Abstract
Pharmaceutical patient assistance programs (PAPs) have the potential to improve prescription drug accessibility for eligible patients, but currently there is limited information regarding their effectiveness. In an attempt to provide a systematic description of primary studies on PAPs, we reviewed 33 unique studies from commercial and grey literature (e.g., government publications, conference abstracts) sources: 15 health care outcome evaluations, seven economic evaluations, seven surveys and four miscellaneous studies. Enrollment assistance for PAPs with additional medication services (e.g., counseling) was significantly associated with improved glycemic (standardized mean difference = −0.40, 95% CI = −0.59,−0.20; k=3 one-group, pre-post-test; 1 comparison-group) and lipid (standardized mean difference = −0.52, 95% CI = 0.78,−0.27; k=3 one-group, pre-post-test; 1 comparison group) control. Inadequately designed economic evaluations suggest free PAP medications offset health care institutions’ costs for uncompensated medications and enrollment assistance programs. More rigorous research is needed to establish the clinical and cost-effectiveness of PAPs from a patient and health care institution perspective.
Keywords: Health insurance, prescription drugs, pharmaceutical services, medical assistance
Patient assistance programs (PAPs), sponsored by pharmaceutical companies, provide certain prescription drugs at low or no cost to patients who lack prescription drug coverage. There are differing views about the value of PAPs. Supporters feel that PAPs are an important resource to eligible patients,1,2 and believe that increased government regulation of these programs would waste time and money3 and might even discourage companies from continuing these programs.4 Others are concerned that PAPs may deflect attention from exploring more comprehensive policy solutions,5 and some question their reach and benefits.6 Some even question whether reliance on PAPs could reinforce existing disparate outcomes for those without prescription drug coverage.7
No formal entity is responsible for tracking utilization of PAPs or evaluating their effectiveness, nor are there readily available public data on the use of PAPs. Individual studies of PAPs have reported cost savings to health care institutions,8–10 programmatic outputs (e.g., number patients enrolled, number of drugs provided),11 and the fact that the PAP application process can be complex and burdensome for both patients and providers.6,12 Despite the landmark Patient Protection and Affordable Health care Act of 2010, millions of U.S. adults will remain uninsured and unable to afford their needed medications. Thus, it is timely to examine the impact that PAPs have on improving access to medications and ultimately patient health outcomes from a clinical, economic and humanistic perspective.13 An initial task must be to assess the current state of knowledge and research on these programs. The purpose of this review is to provide a systematic description of primary studies of PAPs in the commercially published and grey literature.⋆ We investigated, specifically:
What scientific questions have been asked about PAPs, and with what study designs and quality of reporting?
What types of PAPs have been studied (e.g., individual, institutional), in what settings, and with what populations?
What dependent variables have been investigated and with what result?
What are the professional affiliations of the authors and disciplines of the publications where these studies are found, and are these characteristics related to the types of study questions, study designs, and outcomes?
What are the funding sources for these studies, and are funding sources related to the types of study questions, study designs, dependent variables, and study outcomes?
Methods
Inclusion and exclusion criteria
To be included in this review, reports of primary studies had to be: 1) in English; 2) published or reported 1980–June 2009; 3) original research; 4) conducted in the U.S.; and 5) focused on pharmaceutical company-sponsored PAPs used to provide prescription drugs to patients. Studies that focused exclusively on state-based pharmaceutical or prescription assistance programs (SPAPs) (e.g., AIDS Drug Assistance Programs (ADAPs)) were excluded, because the programmatic structure and funding of these programs differ from the individual- and institutional-targeted PAPs of interest in this study. We also excluded studies that did not conduct any type of scientific investigation of PAPs (as confirmed by explicitly stated research questions, objectives, hypotheses or aims, or explicit method of quantitative or qualitative analysis), but merely provided a description of the development or implementation of the use of PAPs within a given institution. Publications were limited to 1980 and after because at that time, the pharmaceutical industry began facing new market challenges in tandem with protests from patient activists about high drug pricing, particularly for life-saving therapies.14 Pharmaceutical companies may have responded to public criticisms by promoting PAPs as a way for patients to avoid high costs while accessing needed therapies. We only included studies conducted in the U.S. because of the differences in health care policies and systems in other countries.
Search strategy
We conducted a systematic search of reports from commercially published and grey literature sources. “Grey literature” refers to “information produced on all levels of government, academics, business and industry in electronic and print formats not controlled by commercial publishing, i.e., where publishing is not the primary activity of the producing body.”15[p.1] Including grey literature increased the likelihood of capturing unpublished reports, conference abstracts, and policy documents from non-academic sources, such as government, non-profit organizations, and the pharmaceutical industry.
For each literature source, search strategies were developed and pilot-tested, using known articles, with the assistance of a health sciences librarian experienced in designing and documenting searches for systematic reviews. For commercially published sources, we began by searching Ovid Medline (1950–2009) using a combination of Medical Subject Headings (e.g., “Medically uninsured,” OR “Medical indigency,” OR “Uncom-pensated care,” AND “Drug Industry,” OR “Pharmaceutical Preparations”) and keyword search terms (e.g., “subsidized prescription,” “patient assistance” OR “pharmaceutical assistance” OR “drug assistance”). We then modified that search using relevant terms for Medline (in-process and non-indexed), International Pharmaceutical Abstracts (IPA, which includes both commercially published and grey literature sources), and Digital Dissertation Abstracts. Scopus and Web of Science were used to search the bibliographies of selected studies for additional relevant cited and citing articles.
For the grey literature, we searched relevant Web resources and databases recommended by the Duke University Medical Center Library:16 the New York Academy of Medicine’s Grey Literature Report, Computer Retrieval of Information on Scientific Projects (CRISP) (replaced by Research Portfolio Online Reporting Tools as of September 2009), OIAster, Health Services Research Projects, Health Services and Sciences Research Resources, National Library of Medicine Gateway, and, in FirstSearch, Papers First, Proceedings, and U.S. Government Publications. We searched using various combinations of general key words (e.g., “patient assistance” OR “pharmaceutical assistance” OR “drug assistance” OR “medication assistance” OR “prescription assistance”) for reports, abstracts, or articles on PAPs.
Study screening
Citations from the searches were imported into and managed with RefWorks (2008 for Windows). Two reviewers independently screened titles and abstracts of retrieved citations for eligibility. If a title-abstract was excluded by both reviewers, it was noted with the first reason and removed from further review. For title-abstracts that either reviewer classified as meeting the inclusion criteria or as having insufficient information provided in the abstract to determine eligibility, the full text was retrieved. For conference abstracts, primary authors were contacted for clarification about their study methods or for additional data to assess the study’s eligibility. Reviewers independently read the full text or evaluated information provided by primary authors. A third reviewer was consulted to resolve disagreements.
Coding of included studies
Eligible studies were coded independently by two reviewers for data relevant to our research questions and for the quality of reporting. Studies were coded for data in three categories: general study characteristics, program delivery, and academic/professional affiliations. General study characteristics addressed Study Questions 1–3, and consisted of standard data about setting, study design, methods, dependent variables, patient characteristics, main results, and limitations. Program delivery also addressed Study Questions 1–3, and consisted of data about how PAPs were used, the type of personnel who assisted patients with PAP enrollment, what types of prescription drugs were procured through PAPs, and any services provided to patients in addition to PAP enrollment (e.g., appointment reminders, medication therapy management). Academic/professional affiliations addressed Study Questions 4–5, and consisted of data about professional affiliations of the author(s) indicated in the publication, study sponsor, and subject focus for the journal or other vehicle where the study was published.
Evaluation of study reporting and study quality
We used the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline to assess the completeness of reporting of surveys and quasi-experimental studies. STROBE is a 22-item checklist (18 core items and four design-specific items) that presents a general set of reporting standards for observational research, developed because articles often fail to present clearly the details needed to fully assess the strengths and weaknesses of the research.17 Two reviewers independently applied all STROBE items to the included, full-text observational and quasi-experimental studies, and one item (specific to abstracts) to the conference abstracts. We used the STROBE checklist and its companion document17 to assess how well the included studies reported the recommended details of the study. As no current rating system exists for the STROBE, we created qualitative categories based on the extent to which the recommendations were followed: sufficient (at least the majority of the recommended information is reported in the item), moderate (some), or poor/insufficient (little to none). We then discussed each article and reached consensus as to the number of items that met each of the categories.
