Abstract
The relationship between advanced age and immunologic deficits is becoming an area of rapidly advancing research. Many of the clinical hurdles in the elderly population result from dysregulation of the immune system leading to the inability of the elderly to swiftly combat infection and to the increased incidence of chronic disease states and autoimmune conditions. Herein, we address the crucial alterations in the innate immune system that occur with advancing age. Specifically, we discuss how the effects of advanced age may lead to functional changes in the neutrophil, macrophage, dendritic cell, natural killer cell, and natural killer T cell populations in human and murine models that translate into aberrant innate immune responses. Furthermore, we elucidate how these changes may contribute to documented deficits in adaptive immunity as well as the pathological conditions and the increased morbidity and mortality seen in the elderly population.
Keywords: Aging, innate immunity, neutrophils, macrophages, dendritic cells, natural killer cells
INTRODUCTION
Advanced age leads to numerous changes in the immune system which results in refractory responses to vaccination and a significant decline in protective immunity. For example, the yearly influenza vaccine is only 40–60% efficacious in older subjects (e.g. ≥ 65 years old) [1]. Hence, the elderly are more susceptible to viral and bacterial infections, opportunistic infections, reactivation of latent viruses, autoimmune diseases and neoplasia, resulting in increased morbidity and mortality [2]. This dysregulation of the immune system with age is referred to as immunosenescence. The development of pulmonary complications, such as pneumonia and acute respiratory distress syndrome, are among the most serious threats to the elderly population. Even in the absence of an immune challenge, healthy, aged individuals have a significantly higher basal inflammatory state where the circulating levels of cytokines including IL-6, IL-1β and tumor necrosis factor-α (TNF-α) are elevated. This progressive proinflammatory status, termed ‘inflamm-aging’ [3], renders the older subjects more susceptible to a poor prognosis following systemic insults including burn trauma [4]. Although it is well documented that both B and T lymphocyte compartments of the adaptive immune system deteriorate with advancing age, the impact of aging on many aspects of the innate immune response remains to be elucidated. Innate immunity is mediated by various cell types functioning through different mechanisms involving cytokines and chemokines, type I interferons, and soluble factors. Cells of the innate immune system include neutrophils, monocytes/macrophages, dendritic cells, natural killer (NK) and natural killer T (NKT) cells, eosinophils and basophils. In this review, we provide an up-to-date summary on the current literature on the effects of aging on innate immunity, with a particular focus on neutrophils, macrophages, DCs, NK and NKT cells.
NEUTROPHIL
A rapid initiation of the inflammatory response is needed to restrict the spread of pathogens at the preliminary stages of infection. Neutrophils play an essential role in the innate immune system as the first line of defense against invading pathogens. Rapid recruitment and activation of neutrophils is mediated through the integration of multiple, successive signaling steps involving selectins, integrins, chemokines and G protein coupled receptors [5, 6]. Upon their recruitment, neutrophilic polymorphonuclear leukocytes (PMNs) clear the site of infection from invading microbes and debris by phagocytosis and intracellular killing mechanisms involving the generation of reactive oxygen species (ROS) and the release of a diverse array of mediators [7]. Granules found in neutrophil contain multiple antimicrobial molecules and proteases, such as elastase, cathepsins, matrix metallopeptidase-9 (MMP-9) and human neutrophil peptide-1 [8]. Neutrophil activation can be brought about by pathogen-associated molecular patterns (PAMPs) binding to their respective pattern recognition receptors (PRRs). Examples of PAMPs include formyl-Methionyl-Leucyl-Phenylalanine lipopolysaccharide (fMLP), lipopolysaccharide (LPS) and other toll-like receptor (TLR) and triggering receptor expressed on myeloid cells-1 (TREM-1) ligands. Cytokines, including granulocyte macrophage-colony stimulating factor (GM-CSF), IL-15 and IL-18, can also prime neutrophils resulting in delayed or decreased apoptosis which subsequently lead to enhanced neutrophil effector functions [9–11]. Activated PMNs, in turn, release cytokines and chemokines locally, including TNF-α, IP-10, macrophage inflammatory protein-1α (MIP-1α), B-Lymphocyte stimulator (BLyS), IL-12, IL-8, vascular endothelial growth factor (VEGF) among others and therefore profoundly impact the elicitation of the subsequent immune response [12].
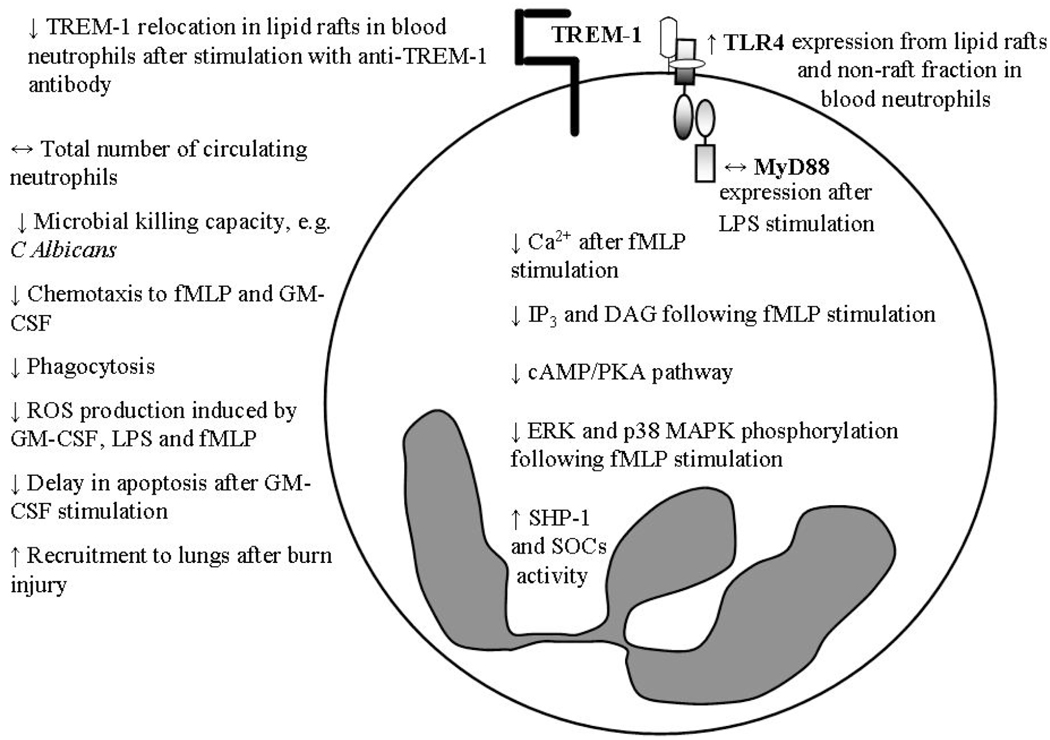
Although there is a general consensus that aging does not impact the total number of circulating neutrophils [13], hematopoietic stem cells from young mice proliferating and differentiating into PMN in an aged microenvironment exhibited decreased effector functions [14]. These results suggest that microenvironmental factors associated with advanced age in mice may be responsible for decreased neutrophil function under certain conditions, for example, impaired killing of C. albicans [15], but not in others such as S. pneumoniae [16]. Neutrophils have a short life span of 16–24 hours after which they undergo apoptosis and are subsequently removed via phagocytosis by macrophages [17]. Analysis of age-related changes in humans have shown that most aspects of neutrophil function in the elderly are decreased, including chemotaxis, phagocytosis of microbes and generation of ROS in response to stimulation by GM-CSF, LPS, fMLP or opsonized bacteria (Fig. 1) [12, 18]. The ability of GM-CSF to prime and to activate respiratory burst, as well as delay apoptosis, is impaired in the elderly. Increased activity of SHP-1, an inhibitor of Src family of tyrosine kinases, and suppressors of cytokine signalling (SOCS) have been attributed to the age-related failure of GM-CSF to induce neutrophil functions via inhibition of Lyn, Phosphoinositide-3 kinase/Akt (PI3K/Akt), extracellular signal-regulated protein kinase (ERK) and Signal Transducers and Activator of Transcription (STAT) signaling pathways [19]. Similarly, reduced phosphorylation of ERK and p38 mitogen-activated protein kinase (MAPK) following stimulation with fMLP is also observed in healthy, aged subjects [20]. A significant decrease in intracellular Ca2+ was also observed in the elderly following fMLP stimulation. This may account for the reduced phagocytic ability and impaired bactericidal activity. Age affected the phospholipase C-protein kinase C (PLC-PKC) pathway since generation of inositol triphosphate (IP3) and diacyl glycerol (DAG) and activation of cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) were decreased in neutrophils from aged humans leading to changes in ROS and inositol 1,4,5-triphosphate production (InsP3) [21, 22]. Contrary to human studies, neutrophils from aged mice did not exhibit any functional deficiency including exocytosis, chemotaxis, respiratory burst and phagocytosis compared to its young counterparts [15].
