Abstract
We investigated the supramolecular structure of the Shigella type III secretion machinery including its major components. Our results indicated that the machinery was composed of needle and basal parts with respective lengths of 45.4 ± 3.3 and 31.6 ± 0.3 nm, and contained MxiD, MxiG, MxiJ and MxiH. spa47, encoding a putative F1-type ATPase, was required for the secretion of effector proteins via the type III system and was involved in the formation of the needle. The spa47 mutant produced a defective, needle-less type III structure, which contained MxiD, MxiG and MxiJ but not MxiH. The mxiH mutant produced a defective type III structure lacking the needle and failed to secrete effector proteins. Upon overexpression of MxiH in the mxiH mutant, the bacteria produced type III structures with protruding dramatically long needles, and showed a remarkable increase in invasiveness. Our results suggest that MxiH is the major needle component of the type III machinery and is essential for delivery of the effector proteins, and that the level of MxiH affects the length of the needle.
Keywords: MxiH/Shigella invasion/supramolecular structure/type III secretion system
Introduction
Invasion of epithelial cells by Shigella is an essential pathogenic feature of bacillary dysentery. The delivery of an effector protein set such as IpaA, IpaB, IpaC, IpaD, IpgD and VirA through the type III secretion system from Shigella into host epithelial cells is a prerequisite for triggering its own internalization process (Allaoui et al., 1993a; Ménard et al., 1993, 1994; Uchiya et al., 1995; Tran Van Nhieu et al., 1997; Tran Van Nhieu and Sansonetti, 1999). Although the precise role of each effector including the secretion mechanism via the type III secretion system is still to be investigated, recent studies have indicated that some of the delivered effector molecules, such as IpaA and IpaC, modulate the host cell actin dynamics, including the signal transduction pathways required for the bacteria involved (Tran Van Nhieu et al., 1997, 1999; Bourdet-Sicard et al., 1999; Tran Van Nhieu and Sansonetti, 1999). Genetic and functional studies have indicated further that the type III secretion machinery of Shigella flexneri is composed of 20 proteins encoded by the mxi and spa genes on the large 230 kb plasmid (Sasakawa et al., 1992, 1993; Venkatesan et al., 1992; Parsot, 1994). The mxi and spa genes in their respective operons exist as part of a 31 kb pathogenicity island where other virulence operons such as ipa, ipg and ics are also present upstream of the mxi operon (Sasakawa et al., 1989; Allaoui et al., 1992a, 1993a; Parsot, 1994).
Mxi and Spa type III secretion proteins share considerable homology with other putative proteins of type III secretion systems of Gram-negative pathogenic bacteria such as Salmonella, Yersinia, enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E.coli (EHEC), Pseudomonas aeruginosa, Bordetella bronchiseptica and Chlamydia, as well as several plant pathogenic bacteria (Lee, 1997; Hueck, 1998; Galán and Collmer, 1999). Recent studies have also suggested that type III secretion systems are specialized devices mediating insertion into the host plasma membrane by forming a pore through which bacterial effectors are injected into the cell cytosol (Cornelis and Wolf-Watz, 1997). More importantly, some of the amino acid sequences of the type III secretion systems also share significant homology with proteins of the bacterial flagellar export system, implying that the type III secretion machinery of Gram-negative bacteria may have evolved from genes associated with flagellar export systems (Hueck, 1998; Galán and Collmer, 1999). Indeed, Kubori et al. (1998) successfully isolated a type III secretion complex from Salmonella typhimurium, and further demonstrated by electron microscopy that the type III apparatus is composed of two major parts: the needle, which is ∼80 nm in length, and the basal part ∼40 nm in width.
The basal portion of the type III machinery, like the flagellar basal body, appeared to possess two sets of rings, namely upper and lower rings. In osmotically shocked S.typhimurium cells, the basal part was found to be located within the membrane by spanning the outer and inner membrane (Kubori et al., 1998). Although the precise structure of the basal part including the needle portion awaits further analysis, this study clearly indicated that the basic feature of the type III basal part is similar to the flagellar basal body. Similarly, Blocker et al. (1999) recently reported that the S.flexneri 5 M90T strain produces similar needle-like appendages protruding from the membrane envelope in osmotically shocked cells; however, the morphological features of the proposed type III secretion structure examined in the osmotically shocked bacteria seemed quite different from those of S.typhimurium observed by Kubori et al. (1998). Therefore, although the genes involved in the type III secretion systems of Shigella and Salmonella share considerable homology, whether or not the precise structure of the Shigella type III machinery is similar to that of Salmonella, including the identified components, remains to be elucidated.
In this context, we purified the intact type III secretion machinery of S.flexneri 2a and extensively characterized its supramolecular structure. The present study provides for the first time detailed structural information on the Shigella type III secretion machinery, including identification of the components of the basal and needle portions. Investigation into the role of the needle will provide a better understanding of the type III secretion system of Shigella and interaction of the host with bacteria, ultimately leading to the development of a vaccine targeted at this system.
Results
Membrane location of the type III machinery
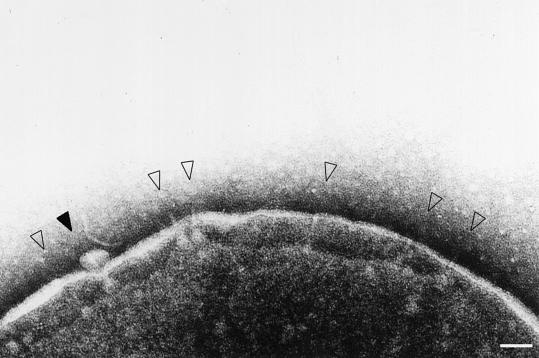
To investigate the membrane location and the morphological features of the type III secretion machinery, S.flexneri (M94) grown to early log phase were osmotically shocked and the envelope was examined via transmission electron microscopy (TEM). As shown in Figure 1, needle-like structures were recognized inside the membrane, with protrusions directed outside the outer membrane, while the basal part was embedded within the envelope. In some cases, a bleb-like moiety associated with the needle tip was observed, whereas no such needle-like structure was recognized in the envelope of the osmotically shocked YSH6200 (a 230 kb plasmid-cured S.flexneri) or del-17 (an ipa-mxi-spa deletion mutant) (data not shown). The needle-like structures present in the bacterial membrane of M94 resembled those of the S.typhimurium or S.flexneri 5 type III machinery (Kubori et al., 1998; Blocker et al., 1999).
Fig. 1. Electron micrograph of osmotically shocked M94 cells. Cells were negatively stained with 2% phosphotungstic acid pH 7.0 and observed under TEM. The open arrowheads indicate the type III secretion complexes on the bacterial envelope and the closed arrowhead denotes the bleb-like moiety associated with the tip of the complex, reminiscent of the secreted proteins through the type III secretion complexes. Scale bar, 100 nm.
