Abstract
Members of the genus Plagiostoma inhabit leaves, stems, twigs, and branches of woody and herbaceous plants predominantly in the temperate Northern Hemisphere. An account of all known species of Plagiostoma including Cryptodiaporthe is presented based on analyses of morphological, cultural, and DNA sequence data. Multigene phylogenetic analyses of DNA sequences from four genes (β-tubulin, ITS, rpb2, and tef1-α) revealed eight previously undescribed phylogenetic species and an association between a clade composed of 11 species of Plagiostoma and the host family Salicaceae. In this paper these eight new species of Plagiostoma are described, four species are redescribed, and four new combinations are proposed. A key to the 25 accepted species of Plagiostoma based on host, shape, and size of perithecia, perithecial arrangement in the host, and microscopic characteristics of the asci and ascospores is provided. Disposition of additional names in Cryptodiaporthe and Plagiostoma is also discussed.
Keywords: Ascomycota, Betulaceae, epitypification, Fraxinus, new species, phylogeny, Salicaceae, Sordariomycetidae
INTRODUCTION
The genus Plagiostoma (Gnomoniaceae, Diaporthales) includes microscopic fungi that inhabit the leaves, stems, twigs, and branches of woody and herbaceous plants from a range of families including the Betulaceae, Euphorbiaceae, Geraniaceae, Hippocastanaceae, Oleaceae, Polygonaceae, Salicaceae, Sapindaceae, and Staphylaceae in temperate regions of the Northern Hemisphere (Sogonov et al. 2008). Although some species of Plagiostoma cause diseases, most do not show symptoms prior to production of perithecia on dead tissues. Described by Fuckel (1870), the morphological concept of Plagiostoma remained relatively unchanged (Barr 1978, Monod 1983) until recently. Multigene phylogenetic studies suggest that the genus Plagiostoma forms a highly supported monophyletic clade that includes the type species of Plagiostoma, P. euphorbiae, and the type species of Cryptodiaporthe, C. aesculi, among others (Mejía et al. 2008, Sogonov et al. 2008). Sogonov et al. (2008) included 13 species in the genus Plagiostoma, several of which were previously placed in Cryptodiaporthe.
A brief historical account of the major taxonomic treatments of Plagiostoma and Cryptodiaporthe illustrates the views of these genera through time. Fuckel (1870) proposed the genus Plagiostoma for sphaericeaous species characterised by flattened perithecia oriented horizontally having short, lateral, erumpent necks. Fuckel (1870) included the genera Ceratostoma, Gnomonia, Linospora, Melanospora, and Rhaphidospora together with Plagiostoma in the tribe Ceratostomeae of the Sphaeriacei. In his original description of Plagiostoma, Fuckel (1870) included four species, P. euphorbiae, P. petiolicola, P devexum, and P. suspecta. Fuckel's concept of Plagiostoma was followed by Höhnel (1917) and von Arx (1951) who, like Fuckel, considered Plagiostoma to be relatively closely related to Gnomonia, the name on which the Gnomoniaceae is based. These authors differentiated Gnomonia from Plagiostoma mainly by orientation of the perithecial neck. Gnomonia was characterised by having central, upright, perithecial necks in contrast to species of Plagiostoma with eccentric, laterally oriented, perithecial necks. In her treatment of the order Diaporthales, Barr (1978) followed Fuckel's concept of Plagiostoma and placed Gnomonia and Plagiostoma in the same suborder Gnomoniineae but in different families, i.e. Gnomonia in the Gnomoniaceae and Plagiostoma in the Valsaceae. The Valsaceae was defined based on having “beaks oblique or lateral, erumpent separately or converging through stromatic disc” (Barr, 1978 p. 15). Barr (1978) made nine new combinations in Plagiostoma expanding the number of species in the genus to 13.
In his monograph of the Gnomoniaceae, Monod (1983) accepted most species treated by Barr (1978). However, Monod considered that the typification of Plagiostoma as P. euphorbiae by Höhnel (1917) was not representative of Plagiostoma because the perithecial necks of this species are eccentric rather than lateral as stipulated by Fuckel (1870). Monod (1983) transferred P. euphorbiae to the genus Gnomonia and re-typified Plagiostoma with P. devexum. In agreement with Barr (1991) and Sogonov et al. (2008) the typification of the genus Plagiostoma with P. euphorbiae by Höhnel (1917) is accepted here because this typification predates Monod (1983) and is in accordance with Article 10 of the International Code of Botanical Nomenclature (McNeill et al. 2006). In addition, the character of perithecial neck orientation has been found not to be phylogenetically informative (Sogonov et al. 2008).
Cryptodiaporthe was described by Petrak (1921) for species with euvalsoid arrangement of perithecia and, in contrast to Diaporthe, lacks a blackened margin in the substratum surrounding the perithecia. In describing Cryptodiaporthe, Petrak (1921) designated C. aesculi as type and included C. hystrix and C. populina. Later, Wehmeyer (1933) recircumscribed Cryptodiaporthe emphasising the lack of a blackened margin within the substratum and made 17 new combinations in this genus for species previously included in Diaporthe expanding the genus to 19 species.
In this study, specimens of Plagiostoma were collected primarily from North America but also from South America, Europe, and China. Among these recent collections eight new species were discovered and a number of described species were recollected, cultured, and sequenced. A multigene phylogenetic analysis is provided of 24 of the 25 species of Plagiostoma accepted here. Eight new species are described and illustrated, four species are redescribed, and four new combinations are proposed. A key to the 25 accepted species of Plagiostoma is provided along with the disposition of additional species names in Cryptodiaporthe and Plagiostoma.
MATERIAL AND METHODS
Collection of specimens, culture preparation, and morphological observations
Collections were made as listed in Table 1 from the following countries mainly during the spring and summers of 2007 and 2008: Argentina (Tucumán), China (Yunnan), France (Deux-Sèvres Département), Germany (Frankfurt), and the United States of America (California, Maryland, New York, Oregon, Washington). Specimens consisting of overwintered, dead, attached, or fallen twigs and branches with perithecia were placed in paper bags, air-dried, and stored at 8–10 °C in sealed plastic bags for a period of 1 wk to 6 mo before processing. All specimens are deposited in the U.S. National Fungus Collections (BPI).
Table 1.
Isolates with sequences included in the phylogenetic analysis of Plagiostoma. Types and epitypes are indicated in bold.
| Taxon | Specimen | Culture | Country | Host | Collector | β-tubulin | ITS | rpb2 | tef1-α |
|---|---|---|---|---|---|---|---|---|---|
| Apiognomonia hystrix | CBS-H 11343 | CBS 911.79 | Switzerland | Acer pseudoplatanus | M. Monod | GU366973 | DQ313549 | EU219260 | GU353957 |
| Apiognomonia veneta | NA | CBS 897.79 | Switzerland | Platanus orientalis | M. Monod | GU377974 | DQ313532 | EU219259 | GU353958 |
| Plagiostoma aesculi | BPI 748430 | CBS 109765 | Austria | Aesculus hippocastaneum | W. Jaklitsch | GU367021 | DQ323530 | EU199138 | GU354004 |
| BPI 878950 | CBS 126127 (= LCM 447.01) | Germany | Aesculus hippocastaneum | L.C. Mejía | GU367019 | GU367076 | GU367110 | GU354002 | |
| BPI 878950 | LCM 447b.01 | Germany | Aesculus hippocastaneum | L.C. Mejía | GU367020 | GU367077 | GU367111 | GU354003 | |
| BPI 840942 | CBS 121905 | Austria | Aesculus hippocastaneum | W. Jaklitsch | GU367022 | EU254994 | EU219269 | GU354005 | |
| Plagiostoma amygdalinae | NA | CBS 791.79 | Switzerland | Euphorbia amygdaloides | M. Monod | GU367030 | EU254995 | GU367113 | GU354012 |
| Plagiostoma apiculatum | BPI 747938 | CBS 109775 (= AR 3455) | Austria | Salix sp. | W. Jaklitsch | GU367008 | DQ323529 | EU199141 | GU353990 |
| BPI 878951 | LCM 393.01 | France | Salix dasyclados | L.C. Mejía | GU367010 | GU367067 | GU367101 | GU353992 | |
| BPI 878952 | CBS 126126 (= LCM 436.01) | USA: WA | Salix sitchensis | L.C. Mejía | GU367009 | GU367066 | GU367100 | GU353991 | |
| Plagiostoma barriae | BPI 878954 | LCM 601.01 | USA: WA | Acer macrophyllum | L.C. Mejía | GU366996 | GU367054 | GU367091 | GU353980 |
| Plagiostoma convexum | BPI 843490 | CBS 123206 | USA: NY | Salix sp. | L. Vasilyeva | GU367011 | EU255047 | - | GU353994 |
| Plagiostoma devexum | BPI 843489 | CBS 123201 | USA: NY | Polygonum sp. | L. Vasilyeva | GU367027 | EU255001 | EU219258 | GU354010 |
| Plagiostoma dilatatum | BPI 878957 | CBS 124976 (= LCM 402.02) | France | Salix irrorata | L.C. Mejía | GU367013 | GU367070 | GU367104 | GU353996 |
| BPI 878958 | LCM 403.02 | France | Salix caprea | L.C. Mejía | GU367012 | GU367069 | GU367103 | GU353995 | |
| Plagiostoma euphorbiaceum | NA | CBS 816.79 | Switzerland | Euphorbia palustris | M. Monod | GU367031 | EU255003 | - | GU354013 |
| Plagiostoma euphorbiae | NA | CBS 340.78 | The Netherlands | Euphorbia palustris | W. Gams | GU367034 | DQ323532 | EU219292 | GU354016 |
| Plagiostoma exstocollum | BPI 878961 | CBS 127662 (= LCM 468.01) | USA: OR | Corylus californica | L.C. Mejía | GU366988 | GU367046 | GU367086 | GU353972 |
| BPI 878959 | LCM 422.01 | USA: OR | Corylus californica | L.C. Mejía | GU366985 | GU367043 | GU367085 | GU353969 | |
| Plagiostoma fraxini | BPI 746412 | CBS 109498 | USA: MD | Fraxinus pennsylvanica | S. Redlin | GU367033 | AY455810 | EU219263 | GU354015 |
| Plagiostoma geranii | NA | CBS 824.79 | Switzerland | Geranium sylvaticum | M. Monod | GU367032 | EU255009 | EU219273 | GU354014 |
| Plagiostoma imperceptibile | BPI 878967 | LCM 456.01 | USA: CA | Salix sp. | L.C. Mejía | GU367002 | GU367059 | GU367094 | GU353984 |
| Plagiostoma oregonense | BPI 878968 | CBS 126124 (= LCM 597.01) | USA: OR | Salix sp. | L.C. Mejía | GU367016 | GU367073 | GU367107 | GU353999 |
| Plagiostoma ovalisporum | BPI 878969 | CBS 124977 (= LCM 458.01) | USA: ID | Salix sp. | L.C. Mejía | GU367015 | GU367072 | GU367106 | GU353998 |
| Plagiostoma petiolophilum | BPI 878970 | CBS 126123 (= LCM 181.01) | USA: NY | Acer spicatum | L.C. Mejía | GU367023 | GU367078 | GU367112 | GU354006 |
| BPI 863769 | AR 3821 | USA: NY | Acer sp. | L. Vasilyeva | GU367025 | EU255039 | EU219257 | GU354008 | |
| Plagiostoma populinum | NA | CBS 144.57 | The Netherlands | Populus trichocarpa | B. Gerrits van den Ende | GU367018 | GU367075 | GU367109 | GU354001 |
| NA | CBS 174.58 | The Netherlands | Populus canadensis | B. Gerrits van den Ende | GU367017 | GU367074 | GU367108 | GU354000 | |
| Plagiostoma pulchellum | BPI 878971 | CBS 126653 (= LCM 365.04) | USA: MD | Salix babylonica | L.C. Mejía | GU367006 | GU367063 | GU367098 | GU353987 |
| BPI 878972 | LCM 371.02 | USA: MD | Salix babylonica | L.C. Mejía | GU367007 | GU367064 | GU367099 | GU353988 | |
| BPI 878973 | LCM 438.04 | USA: WA | Salix lucida | L.C. Mejía | GU366004 | GU367061 | GU367096 | GU353985 | |
| BPI 878974 | LCM 623.01 | Argentina | Salix humboldtiana | L.C. Mejía | GU367005 | GU367062 | GU367097 | GU353986 | |
| NA | CBS 170.69 | The Netherlands | Populus balsamifera | Unknown | - | EU255043 | - | GU353989 | |
| Plagiostoma rhododendri | NA | CBS 847.79 | Switzerland | Rhododendron hirsutum | M. Monod | GU367026 | EU255044 | EU2192578 | GU354009 |
| Plagiostoma robergeanum | BPI 843593 | CBS 121472 | Austria | Staphylea pinnata | W. Jaklitsch | GU367029 | EU255046 | EU219262 | GU354011 |
| Plagiostoma salicellum | BPI 843527 | CBS 121466 (= AR 3828) | Austria | Salix alba | W. Jaklitsch | GU366978 | EU254996 | EU219278 | GU353962 |
| BPI 878975 | CBS 126121 (= LCM 449.01) | Germany | Salix repens | L.C. Mejía | GU366977 | GU367037 | GU367081 | GU353961 | |
| Plagiostoma samuelsii | BPI 878977 | CBS 125668 (= LCM 454.04) | USA: CA | Alnus tenuifolia | L.C. Mejía | GU366993 | GU367051 | GU367089 | GU353977 |
| BPI 878979 | LCM 596.01 | USA: WA | Alnus sp. | L.C. Mejía | GU366994 | GU367052 | GU367090 | GU353978 | |
| Plagiostoma versatile | BPI 878980 | CBS 124978 (= LCM 594.01) | USA: WA | Salix scouleriana | L.C. Mejía | GU366979 | GU367038 | GU367082 | GU393963 |
| BPI 878981 | LCM 595.01 | USA: WA | Salix scouleriana | L.C. Mejía | GU366980 | GU367039 | GU367083 | GU393964 | |
| BPI 878982 | LCM 598.01 | USA: OR | Salix sp. | L.C. Mejía | GU366981 | GU367040 | GU367084 | GU393965 | |
| BPI 877702 | CBS 121251 | Canada | Salix sp. | M.V. Sogonov | GU366982 | EU255059 | EU219268 | GU393966 | |
| Plagiostoma yunnanense | BPI 878983 | CBS 124979 (= LCM 513.03) | China | Salix sp. | L.C. Mejía | GU366975 | GU367035 | GU367079 | GU353959 |
| Plagiostoma yunnanense | BPI 878983 | LCM 513.02 | China | Salix sp. | L.C. Mejía | GU366976 | GU367036 | GU367080 | GU353960 |
Observations, measurements, and digital imaging of morphological characters and isolation of cultures were performed using the same equipment and procedures as in Mejía et al. (2008). AxioVision v. 4.7.2.0 (Carl Zeiss Image Solutions, Carl Zeiss, New York, NY, USA) was used in conjunction with those methods to measure structures. Fresh specimens were mounted in water for microscopic observations; dried specimens were mounted in 3 % potassium hydroxide. Cultural characteristics were observed on Potato Dextrose Agar (PDA, Difco™, Becton, Dickinson & Co., Sparks, MD, USA) 7 d after plating as described in Mejía et al. (2008). Colony diameters were measured twice perpendicularly and averaged and thus are listed as average colony diameter (a.c.d.). Representative cultures of species considered in this study were deposited at the Centraalbureau voor Schimmelcultures (CBS, The Netherlands) as listed in Table 1.
DNA extraction and PCR amplification
DNA extractions were done as described by Mejía et al. (2008) using a Fast Prep FP 120 with Lysing Matrix “A” (MP Biomedicals, Solon, OH, USA) for mechanical lysis. Four gene fragments were amplified and sequenced for the phylogenetic analyses: the complete nuclear ribosomal internal transcribed spacer regions 1 and 2 including 5.8 S rDNA (ITS), regions of the RNA polymerase second largest subunit (rpb2), beta-tubulin (β-tubulin), and translation elongation factor 1-alpha (tef1-α) genes. The ITS and rpb2 genes were amplified and sequenced as described in Mejía et al. (2008) in 25 μL reactions with two internal sequencing primers designed specifically for species of Plagiostoma: rpb2 Plag-F (5' CGT CGC TGC ATY ATC TCR CA 3') and rpb2 Plag-R (5' TGY GAG ATR ATG CAG CGA CG 3'). β-tubulin was amplified using primers T1 and T22 and sequenced with the PCR primers and the internal primers T2 and T12 from O'Donnell & Cigelnik (1997). For some isolates it was necessary to amplify the tef1-α region in two fragments using the following primer combinations: EF1-728F /EF1-1199R and EF1-983F/EF1-1567R (Carbone & Kohn 1999, Castlebury, unpubl. data, for primer 1199R 5' GGG AAG TAC CMG TGA TCA TGT 3', Rehner 2001). The rpb2 gene could not be amplified for P. convexum, P. euphorbiaceum, and P. pulchellum CBS 170.69. In addition, β-tubulin could not be amplified for P. pulchellum CBS 170.69. For the purpose of determining taxonomic affinities of species previously described as Cryptodiaporthe or Plagiostoma but not congeneric with P. euphorbiae (type species), a region of the nuclear ribosomal large subunit (LSU) was amplified as described in Castlebury et al. (2002).