Fig. (1).
Neutrophil defects with advanced age. Arrows (↑, ↓ or ↔) denote increased, decreased or unaltered levels in the aged compared to the young. Abbreviations: fMLP, N-formyl-methionyl-leucyl-phenylalanine; GM-CSF, granulocyte monocyte colony stimulating factor; LPS, lipopolysaccharide; TREM-1, triggering receptor expressed on myeloid cell-1, TLR4, Toll-like receptor 4; MyD88, Myeloid differentiation primary response gene (88); IP3, inositol 1,4,5-triphosphate; DAG, diacyl glycerol; cAMP/PKA, cyclic adenosine monophosphate/protein kinase A; ERK, extracellular signal-regulated protein kinase; MAPK, mitogen activated protein kinase; SHP-1, inhibitor of Src family of tyrosine kinases, SOCs, suppressors of cytokine signaling.
Components of the TLR signaling pathway have also been studied in neutrophils isolated from aged subjects. Basal levels of TLR2 and TLR4 expression on neutrophils are unaltered by age [20]. However, TLR4 expression in PMN from unstimulated lipid rafts and non-raft fractions from aged subjects was elevated compared to young. LPS stimulation did not affect recruitment or redistribution of TLRs between lipid raft and non-raft fractions in the aged which was a marked contrast to the significant increase in TLR recruitment in young mice [20]. Expression of MyD88 on PMN from aged mice after LPS stimulation were comparable to the young; although, the quantity of MyD88 in the membrane of PMN of aged subjects was significantly decreased after stimulation [20] which may impact cell signaling via the TLR4-MyD88-dependent pathway. TREM-1-induced neutrophil functions, including ROS production, degranulation, phagocytosis and production of proinflammatory cytokines and chemokines were impaired with aging. These changes were reflected by alterations in signal transduction that were possibly caused by the failure of TREM-1 to relocate in the lipid rafts upon stimulation in aged subjects [23]. A significant level of soluble TREM-1 in the circulation is an indicator of aberrant inflammatory conditions; and poor outcomes for critically ill patients with sepsis are associated with a sustained presence of soluble TREM-1 in the plasma [24].
Extravasation of PMNs from the circulation was shown to be unaltered by aging, i.e., adhesion to endothelial cells, expression of adhesion molecules and recruitment of PMN are comparable between young and aged donors [25, 26]. However, one piece of contrasting evidence from Damtew et al. 1990 suggested that there is increased adhesion of PMNs from elderly donors to an endothelial cell monolayer following fMLP or phorbol 12-myristate 13-acetate (PMA) stimulation [27]. Chemotaxis of purified peripheral blood neutrophils isolated from healthy, aged subjects conforming to the SENIEUR protocol towards fMLP and GM-CSF were significantly decreased [20]. On the contrary, in vivo studies involving a dermal excisional injury demonstrated comparable neutrophil myeloperoxidase (MPO) activity correlating to similar number of neutrophils in the wound bed and wound homogenates from young and aged mice [28].
Recent work from our group showed that LPS-induced pulmonary inflammation in aged mice was significantly increased compared to its younger counterparts and this was associated with elevated levels of neutrophil chemokines, macrophage inflammatory protein-2 (MIP-2) and KC, along with increased production of IL-1β in lungs of aged mice [29]. Similar results were obtained from further research in our group from aged mice after receiving a systemic insult of 15% TBSA dorsal scald injury. Exacerbated pulmonary inflammation exhibited by the aged mice correlated with increased neutrophil infiltration in the lungs 24 hours after receiving burn trauma. This response was associated with elevated production of KC, but not MIP-2 or IL-1β [30]. Adverse effects of overproduction of cytokines with advanced age have also been reported where elevated levels of IL-17 and IL-6 paired with increased neutrophil activation were associated with mortality in aged mice following systemic viral infection [31]. IL-17 neutralization or neutrophil depletion during viral infection reduced liver necrosis and prevented lethality in aged mice. The aforementioned studies suggest that the cytokine/chemokine milieu during inflammation and the extent of cellular infiltration are dependent on the nature of the trauma, i.e., a local insult may lead to a sequence of controlled, inflammatory events while a systemic insult such as burn trauma or viral infection may be necessary to reveal the intrinsic defects in aging cells which are otherwise masked by the microenvironment. The reported observations herein suggest that fine tuning the neutrophil effector functions through targeting specific chemokines and/or their respective receptors may successfully transform the outcome of inflammation in the elderly.
MACROPHAGES
Macrophages play an indispensable role in innate immunity acting as sentinels in tissues, fighting invading pathogens, as well as orchestrating the attraction and development of the more complex and specific, acquired immune response. Blood monocytes differentiate into macrophages under the influence of cytokines as they enter tissues. Depending on the tissue type, macrophages occupy strategic positions as bone osteoclasts, liver Kupffer cells, brain microglia, and resident cells in the peritoneum, etc. Macrophages play a dual role in host defense initially phagocytosing foreign pathogens including bacteria, protozoa, fungi, parasites and apoptotic cells and subsequently destroying them through oxygen-dependent and oxygen-independent pathways. These phagocytes have the ability to recognize danger signals through receptors capable of inducing specialized activation programs in addition to promoting cell-mediated immunity by antigen presentation to CD4+ T cells. Collaboration of macrophages with T and B cells is further mediated by the release of cytokines, chemokines, enzymes, arachidonic acid metabolites and reactive radicals [32, 33].