Identification of type III complex components
To identify the components of the Shigella type III secretion machinery, we attempted to extract the needle-like structures from the envelope of M94 by modifying the method used for Salmonella (Kubori et al., 1998; see Materials and methods). Proteins of the partially purified membrane fractions from M94 together with the corresponding fractions from YSH6200 or del-17 were separated by 15% SDS–PAGE and examined by immunoblotting with anti-MxiD, anti-MxiG or anti-MxiJ antibodies (Figure 2A). MxiD, MxiG and MxiJ shared significant homology with Salmonella InvG, PrgK and PrgH, respectively, the major components of the type III machinery (Kubori et al., 1998). The results showed that the proteins corresponding to 63, 43 and 27 kDa were recognized by the anti-MxiD, anti-MxiG and anti-MxiJ antibodies, respectively (Figure 2A), suggesting that the partially purified membrane fraction of M94 contained the type III secretion machinery. To isolate this machinery, the membrane fraction was purified further by 40% (w/v) CsCl density gradient centrifugation, followed by fractionation into six aliquots. Proteins present in each aliquot were investigated by 15% SDS–PAGE combined with silver staining, and by immunoblottting with a mixture of anti-MxiD and MxiG antibodies. As shown in Figure 2B, the type III machinery was present mostly in fractions 1 (upper fraction) and 6 (lower fraction), although lesser amounts were also found in fractions 2 and 5. The components of the type III complex in the upper and lower fractions were pelleted by ultracentrifugation, and their structure examined by TEM. It was found that the upper fraction contained the needle-like structures resembling the Salmonella type III machinery (Kubori et al., 1998), although they existed as large aggregates, while the lower fraction contained similar needle-like structures but as individual isolates (Figure 3A). Analysis of the protein profiles in each fraction by SDS–PAGE with silver staining revealed that both the upper and lower fractions contained proteins of 63, 43 and 27 kDa (Figure 2B, arrowheads). Since the proteins were recognized by anti-MxiD, MxiG and MxiJ antibodies, respectively (data not shown), the N-terminal amino acid sequences of stained bands corresponding to 63, 43 and 27 kDa were analyzed. These N-terminal sequences were found to be NNIDSHLLEQ, SEAKNSNLAP and XEQREELISN, respectively, corresponding to the deduced N-terminal sequences of Asn23–Gln32 in MxiD, Ser2–Pro11 in MxiG and Cys18–Asn27 in MxiJ, respectively (Allaoui et al., 1992b, 1993b, 1995). Therefore, these results indicated that the needle-like structures isolated from M94 were the type III secretion complexes composed of at least MxiD, MxiG and MxiJ.
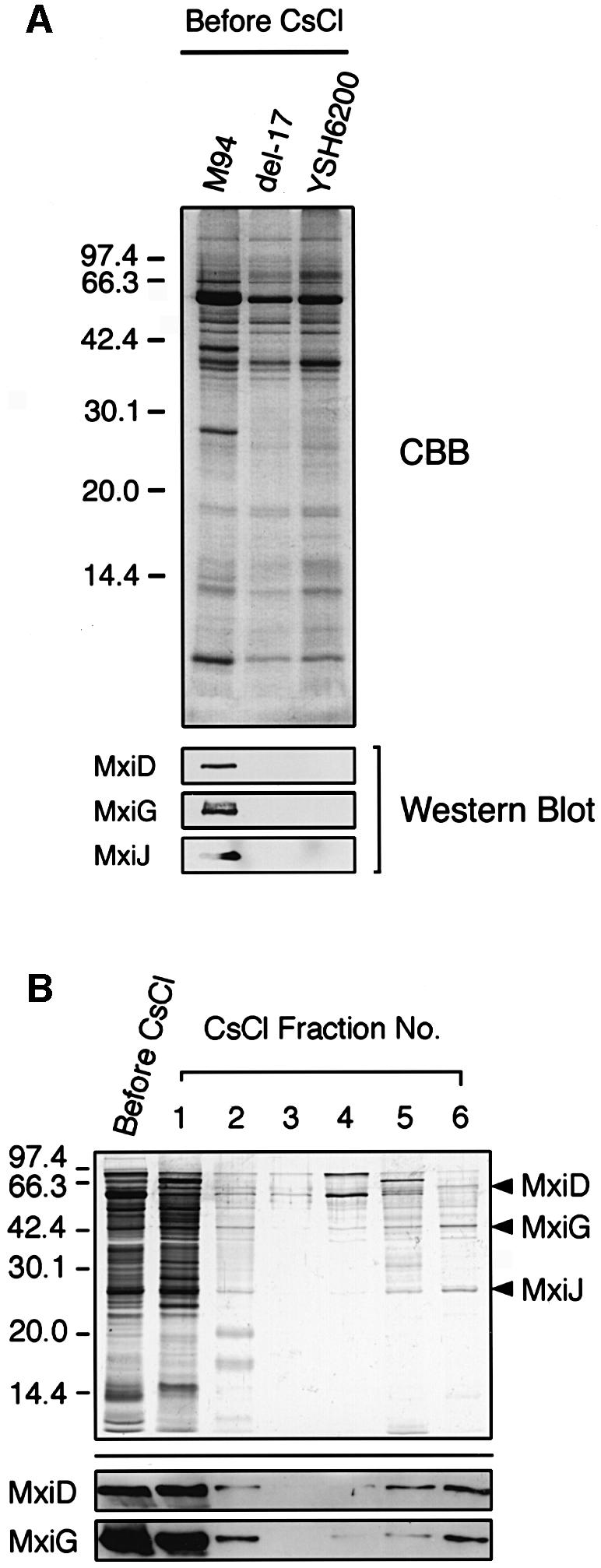
Fig. 2. Purification of type III secretion complexes from M94. (A) Each sample of partially purified type III secretion complexes was separated by 15% SDS–PAGE and the gel was stained with CBB or immuno blotted with antibodies specific for MxiD, MxiG and MxiJ. (B) A partially purified sample of type III secretion complexes from M94 was fractionated by 40% CsCl gradient centrifugation. Each fraction was separated by 15% SDS–PAGE and visualized by silver staining (upper part) or immunoblotted with antibodies specific for MxiD and MxiG (lower part).
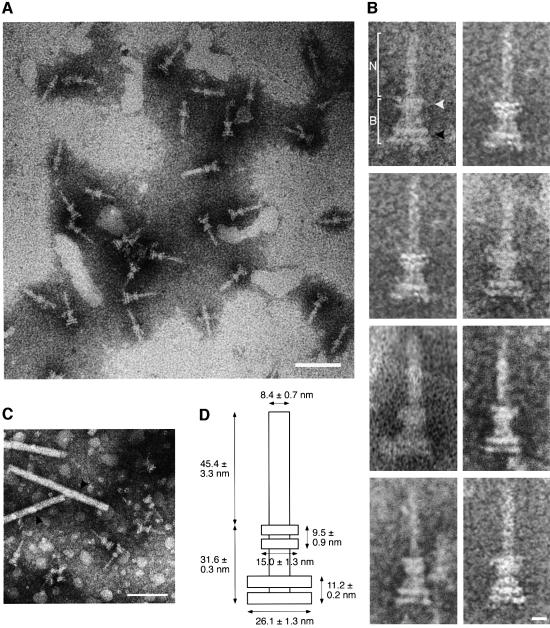
Fig. 3. Electron micrograph of purified type III secretion complexes from M94. (A) Purified type III secretion complexes at a low magnification. The sample was obtained from the lower fraction of a 40% CsCl gradient centrifugation, which corresponds to fraction 6 in Figure 2B. The sample was stained with 2% phosphotungstic acid pH 7.0 and observed by TEM. Scale bar, 100 nm. (B) Type III secretion complexes at a high magnification. N indicates the needle, while B indicates the basal part of the type III secretion complex. The open arrowhead denotes upper (or outer) rings, while the closed arrowhead denotes the lower (or inner) rings. Scale bar, 10 nm. (C) Measurement of the size of type III secretion complexes. The purified type III secretion complexes under TEM with TMV (300 nm long, 20 nm wide, arrowheads). Scale bar, 100 nm. (D) Proposed size of the type III secretion complex of S.flexneri. The size of each portion was measured based on the 28 best preserved type III secretion complexes in comparison with the scale of TMV.
Supramolecular structure of the type III complex
The type III secretion machinery present in fraction 6 of the above experiment was investigated for morphological features by TEM. As shown in Figure 3A, the secretion complex exhibited a needle-like structure with cylindrical symmetry. Individual components of the complex enlarged on electron micrographs further indicated that the basal part comprised two upper and two lower rings (Figure 3B). To estimate the size of each portion, the purified type III machinery was observed by TEM in the presence of tobacco mosaic virus (TMV) (Figure 3C). The TMV particle was shown to be 300 nm long and 20 nm wide (Butler, 1984). Accordingly, the needle portion was 45.4 ± 3.3 nm long and 8.4 ± 0.7 nm wide (n = 28), while the length of the basal portion was 31.6 ± 0.3 nm. The upper rings were estimated to be 9.5 ± 0.9 nm in width and 15.0 ± 1.3 nm in thickness, while the lower rings were 26.1 ± 1.3 nm in width and 11.2 ± 0.2 nm in thickness. The proposed structure of the S.flexneri type III secretion machinery is shown in Figure 3D.