Phylogenetic analyses
Editing and alignment of DNA sequences were performed as described in Mejía et al. (2008). Individual genes were aligned separately and subsequently concatenated into a single alignment. Table 1 includes detailed information about the gene sequences including GenBank numbers. The concatenated sequence alignment includes β-tubulin (1584 bp), ITS (625 bp), rpb2 (1212 bp), and tef1-α (1149 bp) for a total of 4570 bp and 45 isolates. The taxa included in this alignment represent 24 of the 25 accepted species of Plagiostoma with Apiognomonia hystrix and A. veneta as outgroup taxa. Outgroup selection was based on the sister relationship of the genus Apiognomonia with Plagiostoma as inferred by a three-gene phylogeny of the family Gnomoniaceae (Sogonov et al. 2008). Positions with ambiguous alignment were excluded from the analyses.
The concatenated alignment was partitioned by gene and codon position for β-tubulin, rpb2, and tef1-α using PAUP (Swofford 2002). The gene partitions were analysed for conflict with the partition homogeneity test (PHT) as implemented in PAUP (Swofford 2002) using the following settings: 100 homogeneity replicates, 10 random sequence addition replicates, and MULTREES off. Conflict among gene partitions was assessed by reciprocal bootstrap analyses (Reeb et al. 2004) using distance settings for each partition as determined by Modeltest v. 3.7 (Posada & Crandall 1998) following the Bayesian Information Criterion (BIC).
Genes were first analysed individually and then as a combined alignment using maximum parsimony, Bayesian, and maximum likelihood analyses. Trees and bootstrap support of branches were estimated by MP analysis as in Sogonov et al. (2008) with all characters considered unordered with equal weight and an additional analysis with unordered characters weighted as follows: weight = 3 for first and second codon positions and weight = 1 for third codon position. Additionally, trees were estimated using Bayesian analysis with the program MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001) as described in Sogonov et al. (2008) with sampling every 500 generations. Model settings for each gene were determined using the program MrModeltest v. 2 (Nylander 2004) and selected based on the Akaike Information Criterion (AIC). The first 50 000 generations were discarded (burn-in period) based on comparison of tree likelihood scores. A 50 % majority rule consensus tree and a consensus phylogram were constructed from the trees saved after the burn-in period. The Bayesian posterior probabilities (PP) of nodes of the consensus trees are presented in Fig. 1. Trees were also estimated by Maximum Likelihood (ML) analysis using the program PAUP (Swofford 2002) as described in Sogonov et al. (2008) with Modeltest v. 3.7 (Posada & Crandall 1998) used to estimate the best model for the concatenated alignment. Maximum likelihood bootstrap analysis was not conducted.
Fig. 1.
Maximum likelihood phylogenetic tree (ML score = - lnL 13921.12887) estimated from sequences of the β-tubulin, ITS, rpb2, and tef1-α genes for 24 species of Plagiostoma and two species of Apiognomonia. Bayesian posterior probabilities greater than 80 % are shown above each branch and maximum parsimony bootstrap values greater than 70 % are shown below branches. Trees for each gene were also generated; see online Supplementary Information.
RESULTS
Collection of specimens
The following plant species are reported as new hosts for species of Plagiostoma: Alnus tenuifolia, Salix dasyclados, S. humboldtiana, S. irrorata, S. lucida, and S. sitchensis (Table 1). Plagiostoma pulchellum from Argentina and P. yunnanense from southwestern China were collected in regions where no species of Plagiostoma had been previously reported.
Phylogenetic analyses
The partition homogeneity test suggested conflict among the four genes (ITS, rpb2, β-tubulin, and tef1-α) sequenced for this study (P = 0.01) with rpb2 as the source of this conflict. For combinations of the remaining three genes (ITS, β-tubulin, and tef1-α), no incongruence among gene trees was detected when all three were analysed (P = 0.09), with P = 0.07 for ITS and β-tubulin and P = 0.24 for ITS and tef1-α. The following are the likelihood settings estimated for each gene for the reciprocal NJ bootstrap analyses: ITS: Base = equal Nst = 2 TRatio = 2.5434 Rates = equal Pinvar = 0.8337; rpb2: Base = equal Nst = 6 Rmat = (1.0000 4.6961 1.0000 1.0000 13.3827) Rates = gamma Shape = 0.2029 Pinvar = 0; β-tubulin: Base = (0.2006 0.3249 0.2505) Nst = 2 TRatio = 2.1757 Rates = gamma Shape = 0.5017 Pinvar = 0; and tef1-α: Base = (0.1918 0.3110 0.2229) Nst = 2 TRatio = 1.8586 Rates = gamma Shape = 0.6109 Pinvar = 0.
The ITS, β-tubulin, and tef1-α trees individually resolved terminal clades for most of the species analysed. Trees for each gene are provided - see online Supplementary Information. No single gene analysis resolved all the species of Plagiostoma with bootstrap support higher than 70 %. The following numbers of species were resolved by genes with bootstrap > 70 %: ITS = 11, rpb2 = 9, β-tubulin = 12, and tef1-α = 11. In general, rpb2 was not as useful for resolving clades of closely related species as the other three genes. The ITS gene resolved and supported all terminal clades except P. amygdalinae and P. euphorbiaceae for which the sequences were nearly identical. However, it did not support backbone nodes at levels greater than 70 %. In contrast, bootstrap support greater than 90 % for all backbone nodes containing two or more species was obtained in the β-tubulin, rpb2, and tef1-α gene trees. The topology of the individual gene trees differed only slightly. One topological conflict supported by bootstrap values greater than 70 % was observed between the β-tubulin analysis resulting in a clade (97 %) that included all species of Plagiostoma on Salicaceae and the rpb2 analysis resulting in a clade (72 %) that included some but not all the species on Salicaceae with some species on other hosts.
Phylogenetic trees resulting from the combined four-gene dataset (ITS, β-tubulin, rpb2, and tef1-α) were compared with those resulting from the ITS, β-tubulin, tef1-α dataset found to be conflict-free by the PHT. Maximum parsimony analyses of the four-gene combination resulted in 114 equally parsimonious trees (length = 1713, CI = 0.689, RI = 0.809) for the unweighted analysis and 42 equally parsimonious trees (length = 2062, CI = 0.689, RI = 0.807) for the weighted analysis. Fifty percent majority rule consensus trees computed for each analysis did not differ in the terminal species clades but higher bootstrap support was obtained for several clades in the weighted analysis. Maximum parsimony analysis of the three-gene combination composed of ITS, β-tubulin, and tef-α resulted in eight equally parsimonious trees (length = 1275, CI = 0.707, RI = 0.817). The tree topologies obtained by MP analyses of the two alignments did not contradict each other; however, bootstrap support for several nodes increased in analyses of the four-gene combination. Therefore, subsequent analyses were performed on the four-gene combination.
The following models were the best estimates for each gene and were applied during the Bayesian analyses: HKY + I + G for ITS and tef1-α, SYM + G for rpb2, and HKY + G for β-tubulin. The model TrN+G was estimated to be the best for the entire alignment by both hLRT and BIC and those settings were applied to the maximum likelihood analysis: Base = (0.2245 0.2859 0.2454) Nst = 6 Rmat = (1.0000 3.5234 1.0000 1.0000 5.8336) Rates = gamma Shape = 0.2849 Pinvar = 0. Bayesian, ML, MP, and weighted parsimony (WP) analyses of the four-gene alignment all resulted in the same topology. Maximum likelihood analysis of the concatenated alignment of four genes resulted in one tree –lnL score of 13921.12887 and is presented as the inferred phylogeny of Plagiostoma (Fig. 1). Bayesian PP and MP bootstraps are shown above and below the branches. This phylogeny of Plagiostoma supports the recognition of eight new species, which are described in the taxonomic section of this work. Bayesian PP and MP bootstrap supports greater than 90 % were obtained for all the species of Plagiostoma in this multigene phylogeny. Plagiostoma euphorbiae-verrucosae is not included in the multigene phylogeny as only the ITS was available for this species. This species was confirmed as belonging in Plagiostoma by analysis of ITS sequences (tree not shown).
Evaluation of clades and species
Both Bayesian analysis and MP bootstrapping support a clade containing 11 species that occurs exclusively on hosts of the family Salicaceae. All of these species occur on the bark of twigs and branches with one species, Plagiostoma versatile, also occurring in the leaf midvein and petioles. Within the species on Salicaceae, one clade consists of four closely related species characterised by having an expanded perithecial neck: P. apiculatum, P. dilatatum, P. imperceptibile, and P. pulchellum. These species are distinguished by morphological features such as perithecium size, ascospore size and length-to-width (l: w) ratio, and hyphal colour in culture. Plagiostoma imperceptibile is characterised by having ascospores longer than 18 μm but with a length-width ratio (l: w) less than five. Plagiostoma pulchellum is characterised by having ascospores with a l: w greater than five and by producing rosy-coloured hyphae that become dark green on PDA. Plagiostoma apiculatum and P. dilatatum are similar to one another but the perithecia and ascospores of P. dilatatum are larger than those of P. apiculatum. Plagiostoma convexum with a moderately expanded perithecial neck is highly supported (> 95 % MP, PP) as basal to these four species.
Plagiostoma ovalisporum and the pathogenic species P. populinum are closely related and contained within a larger clade including the five species previously mentioned (100 % MP, PP). The remaining species of Plagiostoma on Salicaceae form a weakly supported clade sister to the species on Salicaceae with expanded necks mentioned above. This clade contains three species having cylindrical, usually elongated, perithecial necks, and elongated ascospores: P. salicellum, P. versatile, and P. yunnanense. The remaining member of this clade, P. oregonense, is characterised by short, expanded perithecial necks and short ascospores.
Bayesian PP and MP bootstrapping also support a clade (83 % MP, 100 % PP) of eight species with hosts representing a range of woody and herbaceous plant families. One of the subclades in this group is composed of three species that grow on Euphorbiaceae: P. amygdalinae, P. euphorbiaceum, and P. euphorbiae, type species of the genus. Basal to these species is P. fraxinum on Fraxinus pennsylvanica. A second subclade contains P. exstocollum and P. samuelsii both on betulaceous hosts and P. rhododendri on Rhododendron. The rest of the species included in the tree, namely P. aesculi, P. barriae, P. geranii, P. petiolophilum, and P. robergeanum, are relatively distant from one another and the species previously mentioned. Plagiostoma robergeanum, a species that grows on Staphylea (Staphyleaceae) in Europe, was basal to the other species of Plagiostoma.
Specimens of P. pulchellum were collected in Europe, North America (USA), and South America (Argentina). This species is recognised as the most widely distributed species of Plagiostoma included in this study and is presented here as the first report of Gnomoniaceae for South America. Plagiostoma yunnanense is the first report of Plagiostoma for China.
DISCUSSION
Due to the morphological diversity in species of Plagiostoma, as illustrated in Figs 2, 3, 4, 5, 6, no single morphological character is unique or diagnostic for this genus. The following morphological characters differentiate Plagiostoma from other genera of the Gnomoniaceae as defined by Sogonov et al. (2008, table 2). Unlike Gnomonia, species of Plagiostoma have perithecia that often collapse from the base when dry as illustrated in Sogonov et al. (2008, fig. 43 B–C). In Plagiostoma the neck length is short to long about equal or less than the diameter of perithecia, while in Ophiognomonia the perithecial neck length is usually very long, often pointed, and 2.5–5 times the perithecial diameter. Species of Ophiognomonia occur only on leaves while those of Plagiostoma are found on leaves as well as woody tissues. Except for P. rhododendri, the ascospores of Plagiostoma are not broader at the upper part as in Gnomoniopsis. Ascospores of Plagiostoma are never cylindrical or femuroid as in Cryptosporella. Species of Plagiostoma with ellipsoid, aseptate ascospores similar to those of C. hypodermia do not have a valsoid arrangement of perithecia as do species of Cryptosporella.
Fig. 2.
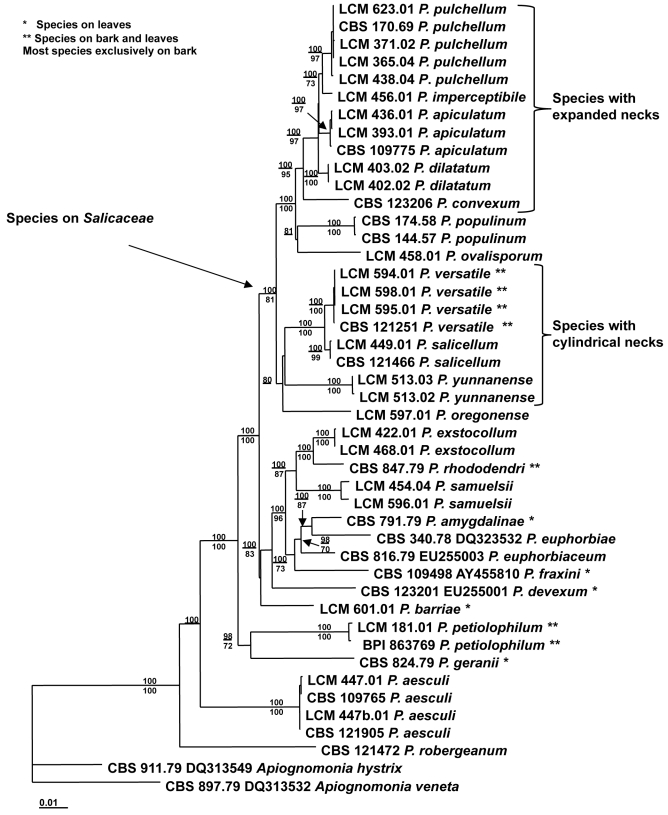
Morphology on natural substrate. A–J: Plagiostoma apiculatum: A,B,I,J = BPI 799002 (lectotype), C–G = BPI 747938 (epitype), H = BPI 878952. K–R. P. convexum: K–M = BPI 799418 (lectotype), L–R = BPI 843490 (epitype). Bars = (A, K) 1 mm; (B–F, L, O–Q) 100 μm; (G–J, M–N, R) 10 μm.
Fig. 3.
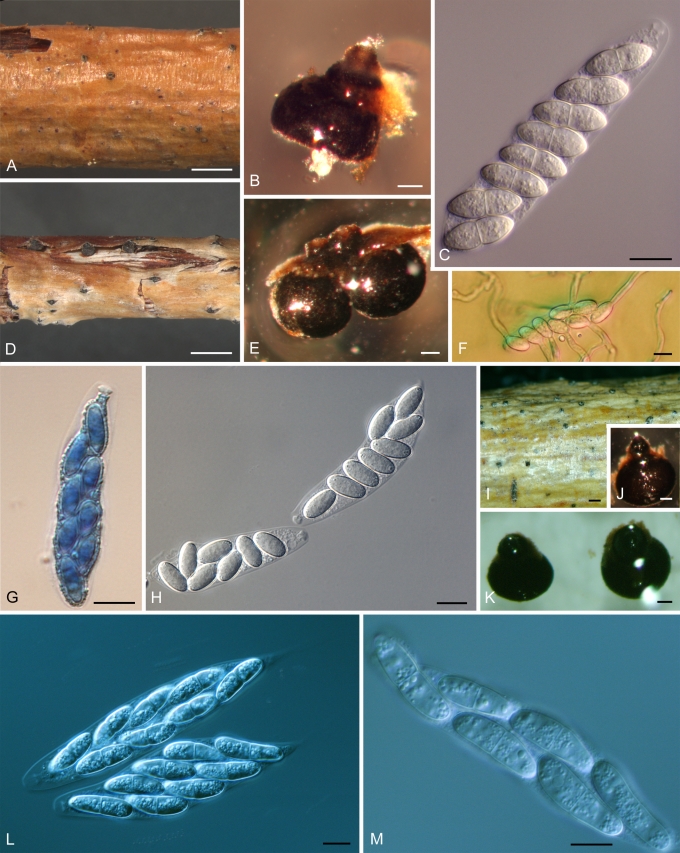
Morphology on natural substrate. A–D: Plagiostoma dilatatum: A–C = BPI 878959 (holotype), D = BPI 878958. E–H: P. exstocollum: E–G = BPI 878961 (holotype), H = BPI 878964. I–M: P. imperceptibile BPI 878967 (holotype). Bars = (A, E, I) 1mm; (M) 200 μm; (B, F, H, J) 100 μm; (C–D) 20 μm; (G, K–L) 10 μm.
