Abstract
Two new pathogens, Guignardia korthalsellae and Rosenscheldiella korthalsellae, are described from New Zealand's pygmy mistletoes (Korthalsella, Viscaceae). Both form ascomata on living phylloclades with minimal disruption of the tissue. Fungal hyphae within the phylloclade are primarily intercellular. Guignardia korthalsellae disrupts a limited number of epidermal cells immediately around the erumpent ascoma, while the ascomata of Rosenscheldiella korthalsellae develop externally on small patches of stromatic tissue that form above stomatal cavities. Rosenscheldiella is applied in a purely morphological sense. LSU sequences show that R. korthalsellae as well as another New Zealand species, Rosenscheldiella brachyglottidis, are members of the Mycosphaerellaceae sensu stricto. Genetically, Rosenscheldiella, in the sense we are using it, is polyphyletic; LSU and ITS sequences place the two New Zealand species in different clades within the Mycosphaerellaceae. Rosenscheldiella is retained for these fungi until generic relationships within the family are resolved. Whether or not the type species of Rosenscheldiella, R. styracis, is also a member of the Mycosphaerellaceae is not known, but it has a similar morphology and relationship to its host as the two New Zealand species.
Keywords: ITS, LSU, Mycosphaerellaceae, Phaeocryptopus, phylogeny
INTRODUCTION
The pygmy mistletoes of New Zealand belong to the genus Korthalsella in the family Viscaceae. Species of Korthalsella are leafless, aerial hemiparasites, having terete or flattened internodes with minute, unisexual flowers borne on the tip of internodes in the axils of rudimentary leaves or on specialised inflorescence branches. Korthalsella has an unusual, scattered, and discontinuous distribution with high levels of species and sectional diversity in Malesia extending from Hawaii, the Marquesas and Henderson Island in the east, to Ethiopia and Madagascar in the west, and from Japan in the north, to Australia and New Zealand in the south (Barlow 1983, Molvray 1997, Burrows 1996). Barlow (1997) estimated that there may be as many as 25 species. Molvray (1997) reduced the number of species to eight; however, her classification is not generally accepted and was not adopted by Barlow (1997) or Wagner et al. (1999) in monographs of the floras of Malesia and Hawaii respectively.
New Zealand is home to three pygmy mistletoe species, Korthalsella salicornioides, K. clavata, and K. lindsayi, although there is debate about the taxonomic status of the latter two as separate species (see Danser 1940, Molvray 1997, Molvray et al. 1999). Korthalsella salicornioides mainly occurs on Leptospermum scoparium and Kunzea spp. (Myrtaceae) and has also been recorded on the introduced Erica lusitanica and E. vagans (Ericaceae, Bannister 1989). Korthalsella clavata is known on Aristotelia fruticosa (Eleocarpaceae), Coprosma propinqua, C. wallii (Rubiaceae), and Discaria toumatou (Rhamnaceae). While the main host for K. lindsayi is Melicope simplex, it also occurs on Coprosma spp., Lophomyrtus obcordata (Myrtaceae), and Myrsine divaricata (Myrsinaceae). Korthalsella salicornioides occurs throughout the North and South Islands and on Stewart Island. Korthalsella clavata and K. lindsayi occur throughout the South Island and the southern half of the North Island. Korthalsella salicornioides may have an even broader range. Barlow (1996) reports it from New Caledonia; Molvray (1997) includes K. madagascarica from Madagascar as a synonym of K. salicornioides. K. salicornioides is classified as “at risk-sparse” in the threatened and uncommon plants list for New Zealand (de Lange et al., 2004) and K. clavata is regarded as a regionally threatened plant in Wellington Conservancy (Anonymous 2001).
The only fungi reported previously from Korthalsella have been from Hawaii, specifically Cucurbitaria obducens (as Teichospora obducens), Echidnodes visci (Petrak 1953), Meliola visci (Stevens 1925), and Pleospora sp. (Kliejunas et al. 1979).
This paper describes two new stem parasites on Korthalsella spp. from New Zealand. The phylogenetic position of Rosenscheldiella korthalsellae sp. nov. and another New Zealand species, R. brachyglottidis, is determined on the basis of ITS and LSU sequences. The genus was placed in the Venturiaceae by Kirk et al. (2008), although Sivanesan & Shivas (2002) referred it to the Mycosphaerellaceae in a paper in which they described R. dysoxyli, a species with erumpent ascomata morphologically reminiscent of typical Mycosphaerella spp.
MATERIALS AND METHODS
Morphological studies
Specimens were examined from dried collections; asci and ascospores are described from squash mounts following rehydration in water or 3 % KOH. Ascomata and conidiomata were sectioned at a thickness of about 10 μm using a freezing microtome and sections were mounted in lactic acid for light microscopy. All collections have been deposited in the New Zealand Fungal and Plant Disease Herbarium (PDD).
Molecular analyses
Guignardia
DNA was extracted using REDExtract-N-Amp Plant PCR Kits (Sigma, USA) from small pieces of tissue taken from within three individual fruiting bodies from three different infected plants stored as dried herbarium specimens, following the removal of the upper surface of the fruiting body. The tissue was ground in extraction buffer with a plastic pestle in the Eppendorf tube, then DNA extraction and PCR were carried out following the manufacturer's instructions. ITS sequences were obtained separately from each extract following the methods of Johnston & Park (2005) using ITS1F and ITS4 amplification primers (White et al. 1990, Gardes & Bruns 1993). Using ClustalW (Larkin et al. 2007) our newly generated ITS sequences were aligned with sequences deposited in GenBank from taxa representing the genetic diversity of Phyllosticta as reported by Okane et al. (2003) and Rodrigues et al. (2004) (Table 1). Botryosphaeria dothidea was selected as the outgroup following Crous et al. (2006b) who showed Phyllosticta sensu stricto to be monophyletic and have a sister relationship with Botryosphaeria. Taxa in more distantly related clades of the Botryosphaeriaceae could not be reliably aligned. The 599-bp-long alignment has been deposited in TreeBase. A 70-bp segment near the start of the alignment could not be reliably aligned and was excluded from the analyses as was the 5.8S part of the alignment, because this was not available for all of the sequences deposited in GenBank, leaving 425 characters in the analyses. Phylogenetic analyses were performed using Bayesian maximum liklelihood in MrBayes 3.1.2 (Huelsenbeck & Ronquist 2001) and a heuristic maximum likelihood analysis in PAUP v. 4.01b (Swofford 2002) with the GTR+I+G model, selected using the AIC method in MrModelTest v. 2.3 (Posada & Crandall 1998, Posada & Buckley 2004). The Bayesian analysis was run with two chains for 10 M generations, trees sampled every 1 000 generations with a burn-in of 10 %. Bayesian posterior probabilities were obtained from 50 % majority rule consensus trees. The PAUP ML analysis used addition sequence random and TBR branch swapping with 100 replicates to avoid local optima. A bootstrap analysis used the ML tree as a starting tree; each of the 100 bootstrap samples run with a single replicate.
