Abstract
Hsp90 has emerged as an important anti-cancer drug target because of its essential role in promoting the folding and maturation of many oncogenic proteins. Here we describe the development of the first high throughput screen, based on AlphaScreen™ technology, to identify a novel type of Hsp90 inhibitors that interrupt its interaction with the cochaperone HOP. The assay uses the 20-mer C-terminal peptide of Hsp90 and the TPR2A domain of HOP. Assay specificity was demonstrated by measuring different interactions using synthetic peptides, with measured IC50s in good agreement with reported values. The assay is stable over 12 hours and tolerates DMSO up to 5%. We first validated the assay by screening against 20,000 compounds in 384-well format. After further optimization into a 1536-well format, it was screened against a NCGC library of 76,134 compounds, with a signal-to-background (S/B) ratio of 78 and Z’ factor of 0.77. The present assay can be used for discovery of novel small molecule Hsp90 inhibitors that can be used as chemical probes to investigate the role of cochaperones in Hsp90 function. Such molecules have the potential to be developed into novel anti-cancer drugs, for use alone or in combination with other Hsp90 inhibitors.
Keywords: heat shock protein 90 (Hsp90), Hsp organizing protein (HOP), tetratricopeptide repeat (TPR), AlphaScreen™, high-throughput screening (HTS)
Introduction
Heat shock protein 90 (Hsp90) has emerged as an important anti-cancer drug target due to its essential activity for the folding and maturation of multiple oncogenic signaling proteins that regulate cancer cell growth and survival 1. Specific inhibition of Hsp90 function leads to the destabilization and eventually the proteasomal degradation of multiple Hsp90 client proteins on which cancer cell growth are highly dependent 2. To date, studies on developing small molecule inhibitors of Hsp90 have exclusively focused on inhibiting its ATPase activity, using both structure based design 3 and high throughput screening 4. Geldenamycin (GM) and its analogue 17-allylaminogeldanamycin (17-AAG), which act by competitively binding to the ATP/ADP pocket at the N-terminal domain of Hsp90, are particularly well characterized examples of such inhibitors. 17-AAG has entered clinical trials in treating cancer patients 5. However, 17-AAG suffers from many limitations in administration, e.g. limited oral bioavailability, poor solubility and liver toxicity 6,7. There is clearly a need to develop new Hsp90 inhibitors which have better pharmacologic and toxicity profiles than 17-AAG, which function by a different mechanism and can be used synergistically with 17-AAG.
Hsp90 does not function alone, but interacts with a cohort of cochaperones to form multichaperone complexes 8, 9. To our knowledge, however, there have been no prior reports of small molecules that inhibit Hsp90 by preventing its interaction with cochaperones. Here we report the development, optimization and implementation of the first in vitro HTS to identify small molecule compounds that disrupt the interaction between Hsp90 and its cochaperone Hsp90/Hsp70-organizing Protein (HOP). HOP mediates the assembly of Hsp70-HOP-Hsp90 multichaperone complex through the interaction between its individual tetratricopeptide repeat (TPR) domains and the C-terminal peptides of Hsp70 and Hsp90 10. The core contact for the Hsp90 and HOP interaction has been identified to be between the C-terminal pentapeptide of Hsp90 (MEEVD) and the TPR2A domain of HOP, with a binding stoichiometry of 1:1 and a dissociation constant (Kd) in the micromolar range 11,12. Exogenous TPR domains act as dominant negative mutants when introduced into cells, leading to decreased activity and levels of Hsp90-depentent client proteins, presumably by competing with endogenous HOP and/or other TPR containing proteins for their interactions with Hsp90 13,14. These observations provide a strong rationale for our approach to seeking for small molecules that inhibit Hsp90 function by preventing the Hsp90-HOP interaction.
