Abstract
Although prostaglandin E2 (PGE2) vasodilates the ductus arteriosus, tocolysis with cyclooxygenase (COX) inhibitors delays postnatal ductus arteriosus closure. We used fetal mice and sheep to determine if PGE2 has a role in the development of ductus contractility that is distinct from its function as a vasodilator. Prolonged exposure of fetal ductus to PGE2, in vitro, increased expression of CaL- and K+-channel genes (CaLα1c, CaLβ2, Kir6.1, Kv1.5) (which regulate oxygen-induced constriction) without affecting genes that regulate Rho-kinase-mediated calcium sensitization. Conversely, chronic exposure to cyclooxygenase inhibitors in utero decreased expression of CaL- and K+-channel genes, without affecting Rho-kinase-associated genes. Chronic cyclooxygenase inhibition in utero decreased the ductus’ in vitro contractile response to stimuli that utilize CaL- and K+-channels (like O2 and K+), whereas the response to stimuli that act through Rho-kinase–mediated pathways (like U46619) was not significantly affected. Phosphodiesterase expression, which decreases the ductus’ sensitivity to cAMP/cGMP-dependent vasodilators, was increased by PGE2 exposure and decreased by cyclooxygenase inhibition, respectively. These studies identify potential downstream effectors of a PGE2-mediated, developmental program, regulating oxygen-induced ductus closure. Alterations in these effectors may explain the increased risk of patent ductus arteriosus (PDA) after in utero cyclooxygenase inhibition.
Prostaglandins (especially prostaglandin E2 (PGE2)) vasodilate the fetal and neonatal ductus arteriosus. When nonselective inhibitors of cyclooxygenase (COX)-1 and -2, the rate-limiting enzymes for prostaglandin synthesis, are given to pregnant women to treat preterm labor, the ductus arteriosus constricts in utero (1). Surprisingly, some preterm infants, who are delivered after in utero exposure to indomethacin, have an increased, rather than decreased, incidence of patent ductus arteriosus (PDA), in the newborn period. The PDA in these infants fails to close with postnatal indomethacin treatment (2,3). Delayed closure of the newborn ductus arteriosus increases an infant’s risk for pulmonary hemorrhage, necrotizing enterocolitis, and chronic lung disease (4).
Delayed closure of the newborn ductus also occurs in mice and sheep that have been exposed to nonselective COX inhibitors during the last 25% of gestation (5–7) and in mice lacking both COX genes (6–8). The basis for this paradoxical response is not entirely clear. Prior studies have suggested that in utero COX inhibition may contribute to delayed closure by increasing nitric oxide (5,9) or decreasing hyaluronic acid production in the ductus (10).
Postnatal closure of the ductus arteriosus requires the presence of specific components of ductus contractility: smooth muscle calcium channels, potassium channels (11–15), Rho-kinase related calcium sensitizing pathways (11,16–18), mature myosin isoforms (19), and cytoskeletal proteins (20). Events or drugs that interfere with these pathways lead to delayed postnatal ductus closure (16,17,21–23). We speculated that prostaglandins may regulate the development of one or more of these contractile pathways, and hypothesized that inhibition of COX activity in utero may alter the contractile apparatus’ development, leading to a persistent PDA after birth.
In the following study, we examined the effects of prostaglandin exposure and inhibition on fetal mice and sheep ductus. We found that, in addition to its known vasodilator effects, PGE2 plays an important role in the expression of specific pathways that are necessary for the ductus’ oxygen-induced closure following delivery.
Materials and Methods
All protocols were approved by the Vanderbilt University and University of California San Francisco Institutional Animal Care and Use Committees.
Mouse studies
Wild type female CD-1 mice (7–8 weeks old; Charles River, Raleigh, NC) were bred to produce timed pregnancies (day 1 = presence of vaginal plug, term =19 days).
Pregnant females received either no drugs (Control) or a combination of a selective COX-1 inhibitor (SC560, 30mg/kg/dose, 0.2ml gavage) and a selective COX-2 inhibitor (SC236, 15 mg/kg/dose, 0.2 ml gavage) (Cayman Chemical Co., Ann Arbor, MI). Both drugs cross the placenta (24). Pregnant mice received the inhibitors either on a single occasion (day 19 of gestation), or chronically from day 15 to day19: (SC560 (30mg/kg/dose, twice daily); SC236 (15 mg/kg/dose, every other day). Both fetuses and newborns were delivered by Caesarian section 4h after the last drug dosage on day 19. Fetuses were euthanized at delivery. Newborn pups were placed in pre-warmed cages (in FiO2=0.8–1.0) to accelerate ductus closure and tissues were harvested 4h later. Tissues were prepared for either RNA analysis or histology as previously described (6,25).
Determination of vessel caliber
Serial sections of fetal and newborn mouse thoraces were examined by an observer that was blind to treatment group (R.I.C). The inner diameters of the ductus arteriosus lumen (DA) and the transverse aortic arch lumen (AO) were determined at their narrowest points. DA diameter was expressed as a ratio of the ductus diameter to the diameter of the transverse aortic arch (DA/AO ratio) (6).
Mouse pressure myography studies
Fetal mouse ductus were isolated and mounted in 4 ml chambers as previously described (26). Distending pressure (in mmHg) was generated by a column of deoxygenated Krebs buffer. Non-recirculating, deoxygenated Krebs buffer (36.5–37.5°C) perfused the chambers at 6 ml/min. The lumen diameter was measured at the point of maximum constriction using an inverted microscope and a video-image capture system; during full vessel closure, measurement was obtained at the optically dense regions of the internal elastic lamina.
