Abstract
We have previously shown that regulatory T cells (Treg) accumulate dramatically in aged animals and negatively impact the ability to control persistent infection. However, the mechanism(s) underlying the age-dependent accrual of Treg remain unclear. Here, we show that Treg accumulation with age is progressive and likely not the result of increased thymic output, increased peripheral proliferation, nor from enhanced peripheral conversion. Instead, we found that Treg from aged mice are more resistant to apoptosis than Treg from young mice. Although Treg from aged mice had increased expression of functional IL-7Rα, we found that IL-7R-signaling was not required for maintenance of Treg in vivo. Notably, aged Treg exhibit decreased expression of the pro-apoptotic molecule Bim compared to Treg from young mice. Further, in the absence of Bim, Treg accumulate rapidly, accounting for more than 25% of the CD4+ T cell compartment by 6 months of age. In addition, accumulation of Treg in Bim-deficient mice occurred after the cells left the transitional recent thymic emigrant compartment. Mechanistically, we show that IL-2 drives preferential proliferation and accumulation of Bimlo Treg. Combined, our data suggest that chronic stimulation by IL-2 leads to preferential expansion of Treg having low expression of Bim, which favors their survival and accumulation in aged hosts.
Introduction
Considerable changes in the function of T cells, in particular decreased responsiveness of CD4+ and CD8+ T cells, have been reported in aged hosts (mice and humans). T cells from aged mice and humans exhibit serious defects in TCR-mediated activation, including changes in the cascades of serine/threonine and tyrosine phosphorylation and trouble forming functional immune synapses with antigen-presenting cells (APC) (1–4). Furthermore, many functional defects have been reported in vivo, as evidenced by the decreased ability of aged hosts to control many infections (rev. in (5)). Although a number of the defects described in senescent CD4+ and CD8+ T cells appear to be intrinsic (rev. in (6)), recent lines of evidence have suggested that the changed balance between effector and regulatory T cells may play a major role in decreased T cell responses in aged hosts.
Regulatory T cells (Treg), a subset of CD4+ T cells, are critical for maintaining self-tolerance (7). Treg dampen effector responses against persistent infections (8) and tumors (9). Treg function by decreasing the level of activation, proliferation and cytokine production of effector T cells in mice and humans, and also by controlling the stimulatory functions of dendritic cells (10) (11). Treg are characterized by their expression of the transcriptional factor FoxP3 (Forkhead box P3), which is critical for their differentiation, function and maintenance (12).
We and other investigators have reported that the frequency of FoxP3+CD4+ T cells significantly increases in multiple lymphoid tissues from aged mice (13–15), and in the blood of or tissues of healthy elderly humans (15–17). Also, we showed that FoxP3+ cells from aged mice have a greater in vitro suppressive activity on a per cell basis than their young counterparts (15). Further, Treg play a critical role in the increased disease severity and reactivation of chronic Leishmania major infection in old mice (15). Notably, depletion of Treg in aged mice leads to enhanced effector T cell responses and increased control of L. major, suggesting that, if relieved from Treg suppression, effector T cells remain functional in vivo (15). Similarly, another group has also reported that Treg accumulation in aged mice inhibits protective anti-tumor responses (13). Thus, increased Treg frequency in aged animals likely contributes to age related immunosuppression. However, the mechanisms underlying their accumulation are not well understood. Here we explored the mechanism(s) contributing to altered Treg homeostasis during aging.
Materials and Methods
Mice
C57BL/6 mice or FoxP3-IRES-GFP knock-in Balb/c were purchased from either Taconic Farms, Jackson Laboratories, or were purchased from the National Institutes of Aging Colony. Bim-deficient (Bim KO) mice were a generous gift from Drs. P. Bouillet and A. Strasser and were backcrossed for 19–20 generations to C57BL/6 (Walter and Eliza Hall Institute, Melbourne, Australia). Rag2p-GFP mice (18) were originally a gift from Dr. Michel Nussenzweig (Rockefeller University, New York) and were backcrossed 10 or more generations to C57BL/6 and then mated to Bim KO mice. Mice were housed under specific pathogen free conditions. All animal protocols were reviewed and approved by our Institutional Animal Care and Use Committee.
Flow Cytometry
Single cell suspensions were generated from thymi, lymph nodes, or spleens and 106 cells/tube were stained with antibodies against CD4, CD44, CD127 (BD Biosciences) and intracellularly for FoxP3 (eBiosciences), Bcl-2 (generated in house), Mcl-1 (Rockland Biochemicals) and Bim (Cell Signaling Technologies). For detection of Mcl-1 and Bim secondary anti-rabbit antibodies (Jackson Immunoresearch) were used. Staining for FoxP3 was performed according to the manufacturer’s instructions. Data were acquired on a FacsCalibur or an LSRII Flow Cytometer and analyzed using CellQuestPro or FacsDIVA software (BD Biosciences).
In vitro conversion
Spleen and pLN were recovered from FoxP3-IRES-GFP knock-in Balb/c mice. Pan-T Cell Isolation kit was used to purify T cells (Miltenyi Biotec). To purify naïve and memory CD4+ T cells, T cells were sorted on their expression of CD4, CD62L and CD44 with a FACSAria (BD Biosciences). 5 × 105 cells/well were stimulated with immobilized anti-CD3e (eBioscience, 2.5 ug) and soluble anti-CD28 (eBioscience, 2 ug), in presence of 100 U/ml IL-2 (NIH) and 5 ng TGF-β1 (PeproTech Inc., Rocky Hill, NJ). As controls, cells were stimulated without TGF-β1 or cultured in IL-2-containing medium. GFP expression was analyzed by flow cytometry in gated CD4+ cells after 5 days.
BrdU administration
C57BL/6 and Bim−/− mice were injected i.p. daily with BrdU (0.6 mg/mouse) for the indicated periods of time. Mice not injected and served as negative controls for BrdU staining. Spleen cells were stained for CD4, BrdU (BD Biosciences), FoxP3 and CD44 expression, and analyzed by flow cytometry.
