Abstract
Roux-en-y gastric bypass (RYGB) surgery is associated with dramatic improvements in obesity-related comorbidity, but also with nutritional deficiencies. Vitamin D concentrations are depressed in the severely obese, but the impact of weight loss via RYGB is unknown. We determined associations between adiposity and systemic 25-hydroxyvitamin D (25(OH)D) during weight loss and the immediate and longer-term effects of RYGB. Plasma 25(OH)D concentrations and fat mass (FAT) were determined by immunoassay and air displacement plethysmography, respectively, at 0 (before RYGB surgery), and at 1, 6, and 24 months in severely obese white and African American (AA) women (n = 20). Decreases in adiposity were observed at 1, 6, and 24 months following RYGB (P < 0.05). Plasma 25(OH)D concentrations increased at 1 month (P = 0.004); a decreasing trend occurred over the remainder months after surgery (P = 0.02). Despite temporary improvement in vitamin D status, a high prevalence of vitamin D insufficiency was observed (76, 71, 67, and 82%, at baseline, 1, 6, and 24 months, respectively), and plasma 25(OH)D concentrations were lower in AA compared to white patients (P < 0.05). Strong positive baseline and 1 month cross-sectional correlations between FAT and plasma 25(OH)D were observed, which remained after adjustment for age and race subgroup (β = 0.76 and 0.61, respectively, P = 0.02). In conclusion, 25(OH)D concentrations increased temporarily and then decreased during the 24 months following RYGB. The acute increase and the positive associations observed between adipose tissue mass and systemic 25(OH)D concentrations suggest storage in adipose tissue and release during weight loss.
INTRODUCTION
The epidemic of severe obesity in adults defined by BMI is a major health concern in the United States. Prevalence has quadrupled from 1976 to 2000 (1,2) and currently 5.7% of the US population is affected (3). Severe obesity is associated with an increased risk of health complications such as: cardiovascular disease, diabetes, and cancer (4–6). Bariatric surgery is the most efficient method in producing maximum weight loss in severely obese patients (7). Roux-en-y gastric bypass (RYGB) is a common bariatric procedure which induces food restriction and as well as malabsorption of macro- and micronutrients. Following RYGB 70% of patients achieve effective weight loss (defined as losing >50% of their excess weight) and resolution of most obesity-related comorbidities (8), but many patients also experience nutritional deficiencies including calcium, iron, vitamin B12, and vitamin D (9).
Vitamin D is an essential nutrient that functions in regulating bone metabolism, cell proliferation, and immunity (10). A high prevalence of vitamin D insufficiency (25(OH)D <75 nmol/l or 30 ng/ml) is found in the United States (11). Many factors affect vitamin D status including latitude, dietary intake and use of supplements, intestinal absorption, sun exposure, and skin color (12).
Past studies have shown decreased levels of serum 25(OH)D in obese individuals, when compared to lean, showing an inverse relationship between BMI or body fatness and serum 25(OH)D (13–15). One explanation for lower levels of 25(OH)D occurring in obesity is that vitamin D, a fat soluble molecule, is stored and possibly sequestered in adipose tissue (16,17). Following ingestion of radiolabeled vitamin D3, high concentrations of the compound were measured in the adipose tissue of rodents relative to serum and to other tissues (18). Substantial amounts of 25(OH)D have also been observed in human adipose tissue (19). Sequestration of vitamin D in adipose tissue is suggested by a single study in humans in which, following UV irradiation in lean vs. obese subjects, equal amounts of vitamin D3 were found in skin samples, but serum vitamin D3 concentrations in obese subjects were reduced (17). In the same study, oral doses of vitamin D2 resulted in lower serum concentrations of vitamin D2 in obese compared to lean subjects. A recent study, in which adipose tissue and plasma samples were obtained from severely obese individuals undergoing bariatric surgery, found a positive relationship between serum and subcutaneous fat vitamin D3 concentrations (20). Thus the issue of whether vitamin D is stored and then released or instead sequestered in obese humans warrants further investigation.
Although it is clear that obesity decreases vitamin D concentrations, the potential benefits of weight loss on vitamin D status have not been shown. Also unknown is the impact of bariatric surgery on vitamin D status, as the malabsorptive types of bariatric surgery promote malabsorption of fat soluble nutrients in the small intestine, changes in dietary intake, and decrease of adipose tissue stores. A high rate of vitamin D deficiency has been identified in preoperative bariatric surgery patients (21). In that study of 312 patients, 57% were deficient in vitamin D preoperatively (25(OH)D ≤50 nmol/l). Of the black patients evaluated, 78% were vitamin D deficient and 37% of the white patients were deficient. Postoperative bariatric surgery patients have a high prevalence of vitamin D deficiency (which has been documented as high as 80%) (22–27), but limited prospective studies have documented both pre- and postoperative levels of 25(OH)D in bariatric surgery patients (27–29). Also, vitamin D status over several months or years following bariatric surgery procedures has been little studied. Thus it is unclear whether bariatric surgery improves or further aggravates the status of vitamin D.