Vessels were initially pressurized to 20 mmHg (in 5 mmHg increments). The vessels were then exposed to 50 mM K+- deoxygenated Krebs buffer (with KCl substituted for NaCl) for 3–5 minutes to stimulate ductus contractility. Following this, N(G)-nitro-L-arginine methyl ester (L-NAME) (10−4 M; Cayman Chemical, Ann Arbor, MI) and indomethacin (5.6 ×10−5 M; Sigma-Aldrich, St. Louis, MO) were added to the perfusion solution and the distending pressure was increased to 25 mmHg for the duration of the experiment. This eliminated any differences between the groups in ongoing nitric oxide and prostaglandin production in vitro (9,27). After a 60-minute equilibration period with L-NAME and indomethacin at 25 mmHg, the vessels were exposed to 50 mM K+- deoxygenated Krebs buffer for 3–5 minutes to stimulate ductus contractility.
Vessels from littermates were then exposed to one of three study protocols: 1) increasing oxygen concentrations (0, 2%, 5%, 12%, and 21% O2; 5% CO2, balance N2), 2) increasing K+ concentrations in deoxygenated (95% N2, 5% CO2) Krebs buffer, or 3) increasing concentrations of U-46619, a thromboxane receptor agonist (Cayman) in deoxygenated Krebs buffer. Each change in concentration was maintained until a stable new diameter was established (10–30 min.). At the completion of each study protocol, vessels were exposed to papaverine (10−4 M; Sigma-Aldrich) to determine the vessel caliber at maximal relaxation.
Sheep studies: in vitro PGE2 incubation
The small size of the mouse ductus presented a great challenge in attempting to acquire sufficient tissue to perform the required number of biochemical analyses from individual ductus incubation studies. Therefore, we used the much larger fetal sheep ductus to examine the effects of PGE2 on isolated ductus arteriosus RNA expression. Immature sheep fetuses (mixed Western breed: 103 ± 2 d gestation, term=145d) were delivered by Cesarean section and anesthetized with intravenous ketamine HCl (30 mg/kg) before rapid exsanguination. The ductus arteriosus was divided into 1 mm-thick rings (2 rings per animal) which were stretched to 5.0 mm in separate 20 ml organ chambers. Throughout the 23 hour experiment, the rings were perfused at 10 ml/hr with non-recirculating modified Krebs buffer (pH=7.4, 37.5°C) containing indomethacin (5 × 10−6 M) (to inhibit endogenous prostaglandin production) (28). Because of the tissue thickness, the chambers were continuously aerated with 30% O2, 5% CO2, balance N2. Following a 3-hour equilibration period, 10−8 M PGE2 (similar to fetal plasma concentrations at late gestation (29)) was added to the indomethacin-containing Krebs buffer perfusing one of the rings. The other ring continued to be perfused with the indomethacin-containing buffer without PGE2. Both rings were incubated for an additional 20 hours before being snap-frozen in liquid nitrogen for RNA analysis.
Preparation of total RNA, reverse transcription and quantitative polymerase chain reaction
Total RNA was isolated from the mouse and sheep ductus and the TaqMan Universal PCR master mix of PE Applied Biosystems was used to quantify the expression of the genes as described elsewhere (25). Taqman probes were designed using the Primer Express program and labeled with fluorophores FAM (6-caboxy-fluorescein) and TAMRA (6 carboxy-tetramethyl-rhodamine) as reporter and quencher dyes, respectively. An ABI PRISM 7700 Sequence detection system was used to determine the cycle threshold (CT). Malate dehydrogenase (MDH) was used as an internal control to normalize the data (11).
Statistics
Statistical analyses of unpaired and paired data were performed by the appropriate t-test and by analysis of variance. Scheffe’s test was used for post hoc analysis. Values are expressed as mean ± standard deviation. Drug concentrations refer to their final molar concentration in the bath.
Results
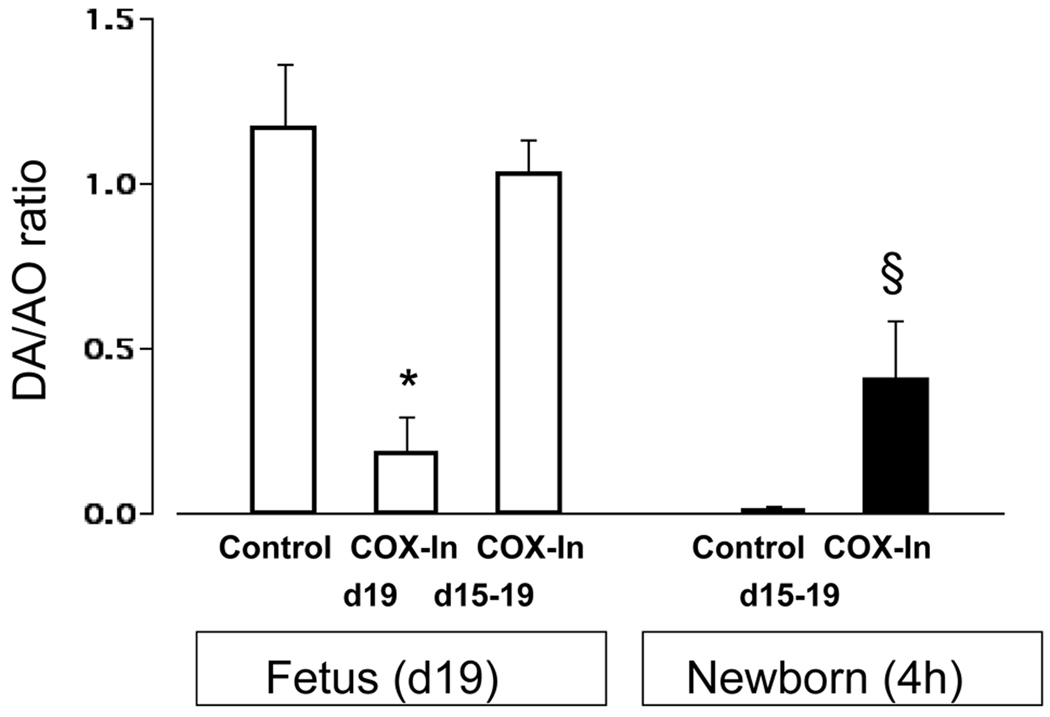
A one-time exposure (on day 19 gestation) to the two selective COX inhibitors (SC560 plus SC236) produced marked constriction in vivo of the fetal mouse ductus arteriosus four hours after administering the drugs (Figure 1).