In vivo cytokine administration and blockade
Human IL-7 was purchased from R&D systems and anti-IL-7 (M25 hybridoma) was grown as ascites in Balb/c mice and purified as described previously (19, 20). Immune complexes (IC) were generated by mixing IL-7 with anti-IL-7 at a 1:2 molar ratio for 5 minutes at room temperature. IC were then diluted in PBS and 200ul (the equivalent of 2.5ug IL-7 and 12.5ug anti-IL-7 per mouse) was injected intraperitoneally every other day for one week. Anti-mouse IL-7Ra antibody (A7R34) or control antibody was purchased from BioXcell (Dartmouth, New Hampshire) and 3mg was injected i.p. into mice every other day for one week. Mouse IL-2 was obtained from the Biological Resource Branch of the Research Reagents Program of the National Cancer Institute. IL-2 was resuspended in PBS and 3×104 U was injected i.p. twice daily for various periods of time. IL-2 “pulse-chase” experiments were performed by injecting groups of C57BL/6 or BimKO mice with IL-2 for 3 days and then were injected with BrdU 3 times over the last 36 hours of IL-2 treatment. On days 1 and 7 after the last IL-2/BrdU injection, mice were sacrificed and spleen cells stained with antibodies against CD4, FoxP3 and intracellularly for BrdU and data acquired by flow cytometry. The percent FoxP3+ BrdU+ cells remaining was calculated as follows: % FoxP3+ cells that were BrdU+ on day 7 / % FoxP3+ cells that were BrdU+ on day 1.
Results
Treg frequency and survival is increased in aged mice
For these studies, young animals were between 1.5–3 months, middle-aged animals were between 9–15 months, and old animals were more than 18 months of age. As we previously showed (15), the frequency and total numbers of Treg are increased in spleens and mesenteric lymph nodes in aged mice (Fig. 1A, Table S1). Treg accrual could be due to increased thymic output, increased peripheral conversion, increased proliferation, and/or altered survival. Although thymic output is directly proportional to thymic size as mice age (21), it remained possible that thymic output of Treg could be selectively increased with age. However, percentages of FoxP3+ CD4+ single positive (SP) thymic T cells were not significantly different in old and young mice (p = 0.35, Mann-Whitney test, Fig. 1B). As expected, the absolute number of aged CD4+FoxP3+ SP thymocytes was reduced to approximately one third compared to young animals, due to decreased thymic cellularity in aged mice (Fig 1B). These data suggest that altered Treg thymic output is not responsible for increased peripheral Treg in aged mice.
Figure 1. Treg accumulate in aged mice and exhibit increased survival in vitro.
(A) The percentages of FoxP3+CD4+ T cells were determined in the spleens and peripheral LNs of 7 young (1.5-month old) and 6 old (22-month old) C57Bl/6 mice. Lines represent means. Statistical differences were evaluated using unpaired t-tests. (B) Thymocytes from 7 young (1.5-month old) and 6 old (22-month old) mice were analyzed by flow cytometry. Cellularity was assessed for each thymus. The percentage of FoxP3+ cells was analyzed in CD4+ SP thymocytes. Lines represent medians. Statistical differences were evaluated using Mann-Whitney tests. (C). Naïve (CD62L+CD44−GFP−CD4+), effector memory (EM, CD62L−CD44+GFP−CD4+) and central memory (CM, CD62L+CD44+GFP−CD4+) non-Treg were sorted from a pool of spleen and pLN from young (2-month old, N=3, full line) and middle-aged (12-month old, N=4, dotted line) FoxP3-IRES-GFP knock-in Balb/c mice. 5×105 naïve, EM or CM cells were stimulated with immobilized anti-CD3 Ab and soluble anti-CD28, in presence of IL-2 and TGF-β1. GFP expression was analyzed by flow cytometry after 5 days of culture in all cell populations. (D) Young (1.5-month old) and old (18-month old) mice were injected with BrdU every day for 3 days. The percentage of BrdU+ cells was analyzed in gated splenic Treg (CD4+FoxP3+). Values shown are mean % (± SE) of BrdU+ cells in each group. (E) LN cells were harvested from 12 young (1.5-month old) and 11 old (20-month old) mice and cultured in S-MEM media containing 10% FBS, in the absence of exogenous cytokines. After 24 hrs in culture, cells were stained for CD4, CD25 and Propidium Iodide (PI). The percentage of dead cells (PI+) was analyzed in gated non-Treg (CD4+CD25−) and Treg (CD4+CD25+). Values shown are mean % (± SE) of PI+ cells in each group. p values represent the difference between old and young mice (unpaired t-tests).
Alternatively, enhanced conversion of non-Treg in the periphery could explain increase Treg frequency in aged mice. TCR-mediated stimulation in presence of TGF-β1 is efficient at inducing Treg conversion from naïve non-Treg in young animals (22). We therefore assessed conversion of naïve non-Treg from middle-aged mice. In vitro conversion was actually less efficient in T cells from middle-aged compared to young mice (21.8% versus 36.7% in middle-aged versus young mice, respectively, Fig. 1C). Such conversion required TGF- β because less than 0.5% GFP+ (FoxP3+) cells were detected when non-Treg were cultured without TGF- β1 (data not shown). It was possible that FoxP3+ T cells in aged mice were converted from memory T cells; however no accrual of FoxP3+ cells was found after 5 days of in vitro culture of memory (both effector and central) T cells from middle-aged mice (Fig. 1C) as found previously in young mice (23, 24). Combined, these data show that although in vitro conversion of non-Treg could occur in aged mice, it was less efficient than in young mice.
