Abstract
Downregulation of cyclin-dependent kinase (Cdk)–mitotic cyclin complexes is important during cell cycle progression and in G1 arrested cells undergoing differentiation. srw1p, a member of the Fizzy-related protein family in fission yeast, is required for the degradation of cdc13p mitotic cyclin B during G1 arrest. Here we show that srw1p is not required for the degradation of cdc13p during mitotic exit demonstrating that there are two systems operative at different stages of the cell cycle for cdc13p degradation, and that srw1p is phosphorylated by Cdk–cdc13p only becoming dephosphorylated during G1 arrest. We propose that this phosphorylation targets srw1p for proteolysis and inhibits its activity to promote cdc13p turnover.
Keywords: Cdk/fission yeast/Fizzy-related/G1 arrest/mitotic cyclin B degradation
Introduction
In all eukaryotes, cyclin-dependent kinases (Cdks) play a central role both in the G1–S and the G2–M cell cycle transitions (reviewed by Nurse, 1990; Nigg, 1995). A single Cdk encoded by cdc2+ in fission yeast regulates both transitions. It is regulated during the cell cycle by several mechanisms including binding to a cyclin subunit, phosphorylation and Cdk inhibitors. A mitotic cyclin B encoded by cdc13+ is essential for G2–M progression (Booher and Beach, 1988; Hagan et al., 1988; Booher et al., 1989; Moreno et al., 1989). Cig2p acts as the major G1–S cyclin (Obara-Ishihara and Okayama, 1994; Martin-Castellanos et al., 1996; Mondesert et al., 1996), although cdc13p can promote G1–S progression in the absence of cig2p (Fisher and Nurse, 1996; Mondesert et al., 1996). Regulation of the cdc13p major mitotic cyclin is important during cell cycle progression. Cells deleted for cdc13p skip mitosis and undergo extra rounds of replication (Hayles et al., 1994), whilst expression of an indestructible cdc13 blocks exit from mitosis (Yamano et al., 1996). Regulation of the activity of Cdks is also important when cells undergo differentiation. Fission yeast cells that are nitrogen starved or treated with pheromone arrest in G1 and undergo conjugation and meiosis. These processes require downregulation of cdc2p–B-type cyclin complexes; pheromone induces G1 arrest by inhibiting cdc2p–cdc13p and cdc2p–cig2p (Stern and Nurse, 1997) due to the degradation of cdc13p and cig2p and the action of the Cdk inhibitor rum1p (Stern and Nurse, 1998).
Mutants with defects in G1 arrest and differentiation have been identified, some of which are involved in regulating Cdk–B-type cyclin activity. The nuc2+ and apc10+ gene products are components of the anaphase promoting complex/cyclosome (APC/C) and are conserved among eukaryotes (Hirano et al., 1988; Kumada et al., 1995; Kominami et al., 1998). Mutants in the nuc2+ and apc10+ genes undergo mitotic arrest at their restrictive temperature because they are unable to degrade mitotic cyclin. At their permissive temperature these mutants fail either to arrest in G1 or to undergo conjugation. Studies of these mutants support the idea that downregulation of B-type cyclins is important both in the cell cycle and in cells undergoing differentiation, although it is not known whether the same system operates for the degradation of cdc13p at mitotic exit as well as during G1 arrest in differentiating cells. In contrast to nuc2 and apc10, both rum1 and srw1/ste9 mutants have no mitotic defects, although they fail to arrest in G1 or to perform conjugation after nitrogen starvation (Moreno and Nurse, 1994; Yamaguchi et al., 1997; Kitamura et al., 1998). rum1+ was isolated as a gene whose overexpression induces extra rounds of replication (Moreno and Nurse, 1994). It is a Cdk inhibitor that directly binds to Cdk–B-type cyclin (Correa-Bordes and Nurse, 1995; Jallepalli and Kelly, 1996; Martin-Castellanos et al., 1996) and is also required for the degradation of cdc13p during G1 (Correa-Bordes et al., 1997). srw1+/ste9+ encodes a Fizzy-related protein that was initially isolated as a multicopy suppressor of hyperactive cdc2p–cdc13p complexes and is also required for the degradation of cdc13p during G1 (Yamaguchi et al., 1997; Kitamura et al., 1998).
In budding yeast the degradation of mitotic cyclins is negatively regulated by CDC28 (the budding yeast cdc2+ homologue) (Amon et al., 1994), and the highly conserved WD repeat proteins Fizzy and Fizzy-related, which activate protein degradation, are important in this regulation. Drosophila fizzy, budding yeast CDC20 and fission yeast slp1+ are all required for the degradation of anaphase inhibitors during the metaphase–anaphase transition (Sethi et al., 1991; Dawson et al., 1995; Sigrist et al., 1995; Matsumoto, 1997; Visintin et al., 1997; Shirayama et al., 1998), while Drosophila fizzy-related, budding yeast HCT1/CDH1 and fission yeast srw1+/ste9+ are required for the degradation of mitotic cyclins mainly during G1 (Schwab et al., 1997; Sigrist and Lehner, 1997; Visintin et al., 1997; Yamaguchi et al., 1997; Kitamura et al., 1998). In budding yeast, Hct1 activity is negatively regulated by Cdc28 and phosphorylation inhibits interaction between Hct1 and APC (Zachariae et al., 1998; Jaspersen et al., 1999).
To understand better the regulation of Cdk–B-type cyclin complex during the mitotic cell cycle and differentiation, we have studied the Fizzy-related protein srw1p function and its regulation in fission yeast. We show that srw1p is not required for cdc13p degradation at mitotic exit demonstrating that there are at least two systems operative for the degradation of cdc13p. We also show that srw1p is phosphorylated during the cell cycle by the cdc2p–cdc13p protein kinase, and that phosphorylation of srw1p by cdc2p affects the stability of srw1p and inhibits its activity to promote cdc13p turnover.
Results
srw1p is not required for cdc13p degradation at mitotic exit
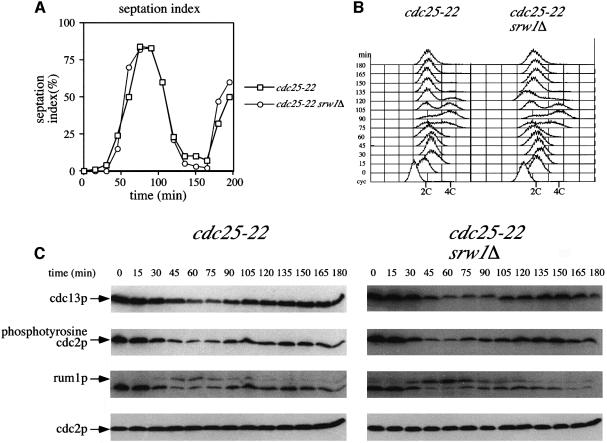
srw1p is required for the degradation of the major fission yeast mitotic cyclin cdc13p when cells are arrested in G1 and this degradation is necessary to prevent further progression through the cell cycle in these circumstances (Correa-Bordes et al., 1997; Yamaguchi et al., 1997; Kitamura et al., 1998; Kominami et al., 1998; Stern and Nurse, 1998). However, srw1Δ mutants are fully viable and show no mitotic defects, suggesting that srw1p may not have a role in the degradation of cdc13p, which is generally required for mitotic exit. To investigate this further, cells were synchronized at the G2–M boundary by incubating the temperature-sensitive mutant cdc25-22 at its restrictive temperature, 36°C. They were then released into mitosis, G1 and S-phase by shifting down to 25°C. A strain with srw1p (cdc25-22) and one lacking srw1p (cdc25-22 srw1Δ) both underwent mitosis, septation (Figure 1A) and S-phase (Figure 1B) with the same kinetics. In wild-type cells, cdc13p becomes degraded at mitotic exit (Figure 1C) (Moreno et al., 1989). In srw1Δ cells, the degradation of cdc13p and tyrosine dephosphorylation of cdc2p occurred to the same extent and with similar kinetics to wild-type cells, as did the appearance of the G1 marker rum1p (Figure 1C). This experiment demonstrates that srw1p has no influence on either the timing of mitotic exit or the kinetics of cdc13p degradation at the mitosis to G1 transition. Therefore, we conclude that there are at least two systems operative for the degradation of cdc13p. One is required for cdc13p degradation at mitotic exit and is independent of srw1p, and the second is required to regulate cdc13p levels when cells are arrested in G1 and this degradation system is dependent upon srw1p.