We have developed a high throughput chemical screening assay based on Amplified Luminescence Proximity Homogenous Assay (AlphaScreen™) technology to identify small molecules that inhibit the Hsp90-TPR2A interaction. We first demonstrated the specificity and utility of this assay by showing that it could distinguish between the interactions of TPR2A domain with its cognate and non-cognate ligands, C-terminal peptides of Hsp90 and Hsp70. When tested against 20,000 chemical compounds in a 384-well format, the assay had an average S/B ratio of 60 and Z’ factor of 0.76, indicating the suitability of this assay for HTS. We further optimized the assay into a 1536-well assay format, and screened against a collection of 76,314 compounds at NCGC. Here we present the detailed description of assay development, HTS and the “hit” compounds that we identified, which will go forward to follow-up assays. Such novel small molecule Hsp90 inhibitors will be useful not only as molecular probes to aid in understanding the functional significance of interactions between Hsp90 and its cochaperones, but also have the potential to be developed into novel anti-cancer drugs, to be used alone or in combination with other chemotherapy agents.
MATERIALS AND METHODS
Reagents for AlphaScreen™ assay
N terminally His6-tagged TPR2A was produced using a bacterial expression system and purified using Ni-NTA superflow resin (Qiagen, Valencia, CA). C-terminal peptides of Hsp70 (FGAQGPKGGSGS-GPTIEEVD), Hsp90 (TEEMPPLEGDDDTSR-MEEVD) with or without an N-terminal biotin group were synthesized using automated solid phase synthesis by the Yale Keck facility. The reaction buffer was 25 mM HEPES, pH 7.4, 100 mM NaCl. 0.1% BSA was added to minimize non-specific interaction between beads. HEPES, NaCl, BSA were purchased from Sigma (St. Louis, MO). Opti-384 plates and AlphaScreen™ Histag fusion detection 10k assay point kit, which includes streptavidin coated Donor beads and Nickle-chelated Acceptor beads; the TrueHits 1k assay point kit, which includes streptavidin coated Donor beads and biotinylated Acceptor beads, and the Biotin-His6 linker peptide for the counter screen were purchased from PerkinElmer (Montreal, Quebec, Canada). We chose the Nickel-chelated Acceptor beads over the anti-histag antibody coated ones based on the consideration that the antibody, upon binding to the His6-tagged TPR2A, might cause steric hindrance and interfere with the interaction between TPR2A protein and Hsp90 peptide. 1536-well polypropylene clear plates (Kalypsys, San Diego, CA) were used as compound plates, 1536-well polystyrene white plates (solid bottom) from Corning Inc. (Corning, NY) were used as assay plates.
Molecular libraries
The compounds tested in this study include 20,000 compounds with diverse chemical structures from Maybridge Inc. (Cornwall, UK) and a NCGC library of 76,174 compounds. All compounds were stored at a stock concentration of 10 mM in 100% DMSO in 384-well plates at − 80 °C.
Assay development and characterization in 384-well format
For the initial assay characterization, the AlphaScreen™ competition assay was performed in white 384-well Opti plates under the following conditions: 10 nM biotin-Hsp90 peptide, 100 nM His6-tagged TPR2A protein, and competitors such as free Hsp90 or Hsp70 peptides at different concentrations were incubated together for 1 hour. Donor and Acceptor beads were then added to a final concentration of 10 µg/ml in 25 µl buffer containing 25 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% BSA. In the assay, the biotin-Hsp90 peptide was attached to streptavidin coated Donor beads and the His6-tagged TPR2A was attached to nickel-chelated Acceptor beads. Non-biotinylated free Hsp90 or Hsp70 C-terminal peptides served as control inhibitors by competing with biotinylated peptide for the binding to TPR2A without bringing the beads together.
The effect of DMSO, a common solvent for small molecule compounds used for HTS, on assay signal was determined by incubating the assay reaction (25µl, 10 nM biotin-Hsp90 and 100 nM His6-tagged TPR2A and 10 µg/ml beads) in different wells at various DMSO concentrations and read at the same time. Competition experiments by free Hsp90 peptide were also performed at 0, 2.5%, 5% DMSO (v/v) to evaluate the solvent effect on the IC50 values.