Table 1.
Isolates included in the phylogenetic analyses of Guignardia.
| Fungus1 | Isolate voucher number2 | GenBank accession number | Host and geographic origin of voucher |
|---|---|---|---|
| Guignardia aesculi | CBS 756.70 | AB095504 | Aesculus hippocastanum, Netherlands |
| Guignardia bidwellii | IFO 9466 | AB095509 | Parthenocissus tricuspidata, Japan |
| Guignardia citricarpa | IMI 304799 | AY042917 | Citrus aurantiacum, India |
| CBS 102374 | FJ824767 | Citrus aurantiacum, Brazil | |
| Guignardia gaultheriae | CBS 447.70 | AB095506 | Gaultheria humifusa, Netherlands |
| Guignardia korthalsellae | PDD 94884 | FJ655899* | Korthalsella lindsayi, New Zealand |
| Guignardia mangiferae | IFO 33119 | AB041233 | Rhododendron sp., Japan |
| ETH 02038 | AY277711 | Anarcardium giganteum, Brazil | |
| Guignardia philoprina | CBS 174.77 | AB095507 | Cryptomeria japonica, Netherlands |
| CBS 447.68 | AF312014 | Taxus baccata, USA | |
| Guignardia vaccinii | CBS 126.22 | AB095508 | Oxycoccus macrocarpos, Netherlands |
| Phyllosticta beaumarisii | CBS 535.87 | AY042927 | Muehlenbeckia adpressa, Australia |
| Phyllosticta eugeniae | CBS 445.82 | AY042925 | Eugenia aromatica, Indonesia |
| Phyllosticta hypoglossi | CBS 434.92 | AY042923 | Ruscus aculeatus, Italy |
| Phyllosticta owaniana | CBS 776.97 | AF312011 | Brabejum stellatifolium, South Africa |
| Phyllosticta podocarpi | CBS 111647 | AF312013 | Podocarpus lanceolata, South Africa |
| Phyllosticta pyrolae | – | AF312010 | Pyrola sp., USA |
| IFO 32652 | AB041242 | Erica carnea, Japan | |
| Phyllosticta spinarum | IMI 070028 | AY042907 and AY042908 | Japan |
| CBS 292.90 | AF312009 | Chamaecyparis pisifera, France | |
| Phyllosticta telopeae | DAR 60749 | AY042909 and AY042910 | Telopea speciosissima, Australia |
Sequences newly generated for this study.
Names used are those cited in GenBank.
CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; DAR, Plant Pathology Herbarium, Orange, Australia; IFO: Inst. for Fermentation Culture Collection, Osaka, Japan; IMI: International Mycological Institute, CABI, United Kingdom; PDD: The New Zealand Fungal Herbarium, Landcare Research, Auckland, New Zealand; ETH: ETH Culture Collection, Swiss Federal Institute of Technology, Zurich, Switzerland.
Rosenscheldiella
DNA was extracted from dried herbarium specimens using the same methods as for Guignardia. Tissue was extracted separately from seven individual pseudothecia taken from several different plants collected from two separate sites for Rosenscheldiella korthalsellae and from individual pseudothecia taken from three different leaves from a fresh collection of R. brachyglottidis (PDD 94939). ITS sequences were generated using the same primers as Guignardia and LSU using LROR and LR5 (Bunyard et al. 1994, Vilgalys & Hester 1990). Our newly generated ITS and LSU sequences were aligned with sequences deposited in GenBank from taxa representing the genetic diversity of Mycosphaerellaceae as reported in recent papers (e.g. Arzanlou et al. 2008, Crous et al. 2006a, 2007) as well as the specimens that formed the closest matches for R. brachyglottidis and R. korthalsellae in a GenBank BLAST search. In all cases the fungi included in the analysis had both ITS and LSU sequences available from the same voucher specimen, and the vouchers are available through public collections (Table 2). Davidiella tassiana was chosen as the outgroup following Crous et al. (2007) who showed the Davidiellaceae to be basal within the Capnodiales to Mycosphaerellaceae plus Teratosphaeriaceae. The alignment has been deposited in TreeBase. Several short segments within the ITS could not be reliably aligned and these were excluded from subsequent analyses, leaving 1384 characters. Phylogenetic analyses followed the procedure described for Guignardia using the GTR+I+G model as selected by MrModelTest.
Table 2.