Fig. 1. srw1p is not required for cdc13p degradation at mitotic exit. (A) Septation index and (B) flow cytometric analysis of G2 block and release experiments. cdc25-22 and cdc25-22 srw1Δ strains were grown to exponential phase at 25°C (cyc), blocked for 4 h at 36°C, released at 25°C and incubated for the time indicated. (C) Western blots of the experiments described above using anti-cdc13p, anti-phosphotyrosine cdc2p, anti-rum1p and anti-cdc2p antibodies. For anti-rum1p antibody blotting, the same blots were used after the anti-phosphotyrosine cdc2p antibody.
srw1p activity is correlated with its state of phosphorylation
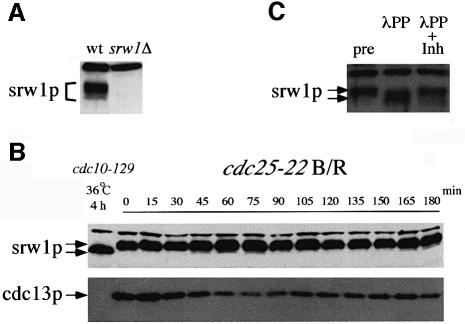
The fact that srw1p has no role at mitotic exit and that its ectopic expression induces G1 arrest suggests that srw1p activity may be maintained at a low level in rapidly growing cells. Antibodies raised against the entire protein produced in bacteria were used to show that the 62 kDa srw1p was present in exponentially growing cells (Figure 2A) and was found at all stages of the cell cycle in a cdc25-22 block and release synchronized culture (Figure 2B). Thus, srw1p activity cannot be regulated during the cell cycle by maintaining its amount at a low level.
Fig. 2. srw1p is phosphorylated throughout the cell cycle. (A) Extracts were prepared from exponentially growing wild-type (wt) cells and srw1Δ cells, western blotted and probed with anti-srw1p antibody. The upper band is a non-specific cross-reacting protein. (B) cdc25-22 strain was blocked and released (B/R) as described in Figure 1. Samples were western blotted and probed with anti-srw1p and anti-cdc13p antibodies. An extract from G1 arrested cdc10-129 cells was loaded as a control. (C) Extracts were prepared from exponentially growing wild-type cells (pre) and incubated with λ phosphatase in the absence (λPP) or presence (λPP + Inh) of phosphatase inhibitors.
Several migration forms could be detected during gel electrophoresis (Figure 2A and C), and treatment with λ phosphatase resulted in the slowest migrating forms disappearing and the fastest forms increasing in amount (Figure 2C), indicating that srw1p is phosphorylated in proliferating cells. To test whether srw1p activity is correlated with its state of phosphorylation, the phosphorylation state of srw1p was monitored in cells arrested in G1 where srw1p has been shown to be required for cdc13p degradation. In fission yeast, the G1 phase of the cell cycle is short in rapidly growing cells. When starved for nitrogen, cells arrest in pre-Start G1 in preparation for differentiation, and G1 arrest can also be brought about by using cdc10 mutants that arrest in pre-Start G1. The cdc10-129 mutant was used, which blocks cells in G1 when incubated for 4 h at 36°C (Figure 3A). By this time point srw1p was observed to have become dephosphorylated (Figure 3B), cdc13p had disappeared from the cells, and cdc2p had become tyrosine dephosphorylated. In contrast, cdc13p and cdc2p tyrosine phosphorylation persisted in cells deleted for srw1p (Figure 3B), even though these cells eventually arrested in G1 (Figure 3A). When wild-type cells were arrested in G1 in response to nitrogen starvation, srw1p also became dephosphorylated and cdc13p disappeared (data not shown). As previously shown, in cells deleted for srw1p, cdc13p remained high even when cells became arrested in G1 (Yamaguchi et al., 1997; Kitamura et al., 1998). Thus, srw1p becomes dephosphorylated in cells arrested in G1, and srw1p is required for cdc13p degradation. At all stages of the cell cycle the mobility of srw1p was unchanged (Figure 2B), indicating that its phosphorylation state probably remains constant in rapidly growing cells. Mobility was retarded compared with the dephosphorylated form of srw1p found in blocked cdc10-129 cells (Figure 2B), showing that srw1p is phosphorylated throughout the cell cycle. We conclude that srw1p is phosphorylated throughout the cell cycle and becomes dephosphorylated during G1 arrest when srw1p is active.
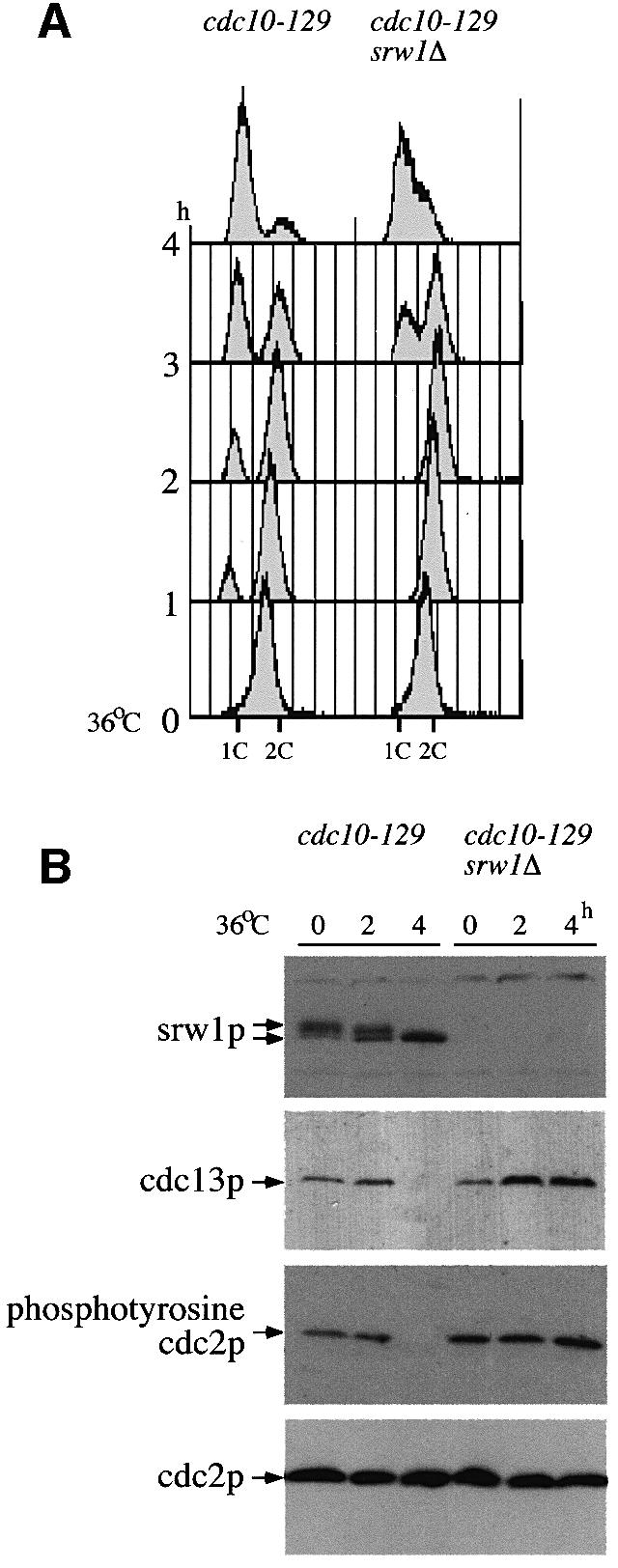
Fig. 3. srw1p becomes dephosphorylated during G1 arrest, which coincides with the degradation of cdc13p. (A) Flow cytometry of G1 block experiments. cdc10-129 and cdc10-129 srw1Δ strains were shifted to 36°C and incubated for the indicated time in hours. (B) Western blots of the experiments above using anti-srw1p, anti-cdc13p, anti-phosphotyrosine cdc2p and anti-cdc2p antibodies.
