Abstract
Objective
Leptin was discovered in 1994 as a hormone produced by adipose tissue with a modulatory effect on feeding behavior and weight control. Recently, the stomach has been identified as an important source of leptin and growing evidence has shown diverse functions for leptin in the gastrointestinal tract.
Methods
Using leptin as a keyword in PubMed, more than 17 000 articles were identified, of which more than 500 articles were related to the role of leptin in the gastrointestinal tract. Available abstracts were reviewed and more than 200 original articles were reviewed in detail.
Results
The available literature demonstrated that leptin can modulate several important functions of the gastrointestinal tract. Leptin interacts with the vagus nerve and cholecystokinin to delay gastric emptying and has a complex effect on motility of the small bowel. Leptin modulates absorption of macronutrients in the gastrointestinal tract differentially in physiologic and pathologic states. In physiologic states, exogenous leptin has been shown to decrease carbohydrate absorption and to increase the absorption of small peptides by the PepT1 di-/tripeptide transporter. In certain pathologic states, leptin has been shown to increase absorption of carbohydrates, proteins, and fat. Leptin has been shown to be upregulated in the colonic mucosa in patients with inflammatory bowel disease. Leptin stimulates gut mucosal cell proliferation and inhibits apoptosis. These functions have led to speculation about the role of leptin in tumorigenesis in the gastrointestinal tract, which is complicated by the multiple immunoregulatory effects of leptin.
Conclusion
Leptin is an important modulator of major aspects of gastrointestinal tract functions, independent of its more well-described roles in appetite regulation and obesity.
Keywords: Colon, Inflammation, Intestine, Leptin, Motility, Nutrient absorption
Leptin physiology and signaling
Leptin is a protein hormone that is a major product of adipose tissue and has well-described roles in the regulation of appetite and metabolism [1,2]. Although leptin was first discovered in adipose tissue, it is also produced in other organs including the stomach, skeletal muscle, and pituitary gland [3,4]. Leptin is a product of the obese (ob) gene, which is located on chromosome 7 in humans and acts through its receptor OB-R. The stomach is the major source of leptin in the gastrointestinal (GI) tract. Endocrine and exocrine cells in gastric mucosa produce leptin; however, exocrine cells play a larger role [5,6]. Endocrine secretion of leptin occurs in various physiologic states, including fasting or refeeding after fasting. It has been shown that during these conditions the concentration of leptin increases in the serum and gastric mucosa [7]. Leptin and the soluble isoform of its receptor are secreted by chief cells in the gastric mucosa and remain stable in the acidic environment of the stomach and reach the duodenum in two forms: protein-bound and free [5]. Leptin receptors are abundant in the GI system, especially in the proximal part of the intestine. These receptors can be found on the luminal and basolateral borders of intestinal cells [8].
There are several OB-R isoforms as a result of the splicing of a single gene transcript [9]. Extracellular domain, transmembrane domain, and the first 29 amino acids of the cytoplasmic domain are identical in all isoforms of leptin receptors. According to the length of the intracellular domain, there is one long isoform (OB-Rb) and four short isoforms (OB-Ra, c, d, f), which differ in their cytosolic carboxy terminals. There is one soluble isoform (OB-Re) that lacks the transmembrane domain and may be involved in leptin transport in the blood [10]. It has been suggested that this type of receptor can indirectly regulate the bioactivity of leptin through modification of the leptin-to-soluble leptin receptor ratio [11]. It has recently been reported that the soluble leptin receptor, when coadministered with leptin, centrally or peripherally decreases the phosphorylation of signal transducer and activator of transcription-3 (STAT-3) and blocks the regulatory effects of leptin on food intake and weight [12]. When leptin binds to the OB-Rb receptor, it activates the Janus kinase/STAT pathway and it activates the mitogen-activated protein kinase signal transduction pathways when attached to short isoforms (OB-Ra, c, d, f) [13]. Janus kinase/STAT activation leads to the phosphorylation of tyrosine residues of leptin receptors at three different sites. Each of the three tyrosine phosphorylation sites recruits specific downstream signaling proteins. One recruits tyrosine phosphatase Src homology-2 domain-containing tyrosine phosphatase-2, which induces extracellular signal-regulated kinases (ERK)-1/2 and suppresses cytokine signaling-3. Another phosphorylation site leads to the activation of STAT-5, and STAT-3 is another downstream signaling pathway activated by leptin receptors [14]. These downstream pathways have been shown to be involved in effects of leptin on various tissues. For example, STAT-3 mediates the effect of leptin on food intake, hepatic glucose production, and gonadotropin secretion [15], whereas control of lipogenesis in adipose tissue, induction of arterial intima formation by leptin, increase of absorption of peptides, and proinflammatory effects of leptin in the colon and liver have been suggested to be independent of STAT-3 [16].
Effect of leptin on GI tract motility
Leptin has complex effects on motility of the GI system. Afferent and efferent vagus nerve endings contain leptin receptors [17]. In rat stomach, two groups of vagal afferent nerve endings based on their sensitivity to leptin and their effect on motility have been identified; one is leptin-responsive and produces a stimulatory response to leptin; the second group is usually leptin-insensitive and produces an inhibitory response to leptin [18]. In the small intestine, leptin can cause excitatory and inhibitory effects on mechanoreceptors and thus has a complex effect on intestinal motility. It has been shown that leptin deficiency increases the rate of gastric emptying [19], increases transit activity in the jejunum, and shortens total transit time in the small intestine [20]. In addition, some studies have shown that central administration of leptin delays gastric emptying [21]. Recently, the fasting plasma level of leptin was positively correlated with myoelectrical abnormalities in the stomach associated with delayed gastric emptying in diabetic patients [22].