The purpose of this study was to determine plasma 25(OH)D concentrations in a cohort of white and African-American women undergoing RYGB surgery and followed prospectively acutely and for up to 24 months. Variables including race, time following surgery, body fat mass (FAT), regional adiposity, and dietary intake were assessed in order to define the potential effects of surgical weight loss on vitamin D status.
METHODS AND PROCEDURES
Patients
The study subjects were 20 severely obese female patients who had RYGB at the Emory Bariatric Center via RYGB (21). Clinical, psychological, and nutritional evaluations were performed before surgery. This was longitudinal study in which each patient served as her own control. Subjects were evaluated at baseline (before surgery) and again at 1, 6, and 24 months postsurgery. Exclusion criteria for this study were (i) male gender, (ii) age <18 years or >65, (iii) BMI <35 kg/ m2, and (iv) current smoking history. All patients were prescribed a multivitamin/multimineral regimen following surgery, but dietary and supplement intake, using 3-day food records, collected at each study visit and analyzed using Food Processor SQL (ESHA Research, Salem, OR), was monitored only for a subset of consecutively enrolled subjects (n = 7). The Emory University Institutional Review Board approved the study and all patients signed informed consent before enrollment.
Anthropometry and body fat composition
Body fat composition was measured by air plethysmography (BOD-POD; Life Measurement Instruments, Concord, CA) (30). Abdominal fat distribution was measured by computed tomography, using a GE High Speed Advantage computed tomography scanner (General Electric Medical Systems, Milwaukee, WI) as described (31). Volumes of visceral adipose tissue and subcutaneous adipose tissue, respectively, were determined from computed tomography scans taken from the L1 to the L5 vertebral region (140 kV, 240–340 mA·s, 10 mm slice thickness). Adipose tissue within an attenuation range of −190 to −30 Hounsfield units was highlighted and computed using software (GE Medical Systems, Waukesha, WI). Body height was measured without shoes. Body weight was measured with subjects in light clothing, in the fasting state, and immediately after voiding in the morning.
Metabolic measures
Plasma samples were obtained at baseline (before surgery), 1, 6, and 24 months following surgery and stored at −80 °C. Analysis of plasma 25(OH)D concentrations from baseline, 1, 6, and 24 months were measured in duplicate samples in complete batches using a commercial human enzyme-linked immunosorbent assay kit (Immunodiagnostics Systems Laboratory, Scottsdale, AZ). Quality control of the 25(OH)D measurements was assured by participation in the Vitamin D External Quality Assessment Scheme (www.deqas.org, site 606). The intra- and interassay coefficient of variation of the 25(OH)D measurements were <8% and <10%, respectively.
Statistical analysis
The statistical software STATISTICA (StatSoft, Tulsa, OK) was used for study analysis. The differences between time points were analyzed using paired Student’s t-tests and repeated measures ANOVA. Relationships between 25(OH)D concentrations and secondary variables, including body FAT, were examined as linear correlations using Pearson correlations. The significance level for the study was set at P < 0.05 and the results are expressed as mean ± s.e. of the mean.
RESULTS
Patient characteristics
Twenty patients who underwent RYGB surgery were assessed from baseline to 6 months following surgery and 10 subjects in this group were analyzed at 24 months following surgery. The mean age was 33.8 ± 1.7 years (range 24–55). Six women were self-described as non-Hispanic African American (AA) in descent, 12 were non-Hispanic–white, and 2 were Hispanic. At baseline, their mean BMI was 48.0 ± 0.9 (range 41.5–55.3). Seven patients (33% of the population) had diabetes at baseline. Of the patients assessed at 24 months, three were non-Hispanic AA, six were non-Hispanic white and one was Hispanic in descent.
Changes in adiposity following bariatric surgery
Significant decreases in BMI, and total body fatness were observed at 1 month following weight loss surgery, and adiposity continued to decrease during 24 months as shown in Table 1. Similarly, visceral and subcutaneous adipose tissue volumes significantly decreased compared to baseline at all time points following surgery. There was no difference in BMI at baseline or in the rate of weight loss in the different race subgroups (data not shown).
Table 1.