The increased frequency and overall numbers of Treg in aged hosts could also be due to an increased rate of peripheral proliferation. There are few data available on Treg in vivo turnover rates in non-lymphopenic animals, particularly in aging. Therefore, to assess Treg proliferation in vivo, we injected mice with bromodeoxy-uridine (BrdU) for 3 days and measured the frequency of Treg that had incorporated BrdU. The percentage of Treg that had incorporated BrdU+ was similar in young and old mice (Fig. 1D, p<0.45). Thus, these data show that enhanced Treg proliferation in aged mice is unlikely to significantly contribute to their accumulation in aging.
It is also possible that increased Treg frequency in old mice is due to enhanced survival of Treg compared to non-Treg. To test this, Treg survival was determined ex vivo, by quantification of the percentage of dead Treg after 24 hrs in culture. For activated and resting T cells, we found that this assay correlates with in vivo cell survival (19). Treg from old animals died significantly less over the course of this assay than did Treg from young mice (Fig. 1E, p = 0.008). In contrast, non-Treg from old animals died significantly more than non-Treg from young mice (Fig. 1E, p = 0.001). Importantly, the ratio of dying Treg/non-Treg, was significantly decreased in old mice (mean ratio of 1-fold versus 2-fold in old versus young mice, p < 10−5). We note that in old mice, most of the non-Treg are likely endogenous memory T cells while in young mice most of the non-Treg are naïve T cells. Thus, increased survival of Treg in aged mice, combined with the decreased survival of non-Treg, likely contributes to the increase in Treg frequency in aged mice.
Expression of Bim strikingly decreases in Treg with aging
The pro-apoptotic molecule, Bim, is critical for survival and homeostasis of conventional non-Treg (19, 25, 26). As age-related changes in Bim expression in Treg could contribute to their increased frequency, we quantified Bim expression in Treg in aged and young mice, using a Bim-specific antibody (Fig. 2A). Strikingly, Bim expression was decreased by ~2-fold in old Treg compared to young Treg (Fig. 2B, C), whereas its expression was only slightly decreased in old non-Treg (Fig 2B, C). For non-Treg cells, we focused on CD4+ CD44hi FoxP3− cells as this population is probably the most comparable to Treg. This process is gradual, as Bim expression was already decreased in FoxP3+ CD4+ T cells from middle-aged mice (Fig. 2D). We also found that levels of the 2 critical anti-apoptotic molecules in T cells, Bcl-2 and Mcl-1 (19, 27), were both decreased ~2-fold in Treg from old mice, suggesting that neither Bcl-2 nor Mcl-1 contributes to increased survival of aged Treg (Table 1).
Figure 2. Bim expression is decreased in Treg from old and middle-age mice.
(A) Spleen cells from either BimKO or C57BL/6 (WT) mice were stained for CD4, FoxP3 and intracellularly for Bim, and analyzed by flow cytometry. Histogram shows the fluorescence signal for Bim in BimKO (light line) and WT (dark line) mice. (B, C) Spleen cells from 12 young (1.5-month old) and 11 old (20-month old) mice were stained for CD4, FoxP3 and Bim, and analyzed by flow cytometry. Bim MFI was analyzed in gated Treg (CD4+FoxP3+) versus gated non-Treg (CD4+FoxP3−CD44hi). Representative Bim staining in (A) Treg and (B) non-Treg in young (thin lines) and old (thick lines) mice. (C) Mean (± SE) Bim MFI in Treg and non-Treg. p values represent differences in Bim expression in young versus old mice, in each cell subset (unpaired t-tests). (D) Spleen cells from 3 young (6–8 wk old) and 3 middle-aged (10-month old) mice were stained for CD4, FoxP3, and Bim. Bim expression in gated Treg (CD4+FoxP3+) is shown in representative animals. The p value represents the difference between young and middle-aged mice (unpaired t-test). Results are representative of two independent experiments.±
Table 1.
Levels of Bcl-2 and Mcl-1 decrease in Tregs from old mice
| Bcl-2 a | Mcl-1 a | |
|---|---|---|
| Young (n= 6) | 118.9 ± 2.0 | 388.7 ± 18.3 |
| Old (n=5) | 57.3 ± 4.8 | 168.4 ± 8.0 |
| t-test | p < 10−6 | p < 10−5 |
Spleen cells from young and old mice were stained for CD4, FoxP3, CD25 and Bcl-2, or CD4, FoxP3, CD25 and Mcl-1. Bcl-2 or Mcl-1 MFI were determined in gated CD4+FoxP3+ cells.