srw1p phosphorylation is cdc2p dependent
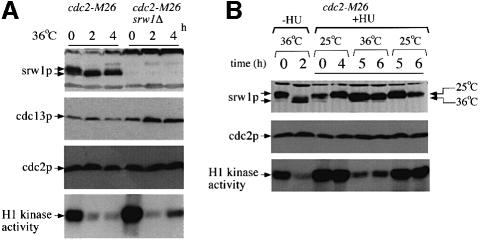
During G1 arrest when srw1p is dephosphorylated, the activity of the cdc2p Cdk is also much reduced. To investigate whether srw1p phosphorylation is dependent upon the cdc2p Cdk, we monitored the mobility state of srw1p in the temperature-sensitive cdc2-M26 mutant. On shift to 36°C, srw1p became dephosphorylated (Figure 4A) even though the cells were mostly arrested in G2, when srw1p would normally be phosphorylated. Next, we assessed the requirement of cdc2p for srw1p phosphorylation in cells arrested with hydroxyurea (HU) after the G1–S transition. srw1p was phosphorylated in cdc2-M26 mutant cells at 25°C arrested with HU, but became dephosphorylated when cdc2p was inactivated by shifting the cells to 36°C (Figure 4B). H1 kinase activity was monitored to determine the level of cdc2p kinase activity in the presence of HU. As previously reported (Knudsen et al., 1996; Rhind and Russell, 1998), cdc2p kinase activity was detected in cells treated with HU and was reduced when cdc2-M26 mutant cells were shifted to 36°C (Figure 4B). Although the srw1p mobility shift was not as great as when cells were shifted in the absence of HU, a clear difference between 36 and 25°C was detected (Figure 4B). This experiment eliminates effects of the cell cycle stage on phosphorylation and establishes that srw1p phosphorylation is at least partly dependent in vivo upon cdc2p activity.
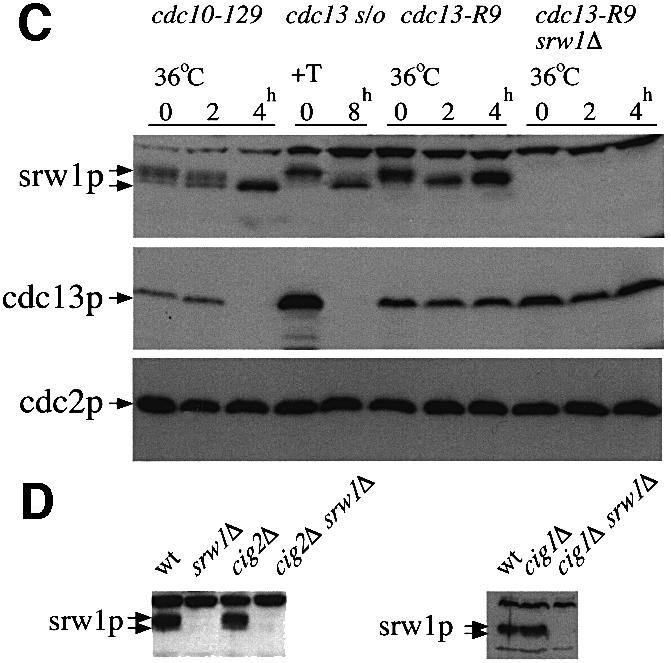
Fig. 4. srw1p phosphorylation is cdc2p dependent. (A) cdc2-M26 and cdc2-M26 srw1Δ strains were shifted to 36°C and incubated for the indicated time in hours. Extracts were western blotted and probed with anti-srw1p, anti-cdc13p and anti-cdc2p antibodies. Extracts were immunoprecipitated with anti-cdc2p antibody, and H1 kinase activity of each immunoprecipitate was measured. (B) cdc2-M26 strain was blocked with 11 mM HU for the indicated time in hours. Further 11 mM HU was added after 4 h to prevent cells from leaking from the block. Cells were incubated for 4 h at 25°C and then incubated for another 2 h at either 36 or 25°C. Extracts from cdc2-M26 cells blocked in the absence of HU were also loaded. The arrows indicate the migration of srw1p in experiments at 36 and 25°C, respectively. Extracts were immunoprecipitated with anti-cdc2p antibody, and H1 kinase activity of each immunoprecipitate was measured. (C) cdc13 was shut off by adding thiamine (+T) to the cdc13Δ REP45-cdc13+ strain (cdc13 s/o) and cells were incubated at 30°C for the indicated time in hours. cdc13-R9 and cdc13-R9 srw1Δ strains were shifted to 36°C and incubated for the indicated time in hours. Extracts from cdc10-129 cells were loaded as controls. Extracts were western blotted and probed with anti-srw1p, anti-cdc13p and anti-cdc2p antibodies. (D) Extracts were taken from exponentially growing wild-type (wt), srw1Δ, cig2Δ, cig2Δ srw1Δ, cig1Δ and cig1Δ srw1Δ cells, western blotted and probed with anti-srw1p antibody.
cdc2p forms Cdk complexes with several cyclins, the major ones being cdc13p (Booher and Beach, 1988; Hagan et al., 1988; Booher et al., 1989; Moreno et al., 1989) and cig2p (Obara-Ishihara and Okayama, 1994; Martin-Castellanos et al., 1996; Mondesert et al., 1996), and a minor one, cig1p (Bueno et al., 1991). To determine which of these Cdks might be responsible for in vivo srw1p phosphorylation, srw1p mobility was monitored in mutant strains that lacked these cyclins. cdc13p activity was removed using both a cdc13 switch off strain and a temperature-sensitive cdc13-R9 mutant, and in both cases srw1p became dephosphorylated (Figure 4C). In contrast, in cig1Δ and cig2Δ mutant strains, srw1p remained phosphorylated (Figure 4D). These experiments establish that the cdc2p–cdc13p mitotic Cdk is largely responsible, either directly or indirectly, for in vivo phosphorylation of srw1p.