Changes in adiposity measures following surgery
| Before surgery | 1 month post-RYGB | 6 month post-RYGB | 24 month post-RYGB | |
|---|---|---|---|---|
| BMI (kg/m2) | 47.5 ± 0.9 | 42.8 ± 0.9 | 33.9 ± 1.1 | 30.9 ± 2.1 |
| FAT (kg) | 70.1 ± 2.2 | 61.4 ± 2 | 40.3 ± 2.5 | 34.2 ± 5.6 |
| VAT (cm3) | 4,500 ± 460 | 3,750 ± 390 | 2,320 ± 310 | 1,630 ± 710 |
| SAT (cm3) | 13,580 ± 430 | 12,700 ± 550 | 8,750 ± 490 | 6,900 ± 1,080 |
Body fat mass (BMI), total adipose tissue mass (FAT), and abdominal visceral (VAT), and subcutaneous (SAT) adipose tissue volumes were measured as described in Methods and Procedures section at 0 (before surgery), and 1, 6 (n = 20), and 24 (n = 10) months following Roux-en-y gastric bypass (RYGB) surgery. Significant decreases in adiposity were observed at all times following surgery (P < 0.000 compared to the baseline measure).
Changes in plasma 25-hydroxyvitamin D (25(OH)D) concentrations
Plasma 25(OH)D concentrations were determined for the study population followed for 6 months and the subgroup followed for 24 months. For patients followed for to 6 months post operatively, there was a transient increase observed in plasma 25(OH)D at 1 month following surgery (P = 0.002), but values at 6 months were not different from those at baseline (54.8 ± 5.4, 65.7 ± 6.7, and 61.2 ± 4.6 nmol/l at baseline, 1, and 6 months, respectively, Figure 1). For the subgroup of subjects followed out to 24 months, plasma 25(OH)D concentrations increased at 1 month (P = 0.004) and then dropped over the remaining time period such that values at 24 months were significantly decreased (P = 0.02 compared to 1 month); values were 63.8 ± 9.1, 76.9 ± 11.1, 67.7 ± 6.5, 51.1 ± 5.5 nmol/l at baseline, 1, 6, and 24 months, respectively (Figure 2). A significant effect of time following surgery on 25(OH)D concentrations was observed (P = 0.02). Plasma 25(OH)D concentrations in AA subjects were reduced compared to white subjects at baseline, and all time points following surgery (P < 0.02). Regardless of race subgroup or time point measured, a high prevalence of vitamin D insufficiency, defined as 25(OH) D <75 nmol/l, was observed (total population; 76, 71, 67, and 82%, for baseline, 1, 6, and 24 months, respectively). Similarly, the prevalence of vitamin D deficiency, defined as 25(OH)D <50 nmol/l, was high (total population; 50, 30, 30, and 60% for baseline, 1, 6, and 24 months, respectively). Vitamin D intake was estimated from 3-day food records obtained from a subgroup of subjects (n = 7). Compared to baseline vitamin D dietary intake, there were no changes observed during 6 months following surgery (111.1 ± 37.4, 104.0 ± 32.7, and 149.5 ± 58.1 IU at baseline, 1, and 6 months, respectively). For these subjects, a transient increase in 25-OH-D was observed at 1 month following surgery as was found in the larger population (54.3 ± 6.5, 72.3 ± 12.3, and 65.9 ± 7.0 nmol/l, at baseline, 1, and 6 months following surgery). Information on sun exposure was not collected.
Figure 1.
Six months changes in plasma 25-hydroxyvitamin D concentrations following surgery. 25-Hydroxyvitamin D (25(OH)D)) was measured in plasma samples obtained at 0 (before surgery), and 1, and 6 months following Roux-en-y gastric bypass surgery as described in Methods and Procedures section. Open squares depicts non-Hispanic white women (n = 12) and open circles depicts non-Hispanic African-American women (n = 6), the thick line depicts the averages of total population (n = 20), the thin line depicts the cutoff for normal vitamin D status (≥75 nmol/l). *Depicts significant different from baseline value (P < 0.05).
Figure 2.
Twenty-four months changes in plasma 25-hydroxyvitamin D concentrations following surgery. 25-Hydroxyvitamin D (25(OH)D) was measured in plasma samples obtained at 0 (before surgery), and 1, 6, and 24 months following Roux-en-y gastric bypass surgery as described in Methods and Procedures section. Open squares depicts non-Hispanic white women (n = 6) and open circles depicts non-Hispanic African-American women (n = 3), the thick line depicts the averages of total population (n = 10), the thin line depicts the cutoff for normal vitamin D status (≥75 nmol/l). *Significantly different from baseline value (P < 0.05); †significantly different from 1 month value (P < 0.05).
