 1.00; maximum parsimony bootstrap proportion (P-BP), 76 %], here called the terminal Fusarium clade. This included various subclades, most corresponding with previously identified holomorph genera or other taxonomic groups with Fusarium-like anamorphs (Fig. 1A, B). The parallel analyses by Gräfenhan et al. (2011) obtained no significant statistical support for the terminal Fusarium clade, but showed that taxa with Fusarium-like macroconidia cannot be regarded monophyletic. In all analyses, the terminal Fusarium clade excludes phylogenetically distantly related Fusarium-like species, most of which are currently classified in Dialonectria, Fusicolla, Macroconia, Microcera, or Stylonectria (Gräfenhan et al. 2011) or as “Nectria” diminuta (Hirooka et al. 2008). Equivocally strong statistical support B-PP, 1.00 and P-BP, 100 % (Fig. 1A, B) or maximum likelihood bootstrap proportions ≥ 75 %, B-PP ≥ 0.95, and P-BP ≥ 75 % (Gräfenhan et al. 2011: fig. 1), was obtained for the subclades nested within the terminal Fusarium clade. These include (i) Fusarium sensu stricto including but not restricted to species with teleomorphs often classified in Gibberella and various species groups (Summerbell & Schroers 2002, O'Donnell et al. 2007, Schroers et al. 2009, O'Donnell et al. 2010), (ii) “Haematonectria” mostly with Fusarium solani like anamorphs (Rossman et al. 1999, O'Donnell 2000, O'Donnell et al. 2008), (iii) Albonectria (Rossman et al. 1999), (vi) the “Fusarium” dimerum species group (Schroers et al. 2009) and “Fusarium” domesticum (anamorphic Rodentomyces Doveri et al. 2010), (vii) Cyanonectria (Samuels et al. 2009, this paper), and (viii) Geejayessia, described below for “N.” desmazieri and its allies.
The genus-level analysis confirmed that several holomorphs in the terminal Fusarium clade previously classified in Nectria (“N.” atrofusca, “N.” cicatricum, “N.” desmazieri and “N.” ventricosa) are distantly related to Nectria sensu stricto. The species of the Fusarium section Macroconia sensu Wollenweber & Reinking (1935) or Gerlach & Nirenberg (1982) belong either to Cyanonectria (as “Fusarium buxicola”) and Geejayessia or to the distantly related genera Microcera (M. coccophila) or Macroconia (M. leptosphaeriae and M. gigas).
The species-level phylogenetic analyses based on introns and exons of the individual (Fig. 2A–C) and combined (Fig. 2D) acl1, tef1 and rpb2 genes were based on a Hasegawa-Kishino-Yano plus Gamma (HKY+G) (acl1) or a General Time Reversible plus Gamma substitution model (GTR+G) (tef1, rpb2) with a proportion of invariable sides set to 0 for all. The shape parameter of the gamma distribution was 0.6150 (acl1), 0.4030 (tef1) and 0.2390 (rpb2) across sites. In modeltest analyses, basefrequencies were calculated as 0.2054, 0.2795, 0.2527, 0.2624 for A, C, G, T, respectively, (acl1), 0.2251, 0.3004, 0.2293, 0.2452 (tef1) and 0.2521, 0.2637, 0.2630, 0.2213 (rpb2); for acl1 a kappa = 5.0494 (ti/tv = 2.5475) was calculated; substitution rates were AC = 0.9777, AG = 1.8456, AT = 1.0869, CG = 0.5516, CT = 3.6992, GT = 1.0000 (tef1) and 0.9601, 3.2151, 0.8718, 0.4166, 7.8738, 1.0000 (rpb2). The most negative likelihood (–lnL) score was –9239.02 for the combined analysis and –3138.52, –2.957.743 and –3.140.872 for the individual data sets of the acl1, tef1 and rpb2, respectively. Parsimony analyses yielded 18240 equally most parsimonious trees 1 083 steps long with a CI of 0.814 and a RI of 0.943 and were based on 711 PIC (Fig. 2D). The following tree scores were retrieved when acl1 (256 PIC), tef1 (223 PIC) and rpb2 (232 PIC) sequences were analysed individually: CI, 0.861, 0.773, 0.822; (RI) 0.965, 0.911, 0.959; number of steps, 353, 388, 338; number of equally parsimonious trees, 156, 28, 28 098. The inferences provided evidence for close relationships among Geejayessia desmazieri, G. cicatricum, G. celtidicola, and G. atrofusca with G. zealandica forming the root of the genus Geejayessia. The strain NRRL 36148 was re-identified as Cyanonectria buxi (Fig. 2C, based on rpb2 sequences).
Analyses of aligned LSU- and ITS rDNA sequences obtained in our study (results not shown) confirmed the equally rDNA based conclusions of Samuels et al. (2009), which showed Geejayessia and Cyanonectria as distinct phylogenetic lineages within the terminal Fusarium clade. The phylogenetic analyses by Samuels et al. (2009) placed Cyanonectria cyanostoma in a moderately supported sister group relationship with Fusarium sensu stricto. According to rDNA based comparisons, we confirm the identity of NRRL 20474 (GenBank U88125) as G. desmazieri, and NRRL 22316 (AF178392), used in phylogenetic analyses by O'Donnell (1993) and Samuels et al. (2009), as G. atrofusca.
Ten to 15 nucleotide substitutions or indels in the ITS rDNA distinguish the new species G. celtidicola from G. zealandica, and the species pair G. desmazieri and G. cicatricum from each other. Geejayessia desmazieri differs from G. cicatricum by 2 substitutions in the ITS rDNA. The ITS rDNA of G. atrofusa differs from that of the other species by 29–33 substitutions or indels.
TAXONOMY
Cyanonectria Samuels & Chaverri, Mycol. Progress 8: 56. 2009.
Anamorph: Fusarium-like
Type species: Cyanonectria cyanostoma (Sacc. & Flageolet) Samuels & Chaverri, Mycol Progress 8: 56. 2009. Basionym: Nectria cyanostoma Sacc. & Flageolet, Rendi Congr. Bot. Palermo 1902: 53. 1902.
Stromata reduced, minute or more or less well developed, prosenchymatous, typically consisting of hypha-like cells. Perithecia gregarious or caespitose, smooth, thin-walled, unevenly coloured, apex darkly pigmented, dark bluish purple or bluish black, main body less intensely dark bluish or red to reddish brown; colours in KOH becoming darker, in lactic acid changing from bluish black to red or from red or reddish brown to yellow. Ascospores 1-septate, ellipsoidal with gently tapering ends, more or less hyaline or pale yellow brown, smooth. Macroconidia (1–)5–7(–8)-septate, gently curved throughout or with a subcylindrical central middle part, pedicellate, with a hooked apical cell; formed in off-white, cream slimy masses, sometimes on sporodochia on branched conidiophores, terminating in whorls of monophialides. Microconidia not observed. Chlamydospores absent or rarely formed in cells of aging macroconidia. Cultures on PDA in C. cyanostoma pale coloured, cream, or somewhat yellowish but at 30 °C with somewhat greyish blue surface or, in C. buxi, dark brown, reddish brown, greenish grey, with a greyish blue, pastel violet or light blue surface.
Notes: When describing the monotypic genus, Samuels et al. (2009) restricted Cyanonectria for a species with spectacularly bicoloured perithecia characterised by a bluish purple papilla and a red perithecial body. The anamorphic characters were narrowly defined in their genus concept. For example, unpigmented, white colonies on SNA and PDA were described. With Gibbera buxi and its Fusarium buxicola anamorph a unicoloured, bluish black or bluish purple perithecial species forming surprisingly dark colonies on PDA is added to the genus necessitating an emended generic concept for Cyanonectria.
Cyanonectria buxi (Fuckel) Schroers, Gräfenhan & Seifert, comb. nov. MycoBank MB519485. Figs 3, 4. Basionym: Gibbera buxi Fuckel, Jahrb. Nassauischen Vereins Naturk. 27–28: 32. 1873.
Fig. 3.
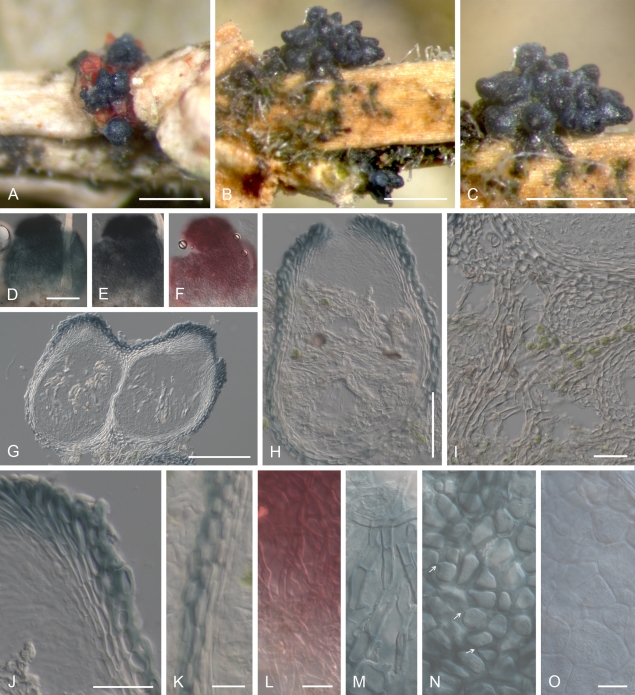
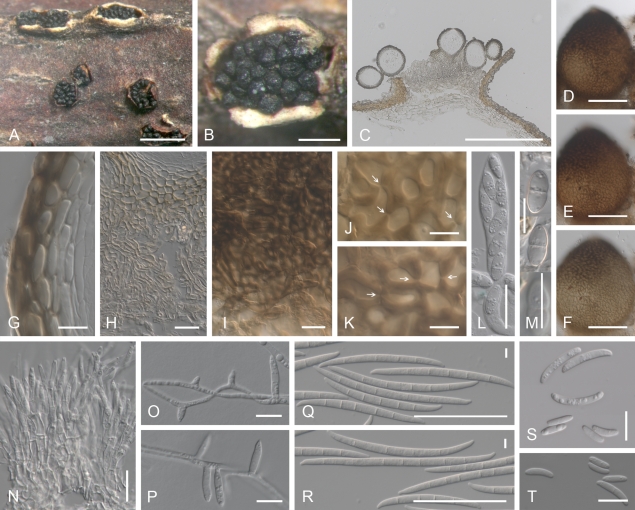
Cyanonectria buxi, perithecia on the natural substrate. A–C. Habit on Buxus twigs. D–F. Colour change in perithecium in water (D), replaced with 2 % KOH (E) and then lactic acid (F), showing the reduced reaction towards the base of perithecium (E, F). G, H, J, K. Median longitudinal section through perithecia (G, H), ostiolar (J) and lateral perithecial walls (K). I. Longitudinal section through hyphal stroma supporting perithecia. L–O. Face view of perithecial wall. L. Hyphae covering perithecia reacting to lactic acid in a similar manner as the perithecium. M. Hypha-like cells on the surface of perithecia. N. Outermost cells of the main perithecial wall region with Samuels pores. O. Innermost cells of the perithecial wall. G–K in Shears; L in 2 % KOH; M–O in water. A, D–F, L–O CBS H-20380; B, C, G–K CBS H-20379. Scale bars: A–C = 500 μm; D (also applies to E, F), G = 100 μm; H = 50 μm; I–L 20 = μm; O (M, N) 10 = μm.
Fig. 4.
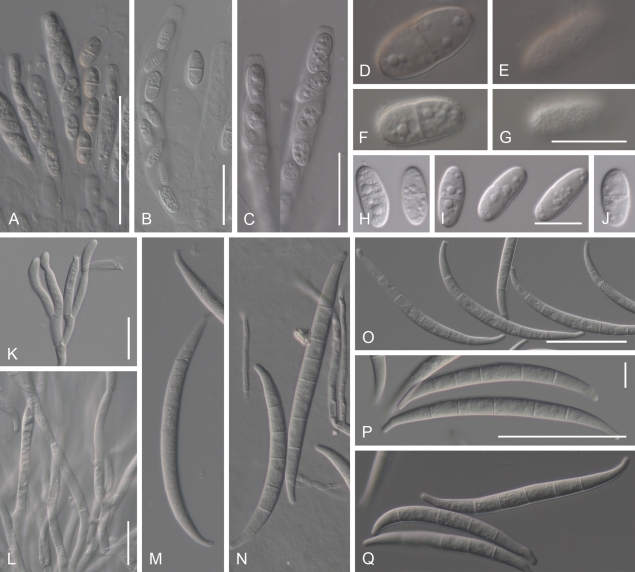
Cyanonectria buxi, spores and spore forming cells. A–D. Asci with a somewhat flattened apex, with or without a visible refractive ring. E, G, I–P. Ascospores. F, H. Ascospore surface. Q–S. Monophialides formed by immersed mycelium. T. Sporodochium. U–Y. Macroconidia. Z. Chlamydospores derived from macroconidium. AA–AC. Surface of PDA colonies after 14 d at 20, 25 and 30 °C. AD. Reverse of colony illustrated in AB. A–P CBS H-20379; Q–S. CBS 130.97; T, Y CBS 109638; U CBS 125554; V CBS 125551; W CBS 30.97; X, AA–AD BBA 64985; Z CBS 125547. A–P, from natural substrate. Q–Z, from SNA/B. Scale bars: B (also applies to A), X (W, Y) 50 = μm; C (D), Q (R, S), T 20 = μm; G (E, F, H), I (J–P), Z 10 = μm; U (V) = 50 and 10 μm; AA–AD = 10 mm.