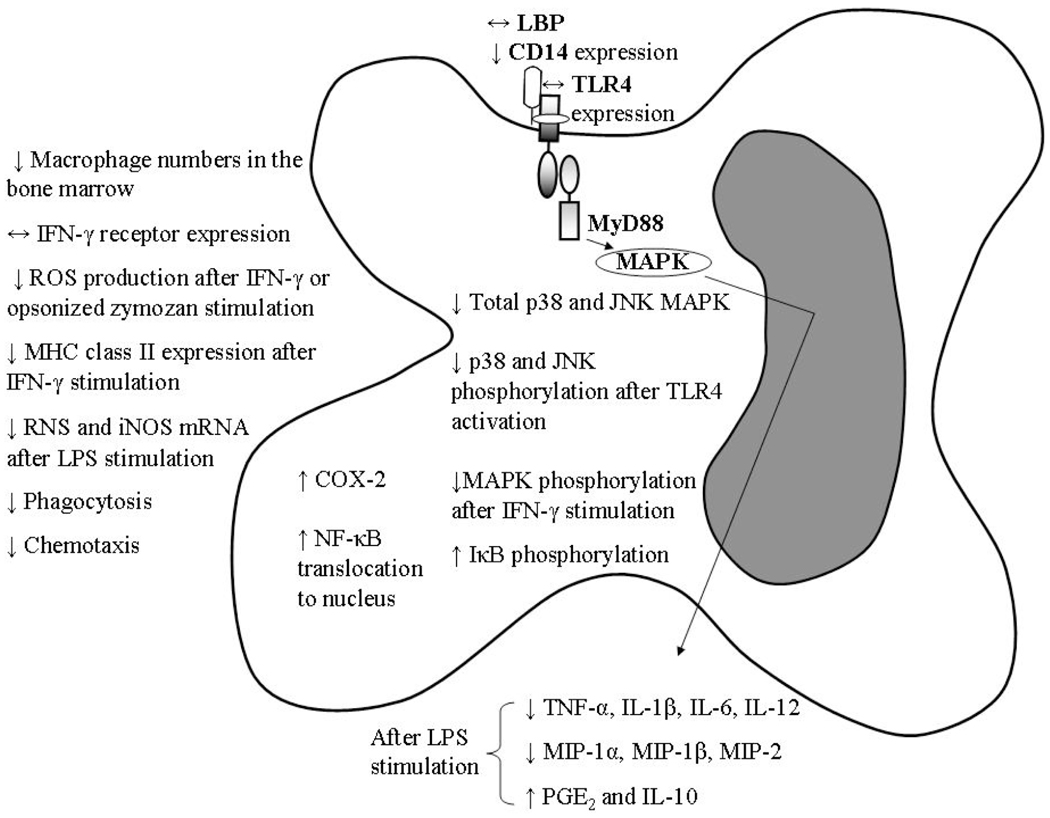
The effect of aging on macrophages appears to be multifaceted, affecting almost every aspect of their normal cellular function (Fig. 2). Myeloid precursors differentiate into macrophages in the presence of growth factors such as macrophage-colony stimulating factor (M-CSF), GM-CSF and IL-3. Activation of macrophages requires interaction with interferon-γ (IFN-γ), a pro-inflammatory cytokine released by activated T cells and NK cells or by the bacterial cell membrane component, LPS. IFN-γ inhibits M-CSF-dependent proliferation and protects macrophages from apoptosis induced by LPS or glucocorticoids [34]. IFN-γ also regulates major histocompatibility complex (MHC) class II gene expression at several levels which are crucial for antigen presentation for T cells [35]. IFN-γ activation of macrophages from aged animals is impaired. Although there was no difference in IFN-γ receptor expression levels in macrophages from aged mice [36], studies have shown a 75% decrease in the capacity of macrophages from aged rats to produce superoxide anion after incubation with IFN-γ or opsonized zymosan [37]. Further investigation by Ding et al. 1994 demonstrated that reduced MAPK phosphorylation after IFN-γ stimulation is responsible for the decreased superoxide production in peritoneal macrophages from aged mice [38]. One study reported reduced STAT-1α phosphorylation in peritoneal macrophages from aged mice compared to their young counterparts [36]. The levels of MHC class II molecules expressed at the cell surface and the intracellular IAβ mRNA and protein levels following IFN-γ stimulation are lower in macrophages from aged mice compared to young [39]. Moreover, LPS-stimulated ROS and nitric oxide (NO) production are significantly reduced in alveolar macrophages from aged rats [40, 41]. Similarly, studies using aged mice showed diminished production of reactive nitrogen species (RNS) and decreased expression of inducible nitric oxide synthase (iNOS) mRNA from splenic and peritoneal macrophages [40, 42]. On the contrary, a recent study identified elevated levels of intracellular ROS and increased susceptibility to oxidative stress as a cause of telomere loss in bone-marrow derived macrophages from aged mice [43]. Moreover, the authors observed that telomere shortening in these macrophages from aged mice is caused by defective GM-CSF- and not M-CSF- dependent proliferation which correlates to reduced STAT5a phosphorylation. Other effector functions, such as chemotactic activity of macrophages, also decreases with advanced age as do their production of chemokines including MIP-1α, MIP-1β and MIP-2. In addition, phagocytosis and clearance of infectious organisms by these innate cells are compromised with advanced age [28,44].
Fig. (2).
Macrophage defects with advanced a ge. Arrows (↑, ↓ or ↔) denote increased, decreased or unaltered levels in the aged compared to the young. Abbreviations: IFN-γ, interferon-gamma; ROS, reactive oxygen species; MHC, major histocompatibility complex; RNS, reactive nitrogen species; iNOS, inducible nitric oxide synthase; LBP, LPS-binding protein; JNK, janus kinase; MAPK, mitogen activated protein kinase; PGE2, prostaglandin E2; I-κB, inhibitor of κB; NF-κB, nuclear factor-kappaB; COX, cyclooxygenase; TNF-α, tumor necrosis factor-alpha; MIP, macrophage inflammatory protein; IL, interleukin.
There is a degree of ambiguity surrounding the age-dependent effects on the generation of macrophages from their monocyte precursor. The number of blood monocytes in the aged and young subjects appears to be comparable; however, there is a significant decrease in macrophage precursors as well as macrophages in the bone marrow of the elderly [45, 46]. In contrast, the macrophage population is enhanced in bone marrow of aged mice as demonstrated by an increase in Mac-1/ CD11b-positive cells [47]. However, it remains unclear whether there is a reduced frequency of macrophage precursors with age in mice. Herrero et al. (2001) established that the number, size, DNA content and cell surface markers expressed during macrophage maturation were similar in macrophages from both young and aged mice. In humans, the percentage of cells expressing CD68, a macrophage marker, is decreased in the bone marrow of adults compared to children [46].
Macrophages play a critical role not only in the initial phases of the inflammatory response during wound healing but also in the regeneration phase, by secreting angiogenic and fibrogenic growth factors that repair damaged tissue. Physical changes observed during the wound healing process which were defective in elderly subjects as well as rodents include enhanced platelet aggregation, delayed re-epithelialization and collagen deposition, turnover and remodeling, delayed healing strength, decreased wound strength and delayed infiltration and function of macrophages. Delayed collagen synthesis and angiogenesis during tissue repair were attributed to a decrease in the secretion of VEGF by macrophages in aged mice [48]. The aforementioned results indicate that a few stages in the wound healing process are markedly altered with advanced age which opens up avenues for therapeutic intervention in the elderly at multiple target points.
The TLR4 signaling pathway is involved in the induction of several pro-inflammatory cytokines including TNF-α and IL-6. There is reduced responsiveness of macrophages from aged mice to the classical TLR4 ligand, LPS, as exemplified by decreased production of TNF-α, IL-6 and IL-1α after stimulation [49]. However, there are no age-related differences in TLR4 expression in macrophages at the protein level [49] although others show contradictory results showing decreased TLR4 and other TLR mRNA from the spleen and peritoneal cavity [50] in a different mouse strain. Renshaw et al. 2002 resorted to isolation of splenic macrophages through adherence of cells to tissue culture plastic. This method of macrophage separation yields a mixture of cells with macrophages along with DCs and B cells, as all these cell types adhere to tissue culture plastic [51]. Hence, the data demonstrating decreased TLR mRNA levels in splenic macrophages may not be a predominantly macrophage population.