Relationships between body fat and serum 25(OH)D concentrations
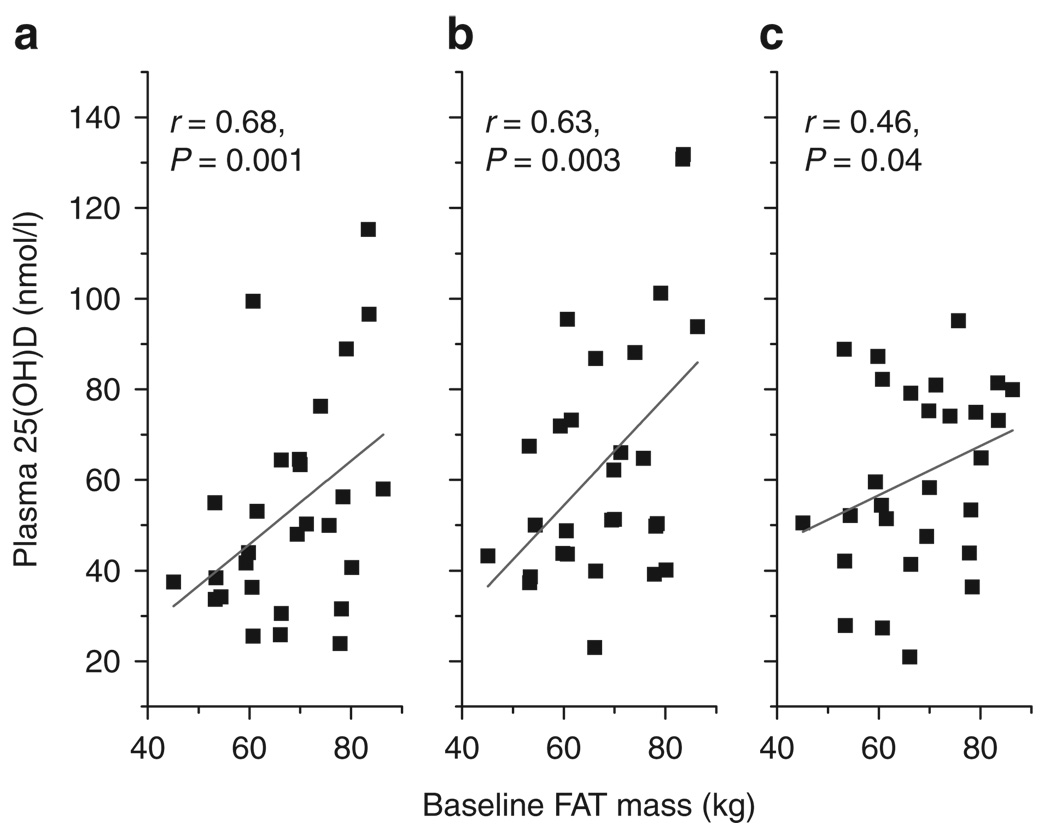
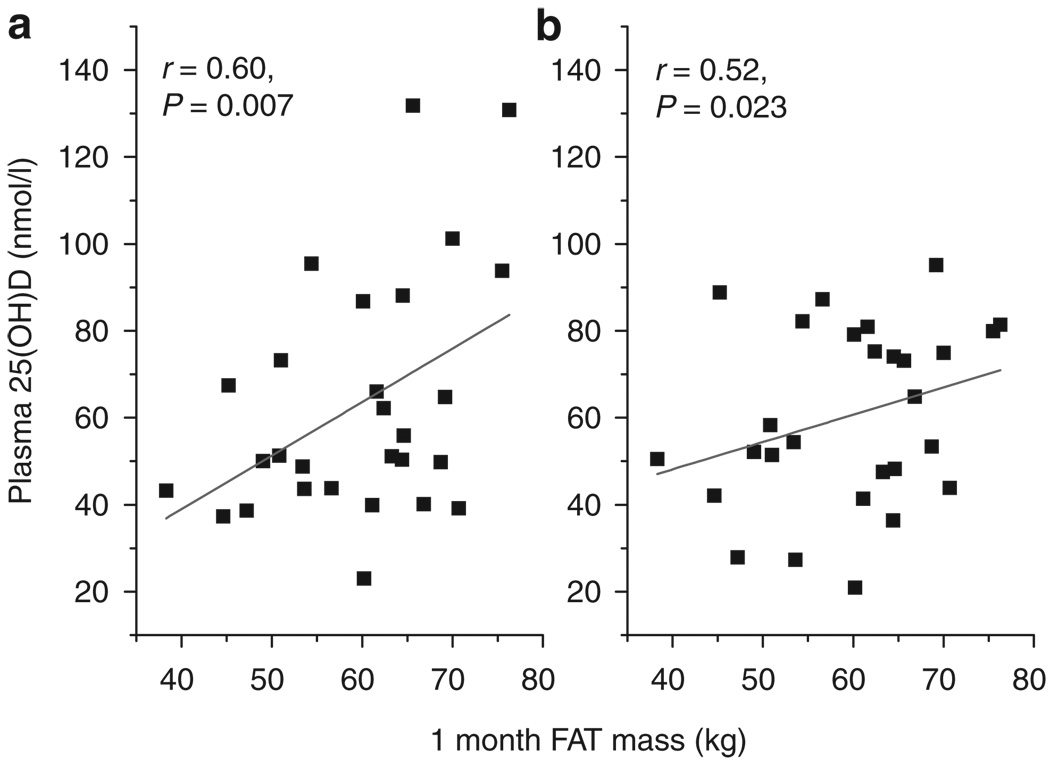
Cross-sectional and longitudinal associations between body FAT and systemic 25(OH)D concentrations at baseline and during the 24-month period following surgery were determined. Significant correlations were observed between adipose tissue mass at baseline and 25(OH)D concentrations at baseline, 1, and 6 months following surgery (r = 0.68, P = 0.001; r = 0.63, P = 0.003; and r = 0.46, P = 0.04, respectively (Figure 3). The relationships between baseline adipose tissue and 25(OH)D concentrations became stronger after adjusting for confounders including age and race subgroup using multiregression analysis (Table 2). Significant correlations were also found between adipose tissue mass at 1 month and concentrations of 25(OH)D at 1 and at 6 months following surgery (r = 0.60, P = 0.007; r = 0.52, P = 0.023, respectively) (Figure 4). These correlations increased after adjustment for age and race subgroup (Table 3). There were no significant cross-sectional correlations between adipose tissue mass at 6 and 24 months and systemic 25(OH)D concentrations at either of those times (data not shown). Consistent decreases in adipose tissue mass over the 24-month period following surgery did not correspond to changes in plasma 25(OH)D (Figure 5). In keeping with this observation, longitudinal correlations between changing adipose tissue mass and changes in 25(OH)D were not significant (correlations between ΔFAT vs. Δ25(OH)D at 1 and 6 months were r = −0.33, P = 0.16 and r = −0.076, P = 0.75, respectively). Finally cross-sectional or longitudinal correlations between 25(OH)D concentrations abdominal visceral or subcutaneous volumes were weak and nonsignificant (data not shown).
Figure 3.