≡ Gibberella buxi (Fuckel) G. Winter, Rabenh. Krypt.-Fl. 2: 103. 1887.
≡ Lisea buxi (Fuckel) Sacc., Syll. Fung. 2: 518. 1883.
= Fusarium buxicola Sacc., Syll. Fung. 2: 518. 1883.
Stromata prosenchymatous, cells 3–5 μm wide, with at least some hypha-like cells, arranged in an irregular textura porrecta. Perithecia solitary or in groups of 20 or more seated on a stroma formed on bark of small twigs, leaf or terminal twig axils; smooth; broadly ampulliform to obpyriform, with a short neck or broadly ellipsoidal; dark bluish purple or bluish black, main body less intensely dark, not red, somewhat darker blue in 2 % KOH, purplish red in lactic acid; in longitudinal section 200–250 μm high, 130–150 μm wide. Hyphae continuous with cells of stroma continous with wall of lower part of perithecia, 4–6(–8) μm wide, with walls to 2 μm thick. Perithecial wall of a single region, 15–20 μm wide or subapically 20–35 μm, consisting of ca. 3 layers of cells; in face view, cell walls of outer and inner layers with pores, 1–1.5 μm wide in outer layers, 0.5 μm thick or less in inner layers; cells in outer layers angular, (8–)11(–14) × (6.5–)8(–9.5) μm, arranged in a textura angularis, in inner layers subglobose to angular, (10–) 14(–22) × (5.5–)10.5(–14.5) μm, arranged in a textura angularis; cells in longitudinal sections subglobose to angular, flatter towards centrum. Asci cylindrical or narrowly clavate, with rounded or flattened apex, with or without visible refractive ring, eight-spored, with mostly overlapping uniseriate or somewhat biseriate above and uniseriate ascospores below, 80–100 × 9–12 μm. Ascospores equally 2-celled, rarely 2-septate, ellipsoidal with somewhat tapering ends, smooth, unpigmented, (12–)13–14–14.5(–17) × (4–)5–5–5.5(–6.5) μm.
Colonies on PDA after 7 d around 12–16 mm diam (20 °C) or 15–20 mm (25 °C); optimum 20–25 °C, maximum between 30 and 34 °C, no growth observed at 35 °C. Colony reverse at 15 °C, 14–21 d on PDA reddish brown to somewhat dark brown (8E7–8F7) or brownish to greenish grey (8F2, 30F2) with or without a reddish brown (8E7) pigment visible outside margin, at 20–25 °C dark green or greyish green (25F4) to brownish black (8F6) or brownish to greenish grey (8F2, 30F2), typically without pigment visible outside margin. Colony surface on PDA with felt-like to cottony mycelium, greyish green to greyish blue or pastel violet (19A4) to light blue (20A5), with or without small or large watery droplets of exudates, with or without off-white sporodochial masses of conidia; on SNA unpigmented or, in older colonies, greenish grey (25B2–25C2), surface smooth or with fine cottony mycelium, greyish blue in centre of colony, with concentrically arranged pale yellow, off-white or somewhat greyish blue, to 5 mm diam conidial masses. Aerial and submersed mycelium and hyphae of sporodochia becoming purplish red in lactic acid. Conidiation on SNA along submersed hyphae or from sporodochia forming within 14 d or later on surface of SNA or on pieces of carnation leaves or Buxus twigs placed on SNA; submersed sporulation by solitary monophialides or on sparsely branched conidiophores. Monophialides cylindrical, 14–21 μm long, 2.5–3.5 μm wide at base, ca. 2.5 μm near aperture; sporodochia of branched conidiophores with solitary or whorls of 2–3 terminal monophialides; base of older sporodochia bluish; phialides of sporodochia cylindrical or bottle-shaped, (9–)15.5–17.5–19.5(–23) μm long, (2.5–)3–3–3.5(–4) μm wide at base, (3–)3.5–4–4(–4.5) μm in middle, (2–)2–2.5–2.5(–3) μm wide near conidiogenous aperture. Microconidia not observed. Macroconidia formed in off-white or pale yellow or somewhat greyish blue slimy masses, typically with central and basal part nearly straight, rarely gently curved throughout, with a more or less pronounced pedicellate foot cell and an inequilateral fusoid or hooked apical cell, (1–4)5–7(–8) septate: 5-septate (46–) 77.5–82–87(–99.5) × (5.5–)6.5–7–7(–8) μm, 6-septate (77.5–)83–87–90.5(–100) × (6–)7–7–7.5(–8) μm, 7-septate 86.5–101 × 6.5–8 μm. Chlamydospores formed from cells of macroconidia, subglobose, 6–11 × 6–8 μm; mycelial chlamydospores not observed.
Characters of holotype, G 00111019, and isotype, G 00111020, of G. buxi, identical to details reported above except as follows: Perithecia turning brownish in KOH, only weakly reddish brown or reddish in lactic acid. Immature ascal ascospores 1-septate. Macroconidia associated with perithecial clusters, 5-septate 70–76.6 × 6.5 μm, 6-septate 75 × 6.5 μm.
Habitat: On decaying or dead terminal twigs still attached to living Buxus sempervirens trees; perithecia sometimes co-occurring with those of G. cicatricum (Figs 3A, 5B).
Fig. 5.
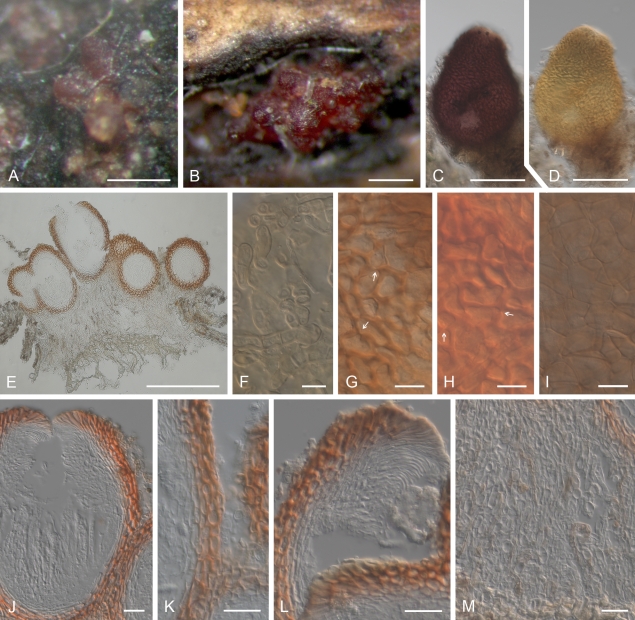
Geejayessia cicatricum, perithecia on the natural substrate. A–C. Habit on decaying buds of Buxus sempervirens. D–F. Colour change in perithecium in water (D), replaced with 2 % KOH (E) and then lactic acid (F). G, H. Longitudinal section through decaying bud. I. Longitudinal section through stroma supporting the perithecia, with hypha-like cells. J–L. Median longitudinal sections through perithecia (J), ostiolar region (K) and lateral perithecial wall (L). M–O. Face views of perithecial wall. M. Cells on the surface of perithecia having hyphal or setose characteristics. N. Outermost cells of the main perithecial wall region with Samuels pores. O. Innermost cells of perithecial wall. C rehydrated in water, G–L in Shears, M, O in water, N in 2 % KOH. A, C, G–L, CBS H-20375; B, D–F, CBS H-20376; M–O, CBS H-20377. Scale bars: A–C, G = 500 μm; E (D, F) = 100 μm; H = 200 μm; I–L = 20 μm; M–O = 10 μm.
Distribution: Europe (Belgium, France, Germany, Slovenia).
Typification: Lectotype of Gibbera buxi and Fusarium buxicola designated here: Germany, Nassau (today, Hesse), Oestrich, K.W.G.L. Fuckel, Herbier Fuckel 1894, G 00111019. Isotypes of Gibbera buxi: DAOM 126623, G 00111020, G00111021, all Herbier Fuckel 1894, Herbier Barbey-Boissier 886. Specimens have sporodochia and clustered, solitary perithecia on a minutely developed stroma; asci of the sampled material are immature and free ascospores were not seen. Epitype for Gibbera buxi designated here: Slovenia, between Domžale and Kamnik, Arboretum Volčji Potok, prealpine zone, on decaying terminal twig still attached to a living Buxus sempervirens var. elegantissima tree, July 2009, H.-J. Schroers 1398 & M. Žerjav, CBS H-20379, filed with dried SNA/B culture of CBS 125551, ex-epitype strain, isolated from ascospore of CBS H-20379.
Additional specimen and strains examined: Belgium, C. Crepel, CBS 109638. France, Dépt. Jura, Bois de la Rochette near Nogna, on leaf litter, 24 Sep 1996, H.-J. Schroers, CBS 130.97. Netherlands, on Buxus sempervirens, 1987, M.E. Noordeloos, BBA 64985. Slovenia, between Domžale and Kamnik, Arboretum Volčji Potok, prealpine zone, on decaying terminal twig with bluish black perithecia still attached to ca. 80 year-old, living Buxus sempervirens tree, July 2009, H.-J. Schroers 1400 & M. Žerjav, CBS H-20380, derived ascospore culture CBS 125554; Ljubljana, nursery, isolated from roots of potted, small bush of Buxus sempervirens, April/March 2009, M. Žerjav 15574, CBS 125548; decaying branch still attached to wilting, small bush of Buxus sempervirens, 2007, H.-J. Schroers, CBS 125547.
DNA sequences generated: ITS rDNA (CBS 125554: HM626660, 125551: HM626661, 125548: HQ728144). LSU rDNA (CBS 125554: HM626672, 125551: HM626673). acl1 (CBS 130.97: HM626622, 125548: HM626623, 125554: HM626629, 125551: HM626630, 125547: HQ728172). tef-1α (CBS 125554: HM626649, 125551: HM626648, 125547: HQ728152, 130.97: HQ728150, BBA 64985: HQ728151). rpb2 (CBS 130.97: HM626690, 125548: HM626687, 125554: HM626688, 125551: HM626689, 125547: HQ728169). See Gräfenhan et al. (2011) for others included in Fig. 1.
Notes: Cyanonectria buxi is characterised by bluish black perithecia that turn somewhat brown in KOH and reddish in lactic acid, 1-septate ascospores, and relatively long, wide macroconidia. Ex-ascospore isolates and several conidial isolates form dark, greyish-blue cultures on PDA. Measurements of macroconidia of C. buxi overlap with those of Geejayessia cicatricum, the latter of which forms pale colonies. The macroconidia are longer than those of G. desmazieri and members of the F. lateritium complex.
Fuckel (1873) described Gibbera buxi as a bluish or violaceous black perithecial fungus with 1-septate ascospores. He considered its anamorph similar and related to that of Nectria gibbera but did not propose anamorph names for either species. Saccardo (1883) accepted G. buxi as distinct and suggested its combination in Lisea in which he placed Gibberella-like species with 1-septate ascospores (see also Rossman et al. 1999). He also described the anamorph of Lisea buxi as Fusarium buxicola, for which he literally copied Fuckel's description of the anamorph of N. gibbera, an act he repeated later for F. fuckelii (Saccardo 1886). Saccardo (1883) clearly attributed the name F. buxicola to Fuckel's bluish black perithecial fungus and referred to the location where Fuckel collected G. buxi. Apparently in error, F. buxicola was later used instead for the anamorph of the orange perithecial G. desmazieri and its synonym Nectria gibbera (Wollenweber & Reinking 1935, Booth 1959, 1971, Gerlach & Nirenberg 1982). Booth (1971) listed Fusarium lateritium var. buxi as the anamorph of the incorrectly cited “Gibberella buxi Fuckel, Symb. Mycol., Nacht. 2: 32, 1873” (apparently confusing Gibberella and Gibbera) but the ascospores of F. lateritium var. buxi reportedly have three septa (Booth 1971).
The genetic and nomenclatural connection between Fusarium buxicola and Gibbera buxi is re-established here, based on recent collections of bluish black, smooth perithecia forming mature asci and 1-septate ascospores (Figs 3A–D, 4A–P). These new specimens exhibit similar characters to those observed on the lectotype and isotypes of G. buxi. The few macroconidia observed associated with the perithecia or stromata in the authentic material are identical to macroconidia formed in cultures of the epitype. Perithecia of G. buxi have an intensely pigmented ostiolar region but their lower parts appear less intensely pigmented (Fig. 3C, D) probably because of relatively thin lateral perithecia walls (Fig. 3G, H, K). Its original material, however, turns somewhat brownish in KOH and only weakly reddish or brownish reddish in lactic acid while our recent gatherings become more intensely bluish black in KOH (Fig. 3E) and bright red in lactic acid (Fig. 3F, L). Perhaps this reflects immaturity of the perithecia on the type specimens of G. buxi, a thought further supported by the fact that no discharged ascospores were visible.
