Abstract
We examined the phylogenetic relationships of two species that mimic Chaetosphaeria in teleomorph and anamorph morphologies, Chaetosphaeria tulasneorum with a Cylindrotrichum anamorph and Australiasca queenslandica with a Dischloridium anamorph. Four data sets were analysed: a) the internal transcribed spacer region including ITS1, 5.8S rDNA and ITS2 (ITS), b) nc28S (ncLSU) rDNA, c) nc18S (ncSSU) rDNA, and d) a combined data set of ncLSU-ncSSU-RPB2 (ribosomal polymerase B2). The traditional placement of Ch. tulasneorum in the Microascales based on ncLSU sequences is unsupported and Australiasca does not belong to the Chaetosphaeriaceae. Both holomorph species are nested within the Glomerellales. A new genus, Reticulascus, is introduced for Ch. tulasneorum with associated Cylindrotrichum anamorph; another species of Reticulascus and its anamorph in Cylindrotrichum are described as new. The taxonomic structure of the Glomerellales is clarified and the name is validly published. As delimited here, it includes three families, the Glomerellaceae and the newly described Australiascaceae and Reticulascaceae. Based on ITS and ncLSU rDNA sequence analyses, we confirm the synonymy of the anamorph genera Dischloridium with Monilochaetes. Consequently Dischloridium laeënse, type species of the genus, and three related species are transferred to the older genus Monilochaetes. The teleomorph of D. laeënse is described in Australiasca as a new species. The Plectosphaerellaceae, to which the anamorph genus Stachylidium is added, is basal to the Glomerellales in the three-gene phylogeny. Stilbella annulata also belongs to this family and is newly combined in Acrostalagmus. Phylogenetic analyses based on ncLSU, ncSSU, and combined ncLSU-ncSSU-RPB2 sequences clarify family relationships within the Microascales. The family Ceratocystidaceae is validated as a strongly supported monophyletic group consisting of Ceratocystis, Cornuvesica, Thielaviopsis, and the type species of Ambrosiella. The new family Gondwanamycetaceae, a strongly supported sister clade to the Ceratocystidaceae, is introduced for the teleomorph genus Gondwanamyces and its Custingophora anamorphs. Four families are accepted in the Microascales, namely the Ceratocystidaceae, Gondwanamycetaceae, Halosphaeriaceae, and Microascaceae. Because of a suggested affinity of a Faurelina indica isolate to the Microascales, the phylogenetic position of the Chadefaudiellaceae is reevaluated. Based on the results from a separate ncLSU analysis of the Dothideomycetes, Faurelina is excluded from the Microascales and placed in the Pleosporales.
Keywords: Australiasca, Australiascaceae, Ceratocystidaceae, Cylindrotrichum, Dischloridium, Gondwanamycetaceae, Reticulascus, Reticulascaceae, phylogeny, Plectosphaerellaceae
INTRODUCTION
The genus Chaetosphaeria (Chaetosphaeriaceae, Chaetosphaeriales) is a cosmopolitan genus of nonstromatic, perithecial ascomycetes (Réblová 2000, Réblová & Winka 2000, Fernández et al. 2006). It is characterised by dark, opaque, usually subglobose to conical perithecia. The asci are unitunicate, short-stipitate with a distinct, inamyloid apical ring. The ascospores are hyaline, rarely bicolorous, 1- to several-septate, ellipsoidal to fusoid, sometimes cylindrical, and rarely fragment into part-spores. Periphyses and paraphyses are persistent, cylindrical, seldom branching, septate, and longer than the asci. The genus has been linked to 13 anamorph genera of phialidic dematiaceous hyphomycetes (Réblová 2000, 2004).
Several distantly related fungi mimic Chaetosphaeria in the morphology of perithecia, asci, ascospores, and phialidic, dematiaceous, hyphomycetous anamorphs. Recognising these species as distinct from Chaetosphaeria is difficult based purely on morphology. In most cases, their systematic placement can be ascertained by DNA sequence data, which suggest that the morphological similarities are a result of convergent evolution.
Chaetosphaeria tulasneorum was experimentally linked to its anamorph Cylindrotrichum oligospermum by Réblová & Gams (1999). Based on ncLSU rDNA sequence data, Ch. tulasneorum was separated from the core species of Chaetosphaeria in the Chaetosphaeriaceae (Réblová & Winka 2000) and tentatively placed in the Microascales, along with Cylindrotrichum hennebertii, a non-setose counterpart of C. oligospermum. Chaetosphaeria tulasneorum colonises decaying wood and forms minute, black perithecia containing unitunicate, short-stipitate asci with an inamyloid apical ring, 2–4-celled ellipsoidal to ellipsoidal-fusoid ascospores, and branching and anastomosing filiform paraphyses forming a “network” within the centrum. The reticulate paraphyses and the 1-septate, cylindrical conidia of the Cylindrotrichum anamorph are the only deviating morphological characters between Ch. tulasneorum and other core Chaetosphaeria species.
The phialidic, dematiaceous hyphomycete Dischloridium laeënse, described originally from dead leaves of Musa paradisiaca in Papua New Guinea (Matsushima 1971), is common on dead palm spathes in Australia. In some respects it is similar to species of Chloridium, a well-established anamorph genus associated with Chaetosphaeria, but the microscopic structures are much larger. On material from Australia and England, perithecia of Australiasca (Sivanesan & Alcorn 2002) were associated with fertile conidiophores of D. laeënse. This teleomorph was first reported from England by Kirk (1986) on stems of Dicksonia antarctica, but never described or illustrated. Sivanesan & Alcorn (2002) erected the monotypic ascomycete genus Australiasca including the type species, A. queenslandica, and named its anamorph Dischloridium camelliae. The fungus was isolated from leaves, stems, and branches of Camellia sinensis and the connection between the morphs was proven experimentally in vitro. They distinguished D. camelliae from D. laeënse by longer conidia and larger conidiophores. Sivanesan & Alcorn (2002) compared Australiasca with genera in the morphologically similar families Chaetosphaeriaceae and Lasiosphaeriaceae. At that time, no molecular data were available to confirm placement in either family.
The Australiasca teleomorph of D. laeënse is morphologically similar to species of Chaetosphaeria in perithecial and anamorph characters. Dischloridium laeënse, the type of its genus, produces effuse colonies of single to fasciculate, macronematous conidiophores with a stromatic base. The conidiophores are dark brown but paler towards the apex. The phialidic conidiogenous cells are terminally integrated bearing an indistinct collarette producing basipetal, broadly ellipsoidal, hyaline, nonseptate conidia with a slightly obtuse base produced in slime. Several of the 15 species described in Dischloridium are remarkably similar to Monilochaetes (Halsted 1890), recently revised and delimited from Exochalara and Dischloridium by Rong & Gams (2000) based on detailed morphology and cultivation studies.
To assess the higher level phylogenetic relationships of Ch. tulasneorum and related species of Cylindrotrichum, Australiasca, Dischloridium, and Monilochaetes, we analysed members from 19 orders or families of perithecial ascomycetes. We used DNA sequence data from the nuclear large (ncLSU rDNA) and small (ncSSU rDNA) subunits in independent analyses and combined these with the second largest subunit of RNA polymerase (RPB2) for a multigene analysis.
Based on the phylogenies presented here, several new and strongly supported families and orders are proposed. The order Glomerellales is phylogenetically well-defined and validated to include three families, the Glomerellaceae and the newly described Australiascaceae and Reticulascaceae. The internal transcribed spacer region (ITS including ITS1, 5.8S and ITS2) was used to further analyse the phylogenetic relationships among species of Dischloridium and Monilochaetes. Within the Microascales, we accept four families, i.e. Ceratocystidaceae, which is validated here, and the newly described Gondwanamycetaceae, Halosphaeriaceae, and Microascaceae. We discuss the family and order affinities of Faurelina attributed to the Chadefaudiellaceae of the Microascales by von Arx (1978) and by Tang et al. (2007). We examined authentic material, specifically the in vitro ex-type and another strain of F. indica, and analysed ITS and ncLSU sequence data. Based on results from a ncLSU analysis of the Dothideomycetes, Faurelina (Chadefaudiellaceae) is excluded from the Microascales and placed in the Pleosporales (Dothideomycetes).
MATERIAL AND METHODS
Morphological observations
All herbarium specimens examined and cultures studied are listed under each treated species. Dried specimens were rehydrated in water; material was examined with an Olympus SZX12 dissecting microscope and centrum material including asci, ascospores, and paraphyses was mounted in Melzer's reagent or 90 % lactic acid. Hand sections of the perithecial wall were studied. When present, conidiophores, conidiogenous cells, and conidia were examined in water, Melzer's reagent, or 90 % lactic acid. All measurements were made in Melzer's reagent. Means ± standard errors (s.e.) based on 25 measurements are given for ascospore, ascal, and conidial dimensions. Images were captured using differential interference (DIC) or phase contrast (PC) microscopy using an Olympus DP70 Camera operated by Imaging Software Cell* on an Olympus BX51 compound microscope or an Evolution MP digital camera operated by ImagePro v. 6.0 on an Olympus BX50 compound microscope. Conidia and conidiogenous cells of Australiasca queenslandica were photographed in the living state using an FEI Quanta 200 Environmental Scanning Electron Microscope (ESEM). A ca. 2 × 2 mm cube of agar with mycelium was observed at 20kV after the sample chamber achieved local thermodynamic equilibrium: chamber pressure 200 Pa, sample temperature from -15 °C to -16 °C. A Gaseous Secondary Electron Detector (GSED) was used for signal detection. Cooling of the specimen in the chamber was achieved using a PC-controlled Peltier cooling stage with external water chiller (JT Manufacturing, Hudson, NH, USA). Images were processed with Adobe Photoshop CS4 Extended or Adobe Photoshop CS2.
Single-ascospore isolates were obtained from fresh material with the aid of a single-spore isolator (Meopta, Prague, Czech Republic). Isolates were grown on potato carrot agar (PCA), oatmeal agar (OA), and 2 % malt extract agar (MEA) (Gams et al. 1998). Colonies were examined after 7, 21, and 30 d at 25 °C in the dark and under near-UV light source (12 h light: 12 h dark). Two strains of Faurelina indica were grown on Blakeslee's malt extract agar (Gams et al. 1998) and OA and incubated under ambient room conditions for two mo to induce the arthroconidial anamorph. Cultures are maintained at BRIP (Plant Pathology Herbarium, Queensland, Australia), CBS (CBS Fungal Biodiversity Center, Utrecht, the Netherlands), DAOM (Canadian Collection of Fungal Cultures, Agriculture and Agri-Food Canada, Ottawa, Canada), and the Institute of Botany, Academy of Sciences, Průhonice, Czech Republic.
DNA extraction, amplification and sequencing
DNA was isolated with an UltraClean Microbial DNA Kit (MoBio Laboratories, Inc., Canada) using mycelium removed from PCA or MEA cultures following the manufacturer's protocol for filamentous fungi. All PCR experiments were carried out using a PTC-200 thermal cycler (MJ Research Inc., Watertown, MA, USA). PCR reactions containing 2–4 mM MgSO4 were performed using Platinum Taq DNA polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA) in 25 mL volume reactions. PCR conditions were as follows: for ncSSU 2 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 55 °C and 150–300 s at 68 °C; for ITS and ncLSU 2 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 55–60 °C, and 165–270 s at 68 °C; and for RPB2 2 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 55–61 °C, and 90–180 s at 68 °C; all amplifications were concluded by incubation for 10 min at 68 °C. Amplicons were purified using the UltraClean PCR Clean-up Kit (MoBio Laboratories, Inc., Canada) following the manufacturer's directions. All nucleotide sequences were obtained by the dideoxy chain-terminating method using automated DNA sequencers ABI PRISM 3100 or ABI PRISM 3130xl (Applied Biosystems, Foster City, CA, USA). For PCR reactions, the following primers were used: ncSSU, NSSU131-NS24 (Kauff & Lutzoni 2002, White et al., 1990); ncLSU, ITS5/NS5/LR0R-LR8 (White et al., 1990, Vilgalys unpublished: www.botany.duke.edu/fungi/mycolab); ITS NS5/ITS5-ITS4 (White et al., 1990); RPB2 fRPB2-5F-fRPB2-7cR (Liu et al., 1999). For sequencing reactions, the following primers were used: ncSSU, NSSU131, SR11R, SR7, SR7R, NSSU897R, NSSU1088, NSSU1088R, NS6, NS24 (White et al., 1990, Gargas & Taylor 1992, Spatafora et al., 1995, Kauff & Lutzoni 2002, Vilgalys unpublished: www.botany.duke.edu/fungi/mycolab); ncLSU LR0R, LR3R, LR6, LR7, LR16, LR5 (Vilgalys & Hester 1990, Rehner & Samuels 1994, Vilgalys & Sun 1994), JS7 and JS8 (Landvik 1996); ITS, ITS5 and ITS4 (White et al. 1990); and RPB2 fRPB2-5F, fRPB2-7cR, RPB2-980R and RPB2-1014F (Reeb et al. 2004). Sequences were edited using Sequencher v. 4.9 software (Gene Codes Corp., Ann Arbor, MI, USA).
Phylogenetic analyses
Accession numbers and isolate information for new ITS, ncLSU, ncSSU rDNA and RPB2 sequences are listed in Table 1. The new sequences were aligned with data retrieved from GenBank, mostly from studies published by Wingfield et al. (1999), Réblová & Winka (2000), Spatafora et al. (2006), and Zhang et al. (2006).
Table 1.
Sources and accession numbers of isolates numbers of isolates analysed in this study. GU1806XX–GU1806YY are sequences newly generated in this study.
