Abstract
Glycogen synthase kinase-3β (GSK3β) role in human immunodeficiency virus (HIV)-associated neurodegeneration has been evidenced by previous investigations. In this study, we investigated the specificity of two GSK3β-specific inhibitors, AR-A014418 (A) and B6B30 (B) to prevent direct neurotoxicity in primary human neurons exposed to HIV (BaL). Neurons were exposed to HIV (500 pg/ml) for 12-h and 6-day periods in the presence and absence of A (1 µM, 100 nM, 10 nM) and B (50 nM, 5 nM, 500 pM) to investigate acute and ongoing mechanisms of HIV neurotoxicity. Using an lactate dehydrogenase (LDH) assay to assess cytotoxicity, we observed a significant neurotoxic effect of HIV from control values (P < .01) that was not restored via coexposures of all concentrations of A and B. Additionally, no change in LDH levels were observed after 6 days. However, activity of the acute proapoptotic markers caspases 3 and 7 using a luminescence assay were measured and found to be increased by exposure to HIV (BaL) compared to controls (P = .022). This effect was ameliorated via coexposure to all concentrations of A and 50 nM B after 12 h (P < .01) and to all concentrations of A and B after 6 days (P < .01). Overall, the results from this study provide further evidence for the ability of GSK3β inhibition to be neuroprotective against HIV-associated neurotoxicity by reducing HIV associated procaspase induction. These data support a role for GSK3β as a potential therapeutic target and may have important clinical implications for treatment of HIV-associated neurocognitive disorder.
Keywords: AR-A014418, B6B30, caspase, GSK3β, HIV, neurotoxicity
Introduction
Human immunodeficiency virus (HIV) is a neurotropic virus that results in a spectrum of inflammatory related brain changes, including HIV encephalitis and HIV leukoencephalopathy (Budka et al, 1991), accompanied by reductions in synaptodendritic markers and neuronal loss (Everall et al, 1991, 1993, 1999; Masliah et al, 1997). Clinically, both inflammatory and synaptodendritic degeneration seen postmortem correlate with the severity of HIV-associated neurocognitive disorder (HAND) seen prior to death (Cherner et al, 2002; Everall et al, 1999; Masliah et al, 1997). Despite the introduction of highly active retroviral treatment (HAART), HAND still remains prominent within the HIV-infected community (Clifford, 2008; Nath et al, 2008). Therefore, although antiretrovirals (ARVs) substantially reduce viral load, their effect on reducing symptoms of HAND is less evident. The pathophysiological process that results in HIV neurodegeneration is complex and mediated by both direct toxic effects of HIV viral proteins as well as indirect toxic effects via release of proinflammatory cytokines and dysregulation of other processes, including trophic factors. Previously, we have noted that synaptodendritic damage is related to brain viral burden (Everall et al, 1999; Masliah et al, 1997) and that the severity of neurodegeneration can be ameliorated when the host brain neurons can maintain expression of the neurotrophic agent fibroblast growth factor-1 (FGF1) (Everall et al, 2001). In further experiments, we noted that the intracellular signaling pathway by which FGF1 mediates this neuroprotection involved the inhibition of glycogen synthase kinase-3β (GSK3β). This was evidenced via blockade of the phosphatidylinositol 3-kinase (PI3)/Akt pathway by LY294002, a pathway that is an upstream regulator of GSK3β (Sagara et al, 2001). GSK3β in the nervous system has been associated with neuronal migration, axonal remodeling, and synaptic plasticity (Packard et al, 2003). In support of a neuroprotective capacity of GSK3β inhibition, up-regulation of GSK3β has been observed following exposure of cerebellar granule neurons to HIV-tat and down-stream mediator platelet-activating factor (PAF), resulting in apoptosis (Maggirwar et al, 1999; Tong et al, 2001).