Free Hsp90 peptide was used as a control inhibitor to test whether its competition for the interaction between biotin-Hsp90 and His6-tagged TPR2A is reversible, and how long it takes for the competition to reach equilibrium. A “dis-aggregation” experiment was performed, in which the biotin-Hsp90, His6-tagged TPR2A protein, the Donor and Acceptor beads were pre-incubated in a stock solution for one hour, 20 µl of the reaction was then aliquoted into individual wells in a 384-well plate, the reaction was initiated by adding 10 µM free Hsp90 peptide in each well. This concentration was chosen because that was the reported Kd for the Hsp90-TPR2A interaction 11,12, and it is about the final compound concentration to be used in the HTS screen. Individual wells were read at different time points to avoid photo-bleaching.
Considering the time (~ 8.5 minutes) the plate reader takes to read one 385-well plate in the AlphaScreen™ mode, we tested the stability of the assay signals using two control populations, positive controls and negative controls. We measured the concentration-response curves for free Hsp90 competition at time periods between 0.5 hour and 24 hours after the addition of Donor and Acceptor beads. A replicate plate was used for each time point to avoid photo bleaching. The signals were stable up to at least 12 hours, while an incubation time of 24 hrs resulted in ~ 30% signal decrease in the maximum, leading to lower S/B ratios. There were no significant differences between the IC50 values at different time periods. These results indicate we can read at least 85 plates (384 well) over a 12-hour period in one experiment.
Assay development into 1536-well format
We further optimized the assay conditions to miniaturize the assay into a 1536-well plate format by including in the assay buffer a reducing reagent dithiothreitol (DTT), which was originally left out of the assay with the concern that it might interfere with the nickel that is chelated to the Acceptor beads. Adding DTT significantly increased the assay signal, suggesting that potential disulfide bonds formation by the two Cys residues in TPR2A may change the protein confirmation and subsequently reduces its binding affinity to Hsp90 C-terminal peptide. We also examined the effects of salts on the assay. While the addition of K+ or Na+ in the assay buffer both produced robust signals, the assay was more sensitive in K+-containing buffer. In addition, it is known that intracellular K+ concentration is approximately 100 mM, whereas Na+ is 10 mM. Thus, we selected 100 mM K+ to use in the assay buffer. In the final optimized 1536-well plate assay, the total assay volume was 5 µL. Briefly, 3 µl/well of His6-tagged TPR2A (5 nM final) and 1 µL biotin-Hsp90 (10 nM final) were dispensed to a white 1536-well plate followed by an addition of 20 nL compound diluted in DMSO and 2 hr incubation. Subsequently, 1µL/well of bead mixture containing both Acceptor and Donor beads was added. After 1 hr incubation at room temperature, the assay plate was measured in a PerkinElmer Envision plate reader. In each 1536-well plate, His6-tagged TPR2A and biotin-Hsp90 were added to the first two columns to act as a positive control. Columns 3 and 4 contained the His6-tagged TPR2A, biotin-Hsp90 and free Hsp90 peptide as a negative control for the basal signal. The remaining wells, the sample area, could contain up to 1408 compounds.
TrueHits kit screen and Counter screen using a Biotin-His6 peptide
In the TrueHits kit screen, the strong binding between streptavidin and biotin brings the Donor and Acceptor beads directly together to generate signals upon excitation at 680 nm under all conditions. In the counter screen, a Biotin-His6 peptide serves as a covalent linker to bring together the streptavidin coated Donor beads and nickel chelated Acceptor beads. Any compound that causes decreased signals in the TrueHits kit screen or the counter screen must be general interfering compounds that are not relevant to the specific target of interest, while those exhibit no effect on the signal are potential true hits. Donor beads and Acceptor beads (final concentration of 10 µg/ml) and each primary “hit” compound were incubated together with (for counter screen), or without (for TrueHits kit screen) the linker peptide at room temperature for 1 hr before the measurement. The screens were performed in the same assay buffer as that used in the HTS.