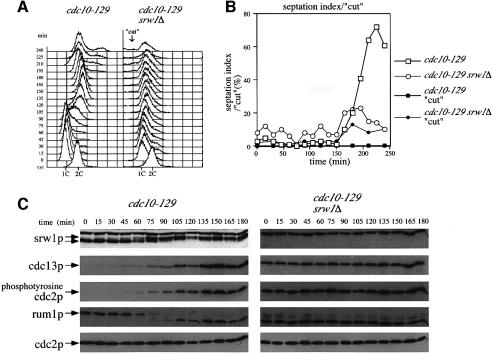
The conclusion that srw1p phosphorylation is dependent upon the cdc2p–cdc13p Cdk is consistent with the cell cycle timing of srw1p phosphorylation, as shown by monitoring srw1p mobility in cdc10-129 mutant cells released synchronously into the cell cycle after a block at 36°C (Figure 5A). S-phase was observed at ∼90 min after release, as monitored either by flow cytometry (Figure 5A) or by the disappearance of the rum1p G1 marker (Figure 5C). At 90 min srw1p also became phosphorylated, and this phosphorylation was correlated with the appearance of cdc13p, the tyrosine phosphorylation of cdc2p (Figure 5C) and the appearance of cdc2p Cdk activity (Baum et al., 1997). Therefore, as cdc2p–cdc13p Cdk activity appears in the cells, srw1p becomes phosphorylated. In a srw1Δ mutant, cdc13p was still found to be present (Figure 5C) and as a consequence S-phase could not be initiated (Figure 5A). In contrast, when srw1Δ mutant cells were starved for nitrogen, they failed to arrest in G1 (Yamaguchi et al., 1997; Kitamura et al., 1998; Kominami et al., 1998). This is expected because initiation of DNA replication occurs in two steps, the first of which is only possible if cdc2p Cdk activity is low and the second requires cdc2p Cdk activity (reviewed by Stern and Nurse, 1996). If Cdk activity is already high, as in this cdc10-129 experiment, then S-phase would not be initiated because the first step requiring low Cdk activity would be unable to take place. Eventually the srw1Δ mutant cells enter mitosis and generate a ‘cut’ phenotype losing viability (Figure 5A and B), as previously reported (Kitamura et al., 1998). This establishes the importance of srw1p in the proper regulation of the cdc2p–cdc13p Cdk during G1 arrest, and during entry into the subsequent S-phase.
Fig. 5. srw1p becomes phosphorylated just before S-phase starts, which coincides with the accumulation of cdc13p. (A) Flow cytometry and (B) septation index and percentage of ‘cut’ cells in G1 block and release experiments. cdc10-129 and cdc10-129 srw1Δ strains were blocked for 4 h at 36°C and released into 25°C and incubated for the times indicated. (C) Western blots of the experiments described above using anti-srw1p, anti-cdc13p, anti-phosphotyrosine cdc2p, anti-rum1p and anti-cdc2p antibodies. For anti-rum1p antibody blotting, the same blots were used after the anti-phosphotyrosine cdc2p antibody.
srw1p stability is regulated by phosphorylation on Cdk consensus sites
Examination of the srw1p protein sequence identified four Cdk consensus sites suggesting that it may be phosphorylated directly by the cdc2p Cdk. This was shown to be the case in vitro by adding immunoprecipitates of cdc2p and cdc13p to glutathione S-transferase (GST)–srw1p fusion protein as a substrate in a Cdk assay (Figure 6). Both immunoprecipitates contained an activity that could phosphorylate srw1p in vitro.
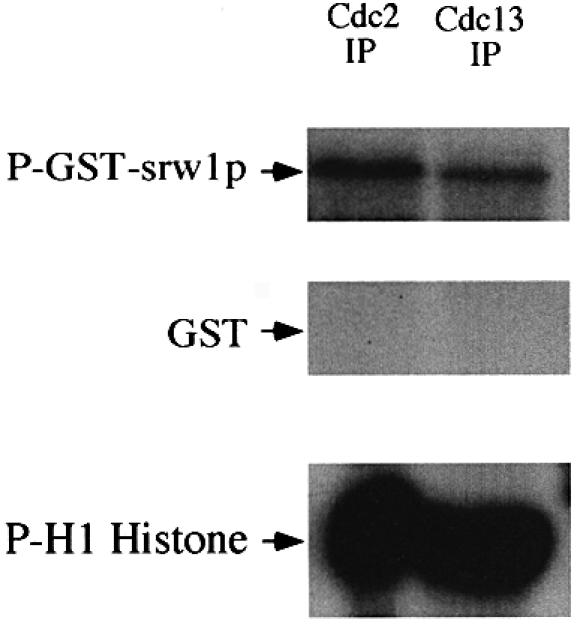
Fig. 6. srw1p is phosphorylated by cdc2p in vitro. cdc2p and cdc13p-associated cdc2p were immunoprecipitated from wild-type extracts and assayed for protein kinase activity using GST–srw1p or histone H1 as substrates. GST was used as a control.
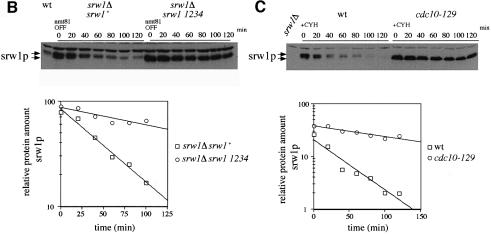
To confirm that srw1p is phosphorylated on these sites in vivo, a quadruple mutant of the srw1 gene was constructed with alanine substitutions at all four of the Cdk consensus sites (S62, S214, T98, T177). When the quadruple mutant was expressed in vivo, the mobility of srw1p was increased, demonstrating that phosphorylation was occurring at the Cdk consensus sites (Figure 7A). cdc2p phosphorylation can bring about the degradation of substrates such as cdc18p and rum1p (Jallepalli et al., 1997; Baum et al., 1998; Benito et al., 1998), and so the effect of cdc2p phosphorylation on srw1p stability was also tested. Switch off strains of wild-type srw1p and the quadruple phosphorylation mutant of srw1p using the nmt81 promoter were constructed for these experiments. When the promoter was switched on, the levels of both wild-type srw1p and mutant srw1p were no more than four times the endogenous level of srw1p. Upon switch off, the wild-type srw1p disappeared with a half-life of ∼40 min, whilst the quadruple mutant was much more stable with a half-life of >120 min (Figure 7B). We conclude that phosphorylation of srw1p on its Cdk consensus sites targets the protein for degradation. To investigate whether srw1p stability is regulated by phosphorylation, we tested the stability of srw1p in rapidly growing cells and in G1 blocked cells, and compared the stability of phosphorylated and dephosphorylated srw1p. After incubating wild-type cells and cdc10-129 cells at 36°C for 4 h, cycloheximide was added to inhibit protein synthesis (Ayscough and Warren, 1994; Jallepalli et al., 1997). In the wild-type cells, srw1p was mostly phosphorylated and disappeared quickly after adding cycloheximide with a half-life of 30 min, whilst in the cdc10-129 cells, srw1p was dephosphorylated and more stable with a half-life of >120 min (Figure 7C). This confirms that srw1p stability is regulated in vivo by phosphorylation.