Furthermore, we found that defective pro-inflammatory cytokine production upon LPS stimulation is due to signaling defects downstream of ligand-receptor interaction, i.e. decreased levels of total MAPK, p38 and janus kinase (JNK), and reduced phosphorylation of these kinases after TLR4 activation [49, 52]. Advanced age does not modulate the basal levels of LPS-binding protein (LBP) [53]. Research also shows that aging affects CD14 levels, a co-receptor for TLR4 signaling, in macrophages from aged mice by a significant decrease in its overall expression [54]. Early studies assessing the effects of aging on cytokine production by human monocytes upon LPS stimulation were contradictory, with some studies showing increased cytokine production and others showing unchanged or decreased production [55–59]. This may be due to the relatively small number of participants in each study, as well as differences in experimental methods including cell isolation techniques. Another restriction could be due to the fact that some studies have limited enrollment as they followed the SENIEUR protocol [60]. A recent study evaluated the effect of various TLR ligands on peripheral blood mononuclear cells (PBMCs) isolated from 159 young and aged individuals and found an age-associated reduction in TNF-α and IL-6 after stimulation with agonists of TLR1/2 correlating with a decrease in surface expression of TLR1, but not TLR2, on monocytes harvested from the aged. An age-associated decrease in TLR4 surface expression and single stranded RNA-induced (TLR7 ligand) IL-6 production was also confirmed in this study [61]. Recent research provided similar evidence for age-associated defects in TLR function in human macrophages in the context of infection with West Nile virus [62]. The influence of aging on LPS-mediated cytokine production selectively impact macrophage responses, such that some functions are depressed while others are elevated. For example, macrophages from aged mice have increased levels of cyclooxygenase (COX)-2, prostaglandin (PG) E2 and IL-10 after LPS stimulation [63–65], whereas TNF-α, IL-6 and IL-1 and IL-12 production are decreased as mentioned above. Defective TLR4 signaling associated with decreased pro-inflammatory cytokine production may significantly contribute to increased susceptibility to infection and elicit a poor adaptive immune response in the elderly.
The cytokine and chemokine milieu during inflammation affects the polarization of macrophages toward an M1, pro-inflammatory, or an M2, anti-inflammatory, phenotype. Aging impacts macrophage polarization as the cytokine microenvironment in aged subjects promotes the M2 phenotype [66]. The decreased production of pro-inflammatory cytokines and increased production of anti-inflammatory cytokines, together with a decrease in MHC class II molecules result in a state of immunosuppression which significantly affects T cell function [67]. A good example of impaired macrophage cytokine production affecting protective immunity in aged subjects has recently been reported by Agius and colleagues, where cutaneous delayed-type hypersensitivity responses to bacterial, fungal or viral antigens by CD4+ memory T cells is significantly decreased in older subjects [68]. The authors linked the defective immunosurveillance by T cells to reduced production of TNF-α by cutaneous macrophages, resulting in decreased endothelial activation and a subsequent decrease in T cell recruitment to the aging skin. Skewing of macrophage polarization in advanced age towards the alternatively activated phenotype may result in insufficient elicitation of protective immunity which may be responsible for the poor prognosis following infections and malignancy.
Other cellular signaling pathways involved in eliciting immune responses have also been shown to be defective in aging. Examples of this include increased phosphorylation of inhibitor of κB (IκB) leading to elevated translocation of nuclear factor-κB (NF-κB) to the nucleus in inflammatory peritoneal macrophages from aged mice [69]. Microarray analysis using RNA from resting and LPS-stimulated macrophages from aged and young mice revealed that genes encoding pro-inflammatory cytokines, chemokines and their receptors were down-regulated in aged mice [70]. Among these were genes in the TLR pathway leading to NF-κB activation, MyD88 and tumor necrosis factor receptor-associated factor 6 (TRAF6). Similarly, Sunil et al. have shown decreased activation of members of the NF-κB family in cells from aged rats [71]. These observations clearly indicate that there are several age-related defects in macrophage function and future research targeting the key molecules of these signaling pathways may provide therapeutic intervention that can lead to restoration of basal, macrophage effector responses.
DENDRITIC CELLS
The dendritic cell (DC) acts as an important bridge between innate and adaptive immunity by sequestration and presentation of antigen to the adaptive immune system and playing a critical role in the mediation of T cell specific self tolerance. DCs are unique in their ability to phagocytose antigen, migrate to secondary lymphoid organs, differentiate into a mature phenotype and activate naïve T cells [72, 73]. Several unique features of DCs are altered in elderly human and older animals, which may contribute to the immunosenescent cellular response, chronic inflammation and autoimmune pathology (Fig. 3) [74].
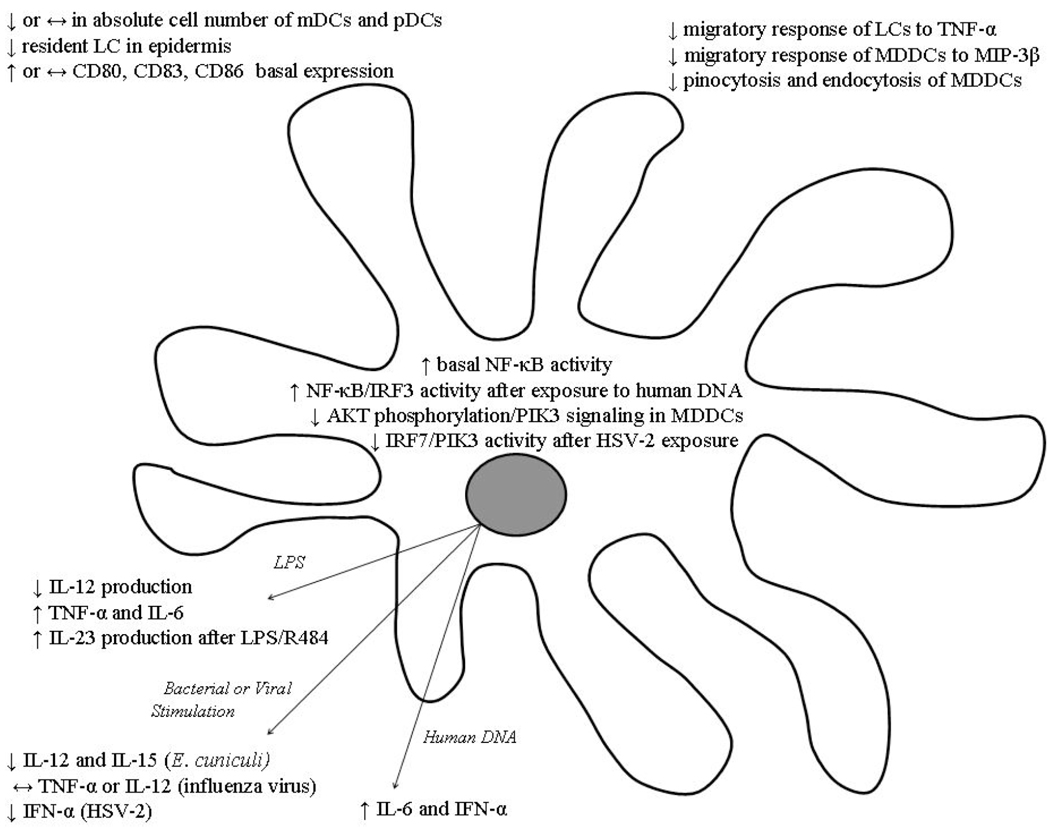
Fig. (3).
Dendritic cell defects with advanced age. Arrows (↑, ↓ or ↔) denote increased, decreased or unaltered levels in the aged compared to the young. Abbreviations: mDC, myleiod dendritic cell; MDDC, monocyte-derived dendritic cell; pDC, plasmacytoid dendritic cell; LC, Langerhan’s cell; LPS, lipopolysaccharide; PI3K, Phosphoinositide-3 kinase; AKT, serine/threonine kinase; MIP, macrophage inflammatory protein; TNF-α, tumor necrosis factor-alpha; IL, interleukin; ssRNA, single-stranded ribonucleic acid; IFN-γ, interferon-gamma; IRF, interferon regulation factor; HSV, herpes simplex virus.