In relation to the treatment of HAND, the mood stabilizers lithium and sodium valproate have both been shown to inhibit GSK3β activity (Klein and Melton, 1996; Rowe et al, 2007). Our group noted that that the HIV envelope glycoprotein (gp120) is capable of causing direct toxicity in primary human neuronal cultures (Everall et al, 2001) and that lithium prevented gp120-associated neurotoxicity (Everall et al, 2002), whereas Dou et al (Dou et al, 2003; Dou et al, 2005) noted a similar neuroprotective effect for both lithium and sodium valproate. Clinically, Letendre et al (Letendre et al, 2006) carried out a single-arm open-label pilot study of lithium 300 mg daily for 12 weeks in eight HIV-infected individuals with documented HAND. Lithium was found to result in improved neuropsychological performance in all individuals. Similarly in a 10-week trial of 16 HIV-infected individuals, in which 6 had no cognitive impairment, administration of 250 mg bid (twice daily) of sodium valproate resulted in a trend for improved neuropsychological performance and related to a significant increase in N-acetylasparate activity in frontal white matter as an indicator of improved activity (Schifitto et al, 2006). As both sodium valproate and lithium can have multiple effects on several intracellular signaling pathways, it is possible that other pathways not involving GSK3β be involved in their therapeutic action. Recently, it has been observed that lithium and sodium valproate work synergistically to prevent glutamate induced neurotoxicity in cerebellar granule cells, an effect that could also be achieved via the transfection of cells with small interfering RNA (siRNA) to GSK3β (Leng et al, 2008).
This study highlights the potential of these compounds in blocking GSK3β and preventing neurodegenerative effects that may be advantageous in alleviating symptoms of HAND. Therefore, the aim of this investigation was to further characterize how GSK3β-specific inhibition may mediate neuroprotection resulting from direct HIV-mediated neurotoxicity in vitro. We investigated the ability of two recently developed specific GSK3β inhibitors, AR-A014418 (A) and BIO-(2′Z,3′E)-6-bromoindirubin-3′-oxime (B6B3O) (B), in ameliorating HIV (BaL)–mediated neurotoxicity, and assessed acute mechanisms for this toxicity via measurement of caspase activation and postnecrotic measurements of lactate dehydrogenase (LDH) released from primary human neurons exposed to HIV (BaL) viral supernatants taken from human primary macrophages.