Fig. 4.
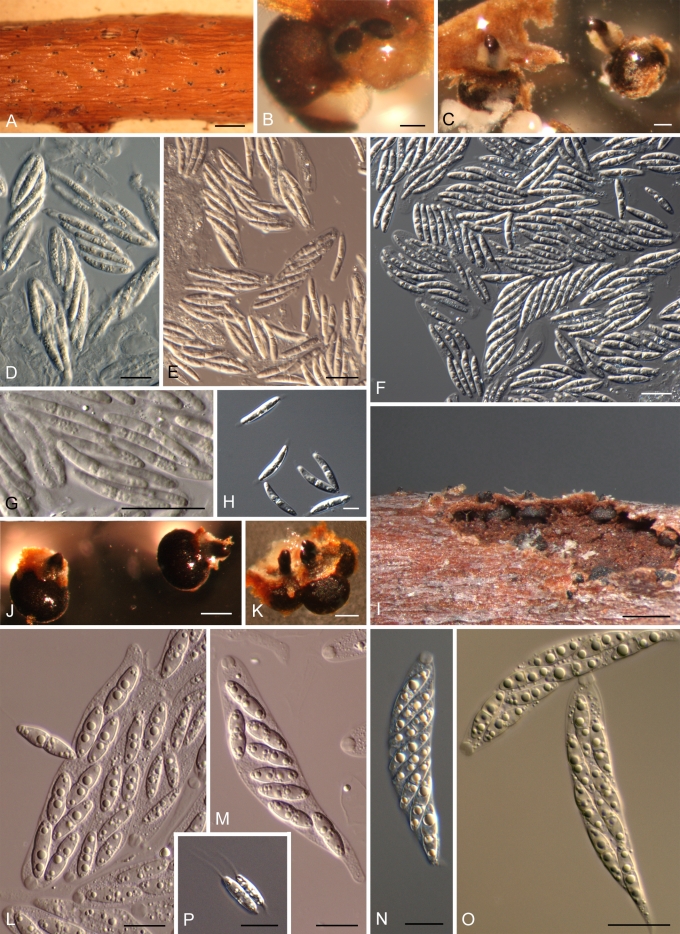
Morphology on natural substrate. A–C: Plagiostoma oregonense BPI 878968 (holotype). D–H. P. ovalisporum: BPI 878969 (holotype). I–M. P. pulchellum: I, M = BPI 878971, J = BPI 878974, K–L = BPI 878972. Bars = (A, D, I) 1 mm; (K) 300 μm; (B, E, J) 100 μm; (H, L–M) 20 μm; (C, F–G) 10 μm.
Fig. 5.
Morphology on natural substrate. A–H: Plagiostoma salicellum: A, B, D, G = Scleromyceti Sueciae 188 (lectotype), C, E, F, H = BPI 843527 (epitype); note whitish stromatic tissue surrounding perithecial neck in Figs 5.5 B and C. I–O: P. samuelsii: I, M, P = BPI 878977 (holotype), N–O = BPI 878979. Bars = (A) 1 mm; (I) 500 μm; (C, J) 200 μm; (B, K) 100 μm; (D–G) 20 μm; (H, L–P) 10 μm.
Fig. 6.
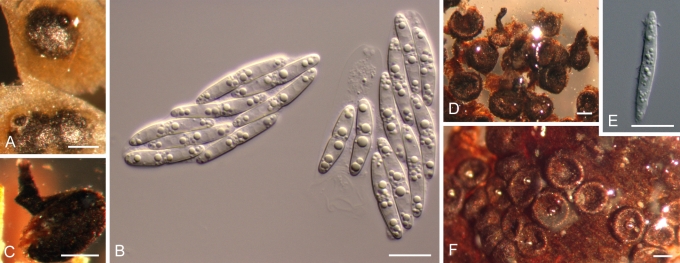
Morphology on natural substrate. A–C. Plagiostoma versatile: A–B = BPI 878980 (holotype), C = BPI 877702. D–F. P. yunnanense BPI 878983 (holotype). Bars = (A, C–D, F) 200 μm; (B, E) 10 μm.
Species of Plagiostoma, except for P. rhododendri, are not apiosporous, differentiating them from species of Apiognomonia except A. hystrix, which possesses flattened perithecial necks. Species of Pleuroceras have elongated ascospores that are quite distinct from those of Plagiostoma. The type and only species of Ditopella, D. ditopa, is characterised by having polysporic asci. Phragmoporthe conformis, a species closely related to Ditopella ditopa, is characterised by phragmosporic ascospores, a character not present in Plagiostoma. The perithecia of Amphiporthe hranicensis, the type species of Amphiporthe, are grouped near the base of the entostroma and, thus, are different from those found in Plagiostoma. In addition, Amphiporthe hranicensis produces perithecia in clusters of up to 20, with perithecial necks protruding as a group from the host periderm and surrounded by gray stromatic tissue.
Perithecial neck characters and ascospore morphology are the most important characters for differentiating Plagiostoma from other genera in the Gnomoniaceae. Host identity, geographic locality, and presence or absence of stroma are secondary characters for the identification of species. For example, the presence of white stromatic tissues surrounding the emerging perithecial necks is diagnostic in species such as P. aesculi, P. salicellum, and P. samuelsii. Within Plagiostoma perithecial neck shape ranges from very short, cylindrical to expanded and thick or thin, cylindrical and elongated with various shapes in the opening area, e.g. conic, flared, or rounded. In one species, P. versatile, the perithecial neck can be both very short when on twigs or elongated when on a leaf midvein suggesting that this structure varies with substrate. Four species, P. apiculatum, P. convexum, P. dilatatum, and P. imperceptibile, are characterised by having an apically expanded perithecial neck. The expanded neck was noticed by Wallroth (1833, as coronatum dilatatis) and Butin (1958, as cushion- or pad-like structure) but neither of these authors used this character to differentiate species. This structure may be involved in rupture of host periderm and release of the ascospores.
The asci of Plagiostoma are clavate, obclavate, ovoidal, cylindrical, or cylindric-fusoid, generally with a short stalk but with a long stalk in P. imperceptibile. Ascospores of Plagiostoma are ellipsoid, ellipsoid-fusoid, oblong-ellipsoid, or ovoid usually with one median septum, although three species, P. euphorbiae-verrucosae, P. fraxini, and P. ovalisporum, have non-septate ascospores and one species, P. rhododendri, is apiosporous. Ascospores vary in size from short, 7.7–13.8 × 2.2–6.6 μm in P. fraxini, to relatively long, 18–27 × 3–4 μm in P. versatile and P. yunnanense. Most species lack appendages although P. salicellum has short, thick, evanescent appendages and P. devexum and P. samuelsii may have long, thin appendages. Morphological characters that are phylogenetically informative for subclades of Plagiostoma include the expanded neck characteristic of species with broadly ellipsoid ascospores versus the cylindrical neck characteristic of species with narrowly ellipsoidal ascospores.
In traditional classification schemes of the Diaporthales, Cryptodiaporthe and Plagiostoma were considered distinct and not closely related genera, each with a specific morphology and arrangement of perithecia (Barr 1978, Kobayashi 1970, Monod 1983). Species of Plagiostoma were characterised by the lack of a stroma and production of a single perithecium, primarily on leaves. Species of Cryptodiaporthe were characterised by production of a rudimentary stroma and grouped perithecia, primarily in the bark of their host branches. The differences between Cryptodiaporthe and Plagiostoma have been emphasised such that some authors placed them in different families or subfamilies (Barr 1978, Wehmeyer 1975).
Monod's (1983) concept of Plagiostoma differed significantly from the concept presented here. Of the 13 species treated by Monod (1983) as Plagiostoma, only Plagiostoma devexum is accepted here in that genus. Of the 13 species of Plagiostoma accepted by Sogonov et al. (2008), only P. euphorbiae and P. devexum were originally described as Plagiostoma with Plagiostoma barriae newly described in that work. Plagiostoma aesculi and P. salicellum were previously regarded by Wehmeyer (1933) as Cryptodiaporthe. Four additional species of Cryptodiaporthe are here formally combined in Plagiostoma, namely P. apiculatum, P. convexum, P. populinum, and P. pulchellum. The recognition of these four species formerly classified as Cryptodiaporthe salicina broadens the range of morphological and ecological traits of the genus Plagiostoma. The pathogenic species, P. apiculatum, P. fraxini, and P. populinum, contrasts with the concept of Plagiostoma as primarily saprobic.
The economically important species of Plagiostoma are pathogens that cause cankers on willows and poplars. Plagiostoma apiculatum (synonym Cryptodiaporthe salicella) is here determined to be the correct name for the fungus causing a canker disease of willow (Sinclair & Lyon 2005). This species, referred to by the anamorph Diplodina microsperma, has been reported as the most abundant endophyte in healthy twigs of Salix fragilis in England (Petrini & Fisher 1990) and is thus an important component of the host microbiota. Similarly, the closely related species P. dilatatum, P. imperceptibile, and P. pulchellum form a black halo or spot on the host surface, a feature that may be associated with the early stages of canker development. Whether or not these species are primarily pathogenic or establish an asymptomatic infection that later develops into cankers needs to be determined. These species form a highly supported monophyletic group (Fig. 1) characterised by having an expanded perithecial neck and broad ellipsoid to renoid ascospores. This group of species is part of a larger, highly supported clade that also includes P. convexum, P. ovalisporum, and P. populinum (synonym Cryptodiaporthe populea), the pathogen causing a canker of poplars. Plagiostoma fraxini causes anthracnose on ash (Fraxinus pennsylvanica) and fringetree (Chionanthus retusis) (Gregory et al. 2004, Sinclair & Lyon 2005), and is sister to the clade containing three species on Euphorbiaceae.
Species of Plagiostoma occur on a broad range of host plant families within the Eudicots, although most species are associated with Rosids. This study shows an association between a clade composed of 11 species of Plagiostoma and the host family Salicaceae (Fig. 1) especially on the genus Salix. Most of the species of Plagiostoma on Salix have expanded necks. These findings agree with those for other genera within the Gnomoniaceae that are associated primarily with specific host genera in the Betulaceae such as Cryptosporella on Alnus and Betula (Mejía et al. 2008) and Gnomonia on the Coryloideae (Sogonov et al. 2008).
TAXONOMY
KEY TO SPECIES OF PLAGIOSTOMA
1. Ascospores non-septate............................................................................................................................................................................ 2 1'. Ascospores 1-septate................................................................................................................................................................................ 4
2. Ascospores ovoid, (12–)14–16(–17) × 7–8(–9) μm. On twigs of Salix sp., in North America (USA: ID).............................. P. ovalisporum 2'. Ascospores ellipsoid-fusoid. Not on Salix, in Europe and North America................................................................................................. 3
3. Ascospores 20–25.5 × 5.3–6 μm fide Monod (1983), with pointed ends. On Euphorbia, in Europe.................. P. euphorbiae-verrucosae 3'. Ascospores (7.7–)8.6–12.7(–13.8) × (2.2)2.8–5.9(–6.6) μm fide Redlin & Stack (1988). On Chionanthus and Fraxinus (Oleaceae), in Canada and USA....................................................................................................................................................................... P. fraxini
4. On Salicaceae........................................................................................................................................................................................... 5 4'. On hosts other than the Salicaceae........................................................................................................................................................ 14
5. Perithecia neck cylindric. On woody substrates except P. versatile, which occurs on both leafy and woody substrates.......................... 6 5'. Perithecial neck dilated i.e. with an expanded or thickened area that appears disk-like when seen from above, like a thick collar in section, usually appearing with a black halo or black spot in host surface where perithecial necks protrude. On woody substrates..... 10
6. Perithecial neck surrounded by a whitish stroma. On Salix, in Europe................................................................................... P. salicellum 6'. Perithecial neck without a whitish stroma. On Salix or Populus, in Europe and elsewhere...................................................................... 7
7. On twigs and branches of Populus, in Europe and North America (USA). Ascospores 14–16 × 6–9 μm fide Butin (1958)................................................................................................................................................................................................. P. populinum 7'. On twigs and branches of Salix, in China, Europe, and North America. Ascospores greater than 16 μm long........................................ 8
8. Ascospores ellipsoid-fusoid, constricted, curved, tapering to acute ends, (16–)18–20 (–22) × 4–5 μm. In Europe and North America (USA: NY)................................................................................................................................................................................ P. convexum 8'. Ascospores ellipsoid-elongated, slightly constricted, straight to slightly curved, rounded ends, generally longer than 20 μm. In China or North America......................................................................................................................................................................... 9
9. Perithecial neck slightly twisted in upper half, of constant length. Ascospores (19–)23–26(–27) × 3–4 μm. On Salix sp., in China (Yunnan)................................................................................................................................................................ P. yunnanense 9'. Perithecial neck straight, of variable length, very short in twigs, longer in leaves. Ascospores (18–)20–23(–25) × 3–4 μm. On Salix spp., in North America (Pacific Northwest region)....................................................................................................... P. versatile
10. Ascospores ellipsoid to broadly ellipsoid, constricted, tapering to narrowly rounded ends, (16–)17–19(–22) × (4–)6(–7) μm. On Salix, in North America (USA: OR)................................................................................................................................. P. oregonense 10'. Ascospores oblong-ellipsoid to renoid, not or slightly constricted, rounded ends, size different than above. On Populus and Salix, in North America and elsewhere............................................................................................................................................................. 11
11. Ascospores usually straight, sometimes slightly curved, l: w > 5, (17–)18–22(–27) × (5–)6–7(–7.5) μm. On Populus and Salix, in Europe, North and South America (Argentina)................................................................................................................... P. pulchellum 11'. Ascospores slightly curved, l: w < 5. On Salix spp., in Europe and North America................................................................................ 12
12. Asci ovoid elongated, with long, usually persistent stalk. Ascospores (18–)19–20(–21) × (5–)6–7(–8) μm, l: w (2.5–)2.9–3.1(–3.8). On Salix sp., in North America (USA: CA)......................................................................................................................... P. imperceptibile 12'. Asci cylindric, often with long but not persistent stalk. Ascospores averaging < 18 μm long. In Europe and North America.................. 13
13. Ascospores (12–)13–15(–22) × 4–5(–7) μm, mean = 15 × 5 μm, l: w (2.6–)3.0–3.3(–3.8). On Salix, in Europe (France)..... P. dilatatum 13'. Ascospores (12–)16–18.5(–21) × (3–)5–6(–7) μm, mean = 17 × 6 μm, l: w (2.4–)2.9–3.2(–4.0). On Salix, in Europe and North America......................................................................................................................................................................... P. apiculatum
14. On hosts in the Euphorbiaceae............................................................................................................................................................... 15 14'. On hosts other than Euphorbiaceae........................................................................................................................................................ 17
15. On leaves of Euphorbiaceae, specifically Euphorbia amygdaloides and E. stepposa. Ascospores 13–15.5 × 2.3–3 μm fide Monod (1983 as Gnomonia amygdalinae), with a thin appendage at each end............................................................................. P. amygdalinae 15'. On twigs, branches, or stems of the Euphorbiaceae. Ascospores without appendages......................................................................... 16
16. Perithecial neck less than 100 μm. Ascospores (12–)13–13.5(–15.5) × (3–)3.5(–4) μm fide Sogonov et al. (2008)............ P. euphorbiae 16'. Perithecial neck 100–150 μm. Ascospores 14–17.5 × 3.5–4.5 μm fide Monod (1983 as Gnomonia euphorbiacea)..... P. euphorbiaceum
17. On Acer................................................................................................................................................................................................... 18 17'. On hosts other than Acer........................................................................................................................................................................ 19
18. On leaves, twigs, and branches of Acer spp., in the Pacific Northwest region of USA. Ascospores (11.5–)14–15.5(–17.5) × (2.5–)3.5–4(–4.5) μm fide Sogonov et al. (2008).............................................................. P. barriae 18'. On leaves, twigs, and branches of Acer saccharum and A. spicatum, in eastern USA and Canada. Ascospores 7–12 × 1–2.5 μm fide Barr (1978).................................................................................................................................................................. P. petiolophilum
19. Ascospores with thin, deliquescent appendages.................................................................................................................................... 20 19'. Ascospores without appendages............................................................................................................................................................ 21
20. Necks eccentric, stout, cone-shaped, surrounded by a whitish stroma. Ascospores (10–)11–12(–19) × 3–4 μm. On Alnus spp., in the Pacific Northwest region of USA............................................................................................................ P. samuelsii 20'. Necks marginal, cylindrical, without whitish stroma. Ascospores 8–10 × 2–3 μm fide Monod (1983). On Persicaria and Polygonum, rarely on Rumex and Vitis, in Europe and USA (NY)............................................................................................................... P. devexum
21. Ascospore upper cell rounded, basal cell short-conic, 13–16 × 4–5 μm/12–16 × 5–7 μm fide Monod 1983 & Remler 1979 as Apiognomonia rhododendri. In pedicels and branches of Rhododendron spp., in Europe.................................................. P. rhododendri 21'. Ascospores not as above........................................................................................................................................................................ 22
22. In dead stems of herbaceous plants, specifically Geranium spp., in Europe. Ascospores 13–18 × 1.8–2.5 μm fide Monod (1983)........................................................................................................................................................................ P. geranii 22'. In twigs and branches of woody plants, in Europe or North America...................................................................................................... 23
23. Perithecia in groups, with necks closely appressed as a mass emerging together or in a row, surrounded by a white stroma. On Aesculus hippocastanum, in Europe...................................................................................................................................... P. aesculi 23'. Perithecia in groups or solitary, with necks emerging together or not, surrounded or not by a brownish stroma. On hosts other than Aesculus hippocastanum, in Europe or North America.......................................................................................... 24
24. Stroma brownish, covering perithecia but not surrounding necks. Perithecia arranged in groups, with necks emerging together but oriented in different directions where they protrude through host epidermis. On Corylus californica, in the Pacific Northwest region of USA...................................................................................................................................................................................... P. exstocollum 24'. Stroma absent. Perithecia solitary or in groups with convergent, protruding necks. On Staphylea, in Europe.................. P. robergeanum
DESCRIPTIONS
Plagiostoma Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 118. 1870.