Figure 1.
Response of the fetal and newborn ductus arteriosus to acute (COX-In d19) and chronic (COX-In d15–19) COX inhibition in vivo. DA=ductus and AO=transverse aorta lumen diameters. Acute COX inhibition constricted the fetal ductus at term gestation (n = 17 fetuses; 7 litters) compared with Control fetuses (n = 9; 6 litters). Chronic COX inhibition (n = 17; 6 litters) did not constrict the fetal ductus. Ductus closure in 4 hour-old newborn pups (chronically exposed to COX inhibitors in utero (n = 26; 11 litters) was significantly reduced (resulting in a PDA) compared to Control newborns (n = 12; 4 litters). *p<0.05 compared to fetal controls; § p<0.05 compared to newborn control.
We wanted to examine the effects of chronic COX inhibition, which were independent of the initial acute constriction. Therefore, we started the chronic treatment (SC560 plus SC236) at a point in gestation (day 15) when the inhibitors had no acute contractile effect on the fetal mouse ductus (6) and continued the treatment regimen through day 19 of gestation. The ductus of fetuses that were exposed to the inhibitors (SC560 plus SC236) from days 15 through 19 of gestation did not constrict in utero and had the same degree of patency as control fetuses on days 16, 17, 18 (data not shown) and 19 (Figure 1). Chronic exposure to the COX inhibitors, in utero, resulted in incomplete closure of the newborn ductus after birth (Figure 1).
We next examined the effects of chronic COX inhibition on ductus contractility using isolated pressurized ductus from Control fetal mice and from mice exposed to the combined COX inhibitors (from days 15 through 19) in utero. Ductus smooth muscle contractility is determined by developmentally regulated pathways that control both the concentration of intracellular calcium and the sensitivity of the contractile proteins to changes in intracellular calcium. Intracellular calcium concentrations are determined by calcium influx through L-type calcium channels; similarly, Rho/Rho-kinase activity plays an important role in regulating calcium sensitization during the sustained phase of ductus contraction (11–17). Stimuli like oxygen and elevated K+ affect ductus contractility primarily by increasing calcium entry through voltage-dependent CaL channels (11,15,16,18,30,31). However, most agonists that alter vascular tone affect both aspects of ductus contractility. For example, in other vessels, U46619, a thromboxane-mimetic, appears to affect both calcium entry and Rho-kinase–mediated calcium sensitization (32,33).
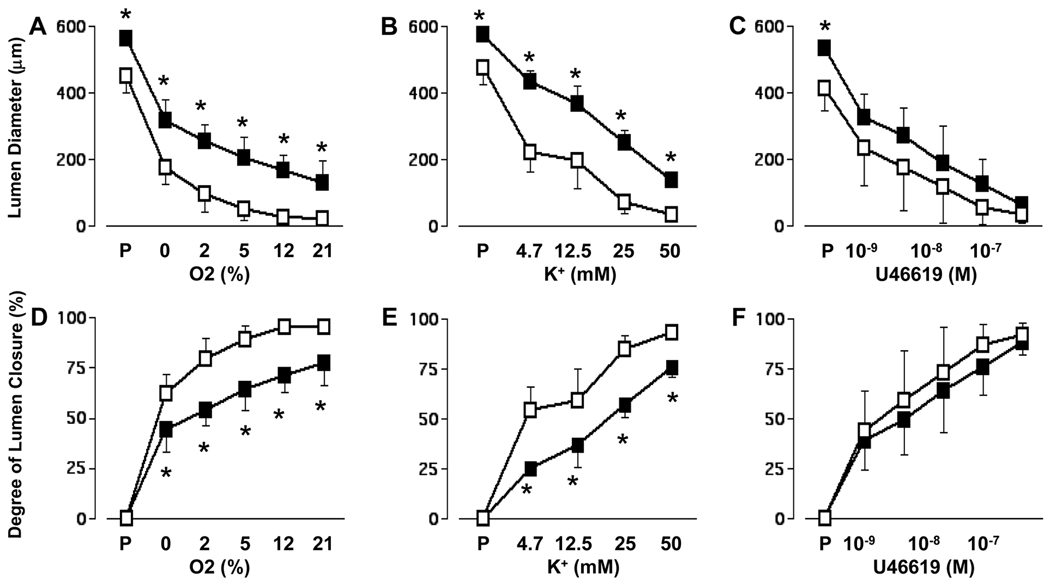
When incubated at the same pressures, under baseline conditions (buffer bubbled with 0% O2), ductus obtained from term fetuses that were chronically exposed to COX inhibitors in utero, had significantly larger lumina than Control ductus (lumen diameter: COX-inhibitor exposed = 360±70 µm, n=17; Control = 223±74 µm, n=22, p<0.05) (Figure 2). Similarly, when the ductus were maximally relaxed by papaverine, the lumina of the chronically COX inhibited ductus were significantly larger than those of the Control ductus (lumen diameter: COX-inhibitor exposed = 559±31 µm; Control = 449±61 µm, p<0.05). Ductus from both groups constricted with increasing concentrations of O2, K+, and U46619 (Figure 2). However, O2 and K+ produced a lesser degree of constriction in ductus that were chronically exposed to COX inhibitors in utero than they did in Control ductus (Figure 2). On the other hand, the thromboxane-mimetic, U46619, constricted both groups of ductus to a similar degree (Figure 2).
Figure 2.