Identification of the needle portion of the type III complex
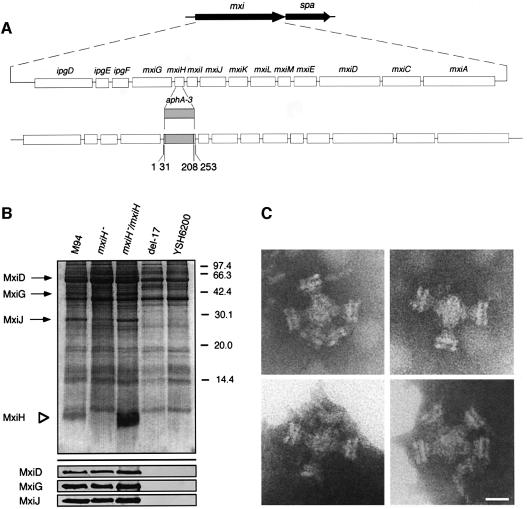
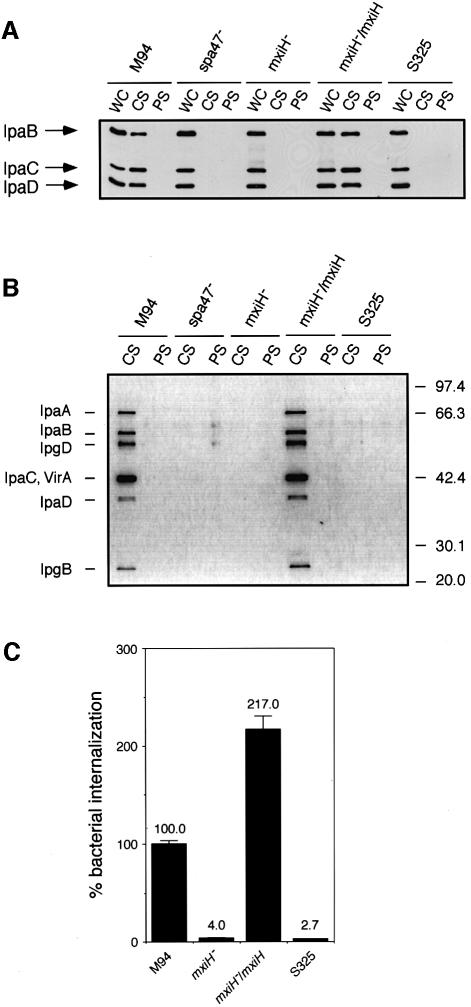
Studies of the flagellar export system of S.typhimurium have indicated that the hook assembly (FlgE) of the flagella can be formed following the transport of FlgE through the hollow conduit of the basal body (Macnab, 1992; Aizawa and Kubori, 1998), for which the activity of FliI, an F1-type ATPase, was shown to be required (Dreyfus et al., 1993; Fan and Macnab, 1996; Aizawa and Kubori, 1998). Since Spa47 of Shigella shared significant amino acid homology with FliI, and the spa47 mutant of S.flexneri was shown to be defective in the transport of type III secretion proteins (Venkatesan et al., 1992), we investigated whether Spa47 would be required for transport of the needle component on the type III secretion machinery. We constructed a non-polar spa47 mutant from YSH6000 (wild-type S.flexneri) (Figure 4A) and examined the components of the type III system using TEM, which showed that the bacteria produced defective type III particles lacking the needle (Figure 4B), suggesting that Spa47 is required for the formation of the needle structure on the type III basal body. Determination of the protein compositions of the partially purified type III structures on 20% SDS–PAGE with Coomassie Brilliant Blue (CBB) staining and immunoblotting with anti-MxiD, anti-MxiG and anti-MxiJ antibodies revealed that although the type III machinery extracted from the spa47 mutant and M94 possessed MxiD, MxiG and MxiJ, that of the spa47 mutant lacked an ∼9 kDa protein (Figure 4C). Examination of the corresponding membrane fractions prepared from del-17 or YSH6200 by 20% SDS–PAGE with CBB staining also showed lack of a 9 kDa protein (Figure 4C). We therefore analyzed the N-terminal amino acid sequence of the missing protein. The sequence, SVTVPNDDWT, corresponded to the deduced N-terminal sequence of Ser2–Thr11 of MxiH (Allaoui et al., 1992b), which is encoded by the mxiH gene in the mxi operon on the large plasmid of S.flexneri (Figure 5A).
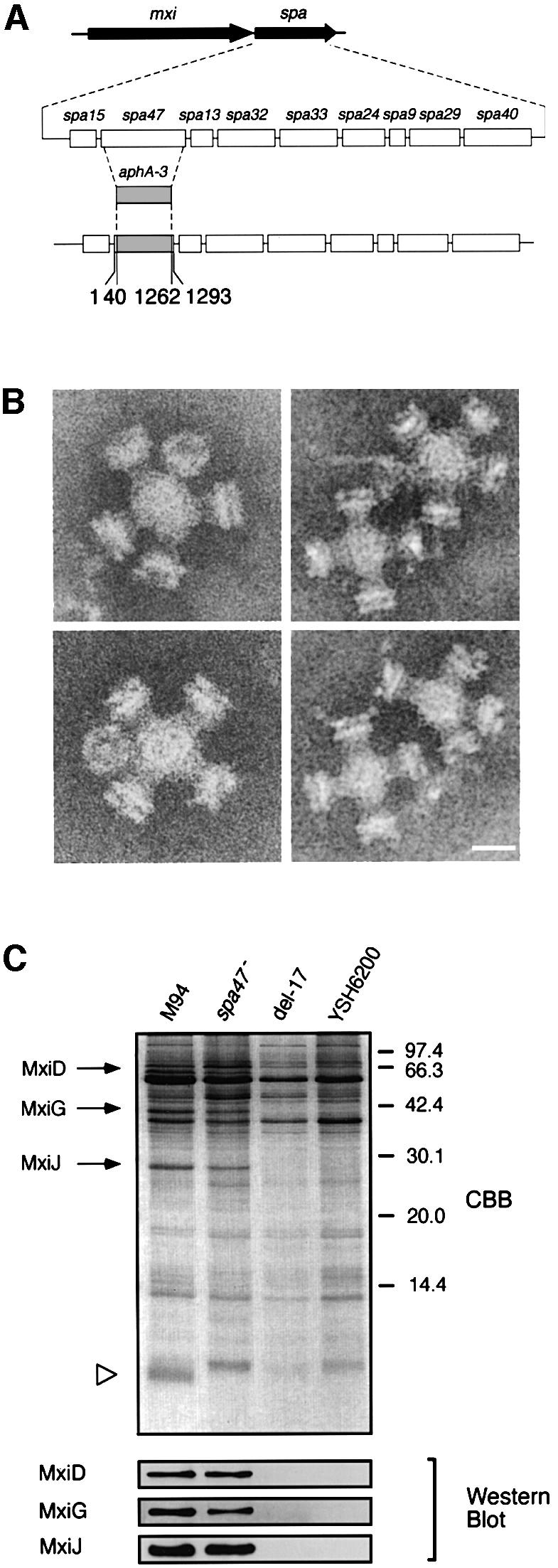
Fig. 4. Effect of the spa47 mutation on the formation of type III secretion complexes. (A) Genomic organization of the spa operon and construction of an spa47::aphA-3 mutant. (B) Electron micrographs of the purified type III secretion complexes from the spa47 mutant. Scale bar, 20 nm. (C) Pattern on the 20% SDS–polyacrylamide gel and immunoblot with antibodies specific for MxiD, MxiG and MxiJ of partially purified type III secretion complexes of M94, the spa47 mutant (spa47–) and non-invasive strains del-17 and YSH6200. The gel was stained with CBB.