Lectotype designated by Höhnel (1917): Plagiostoma euphorbiae (Fuckel) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 118. 1870.
= Cryptodiaporthe Petr., Ann. Mycol. 19: 118. 1921. Lectotype designated by Clements and Shear (1931): Cryptodiaporthe aesculi (Fuckel) Petr., now Plagiostoma aesculi (Fuckel) Sogonov, Stud. Mycol. 62: 69. 2008.
= Rostrocoronophora Munk, Dansk Bot. Arkiv 15: 98. 1953. Type: R. geranii (Hollós) Munk, now Plagiostoma geranii (Hollós) Sogonov, Stud. Mycol. 62: 72. 2008.
Anamorph: Diplodina Westend., Bull. Acad. Roy. Sci. Belgique, sér. 2, 2: 562. 1857.
Anamorph type species: Diplodina salicis Westend., Bull. Acad. R. Sci. Belg., Cl. Sci., sér. 2 12(7) (1857), now recognised as Diplodina microsperma (Johnst.) B. Sutton, Mycol. Pap. 141: 69. 1977 fide Sutton (1980).
Perithecia produced in dead, fallen or still attached host organs, immersed in bark of stems, branches, and twigs, in midvein or petiole of leaves (P. fraxini on leaf lamina), on stalks of herbaceous plants, and on peduncles (P. rhododendri). Most species initially appearing as conic-shaped or rounded elevations, usually 0.2–0.5 mm high × 1–2 mm diam, produced where a single perithecium or group of perithecia push up host surface from below. Perithecial necks protrude through epidermis or periderm making a small hole or slit, with perithecia partially or completely exposed by peeling host periderm. In bark, perithecia arranged in groups or solitary, scattered, numerous; in leaves, perithecia discrete, but growing close together. Stroma scanty, flocculose, gray, brownish, cream, yellowish white, or whitish. Perithecia black, globose, slightly flattened or suboblate, usually collapsed from base when dry, with or without stromatic tissue surrounding neck. Neck central to marginal, mostly cylindrical, also flattened, short and stout, upright, straight or contorted, or slanted and straight; 30–150 μm diam not including expanded area, with or without a disk-like expansion, up to 450 μm diam; apex rounded, acute, flared, cupulate, papillate, or conic, black, brown, yellow or hyaline, with or without furrows. Asci clavate, obclavate, ovoidal to cylindrical and cylindric-fusoid, usually with a short stalk, with a long stalk in P. imperceptibile, with a conspicuous apical ring that may appear single and thick or as two refractive bodies, eight ascospores arranged obliquely parallel, biseriate, multiseriate, or twisted. Ascospores ellipsoid, ellipsoid-fusoid, oblong-ellipsoid, ovoid, hyaline, non- or 1-septate, constricted or not at median to submedian septum, apiosporous in P. rhododendri, often with four or more rounded guttules, or appearing granulated, with or without an appendage at each end. Cultures of Plagiostoma generally grow moderately (4 cm) to fast (5–6 cm) diam after 7 d on PDA, velvety, granular, with concentric halo, with scant aerial mycelium, translucent, white, pale to very dark gray, hazel, dark green, olive or with various dark yellow to orange pigmentation, margins fringed, stringed, or root-like.
Species of Plagiostoma
Plagiostoma aesculi (Fuckel) Sogonov, Stud. Mycol. 62: 69. 2008. Basionym: Cryptospora aesculi Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 193. 1870.
≡ Cryptosporella aesculi (Fuckel) Sacc., Michelia 1: 30. 1877.
[≡ Diaporthe aesculi (Fuckel) Höhn., Ann. Mycol. 16: 116. 1918, nom. illeg. non Cooke & Harkn. 1881]
≡ Cryptodiaporthe aesculi (Fuckel) Petr., Ann. Mycol. 19:119. 1921.
Note: Sogonov et al. (2008) provided a description and illustrations of this species. Cultures are illustrated here in Fig. 7A–B.
Fig. 7.
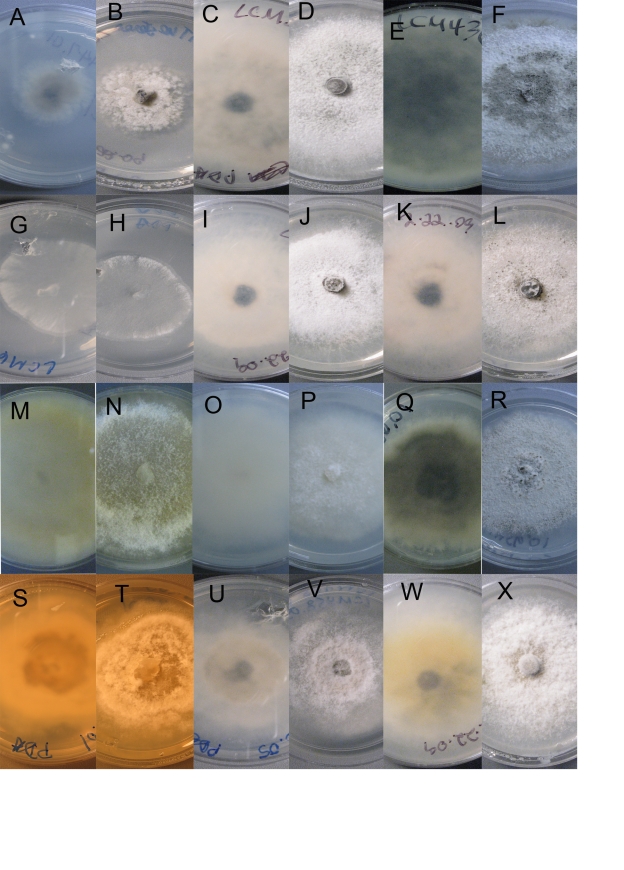
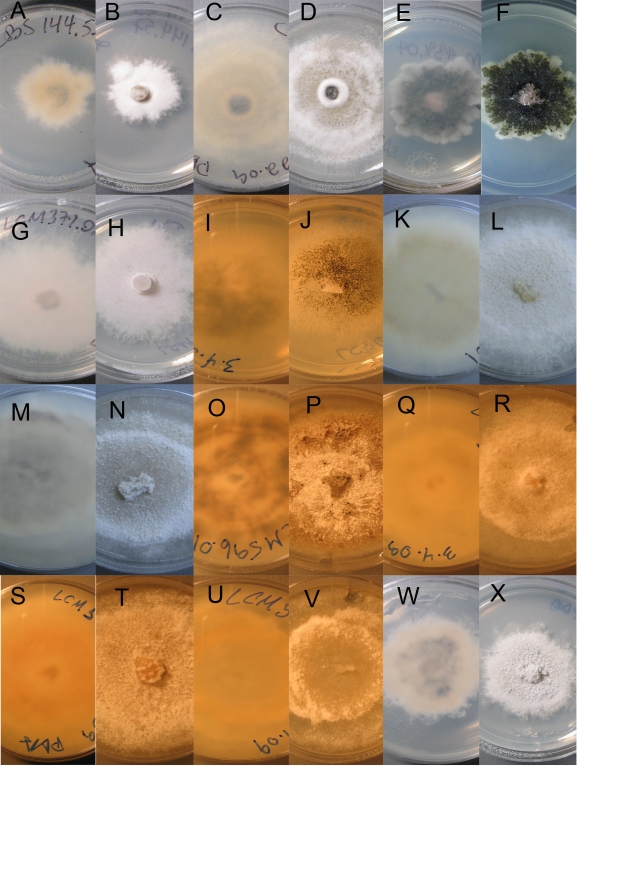
Culture morphology. A–B. Plagiostoma aesculi. CBS 126127 = LCM447.01. C–F. P. apiculatum. C–D. LCM 393.01. E–F. CBS 126126 = LCM436.01. G–H. P. barriae. LCM 601.02. I–L. P. dilatatum. I–J. LCM 402.01. K–L. LCM 403.01. M–P. P. exstocollum. M–N. LCM 422.02. O–P. LCM 468.02. Q–R. P. imperceptibile. LCM 456.01. S–T. P. oregonense. Ex-type CBS 126124 = LCM 597.01. U–V. P. ovalisporum.LCM 458.05. W–X. P. petiolophilum. CBS 126123 = LCM 181.01. A–D, I–L, W–X. Colony habit, 10 d, 23 °C. E–H, M–R, U–V. Colony habit, 9 d, 23 °C. S–T. Colony habit, 7 d, 23 °C. A, C, E, G, I, K, M, O, Q, S, U, W. Reverse. B, D, F, H, J, L, N, P, R, T, V, X. Surface.
Specimen examined: Germany, Langen, on branches of Aesculus hippocastaneum, L.C. Mejía, BPI 878950, culture LCM 447.01 = CBS 126127.
Plagiostoma amygdalinae (Fuckel) Sogonov, Stud. Mycol. 62: 70. 2008. Basionym: Gnomonia amygdalinae Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 121. 1870.
≡ Gnomoniella amygdalinae (Fuckel) Sacc., Syll. Fung. 1: 418. 1882.
= Gnomoniella amygdalinae f. euphorbiae-stepposae Sandu, Stud. Cercet. Biol., Bot. 18: 18. 1966 fide Monod (1983).
Note: Monod (1983) provided a detailed description of this species as Gnomonia amygdalinae. Although ITS sequences of P. amygdalinae (Monod 207 = CBS 791.79) and P. euphorbiaceum (MS196 = CBS 121241, Monod 465 = CBS 816.79) suggest that these taxa are the same, the multigene phylogeny obtained here reveals that P. amygdalinae and P. euphorbiaceum are distinct species. Plagiostoma amygdalinae occurs on leaves and has a longer and thinner perithecial neck, shorter asci, thinner apical ring, and ascospores not constricted at septum and thinner than P. euphorbiaceum that occurs on twigs, stems, and branches (also see Monod 1983).
Plagiostoma apiculatum (Wallr.) L.C. Mejía, comb. nov. MycoBank MB515689. Figs 2A–J, 7C–F Basionym: Sphaeria apiculata Wallr., Fl. Crypt. Germ. 2: 778. 1833.
≡ Metasphaeria apiculata (Wallr.) Sacc., Syll. Fung. 2: 166. 1883.
≡ Gnomonia apiculata (Wallr.) G. Winter, Rabenh., Kryptog.- Fl., ed. 2, vol. 1(2): 589. 1887.
≡ Diaporthe spina Fuckel var. apiculata (Wallr.) Rehm, Ann. Mycol. 7: 404. 1909.
≡ Cryptodiaporthe apiculata (Wallr.) Petr., Ann. Mycol. 19: 177. 1921.
Anamorph: Diplodina microsperma (Johnst.) B. Sutton, Mycol. Pap. 141: 69. 1977.
Perithecia immersed in bark, solitary, scattered, appearing initially as slight punctiform elevations of periderm surrounded by a black halo with tip of neck protruding through slit, usually with three short radiating slits, halo paler in some collections, later becoming completely black, globose, (223–)252–364(–440) μm high × (349–)370–476(–477) μm diam (mean = 314 × 429 μm, SD 59, 77, n = 8), each with one neck. Neck central to eccentric, straight to oblique, with a pale brown papilla, with an expanded area that appears disk-like, sometimes evident only as a thick neck, initially below epidermis, becoming exposed, producing a black halo at surface, (115–)159–256(–351) μm long (mean = 208, SD 78, n = 8), expanded area (187–)224–340(–389) μm diam (mean = 284, SD 74, n = 8), (62.5–)81–128(–134) μm diam at apex (mean = 104, SD 29, n = 7). Asci cylindrical, (45–)51–80(–86) × 10–16(–18) μm (mean = 68 × 13, SD 15, 4, n = 19), apical ring 2.5–5.0 μm diam, variable in shape e.g. elongated as two bodies or hexagonal, with eight ascospores arranged biseriate to multiseriate. Ascospores oblong-ellipsoid, slightly tapering to rounded ends, straight to slightly curved, one median to submedian septum, not constricted, (12–)16–18.5(–21) × (3–)5–6(–7) μm (mean = 16.5 × 5.5, SD 2.5, 1.0, n = 106), l: w (2.4–)2.9–3.2(–4) (mean = 3.0, SD 0.3, n = 106), with granular cytoplasm.
Cultures: Moderate to fast growth on PDA after 7 d a.c.d. 5 cm (SD 0.4, n = 4), thin aerial mycelium of velvety granular texture, central area vinaceous buff 45, with scattered black mycelial clumps of 0.5 mm diam in central area, margin white, stringy; reverse similar but slightly darker.
Habitat and host: On dead twigs and branches of Salix spp., Salix alba, S. alba subsp. vitellina, S. dasyclados, and S. sitchensis (Salicaceae).
Distribution: Europe and North America.
Lectotype of Sphaeria apiculata designated here: BPI 799092, labelled Sphaeria apiculata Wallr., ex. Herb. Strasbourg.
Epitype of Sphaeria apiculata designated here:Austria, Vienna, 21st district, Marchfeldkanalweg, MTB 7764/1, on Salix sp., 20 May 2000, W. Jaklitsch 1463, BPI 747938, derived culture CBS 109775 = AR3455.
Exsiccatus examined: Fungi Rhenani 918, as Sphaeria apiculata, from Salix vitellina, BPI bound.
Additional specimens examined: Austria, Vienna, St. Margareten im Rosental, Kaernten, Drau-Auen, 9452/1, on Salix alba, 2 May 2002, W. Jaklitsch 1890, BPI 843511, derived culture AR 3826; St. Margareten im Rosental, Drau-Auen, Kaernten. 9452/2, on Salix alba, 14 Apr. 2001, W. Jaklitsch 1741, BPI 872037. France, Deux-Sèvres Département, Melle, Melle Arboretum, 15 Apr. 2008, on twigs of Salix dasyclados, L.C. Mejía 393, BPI 878951, derived cultures L.C. Mejía 393.01 and CBS 124974 = LCM393.03. USA, Washington, Kitsap County, Kitsap Memorial State Park, on twigs of Salix sitchensis, 28 May 2008, L.C. Mejía 436, BPI 878952, derived culture CBS 126126 = LCM436.01.
Notes: The specimen designated here as lectotype is part of the collection of Sphaeria apiculata referred to in the protologue. It agrees with Wallroth's description of S. apiculata. Fuckel (1870) recircumscribed Sphaeria apiculata Wallr. based on Fungi Rhenani 918. The original Latin description of Sphaeria apiculata includes morphological characters of the perithecia such as an apiculate papilla, i.e. “coronatum dilatatis”, here interpreted as the disk-shaped expansion of the perithecial neck, and “nucleo atro” at the apex. These morphological characters are present in the type specimen BPI 799092 of S. apiculata designated here as the lectotype. The protologue of S. apiculata by Wallroth (1833) does not include a description of the ascospores, however, the fungus on this specimen contains broadly ellipsoid ascospores. This specimen and thus Plagiostoma apiculatum is distinctive and differs from Plagiostoma salicellum as discussed under that species.