Isolates included in the phylogenetic analyses of Rosenscheldiella.
| Fungus1 | Isolate voucher number2 | GenBank accession number (ITS, LSU) | Host and geographic origin of voucher |
|---|---|---|---|
| Cercospora apii | CBS 536.71 | AY752133, AY152629 | - |
| Cercospora beticola | CBS 116456 | AY840527, DQ678091 | Beta vulgaris, Italy |
| Davidiella tassiana | STE-U 5101 | AY251078, AY342092 | CCA-treated Douglas-fir pole, USA |
| Mycosphaerella aurantia | CBS 110500 | AY725531, DQ246256 | Eucalyptus globulus, Australia |
| Mycosphaerella buckinghamiae | CBS 112175 | EU707856, EU707856 | Buckinghamia sp., Australia |
| Mycosphaerella colombiensis | CMW 11255 | DQ239993, DQ204745 | Eucalyptus sp., Colombia |
| Mycosphaerella communis | CBS 110976 | AY725537, DQ246261 | Eucalyptus sp., South Africa |
| Mycosphaerella fori | CMW 9096 | DQ267581, DQ204749 | Eucalyptus grandis, South Africa |
| Mycosphaerella graminicola | CBS 100335 | EU019297, EU019297 | Triticum aestivum, The Netherlands |
| Mycosphaerella grandis | CMW 8554 | DQ267584, DQ246240 | Eucalyptus globulus, Chile |
| Mycosphaerella heimii | CPC 15429 | EU882122, EU882141 | Eucalyptus sp., Thailand |
| Mycosphaerella lateralis | CBS 111169 | AY725550, DQ246260 | Eucalyptus globulus, Zambia |
| Mycosphaerella pini | ATCC 28973 | EF114684, EF114697 | Pinus ponderosa, USA |
| Mycosphaerella punctata | CBS 113315 | EU167582, EU167582 | Syzygium cordatum, South Africa |
| Mycosphaerella walkeri | CMW 20333 | DQ267593, DQ267574 | Eucalyptus globulus, Chile |
| Phaeocryptopus gaeumannii | CBS 267.37 | EF114685, EF114698 | Pseudotsuga menziesii, Germany |
| Pseudocercospora natalensis | CBS 111069 | DQ303077, DQ267576 | Eucalyptus nitens, South Africa |
| Pseudocercospora paraguayensis | CBS 111286 | DQ267602, DQ204764 | Eucalyptus nitens, Brasil |
| Pseudocercospora vitis | CPC 11595 | DQ073923, DQ073923 | Vitis vinifera, South Africa |
| Readeriella novae-zelandiae | CBS 114357 | DQ267603, DQ246239 | Eucalyptus botryoides, New Zealand |
| Rosenscheldiella brachyglottidis | PDD 94939 | GQ355335*, GQ355334* | Brachyglottis repanda, New Zealand |
| Rosenscheldiella korthalsellae | PDD 94885 | GQ355332*, GQ355333* | Korthalsella lindsayi, New Zealand |
| Teratosphaeria mexicana | CBS 110502 | AY725558, DQ246237 | Eucalyptus globulus, Australia |
| Teratosphaeria nubilosa | CBS 116005 | AY725572, EU019304 | Eucalyptus globulus, Australia |
Sequences newly generated for this study.
Names used are those cited in GenBank.
ATCC: American Type Culture Collection, Virginia, USA; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; CMW: Culture collection of Mike Wingfield, housed at FABI, Pretoria, South Africa; PDD: The New Zealand Fungal Herbarium, Landcare Research, Auckland, New Zealand; STE-U: Culture collection of Stellenbosch University, South Africa.
RESULTS AND DISCUSSION
Phylogenetic analyses
Guignardia
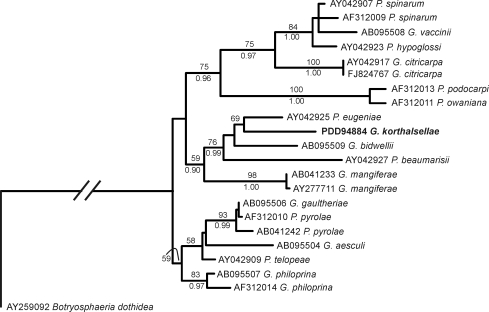
Identical sequences were obtained from all three fruiting bodies of Guignardia korthalsellae; one has been deposited as GenBank FJ655899. Phylogenetic analysis shows that G. korthalsellae groups most closely with the Vitaceae-associated G. bidwellii, the Eugenia-associated P. eugeniae, and the Muehlenbeckia-associated P. beaumarisii (Fig. 1).
Fig. 1.
Maximum likelihood tree from the PAUP analysis (Ln = –2273.33147) based on ITS sequences from Guignardia korthalsellae (PDD 94884, GenBank FJ655899) and GenBank data from other species of Guignardia and Phyllosticta, (Table 1) representing the genetic diversity accepted for these fungi by Okane et al. (2003) and Rodrigues et al. (2004). Bootstrap values shown above the branches where greater then 50 % and Bayesian posterior probabilities below the branches where 0.90 or above. Tree rooted with Botryosphaeria dothidea as outgroup.
Rosenscheldiella
For both of the species of Rosenscheldiella sequenced, all samples from each of the species had matching DNA sequences, making it unlikely that a contaminating fungus had been sequenced.
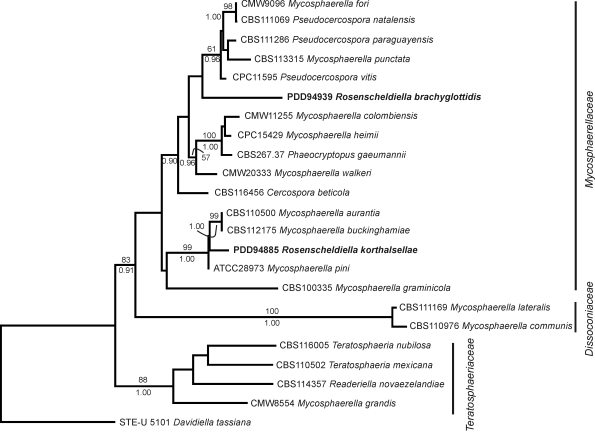
Based on these sequences R. brachyglottidis and R. korthalsellae are members of the Mycosphaerellaceae sensu Crous et al. (2007, 2009). Micromorphologically these two species are similar to Mycosphaerella, with fissitunicate, fasciculate asci, hamathecial elements lacking or poorly developed, and ascospores 1-septate, slightly constricted at septum, upper cell slightly wider than the lower. However, the anatomy of the ascomata and their relationship to the host tissue is unusual for the Mycosphaerellaceae. In both R. brachyglottidis and R. korthalsellae, as well as R. styracis, the type species of the genus, groups of pseudothecia develop externally to the host leaf or phylloclade on small pads of stromatic tissue that develop superficially from hyphae growing through the stomata. Within the leaf, the substomatal cavity is tightly packed with hyphae, but otherwise the hyphae are confined to leaf tissue in the immediate vicinity of the fruiting body and are always intercellular. Unusual for Mycosphaerellaceae, the host leaves show little or no symptoms beyond the presence of the fruiting bodies. This relationship to the host leaf with development of pseudothecia superficially on small pads of stromatic tissue growing from stomata is the same as has been described for Phaeocryptopus gaeumannii, a pathogen of Douglas fir (Stone et al. 2008). Like Rosenscheldiella, Phaeocryptopus is a member of the Mycosphaerellaceae (Winton et al. 2007).