Dendritic cells can best be considered in the context of two broad subpopulations: myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC). Plasmacytoid DCs, which do not express the typical DC surface marker CD11c and resemble plasma B cells in circulation, have been shown to play a role in autoimmunity and are the major subset of DCs responsible for IFN-α generation in response to infectious challenge [73, 75–78]. On the other hand, mDCs, a population that can be further subdivided to include Langerhans cells (LC), interstitial dendritic cells and monocyte-derived dendritic cells (MDDC), have been shown to have a major role in IL-12 secretion and induction of the Th1 immune response [72, 73, 79]. Advanced age alters the relative population balance of these two DC subsets. In one report, the absolute number of mDCs and their CD34+ precursors in human peripheral blood decline with age while the pDC subpopulation is maintained [79]. However, another study found that there is a decline in the pDC population with no change in the mDC subset [75]. While these divergent findings need to be reconciled, functional and phenotypic changes in mDCs from aged subjects have been described. Myeloid DCs isolated directly from aged human peripheral blood have shown an increased basal expression of the co-stimulatory molecule CD86 and marker of DC activation CD83 [79, 80]. However, when PBMCs from young and aged subjects are induced with IL-4 and GM-CSF to differentiate into MDDCs in vitro, studies have shown no difference in baseline expression of DC surface markers [80,81]. Additionally, following stimulation with either LPS or inactivated influenza viral components, there was no difference in expression of these markers between MDDCs differentiated in vitro from young and aged subjects [81]. These findings suggest that in vitro, DCs are capable of undergoing appropriate lineage specific differentiation and highlights the importance of in vivo modeling to better understand how the aging microenvironment may contribute to a skewed cellular phenotype.
Functional changes associated with advanced age have also been found in the MDDC subset [80, 82]. MDDCs from healthy elderly human donors have deficient pinocytic and endocytic capabilities in response to antigen and a compromised migratory response to macrophage inflammatory protein-3β (MIP-3β) and stromal cell-derived factor-1 (SDF-1) [80]. The cause of these phagocytic and migratory defects has been attributed to abnormalities in the PI3K, a critical feed-forward mediator of both phagocytosis and migration. The PI3K pathway was shown to be compromised by a decrease in the phosphorylation of AKT, a serine/threonine kinase, suggesting a possible mechanism for disruption in MDDC function [80]. AKT also acts as an inhibitory mediator of downstream NF-κB activation, an important moderator of cellular homeostatic and immune responses that is elevated in DCs from aged subjects [82]. Disruption of this pathway not only appears to contribute to the functional defects seen in the MDDC subset but also suggests a possible role of DCs in the heightened NF-κB signaling tied to aging and cellular immunosenescence [83]. These signaling alterations may be the root of aberrant cytokine production observed in aging studies. Monocyte derived DCs differentiated from PBMCs in vitro from aged subjects have been shown to generate higher levels of TNF-α and IL-6 in response to LPS stimulation [80], which may contribute to the increased systemic levels of pro-inflammatory cytokines associated with advance aged [84, 85]. Interestingly, mDCs obtained from elderly humans cultured in vitro with LPS produced less IL-12 than mDCs from young subjects, while IL-10 production by mDCs from aged subjects was comparable to their younger counterparts [79]. In contrast, there was no difference in the production of pro-inflammatory cytokines by MDDCs differentiated in vitro in the presence of inactivated influenza virus [81], implicating that specific TLR signaling pathways may be differentially affected in DCs with advanced age and, as previously mentioned, emphasizing the importance of the aged environmental milieu in these studies.
The effect of aging on LCs, a tissue specific subset of mDCs, is characterized by an overall decline in LC density in epidermal and epithelial sites [74, 86, 87] in addition to functional defects. Studies in humans confirm a decreased density of resident LC expressing CD1a, a characteristic membrane marker, in the skin of elderly individuals as compared to young [87]. Furthermore, the authors also demonstrate a deficient migratory response of these LCs to TNF-α with advanced age. In murine models, TNF-α has been shown to play an important role in mobilization and migration of LCs to lymph nodes [87–89] and systemic administration of TNF-α antibody has been shown to inhibit this migratory response [90]. Interestingly, TNF-α and other pro-inflammatory cytokines have been shown to be systemically elevated in aging [84, 85]. This enhanced expression of TNF-α with advanced age may desensitize aged LCs to the chemotatic effects of TNF-α by downregulating cell surface receptors and overall signal transduction. Moreover, the impaired migratory ability of LCs to regional lymph nodes might hinder antigen presentation and ability to effectively combat dermal infection.
As DCs are important in both the initiation and regulation of subsequent adaptive immune responses, the emergence of the autoimmune-linked Th17 subset of T lymphocytes has lead to the implication of DC dysregulation as a contributor to autoimmune pathology [91]. Along with macrophages, DCs are the principal cell type that produce IL-23, a cytokine critical to the maturation and maintenance of Th17 cells. In elderly humans, bone marrow derived DCs have been shown to have increased mRNA expression of p19, a subunit of IL-23, in addition to increased protein production of IL-23 following stimulation with LPS and R484, a TLR7/8 agonist [91]. These changes are associated with age-specific epigenetic modifications in histone methylation that promote preferential binding of the NF-κB transcription factor c-Rel, resulting in upregulation of IL-23 production from aged DCs. In concert with these data, basal elevation of NF-κB activity in DCs from aged humans has also been described [82]. Furthermore, exposure of DCs from aged subjects to human DNA in vitro resulted in heightened activity of interferon regulatory factor 3 (IRF3) and NF-κB, providing further insight into the age-associated changes in DC signaling pathways, which can contribute to an autoimmune phenotype at a cellular level [82]. Additionally, DCs from aged subjects also demonstrated an elevated expression of co-stimulatory molecules, increased IL-6 secretion and IFN-α production [82]. These findings supply further evidence for the role of the innate immune system, and specifically that of DCs, as a mediator of autoimmune pathology and chronic inflammatory conditions.
Heightened sensitivity of DCs to self antigen may prime the body to mount an attack against endogenous tissue [77, 78], while additional functional defects in pDCs may compromise the ability of aged individual to effectively fight infection [75, 76]. Although pDCs are generally associated with high levels of IFN-α generation in response to infectious challenge, when aged pDCs were exposed to herpes simplex virus (HSV) infection, they produced decreased levels of IFN-α that can be associated with defective initiation of IFN pathway signaling via TLR9 [75, 76]. Specifically, these signaling defects were attributed to inadequate upregulation of interferon regulation factor 7 (IRF7) and deficits in the PI3K signaling pathway [76]. Activation of TLR9 also increased oxidative stress in pDCs from aged mice compared to young mice with reduction of this stress allowing partial recovery of IFN-α production [76]. Additionally, DCs isolated from the mesenteric lymph nodes (MLN) of aged mice demonstrate an impaired ability to generate a sufficient T cell response after exposure to Encephalitozoon cuniculi [92]. After antigenic exposure, MLN DCs of aged mice exhibited impaired IL-12 and IL-15 generation and poorly induced proliferation of young T cells in vitro. Furthermore, adoptive transfer of DCs isolated from the MLN of aged mice showed impaired cytolytic activity in vivo. Interestingly, administration of IL-15 restored the cytolytic activity of aged MLN DCs and increased expression of CD80 and CD86, providing a potential means to enhance the immune response to vaccination and opportunistic infections in the elderly [92].
As it becomes clear that age-associated deficits in pDC and mDC subsets may play a role in the increased susceptibility to infection [75, 76, 92], chronic inflammatory states [74, 85, 93] and autoimmune pathology [77, 78, 82, 91] in the elderly, further research involving DC population dynamics, TLR expression, intracellular signaling pathways, cytokine production and the interaction between DCs and T cells should provide additional insight into the pathophysiology of clinical disease states associated with aging.
NATURAL KILLER CELLS
Natural killer cells are crucial modulators of tumor, microbial, and viral immunogenicity that also demonstrate alterations in immune function and cellular phenotype with advanced age (Table 1). NK cells are characterized phenotypically by surface expression of CD56 (NCAM) and CD16 (FcγRIIIa) in humans and expression of NK1.1/1.2 in mice. These unique cells can be distinguished from T and NKT cells by their lack of T-cell antigen receptor (TCR) and thymic independent maturation [94, 95]. Although NK cells share a common T cell precursor in the bone marrow, they, unlike thymic T and NKT cells, undergo maturation in the bone marrow, driven by a poorly described mechanism that may involve IL-12, IL-15, and IL-21 [96, 97]. While the precise nature of NK maturation is not fully understood, NK cells from aged individuals have decreased peak proliferation of cellular DNA after administration of deuterium-enriched glucose when compared to young subjects [98].