results are expressed as mean MFI ± SE
Decreased expression of Bim contributes to Treg accumulation in aged mice
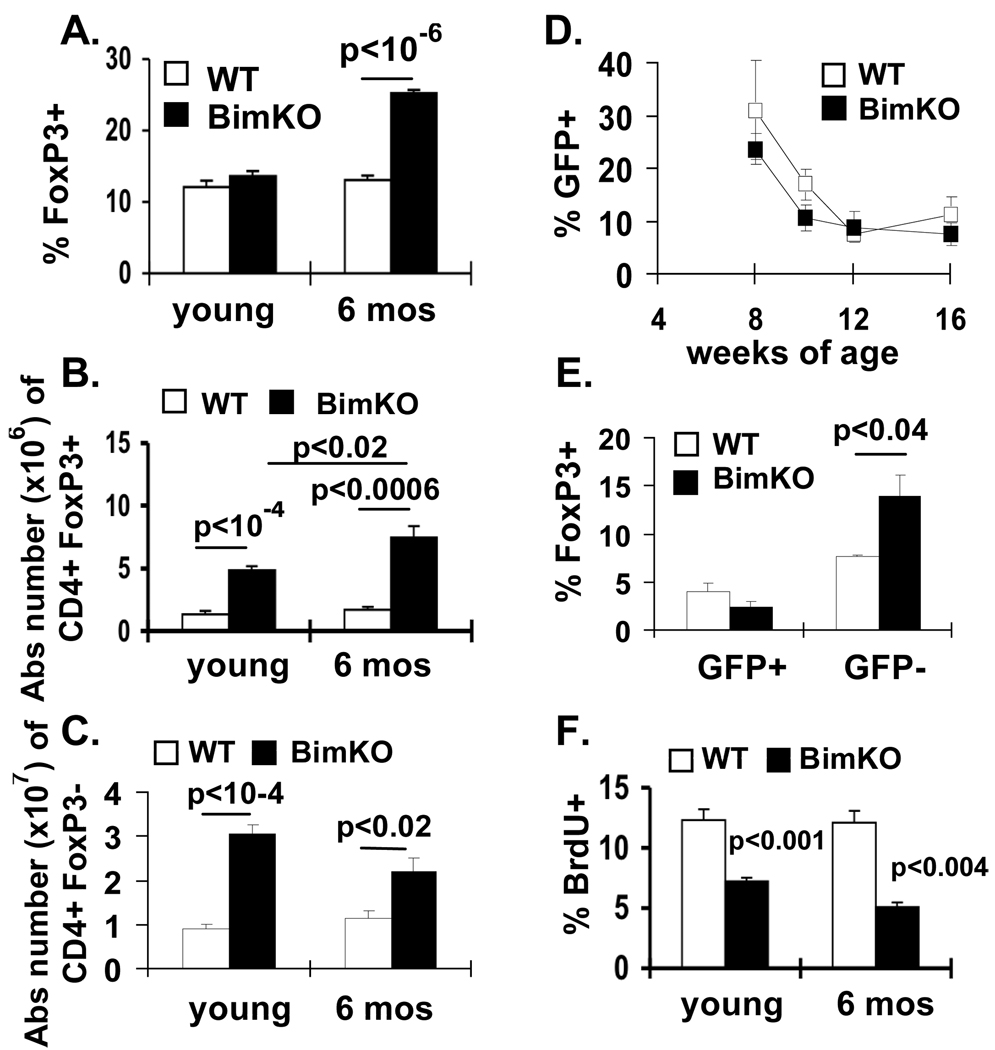
If Bim plays a central role in age-related Treg accumulation, the absence of Bim should lead to a more rapid accumulation of Treg. We thus quantified Treg frequency and total numbers in wild-type (WT) and Bim KO mice at either 2 or 6 months of age. Treg frequency was strikingly increased in 6-month old Bim KO animals (Fig. 3A), compared to age-matched WT mice. While the total numbers of Treg were increased in Bim KO animals at 2 months of age, they accumulated even further by 6 months of age (Fig. 3B). In contrast, the numbers of non-Treg did not increase in Bim KO mice between 2 and 6 months of age (Fig 3C).
Figure 3. Increased frequency of FoxP3+ T cells in middle-aged Bim KO mice.
(A, B, C) Groups of 2 month and 6 month old C57BL/6 (N=9 total; white symbols) and Bim KO mice (N==11 total; black symbols), were injected i.p. with BrdU for 6 days. Results show the mean (±SE) (A) frequency and total numbers of (B) CD4+ FoxP3+ vs (C) CD4+FoxP3− T cells in the spleen. p-values represent the differences between WT and Bim KO mice (unpaired t-test). (D,E) Groups of Rag-2p-GFP transgenic mice (N=3) and Bim KO-Rag-2-GFP (N=3) mice were bled every 2 weeks. Results show the mean (±SE) percent of CD4+ FoxP3+ cells that were GFP+ from Rag-2GFP or Bim KO-Rag-2-GFP in the peripheral blood. (E) Mice were sacrificed at 17 weeks of age. Results show the percent (±SE) of GFP+ versus GFP− CD4+ LN T cells that were FoxP3+. (F) Results show the percent (±SE) of FoxP3+ cells that are BrdU+ in the spleens of Bim KO versus C57BL/6 mice after in vivo treatment with BrdU.
Defects in negative selection in Bim KO mice (28) could contribute to increased Treg production. To test whether defects in negative selection affected Treg production, we crossed Bim KO mice to Rag2p-GFP transgenic mice and tracked thymic output of Treg in Bim KO and WT mice. In Rag2p-GFP transgenic mice, GFP expression is an excellent marker of recent thymic emigrants (RTE) (29). No significant difference was found in the frequency of circulating CD4+FoxP3+ T cells that were recent thymic emigrants (GFP+) between WT-Rag2p-GFP and Bim KO-Rag2p-GFP mice (Fig. 3D). Further, in the LN of Bim KO-Rag2p-GFP mice, the increase in FoxP3+ cells was confined to the GFP− cells (Fig. 3E), suggesting that Treg start accumulating in Bim KO mice in the periphery, after they left the transitional RTE compartment.
As the decreased proportion of GFP+ FoxP3+ cells in Bim KO-Rag2p-GFP mice could be due to their enhanced proliferation, and thus GFP dilution, we monitored in vivo incorporation of BrdU in Treg. However, Treg from Bim KO mice proliferate significantly less than their WT counterparts (Fig. 3F). Together, these data show that Bim controls normal Treg homeostasis by mechanisms that do not involve increased thymic output, nor peripheral proliferation, but instead likely involve survival.