Although Rosenscheldiella brachyglottidis, R. korthalsellae, and Phaeocryptopus gaeumannii share a similar morphology, they are phylogenetically distinct within the Mycosphaerellaceae. Of the taxa sampled in this study, R. korthalsellae forms a sister relationship with Mycosphaerella aurantii, M. buckinghamiae and M. pini, P. gaeumannii forms a sister relationship with Mycosphaerella heimii, and R. brachyglottidis forms no close relationship with other sampled species (Fig. 2). The distinctive biology and morphology shown by these three species has evolved several times within the Mycosphaerellaceae. These fungi are retained in Rosenscheldiella and Phaeocryptopus for the time being, awaiting resolution of generic relationships within the family.
Fig. 2.
Maximum likelihood tree from the PAUP analysis (Ln = –5475.66915) based on LSU and ITS sequences from Rosenscheldiella brachyglottidis and R. korthalsellae, together with GenBank data from related Mycosphaerella species (Table 2). Bootstrap values shown above the branches where greater than 50 % and Bayesian posterior probabilities below the branches where 0.90 or above. Tree rooted with Davidiella tassiana as outgroup.
TAXONOMY
Guignardia korthalsellae A. Sultan, P.R. Johnst., D.C. Park & A.W. Robertson, sp. nov. MycoBank MB514115. Figs 3, 4.
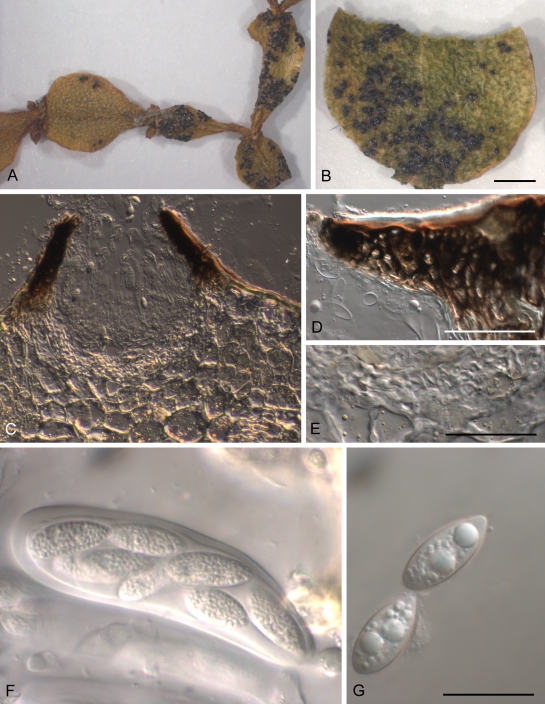
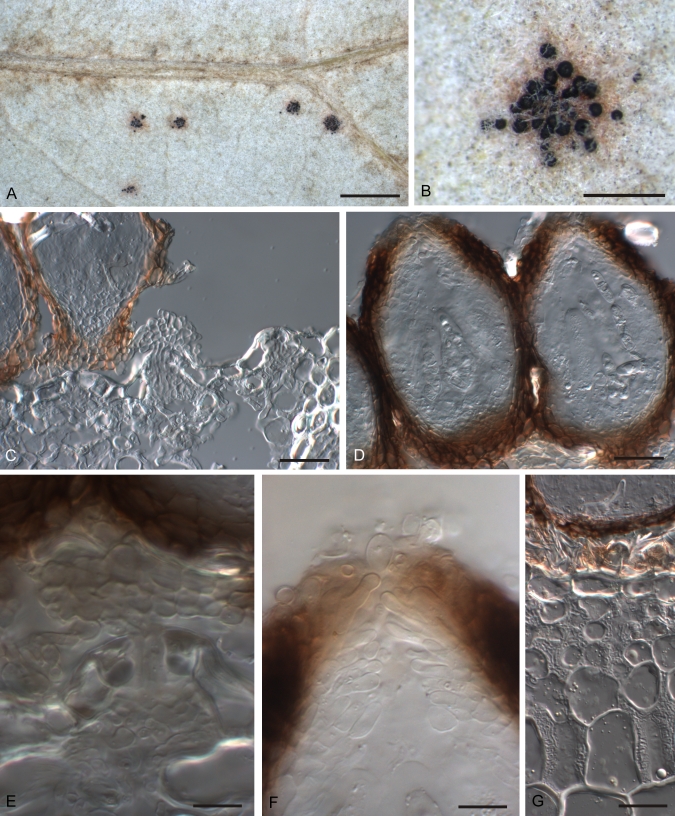
Fig. 3.
Guignardia korthalsellae A. Infected plant. B. Detail of infected internode showing gregarious fruiting bodies. C. Ascoma in vertical section. D. Detail of dark, upper part of wall of ascoma near ostiole. E. Detail of lower, pale part of wall of ascoma. F. Ascus and developing ascospores. G. Released ascospores. A, F = PDD 65953, B–E, G = PDD 94922. Scale bars: A–B = 1 mm, C = 50 μm, D–G = 20 μm.