Fig. 5. Analysis of the mxiH mutant and its complemented mutant for the type III secretion complexes. (A) Genomic organization of the mxi operon and construction of the mxiH::aphA-3 mutant. (B) Pattern on the 20% SDS–polyacrylamide gel (upper part) and immunoblot (lower part) with antibodies specific for MxiD, MxiG and MxiJ of partially purified type III secretion complexes of M94, the mxiH mutant (mxiH–), the mxiH mutant harboring pKT001 (mxiH–/mxiH), del-17 and YSH6200. The gel was stained with CBB. (C) Electron micrographs of the purified type III secretion complexes from the mxiH mutant. Scale bar, 20 nm.
Since the above results suggested MxiH to be a component of the needle of the type III secretion machinery, we constructed a non-polar mxiH mutant from YSH6000 (Figure 5A) and examined both the components and structure of the type III complex. The partially purified type III machinery prepared from the mxiH mutant contained proteins corresponding to 63, 43 and 27 kDa, but lacked the 9 kDa protein, while that from M94 contained all four proteins (Figure 5B). Immunoblotting with anti-MxiD, anti-MxiG and anti-MxiJ antibodies indicated that the 63, 43 and 27 kDa proteins present in the type III machinery from M94 were MxiD, MxiG and MxiJ, respectively (Figure 5B). Analysis of the type III structure by TEM revealed that the mxiH mutant produced defective type III particles similar to those produced by the spa47 mutant (Figure 5C). Since the needles of the type III secretion machinery protruded towards the outside of M94, the spa47 and mxiH mutants were osmotically shocked in order to examine the surface protrusions by TEM. The results showed that although the needles were detected on the surface of M94, they were undetectable on the bacterial surface of the spa47 or mxiH mutant (data not shown).
Levels of MxiH in Shigella can affect the needle length
To confirm the above notion further, we cloned the wild-type mxiH gene from S.flexneri into pTB101 (pKT001), which in turn was introduced into the mxiH mutant. The bacteria were grown in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; see Materials and methods), and the type III machinery extracted from the bacteria was examined for protein composition by 20% SDS–PAGE with CBB staining. As shown in Figure 5B, although the protein bands were similar to those of M94, the amount of 9 kDa protein was increased compared with that of M94. The N-terminal amino acid sequencing confirmed the 9 kDa protein to be MxiH.
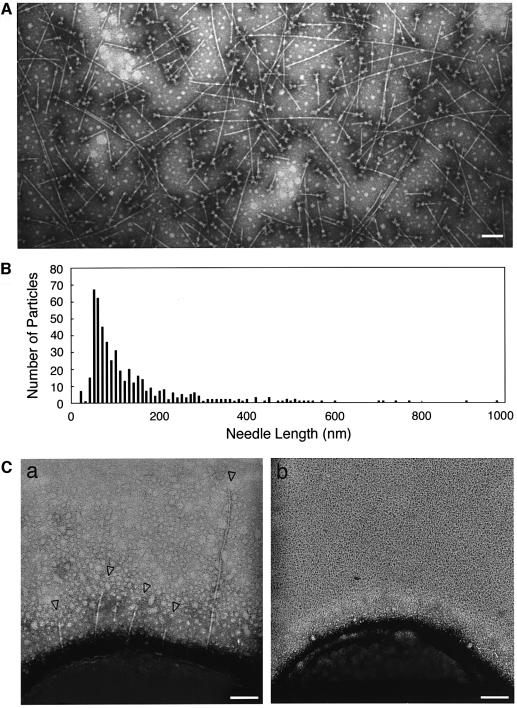
To ensure the restoration of needle formation on the type III machinery, we investigated the type III complex extracted from bacteria overexpressing MxiH using TEM. Surprisingly, long needles protruded from the type III machinery, although the lengths varied considerably (Figure 6A). The distribution of the lengths of needles was examined using 497 randomly chosen type III structures. As shown in Figure 6B, the lengths varied greatly, ranging from 45 nm to 1 µm with a peak at 40–50 nm. Indeed, the bacterial surface exhibited needle protrusions of various lengths (Figure 6C, panel a) that were not detected on bacteria grown in medium without IPTG (Figure 6C, panel b), suggesting that the long needles extending from the purified type III machinery were not assembled during extraction. To investigate further whether the length of needles was affected by the level of MxiH production, bacteria were grown in the presence of 1.0, 0.1, 0.01 or 0 mM IPTG. As the level of MxiH production decreased, the length of the needles was also reduced. In the absence of IPTG, needles shorter than those of M94 were observed from the type III particles, possibly due to the leakage of MxiH from the tac promoter (data not shown). Thus, these results strongly indicate that MxiH is the major needle component, and that the length of the needle could depend on the level of MxiH production.
Fig. 6. Effect of overexpression of MxiH in Shigella on the length of type III secretion needles. (A) Electron micrograph of the purified type III secretion complexes from the mxiH mutant harboring pKT001 (cloned mxiH gene). Bacteria were grown in L-broth and induced with 1 mM IPTG. Scale bar, 100 nm. (B) Distribution of needle lengths in the type III secretion complexes purified from the mxiH mutant harboring pKT001 grown in L-broth with 1 mM IPTG. (C) Electron micrographs of the mxiH mutant harboring pKT001 grown in L-broth with 1 mM IPTG (a) or without IPTG (b). Arrowheads are indicative of the long needle structures in type III secretion complexes protruding from the cell surface. Scale bars, 100 nm.
Effect of mxiH mutation on the activity of the type III secretion system
Although the precise mechanisms underlying the secretion of type III effector proteins such as IpaB and IpaC into the bacterial environment and the host cell cytosol remain unclear, recent studies have indicated that the IpaB–IpaC complex formed a membrane pore in the target host membrane required for delivery of other effector proteins such as IpaA, IpgD and VirA (Demers et al., 1998; Blocker et al., 1999; Boudet-Sicard et al., 1999; Tran Van Nhieu and Sansonetti, 1999). In this context, we examined the involvement of the MxiH needle in mediating secretion of Ipa proteins. The mxiH and spa47 mutants were investigated together with M94 for their ability to secrete Ipa proteins into the environmental medium when incubated in phosphate-buffered saline (PBS) containing 0.003% Congo red, a conditional medium for stimulating secretion of Shigella effector proteins through the type III secretion system (Parsot et al., 1995). The results showed that secretion of IpaB, IpaC and IpaD from the mxiH or the spa47 mutant but not M94 was almost completely abolished, as revealed by immunoblotting with anti-IpaB, anti-IpaC and anti-IpaD antibodies (Figure 7A). The mxiH mutant harboring pKT001 (cloned mxiH gene) grown in the presence of 1 mM IPTG, which formed various lengths of long needles, resulted in restoration of the ability to secrete Ipa proteins (Figure 7A). Indeed, the amounts of other secreted effector proteins including IpaB, IpaC and IpaD, as examined by SDS–PAGE with silver staining, indicated that the capacity of bacteria overexpressing MxiH was also restored (Figure 7B). The invasiveness of the mxiH mutant or the mutant harboring pKT001 was also examined by a conventional gentamicin protection assay with HeLa cells. The results showed that although invasiveness of the mxiH mutant was reduced to <5% of the wild-type level (100%), the invasiveness of the mxiH mutant carrying pKT001 grown in the presence of 1 mM IPTG was greatly increased to a higher level (217%) than the wild-type level (100%) (Figure 7C). These results further confirm that MxiH is an essential component in the secretion of Shigella effector proteins.
Fig. 7. Effect of the mxiH mutation on secretion of effector proteins and invasion of HeLa cells. (A) Immunoblot analysis of secreted proteins from M94, spa47–, mxiH–, mxiH– harboring pKT001 (mxiH–/mxiH) or the S325 strain by addition of Congo red (0.003% final concentration) with antibodies specific for IpaB, IpaC and IpaD. S325 is a mxiA::Tn5 mutant used for negative control of Ipa secretion. WC, CS and PS denote the whole-cell lysate, Congo red-induced supernatant and supernatant in PBS, respectively. (B) Band pattern on a 10% SDS–polyacrylamide gel of secreted proteins from M94, spa47–, mxiH–, mxiH– harboring pKT001 (mxiH–/mxiH) or the S325 strain by addition of Congo red. The gel was silver stained. (C) Invasiveness of the mxiH mutant (mxiH–) or the mxiH mutant harboring pKT001 (mxiH–/mxiH) in HeLa cells by the gentamicin protection assay.