We used the Drummond Checklist to assess the quality of reporting and validity of results from economic evaluations. The checklist consists of 10 broad questions, each with its own set of sub-questions, to provide a framework for critically assessing the key elements of a well-executed economic study,18 with possible responses of Yes, Cannot tell, and No. One author and a reviewer with doctoral training in health policy sciences and economics independently assessed all included studies that conducted an economic evaluation. The two then reached consensus on the score.
Results
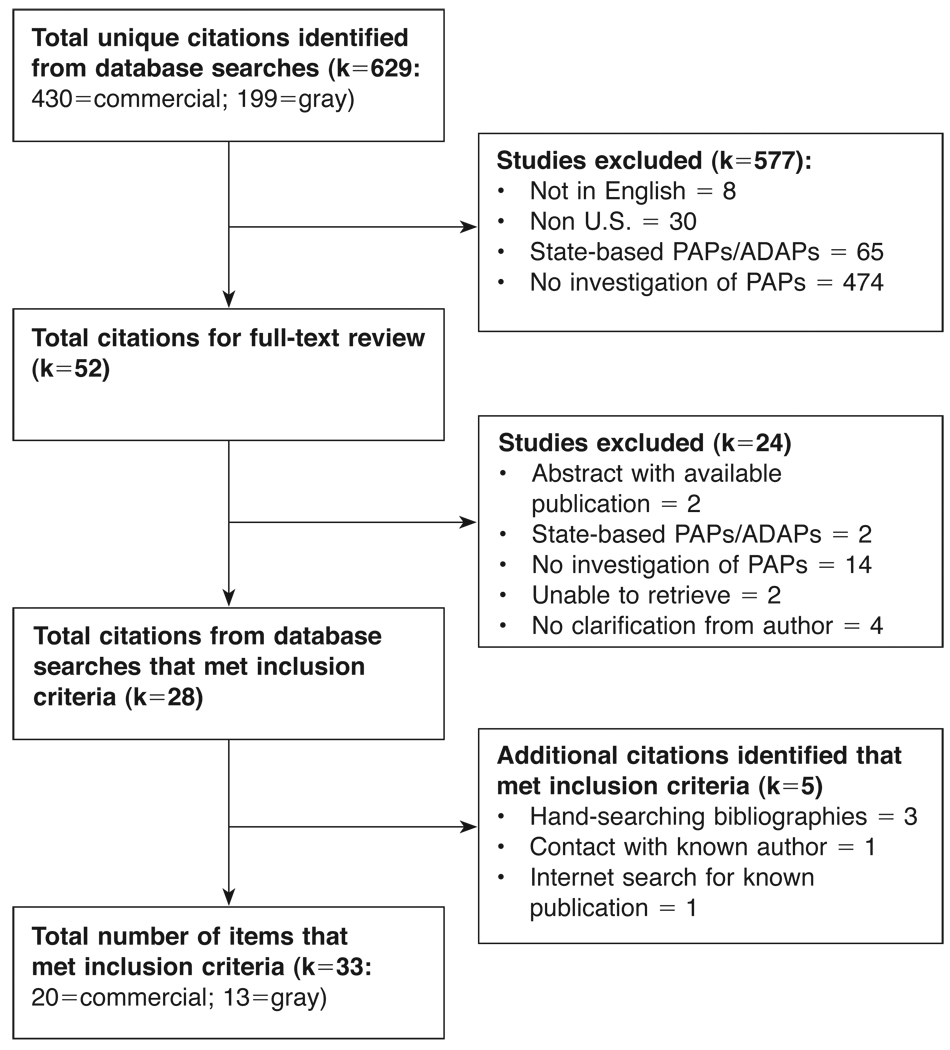
A total of 33 studies met the inclusion criteria for our review (Figure 1). Authors of the included studies investigated scientific questions about: 1) the effect of PAP use on patient health care outcomes (k=15, Table 1); 2) the costs of implementing an institutional program to assist patients with PAP enrollment (k=7, Table 2); 3) the use and perceptions of PAPs (k=7, Table 3); or 4) other study aims (Table 4). Within each of these four types of studies, we compared and contrasted their study designs, settings, populations, dependent variables (when applicable), study results and level of quality. Across studies, we summarized patterns between the types of PAP studies and author and publication affiliations, and study funding sources.
Figure 1.
Flow-chart of search results and study eligibility.
Table 1.
Health outcomes of patient assistance programs (PAP): included studies’ characteristics and outcomes (K=15)
| PUBLICATION 1st Author (Year) Au: Author affiliation(s) Pub: Publication discipline $: Funding source |
DESIGN Setting, location n=number of subjects (follow-up): sample description STROBE score = # sufficient items/total |
PAP + ADDITIONAL SERVICESa Enrollment personnel PAP medications + Additional services |
OUTCOMES Reported |
ESTIMATED EFFECT SIZEb Clinical indicators (95% CI) |
|---|---|---|---|---|
| Commercially published articles (k=10) | ||||
| Single group, pre-post design with clinical indicators data (k=6) | ||||
| Chisholm (2007)19 Au: Univ: pharmacy, medicine Pub: Pharmacy $: Private trust |
Renal transplant clinic, Georgia n = 36 renal transplant pts (12 mos): m age = 52.8 ± 13.4yr, 39%, 5 0%Cauc, 44% Af Am STROBE score = 19/22 |
Clinical pharmacist Meds: diabetes, dyslipidemia, hypertension, immunosuppressants + medication therapy mgt |
Clinical indicators: Glucose, lipids, BP, other # meds QOL: SF-12v2 |
HbA1c: −0.91 (−1.882, 0.069) LDL: −2.24 (−3.078, −0.411) SBP: −0.81 (−1.299, −0.321) |
| Horswell (2008)20 Au: Univ: pharmacy, medicine, public health Pub: Health policy/services $: Federal gov’t |
Medical center pharmacy, Louisiana n=289 type II diabetes pts (varied): m age = 52 yrs, 71%, 80% Af Am STROBE score = 20/22 |
Pharmacist Meds: diabetes |
Clinical indicators: Glucose |
HbA1c: −0.20 (−0.328, −0.072)c |
| Patel (2006)21 Au: Univ pharmacy, hospital Pub: Pharmacy $: not reported |
Outpatient hospital pharmacy, Connecticut n = 50 diabetes pts (6 mos): m age = 58.9 ± 8.0yrs, 48% , 52% Hisp, 20% Black STROBE score = 18/22 |
Pharmacy research fellow Meds: diabetes, hyperlipidemia + appt reminder |
Clinical indicators: Glucose, lipids |
HbAlc: −0.38 (−0.701, −0.060) LDL: −0.23 (−0.643,0.184) |
| Sauvegeot (2008)22 Au: Univ pharmacy Pub: Pharmacy $: not reported |
Non-profit pharmacy, Virginia n = 84 diabetes, dyslipidemia or hypertension pts (unclear): m age = 72.7 ± 10.6yrs 73.8% STROBE score = 17/22 |
Patient advocate Meds: diabetes, hyperlipidemia, hypertension |
Clinical indicators: Glucose, lipids, BP |
HbA1c: −0.35 (−1.031, 0.327) LDL: −0.38 (−0.896, 0.131) SBP: −0.12 (−0.492, 0.251) |
| Schoen (2001)23 Au: Univ: pharmacy, medicine Pub: Pharmacy $: not reported |
Inner-city medical center, Illinois n = 163 heart disease pts (6, 12, 18, 24 mos)d: m age = 61 ± 12.3yrs*, 46%, 59% Afr Am, 24% Hisp STROBE score = 18/22 |
Pharmacist Meds: cardiovascular, other + public aid or insurance enrollment, medication samples, counseling |
Clinical indicators: lipids, BP, other Adherence: physician and/or self-report Healthcare use: hospitalization rate Financial: avg price |
LDL: −0.47 (−0.713, −0.222) SBP: 0.00 (−0.186, 0.186) |
| Strum (2005)24 Au: Univ: pharmacy, medicine Pub: Pharmacy $: not reported |
Internal medicine clinic, Arkansas n = 52 diabetes pts (12 mos): m age = 59, 73%, 50% Afr Am, 48% White STROBE score = 18/22 |
Pharmacist, case coordination staff Meds: ACE inhibitors, antilipidemics, antihyper- glycemics, antihypertensives + 340B pricing program |
Clinical indicators: Glucose, lipids, BP, other # meds, # pts taking each med Vaccine use: flu |
HbA1c: −0.40 (−0.715, −0.085) LDL: −0.48 (−0.795, −0.157) SBP: −0.004 (−.0.309, 0.301) |
| Two group comparison design with clinical indicators (k=2) | ||||