Treg exhibit dysregulated expression of CD127 with aging
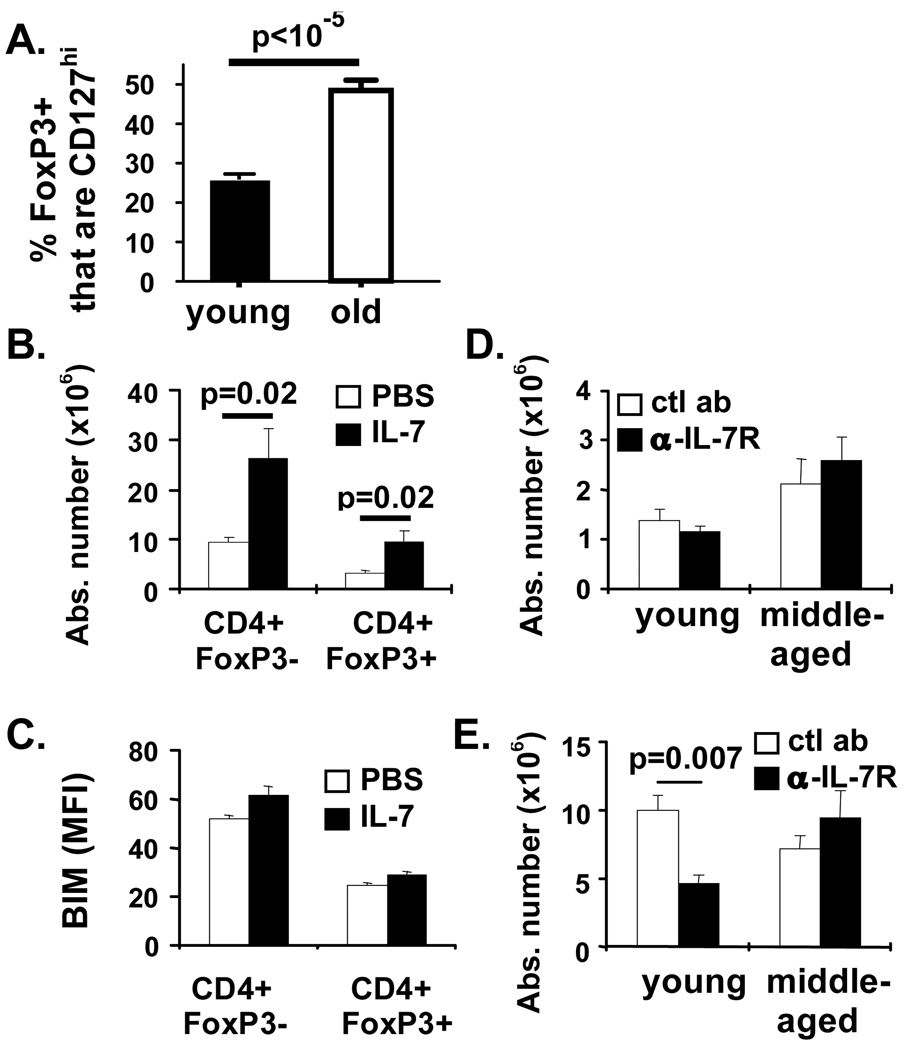
IL-7 is critical for survival of peripheral CD4+ T cells (30). However, few studies have examined CD127 expression on, and the role of IL-7 in, maintaining Treg in aged mice. In young animals, some FoxP3+ T cells were CD127hi (Fig. 4A), similar to previous reports (31, 32), but, the frequency of FoxP3+ T cells expressing CD127 increased by more than 2-fold in aged mice (Fig. 4A). Increased CD127 expression was found in peripheral LN, spleen and mesenteric LN. Most of the FoxP3+CD127+ T cells in aged mice also expressed CD25 (82.3 ± 3.9% in the peripheral LN, 51.9 ± 6.8% in the spleen, 69.9 ± 4.4% in the mesenteric LN).
Figure 4. CD127 expression is increased and functional on Treg from aged mice but is not required for their survival.
Spleen cells from 7 young (1.5-month old) and 6 old (22-month old) mice were stained with antibodies against CD4, FoxP3, CD127, and CD25 and analyzed by flow cytometry. (A) Mean % (± SE) of CD127+ cells in gated Treg (CD4+FoxP3+) are shown. p-value denotes significant difference between young and old mice (student’s t-tests). (B, C) Old (18-month old, n=5) mice were treated with IL-7 IC every other day for one week. Results show the mean (± SE) absolute numbers of CD4+FoxP3− and CD4+FoxP3+ cells. Spleen cells were harvested and stained with antibodies against CD4, FoxP3 and Bim and analyzed by flow cytometry. (C) Results show the mean fluorescence intensity of the Bim stain in CD4+ FoxP3-vs CD4+FoxP3+ cells from PBS (open bars) vs IL-7-treated (filled bars) mice. p-values represent the difference between untreated and IL-7 IC-treated mice (student’s t-test). Results are representative of 2 independent experiments. (D, E) Groups of 2 month (N=4) or 12 month old (N=4) C57BL/6 mice were injected with either anti-IL7R (black bars) or control antibody (ctl Ab; white bars). Results show the mean (±SE) total numbers of splenic (D) CD4+FoxP3+ and (E) CD4+FoxP3−. p-values represent the difference between control and anti-IL-7R-treated mice (student’s t-test).
We next determined whether the CD127 expressed by old Treg was functional by treating 18-month old mice with IL-7 immune complexes (IC), a method to deliver IL-7 in vivo (19). As expected, the absolute number of splenic CD4+ T cells was significantly increased in IL-7 IC-treated old mice (Fig. 4B). Importantly, IL-7 IC treatment also significantly increased Treg absolute number (Fig. 4B), although it did not alter their frequency (25.1 ± 1.7% and 27.1 ± 1.7% of CD4+ T cells expressed FoxP3 in untreated and treated animals, respectively; p = 0.23, unpaired t-test). Similarly, IL-7 significantly improved in vitro Treg survival after 24 hrs (25.6 ± 6.9 % increased survival compared to untreated cultures, p = 0.003). However, in vivo IL-7 administration did not affect expression of Bim in either Treg or non-Treg (Fig. 4C).
We next determined if IL-7 was required for Treg survival in middle-aged animals by preventing IL-7 signaling in vivo. Blockade of IL-7R signaling in vivo did not decrease the numbers of Treg in young or old mice (Fig. 4D). As expected, IL-7R blockade significantly decreased the numbers of non-Treg in young mice (Fig. 4E) and substantially decreased immature B cells in the bone marrow (Fig. S1). Interestingly, IL-7R blockade did not decrease non-Treg in middle-aged mice (Fig. 4E). Together, these data suggest that in both young and middle-aged mice short-term survival of Treg does not strictly require IL-7.