The leptin-induced inhibition of food intake and the stimulation of pancreatic exocrine secretions can be blocked by a cholecystokinin-1 (CCK-1) receptor antagonist [23]. One in vitro study showed that STC-1 cells that secrete CCK have leptin receptors and are stimulated by the presence of leptin. Duodenal delivery of leptin in vivo increases the concentration of CCK in the serum. Feeding decreases the amount of leptin in the gastric juice and increases the amount of leptin in the duodenum even in leptin receptor-deficient mice; however, a surge of serum CCK after feeding is not observed in such mice [24]. CCK itself increases the release of leptin from gastric glands, suggesting that leptin and CCK comprise a positive feedback loop. Locally injected intra-arterial leptin stimulated motility in the small intestine in cats pretreated with CCK, whereas leptin alone did not induce such activity [24,25]. This suggests that modulation of vagal fibers in the presence of CCK plays a key role in the observed effects of leptin on intestinal motility. Gaigé et al. [25] also suggested that, depending on the absence or presence of CCK, leptin can switch its regulatory role between feeding behavior and motility. Leptin, in the absence of CCK, regulates the feeding behavior and appetite by exciting type 1 vagal nerves and inhibiting type 2 vagal nerves while in the presence of CCK, enhancing the motility of the GI tract.
Effect of leptin on nutrient absorption
Available data suggest that leptin has complex effects on macronutrient absorption in physiologic and pathologic states. In physiologic states, leptin has been shown to decrease carbohydrate absorption by inhibiting d-glucose transport in the preprandial state and to upregulate glucose absorption in the postprandial state. Sodium–glucose transporter-1 is expressed in small and large intestinal mucosa and is responsible for absorption of glucose. Luminal and systemic administrations of leptin decrease the activity of this cotransporter through various mechanisms. It has been suggested that luminal leptin, most likely produced by gastric mucosa, rapidly decreases the expression and activity of sodium–glucose transporter-1 through a direct effect at the brush border, whereas systemic leptin has a slower indirect effect that is at least in part mediated by CCK [26]. Sodium–glucose transporter-1 is the major transporter of glucose during the preprandial state. In the postprandial state, glucose transporter (GLUT)-5 and GLUT-2 become upregulated and their activity increases. Recently, it has been reported that luminal leptin increases the activity of GLUT-2 and GLUT-5 transports through the activation of protein kinase C and adenosin mono phosphate activated protein kinase (AMPK)-α, whereas oral fructose rapidly induces the release of leptin in the GI lumen without significantly affecting plasma leptin levels [27]. This observation suggests that in preprandial state leptin produced by gastric cells decreases the absorption of glucose, whereas in the postprandial state leptin has an important role in increasing the uptake of glucose.
Leptin has also been shown to increase the absorption of small peptide products of protein digestion through the di-/tripeptide transporter PepT1 [28]. Leptin produced from gastric mucosa and present in the proximal small bowel lumen appears to recruit an intracellular pool of PepT1 to be expressed on the brush border of intestinal cells [28]. In the long term, leptin appears to increase the translation and mRNA production of PepT1 in the small intestine [29,30]. However, in diet-induced obesity in mice (a chronic hyperleptinemia state), leptin receptors are downregulated, which in turn leads to a decrease in small intestinal expression of PepT1 and absorption of small peptides [31]. This effect of leptin is independent of STAT-3 or STAT-5 and is modulated by the ERK-1/2 pathway [31]. Leptin-deficient mice have significantly decreased PepT1 expression and activity, which is reversible by peripheral administration of leptin. In such mice, activities of the jejunal aminopeptidase and dipeptidyl peptidase are also decreased compared with wild-type mice [32]. Because PepT1 has an important role in absorption of nutrients, as well as several drugs, a better understanding of the role of leptin in the regulation of this peptide transporter may have important clinical applications.
Intravenous infusion of leptin decreases apolipoprotein A-IV, whose synthesis is stimulated by fat absorption [33]. Leptin administered to the basolateral side of Caco-2 cells was found to inhibit the secretion of triacylglycerols, the biosynthesis of apolipoproteins B-100 and B-48, and the output of chylomicron and low-density lipoproteins [34]. In the same cells, luminal leptin can increase the intracellular pool of proton-linked monocarboxylate transporter-1 and its translocation to the luminal membrane and, in consequence, increases the uptake of butyrate by colonic cells [34]. Butyrate is a short-chain fatty acid produced by microbial fermentation of carbohydrate, which is a main source of energy in intestinal cells and has important anti-inflammatory and cell regulatory functions. Leptin also has been shown to increase the expression of liver and intestinal fatty acid-binding proteins in vitro, which might participate in the uptake, intracellular metabolism, and transport of long-chain fatty acids [35].
In a mouse model of short bowel syndrome after massive small bowel resection, exogenous leptin administration increased the absorption of sucrose [32]. Leptin also increases the expression and activity of GLUT-5, which is a glucose and galactose transporter in enterocytes after partial small bowel resection [36]. After bowel resection, leptin administration induces differential effects on carboxy- and aminopeptidases in the ileum, increasing the activity of aminopeptidase and decreasing the activity of carboxypeptidase. Exogenous leptin also was shown to increase the intestinal absorption of fat in this model of short bowel syndrome [32]. Although data are very limited in humans, one study failed to find any significant difference between serum levels of leptin in patients with short bowel syndrome after massive small bowel resection and unresected controls, regardless of the route of nutrition (i.e., parenteral versus oral as the primary route of feeding) [37].