| Marrs (2008)25 Au: Univ pharmacy Pub: Pharmacy $: not reported |
Outpatient pharmacy, Colorado n=200 indigent vs 40 insured dyslipidemia pts (12 mos): m age = 63 ± 11 yrs vs 56 ± 9 yrs** 64% vs 60%** STROBE score = 18/22 |
Pharmaceutical manufacturer assistance program specialist Meds: statins + indigent care program (Fed, State-funded) |
Clinical indicators: lipids Adherence: refill records # pts using a statin |
LDL: −0.27 (−0.74, 0.20)e |
| Trompeter (2009)26 Au: Univ: pharmacy, medicine Pub: Pharmacy $: not reported |
Private family practice, Virginia n=250 insured vs 208 PAP pts w/ diabetes, hypertension or dyslipidemia (12 mos): m age = 53.61 ± 11.0 vs 67.3 ± 10.4***, 41.2% vs 71.2%*** STROBE score = 18/22 |
Clinical pharmacist Meds: diabetes, dyslipidemia, hypertension + counseling, disease info, medication reminders |
Clinical indicators: Glucose, lipids, BP # meds, # pts taking each med |
HbA1c: −0.70 (−1.04, −0.350) LDL: −0.49 (−0.720, −0.250) SBP: 0.32 (0.120, 0.520) |
| Two group comparison design without clinical indicators (k=2) | ||||
| Paris (1999)27 Au: Univ pharmacy, healthcare organization Pub: Medicine $: not reported |
Outpatient clinic, Oklahoma n = 50 liver (current study) vs 100 heart transplant pts (previous study): m age = 49 vs 53 yrs, 45% vs 10% ** STROBE score = 13/22 |
Drug replacement specialist Meds: immuno-suppressants, others + Medicaid enrollment |
Adherence: pharmacy refills confirm medical records and self-report |
– |
| Spiker (2005)28 Au: Univ pharmacy, pharma-ceutical company Pub: Pharmacy $: not reported |
Phone survey, Ohio n = systematic selection of 104 enrollees (9 mos): m age = 70.6 ± 12.4 yr, 78% STROBE score = 17/22 |
Pharmacist Meds: not reported + county medication program enrollment, provider referrals |
Adherence: self-report Healthcare use: self- report # of unscheduled visits Other: Med safety |
– |
| Grey literature (k=5) | ||||
| Single group, pre-post design with insufficient clinical indicator data (k=3) | ||||
| Divine (2001)29 Au: Univ pharmacy Pub: Pharmacy conf abstract $: not reported |
Ambulatory care clinic, Kentucky n = 120 diabetes & hypertension pts (≥4 mos) STROBE score = 1/1 |
Pharmacist Meds: not reported + counseling, disease mgt, alt medication recommendations, discount/sample medications |
Clinical indicators: Glucose, BP |
• Decrease in HbA1c • Decrease in SBP |
| King (2000)30 Au: Univ medicine Pub: Medical conf abstract $: not reported |
Medical center hypertension clinic, Mississippi n = 38 pts w/ metabolic syndrome (6 mos) STROBE score = 1/1 |
not reported | Clinical indicators: BP | • Decrease in SBP |
| Prutting (2003)31 Au: Univ: pharmacy, medicine; hospital Pub: Pharmacy conf abstract $: not reported |
Adult primary care center, South Carolina n = 87 diabetes, hyperlipidemia, or hypertension pts (6 mos) STROBE score = 1/1 |
Pharmacy technician Meds: Diabetes, hyperlipidemia, hypertension |
Clinical indicators: Glucose, lipids, BP Adherence: refill records Healthcare use: # of visits Financial: costs saved |
• Decrease in HbAlc • Increase in LDL • Decrease in SPB |
| Single group, pre-post design without clinical indicators (k=2) | ||||
| Kiser (2001)32 Au: Hospital Pub: Pharmacy conf abstract $: not reported |
Hospital, North Carolina n, sample = not reported STROBE score = 1/1 |
not reported | Healthcare use: # of hospital re-admissions Financial: monetary loss |
– |
| Schoen (1997)33 Au: Univ: not reported Pub: Medical conf abstract $: not reported |
Academic health center, not reported n = 110 indigent pts w/ cardiovascular disease (≤6 mos): m age = 60.2 ± 13.3 yrs, 45% STROBE score = 1/1 |
Personnel: not reported Meds: Chronic diseases + public aid or insurance enrollment, medication samples |
Adherence: not defined Financial: cost savings |
– |
p<.05
p<.01
p<.001
Describes the institutional program that assists with PAPs and its components. Services include those in addition to PAP enrollment.
Standardized measure < 0 = improvement in clinical indicator following PAP enrollment (single group) or greater improvement for PAP versus comparison group (two groups)
Effect size calculated on reported single group, pre/post mean and p-value; standard deviation was not reported.
Study used a prospective cohort design with 4 follow-up time points
Effect size calculated based on reported number and percent of patients in each group achieving a goal of a 30% reduction in LDL level
ACE inhibitor = Angiotensin-Converting Enzyme inhibitors, BP = Blood Pressure, Conf = conference, Gov’t = government, HbA1c = Glycosylated hemoglobin, LDL = Low-Density Lipoprotein, Mos = months, Med(s) = medication(s), SBP = Systolic Blood Pressure, Pts = patients, QOL = Quality Of Life, Univ = University or College affiliation, Yr = years, CI = Confidence Interval
Table 2.