Results
LDH
A significant increase (P < .01) in LDH was observed in primary human neurons following 12-h exposure to 20% conditioned HIV macrophage supernatants (equivalent to 500 pg/ml), demonstrating toxicity of HIV to primary human neurons. This acute effect was not ameliorated with coexposure of either compound A or B, which were also elevated from control values (P < .01) and not significantly different from HIV alone (P > .10). No significant differences in LDH values between conditions were seen in 6-day exposures.
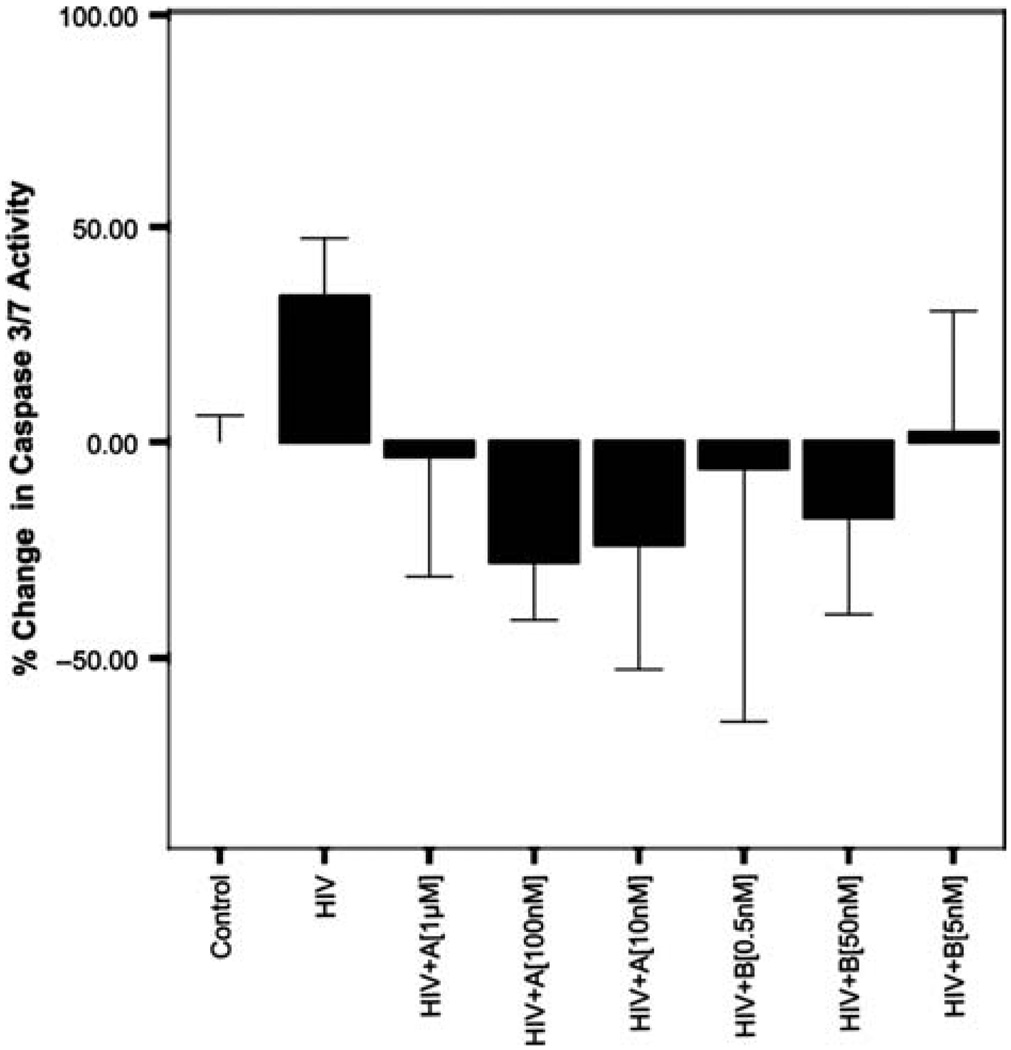
12-Hour HIV exposure and caspase 3,7
Exposure of human primary neurons to 20% conditioned HIV macrophage supernatants (equivalent to 500pg/ml) produced a 34% increase in caspase 3,7 activity and compared to controls (P = .022). Coexposure of primary human neurons with HIV and compound A at 1 µM, 100 nM, and 10nM produced 38%, 62%, and 58% decreases in caspase 3,7 activity, respectively, when compared to HIV alone (P < .01). Although all concentrations of A reduced caspase 3,7 activity below even that for controls, these effects were not significant. Coexposure of neurons with HIV and compound B at 50 nM produced a 52% decrease in caspase 3,7 activity when compared to HIV alone (P < .01). Although other concentrations of B decreased activity of caspase 3,7 from HIV alone, these effects did not reach significance. Figure 1 below illustrates these results.
Figure 1.
Twelve-hour caspase 3/7 activity of HIV (BaL) (500 pg/ml)–exposed human primary neurons in the presence and absence of GSK3β inhibitors A and B. Human primary neurons were incubated for 12 h in the presence and absence of HIV (BaL) at 500 pg/ml with and without A (1 µM,100 nM,10nM) and B (500 pM, 5 nM, and 50nM). Exposure to HIV (BaL) resulted in a significant increase in caspase 3/7 activity (P= .022). Coexposure of HIV with all concentrations of A significantly decreased caspase 3,7 activity from HIV alone (P <.01). Coexposure of compound B at 50 nM also produced significant decreases in caspase 3,7 activity (P < .01). Data represented are% change in caspase 3,7 activity from controls. *Significant at P < .05.