The concept of the name Sphaeria apiculata has been confused. The following is an account of this species and its various synonyms based on the results of our study of the original description, type specimens, and relevant later specimens. Höhnel (1917), Petrak (1921), and later authors considered Sphaeria apiculata to have narrowly elongated ascospores while Wehmeyer (1933) recognised this species as having broadly ellipsoid ascospores and considered Cryptodiaporthe salicina to be a synonym. Höhnel (1917) examined specimens made by Rehm, Krieger, and his own of Diaporthe spina and considered this name to be a synonym of Sphaeria apiculata. He acknowledged differences in perithecial neck length among collections of these two species. To determine the synonymy of these two species we compared the original description of D. spina with the original description of S. apiculata by Wallroth (1833) as well as the recircumscription by Fuckel (1870). In his original description of D. spina Fuckel (1870) provided a drawing that is quite unlike the original description of S. apiculata. Based on the comparison of descriptions and the specimens observed, we do not consider S. apiculata and D. spina to be synonyms. The synonymy of these two species proposed by Höhnel (1917) and accepted by Petrak's (1921) who provided a description of Cryptodiaporthe apiculata (≡ Sphaeria apiculata) may be the reason that later authors considered S. apiculata to be characterised by narrow, elongated ascospores as described and observed for D. spina.
Plagiostoma barriae Sogonov, Stud. Mycol. 62: 69. 2008.
Note: Sogonov et al. (2008) provided a description and illustrations of this species. Cultures of isolates used in this study are illustrated in Fig. 7G–H. Originally described from the state of Washington (USA), this species is here reported from Oregon.
Specimens examined: USA, Oregon, on Acer sp., coll. L.C. Mejía LCM 484.01, BPI 878953, derived culture CBS 126125 = LCM 484.01; Washington, on Acer macrophyllum, L.C. Mejía 601, BPI 87895, derived culture LCM 601.01.
Plagiostoma convexum (Preuss) L.C. Mejía, comb. nov. MycoBank MB515690. Fig. 2K–R. Basionym: Sphaeria convexa Preuss, Linnea 26: 714. 1853.
≡ Diaporthe convexa (Preuss) Sacc., Syll. Fung. 1: 630. 1882.
= Cryptodiaporthe salicina Wehm. as (Curr.) Wehm., The Genus Diaporthe Nitschke and its Segregates p. 194. 1933.
[≡ Sphaeria salicina Curr., Trans. Linn. Soc. Lond., 22: 279, 1858 non Sphaeria salicina Pers., 1796]
≡ Diaporthe punctata (Cooke) Berl. & Voglino, Syll. Fung., Add. 108. 1886.
Perithecia immersed in bark, solitary or in groups of up to four, appearing initially as slight conic elevation of periderm with apex protruding through a small hole, black, globose, (180–)213–258(–326) μm high × (282–)303–352(–415) μm diam (mean = 238 × 329, SD 38, 44, n = 13), each with one neck. Neck central to eccentric, cylindrical, thick, usually thicker toward apex, some thicker elsewhere on neck, upright, diagonally straight, or curved, closely appressed when in groups, (82–)161–204(–222) μm long (mean = 176, SD 36, n = 13), (71–)82–104(–121) μm diam at base (mean = 95, SD 16, n = 13), (64–)78–108(–128) μm diam at apex (mean = 93, SD 21, n = 13), apex usually paler. Asci clavate, (54–)60–63(–69) × (14–)15–18(–20) μm (mean = 61 × 17, SD 4.5, 2.2, n = 8) apical ring 3.0–4.0 μm diam, with eight ascospores arranged obliquely parallel to multiseriate. Ascospores ellipsoid-fusoid, tapering toward rounded ends, curved or straight, one median to submedian septum, constricted, (16–)18–20(–22) × 4–5 μm (mean = 18.5 × 4.5, SD 1.0, 0.4, n = 51), l: w (3.2–)3.9–4.5(–4.9) (mean = 4.2, SD 0.4, n = 51), with four refractive bodies of various shapes, often globose.
Habitat and host: On twigs of Salix spp.
Distribution: Germany, USA (New York).
Lectotype specimen of Sphaeria convexa designated here: Sphaeria convexa Preuss, without other data, ex. Herb. Brussels in Shear study collection types and rarities, BPI 799418.
Epitype specimen of Sphaeria convexa designated here: USA, New York, Tompkins Co., near Ithaca, Arnot Forest, on Salix sp., 12 Jul 2002, L. Vasilyeva, BPI 843490, derived culture CBS 123206.
Notes: Plagiostoma convexum as Sphaeria convexa was considered a synonym of Cryptodiaporthe salicina by Wehmeyer (1933). Plagiostoma convexum has ascospores that agree with those drawn by Wehmeyer (1933 as C. salicina, plate XIII, figs 3–5). In his description of S. salicina, Currey (1858) mentions that the septum in the sporidia (ascospores) is “often very difficult to make out”, but the ascospores in his drawing have a septum. The rest of his description agrees with the description of S. convexa. It also agrees with the lectotype specimen of S. convexa, BPI 799418 ex. Herb. Brussels with a note on the label saying apparently from Preuss). This evidence suggests that S. salicina Curr. 1858 and S. convexa Preuss 1852 represent the same species. Because S. salicina Curr. is a later homonym of Sphaeria salicina Pers. 1796, this basionym cannot be used and the next available epithet is S. convexa; hence the correct name for this taxon is Plagiostoma convexum. The specimen BPI 799418 is here designated the lectotype and the specimen BPI 843490 with the ex-epitype culture CBS 123206 is designated the epitype of Sphaeria convexa.
Wehmeyer (1933) listed 28 synonyms of Cryptodiaporthe salicina. Among specimens that Wehmeyer (1933) recognised under that name, Butin (1958) elaborated differences in ascospore morphology, conidial state, host, and ecological characteristics and distinguished three species: C. apiculata (Wallr.) Petr., C. populea (Sacc.) Butin, and C. pulchella (Sacc.) Butin, here accepted as Plagiostoma apiculatum, P. populinum, and P. pulchellum. Butin (1958) did not consider any of these species to be conspecific with Sphaeria salicina Curr. On the contrary he listed Cryptodiaporthe salicina based on Sphaeria salicina Curr. as a synonym of Cryptodiaporthe salicella, here recognised as Plagiostoma salicellum. Although Wehmeyer (1933) listed Sphaeria sphingiophora Oudem. 1873 [≡ Diaporthe sphingiophora (Oudem.) Sacc.] as a synonym of C. salicella, S. sphingiophora occurs on Cornus. It is unlikely to be the same species as P. convexum. Diaporthe cupulata Berl. & Destrée was considered a synonym of Sphaeria convexa by Wehmeyer (1933), however, the ascospore sizes of these species are different. We do not consider them to be synonymous. The specimen at BPI of Sphaeria salicina Pers., Scleromyceti Sueciae 10, was examined and determined to be a species of Valsa.
Plagiostoma devexum (Desm.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 119. 1870. Basionym: Sphaeria devexa Desm., Pl. Cryptog. Nord. de France, Edit. II, Ser. II, No. 367. 1856.
≡ Gnomonia devexa (Desm.) Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 23. 1869.
≡ Gnomoniella devexa (Desm.) Sacc., Syll. Fung. 1: 417. 1881.
≡ Gnomoniopsis devexa (Desm.) Moesz & Smarods, Bot. Közlem. 38: 68. 1941.
= Sphaeria excentrica Cooke & Peck, Annual Rep. New York State Mus. 25: 105. 1873 fide Monod (1983).
≡ Gnomoniella excentrica (Cooke & Peck) Sacc., Syll. Fung. 1: 418. 1882.
= Diaporthe sechalinensis Sacc., Atti Del Congr. Bot. Di Palermo 1902: 52. 1902 fide Monod (1983).
= Ceriosporella polygoni A. L. Sm. & Ramsb., Trans. Brit. Mycol. Soc. 4: 325. 1914 fide Monod (1983).
Note: Barr (1978) and Monod (1983) provided detailed descriptions of this species.
Plagiostoma dilatatum L.C. Mejía, sp. nov. MycoBank MB515700. Figs 3A–D, 7I–L.
Etymology: dilatatum - dilate; referring to the dilated or expanded area of the perithecial neck that appears disk-like when seen from above, and like a thick collar in section.
Perithecia globosa, (277–)320–442(–502) μm elata, (382–)475–572(–642) μm diametro; rostrum breve, apice punctatum, (152–)257–308(–327) μm longum, cum expansa area disciformi vertice visu, simili collo in sectione, (217–)352–401(–452) μm diametro ubi latissima, (92–)95–108(–122) μm diametro apice. Ascosporae reniformes vel oblongo-ellipticae, uni-septatae, constrictae ubi medianae vel submedianae septatae, (12–)13–15(–22) × (4–)4–5(–7) μm, L:l (2.6–)3.0–3.3(–3.8).
Perithecia immersed in bark, solitary or aggregated, appearing initially as slight elevation of periderm surrounded by a black halo, later developing into a black circular spot, apex protruding through a tiny slit, globose, (277–)320–442(–502) μm high × (382–)475–572(–642) μm diam (mean = 383 × 515, SD 78, 79, n1 = 11, n2 = 10), each with one neck. Neck central to eccentric, relatively short, with punctate ostiolar opening, expanded, initially below epidermis, appearing disk-like when seen from above, like a thick collar in section, becoming exposed, with black halo or circular area below epidermis, when epidermis removed, exposing expanded neck and apex, sometimes two necks joined at expanded area; sometimes black mycelium of developing conidioma above perithecia; neck (152–)257–308(–327) μm long (mean = 263, SD 61, n = 10), (217–) 352–401(–452) μm diam at base (mean = 367, SD 67, n = 10), (92–)95–108(–122) μm diam at apex (mean = 103, SD 10, n = 9). Asci cylindric, (48–)54–62(–77) × (8–)12–14(–18) μm (mean = 58 × 13, SD 7.2, 2.5, n = 15), long stalked, apical ring 2.1–4.3 μm diam, appearing rectangular, with eight ascospores arranged obliquely parallel to multiseriate. Ascospores renoid to oblong-ellipsoid, slightly tapering to rounded ends, slightly curved, one, median to submedian septum, slightly constricted, (12–)13–15(–22) × 4–5(–7) μm (mean = 15 × 5, SD 2.5, 1.0, n = 48), l: w (2.6–)3.0–3.3(–3.8) (mean = 3.2, SD 0.3, n = 48), with granular cytoplasm.
Cultures: Moderate to fast growth on PDA after 7 d a.c.d. 5.2 cm (SD 0.2, n = 8), thin aerial mycelium of velvety to granular texture, whitish to vinaceous buff 86, becoming olivaceous 48 toward margin; fasiculate mycelium buff 45 developing from concave central area; reverse same; 7 d a.c.d. denser mycelium hazel 88 in centre, with vinaceous 86, black droplets on surface, with immersed mycelium dark, reverse dark, with a lighter halo and whitish to translucent margin.
Habitat and host: On dead, still attached twigs of Salix caprea and S. irrorata (Salicaceae).
Distribution: France (Melle).
Holotype: France, Deux-Sèvres Département, Melle, Melle Arboretum, on Salix irrorata, 15 Apr 2008, L.C. Mejía 402, BPI 878959, derived cultures CBS 124976 = LCM 402.02, = LCM402.01.
Additional specimens examined: France, Deux-Sèvres Département, Foret del' Hermitain, on Salix caprea, 17 Apr. 2008, L.C. Mejía 403, BPI 878958, derived cultures LCM403.01, LCM403.02.
Notes: The intricate mycelium that develops above the body of the perithecia in some pustules resembles the conidioma of Diplodina, the anamorph of Plagiostoma.
Plagiostoma euphorbiae (Fuckel) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 118. 1870. Basionym: Sphaeria euphorbiae Fuckel, Enumeratio Fung. Nassoviae p. 69. 1860.
≡ Gnomonia euphorbiae (Fuckel) Sacc., Michelia 2: 312. 1881.
≡ Gnomoniella euphorbiae (Fuckel) Sacc., Syll. Fung. 1: 418. 1882.
= Gnomoniella tithymalina Sacc. & Briard, Rev. Mycol. (Toulouse) 7: 209. 1885 fide Monod (1983).
Note: This species was fully described and illustrated by Fröhlich & Hyde (1995) and Sogonov et al. (2008).
Plagiostoma euphorbiaceum (Sacc. & Briard) Sogonov, Stud. Mycol. 62: 72. 2008. Basionym: Gnomonia euphorbiacea Sacc. & Briard, Rev. Mycol. (Toulouse) 7: 208. 1885.
Note: Monod (1983) provided a detailed description of this species. Plagiostoma euphorbiaceum is phylogenetically related to P. amygdalinae as discussed under that species.
Plagiostoma euphorbiae-verrucosae (M. Monod) Sogonov, Stud. Mycol. 62: 72. 2008 Basionym: Gnomoniella euphorbiae-verrucosae M. Monod, Beih. Sydowia 9: 42. 1983.
Note: Monod (1983) provided a detailed description of this species.
Plagiostoma exstocollum L.C. Mejía, sp. nov. MycoBank MB515701. Figs 3E–H, 7M–P.
Etymology: exsto –standing out; collus –neck, referring to the perithecial neck that emerges from the host periderm.
Perithecia suboblata, (186–)194–227(–278) μm etata, (219–)269–336(–341) μm diámetro, rostrum (197–)247–281(–382) μm longum, (50–)53–63(–67) μm diamwtro basi, (39–)44–49(–50) μm diametro apice. Ascosporae ellipsoideae, uni-septatae, constrictae ubi submedianae septatae, (9–)10–15(–16) × (2–)2–3(–4) μm, L:l (3–)4–4.5(–6).
Perithecia immersed in bark, aggregated in groups up to 12, joined by a scanty, brownish to cream stroma, occasionally solitary, appearing as elevations in bark where perithecial necks emerge through slit or crack in periderm, usually ellipsoid in shape when seen from top, black, suboblate, (186–)194–227(–278) μm high × (219–)269–336(–341) μm diam (mean = 216 × 293, SD 31, 49, n = 9), each with one neck. Neck marginal, slightly sulcate, long, (197–)247–281(–382) μm long (mean = 270, SD 54, n = 9), (50–) 53–63(–67) μm diam at base (mean = 59, SD 6.1, n = 9), (39–)44–49(–50) μm diam at apex (mean = 46, SD 3.7, n = 9). Asci cylindric to clavate, (15–)39–57(–76) × (3.5–)6.5–11(–13) μm (mean = 49.5 × 8.5, SD 15.1, 2.6, n = 26), apical ring 1.5–3.5 μm diam, with eight ascospores arranged biseriate. Ascospores ellipsoid, tapering to rounded ends, 1-septate, constricted at submedian septum, (9–) 10–15(–16) × 2–3(–4) μm (mean = 12.5 × 3.0, SD 2.4, 0.7, n = 49), l: w (3–)4–4.5(–6) (mean = 4.3, SD 0.4, n = 49), usually with at least four refractive circular bodies in each ascospore, two large ones on each side of septum, one smaller one at end of each cell.
Cultures: Moderate to fast growth on PDA after 7 d a.c.d. 4.3 cm (SD 1, n = 16), thin aerial mycelium appearing velvety, margin fringed, stringy, whitish to buff 45 or vinaceous buff 86 from top, with a slightly to pronounced halo of thick, white mycelium extending about 2 cm from centre, reverse whitish to buff 45.
Habitat and host: On dead, still attached, overwintered twigs of Corylus californica (Betulaceae).
Distribution: USA (Oregon).
Holotype: USA, Oregon, Jackson Co., Upper Rogue River, River Bridge Campground, on Corylus californica, 20 May 2008, L.C. Mejía 468, BPI 878961, derived culture CBS 127663 = LCM468.01.
Specimens examined: USA, Oregon, Jackson Co., River Bridge Campground, Upper Rogue River, on Corylus californica, 20 May 2008, L.C. Mejía 469, BPI 878962; on Corylus californica, 21 May 2008, L.C. Mejía 422, BPI 878959, derived culture LCM422.02; on Corylus californica, 21 May 2008, L.C. Mejía 472, BPI 878963, derived culture LCM472.01; Upper Rogue River trail, on Corylus californica, 21 May 2008, L.C. Mejía 473, BPI 878964, derived culture LCM473.01; Oregon, Lane Co., Willamette National Forest, Salmon Creek, 22 May 2008. L.C. Mejía 483, BPI 878965, derived culture LCM483.01; on Corylus californica, 23 May 2008, L.C. Mejía 464, BPI 878960, derived culture LCM464.
Plagiostoma fraxini (Redlin & Stack) Sogonov, Stud. Mycol. 62: 72. 2008. Basionym: Gnomoniella fraxini Redlin & Stack, Mycotaxon 32:185. 1988.
Note: This species, often as its anamorph referred to as Discula fraxinea (Peck) Redlin & Stack, causes an anthracnose disease of ash and fringetree (Oleaceae) known most commonly in the eastern and midwestern United States, rarely from Oregon (Gregory et al. 2004, Rossman et al. 2004). Redlin & Stack (1988) provided a detailed description of this species as Gnomoniella fraxini.
Plagiostoma geranii (Hollós) Sogonov, Stud. Mycol. 62: 72. 2008. Basionym: Gnomonia geranii Hollós, Annls. Mus. Nat. Hung. 7: 52. 1909.