| Teleomorph | Anamorph | M | Source* |
Substrate and Locality |
GenBank accession numbers** | |||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | RPB2 | |||||
| Australiasca laeënsis | Monilochaetes laeënsis | • | DAOM 226788 | Australia, dead fronds of a tree fern | GU180623 | GU180641 | GU180610 | – |
| Monilochaetes laeënsis | • | PRM 915720 | UK, stem of Dicksonia antarctica | GU180624 | GU180642 | – | – | |
| Australiasca queenslandica | Monilochaetes camelliae | • | BRIP 24607a | Australia, branch of Camellia sinensis | HM237327 | HM237324 | – | – |
| Monilochaetes camelliae | ○ | BRIP 24334c | Australia, branch of Camellia sinensis | HM237326 | HM237323 | – | – | |
| Calosphaeria pulchella | Calosphaeriophora pulchella | • | CBS 115999 | France, wood and bark of Prunus avium | – | AY761075** | AY761071** | GU180661 |
| Ceratosphaeria lampadophora | Harpophora-like | • | CBS 117555 | France, decayed wood | – | – | GU180618 | – |
| Chaetosphaeria ciliata | Menispora ciliata | • | ICMP 18253 | New Zealand, decayed wood | – | GU180637 | GU180614 | GU180659 |
| Chaetosphaeria curvispora | Chloridium-like | • | ICMP 18255 | New Zealand, decayed wood | – | GU180636 | AY502933** | GU180655 |
| Faurelina indica | Arthrographis sp. | • | CBS 126.78 | India, dung of goat | GU291802 | GU180653 | – | – |
| Arthrographis sp. | • | CBS 301.78 | India, dung of cow | – | GU180654 | – | – | |
| Reticulascus clavatus | Cylindrotrichum clavatum | • | CBS 125296 | France, submerged wood of Alnus glutinosa | GU180627 | GU180643 | GU180622 | – |
| Cylindrotrichum clavatum | ○ | CBS 125239 | France, submerged wood of Platanus sp. | GU180633 | GU180649 | GU180615 | – | |
| Cylindrotrichum clavatum | ○ | CBS 125297 | France, submerged wood of Fraxinus sp. | GU180634 | GU180650 | – | – | |
| Cylindrotrichum clavatum | ○ | CBS 428.76 | Sweden, decayed wood of Ulmus scabra | GU291799 | – | – | – | |
| Reticulascus tulasneorum | Cylindrotrichum oligospermum | ○ | CBS 561.77 | Netherlands, twig of Fraxinus excelsior | GU291801 | – | – | – |
| Reticulascus tulasneorum | Cylindrotrichum oligospermum (as hennebertii) | ○ | CBS 570.76 | Germany, dead twig of Symphoricarpos albus | AF178560** | AF178560** | – | – |
| Reticulascus tulasneorum | Cylindrotrichum oligospermum | ○ | CBS 557.74 | Czech Republic, wood of Salix purpurea | GU291798 | – | – | – |
| Cylindrotrichum oligospermum | • | CBS 101319 | Czech Republic, wood of Sambucus nigra | AF178547** | AF178547** | – | – | |
| Togniniella acerosa | Phaeocrella acerosa | • | ICMP 18256 | New Zealand, decayed wood of Nothofagus sp. | – | AY761076** | AY761073** | GU180660 |
| CBS 125298 | ||||||||
| tu | Acrostalagmus annulatus | ○ | DAOM 212126 | Germany, soil and roots | GU180632 | GU180646 | GU180611 | GU180662 |
| tu | Cylindrotrichum gorii | ○ | CBS 879.85 | Sweden, dead stem of Urtica dioica | HM237328 | HM237322 | – | – |
| tu | Cylindrotrichum setosum | ○ | DAOM 229246 | Australia, wood and bark mulch on the ground | GU180635 | GU180652 | GU180617 | – |
| tu | Custingophora olivacea | ○ | CBS 335.68 | Germany, compost | – | – | – | GU180665 |
| tu | Kylindria peruamazonensis | ○ | CBS 838.91 | Cuba, leaf litter of Bucida palustris | GU180628 | GU180638 | GU180609 | GU180656 |
| tu | Kylindria peruamazonensis | ○ | CBS 421.95 | Cuba, leaf of Bucida palustris | GU291800 | HM237325 | – | – |
| tu | Gibellulopsis nigrescens | ○ | DAOM 226890 | Canada, Ontario, soil | GU180631 | GU180648 | GU180613 | GU180664 |
| tu | Monilochaetes guadalcanalensis | ○ | CBS 346.76 | Solomon Islands, leaf of Musa | GU180625 | GU180640 | – | – |
| tu | Monilochaetes infuscans | ○ | CBS 379.77 | New Zealand, Ipomoea batatas | – | GU180645 | GU180619 | GU180658 |
| tu | Monilochaetes infuscans | ○ | CBS 869.96 | South Africa, Ipomoea batatas | GU180626 | GU180639 | GU180620 | GU180657 |
| tu | Monilochaetes infuscans | ○ | CBS 870.96 | South Africa, Ipomoea batatas | – | GU180644 | GU180621 | – |
| tu | Plectosporium tabacinum | ○ | DAOM 229828 | Canada, Ontario, soil | GU180630 | GU180647 | GU180612 | GU180663 |
| tu | Stachylidium bicolor | ○ | DAOM 226658 | straw of Oryza sativa imported from India into Canada | – | GU180651 | GU180616 | – |
M: morph of material available: • = teleomorph, ○ = anamorph.
tu = teleomorph unknown
BRIP = Plant Pathology Herbarium, Queensland, Australia; CBS = Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands; DAOM = Canadian Collection of Fungal Cultures, Agriculture and Agri-Food Canada, Ottawa.
These sequences were published elsewhere (Réblová & Winka 1999, Réblová & Seifert 2004, Réblová et al. 2004).
All sequences were manually aligned in BioEdit v. 7.0.9.0 (Hall 1999). Predicted models of the secondary structure of the ncLSU and ncSSU molecules of Saccharomyces cerevisiae (Gutell 1993, Gutell et al. 1993) were used to improve decisions on homologous characters. To assist with decisions on homologous characters in the ITS alignment, we used the predicted models of the secondary structure designed for species of Chaetosphaeria (Réblová & Winka 2000). They included a model for the whole ITS2 region and a model for long duplex structures located in the middle of the ITS1 region. The long duplex represents the most variable part of the ITS1 alignment because of its variable lengths and irregular occurrence of internal asymmetrical loops.
Phylogenetic relationships were examined using ncLSU, ncSSU, ITS rDNA, and RPB2 sequences of species from 19 orders or families of the Sordariomycetes. For all analyses rooting was accomplished by the outgroup method (Nixon & Carpenter 1993). Two outgroup taxa, Leotia lubrica and Microglossum rufum (Leotiaceae, Helotiales, Leotiomycetes), were used in the ncLSU, ncSSU, and the three-gene (ncLSU-ncSSU-RPB2) analyses of the Sordariomycetes; two outgroup taxa, Vanderwaltozyma polyspora and Saccharomyces cerevisiae (Saccharomycetaceae, Saccharomycetales, Saccharomycetes), were used for the ncLSU phylogeny of Faurelina in the Dothideomycetes; two Chaetosphaeria species (Chaetosphaeriaceae, Chaetosphaeriales, Sordariomycetes) were used as outgroups for the ITS phylogeny.
Maximum parsimony and Bayesian analyses were used to estimate phylogenetic relationships. Four alignments for ITS, ncLSU, ncSSU, and the combined set were constructed. The lengths of the alignments were determined after introduction of gaps. All characters in the ITS alignment were included. Bases 1–75 were excluded from analyses of the ncLSU and ncSSU alignments and bases 1–59 were excluded from the analysis of the RPB2 alignment, because of the incompleteness of the 5′-end of the majority of the available sequences. An additional 69 bases in the RPB2 part of the alignment, which were difficult to identify as homologous, were also excluded. All alignments are deposited in TreeBase (10538).
The three genes for the combined analysis (ncLSU-ncSSU-RPB2) were tested for heterogeneity among data partitions before combining them for the total evidence analysis. We used the partition homogeneity/incongruence-length difference test implemented in PAUP (Swofford 2002) to determine if different partitions of the data gave significantly different signals. Because combining data with value P > 0.01 generally improves phylogenetic accuracy (Cunningham 1997) and our data did not show significant heterogeneity (P = 0.01), the sequences were combined for further analysis.
Maximum parsimony analyses were conducted with PAUP v. 4.0b10 (Swofford 2002). A heuristic search was performed with the stepwise-addition option with 1 000 random taxon addition replicates and TBR branch swapping. All characters were unordered and given equal weight. Gaps were treated as missing data. Branch support was estimated on the recovered topologies by performing a heuristic search of 1 000 bootstrap replicates consisting of ten random-addition replicates for each bootstrap replicate.
Bayesian analysis was performed in a likelihood framework, as implemented by the MrBayes v. 3.0b4 software package, to reconstruct phylogenetic trees (Huelsenbeck & Ronquist 2001). The program MrModeltest2 v. 2.3. (Nylander 2008) was used to infer the appropriate substitution model that would best fit the model of DNA evolution for our sequence data sets. Bayesian searches using Metropolis-coupled Markov chain Monte Carlo sampling were conducted. One cold and three heated Markov chains were used in the analysis. Bayesian analyses were run for 5 M generations with trees sampled every 1 000 generations. The first 20 000 trees representing the “burn-in” phase were discarded. To estimate posterior probabilities (PP) of recovered branches (Larget & Simon 1999), 50 % majority rule consensus trees were produced from the remaining trees using PAUP.
PHYLOGENETIC RESULTS
The first analysis was restricted to the ncLSU. The alignment consisted of the two first thirds of the ncLSU region for 99 sequences representing 91 species in 19 ascomycetous families and orders and 1 283 total characters: 615 constant, 140 not parsimony-informative, and 453 parsimony-informative. A maximum parsimony (MP) heuristic search produced 16 most parsimonious trees (MPTs) with a length of 3 303 steps (CI = 0.303, RI = 0.665, HI = 0.696). One of these trees is shown in Fig. 1. The GTR+I+G substitution model was selected for the Bayesian analysis. The order Glomerellales forms a monophyletic clade (82 % bootstrap support /0.7 posterior probability) with three families recognised, the Australiascaceae (90/1.0), Glomerellaceae (85/0.83), and Reticulascaceae (97/1.0). Within the Reticulascaceae, Cylindrotrichum setosum is sister to the Reticulascus clavatus clade (95/1.0), R. tulasneorum forms a well-supported clade (75/1.0), and Kylindria peruamazonensis and Porosphaerellopsis are nested at the base of the Reticulascaceae (97/1.0). The order Microascales as presently conceived appears to be polyphyletic. The monophyletic Ceratocystidaceae (100/1.0) and Gondwanamycetaceae (100/1.0) form a clade (92/1.0) as a sister to the Plectosphaerellaceae (100/1.0). The other two families of the Microascales form a separate clade (82/1.0) containing the Microascaceae (97/1.0) and the Halosphaeriaceae (88/1.0). The second analysis was restricted to the ncSSU. The alignment consisted of the whole gene for ncSSU for 71 sequences representing 67 species in 19 ascomycetous orders and families and 1 777 total characters: 1 102 constant, 195 not parsimony-informative, and 405 parsimony-informative. A maximum parsimony heuristic search produced 14 MPTs with a length of 2 267 steps (CI = 0.390, RI = 0.697, HI = 0.609), one of which is shown in Fig. 2. For the Bayesian analysis, the GTR+I+G substitution model was inferred. The Glomerellales form a monophyletic, strongly supported clade (81/0.98) containing representatives of three families, the Australiascaceae (78/1.0), Glomerellaceae (100/1.0), and Reticulascaceae (94/1.0). The Plectosphaerellaceae form a separate, strongly supported clade (100/1.0) basal to the Microascales. The Microascales appear as a monophyletic clade (100/1.0) including two strongly supported subclades. The first subclade (78/1.0) contains the Halosphaeriaceae (65/-) and Microascaceae (81/1.0) and the second subclade (83/1.0) contains the Ceratocystidaceae (80/1.0) and Gondwanamycetaceae (100/1.0).
Fig. 1.
One of 16 most parsimonious trees from a heuristic analysis of ncLSU rDNA sequences. Thickened branches indicate posterior probability values = 1.0 PP and 100 % bootstrap support. Bootstrap support values ≥ 50 % and Posterior probability values ≥ 0.5 are included at the nodes. Branch lengths are drawn to scale. An asterisk above or below a branch marks branches that collapse in the strict consensus tree.
Fig. 2.
One of the 14 most parsimonious trees from a heuristic analysis of ncSSU rDNA sequences. Details as in Fig. 1.
In the third analysis, a combination of the ncLSU and ncSSU data sets plus RPB2 sequences was assessed for 54 taxa representing 52 species in 18 ascomycetous orders and families. The alignment of the combined set of ncLSU-ncSSU-RPB2 DNA sequences consisted of 4 224 total characters: 2 148 constant, 314 not parsimony-informative, and 1 484 parsimony-informative. A maximum parsimony heuristic search produced two MPTs with a length of 11 688 steps (CI = 0.278, RI = 0.476, HI = 0.722); one is shown in Fig. 3. For the Bayesian analysis the GTR+I+G substitution model was selected. The Glomerellales are a monophyletic, well-supported clade (100/0.81) with the Plectosphaerellaceae as a sister group (100/1.0). The Microascales appear as a monophyletic, strongly supported clade (88/1.0), again with two subclades; the first (87/1.0) contains the Halosphaeriaceae (94/1.0) and Microascaceae (88/1.0) while the other (100/1.0) comprises the Ceratocystidaceae (100/1.0) and Gondwanamycetaceae (99/1.0).
Fig. 3.
One of the two most parsimonious trees from a heuristic analysis of the three-gene combined data set (ncLSU-ncSSU-RPB2). Details as in Fig. 1. The GenBank accession numbers given after the names are those of ncLSU/ncSSU/RPB2 genes. Missing sequences are indicated by “–“.
The fourth analysis included ITS1, 5.8S, and ITS2 regions of species of the Glomerellales and Plectosphaerellaceae. The alignment consisted of 38 sequences representing 29 species in five families and 574 total characters: 277 constant, 75 not parsimony-informative, and 222 parsimony-informative. A maximum parsimony heuristic search produced nine MPTs with a length of 786 steps (CI = 0.592, RI = 0.821, HI = 0.407). One is shown in Fig. 4. The GTR+G substitution model was inferred for the Bayesian analysis. Following the results of our other analyses, two Chaetosphaeria species (Chaetosphaeriales) were used as outgroups. The order Glomerellales is a strongly supported monophylum (95/0.99) containing three strongly supported families, the Australiascaceae (99/1.0), Glomerellaceae (99/0.97), and Reticulascaceae (96/1.0). The Plectosphaerellaceae (100/1.0) appears as a strongly supported sister clade to the Glomerellales. Six strains represent the Australiascaceae in the analysis: two strains of Australiasca queenslandica, two strains of A. laeënsis, and one strain each of Monilochaetes infuscans and M. guadalcanalensis. The Reticulascaceae are represented by four strains of Reticulascus clavatus (anamorph C. clavatum), four strains of R. tulasneorum (anamorph Cylindrotrichum oligospermum), C. setosum, C. gorii, and two strains of Kylindria peruamazonensis. The two conidial (CBS 125239, CBS 125297) and single ascospore (ex-type strain CBS 125296) isolates of freshwater R. clavatus plus one terrestrial isolate (CBS 428.76) formed a strongly supported monophylum (100/1.0). Another strongly supported monophyletic clade (97/1.0) included one ascospore-(ex-type strain CBS 101319) and three conidial isolates of R. tulasneorum (CBS 557.74, CBS 561.77, ex-type strain of C. hennebertii CBS 570.76). The anamorphic C. setosum (ex-type strain DAOM 229246), C. gorii (CBS 879.85), and K. peruamazonensis (CBS 421.95, CBS 838.91) were basal to the rest of the clade on separate branches.
Fig. 4.
One of the nine most parsimonious trees from a heuristic analysis of ITS rDNA operon of the Glomerellales and the Plectosphaerellaceae. The accession numbers of strains of the newly described Australiascaceae and Reticulascaceae are indicated. Details as in Fig. 1.
A fifth analysis of the ncLSU rDNA sequences was run to determine the relationship of two strains of Faurelina indica with members of the Dothideomycetes and Eurotiomycetes. The alignment consisted of the first two thirds of the ncLSU for 68 sequences representing 66 species in 11 orders and families and 1 229 total characters: 716 constant, 76 not parsimony-informative, and 362 parsimony-informative. A maximum parsimony heuristic search produced 66 MPTs with a length of 1 593 steps (CI = 0.433, RI = 0.760, HI = 0.567). One is shown in Fig. 5. The GTR+I+G substitution model was selected for the Bayesian analysis. The two strains of Faurelina form a monophyletic clade (88/0.9), which is a sister to the Didymellaceae (96/1.0). The suggested relationship of Faurelina with the Eremomycetaceae and Testudinaceae could not be confirmed; the families grouped on separate branches with no close relationship to each other. Faurelina appears to be a member of the Pleosporales within the Dothideomycetes unrelated to the Microascales.
Fig. 5.
One of 66 most parsimonious trees from a heuristic analysis of ncLSU rDNA of Faurelina indica and Dothideomycetes. Details as in Fig. 1.
TAXONOMY
Glomerellales
Chadefaud (1960) proposed the order “Glomérellales” for a group of endophytic fungi and parasites of living plants with ascomata varying from endostromatal to apostromatal and ascospores that are often unicellular and hyaline. No Latin diagnosis was provided for the order. Within the order he suggested an evolution of the apical apparatus from an initial condition of the periocular thickening of the apical dome lacking a pronounced chitinoid or amyloid ring to derived conditions of either the apical thickening converted into an apical cushion reduced to a simple lens-shaped disc or with the initial of a chitinoid ring developing in the periocular thickening. According to the texture and pigmentation of the ascomata, he further divided the order into two groups: a) “Eu-Glomérellales”, which included genera with a non-fleshy black stroma i.e. Gibellina, Glomerella, Phyllachora, and Physalospora; and b) “Polystigmatales” as “Glomérellales nectrioïdes”, which comprised one genus, Polystigma, with a orange to red, fleshy stroma. After this invalid introduction of the name Glomerellales, the order was also cited by Lanier et al. (1978) and later by Locquin (1984), when he listed the Glomerellales and Polystigmatales as separate orders, again without a Latin diagnosis. After the validation of the Glomerellaceae in Zhang et al. (2006), we validate here the phylogenetically delimited order Glomerellales, excluding the earlier validated but unrelated Phyllachorales.