Economic evaluations of patient assistance programs (PAP): included studies’ characteristics and quality (K=7)
| PUBLICATION 1st Author (Year) Au: Author affiliation(s) Pub: Publication discipline $: Funding source |
DESIGN Setting, location Design, perspective, time period n=number of subjects Sample description Drummond score = # yes items/total |
PAP + ADDITIONAL SERVICESa Enrollment Personnel PAP medications + Additional services |
OUTCOMES Reported |
MAIN FINDINGS Reported by author |
|---|---|---|---|---|
| Commercially published articles (k=5) | ||||
| Chisholm (2000)34 Au: Univ: pharmacy, medicine Pub: Surgery $: Private Trust, Professional org, Pharmaceutical co. |
Renal transplant clinic, Georgia Cost-benefit analysis, institutional, 12 mos n = 61 renal transplant pts 50% Medicare* Drummond score = 4/10 |
Clinical pharmacist Meds: immuno-suppressants + state assistance |
Benefit: (ACQ of PAP meds with/without Medicare reimburse- ment) — (program costs) Sensitivity analysis: not specified |
|
| Clay et al (2007)35 Au: Univ medicine, Healthcare organization Pub: Healthcare sciences/ policy, pharmacy $: not reported |
Physician office, Kansas Time-motion study, institutional 12 mos n = 32 pharmaceutical companies Drummond score = 2/10 |
Medical assistant Meds: 143 meds; type not reported |
Cost: personnel, supply, submission |
|
| Coleman (2003)8 Au: Univ pharmacy, Hospital Pub: Pharmacy $: not reported |
Inpatient hospital pharmacy, Connecticut Cost-benefit analysis, institutional, 6 mos n = 96 apps filed; 95% of apps approved Drummond score = 4/10 |
Pharmacy Research Fellows Meds: cardiology, hematology/ oncology, endocrinology, pain, other |
Benefit: (ACQ of PAP meds) — (program costs) Sensitivity analysis: Monte Carlo, threshold |
|
| Gillespie (2006)36 Au: Univ pharmacy, Hospital Pub: Pharmacy $: not reported |
Outpatient care clinic, Connecticut Cost-benefit analysis, institutional, 24 mos n = 143 PAP apps; 338 PAP meds, 90% of apps approved Drummond score = 3/10 |
Pharmacy Research Fellows Meds: hypertension, diabetes, asthma + renewal appt contact |
Benefit: (AWP of PAP meds) — (program costs) Sensitivity analysis: Monte Carlo, threshold |
|
| Richardson (2002)37 Au: Univ pharmacy, Non-profit org Pub: Pharmacy $: not reported |
National (U.S.) Fax/mail survey, institutional, 4 mos n = 118 safety-net providers from population of 1,204 340B participants Drummond: score = 5/10 |
Pharmacist, pharmacy technicians, physicians, nurses Meds: diabetes, lipids |
Benefit: (provider fees) + (340B PAP meds) – (program costs) Program costs: personnel, equipment, miscellaneous Other: # PAP meds requested, received |
|
| Grey literature (k=2) | ||||
| Chisholm (2000)38 Au: Univ medicine Pub: Pharmacy conf abstract $: not reported |
Renal transplant clinic, Georgia Cost-benefit analysis, institutional, 9 mos n = Over 70 pts Drummond score = not applicable |
Personnel: not reported | Benefit: (AWP of PAP meds) - (program costs) |
|
| Meds: immuno-suppresants, cardio-vasular, gastrointestinal, other | ||||
| Kokko (2003)39 Au: Univ medicine Pub: Pharmacy conf abstract $: not reported |
Outpatient cancer center, South Carolina Cost-benefit analysis, institutional; time=not reported n= 79 indigent pts Drummond score = not applicable |
Pharmacy technician | Benefit: (value of PAP meds based on AWP and PHS) - (program costs) |
|
| Meds: anemia, infection, cardiovascular, other | ||||
p<.05
p<.01
p<.001
Describes the institutional program that assists with PAPs and its components. Services include those in addition to PAP enrollment.
Refers to the ratio of the benefits gained to the program costs.
340B = federal program pricing, ACQ = Actual Acquisition Costs, AWP = Average Wholesale Price, Conf = conference, Mos = months;, PHS = Public Health System, Pricing, Univ = University or College, affiliation
Table 3.
Surveys of patient assistance programs (PAP): included studies’ characteristics and reporting quality (K=7)
| PUBLICATION 1st Author (Year) Au: Author affiliation(s) Pub: Publication discipline $: Funding source |
STUDY AIM(S) | DESIGN Survey Mode (Response Rate), Sampling Strategy Sample Description + 2nd method, dates STROBE score = # sufficient items/total |
MAIN FINDINGS Reported by author |
|---|---|---|---|
| Commercially-published articles (k=3) | |||
| Choudhry (2009)6 Au: Univ medicine; Hospital Pub: Health policy/services $: unfunded |
Describe the benefits offered by and eligibility/application process for PAPs |
Phone (91%), non-random sample of 165 pharmaceutical PAP representatives + Internet review of PAPs, Sep–Nov 2007 STROBE score = 17/22* |
|
| Duke (2005)12 Au: Univ: pharmacy, business; non-profit Pub: Pharmacy $: Private foundation |
Examine safety-net clinics’ use and assessment of PAPs |
Phone (63%), non-random sample of 215 safety- net clinic staff + 10 case studies, Jul–Sep 2002 STROBE score = 15/22 |
|
| Pisu (2009)43 Au: Univ medicine Pub: Medicine $: Federal gov’t |
Explore physicians’ perceptions of the usefulness of and barriers to using PAPs |
Fax (10%), non-random sample of 364 physicians from Tennessee, Mississippi, Georgia, and Florida, 2003 STROBE score = 18/22 |
|
| Grey literature (k=4) | |||
| Gov’t Accounting Office (2000)44 Au: Federal gov’t Pub: Federal gov’t $: not reported |
Describe the scope and design of PAPs |
Mode: not reported (82%), non-random sample of 72 pharmaceutical PAP representatives + select interviews, dates = not reported STROBE score = 9/22 |
|
| Sagall (2009)45 Au: Non-profit Pub: Non-profit $: not reported |
Examine the pros/cons of PAPs and propose specific recommendations for improvement |
Internet (rate=not reported), non-random sample of 1,121 visitors to Needy Meds website or newsletter recipients, Sept-Oct 2008 STROBE score = not applicable |
|
| Sorenson (2002)46 Au: Univ pharmacy Pub: Pharmacy conf abstract, Pharmacy web report $: not reported |
Determine uninsured patients’ perceptions of medication costs and their willingness to pay for medications within the network of FQHCs |
In-person (55%) random sample of 11 uninsured pts at a FQHC, dates=not reported + 2 focus groups STROBE score = 14/22 |
|
| Zerzan (2004)47 Au: Univ medicine Pub: Health policy/services conf abstract $: not reported |
Determine how the loss of program benefits affected former program enrollees; explore the use of PAPs among enrollees |
Phone (58%), strategy: not reported, sample of 439 former state medically needy program beneficiaries, after Jan 2003 STROBE score = 1/1 |
|
p<.05
p<.01
p<.001
Conf = conference, FQHC = Federally Qualified Health Center, Gov’t = government
Table 4.
Other studies of patient assistance programs (PAP): included studies’ characteristics and reporting quality (K=4)
| PUBLICATION 1st Author (Year) Au: Author affiliation(s) Pub: Publication discipline $: Funding source |
STUDY AIMS | DESIGN Setting, location Design + 2nd method, dates n = number of subjects: sample description STROBE score = # sufficient items/total |
MAIN FINDINGS Reported by author |
|---|---|---|---|
| Commercially-published articles (k=2) | |||
| Chauncey (2006)48 Au: Univ pharmacy; non- profit; consulting firm Pub: Pharmacy $: not reported |
Describe PAPs and their enrollment processes; demonstrate the complexity of accessing PAPs using a convenience sample |
Non-profit, MD Cross-sectional analysis + internet review of PAPs, Jan 2001–Apr 2004 n = 15,925 MedBank pts: m age = 64.5 ± 15.9 yr, 69% ♀, 65.5% Caucsn, 25% Afr Am STROBE score = 14/22 |
|
| Chisholm (2002)49 Au: Univ: pharmacy, medicine Pub: Medicine $: Private trust |
Facilitate awareness of PAPs, their availability and enrollment process |
National (U.S.) Cross-sectional analysis + internet and literature review of PAPs, dates = not reported n=200 most prescribed medications in U.S. in 1999 STROBE score = 4/22 |
|
| Grey literature (k=2) | |||
| Gov’t Accouting Office (2008)50 Au: Federal gov’t Pub: Federal gov’t $: not reported |
Examine (1) the importance of assets and income in LIS denials; (2) SPAPs and PAPs that provide access to medications for Medicare beneficiaries |
National (U.S.) Pop-based, cross-sectional analysis + literature review on SPAPs and PAPs and interviews, 2006 & 2007 n=2,252,412 Medicare Part D LIS applicants in 2006 vs 366,183 in 2007 STROBE score = 6/22 |
|
| Hebert (2006) Au: Hospital Pub: Medical conf abstract $: not reported |
Quantify the magnitude of Medicare Part D on a disease management program supplying care primarily to indigent pts |
Safety-net hospital, LA Cross-sectional analysis, Jan–Dec 2005 n = 382 pts w/ systolic heart failure: m age = 66.7 yr, 41%, 36% Afr Am STROBE score = 1/1 |
|
FQHC = Federally Qualified Health Center, LIS = Low-Income Subsidy, SPAP = State Prescription Assistance Program
Types of PAP studies
Health care outcome studies (k=10 articles, 5 abstracts)
Most health care outcome studies were from the commercially published literature (k=10) and used single-group, pre/post designs (k=8), and combined PAP enrollment with additional services (k=10). All studies reported on an institutional PAP program in an outpatient setting (k=15), where pharmacists (k=8) commonly assisted patients with diabetes (k=3 diabetes only, k=5 with other diseases) in enrolling in PAPs and provided additional medication services (k=10). All but one full article (k=9) reported at least 17 of 22 STROBE items sufficiently.