Contractile response of isolated ductus from19 day-old fetal mice treated with or without chronic COX inhibitors. Ductus were incubated in deoxygenated buffer containing L-NAME and indomethacin and exposed to increasing oxygen (A, D), K+ (B, E), or U-46619 (C, F) concentrations. P = maximal relaxation with papaverine. Steady state oxygen tensions were 42.5±2., 57.3±2.1, 75.1±1.8, 118.8±1.7, and 181.0±5.6 mmHg when the buffer was bubbled with 0, 2, 5, 12, and 21% oxygen, respectively. The K+ and U-46619 dose-response experiments were performed in 0% oxygen. Degree of lumen closure = 1-(measured diameter/ diameter after papaverine relaxation). Open squares = Controls, solid squares = chronic COX inhibition. Animal numbers: O2 (A, D): Control=6, COX inhibition=6; K+ (B,E): Control=9, COX inhibition=5; U-46619 (C, F): Control=7, COX inhibition=6. *p<0.05 compared to Control.
In the Control ductus, peak concentrations of O2 (21%), K+ (50mM) and U46619 (10−7 M) produced the same maximal degree of constriction (% closure: 95±2%, 93±4%, and 92±6%, respectively). In contrast, following chronic COX inhibition, U46619 (10−7 M) produced a significantly greater degree of ductus closure (% closure = 88±5) than either 21% O2 (% closure = 77±11, p<0.05) or 50mM K+ (% closure = 77±5, p<0.05) (Figure 2).
We examined ductus obtained from 19d gestation mice fetuses to determine if chronic in utero COX inhibition altered the developmental expression of genes known to affect ductus contractility. Chronic treatment with SC560 plus SC236 altered the expression of genes that regulate CaL and K+ channels (CaLα1c, CaLβ2, CaLβ3, Kir6.1, Kv1.5), actin/myosin interactions (caldesmon, myocardin, tropomyosin), matrix production (tenascin C, TGFβ), and vasodilator activity (hemeoxygenase-1, PDE 1A, PDE 3A, VEGF) (Table 1).
Table 1.
Real Time polymerase chain reaction measurements of genes involved with ductus closure and remodeling: ductus obtained from 19d gestation fetuses.
| ΔCT(MDH-gene) | ΔCT(MDH-gene) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Control | COX Inhibition |
P | Gene | Control | COX Inhibition |
P | ||||
| mean | sd | mean | sd | mean | sd | mean | sd | ||||
| Ca++ L-channels | Vasodilation | ||||||||||
| CaLα1c | −2.33 | ±0.52 | −3.09 | ±0.31 | * | COX1 | −5.34 | ±0.37 | −5.29 | ±0.69 | |
| CaLβ2 | −2.52 | ±0.34 | −3.26 | ±0.53 | * | COX2 | −5.53 | ±0.58 | −4.75 | ±0.89 | |
| CaLβ3 | −2.71 | ±0.41 | −3.48 | ±0.52 | * | eNOS | −8.86 | ±0.91 | −9.68 | ±1.43 | |
| EP2 | −8.16 | ±1.00 | −7.76 | ±0.36 | |||||||
| K+ channels | EP3 | −5.46 | ±0.27 | −5.63 | ±0.62 | ||||||
| BKCa | −9.34 | ±0.93 | −7.99 | ±1.31 | EP4 | −0.20 | ±1.20 | −0.71 | ±0.87 | ||
| BKCaβ1 | −3.70 | ±0.52 | −4.30 | ±0.36 | * | HO1 | −3.52 | ±0.31 | −1.48 | ±0.97 | * |
| Kir6.1 | −2.44 | ±0.72 | −3.52 | ±0.87 | * | HO2 | −2.51 | ±0.42 | −2.51 | ±0.28 | |
| SUR2 | −3.21 | ±0.43 | −3.63 | ±0.61 | PDE1A | −5.22 | ±0.40 | −5.89 | ±0.19 | * | |
| Kv1.2 | −4.68 | ±0.53 | −4.99 | ±0.76 | PDE1B | −2.52 | ±1.03 | −2.83 | ±0.48 | ||
| Kv1.5 | −3.11 | ±0.36 | −3.99 | ±0.48 | * | PDE3A | −2.38 | ±0.28 | −3.41 | ±0.54 | * |
| Kv2.1 | −3.53 | ±0.83 | −3.69 | ±0.71 | PDE3B | −2.80 | ±0.52 | −3.05 | ±0.13 | ||
| Kv9.3 | −1.47 | ±0.41 | −2.18 | ±0.53 | PDE4D | −6.22 | ±0.69 | −6.51 | ±0.30 | ||
| Kvβ1.2 | −3.96 | ±0.63 | −4.36 | ±0.36 | PDE5A | −2.75 | ±0.36 | −3.26 | ±0.55 | ||
| Kvβ1.3 | −1.96 | ±0.36 | −2.29 | ±0.27 | |||||||
| Remodeling | |||||||||||
| Contractility | Ang−1 | −4.44 | ±0.54 | −4.76 | ±0.36 | ||||||
| Caldesmon | −0.03 | ±0.21 | −0.76 | ±0.41 | * | Ang-2 | −5.91 | ±0.47 | −5.82 | ±0.74 | |
| Calponin | −1.58 | ±0.55 | −2.01 | ±0.49 | ATIIR-1 | −4.96 | ±0.72 | −5.08 | ±0.45 | ||
| Myocardin | −3.17 | ±0.38 | −3.97 | ±0.31 | * | HAS2 | −4.69 | ±0.45 | −4.99 | ±0.19 | |
| MYH1 | 0.41 | ±0.68 | 0.05 | ±0.81 | HIF1α | −3.75 | ±0.87 | −3.99 | ±0.25 | ||
| MYH2 | −1.97 | ±0.93 | −2.34 | ±0.48 | HIF2α | −2.71 | ±0.66 | −2.76 | ±0.44 | ||
| RhoA | −0.94 | ±0.74 | −0.80 | ±0.44 | IFNγ | −3.07 | ±0.54 | −3.18 | ±0.25 | ||
| RhoB | −2.36 | ±0.27 | −2.87 | ±0.51 | IL6 | −3.66 | ±0.37 | −4.06 | ±0.26 | ||
| ROCK1 | −4.02 | ±0.81 | −4.27 | ±0.64 | PDGF-B | −5.06 | ±0.45 | −5.19 | ±0.54 | ||
| NCX1 | −4.29 | ±0.95 | −4.92 | ±0.60 | Tenascin-C | −1.60 | ±0.30 | −2.13 | ±0.36 | * | |
| PHLBN | −2.88 | ±0.68 | −2.98 | ±0.51 | TFAP2b | −4.81 | ±1.34 | −5.36 | ±1.05 | ||
| SERCA3 | −6.58 | ±0.42 | −6.64 | ±0.23 | TGFβ1 | −4.17 | ±0.37 | −4.67 | ±0.29 | * | |
| Tropomyosin | −3.00 | ±0.39 | −3.80 | ±0.54 | * | TGFβ3 | −2.71 | ±0.23 | −3.43 | ±0.21 | * |
| TNFα | −2.38 | ±1.00 | −2.69 | ±0.29 | |||||||