Leptin as a trophic factor in the GI tract
Although there is some evidence in the literature suggesting that leptin does not stimulate growth in the GI tract mucosa, several studies have shown that leptin acts as a trophic factor in the GI tract and can stimulate gut epithelial cell proliferation when given exogenously [38,39]. In neonatal piglets, leptin has been shown to have a stimulatory effect on the development of intestinal mucosal morphometry, proliferation of mucosal epithelial cells, enzymatic activity in the brush border of enterocytes, and, as outlined earlier, in nutrient absorption [40]. After massive small bowel resection, the leptin receptor gene and protein expression were upregulated in residual jejunal and ileal mucosa, concomitant with adaptively increased villus and crypt growth [41,42]. Treatment with exogenous leptin enhanced all of these effects [41,42]. Leptin-deficient ob/ob mice demonstrated decreased cellular proliferation and increased apoptosis in intestinal cells after massive small bowel resection [43]. This study and a study reported by Lin et al. [44] suggested that leptin increases apoptosis in intestinal cells, in contrast to the decrease in apoptosis observed by other investigators [42].
The mitogenic and antiapoptotic effects of leptin make it a potential tumorigenic factor. The association between a high-fat diet and obesity with colon cancer and the association between a high-fat diet and high concentrations of leptin in the serum were suggestive of a role for leptin in colon carcinogenesis [45]. However, experimental data have been controversial and there is no conclusive evidence, to date, for the role of leptin in carcinogenesis in colorectal cancer. It has been suggested that leptin and its receptor are expressed in cancer tissues and that leptin may promote cancer progression in an autocrine and paracrine manner. Leptin can stimulate mitogen-activated protein kinase activity in vitro and increase the proliferation of gastric mucosa cell lines [46]. Leptin also induces cell proliferation and migration in normal and malignant colorectal cells [39,47]. Leptin expression has been found in gastric adenocarcinoma, colorectal polyps, and adenocarcinoma of the colon [46,48,49]. An association between high leptin concentration and increased risk of Barret’s esophagus and squamous cell carcinoma of esophagus has been reported [50]. Leptin may accelerate fibrosis in the setting of chronic liver injury [51] and might precipitate fatty changes in non-alcoholic liver disease [52]. Outside the GI tract, leptin expression has been reported to be upregulated in breast carcinomas [53]. Although some studies have reported that high levels of leptin in the serum correlate with increased proliferation of colonic cells [48] or the risk of cancer in rats consuming a high-fat diet [45], other studies have not shown a significant difference in the concentration of leptin in the serum of patients with colorectal cancers compared with normal controls [45]. Some studies have even showed lower levels serum leptin in patients with advanced cancer, but the role of food intake was unclear in such observational studies [54]. In rats, leptin decreased the precancerous colonic mucosal lesions induced by azoxymethane [55]. Expression of leptin was found to be increased in human colorectal carcinomas and even in the normal colonic mucosa adjacent to the tumors [49]. The investigators also showed that the level of leptin expression correlated with the degree of differentiation of the tumor, with poorly differentiated tumors expressing less leptin [49]. Transgenic mice lacking leptin receptors were shown to be prone to azoxymethane-induced premalignant lesions in colonic mucosa [56]. Moreover, this picture has become more complex after studies indicating that leptin might have a role in the regulation of the antitumor immune response in colonic cells [57,58]. On the one hand, leptin induces colonic cell proliferation and has antiapoptotic effects; on the other hand, leptin has been shown to enhance antitumor immune responses, suggesting that the observed increase in expression of leptin receptors in tumor cells might be a homeostatic response.
Leptin and immune function and gut mucosal inflammation and injury
Leptin has important modulatory effects on the immune system. Congenital leptin deficiency increases the incidence of infection-related death during childhood [59]. Leptin modifies the cytokine production pattern toward a type 1 T-cell helper response by promoting the release of interleukin (IL)-2 and interferon and inhibiting IL-4 secretion, reverses starvation-induced immunosuppression [60], and directly stimulates the expression and release of IL-1 and tumor necrosis factor-α by T cells [61]. In addition, leptin facilitates type 1 T-cell helper responses by promoting the differentiation of antigen-presenting dendritic cells [62]. However, leptin has also been shown to exhibit anti-inflammatory properties by stimulating the expression and production of the IL-1 receptor antagonist in human monocytes [63]. Leptin receptor-deficient mice exhibit impaired natural killer cell activity and leptin has been shown to enhance the proliferation and cytotoxicity of natural killer cells [64] and protect them from apoptosis [65]. Leptin induces the production of nitric oxide and proinflammatory cytokines in macrophages and monocytes [66] and enhances the release of reactive oxygen by neutrophils [67]. Leptin deficiency results in an attenuated adaptive immune-mediated response and causes inadequate control of inflammation [59].