Three families are accepted in the Glomerellales, namely the Glomerellaceae, Australiascaceae, and Reticulascaceae. The latter two families are newly described below based on cultural studies, detailed morphological comparisons of the holomorphs, and newly generated ITS, ncLSU, ncSSU, and RPB2 sequences.
Glomerellales Chadef. ex Réblová, W. Gams & Seifert, ord. nov. MycoBank MB515429.
Glomerellales Chadef., Traité de botanique systématique. Tome I, p. 613. 1960 (also in Lanier et al., Mycol. Pathol. Forest. I: 292. 1978; Locquin, Mycol. Gén. Struct., p. 170. 1984) nom. inval., Art. 36.
Ascomata perithecia, brunnea usque nigra, nonnumquam sclerotioidea, ostiolum periphysatum. Pariete ascomatum 2-3-stratoso. Hamathecium paraphyses verae. Asci unitunicati, brevi-stipitati, parte apicali iodo non reagente. Ascosporae hyalinae vel pallide pigmentatae, 0-pluri-cellulares. Anamorphe: conidia modo phialidico orientia.
Typus: Glomerellaceae Locq. ex Seifert & W. Gams, Mycologia 98: 1083. 2007 [2006].
Perithecia darkly pigmented, sometimes becoming ± sclerotial. Perithecial wall 2–3-layered, ostiolum periphysate. Interascal tissue of thin-walled, tapering paraphyses. Asci unitunicate, thin-walled, ascal apex thickened without visible discharge mechanism or thin-walled with a distinct apical annulus, inamyloid, 8-spored. Ascospores hyaline or pigmented, 0–several-septate. Anamorphs with phialidic conidiogenesis.
Families: Australiascaceae Réblová & W. Gams, Glomerellaceae Locq. ex Seifert & W. Gams, and Reticulascaceae Réblová & W. Gams.
This order is phylogenetically distinct from the Phyllachorales, in which its members were formerly classified. The original classification proposed by Chadefaud (1960), who included Phyllachora in this order, is untenable based on our molecular data (Fig. 2). In the ncSSU phylogeny, the Phyllachorales represented by Phyllachora graminis (ncSSU rDNA sequence: AF064051, Winka & Eriksson 2000) are clearly separated from the Glomerellales; the former is nested within a clade (91/1.0) sister to the Chaetosphaeriales (100/1.0).
Glomerellaceae
This family accommodates the teleomorph genus Glomerella and its Colletotrichum anamorphs. For discussion and description refer to Zhang et al. (2006).
Glomerellaceae Locq. ex Seifert & W. Gams in Zhang et al., Mycologia 98: 1083. 2007. [2006].
Australiascaceae Réblová & W. Gams, fam. nov. MycoBank MB515430.
Stromata absentia. Ascomata perithecia, brunnea usque nigra, ostiolum periphysatum. Pariete ascomatum fragili, 2-stratoso. Hamathecium paraphyses verae. Asci unitunicati, 8-spori, cylindraceo-clavati, annulo apicali iodo non reagente. Ascosporae hyalinae, septatae. Anamorphe Monilochaetes; conidiis 0(–3)-septatis, hyalinis modo phialidico orientibus.
Typus: Australiasca Sivan. & Alcorn, Aust. Syst. Bot. 15: 742. 2002.
Stroma absent. Perithecia brown to black, ostiolum periphysate. Perithecial wall 2-layered, fragile. Interascal tissue of thin-walled, tapering paraphyses. Asci unitunicate, 8-spored, cylindrical-clavate, apical ring distinct, inamyloid. Ascospores hyaline, septate. Anamorph: Monilochaetes; conidiogenesis phialidic, with hyaline 0(–3)-septate conidia, aggregated in slime or in chains.
The Australiascaceae accommodates the holomorphic genus Australiasca and anamorphic Monilochaetes. The molecular data for Australiasca (Figs 1, 2, 3) confirm that the genus is unrelated to the Chaetosphaeriaceae or Lasiosphaeriaceae as suggested by Sivanesan & Alcorn (2002). However, the Australiascaceae, like the Reticulascaceae, accommodates teleomorphs that mimic Chaetosphaeria and which are almost indistinguishable from its perithecia on morphological grounds. The anamorphs are phialidic, dematiaceous hyphomycetes with hyaline, slimy conidia, which are also similar to anamorphs of Chaetosphaeria.
The dematiaceous hyphomycete genus Monilochaetes was described and illustrated for a single species, M. infuscans (Halsted 1890, Harter 1916), which causes scurf disease or soil stain of Ipomoea batatas (sweet-potato). Another saprobic species, M. guadalcanalensis, collected on leaves of Musa sp., originally described in Catenularia, was recently added (Rong & Gams 2000) and this classification is confirmed here by molecular data. Monilochaetes includes species with solitary, erect, sometimes curved or geniculate, macronematous conidiophores, darker near the base, becoming paler towards the apex, with prominently darkened septa, terminal, wide monophialides with a shallow collarette, and aseptate, rarely septate, hyaline conidia adhering in basipetal chains or heads. Rong & Gams (2000) distinguished Monilochaetes from the other two similar dematiaceous hyphomycete genera Dischloridium (Sutton 1976) and Exochalara (Gams & Holubová-Jechová 1976) by aspects of conidiophore branching and fasciculation and conidial shapes and dimensions. The present ITS and ncLSU phylogenies confirm that Dischloridium and the morphologically similar older genus Monilochaetes, which up to now was only known as asexual, are congeneric. Therefore, Dischloridium laeënse, type of the genus, is transferred to Monilochaetes and Dischloridium becomes a generic synonym of Monilochaetes.
The teleomorph-anamorph connections of Australiasca queenslandica, type species of the genus, with M. camelliae and of the newly described A. laeënsis with M. laeënsis were experimentally established (Sivanesan & Alcorn 2002, this study). The other four species accepted in Monilochaetes are presently only known to be anamorphic. A further species of Monilochaetes is described by Réblová et al. (2011)
KEY TO THE SPECIES OF AUSTRALIASCA AND MONILOCHAETES IN THE AUSTRALIASCACEAE
1. Conidia hyaline, ellipsoidal, aseptate, rarely 1–3-septate, longer than 26 μm.......................................................................................... 2 1. Conidia hyaline, ellipsoidal, aseptate, rarely 1-septate, shorter than 26 μm............................................................................................. 3
2. Conidia ellipsoidal with an obtuse base, sometimes with laterally displaced hilum, aseptate, rarely 1–3-septate at maturity, 18–35 × 8–13 μm in vitro; 20.5–24(–26.5) × (10–)11–12(–13) μm on PCA; asci 65–140 × 12.5–17.5 μm; ascospores 18–31 × 7.5–10.5 μm.................................................................................................. A. camelliae (anamorph M. camelliae) 2. Conidia cylindrical to ellipsoidal with an obtuse base, 25–38 × 12–16 μm in vivo; teleomorph unknown ............................ M. regenerans
3. Conidia aseptate, ellipsoidal to oblong, usually in small clusters, aggregated in slimy droplets............................................................... 4 3. Conidia 0–1-septate, rhomboid–ellipsoidal to obovoidal, usually forming chains..................................................................................... 5
4. Conidia oblong, apically rounded, with an obtuse base, 9–25 × 3.5–6(–7) μm; teleomorph unknown............................... M. basicurvata 4. Conidia ellipsoidal to oblong with an obtuse base, 22–26 × 10–12 μm in vivo, (15.5–)18–22.5(–23.5) × 7.5–9(–10) μm in vitro (PCA); asci 130–148 × 12.5–17.5 μm; ascospores (20–)24.5–31.5(–33) × (7.5–)9–9.5 μm......................... A. laeënsis (anamorph M. laeënsis)
5. Conidia rhomboid–ellipsoidal to obovoidal on host, rarely 1-septate, ellipsoidal with an obtuse base in culture, 15–20 × 4–6 μm...................................................................................................................................................................... M. infuscans 5. Conidia ellipsoidal with an obtuse base in culture, 18–21 × 6–9 μm.......................................................................... M. guadalcanalensis
Australiasca laeënsis Réblová & W. Gams, sp. nov. MycoBank MB518384, Fig. 6A–K.
Fig. 6.
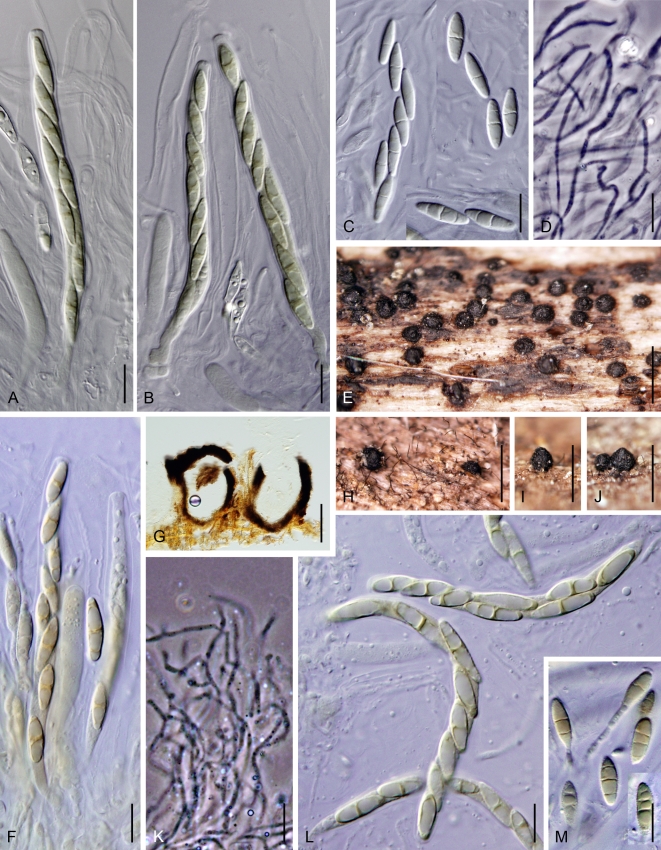
A–K. Australiasca laeënsis. A. Perithecium. B. Ascospores. C. Asci with ascospores, some ascospores with a developed median septum. D, E. Conidia and F–K. Conidiophores of the Monilochaetes laeënsis anamorph, in culture. A–C from PRM 915720; D–K DAOM 226788 (PCA, 14 d old). Scale bars: A = 100 μm; B, D–K = 10 μm; C = 50 μm. DIC: A, D, G–K; PC: B, C, E, F.
Anamorph: Monilochaetes laeënsis (Matsush.) Réblová, W. Gams & Seifert, comb. nov. MycoBank MB515431. Basionym: Chloridium laeënse Matsush., Bull. Natl. Sci. Mus. Tokyo 14: 462. 1971.
≡ Dischloridium laeënse (Matsush) B. Sutton, Kavaka 4: 47. 1976.
Etymology: Epithet from the anamorph species, originally derived from the type locality, Lae in Papua-New Guinea (Matsushima 1971).
Stromata absentia. Perithecia superficialia, gregaria vel solitaria, atra, conica usque obpyriformia, 200–320 μm diam, 340–450 μm alta, ostiolum periphysatum. Paries ascomatum fragilis, 2-stratosus. Paraphyses septatae, hyalinae, sursum angustatae, ascos superantes. Asci unitunicati, cylindraceo-clavati, 130–148 × 18–20 μm (in medio ± s.e. = 137.6 ± 5.3 × 19.3 ± 0.6 μm), 8-spori, brevi-stipitati, apice truncato. Ascosporae ellipsoideae usque ovoideae, 24.5–31.5(–33) × (8–)9–9.5 μm (in medio ± s.e. = 15.7 ± 0.2 × 4.4 ± 0.04 μm), hyalinae, 0–1-septatae. Anamorphe Monilochaetes laeënsis.
Perithecia 200–320 μm diam, 340–450 μm high, gregarious to solitary among conidiophores, superficial, base slightly immersed, conical to obpyriform, with a short beak, black, glabrous or with setae. Setae scanty, acute, thick-walled, septate, dark brown, paler to subhyaline towards apex, sometimes on upper half of perithecium, 90–155 × 5–7 μm; longer, thicker-walled setae, arising from base of perithecium, 300–420 × 10–11 μm. Perithecial wall 18–22 μm thick, becoming 45–54 μm thick towards base, fragile, 2-layered: outer layer of textura prismatica consisting of thick-walled, brick-like cells, cells becoming polyhedral towards base; inner layer of hyaline, compressed cells. Paraphyses ca. 2.5–3 μm wide, persistent, hyaline, septate, branching, longer than asci. Asci 130–148 × 18–20 μm (mean ± s.e. = 137.6 ± 5.3 × 19.3 ± 0.6 μm), unitunicate, cylindrical-clavate, short-stipitate, apex truncate, with a distinct, shallow annulus, ca. 6 μm wide, 1–1.5 μm high, 8-spored. Ascospores 24.5–31.5(–33) × (8–)9–9.5 μm (mean ± s.e. = 28.4 ± 0.6 × 8.9 ± 0.1 μm), ellipsoidal to oblong, apiculate at both ends, 1-celled, becoming transversely 1–3-septate after discharge, smooth, germinating with germ tubes at both ends, hyaline, irregularly 2-seriate in ascus.
Colonies in vivo dark, hairy, effuse. Conidiophores 200–600 μm long, 6.5–9 μm wide, arising in small fascicles or small loose groups of 2–6 or solitary from a minute stromata, macronematous, percurrently proliferating, dark brown, 5–15-septate; base occasionally bulbous with smaller, thick-walled, adjacent pseudoparenchymatous cells forming stromatic tissue in substratum. Conidiogenous cells monophialidic, 50–70 × 4.5–8(–10) μm, terminal, cylindrical, hardly tapering at apex, subhyaline; collarette ca. 1–2 μm high, minute, conidiogenous locus located at base of collarette. Conidia 22–26 × 10–12 μm (mean ± s.e. = 23.2 ± 0.2 × 10.8 ± 0.2 μm), ellipsoidal to cylindrical-ellipsoidal, broadly rounded, sometimes obtuse at base, hyaline, basal scar 3.5–4 μm diam, smooth-walled.
Colonies in vitro after 14 d on PCA at 25 °C 15–20 mm diam, felty, stromatic tissue absent, aerial mycelium olive-brown, margin entire; reverse pale greyish-brown. Colonies readily sporulating, beginning after 5 d on PCA at 25 °C under near-UV light (12 h light: 12 h dark). Conidiophores, phialides, and conidia morphologically identical to those on natural substratum. Conidiophores 40–160 × 7–8 μm, pale brown throughout, with none or 1 percurrent proliferation, 2–5-septate; in about 28 d, longer conidiophores developing, ca. 160-280 μm long, dark brown, subhyaline towards apex, with 1–4 percurrent proliferations, up to 2–15-septate. Conidiogenous cells monophialides 31–58 × (6–)7–9 μm, tapering to 4–6.5 μm just below collarette; collarette ca. 1.5 μm high and (5.5–)6–8 μm wide. Conidia (15.5–)18–22.5(–23.5) × 7.5–9(–10) μm (mean ± s.e. = 20.9 ± 1 × 8.2 ± 0.3 μm), ellipsoidal to cylindrical-ellipsoidal, broadly rounded, sometimes obtuse at base, hyaline, basal scar 2–3.5 μm diam, smooth-walled.