Discussion
In this study, we have attempted to elucidate the supra molecular structure of the type III secretion machinery of S.flexneri 2a, and report for the first time on its precise size including its major structural components. Our results indicate that the type III machinery is composed of two major parts, a basal body and a needle, and that the morphological feature including the membrane localization resemble those of S.typhimurium (Kubori et al., 1998; Galán and Collmer, 1999). We have identified MxiD, MxiG, MxiJ and MxiH as the components of the type III machinery, in which the basal body is composed of at least MxiD, MxiG and MxiJ, while the needle is composed of at least MxiH. MxiD, MxiG and MxiJ share significant amino acid similarity with Salmonella InvG, PrgH and PrgK, respectively, known as the major components of Salmonella type III secretion machinery (Galán, 1996; Kubori et al., 1998; Galán and Collmer, 1999). Although no direct evidence has yet been obtained, the same could be true for MxiH and PrgI, a MxiH homolog of Salmonella (Galán, 1996). Genetic and functional studies have indicated that the putative components of the type III secretion systems of Shigella and Salmonella share significant amino acid similarity (Galán, 1996), and some of them also share significant amino acid similarity with putative components of other type III secretion systems including bacterial flagellar export systems (Lee, 1997; Hueck, 1998; Galán and Collmer, 1999). Although no significant amino acid similarity of MxiD, MxiG, MxiJ and MxiH to any of the known flagellar export components is found, our structural and functional analyses of the Shigella type III machinery, including the study by Kubori et al. (1998), strongly indicate that the basal body is similar to that of the flagellar export machinery. For example, both type III and flagellar basal bodies possess two upper and lower rings. Furthermore, the estimated size of the lower rings of the Shigella type III machinery is 26 nm wide and 11 nm thick, while the flagellar MS ring is estimated to be 25 nm wide and 11 nm thick (Katayama et al., 1996). Therefore, we assume that the morphological characteristics of the basal body of the S.flexneri 2a type III machinery indicated by this study is substantially similar to that of the flagellar export machinery.
It is worth noting that Blocker et al. (1999) recently observed that the type III secretion complex interacted with the envelope of osmotically shocked S.flexneri 5 MT90. Based on the structural analysis, they proposed that the type III complex formed a tripartite structure composed of an external needle, a neck and a large proximal bulb. Their type III structure possessed a large globular basal part called the ‘bulb’, which was estimated to be 44 nm wide and 42 nm thick, almost twice the width of our type III structure (26.1 nm wide and 31.6 nm thick). In our electron microscopic images, the features of the type III secretion complex closely resembled those of Salmonella observed by Kubori et al. (1998). Although the reason for the different morphological features of S.flexneri type III machinery shown by this and the previous study is unclear, they are most likely to be due to the different experimental design; Blocker et al. (1999) only made observations on the type III particles associated with the envelope of the osmotically shocked bacteria, while we observed the entire purified type III machinery. We assume that the ‘bulb structure’ observed by Blocker et al. (1999) is composed of highly conserved constituents of the type III secretion apparatus.
The needle structure of the type III secretion system of S.flexneri 2a, similarly to those of S.typhimurium or S.flexneri 5 (Kubori et al., 1998; Blocker et al., 1999; this study), protruded straight outwards from the outer membrane as revealed by examination of the osmotically shocked cell envelope (Figure 1). Extensive analysis of the purified Shigella type III system in the presence of TMV estimated the length of the needle to be 45.4 nm and its diameter 8.4 nm. As has been demonstrated for Salmonella (Kubori et al., 1998), the needle portion of the Shigella type III machinery corresponds to the flagellar hook, although the morphological feature of the hook represents a slightly curved cylindrical structure protruding from the basal body (Katayama et al., 1996). The flagellar hook is composed of FlgE, which is thought to be assembled along the rod over the MS ring, the lower rings of the flagellar basal body (Kubori et al., 1992; Macnab, 1992; Katayama et al., 1996; Aizawa and Kubori, 1998). The hook proteins, as well as flagellin and its capping proteins, are thought to be exported through the hollow conduit of the basal body (Macnab, 1992; Aizawa and Kubori, 1998), for which energy supplied through hydrolysis of ATP with FliI, an F1-type ATPase, is required (Dreyfus et al., 1993; Fan and Macnab, 1996). Spa47 of S.flexneri shares significant amino acid similarity with FliI, and has been suggested to be required for secretion of effector proteins through the type III secretion system (Venkatesan et al., 1992), although whether the Spa47 activity would be involved in the assembly of the type III secretion system had remained unclear. Thus, in this study, we constructed a non-polar spa47 mutant of S.flexneri to investigate the effect on the assembly of the type III machinery. Our results indicate that the spa47 mutant produces defective type III particles, which seem to lack the needle (Figure 4B), suggesting that Spa47 activity is not involved in the assembly of the basal body, but is required for the export of needle components through its type III secretion structure. Examination of the protein composition of the defective type III particles produced by the spa47 mutant showed that although the particles contained MxiD, MxiG and MxiJ, they lacked an ∼9 kDa protein band. Since the 9 kDa protein including MxiD, MxiG and MxiJ was never detected in any of the membrane fractions prepared from the del-17 mutant or YSH6200, we presumed that the 9 kDa protein is a component of the type III system. N-terminal amino acid sequencing of the 9 kDa protein indicated that it corresponded to MxiH encoded by the mxiH gene in the mxi operon on the large plasmid of S.flexneri (Allaoui et al., 1992b). A constructed non-polar mxiH mutant of S.flexneri demonstrated production of defective type III particles similar to those observed in the spa47 mutant. Indeed, the type III particles lacked the needle structures (Figures 4B and 5C), and the partially purified type III structure from the mxiH mutant lacked the 9 kDa protein but possessed MxiD, MxiG and MxiJ (Figure 5B), further indicating that the MxiH protein is a component of the needle. Importantly, the deduced amino acid sequence of MxiH has no signal sequence at the N-terminus nor a putative internal transmembrane domain; rather, it contains two predictable coiled-coil domains at amino acid residues Leu26–Lys39 and Gln45–Thr58 as analyzed by the program COILS on http://www.ch.embnet.org/software/COILS_form.html. As coiled-coil domains have been indicated to be characteristic of proteins capable of protein–protein interactions and secretion through type III secretion systems (Pallen et al., 1997), these results together strongly suggest that MxiH is exported through the type III secretion machinery and is involved in the needle structure.
It is worth noting that the electron microscopic images of the purified defective type III particles from both the spa47 mutant and the mxiH mutant revealed the formation of oligomers (Figures 4B and 5C). Similar oligomeric forms were also noted in the purified defective type III structure from S.typhimurium lacking the needle structure (Kubori et al., 1998). Therefore, although the reason for the formation of oligomers is unclear, some proteins exposed on the defective type III particles might result in the formation of such an assembly through a process of extraction from the bacterial envelope.
The mxiH mutant, like the spa47 mutant (Venkatesan et al., 1992), shows not only failure to form a needle on the type III basal body, but also the inability to secrete Ipa proteins into the environmental medium, thus becoming non-invasive upon infection of HeLa cells. Although we do not yet know the exact reason for the presence of the defect during the secretory process in the absence of MxiH, the protein may also be involved as an essential component in the putative hollow conduit of the basal body of the type III machinery. Alternatively, the absence of MxiH from the type III machinery may somehow lead to a closure of the putative channel of the basal body. It has been indicated that upon contact with the target host cell, Shigella secrete Ipa proteins including other effector proteins via the type III machinery, where the secreted IpaB and IpaC form a membrane pore in the host cell membrane, thus allowing the other effector proteins to be delivered into the host cytosol (Ménard et al., 1994; Parsot, 1994; Watarai et al., 1995; Blocker et al., 1999). Similar systems have also been indicated in the other type III secretion systems, such as in Yersinia and Salmonella (Kaniga et al., 1995; Håkansson et al., 1996; Cornelis and Wolf-Watz, 1997; Cornelis, 1998). In this context, the needle assembled on the type III basal body could serve as the bridge between the contact bacterial surface and the host cell, in which the needle together with the IpaB–IpaC pore would act as the functional channel mediating export of the effector proteins into the target host cells. Therefore, the absence of a needle portion on the type III structure must cause another serious problem in the delivery of effector proteins from the contact bacterium into the host cell.