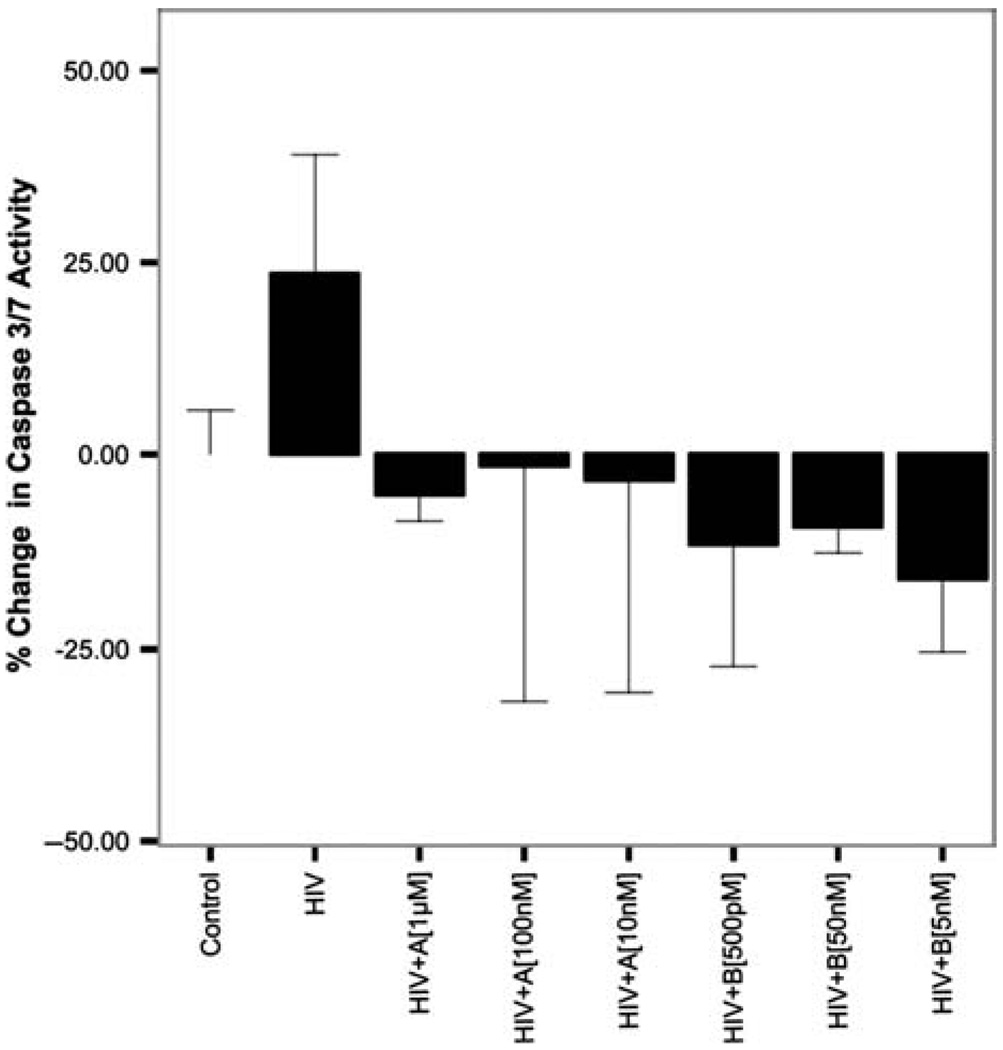
6-Day HIV exposure and caspase 3,7
Exposure of primary human neurons to 20% conditioned HIV macrophage supernatants (equivalent to 500 pg/ml) for a period of 6 days produced a 24% increase in caspase 3,7 activity compared to controls (P < .01). Coexposure of primary human neurons with HIV and compound A at 1 µM, 100 nM, and 10nM produced 29%, 25%, and 27% decreases in caspase 3,7 activity, respectively, when compared to HIV alone (P < .01). Coexposure of primary human neurons with HIV and compound B at 50 nM, 5 nM, and 500 pM produced 27%, 33%, and 39% decreases in caspase 3,7 activity, respectively, when compared to HIV alone (P < .001). Figure 2 below illustrates these results.
Figure 2.
Six-day caspase 3/7 activity of HIV (BaL) (500 pg/ml)–exposed human primary neurons in the presence and absence of GSK3β inhibitors A and B. Human primary neurons were incubated for 6 days in the presence and absence of HIV (BaL) at 500 pg/ml with and without A (1 µM,100 nM,10 nM) and B (500 pM, 5 nM and 50 nM). Exposure to HIV (BaL) resulted in a significant increase in caspase 3/7 activity (P < .01). Coexposure of HIV with all concentrations of A and B significantly decreased caspase 3,7 activity from HIV alone (P < .01). Data represented are% change in caspase 3,7 activity from controls. *Significant at P < .05.