Specimens examined (anamorph and teleomorph): Australia, New South Wales, Blue Mountains, Mt. Tomah Botanical Garden, S 33 32.4, E 150 25.4, 1197 m alt., on dead stipes and spathes of a tree fern in a rain forest, 17 Aug. 1999, K.A. Seifert no. 884 and G.J. Samuels, DAOM 226788. UK, England, West Cornwall, Penjerrick House Gardens, 22 June 2000, dead stipes of Dicksonia antarctica, B. Candy, PRM 915720, holotype of A. laeënsis.
Notes: Based on the results from ITS and LSU rDNA phylogenies, Australiasca laeënsis and A. queenslandica are distinct species, although they are morphologically similar. Australiasca queenslandica and its M. camelliae anamorph were originally described and isolated into culture from leaves, stems and branches of Camellia sinensis; perithecia containing mature asci and ascospores formed in vitro (Sivanesan & Alcorn 2002). The ascospores released by A. queenslandica were often observed to be 1–3-septate, becoming dictyoseptate, and some produced phialides with hyaline microconidia in vitro. The recently collected material of A. laeënsis from England and Australia documents perithecia produced on the host associated with the conidiophores of its M. laeënsis anamorph. The ascospores were observed to be transversely 1–3-septate after discharge, but never became dictyoseptate or exhibited phialidic germination. Australiasca laeënsis is described here based on our observations on the host and the anamorph in culture.
The range of conidial lengths of M. camelliae and M. laeënsis overlap, but those of the former species are usually longer. Monilochaetes camelliae produces conidia 18–35 × 8–13 μm in culture on Sachs agar + maize leaves (Sivanesan & Alcorn 2002) or 20.5–24(–26.5) × (10–)11–12(–13) μm on PCA (this study). The conidia of M. laeënsis are 22–26 × 10–12 μm on the host and (15.5–)18–22.5(–23.5) × 7.5–9(–10) μm on PCA (this study). Therefore, the conidia of M. camelliae from Sachs agar overlap in length with conidia of M. laeënsis, but exceed its upper range by nearly 10 μm, while on PCA the conidia of M. camelliae are only slightly longer than those of M. laeënsis. The conidial dimensions for M. laeënsis from our collections correspond with measurements of the type and other specimens on host substrata from different localities, e.g. Sutton (1976, conidia 15–20 × 8–10 μm), Matsushima (1971, 17–26 × 8–12 μm), and Holubová-Jechová (1982, 14.5–24 × 6.5–10 μm). The conidia of M. laeënsis are hyaline, aseptate, and arise singly from the conidiogenous locus, usually in slimy heads. The conidia of M. camelliae were described as occasionally 1–3-septate, produced in heads or chains (Sivanesan & Alcorn 2002). The conidiophores of M. camelliae are also slightly longer, often swollen subapically (Sivanesan & Alcorn 2002).
Monilochaetes laeënsis has been collected on dead leaves in Papua New Guinea (Matsushima 1971), Sri Lanka (Sutton 1976, Bhat & Sutton 1985), and Cuba (Holubová-Jechová 1982), dead leaves or twigs and dead palm spathes in Australia, Ethiopia, India and Malaysia (Bhat & Sutton 1985), and dead fern stipes in the United Kingdom (Kirk 1986). Only the European and recent Australian material contained perithecia with mature asci and ascospores. Kirk (1986) noted that Dischloridium does not occur naturally in the British Isles but was probably introduced into gardens where it was found along with its host Dicksonia antarctica. He also suggested that the prevailing colder temperatures may have triggered sexual reproduction in nature; our own teleomorph specimen was collected in a cool, humid valley in the Australian winter.
Australiasca queenslandica Sivan. & Alcorn, Aust. Syst. Bot. 15: 742. 2002. Figs 7A–R, 8A–G.
Fig. 7.
A–R. Monilochaetes camelliae anamorph of Australiasca queenslandica. A–D. Conidiogenous cells. E. Conidiophores. F–J. Conidia. K–M. Microconidia. N–R. Minute conidiophores that produce microconidia. A–J from BRIP 24607 (PCA, 14 d old), K–R from BRIP 24334c (PCA, 6 mo old). Scale bars: A–D, F–J, K–R = 10 μm; E = 50 μm. A–R: DIC.
Fig. 8.
A–G. Environmental Scanning Electron Microscopy photographs of Monilochaetes camelliae anamorph of Australiasca queenslandica. A, B, F, G. Chains of conidia; arrow indicates the tip with a porus of the conidiogenous cell after the liberation of the conidial chain. C. Conidiogenous cells with collarette. D. Conidium with laterally displaced hilum. E. Conidiogenous cell with conidia. A–G from BRIP 24607 (PCA, 14 d old). A, B, E, G = 20 μm; C, D = 10 μm; F = 50 μm.
Anamorph: Monilochaetes camelliae (Alcorn & Sivan.) Réblová, W. Gams & Seifert, comb. nov. MycoBank MB518385. Basionym: Dischloridium camelliae Sivan. & Alcorn, Aust. Syst. Bot. 15: 743. 2002.
Colonies in vitro on MEA after 14 d at 25 °C with 22–25 mm radial growth, more or less planar, surface dark brown, covered with abundant, pale grey, lanose to cottony aerial mycelium, margin smooth and entire, reverse grey, sterile. Colonies on PCA after 14 d at 25 °C with 23–25 mm radial growth, planar, surface brown, covered with pale grey, lanose to cottony aerial mycelium, margin smooth and entire, reverse dark grey, sterile.
Colonies in vitro on PCA sporulating in 14 d at 25 °C in darkness. Setae absent. Conidiophores 200–720 μm long, 9–10(–10.5) μm wide near base and 6.5–7.5(–8.5) μm wide in middle, pale to dark brown, subhyaline towards apex, with none or 1 percurrent proliferation, up to 20-septate. Conidiogenous cells monophialidic, subhyaline, paler towards collarette, ampulliform to cylindrical, slightly swollen, 36–45(–60) μm long, 6.5–8(–9) μm wide at widest part, tapering to ca. 3–4 μm just below collarette; collarette 4.5–5.5 μm wide and ca. 1.5–2 μm high. Conidia 20.5–24(–26.5) × (10–)11–12 μm (mean ± s.e. 22.5 ± 0.3 × 11.8 ± 0.1), 0–1-septate, ellipsoidal to cylindrical-ellipsoidal, broadly rounded at end, obtuse at base, basal scar 3–3.5 μm diam, some conidia with a laterally displaced hilum, hyaline, smooth-walled.
After 6 mo on PCA at 25 °C in darkness, producing minute conidiophores with microconidia. Setae absent. Conidiophores more or less erect, arising from aerial mycelium, simple or sparingly branched, pale brown to subhyaline, 40–60 μm long and 2–2.5 μm wide, with terminally integrated or intercalary conidiogenous cells. Conidiogenous cells monophialidic, subhyaline to pale brown, usually paler towards apex, ampulliform to cylindrical, 8–20 μm long, 2.5–3.5 μm wide at widest part, tapering to ca. 1.5 μm just below collarette; collarette 2.5–3 wide, ca. 2 μm high. Conidia 4–5.5 × 3–3.5 μm (mean ± s.e. 4.5 ± 0.1 × 3.2 ± 0.1), aseptate, thick-walled, broadly ellipsoidal to subglobose, rounded at ends, base slightly tapering, obtuse with a minute abscission scar, accumulating in small, clear to whitish droplets, hyaline, smooth-walled. Chlamydospores not observed.
Specimens examined (anamorph only): Australia, Queensland, Malanda, isolated from branch of Camellia sinensis, 19 Feb. 1997, D. Steel M. 8982c, BRIP 24334c; Queensland, Brisbane, S 27 30, E 152 58, isolated from branch of Camellia sinensis, 10 July 1997, J.L. Alcorn, BRIP 24607a).
Notes: Two isolates of M. camelliae were examined and ITS and ncLSU sequences were generated (Table 1). One of these is an authentic, single-ascospore isolate listed among specimens examined in the protologue of Dischloridium camelliae (Sivanesan & Alcorn 2002).
The ESEM photographs of conidia of M. camelliae (Fig. 8A, B, F, G) demonstrate well that there is a continuum between conidial chains and slimy heads on the phialides. The osmolarity of the medium may influence the relative proportion of chains and slimy heads as seen particularly in Chloridium, where chains, cirrhi, and slimy heads are all observed in one genus or even one species (W. Gams, unpubl. data). The conidial chains of M. camelliae were difficult to observe in squash mounts from agar, but were visible directly in the Petri dish by light microscopy.
Additional species of Monilochaetes
Since its description the original generic concept of Dischloridium has been expanded with the addition of fifteen species having variable morphology of conidia and conidiophores including several species with brown, distoseptate conidia. To be consistent with the morphological delimitation of Monilochaetes indicated by phylogeny, we accept only two of the fourteen remaining species previously included in Dischloridium for transfer to Monilochaetes, namely D. basicurvatum and D. regenerans. Other species are newly transferred to or accepted in other hyphomycete genera, such as Craspedodidymum, Hyalocylindrophora, or Paradischloridium, and a few cannot presently be reassigned.
After revising type material, cultivation studies, and molecular data of Exochalara longissima, the type species of that genus, we confirm that the species is unrelated to Monilochaetes (material and isolates examined: IMI 18047 holotype of Chalara longissima; IMI 167413 holotype of Catenularia piceae; CBS 980.73, cited as the only strain in the description of E. longissima by Gams & Holubová-Jechová 1976, and CBS 393.82). The true relationship of the genus Exochalara lies with the Helotiales of the Leotiomycetes (Réblová et al. 2011). The strain studied by Rong & Gams (2000), CBS 662.82, with pronounced branching of the short conidiophores, is not conspecific with or related to E. longissima.
Monilochaetes Halst., New Jersey Agric. Exp. Stn. Bull. 76: 27. 1890.
= Dischloridium B. Sutton, Kavaka 4: 47. 1976.
Monilochaetes basicurvata (Matsush.) Réblová & Seifert, comb. nov. MycoBank MB515432. Basionym: Dischloridium basicurvatum Matsuh., Matsush. Mycol. Mem. 8: 18. 1995.
Monilochaetes guadalcanalensis (Matsush.) I.H. Rong & W. Gams, Mycotaxon 76: 455. 2000. Basionym: Catenularia guadalcanalensis Matsush., Microfungi of the Salomon Islands and Papua New Guinea, Kobe, p. 10. 1971.
≡ Exochalara guadalcanalensis (Matsush.) W. Gams & Hol.-Jech., Stud. Mycol. 13: 58. 1976.
Monilochaetes infuscans Ellis & Halst., New Jersey Agric. Exp. Stn. Bull. 76: 27. 1890. Fig. 9A–I.
Fig. 9.
A–I. Monilochaetes infuscans. A–F. Conidiophores; arrow indicates a percurrent regeneration of the conidiophore. G–I. Conidia. A–I from CBS 379.77 (PCA, 14 d old). Scale bars: A = 25 μm; B–I = 10 μm. DIC: A, C–I. PC: B.
= Dischloridium cylindrospermum S.K. Srivast., Sydowia 39: 217. 1986.
Monilochaetes regenerans (Bhat & W.B. Kendr.) Réblová & Seifert, comb. nov. MycoBank MB515433. Basionym: Dischloridium regenerans Bhat & W.B. Kendr., Mycotaxon 49: 48. 1993.
Species excluded from Dischloridium and Monilochaetes, but not reclassified
Accepted names are printed in bold.
Dischloridium keniense P.M. Kirk, Mycotaxon 23: 30. 1985. Basionym: Craspedodidymum keniense (P.M. Kirk) Bhat & W.B. Kendr., Mycotaxon 49: 37. 1993.
Dischloridium roseum (Petch) Seifert & W. Gams, Mycotaxon 24: 459. 1985. Basionym: Acremonium roseum Petch, Ann. Royal Bot. Gard. Peradeniya 7: 317. 1922.
≡ hyalocylindrophora rosea (Petch) Réblová & W. Gams, comb. nov. MycoBank MB515434
-
= Hyalocylindrophora venezuelensis J.L. Crane & Dumont, Canad. J. Bot. 56: 2616. 1978.
≡ Dischloridium venezuelense (J.L. Crane & Dumont) Bhat & B. Sutton, Trans. Brit. Mycol. Soc. 84: 725. 1985.
Notes: With this new combination the combining authors accept the argument by Holubová-Jechová (1990) that this hyaline species should not be considered congeneric with similar pigmented species. The species has not been cultured or sequenced.
Dischloridium triseptatum Hol.-Jech, Česká Mykol. 41: 110. 1987. Fig. 10A–J.
Fig. 10.
A–J. Paradischloridium ychaffrei. A–H. Conidiophores. I. Conidia. J. Base of the conidiophore. K–T. Dischloridium tenuisporum. K–P. Conidiophores with conidia. Q. Conidia. R–T. Stromatic spots erumpent through the epidermis of the host bearing clusters of conidiophores. A–J from PRM 842733 (holotype of Dischloridium triseptatum), on the host; K–T from PRM 842727 (holotype), on the host. Scale bars: A–J, Q = 10 μm; K–P = 25 μm; R, T = 50 μm; S = 250 μm. DIC: A–J.
= Paradischloridium ychaffreiBhat & B. Sutton, Trans. Brit. Mycol. Soc. 84: 723. 1985.
Specimens examined: Cuba, Oriente, Gran Piedra Mts., Nature Reserve Isabelica Norte, near Santiago de Cuba, on dead branches of an unidentified tree, 22 May 1985, V. Holubová-Jechová, PRM 842733, holotype of D. triseptatum.
Dischloridium venezuelense (J.L. Crane & Dumont) Bhat & B. Sutton, Trans. Brit. Mycol. Soc. 84: 725. 1985.
= hyalocylindrophora rosea (Petch) Réblová & W. Gams (see above).
Dischloridium ychaffrei (Bhat & B. Sutton) Hol.-Jech., Česká Mykol. 42: 204. 1988. Basionym: Paradischloridium ychaffrei Bhat & B. Sutton, Trans. Brit. Mycol. Soc. 84: 723. 1985. Fig. 10A–J.
= Dischloridium triseptatum Hol.-Jech, Česká Mykol. 41: 110. 1987.
Notes: Paradischloridium was erected for phialidic dematiaceous hyphomycetes reminiscent of Dischloridium, but with conidiophores that are not fasciculate and do not arise from stromatic tissue. The phialides lack even remnants of a collarette and conidia are brown with 3-distosepta (Bhat & Sutton 1985). The conidiogenesis of P. ychaffrei is particularly interesting. Fig. 10C–F show the conidiogenous locus sitting deeper in the venter of the cylindrical phialide than is typical for M. laeënsis or other Monilochaetes species; it is located more towards the bottom of the conidiogenous cells. Fig. 10C, D show young, hyaline conidia formed within the venter. In Fig. 10B, F, the top of a new phialide appears to be proliferating through the old phialide and collarette to form a new functional phialide. Similar phialidic structures and conidium ontogeny were described, for example, in species of Catenularia, Chloridium, and Sporoschismopsis (Holubová-Jechová & Hennebert 1972). No living culture of P. ychaffrei was available to further assess the phylogenetic relationships of this genus.
Dischloridium species of uncertain status
A few Dischloridium species remain that cannot be transferred to Monilochaetes or other genera. Three of these form a group of morphologically similar taxa with features intermediate between Monilochaetes and Colletotrichum, viz. Dischloridium gloeosporioides, D. livistoniae, and D. tenuisporum. The former two species were transferred into Dischloridium from Cladosporium and Fusicladium when Schubert & Braun (2005) observed terminal, monophialidic conidiogenous cells, stromata, and brown fasciculate conidiophores with paler tips. Dischloridium gloeosporioides was described from living stems and leaves, while the other two species were collected on dead leaves or petioles. All three produce well-delimited, subcircular to irregular spots, caused by emerging stromatic tissue that ruptures the epidermis (Fig. 10R–T), unlike the effuse colonies of D. laeënse. Conidia of D. gloeosporioides and D. livistoniae are obovoidal or ellipsoid-ovoidal, whereas conidia of D. tenuisporum are ellipsoidal to elongate-ellipsoidal, sometimes with a basal papilla. The single conidiogenous locus is at the base of the indistinct collarette. Although characters such as discrete stromatic tissue, fasciculate conidiophores, and terminal monophialides with hyaline conidia match the profile of dematiaceous hyphomycetes associated with the Glomerellales, transferring them to Monilochaetes or describing a new genus for them seems ill-advised until cultures and molecular data are available. Therefore, these species remain as incertae sedis.