Leptin modulates inflammatory and anti-inflammatory responses in the GI tract. Acute administration of leptin stimulates the production and secretion of corticotropin [68]. Conversely, chronic exogenous leptin can inhibit production of corticotropin-releasing hormone and corticotropin, block the physiologic surge of corticosteroids in response to stress, and decrease the production of glucocorticoids by the adrenal glands [69]. Therefore, at the systemic level, acute exogenous administration of leptin was shown to prevent experimental induced colitis, an effect that has been shown to be reversed by blockage of corticosteroids receptors [70]. Leptin might prevent gastric ulcer formation by increasing the activities of the cyclooxygenase and nitric oxide pathways and by enhancing mucus secretion [71]. Serum leptin concentrations are increased in experimental animals with intestinal inflammation and human patients with inflammatory bowel disease [72]. It has been suggested that inflammation-induced anorexia is the major reason for hyperleptinemia and it was shown that leptin levels correlated with the level of inflammation and anorexia [73]. Others have reported that serum concentrations of leptin decrease in patients with Crohn’s disease, whereas treatment with infliximab (tumor necrosis factor-α inhibitor) had the opposite effect [74,75].
It has also been suggested that leptin may play a role in the inflammatory process in the colon. Leptin-deficient mice are resistant to acute and chronic intestinal inflammations induced by chemical agents [76]. Levels of proinflammatory cytokines and neutrophil infiltration into the colonic tissue are significantly suppressed in leptin-deficient mice compared with wild mice, and these effects are reversible by the administration of leptin [76]. Leptin-deficient mice are also resistant to type 2 helper T-cell–mediated chemically induced colitis, and this resistance is reversible by leptin administration [58]. The human [h] PepT1 is suggested to have a role in inflammatory bowel disease by transport of bacterially derived proinflammatory peptides [77,78]; leptin enhances hPepT1 promoter activity and increases hPepT1 mRNA and protein expression in the gut [17]. In patients with ulcerative colitis, inflamed colonic epithelial cells expressed larger amounts of leptin and released leptin into the intestinal lumen; this in turn induced epithelial wall damage and neutrophil infiltration [79]. Luminal leptin concentrations during inflammation in patients with inflammatory bowel disease are much higher than those observed in normal colonic luminal fluid [79], and leptin is overexpressed in creeping fat in patients with Crohn’s disease [80]. In contrast, Valentini et al. [81] did not find any significant increase in serum leptin levels in patients with inflammatory bowel disease or a correlation between inflammation or disease activity and serum levels of leptin. It has been suggested that leptin might produce proinflammatory effects in mice by the STAT-3 action pathway [76]. Increased levels of STAT-3 have been detected in mice after experimentally induced colitis and in colon samples of humans with Crohn’s disease or ulcerative colitis [82]. Luminal leptin was shown to activate mucin-secreting goblet cells in the colon and stimulate expression of secreted and membrane-bound mucin [83,84]. Because mucin is an important part of the colonic defense mechanism and protects epithelial cells from physical, microbial, and chemical injuries, leptin secretion during intestinal inflammation may represent an adaptive beneficial response in this setting.
The role of leptin in ischemic injury and healing of the GI tract has also been studied. Leptin administration accelerated the healing of colonic anastomosis [85]. In an intestinal ischemic injury model in mice, exogenous leptin was shown to decrease the level of intestinal tissue injury [86]. Other studies have shown that leptin controls the production of nitric oxide by activating nitric oxide synthase in a dose-dependent manner in rats [87]. Nitric oxide has a vasodilatory effect and leptin was shown to relax the rat mesenteric artery through its effect on nitric oxide [88].
Leptin and its interaction with ghrelin in the GI tract
Ghrelin, first identified in 1999 as primarily produced in the stomach, is a 28-amino acid peptide and its receptor is a member of the growth hormone secretagogue receptor family. Ghrelin induces secretion of growth hormone from the pituitary gland in vivo and in vitro [89]. Fasting can increase expression and secretion of ghrelin, whereas refeeding decreases these [90,91]. Ghrelin-synthesizing cells are abundant in the GI tract, especially in the stomach [92,93].
Soon after its discovery, it was shown that ghrelin has a major role in regulating feeding behavior [90,91]. Effects of ghrelin on appetite and observed changes in ghrelin levels in response to fasting or eating are opposite those of leptin [94]. Ghrelin induces adiposity in adipose tissues [95], increases appetite [93], and initiates eating behavior [96]. Circadian changes in the level of circulating ghrelin and leptin are reciprocal [97]. Leptin and ghrelin also have contradictory effects on intestinal inflammation. For example, ghrelin has been shown to ameliorate inflammation in the GI tract in a mouse model of colitis [98]. Ghrelin’s effect on GI tract motility is also generally opposite that of leptin. Ghrelin stimulates gastric acid secretion and motility [99] and accelerates gastric emptying and small intestinal transit [100]. Leptin has been shown to decrease the expression and secretion of ghrelin from gastric mucosa [101]. Interestingly, leptin cells are adjacent to ghrelin cells in the gastric mucosa, surrounding ghrelin cells in the lower half of stomach, possibly providing a paracrine regulation of ghrelin secretion [94]. However, ghrelin shows some effects on the gut that are similar to those of leptin. For example, ghrelin decreases the rate of apoptosis in intestinal cells [102], ameliorates ischemic–reperfusion injury [103], and decreases the permeability of the intestine in case of shock (partly by increasing mucin secretion) [104]. Further studies are required to completely elucidate the nature of the interaction between ghrelin and leptin in health and disease.