Clinical disease indicators (k=11) and adherence (k=6) were the most frequently reported outcomes. Studies typically assessed multiple (range: 1–10) clinical disease indicators. None reported using a correction for multiple tests (e.g., Bonferroni correction), but one study mentioned setting statistical significance at p<.01 instead of p<.05 to reduce the possibility of committing a Type I error. Studies examining adherence used a wide range of indicators (medical records and self report = 3 studies, pharmacy refill records = 2, not reported = 1) and time frames (e.g., no more than 14 days without a refill, 12-month medication possession ratio).
Effect size calculations. For select health care outcomes study, we reported standardized mean differences with 95% confidence intervals (CIs) calculated using STATA version 11 (STATA Corp., College Station, Texas) and a DerSimonian and Laird random-effects model. Statistical heterogeneity was measured using the Q statistic (p<.10 was considered representative of significant statistical heterogeneity). We selected the most common clinical indicators for diabetes, dyslipidemia, and hypertension based on recommendations by relevant guidelines and frequency of use in the included studies. For diabetes, HbA1c is the key measure to assess glycemic control40 and it was measured in all eight studies that included a diabetes measure. For dyslipidemia, LDL alone was the preferred measure,41 but LDL goal attainment (1 of 8 studies) was used when LDL alone (7 of 8 studies) was not available. For hypertension, systolic blood pressure (SBP) was either reported alone (5 of 8 studies) or as part of total blood pressure (3 of 8 studies). Because SBP increases with age, national guidelines suggest greater emphasis on its control.42
Means and standard deviations from single group pre- and post-PAP were the primary choice of measurement and comparison. If there were more than two time points of measurement, the baseline and the measure immediately following baseline was used. When there were two groups (PAP versus comparison), the PAP group’s post-enrollment measure was compared with the comparison group’s measure. Selected measures could not be sub-categorized or stratified by any other variable or value. Conference abstracts reported insufficient disease indicator statistics for inclusion in estimate calculations.
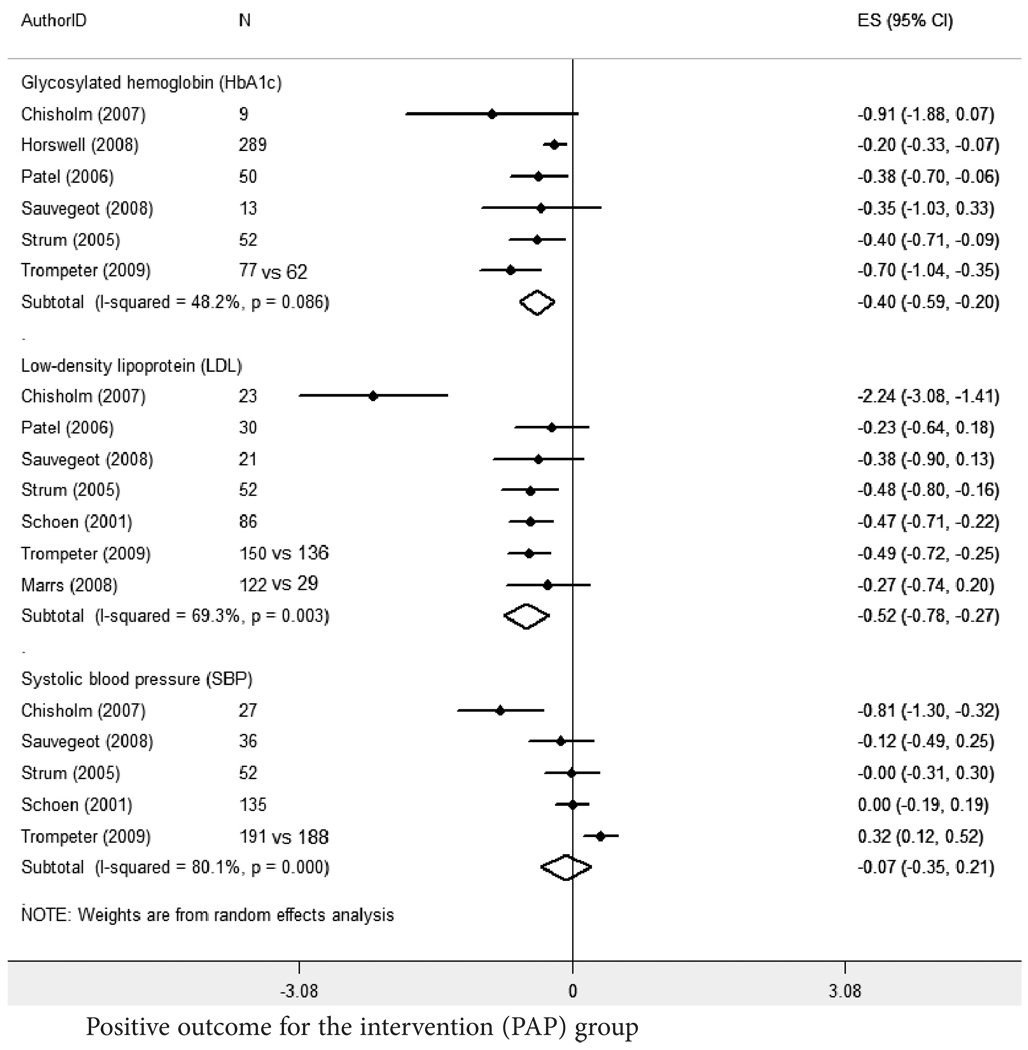
Weighted average effect size estimates showed significant improvements in HbA1c (ES=−0.40, 95% CI: −0.59,−0.20) and LDL (ES: −0.52, 95% CI: −0.78,−0.27) (random effects model) (Figure 2). Significant heterogeneity was found in all three groups (Q statistics = 9.7, df(5) for HbA1c, p=0.086; 19.5, df(6) for LDL, p=.003; and 20.1, df(4) for SBP, p<.001). Study heterogeneity and the additional services that could not be separated from the PAP enrollment assistance alone make these estimates problematic to interpret.
Figure 2.
Estimated effect size (ES) calculations for Health Outcomes Studies: standardized mean differences for select clinical indicators.
Survey studies (5 reports, 1 abstract, 1 oral presentation)
Survey studies were primarily telephone surveys (k=4) of non-random samples (k=5) of potential PAP users (k=3), health care providers (k=2), or pharmaceutical company representatives (k=2) about their use and perceptions of PAPs. Response rates ranged from 10% to 91%. Respondents reported both positive (k=2) and negative (k=2) perceptions of PAPs. Suggested improvements for PAPs included universal application forms for potential users (k=2) and reducing the role of providers in the completion of PAP applications (k=2). The quality of reporting was higher for survey studies that were from commercially published (STROBE: m=16.7 ± 1.53) than for full-text grey literature sources (STROBE: m=11.5 ± 3.53).
Economic evaluations (5 reports, 2 abstracts)
All evaluations were from an institutional perspective; no additional perspectives were considered. Five evaluations were mislabeled as cost-benefit analyses (CBAs); these evaluations did not include a valuation of the consequences or effects of the PAP enrollment assistance programs or compare the PAPs to an alternative. Findings from authors indicated that the sum of the cost, often based on the average wholesale price (k=3) for each PAP medication received, outweighed program costs (time spent on the program multiplied by health care personnel wages (k=5); highest ratio = 11:1, lowest = 4:1). These findings were robust to sensitivity analyses (k=3), when the value of PAP medication, time spent on program, and/or personnel wage rates were varied. The two evaluations with the highest cost-savings ratios provided a medication service in combination with PAPs. Cancer drugs and immunosuppressants accounted for the majority of institutional cost-savings in their respective studies. Overall quality scores (m=3.6 ± 1.14) reflected the fact that the economic evaluations consistently did not adequately address the Drummond checklist items.
Other studies (3 reports, 1 abstract)
Each of the four other studies conducted a cross-sectional analysis, and three also included some type of Internet or literature review of PAP eligibility and applications. Nearly the same number of STROBE items were sufficiently reported (m=8.0 ± 5.29) as not reported (m=9.0 ± 3.0; data not shown) by these studies.