Relationship between plasma 25-hydroxyvitamin D and total adiposity before surgery. Cross-sectional Pearson correlations were determined between total fat mass (FAT) before surgery (baseline), and 25-hydroxyvitamin D (25(OH)D) at (a) baseline, (b) 1 and (c) 6 months following RYGB (n = 20). FAT, fat mass; RYGB, Roux-en-y gastric bypass.
Table 2.
Multiple linear regression analysis of relationships between baseline adiposity and 25(OH)D
| FAT baseline | Model 1 (unadjusted) | Model 2 (age, race) |
|---|---|---|
| 25(OH)Dbaseline | 0.68 | 0.76 |
| 25(OH)D1 month | 0.63 | 0.69 |
| 25(OH)D6 months | 0.46 | 0.63 |
Multiple linear regression was performed to assess the effects of age and race as potential confounding variables on the relationships between baseline adipose tissue mass (FAT) and plasma 25-hydroxyvitamin D concentrations (25(OH)D) at baseline, 1, and 6 months following surgery. β coefficients are depicted for either unadjusted or adjusted (for age and race) subgroup correlations. All values are significant (P < 0.05).
25(OH)D, 25-hydroxyvitamin D.
Figure 4.
Relationship between plasma 25-hydroxyvitamin D and adiposity at 1 month following surgery. Cross-sectional Pearson correlations were determined between total fat mass (FAT) at 1 month following surgery and 25-hydroxyvitamin D (25(OH)D) at (a) 1 and (b) 6 months following RYGB (n = 20). RYGB, Roux-en-y gastric bypass.
Table 3.
Multiple linear regression analysis of relationships between adiposity at 1 month following surgery and 25(OH)D
| FAT1 month | Model 1 (unadjusted) | Model 2 (age, race) |
|---|---|---|
| 25(OH)D1 month | 0.6 | 0.61 |
| 25(OH)D6 months | 0.52 | 0.63 |
Multiple linear regression was performed to assess the effects of age and race as potential confounding variables on the relationships between 1 month adipose tissue mass (FAT) and 25-hydroxy-vitamin D (25(OH)D) concentrations at 1 and 6 months following surgery. β coefficients are depicted for either unadjusted or adjusted (for age and race subgroup) correlations. All values are significant, P < 0.05.
Figure 5.