Surprisingly, the mxiH mutant overexpressing MxiH, as induced with 1 mM IPTG, produced aberrant but functional type III machinery possessing remarkably long needles (Figure 6). Interestingly, the lengths of wild-type needles were distributed in a narrow range with a peak at 42.7 ± 3.3 nm (Figure 3D). However, the lengths of aberrant needles were distributed over a wider range (from 45 nm to 1 µm), with a noticeable peak at 40–50 nm (Figure 6B). Since the addition of 0.1 mM IPTG resulted in a significant reduction in the distribution range of needle length, the level of MxiH can possibly affect the needle length. In the case of the flagellar hook of S.typhimurium, the fliK mutation was shown to produce long hooks (called polyhooks) (Patterson-Delafield et al., 1973). FliK has been assumed to regulate hook length, but not the hook component itself. Hirano et al. (1994) previously reported that the lengths of wild-type hooks were distributed over a narrow range with a peak at 55 ± 6 nm, but the polyhooks produced from the fliK mutant were distributed in a wider range (from 20 nm to 1 µm) (Koroyasu et al., 1998). Although FliK and MxiH share no amino acid homology at all, we cannot rule out the possibility that MxiH may have a regulatory role rather than being a structural component of the needle complex. In any case, the type III secretion needle and the flagellar hook share the dynamic aspect of changeable lengths.
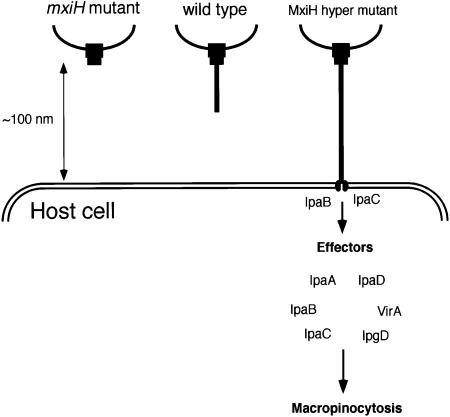
Interestingly, bacterial invasiveness under high level MxiH expression also increased dramatically to 2.5-fold wild-type invasiveness. Since the level of Ipa secretion by the bacteria into the culture medium was not markedly elevated, it is not clear whether the long needles generated by the type III machinery in Shigella can solely lead to the efficient invasiveness. However, Blocker et al. (1999) recently suggested that the translocation efficiency of effector proteins via the type III machinery in Shigella in contact with the host cells can certainly be affected by the distance between the bacterial and host cell surfaces, including the strength of bacterial contact. In that study, they indicated that the maximum distance required for functional contact of type III machinery with the host cells, including the formation of the IpaB–IpaC pore in the host plasma membrane, would be ∼100 nm. In this regard, the length of the needle of the type III machinery, estimated in this study as 45.4 nm, is clearly too short to allow direct contact with the host cell (Figure 3D). Therefore, the dramatic increase in invasiveness of the bacteria expressing a high level of MxiH, which leads to formation of long needles (maximum 22 times longer than wild type), may be accounted for by the easier access of the long needles to the surface of host cells compared with that of wild-type needles. Although we must await further investigation into the control systems for the length of type III needles, the fact that the needles can be extended in length dramatically may suggest the potency to protrude and retract, which might take place during attachment of a bacterium to and detachment from the host cell surface in vivo (Figure 8).
Fig. 8. A schematic representation of the interaction between the extended needles of the Shigella type III secretion machinery and the target host cell. The length of the wild-type needle of the type III secretion machinery of S.flexneri was estimated as 45.4 ± 3.3 nm (Figure 3D), while the maximum distance between the contact bacterium and the target epithelial cells for efficiently delivering the effector proteins into the host cell was proposed to be ∼100 nm (Blocker et al., 1999). Although Shigella expressing wild-type type III machinery may be too short to achieve direct contact, because the length of type III needles can be extended by elevated MxiH (Figure 6), wild-type Shigella may have the ability to extend long needles by controlling mxiH expression under in vivo conditions.
Materials and methods
Bacterial strains, plasmids and media
Shigella flexneri 2a YSH6000 is the wild type and YSH6200 is a large plasmid-less derivative of YSH6000 (Sasakawa et al., 1986). M94 is invasive but defective in intra- and intercellular spreading due to a Tn5 insertion in the virG gene of YSH6000 (Makino et al., 1986). del-17 is a spontaneous deletion derivative of YSH6000 lacking the 31 kb pathogenicity island containing ipa, mxi and spa operons (Sasakawa et al., 1986, 1988). S325 is a mxiA::Tn5 derivative of YSH6000 used as the negative control for the type III secretion-defective mutant (Watarai et al., 1995). Escherichia coli K-12 MC1061 was used as the host for constructing various plasmids. pBluescriptII SK+ (pBS) (Stratagene) was used as the cloning vector. pCACTUS-Tpr is a temperature- and sucrose-sensitive suicide vector constructed by inserting a trimethoprim resistance gene in the AseI site of pCACTUS. The trimethoprim resistance gene was derived from pMY6003 digested at its EcoRI and PvuII sites and filled in using Klenow enzyme (Makino et al., 1986). pTB101 is an expression vector used to express the mxiH gene in S.flexneri (Tobe et al., 1992). All S.flexneri-derived strains were grown routinely in brain–heart infusion (BHI) broth (Difco). Mueller–Hinton (MH) broth (Difco) was used for selection by trimethoprim resistance. HeLa cells were maintained in minimal essential medium (MEM) (Sigma) with 10% fetal calf serum (FCS) (Nichirei, Tokyo, Japan) in a 37°C incubator supplemented with 5% CO2.
Preparation of osmotically shocked cells of S.flexneri
Bacteria were collected after centrifugation of 1.5 ml of bacterial culture in L-broth grown to early log phase at 37°C, followed by suspension in 0.15 ml of ice-cold sucrose solution (0.5 M sucrose, 0.15 M Tris; pH not adjusted). After incubation on ice for 30 min, 1.5 ml of ice-cold 0.1 M EDTA-2Na pH 8.0 was added to the cell suspension. At this stage, cells were osmotically shocked, and intact cells were subsequently removed by low-speed centrifugation (5000 g for 10 min at 4°C), followed by collecting osmotically shocked cells in the supernatant by high-speed centrifugation (18 500 g for 20 min at 4°C). Pelleted cells were resuspended in 10 µl of ice-cold 0.1 M EDTA-2Na (pH 8.0) and immediately examined by TEM.
Isolation of type III secretion complexes from S.flexneri
A 10 ml aliquot of overnight culture grown at 30°C in L-broth was inoculated into 1 l of L-broth, and the bacteria were grown until late log phase (∼1 × 109 cells/ml) at 37°C. For spheroplast formation, the bacteria collected by centrifugation were suspended in 100 ml of ice-cold sucrose solution containing 1 mM phenylmethylsulfonyl fluoride, 2 mM EDTA-2Na (pH 8.0) and 0.2 mg/ml lysozyme, followed by incubation for 15 min at 4°C and for a further 15 min at 30°C. The resulting spheroplasts were then lysed by addition of 10% Triton X-100 and 1 M MgCl2 (the final concentration of Triton X-100 was 0.1% and MgCl2 4 mM) followed by addition of 8 mg of DNase I (Sigma, Code No. DN-25). After the cell debris was removed by centrifugation at 20 000 g for 20 min at 4°C, NaCl was added to the cleared lysate to a final concentration of 0.3 M and the lysate was incubated further at 4°C for 30 min. At this stage, since the alkaline treatment for extracting type III secretion complexes from Salmonella membrane caused degradation of type III secretion complexes from M94, this treatment was omitted in this experiment. To collect type III secretion complexes, the lysate was subjected to ultracentrifugation at 110 000 g for 60 min at 4°C. The pellet was then suspended in 1 ml of TET buffer (10 mM Tris–HCl pH 8.0, 1 mM EDTA-2Na pH 8.0, 0.1% Triton X-100) and stored overnight at 4°C. The insoluble materials appeared in the solution after overnight stock was removed by a low-speed centrifugation, and the solution was then fractionated by 40% (w/v) CsCl density gradient centrifugation at 38 000 g for 10 h at 24°C. Each of the fractions was diluted in a small amount of TET buffer, and fractions containing type III secretion complex were centrifuged further at 225 000 g for 60 min at 4°C. The pelleted type III secretion complexes were suspended in a small amount of TET buffer.