≡ Rostrocoronophora geranii (Hollós) Munk, Dansk Bot. Arkiv 15: 98. 1953.
Note: Müller & Arx (1962) and Monod (1983) provided detailed descriptions of this species as Gnomonia geranii.
Plagiostoma imperceptibile L.C. Mejía, sp. nov. MycoBank MB515702. Figs 3I–M, 7Q–R.
Etymology: imperceptibile referring to the very short, non-protruding neck, thus the species is difficult to see in nature.
Perithecia globosa, (289–)309–356(–414) μm elata, (385–)412–462(–504) μm diametro, rostrum breve, (136–)175–211(–225) μm longum, cum expansa area, disciformi vertice visu, simili collo in sectione, (251–)301–318(–351) μm diametro ubi latissima, (87–)89–100(–113) μm diametro apice. Ascosporae reniformes vel oblongo-ellipticae, uniseptatae, constrictae ubi septatae, (18–)19–20(–21) × (5–)6–7(–8) μm, L:l (2.5–)2.9–3.1(–3.8).
Perithecia immersed in bark, solitary, appearing as slight elevations of periderm, central area pale, delimited by black halo from which apex of neck protrudes, black, globose, (289–)309–356(–414) μm high × (385–)412–462(–504) μm diam (mean = 338 × 437, SD 44, 41, n = 7), each with one neck. Neck central to eccentric, short, with apex scarcely protruding through a tiny slit, with neck expanded below epidermis, disk-like when seen from above, like a thick collar in section, with black halo or circular black spot through epidermis or black when exposed, (136–)175–211(–225) μm long (mean = 189, SD 38, n = 4), (251–)301–318(–351) μm diam at widest point (mean = 307, SD 36.3, n = 5), (87–)89–100(–113) μm diam at apex (mean = 97.5, SD 10.5, n = 5). Asci ovoid elongate, often with long, slender, persistent stalk, (67–)76–80(–87) × (13–)18–21(–24) μm (mean = 77.5 × 19.5, SD 4.9, 3.1, n = 11), apical ring 3.0–4.5 μm diam, with eight ascospores arranged obliquely parallel to multiseriate. Ascospores renoid to oblong-ellipsoid, slightly tapering to broadly rounded ends, slightly curved, one median septum, slightly constricted, (18–)19–20(–21) × (5–)6–7(–8) μm (mean = 19.5 × 6.5, SD 0.9, 0.6, n = 45), l: w (2.5–)3(–4) (mean = 3, SD 0.3, n = 45), with granular cytoplasm.
Cultures: Moderate growth on PDA after 7 d a.c.d. 4 cm (SD 0.4, n = 4), thin aerial mycelium of velvety, powdery texture, margin stringy, colour grey becoming vinaceous buff 86 from the top, reverse isabelline 65.
Habitat and host: On twigs of Salix sp. (Salicaeae).
Distribution: USA (California).
Holotype: USA, California, Shasta Co., Cow Creek, close to Old Station, on Salix sp., 18 May 2008, L.C. Mejía 456, BPI 878967, derived cultures LCM456.01 and LCM456.02 = CBS 127495.
Note: Plagiostoma imperceptibile has an expanded neck similar to other species in the clade, specifically P. apiculatum, P. convexum, P. dilatatum, and P. pulchellum (Fig. 1).
Plagiostoma oregonense L.C. Mejía, sp. nov. MycoBank MB515703. Figs 4A–C, 7S–T.
Etymology: oregonense –from Oregon, referring to the only state in the USA where it was collected.
Perithecia subglobosa, (261–)270–326(–373) μm elata, (369–)381–400(–407) μm diametro; rostrum breve, (156–)168–182(–185) μm longum, cum expansa area, disciformi vertice visu, simili collo in sectione, (176–)182–204(–221) μm diametro ubi latissima, 119–120(–121) μm diametro apice. Ascosporae latoellipticae vel ellipticae, uni-septatae, constrictae medianae vel submedianae septatae, (16–)17–19(–22)×(4–)6(–7) μm, L:l (2.6–)2.9–3.2(–4.0).
Perithecia immersed in bark, solitary, evident as conic-shaped elevation of periderm with neck protruding, black, globose to subglobose, (261–)270–326(–373) μm high × (369–)381–400(–407) μm diam (mean = 304 × 389, SD 60, 19, n = 3), each with one neck. Neck eccentric or lateral, expanded, usually attached to periderm, (156–)168–182(–185) μm long (mean = 173, SD 16, n = 3), (176–)182–204(–221) μm diam at base (mean = 195, SD 23, n = 3), (119–)119–120(–121) μm diam at apex (mean = 120, SD 1.0, n = 3). Asci cylindric, (74–)78–92(–95) × (12–)15–17(–19) μm (mean = 86 × 16, SD 8, 2, n = 10), apical ring 2.8–4.0 μm diam, looks like a stretched hexagon, with eight ascospores arranged obliquely parallel or biseriate. Ascospores broadly ellipsoid to ellipsoid, with rounded ends, 1-septate, constricted at median to submedian septum, (16–)17–19(–22) × (4.5–)5.5–6(–7) μm (mean = 18.0 × 6.0, SD 1.5, 0.5, n = 36), l: w (2.6–)2.9–3.2(–4.0) (mean = 3.1, SD 0.3, n = 36), with granular cytoplasm.
Cultures: Moderate growth on PDA after 7 d a.c.d. 4.6 cm (SD 0.1, n = 2), thin aerial mycelium of felty texture, margin fringed, stringy, central area white, with a halo of aerial mycelium 1.5 cm from centre, marginal area buff 45m, reverse with a central circular area of 2 cm diam fawn 87.
Habitat and host: On overwintered branches of Salix sp. (Salicaceae).
Distribution: USA (Oregon).
Holotype: USA, Oregon, Lincoln Co., Fogarty Creek, on Salix sp., 24 May 2008, L.C. Mejía 597, BPI 878968, derived culture LCM597.01 = CBS 126124.
Plagiostoma ovalisporum L.C. Mejía, sp. nov. Figs 4D–H, 7U–V.
Etymology: ovalis - ovoid; sporum - spore, referring to the ovoid shape of the ascospores.
Perithecia globosa, (246–)277–363(–385) μm elata, (394–)403–414(–427) μm diametro; rostrum breve, (131–)146–159(–162) μm longum, apice cupulatum, (125–)136–153(–194) μm basi, (113–)117–160(–168) μm diametro apice. Ascosporae ovoideae, non-septatae, (12–)14–16(–17) × (7–)7–8(–9) μm, L:l (1.6–) 1.8–2.0(–2.2). Perithecia immersed in bark, solitary, or in groups up to five, usually in a row, scattered, erumpent, appearing as raised, conical area of bark periderm, with neck protruding through slit or hole, black, globose, (246–)277–363(–385) μm high × (394–)403–414(–427) μm diam (mean = 322 × 409, SD 57, 11, n = 6), each with one neck. Neck lateral, short and thick, apex cupulate, (131–)146–159(–162) μm long (mean = 151, SD 12, n = 6), (125–)136–153(–194) μm diam at base (mean = 150, SD 24, n = 6), (113–)117–160(–168) μm diam at apex (mean = 139, SD 24, n = 6). Asci cylindric to obclavate, (63.5–) 68.5–75.5(–87.5) × (12.5–)14.5–17(–18) μm (mean = 72 × 15.5, SD 6.5, 1.5, n = 19), apical ring 3.5–4.5 μm diam, with eight ascospores arranged obliquely parallel to biseriate. Ascospores ovoid, non-septate, appearing double-walled, more evident when stained with cotton blue lactophenol or Melzer's reagent, (12–)14–16(–17) × 7–8(–9) μm (mean = 15 × 7.5, SD 1.2, 0.5, n = 35), l: w (1.6–)1.8–2(–2.2) (mean = 1.9, SD 0.1, n = 35).
Cultures: Moderate growth on PDA after 7 d a.c.d. 4.2 cm (SD 0.1, n = 2), thin aerial mycelium of felty texture, margin fringed, like roots, whitish, with denser mycelium in centre within a radius of 1 cm, reverse buff 45 becoming dark grey, whitish in the margin.
Habitat and host: On dead twigs of Salix sp. (Salicaceae).
Holotype:USA, Idaho, Idaho Co., near Burgdorf, Burgdorf Rd. FR246, parking area at camping site at Three Mile Creek, approx. GPS: N45° 18.139 W 115° 55.782, elevation 6309 ft, on dead twigs of Salix sp., 5 Sep. 2008 (NAMA Annual Foray, Orson K. Miller Jr. Memorial Foray), A.M. Minnis s.n., BPI 878969, derived culture CBS 124977 = LCM458.01.
Notes: This species differs from other species of Plagiostoma by having ovoid, non-septate ascospores. The other two known species of Plagiostoma with non-septate ascospores, P. euphorbiae-verrucosae and P. fraxini, occur on hosts other than Salix and their ascospores are ellipsoid-fusoid. Unlike P. dilatatum, P. ovalisporum does not have a circular black halo or spot at the point where the perithecial necks emerge through the periderm.
Plagiostoma petiolophilum (Peck) Sogonov, Stud. Mycol. 62: 72. 2008. Basionym: Sphaeria petiolophila Peck, Annual Rep. New York State Mus. 35: 144. 1884.
≡ Gnomonia petiolophila (Peck) Berl. & Voglino, Syll. Fung. Addit. 1–4: 90. 1886.
≡ Cryptodiaporthe petiolophila (Peck) M.E. Barr, Mycol. Mem. 7: 136. 1978.
Notes: Barr (1978) provided a detailed description of this species as Cryptodiaporthe petiolophila.
Plagiostoma populinum (Fuckel) L.C. Mejía, comb. nov. MycoBank MB515705. Basionym: Cryptospora populina Fuckel, Jahrb. Nassauischen Vereins Naturk. 23/24: 193. 1870.
= Diaporthe populea Sacc. in Mouton, Bull. Soc. Roy. Bot. Belgique 26: 174. 1887 fide Butin (1958).
≡ Cryptodiaporthe populea (Sacc.) Butin, Sydowia 11: 31. 1958 [1957].
Moderate to fast growth on PDA after 7 d a.c.d. 3.3 cm (SD 1.2, n = 8), thin aerial mycelium of velvety or felty texture, whitish to buff 45 or rosy buff 6 in central area and isabeline 65 in the margin, with some droplets (honey 64) in the centre, with fringed margin appearing like roots, reverse whitish to fawn 87 or honey 64, in some cultures becoming dark and with a concentric halo light. Cultures are illustrated in Fig. 8A–D.
Fig. 8.
Culture morphology. A–D. Plagiostoma populinum. A–B. CBS 144.57. C–D. CBS 174.58. E–J. P. pulchellum. E–F. LCM 438.04. G–H. LCM 371.02. I–J. LCM 623.01. K–L. P. salicellum. CBS 126121 = LCM449.01. M–P. P. samuelsii. M–N. Ex-type CBS 125668 = LCM 454.04. O–P. LCM 596.01. Q–V. P. versatile. Q–R. Ex-type CBS 124978 = LCM 594.01. S–T. LCM 595.01. U–V. LCM 598.01. W–X. P. yunnanense. Ex-type CBS 124979 = LCM 513.03. A–D, G–H, W–X. Colony habit, 10 d, 23 °C. E–F, K–N. Colony habit, 9 d, 23 °C. I–J, O–V. Colony habit, 7 d, 23 °C. A, C, E, G, I, K, M, O, Q, S, U, W. Reverse. B, D, F, H, J, L, N, P, R, T, V, X. Surface.
Notes: Butin (1958) presented a full description with illustrations of this species as Cryptodiaporthe populea. Because the name Cryptodiaporthe populina (Fuckel) Petr. based on Valsa populina Fuckel was already occupied in Cryptodiaporthe, Butin (1958) based his new combination on Diaporthe populea Sacc. When placed in Plagiostoma the basionym Cryptospora populina Fuckel provides the oldest epithet for this species.
Plagiostoma pulchellum (Sacc. & Briard) L.C. Mejía, comb. nov. MycoBank MB515706. Figs 4I–M, 8E–J. Basionym: Diaporthe pulchella Sacc. & Briard in Sacc., Atti Ist. Veneto Sci. 2, Ser. 6, 437. 1884.
≡ Cryptodiaporthe pulchella (Sacc. & Briard) Butin, Phytopathol. Z. 32: 407. 1958.
= Diaporthe recedens Sacc., Ann. Mycol. 12: 290 1914 fide Butin (1958).
Perithecia immersed in bark, solitary, often growing close together, appearing initially as slight elevation of periderm, with black halo or black spot where apex protrudes through a small hole, black, globose, (311–)371–473(–613) μm high × (467–)483–642(–660) μm diam (mean = 435 × 563, SD 128, 99, n = 4), each with one neck. Neck central to eccentric, straight to oblique, with an expanded disk-like area, initially below epidermis, becoming exposed with time, producing black halo or spot at surface, (169–)173–319(–388) μm long (mean = 257, SD 105, n = 4), (153–)209–256(–306) μm diam at widest point (mean = 231, SD 62.5, n = 4), (93–)99–160(–212) μm diam at apex (mean = 137, SD 54, n = 4). Asci ovoid elongated, (75–)85–107(–117) × (15–)17–21(–24) μm (mean = 95 × 19, SD 13.5, 3.0, n = 15), apical ring 4–4.8 μm diam, very thick, with eight ascospores arranged obliquely parallel to multiseriate. Ascospores oblong ellipsoid-elongated, slightly tapering, with rounded ends, straight to slightly curved, one median to submedian septum, not constricted, (17–)18–22(–27) × (5–)6–7(–7.5) μm (mean = 20.3 × 6.3, SD 2.9, 0.6, n = 39), l: w (2.5–)2.9–3.4(–4.4) (mean = 3.2, SD 0.4, n = 39), with granular cytoplasm.
Cultures: Moderate growth on PDA after 7 d a.c.d. 3.9 cm (0.8 n = 6), thin aerial mycelium whitish to rosy vinaceous 58 colour, of velvety, granular texture due to mycelial clumps ca. 500 um diam, isabeline 65, produced in central area of 2.4 cm diam, central area appearing often moist, margin translucent to buff 45, with hyphae extending radially, stringy, becoming fringed toward margin; reverse whitish to rosy vinaceous 58 or olivaceous. At 14 d small black and dark green slimy droplet surrounded by a second halo rosy vinaceous 58 with white greyish margin, reverse same colour pattern.
Habitat and host: On dead, still attached branches of Populus balsamifera, Populus sp., Salix babylonica, S. humboldtiana and S. lucida (Salicaceae).
Distribution: North America, South America (Argentina), and Europe.
Type specimen: France, Troyes, on branch of Populus alba cv. pyramidalis, Briard n. 5, PAD-not available.
Specimens examined: Argentina, Tucumán, vicinity of Villa Nougués, on twigs of Salix humboldtiana, 16 Nov 2008, L.C. Mejía 623, BPI 878974, derived cultures LCM623.01 and LCM623.03.Netherlands, ex leaf of Populus balsamifera, (CBS 170.69 as Cryptodiaporthe pulchella. USA, Maryland, Prince George's Co., Beltsville, USDA-BARC, outside of B011A, on twigs of Salix babylonica, 3 Mar 2008, Amy Y. Rossman & L.C. Mejía 365, BPI 878971, derived culture LCM365.04 = CBS 126653; Maryland, Prince George's Co., Greenbelt, Lake Artemisia, on twigs of Salix babylonica, 15 Mar 2008, L.C. Mejía 371, BPI 878972, derived culture LCM371.02; Washington, Kitsap Co., Kitsap Memorial State Park, on twigs of Salix lucida, 28 May 2008, L.C. Mejía 438, BPI 878973, derived cultures LCM438.03 (= CBS 126122) and LCM438.04.
Notes: Butin (1958) refers to this species as saprobic on Populus spp. The evidence presented in this study shows that this species also infects Salix spp. and has a more extensive geographic distribution than previously reported.
Plagiostoma rhododendri (Auersw.) Sogonov, Stud. Mycol. 62: 72. 2008. Basionym: Gnomonia rhododendri Auersw. in Gonn. & Rabenh., Mycol. Europ. 5/6: 26. 1869.
≡ Apiognomonia rhododendri (Auersw.) Remler, Bibliotheca Mycologica 68: 74. 1979.
Note: Remler (1979 as A. rhododendri) and Monod (1983 as G. rhododendri) presented descriptions of this species.
Plagiostoma robergeanum (Desm.) Sogonov, Stud. Mycol. 62: 73. 2008. Basionym: Sphaeria robergeana Desm., Ann. Sci. Nat. Bot. ser. 3, 16: 306. 1851.
≡ Diaporthe robergeana (Desm.) Niessl. in Rabenh., Fungi Europ. 2222. 1882.