Dischloridium gloeosporioides (G.F. Atk.) U. Braun & K. Schub., Fung. Diversity 20: 189. 2005. Basionym: Cladosporium gloeosporioides G.F. Atk., Cornell Univ. Sci. Bull. 3: 39. 1897.
Dischloridium inaequiseptatum (Matsush.) Hol.-Jech., Česká Mykol. 41: 111. 1987. Basionym: Endophragmia inaequiseptata Matsush., Icones Microfungorum a Matsushima lectorum, Kobe, p. 69. 1975.
Notes: This species is not accepted in Monilochaetes because of its 3-septate, cylindrical, slightly curved conidia with a subhyaline basal cell and the remainder of the conidial cells dark brown. Conidiophores are fasciculate without stromatic tissue; a collarette is absent or very indistinct.
Dischloridium livistoniae (P. Karst.) U. Braun & K. Schub., Fung. Diversity 20: 192. 2005. Basionym: Fusicladium livistoniae P. Karst., Hedwigia 30: 302. 1891.
Dischloridium microsporum R.F. Castañeda & W.B. Kendr., Univ. Waterloo Biol. Ser. 35: 50 (fig. 29). 1991.
Specimen examined: Cuba, La Estrella, Buey Arriba, Granma, on dead leaves of Trophis racemosa, 14 Mar. 1991, R.F. Castañeda, INIFAT C91/98-2, holotype, ex-type strain CBS 498.92.
Notes: The ex-type strain (CBS 498.92) looks Acremonium-like; it formed a Nectria-like teleomorph in vitro (W. Gams, unpubl. data).
Dischloridium tenuisporum Hol.-Jech., Česká Mykol. 41: 31. 1987. Fig. 10K–T.
Specimen examined: Cuba, Habana province, Jaruco, Loma de la Coca, south from Campo Florido, 142 m a. s. l., on dead leaves of Clusia rosea, 13 Feb. 1981, V. Holubová-Jechová, PRM 842727, holotype.
Reticulascaceae
This family contains two holomorph genera, Reticulascus and Porosphaerellopsis. Although these genera differ morphologically, ontogeny and morphology of the centrum and interthecial filaments unite them and partially define the family. The interthecial tissue is formed of filiform branching and anastomosing filaments, forming a “network” among the asci. They are attached to the hymenium and to the top of the ascomatal wall. This structure was first described and illustrated by Samuels & Müller (1978) for Porosphaerellopsis sporoschismophora and is documented here for Reticulascus tulasneorum and R. clavatus. The second species in the genus Porosphaerellopsis, P. bipolaris (Ranghoo et al. 2001), collected on submerged wood in a stream in China, does not form this “network”, and has paraphyses that are wider and simple. The link between P. bipolaris and a Sporoschismopsis anamorph suggested by Ranghoo et al. (2001) has not been established convincingly.
Reticulascaceae Réblová & W. Gams, fam. nov. MycoBank MB515435.
Stromata minuta nonnumquam formata. Ascomata perithecia, fusca usque nigra, ostiolum periphysatum. Pariete ascomatum 2-stratoso. Hamathecium paraphyses verae; paraphyses septatae, hyalinae, ramosae, anastomosantes, sursum angustatae, ascos superantes. Asci unitunicati, cylindraceo-clavati, 8-spori, annulo apicali iodo non reagente. Ascosporae hyalinae vel atrobrunneae, ellipsoideae usque fusiformes, septatae, nonnumquam utrinque poro praeditae. Anamorphae: Cylindrotrichum, Sporoschismopsis; conidia modo phialidico formata.
Typus: Reticulascus Réblová & W. Gams.
Stromata minute, sometimes present. Perithecia brown to black. Ostiolum periphysate. Perithecial wall 2-layered. Interascal tissue of thin-walled, tapering, branching and anastomosing paraphyses. Asci unitunicate, 8-spored, cylindrical-clavate, apical ring inamyloid. Ascospores hyaline or dark brown, ellipsoid to fusiform, sometimes with end pores. Anamorphs: Cylindrotrichum, Sporoschismopsis; conidiogenesis phialidic.
Reticulascus Réblová & W. Gams, gen. nov. MycoBank MB515436.
Etymology: from the Latin ascus and reticulum, referring to the network of interthecial filaments.
Stromata absentia. Perithecia superficialia, solitaria vel aggregata, fusca, venter subglobosus usque conicus, ostiolum periphysatum. Paries perithecii fragilis, bistratosus. Paraphyses septatae, hyalinae, filiformes, ramosae, anastomosantes, reticulum formantes, sursum angustatae, ascos superantes. Asci unitunicati, cylindraceo-clavati, 8-spori, brevi-stipitati. Ascosporae ellipsoideae usque fusiformes, hyalinae, septatae. Anamorphe Cylindrotrichum.
Typus: Reticulascus tulasneorum (Réblová & W. Gams) Réblová & W. Gams.
Stroma absent. Perithecia superficial, solitary, or gregarious, brown, venter subglobose to conical. Ostiolum periphysate. Perithecial wall fragile, 2-layered. Paraphyses septate, hyaline, filiform, forming a branching and anastomosing “network”. Asci unitunicate, cylindrical-clavate, 8-spored, short-stipitate. Ascospores ellipsoidal to fusiform, hyaline, septate. Anamorph Cylindrotrichum.
Notes: Based on the results of our ITS, ncLSU, ncSSU analyses, and the combined three-gene phylogeny, the new holomorph genus Reticulascus is introduced below for two holomorph species. Chaetosphaeria tulasneorum with the anamorph Cylindrotrichum oligospermum including C. hennebertii as a synonym, is recombined as the type of Reticulascus; the second species, R. clavatus, is introduced as a new species with C. clavatum as its anamorph.
Several anamorphic species are related to this clade. The delimitation of Cylindrotrichum, typified by C. oligosporum, and morphologically similar genera of dematiaceous hyphomyces has been controversial, with varying concepts proposed by Gams & Holubová-Jechová (1976), DiCosmo et al. (1983), Rambelli & Onofri (1987), Arambarri & Cabello (1989), and Holubová-Jechová (1990). The Cylindrotrichum anamorphs of Reticulascus species generally resemble the dematiaceous, phialidic hyphomycetous anamorphs linked with Chaetosphaeria (Réblová 2000, 2004), but the presence of cylindrical, 1-septate conidia seems to be a deviating character. The conidia are formed from conspicuously sympodially proliferating, terminally integrated phialides within shallow collarettes (Gams & Holubová-Jechová 1976: 48, figs 23, 24; Réblová & Gams 1999: 34, fig. 16). Based on a ncLSU phylogeny, Réblová & Winka (2000) showed that several species included in Cylindrotrichum by Gams & Holubová-Jechová (1976) belong to the Chaetosphaeriaceae (Chaetosphaeriales), but others are phylogenetically unrelated with a possible affinity with the Microascales. Our molecular analyses of ITS, ncLSU, ncSSU, and the combined data set of three genes (Figs 1, 2, 3, 4) confirm that Reticulascus tulasneorum/C. oligospermum, R. clavatus/C. clavatum, the newly described anamorphic C. setosum, previously known species C. gorii, Kylindria peruamazonensis, and Porosphaerellopsis (anamorph Sporoschismopsis) group outside the Chaetosphaeriales and Microascales. They form a monophyletic group that we recognise as a new family within the Glomerellales.
The dematiaceous hyphomycete genera Cylindrotrichum, Kylindria, and Sporoschismopsis, linked as anamorphs with the Reticulascaceae, possess conidia that vary in shape, colour, and size, and, although conidiogenesis is phialidic, the position of the conidiogenous locus within the collarette also varies. In Sporoschismopsis, the first and a few subsequent conidia arise endogenously and are formed in basipetal succession from the apical portion of the phialide from deep-set conidiogenous loci within a deep collarette. After formation of several conidia, the phialide proliferates through the collarette to form a new functional phialide (Holubová-Jechová & Hennebert 1972: 385, fig. 1). Similar conidium ontogeny also occurs in Catenularia and Chloridium anamorphs of Chaetosphaeria species and in species of Cadophora or Phialophora.
The remaining 18 species previously classified in Cylindrotrichum, including those transferred to Kylindria (13 species) and Xenokylindria (3 species) by DiCosmo et al. (1983), are putative members of the Chaetosphaeriales for which the name Kylindria was given preference by Réblová (2000). In fact, the Cylindrotrichum-like anamorphs linked with Chaetosphaeria are variations on the Chloridium theme and do not represent a unique or unusual pattern within the Chaetosphaeriaceae. If future analyses confirm the placement of K. triseptata in the Reticulascaceae, Kylindria will be excluded from the anamorphs linked with Chaetosphaeria and separated from Xenokylindria.
Kylindria peruamazonensis did not group in the same clade as the four Cylindrotrichum species, rather it formed a poorly supported branch with Porosphaerellopsis at the base of the Reticulascus/Cylindrotrichum clade (Figs 1, 4). This species is discussed and illustrated below and is the only typical representative of the genus Kylindria included in our analysis. Unlike Cylindrotrichum species of Kylindria have oblong, longer, and wider, 1-several-septate, often asymmetrical conidia and wider and shorter conidiophores terminating with a monophialide swollen in its upper part with or without a collarette. The phialides occasionally elongate above the collarette with several percurrent extensions. These characters contrast with Cylindrotrichum having 1-septate, symmetrical, cylindrical conidia and narrower, longer, and often seta-like conidiophores with cylindrical mono- or polyphialides that never elongate above the collarette. Because of the morphological characters distinguishing Kylindria and Cylindrotrichum and results from the ITS and ncLSU phylogenetic analyses, we prefer to keep these anamorph genera separate.
Reticulascus tulasneorum (Réblová & W. Gams) Réblová & W. Gams, comb. nov. MycoBank MB515437. Fig. 11. Basionym: Chaetosphaeria tulasneorum Réblová & W. Gams, Czech Mycol. 51: 32. 1999.
Fig. 11.
A–E. Reticulascus tulasneorum. A, B. Asci containing ascospores. C. Ascospores. D. Interthecial filaments. E. Perithecia on the host. F–M. Reticulascus clavatus. F, L. Asci with ascospores. G. Vertical section of the perithecial wall. H–J. Perithecia with conidiophores of the anamorph on the host. K. Interthecial filaments. M. Ascospores. A–E from PRM 842978 (holotype); F–M from PRM 915717 (holotype). Scale bars: A = A–D, F, K–M = 10 μm; E, H–J = 250 μm; G = 50 μm. DIC: A–C, E–J, L, M; PC: D, K.
Anamorph: Cylindrotrichum oligospermum (Corda) Bonord., Handb. Allg. Mykol. p. 88. 1851.
= Cylindrotrichum hennebertii W. Gams & Hol.-Jech., Stud. Mycol. 13: 50. 1976.
For a full description and more information, refer to Réblová & Gams (1999).
Specimen examined: Czech Republic, South-western Bohemia, Javornická hornatina Mts., Strašín near Sušice, on dead branch of Sambucus nigra, 21 Oct. 1997, M. Svrček, PRM 842978, holotype of Chaetosphaeria tulasneorum, ex-type strain CBS 101319.
Notes: Reticulascus tulasneorum produces minute, black, nonstromatic ascomata growing on decaying wood. The ascospores are hyaline, narrowly ellipsoidal, 1- to rarely 3-septate, and glabrous at maturity, similar to those of R. clavatus having slightly verruculose ascospores. In the features of asci, interthecial filaments, and perithecial wall, these species are indistinguishable. The morphological characters of the associated anamorphs are diagnostic. The teleomorph is known from only one locality (Réblová & Gams 1999).
Cylindrotrichum hennebertii (ex-type strain CBS 570.76) groups with R. tulasneorum including its anamorph C. oligospermum. The former taxon was described for specimens with only a short layer of conidiophores (Gams & Holubová-Jechová 1976: 50, fig. 24), contrasting with the development of two strata of conidiophores for the latter species. The layering of the conidiophores described in the protologue of C. hennebertii seems to be quite variable depending on substrate and age of the material. With the further evidence of their identical ITS sequences, C. hennebertii is now regarded as a synonym of C. oligospermum, the anamorph of Reticulascus tulasneorum.
Reticulascus clavatus Réblová & Fournier, sp. nov. MycoBank MB515652. Figs 11F–M, 12A–F.
Fig. 12.
A–F. Cylindrotrichum clavatum anamorph of Reticulascus clavatus. A. Conidia. B–D. Conidiophores of the lower layer (shorter conidiophores) with sympodially extending sporiferous apices, in culture. E–F. Conidiophores ending into a monophialide on the host. A–C from ex–type strain CBS 125296 (PCA, 14 d old), E–F from PRM 915717 (holotype). G–M. Cylindrotrichum gorii. G, H. Conidiophores, in culture. I. Conidia, in culture. J–L. Conidiophores, on the host. M. Conidia, on the host. G–M from CBS 879.85 (PCA, 14 d old). Scale bars: A = 10 μm; B–F = 20 μm; G, H, J–L = 20 μm; I, M = 10 μm. DIC: A–F, G–M.
Anamorph: Cylindrotrichum clavatum W. Gams & Hol.-Jech., Stud. Mycol. 43: 54. 1976.
Etymology: Epithet taken from that of the anamorph species, derived from the shape of conidia.
Perithecia 150–170 μm alta, 120–200 μm diam, superficialia, solitaria, subglobosa vel conica, minute papillata, ostiolata, glabra. Canalis ostiolaris periphysatus. Paries perithecii fragilis, ad latus et apicem sclerotialis, deorsum attenuatus; paries lateralis 15 μm crassus, bistratosus. Paraphyses copiosae, filiformes, septatae, ramosae, anastomosantes, reticulum formantes, hyalinae, 1.5 μm latae, ultra ascorum apices protrudentes. Asci 87–108 × 7–8.5 μm (in medio ± s.e. = 95.5 ± 0.2 × 7.5 ± 0.2 μm), cylindrici vel clavati, breviter stipitati. Ascosporae 14–18(–19) × 4–4.5 μm (in medio ± s.e. = 15.7 ± 0.2 × 4.4 ± 0.04 μm), fusiformes, bi- vel quadri-cellulares, verruculosae, hyalinae, 1–2-seriatae in asco.
Perithecia 150–170 μm high, 120–200 μm diam, scattered among conidiophores, superficial, solitary, subglobose to conical, with minute papilla, glabrous, ostiolum lined with periphyses. Perithecial wall brittle, heavily sclerotised in upper part, sclerotisation weakens towards base. Lateral wall ca. 15 μm thick, 2-layered: outer layer of thin-walled, dark brown, brick-like cells; inner layer of flattened, elongated hyaline cells. Paraphyses ca. 1.5 μm wide, copious, filiform, sparsely septate, not constricted at septa, forming a network, hyaline, longer than asci. Asci 87–108 × 7–8.5 μm (mean ± s.e. = 95.5 ± 0.2 × 7.5 ± 0.2 μm), cylindrical to clavate, slightly truncate to broadly rounded at apex, short–stipitate, ascal apex with inamyloid apical annulus, 3–3.5 μm wide, 1–1.5 μm deep, 8-spored. Ascospores 14–18(–19) × 4–4.5 μm (mean ± s.e. = 15.7 ± 0.2 × 4.4 ± 0.04 μm), fusiform, 2–4-celled, with a delayed formation of second and third septa, slightly constricted at septa, mature ascospores finely verruculose, 1–2-seriate in ascus.