Summary
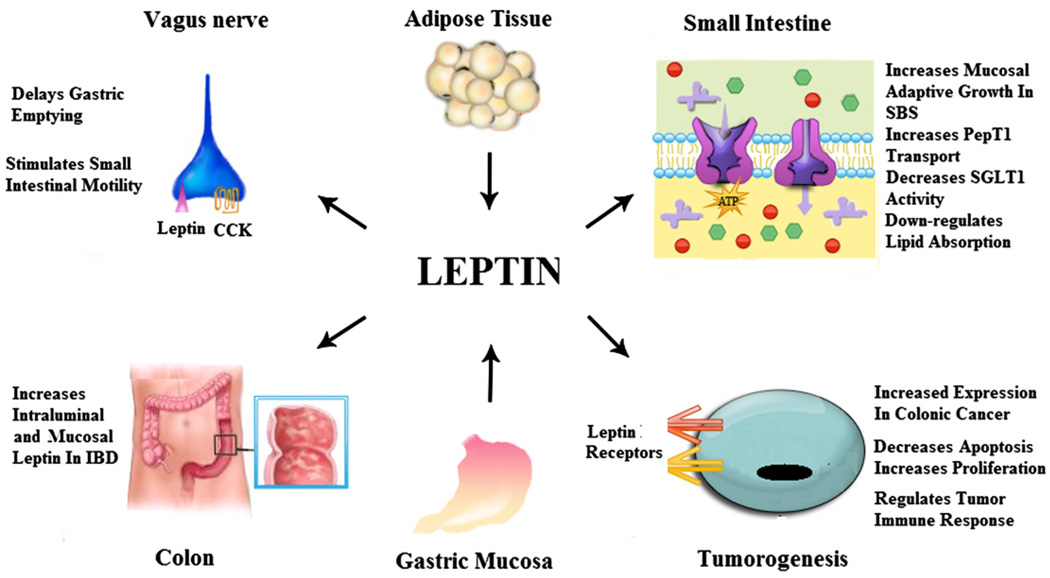
Leptin has multiple complex roles in the GI tract (Fig.1). It was initially described as a promoter of satiety and a regulator of food intake. However, the mechanism of leptin’s effect on satiety and the complexity of the interactions among leptin, other recently discovered appetite regulatory polypeptides, and pancreatic exocrine hormones (CCK, etc.) continue to be explored and may ultimately provide insight into obesity and appetite. Motility of the GI tract may be one aspect in which leptin induces satiety. Furthermore, leptin clearly has a role in nutrient absorption, which may differ in the physiologic and pathologic disease states. Leptin’s complex role in GI motility and nutrient absorption is a key to understanding the role of leptin in linking nutritional status, obesity, and metabolism. Recent data about interaction between leptin and neuropeptide Y in the regulation of absorption might help to elucidate this role [105,106].
Fig. 1.
Effects of leptin on the gastrointestinal tract. Leptin, produced by adipose tissue and gastric mucosa, modulates numerous aspects of gastrointestinal function. Leptin, mainly produced by the gastric mucosa, regulates motility of the stomach and small intestine through its interaction with CCK and the vagus nerve. Leptin can act as a proinflammatory cytokine in colonic IBD. Leptin influences macronutrient transport in the small intestine in part by its action to regulate PepT1 and SGLT-1 transports of di-/tripeptides and glucose, respectively. Leptin exerts proliferative and antiapoptotic effects, suggesting that leptin is a potential tumorigenic factor. Several immunoregulatory effects relevant to colon cancer have been attributed to leptin. CCK, cholecystokinin; IBD, inflammatory bowel disease; SBS, short bowel syndrome; SGLT-1, sodium–glucose transporter-1.
Leptin also has significant effects as a local trophic factor in the GI tract and as an immune system modulator. These effects not only suggest a role in GI cancers but also inflammatory bowel disease and likely other autoimmune diseases. Gastric production of leptin contributes to trophic control of GI cells, but it is unknown whether gastric production of leptin has significant effects on the GI immune system. Clearly, leptin itself alters the innate and adaptive systemic immune response and may participate in diseases of the intestine and colon systemically or even locally.
In this review, we highlight the GI effects of leptin and its relation to GI motility, nutrient absorption, trophic effects on GI cells, and the immune system. The future directions in the study of leptin are broad and specifically should be focused on the interplay between leptin and other polypeptides in the regulation of motility, absorption, and satiety to better understand the effects of leptin on nutritional status and metabolism, which are major contributors to obesity. Furthermore, trophic effects in the GI tract and immune system modulation effects of leptin can provide insight into GI cancers and inflammatory disorders of the GI tract including Crohn’s disease and ulcerative colitis. Ultimately, the many effects of leptin are only beginning to be understood and the effects on the GI tract likely affect many pathologic states including obesity, GI cancers, and inflammatory bowel disease.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–453. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 3.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 4.Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem. 2005;53:851–860. doi: 10.1369/jhc.5A6620.2005. [DOI] [PubMed] [Google Scholar]
- 6.Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006;290:G242–G249. doi: 10.1152/ajpgi.00334.2005. [DOI] [PubMed] [Google Scholar]
- 7.Cinti S, de Matteis R, Ceresi E, Picó C, Oliver J, Oliver P, et al. Leptin in the human stomach. Gut. 2001;49:155–159. doi: 10.1136/gut.49.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrenetxe J, Villaro AC, Guembe L, Pascual I, Munoz-Navas M, Barber A, Lostao MP. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 2008;50:797–809. doi: 10.1136/gut.50.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 10.Wang MY, Zhou YT, Newgard CB, Unger RH. A novel leptin receptor isoform in rat. FEBS Lett. 1996;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem. 2001;276:6343–6349. doi: 10.1074/jbc.M009795200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Scarpace PJ. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol. 2009;158:475–482. doi: 10.1111/j.1476-5381.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor OB-R. Cell. 1995;81:1263–1272. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 14.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 15.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci. 2001;14:64–73. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol Regul Integr Comp Physiol. 1997;273:R833–R837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- 19.Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology. 1999;116:1287. doi: 10.1016/s0016-5085(99)70491-9. [DOI] [PubMed] [Google Scholar]