Cyanonectria buxi is well characterised by its greyish blue colonies on PDA, which we observed in all strains. Gerlach & Nirenberg (1982) observed only cream, amber, or fawn to brown and noted blue or verdigris, spotted pigmentation as seldom occurring. It is therefore possible that their concept of F. buxicola was based on a heterogeneous selection of strains, probably including G. cicatricum and G. desmazieri, or that some degeneration had occurred. Cyanonectria buxi forms longer and wider and partly more-septate macroconidia than G. celtidicola and G. desmazieri. Macroconidia of C. buxi and G. cicatricum are similar in size and number of septa.
Cyanonectria buxi has been reported rarely. We collected its teleomorph in July; Fuckel (1873) reported it as very rare and also found perithecia in the summer. Several isolations from conidia or mycelium and one from surface sterilised roots indicate that it is commonly associated with Buxus sempervirens. A surprising observation in our study is that perithecia of C. buxi can apparently co-occur with those of G. cicatricum on what appears to be the same perithecial stroma (Figs 3A, 5B).
Cyanonectria cyanostoma (Sacc. & Flageolet) Samuels & Chaverri, Mycol. Progr. 8: 56. 2009. Basionym: Nectria cyanostoma Sacc. & Flageolet, Atti del Congr. bot. di Palermo: 53. 1902.
Description and illustrations: Samuels et al. (2009).
Material studied: CBS 101734 = BBA 70964, GJS 98-127, ex epitype strain, see Samuels et al. (2009).
DNA sequences generated: LSU rDNA (CBS 101734: HM626671). tef-1α (CBS 101734: HM626647). See Gräfenhan et al. (2011) for others included in Fig. 1.
Geejayessia Schroers, Gräfenhan & Seifert, gen. nov. MycoBank MB519479.
Anamorph: Fusarium-like
Etymology: In honour of Gary J. Samuels, in recognition of his contributions to our knowledge of hypocrealean holomorphs, acknowledging the thousands of specimens and strains he collected and isolated, known universally by their G.J.S. collecting numbers, which he made freely available to his many colleagues.
Perithecia e stromate in substratis erumpente exorientia, superficialia dense coarctata, subglobosa, ovoidea vel obpyriformia, superficie levia vel minute verrucosa, coccinea, aurantiaca vel atra, KOH–vel KOH+. Tunica perithecii ex uno strato composite. Asci 8 spori, cylindrici vel clavate. Ascosporae ellipsoideae, uniseptatae, verruculosae ali leviae, hyalinae vel pallide brunneae. Coloniae fere celeriter crescentes, incoloratae, pallide luteae, pallide aurantiacae veil pallide ochraceae; reversum pigmento rubro carens. Mycelium aerium in agaro parcum, albidum. Sporodochia ad superficiem agari SNA, in foliis Dianthi caryophylli vel foliis et ramis Buxi sempervirentis formata. Monophialides sporodochiales plus minusve cylindricae. Microconidia absentia vel praesentia, 0–1 septata, ovoidea vel ellipsoidea, allantoidea vel fusiformia. Macroconidia sporodochialia 3–multi septata, modice curvata vel quasi recta et apicales rostrata et curvata. Chlamydosporae absentes.
Stromata erumpent, byssoid or densely prosenchymatous, typically of densely packed hyphae, bearing either perithecia or well-developed sporodochia. Perithecia caespitose on bark of decaying twigs or dead buds of woody hosts, often on dead twigs still attached to living host, mostly smooth, smooth to warted in one species, thin-walled, uniformly coloured or with a darker ostiolar region when dry, pale orange, brownish to reddish orange, bright red or black, reacting to KOH and lactic acid, unless black, then hardly reacting. Ascospores 1-septate, ellipsoidal, with gently tapering or broadly rounded ends, pale brown or yellowish brown, smooth or verruculose at maturity. Macroconidia observed in all species, 3- to multi-septate, relatively long when 3-septate, either gently curved throughout with dorsal wall somewhat more curved or with a subcylindrical middle part, always conspicuously pedicellate, with an inequilaterally fusoid and more or less hooked apical cell; formed in slimy yellowish or orange masses on branched, frequently sporodochial conidiophores, terminating in whorls of monophialides. Microconidia usually absent; when present, then oblong ellipsoidal, gently curved, rounded at both ends or with an asymmetrical hilum. Chlamydospores not seen. Cultures on nutritionally rich media such as PDA about 15–20 mm diam after 7 d at 20–25 °C, pale coloured, cream, yellowish, orange, brownish orange or with some greyish hues.
Type species: Geejayessia cicatricum (Berk.) Schroers, Stud Mycol. 68: 124. 2011.
Geejayessia cicatricum (Berk.) Schroers, comb. nov. MycoBank MB519481. Figs 5, 6. Basionym: Sphaeria sanguinea var. cicatricum Berk., Mag. Zool. Bot. 1: 48. 1837.
Fig. 6.
Geejayessia cicatricum, spores and spore forming cells. A–C. Asci with broadly rounded or slightly flattened apex, with visible refractive ring. D–J. Ascospores, with E, G showing surface roughening. K, L. Branched conidiophores and monophialides from sporodochia with anastomosing cells. M–Q. Macroconidia. A–J, M from natural substrate. K, N–Q from SNA, SNA/CL or SNA/B. L from PDA. A–J, L, CBS H-20374; K, N, P, Q, CBS 125549; M, CBS 125552; O, CBS 125550. Scale bars: A, O 50 = μm, B, C, K, L = 20 μm, G (applies also to D–F), I (H, J) = 10 μm, P (M, N, Q) = 50 and 10 μm.
≡ Nectria cicatricum (Berk.) Tul. & C. Tul., Selecta Fungorum Carpologia: Nectriei- Phacidiei- Pezizei 3: 77. 1865.
Stromata formed within bud leaves or erumpent through substrate, prosenchymatous, cells 3–5 μm wide, with at least some hypha-like cells, arranged in an irregular textura porrecta; hyphae connecting cells of stroma and wall of lower part of perithecia, 2.5–7 μm wide, with walls less than 1 μm thick. Perithecia crowded in groups of 5 to > 50, smooth, broadly ampulliform with a short neck or broadly ellipsoidal, bright red with concolourous ostiolar region, deep violet in 2 % KOH, yellowish orange in lactic acid; in longitudinal section 160–260 μm high, 125–250 μm wide. Perithecial wall with a single region, (12–)13.5–18(–21) μm wide or, subapically 20–30 μm wide, consisting of 3–5 layers of cells; in face view, cell walls 1–1.5 μm thick in outer layers, 0.5 μm thick or less in inner layers, with pores in all layers; cells in outer layers angular to lobed, (9–) 12.5(–18) × (6–)9(–13.5) μm, arranged in a textura epidermoidea or t. angularis, subglobose to angular in inner layers, (10–)16.5(–23.5) × (7.5–)10–11.5(–16) μm, arranged in a textura angularis; in longitudinal section, cells subglobose to angular, narrow towards centrum. Asci cylindrical or clavate, with a broadly rounded or flattened apex, with a minute refractive ring, eight-spored, mostly overlapping uniseriate or biseriate above and uniseriate below, (65.5–)73–92.5(–103) × (8–)10–11(–13.5) μm. Ascospores equally 2-celled, broadly ellipsoidal to ellipsoidal, slightly constricted at septum, verruculose, hyaline or pale brown, (9.5–)11.5–12–13(–14.5) × (4.5–)5.0–5.5–6(–6.5) μm.
Colonies on PDA after 7 d at 20 and 25 °C 15–20 mm diam; optimum for growth 25 °C, maximum 30–34 °C, no growth at 35 °C. Colony reverse lacking red pigments, after 14–21 d on PDA at 15–25 °C with weak pigment production, pale to light yellow (4A3-4A5), at 30 °C somewhat pale orange. Colony surface on PDA with pustules or cushions of white aerial mycelium to 15 mm diam, with scattered sporodochia covered with pale yellow conidial masses, smooth at margin, wax-like, pale yellow (4A2–4A3); on SNA hyaline, typically smooth or occasionally with pustules of white mycelium. Conidiation on SNA inconspicuous, first along submersed hyphae, within 14 d or later from sporodochia formed on the agar surface or on CL or B. Sporodochia with a hymenium of branched conidiophores with solitary phialides or whorls of 2–3 terminal monophialides; metulae anastomosing; cells of stroma densely packed, arranged in an irregular textura porrecta. Phialides more or less cylindrical, tapering towards apex, on SNA (18.5)–22–26.5(–31) μm long, 3–4 μm wide at base and in middle, 2–2.5 μm wide near the conidiogenous aperture; on PDA to 45 μm long, 3–4.5 μm wide at base and 2.5–3.5 μm wide near conidiogenous aperture. Microconidia not observed. Macroconidia formed in pale yellow slimy masses, typically gently curved throughout, less commonly almost straight, with pronounced pedicellate foot cell, and a more or less inequilaterally fusoid, hooked apical cell, (2–)5–7(–8) septate: 5-septate (55–)73–81–92(–107) × (6–)6.5–7–7.5(–8.5) μm; 6-septate (88–)98.5–103–107(–124) × (7–)7.5–7.5–8(–8.5) μm; 7-septate 88–125 × 6.5–9 μm. Chlamydospores not observed.
Habitat: On decaying or dead buds, axils of dead leaves or twigs or sometimes on decaying, subterminal twigs still attached to living Buxus sempervirens trees; perithecia sometimes co-occurring with those of C. buxi (Figs 3A, 5B).
Distribution: Europe (Slovenia, England).
Typification: Isotype of Sphaeria sanguinea var. cicatricum: Sine loco but presumably England based on the name of the publication, on stems of?B. sempervirens, ex herb. M.J. Berkeley, K(M) 160064. Epitype of Sphaeria sanguinea var. cicatricum designated here: Slovenia, between Domžale and Kamnik, Arboretum Volčji Potok, prealpine zone, on dead buds or bark of decaying, terminal twig still attached to ca. 80 year-old, living B. sempervirens tree, July 2009, H.-J. Schroers & M. Žerjav, CBS H-20374, twig with perithecial stromata filed together with dried SNA culture of ex-epitype ascospore isolate CBS 125549.
Additional specimen and strains examined: Same location as the epitype. On dead buds or decaying terminal twig still attached to living B. sempervirens tree, CBS H-20376, ascospore culture CBS 125552; CBS H-20377, ascospore culture CBS 125553; CBS H-203801, ascospore culture CBS 125740; on B. sempervirens var. elegantissima, July 2009, H.-J. Schroers & M. Žerjav, CBS H-20375, ascospore culture CBS 125550.
DNA sequences generated: ITS rDNA (CBS 125553: HM626653, 125550: HM626654, 125740: HM626655, 125552: HQ728145). LSU rDNA (CBS 125553: HM626665, 125550: HM626666, 125740: HM626667). acl1 (CBS 125740: HM626635, 125549: HM626636, 125552: HQ728171, 125553: HQ728170). tef-1α (CBS 125553: HM626645, 125550: HM626642, 125549: HM626643, 125552: HM626644, 125740: HM626646). rpb2 (CBS 125740: HM626680, 125549: HM626679, 125552: HQ728153). See Gräfenhan et al. (2011) for other strains included in Fig. 1.
Notes: The morphological distinctions between G. cicatricum and G. desmazieri are discussed in the notes for G. desmazieri. Based on the collections available including the isotype, G. cicatricum occurs on dead buds specifically on decaying or dead terminal branches of Buxus sempervirens, whereas the majority of G. desmazieri specimens suggest a habitat on thicker, subterminal branches, with perithecia forming on bark. Although the niche of these species may overlap, there are no indications that they co-occur. The width of macroconidia from the type specimen of Sphaeria sanguinea var. cicraticum (K 160064) were wider than macroconidia of G. desmazieri, confirming the usefulness of this character for distinguishing the species.
Geejayessia atrofusca (Schw.) Schroers & Gräfenhan, comb. nov. MycoBank MB519483. Fig. 7. Basionym: Sphaeria atrofusca Schw., Trans. Amer. Philos. Soc. ser. 2. 4: 206. 1832.
Fig. 7.