Colonies in vivo brown to black, hairy, effuse. Setae absent. Conidiophores macronematous, mononematous, cylindrical, straight, forming two layers. Conidiophores of lower layer shorter, 60–135 × 4.5–5 μm, pale brown, subhyaline towards apex, 2–5-septate; longer conidiophores forming an upper layer, 200–360 × 5–5.5 μm, mid to dark brown, subhyaline towards apex, up to 10-septate; conidiophores of both layers ending in a monophialide or polyphialide. Conidiogenous cells 25–37 × 3.5–5 μm, usually monophialidic, rarely polyphialidic with up to two lateral openings; collarette hyaline to subhyaline, 1.5–2 μm wide, ca. 1.5 μm high. Conidia 10.5–11 × 4–4.5 μm (mean ± s.e. = 10.2 ± 0.2 × 4.2 ± 0.04 μm), cylindrical, rounded at apex, slightly tapering, obtuse at base, 1-septate, not constricted at septum, hyaline, smooth.
Colonies in vitro after 14 d on PCA at 25 °C 14–17 mm diam, cushion–like, aerial mycelium greyish brown, margin entire, reverse dark brown. Colonies sporulating after 7–10 d on PCA at 25 °C in darkness. Conidiophores macronematous, mononematous, solitary, erect, forming two layers: conidiophores of lower layer 50–100 × 2.5–3 μm, cylindrical, straight or slightly flexuous, 2–10-septate, pale brown, subhyaline to hyaline towards apex; conidiophores of upper layer up to 260 μm long, 3.5–4 μm wide, mid brown, subhyaline towards apex. Conidiogenous cells integrated, terminal or intercalary, with up to 30 lateral phialidic openings arising from sympodial elongation, fertile apices 15–70 μm long; collarettes hyaline to subhyaline, 1–1.5 μm wide, ca. 1.5 μm high. Conidia 9–12.5(–13.5) × 2.5–3 μm (mean ± s.e. = 11.6 ± 0.3 × 2.7 ± 0.05 μm), cylindrical, rounded at apex, slightly tapering, obtuse at base, 1-septate, not constricted at septum, hyaline, smooth. In PDA culture, conidia slightly smaller, 8.5–10.5 × 2.5(–3) μm (mean ± s.e. = 9.3 ± 0.1 × 2.6 ± 0.03 μm).
Specimens examined: France, Haute Garonne, Mancioux, along road D635 on the way to Frechet, on submerged wood of Alnus glutinosa, 28 Feb. 2009, J. Fournier no. J.F. 09009, PRM 915717, holotype, ex-type strain CBS 125296; Rimont, Le Baup, on submerged wood of Fraxinus sp., 12 June 2009, J. Fournier no. J.F. 09154, PRM 915718, living culture CBS 125297; Ariège, Rimont, road D18, 1.5 km south of the village, Le Baup, 500 m a. s. l., on submerged wood of Platanus sp., associated with Achroceratosphaeria potamia, Cosmospora sp., Savoryella limnetica, 23 May 2008, J. Fournier & M. Delpont no. J.F. 08139, PRM 915719, living culture CBS 125239.
Notes: Reticulascus clavatus is a common dweller of submerged wood in lotic sites in France. The anamorph does not always occur on freshly collected material, although fertile conidiophores usually appear after incubation in a moist chamber for 1–2 wk (J. Fournier, unpubl. data).
Reticulascus clavatus differs from the closely related R. tulasneorum and its C. oligospermum anamorph by verruculose mature ascospores, absence of setae among the conidiophores, which terminate with a monophialide in vivo and only rarely a polyphialide. In axenic culture (PCA, PDA) of R. clavatus, the lower layer of conidiophores terminates in polyphialides with up to 30 lateral openings (Fig. 12C, D).
Cylindrotrichum setosum Seifert, sp. nov. MycoBank MB515589. Fig. 13A–L.
Fig. 13.
A–L. Cylindrotrichum setosum. A–C. Conidiophores with setae and polyphialidic conidiogenous cells. D, F–I. Polyphialides. E. Seta with unbranched basal conidiogenous cells. J, K. Macroconidia. L. Microconidia. A–L from ex-type strain DAOM 229246 (OA, 3wk old). Scale bars: A–C = 20 μm; D–I = 10 μm. DIC: all PC except E, DIC.
Coloniae in agaro farina avenae confecto post 20 dies radium 6–7 mm attingentes, in agaro maltoso 4–5 mm. Conidiophora simplicia vel raro ramosa, stipite subhyalino vel dilute brunneo ad 200 μm longo, 1.5–2.5 μm lato, vel cellulae conidiogenae ex hyphis fasciculatis dilute brunneis, 3.5–8.5 μm latis singulae vel acervatae oriundae; setae seu conidiophora superantes seu ex hyphis aggregatis perpendiculariter oriundae, 45–80 μm longae, simplices, brunneae vel fuscae, aciculares, in parte inferiore 3.5–4 μm latae, sursum acutatae. Cellulae conidiogenae mono- vel polyphialides, subhyalinae vel dilute brunneae, ampulliformes vel subulatae, 6–13 μm longae, parte inferiore ellipsoidea, 3.5–7 × 2–4 μm, rachide recta vel geniculata ad 7 × 1.5–2.5 μm, 1–6 foramina conidiogena sessilia vel < 3 μm longa ferente, collare inconspicuum, vix periclinaliter inspissatum. Conidia cylindrica, 1-septata, 12–16.5 × 1.5–2.5 μm; microconidia rara, continua, ellipsoidea vel oblonge ellipsoidea, 3.5–5 × 1.5–2.5 μm. Colonies on OA after 3 wk about 6–7 mm radial growth, more or less planar, surface pale yellow, with little aerial mycelium but diffusely hirsute because of setae and conidiophores, margin smooth, entire, reverse unpigmented, lacking soluble pigments. Colonies on Blakeslee's malt-peptone agar after 3 wk 4–5 mm radial growth, convex, surface dark brown to black, covered with pale grey, lanose to cottony aerial mycelium that gives the central part of the colony a greyish aspect, with embedded small droplets of black exudates, with some sporulation but setae not seen, reverse grey, margin somewhat uneven.
Conidiophores on OA more or less erect, usually undulating, unbranched or sparingly branched, subhyaline to pale brown with stipes up to 200 μm long, 1.5–2.5 μm wide, or conidiogenous cells directly on erect or lax fascicles of pale brown hyphae 3.5–8.5 μm wide, more or less at right angles, single, in pairs or clusters, sometimes on a metula, with setae either terminating conidiophores or emerging from fascicles more or less at right angles. Setae 45–80 μm long, unbranched, brown to dark brown, acicular, 3.5–4 μm wide at base, slightly thick-walled, with 3–7 septa and acute apex, sometimes terminating with a monophialide. Conidiogenous cells monophialidic or polyphialidic, subhyaline to pale brown, concolourous with subtending hypha or conidiophore, usually ampulliform, sometimes subulate, 6–13 μm long, with an ellipsoidal base about 3.5–7 × 2–4 μm, slightly thick-walled, and then a narrower neck 1.5–2.5 μm wide, up to 7 μm long, straight or becoming geniculate with proliferation; lateral conidiogenous apertures usually sessile, but sometimes up to 3 μm long, with 1–6 conidiogenous openings about 1 μm wide with minute frills and inconspicuous periclinal thickening. Conidia 12–16.5 × 1.5–2.5 μm (mean ± s.e. 14.4 ± 0.2 × 2.1 ± 0.1), L/W 5.5–8, 1-septate, cylindrical, straight or rarely slightly bent, with rounded ends, base sometimes with an inconspicuous papilla-like abscission scar, accumulating in small, clear to whitish droplets. Microconidia rare, less than 1 % abundance, 3.5–5 × 1.5–2.5 μm, l/w ∼1.7–3, aseptate, ellipsoidal to oblong-ellipsoidal, with a minute basal papilla, hyaline, with no obvious abscission scar.
Specimen examined: Australia, New South Wales, Mt. Annan Botanical Garden, S 34° 05.118', E 150° 45.438', 315 m alt., on wood and bark mulch on the ground, 26 Aug. 1999, K.A. Seifert no. 1228, DAOM 229246 holotype, ex-type strain in CCFC.
Notes: Cylindrotrichum setosum is unique in the genus because of the physical separation of the conidiogenous cells and the setae. In other species, the conidiophores are seta-like and have a terminal phialide or polyphialide at the apex. In C. setosum, the conidiogenous cells tend to be clustered at the base of the setae in a manner reminiscent of species of Circinotrichum or Gyrothrix. However, the proliferation of the conidiogenous cells and the morphology of the 1-septate conidia resemble other species of Cylindrotrichum. Unlike C. setosum, microconidia have not been reported in other Cylindrotrichum species.
KEY TO ACCEPTED SPECIES OF CYLINDROTRICHUM
1. Conidia cylindrical to slightly clavate, usually wider than 2.5 μm; conidiophores seta–like but sterile setae absent................................ 2 1. Conidia cylindrical, not wider than 2.5 μm, usually 1.5–2.5 μm in vivo; setae present or absent.............................................................. 3
2. Conidia longer than 9 μm; 8.5–13 × 3–4 μm in vivo and 9–12.5(–13.5) × 2.5–3 μm in vitro; teleomorph R. clavatus............. C. clavatum 2. Conidia shorter than 9 μm; (5–)5.5–7.5(–9) × 3–3.5 in vivo and 7–9(–9.5) × 3–3.5 μm in vitro; teleomorph unknown............................................................................................................................................. C. gorii (Lunghini 1979)
3. Setae sterile, pointed, distinct from the ampulliform to subulate conidiogenous cells; teleomorph unknown........................... C. setosum 3. Conidiophores often seta-like, with a terminal mono- or polyphialide at the apex; teleomorph R. tulasneorum.............. C. oligospermum
Species phylogenetically related to cylindrotrichum
Kylindria peruamazonensis Matsush., Matsush. Mycol. Mem. 7: 56. 1993. Fig. 14.
Fig. 14.
A–P. Kylindria peruamazonensis. A–I. Conidiophores, in culture. J–P. Conidia, in culture. A–D, G–K, M, N, P from CBS 421.95 (PCA, 14 d old); E, F, L, O from CBS 838.91 (PCA, 3 wk old). Scale bars: A–D, G–K, M, N, P = 10 μm; E, F, L, O = 15 μm. DIC: A–D, G–K, M, N, P; PC: E, F, L, O.
Specimens examined: Cuba, Ciénaga de Zapata Matanzas, on leaf litter of Bucida palustris, Dec. 1991, R.F. Castañeda, INIFAT C91/111, living culture CBS 838.91; Sancti Spiritus. Las V, on leaf of B. palustris, 25 Aug. 1994, R.F. Castañeda, INIFAT C94/84R, living culture CBS 421.95.
Notes: Two strains identified as Kylindria triseptata were analysed (CBS 838.91, 421.95), but neither matches the fungus described by Matsushima (1975) as Cylindrotrichum triseptatum Matsush. in the morphology of conidiogenous cells and conidial dimensions. Our cultural observations suggest that the conidiophores, conidiogenous cells, and conidia of these strains match the description of Kylindria peruamazonensis. The apex of the phialide terminates with a funnel-shaped collarette unlike that of C. triseptatum, in which the monophialides lack a collarette and may elongate slightly and possess apical densely annellate proliferations. Previously such proliferations were observed in Cacumisporium capitulatum, the anamorph of Chaetosphaeria decastyla, and in the so-called Cylindrotrichum anamorph of Ch. acutata (Réblová & Gams 1999); these were considered a diagnostic character of Xenokylindria (DiCosmo et al. 1983). Kylindria ellisii also has 3-septate hyaline conidia, but differs from K. peruamazonensis by a hardly visible collarette and symmetrical, 3-septate conidia rounded at both ends, without an apiculus.
Unlike K. peruamazonensis, cylindrical to oblong, septate conidia with a tapering, obtuse to papillate base with a laterally displaced hilum are typical of several Kylindria species, namely K. excentrica, K. pluriseptata, and K. triseptata. Kylindria excentrica has 3-septate conidia, but differs from K. peruamazonensis in absence of a collarette and much larger conidia (27.5–35 × 7.5–8 μm; Bhat & Sutton 1985). Kylindria peruamazonensis is probably the species morphologically most similar to K. triseptata; it differs from the latter by the presence of a collarette, either lacking or with a very short elongation of the phialides above the collarette, with several percurrent proliferations, the unusual formation of imbricate conidial chains, and production of a microconidial form in vitro (macroconidia 12.5–23 × 4–7.5 μm; Matsushima 1993). Sympodial proliferation of the apex of the conidiogenous cell was not observed in cultures of K. peruamazonensis and K. triseptata (Matsushima 1975, 1993). Unlike K. peruamazonensis, K. pluriseptata has 6–8-septate and much longer conidia (35–40 × 5–6 μm; Castañeda 1987).
Additional anamorph species affiliated with the Plectosphaerellaceae
Our phylogenetic analyses place the anamorph species Stachylidium bicolor (DAOM 226658) in a basal position in the family Plectosphaerellaceae (Figs 1, 2, 3). Several anamorph genera in this family have verticillate conidiophores such as Acrostalagmus and Verticillium. Stachylidium bicolor, the type of its genus, produces erect, roughened, verticillate conidiophores, often with additional verticillate axes emerging from the main stipe; this results in a more complex conidiophore than in other similar genera. As with the species of the other genera, the conidiogenous cells are phialidic but taper strongly near the tip, and the conidia are oblong-ellipsoidal and accumulate in slime. We consider S. bicolor sufficiently distinct both morphologically and phylogenetically from Acrostalagmus and Verticillium to continue to be recognised as a distinct genus.
The phylogenetic analyses demonstrate that the common tropical hyphomycete described and illustrated by Seifert (1985) as Stilbella annulata is a member of the Plectosphaerellaceae and a sister species to Acrostalagmus luteoalbus, the type of the genus. Both S. annulata and A. luteoalbus produce ameroconidia in bright orange to reddish slimy masses; in both species the reddish pigmentation sometimes also colours the phialides. The conidiophore branching of S. annulata lacks the regular verticillate aspect of A. luteoalbus, being intermediate between verticillate and penicillate. The synnemata of S. annulata and their conspicuously lobed marginal hyphae are also deviating characters from the present generic concept of Acrostalagmus. Given the well-supported phylogenetic relationship between these two species, it seems preferable to focus on the similarities between these species rather than the differences and to transfer S. annulata to Acrostalagmus rather than propose a new genus. This modifies the generic concept of Acrostalagmus to include synnematous species:
Acrostalagmus annulatus (Berk. & Broome) Seifert, comb. nov. MycoBank MB518663. Basionym: Stilbum annulatum Berk. & Broome, Grevillea 3: 63. 1874. (holotype: no. 6045, on Brassica sp., Car. Inf., herb. Berkeley, 1879, K.
≡ Stilbella annulata (Berk. & Broome) Seifert, Stud. Mycol. 27: 58. 1985
Note: For full synonymy and examined material, refer to Seifert (1985).
MICROASCALES
Kirk et al. (2008) and Cannon & Kirk (2007) included four families in the Microascales, i.e. Ceratocystidaceae, Chadefaudiellaceae, Halosphaeriaceae, and Microascaceae, although the Ceratocystidaceae is not validly published and was not listed among accepted fungal families by Hawksworth & David (1988). On the basis of our results from ncSSU rDNA and three-gene phylogenies (Figs 2, 3), the following families are accepted in the order, Microascaceae, Halosphaeriaceae, Ceratocystidaceae, which is validated here, and Gondwanamycetaceae fam. nov. We accept the Halosphaeriaceae as a family of the Microascales (Kirk et al. 2008), although they are often placed separately in their own order (Spatafora et al. 1998).