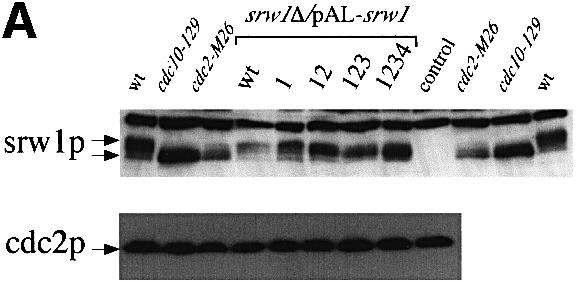
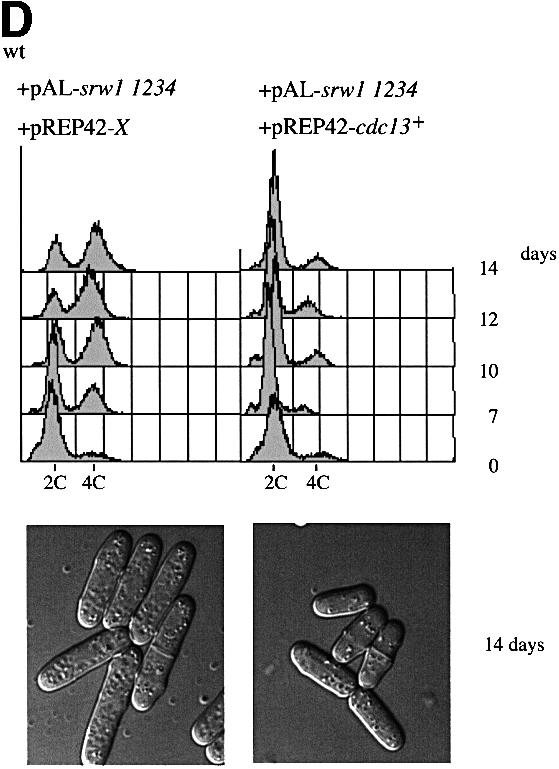

Fig. 7. srw1p stability is regulated by phosphorylation on Cdk consensus sites. (A) Samples were taken from exponentially growing srw1Δ cells carrying pAL-srw1 with no (wt), single (1), double (12), triple (123) or quadruple (1234) mutations in Cdk consensus sites or empty vector (pAL-X). Extracts from wild-type cells, cdc10-129 blocked cells and cdc2-M26 blocked cells were loaded as controls. Extracts were western blotted and probed with anti-srw1p and anti-cdc2p antibodies. (B) srw1 was shut off by adding thiamine (+T) to srw1Δ cells expressing wild-type srw1 (srw1Δ nmt81-srw1+) or quadruple mutant srw1 (srw1Δ nmt81-srw1 1234) and cells were incubated at 30°C for the times indicated. Extracts from wild-type cells were loaded to show the endogenous level of srw1p. Extracts were western blotted and probed with anti-srw1p antibody, the srw1p protein was quantified with NIH image analyser. (C) Wild-type cells and cdc10-129 cells were incubated for 4 h at 36°C, cycloheximide (CYH) was added, and cells were incubated at 36°C for the times indicated. (D) Flow cytometry and morphology of wild-type cells co-expressing quadruple mutant srw1 (pAL-srw1 1234) and either pREP42-X or pREP42-cdc13+. Cells were incubated for the number of days indicated at 30°C in minimal medium in the absence of thiamine. (E) cdc13 expression was turned off by adding thiamine to cdc13Δ REP41-cdc13+ and cdc13Δ REP41-cdc13+ srw1:: srw1 1234 strains and cells were further incubated at 32°C for the time indicated in minutes. Extracts were western blotted and probed with anti-cdc13p, anti-srw1p and anti-cdc2p antibodies.
When cells were grown continually in the presence of the srw1p quadruple mutant, diploid cells were found to accumulate in the culture (Figure 7D), which was not observed when wild-type srw1+ was expressed under the same conditions (data not shown), indicating that phosphorylation of srw1p by cdc2p is important for genomic stability. Increasing the levels of cdc13p suppressed this effect (Figure 7D), suggesting that it might be a reduction of endogenous cdc13p levels caused by the constitutive srw1p activity that was responsible for the observed diploidization. To test whether there is an increased activity of srw1p in the quadruple mutant, cdc13p turnover was examined using a cdc13 switch off strain. Upon switch off, cdc13p was degraded more quickly in the quadruple mutant than in wild type (Figure 7E). These results suggest that phosphorylation of srw1p inhibits its activity to promote cdc13p turnover.
Discussion
Earlier work has shown that degradation of the cdc13p mitotic cyclin occurs at two phases of the fission yeast cell cycle, both at mitotic exit and during G1 arrest (Moreno et al., 1989; Hayles and Nurse, 1995; Correa-Bordes et al., 1997). In this paper we have shown that the mechanisms bringing about the degradation of cdc13p mitotic cyclin B differ at these two different phases of the cell cycle. During G1 arrest, degradation is dependent on the WD repeat Fizzy-related protein srw1p (Yamaguchi et al., 1997; Kitamura et al., 1998; Figure 3) but this protein is not required for the degradation of cdc13p at exit from mitosis (Figure 1). We have also shown that srw1p is phosphorylated by Cdk and that this phosphorylation targets srw1p for proteolysis.
Fizzy-related proteins are highly conserved in eukaryotes, including Hct1/Cdh1 in budding yeast, Fizzy-related in Drosophila and Cdh1 in mammalian cells (Schwab et al., 1997; Sigrist and Lehner, 1997; Visintin et al., 1997; Fang et al., 1998; Kramer et al., 1998). In fission yeast, both srw1p and the Cdk inhibitor rum1p are required during G1 arrest and in cells differentiating from the G1 phase of the cell cycle, and the double deletion mutant of srw1 and rum1 is fully viable and shows no mitotic defects (our unpublished data; Kitamura et al., 1998). In Drosophila, the Fizzy-related protein is not found in cells undergoing rapid mitotic proliferation and is only observed later during development when cells develop an extended G1 and begin differentiation (Sigrist and Lehner, 1997). Fizzy-related and Cdk inhibitor rux mutants both undergo an additional mitotic cycle before arresting in G1 prior to differentiation (Gonczy et al., 1994; Thomas et al., 1994; Sigrist and Lehner, 1997). These results indicate that these Drosophila mutant cells have difficulty in maintaining G1 arrest, a phenotype which is similar to that of the fission yeast srw1 and rum1 mutants. In Xenopus embryos, the Fizzy-related FZR protein was also not detected until a G1 phase appeared around the time of the mid-blastula transition (Lorca et al., 1998). Therefore, Fizzy-related proteins appear to be required for the degradation of mitotic cyclins in a number of eukaryotic cells undergoing G1 arrest and differentiation.