Homeostatic and IL-2-driven proliferation promotes accrual of Treg cells that have decreased expression of Bim
IL-2 is a major survival/proliferative factor for Treg (33) and has been reported to control Bim expression (34). Therefore, we first examined whether levels of Bim would be altered in cells undergoing normal homeostatic proliferation. Interestingly, levels of Bim were significantly decreased in FoxP3+BrdU+ compared to FoxP3+BrdU− cells, whilst in FoxP3− cells, Bim levels were not different between BrdU+ and BrdU− populations (Figure 5A). We next determined the effect of exogenous IL-2 on Treg homeostasis and Bim expression. IL-2 drove a progressive 7-fold increase in CD4+FoxP3+ T cells (Fig. 5B), compared to a 3-fold increase in CD4+FoxP3− T cells (Fig. 5C). Notably, within the Treg population, IL-2 preferentially increased the numbers of Bimlo Treg (Fig. 5D). The effect of IL-2 on Bim was clearly seen only after repeated IL-2 injection (Fig. 5D). As cell proliferation in response to IL-2 may have contributed to the accumulation of Bimlo Treg, we assessed their in vivo proliferation by BrdU labeling along with intracellular staining for Bim. We found that IL-2 drove substantial BrdU incorporation in FoxP3+ compared to FoxP3− cells (Fig. 5E). In addition, similar to our previous data under basal homeostatic conditions, most proliferating Treg expressed low levels of Bim (Fig. 5F).
Figure 5. Proliferation and accrual of Bimlo Treg.
(A). Young BL/6 mice were injected with BrdU for 3 days, sacrificed and spleen cells analyzed for Bim expression and BrdU incoporation by intracellular flow cytometric staining. Results show the mean fluorescence intensity of the Bim signal in either CD4+FoxP3+ or CD4+FoxP3− cells that were either BrdU− (open bars) or BrdU+ (closed bars) (± SE) (B–D) Young BL/6 mice (N = 3/group) were injected i.p., with either 3×104 U hIL-2 or PBS twice daily for 4 days. Results show the mean (±SE) absolute number of (B) CD4+ FoxP3+ T cells; (C) CD4+FoxP3− T cells; or (D) CD4+FoxP3+ T cells that are either Bimlo or Bimhi. (E, F) Groups of 8 week old C57BL/6 mice were injected twice daily with either PBS or IL-2 for 3 days and on the day before sacrifice were injected twice with 0.6mg BrdU. (E) Results show percent (±SE) of FoxP3+ versus FoxP3− cells that are BrdU+ (F). Results show the percent (±SE) of BrdU− or BrdU+ cells that were Bimlo in either FoxP3− (open bars) versus FoxP3+ (closed bars) T cells in IL-2 treated mice. (G). Groups of BL/6 and BimKO mice (n=4 mice/group/timepoint) were injected with IL-2 twice daily for three days and with BrdU the last 36 hours of IL-2 treatment. Groups of BL/6 and BimKO mice were sacrificed either one or seven days after the last IL-2/BrdU injection. Results show the percentage of FoxP3+BrdU+ cells (± SE) remaining on day 7 relative to the amount observed on day 1 after cessation of IL-2/BrdU treatment.
We next determined whether deficiency in Bim promoted Treg survival following brief treatment with IL-2, by performing an IL-2/BrdU “pulse-chase” experiment. We treated groups of C57BL/6 and BimKO mice with IL-2 and BrdU and then sacrificed groups of mice one or 7 days after the last IL-2/BrdU treatment. One day after cessation of IL-2/BrdU, there was no significant difference in the frequency of Treg that were BrdU+ between BL/6 and BimKO mice (42.8 +/− 2.8% versus 39.4 +/− 1.9%, respectively; p<0.365). However, one week later, significantly more BrdU+ Treg remained in BimKO compared to C57BL/6 mice (Figure 5G). Together, our data suggest that the progressive accrual of Treg in aged animals is due to increased IL-2-driven proliferation, combined with enhanced survival of Bimlo Treg.
Discussion
Age-related immunosuppression is likely driven by several factors. While it is clear that cell-intrinsic mechanisms contribute, less is known about the contribution of Treg to age-related immunosuppression. We previously showed that Treg accumulate dramatically with age and that their depletion led to significantly increased effector T cell responses against Leishmania major (15). These data strongly suggest that, under the conditions of this in vivo model, Treg contribute to age-related immunosuppression. In addition, other investigators have shown that Treg were functionally suppressive in aged hosts (13, 17, 35). Collectively, these data incriminate the accrual of Treg as one of the mechanisms involved in the immune suppression associated with aging.
However, the mechanisms underlying Treg accumulation in aging are not well understood. Recent studies of Treg biology have identified several critical steps. It is now well established that Treg can emerge from two different pathways, either the generation of CD4+FoxP3+ cells directly in the thymus, or the peripheral induction of FoxP3 in naïve T cells that did not express FoxP3 (rev. in (36)). Treg generated through these two pathways express similar phenotype, although thymic-derived Treg appear to more specifically express the transcription factor Helios (37). Thus, Treg accrual in aging could be due to changes in one or several of these pathways, i.e. increased thymic output, increased peripheral conversion, and/or altered homeostasis, such as a changed capacity to proliferate or a decreased susceptibility to death. In depth investigation of these pathways was thus the primary objective of our present studies.