Table 1.
Natural Killer and Natural Killer T Cells
| Age-Associated Defects | Refs. | |
|---|---|---|
| NK Cells | ↓ bone marrow production | [98] |
| ↑ CD56dim cell population | [95, 99–102] | |
| ↓ cytotoxicity | [94, 100, 103–106] | |
| ↓ or ↔perforin | [107–108] | |
| ↓ IFN-γ, TNF-α, IL-2 and IL-12 production | [95] | |
| ↓ chemokine production in response to IL-2 and IL-12 | [109] | |
| Improved cytotoxicity and IFN-γ production after zinc or hormone treatment | [111–113] | |
| NKT Cells | ↓ iNKTpopulation in human peripheral blood and liver | [114–115] |
| ↑ iNKTpopulation in murinespleen and lymph node | [117] | |
| ↑ NKT-like cell population in human peripheral blood | [114, 116–119] | |
| ↓ iNKTcell proliferation after α-Gal Cerstimulation | [116] | |
| ↓ IFN-γafter IL-12 stimulation | [113] | |
| ↑ IL-4, IL-10 and IL-17 | [31, 120] | |
| Role in age-associated defective T cell proliferation | [117] | |
Arrows (↑, ↓ or ↔) denote increased, decreased or unaltered levels in the aged compared to the young.
Abbreviations: NK, natural killer cell; NKT, natural killer T cell; iNKT, invariant natural killer T cell; NKT-like, natural killer like T cells; TNF-α, tumor necrosis factor-alpha; IL, interleukin; IFN-γ, interferon-gamma; α-GalCer, a CD1d ligand.
In spite of decreased bone marrow production, it is generally accepted that the mature, highly cytotoxic CD56dim peripheral NK cell population expands with age [95, 99–102]. This shift to the mature, cytotoxic NK cell phenotype with advanced age suggested the possibility of functional alterations in NK cytotoxicity, however, studies following the strict exclusion criteria of the SENIUER protocol in humans did not indicate any difference in NK cytoxicity in peripheral blood [103, 104]. Indeed, when NK cells from elderly subjects were examined on an individual basis for lytic activity, they actually had lower cytotoxicity [105]. The deficits in NK cytotoxicity have, in part, been attributed to defective IP3 release following receptor binding which subsequently decreases signal transduction via the PKC1 pathway [106]. Additionally, aged human NK cells responding to IL-2, IL-12, IFN-α and IFN-γ stimulation demonstrate preserved cytotoxic activity against the NK-sensitive K562 cell line [103, 104]. Conversely, cytokine-induced cytolytic capabilities against Daudi, a NK resistant cell line, are decreased in NK cells from elderly subjects [94, 100, 103, 104]. Perforin, an intracytoplasmic cytolytic enzyme found in NK cells, has also been examined to determine whether it plays a role in the diminished NK cell cytolytic activity seen with advanced age. While no decrease in perforin production in elderly subjects has been found in vitro [107], a decrease in intracellular perforin expression by NK cells as measured by flow cytometry has been described [108].
In response to cytokine stimulation, NK cells play an important role in not only direct cytolytic activity of infected cells but also in release of pro-inflammatory cytokines and chemokines that facilitate the Th1 driven immune response [94]. NK cell production of IFN-γ, TNF-α, IL-2 and IL-12 is decreased in elderly individuals and may contribute to T cell deficits associated with aging [95]. After IL-2 or IL-12 stimulation, NK cells from aged subjects display increased production of the pro-inflammatory chemokines, MIP-1α, RANTES and IL-8, but the level of these mediators fail to reach peak amounts produced by cells from young subjects [109]. The attenuated response to IL-2 cytokine stimulation may be due to lower surface expression of IL-2 receptors on NK cells from aged subjects [94] and may be the reason that IL-12 stimulation of aged NK cells generated more chemokine production when compared to IL-2 stimulation [109].
Clinically, NK cell deficits in cytokine stimulation, cytotoxicity and cytokine/chemokine production directly translate into increased risks of infection, morbidity and mortality encountered in both aged humans and rodents [102, 110, 111]. Interestingly, NK cytotoxicity can be modulated by external agents, for example, hormonal treatments or zinc administration [112]. Physiological doses of zinc in infected, elderly patients act to improve NK cytotoxicity, restore IFN-γ production, and improve clinical outcomes [111]. Furthermore, administration of thyroid hormone (T3 and T4), melatonin, growth hormone and insulin-like growth factor-1 (IGF-1) also restored NK cell cytotoxicity and IFN-γ production. Whether hormones act through well-described hormone receptors on NK cells that induce cytokine production or by activation of mucosal gut receptors that enhance zinc absorption, or both mechanisms, is unknown [113]. Hormonal and nutritional enhancement of NK function in the elderly offer the potential for clinical interventions and provide a strong rationale for further study of NK cells in the elderly.
NATURAL KILLER T CELLS
Natural killer T cells are considered to be a unique T cell subset that express TCRs and NK1.1, undergo maturation in the thymus and/or at extrathymic sites, and play an important role in viral and antitumor cytolytic activity [94, 112]. Two specific NKT cell subsets, the invariant CD1d-restricted NKT cells (iNKT) and the CD8+ T lymphocytes with elevated expression of NK cell markers (NKT-like cells), undergo alterations in population size and function with aging (summarized in Table 1) [114–116]. Invariant NKT cells have been shown to be increased in the spleen and lymph nodes in aged mice [117], however, this subset is decreased in peripheral blood and liver in aged humans [114, 115]. Additionally, in aged human subjects, iNKT cells demonstrate decreased proliferation following stimulation with CD1d ligand α-GalCer [116], which may contribute to differences in the iNKT cell population with advanced age. Interestingly, the NKT-like cell population reportedly increases in peripheral blood from healthy elderly humans [114, 116, 118, 119], suggesting that advanced aged may alter the population dynamics of NKT cells in the peripheral pool.
In addition to these age related changes in the NKT cell faction, NKT cells demonstrate a propensity to skew the environmental cytokine milieu, promoting chronic inflammatory injury and altering T lymphocyte polarization [31, 120]. Compared to iNKT cells from young mice, iNKT cells from aged mice infected with HSV-2 demonstrated elevated expression and production of IL-17A that was associated with increased hepatic damage [31]. Adoptive transfer of iNKT cells from aged mice into young mice subsequently challenged with HSV-2 resulted in overproduction of IL-17A and exhibited more hepatic necrosis, supporting the concept that excessive IL-17A contributes to persistent inflammatory pathology and ensuing morbidity in the elderly [31]. Invariant NKT cells are not only involved in promotion of an IL-17 driven immune response, but aged iNKT cells also impact T cell polarization by releasing elevated levels of Th2 cytokines, IL-4 and IL-10 [120] and decreased levels of the Th1 cytokine IFN-γ before and after stimulation by IL-12 as compared to young mice [113].
Aside from differential cytokine production, NKT cells from aged subjects also decrease the proliferative response of the T lymphocyte population [117]. Removal of NK/NKT cells from splenocyte suspensions and systemic administration of anti-CD1d antibody, an inhibitor of iNKT TCR/CD1d-mediated activation, restored T cell proliferation in aged mice [117], implicating NK/NKT cells in the dysregulation of cell-mediated immunity with advanced aged. Taken together, the effects of NKT cells exhibiting an aged phenotype would influence T cell proliferation and skew T cell polarization, contributing to chronic pathological conditions, decreased microbial immunity and altered anti-tumor cytolytic activity with advance age.