Author and publication affiliations
Across all study categories and literature sources, pharmacy was the predominant discipline among both authors (k=20) and publications (k=18). Studies with at least one author affiliated with a school or college of pharmacy most frequently investigated the effect of PAP use on health care outcomes (k=12 of 20 included studies), often clinical indicators (k=10 of 12), using single-group, pre-post study designs (k=7 of 10). The majority of included studies published in the pharmacy literature were either health care outcomes studies (k=11 of 18), or economic evaluations (k=6 of 18), all of which reported positive outcomes. Full-text studies published in the pharmacy literature reported more STROBE items sufficiently (m=16.9 ± 1.758) than non-pharmacy studies (m=12.4 ± 6.241). Within study categories, economic evaluations had an equal number of studies with authors affiliated with medicine (k=4) and pharmacy (k=4). Among the grey literature reports (k=13), authors were frequently affiliated with medicine (k=5) and publications were often pharmacy conference abstracts (k=6).
Study funding sources
Most of the six commercially published studies reported study funding from private sources (k=3 private trust fund, all by the same primary author; k=1 private foundation). Studies were most frequently published in medical journals (k=3) by authors affiliated with pharmacy and medicine (k=4). Funded observational studies had a similar number of sufficiently reported items (m=15.2 ± 6.53) but fewer not reported items (m=3.4 ± 3.13) than unfunded studies (m=15.2 ± 3.90 and m=5.5 ± 2.50, respectively). Only economic evaluations reported pharmaceutical company funding sources (k=1).
Discussion
This systematic review identified and described a broad range of reports of studies from commercially published and grey literature sources that examined the use of PAPs, often in combination with various medication services (e.g., medication therapy management, counseling). Fifteen studies investigated questions about the impact of using PAPs on patient health care outcomes, seven surveyed PAP stakeholders, and seven evaluated the costs of providing PAP enrollment assistance.
Limitations of study designs
While the authors of the individual studies generally presented balanced interpretations of their findings, few inferences can be made from our descriptive synthesis regarding the effectiveness, use, or value of PAPs because of limitations in the studies’ designs. For example, effect size calculations showed statistically significant reductions in most clinical disease indicators, suggesting positive effects of PAPs on these health care outcomes; however, there was a high level of study heterogeneity. Also because of limitations in the studies’ designs (e.g., retrospective pre/ post-test designs, no control group), the effects of PAPs on patient health care outcomes may have been overestimated: PAPs were consistently provided in combination with various medication services (e.g., patient counseling, reminder services). Because PAP applications require the involvement of a health care professional, the effect that the patient-professional interaction has on a patient’s health care outcomes also cannot be ignored, particularly when the professionals are pharmacists who have specialized training and knowledge in medication therapy.22
Similarly, economic evaluations, inaccurately labeled as CBAs, reported positive findings. These findings should be taken with caution, however. True CBAs require the monetary valuation of health outcomes and comparison of alternatives,18 which were not included in these mislabeled evaluations. Therefore it remains unclear whether PAP enrollment assistant programs are the most cost-beneficial service health care institutions can use to improve patient access to needed medications. Generalizations about the survey studies results are also difficult to make due to the frequent use of non-random samples and low response rates.
It has been suggested that pharmaceutical companies use PAPs as a marketing tool to promote the use of their newer, brand-name therapies5,52 to low-income patients; therefore, applying rigorous study designs and methods is essential to substantiate the true clinical and financial effectiveness of these programs. Specifically, cost-effectiveness studies must be conducted taking into account the differences in health care outcomes (which do not require the complicated valuation process) and costs. Comparisons of the cost-effectiveness for patients using PAPs to those for patients using other options that minimize medication costs, such as the use of generics or from discount programs (e.g., WalMart $4 program), should be conducted. Such cost-effectiveness ratios would be valuable to both health care providers and policymakers.
Lack of study funding
One likely explanation for the use of less rigorous study designs is a lack of study funding. For example, one survey study that did not report a funding source had a 10% response rate and specifically cited limited funds as the reason for being unable to follow up with non-responders and attempt to address bias.37 Less than one-fourth of the included studies in our review reported any source of study funding, and one source funded nearly half of the studies. Among the funded studies, pharmacy was consistently represented in the author and publication affiliations, and such authors collaborated most frequently with co-authors affiliated with medicine. In general, pharmacists view PAPs as a means to implement cost-avoidance measures as well as improve medication access for patients.53 The problem of medication access is multi-factorial, and yet people from pharmacy and medicine—the disciplines that are often focused on clinical practice and patient care—may have limited time and resources for conducting research. Previous research has shown associations between study quality and study funding;54,55 therefore clinical practitioners need multidisciplinary collaborations with researchers from other disciplines (e.g., public health) concerned with medication access and affordability issues to be competitive scientists and successful in obtaining research funding.56 More rigorous, multidisciplinary research is needed to determine the true impact of PAPs on overall health care costs and on patient health outcomes, rather than simply conducting programmatic evaluations for individual health care institutions.
Value of the grey literature
Although the health care outcome studies from the grey literature reported inadequate statistics for calculating effect sizes, the overall value of including this literature in our review should not be minimized. Rothstein and Hopewell argue that the most essential feature of a reliable and valid research synthesis is a lack of bias in the search for relevant studies.57 Including the grey literature in the search is essential to minimize publication bias. Within the context of health services research and health policy, the grey literature is considered a valuable barometer of current public interests and emerging priorities.58 In our review, there were more survey studies from the grey than from the commercially-published literature; these surveys tended to address contemporary concerns. For example, one survey was the result of a request from Congressional representatives to the Government Accounting Office in 2000 to examine Medicare beneficiaries’ ability to access needed medications through PAPs prior to the implementation of Medicare Part D.44
Future directions
2010 passage of the Patient Protection and Affordable Health care Act, which extends health insurance, including prescription drug coverage, to 32 million people in the U.S.,59 raises this question: will PAPs remain relevant? Recent reports of the increasing demand for PAPs, particularly among the underinsured,52,60 suggests that the answer may be yes. Findings from this systematic review suggests PAP enrollment assistance plus additional medication services (e.g., counseling, free samples) is associated with improved disease indicators for patients with chronic diseases. Safety-net and other health care providers may also avoid potential uncompensated prescription drug costs and recoup costs incurred in implementing PAP enrollment assistance programs. Patient assistance programs have not been adequately evaluated from a patient or health care institution perspective, however. Even when prescription drugs are made available to patients free of charge through PAPs, patients may face other costs (e.g., transportation) that can restrict their access.20 Pharmaceutical patient assistance programs also may lead patients to use particular brand products when there are less costly alternatives available.5,61 Once the formerly uninsured have health care coverage, they may prefer to continue using these brand-name products, which could lead to higher drug spending.6 The conduct of full economic evaluations, particularly cost-effectiveness studies from a health care institution perspective, that compare PAP enrollment assistance programs to other medication access strategies would fill a gap in our understanding of which programs, given finite resources, offer the greatest benefits to patients and society. Future studies should also be conducted by multidisciplinary teams, because problems of medication access and use cannot be solved by a single discipline. The combined expertise of these teams should yield studies that apply more rigorous designs and methods, including larger sample sizes and the use of validated instruments for assessing health outcomes, in order to establish the effectiveness of PAPs.
Acknowledgments
We acknowledge Dr. Brandon Kimmins for his support with coding, Dr. Gil Ramirez for his assistance with effect size calculations, Ms. Helena Vonville for her assistance with development of the literature search strategy, and Ms. Karyn Popham for her editorial support. We also appreciate Dr. Anthony Alberg for his thoughtful suggestions.
At the time of study, TMF and NRP were supported by a Pre-doctoral Fellowship, University of Texas School of Public Health, Cancer Education and Career Development Program; National Cancer Institute Grant R25CA57712. NRP is currently supported by the Comprehensive Cancer Center of Wake Forest University Cancer Control Traineeship–National Cancer Institute/National Institutes of Health Grant R25CA122061.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
The term grey literature refers to “information produced on all levels of government, academics, business and industry in electronic and print formats not controlled by commercial publishing, i.e., where publishing is not the primary activity of the producing body.” Source: New York Academy of Medicine, 1999.15[p.1]
Contributor Information
Tisha M. Felder, Postdoctoral Fellow jointly appointed to the Department of Clinical Pharmacy and Outcomes Sciences in the South Carolina College of Pharmacy and the Statewide Cancer Prevention and Control Program of the Arnold School of Public Health at the University of South Carolina in Columbia, South Carolina.