Our data show that altered Treg thymic output is not responsible for the increased frequency of peripheral Treg in aged mice. Indeed, we did not find a selectively increased thymic output of Treg with age, as percentages of FoxP3+ CD4+ SP thymic T cells were similar in old and young mice, a result in agreement with a previous study (14). Our data also do not support a major role for increased peripheral conversion in aged mice. This is in contrast to another study, which showed that both Treg and non-Treg from elderly CMV seropositive patients were enriched for Vβ2-bearing T cells. Although the authors of this study suggested that this commonality of T cells with similar TCRs resulted from conversion of conventional T cells into Treg, direct evidence of peripheral conversion was not shown (35). An alternative explanation is that, within the thymic derived Treg compartment, there exists Vβ2-bearing Treg with specificity to CMV and these cells expanded during chronic CMV infection. Recent data have suggested that Treg have a fairly broad TCR repertoire (38). To our knowledge, our study is the first one to examine the ability of naïve versus memory T cells from aged mice to undergo conversion. Our finding that naïve non-Treg from old mice exhibited a lesser capacity to start expressing FoxP3, while old memory T cells remained resistant to conversion, do not support increased conversion as a major mechanism underlying age-associated Treg accrual. Further, Thornton et al have shown that the frequency of FoxP3+ T cells expressing Helios (a marker of thymically derived Treg) do not change with age, arguing against peripheral conversion being a major mechanism of Treg accrual (37).
Importantly, our study shows that aged Treg exhibit better ex vivo survival and strikingly decreased expression of Bim. We found that Bim levels in Treg are progressively and normally decreased with age in wildtype mice and that Treg accumulate significantly faster in Bim KO mice after they have left the RTE compartment, despite decreased in vivo proliferation. During our studies, it was shown that Bim also contributes to the longer lifespan of aged naïve CD4+ T cells (39). Our data are consistent with these data, although they also suggest that the effects of Bim are more profound on Treg than on conventional non-Treg. Our data also broaden and expand on a previous study showing increased Treg frequency in Bim KO mice, although the age of the mice was not indicated in that study (40). Neither Bcl-2 nor Mcl-1 appeared to contribute to increased survival of aged Treg, as their levels were also significantly decreased in old Treg. Decreased Bcl-2 levels had already been described in Treg (35); however, levels of Bim were not assessed in that study. The significance of Bcl-2 levels needs to be interpreted in concordance with Bim levels, as Bcl-2 functions as the major Bim antagonist in T cells (19). We acknowledge that molecules other than Bim may contribute to Treg homeostasis, however, our data strongly indicate that decreased expression of Bim contributes significantly to Treg accrual with age.
Bim is normally counteracted by IL-7 signaling in non-Treg (26). The frequency of FoxP3+ T cells expressing CD127 increased by more than 2-fold in aged mice, and this increased CD127 expression was found in peripheral LN, spleen and mesenteric LN. As it had been shown that IL-7 signaling allows for survival of peripheral Treg in IL-2Rβ KO mice (41), we determined whether increased CD127 expression was involved in enhanced survival of old Treg. Although CD127 was clearly functional in old Treg, because administration of IL-7 IC led to increased Treg numbers in old mice; in vivo neutralization of IL-7 did not alter Treg frequency. Furthermore, IL-7 signaling did not affect Bim expression in Treg (Fig. 4C). Thus, despite Treg increased expression of CD127, IL-7 is not required for their survival in old animals. However, these data do not necessarily exclude the possibility that IL-7 plays a redundant role with IL-2 in Treg survival in aged mice.
We show a novel intersection between IL-2 and Bim leading to the age-related persistence of Treg in vivo. It is possible that IL-2 drives Treg proliferation on one hand and regulates expression of Bim mRNA or Bim protein half-life in Treg on the other (42). Regulation of Bim expression is complex and is controlled at both the transcriptional and post-transcriptional levels. Post-transcriptionally, Bim protein levels can be controlled by the microRNA 17–92 cluster in B cells (43) and by phosphorylation and turnover downstream of ERK signaling (44). At the transcriptional level, Foxo family members have been implicated as potential regulators of Bim expression (34, 45). Foxo activity is controlled by AKT-dependent phosphorylation, which sequesters Foxo molecules in the cytoplasm, preventing them from promoting Bim transcription (34, 45). However, additional, AKT-independent, mechanisms can modify Foxo regulation of transcriptional targets (46). Recent work has shown that IL-2-driven, AKT-activation in Treg cells is impaired due to their high expression of PTEN (phosphatase and tensin homologue deleted on chromosome 10) (47), arguing that classical Foxo regulation of Bim in response to IL-2 in Treg may involve additional pathways.
Alternatively, IL-2 may not directly control Bim expression, but instead could drive the selective accumulation of Bimlo cells because these cells survive better than their Bimhi counterparts. Three key pieces of data support this model. First, we did not observe changes in Bim expression after short-term treatment with IL-2; instead, Bim levels were decreased only after IL-2 has started driving Treg expansion. If IL-2 were directly controlling Bim expression, we would have expected to observe changes in Bim prior to Treg expansion. Second, under both homeostatic and IL-2-driven proliferation, the majority of the proliferating (BrdU+) cells were Bimlo. Third, after IL-2 withdrawal in vivo, Bim-deficient cells survive better. Combined, these data are supportive of a model in which IL-2 promotes homeostatic proliferation of Bimlo Treg and these cells have a survival advantage over Bimhi Treg which leads to their progressive accumulation with age. We acknowledge that we cannot definitively determine if a small preexisting Bimlo cell population has a proliferative advantage as well as a survival advantage as we would need to sort cells based on their expression of Bim to perform this experiment. In the absence of Bim-reporter mice, this is technically infeasible.