CONCLUDING REMARKS
Age-associated impairment in the various components of the immune system has been extensively studied. However, it is evident from the recent studies reported herein that some aspects of innate immunity require more investigation than others. While the murine model of aging can be successfully used to study some cell types (e.g., macrophages and DCs), the model does not accurately reflect the role of other immune cells (e.g., neutrophils) in the elderly. Tackling immunosenescence as it affects the innate arms of the immune system is a logical step forward as efficient antigen presentation and co-stimulation by macrophages and DCs may promote a ‘normal’ humoral and cell-mediated immune response in aged individuals. Moreover, future aging research should focus on the key signaling molecules modulating the various signaling pathways involved in phagocyte and APC effector responses and on cross-talk between these pathways. Furthermore, studying the intrinsic defects of a cell without the interference of the aged microenvironment is as crucial as focusing on the overall aged phenotype. In vitro research coupled with in vivo studies on the complex physiological interactions between immune cell types and its surrounding milieu can hold a promising future for aging research.
ACKNOWLEDGEMENTS
We would like to thank Pamela Witte, Director of the Immunology and Aging program at Loyola University Medical Center. We would also like to thank Dr. Melanie Bird and Cory Deburghgraeve for assistance with the preparation of this manuscript. This work was supported by the National Institute of Health R01 AG018859 (EJK).
REFERENCES
- 1.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20(13–14):1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 2.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 4.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31(1):11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowell CA, Berton G. Integrin signal transduction in myeloid leukocytes. J Leukoc Biol. 1999;65(3):313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 6.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 7.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28(8):340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Chilvers ER, Cadwallader KA, Reed BJ, White JF, Condliffe AM. The function and fate of neutrophils at the inflamed site: prospects for therapeutic intervention. J R Coll Physicians Lond. 2000;34(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchard A, Ratthe C, Girard D. Interleukin-15 delays human neutrophil apoptosis by intracellular events and not via extracellular factors: role of Mcl-1 and decreased activity of caspase-3 and caspase-8. J Leukoc Biol. 2004;75(5):893–900. doi: 10.1189/jlb.1103585. [DOI] [PubMed] [Google Scholar]
- 11.Hirata J, Kotani J, Aoyama M, et al. A role for IL-18 in human neutrophil apoptosis. Shock. 2008;30(6):628–633. doi: 10.1097/SHK.0b013e31817c0c69. [DOI] [PubMed] [Google Scholar]
- 12.Fortin CF, McDonald PP, Lesur O, Fülöp T., Jr Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11(5):873–882. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- 13.Joseph FA, Julia WA. Aging, Immunity, and Infection. Totowa, NJ: Humana Press; 2003. [Google Scholar]
- 14.Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30(7):360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Murciano C, Yanez A, O'Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53(2):214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs EJ, Palmer JL, Fortin CFTF, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30(7):319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savill JS, Henson PM, Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel "charge-sensitive" recognition mechanism. J Clin Invest. 1989;84(5):1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122(14):1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 19.Tortorella C, Simone O, Piazzolla G, Stella I, Antonaci S. Age-related impairment of GM-CSF-induced signalling in neutrophils: role of SHP-1 and SOCS proteins. Ageing Res Rev. 2007;6(2):81–93. doi: 10.1016/j.arr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Fulop T, Larbi A, Douziech N, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3(4):217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 21.Lipschitz DA, Udupa KB, Indelicato SR, Das M. Effect of age on second messenger generation in neutrophils. Blood. 1991;78(5):1347–1354. [PubMed] [Google Scholar]
- 22.Chaves MM, Costa DC, Pereira CC, Andrade TR, Horta BC, Nogueira-Machado JA. Role of inositol 1, 4, 5-triphosphate and p38 mitogen-activated protein kinase in reactive oxygen species generation by granulocytes in a cyclic AMP-dependent manner: an age-related phenomenon. Gerontology. 2007;53(4):228–233. doi: 10.1159/000100960. [DOI] [PubMed] [Google Scholar]
- 23.Fortin CF, Lesur OTF., Jr Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int Immunol. 2007;19(1):41–50. doi: 10.1093/intimm/dxl119. [DOI] [PubMed] [Google Scholar]
- 24.Routsi C, Giamarellos-Bourboulis EJ, Antonopoulou A, et al. Does soluble triggering receptor expressed on myeloid cells-1 play any role in the pathogenesis of septic shock? Clin Exp Immunol. 2005;142(1):62–67. doi: 10.1111/j.1365-2249.2005.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70(6):881–886. [PubMed] [Google Scholar]
- 26.Biasi D, Carletto A, Dell'Agnola C, et al. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation. 1996;20(6):673–681. doi: 10.1007/BF01488803. [DOI] [PubMed] [Google Scholar]
- 27.Damtew B, Spagnuolo PJ, Goldsmith GG, Marino JA. Neutrophil adhesion in the elderly: inhibitory effects of plasma from elderly patients. Clin Immunol Immunopathol. 1990;54(2):247–255. doi: 10.1016/0090-1229(90)90086-6. [DOI] [PubMed] [Google Scholar]
- 28.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117(5):1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 29.Gomez CR, Hirano S, Cutro BT, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35(1):246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 30.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83(6):1493–1501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;6(5):446–456. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Xaus J, Mirabet M, Lloberas J, et al. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol. 1999;162(6):3607–3614. [PubMed] [Google Scholar]
- 35.Cullell-Young M, Barrachina M, Lopez-Lopez C, et al. From transcription to cell surface expression, the induction of MHC class II I-A alpha by interferon-gamma in macrophages is regulated at different levels. Immunogenetics. 2001;53(2):136–144. doi: 10.1007/s002510100312. [DOI] [PubMed] [Google Scholar]
- 36.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125(2):137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Davila DR, Edwards CK, 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4(11):2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 38.Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153(5):2146–2152. [PubMed] [Google Scholar]
- 39.Herrero C, Marques L, Lloberas J, Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest. 2001;107(4):485–493. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175(4):1004–1007. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- 41.Tasat DR, Mancuso R, O'Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2(3):159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 42.Lu Q, Ceddia MA, Price EA, Ye SM, Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol. 1999;276(2 Pt 2):R482–R489. doi: 10.1152/ajpregu.1999.276.2.R482. [DOI] [PubMed] [Google Scholar]
- 43.Sebastian C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere Shortening and Oxidative Stress in Aged Macrophages Results in Impaired STAT5a Phosphorylation. J Immunol. 2009;183(4):2356–2364. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 44.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79(12):1479–1487. [PubMed] [Google Scholar]
- 45.Takahashi I, Ohmoto E, Aoyama S, et al. Monocyte chemiluminescence and macrophage precursors in the aged. Acta Med Okayama. 1985;39(6):447–451. doi: 10.18926/AMO/31506. [DOI] [PubMed] [Google Scholar]
- 46.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117(1–3):57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 47.Wang CQ, Udupa KB, Xiao H, Lipschitz DA. Effect of age on marrow macrophage number and function. Aging (Milano) 1995;7(5):379–384. doi: 10.1007/BF03324349. [DOI] [PubMed] [Google Scholar]
- 48.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28(3):321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 49.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75(2):342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 50.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 51.Rowland-Jones SL, McMichael AJ. Lymphocytes: A Practical Approach. 2nd ed. US: Oxford University Press; 2000. [Google Scholar]
- 52.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76(2):291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 53.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25(6):581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 54.Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77(4):503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 55.Clark JA, Peterson TC. Cytokine production and aging: overproduction of IL-8 in elderly males in response to lipopolysaccharide. Mech Ageing Dev. 1994;77(2):127–139. doi: 10.1016/0047-6374(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 56.Delpedro AD, Barjavel MJ, Mamdouh Z, Faure S, Bakouche O. Signal transduction in LPS-activated aged and young monocytes. J Interferon Cytokine Res. 1998;18(6):429–437. doi: 10.1089/jir.1998.18.429. [DOI] [PubMed] [Google Scholar]