Construction of non-polar mutants of spa47 or mxiH of S.flexneri
For construction of non-polar mutants of spa47 or mxiH, the aphA-3 (the kanamycin resistance gene) cassette specifically designed for the construction of non-polar mutants was used (Ménard et al., 1993). Non-polar mutants of spa47 were constructed as follows. A DNA fragment encompassing nucleotides from position 1837 upstream of the 5′ end of the spa47 gene through to nucleotide 40 downstream from the 5′ end was amplified by PCR using primers 5′-CCAAACTGCAGGGTAT GAGTCAACATGGAATG-3′ containing a PstI site and 5′-GCTCCCCC GGGTAGGAAAAGATAATTGAGTG-3′ containing a SmaI site. The PstI–SmaI fragment was cloned into pBS, resulting in plasmid p47N. Another DNA fragment encompassing nucleotides from position 1262 downstream from the 5′ end of the spa47 gene through to nucleotide 3062 was amplified using primers 5′-GCTCCCCCGGGAATGGAGCTTATT GGTGAAAC-3′ containing a SmaI site and 5′-GCGGCTCTAGAAGCC AGCCTTACATTAGATG-3′ containing an XbaI site, and the SmaI–XbaI fragment was cloned into p47N using the SmaI and XbaI sites, yielding plasmid p47NC. The aphA-3 cassette was cloned at the SmaI site of p47NC in the correct orientation, resulting in plasmid p47NCK. The inactivated spa47 gene digested from p47NCK at pBS-derived SalI and SacI sites was subcloned into pCACTUS-Tpr, followed by introduction of the resultant plasmid into YSH6000 by electroporation. The transformants were grown on L-agar plates supplemented with 5% sucrose without NaCl at 42°C. The resulting sucrose- and kanamycin-resistant colonies were tested for trimethoprim sensitivity, indicative of the loss of suicide vector. One of the trimethoprim-sensitive colonies thus selected was confirmed to contain an insertion of the aphA-3 gene in the spa47 gene, as determined from restriction enzyme digestion of the PCR-amplified segment.
Non-polar mutants of mxiH were constructed as follows. A DNA fragment encompassing nucleotides from position 1744 upstream of the 5′ end of the mxiH gene through to nucleotide 31 downstream from the 5′ end was amplified using primers 5′-CAAAACTGCAGGCTTAGCTAC TGATGATGAAG-3′ and 5′-GCTCCCCCGGGTCCAATCATCATTC GGTACTG-3′ containing a SmaI site. The amplified DNA has the PstI site at nucleotide 1448 upstream of the 5′ end of the mxiH gene, and the PstI–SmaI fragment was cloned into pBS, resulting in plasmid pHN. Another DNA fragment encompassing nucleotides from position 208 downstream from the 5′ end of the mxiH gene through to nucleotide 2025 was amplified using primers 5′-GCTCCCCCGGGTGATTAAGGATGT TGATGCTG-3′ containing a SmaI site and 5′-GGCGCGGATCCTGCT TTAACTTCCATTCAC-3′. The amplified DNA has the PvuII site at nucleotide 1508 downstream from the 5′ end of mxiH, and the SmaI–PvuII fragment of this DNA was cloned at the SmaI site of pHN, yielding plasmid pHNC. The aphA-3 cassette was cloned at the SmaI site of pHNC in the correct orientation, resulting in plasmid pHNCK. The inactivated mxiH gene digested from pHNCK at PstI and pBS-derived XbaI sites was subcloned into pCACTUS-Tpr, followed by introduction of the resultant plasmid into YSH6000. Integration and selection of mxiH mutants were achieved as described for the spa47 mutant.
Preparation of antibodies and immunoblotting
Each peptide encompassing residues 554–566 (LEDEKSLVSYLNY), 359–371 (LNDKHWFFLDKNK) and 229–241 (WAFKTGWFKRNKI), which corresponded to the C-terminal amino acids of MxiD, MxiG and MxiJ, respectively, was synthesized. The antiserum specific for each oligopeptide was obtained by immunization of rabbits with the peptide coupled to keyhole limpet hemocyanin using m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS). For immunoblotting, anti-MxiD and anti-MxiG antibodies were used at a dilution of 1:1000, and anti-MxiJ antibody at 1:100. Antibodies specific for IpaB, IpaC and IpaD were used at dilutions of 1:1000, 1:20 000 and 1:100, respectively (Watarai et al., 1995).
Electron microscopy
Samples were negatively stained with 2% phosphotungstic acid pH 7.0 on the carbon-coated copper grids and observed under a JEM-1200EXII or JEM-2000EX transmission electron microscope (JEOL, Tokyo). Micrographs were taken at an accelerating voltage of 80 kV.
Measurement of type III secretion complexes of S.flexneri
Purified type III secretion complexes of S.flexneri were observed by TEM with TMV, the size of which was known (300 nm long, 20 nm wide). The length of each of the 28 best preserved type III secretion complexes was measured on microphotographs and the average and standard deviation were calculated as compared with TMV.
N-terminal amino acid sequencing
Proteins were separated by SDS–PAGE and blotted onto PVDF membrane. The bands were stained with CBB and each was sequenced using an amino acid sequence analyzer (LF3000; Beckman).
Assay for Ipa secretion
Aliquots of 2.5 ml of Shigella cultures in BHI broth at 37°C were washed in ice-cold PBS and resuspended in 1 ml of PBS. After incubation at 37°C for 5 min, 3 µl of 1% Congo red were added to the bacterial suspension, which was incubated for 10 min at 37°C and centrifuged at 14 000 g for 5 min at 4°C. The supernatant was passed through a 0.45 µm pore size filter, and trichloroacetic acid was added to the resultant supernatant (0.5 ml) at a final concentration of 6%. The secreted proteins present in PBS containing 0.003% Congo red were pelleted down at 14 000 g for 5 min at 4°C. Each pellet from the same number of bacteria was separated by 10% SDS–PAGE and immunoblotted with antibodies specific for IpaB, IpaC and IpaD.
Invasion assay
Bacterial invasion of HeLa cells was tested using the gentamicin protection assay. Briefly, HeLa cells were grown on glass coverslips to ∼60% confluency in antibiotic-free MEM containing 10% FCS. Cells were then infected with bacteria grown in BHI broth at 37°C at a multiplicity of infection of 100 per cell, and centrifuged at 700 g for 10 min. After cells were incubated at 37°C in a CO2 incubator for 20 min, gentamicin was added to the culture medium at a final concentration of 200 µg/ml and cells were incubated further at 37°C in a CO2 incubator for 15 min. After incubation, cells were washed twice with PBS and lysed in PBS containing 0.5 ml of 0.5% Triton X-100. A 50 µl aliquot of the lysates was plated onto L-agar plates and incubated at 37°C overnight. Colonies grown on L-agar plates were counted. For the reliability of results, each sample determination was performed in triplicate, and assays repeated three times.
Acknowledgments
Acknowledgements
We are grateful to Toru Tobe, Toshihiko Suzuki, Ichiro Tatsuno, Hiroyuki Abe, Hitomi Mimuro and Sei Yoshida for advice and helpful discussions. We are also grateful to the members of the Aizawa laboratory for cooperating in this work. We thank Kumar Rajakumar for providing the suicide vector pCACTUS, and Masayuki Kajitani, Teikyo University, for providing TMV. This work was supported by the Research for the Future Program of the Japanese Society for the Promotion of Science.