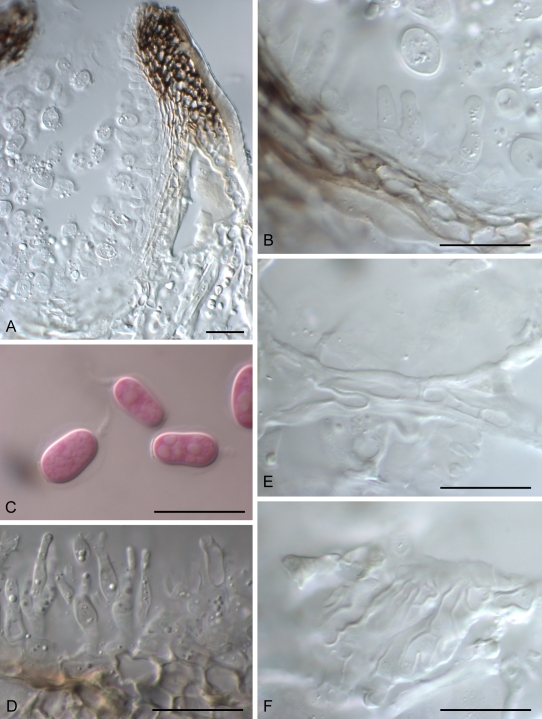
Fig. 4.
Guignardia korthalsellae A. Conidioma in vertical section. B. Wall of conidioma with conidiogenous cells. C. Conidia. D. Conidiogenous cells and conidia of spermatial Leptodothiorella state. E. Fungal hyphae within phylloclade between hypodermal cells. F. Fungal hyphae within phylloclade, showing broad, plate-like layer of hyphae between hypodermal cells. A, B, E, F = PDD 65953; C, D = PDD 94922. Scale bars: A = 50 μm, B–F = 20 μm.
Etymology: korthalsellae refers to the genus of the host plant. Ab G. bidwellii ascosporis 19–27.5 × 8–11 μm, habitanti Korthalsella lindsayi differens.
Holotypus: New Zealand, Wanganui, vic. Palmerston North, Coles Bush, living internodes of Korthalsella lindsayi, 22 Nov. 2008, A. Sultan, PDD 94922.
Ascomata and conidiomata 0.15–0.40 mm diam, black, globose, erumpent, with a single, round, apical, nonpapillate ostiole, solitary or often coalescing, gregarious, developing on flattened, leaf-like internodes of living plants, rarely associated with obvious chlorotic or necrotic symptoms, indistinct chlorotic halos sometimes seen on heavily infected leaves. Fruiting bodies develop within epidermal layer, breaking down 3–4 epidermal cells, with immediately adjacent hypodermal cells pushed aside. Otherwise, host tissue disrupted to a minimal extent, fungal hyphae within plant restricted to plate-like layers of hyaline, thin-walled hyphae developing between 2–3 layers of host hypodermal cells and between cuticle and epidermal cells adjacent to fruiting bodies. Ascomatal and conidiomatal wall 20–25 μm thick, comprising 4–5 layers of short-cylindric cells 3–5 μm diam, with cell walls slightly thickened, hyaline in lower part, darkened in upper part, innermost layers of cells narrower, thinner-walled; cells adjacent to ostiole angular to globose with walls thick and dark. Groups of fruiting bodies may be entirely ascomatal, entirely conidiomatal, or have a mixture of both forms. Spermatial conidiomata sometimes also present. Asci clavate, bitunicate, 65–100 × 18–20 μm, attenuated at base to a short stipe, 8-spored. Ascospores ellipsoid, 19–27.5 × 8–11 μm, hyaline, 0-septate, no gelatinous sheaths or caps observed. Pseudoparaphysoid-like elements broad-cylindric, thin-walled, absent in mature ascomata.
Conidiogenous cells lining entire inner layer of conidiomata, solitary, cylindrical to lageniform, 6–14 × 4.5–5 μm, wall not thickened at single, apical conidiogenous locus. Conidia 13–15 × 7–9 μm, ellipsoid to clavoid, apex broadly rounded, base truncate, 0-septate, hyaline, surrounded by a thin gelatinous sheath, with a gelatinous, tapering apical appendage (4–)6–9(–20) μm long.
Spermatial state Leptodothiorella with conidiogenous cells cylindric, 7.5–10 × 3–5 μm, wall thickened at single, apical conidiogenous locus, in groups of 2–3 on a single, short-cylindric basal cell. Microconidia 5.5–7 × 2 μm, straight, dumbbell-shaped, 0-septate, hyaline.
Habitat: On living internodes of Korthalsella lindsayi. Ascomata more common in summer, conidiomata in winter and spring. A macroscopically similar fungus was observed on K. clavata, but no collections were made and its identity was not confirmed.
Distribution: New Zealand.
Additional specimens examined. New Zealand, Wanganui, vic. Palmerston North, Coles Bush, living internodes of Korthalsella lindsayi, Oct. 2008, A. Sultan, PDD 94884; Wanganui, vic. Palmerston North, Coles Bush, living internodes of K. lindsayi, 23 Dec. 2008, A. Sultan, PDD 95152; Bay of Plenty, Paengaroa Scenic Reserve, living internodes of K. lindsayi, Oct./Nov. 2008, A. Sultan, PDD 94900; Mid Canterbury, Christchurch, Riccarton Bush, on living internodes of K. lindsayi, 11 Apr. 1996, R.C. Close, PDD 65953.
Notes: this fungus is probably common on Korthalsella lindsayi throughout its range. No Guignardia or Phyllosticta spp. have been previously reported from Korthalsella. Phyllosticta phoradendri reported on Phoradendron, another member of the Viscaceae from California (Bonar 1942), was not accepted in the genus by van der Aa & Vanev (2002); these authors considered it to be probably an Asteromella-like species. De Lange (1997) reported a Phyllosticta sp. on the loranthaceous mistletoe Ileostylus micranthus in New Zealand. We could find no literature report or voucher specimen to support this record and suspect it may have been a miscitation of the host of PDD 65953, K. lindsayi, the only mistletoe-associated specimen of Phyllosticta available in 1997.
Guignardia and its anamorph Phyllosticta is monophyletic within the Botryosphaeriaceae (Crous et al. 2006b). Within Guignardia, G. korthalsellae is genetically distinct from the geographically widespread, biologically unspecialised G. mangiferae and from all other Guignardia spp. represented in recent phylogenetic studies (Fig. 1). However, many species have no molecular data available, and acceptance of the species described here as new is based in part on its host preference. Although recent studies (e.g. Rodrigues et al. 2004) have shown that some Phyllosticta species isolated as symptomless endophytes may have a broad host range, the biological relationship between these fungi and the hosts from which they have been isolated is poorly understood. In a study based on herbarium specimens, van der Aa (1973) considered most Phyllosticta species to be host specialised pathogens. Guignardia korthalsellae develops within living host tissue but causes minimal damage. This apparently highly developed biological relationship supports the likelihood of host specialisation in this case.