| Endothelin | TRAF1 | −5.96 | ±1.05 | −6.31 | ±0.53 | ||||||
| ECE1 | −1.18 | ±0.68 | −1.86 | ±0.26 | VEGF | −3.57 | ±0.50 | −2.78 | ±0.42 | * | |
| ET1 | −4.43 | ±0.95 | −4.55 | ±0.62 | |||||||
| ETAr | −5.99 | ±1.52 | −6.27 | ±1.67 | |||||||
| ETBr | −2.66 | ±0.79 | −2.77 | ±0.37 | |||||||
ΔCT(MDH-gene) = difference in cycle threshold (CT) between the expression of the housekeeping gene Malate dehydrogenase (MDH) and the gene of interest. Each unit of ΔCT(MDH-gene) represents a 2-fold increase in a gene’s mRNA. The more negative the ΔCT(MDH-gene), the fewer the number of starting copies of a gene (mRNA). Number of separate litters used: Control (day 19) = 9, COX-inhibition (days 15–19) = 7. From each litter of mice we obtained and pooled between 10–12 ductus.
p<0.05.
MYH, myosin heavy chain; ROCK1, Rho kinase 1; NCX1, Na+/Ca++ exchanger; PHLBN, phospholamban; SERCA, sarcoplasmic reticulum Ca++-ATPase; ECE, endothelin converting enzyme; ET, endothelin; ETAr, endothelin receptor A; eNOS, endothelial nitric oxide synthase; EP, prostaglandin E receptor; HO, heme oxygenase; PDE, phosphodiesterase; ang, angiopoietin; ATIIR-1, angiotensin II receptor; HAS, hyaluronic acid synthase; HIF, hypoxia inducible factor; IFN, interferon; IL, interleukin; PDGF, platelet derived growth factor.
Since chronic COX inhibition decreased the expression of genes that facilitate ductus closure late in gestation, we hypothesized that prolonged PGE2 exposure (early in gestation) may have the opposite effect, and promote their developmental expression. To test this hypothesis, we used isolated ductus from immature fetal sheep, mounted in organ culture baths. The isolated ductus were incubated in media containing indomethacin to inhibit the increase in tissue prostaglandin production that follows ductus dissection and manipulation (28). Parallel rings from the same ductus were incubated either with or without PGE2 (10−8 M) for 20 hours. In vitro PGE2 exposure increased the expression of genes that regulate CaL channels (CaLα1c, CaLβ2), K+ channels (Kir6.1, Kv1.5), and phosphodiesterase activity (PDE 1A, PDE 3A) (Table 2). This is consistent with PGE2 having a direct, positive effect on the in utero expression of these genes. On the other hand, PGE2 exposure did not affect the expression of genes that regulate actin/myosin interactions (caldesmon, myocardin, tropomyosin) or matrix production (tenascin C, TGFβ). We also examined the expression of several genes that were not affected by chronic COX inhibition in utero (large conductance BKCa channel, endothelin-1, platelet derived growth factor-B chain, Rho A, Rho B, and ROCK-1). As might be anticipated, PGE2 exposure did not affect their expression.
Table 2.
Real Time PCR measurements in sheep ductus.
| ΔCT(MDH-gene) | ΔCT(MDH-gene) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Control | PGE2 incubation |
P | Gene | Control | PGE2 incubation |
P | ||||
| mean | sd | mean | sd | mean | sd | mean | sd | ||||
| Genes affected by chronic COX inhibition in utero | Genes un-affected by chronic COX inhibition in utero | ||||||||||
| CaLα1c | −0.24 | ±0.49 | 0.59 | ±0.54 | * | BKCa | −7.13 | ±0.98 | −7.27 | ±0.55 | |
| CaLβ2 | −2.31 | ±1.01 | −1.33 | ±0.83 | * | COX2 | 0.77 | ±0.96 | 1.06 | ±1.36 | |
| CaLβ3 | −1.28 | ±0.38 | −1.12 | ±0.31 | EP2 | −1.86 | ±0.43 | −1.96 | ±0.85 | ||
| Caldesmon | 1.12 | ±0.78 | 1.11 | ±0.63 | EP3 | −5.44 | ±1.03 | −4.99 | ±0.98 | ||
| BKCaβ1 | −4.40 | ±0.26 | −4.06 | ±0.44 | ET1 | −4.52 | ±1.33 | −4.74 | ±1.22 | ||
| HO1 | −2.08 | ±0.38 | −1.59 | ±0.47 | * | PDGF−B | −3.95 | ±0.83 | −3.91 | ±0.71 | |
| Kir6.1 | −5.21 | ±1.00 | −4.31 | ±0.92 | * | RhoA | 1.87 | ±0.41 | 2.07 | ±0.39 | |
| Kv1.5 | −1.99 | ±1.40 | −0.96 | ±0.87 | * | RhoB | 2.42 | ±0.61 | 2.80 | ±0.78 | |
| Myocardin | −3.40 | ±0.47 | −3.29 | ±0.77 | ROCK1 | −2.82 | ±0.75 | −2.49 | ±0.59 | ||
| PDE1A | −5.79 | ±1.02 | −4.72 | ±0.95 | * | SUR2 | −1.04 | ±0.41 | −0.78 | ±0.55 | |
| PDE3A | −2.58 | ±0.66 | −1.62 | ±0.96 | * | ||||||
| Tenascin-C | 1.00 | ±0.81 | 0.64 | ±1.18 | |||||||
| TGFβ1 | −0.61 | ±0.34 | −0.35 | ±0.31 | |||||||
| TGFβ3 | −4.09 | ±0.76 | −3.76 | ±0.64 | |||||||
| Tropomyosin | −0.25 | ±1.01 | −0.50 | ±0.58 | |||||||
| VEGF | 0.31 | ±0.72 | 1.61 | ±0.86 | * | ||||||
Rings from the same sheep ductus were incubated with or without PGE2 (10−8 M) for 20 hours. We examined the expression of genes that were altered by chronic COX inhibition in utero in Table 1; we also examined the expression of several of the genes that were not affected by chronic COX inhibition (see Table 1). Number of separate animals used = 7.