≡ Cryptodiaporthe robergeana (Desm.) Wehm., The Genus Diaporthe Nitschke and its Segregates p. 200. 1933.
Note: Wehmeyer (1933) provided a description of this species as Cryptodiaporthe robergeana.
Plagiostoma salicellum (Fr.) Sogonov, Stud. Mycol. 62: 73. 2008. Fig. 5A–H. Basionym: Sphaeria salicella Fr., Syst. Mycol. 2: 377. 1823.
≡ Diaporthe salicella (Fr.) Sacc., Mycotheca Venet. 135. 1873.
≡ Gnomonia salicella (Fr.) J. Schröt., Pilze Schles. 3, 2: 392.1897.
≡ Chorostate salicella (Fr.) Traverso, Fl. Ital. Crypt. 2: 203. 1906.
≡ Cryptodiaporthe salicella (Fr.) Wehm., The Genus Diaporthe Nitschke and its Segregates p. 193. 1933.
Perithecia immersed in bark, solitary or in groups up to five, scattered, evident as slight elevation of periderm, black, subglobose, (157–)208–308(–331) μm high × (339–)372–410(–507) μm diam (mean = 257 × 397, SD 60, 43, n = 11), each with one neck. Neck cylindrical, eccentric to lateral, surrounded by a whitish stroma, (96–)147–202(–308) μm long (mean = 177, SD 67, n = 11), (61–)80–84(–95) μm diam at base (mean = 81, SD 8.8, n = 11), (54–)74–85(–91) μm diam at apex (mean = 79, SD 10, n = 11). Asci cylindric to clavate, (40–)51.5–59(–63) × (11–)13–14(–15) μm (mean = 55 × 13, SD 5.8, 1.4, n = 15), with apical ring 2.0–3.5 μm diam, with eight ascospores arranged obliquely parallel or irregularly seriate. Ascospores ellipsoid-elongated, slightly tapering toward rounded ends, 1-septate, often with short appendages 1.5–2 μm, slightly constricted at median to submedian septum, (14–)17–20(–27) × 3–4(–5) μm (mean = 18.5 × 3.5, SD 2.3, 0.5, n = 57), l: w (3.2–)5.2–6.6(–8.7) (mean = 5.9, SD 1.1, n = 57), with granular cytoplasm.
Habitat and host: On dead, still attached branches of Salix alba, S. repens, and Salix sp. (Salicaceae).
Distribution: Europe.
Lectotype of Sphaeria salicella designated here: Scleromyceti Sueciae 188 issued 1821, Sbarbaro Collection, BPI exsiccati).
Epitype of Sphaeria salicella designated here: Austria, St. Margareten im Rosental, Kaernten, Drau-Auen. 9452/1, as Cryptodiaporthe apiculata on Salix alba, 2 May 2002, W. Jaklitsch 1889, BPI 843527, derived culture CBS 121466.
Additional specimen examined: Germany, Langen, on Salix repens, L.C. Mejía, BPI 878975, derived culture CBS 126121 = LCM 449.01.
Notes: The application of the name Sphaeria salicella Fr. has been the source of confusion and the subject of taxonomic studies since the 1800's. It was clearly specified by Fries (1823) that this name is typified by Fries: Scleromyceti Sueciae 188 issued in 1821. According to Wehmeyer (1933) and Butin (1958) confusion about this name was in part due to the fact that different parts of exsiccati Scleromyceti Sueciae 188 contain different species. One of the two species has the narrowly ellipsoid, elongated ascospores of P. salicellum while the other has the broadly ellipsoid ascospores of P. apiculatum. Not recognising the confusion regarding Scleromyceti Sueciae 188, Petrak (1921) wrongly suggested that S. salicella was characterised by having broadly ellipsoid ascospores and made a new combination Cryptodiaporthe salicella (Fr.) Petr. in addition to the new combination C. apiculata (Wallr.) Petr. based on S. apiculata Wallr. The latter species was wrongly considered to have narrowly ellipsoid, elongated ascospores. It is not clear if Petrak (1921) looked at the type specimen of S. apiculata or if he based his conclusions solely on the description of S. apiculata by Wallroth (1833).
Wehmeyer (1933) arrived at a conclusion different from that of Petrak (1921). Wehmeyer (1933) studied the exsiccati Scleromyceti Sueciae 188 at the Farlow Herbarium and determined that this number and hence S. salicella Fr. were characterised by having narrowly ellipsoid, elongated ascospores. To use his words, S. salicella represents “the narrow-spored species”. He synonymised S. salicella Fr. with C. apiculata (Wallr.) Petr. and published the combination C. salicella (Fr.) Wehm. (1933) non Petrak (1921). In addition, Wehmeyer (1933) made the new combination C. salicina (Curr.) Wehm. based on S. salicina Curr. for species having broadly ellipsoid ascospores (see notes under P. convexum). Later Butin (1958) studied species of Cryptodiaporthe on Populus and Salix, examined Scleromyceti Sueciae 188 at Uppsala Herbarium, and suggested that S. salicella should be understood as the species with broadly ellipsoid ascospores and followed Petrak's concept of S. salicella.
We studied the Scleromyceti Sueciae 188 (Sbarbaro collection) available at the BPI Herbarium as well as other exsiccati of taxa that have been synonymised with S. salicella and C. salicina including S. apiculata. In doing so we paid close attention to the original descriptions of S. apiculata Wallr. and S. salicella Fr. In referring to Scleromyceti Sueciae 188 Fries (1823) described S. salicella as having a powdery “albicant” (whitish) stroma and that the multiple ostioles are “erumpent simultaneously”. The specimen of Scleromyceti Sueciae 188 at BPI includes all of these morphological characters and has ascospores that are ellipsoid elongated (see Fig. 5A, B, D, and G).
In their treatment of Plagiostoma, Sogonov et al. (2008) made the combination Plagiostoma salicellum (Fr.) Sogonov. In our study of P. salicellum we noticed that ascospore length and width can be quite variable, even within an ascus, but with a prevalence of elongated ascospores (Fig. 5A–H). A second specimen, BPI 878975 from Germany on Salix repens, was sequenced and determined to be conspecific with the epitype of P. salicellum within a major clade containing two other species having cylindrical ostioles and ellipsoid elongated ascospores. Unlike the lectotype and epitype of P. salicellium, the ascospores of BPI 878975 are ellipsoid but not elongated. In spite of this difference in ascospore morphology, BPI 878975 is P. salicellum based on the cylindrical perithecial neck surrounded by a whitish stroma as well as DNA sequence data.
In summary Plagiostoma salicellum is characterised by having cylindrical perithecial necks surrounded by a whitish stroma and ascospores predominantly ellipsoid-elongated, less commonly ellipsoid, tapering to slightly acute, rounded ends (Fig. 5D–G), unlike P. apiculatum that has oblong ellipsoid to renoid, broadly rounded ascospores (Fig. 2H–J).
Plagiostoma samuelsii L.C. Mejía, sp. nov. MycoBank MB515707. Figs 5I–O, 8M–P.
Etymology: Named in honour of distinguished mycologist Gary J. Samuels for his outstanding contributions to the systematics of Pyrenomycetes.
Perithecia subglobosa, (192–)204–258(–305) μm elata, (295–)302–327(–334) μm diametro, rostrum conicum, (114–)128–161(–170) μm longum, (69–)72–74(–81) μm diametro basi, (58–)62–73(–78) μm diametro apice. Ascosporae ellipticae, uni-septatae, constrictae ubi medianae vel submedianae septatae, (10–)11–12(–19) × 3–4 μm, appendiculatae duabus extremitatibus, anguste filiformes, ascosporis vulgo 2-plo longiores, appendices deliquescentes.
Perithecia immersed in bark, solitary or in groups up to five, scattered on substrate, evident as conical shaped elevations of host periderm with necks protruding through small holes in periderm, black, subglobose, (192–)204–258(–305) μm high × (295–)302–327(–334) μm diam (mean = 239 × 313, SD 43, 16, n = 6), each with one neck. Neck eccentric to lateral, surrounded by a whitish stroma, cone-shaped with rounded apex, (114–)128–161(–170) μm long (mean = 145, SD 23, n = 6), (69–)72–74(–81) μm at base (mean = 73.8, SD 3.9, n = 6), (58–)62–73(–78) μm at apex (mean = 67.5, SD 8.0, n = 6). Asci cylindric to clavate, (32–)42–62(–79) × (6–)7–11(–12) μm (mean = 53 × 9, SD 13, 2.1, n = 24), apical ring 1.8–3.6 μm diam, with eight ascospores arranged obliquely parallel or biseriate. Ascospores ellipsoid, 1-septate, constricted at median to submedian septum, with two deliquescent appendages, one at end of each cell, narrowly filiform, usually twice the length of ascospores, (10–)11–12(–19) × 3–4 μm (mean = 12 × 3.5, SD 1.4, 0.2, n = 48), l: w (2.8–)3.2–3.5(–4.8) (mean = 3.4, SD 0.4, n = 48), with four refractive bodies in each cell, two big ones near septum, one smaller one at end of each cell.
Cultures: Fast growth on PDA after 7 d reaching edge of petri plates of 6 cm diam (n = 8), thin aerial mycelium of felty to granular texture, fringed, stringy margin, with concentric halo of dense mycelium 1.4 cm from centre, buff 45 inside halo or central region, white toward margin, some cultures with depression at concentric halo, reverse honey 64 developing a halo fawn 87 near margin.
Habitat and host: On dead, still attached twigs and branches of Alnus incana var. tenuifolia, A. rubra, and Alnus spp. (Betulaceae).
Distribution: USA (California, Oregon, Washington).
Holotype: USA, California, Plumas Co., Little Last Chance Creek, Chilcook Campground, on Alnus incana var. tenuifolia, 17 May 2008, L.C. Mejía 454, BPI 878977, derived CBS 125668 = LCM454.04.
Specimens examined: USA, Oregon, Jackson Co., Upper Rogue River, on Alnus (tenuifolia?), 21 May 2008, L.C. Mejía 419, BPI 878976, derived culture LCM419.01 and LCM419.02; Upper Rogue River trail on Alnus sp. 21 May 2008, L.C. Mejía 474, BPI 878978, derived culture LCM474.01; Washington, Clallam Co., Crescent Lake, on Alnus rubra (branch on soil), 27 May 2008, L.C. Mejía 596, BPI 878979, derived culture LCM596.01.
Notes: Plagiostoma samuelsii is the only species of Plagiostoma on leaves of Alnus except for P. jensenii M.E. Barr. Unlike P. samuelsii, P. jensenii lacks a stroma and perithecial neck and has longer and wider ascospores (20–30 × 4–6 μm) with very short pulvinate appendages (Barr 1991). Most likely P. jensenii does not belong in Plagiostoma.
Plagiostoma versatile L.C. Mejía & Sogonov, sp. nov. MycoBank MB515708. Figs 6A–C, 8Q–V.
Etymology: versatile –versatile, referring to the occurrence of this species on different plant organs, twigs, branches, and leaves; and to the variable nature of the perithecia that grow with short necks on twigs and branches and with medium to long necks on leaves.
Perithecia subglobosa, (178–)194–317(–345) μm elata, (232–)264–378(–444) μm diametro; rostrum cylindricum, (60–)77–137(–226) μm longum, (51–)56–80(–87) μm diamtro basi, (37–)49–60(–76) μm apice. Ascosporae elliptico-elongatae, uni-septatae, constrictae ubi medianae vel submedianae septatae, (18–)20–23(–25) × 3–4 μm, L:l (4.9–)5.6–6.8(–8.6).
Perithecia immersed in bark of twigs or in midvein and petioles of adaxial and abaxial side of leaves, solitary or in pairs, scattered, on twigs evident as slight elevations of periderm that appear black, upper part of perithecia showing a few cell layers below epidermis, on leaves producing swollen, raised areas on midvein, becoming highly erumpent, cracking periderm and leaving an ellipsoidal cavity, with longer neck on leaves than on twigs, black, subglobose, (178–)194–317(–345) μm high × (232–)264–378(–444) μm diam (mean = 248 × 323, SD 68.5, 78, n = 8), each with one neck. Neck eccentric to lateral, short, ostiolar opening sulcate with four grooves, (60–)77–137(–226) μm long (mean = 115, SD 59.7, n = 8), (51–)56–80(–87) μm diam at base (mean = 68.5, SD 13.4, n = 8), (37–)49–60(–76) μm diam at apex (mean = 55, SD 11.8, n = 8). Asci cylindric to clavate, (49–)54–66(–71) × (11–)13–16(–20) μm (mean = 60 × 15, SD 7.5, 2.5, n = 15), apical ring 2.0–3.5 μm diam, with eight ascospores arranged biseriate. Ascospores ellipsoid elongated, slightly tapering toward rounded ends, 1-septate, constricted at median to submedian septum, (18–)20–23(–25) × 3–4 μm (mean = 21.5 × 3.5, SD 2.0, 0.4, n = 36), l: w (4.9–)5.6–6.8(–8.6) (mean = 6.2, SD 1.0, n = 36), usually with four large refractive bodies, two near septum, one in each end of cells.
Cultures: Fast growth on PDA after 7 d reaching the edge of petri plates of 6 cm diam (n = 12), thin aerial mycelium of felty to granular texture, margin fringed, like roots, buff 45 with clumps of white mycelium, with a halo of elevated mycelium at 1.5 cm from centre, reverse buff 45 becoming dark, with halo visible from reverse.
Habitat and hosts: On dead twigs of Salix scouleriana and Salix sp., on overwintered leaves of Salix sp. (Salicaceae).
Distribution: USA (Oregon, Washington); Canada (British Columbia).
Holotype: USA, Washington, Jefferson Co., intersection of Upper Hoh River Road & Route 101, on Salix scouleriana, 27 May 2008, L.C. Mejía 594, BPI 878980, derived culture CBS 124978 = LCM594.01.
Additional specimens examined: Canada, British Columbia, Vancouver, on overwintered dead leaves of Salix sp., 12 May 2006, M. V. Sogonov 379, BPI 877702, derived culture CBS 121251 = AR4294. USA, Oregon, Lane Co., Willamette Pass, on Salix sp., 22 May 2008, L.C. Mejía 598, BPI 878982, derived culture LCM598.01; Washington, Jefferson Co., Hoh River Campground, on Salix scouleriana, 27 May 2008, L.C. Mejía 595, BPI 878981, derived culture LCM595.01.
Notes: The ascospores of this species are similar to those of Plagiostoma salicellum, however, the perithecial necks of P. versatile lack the whitish stroma characteristic of P. salicellum.
Plagiostoma yunnanense L.C. Mejía & Zhu L. Yang, sp. nov. MycoBank MB515709. Figs 6D–F, 8W–X.
Etymology: referring to the place where this species was collected: Yunnan, China.
Perithecia globosa, (231–)267–311(–318) μm elata, (282–)312–352(–362) μm diametro, rostrum cylindricum, contortum, (315–)318–321(–322) μm longum, (77–) 78–81(–82) μm diametro basi, (57–)59–63(–66) μm diametro apice. Ascosporae elliptico-elongatae, uni-septatae, leviter vel non constrictum ubi septatae, (19–)23–26(–27) × 3–4, L:l (6. 6–)6.8–7.9(–8.2).
Perithecia immersed, solitary or in groups, numerous, appearing as conical elevations of periderm where necks protrude, black, globose, (231–)267–311(–318) μm high × (282–)312–352(–362) μm diam (mean = 284 × 328, SD 48, 42, n = 3), each with one neck. Neck eccentric, contorted, (315–)318–321(–322) μm long (mean = 319, SD 3.8, n = 3), (77–)78–81(–82) μm diam at base (mean = 79, SD 2.4, n = 3), (57–)59–63(–66) μm diam at apex (mean = 61, SD 4.8, n = 3). Asci not observed. Ascospores ellipsoid-elongate, with rounded ends, 1-septate, slightly or not constricted at median to submedian septum, (19–)23–26(–27) × 3–4 μm (mean = 24 × 3.3, SD 2.7, 0.4, n = 6), l: w (6.6–)6.8–7.9(–8.2) (mean = 7.3, SD 0.7, n = 6), with granular cytoplasm.
Cultures: Moderate growth on PDA after 7 d a.c.d. 3.4 cm (SD 0.2, n = 4). Mycelium of granular texture, margin stringy, whitish with granules, becoming grey or vinaceous buff, reverse with dark inclusions near centre, most of colony whitish.
Habitat and host: On dead, still attached branches of Salix sp. (Salicaceae).
Distribution: China (Yunnan).
Holotype: China, Yunnan, Ailoshan, on Salix sp., 14 Jul. 2008, L.C. Mejía 513, BPI 878983, derived cultures CBS 124979 = LCM513.03 and LCM513.02.