Geejayessia atrofusca. A, B. Habit of perithecia on twigs of Staphylea trifolia. C, G, H. Longitudinal section through perithecial stroma (C), lateral perithecial wall (G), stroma composed of hypha-like cells (H). D–F. Colour change in perithecium in water (D), replaced with 2 % KOH (E) and then lactic acid (F). I. Hypha-like cells continuous with cells of the stroma covering base of perithecium. J, K. Face view of outermost cells of perithecial wall with Samuels pores (arrows). L. Ascus. M. Ascospores, the bottom one showing surface. N. Sporodochium. O, P. Mononematious, simple conidiophores, phialides. Q, R. Macroconidia. S, T. Aseptate or 1-septate microconidia. A–M, CBS H-20381, from natural substrate; N–T, CBS 125505 ex ascospores of CBS H-20381 on SNA/CL. Scale bars: A = 1 mm; B, C = 500 μm; D–F = 100 μm; G, J, K, M, O, P, S, T = 10 μm; H, I, L, N = 20 μm; Q, R = 50 and 5 μm.
≡ Nectria atrofusca (Schw.) Ellis & Everhart, N. Amer. Pyrenomyc.: 99. 1892.
= Fusarium staphyleae Samuels & Rogerson, Brittonia 36: 84. 1984.
Habitat: On bark of twigs of Staphylea trifolia, associated with twig blight (Samuels & Rogerson 1984).
Description and illustrations: Samuels & Rogerson (1984).
Material studied: Canada, Ontario, Ottawa, Petrie Island, riverine forest, on twig of Staphylea trifolia, Oct. 2006, T. Gräfenhan T.G. 2006-01, DAOM 238118, ascospore isolate CBS 125482; same as above: T.G. 2006-01A, conidial isolate CBS 125503; Nov. 2008, T.G. 2008-34, ascospore isolate CBS 125505. USA, New Jersey, Palisade Interstate Parkway, Staphylea trifolia, C.T. Rogerson 81-53, BBA 66968.
DNA sequences generated: ITS rDNA (CBS 125505: HM626659). LSU rDNA (CBS 125505: HM626674). acl1 (CBS 125503: HM626627, 125505: HM626628, BBA 66968: HM626637). rpb2 (CBS 125503: HM626683, 125505: HM626682, BBA 66968: HM626681). See Gräfenhan et al. (2011) for other strains included in Fig. 1.
Notes: The almost black perithecia and weak reaction of their pigments to KOH are distinctive features of Geejayessia atrofusca. As noted in the discussion of phylogeny, this species does not belong to Gibberella, despite the similar colouration of perithecia on the natural substratum. Geejayessia atrofusca is clearly a member of this new genus based on combined LSU- and ITS analysis (Samuels et al. 2009) and the combined rpb2 and acl1 analysis (Fig. 1). Geejayessia atrofusca forms non-septate or sparsely septate microconidia on SNA. In the other species of Geejayessia, no such microconidia were observed. Samuels & Rogerson (1984) described F. staphyleae from cultures grown from ascospores isolated from G. atrofusca; thus the genetic connection between the teleomorph and anamorph covered by these two names is clear.
Geejayessia celtidicola Gräfenhan & Schroers, sp. nov. MycoBank MB519482. Figs 8, 9.
Fig. 8.
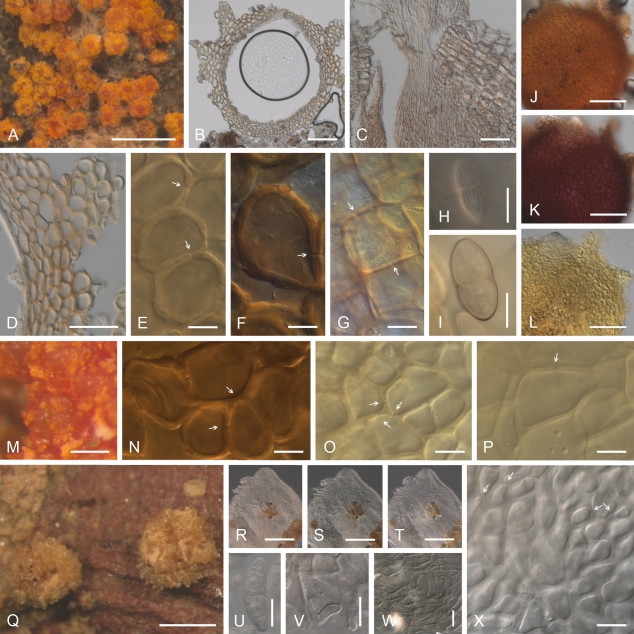
Geejayessia celtidicola, perithecia on the natural substrate, holotype. A, B. Habit on bark of Celtis occidentalis. C, D. Colour change in perithecium in 2 % KOH (C) then replaced by lactic acid (D). E. Longitudinal section of erumpent stroma and perithecia. F–I. Face view of perithecial wall. F. Cells on the surface of perithecia showing hyphal or setose characteristics. G, H. Outermost cells of the main perithecial wall with Samuels pores. I. Intermediate cells of the perithecial wall. J–L. Median longitudinal sections of perithecia or perithecial walls. M. Longitudinal section through stroma supporting the perithecia showing hypha-like cells. B. after rehydration in water; E, H, J–M in Shears; F, G, I in water. Scale bars: A = 500 μm; B, E = 200 μm; C, D = 100 μm; F–I = 10 μm; J–M = 20 μm.
Fig. 9.
Geejayessia celtidicola, spores and spore forming cells. A–C. Asci with rounded apex, lacking refractive ring. D. Ascospore surface. E, F. Ascospores. G. Longitudinal section of sporodochium. H. Hymenial tissue of base of sporodochial stroma. I–K. Monophialides on branched conidiophores from sporodochial hymenium, I, J. With anastomoses. L–P. Macroconidia. A–F. Holotype CBS H-20378; G–K, P CBS 125502; L, M, O, CBS 125504; N, CBS 125481. Scale bars: A, B (applies also to C), N (L, M, O, P) = 50 μm; D (E, F) = 10 μm; G =100 μm; H, J (I, K) = 20 μm.
Etymology: In reference to the substrate of this species, Celtis occidentalis.
Perithecia e stromate in substratis lignosis erumpente exorientia, superficialia vel interdum partim semi-immersa, dense coarctata, ovoidea vel obpyriformia, breviter papillata, levia, intense rubida, ca. 200–250 μm alta, 120–210 μm lata in 2 % KOH purpurascentia, luteo-aurantia in acido lactico. Stromata prosenchymatica cellulae partim hyphales. Tunica perithecii ex uno strato composita, 15–25 μm crassa ad latus, cellulae exteriores angulares vel lobatae, (6.5–)9.5(–14) × (5–)6.5(–11) μm, interiores subglobosae vel angulares, (9.5–)14(–18.5) × (6.5–)10(–13.5) μm; cellulae contiguae pseudoporis connexae. Asci cylindrici vel clavati, apice anulo refringente carentes, 70–87.5–94 × 6–10–13.5 μm. Ascosporae ellipsoidea, uniseptatae, leves vel eximie verruculosae, hyalinae vel pallide brunneae, (10.5–)12.5–13.5–14(–16.5) × (4.5–) 5–5.5–6(–6.5) μm. Coloniae fere celeriter crescentes, in agaro PDA incoloratae vel pallide luteae 15–25 °C, pallide brunneoaurantiacae 30 °C, reversum pigmento rubro carens. Mycelium aerium in agaro PDA et SNA parcum vel in parte media pulvinos albos formans. Sporodochia post 14 dies vel postea in foliis Dianthi caryophylli vel ad superficiem agari SNA formata, hemisphaerica. Monophialides sporodochiales plus minusve cylindricae, (12–)20–22–25(–34) μm longae, 2–3 μm latae ad basim, 1.5–2 μm latae ad apicem. Microconidia absentia. Massae conidiorum sporodochialium in agaro SNA hemisphaericae, albidae vel pallide aurantiacae. Macroconidia sporodochialia (1–)3–5(–8)-septata, cellulae basilares pediformes, latissimae in medio, cellulae centrales et basilares quasi rectae, apicales rostratae et curvatae vel utrinque modice curvata; conidia 3-septata (34.5–)54–58–62(–70) × (3.5–)4–4.5–4.5(–5) μm; 4-septata (56–)60.5–64–67.5(–75) × (4–)4.5–4.5–4.5(–5) μm; 5-septata (55–)63–67.5–74(–78.5) × (4–)4.5–5–5(–5.5); 6-septata 66–82.5 × 4.5–5.5; 7-septata 71–84 × 5–5.5; 8-septata 74.5–93 × 5–5.5 μm. Chlamydosporae absentes.
Stromata erumpent through bark, prosenchymatous, cells 3–5 μm wide, with at least some hypha-like cells, arranged in an irregular textura porrecta; hyphae connecting cells of stroma to wall of lower part of perithecia, either arranged in a network or as terminal hyphae, 18.5–45 long, 6–7.5 μm wide near base, with walls to 2 μm wide. Perithecia crowded in groups of up to 15, seated on surface of or with base partly immersed in stroma, smooth, broadly ampulliform with a short neck or broadly ellipsoidal, dark red with a darker red ostiolar region, dark purple red in 2 % KOH, yellowish orange in lactic acid; in longitudinal sections 200–250 μm high, 120–210 μm wide. Perithecial wall consisting of a single region, 15–25 μm thick or, subapically, 30–35 μm, of 3–5 layers of cells; in face view, cell walls 1–1.5 μm thick in outer layers, 0.5 μm thick or less in inner layers, with pores in all layers; cells in outer layers lobed to angular, (6.5–)9.5(–14) × (5–)6.5(–11) μm, arranged in a textura angularis to t. epidermoidea, in inner layers subglobose to angular, (9.5–) 14(–18.5) × (6.5–)10(–13.5) μm, arranged in a textura angularis; in longitudinal section, cells subglobose to angular, flatter towards centrum. Asci cylindrical or clavate, with rounded apex, without a visible refractive ring, eight-spored, overlapping uniseriate or biseriate above and uniseriate below, 70–87.5–94 × 6–10–13.5 μm. Ascospores equally 2-celled, ellipsoidal, slightly constricted at septum, smooth or finely verruculose, hyaline or pale brown, (10.5–)12.5–13.5–14(–16.5) × (4.5–)5–5.5–6(–6.5) μm.
Colonies on PDA after 7 d at 20 and 25 °C 25 mm diam; optimum for growth 20–25 °C, maximum 30–34 °C, no growth at 35 °C. Colony reverse lacking red pigments, after 14–21 d on PDA at 15–25 °C without obvious pigment production, yellowish white to pale yellow (3–4A2–3), at 30 °C weakly greyish to brownish orange (5B4, 6C7). Colony surface on PDA smooth, wax-like because of dense spreading mycelium; aerial mycelium sparse, felt-like, produced in central half of colony or restricted to pustules; aerial mycelium on SNA present in central half of colony, white, loosely branched or felt-like, absent towards margin. Conidiation on SNA beginning within 14 d or later, inconspicuous, along submersed hyphae or from sporodochia on CL, later on surface of SNA and from aerial mycelium. Sporodochia on CL consisting of a well-developed stroma covered with dense, ca. 100 μm high hymenium of phialides and anastomosing cells of conidiophores; cells of subhymenium densely packed, prosenchymatous. Conidiogenous cells monophialidic, 2-level or twice monochasial, more or less cylindrical but tapering towards apex, (12–)20–22–25(–34) μm long, 2–3 μm wide at base, 1.5–2 μm wide near conidiogenous aperture. Microconidia not observed. Macroconidia formed in off-white or pale yellow slimy masses, with pronounced pedicellate foot cell and almost equilateral fusoid, hooked apical cell, gently and equally curved towards both ends or with central and basal part nearly straight, (1–)3–5(–8) septate: 3-septate (34.5–)54–58–62(–70) × (3.5–)4–4.5–4.5(–5) μm; 4-septate (56–)60.5–64–67.5(–75) × (4–)4.5–4.5–4.5(–5) μm; 5-septate (55–)63–67.5–74(–78.5) × (4–)4.5–5–5(–5.5) μm; 6-septate 66–82.5 × 4.5–5.5; 7-septate 71–84 × 5–5.5 μm; 8-septate, 74.5–93 × 5–5.5 μm. Chlamydospores not observed.
Habitat: On bark of dead twigs and branches in the canopy of living Celtis occidentalis.
Distribution: North America (Canada: Ontario).
Typification: Holotype of Geejayessia celtidicola: Canada, Ontario, Ottawa, Petrie Island, riverine forest, on dead branches in the canopy of a living Celtis occidentalis tree, Nov. 2008, T. Gräfenhan 2008-32, CBS H-20378, twig with perithecial stromata; ex-type culture CBS 125502.
Additional specimens and strains examined: Canada, Ontario, same general location and habit as the holotype, Nov. 2006., T. Gräfenhan 2006-29, DAOM 238129, ascospore isolate CBS 125481; Ontario, Carleton Place, riverine forest, Nov. 2006, T. Gräfenhan 2006-35, DAOM 238130, ascospore isolate CBS 125504.
DNA sequences generated: ITS rDNA (CBS 125504: HM626656, 125502: HM626657). LSU rDNA (CBS 125504: HM626668, 125502: HM626669). acl1 (CBS 125504: HM626624, 125502: HM626625). tef-1α (CBS 125504: HM626639, 125502: HM626638, 125481: HQ728149). rpb2 (CBS 125504: HM626686, 125502: HM626685). See Gräfenhan et al. (2011) for other strains included in Fig. 1.