Changes in adiposity and 25-hydroxyvitamin D during 24 months following surgery. Absolute changes, compared to baseline measures, in total adipose tissue mass and 25-hydroxyvitamin D (25(OH)D) were determined at 1 (Δ1 month, n = 20), 6 (Δ6 months, n = 20) and 24 (Δ24 months, n = 10) months following Roux-en-y gastric bypass surgery. *Significantly different from baseline value (P < 0.05), †significantly different from 1 month value (P < 0.05).
DISCUSSION
The present study demonstrates the early and longer-term impact of RYGB surgery on vitamin D status as well as relationships between 25(OH)D concentrations and adiposity. Interestingly, an acute and transient increase in systemic 25(OH)D concentrations was observed at 1 month followed by a decreasing trend during the remaining 23-months post-surgery. Before and after surgery, a high prevalence of vitamin D deficiency was found in both AAs and whites. Finally, strong correlations were demonstrated between adipose tissue mass and plasma 25(OH)D, which suggest that adipose tissue is a strong contributor to plasma 25(OH)D concentrations.
The high prevalence of vitamin D deficiency in bariatric surgery patients before and following surgery is well documented (22–26), but only by a few prospective studies (27–29). We found similar prevalence of vitamin D deficiency preoperatively (50%) and postoperatively (30–60%) to what has been reported previously (23,24,29). Only one study in Sweden, has reported the immediate and longer-term impact of gastric bypass surgery on vitamin D status in obese individuals (28) and consistent with the present study, they reported an increase in systemic 25(OH)D at early following surgery. However, in contrast, the Swedish study found that the increases in 25(OH)D were sustained at 6 months following RYGB. Potential reasons for the differences between our study and the Swedish study include that the subjects were superobese (BMI ≥50 kg/ m2) having an average BMI which was 7.3 points higher than subjects in the present study and subjects increased vitamin D supplementation following surgery(subjects were prescribed 400 IU vitamin D/day). However, similar to our study, the Swedish study reported decreased 25(OH)D at 12 months after bariatric surgery. These data taken together suggests that the initial benefits of surgery on 25(OH)D levels are temporary and do not result in normalization of vitamin D status in most patients despite achieving lower fat mass. Subjects in the present study had below recommended intakes of vitamin D (<200 IU/day) and this could be a reason—independent of gut malabsorption—for the reduced vitamin D status observed over the longer term following surgery. However, our findings suggest that patients who are undergoing RYGB should be serially monitored over the longer term as the risk of vitamin D deficiency increases with time following surgery. Deleterious consequences of low vitamin D status include increased risk of postoperative infection (32,33), hypertension (34), and bone density loss (35,36).
Strong correlations that were independent of age and race were observed between adipose tissue mass at baseline and at 1 month and 25(OH)D concentrations. This finding suggests that adipose tissue is a strong contributor to vitamin D status. Biochemical analysis of adipose tissue obtained from humans (19,20) and rodents (18,37) demonstrated that adipose tissue is a storage depot for vitamin D. Rapid redistribution of vitamin D in adipose tissue following oral pharmacological dosing, slow release into the systemic circulation, and accelerated release during weight loss has been shown (18,37). Thus, we speculate that the increase in plasma 25(OH)D rapidly following RYGB observed in the present study, and in the study by Aasheim and colleagues (28) may be due to the initial release of vitamin D from adipose tissue stores. Using direct measurements of human adipose tissue biopsies obtained from severely obese individuals, Blum et al. showed strong correlations between concentrations of 25(OH)D in subcutaneous fat and in serum (20). Although we did not measure 25(OH)D in adipose tissue directly, our finding of positive relationship among body FAT and plasma 25(OH)D is consistent with the study by Blum and colleagues (20). Paradoxically, several studies have shown inverse correlations between adiposity and blood concentrations of 25(OH)D (13–15,38) and also that obesity is associated with decreased vitamin D status. An explanation of the discrepancy between the current study finding of a positive relationship between FAT and circulating 25(OH)D, and the negative relationship reported by others may be found by examining subjects’ recent energy balance history. Unlike the latter studies, patients in our study, and in the study by Blum et al., were about to undergo bariatric surgery. Although our study subjects were currently weight stable at the time of testing, they are often counseled to undergo weight loss as part of preoperative preparation and they also may be chronic dieters (39,40). We therefore speculate that 25(OH)D, which has a half-life of 2 months, was released into the circulation during the preoperative period. Wortsman and colleagues showed that following equal oral intakes of vitamin D2, lower peak concentrations were observed in obese vs. lean individuals (17). Thus in patients who are weight stable, dietary or endogenously produced 25(OH)D, which is lipid-soluble, is sequestered and accumulates in adipose tissue thus limiting its bioavailability and resulting in a negative association observed between adiposity and vitamin D status. However, during weight loss and adipose tissue lipolysis, release of 25(OH)D from adipose tissue may occur, which in turn, may contribute to systemic 25(OH)D concentrations. In our study, plasma 25(OH)D concentrations peaked at 1 month despite continued decrease of adipose tissue over the 24-month postsurgery period, and correlations between adipose tissue mass and plasma 25(OH)D were only found at earlier timepoints (baseline and 1 month), but not later time points (6 and 24 months). These observations suggest that the bioavailability of adipose tissue stores of vitamin D during weight loss is limited, perhaps due to limited storage or quick release from specific fat depots that remain to be identified. Although others have reported differential associations between abdominal visceral and subcutaneous adipose tissue depots and 25(OH)D (41,42), we did not find these to be substantial in the present study.