Recent studies by Spatafora et al. (1998), Kong et al. (2000), and Zhang et al. (2006) suggested that the Microascales may prove to be paraphyletic or polyphyletic. In our study, the cladogram based on ncLSU rDNA sequences (Fig. 1) provided no support for any of the backbone branches of the four families that we accept in the order. In the phylogenies based on ncSSU rDNA and the combined ncLSU-ncSSU-RPB2 data sets (Figs 2, 3), the Microascales appear as a monophyletic grouping of four families, all with high branch support. In both phylogenies the Microascales are divided into two major subclades, one containing the Halosphaeriaceae and Microascaceae and a second subclade with the Ceratocystidaceae and Gondwanamycetaceae. The ncSSU and the three-gene phylogeny did not support the putative para- or polyphyly of the Microascales.
The family Microascaceae and order Microascales were introduced by Luttrell (1951) and were later validated with Latin descriptions by Malloch (1970) and Benny & Kimbrough (1980), respectively. Luttrell (1951) described the Microascaceae for taxa with beaked ascomata and evanescent, nonstipitate asci disposed irregularly throughout the filamentous centrum. Corlett (1963, 1966) confirmed the observations of Luttrell (1951) and described the asci of Microascus and Petriella as developing directly from the cells of the ascogenous hyphae and not from croziers. Members of the Microascaceae appear to have evolved away from a hymenial configuration; in the microascaceous centrum a peripheral layer of paraphysoidal elements develops that grows inward towards the ascogenous hyphae (Benny & Kimbrough 1980). Malloch (1970) redefined the Microascaceae to include both ostiolate and nonostiolate taxa; ascocarps are darkly pigmented, usually hairy, rarely glabrous; asci arise singly or in chains, without croziers, evanescent, irregularly disposed throughout the centrum; ascospores are reddish brown to copper-coloured with germ pores, dextrinoid when young and smooth. The genera of the Microascaceae differ in the manner of ramification of ascogenous hyphae and the formation of asci among the interthecial elements. The associated anamorphs are of the annellidic type, e.g. Cephalotrichum and Scopulariopsis. Aleurioconidia as in Petriella and arthroconidia as in Kernia also occur (Malloch 1970, 1971).
Ceratocystidaceae
The family level classification of Ceratocystis has been discussed since the genus was removed from the Ophiostomatales (Barr 1990, Samuels 1993). In recent literature the genus has sometimes been placed in the Chadefaudiellaceae, while other authors placed it in its own family, the Ceratocystidaceae, as proposed by Locquin (1972, as “Ceratocystaceae”). The name Chadefaudiellaceae predates the Ceratocystidaceae, but these families are phylogenetically distinct (see below). The Ceratocystis clade is a monophyletic group centred on species of Ceratocystis or anamorphic species of the Chalara-like genus Thielaviopsis. Ambrosiella xylebori, type of this anamorph genus, occurs in a monophyletic clade together with Ceratocystis, now separated from similar anamorphs of the Ophiostomatales that are classified in Raffaelea (Cassar & Blackwell 1996, Jones & Blackwell 1998, Harrington et al. 2010).
The teleomorph genus Cornuvesica shares similar characters of centrum ontogeny, ascospore morphology, evanescent asci, and associated anamorphs with Ceratocystis, and may belong to the same clade. Because there are no available ncLSU sequences for Cornuvesica, its relationship to Ceratocystis and A. xylebori could only be explored with the ncSSU rDNA phylogeny (Fig. 2). Cornuvesica falcata, with a Chalara-like anamorph (Viljoen et al. 2000), falls in a basal position with these taxa in a monophyletic clade.
These four genera, Ambrosiella, Ceratocystis, Cornuvesica, and Thielaviopsis, constitute a family of their own, which has no valid name. The family name Ceratocystidaceae (as “Ceratocystaceae”) proposed by Locquin (1972) was never validly published. It is phylogenetically well-established and is validated here.
Ceratocystidaceae Locq. ex Réblová, W. Gams & Seifert, fam. nov. MycoBank MB515438.
Ceratocystaceae Locq., Rev. Mycol., Supplément, 1 Table. 1972, nom. inval., Art. 36.
Stromata absentia. Ascomata perithecia, fusca usque nigra, saepe aggregata, collo longo angustato et hyphis ostiolaribus protrudentibus, divergentibus praedita. Paries tenuis. Structura interascalis nulla. Asci unitunicati, catenati, saccati, evanescentes, 8-spori. Ascosporae hyalinae, forma variabiles, 0–1-septatae, saepe pariete partim inspissato vel lamina superficiali circumdatae. Anamorphe: aleurioconidia vel conidia modo phialidico orientia; Thielaviopsis vel Chalara similia.
Typus: Ceratocystis Ellis & Halst., New Jersey Agric. Coll. Exp. Sta. Bull. 76: 14. 1890.
Stromata absent. Perithecia dark brown to black, often aggregated, long-necked, usually with divergent ostiolar setae. Perithecial wall thin. Interascal tissue absent. Asci unitunicate, formed in chains, saccate, evanescent, 8-spored. Ascospores hyaline, varied in shape, 0–1-septate, often with eccentric wall thickening or sheaths. Anamorphs with phialidic conidiogenesis and aleurioconidia; Thielaviopsis and Chalara-like.
The holomorph taxa of the Ceratocystidaceae share common diagnostic characters of centrum ontogeny, evanescent catenate asci, ascospores, and associated anamorphs that are referred to Thielaviopsis (Fig. 1) or as Chalara-like, producing ameroconidia from phialides and in some cases also aleurioconidia. Ascospores are hyaline, often with eccentric wall thickening or sheaths, aseptate or 1-septate, hat-shaped in Ceratocystis or acicular in face view and falcate in side view with a hyaline sheath in Cornuvesica. Parguey-Leduc (1977) described the ontogeny of asci of this group with the examples of Ceratocystis, Faurelina, and Sphaeronaemella, Asci arise from the basal hymenium and, as the ascogenous hyphae ramify upward, asci differentiate, dissolve basally from the ascogenous hyphae, and become free within the centrum. Interascal tissue is lacking.
Faurelina and Chadefaudiella (Chadefaudiellaceae) are discussed below.
Gondwanamycetaceae
Species of Gondwanamyces and their Custingophora anamorphs form a strongly supported monophyletic clade (Figs 1, 2, 3) that is sister to the Ceratocystidaceae. The diagnostic characters of this clade include the apparent absence of interascal filaments in the ascomatal centrum and hyaline, allantoid ascospores with a hyaline sheath giving the spore a falcate to lunate appearance. The teleomorphs, described either from infructescences of Protea (Wingfield et al. 1988, Marais et al. 1998) or from sapwood associated with Scolytidae (bark beetles) (Bright & Torres 2006, Kolařík & Hulcr 2008), produce dark, globose perithecia with a long, filiform neck, evanescent asci, and hyaline, fusiform ascospores with or without a gelatinous sheath.
Detailed observations on the ontogeny of asci and centrum of Gondwanamyces are lacking. Based on the phylogenetic position of the genus, it is likely to be similar to that of the Ceratocystidaceae. The morphology of the anamorphs of Gondwanamyces is distinctive. The conidiophores are erect, darkly pigmented, paler towards the apex, and either monoverticillate, sometimes with a terminal vesicle or divergently penicillate with whorls of phialides producing hyaline conidia. The conidiogenous locus is located at the base of the shallow collarette. The terminal vesicle was not observed in the anamorph of Gondwanamyces scolytodis and Custingophora cecropiae, both associated with bark beetles in Cecropia (Kolařík & Hulcr 2008). The conidiogenesis of Custingophora (as Knoxdaviesia proteae), the anamorph of Gondwanamyces proteae, observed with fluorescence microscopy, TEM, and SEM, was illustrated by Mouton et al. (1993). After discharge, conidia adhere in slimy droplets on the phialide apices. In contrast, the phialidic conidia of species of the Ceratocystidaceae are formed in long chains deep within the venter of the cylindrical phialide.
The taxonomic relationships of the anamorph genera Knoxdaviesia and Custingophora, both phylogenetically related to this family, have been discussed by others, e.g. Viljoen et al. (1999), Kolařík & Hulcr (2008). Although the genera appear morphologically identical as originally described, they differ in their ecological behaviour. Species of Custingophora occur in compost, whereas species of Knoxdaviesia associated with Gondwanamyces were first observed in infructescences of Protea spp. infested by insects (Wingfield et al. 1988, Marais et al. 1998). The fact that some recently described species of Gondwanamyces and Custingophora are associated with Scolytidae (bark beetles) (Bright & Torres 2006, Kolařík & Hulcr 2008) raises the possibility that the originally reported ecological distinction might have been an artifact of intense sampling of Protea in a relatively narrow geographical area in the Western Cape Province of South Africa. Based on molecular and morphological features, Kolařík & Hulcr (2008) considered Knoxdaviesia and Goidanichiella to be synonyms of Custingophora. We prefer to recognise Goidanichiella as distinct because of the Aspergillus-like vesicles on the conidiophores of the only species, G. barronii.
We recognise this clade as a distinct family in the Microascales, proposed here as the Gondwanamycetaceae.
Gondwanamycetaceae Réblová, W. Gams & Seifert, fam. nov. MycoBank MB515439.
Stromata absentia. Ascomata perithecia, nigra, collo comparate longo praedita, apicem versus angustata, ostiolum hyphis divergentibus praeditum. Paries ascomatum fragilis. Filamenta interthecialia nulla. Asci unitunicati, evanescentes. Ascosporae hyalinae, aseptatae, fusiformes, lunatae vel falcatae, vagina gelatinosa praesens vel absens. Anamorphe Custingophora; conidiophora monoverticillata vel penicillata, fusca, conidiis aseptatis modo phialidico orientibus, in massa mucida aggregatis.
Typus: Gondwanamyces G.J. Marais & M.J. Wingf., Mycologia 90: 139. 1998.
Stromata absent. Ascomata perithecioid, black, necks relatively long, tapered toward apex; ostiolar hyphae present. Perithecial wall fragile, thin-walled. Interascal tissue absent. Asci evanescent. Ascospores hyaline, aseptate, fusiform to lunate to falcate, with or without a gelatinous sheath. Anamorph: Custingophora; conidiophores monoverticillate or penicillate, brown, conidiogenesis phialidic, conidia aseptate, slimy.
Chadefaudiellaceae
Chadefaudiellaceae was described and validly published by Benny & Kimbrough (1980) for the coprophilous genus Chadefaudiella. Cannon & Kirk (2007) added a second genus to the family, Faurelina (Locquin-Linard 1975). Locquin-Linard (1973) and Parguey-Leduc (1977) placed the Chadefaudiella in the Microascales because of its perithecial acomata, catenate asci, and characteristic centrum structures, i.e. asci arising from a fertile layer lining the bottom of the cavity, ascogenous hyphae ramifying upwards, asci differentiated without croziers and liberated by basal dissolution to float free in the centrum (Benny & Kimbrough 1980). Ascospores are 1-celled, nondextrinoid, striate, and lack germ pores. No anamorph has been reported. Faurelina was described for coprophilous, cleistothecial fungi, otherwise reminiscent of Chadefaudiella, but differing by dextrinoid ascospores, and the absence of apical anastomosing setae on its ascomata. The ascomatal wall of Faurelina is cephalothecoid and the asci are catenate, irregularly disposed in the centrum at maturity, characters reminiscent of Chadefaudiella (Udagawa & Furuya 1973, Furuya 1978, von Arx et al. 1981). Von Arx (1978) and von Arx et al. (1981) regarded the anamorph of Faurelina as similar to the Arthrographis anamorph of Pithoascus langeronii, producing arthroconidia and secondary small blastoconidia in axenic culture (CBS 126.78).
The classification of Faurelina has been problematic. Despite the similarities with Chadefaudiella noted by Locquin-Linard (1975), Parguey-Leduc & Locquin-Linard (1976) concluded that Faurelina should be placed in the Loculoascomycetes. Faurelina was later transferred by von Arx (1978) to the Microascaceae because of its dextrinoid ascospores, which lack germ pores. He speculated on a relationship with Neurospora in the Sordariaceae (Sordariomycetes), which is characterised by elongate, striate ascospores with apical germ pores, and an anamorph with 1-celled, inflated arthroconidia or perhaps even with the Testudinaceae (Dothideomycetes). Benny & Kimbrough (1980) accepted Faurelina in the Pithoascaceae (= Microascaceae fide Kirk et al. 2008), a family erected for members of the Microascales with arthroconidial anamorphs and narrowly fusoid or naviculate ascospores. Recently both genera were placed in the Chadefaudiellaceae, Microascales (Cannon & Kirk 2007). This was in part based on the conclusions of Tang et al. (2007), who sequenced a single strain of Faurelina indica (CBS 126.78) and obtained ncLSU, ncSSU, and RPB2 sequences identical to those of Ceratocystis fimbriata, the type species of Ceratocystis.
We studied two authentic strains of Faurelina indica, the ex-type strain CBS 126.78 and CBS 301.78. They both grew slowly and mature ascomata did not develop on OA after 2 mo, but an arthroconidial anamorph with 0–1-septate conidia was observed similar to that illustrated by von Arx et al. (1981). No structures resembling phialides or Ceratocystis-type ascomata were produced. We generated new ITS and ncLSU sequences (ITS: GU291802; ncLSU: GU180653, GU180654) for these two strains. Phylogenetic analysis of ncLSU sequences (Fig. 5) suggests a relationship with the Didymellaceae (Pleosporales, Dothideomycetes). ITS sequences (phylogeny not shown) were similar to those of Eremomyces and Arthrographis species (90–91 % overall similarity), which also have arthroconidial anamorphs. We are confident that our sequences represent the fungus described by von Arx et al. (1981); those reported by Tang et al. (2007) were based on a different fungus. Our morphological and molecular studies fail to support the phylogenetic relationship of Faurelina with Ceratocystis suggested by Tang et al. (2007).
Based on these results, we confirm the hypothesis originally proposed by Parguey-Leduc & Locquin-Linard (1976) that Faurelina originated in the group of fungi with ascolocular development. Based on ncLSU sequences, we cannot confirm a close relationship of Faurelina with the Testudinaceae (von Arx 1978) or the Eremomycetaceae; the latter includes the morphologically similar Arthrographis (Fig. 5).
This phylogenetic reevaluation eliminates the Chadefaudiellaceae as an appropriate family name for the Ceratocystis clade. Chadefaudiella is morphologically slightly different from Faurelina. A further molecular analysis may lead to a re-establishment of the Chadefaudiellaceae in the Microascales, but with the exclusion of Faurelina from the family and distinct from the Ceratocystidaceae.
Acknowledgments
This study was supported by the Project of the National Foundation of the Czech Republic (GA206/09/0547) and Institutional Research Concept No. AV0Z60050516 (Institute of Botany of the ASCR). We would like to thank Jean Mouchacca and Johannes Groenewald for help with obtaining old French literature, Paul Kirk for providing fresh material of Australiasca laeënsis from England, Jacques Fournier for providing fresh material of Cylindrotrichum clavatum and Reticulascus clavatus from freshwater habitats in France, John L. Alcorn and B. McNeil for providing strains of Australiasca queenslandica, and Jan Holec from PRM herbarium in Prague for loans of type material. Brett Summerell arranged collecting expeditions to different campuses of the Australian Royal Botanic Gardens during a visit by KAS in 1999, which resulted in the collection of two of the taxa described here. We would like to thank the culture collections CBS (CBS Fungal Diversity Centre, Utrecht) and DAOM (Agriculture and Agri-Food Canada, Ottawa) for providing valuable strains. We are grateful for help of Václav Štěpánek and Gerry Louis-Seize who undertook the DNA sequencing of several members of the Australiascaceae, Plectosphaerellaceae, and Reticulascaceae and provided other sequences newly presented in this study.