Discussion
In this study we observed the ability of the specific GSK3β inhibitors AR-A014418 (1 µM, 500 nM, 50 nM) and B6B3O (50 nM, 5 nM, 500 pM) to significantly reduce HIV-induced caspase 3/7 activity back to control values at both 12 hours and 6 days of exposure. Whereas we noted that these two inhibitors were not associated with neurotoxicity at IC50 concentrations or above, we observed reduced HIV-mediated caspase activation, insignificantly different from control values, at both log10 order concentrations above and below IC50. This strongly implies that GSK3β inhibitors may be effective in continually and selectively mitigating proapoptotic HIV-induced caspase cascades (Gwenn et al, 2002). However, it should be noted that LDH levels remained significantly elevated in HIV at 12 hours without amelioration by GSK3β inhibitors. Furthermore, LDH levels were not significantly elevated among any exposure condition at 6 days. Although we acknowledge that elevated LDH levels at 12 hours could be an artifact from using macrophage supernatant, other acute HIV-mediated neurodegenerative processes may be at work. This implies that GSK3β inhibitors may be effective in selectively mitigating proapoptotic HIV-induced caspase cascades (Gwenn et al, 2002), but not other possible acute neurodegenerative factors caused by HIV such as oxidative stress (Kaul et al, 2001). Although caspase 3/7 and supernatant LDH levels did not correlate at 6 days, it has been shown that activation of caspases do not commit neuronal cells to immediate cell death that would be detected by the LDH assay but may induce a protracted or reversible form of apoptosis (Zhang et al, 2000).
Our findings indicate that acute and long-term HIV-mediated neurotoxicity via induction of proapoptotic cascades can be abrogated by inhibition of GSK3β. However, because LDH levels measure the end point of cell death, the discrepancy between caspase and LDH results suggest that further study is warranted on other distinct pathways of neurodegeneration. It is possible that other viral proteins such as gp120, tat, and nef, which are known to be neurotoxic (Bansal et al, 2000; Gurwell et al, 2001; Trillo-Pazos et al, 2000), may also contribute to the activation of caspase 3/7 that we observed in HIV (BaL) exposures as well as cytokines and proinflammatory markers released by microglia. Preventing HIV-mediated neurotoxicity and neurodegenerative effects have implications in alleviating symptoms of HIV within the brain and treatment of HAND. This is consistent with a growing body of in vitro and clinical literature demonstrating the neuroprotective effects of inhibiting the GSK3β pathway with therapeutic agents such as lithium and sodium valproate (Dou et al, 2003,2005; Everall et al, 2002; Letendre et al, 2006). AR-A014418 neuroprotection demonstrated in our study is consistent with a previous finding also demonstrating this effect in neuroblastoma N2A cells exposed to β-amyloid neurodegenerative effects (Bhat et al, 2003). Our utilization of specific GSK3β inhibitors to demonstrate neuroprotective effects against direct HIV-induced neurotoxicity contribute to the rational of developing therapeutic agents that target GSK3β activity (Cohen and Goedert, 2004; Dewhurst et al, 2007) and may provide a novel approach to preventing the development and progress of HAND in individuals stabilized on ARVs (Clifford, 2008; Nath et al, 2008).
Materials and methods
Establishment of primary human neuronal cultures
Human primary neuronal cultures were prepared as previously described from human fetal brain tissue, 14 to 18 weeks’ gestation, collected from consenting patients undergoing terminations without any clinical identifiers (Advanced Bioscience Resources, CA, USA) (Trillo-Pazos et al, 2004). Cultures were maintained for a period of 30 days prior to experimentation. This time period corresponds to the beginning of N-methyl-d-asparate (NMDA) receptor expression in these cultures and as such is representative of a functionally mature phenotype (Mattson et al, 1991).
12-Hour HIV exposure
Human primary neurons were incubated in the presence and absence of 20% conditioned HIV (BaL) macrophage supernatants (equivalent to 500 pg/ml) with and without GSK3β inhibitors AR-A014418 (A) (1 µM, 100nM, 10 nM) and B6B3O (B) (50 nM, 5 nM, 500 pM) (both obtained from Sigma Aldrich, USA). IC50 values have been previously determined to be 100nM for A and 5 nM for B from dose-response curves of inhibitor and substrate affinity binding by nuclear magnetic resonance spectroscopy (Meijer et al, 2004). Concentrations used were shown to be nontoxic to neuronal cultures in a prior 6-day exposure period measured using a LDH cytotoxicity assay (CytoTox96, Promega, USA), which measures extracellular leakage of this enzyme when the cell membrane is compromised. HIV (BaL) is a macrophage-, monocyte-, and CD4+ T cell–tropic HIV-1 strain obtained from the NIH AIDS Research and Reference Reagent Program (catalogue number 510). After 12 h, cells were scraped and lysed using the the caspase-glo 3/7 luminscence assay (Promega) and levels of caspases 3 and 7 were measured as an early indicator of neurodegenerative changes related to apoptosis. Results were expressed as arbitrary relative luminescence units and normalized into percent changes of caspase luminescence from controls. LDH levels from cellular supernatant were also measured after 12 h and normalized into percent change of LDH released from controls.