The peak of plasma 25(OH)D concentrations in the early phase following RYGB surgery was followed by a decreasing trend in levels from 6 to 24 months. A decline in vitamin D status following RYGB has also previously been observed by other studies (23,28) which may be due to the malabsorptive effects of the procedure. Compliance to vitamin supplementation regimens has been reported to be low in postbariatric surgery patients (43) and it is not yet clear from this study or from others (25,28) whether low intake of dietary vitamin D or malabsorption of vitamin D in the intestine is responsible for the high prevalence of deficiency following surgery in long-term follow-up. Also some studies have shown that supplementation of vitamin D at doses close to the recommended daily intakes (400 IU/day) has not been effective for repletion of vitamin D status in postoperative RYGB patients (25,44–46).
The strengths of our study were its prospective cohort design with serial determinations before, and immediately following and during 24 months postgastric bypass in a racially diverse population. Also, direct measures of body FAT allowed us to determine the relationship between 25(OH)D concentrations and adiposity during weight loss. Limitations of our study are the small sample size which included only women, and there was none or limited information regarding other confounding variables including sun exposure and dietary vitamin D intake. Although vitamin D deficiency was highly prevalent before and following surgery, we did not screen for functional consequences such as increased parathyroid hormone or decreased bone density. Larger prospective studies, which include such assessments, information on sun exposure and concomitantly assess adipose tissue and plasma 25(OH)D concentrations before and after RYGB are needed.
The findings from this study show that patients undergoing gastric bypass surgery are at high risk for vitamin D deficiency preoperatively and the condition worsens with increasing time following surgery. Associations between adipose tissue mass and systemic 25(OH)D concentrations, and a temporary increase acutely following surgery in plasma 25(OH)D levels were observed. These findings point to increased storage and sequestration of vitamin D with adiposity and release of vitamin D from adipose tissue during initial weight loss. This study confirms that vitamin D status further worsens following gastric bypass, which is a significant concern, given the associations of vitamin D deficiency and the risk of disease including osteoporosis, cancer, and atherosclerosis.
ACKNOWLEDGMENTS
We thank all the study participants. Adeola T. Ayeni assisted with clinical research coordination of the study participants. This work was supported by National Institute of Health grants R03 DK067167 (to N.G.-M.), K24 RR023356 (to T.R.Z.), K23 AR054334 (to V.T.), General Clinical Research Center Grant M01 RR00039 and the Atlanta Clinical and Translational Science Institute grant UL1 RR025008.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mondolfi RN, Jones TM, Hyre AD, Raggi P, Muntner P. Comparison of percent of United States adults weighing >300 pounds (136 kilograms) in three time periods and comparison of five atherosclerotic risk factors for those weighing >300 pounds to those <300 pounds. Am J Cardiol. 2007;100:1651–1653. doi: 10.1016/j.amjcard.2007.06.072. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 8.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7:569–575. doi: 10.1097/00075197-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66:S182–S194. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 13.Ernst B, Thurnheer M, Schmid SM, Wilms B, Schultes B. Seasonal variation in the deficiency of 25-hydroxyvitamin D3 in mildly to extremely obese subjects. Obes Surg. 2009;19:180–183. doi: 10.1007/s11695-008-9636-2. [DOI] [PubMed] [Google Scholar]
- 14.Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 15.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 16.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 17.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstreich SJ, Rich C, Volwiler W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest. 1971;50:679–687. doi: 10.1172/JCI106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson DE, Douglas J, Lean M, Sedrani S. Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clin Chim Acta. 1986;157:175–181. doi: 10.1016/0009-8981(86)90223-8. [DOI] [PubMed] [Google Scholar]