- 20.Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124:98–103. doi: 10.1016/j.jss.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Martinez V, Barrachina MD, Wang L, Tache Y. Intracerebroventricular leptin inhibits gastric emptying of a solid nutrient meal in rats. Neuroreport. 1999;10:3217. doi: 10.1097/00001756-199910190-00017. [DOI] [PubMed] [Google Scholar]
- 22.Hata N, Murata S, Maeda J, Yatani H, Kohno Y, Yokono K, Okano H. Predictors of gastric myoelectrical activity in type 2 diabetes mellitus. J Clin Gastroenterol. 2009;43:429–436. doi: 10.1097/MCG.0b013e31818337f1. [DOI] [PubMed] [Google Scholar]
- 23.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl Acad Sci U S A. 1997;94:10455–10466. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilmeau S, Buyse M, Tsocas A, Laigneau JP, Bado A. Duodenal leptin stimulates cholecystokinin secretion evidence of a positive leptin-cholecystokinin feedback loop. Diabetes. 2003;52:1664–1672. doi: 10.2337/diabetes.52.7.1664. [DOI] [PubMed] [Google Scholar]
- 25.Gaigé S, Abysique A, Bouvier M. Effects of leptin on cat intestinal motility. J Physiol. 2003;546:267–277. doi: 10.1113/jphysiol.2002.029462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose-mediated by sodium-glucose cotransporter 1. Diabetes. 2005;54:348–354. doi: 10.2337/diabetes.54.2.348. [DOI] [PubMed] [Google Scholar]
- 27.Sakar Y, Nazaret C, Lettéron P, Ait Omar A, Avenati M, Viollet B, et al. Positive regulatory control loop between gut leptin and intestinal GLUT2/GLUT5 transporters links to hepatic metabolic functions in rodents. PLoS One. 2007;4:e7935–e7940. doi: 10.1371/journal.pone.0007935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108:1483. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindlet P, Bado A, Farinotti R, Buyse M. Long-term effect of leptin on H+-coupled peptide cotransporter 1 activity and expression in vivo: evidence in leptin-deficient mice. J Pharmacol Exp Ther. 2007;323:192–201. doi: 10.1124/jpet.107.125799. [DOI] [PubMed] [Google Scholar]
- 30.Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem. 2007;282:1359–1373. doi: 10.1074/jbc.M604267200. [DOI] [PubMed] [Google Scholar]
- 31.Hindlet P, Bado A, Kamenicky P, Deloménie C, Bourasset F, Nazaret C, et al. Reduced intestinal absorption of dipeptides via PepT1 in mice with diet-induced obesity is associated with leptin receptor down-regulation. J Biol Chem. 2009;284:6801–6808. doi: 10.1074/jbc.M805564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiely JM, Noh JH, Svatek CL, Pitt HA, Swartz-Basile DA. Altered small intestinal absorptive enzyme activities in leptin-deficient obese mice: influence of bowel resection. J Pediatr Surg. 2006;41:1243–1249. doi: 10.1016/j.jpedsurg.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 33.Doi T, Liu M, Seeley RJ. Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am J Physiol Regul Integr Comp Physiol. 2001;281:R753–R759. doi: 10.1152/ajpregu.2001.281.3.R753. [DOI] [PubMed] [Google Scholar]
- 34.Stan S, Levy E, Bendayan M, Zoltowska M, Lambert M, Michaud J, et al. Effect of human recombinant leptin on lipid handling by fully differentiated Caco-2 cells. FEBS Lett. 2001;508:80–84. doi: 10.1016/s0014-5793(01)03032-0. [DOI] [PubMed] [Google Scholar]
- 35.Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem. 2002;277:28182–28190. doi: 10.1074/jbc.M203281200. [DOI] [PubMed] [Google Scholar]
- 36.Pearson PY, O’Connor DM, Schwartz MZ. Novel effect of leptin on small intestine adaptation. J Surg Res. 2001;97:192–195. doi: 10.1006/jsre.2001.6153. [DOI] [PubMed] [Google Scholar]
- 37.Molina A, Pita A, Farriol M, Virgili N, Soler J, Gómez JM. Serum leptin concentrations in patients with short-bowel syndrome. Clin Nutr. 2000;19:333–338. doi: 10.1054/clnu.2000.0110. [DOI] [PubMed] [Google Scholar]
- 38.FitzGerald AJ, Mandir N, Goodlad RA. Leptin, cell proliferation and crypt fission in the gastrointestinal tract of intravenously fed rats. Cell Prolif. 2005;38:25–33. doi: 10.1111/j.1365-2184.2005.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 40.Woliński J, Biernat M, Guilloteau P, Weström BR, Zabielski R. Exogenous leptin controls the development of the small intestine in neonatal piglets. J Endocrinol. 2003;177:215–222. doi: 10.1677/joe.0.1770215. [DOI] [PubMed] [Google Scholar]
- 41.Sukhotnik I, Vadasz Z, Coran AG, Lurie M, Shiloni E, Hatoum OA, Mogilner JG. Effect of leptin on intestinal re-growth following massive small bowel resection in rat. Pediatr Surg Int. 2006;22:9–15. doi: 10.1007/s00383-005-1572-9. [DOI] [PubMed] [Google Scholar]
- 42.Sukhotnik I, Coran AG, Mogilner JG, Shamian B, Karry R, Lieber M, Shaoul R. Leptin affects intestinal epithelial cell turnover in correlation with leptin receptor expression along the villus-crypt axis after massive small bowel resection in a rat. Pediatr Res. 2009;66:648–653. doi: 10.1203/PDR.0b013e3181be9f84. [DOI] [PubMed] [Google Scholar]
- 43.Kiely JM, Noh JH, Pitt HA, Swartz-Basile DA. Impaired intestinal cell proliferation and cell death in leptin-deficient obese mice. JPEN. 2005;9:30–35. doi: 10.1177/014860710502900130. [DOI] [PubMed] [Google Scholar]