- 57.Mariani E, Pulsatelli L, Neri S, et al. RANTES and MIP-1 alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37(2–3):219–226. doi: 10.1016/s0531-5565(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 58.Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106(1):120–126. [PubMed] [Google Scholar]
- 59.van Duin D, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc. 2007;55(9):1438–1444. doi: 10.1111/j.1532-5415.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- 60.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28(1):47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 61.van Duin D, Mohanty S, Thomas V, et al. Age-Associated Defect in Human TLR-1/2 Function. J Immunol. 2007;178(2):970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 62.Kong KF, Delroux K, Wang X, et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82(15):7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayek MG, Mura C, Wu D, et al. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159(5):2445–2451. [PubMed] [Google Scholar]
- 64.Spencer NF, Norton SD, Harrison LL, Li GZ, Daynes RA. Dysregulation of IL-10 production with aging: possible linkage to the age-associated decline in DHEA and its sulfated derivative. Exp Gerontol. 1996;31(3):393–408. doi: 10.1016/0531-5565(95)02033-0. [DOI] [PubMed] [Google Scholar]
- 65.Wu D, Mura C, Beharka AA, et al. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol. 1998;275(3 Pt 1):C661–C668. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- 66.Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11(1):177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 67.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 68.Agius E, Lacy KE, Vukmanovic-Stejic M, et al. Decreased TNF-{alpha} synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206(9):1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu D, Marko M, Claycombe K, Paulson KE, Meydani SN. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J Biol Chem. 2003;278(13):10983–10992. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- 70.Chelvarajan RL, Liu Y, Popa D, et al. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79(6):1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 71.Sunil VR, Laumbach RJ, Patel KJ, et al. Pulmonary effects of inhaled limonene ozone reaction products in elderly rats. Toxicol Appl Pharmacol. 2007;222(2):211–220. doi: 10.1016/j.taap.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 73.Steinman RM. Dendritic Cells In Vivo: A Key Target for a New Vaccine Science. Immunity. 2008;29(3):319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2008;28(1):14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 75.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56(5):518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 76.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181(10):6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003;15(5):548–556. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Della Bella S, Bierti L, Presicce P, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122(2):220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178(11):6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 81.Lung TL, Saurwein-Teissl M, Parson W, Schönitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18(16):1606–1612. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 82.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182(2):1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salminen A, Kaarniranta K. NF-kB signaling in the aging process. J Clin Immunol. 2009;29:397–405. doi: 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 84.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17(5):457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 85.Nomellini V, Gomez CR, Kovacs EJ. Aging and impairment of innate immunity. Contrib Microbiol. 2008;15:188–205. doi: 10.1159/000136358. [DOI] [PubMed] [Google Scholar]
- 86.Steuhl KP, Sitz U, Knorr M, Thanos S, Thiel HJ. Age-dependent distribution of Langerhans cells within human conjunctival epithelium. Ophthalmologe. 1995;92(1):21–25. [PubMed] [Google Scholar]
- 87.Bhushan M, Cumberbatch M, Dearman RJ, Andrew SM, Kimber I, Griffiths CE. Tumour necrosis factor-alpha-induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol. 2002;146(1):32–40. doi: 10.1046/j.1365-2133.2002.04549.x. [DOI] [PubMed] [Google Scholar]
- 88.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans' cell frequency by tumour necrosis factor-alpha. Immunology. 1994;81(3):395–401. [PMC free article] [PubMed] [Google Scholar]
- 89.Cumberbatch M, Dearman RJ, Kimber I. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin-1beta. Immunology. 2002;105(4):466–477. doi: 10.1046/j.1365-2567.2002.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cumberbatch M, Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- 91.El Mezayen R, El Gazzar M, Myer R, High KP. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8(5):553–565. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181(11):7977–7984. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43(8):718–728. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18(16):1613–1620. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 95.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3(4):177–184. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 96.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3(4):459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 97.Toomey JA, Gays F, Foster D, Brooks CG. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol. 2003;74(2):233–242. doi: 10.1189/jlb.0303097. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Wallace DL, de Lara CM, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34(2):253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 100.Krishnaraj R. Senescence and cytokines modulate the NK cell expression. Mech Ageing Dev. 1997;96(1–3):89–101. doi: 10.1016/s0047-6374(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 101.Krishnaraj R. Immunosenescence of human NK cells: effects on tumor target recognition, lethal hit and interferon sensitivity. Immunol Lett. 1992;34(1):79–84. doi: 10.1016/0165-2478(92)90030-r. [DOI] [PubMed] [Google Scholar]
- 102.Comin F, Speziali E, Martins-Filho OA, et al. Ageing and Toll-like receptor expression by innate immune cells in chronic human schistosomiasis. Clin Exp Immunol. 2007;149(2):274–284. doi: 10.1111/j.1365-2249.2007.03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kutza J, Murasko DM. Effects of aging on natural killer cell activity and activation by interleukin-2 and IFN-alpha. Cell Immunol. 1994;155(1):195–204. doi: 10.1006/cimm.1994.1112. [DOI] [PubMed] [Google Scholar]
- 104.Kutza J, Murasko DM. Age-associated decline in IL-2 and IL-12 induction of LAK cell activity of human PBMC samples. Mech Ageing Dev. 1996;90(3):209–222. doi: 10.1016/0047-6374(96)01772-1. [DOI] [PubMed] [Google Scholar]
- 105.Mariani E, Roda P, Mariani AR, et al. Age-associated changes in CD8+ and CD16+ cell reactivity: clonal analysis. Clin Exp Immunol. 1990;81(3):479–484. doi: 10.1111/j.1365-2249.1990.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mariani E, Mariani AR, Meneghetti A, Tarozzi A, Cocco L, Facchini A. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int Immunol. 1998;10(7):981–989. doi: 10.1093/intimm/10.7.981. [DOI] [PubMed] [Google Scholar]
- 107.Mariani E, Sgobbi S, Meneghetti A, et al. Perforins in human cytolytic cells: the effect of age. Mech Ageing Dev. 1996;92(2–3):195–209. doi: 10.1016/s0047-6374(96)01829-5. [DOI] [PubMed] [Google Scholar]
- 108.Rukavina D, Laskarin G, Rubesa G, et al. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92(7):2410–2420. [PubMed] [Google Scholar]
- 109.Mariani E, Meneghetti A, Neri S, et al. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32(6):1524–1529. doi: 10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 110.Ogata K, An E, Shioi Y, et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol. 2001;124(3):392–397. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mocchegiani E, Muzzioli M, Giacconi R, et al. Metallothioneins/PARP-1/IL-6 interplay on natural killer cell activity in elderly: parallelism with nonagenarians and old infected humans. Effect of zinc supply. Mech Ageing Dev. 2003;124(4):459–468. doi: 10.1016/s0047-6374(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 112.Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29(4):416–425. doi: 10.1007/s10875-009-9298-4. [DOI] [PubMed] [Google Scholar]
- 113.Mocchegiani E, Giacconi R, Cipriano C, et al. The variations during the circadian cycle of liver CD1d-unrestricted NK1.1+TCR gamma/delta+ cells lead to successful ageing. Role of metallothionein/IL-6/gp130/PARP-1 interplay in very old mice. Exp Gerontol. 2004;39(5):775–788. doi: 10.1016/j.exger.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 114.Peralbo E, Alonso C, Solana R. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42(8):703–708. doi: 10.1016/j.exger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 115.DelaRosa O, Tarazona R, Casado JG, et al. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37(2–3):213–217. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 116.Peralbo E, DelaRosa O, Gayoso I, Pita ML, Tarazona R, Solana R. Decreased frequency and proliferative response of invariant Valpha24Vbeta11 natural killer T (iNKT) cells in healthy elderly. Biogerontology. 2006;7(5–6):483–492. doi: 10.1007/s10522-006-9063-5. [DOI] [PubMed] [Google Scholar]
- 117.Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol. 2005;175(5):3102–3109. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- 118.Tarazona R, DelaRosa O, Alonso C, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121(1–3):77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 119.Panda A, Arjona A, Sapey E, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jing Y, Gravenstein S, Chaganty NR, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42(8):719–732. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]