There are two conserved members of WD repeat proteins in all eukaryotes that are involved in the degradation of APC substrates, Cdc20/slp1p/Fizzy/p55CDC (Sethi et al., 1991; Weinstein et al., 1994; Dawson et al., 1995; Sigrist et al., 1995; Matsumoto, 1997) and Hct1/srw1p/Fizzy-related/Cdh1 (Schwab et al., 1997; Sigrist and Lehner, 1997; Visintin et al., 1997; Yamaguchi et al., 1997; Fang et al., 1998; Kitamura et al., 1998; Kramer et al., 1998). In a number of these organisms the Fizzy and Fizzy-related protein families appear to act as specific cell cycle phase activators of mitotic cyclin degradation, with Fizzy acting during mitosis and Fizzy-related acting during G1. Fizzy is required for the degradation of mitotic cyclins and for exit from mitosis in rapidly growing Drosophila (Sigrist and Lehner, 1997) and Xenopus (Lorca et al., 1998) cells that do not express Fizzy-related proteins. In budding yeast, Cdc20 and Hct1 had been thought to act as substrate-specific activators of APC, with Cdc20 activating the degradation of Pds1, and Hct1 activating the degradation of Clb2 (Schwab et al., 1997; Visintin et al., 1997; Shirayama et al., 1998). However, more recently it has been shown that Clb2 degradation in mitosis is dependent on Cdc20, not Hct1 (Yeong et al., 2000). In fission yeast, Fizzy protein slp1p activates the degradation of cut2p at the metaphase–anaphase transition, and so it is likely that slp1p is responsible for the degradation of mitotic cyclin at the exit from mitosis.
We have shown that srw1p is phosphorylated throughout the cell cycle, only becoming dephosphorylated during G1 arrest (Figures 2 and 3) when srw1p is required for the degradation of cdc13p. srw1p phosphorylation requires the cdc2p protein kinase in vivo (Figure 4A and B), and in vitro kinase assays revealed that cdc2p can phosphorylate srw1p directly (Figure 6). Analysis of srw1 phosphorylation-deficient mutants demonstrated that srw1p is phosphorylated at Cdk consensus sites in vivo (Figure 7A). Mutant forms of srw1 that could not be phosphorylated on the four Cdk consensus sites in srw1p were more stable than wild-type srw1p: the half-life of the unphosphorylatable srw1p was >120 min, compared with 40 min for wild-type srw1p (Figure 7B). This establishes that cdc2p phosphorylation stimulates proteolysis of srw1p. Consistent with this view, when protein synthesis was inhibited, srw1p in G1 arrested cells was more stable than srw1p in proliferating cells (Figure 7C). Although phosphorylation of srw1p affects its stability, srw1p levels did not change significantly during G1 arrest when srw1p was dephosphorylated (Figures 3 and 5), suggesting that cdc2p-regulated proteolysis is not the only mechanism by which srw1p abundance is controlled. The unphosphorylatable srw1 mutant causes diploidization (Figure 7D), indicating that the proper regulation of srw1p by cdc2p-mediated phosphorylation is required to maintain genomic stability. This effect was suppressed by increased levels of cdc13p (Figure 7D). Loss of cdc13p and overexpression of srw1p have both been shown to induce extra rounds of DNA replication (Hayles et al., 1994; Yamaguchi et al., 1997; Kitamura et al., 1998), probably by re-initiating S-phase in G2 cells due to reduced cdc2p activity. The observed diploidization might be due to some effect on cdc13p caused by increased srw1p activity in unphosphorylatable srw1 mutant cells. Consistent with this view, cdc13p was degraded more quickly in unphosphorylatable srw1 mutant cells (Figure 7E), indicating that phosphorylation leads to the inhibition of srw1p activity. However, no significant difference in endogenous cdc13p levels was detected between unphosphorylatable srw1 mutant cells and wild-type cells, indicating that there must be some other homeostatic mechanism operative that maintains cdc13p at a constant level. It is possible that cdc2p phosphorylation of srw1p may have two regulatory effects: to stimulate the proteolysis of srw1p and to inhibit srw1p activity directly.
We have also shown that the phosphorylation of srw1p is mainly dependent on the cdc2p–cdc13p complex. srw1p became dephosphorylated when the mitotic cyclin cdc13p was inactivated but remained phosphorylated in mutants deleted for the G1 cyclin cig2 or cig1 (Figure 4C and D), and in vitro kinase assays showed that the cdc13p-associated kinase could phosphorylate srw1p (Figure 6). The timing of srw1p phosphorylation at the G1–S transition correlates with the accumulation of cdc13p in the cell (Figure 5). When cdc2p–cdc13p Cdk activity is low at mitotic exit or in a cdc25 block (Moreno et al., 1989), srw1p is still phosphorylated (Figure 2B), suggesting that a low activity of cdc2p–cdc13p Cdk is sufficient to phosphorylate srw1p, and also that a phosphatase exists that brings about the dephosphorylation of srw1p during G1 arrest. The phosphorylation of srw1p by the cdc2p–cdc13p complex might render srw1p inactive by targeting it for proteolysis and by directly inhibiting its activity so that the cdc13p level is sufficient for cells to proceed through the cell cycle. Such a regulatory mechanism has positive feedback characteristics providing a point of no return so that cells become committed to further cell cycle progression. An interesting as yet unanswered question that will require further study is which occurs first, cdc13p accumulation or the phosphorylation of srw1p.
We conclude that the Fizzy-related protein srw1p is a G1-specific promoter of mitotic cyclin cdc13p degradation. It plays no role in mitotic exit but is necessary to block cell cycle progression in cells arrested in G1 and has an important role in ensuring proper sexual differentiation that occurs from cells arrested in the G1 phase of the cycle. srw1p is phosphorylated by cdc2p–cdc13p Cdk activity and this phosphorylation targets srw1p for proteolysis. On exit from a G1 block srw1p phosphorylation occurs as cdc2p–cdc13p Cdk activity increases in the cells. The involvement of Fizzy-related proteins in properly regulating G1 arrest in differentiating cells may also be conserved in metazoan cells, given that these proteins have been shown to be necessary for proper G1 regulation in Drosophila and Xenopus embryonic cells arrested in G1 or undergoing differentiation from G1.
Materials and methods
Fission yeast strains and methods
All experiments were carried out in EMM2 minimal media, and growth conditions are as described previously (Moreno et al., 1991). Strains used in this study are shown in Table I. Thiamine-repressible promoters nmt45 and nmt81 were used (Maundrell, 1990, 1993), with thiamine being used at 5 µg/ml. Cycloheximide was used at 100 µg/ml. Temperature-sensitive strains were grown exponentially at 25°C and shifted to 36°C, and other strains were grown at 30°C. Yeast transformation was performed as described previously (Bähler et al., 1998).