Additional names in Cryptodiaporthe and Plagiostoma
Cryptodiaporthe acerinum J. Reid & Cain, Canad. J. Bot. 40: 839. 1962.
Notes: A fresh specimen determined to be this species was cultured and sequenced. Analyses of LSU and RPB2 sequences place this species in a basal branch of the Gnomoniaceae.
Specimen examined: USA, New York, Adirondacks, Cranberry Lake, on dead branch of Acer sp., 13 Jun. 2002, L. Vasilyeva, BPI 870989, culture CBS 121465 = AR 3822.
Cryptodiaporthe aculeans (Schwein.) Wehm., The Genus Diaporthe Nitschke and its Segregates p. 212. 1933. Basionym: Sphaeria aculeans Schwein., Trans. Am. Phil. Soc., New Series 4(2): 204. 1834,. [1832].
Notes: The only available culture of this species was sequenced. Analyses of LSU sequences place this species in a clade sister to the Melanconidaceae.
Culture sequenced: Japan, on branch of Rhus javanica, isol. G. Okada, CBS 525.85.
Cryptodiaporthe aubertii (Westend.) Wehm., The Genus Diaporthe Nitschke and its Segregates 202. 1933. Basionym: Sphaeria aubertii Westend., Bull. Acad. R. Sci. Belg., Cl. Sci., sér. 2: tab. 7, no. 5. 1859.
Notes: A culture of this species was sequenced. Analyses of this LSU sequence suggests that this species is related to the genus Cryptosporella within the Gnomoniaceae.
Culture sequenced:Sweden, Småland, on Myrica gale, 14 Apr. 1989, K. & L. Holm, isol. O. Constantinescu 89–53, CBS 114196.
Cryptodiaporthe galericulata (Tul. & C. Tul.) Wehm., The Genus Diaporthe Nitschke and its Segregates p. 211. 1933. Basionym: Valsa galericulata Tul. & C. Tul., Select. Fung. Carpol. (Paris) 2: 203. 1863.
Notes: A fresh specimen determined to be this species was cultured and sequenced. Analyses of LSU sequences suggest this species belongs in the Sydowiellaceae.
Specimen examined: USA, Tennessee, Great Smoky Mts. National Park, near Cosby, Horse Trail, on Fagus grandifolia, 25 Mar. 2002, L. Vasilyeva, BPI 863767 ex culture AR 3811.
Cryptodiaporthe liquidambaris Petr., Sydowia 5: 236. 1951.
Notes: A fresh specimen determined to be this species was cultured and sequenced. Analyses of LSU sequences place this species within the Diaporthales but not within any described family.
Specimen examined: USA, Maryland, Beltsville, on overwintered twig of Liquidambar styraciflua, 15 May 2001, M. Barr, isol. A. Rossman, BPI 749123 culture AR 3648 (now dead).
Cryptodiaporthe macounii (Dearn.) Wehm., The Genus Diaporthe Nitschke and its Segregates p. 191. 1933. Basionym: Diaporthe macounii Dearn., Mycologia 8: 100. 1916.
Note: This species was included in the genus Gnomoniopsis (Gnomoniaceae) by Sogonov et al. (2008).
Cryptodiaporthe vepris (Delacr.) Petr., Ann. Mycol. 32: 445. 1934. Basionym: Sphaeria vepris Delacr., Fungi europ. 443. 1862.
Notes: A fresh specimen determined to be this species was cultured and sequenced. Analyses of LSU sequences place this species within the Diaporthales but not in any described family.
Specimen examined: Austria, Wograda, St. Margareten, Kaernten, on Rubus idaeus, 27 Oct. 2000, W. Jaklitsch 1661, isol. A. Rossman, BPI 749132, culture AR 3559.
Plagiostoma acerophilum (Dearn. & House) M.E. Barr, Mycol. Mem. 7: 113. 1978. Basionym: Gnomoniopsis acerophila Dearn. & House, Bull. New York State Mus. 233–234: 36. 1921.
Notes: A fresh specimen determined to be this species was cultured and sequenced. This species falls within the Gnomoniaceae according to analyses of ITS sequences but not within any known genus. The perithecial neck of this species is lateral, upright, and slightly curved at the apex.
Specimens examined: USA, New York, Sullivan Co., Roscoe, on overwintered leaves of Acer pensylvanicum, Jul. 2005, M.V. Sogonov MS 0302, BPI 877681; Tennessee, Blount Co., Great Smoky Mountains National Park, Cades Cove, on overwintered petioles of Acer pensylvanicum, 24 May 2006, M.V. Sogonov MS 0467, BPI 877679; Sevier Co., Great Smoky Mountains National Park, on overwintered leaves and petioles of Acer pensylvanicum, 22 May 2006, M.V. Sogonov MS 0473, BPI 877682.
Plagiostoma alneum (Fr.) Arx, Antonie van Leeuwenhoek 17: 264. 1951.
= Sphaeria alnea Fr.: Fr., Observ. mycol. 1: 185. 1815: Syst. Mycol. 2: 520. 1823.
Notes: This species is now regarded as Gnomonia alnea (Fr.) Sogonov and was described and illustrated in Sogonov et al. (2008).
Plagiostoma arnstadtiense (Auersw.) M. Monod, Beihefte Sydowia 9: 143. 1983. Basionym: Gnomonia arnstadtiensis Auersw. in Gonnerm. & Robenh., Mycol. Europ. 5/6: 22. 1869.
Notes: This species is now accepted in Gnomonia according to Sogonov et al. (2008).
Plagiostoma bavaricum (Rehm) M.E. Barr, Mycol. Mem. 7: 112. 1978. Basionym: Hypospila bavarica Rehm, Ann. Mycol. 6:322. 1908.
Note: Based on an LSU sequence, this species belongs in the Gnomoniaceae but it cannot be placed in a genus.
Culture sequenced: Switzerland, on Acer opalus, M. Monod, CBS 772.79.
Plagiostoma conradii (Ellis) M.E. Barr, Mycol. Mem. 7: 107. 1978. Basionym: Diaporthe conradii Ellis, Am. Nat. 17: 316. 1883.
Notes: A fresh specimen determined to be this species was cultured and sequenced. Analysis of the LSU sequence suggests this species is closely related to Cryptodiaporthe aubertii and Cryptosporella but not within any known genus in the Gnomoniaceae. The perithecial neck of this species is lateral and upright.
Specimen examined: USA, New Jersey, on living stems of Hudsonia tomentosa, G. Bills, BPI 746482, culture CBS 109761 = AR 3488.
Plagiostoma inclinatum (Auersw.) M.E. Barr, Mycol. Mem. 7: 115. 1978. Basionym: Gnomonia inclinata Auersw. in Rabenh., Mycol. Europ. 5/6: 27. 1869.
[≡ Sphaeria inclinata Desm., Ann. Sci. Nat. Bot. III, 16: 315. 1851 non Schwein. 1832]
Notes: Two isolates of this species were sequenced. This species apparently belongs in the Gnomoniaceae but it cannot be placed in a genus.
Cultures sequenced: The Netherlands, on dead leaf of Acer pseudoplatanus, CBS 209.67. Switzerland, on Acer platanoides, M. Monod, CBS 830.79.
Plagiostoma jensenii M.E. Barr, Mycotaxon 41: 298. 1991.
Note: Based on the lack of a perithecial neck and the pulvinate appendages on the ascospores, P. jensenii most likely does not belong in Plagiostoma.
Plagiostoma lugubre (P. Karst.) Bolay, Ber. Schweiz. Bot. Ges. 81: 436. 1972. Basionym: Gnomonia lugubris P. Karst., Bidr. Känn. Finl. Nat. Folk 23: 121. 1873.
Note: The disposition of this species was not investigated in this study.
Plagiostoma magnoliae (Ellis) M.E. Barr, Mycol. Mem. 7: 117. 1978. Basionym: Gnomonia magnoliae Ellis, Amer. Nat. 17: 318. 1883.
Notes: This species has been reported in leaves of Magnolia virginiana in North America. The perithecial neck of this species is lateral and obliquely upright as drawn by Barr (1978). The disposition of this species was not investigated in this study.
Plagiostoma micromegalum (Ellis & Everh.) M.E. Barr, Mycol. Mem. 7: 112. 1978. Basionym: Diaporthe micromegala Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 1893: 449. 1894.
Notes: This species is now placed in Ophiognomonia as O. micromegala (Ellis & Everh.) Sogonov according to Sogonov et al. (2008).
Plagiostoma petrakii (E. Müll.) M. Monod, Beihefte Sydowia 9: 146. 1983. Basionym: Plagiostigme petrakii E. Müll., Sydowia 18:90. 1965.
Note: No material of this species was located.
Plagiostoma pseudobavaricum M. Monod, Beihefte Sydowia 9: 151. 1983.
Notes: ITS sequences of this species were included in a phylogenetic analysis. Although closely related to Apiognomonia and Plagiostoma, the results suggest that this species may represent an undescribed genus within the Gnomoniaceae.
Specimens examined: USA, New York, Adirondack Mts., Cranberry Lake, on petioles of Acer sp., 22 Jun. 2002, L. Vasilyeva, BPI 843494, ex culture AR 3819; ibid., 23 Jun. 2002, L. Vasilyeva, BPI 843495, ex culture AR 3894; Tennessee, Sevier Co., Great Smokey Mountains National Park, on overwintered petioles of Acer saccharum, 22 May 2006, M.V. Sogonov MS 0483, BPI 877700.
Plagiostoma robertiani (Petr.) M.E. Barr, Mycol. Mem. 7: 113. 1978. Basionym: Gnomonia robertiani Petr., Ann. Mycol. 23: 122. 1925.
Note: No material of this species was located.
Plagiostoma tormentillae (Lind) Bolay, Ber. Schwiz. Bot. Ges. 81: 436. 1971. Basionym: Gnomoniella tormentillae Lind, Bot. Tidsskr. 41: 217. 1931.
Note: This species is now recognised as Gnomoniopsis tormentillae (Lind) Sogonov according to Sogonov et al. (2008).
Acknowledgments
This work was funded by the National Science Foundation Partnerships for Enhancing Expertise in Taxonomy (NSF 03-28364). Additional funding for field work by LCM was received through Rutgers University, New Brunswick, NJ, from the Neyra Fund, the Spencer Davis Research Award from the Department of Plant Biology and Pathology, a Special Study Support Award, and a Graduate School Travel Award and through the Backus Award from the Mycological Society of America. Much appreciation is extended to Lena Struwe for her valuable comments and insights during the course of this work. We acknowledge and appreciate Andrew Minnis for providing a specimen from which the new species Plagiostoma ovalisporum was described and for nomenclatural advice. Christian Feuillet kindly provided translations of the diagnoses into Latin. Tunesha Phipps and Sasha Allen are sincerely acknowledged for their technical expertise in the molecular laboratory. LCM appreciates the following people for graciously hosting visits for collecting and facilitating the shipment of specimens: Michael Giraurd, Adriana Hladki, Tina Hoffman, Christian Lechat, Christophe Lecuru, and Ralph Mangelsdorff. Special thanks to Zhu LiangYang for hosting LCM and providing superb logistical support during his collecting trip in China.
Taxonomic novelties: Plagiostoma dilatatum L.C. Mejía, sp. nov., Plagiostoma exstocollum L.C. Mejía, sp. nov., Plagiostoma imperceptibile L.C. Mejía, sp. nov., Plagiostoma oregonense L.C. Mejía, sp. nov., Plagiostoma ovalisporum L.C. Mejía, sp. nov., Plagiostoma samuelsii L.C. Mejía, sp. nov., Plagiostoma versatile L.C. Mejía & Sogonov, sp. nov., Plagiostoma yunnanense L.C. Mejía & Zhu L. Yang, sp. nov., Plagiostoma apiculatum (Wallr.) L.C. Mejía, comb. nov., Plagiostoma convexum (Preuss) L.C. Mejía, comb. nov., Plagiostoma populinum (Fuckel) L.C. Mejía, comb. nov., Plagiostoma pulchellum (Sacc. & Briard) L.C. Mejía, comb. nov.
References
- Arx JA von (1951). Ueber die Gattung Laestadia und die Gnomoniaceen. Antonie van Leeuwenhoek 17: 259–272. [PubMed] [Google Scholar]
- Barr ME (1978). The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycological Memoir 7: 1–232. [Google Scholar]
- Barr ME (1991). Revisions and additions to the Diaporthales. Mycotaxon 41: 287–305. [Google Scholar]
- Butin H (1958). Über die auf Salix und Populus vorkommenden Arten der Gattung Cryptodiaporthe Petrak. Phytopathologische Zeitschrift 32: 399–415. [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002). A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Clements FE, Shear CL (1931). The Genera of Fungi. H.W. Wilson Co., New York.
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004). MycoBank: an online initiative to launch MycoBank to the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Currey F (1858). Synopsis of the fructification of the compound Sphaeriae of the Hookerian herbarium. Transactions of the Linnean Society of London 22: 257–287. [Google Scholar]
- Fries E (1823). Systema Mycologicum 2. Ex. Officina Berlingiana, Lund.
- Fröhlich J, Hyde KD (1995). Maculatipalma fronsicola gen. et sp. nov. causing leaf spots on palm species in north Queensland with descriptions of the related genera: Apioplagiostoma and Plagiostoma. Mycological Research 99: 727–734. [Google Scholar]
- Fuckel L (1870). Symbolae Mycologicae. Julius Niedner, Wiesbaden.
- Gregory NF, Mulrooney RP, Rossman AY, Castlebury LA (2004). Anthracnose caused by Discula fraxinea on the new host Chinese fringetree and white ash in Delaware. Plant Disease 88: 427. [DOI] [PubMed] [Google Scholar]
- Höhnel F von (1917). System der Diaportheen. Sonderabruck aus den Berichten der Deutschen Botanischen Gesellschaft 35: 631–638. [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Kobayashi T (1970). Taxonomic studies of Japanese Diaporthaceae with special reference to their life histories. Bulletin of the Government Forest Experiment Station (Japan) 226: 1–242. [Google Scholar]
- McNeill J, Barrie FR, Burdet HM, DeMoulin V, Hawksworth DL, et al. (2006). International Code of Botanical Nomenclature (Vienna Code). Regnum Vegetabile 146. A.R.G. Gantner Verlag KG.
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF (2008). Phylogenetic placement and taxonomic review of the genus Cryptosporella and its synonyms Ophiovalsa and Winterella (Gnomoniaceae, Diaporthales). Mycological Research 112: 23–35. [DOI] [PubMed] [Google Scholar]
- Monod M (1983). Monographie taxonomique des Gnomoniaceae. Beihefte zur Sydowia. Annales Mycologici, Ser. 2. 9: 1–315. [Google Scholar]
- Müller E, Arx JA von (1962). Die Gattungen der didymosporen Pyrenomyceten. Beitr. Kryptogamenfl. Schweiz 11(2): 1–922. [Google Scholar]
- Nylander JAA (2004). MrModeltest v. 2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- O'Donnell K, Cigelnik E (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Petrak F (1921). Mycologische Notizen. II. Annales Mycologici 19: 17–128. [Google Scholar]
- Petrini O, Fisher PJ (1990). Occurrence of fungal endophytes in twigs of Salix fragilis and Quercus robur. Mycological Research 94: 1077–1080. [Google Scholar]
- Posada D, Crandall KA (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Redlin SC, Stack RW (1988). Gnomoniella fraxini sp. nov., teleomorph of the ash anthracnose fungus and its connection to Discula fraxinea comb. nov. Mycotaxon 32: 175–198. [Google Scholar]
- Reeb V, Lutzoni F, Roux C (2004). Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution 32: 1036–1060. [DOI] [PubMed] [Google Scholar]
- Rehner SA (2001). EF1 alpha primers. Available online at http://ocid.nacse.org/research/deephyphae/EF1primer.pdf
- Remler P (1979). Ascomyceten aur Ericaceen in den Ostalpen. Bibliotheca Mycologica 68: 1–321. [Google Scholar]
- Rossman AY, Castlebury LA, Putnam ML (2004). First report of ash anthracnose caused by Discula fraxinea in Oregon. Plant Disease 88: 222. [DOI] [PubMed] [Google Scholar]
- Sinclair WA, Lyon H (2005). Diseases of trees and shrubs, second ed. Cornell University Press, Ithaca.
- Sogonov MV, Castlebury LA, Rossman AY, Mejía LC, White JF, Jr (2008). Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton BC (1980). The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. Commonwealth Mycological Institute, Kew, Surrey, England.
- Swofford DL (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and OtherMethods). Version 4.0b10 Sinauer Associates, Sunderland.
- Wehmeyer LE (1933). The genus Diaporthe Nitschke and its segregates. University of Michigan Press, Ann Arbor.
- Wehmeyer LE (1975). The Pyrenomycetous Fungi. Edited by Hanlin, RT. Mycological Memoir 6: 1–250. [Google Scholar]