References
- Aizawa S.-I. and Kubori,T. (1998) Bacterial flagellation and cell division. Genes Cells, 3, 625–634. [DOI] [PubMed] [Google Scholar]
- Allaoui A., Mounier,J., Prevost,M.C., Sansonetti,P.J. and Parsot,C. (1992a) icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol. Microbiol., 6, 1605–1616. [DOI] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti,P.J. and Parsot,C. (1992b) MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J. Bacteriol., 174, 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaoui A., Ménard,R., Sansonetti,P.J. and Parsot,C. (1993a) Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect. Immun., 61, 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti,P.J. and Parsot,C. (1993b) MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol. Microbiol., 7, 59–68. [DOI] [PubMed] [Google Scholar]
- Allaoui A., Sansonetti,P.J., Ménard,R., Barzu,S., Mounier,J., Phalipon,A. and Parsot,C. (1995) MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol. Microbiol., 17, 461–470. [DOI] [PubMed] [Google Scholar]
- Blocker A., Gounon,P., Larquet,E., Niebuhr,K., Cabiaux,V., Parsot,C. and Sansonetti,P. (1999) The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol., 147, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdet-Sicard R., Rüdiger,M., Jockusch,B.M., Gounon,P., Sansonetti,P.J. and Tran Van Nhieu,G. (1999) Binding of the Shigella protein IpaA to vinculin induces F-actin depolymerization. EMBO J., 18, 5853–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P.J.G. (1984) The current picture of the structure and assembly of tobacco mosaic virus. J. Gen. Virol., 65, 253–279. [DOI] [PubMed] [Google Scholar]
- Cornelis G.R. (1998) The Yersinia deadly kiss. J. Bacteriol., 180, 5495–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G.R. and Wolf-Watz,H. (1997) The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol., 23, 861–867. [DOI] [PubMed] [Google Scholar]
- Demers B., Sansonetti,P.J. and Parsot,C. (1998) Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. EMBO J., 17, 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus G., Williams,A.W., Kawaguchi,I. and Macnab,R.M. (1993) Genetic and biochemical analysis of Salmonella typhimurium FliI, a flagellar protein related to the catalytic subunit of the F0F1 ATPase and to virulence proteins of mammalian and plant pathogens. J. Bacteriol., 175, 3131–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F. and Macnab,R.M. (1996) Enzymatic characterization of FliI. J. Biol. Chem., 271, 31981–31988. [DOI] [PubMed] [Google Scholar]
- Galán J.E. (1996) Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol., 20, 263–271. [DOI] [PubMed] [Google Scholar]
- Galán J.E. and Collmer,A. (1999) Type III secretion machines: bacterial devices for protein delivery into host cells. Science, 284, 1322–1328. [DOI] [PubMed] [Google Scholar]
- Håkansson S., Schesser,K., Persson,C., Galyov,E.E., Rosqvist,R., Homble,F. and Wolf-Watz,H. (1996) The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J., 15, 5812–5823. [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yamaguchi,S., Oosawa,K. and Aizawa,S.-I. (1994) Roles of FliK and FlhB in determination of flagellar hook length in Salmonella typhimurium. J. Bacteriol., 176, 5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueck C.J. (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev., 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga K., Tucker,S., Trollinger,D. and Galán,J.E. (1995) Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol., 177, 3965–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama E., Shiraishi,T., Oosawa,K., Baba,N. and Aizawa,S.-I. (1996) Geometry of the flagellar motor in the cytoplasmic membrane of Salmonella typhimurium as determined by stereo-photogrammetry of quick-freeze deep-etch replica images. J. Mol. Biol., 255, 458–475. [DOI] [PubMed] [Google Scholar]
- Koroyasu S., Yamazato,M., Hirano,T. and Aizawa,S.-I. (1998) Kinetic analysis of the growth rate of the flagellar hook in Salmonella typhimurium by the population balance method. Biophys. J., 74, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori T., Shimamoto,N., Yamaguchi,S., Namba,K. and Aizawa,S.-I. (1992) Morphological pathway of flagellar assembly in Salmonella typhimurium. J. Mol. Biol., 226, 433–446. [DOI] [PubMed] [Google Scholar]
- Kubori T., Matsushima,Y., Nakamura,D., Uralil,J., Lara-Tejero,M., Sukhan,A., Galán,J.E. and Aizawa,S.-I. (1998) Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science, 280, 602–605. [DOI] [PubMed] [Google Scholar]
- Lee C.A. (1997) Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol., 5, 148–156. [DOI] [PubMed] [Google Scholar]
- Macnab R.M. (1992) Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet., 26, 131–158. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa,C., Kamata,K., Kurata,T. and Yoshikawa,M. (1986) A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S.flexneri 2a. Cell, 46, 551–555. [DOI] [PubMed] [Google Scholar]
- Ménard R., Sansonetti,P.J. and Parsot,C. (1993) Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol., 175, 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard R., Sansonetti,P.J. and Parsot,C. (1994) The secretion of Shigella flexneri invasins is induced by the epithelial cell and controlled by IpaB and IpaD. EMBO J., 13, 5293–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen M.J., Dougan,G. and Frankel,G. (1997) Coiled-coil domains in proteins secreted by type III secretion systems. Mol. Microbiol., 25, 423–425. [DOI] [PubMed] [Google Scholar]
- Parsot C. (1994) Shigella flexneri: genetics of entry and intercellular dissemination in epithelial cells. Curr. Top. Microbiol. Immunol., 192, 217–241. [DOI] [PubMed] [Google Scholar]
- Parsot C., Ménard,R., Gounon,P. and Sansonetti,P.J. (1995) Enhanced secretion through the Shigella flexneri Mxi–Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol. Microbiol., 16, 291–300. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez,R.J., Stocker,B.A. and Yamaguchi,S. (1973) A new fla gene in Salmonella typhimurium flaR and its mutant phenotype—superhooks. Arch. Mikrobiol., 90, 107–120. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata,K., Sakai,T., Murayama,S.Y., Makino,S. and Yoshikawa,M. (1986) Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect. Immun., 51, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata,K., Sakai,T., Makino,S., Yamada,M., Okada,N. and Yoshikawa,M. (1988) Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J. Bacteriol., 170, 2480–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Adler,B., Tobe,T., Okada,N., Nagai,S., Komatsu,K. and Yoshikawa,M. (1989) Functional organization and nucleotide sequence of virulence region-2 on the large virulence plasmid in Shigella flexneri 2a. Mol. Microbiol., 3, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Buysse,J. and Watanabe,H. (1992) The large virulence plasmid of Shigella. Curr. Top. Microbiol. Immunol., 180, 21–44. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Komatsu,K., Tobe,T., Suzuki,T. and Yoshikawa,M. (1993) Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J. Bacteriol., 175, 2334–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T., Sasakawa,C., Okada,N., Honma,Y. and Yoshikawa,M. (1992) vacB, a novel chromosomal gene required for expression of virulence genes on the large plasmid of Shigella flexneri. J. Bacteriol., 174, 6359–6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G. and Sansonetti,P.J. (1999) Mechanism of Shigella entry into epithelial cells. Curr. Opin. Microbiol., 2, 51–55. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Ben-Ze’ev,A. and Sansonetti,P.J. (1997) Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J., 16, 2717–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Caron,E., Hall,A. and Sansonetti,P.J. (1999) IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J., 18, 3249–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiya K.-i., Tobe,T., Komatsu,K., Suzuki,T., Watarai,M., Fukuda,I., Yoshikawa,M. and Sasakawa,C. (1995) Identification of a novel virulence gene, virA, on the large plasmid of Shigella, involved in invasion and intercellular spreading. Mol. Microbiol., 17, 241–250. [DOI] [PubMed] [Google Scholar]
- Venkatesan M.M., Buysse,J.M. and Oaks,E.V. (1992) Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J. Bacteriol., 174, 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M., Tobe,T., Yoshikawa,M. and Sasakawa,C. (1995) Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J., 14, 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]