Rosenscheldiella brachyglottidis G.F. Laundon & Sivan. in Laundon, New Zealand J. Bot. 9: 619. 1972 [1971]. Fig. 5.
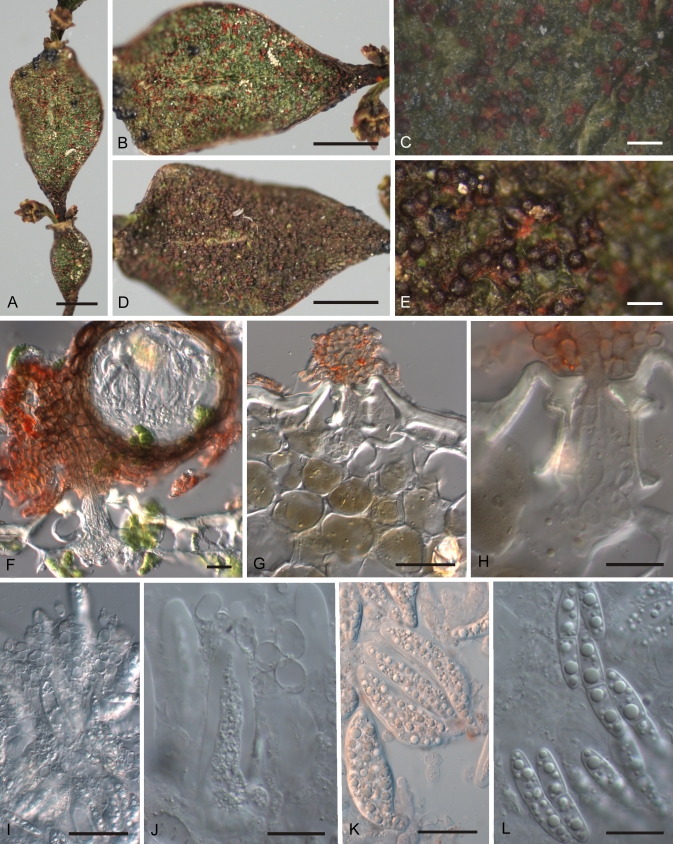
Fig. 5.
Rosenscheldiella brachyglottidis A. Infections on underside of leaf, several patches of gregarious pseudothecia. B. Single infection, pseudothecia associated with reddish patch amongst leaf hairs on underside of leaf. C. Pseudothecia in vertical section, fungal hyphae arising from several adjacent stomata, guard cells of stomata indicated by asterisks, and forming poorly differentiated stromatic base on which pseudothecia are held. D. Pseudothecia in vertical section. E. Detail of base of ascoma in vertical section, fungal hyphae emerging from substomatal cavity, guard cells of stoma indicated by asterisks, and forming stromatic pad on leaf surface. F. Detail of ostiole in vertical section. G. Extensive network of intercellular hyphae within host leaf; base of pseudothecia visible amongst leaf hairs above intact epidermal layer of leaf. A, B = PDD 94939; C–G = PDD 50727. Scale bars: A = 5 mm; B = 1 mm; C, D, G = 20 μm; E, F = 10 μm.
Ascomata develop within dense tomentum of hairs on underside of leaves, no visible symptoms on upper surface. Ascomata with one or a small number of black-walled pseudothecia, up to about 0.1 mm diam, held on a stroma-like structure comprising a small group of hyaline to pale brown, globose to angular cells that arise from hyphae growing through leaf stomata. Ascomata generally develop in gregarious groups of 10–20, forming patches up to about 5 mm diam. Hyaline, thin-walled fungal hyphae ramify amongst leaf tomentum adjacent to ascomata. Internally, host substomatal cavity packed with hyaline, thin-walled fungal hyphae, extensive plates of hyphae between host cells close to substomatal cavity. Hamathecium lacking. Asci fissitunicate, subsaccate with a small basal foot, 8-spored. Ascospores 16–22 × 5–6 μm, ellipsoid, 1-septate, slightly constricted at median septum, hyaline.
Specimens examined: New Zealand, Wellington, Levin, Waiopehu Reserve, on Brachyglottis repanda, 27 Dec. 1969, G.F. Laundon, PDD 50728, holotype; Wellington, Levin, Waiopehu Reserve, on B. repanda, 5 Apr. 1969, G.F. Laundon, PDD 50727; Auckland, Waitakere Ranges, roadside near Rose Hellaby House, on B. repanda, 8 Dec. 2008, P.R. Johnston & E.M. Gibellini, PDD 94939.
Rosenscheldiella korthalsellae A. Sultan, P.R. Johnst., D.C. Park & A.W. Robertson, sp. nov. MycoBank MB514116, Fig. 6.
Fig. 6.
Rosenscheldiella korthalsellae A. Infected internodes. B. Detail showing immature, reddish ascomata. C. Detail of B. D. Infected internode densely covered with mature, blackish ascomata. E. Detail of D. F. Ascoma in vertical section, pseudothecium on pad of stromatic tissue developing above stoma. G. Pad of stromatic tissue above stoma, fungal hyphae packing substomatal cavity but otherwise sparse within the internode. H. Detail of G. I. Hymenium, squash mount showing loose, more or less globose cells of hamathecial tissue. J. Detail of hamathecial cells. K. Asci. L. Ascospores. PDD 94885. Scale bars: A, B, D = 2 mm; C, E = 0.5 mm; F = 100 μm; G, I, K = 20 μm; H, J, L = 10 μm.
Etymology: korthalsellae refers to genus of the host plant.
Ab R. styracis ascosporis 21.5–27 × 4.5–6 μm, habitanti Korthalsella lindsayi differens.
Holotypus: New Zealand, Rangitikei, vic. Taihape, Paengaroa Scenic Reserve, on living internodes of Korthalsella lindsayi, 3 Nov. 2008, A. Sultan, PDD 94885.