6-Day HIV exposure
Exposure of human primary neurons to HIV with and without A and B were repeated as above. Media, including HIV and A and B, were changed once on day 3. Caspase 3/7 and LDH measurements were repeated after 6 days.
Statistical Analysis
An one-way analysis of variance (ANOVA) was performed on the data to compare caspase 3/7 activation and LDH levels between control and treatment groups using a Tukey-HSD (highly significant difference) post hoc analysis for multiple comparisons.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Bhat R, Xue Y, Berg S, Hellberg S, Ormo M, Nilsson Y, Radesater AC, Jerning E, Markgren PO, Borgegard T, Nylof M, Gimenez-Cassina A, Hernandez F, Lucas JJ, Diaz-Nido J, Avila J. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278:45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16:94–98. [PubMed] [Google Scholar]
- Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Dewhurst S, Maggirwar SB, Schifitto G, Gendelman HE, Gelbard HA. Glycogen synthase kinase 3 beta (GSK-3 beta) as a therapeutic target in neuroAIDS. J Neuroimmune Pharmacol. 2007;2:93–96. doi: 10.1007/s11481-006-9051-1. [DOI] [PubMed] [Google Scholar]
- Dou H, Birusingh K, Faraci J, Gorantla S, Poluektova LY, Maggirwar SB, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis. J Neurosci. 2003;23:9162–9170. doi: 10.1523/JNEUROSCI.23-27-09162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Ellison B, Bradley J, Kasiyanov A, Poluektova LY, Xiong H, Maggirwar S, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective mechanisms of lithium in murine human immunodeficiency virus-1 encephalitis. J Neurosci. 2005;25:8375–8385. doi: 10.1523/JNEUROSCI.2164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21(3):493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal number and volume alterations in the neocortex of HIV infected individuals. J Neurol Neurosurg Psychiatry. 1993;56:481–486. doi: 10.1136/jnnp.56.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Trillo-Pazos G, Bell C, Mallory M, Sanders V, Masliah E. Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J Neuropathol Exp Neurol. 2001;60:293–301. doi: 10.1093/jnen/60.3.293. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102(3):555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden G, Budd S, Tsai E, Hanson L, Kaul M, Emilia D, Friedlander R, Yuan J, Masliah E, Lipton S. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Langford D, Everall IP, Mallory M, Everson A, Digicaylioglu M, Masliah E. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3beta pathway. Implications for neuroprotection. J Biol Chem. 2001;277(36):32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden G, Lipson SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, Durelle J, Grant I, Everall I. Lithium improves HIV-associated neurocognitive impairment. AIDS. 2006;20:1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for Human Immunodeficiency Virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wang H, Michaelis EK. Developmental expression, compartmentalization, and possible role in excitotoxicity of a putative NMDA receptor protein in cultured hippocampal neurons. Brain Res. 1991;565:94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V. Wnts and TGF beta in synaptogenesis: old friends signalling at new places. Nat Rev Neurosci. 2003;4:113–120. doi: 10.1038/nrn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Peterson DR, Zhong J, Ni H, Cruttenden K, Gaugh M, Gendelman HE, Boska M, Gelbard H. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology. 2006;66:919–921. doi: 10.1212/01.wnl.0000204294.28189.03. [DOI] [PubMed] [Google Scholar]
- Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, Gelbard HA. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci. 2001;13:1913–1922. doi: 10.1046/j.0953-816x.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, Kandanearatchi A, Eyeson J, King D, Vyakarnam A, Everall I. Infection of stationary human brain aggregates with HIV-1 sf162 and IIIB results in transient neuronal damage and neurotoxicity. Neuropathol Appl Neurobiol. 2004;30:136–147. doi: 10.1046/j.0305-1846.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP. Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res. 2000;864:315–326. doi: 10.1016/s0006-8993(00)02213-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goodyer C, LeBlanc A. Selective and protracted apoptosis in human primary neurons microinjected with active caspase −3, −6, −7, and −8. J Neurosci. 2000;20:185–200. doi: 10.1523/JNEUROSCI.20-22-08384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]