Notes: In addition to host differences, the dark red perithecia of Geejayessia celtidicola distinguish this species from its phylogenetic relatives G. cicatricum and G. desmazieri. The shape and size of its macroconidia are reminiscent of G. desmazieri, but they are longer and more frequently septate. PDA cultures of G. celtidicola are relatively darkly pigmented at 30 °C, compared with those of G. cicatricum and G. desmazieri. On PDA, cultures form comparatively little aerial mycelium. The shapes and sizes of the macroconidia in G. celtidicola and G. zealandica are similar. The 3-septate macroconidia in G. zealandica are mostly less than 4 μm and its 5-septate macroconidia can be up to 5 μm wide (Nirenberg & Samuels 2000), whereas the 3-septate macroconidia of G. celtidicola are mostly 4–5 μm wide and have a similar width to the 5-septate macroconidia formed on SNA.
Fusarium celtidis produces almost straight macroconidia on fruits of C. occidentalis in North America (Ellis & Tracy 1890), whereas those of G. celtidicola are frequently gently curved throughout. The shape and substratum of F. celtidis suggest the F. lateritium complex as noted by Booth (1971) following Wollenweber & Reinking (1935). Fusarium sphaeriaeforme, described from bark of Celtis australis in Italy, differs from G. celtidicola by its shorter macroconidia (Saccardo 1892).
Geejayessia desmazieri (Becc. & De Not.) Schroers, Gräfenhan & Seifert, comb. nov. MycoBank MB519480. Figs 10, 11. Basionym: Nectria desmazieri Becc. & De Not., Schem. di Classif. Sferiacei: 10. 1863.
Fig. 10.
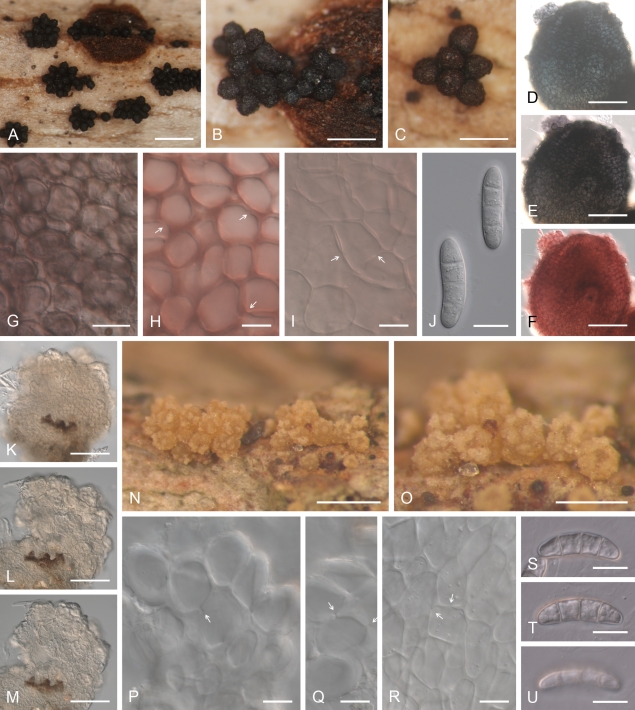
Geejayessia desmazieri, perithecia on the natural substrate. A–D. Habit on twigs of Buxus sempervirens. E–G. Colour change in perithecium in water (E), replaced with 2 % KOH (F) and then lactic acid (G). H–L. Longitudinal section through perithecial stroma (H), single perithecium (I), lateral perithecial wall (J), stroma with hypha-like cells (K), perithecial wall near ostiole (L). M, N. Hyphal or setose cells on the surface of perithecial wall. O–Q. Face view of outermost (O), intermediate (P) and innermost (Q) cell layers of perithecial wall. Arrows in O, P indicate Samuels pores in cell walls. H–L in Shears; M–Q in water. A, BPI 798402, isotype of N. desmazieri; B, M–O CBS H-20373; C, Q W-10342, isotype of N. gibbera; D–L, P, CBS H-20372, epitype; Q, “Funghi. rh. 2357, Fuckel 852”. Scale bars: A–D = 500 μm; E–G = 100 μm; H = 200 μm; I = 50 μm; J–L = 20 μm; M–Q = 10 μm.
Fig. 11.
Geejayessia desmazieri, spores and spore forming cells. A–C. Asci with broadly rounded or slightly flattened apex, lacking or with inconspicuous refractive rings. D. Ascospore surface. E. Ascospores. F. Branched conidiophores from sporodochia. G, N. Hymenium of monophialides from sporodochia. H–M, O–V. Macroconidia from sporodochia. A–E, N, O–V, from natural substrate. F–M, from SNA/B or SNA/CL. A–C, CBS H-20372, epitype of G. desmazieri; D, E, CBS H-20373; F, G, L, M, CBS 840.85; H–J, BBA 67515; K, CBS 125507, ex-epitype strain; N–V, G00110886, lectotype of N. gibbera. N, S–V, mounted in lactic acid, all others in water. Scale bars: B (also applies to A, C), H (I–M, O–V) = 50 μm; D, E = 10 μm; F, G (N) = 20 μm; O (H–M, P–V) = 5 μm.
≡ Dialonectria desmazieri (Becc. & De Not.) Petch, Naturalist (London): 281. 1937.
= Nectria coccinea var. cicatricum Desm., Ann. Sci. Nat., Bot. 10: 351. 1848 fide Wollenweber & Reinking 1935, Booth 1971. Type not seen.
= Nectria gibbera Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 177. 1870.
= Fusarium fuckelii Sacc., Syll. Fung. 4: 695. 1886.
Stromata erumpent, prosenchymatous, cells 3.5–5.5(–6.5) μm wide, with at least some hypha-like cells, arranged in an irregular textura porrecta; hyphae continuous with cells of stroma clinging to wall of the perithecial base, either arranged like a network or as terminal hyphae 25–55 μm long, 5–8 μm wide, with walls 2–3 μm wide. Perithecia typically crowded in groups of 3 to > 50, sometimes solitary or gregarious, either formed superficially or with base somewhat immersed in stroma, smooth, broadly ampulliform with a short neck or broadly ellipsoidal, pale to brownish orange, reddish brown or brownish to greyish red, less often bright to dark red, frequently with a slightly darker ostiolar region when dry, brownish to deep violet in 2 % KOH, yellowish orange in lactic acid; in longitudinal section 200–300 μm high, 150–220 μm wide. Perithecial wall of a single region, 20–30 μm thick or, subapically 30–40 μm, consisting of 3–5 layers of cells; in face view, cell walls to 1.5 μm in outer layers thick, 0.5 μm thick or less in inner layers, in all layers with pores; cells in outer layers angular to somewhat lobed, (9.5–)13(–18.5) × (5.5–)9(–13.5) μm, arranged in a textura epidermoidea or t. angularis; cells of inner layers subglobose to angular, (10–)15(–23) × (8.5–)11.5(–16) μm, arranged in a textura angularis; in longitudinal sections, cells subglobose to angular, flatter towards centrum. Asci cylindrical or clavate, with a rounded or flattened apex, lacking or with an inconspicuous refractive ring, eight-spored, mostly overlapping uniseriate or biseriate above and uniseriate below, (75.5–)85(–100) × (8–)9(–11) μm. Ascospores equally 2-celled, broadly ellipsoidal with broadly rounded, rarely somewhat tapering ends, verruculose, hyaline or pale brown, (9.5–)11–12–12.5(–15) × (4.5–)5.5–5.5–6(–7) μm.
Colonies on PDA after 7 d at 20 °C 15 mm diam, 20 mm at 25 °C; optimum for growth 20–25 °C, maximum 30–34 °C, no growth at 35 °C. Colony reverse lacking red pigments, after 14–21 d on PDA at 15 °C without obvious pigment production, at 20–25 °C pale yellow, light yellow to greyish yellow (4A5–4B5), at 30 °C with a pale yellow soluble pigment. Colony surface on PDA with felt-like, white or somewhat greyish green aerial mycelium, smooth towards margin, wax-like or with sparse aerial mycelium; on SNA unpigmented or pale yellow, typically smooth or with scant cottony, white aerial mycelium near inoculum or CL or B plant material. Conidiation on SNA within 14 d or later inconspicuously submersed along hyphae or from sporodochia on surface or on CL or B; on PDA also sporulating in aerial mycelium. Sporodochia with a hymenium of branched conidiophores with single phialides, or whorls of 2–3 terminal monophialides; cells of stroma densely packed, arranged in an irregular textura porrecta. Phialides more or less cylindrical but tapering towards apex, on SNA (11–)18–21.5–24(–34) μm long, (2.5–)3–3–3.5(–4) μm wide at base, (2.5–)3–3.5–4(–4.5) μm in middle, (1.5–)2–2.5–2.5(–2.5) μm wide near the conidiogenous aperture. Microconidia not observed. Macroconidia formed in pale yellow to pale orange, slimy masses to 3 mm diam, gently curved throughout or with central part almost straight and cylindrical, with a pronounced pedicellate foot cell and an inequilaterally fusoid, hooked apical cell, (1–)3–5(–7) septate: 3-septate (41.5–)50–52.5–55.5(–63.5) × (4.5–)5–5–5(–5.5) μm; 4-septate (51–)57(–64.5) × (4.5–)5(–5.5) μm; 5-septate (55–)62(–72.5) × (4.5–)5(–5.5) μm; 6-septate 63–74 × 5.5–6 μm. Chlamydospores not observed.
Sporodochia on G 00110886, lectotype of N. gibbera, erumpent through bark, with pale yellow to off-white conidial masses. Macroconidia (1–)3–5 septate, when measured in water: 1-septate macroconidia 59–61 × 5–6 μm (n = 3); 3-septate 58–68.5 × 5–6.5 μm (n = 14); 4-septate 60.5 × 6 μm (n = 1); 5-septate 58–68 × 5.5–6.5 μm (n = 10); in lactic acid: 1-septate 61 × 5.5 μm (n = 1); 3-septate 53–61 × 5–6 μm (n = 3); 5-septate 59–66 × 5.5–6 μm (n = 5). Macroconidia on BPI 798402, isotype of N. desmazieri, measured in lactic acid/cotton blue, 3-septate 36.5–43.5 × 4.0–4.5 μm (n = 5).
Habitat: On decaying or small, dead branches or twigs of Buxus balearica and B. sempervirens, often on bark near or on scars of subterminal twigs or in axils of leaves and twigs, less frequently on dead buds.
Distribution: Europe (Belgium, France, Germany, Italy, Spain).
Typification: Lectotype of Nectria desmazieri designated here: Italy, Pisa, Botanical Gardens, on twig of Buxus balearica, 1862, O. Beccari, Erbar. Crittogam. Ital. Cent. X. n. 983, BPI 798402; ex herb. Bot. Gard. Pisa, a specimen from Shear Study Collection Types & Rarities where it was noted as an isotype, consisting of a single twig, ca. 4.5 cm long and 3–5 mm wide comprising several clustered perithecia and sporodochia with macroconidia. Syntype: K, a small fragment unsuitable for slide preparation. Epitype of Nectria desmazieri designated here: Italy, Latio, Bagnaia, Villa Lante, in park, on twig of Buxus sempervirens, Nov. 2007, W. Gams TG2007-87, CBS H-20372, ex-epitype strain, isolated from ascospores, CBS 125507; the specimen comprises several clustered perithecia but macroconidia and sporodochia were not seen. Lectotype of Fusarium fuckelii and Nectria gibbera designated here: Germany, Nassau (now Hesse), Oestrich, K.W.G.L. Fuckel Fungi rh. 2357, originally labelled “Nectria desmazieri + F. integr.; I. & II.”, G 00110886, Herbier Fuckel 1894, Herbier Boissier. This is the specimen to which Fuckel added drawings showing (i) a Fusarium macroconidium 68 × 8 μm, (ii) an ascus 72 × 8 μm, (iii) an ascospore 11 × 5 μm, (iv) the habitat of a Fusarium sporodochium, and (v) the habitat of a “roth durchscheinend” perithecium. The lectotype consists of a twig ca. 5.5 cm long and ca. 4 mm thick with a few perithecia and sporodochia. Isolectotypes for Nectria gibbera: all labelled “Fungi rh. 2357” (see also lectotype of Nectria gibbera): Fuckel 852, as Nectria gibbera, G 00110885, Herbier Fuckel 1894, Herbier Barbey-Boissier; W 10342, Herbier Fuckel 1894, Herbier Barbey-Boissier. As Nectria gibbera II, G 00110888, G 00110887. As Nectria desmazieri det. Fuckel and N. gibbera (non N. desmazieri) det. J. Weese, 16 March 1910, W 2009-01115. As “Nectria desmazieri + F. integr.; I. & II.”, M-0155489.