Table I. Schizosaccharomyces pombe strains used in this study.
| Strain | Genotype |
|---|---|
| ATCC36399 | h– leu1-32 |
| PN513 | h– ura4-D18 leu1-32 |
| SY1 | h– srw1Δ::ura4+ ura4-D18 leu1-32 |
| PN1419 | h– cdc25-22 leu1-32 |
| SY15 | h– cdc25-22 srw1Δ::ura4+ ura4-D18 leu1-32 |
| PN9 | h– cdc10-129 leu1-32 |
| SY24 | h– cdc10-129 srw1Δ::ura4+ ura4-D18 leu1-32 |
| PN30 | h– cdc2-M26 leu1-32 |
| SY9 | h– cdc2-M26 srw1Δ::ura4+ ura4-D18 leu1-32 |
| PN143 | h– cdc13-R9 leu1-32 |
| SY16 | h– cdc13-R9 srw1Δ::ura4+ ura4-D18 leu1-32 |
| PN1414 | h– cdc13Δ::ura4+ ade6-704 leu1-32 ura4-D18 pREP45::cdc13+ (Hayles et al., 1994) |
| SY124 | h+s cig2Δ::ura4+ ura4-D18 leu1-32 |
| SY5 | h– cig2Δ::ura4+ srw1Δ::ura4+ ura4-D18 leu1-32 |
| SY127 | h– cig1Δ::ura4+ ura4-D18 leu1-32 (Bueno et al., 1991) |
| SY21 | h+s cig1Δ::ura4+ srw1Δ::ura4+ ura4-D18 leu1-32 |
| SY51 | h– srw1Δ::ura4+ pREP81::srw1+ int ura4-D18 leu1-32 |
| SY52 | h– srw1Δ::ura4+ pREP81::srw1 1234 int ura4-D18 leu1-32 |
| PN1260 | h+ cdc13Δ::ura4+ ura4-D18 leu1-32 pREP41::cdc13+ int |
| SY60 | h– cdc13Δ::ura4+ ura4-D18 leu1-32 pREP41::cdc13+ int srw1::srw1 1234 |
Purification of GST–srw1p
The srw1+ gene was subcloned into pGEX5X-1 (Pharmacia) and the GST–srw1p fusion protein was produced in Escherichia coli carrying pGEX5X-1-srw1+ and the protein purified with glutathione–Sepharose 4B (Pharmacia).
Anti-srw1p antibody
Purified GST–srw1p protein was used to raise anti-srw1p polyclonal antibodies. Anti-GST–srw1p serum was purified by blot affinity purification for western blotting.
Western blotting
Cells were boiled for 6 min after being washed once in Stop buffer (150 mM NaCl, 50 mM NaF, 1 mM NaN3, 10 mM EDTA pH 8.0). Protein extracts were made by vortexing with glass beads in HB buffer (Moreno et al., 1991) and protein concentration determined with the BCA assay kit (Sigma). Cell extracts were re-boiled in 2× sample buffer and 50 µg of each sample were run in an 8% SDS–polyacrylamide gel (Laemmli, 1970). The protein was blotted to Immobilon™-P membrane (Millipore) and detected using ECL (Amersham). The antibodies used were purified anti-srw1p polyclonal antibody (1:200), anti-cdc13p monoclonal antibody 6F (1:500; made by Hayles and Steel), anti-phosphotyrosine cdc2p polyclonal antibody (1:1000; New England Biolabs), purified anti-rum1p polyclonal antibody R4 (1:500; Correa-Bordes and Nurse, 1995) and anti-cdc2p monoclonal antibody Y63 (1:100; Yamano et al., 1996).
Phosphatase treatment
Extracts were prepared from exponentially growing wild-type cells, followed by boiling. Cells were broken using glass beads and recovered in HB without any phosphatase inhibitors. λ phosphatase (New England Biolabs), λ phosphatase buffer (New England Biolabs) and 2 mM MnCl2 were added, and the extracts were incubated at 30°C for 20 min with or without phosphatase inhibitors (60 mM β-glycerophosphate, 15 mM p-nitrophenylphosphate, 0.1 mM sodium vanadate). The reactions were stopped by the addition of 2× sample buffer before boiling for 3 min.
Flow cytometric analysis
Flow cytometric analysis was performed on a Becton-Dickinson FACScan using propidium iodide staining of cells as described previously (Sazer and Sherwood, 1990).
Phosphorylation experiments in vitro
Protein A and 5 µl of anti-cdc2p polyclonal antibody C2 (Simanis and Nurse, 1986) or protein G and 10 µl of anti-cdc13p monoclonal antibody 6F were rotated in HB buffer at room temperature for 30 min. After being washed three times with HB, these antibodies were rotated with 5 mg of cell extract at 4°C for 2 h. Immunoprecipitates were incubated with 200 µM ATP, 40 µCi/ml [γ-32P]ATP, and 0.2 mg/ml GST–srw1p, 0.2 mg/ml GST (Sigma) or 1 mg/ml histone H1 (Calbiochem) at 30°C for 30 min. The reactions were stopped by boiling for 3 min after the addition of 2× SDS sample buffer and samples were analysed by 15% SDS–polyacrylamide gel.
Site-directed mutagenesis of Cdk phosphorylation consensus sites
The serine and threonine residues of the four Cdk phosphorylation consensus sites S/T-P-R-K (S62, S214, T98, T177) were changed to alanine using the Quickchange site-directed mutagenesis kit (Stratagene). The mutated clones were confirmed by sequencing of the open reading frame. srw1 shut off strains were constructed by transforming linearized pREP81-srw1+ or pREP81-srw1 1234 (quadruple mutant) into srw1Δ mutant. Cells were grown in rich medium and integrants were selected for leucine stability. For the srw1::srw1 1234 strain, the 6 kb genomic fragment carrying the four mutations was transformed into srw1Δ cells selecting for 5-fluororotic acid resistance, and strain construction was confirmed by colony PCR.
Acknowledgments
Acknowledgements
We thank Jacky Hayles, Shigeki Jinno, Zoi Lygerou, Hiroshi Murakami and Patrick Zarzov for helpful comments and suggestions. We thank Takashi Toda for critical reading of the manuscript, Paul Russell for supplying the cig1Δ strain, Hiroyuki Yamano for supplying the Y63 antibody and all the members of the Cell Cycle Laboratory for their help and support. S.Y. is supported by a JSPS doctoral fellowship.