- 44.Lin T, Sakata H, Ootani A, Fujise T, Tsunada S, Amemori S, et al. Apoptosis in rat jejunal mucosa is regulated partly through the central nervous system, which controls feeding behavior. J Gastroenterol Hepatol. 2005;20:1285–1289. doi: 10.1111/j.1440-1746.2005.03921.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Uesaka T, Watanabe H, Kato N. High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int J Oncol. 2005;19:1009–1014. doi: 10.3892/ijo.19.5.1009. [DOI] [PubMed] [Google Scholar]
- 46.Hong SJ, Kwon KW, Kim SG. Variation in expression of gastric leptin according to differentiation and growth pattern in gastric adenocarcinoma. Cytokine. 2006;33:66–71. doi: 10.1016/j.cyto.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Aparicio T, Kotelevets L, Tsocas A. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc (Min/+) mice. Gut. 2005;54:1136–1145. doi: 10.1136/gut.2004.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–426. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 49.Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E. Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol. 2007;60:902–906. doi: 10.1136/jcp.2006.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francois F, Roper J, Goodman AJ, Pei Z, Ghumman M, Mourad M, et al. The association of gastric leptin with oesophageal inflammation and metaplasia. Gut. 2008;57:16–24. doi: 10.1136/gut.2007.131672. [DOI] [PubMed] [Google Scholar]
- 51.Tsochatzis E, Papatheodoridis GV, Archimandritis AJ. The evolving role of leptin and adiponectin in chronic liver diseases. Am J Gastroenterol. 2006;101:2629–2640. doi: 10.1111/j.1572-0241.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 52.Huang XD, Fan Y, Zhang H, Wang P, Yuan JP, Li MJ, Zhan XY. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World J Gastroenterol. 2008;14:2888–2893. doi: 10.3748/wjg.14.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–993. [PubMed] [Google Scholar]
- 54.Bolukbas FF, Kilic H, Bolukbas C, Gumus M, Horoz M, Turhal NS, et al. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer. 2004;4:4–29. doi: 10.1186/1471-2407-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aparicio T, Guilmeau S, Goiot H, Tsocas A, Laigneau JP, Bado A. Leptin reduces the development of the initial precancerous lesions induced by azoxymethane in the rat colonic mucosa. Gastroenterology. 2004;126:499–510. doi: 10.1053/j.gastro.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Hirose Y, Hata K, Kuno T. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis. 2004;25:821–825. doi: 10.1093/carcin/bgh059. [DOI] [PubMed] [Google Scholar]
- 57.Abolhassani M, Aloulou N, Chaumette MT, Aparicio T, Martin-Garcia N, Mansour H, et al. Leptin receptor-related immune response in colorectal tumors: the role of colonocytes and interleukin-8. Cancer Res. 2008;68:9423–9432. doi: 10.1158/0008-5472.CAN-08-1017. [DOI] [PubMed] [Google Scholar]
- 58.Aloulou N, Bastuji-Garin S, Le Gouvello S, Abolhassani M, Chaumette MT, Charachon A, et al. Involvement of the leptin receptor in the immune response in intestinal cancer. Cancer Res. 2008;68:9413–9422. doi: 10.1158/0008-5472.CAN-08-0909. [DOI] [PubMed] [Google Scholar]
- 59.Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin—implications for inflammatory bowel disease. Mol Nutr Food Res. 2008;52:855–866. doi: 10.1002/mnfr.200700050. [DOI] [PubMed] [Google Scholar]
- 60.Batra A, Okur B, Glauben R, Erben U, Ihbe J, Stroh T, et al. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010;151:56–62. doi: 10.1210/en.2009-0565. [DOI] [PubMed] [Google Scholar]
- 61.De Rosa V, Procaccini C, Cali G, Pirozzi G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 63.Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- 64.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 65.Lo CK, Lam QL, Yang M, Ko KH, Sun L, Ma R, et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol Immunol. 2009;6:353–360. doi: 10.1038/cmi.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raso GM, Pacilio M, Esposito E, Coppola A, Di Carlo R, Meli R. Leptin potentiates IFN-γ–induced expression of nitric oxide synthase and cyclooxygenase-2 in murine macrophage. Br J Pharmacol. 2002;137:799–804. doi: 10.1038/sj.bjp.0704903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caldefie-Chezet F, Poulin A, Vasson MP. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2007;37:809–814. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 68.Nowak KW, Pierzchala-Koziec K, Tortorella C, Nussdorfer GG, Malendowicz LK. Effects of prolonged leptin infusion on rat pituitaryadrenocortical function. Int J Mol Med. 2002;9:61–64. [PubMed] [Google Scholar]
- 69.Oates M, Woodside B, Walker CD. Chronic leptin administration in developing rats reduces stress responsiveness partly through changes in maternal behaviour. Horm Behav. 2004;37:366–376. doi: 10.1006/hbeh.2000.1578. [DOI] [PubMed] [Google Scholar]
- 70.Cakir B, Bozkurt A, Ercan F, Yegen BC. The anti-inflammatory effect of leptin on experimental colitis: involvement of endogenous glucocorticoids. Peptides. 2004;25:95–104. doi: 10.1016/j.peptides.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Adeyemi EO, Bastaki SA, Chandranath IS. Mechanisms of action of leptin in preventing gastric ulcer. World J Gastroenterol. 2005;11:4154–4160. doi: 10.3748/wjg.v11.i27.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuzun A, Uygun A, Yesilova Z, Ozel AM. Leptin levels in the acute stage of ulcerative colitis. J Gastroenterol Hepatol. 2008;19:429–432. doi: 10.1111/j.1440-1746.2003.03300.x. [DOI] [PubMed] [Google Scholar]