p<0.05.
Discussion
Although acute COX inhibition in near-term mice led to fetal ductus constriction (Figure 1), prolonged COX inhibition during the last 25% of gestation led to an impaired contractile response and an incomplete closure in the newborn (Figure 1). The effects of prolonged COX inhibition, in utero, are similar to the effects observed after deletion of both COX genes in utero (6–8). Similar findings have also been observed in larger species, (e.g., humans (2) and sheep (5)) following indomethacin exposure in utero. In larger species, however, the loss of ductus contractility, following COX inhibition, appears to be due to ischemia of the ductus wall secondary to in utero ductus constriction and loss of vasa vasorum blood flow to the muscle media (5). Ductus wall ischemia does not appear to be the explanation for our findings in mice. The mouse ductus is so thin that luminal flow sustains all of its nutrient needs. As a result, the mouse ductus has no need for muscle media vasa vasorum (34,35). In our study, COX inhibition was started on day 15, when COX inhibitors do not contract the mouse ductus (6). Chronic COX inhibition (between days 15 and 19) does not affect the in utero dimensions of the ductus lumen (6) (Figure 1). Consistent with these findings, surrogate markers of hypoxia, like HIF1α and HIF2α, whose expression increases during ductus hypoxia (36), are unaffected by chronic COX inhibition in utero (Table 1).
We hypothesized that PGE2 may play a unique role in the development of ductus contractility that is distinct from its function as a vasodilator. Previous studies found that gene deletion of the PGE2 receptor, EP4, produced a persistent PDA phenotype in newborn mice (37) that was similar to what occurs after COX gene deletion or chronic in utero COX inhibition. Our findings are consistent with PGE2 having a direct effect on ductus contractility by increasing the developmental expression of genes that regulate calcium availability. The ductus’ developmentally regulated, oxygen-induced constriction appears to be due in large part to increased expression of CaL channels (11,15) and oxygen-sensitive K+ channels (11,14,15). In our experiments, in vitro exposure to PGE2 increased the expression of CaL channel (CaLα1c, CaLβ2) and K+ channel (Kir6.1, Kv1.5) genes without affecting genes that regulate Rho-kinase-mediated calcium sensitization (Table 2). Conversely, inhibition of prostaglandin production, by chronic in utero COX inhibition, decreased the expression of the same CaL and K+ channel genes, without affecting Rho-kinase-associated genes (Table 1).
The effects of chronic in utero COX inhibition on gene expression are consistent with the effects we found on ductus contractility. Contractile stimuli, which act primarily on ductus K+ and CaL channels (like O2 and K+ (11–14,17,32)), have a diminished contractile effect, whereas, U46619, which has been shown to affect both calcium entry and Rho-kinase–mediated calcium sensitization in other vascular tissues (32,33), constricts Control and COX-inhibited ductus to a similar degree (Figure 2).
Several other genes were also affected by chronic COX inhibition and may contribute to the delayed ductus closure after birth (Table 1). Prior studies have shown that a developmental increase in phosphodiesterase (PDE 1A and PDE 3A) expression and activity decreases the sensitivity of the late gestation ductus to vasodilators, like PGE2 (38). We found that the phosphodiesterase genes were down-regulated by chronic COX inhibition in utero (Table 1) and up-regulated by PGE2 exposure in vitro (Table 2). This is consistent with endogenous PGE2 having a direct, positive effect on PDE 1A and PDE 3A expression in utero.
On the other hand, the effects of chronic COX inhibition on other genes (like caldesmon, myocardin, tropomyosin, tenascin C, TGFβ, hemeoxygenase-1, and VEGF) may not be due to direct effects of PGE2 on the ductus. For example, the genes that regulate actin/myosin interactions (caldesmon, myocardin, tropomyosin) and matrix production (tenascin C, TGFβ) (which were decreased by chronic COX inhibition in utero) were not affected by incubation with PGE2 in vitro; and the hemeoxygenase-1 and VEGF genes, which were increased by chronic COX inhibition in utero, were not decreased by incubation with PGE2 in vitro. The effects of chronic COX inhibition on these genes may be due to distal effects of COX inhibition on other maternal, placental or fetal organs. The fact that PGE2 incubation did not produce the opposite effect as chronic COX inhibition for this set of genes may also be due to differences in experimental design (in vitro versus in vivo) or species used (sheep versus mice).