References
- Amon A., Irniger,S. and Nasmyth,K. (1994) Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell, 77, 1037–1050. [DOI] [PubMed] [Google Scholar]
- Ayscough K. and Warren,G. (1994) Inhibition of protein synthesis disrupts the Golgi apparatus in the fission yeast, Schizosaccharomyces pombe. Yeast, 10, 1–11. [DOI] [PubMed] [Google Scholar]
- Bähler J., Wu,J.Q., Longtine,M.S., Shah,N.G., McKenzie,A.,III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Baum B., Wuarin,J. and Nurse P. (1997) Control of S-phase periodic transcription in the fission yeast mitotic cycle. EMBO J., 16, 4676–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B., Nishitani,H., Yanow,S. and Nurse,P. (1998) Cdc18 transcription and proteolysis couple S phase to passage through mitosis. EMBO J., 17, 5689–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito J., Martin-Castellanos,C. and Moreno,S. (1998) Regulation of the G1 phase of the cell cycle by periodic stabilization and degradation of the p25rum1 CDK inhibitor. EMBO J., 17, 482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R. and Beach,D. (1988) Involvement of cdc13+ in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J., 7, 2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R.N., Alfa,C.E., Hyams,J.S. and Beach,D.H. (1989) The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell, 58, 485–497. [DOI] [PubMed] [Google Scholar]
- Bueno A., Richardson,H., Reed,S.I. and Russell,P. (1991) A fission yeast B-type cyclin functioning early in the cell cycle. Cell, 66, 149–159. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J. and Nurse,P. (1995) p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell, 83, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J., Gulli,M.P. and Nurse,P. (1997) p25rum1 promotes proteolysis of the mitotic B-cyclin p56cdc13 during G1 of the fission yeast cell cycle. EMBO J., 16, 4657–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson I.A., Roth,S. and Artavanis-Tsakonas,S. (1995) The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J. Cell Biol., 129, 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Yu,H. and Kirschner,M.W. (1998) The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev., 12, 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.L. and Nurse,P. (1996) A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J., 15, 850–860. [PMC free article] [PubMed] [Google Scholar]
- Gonczy P., Thomas,B.J. and DiNardo,S. (1994) roughex is a dose-dependent regulator of the second meiotic division during Drosophila spermatogenesis. Cell, 77, 1015–1025. [DOI] [PubMed] [Google Scholar]
- Hagan I., Hayles,J. and Nurse,P. (1988) Cloning and sequencing of the cyclin-related cdc13+ gene and a cytological study of its role in fission yeast mitosis. J. Cell Sci., 91, 587–595. [DOI] [PubMed] [Google Scholar]
- Hayles J. and Nurse,P. (1995) A pre-start checkpoint preventing mitosis in fission yeast acts independently of p34cdc2 tyrosine phosphorylation. EMBO J., 14, 2760–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles J., Fisher,D., Woollard,A. and Nurse,P. (1994) Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2–mitotic B cyclin complex. Cell, 78, 813–822. [DOI] [PubMed] [Google Scholar]
- Hirano T., Hiraoka,Y. and Yanagida,M. (1988) A temperature-sensitive mutation of the Schizosaccharomyces pombe gene nuc2+ that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J. Cell Biol., 106, 1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallepalli P.V. and Kelly,T.J. (1996) Rum1 and Cdc18 link inhibition of cyclin-dependent kinase to the initiation of DNA replication in Schizosaccharomyces pombe. Genes Dev., 10, 541–552. [DOI] [PubMed] [Google Scholar]
- Jallepalli P.V., Brown,G.W., Muzi-Falconi,M., Tien,D. and Kelly,T.J. (1997) Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev., 11, 2767–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen S.L., Charles,J.F. and Morgan,D.O. (1999) Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol., 9, 227–236. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Maekawa,H. and Shimoda,C. (1998) Fission yeast Ste9, a homolog of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1-phase. Mol. Biol. Cell, 9, 1065–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K.E., Knudsen,E.S., Wang,J.Y. and Subramani,S. (1996) p34cdc2 kinase activity is maintained upon activation of the replication checkpoint in Schizosaccharomyces pombe. Proc. Natl Acad. Sci. USA, 93, 8278–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami K., Seth-Smith,H. and Toda,T. (1998) Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell-cycle arrest in fission yeast. EMBO J., 17, 5388–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E.R., Gieffers,C., Holzl,G., Hengstschlager,M. and Peters,J.M. (1998) Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr. Biol., 8, 1207–1210. [DOI] [PubMed] [Google Scholar]
- Kumada K., Su,S., Yanagida,M. and Toda,T. (1995) Fission yeast TPR-family protein nuc2 is required for G1-arrest upon nitrogen starvation and is an inhibitor of septum formation. J. Cell Sci., 108, 895–905. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lorca T., Castro,A., Martinez,A.M., Vigneron,S., Morin,N., Sigrist,S., Lehner,C., Doree,M. and Labbe,J.C. (1998) Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J., 17, 3565–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Castellanos C., Labib,K. and Moreno,S. (1996) B-type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. EMBO J., 15, 839–849. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. (1997) A fission yeast homolog of CDC20/p55CDC/Fizzy is required for recovery from DNA damage and genetically interacts with p34cdc2. Mol. Cell. Biol., 17, 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. (1990) nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem., 265, 10857–10864. [PubMed] [Google Scholar]
- Maundrell K. (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene, 123, 127–130. [DOI] [PubMed] [Google Scholar]
- Mondesert O., McGowan,C.H. and Russell,P. (1996) Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol. Cell. Biol., 16, 1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S. and Nurse,P. (1994) Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature, 367, 236–242. [DOI] [PubMed] [Google Scholar]
- Moreno S., Hayles,J. and Nurse,P. (1989) Regulation of p34cdc2 protein kinase during mitosis. Cell, 58, 361–372. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays, 17, 471–480. [DOI] [PubMed] [Google Scholar]
- Nurse P. (1990) Universal control mechanism regulating onset of M-phase. Nature, 344, 503–508. [DOI] [PubMed] [Google Scholar]
- Obara-Ishihara T. and Okayama,H. (1994) A B-type cyclin negatively regulates conjugation via interacting with cell cycle ‘start’ genes in fission yeast. EMBO J., 13, 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N. and Russell,P. (1998) Tyrosine phosphorylation of cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol. Cell. Biol., 18, 3782–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S. and Sherwood,S.W. (1990) Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J. Cell Sci., 97, 509–516. [DOI] [PubMed] [Google Scholar]
- Schwab M., Lutum,A.S. and Seufert,W. (1997) Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell, 90, 683–693. [DOI] [PubMed] [Google Scholar]
- Sethi N., Monteagudo,M.C., Koshland,D., Hogan,E. and Burke,D. (1991) The CDC20 gene product of Saccharomyces cerevisiae, a β-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol. Cell. Biol., 11, 5592–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M., Zachariae,W., Ciosk,R. and Nasmyth,K. (1998) The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J., 17, 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S.J. and Lehner,C.F. (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell, 90, 671–681. [DOI] [PubMed] [Google Scholar]
- Sigrist S., Jacobs,H., Stratmann,R. and Lehner,C.F. (1995) Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J., 14, 4827–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V. and Nurse,P. (1986) The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell, 45, 261–268. [DOI] [PubMed] [Google Scholar]
- Stern B. and Nurse,P. (1996) A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet., 12, 345–350. [PubMed] [Google Scholar]
- Stern B. and Nurse,P. (1997) Fission yeast pheromone blocks S-phase by inhibiting the G1 cyclin B–p34cdc2 kinase. EMBO J., 16, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern B. and Nurse,P. (1998) Cyclin B proteolysis and the cyclin-dependent kinase inhibitor rum1p are required for pheromone-induced G1 arrest in fission yeast. Mol. Biol. Cell, 9, 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.J., Gunning,D.A., Cho,J. and Zipursky,L. (1994) Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell, 77, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Visintin R., Prinz,S. and Amon,A. (1997) CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science, 278, 460–463. [DOI] [PubMed] [Google Scholar]
- Weinstein J., Jacobsen,F.W., Hsu-Chen,J., Wu,T. and Baum,L.G. (1994) A novel mammalian protein, p55CDC, present in dividing cells is associated with protein kinase activity and has homology to the Saccharomyces cerevisiae cell division cycle proteins Cdc20 and Cdc4. Mol. Cell. Biol., 14, 3350–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Murakami,H. and Okayama,H. (1997) A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol. Biol. Cell, 8, 2475–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H., Gannon,J. and Hunt,T. (1996) The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J., 15, 5268–5279. [PMC free article] [PubMed] [Google Scholar]
- Yeong F.M., Lim,H.H., Padmashree,C.G. and Surana,U. (2000) Exit from mitosis in budding yeast; biphasic inactivation of the Cdc28–Clb2 mitotic kinase and the role of Cdc20. Mol. Cell, 5, 501–511. [DOI] [PubMed] [Google Scholar]
- Zachariae W., Schwab,M., Nasmyth,K. and Seufert,W. (1998) Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science, 282, 1721–1724. [DOI] [PubMed] [Google Scholar]