It is also possible that Treg in old mice may not require constant exposure to IL-2 to maintain their Bimlo status, and thus, survive. In fact our data is supportive of such a model as we showed that the lack of Bim increases survival of Treg after IL-2 withdrawal in vivo. The source and availability of IL-2 in aged mice is unclear. Previous work has suggested that IL-2 levels decline with age, however, these data were based on T cell production of IL-2 after mitogenic stimulation in vitro (48). Whether or not in vivo IL-2 levels decline with age and whether T cells are a major source of such IL-2 is unclear. However, defining the active levels of IL-2 in vivo is complicated by the fact that bioactive IL-2 binds to heparan sulfate, a glycosaminoglycan found on cell surfaces and within extracellular matrices (49). Nonetheless, it is possible that IL-2 is critical to initiate the expansion of Bimlo cells, but IL-2 is not critical for the maintenance of these cells thereafter. A potential scenario for such a model is that the accumulation of Bimlo Treg is due to genetic modifications that control Bim expression. For example, the Bim promoter has CpG-rich areas and Bim gene expression can be controlled by promoter methylation (50–52). Further, increased methylation of CpG-rich areas is a common finding in aged cells (53, 54). As such methylation is an inherited trait, methylation of the Bim promoter in Treg would be inherited and selected for, as Treg from Bim-deficient mice survive better than WT Treg (40). Control of Bim expression may be indirect as well, transcription factors controlling basal Bim expression may themselves be regulated and therefore not be available to promote Bim expression in aged mice. Future work will uncover the mechanistic interplay between IL-2, Treg accrual, and regulation of Bim expression in Treg.
In summary, our data strongly suggest that age-related Treg accumulation is not due to overproduction of Treg late in life, nor increased peripheral conversion, but rather due to enhanced survival of Treg. Together, these data have implications for the immunosuppression that occurs with aging. Limiting or reducing Treg frequency in the elderly may enhance their ability to maintain immune competence. On the other hand, limiting Treg in the elderly may enhance autoimmune responses. Nonetheless, with a better understanding of cytokine-mediated regulation of Treg homeostasis, acute and/or chronic manipulation of Treg numbers may become feasible when increased immune function is required to counteract infectious diseases or tumors in the elderly.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. F. Finkelman and C. Perkins for help purifying anti-IL-7 antibody; Drs. M. Wills-Karp, C. Karp, and A. Lentsch for their donations of aged mice; and Drs. K. Hoebe, C. Karp and J. Katz for critical reading of the manuscript.
Non-standard abbreviations
- FoxP3
Forkhead box P3
- IC
immune complexes
- Treg
regulatory T cells
Footnotes
This work was supported by funds from the Divisions of Immunobiology and Molecular Immunology, and NIH Grants AI068524 (C.C.), AG033057 (C.C. and D.H.), AI057753 (D. H.), and ACS RSG-08-293-01-CCG (D.P.).
References
- 1.Miller RA. Effect of aging on T lymphocyte activation. Vaccine. 2000;18:1654–1660. doi: 10.1016/s0264-410x(99)00502-2. [DOI] [PubMed] [Google Scholar]
- 2.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 3.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 4.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 5.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 14.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 15.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, Salmon M, Taams LS, Krueger J, Greenwood J, Klein N, Rustin MH, Akbar AN. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W, Nagaoka H, Misulovin Z, Meffre E, Suh H, Jankovic M, Yannoutsos N, Casellas R, Besmer E, Papavasiliou F, Qin X, Nussenzweig MC. RAG expression in B cells in secondary lymphoid tissues. Cold Spring Harb Symp Quant Biol. 1999;64:207–210. doi: 10.1101/sqb.1999.64.207. [DOI] [PubMed] [Google Scholar]
- 19.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting Edge: Limiting amounts of IL-7 do not control contraction of CD4+ T cell responses. J Immunol. 2007;178:4027–4031. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyzik M, Piccirillo CA. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leukoc Biol. 2007;82:335–346. doi: 10.1189/jlb.1006644. [DOI] [PubMed] [Google Scholar]
- 25.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 28.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 29.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 30.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 32.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 34.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 35.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 37.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz. L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinjyo T, Kuribara R, Inukai T, Hosoi H, Kinoshita T, Miyajima A, Houghton PJ, Look AT, Ozawa K, Inaba T. Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol Cell Biol. 2001;21:854–864. doi: 10.1128/MCB.21.3.854-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 45.Riou C, Yassine-Diab B, Van grevenynghe J, Somogyi R, Greller LD, Gagnon D, Gimmig S, Wilkinson P, Shi Y, Cameron MJ, Campos-Gonzalez R, Balderas RS, Kelvin D, Sekaly RP, Haddad EK. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 47.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoman ML, Weigle WO. Lymphokines and aging: interleukin-2-production and activity in aged animals. J Immunol. 1981;127:2102–2106. [PubMed] [Google Scholar]
- 49.Wrenshall LE, Platt JL. Regulation of T cell homeostasis by heparin sulfate-bound IL-2. J Immunol. 1999;163:3793–3800. [PubMed] [Google Scholar]
- 50.De Bruyne E, Bos TJ, Schuit F, Van Valckenborgh E, Menu E, Thorrez L, Atadja P, Jernberg-Wiklund H, Vanderkerken K. IGF-1 suppresses Bim expression in multiple myeloma via epigenetic and posttranslational mechanisms. Blood. 2010;115:2430–2440. doi: 10.1182/blood-2009-07-232801. [DOI] [PubMed] [Google Scholar]
- 51.Paschos K, Smith P, Anderton E, Middeldorp JM, White RE, Allday MJ. Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog. 2009;5:e1000492. doi: 10.1371/journal.ppat.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.San Jose-Eneriz E, Agirre X, Jimenez-Velasco A, Cordeu L, Martin V, Arqueros V, Garate L, Fresquet V, Cervantes F, Martinez-Climent JA, Heiniger A, Torres A, Prosper F, Roman-Gomez J. Epigenetic down-regulation of BIM expression is associated with reduced optimal responses to imatinib treatment in chronic myeloid leukaemia. Eur J Cancer. 2009;45:1877–1889. doi: 10.1016/j.ejca.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Issa JP. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109:103–108. doi: 10.1016/s1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]
- 54.Golbus J, Palella TD, Richardson BC. Quantitative changes in T cell DNA methylation occur during differentiation and ageing. Eur J Immunol. 1990;20:1869–1872. doi: 10.1002/eji.1830200836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.