Ascomata develop superficially, with one to several globose, dark-walled pseudothecia forming on small pads of reddish stromatic tissue above stomata in host phylloclade, stroma forming from hyphae that emerge through stomata. Infected areas of host with large numbers of stromatic pads and their associated pseudothecia. Basal stroma comprising more or less globose cells 5–7 μm diam, with walls thin, pale, encrusted with small, reddish crystals. Irregular, short strands of hyphae, 3–5 μm diam with reddish contents, radiating away from stromata across host surface. Stromata arise from hyphae that extend through phylloclade stomata from substomatal cavity packed with hyaline, thin-walled fungal cells. Hyphae within phylloclade otherwise sparse and intercellular, confined to immediate area around ascomata. Pseudothecia initially reddish, darker with age, 100–150 μm diam, in vertical section wall 15–20 μm thick, comprising 4–5 layers of short-cylindric cells, towards inside of wall cells narrower and hyaline, towards outside wider, walls encrusted with dark brown material. Ostiole apical, round, non-papillate, surrounded by a few short-cylindric, outwardly projecting cells. Hamathecium of short-cylindric to globose cells arranged amongst asci more or less in loose columns. Asci 55–70 × 12–14 μm, fissitunicate, cylindric to clavate with a short, foot-like base, 8-spored, overlapping 2–3 seriate. Ascospores 21.5–27 × 4.5–6 μm, cylindric, straight, hyaline, 1-septate, upper cell slightly wider than lower, slightly constricted at septum.
Habitat: On living internodes of all three Korthalsella spp. in New Zealand.
Distribution: New Zealand, probably common throughout the range of its host species.
Other specimens examined. New Zealand, Mackenzie, Lake Ohau, on Korthalsella clavata, Jan. 2009, A.W. Robertson, PDD 95153; Mid Canterbury, Banks Peninsula, Price's Valley, on K. lindsayi, 2 Sep. 1995, J.E. Braggins, PDD 65042; Mid Canterbury, Castle Hill, on K. clavata, 17 Jan. 2008, A. Sultan & A.W. Robertson, PDD 95150; South Canterbury, Peel Forest, on K. lindsayi, 22 June 1995, B.P.J. Molloy, PDD 35039; Taupo, vic. Motuoapa, on K. salicornioides, 3 Apr. 2008, A. Sultan, PDD 95151; Wanganui, vic. Palmerston North, Coles Bush, living internodes of K. lindsayi, 22 Nov. 2008, S. Amir, PDD 94923.
Notes: The only species of Rosenscheldiella previously reported from a viscaceous mistletoe is R. phoradendri known from El Salvador on Phoradendron robustissimum. Based on the published description (Jenkins & Limber 1952), R. phoradendri has larger ascospores, 35–48 × 13–16 μm, than our new species.
Rosenscheldiella styracis (Henn.) Theiss. & Syd., Ann. Mycol. 13: 645. 1915. Fig. 7.
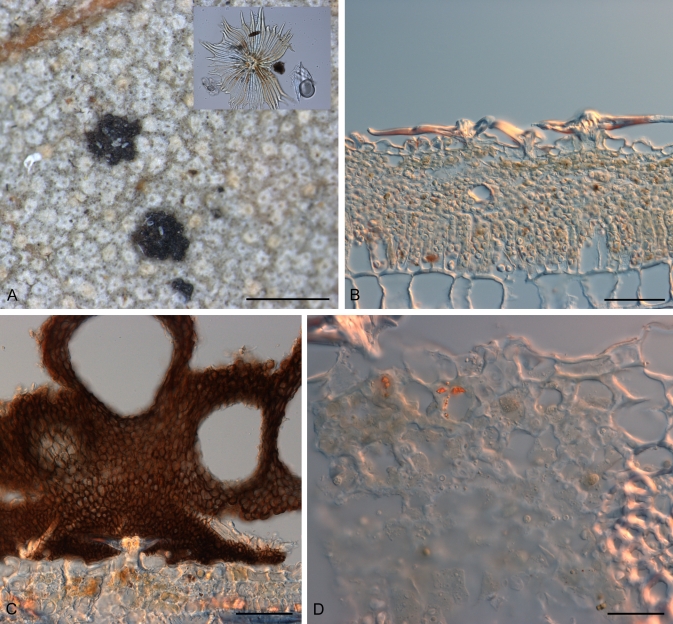
Fig. 7.
Rosenscheldiella styracis A. Ascomata, comprising groups of superficial pseudothecia amongst leaf hairs on underside of host leaf, detail of leaf hair inset. B. Uninfected host leaf in vertical section showing leaf hairs. C. Ascoma in vertical section, several pseudothecia held on extensive basal stroma, superficial amongst leaf hairs. D. Intercellular fungal hyphae within leaf immediately below ascoma. PDD 38182. Scale bars: A = 1 mm; B, C = 50 μm; D = 10 μm.
Ascomata develop on lower surface of leaf, comprising 10–30 globose, dark-walled pseudothecia in confluent groups up to 0.7 mm across. Pseudothecia develop on extensive stromatic pads of globose cells with thick, dark walls that form amongst thick-walled, multi-lobed hairs on lower surface of leaves. Stromatic pads appear to arise from hyphae growing through stomata, but this not clearly seen. Within leaf, fungal hyphae confined to area immediately adjacent to ascomata. Hamathecium lacking. Asci fissitunicate, cylindric, about 110 × 20 μm, 8-spored. Ascospores 32.5–36 × 7–7.5 μm, cylindric, tapering slightly to rounded ends, 1 median septum, slightly constricted at septum, hyaline.
Specimen examined: Uruguay, Dept. Treinta y Tres, Tacuari, on Styrax leprosus, Nov. 1933, W.G. Herter, Reliquiae Petrakianae 105, PDD 38182. Although not the type, this specimen is considered authentic.