Increased nitric oxide production has been implicated in delayed ductus closure following prolonged COX inhibition in utero (9). Functional coupling of COX and NOS systems is a well-recognized phenomenon (5,9). In the mouse ductus arteriosus, eNOS is the predominant isoform for nitric oxide production (35). We did not observe a change in ductus eNOS expression after chronic COX inhibition; however, we did observe changes in PDE1A and VEGF expression, which could contribute to increased nitric oxide activity or production (Table 1). Our contractility experiments were not designed to examine the effects of chronic COX inhibition on the production of nitric oxide or other vasodilators. We were primarily interested in examining the effects of COX inhibition on the contractile apparatus. Therefore, we specifically incubated the isolated ductus with inhibitors of prostaglandin and nitric oxide production to eliminate any differences between the groups in in vitro prostaglandin and nitric oxide production.
Our studies examine the chronic effects of COX inhibition and PGE2 stimulation on ductus gene expression and contractility. They do not identify the mechanism(s) by which PGE2 is able to affect these changes. cAMP has previously been shown to regulate K+ channel activity and expression in excitable cells (39); a similar mechanism may mediate the effects of PGE2 on CaL and K+ channel gene expression in the current experiments. Future studies, designed to measure protein expression and intracellular ion fluxes, will be necessary to identify the exact pathways that have been altered by our pharmacologic manipulations.
In summary, we speculate that the paradoxical effects of acute and chronic COX inhibition are consistent with the existence of two complementary roles for PGE2 during ductus development: one that promotes the expression of pathways necessary for its oxygen-induced closure following delivery, and, a second, which maintains ductus patency, for fetal wellbeing. A better understanding of these two processes will be important for the development of new strategies to treat preterm labor without affecting fetal vascular development.
Acknowledgments
Financial Support: This work was supported by grants from U.S. Public Health Service (NIH grants HL46691, HL77395) and by a gift from the Jamie and Bobby Gates Foundation.
Abbreviations
- COX
cyclooxygenase
- PGE2
prostaglandin E2
- PDA
patent ductus arteriosus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huhta JC, Moise KJ, Fisher DJ, Sharif DS, Wasserstrum N, Martin C. Detection and quantitation of constriction of the fetal ductus arteriosus by Doppler echocardiography. Circulation. 1987;75:406–412. doi: 10.1161/01.cir.75.2.406. [DOI] [PubMed] [Google Scholar]
- 2.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 3.Hammerman C, Glaser J, Kaplan M, Schimmel MS, Ferber B, Eidelman AI. Indomethacin tocolysis increases postnatal patent ductus arteriosus. Pediatrics. 1998;102:E56. doi: 10.1542/peds.102.5.e56. [DOI] [PubMed] [Google Scholar]
- 4.Clyman RI, Chorne N. Patent ductus arteriosus: evidence for and against treatment. J Pediatr. 2007;150:216–219. doi: 10.1016/j.jpeds.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clyman RI, Chen YQ, Chemtob S, Mauray F, Kohl T, Varma DR, Roman C. In utero remodeling of the fetal lamb ductus arteriosus: the role of antenatal indomethacin and avascular zone thickness on vasa vasorum proliferation, neointima formation, and cell death. Circulation. 2001;103:1806–1812. doi: 10.1161/01.cir.103.13.1806. [DOI] [PubMed] [Google Scholar]
- 6.Reese J, Anderson JD, Brown N, Roman C, Clyman RI. Inhibition of cyclooxygenase isoforms in late- but not midgestation decreases contractility of the ductus arteriosus and prevents postnatal closure in mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1717–R1723. doi: 10.1152/ajpregu.00259.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loftin CD, Trivedi DB, Tiano HF, Clark JA, Lee CA, Epstein JA, Morham SG, Breyer MD, Nguyen M, Hawkins BM, Goulet JL, Smithies O, Koller BH, Langenbach R. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc Natl Acad Sci USA. 2001;98:1059–1064. doi: 10.1073/pnas.031573498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA. 2000;97:9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baragatti B, Brizzi F, Ackerley C, Barogi S, Ballou LR, Coceani F. Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase. Br J Pharmacol. 2003;139:1505–1515. doi: 10.1038/sj.bjp.0705391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, Iwasaki S, Iwamoto M, Misra S, Tamura K, Hori H, Yokota S, Toole BP, Sugimoto Y, Ishikawa Y. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clyman RI, Waleh NS, Kajino H, Roman C, Mauray F. Calcium-dependent and calcium-sensitizing pathways in the mature and immature ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1650–R1656. doi: 10.1152/ajpregu.00300.2007. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi T, Gu H, Hagiwara N, Momma K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res. 1993;72:1218–1228. doi: 10.1161/01.res.72.6.1218. [DOI] [PubMed] [Google Scholar]
- 13.Tristani-Firouzi M, Reeve HL, Tolarova S, Weir EK, Archer SL. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest. 1996;98:1959–1965. doi: 10.1172/JCI118999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thebaud B, Michelakis ED, Wu XC, Moudgil R, Kuzyk M, Dyck JR, Harry G, Hashimoto K, Haromy A, Rebeyka I, Archer SL. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: implications for infants with patent ductus arteriosus. Circulation. 2004;110:1372–1379. doi: 10.1161/01.CIR.0000141292.28616.65. [DOI] [PubMed] [Google Scholar]
- 15.Thebaud B, Wu XC, Kajimoto H, Bonnet S, Hashimoto K, Michelakis ED, Archer SL. Developmental absence of the O2 sensitivity of L-type calcium channels in preterm ductus arteriosus smooth muscle cells impairs O2 constriction contributing to patent ductus arteriosus. Pediatr Res. 2008;63:176–181. doi: 10.1203/PDR.0b013e31815ed059. [DOI] [PubMed] [Google Scholar]