Additional specimens and strains examined: All from twigs of Buxus sempervirens. Belgium, as Fusarium buxicola, CBS 840.85 = BBA 64557. France, Jardin Public 64 Eaux Chaudes, 14 June 1992, F. Candoussau 4856-4, as Nectria desmazieri, BPI 747855; culture BBA 67515 = GJS 92-65; Cappenberg, G 00110881. Italy, Treviso, Selva, Saccardo Mycotheca Veneta 116, Sept. 1874, BPI 551667, annotated by G.J. Samuels, Nov. 1989: “This exsiccata was cited by Booth (1971: Fusarium) as Nectria desmazier(es)i. The perithecia here are immature and do not contain asci. Perithecia are orange-yellow, cells at the surface of the perithecial wall are angular. This is definitely not a member of the Nectria episphaeria group. Fusarium present”. Spain, Montserrat, near Barcelona, Sept. 2007, W. Gams TG2007-69, CBS H-20373, culture CBS 125506. UK, England, Norfolk, Overstrand Woods near Norwich, as Nectria desmazieri, CBS 313.34.
DNA sequences generated: ITS rDNA (CBS 125507: HM626651, 840.85: HM626650, BBA 67515: HM626652). LSU rDNA (CBS 125507: HM626663, 840.85: HM626662, BBA 67515: HM626664). acl1 (CBS 125506: HM626632, 125507: HM626633, 840.85: HM626634, BBA 67515: HM626631). rpb2 (CBS 125506: HM626676, 125507: HM626675, 840.85: HM626678, BBA 67515: HM626677). tef-1α (CBS 125507: HQ728146, 840.85: HQ728147, BBA 67515: HM626641). See Gräfenhan et al. (2011) for other strains included in Fig. 1.
Notes: The syntype of N. desmazieri (K), possibly studied by Booth (1959, 1971), is in poor condition (B. Aguirre-Hudson, pers. comm.) and was unavailable. Booth (1959, 1971) reported perithecia and sporodochia on this specimen but it is unclear whether the microscopic details in his descriptions were based on original or secondary material. The label information on BPI 798402, “Erb. Critt. Ital. n. 983, ex Herb. Bot. Gard. Pisa” identifies this specimen as an isotype of N. desmazieri (De Notaris 1863).
Geejayessia desmazieri is characterised by pale orange to reddish brown, less typically reddish perithecia that turn brownish to deep violet in 2 % KOH. Perithecia on the isotype exhibit these colours, and are clustered in small or large groups, similar to what we observed on authentic material of N. gibbera. The perithecia of G. desmazieri are typically associated with scars on the bark of twigs or formed on the bark. By means of contrast, the perithecia of G. cicraticum, redescribed above, are bright red and associated with decaying buds.
The morphological characters of the anamorph support the distinction of G. desmazieri and G. cicatricum. These include sizes and shapes of macroconidia produced in culture and as observed on the lectotype and additional specimens. Macroconidia or fragments of macroconidia encountered on reference specimens were compared with macroconidia from pure cultures of recently collected material. Measurements of the length and width of these macroconidia confirmed the identity of the recently collected specimens and the authentic material of N. gibbera as G. desmazieri. Sporodochial macroconidia on the lectotype of N. gibbera were used to recharacterise and lectotypify Fusarium fuckelii, which we consider a synonym of G. desmazieri. The macroconidia from these exsiccatae are typically less than 6.5 μm wide; on the isotype of G. desmazieri, they are 3-septate, while on the lectotype of N. gibbera, they are 3–5 septate (Fig. 11O–V) similar to macroconidia in recently isolated cultures (Fig. 11H–M). The few macroconidia encountered on the isotype of G. cicatricum were wider than those of G. desmazieri, 6.5–7.5 μm and at least one had 8 septa; similarly broad and septate macroconidia were produced by freshly collected strains of G. cicraticum.
De Notaris (1863) originally described G. desmazieri from Buxus balearica. The identification of the herbarium specimens noted above as G. desmazieri, all originating from B. sempervirens, is based on their morphological similarities to the lectotype of N. desmazieri designated above, and the concept is formalised by the designation of an ex-epitype strain tied to DNA sequences.
There has been confusion between Fusarium fuckelii (applicable to the anamorph of G. desmazieri) and F. buxicola (applicable to the anamorph of Cyanonectria buxi, see above), but the differences in macroconidial dimensions allow the relevant specimen to be reidentified and the names to be clarified. Saccardo (1886) copied Fuckel's description of the anamorph of N. gibbera for his diagnosis of F. fuckelii and explicitly referred to Fuckel (1870: 177) where the anamorph of N. gibbera is described. Therefore, F. fuckelii was unequivocally established as the anamorph of N. gibbera, and, following our concepts, is a synonym of G. desmazieri. Fuckel (1870) described the macroconidia of Nectria gibbera, which he later accepted as a synonym of N. desmazieri (Fuckel 1872), as “5–6-septatis, hyalinis, 68 × 8” μm. Our observations of Fuckel's original material of N. gibbera (G 00110886 and isotypes listed above) correct the width to 5–6.5 μm, measurements that correspond to macroconidia of G. desmazieri. As discussed above, Wollenweber & Reinking (1935) and Booth (1959, 1971) followed Fuckel's synonymy of N. gibbera with G. desmazieri, but wrongly adopted Saccardo's F. buxicola for the anamorph. Booth (1959) reported 5–6 septate macroconidia measuring 30–55 × 4–5 μm, but later (Booth, 1971) reported 3–5 septate macroconidia measuring 56–73 × 6–7 μm. These macroconidial measurements correlate with our concept of G. desmazieri; macroconidia of Cyanonectria buxi and G. cicatricum are mostly longer, wider, and typically have more than 5 septa. His observation of “yellow to orange” perithecia suggests that Booth (1959, 1971) saw only specimens and strains of G. desmazieri, and did not see the species redescribed above as C. buxi.
Geejayessia zealandica (Cooke) Schroers, comb. nov. MycoBank MB519484. Basionym: Nectria zealandica Cooke, Grevillea 8: 65. 1879.
≡ Cosmospora zealandica (Cooke) Samuels & Nirenberg, Canad. J. Bot. 78: 1483. 2000.
= Fusarium zealandicum Nirenberg & Samuels, Canad. J. Bot. 78: 1483. 2000.
Description and illustrations: Nirenberg & Samuels (2000).
Material studied: New Zealand, North Island, Auckland, Waitemata City, Waitakere Ranges, Cascades, on bark of Hoheria populnea, 3 June 1983, J.M. Dingley & A.Y. Rossman, CBS 111.93 = BBA 64792, GJS 83-235, ex holotypus anamorphicus; specimen PDD 46436 = BPI 802574; Canterbury, Christchurch, Riccarton, on bark of Plagianthus sp., J.M. Dingley, May 1986, BPI 802575, culture CBS 101913 = BBA 65034, GJS 86-509.
DNA sequences generated: ITS rDNA (CBS 111.93: HM626658). LSU rDNA (CBS 111.93: HM626670). acl1 (CBS 111.93: HM626626). tef-1α (CBS 111.93: HQ728148, 101913: HM626640). rpb2 (CBS 111.93: HM626684).
Notes: The perithecial wall anatomy, stromata development for perithecia and sporodochia, and overall morphological characters of the anamorph and teleomorph (Nirenberg & Samuels 2000) justify the classification of N. zealandica in Geejayessia. It differs from other members of the genus by relatively thick perithecial walls.
DISCUSSION
Supraspecific classification
Fusarium typified by F. sambucinum is firmly linked taxonomically to the teleomorphic genus Gibberella typified by G. pulicaris. Both names refer to the same genetic and phylogenetic species. Fusarium sambucinum is closely related to the Fusarium fujikuroi, F. graminearum, F. incarnatum-equiseti, F. oxysporum species groups and some others. Their close relationships are confirmed by studies using ribosomal DNA sequences or protein-encoding genes for phylogenetic analyses suggesting that these taxa form a monophyletic group (Summerbell & Schroers 2002, O'Donnell et al. 2007, Schroers et al. 2009, O'Donnell et al. 2010). The known teleomorphs of these species groups almost always correspond to the modern concept of Gibberella; they form homogeneously bluish black pigmented, KOH+, warted perithecia and mostly multiseptate ascospores (Rossman et al. 1999, Samuels et al. 2001). Therefore, the strongly supported phylogenetic clade accommodating Fusarium sambucinum and its closely related sister clades has been referred to as the “Gibberella clade” (O'Donnell et al. 2010). Applying the philosophy of Article 59 of the International Code of Botanical Nomenclature (McNeill 2006), Gibberella is the appropriate name for the holomorphs in this clade. However, following the logic argued in this volume by Gräfenhan et al. (2011) and earlier by Seifert & Samuels (2000), Cannon & Kirk (2000), Rossman & Samuels (2005), Fusarium Link (1809) has priority over Gibberella Sacc. (1877) and we prefer to use the earlier name for this clade.
Samuels et al. (2009) assigned the term “Fusarium group” to the moderately supported phylogenetic clade encompassing several statistically strongly supported subclades, whose relationships among each other remained unresolved. They accepted the subclades of the Fusarium group as genera, for example, Albonectria, Cyanonectria, Gibberella, Neocosmospora/Haematonectria (the latter to be replaced with an earlier, legitimate genus name), following the underlying principles applied to the generic concepts of nectriaceaous fungi presented by Rossman et al. (1999). Our phylogenetic studies based on protein-encoding genes (Fig. 1, Gräfenhan et al. 2011) extend the results shown by Samuels et al. (2009). They identify up to eight strongly supported clades with Fusarium-like anamorphs apart from Dialonectria, Fusicolla, Macroconia Microcera, and Stylonectria that comprise the distantly related, basal Fusarium clade.
Our recognition of the distinctiveness of Cyanonectria, Geejayessia, and other genera of the terminal Fusarium clade aims to encapsulate a similar degree of divergence at the generic rank across the Nectriaceae (cf. Chaverri et al. 2011, Hirooka et al. 2011) and in other ascomycetous families or orders (cf. Crous et al. 2007, Tanaka et al. 2009). These conclusions also affirm the importance of applying monophyletic concepts to fungi at the generic level. As discussed by Gräfenhan et al. (2011), the falcate macroconidia of Fusarium and morphologically similar genera cannot be interpreted as a genus delineating structure, but are probably a plesiomorphic character present in unrelated taxa of the Nectriaceae. At the generic level, the recent name Cyanonectria is accepted here and the new genus Geejayessia is proposed because they have apparently evolved within the terminal Fusarium clade independently, and no other generic name is available.
Clarification of the concepts and nomenclature of the species of two of these genera, Cyanonectria and Geejayessia, is the main focus of this paper. Often misidentified as Fusarium buxicola, representatives of both genera were previously classified in Fusarium section Macroconia (Wollenweber & Reinking 1935, Gerlach & Nirenberg 1982).
Analysis of morphological characters
Delimitation of the genera Cyanonectria and Geejayessia using morphological characters requires reinterpretation of the shape, disposition, colour, and anatomical features of perithecia, ascal apex, stroma, and anamorphic characters.
Species of Geejayessia and Cyanonectria buxi form perithecia on a hyphal or byssoid tissue (Figs 3I; 5G–I; 7C, H; 8E, M; 10H, K) as observed in previous studies (Booth 1959, Samuels & Rogerson 1984, Nirenberg & Samuels 1990, Samuels et al. 1991). We interpret these cushions as stromata because they consist of densely aggregated, hypha-like cells that emerge through cracks of woody parts or plant hosts or through leaflets of decaying buds. Similar erumpent stromata were also seen in representatives of “Haematonectria” (Fig. 12C) and Fusarium sensu stricto, while well-developed stromatal structures are typically absent in genera such as Cosmospora, Dialonectria, Macroconia, and Microcera (Samuels et al. 1991, Gräfenhan et al. 2011). Booth (1971: 59) concluded that the presence or absence of stromata is “hardly worthy of generic rank”, which explains why he placed G. desmazieri in the otherwise astromatic “N.” episphaeria group (Dialonectria sensu Gräfenhan et al. 2011). Phylogenetic analyses across the Nectriaceae and the phylogenetic distinction of stromata forming and astromatous taxa does not support Booth's view (Fig. 1, Samuels et al. 2009, Gräfenhan et al. 2011). Stromata erumpent through plant tissue and supporting sporodochia and perithecia occur in diverse groups of the Hypocreales (Samuels 1976, Rossman et al. 1999). However, they frequently occur in taxa of the terminal Fusarium clade, which is rich in plant parasitic species. In contrast, the nearly astromatous genera Cosmospora, Dialonectria, Macroconia, and Microcera, distantly related to the terminal Fusarium clade (Fig. 1, Gräfenhan et al. 2011), seem to be mostly associated with insects, lichens, and other fungi. In Geejayessia species with their frequent occurrence on dead branches of living trees, the stroma may be the interface between an endophytic or endoparasitc lifestyle and the exposed fruiting phase (Figs 7C, 8E, 10H for perithecia; Nirenberg & Samuels 2000, fig. 3 and Samuels & Rogerson 1984, fig. 5 for sporodochia). Even in species of the Fusarium clade associated with more ephemeral gramineous hosts, perithecia are typically firmly connected to the substrate by weakly developed stromata embedded in the plant tissue.