Data analyses
Data analysis for the 20,000 compounds screen and following IC50 measurements at Yale Chemical Genomics Screening Facility was performed using Prism software (Graphpad Software, Inc. San Diego, CA). The concentration-response (CR) screening results from the NCGC screen of 76,174 compounds were analyzed using the NCGC CurveFit software (www.ncgc.gov). A four parameter Hill equation was fitted to the CR data by minimizing the residual error between the modeled and observed responses. Outliers were masked if the difference with the modeled Hill equation exceeded the noise in the assay which was calculated from the standard deviation of the activity at the lowest tested compound concentration. Additionally, data from higher concentrations were preferentially masked if doing so allowed the fit of the lower-concentration data to achieve significance as judged by efficacy and r2 requirements. Concentration-response curves were plotted using Prism software. The Z' factor, an index for assay quality control, was determined by
All values are expressed as mean ± SD.
RESULTS AND DISCUSSION
Characterization of AlphaScreen™ competition assay to measure different protein-ligand interactions
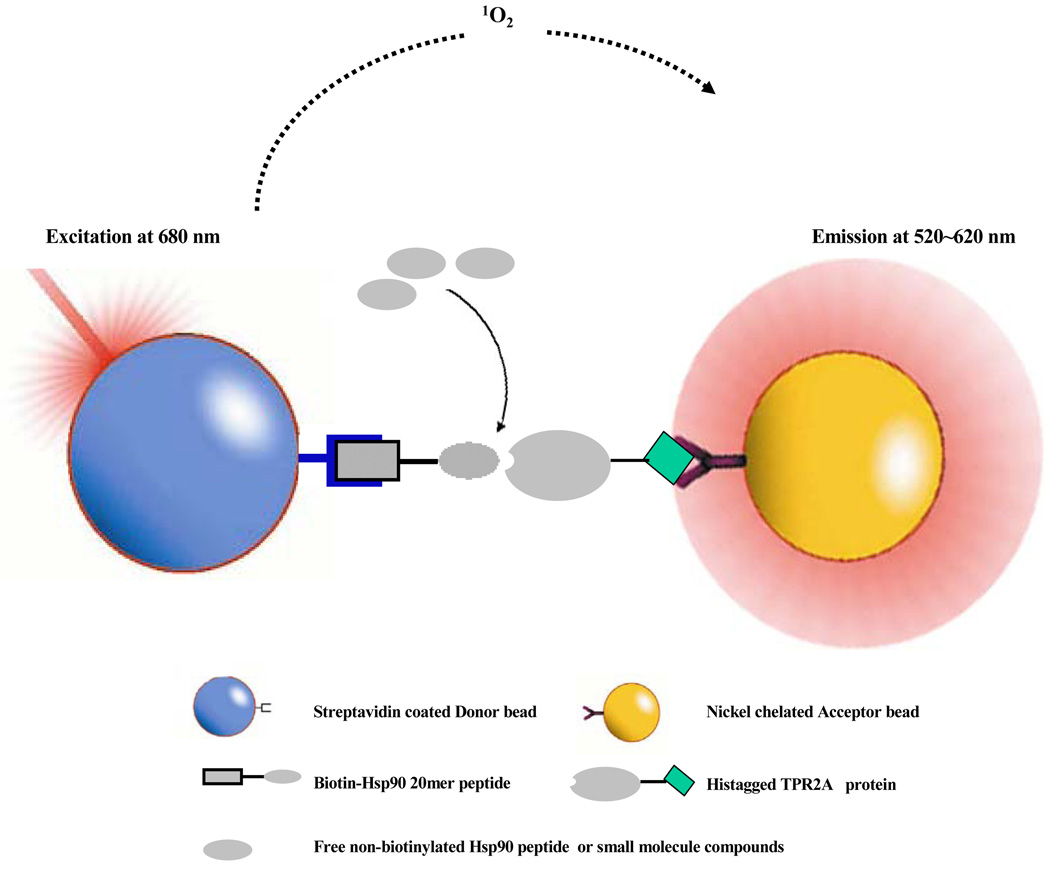
AlphaScreen™ is a beads-based assay which relies on the proximity of “Donor” and “Acceptor” beads conjugated to different biomolecules of interest 15,16. The streptavidin coated Donor beads and nickel-chelated Acceptor beads are brought together through the biotin-Hsp90/His6-tagged TPR2A interaction (Figure 1). Upon laser excitation at 680nm, the photosensitizers inside the Donor beads convert ambient oxygen to a singlet oxygen state. Only when the Donor and Acceptor beads are brought within 200 nm, can the singlet oxygen molecules diffuse to the Acceptor beads, resulting in extensive emission at 520–620 nm. To demonstrate appropriate binding, and the sensitivity of the assay, His6-tagged TPR2A protein and biotin-Hsp90 peptide were titrated to determine the optimal reagent concentrations (Figure 2). At each of the three TPR2A concentrations tested, a plateau of maximum signal was reached at ~50 nM biotin-Hsp90, indicating the Donor beads became saturated by biotin. A TPR2A concentration of 3.25 nM gave a maximum signal of only 2000 and a S/B ratio of 4, thus is too low for the following characterization, while the S/B ratios for