- 16.Kajimoto H, Hashimoto K, Bonnet SN, Haromy A, Harry G, Moudgil R, Nakanishi T, Rebeyka I, Thebaud B, Michelakis ED, Archer SL. Oxygen Activates the Rho/Rho-Kinase Pathway and Induces RhoB and ROCK-1 Expression in Human and Rabbit Ductus Arteriosus by Increasing Mitochondria-Derived Reactive Oxygen Species. A Newly Recognized Mechanism for Sustaining Ductal Constriction. Circulation. 2007;115:1777–1788. doi: 10.1161/CIRCULATIONAHA.106.649566. [DOI] [PubMed] [Google Scholar]
- 17.Hong Z, Hong F, Olschewski A, Cabrera JA, Varghese A, Nelson DP, Weir EK. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation. 2006;114:1372–1379. doi: 10.1161/CIRCULATIONAHA.106.641126. [DOI] [PubMed] [Google Scholar]
- 18.Costa M, Barogi S, Socci ND, Angeloni D, Maffei M, Baragatti B, Chiellini C, Grasso E, Coceani F. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genomics. 2006;25:250–262. doi: 10.1152/physiolgenomics.00231.2005. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Aikawa M, Kimura K, Kuro-o M, Nakahara K, Suzuki T, Katoh H, Okamoto E, Yazaki Y, Nagai R. Ductus arteriosus. Advanced differentiation of smooth muscle cells demonstrated by myosin heavy chain isoform expression in rabbits. Circulation. 1993;88:1804–1810. doi: 10.1161/01.cir.88.4.1804. [DOI] [PubMed] [Google Scholar]
- 20.Slomp J, Gittenberger-de Groot AC, Glukhova MA, Conny van Munsteren J, Kockx MM, Schwartz SM, Koteliansky VE. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol. 1997;17:1003–1009. doi: 10.1161/01.atv.17.5.1003. [DOI] [PubMed] [Google Scholar]
- 21.Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, Haase H, Bader M. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol. 2000;2:371–375. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest. 2008;118:515–525. doi: 10.1172/JCI33304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takizawa T, Oda T, Arishima K, Yamamoto M, Masaoka T, Somiya H, Akahori F, Shiota K. A calcium channel blocker verapamil inhibits the spontaneous closure of the ductus arteriosus in newborn rats. J Toxicol Sci. 1994;19:171–174. doi: 10.2131/jts.19.3_171. [DOI] [PubMed] [Google Scholar]
- 24.Loftin CD, Trivedi DB, Langenbach R. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J Clin Invest. 2002;110:549–557. doi: 10.1172/JCI14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouayad A, Kajino H, Waleh N, Fouron JC, Andelfinger G, Varma DR, Skoll A, Vazquez A, Gobeil F, Jr, Clyman RI, Chemtob S. Characterization of PGE2 receptors in fetal and newborn lamb ductus arteriosus. Am J Physiol Heart Circ Physiol. 2001;280:H2342–H2349. doi: 10.1152/ajpheart.2001.280.5.H2342. [DOI] [PubMed] [Google Scholar]
- 26.Reese J, O'Mara PW, Poole SD, Brown N, Tolentino C, Eckman DM, Aschner JL. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 2008 doi: 10.1016/j.prostaglandins.2008.11.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sodini D, Baragatti B, Barogi S, Laubach VE, Coceani F. Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br J Pharmacol. 2008;153:1631–1640. doi: 10.1038/bjp.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajino H, Chen YQ, Seidner SR, Waleh N, Mauray F, Roman C, Chemtob S, Koch CJ, Clyman RI. Factors that increase the contractile tone of the Ductus Arteriosus also regulate its anatomic remodeling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R291–R301. doi: 10.1152/ajpregu.2001.281.1.R291. [DOI] [PubMed] [Google Scholar]
- 29.Challis JR, Dilley SR, Robinson JS, Thorburn GD. Prostaglandins in the circulation of the fetal lamb. Prostaglandins. 1976;11:1041–1052. doi: 10.1016/0090-6980(76)90011-3. [DOI] [PubMed] [Google Scholar]
- 30.Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol Cell Physiol. 2003;285:C1377–C1385. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- 31.Janssen LJ, Tazzeo T, Zuo J, Pertens E, Keshavjee S. KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L852–L858. doi: 10.1152/ajplung.00130.2004. [DOI] [PubMed] [Google Scholar]
- 32.Nobe K, Paul RJ. Distinct pathways of Ca(2+) sensitization in porcine coronary artery: effects of Rho-related kinase and protein kinase C inhibition on force and intracellular Ca(2+) Circ Res. 2001;88:1283–1290. doi: 10.1161/hh1201.092035. [DOI] [PubMed] [Google Scholar]
- 33.Cogolludo A, Moreno L, Lodi F, Tamargo J, Perez-Vizcaino F. Postnatal maturational shift from PKCzeta and voltage-gated K+ channels to RhoA/Rho kinase in pulmonary vasoconstriction. Cardiovasc Res. 2005;66:84–93. doi: 10.1016/j.cardiores.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Tada T, Kishimoto H. Ultrastructural and histological studies on closure of the mouse ductus arteriosus. Acta Anat (Basel) 1990;139:326–334. doi: 10.1159/000147020. [DOI] [PubMed] [Google Scholar]
- 35.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, Reese J. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004;287:R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 36.Levin M, McCurnin D, Seidner SR, Yoder B, Waleh N, Goldbarg S, Roman C, Liu BM, Boren J, Clyman RI. Postnatal constriction, ATP depletion, and cell death in the mature and immature ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R359–R364. doi: 10.1152/ajpregu.00629.2005. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Manganiello VC, Clyman RI. Expression, activity and function of cAMP and cGMP phosphodiesterases in the mature and immature ductus arteriosus. Pediatr Res. 2008;64:477–481. doi: 10.1203/PDR.0b013e3181827c2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori Y, Matsubara H, Folco E, Siegel A, Koren G. The transcription of a mammalian voltage-gated potassium channel is regulated by cAMP in a cell-specific manner. J Biol Chem. 1993;268:26482–26493. [PubMed] [Google Scholar]