Nynikka R. Palmer, Postdoctoral Fellow in the Division of Public Health Sciences–Social Sciences & Health Policy at the Wake Forest University School of Medicine in Winston-Salem, North Carolina.
Lincy S. Lal, Senior Research Consultant for Ingenix Consulting in Missouri City, Texas.
Patricia Dolan Mullen, Professor in the Division of Health Promotion and Behavioral Sciences, and a Senior Investigator in Center for Health Promotion and Prevention Research at the University of Texas School of Public Health in Houston, Texas. Please address correspondence to Tisha M. Felder, PhD, MSW, South Carolina College of Pharmacy & Statewide Cancer Prevention and Control Program, University of South Carolina, 915 Greene Street, Suite 233, Columbia, SC 29208.
Notes
- 1.U.S. Department of Health and Human Services. HHS applauds pharmaceutical patient assistance programs. Washington, DC: U.S. Department of Health and Human Services; 2005. Available at: http://www.hhs.gov/news/press/2006pres/20060418a.html. [Google Scholar]
- 2.U.S. Food and Drug Administration. Saving money on prescription drugs. Washington, DC: U.S. Food and Drug Administration; 2005. Available at: http://www.fda.gov/fdac/features/2005/505_save.html. [Google Scholar]
- 3.Weinberg M. Reforming patient assistance programs: perfect world meets real world. Health Aff (Millwood) 2009 May–Jun;28(3):839–842. doi: 10.1377/hlthaff.28.3.839. [DOI] [PubMed] [Google Scholar]
- 4.Johnson K. Voluntary patient assistance programs: additional federal oversight unwarranted. Health Aff (Millwood) 2009 May–Jun;28(3):835–838. doi: 10.1377/hlthaff.28.3.835. [DOI] [PubMed] [Google Scholar]
- 5.Carroll NV. Pharmaceutical patient assistance programs: don’t look a gift horse in the mouth or there’s no such thing as a free lunch. J Manag Care Pharm. 2007 Sep;13(7):614–616. doi: 10.18553/jmcp.2007.13.7.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhry NK, Lee JL, Agnew-Blais J, et al. Drug company-sponsored patient assistance programs: a viable safety net? Health Aff (Millwood) 2009 May–Jun;28(3):827–834. doi: 10.1377/hlthaff.28.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorenson TD, Song J, Westberg SM. The limitations of good intentions: prescribing medications for the uninsured. J Health Care Poor Underserved. 2004 May;15(2):152–160. doi: 10.1353/hpu.2004.0029. [DOI] [PubMed] [Google Scholar]
- 8.Coleman CI, Reddy P, Quercia RA, et al. Cost-benefit analysis of a pharmacy-managed medication assistance program for hospitalized indigent patients. Am J Health Syst Pharm. 2003 Feb 15;60(4):378–382. doi: 10.1093/ajhp/60.4.378. [DOI] [PubMed] [Google Scholar]
- 9.Weiner S, Dischler J, Horvitz C. Beyond pharmaceutical manufacturer assistance: broadening the scope of an indigent drug program. Am J Health Syst Pharm. 2001 Jan 15;58(2):146–150. doi: 10.1093/ajhp/58.2.146. [DOI] [PubMed] [Google Scholar]
- 10.Williams K. Accessing patient assistance programs to meet clients’ medication needs. J Am Acad Nurse Pract. 2000 Jun;12(6):233–235. doi: 10.1111/j.1745-7599.2000.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 11.Mounts VL, Ringenberg DG, Rhees K, et al. Implementation of a patient medication assistance program in a community pharmacy setting. J Am Pharm Assoc (2003) 2005 Jan–Feb;45(1):76–81. doi: 10.1331/1544345052843039. [DOI] [PubMed] [Google Scholar]
- 12.Duke KS, Raube K, Lipton HL. Patient-assistance programs: assessment of and use by safety-net clinics. Am J Health Syst Pharm. 2005 Apr 1;62(7):726–731. doi: 10.1093/ajhp/62.7.726. [DOI] [PubMed] [Google Scholar]
- 13.Chen JT, Summers KH. Pharmaceutical manufacturer prescription assistance programs: are they worth it? J Manag Care Pharm. 2007 Sep;13(7):611–613. doi: 10.18553/jmcp.2007.13.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daemmrich A, Bowden ME. Top pharmaceuticals: a look at drugs that changed our world. Chemical & Engineering News. 2005 June 20;83(25):3–5. [Google Scholar]
- 15.New York Academy of Medicine. Grey literature report: what is grey literature? New York, NY: New York Academy of Medicine; 2009. Available at: http://www.nyam.org/library/greywhat.shtml#gl. [Google Scholar]
- 16.Duke University Medical Center Library. Searching for grey literature. Durham, NC: Duke University Medical Center Library; 2008. Available at: http://www.mclibrary.duke.edu/pubsupport/greyliterature. [Google Scholar]
- 17.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007 Oct 16;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. Cary, NC: Oxford University Press; 2005. [Google Scholar]
- 19.Chisholm MA, Spivey CA, Mulloy LL. Effects of a medication assistance program with medication therapy management on the health of renal transplant recipients. Am J Health Syst Pharm. 2007;64(14):1506–1512. doi: 10.2146/ajhp060634. [DOI] [PubMed] [Google Scholar]
- 20.Horswell RL, Wascom CK, Cerise FP, et al. Diabetes mellitus medication assistance program: relationship of effectiveness to adherence. J Health Care Poor Underserved. 2008 Aug;19(3):677–686. doi: 10.1353/hpu.0.0062. [DOI] [PubMed] [Google Scholar]
- 21.Patel AA, Kuti EL, Dale KM, et al. Effect of a medication assistance program on clinical outcomes in patients with diabetes. Formulary. 2006;41(10):518. 520, 522. [Google Scholar]
- 22.Sauvageot J, Kirkpatrick MA, Spray JW. Pharmacist-implemented pharmaceutical manufacturers’ assistance programs: effects on health outcomes for seniors. Consult Pharm. 2008 Oct;23(10):809–812. doi: 10.4140/tcp.n.2008.809. [DOI] [PubMed] [Google Scholar]
- 23.Schoen MD, DiDomenico RJ, Connor SE, et al. Impact of the cost of prescription drugs on clinical outcomes in indigent patients with heart disease. Pharmacotherapy. 2001 Dec;21(12):1455–1463. doi: 10.1592/phco.21.20.1455.34473. [DOI] [PubMed] [Google Scholar]
- 24.Strum MW, Hopkins R, West DS, et al. Effects of a medication assistance program on health outcomes in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62(10):1048–1052. doi: 10.1093/ajhp/62.10.1048. [DOI] [PubMed] [Google Scholar]
- 25.Marrs JC, Saseen JJ. Dyslipidemia control in indigent patients receiving medication assistance compared with insured patients. Pharmacotherapy. 2008;28(5):562–569. doi: 10.1592/phco.28.5.562. [DOI] [PubMed] [Google Scholar]
- 26.Trompeter JM, Havrda DE. Impact of obtaining medications from pharmaceutical company assistance programs on therapeutic goals. Ann Pharmacother. 2009 Mar;43(3):469–477. doi: 10.1345/aph.1L420. [DOI] [PubMed] [Google Scholar]
- 27.Paris W, Dunham S, Sebastian A, et al. Medication nonadherence and its relation to financial restriction. J Transpl Coord. 1999 Sep;9(3):149–152. doi: 10.7182/prtr.1.9.3.j28354x695735514. [DOI] [PubMed] [Google Scholar]
- 28.Spiker EC, Giannamore MR, Nahata MC. Medication use patterns and health outcomes among patients using a subsidized prescription drug program. J Am Pharm Assoc. 2005 Nov–Dec;45(6):714–719. doi: 10.1331/154434505774909616. [DOI] [PubMed] [Google Scholar]
- 29.Divine HS, Millheim ET, Adams JS. Clinic pharmacist’s use of prescription assistance programs to impact patient compliance. ASHP Midyear Clinical Meeting. 2001;36 (Dec):P-518. [Google Scholar]