- 73.Nishi Y, Isomoto H, Ueno H, Ohnita K. Plasma leptin and ghrelin concentrations in patients with Crohn’s disease. World J. Gastroenterol. 2005;11:7314–7317. doi: 10.3748/wjg.v11.i46.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M. Circulating levels of leptin, adiponectin, resistin and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 75.Franchimont D, Roland S, Gustot T, Quertinmont E. Impact of infliximab on serum leptin levels in patients with Crohn’s disease. J Clin Endocrinol Metab. 2005;90:3510–3519. doi: 10.1210/jc.2004-1222. [DOI] [PubMed] [Google Scholar]
- 76.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 77.Merlin D, Si-Tahar M, Sitaraman SV, Eastburn K, Williams I, Liu X, et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120:1666–1679. doi: 10.1053/gast.2001.24845. [DOI] [PubMed] [Google Scholar]
- 78.Charrier L, Merlin D. The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest. 2006;86:538–546. doi: 10.1038/labinvest.3700423. [DOI] [PubMed] [Google Scholar]
- 79.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004;18:696–698. doi: 10.1096/fj.03-0422fje. [DOI] [PubMed] [Google Scholar]
- 80.Paul G, Schaffler A, Neumeier M, Furst A, Bataillle F, Buechler C. Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm Bowel Dis. 2006;12:471–477. doi: 10.1097/00054725-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Valentini L, Wirth EK, Schweizer U, Hengstermann S, Schaper L, Koernicke T, et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. 2009;25:172–181. doi: 10.1016/j.nut.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 82.Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 83.Plaisancie P, Ducroc R, El Homsi M, Tsocas A, Guilmeau S, Zoghbi S, et al. Luminal leptin activates mucin-secreting goblet cells in the large bowel. Am J Physiol Gastrointest Liver Physiol. 2006;290:G805–G812. doi: 10.1152/ajpgi.00433.2005. [DOI] [PubMed] [Google Scholar]
- 84.El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, et al. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am J Physiol Gastrointest Liver Physiol. 2007;293:G365–G373. doi: 10.1152/ajpgi.00091.2007. [DOI] [PubMed] [Google Scholar]
- 85.Tasdelen A, Algin C, Ates E, Kiper H, Inal M, Sahin F. Effect of leptin on healing of colonic anastomoses in rats. Hepatogastroenterology. 2004;51:994–997. [PubMed] [Google Scholar]
- 86.Hacioglu A, Algin C, Pasaoglu O, Pasaoglu E, Kanbak G. Protective effect of leptin against ischemia-reperfusion injury in the rat small intestine. BMC Gastroenterol. 2005;21:5–37. doi: 10.1186/1471-230X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mastronardi CA, Yu WH, McCann SM. Resting and circadian release of nitric oxide is controlled by leptin in male rats. Proc Natl Acad Sci U S A. 2002;99:5721–5726. doi: 10.1073/pnas.082098499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, et al. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun. 2000;273:745–749. doi: 10.1006/bbrc.2000.3005. [DOI] [PubMed] [Google Scholar]
- 89.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 90.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 91.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 92.Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531–536. doi: 10.1016/s0196-9781(01)00633-7. [DOI] [PubMed] [Google Scholar]
- 93.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Z, Sakai T. Characteristic features of ghrelin cells in the gastrointestinal tract and the regulation of stomach ghrelin expression and production. World J Gastroenterol. 2008;14:6306–6311. doi: 10.3748/wjg.14.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 96.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 97.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 99.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 100.Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 101.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119:77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Park JM, Kakimoto T, Kuroki T, Shiraishi R, Fujise T, Iwakiri R, Fujimoto K. Suppression of intestinal mucosal apoptosis by ghrelin in fasting rats. Exp Biol Med. 2008;233:48–56. doi: 10.3181/0706-RM-169. [DOI] [PubMed] [Google Scholar]
- 103.Wu R, Dong W, Qiang X, Wang H, Blau SA, Ravikumar TS, Wang P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit Care Med. 2009;37:2421–2426. doi: 10.1097/CCM.0b013e3181a557a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, Wang P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS One. 2007;3:e2026. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee NJ, Enriquez RF, Boey D, Lin S, Slack K, Baldock PA, Herzog H, Sainsbury A. Synergistic attenuation of obesity by Y2- and Y4-receptor double knockout in ob/ob mice. Nutrition. 2008;24:892–899. doi: 10.1016/j.nut.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 106.Cox HM. Endogenous PYY and NPY mediate tonic Y1- and Y2-mediated absorption in human and mouse colon. Nutrition. 2008;24:900–906. doi: 10.1016/j.nut.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]