Fig. 12.
Comparison of teleomorph characters of “Haematonectria” illudens, “Haematonectria” sp. and “Nectria” albida. A–I. “Haematonectria” illudens on the natural substrate. A. Habit of perithecia. B–D. Longitudinal sections of perithecia, erumpent stroma and lateral perithecial wall. E–G. Front view of cell layers of the perithecial wall, E, F showing cells of perithecial warts. H, I. Ascospore, H showing surface striations. J–P. “Haematonectria” sp. J–L. Colour changes in perithecium mounted in water (J), replaced with 2 % KOH (K) and then lactic acid (L). M. Habit of perithecia. N. Cells of perithecial warts. O, P. Intermediate cells of the perithecial wall. Q–X. “Nectria” albida. Q. Habit of perithecia. R–T. Colour changes in perithecium mounted in water (R), replaced with 2 % KOH (S) and then lactic acid (T). U. Ascospore. V. Short seta like cells emerging from perithecial wall. W. Hypha-like cells probably continuous with cells of the stroma covering base of perithecium. X. Outermost cell layer of perithecial wall. Arrows in E–G, N–P, X indicate Samuels pores in cell-walls. A–I BPI 802461; J–P BPI 745186; Q–X BPI 1108875. Scale bars: A, Q = 500 μm; B, J–L, R–T = 100 μm; C, D = 50 μm; E–I, N–P, U–X = 10 μm; M = 200 μm.
Perithecia of C. buxi and Geejayessia spp. can be at least partly covered by a network of hyphae that emerges from the byssoid stroma (Figs 3L, M; 5M; 7I) and terminal hyphae, apparently originating from this network, may appear as short setae (Figs 8F; 10M, N). A similar situation occurs in “Nectria” albida, where bases of the smooth perithecia seem to be covered by a hyphal network (Fig. 12W) from which some terminal hyphae emerge (Fig. 12V).
Perithecia of Cyanonectria and Geejayessia are obpyriform but those of the former have a somewhat widened, broadly rounded ostiolar neck, described by Samuels et al. (2009) as “knobby” for C. cyanostoma (Fig. 3B–D). On drying, the perithecia collapse laterally. In contrast, perithecia of G. atrofusca and G. zealandica are rather globose (Fig. 7B–D, Rogerson & Samuels 1984, fig. 1; Nirenberg & Samuels 2000, fig. 4).
Perithecial colour characters are often used to delineate teleomorphically typified genera with Fusarium-like anamorphs (Rossman et al. 1999). Although the Nectriaceae are reasonably well characterised by red or bluish black perithecia with positive colour changes in KOH and lactic acid, our phylogenetic results suggest independent, apomorphic losses or modifications of pigments. Red pigments have probably been lost independently in “N.” albida (Fig. 12Q–T), and Albonectria (Fig. 13K–O), and bluish black in “Albonectria” verrucosa, which has pale KOH–perithecia but is phylogenetically within Fusarium sensu stricto (Rossman et al. 1999, Gräfenhan et al. 2011). Colour changes of perithecia in Cyanonectria and Geejayessia correlate with the basic phenology of the Nectriaceae. However, C. cyanostoma has remarkable bicoloured perithecia (Samuels et al. 2009) having a bluish black apex that reacts in KOH in identical manner to the teleomorph of F. sambucinum (Fig. 13A–F) and other species of Fusarium sensu stricto, while the main part of the red perithecial body reacts identically to the red perithecia of other genera of the Nectriaceae. Although the perithecia of C. buxi lack red colours and thus do not obviously correspond to the generic concept of Cyanonectria, they are heterogeneously pigmented, intensely in their upper part and faintly in their lower part (Fig. 3C–G). The heterogeneous aspect of perithecial pigmentation supports segregation of Cyanonectria from other genera of the terminal Fusarium clade.
Fig. 13.
Comparison of teleomorph characters of Fusarium sambucinum and Albonectria rigidiuscula. A–J. Fusarium sambucinum on the natural substrate. A–C. Habit of perithecia. D–F. Colour changes in perithecium mounted in water (D), replaced with 2 % KOH (E) and then with lactic acid (F). G–I. Face view of cell layers of the perithecial wall. G. Cells of perithecial warts. I. Innermost cells of the perithecial wall. K–U. Albonectria rigidiuscula. K–M. Colour changes in perithecium mounted in water (K), replaced with 2 % KOH (L) and then with lactic acid (M). N, O. Habit of perithecia. P, Q. Cells of perithecial warts. R. Cells of the main perithecial wall. S–U. Ascospores, U showing wall surface. Arrows in H, I, P–R indicate Samuels pores in cell walls. A, B, D–F HJS 1459; C, G–J BPI 1109327; K–U HJS 0109. Scale bars: A = 1 mm; B, C, N, O = 500 μm; D–F, K–M = 100 μm; G = 20 μm; H–J, P–U = 10 μm.
The spectrum of perithecial colours exhibited by G. celtidicola and G. cicatricum is also quite characteristic; their perithecia are bright or dark red on the natural substrate and become darker red or purple in KOH and pale yellowish in lactic acid (Figs 5A–F, 8A–D). In contrast, perithecia of G. desmazieri are mostly orange or brownish orange on the natural substrate (Fig. 10A–C, Samuels et al. 1991, Booth 1959). The perithecia of G. atrofusca are somewhat Gibberella-like because they appear black on the natural substrate. They seem not to change colour in KOH and only inconspicuously so in lactic acid (Fig. 7D–F; Samuels & Rogerson 1984). This behaviour differs not only from species of Fusarium, but also from its closely related, KOH+ sister species in Geejayessia.
Species of Geejayessia have outer perithecial wall layers differing from those of species of Albonectria, Fusarium, and “Haematonectria”. Perithecial walls of these species are rather narrow, which led Booth (1959) to classify N. desmazieri in the N. episphaeria group. The wall consists of a single region, comprising several layers of morphologically similar cells that gradually change shape and size across the wall, but cannot be recognised as forming distinct regions. This pattern is also shared by species of Cyanonectria (Fig. 3H, K, N), “N.” albida (Fig. 12X), and the distantly related species of Dialonectria, Cosmospora, Microcera, and Macroconia. In contrast, Albonectria, Fusarium, and most species of “Haematonectria” have rather strongly warted perithecia and thicker perithecial walls.
In species of Cyanonectria and Geejayessia, the cell walls in all layers have locally and abruptly thinned areas ca. 1 μm diam or less, but which do not become complete pores (Figs 3N; 5N; 7J, K; 8G, H; 10O–P). We propose the name “Samuels pores” for these structures.They appear to differ from the so-called “Munk pores” in the Nitschkiaceae and other related Sordariomycetes (Carroll & Munk 1964), which are typically surrounded by a distinctly thickened and morphologically conspicuous rim (Samuels et al. 1993, figs 6, 12; Vasilyeva et al. 2010, fig. 7 j, k). In contrast, the cell wall surrounding Samuels pores in the Hypocreales is typically strongly and abruptly attenuated and is probably never completely perforated; no rim surrounds these pores. Samuels pores were seen in species of Microcera and, for example, Cosmospora joca (Samuels et al. 1991, fig. 23), C. lasiodiplodiae (ibid., fig. 25), C. pseudepisphaeria (Rossman et al. 1999, plate 28 i), Chaetopsinectria chaetopsinae (Samuels 1985, fig. 1B), Ch. chaetopsinae-penicillatae (ibid., fig. 4D), and Ch. chaetopsinae-catenulatae (ibid., fig. 5B). At least rarely, they occur in the outermost layers of perithecial cells or in the cells of perithecial warts of representatives also of “Haematonectria” (Fig. 12E–G, N–P), “Nectria” albida (Fig. 12X), Fusarium sambucinum (Fig. 13H, I), and Albonectria rigidiuscula (Fig. 13P–R).
Perithecial walls of species of Cyanonectria and Geejayessia are also similar to the innermost of the two or three regions observed in species of the distantly related genus Bionectria (Bionectriaceae). Samuels pores in Bionectria were only seen in the innermost anatomical region of perithecial walls (Schroers 2001, figs 31i, 42k, 44h, 46k). Samuels pores may occur only in the inner region of perithecial walls that consist of multiple regions or throughout the entire perithecial wall when there is only a single wall region. Accordingly, the presence or absence of Samuels pores could be used as an additional criterion to identify homologous perithecial wall regions among hypocrealean taxa. Their observed presence in the innermost and also in the more or less outermost cell layers in “Haematonectria” (Fig. 12E–G, N–P), “N.” albida (Fig. 12X) and Albonectria (Fig. 13P–R) suggests the perithecial walls may have been originally derived from, or consist of, only one wall region. This wall region may be homologous to the entire wall of Geejayessia species, but to the inner wall region only of Bionectria species. This interpretation is discordant with the prevailing view. For example, Rossman et al. (1999) distinguished three distinct perithecial wall regions in Albonectria and two in “Haematonectria”, emphasising characters seen in longitudinal sections such as shape of cells and thickness and pigmentation of cell-walls.
The asci of Geejayessia species are clavate and either lack or have an inconspicuous refractive ring (Figs 6A–C, 7L, 9A–C, 11A–C). In Cyanonectria buxi refractive rings were observed (Fig. 11A–D) comparable to those reported for C. cyanostoma (Samuels et al. 2009). The ascospores of all known Cyanonectria and Geejayessia species are 1-septate. They are initially more or less smooth, but, perhaps as a function of maturity, are sometimes finely warted in Cyanonectria, G. atrofusca, and G. celtidicola (Figs 4E–H, 7M, 9D, Samuels et al. 2009, fig. 2j), and clearly warted in G. cicatricum and G. desmazieri (Figs 6D–G; 11D, E). At maturity and when clearly warted, the ascospores were somewhat yellowish brown in Geejayessia but hyaline in Cyanonectria. Our generic concept emphasises the meaning of ascospore septation as a delimiting character. By means of contrast, “N.” albida, Fusarium sensu stricto, and Albonectria have multi-septate ascospores (Figs 12U; 13J, S, T).
The macroconidia in Cyanonectria and Geejayessia species correspond to Nectria section Macroconia, characterised by an almost cylindrical main body and relatively conspicuous or thick walls and septa (Wollenweber & Reinking 1935, Gerlach & Nirenberg 1982). They have moderately or well-developed, pedicellate basal cells and gently curved or clearly hooked and tapering apical cells. Most macroconidia are only gently curved (Figs 4U–Y; 7Q, R; 9L–P; 11H–M, O–V; Nirenberg & Samuels 2000, figs 8–10) but more arced macroconidia were seen in G. cicatricum (Fig. 6M–Q). Distal and proximal cells of macroconidia are usually longer than intercalary cells. A few macroconidia can be observed in squash mounts of perithecial stromata from herbarium specimens. Erumpent sporodochia sometimes occur, emerging through the outer cortex of the bark. Sporodochia of C. buxi were seen on specimens lacking perithecial stromata, e.g. CBS 125547. In culture, macroconidia are formed first on verticillately branched conidiophores that correspond to structures called macroconidial “sporodochia” by Wollenweber & Reinking (1935) and Gerlach & Nirenberg (1982) (Figs 6K, L; 9I, J; 11F). Conidiophore cells beneath the phialides in sporodochia of Geejayessia frequently have anastomosing bridges between neighbouring cells (Figs 6L; 9I, J). On SNA/CL or SNA/B, sporodochia with a well-developed subhymenium occur in Cyanonectria and Geejayessia (Figs 4T; 7N; 9G, H; 11G) similar to those observed in nature for G. zealandica (Nirenberg & Samuels 2000, fig. 3). In culture, species of Cyanonectria and Geejayessia are faster growing than the species originally included in Nectria section Macroconia (Wollenweber 1926), transferred to Macroconia or Microcera (Gräfenhan et al. 2011). The anamorph of G. cicatricum may have been confused with C. buxi or G. desmazieri in the past. It has similarly wide and long macroconidia as C. buxi and forms similarly pale colonies on SNA or PDA as G. desmazieri. Geejayessia celtidicola forms comparably little aerial mycelium on PDA and, at 30 °C, produces greyish colonies dissimilar to those of G. atrofusca, G. desmazieri, and G. zealandica. On PDA, Cyanonectria buxi forms a dark colony reverse and surface showing bluish hues (Fig. 4AA–AD). This combination of characters is not seen in other fusaria. A dark reverse is not produced in cultures of its closest relative, C. cyanostoma; Samuels et al. (2009) described its PDA cultures as white to cream. When describing F. buxicola, Gerlach & Nirenberg (1982) mentioned fawn to brown colours, rarely blue or verdigris spotted colonies. We encountered no such spotted colouration in G. cicatricum or G. desmazieri, which suggests that Gerlach & Nirenberg (1982) may have included C. buxi and G. desmazieri in their description of F. buxicola. No red pigments were observed in Geejayessia, while in Albonectria, Fusarium sensu stricto, and to some extent also “Haematonectria”, red, violet, or purple pigments are commonly seen.