- 30.King DS, Wyatt SB, King NS, et al. Improvement in blood pressure control through interdisciplinary medication assistance efforts in hypertensive patients with multiple metabolic syndrome. Am J Hypertens. 2000 Apr;13(13) 18A. [Google Scholar]
- 31.Prutting SM, Mazur JN, Johnson CB, et al. Evaluation of outcomes for patients enrolled in pharmaceutical assistance programs. ASHP Midyear Clinical Meeting. 2003;38 (Dec):P-536E. [Google Scholar]
- 32.Kiser SN, Patel NH. Improving patient outcomes in a medication assistance program: study in pharmaceutical care. ASHP Midyear Clinical Meeting. 2001;36 (Dec): P-342. [Google Scholar]
- 33.Schoen MD, Dischler JE, Connor SW, et al. Economic outcomes and medication compliance in a prescription procurement clinic for indigent patients with cardiovascular disease. Circulation. 1997;96(8 Suppl):1–617. [Google Scholar]
- 34.Chisholm MA, Vollenweider LJ, Mulloy LL, et al. Cost-benefit analysis of a clinical pharmacist-managed medication assistance program in a renal transplant clinic. Clin Transplant. 2000 Aug;14:304–307. doi: 10.1034/j.1399-0012.2000.140405.x. [DOI] [PubMed] [Google Scholar]
- 35.Clay P, Vaught E, Glaros A, et al. Costs to physician offices of providing medications to medically indigent patients via pharmaceutical manufacturer prescription assistance programs. J Manage Care Pharm. 2007 Jul–Aug;13(6):506–514. doi: 10.18553/jmcp.2007.13.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillespie EL, Patel AA, Henyan NN, et al. Economic analysis of a medication assistance program for ambulatory care patients at an urban teaching clinic. Formulary. 2006;41(1):31–34. [Google Scholar]
- 37.Richardson K, Basskin LE. Use of drug manufacturers’ patient assistance programs by safety net providers. Am J Health-Syst Pharm. 2002 Jun;59(11):1105–1109. doi: 10.1093/ajhp/59.11.1105. [DOI] [PubMed] [Google Scholar]
- 38.Chisholm MA, Kendrick BD, Vollenweider LJ, et al. Cost benefit analysis of a medication access program from a tertiary care institution’s perspective. ASHP Midyear Clinical Meeting. 2000;35 (Dec):P-156E. [Google Scholar]
- 39.Kokko H, Uber LA, Priester S, et al. Development of a technician driven medication assistance program. ASHP Midyear Clinical Meeting. 2003;38 (Dec):TEH-17. [Google Scholar]
- 40.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010 Jan;33 Suppl 1:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Health Institute. Third report of the national cholestrol education program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III, Publication No. 02-5215) Bethesda, MD: National Health Institute; 2002. [Google Scholar]
- 42.National Health Institute. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure (Publication No. 04-5230) Bethesda, MD: National Health Institute; 2009. [Google Scholar]
- 43.Pisu M, Richman J, Allison JJ, et al. Pharmaceuticals companies’ medication assistance programs: potentially useful but too burdensome to use? South Med J. 2009 Feb;102(2):139–144. doi: 10.1097/SMJ.0b013e31818bbe5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Government Accountability Office. Prescription drugs drug company programs help some people who lack coverage: report to congressional requesters (GAO-01-137) Washington, DC: U.S. General Accounting Office; 2000. Available at http://www.gao.gov/new.items/d01137.pdf. [Google Scholar]
- 45.Sagall R. The good, the bad, and the ugly of PAPs [PowerPoint slides]. Paper presented at ExL Pharma Patient Assistance Programs Conference; Washington, DC. 2009. [Google Scholar]
- 46.Sorensen TD, Kelley K. Determination of uninsured patients’ perceptions of medication access, costs and pharmacy services in federally qualified health centers. ASHP Midyear Clinical Meeting. 2002;37 (Dec):PP-10. [Google Scholar]
- 47.Zerzan J, Edlund T Academy Health. Meeting (2004): San Diego, Calif.) The demise of Oregon’s medically needy program: effects of losing prescription drug coverage and pharmaceutical company drug assistance programs. Academy Health Meeting. 2004;21 abstract no. 980. [Google Scholar]
- 48.Chauncey D, Mullins CD, Tran BV, et al. Medication access through patient assistance programs. Am J Health-Syst Pharm. 2006 Jul 1;63(13):1254–1259. doi: 10.2146/ajhp050457. [DOI] [PubMed] [Google Scholar]
- 49.Chisholm MA, DiPiro JT. Pharmaceutical manufacturer assistance programs. Arch Intern Med. 2002 Apr 8;162(7):780–784. doi: 10.1001/archinte.162.7.780. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Government Accountability Office. Medicare Part D low-income subsidy assets and income are both important in subsidy denials, and access to state and manufacturer drug programs is uneven: report to congressional committees (GAO-08-824) Washington, DC: U.S. General Accounting Office; 2008. Available at http://www.gao.gov/new.items/d08824.pdf. [Google Scholar]
- 51.Hebert K, Arcement LM, Horswell R. Pharmaceutical-sponsored medication assistance program utilization by an indigent rural heart failure disease management clinic: magnitude and potential impact of Medicare Part D. Chest Meeting. 2006:100S. Abstracts. [Google Scholar]
- 52.Fuhrmans V. Patients seek financial aid to buy medicine: layoffs drive rise in interest in programs aimed at the poor; help navigating the choices. New York, NY: The Wall Street Journal (Health D1); 2008. Oct 21, [Google Scholar]
- 53.Fijalka S, Fye D, Johnson PE. Current issues in pharmaceutical reimbursement. A J Health Syst Pharm. 2008 Jan 15;65(2 Suppl 1):S11–S26. doi: 10.2146/ajhp070620. [DOI] [PubMed] [Google Scholar]
- 54.Thomas O, Thabane L, Douketis J, et al. Industry funding and the reporting quality of large long-term weight loss trails. Int J Obes (Lond) 2008 Oct;32(10):1531–1536. doi: 10.1038/ijo.2008.137. Epub 2008 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reed DA, Cook DA, Beckman TJ, et al. Association between funding and quality of published medical education research. JAMA. 2007 Sep 5;298(9):1002–1009. doi: 10.1001/jama.298.9.1002. [DOI] [PubMed] [Google Scholar]
- 56.Fagan SC, Touchette D, Smith JA, et al. The state of science and research in clinical pharmacy. Pharmacotherapy. 2006;26(7):1027–1040. doi: 10.1592/phco.26.7.1027. [DOI] [PubMed] [Google Scholar]
- 57.Rothstein HR, Hopewell S. The grey literature. In: Cooper H, Hedges LV, Valentine JC, editors. Handbook of research synthesis and meta-analysis. 2nd ed. New York, NY: Russell Sage Foundation; 2009. [Google Scholar]
- 58.National Library of Medicine. Health services research and health policy grey literature project: summary report. Bethesda, MD: National Library of Medicine; 2008. Available at: http://www.nlm.nih.gov/nichsr/greylitreport_06.html#_Toc124061657. [Google Scholar]
- 59.Kaiser Family Foundation. Side-by-side comparison of major health care reform proposals. Washington, DC: Kaiser Family Foundation; 2010. Available at: http://www.kff.org/healthreform/upload/housesenatebill_fnal.pdf. [Google Scholar]
- 60.Jack A. Downturn fuels demand for free drugs in U.S. London, United Kingdom: Financial Times. 2010 Apr 5; [Google Scholar]
- 61.Fairman KA, Curtiss FR. The elephant in the pharmacy: patient choice is the big challenge that no one talks about in affordability of prescription drugs. J Manag Care Pharm. 2007 Sep;13(7):620–622. doi: 10.18553/jmcp.2007.13.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]