Acknowledgments
Acknowledgements are extended to Bevan Weir (Landcare Research) for advice on the phylogenetic analyses, Don Reynolds (Curator of Fungi at University of California Herbarium) for advice on the generic placement of Rosenscheldiella korthalsellae, and the Department of Conservation (Wanganui Conservancy, Palmerston North office, and Tongariro-Taupo Conservancy, Turangi office) for collecting permits at the Paengaroa and Motuoapa sites. Sultan and Robertson were supported financially by Massey University and the Higher Education Commission, Government of Pakistan, and Johnston and Park by the New Zealand Foundation for Research Science and Technology through the Defining New Zealand's Land Biota OBI.
Taxonomic novelties: Guignardia korthalsellae A. Sultan, P.R. Johnst., D.C. Park & A.W. Robertson, sp. nov.; Rosenscheldiella korthalsellae A. Sultan, P.R. Johnst., D.C. Park & A.W. Robertson, sp. nov.
References
- Aa HA van der (1973). Studies in Phyllosticta I. Studies in Mycology 5: 1–110. [Google Scholar]
- Aa HA van der, Vanev S (2002). A revision of the species described in Phyllosticta. CBS, Utrecht.
- Anonymous (2001). Mistletoes in Wellington Conservancy: current status and management requirements. Department of Conservation, Wellington Conservancy.
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, et al. (2008). Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister P (1989). Nitrogen concentration and mimicry in some New Zealand mistletoes. Oecologia 79: 128–132. [DOI] [PubMed] [Google Scholar]
- Barlow BA (1983). A revision of the Viscaceae of Australia. Brunonia 6: 25–57. [Google Scholar]
- Barlow BA (1996). Viscaceae. Flore de la Nouvelle-Calédonie et Dépendances. Muséum National d'Histoire Naturelle, Paris 20: 92–99. [Google Scholar]
- Barlow BA (1997). Viscaceae. Flora Malesiana 13: 403–442. [Google Scholar]
- Bonar L (1942). Studies of some Californian fungi II. Mycologia 34: 180–192. [Google Scholar]
- Bunyard BA, Nicholson MS, Royse DJ (1994). A systematic assessment of Morchella using RFLP analysis of the 28S ribosomal RNA gene. Mycologia 86: 762–772. [Google Scholar]
- Burrows C (1996). Dispersal of Korthalsella seeds. Canterbury Botanical Society Journal 31: 69–70. [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenwald JZ (2006a). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs on Eucalyptus II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. (2006b). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Groenwald JZ (2007). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. (2009). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danser BH (1940). A supplement to the revision of the genus Korthalsella. Bulletin du Jardin Botanique Buitenzorg 16: 329–342. [Google Scholar]
- Gardes M, Bruns TD (1993). ITS primers with enhanced specificity for basidiomycetes — application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jenkins AE, Limber DP (1952). Two new ascomycetes on Phoradendron. Mycologia 44: 557–560. [Google Scholar]
- Johnston PR, Park D (2005). Chlorociboria (Fungi, Helotiales) in New Zealand. New Zealand Journal of Botany 43: 679–719. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Ainsworth and Bisby's Dictionary of the Fungi. 10th Ed. CAB International: Wallingford, UK.
- Kliejunas JT, Scharpf RF, Smith RS (1979). Teichospora obducens and a Pleospora species on Korthalsella in Hawaii. Plant Disease Reporter 63: 1060–1062. [Google Scholar]
- Lange PJ de (1997). Decline of New Zealand loranthaceous mistletoes –a review of non-possum (Trichosurus vulpecula) threats. In: New Zealand's Loranthaceous mistletoes (de Lange PJ, Norton DA, eds). Proceedings of a workshop hosted by Threatened Species Unit, Cass, 17–20 July 1995. Department of Conservation, Wellington, New Zealand: 155–163.
- Lange PJ de, Norton DA, Heenan PB, Courtney SP, Molloy BPJ, et al. (2004). Threatened and uncommon plants of New Zealand. New Zealand Journal of Botany 42: 45–76. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Molvray M (1997). A synopsis of Korthalsella (Viscaceae). Novon 7: 268–273. [Google Scholar]
- Molvray M, Kores PJ, Chase MW (1999). Phylogenetic relationships within Korthalsella (Viscaceae) based on nuclear ITS and plastid trnL-F sequence data. American Journal of Botany 86: 249–260. [PubMed] [Google Scholar]
- Okane I, Lumyong S, Nakagiri A, Ito T (2003). Extensive host range of an endophytic fungus, Guignardia endophyllicola (anamorph: Phyllosticta capitalensis). Mycoscience 44: 353–363. [Google Scholar]
- Petrak F (1953). Beitrage zur pilzflora von Hawaii. Sydowia 7: 381–409. [Google Scholar]
- Posada D, Buckley TR (2004). Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Rodrigues KF, Sieber TN, Grünig CR, Holdenrieder O (2004). Characterization of Guignardia mangiferae isolated from tropical plants based on morphology, ISSR-PCR amplifications and ITS1-5.8S-ITS2 sequences. Mycological Research 108: 45–52. [DOI] [PubMed] [Google Scholar]
- Sivanesan A, Shivas RG (2002). New species of foliicolous Loculaoascomycetes on Dysoxylum, Melaleuca and Syzygium from Queensland, Australia. Fungal Diversity 11: 151–158. [Google Scholar]
- Stevens FL (1925). Hawaiian Fungi. Bishop Museum Bulletin 19: 1–189. [Google Scholar]
- Stone JK, Capitano BR, Kerrigan JL (2008). The histopathology of Phaeocryptopus gaeumannii on douglas-fir needles. Mycologia 100: 431–444. [DOI] [PubMed] [Google Scholar]
- Swofford DL (2002). PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sun derland, Massachusetts, Sinauer Associates.
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner WL, Herbst DR, Sohmer SH (1999). Manual of the flowering plants of Hawai'i. Revised edition. Bishop Museum Special Publication 97. University of Hawaii Press, Honolulu.
- White TJ, Bruns T, Lee S, Taylor JW (1990). Amplification of direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: A guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ eds). Academic Press, San Diego: 315–322.
- Winton LM, Stone JK, Hansen EM, Shoemaker RA (2007). The systematic position of Phaeocryptopus gaeumannii. Mycologia 99: 240–252. [DOI] [PubMed] [Google Scholar]