- 20.Blum M, Dolnikowski G, Seyoum E, et al. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gemmel K, Santry HP, Prachand VN, Alverdy JC. Vitamin D deficiency in preoperative bariatric surgery patients. Surg Obes Relat Dis. 2009;5:54–59. doi: 10.1016/j.soard.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Clements RH, Katasani VG, Palepu R, et al. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72:1196–1202. doi: 10.1177/000313480607201209. discussion 1203. [DOI] [PubMed] [Google Scholar]
- 23.Clements RH, Yellumahanthi K, Wesley M, Ballem N, Bland KI. Hyperparathyroidism and vitamin D deficiency after laparoscopic gastric bypass. Am Surg. 2008;74:469–474. discussion 474. [PubMed] [Google Scholar]
- 24.de-Campos CD, Dalcanale L, Pajecki D, Garrido ABJ, Halpern A. Calcium intake and metabolic bone disease after eight years of roux-en-y-gastric bypass. Obes Surg. 2008;18:386–390. doi: 10.1007/s11695-007-9393-7. [DOI] [PubMed] [Google Scholar]
- 25.Gasteyger C, Suter M, Gaillard RC, Giusti V. Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr. 2008;87:1128–1133. doi: 10.1093/ajcn/87.5.1128. [DOI] [PubMed] [Google Scholar]
- 26.Gómez JM, Vilarrasa N, Masdevall C, et al. Regulation of bone mineral density in morbidly obese women: a cross-sectional study in two cohorts before and after bypass surgery. Obes Surg. 2009;19:345–350. doi: 10.1007/s11695-008-9529-4. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Hernández J, Ybarra J, Gich I, et al. Effects of bariatric surgery on vitamin D status and secondary hyperparathyroidism: a prospective study. Obes Surg. 2005;15:1389–1395. doi: 10.1381/096089205774859182. [DOI] [PubMed] [Google Scholar]
- 28.Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90:15–22. doi: 10.3945/ajcn.2009.27583. [DOI] [PubMed] [Google Scholar]
- 29.Jin J, Stellato TA, Hallowell PT, et al. Utilization of preoperative patient factors to predict postoperative vitamin D deficiency for patients undergoing gastric bypass. J Gastrointest Surg. 2009;13:1052–1057. doi: 10.1007/s11605-009-0847-1. [DOI] [PubMed] [Google Scholar]
- 30.Gletsu-Miller N, Hansen JM, Jones DP, et al. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity (Silver Spring) 2009;17:439–446. doi: 10.1038/oby.2008.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kvist H, Sjöström L, Tylén U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10:53–67. [PubMed] [Google Scholar]
- 32.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlin AM, Yager KM, Rao DS. Vitamin D depletion impairs hypertension resolution after Roux-en-Y gastric bypass. Am J Surg. 2008;195:349–352. doi: 10.1016/j.amjsurg.2007.12.016. discussion 352. [DOI] [PubMed] [Google Scholar]
- 35.Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–3740. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahdy T, Atia S, Farid M, Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg. 2008;18:1526–1531. doi: 10.1007/s11695-008-9653-1. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer DA, van Beek J, Ferwerda H, et al. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr. 1998;79:527–532. doi: 10.1079/bjn19980091. [DOI] [PubMed] [Google Scholar]
- 38.Bolland MJ, Grey AB, Ames RW, et al. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007;86:959–964. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 39.Liu RC, Sabnis AA, Forsyth C, Chand B. The effects of acute preoperative weight loss on laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15:1396–1402. doi: 10.1381/096089205774859155. [DOI] [PubMed] [Google Scholar]
- 40.Roehrig M, Masheb RM, White MA, et al. Chronic dieting among extremely obese bariatric surgery candidates. Obes Surg. 2009;19:1116–1123. doi: 10.1007/s11695-009-9865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94:67–73. doi: 10.1210/jc.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toussi R, Fujioka K, Coleman KJ. Pre- and postsurgery behavioral compliance, patient health, and postbariatric surgical weight loss. Obesity (Silver Spring) 2009;17:996–1002. doi: 10.1038/oby.2008.628. [DOI] [PubMed] [Google Scholar]
- 44.Nelson ML, Bolduc LM, Toder ME, Clough DM, Sullivan SS. Correction of preoperative vitamin D deficiency after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2007;3:434–437. doi: 10.1016/j.soard.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Goldner WS, Stoner JA, Lyden E, et al. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: a prospective, randomized pilot clinical trial. Obes Surg. 2009;19:173–179. doi: 10.1007/s11695-008-9680-y. [DOI] [PubMed] [Google Scholar]
- 46.Mahlay NF, Verka LG, Thomsen K, Merugu S, Salomone M. Vitamin D status before Roux-en-Y and efficacy of prophylactic and therapeutic doses of vitamin D in patients after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:590–594. doi: 10.1007/s11695-008-9698-1. [DOI] [PubMed] [Google Scholar]