Taxonomic novelties: New order: Glomerellales Chadef. ex Réblová, W. Gams & Seifert, ord. nov. New families: Australiascaceae Réblová & W. Gams, fam. nov., Ceratocystidaceae Locq. ex Réblová, W. Gams & Seifert, fam. nov., Gondwanamycetaceae Réblová, W. Gams & Seifert, fam. nov., Reticulascaceae Réblová & W. Gams, fam. nov. New genera: Reticulascus Réblová & W. Gams, gen. nov., New species: Australiasca laeënsis Réblová & W. Gams, sp. nov., Cylindrotrichum setosum Seifert, sp. nov., Reticulascus clavatus Réblová & Fournier, sp. nov. New combinations: Acrostalagmus annulatus (Berk. & Broome) Seifert, comb. nov., Hyalocylindrophora rosea (Petch) Réblová & W. Gams, comb. nov., Monilochaetes basicurvata (Matsush.) Réblová & Seifert, comb. nov., Monilochaetes camelliae (Alcorn & Sivan.) Réblová, W. Gams & Seifert, comb. nov., Monilochaetes laeënsis (Matsush.) Réblová, W. Gams & Seifert, comb. nov., Monilochaetes regenerans (Bhat & W.B. Kendr.) Réblová & Seifert, comb. nov., Reticulascus tulasneorum (Réblová & W. Gams) Réblová & W. Gams, comb. nov.
References
- Arambarri AM, Cabello MN (1989). A numerical taxonomic study of some phialidic genera of hyphomycetes: cluster analysis. Mycotaxon 34: 679–696. [Google Scholar]
- Arx JA von (1978). Notes on Microascaceae with the description of two species. Persoonia 10: 23–31. [Google Scholar]
- Arx JA von, Mukerji KG, Singh N (1981). Faurelina indica sp. nov. Sydowia, Series II, 34: 39–41. [Google Scholar]
- Barr ME (1990). Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 39: 43–184. [Google Scholar]
- Benny GL, Kimbrough JW (1980). A synopsis of the orders and families of Plectomycetes with keys to genera. Mycotaxon 12: 1–91. [Google Scholar]
- Bhat DJ, Sutton BC (1985). Some `phialidic' hyphomycetes from Ethiopia. Transactions of the British Mycological Society 84: 723–730. [Google Scholar]
- Bright DE, Torres JA (2006). Studies on West Indian Scolytidae (Coleoptera). 4. A review of the Scolytidae of Puerto Rico, USA, with descriptions of one new genus, fourteen new species and notes on new synonymy. Koleopterologische Rundschau 76: 389–428. [Google Scholar]
- Cannon PF, Kirk PM (2007). Fungal Families of the World. CAB International: Wallingford, UK.
- Cassar S, Blackwell M (1996). Convergent origins of ambrosia fungi. Mycologia 88: 596–601. [Google Scholar]
- Castañeda RF (1987). Fungi Cubenses II. Instituto de Investigaciones Fundamentales en Agricultura Tropical “Alejandro Humboldt”, Academia de Ciencas de Cuba: la Habana, Cuba.
- Chadefaud M (1960). Les végétaux non vasculaires (Cryptogamie). In: Traité de botanique systématique. Tome I (Chadefaud M, Emberger L, eds), Masson et Cie, Paris, France.
- Corlett M (1963). The developmental morphology of two species of Microascus. Canadian Journal of Botany 41: 253–266. [Google Scholar]
- Corlett M (1966). Developmental studies in the Microascaceae. Canadian Journal of Botany 44: 79–88. [Google Scholar]
- Cunningham CW (1997). Can three incongruence tests predict when data should be combined? Molecular Phylogenetics and Evolution 14: 733–740. [DOI] [PubMed] [Google Scholar]
- DiCosmo F, Berch S, Kendrick WB (1983). Cylindrotrichum, Chaetopsis, and two new genera of hyphomycetes, Kylindria and Xenokylindria. Mycologia 75: 949–973. [Google Scholar]
- Fernández FA, Miller AN, Huhndorf SM, Lutzoni FM, Zoller S (2006). Systematics of the genus Chaetosphaeria and its allied genera: morphological and phylogenetic diversity in north temperate and neotropical taxa. Mycologia 98: 121–130. [DOI] [PubMed] [Google Scholar]
- Furuya K (1978). Note concerning Leuconeurospora elongata. Transactions of the Japanese Mycological Society 19: 149–150. [Google Scholar]
- Gams W, Hoekstra ES, Aptroot A (eds), 1998. CBS Course of Mycology. Centraalbureau voor Schimmelcultures: Baarn, The Netherlands.
- Gams W, Holubová-Jechová V (1976). Chloridium and some other dematiaceous hyphomycetes growing on decaying wood. Studies in Mycology 13: 1–99. [Google Scholar]
- Gargas A, Taylor JW (1992). Polymerase chain reaction (PCR) primers for amplifying and sequencing nuclear 18S rDNA from lichenized fungi. Mycologia 84: 589–592. [Google Scholar]
- Gutell RR (1993). Collection of small subunit (16S- and 16S-like) ribosomal RNA structures. Nucleic Acids Research 21: 3051–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell RR, Gray MW, Schnare MN (1993). A compilation of large subunit (23S and 23S-like) ribosomal RNA structures. Nucleic Acids Research 21: 3055–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA (1999). BioEdit 5.0.9: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Halsted BD (1890). Some fungus disease of the sweet potato. New Jersey Agricultural Experiment Station Bulletin 76: 25–27. [Google Scholar]
- Harrington TC, Aghayeva DN, Fraedrich, SW (2010). New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111: 337–361. [Google Scholar]
- Harter LL (1916). Sweet-potato scurf. Journal of Agricultural Research 5: 787–795. [Google Scholar]
- Hawksworth DL, David JC (1988). Family names. Index of Fungi Supplement. CAB International: Wallingford, UK.
- Holubová-Jechová V (1982). New or interesting phialidic hyphomycetes from Cuba. Mycotaxon 15: 277–292. [Google Scholar]
- Holubová-Jechová V (1990). Problems in the taxonomy of the dematiaceous hyphomycetes. Studies in Mycology 32: 41–48. [Google Scholar]
- Holubová-Jechová V, Hennebert GL (1972). Sporoschismopsis, a new genus of lignicolous hyphomycetes. Bulletin du Jardin Botanique National de Belgique 42: 385–391. [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jones KG, Blackwell M (1998). Phylogenetic analysis of ambrosia species in the genus Raffaelea based on 18S rDNA sequences. Mycological Research 102: 661–665. [Google Scholar]
- Kauff F, Lutzoni F (2002). Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution 25: 138–156. [DOI] [PubMed] [Google Scholar]
- Kirk PM (1986). New or interesting microfungi XV. Miscellaneous hyphomycetes from the British Isles. Transactions of British Mycological Society 86: 409–428. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Dictionary of the Fungi. CAB International: Wallingford, UK.
- Kolařík M, Hulcr J (2008). Mycobiota associated with the ambrosia beetle Scolytodes unipunctatus (Coleoptera: Curculionidae, Scolytinae). Mycological Research 113: 44–60. [DOI] [PubMed] [Google Scholar]
- Kong RYC, Chan JYC, Mitchell JI, Vrijmoed LLP, Jones EBG (2000). Relationships of Halosphaeria, Lignincola and Nais inferred from partial 18S rDNA. Mycological Research 104: 35–43. [Google Scholar]
- Landvik S (1996). Neolecta, a fruit-body-producing genus of the basal ascomycetes, as shown by SSU and LSU DNA sequences. Mycological Research 100: 199–202. [Google Scholar]
- Lanier L, Bodoux P, Joly P, Bellemère A (1978). Mycologie et pathologie forestières. I. Mycologie forestière. Masson: Paris.
- Larget B, Simon DL (1999). Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16: 750–759. [Google Scholar]
- Liu YJ, Whelen S, Hall BD (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Locquin MV (1972). Synopsis generalis fungorum, excerpta ex libro'De Taxia Fungorum'. Revue de Mycologie, Supplément.
- Locquin MV (1984). Classification Générale des Mycota. In: Mycologie Générale et Structurale. Masson S.A.: Paris: 169–175.
- Locquin-Linard M (1973). Recherches sur le genre Chadefaudiella. Revue de Mycologie 38: 129–146. [Google Scholar]
- Locquin-Linard M (1975). Faurelina, nouveau genre d'Ascomycètes (Chadefaudiellaceae?) Revue de Mycologie 39: 125–129. [Google Scholar]
- Lunghini D (1979). Cylindrotrichum gorii, una nuova specie di ifale demaziaceo. Micologia italiana 8: 25–29. [Google Scholar]
- Luttrell ES (1951). Taxonomy of the Pyrenomycetes. University of Missouri Studies, Scientific Series 24(3): 1–120. [Google Scholar]
- Malloch D (1970). New concepts in the Microascaceae illustrated by two new species. Mycologia 62: 727–740. [Google Scholar]
- Malloch D (1971). The genus Kernia. Canadian Journal of Botany 49: 855–867. [Google Scholar]
- Marais GJ, Wingfield MJ, Viljoen CD, Wingfield BD (1998). A new ophiostomatoid genus from Protea infructescences. Mycologia 90: 136–141. [Google Scholar]
- Matsushima T (1971). Microfungi of the Solomon Islands and Papua-New Guinea. Kobe, Japan.
- Matsushima T (1975). Icones Microfungorum a Matsushima Lectorum. Kobe, Japan.
- Matsushima T (1993). Matsushima Mycological Memoirs 7: 1–75, 131 plates. [Google Scholar]
- Mouton M, Wingfield MJ, Wyk PS van (1993). Conidium development in the Knoxdaviesia anamorph of Ceratocystiopsis proteae. Mycotaxon 44: 363–370. [Google Scholar]
- Nixon KC, Carpenter JM (1993). On outgroups. Cladistics 9: 413–426. [DOI] [PubMed] [Google Scholar]
- Nylander J (2008). MrModeltest2 v. 2.3. C program for selecting DNA substitution models using PAUP*. Program distributed by the author. Evolutionary Biology Centre, Uppsala, Sweden.
- Parguey-Leduc A (1977). Les asques des Pyrénomycètes. Revue de Mycologie 41: 281–338. [Google Scholar]
- Parguey-Leduc A, Locquin-Linard M (1976). L'ontogénie et la structures des périthèces de Faurelina fimigenes Locquin-Linard. Revue de Mycologie 40: 161–175. [Google Scholar]
- Rambelli A, Onofri S (1987). New species of Kylindria and Xenokylindria and notes on Cylindrotrichum (Hyphomycetes). Transactions of the British Mycological Society 88: 393–397. [Google Scholar]
- Ranghoo VM, Tsui CKM, Hyde KD (2001). Brunneosporella aquatica gen. et sp. nov., Aqualignicola hyalina gen. et sp. nov., Jobellisia viridifusca sp. nov. and Porosphaerellopsis bipolaris sp. nov. (ascomycetes) from submerged wood in freshwater habitats. Mycological Research 105: 625–633. [Google Scholar]
- Réblová M (2000). The genus Chaetosphaeria and its anamorphs. Studies in Mycology 45: 149–168. [Google Scholar]
- Réblová M (2004). Four new species of Chaetosphaeria from New Zealand and redescription of Dictyochaeta fuegiana. Studies in Mycology 50: 171–186. [Google Scholar]
- Réblová M, Gams W (1999). Teleomorph-anamorph connections in Ascomycetes 1. Cylindrotrichum and Cacumisporium anamorphs of Chaetosphaeria. Czech Mycology 51: 1–40. [Google Scholar]
- Réblová M, Gams W, Štěpánek V (2011). The new hyphomycete genera Brachyalara and Infundichalara, the similar Exochalara and species of `Phialophora sect. Catenulatae' (Leotiomycetes). Fungal Diversity 46: 67–86. [Google Scholar]
- Réblová M, Winka K (2000). Phylogeny of Chaetosphaeria and its anamorphs based on morphological and molecular data. Mycologia 92: 939–954. [Google Scholar]
- Reeb V, Lutzoni F, Roux C (2004). Contribution of RPB2 to multilocus phylogenetic studies of the Pezizomycotina (euascomycetes, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution 32: 1036–1060. [DOI] [PubMed] [Google Scholar]
- Rehner S, Samuels GJ (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Rong IH, Gams W (2000). The hyphomycete genera Exochalara and Monilochaetes. Mycotaxon 76: 451–462. [Google Scholar]
- Samuels GJ (1993). The case for distinguishing Ceratocystis and Ophiostoma. In: Ceratocystis and Ophiostoma. In: Taxonomy, Ecology and Pathogenicity. (Wingfield MJ, Seifert KA, Webber JF, eds.). American Phytopathological Society: St. Paul, MN, USA: 15–25.
- Samuels GJ, Müller E (1978). Life-history studies of Brazilian ascomycetes 1. Two new genera of the Sphaeriaceae having, respectively, Sporoschisma-like and Codinaea anamorphs. Sydowia 31: 126–136. [Google Scholar]
- Schubert K, Braun U (2005). Taxonomic revision of the genus Cladosporium s.l. 4. Species reallocated to Asperisporium, Dischloridium, Fusicladium, Passalora, Pseudoasperisporium and Stenella. Fungal Diversity 20: 187–208. [Google Scholar]
- Seifert KA (1985). A monograph of Stilbella and some allied hyphomycetes. Studies in Mycology 27: 1–238. [Google Scholar]
- Sivanesan A, Alcorn JL (2002). Australiasca queenslandica gen. et sp. nov. (Chaetosphaeriaceae: Ascomycota) and its anamorph Dischloridium camelliae sp. nov. from Australia. Australian Journal of Botany 15: 741–747. [Google Scholar]
- Spatafora JW, Mitchell TG, Vilgalys R (1995). Analysis of genes coding for small-subunit rRNA sequences in studying phylogenetics of dematiaceous fungal pathogens. Journal of Clinical Microbiology 33: 1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora JW, Sung GH, Johnson D, Hesse C, O`Rourke B, et al. (2006). A five-gene phylogeny of Pezizomycotina. Mycologia 98: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Spatafora JW, Volkmann-Kohlmeyer B, Kohlmeyer J (1998). Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). American Journal of Botany 85: 1569–1580. [PubMed] [Google Scholar]
- Sutton BC (1976). Species of Hemibeltrania Piroz. and Dischloridium gen. nov. Kavaka 4: 43–50. [Google Scholar]
- Swofford DL (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates: Sunderland, MA, USA.
- Tang AMC, Jeewon R, Hyde KD (2007). Phylogenetic utility of protein (RPB2, beta-tubulin) and ribosomal (LSU, SSU) gene sequences in the systematics of Sordariomycetes (Ascomycota, Fungi). Antonie van Leeuwenhoek 91: 327–349. [DOI] [PubMed] [Google Scholar]
- Udagawa S, Furuya K (1973). The genus Leuconeurospora. Journal of Japanese Botany 48: 111–116. [Google Scholar]
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Sun BL (1994). Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of National Academy of Sciences 91: 4599–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen CD, Wingfield MJ, Jacobs K, Wingfield BD (2000). Cornuvesica, a new genus to accommodate Ceratocystiopsis falcata. Mycological Research 104: 365-367. [Google Scholar]
- Viljoen CD, Wingfield BD, Wingfield MJ (1999). Relatedness of Custingophora olivacea to Gondwanamyces spp. from Protea spp. Mycological Research 103: 497–500. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: A guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds.). Academic Press: San Diego, CA, USA: 315–322.
- Wingfield BD, Viljoen CD, Wingfield MJ (1999). Phylogenetic relationships of ophiostomatoid fungi associated with Protea infructescences in South Africa. Mycological Research 103: 1616–1620. [Google Scholar]
- Wingfield MJ, Wyk PS van, Marasas WFO (1988). Ceratocystiopsis proteae sp. nov., with a new anamorph genus. Mycologia 80: 23–30. [Google Scholar]
- Winka K, Eriksson OE (2000). Papulosa amerospora accommodated in a new family Papulosaceae, Sordariomycetes, Ascomycota) inferred from morphological and molecular data. Mycoscience 41: 97–103. [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch C, et al. (2006). An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98: 1077–1088. [DOI] [PubMed] [Google Scholar]