We did not see microconidia in our study of cultures of C. buxi and G. desmazieri, but Gerlach & Nirenberg (1982) reported “ellipsoid, spindle- or comma-shaped (according to Wollenweber & Reinking 1935 and J. Ehrlich, cited by Booth 1959)” macroconidia for C. buxi. Nirenberg & Samuels (2000) did not mention microconidia when describing G. zealandica. The microconidia we observed in G. atrofusca (Fig. 7S, T) largely confirm the observations made by Samuels & Rogerson (1984).
Life style and ecology
Cyanonectria and Geejayessia are associated only with woody hosts. Remarkably, we are not aware of any Geejayessia isolated from bulk soil, while representatives of several other taxa in the terminal Fusarium clade are well known as soil inhabitants or soil-borne plant pathogens. With the exception of G. zealandica, the species of Geejayessia and Cyanonectria have never been isolated from substrates other than Buxus sempervirens, B. balearica (Buxales, Buxaceae), Celtis occidentalis (Rosales, Ulmaceae), or Staphylea trifolia (Crossosomatales, Staphyleaceae).
Acknowledgments
We are grateful to the curators of the CBS and DAOM culture collections for providing and preserving cultures for our studies, and the herbaria BPI, DAOM, G, K, M, and W for loans of specimens. H.-J.S. extends his gratitude to Walter Gams (Baarn, the Netherlands) for assistance with the Latin diagnosis and Silvester Žgur (University of Ljubljana, Biotechnical Faculty, Institute for Animal Breeding) for kindly providing access to a microtome. We thank Amy Rossman (Systematic Mycology & Microbiology Laboratory (SMML), USDA, Beltsville, USA) and Brett Summerell (Royal Botanic Gardens, Sydney, Australia) for providing helpful comments on earlier versions of this text. The illustrations in Fig. 12B, C and D were made from longitudinal sections prepared by Gary J. Samuels (SMML).
Taxonomic novelties: Geejayessia Schroers, Gräfenhan & Seifert, gen. nov., Geejayessia celtidicola Gräfenhan & Schroers, sp. nov., Cyanonectria buxi (Fuckel) Schroers, Gräfenhan & Seifert, comb. nov., Geejayessia atrofusca (Schw.) Schroers & Gräfenhan, comb. nov., Geejayessia cicatricum (Berk.) Schroers, comb. nov., Geejayessia desmazieri (Becc. & De Not.) Schroers, Gräfenhan & Seifert, comb. nov., Geejayessia zealandica (Cooke) Schroers, comb. nov.
References
- Booth C (1959). Studies of pyrenomycetes: IV. Nectria (part I). Mycological Papers 73: 1–115. [Google Scholar]
- Booth C (1971). The genus Fusarium. Commonwealth Mycological Institute, Kew.
- Cannon PF, Kirk PM (2000). The philosophy and practicalities of amalgamating anamorph and teleomorph concepts. Studies in Mycology 45: 19–25. [Google Scholar]
- Carroll G, Munk A (1964). Studies on lignicolous Sordariaceae. Mycologia 56: 77–98. [Google Scholar]
- Chaverri P, Salgado C, Hirooka Y, Rossman AY, Samuels GJ (2011). Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology 68: 57–78 (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ (2007). Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaris G de (1863). Sferiacei Italici. Genova, Tipi del R.I. de' Sordo-Muti: 10–11.
- Domsch KH, Gams W, Anderson T (2007). Compendium of soil fungi. 2nd ed. IHW-Verlag, Eching, Germany.
- Doveri F, Pecchia S, Sarrocco S, Minnocci A, Vannacci G (2010). Rodentomyces, a new hypocrealean genus from Italy. Fungal Diversity 42: 57–69. [Google Scholar]
- Ellis JB, Tracy SM (1890) A few new fungi. Journal of Mycology 6(2): 76–77. [Google Scholar]
- Fuckel L (1870). Symbolae mycologicae. Beiträge zur Kenntnis der rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde 23–24: 1–459. [Google Scholar]
- Fuckel L (1872). Symbolae mycologicae. Beiträge zur Kenntnis der rheinischen Pilze. Erster Nachtrag. Jahrbücher des Nassauischen Vereins für Naturkunde 25–26: 1–459. [Google Scholar]
- Fuckel L (1873). Symbolae mycologicae. Beiträge zur Kenntnis der rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde 27–28: 1–99. [Google Scholar]
- Gams W, Hoekstra ES, Aptroot A (1998). CBS Course of Mycology, 4th edn. Centraalbureau voor Schimmelcultures, Baarn, the Netherlands.
- Gams W, Nirenberg HI, Samuels GJ, Seifert KA, Thrane U, Neish GA (1997). Database of Fusarium names. http://www.cbs.knaw.nl/databases/fusarium/database.aspx
- Gerlach W, Nirenberg HI (1982). The genus Fusarium—a pictorial atlas. Mitteilungen aus der Biologischen Bundesanstalt fur Land- und Forstwirtschaft 209: 1–406. [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI, Seifert KA (2011). An overview of the taxonomy, phylogeny and typification of some nectriaceous fungi classifed in Cosmospora, Acremonium, Fusarium, Stilbella and Volutella. Studies in Mycology 68: 79–113 (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Kobayashi T, Samuels GJ (2008). Taxonomic studies of nectrioid fungi in Japan. III. The genus Cosmospora. Mycoscience 49: 281–290. [Google Scholar]
- Hirooka Y, Rossman AY, Chaverri P (2011). Morphological and phylogenetic analyses of the Nectria cinnabarina species complex. Studies in Mycology 68: 35–56 (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren, PK, Holmgren NH, Barnett LC (1990) Index herbariorum, 8th ed. Regnum vegetabile vol. 120. Gantner Verlag, Ruggell, Liechtenstein.
- Kornerup A, Wanscher JH (1978). Methuen handbook of colour, 3rd edition. 1–252. Eyre Methuen, London.
- Leslie JF (1991) Mating populations in Gibberella fujikuroi (Fusarium Section Liseola). Phytopathology 81: 1058–1060. [Google Scholar]
- Leslie JF, Summerell BA (2006). The Fusarium laboratory manual. Blackwell Publishing Professional, Ames, Iowa.
- Link JHF (1809). Observationes in ordines plantarum naturales. Dissertatio Ime. Gesellschaft Naturforschender Freunde zu Berlin 3: 3–42. [Google Scholar]
- Marasas WFO, Nelson PE, Toussoun TA (1984). Toxigenic Fusarium species: Identity and mycotoxicology. Pennsylvania State University Press, University Park, PA.
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, Marhold K, Nicolson DH, Prado J, Silva PC, Skog JE, Wiersema JH, Turland NJ (2006). International Code of Botanical Nomenclature (Vienna Code). Regnum Vegetabile, vol. 146, Gantner Verlag, Ruggell, Liechtenstein.
- Nelson PE, Toussoun TA, Marasas WFO (1983). Fusarium species. An illustrated manual for identification. The Pennsylvania State University Press, University Park and London.
- Nirenberg HI (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt fur Land- und Forstwirtschaft 169: 1–117. [Google Scholar]
- Nirenberg HI, Samuels GJ (2000). Nectria and Fusarium. II. Cosmospora zealandica comb.nov. and its anamorph, Fusarium zealandicum sp. nov. Canadian Journal of Botany 78: 1482–1487. [Google Scholar]
- O'Donnell K (1993). Fusarium and its near relatives. In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (Reynolds DR, Taylor JW, eds), CAB International, Wallingford, England: 225–233.
- O'Donnell K (2000). Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92: 919–938. [Google Scholar]
- O'Donnell K, Sarver BAJ, Brandt M, Chang DC, Noble-Wang J, Park BJ, Sutton DA, Benjamin L, Lindsley M, Padhye A, Geiser DM, Ward TJ (2007). Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the 2005–06 multistate contact lens-associated U.S. keratitis outbreaks. Journal of Clinical Microbiology 45: 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM (2008). Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. Journal of Clinical Microbiology 46: 2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K, Sutton DA, Rinaldi MG, Sarver BAJ, Balajee SA, Schroers H-J, Summerbell RC, Robert VARG, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee Y-H, Kang S, Park B, Geiser DM (2010). Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. Journal of Clinical Microbiology 48: 3708–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM (1996). TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Posada D (2008). jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Rossman AY, Samuels GJ (2005). Towards a single scientific name for species of fungi. Inoculum 56: 3–6. [Google Scholar]
- Saccardo PA (1877). Fungi Veneti novi vel critici vel Mycologiae Venetae addendi. Ser. 6. Michelia 1: 1–72. [Google Scholar]
- Saccardo PA (1883). Sylloge Fungorum omnium hucusque cognitorum. Vol. 2. Pyrenomycologiae universae continuation et finis. Padova.
- Saccardo PA (1886). Sylloge Fungorum omnium hucusque cognitorum. Vol. 4. Padova.
- Saccardo PA (1892). Sylloge Fungorum omnium hucusque cognitorum. Vol. 10. Supplementum universal. Padova.
- Samuels GJ (1976). A revision of the fungi formerly classified as Nectria subgenus Hyphonectria. Memoirs of the New York Botanical Garden 26: 1–126. [Google Scholar]
- Samuels GJ (1985). Four new species of Nectria and their Chaetopsina anamorphs. Mycotaxon 22: 13–32. [Google Scholar]
- Samuels GJ, Barr ME, Lowen R (1993). Revision of Schizoparme (Diaporthales, Melanconidaceae). Mycotaxon 46: 459–483. [Google Scholar]
- Samuels GJ, Brayford D (1994). Species of Nectria (sensu lato) with red perithecia and striate ascospores. Sydowia 46: 75–161. [Google Scholar]
- Samuels GJ, Lu B-S, Chaverri P, Candoussau F, Fournier J, Rossman AY (2009). Cyanonectria, a new genus for Nectria cyanostoma and its Fusarium anamorph. Mycological Progress 8: 49–58. [Google Scholar]
- Samuels GJ, Nirenberg HI, Seifert KA (2001). Perithecial species of Fusarium. In: Fusarium. Paul E. Nelson memorial symposium (Summerell BA, Leslie JF, Backhouse D, Bryden WL, Burgess LW, eds). American Phytopathological Society, St. Paul, MN: 1–15.
- Samuels GJ, Doi Y, Rogerson CT (1990). Hypocreales. Memoirs of the New York Botanical Garden 59: 6–108. [Google Scholar]
- Samuels GJ, Rogerson CT (1984). Nectria atrofusca and its anamorph, Fusarium staphyleae, a parasite of Staphylea trifolia in Eastern North America. Brittonia 36: 81–85. [Google Scholar]
- Samuels GJ, Rossman AY, Lowen R, Rogerson CT (1991). A synopsis of Nectria subgen. Dialonectria. Mycological Papers 164: 1–47. [Google Scholar]
- Schroers H-J (2001). A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Studies in Mycology 46: 1–214. [Google Scholar]
- Schroers H-J, Geldenhuis MM, Wingfield MJ, Schoeman MH, Yen YF, Shen WC, Wingfield BD (2005). Classification of the guava wilt fungus Myxosporium psidii, the palm pathogen Gliocladium vermoesenii and the persimmon wilt fungus Acremonium diospyri in Nalanthamala. Mycologia 97: 375–95. [DOI] [PubMed] [Google Scholar]
- Schroers H-J, O'Donnell K, Lamprecht S, Kammeyer PL, Johnson S, Sutton DA, Rinaldi MG, Geiser DM, Summerbell RC (2009). Taxonomy and phylogeny of the Fusarium dimerum species group. Mycologia 101: 44–70. [DOI] [PubMed] [Google Scholar]
- Seifert KA, Samuels GJ (2000). How should we look at anamorphs? Studies in Mycology 45: 5–18. [Google Scholar]
- Summerbell RC, Schroers H-J (2002). Analysis of phylogenetic relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. Journal of Clinical Microbiology 40: 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC (2003). Aspergillus, Fusarium, Sporothrix, Piedraia and their relatives. In: Pathogenic Fungi in Humans and Animals (Howard DH, ed) Marcel Dekker Press, New York: 237–498.
- Swofford DL (2003). PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sunderland, Massachusetts: Sinauer Associates.
- Tamura K, Dudley J, Nei M, Kumar S (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, Sano T, Shirouzu T, Hosoya T (2009) Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Studies in Mycology 64: 175–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyeva L, Chernyshev A, Stephenson S (2010). Pyrenomycetes of the Russian far East 4: family Nitschkiaceae (Coronophorales, Ascomycota). Mycologia 102: 233–247. [DOI] [PubMed] [Google Scholar]
- Wollenweber HW (1926). Pyrenomyceten-Studien. II. Angewandte Botanik 8: 168–212. [Google Scholar]
- Wollenweber HW, Reinking OA (1935). Die Fusarien, ihre Beschreibung, Schadwirkung und Bekämpfung. Paul Parey, Berlin.