the binding using 325 nM and 32.5 nM His6-tagged TPR2A were 144 and 133 respectively. With the intention to keep the biotin-Hsp90 peptide concentration as low as possible for the competition assay, while still maintaining a high assay signal and sensitivity, we used 10 nM biotin-Hsp90 peptide and 100 nM His6-tagged TPR2A, with an estimated S/B ratio around 60, for the following assay characterization.
Figure 1. Schematic diagram of AlphaScreen™ monitored Hsp90 competition assay.
The biotin-Hsp90 peptide and His6-tagged TPR2A interaction brings the beads together by conjugating to Streptavidin coated Donor beads and Nickel chelated Acceptor beads respectively. Upon excitation at 680 nm, a donor bead produces singlet oxygen which diffuses to Acceptor beads within 200 nm, initiating a chemiluminecent reaction. Any non-biotinylated molecules (peptides or compounds) that can bind to TPR2A will inhibit the signals by competing with biotinylated Hsp90 peptide.
Figure 2. Dose-dependent binding of biotinylated Hsp90 C-terminal peptide to TPR2A.
Increasing concentrations of biotin-Hsp90 peptide were incubated with 325 nM (■), 32.5 nM (●) or 3.25 nM (♦) histag-TPR2A, and acceptor beads (20 µg/ml) for 1 hour, donor beads were then added into each well to a final concentration of 20µg/ml in 25 µl assay volume. After one hour incubation, the plate was read on an Envision plate reader.
We then assessed the kinetics of the binding reaction that gives rise to the AlphaScreen™ signal. Time course experiments demonstrated that maximal signal was reached 30 minutes after incubating the assay components (Figure 3A). Fitting the data to a single phase exponential decay gave a half-life for the dis-aggregation reaction of 35 minutes (Figure 3B). This represents the upper limit for the equilibration time necessary for the competition assays, because in this experiment, the free Hsp90 peptide was added after the bead complexes were pre-incubated, while in the actual screen the compound competitors would be added at the same time as the biotin-Hsp90 peptide. Based on these results, biotin-Hsp90, compounds and TPR2A were incubated for at least one hour to ensure the competition reached equilibrium before addition of the beads.
Figure 3.
(A) Time course of the biotin-Hsp90 peptide binding to His6-tagged TPR2A protein. His6-tagged TPR2A (100 nM) and 10 µg/ml Donor and Acceptor beads were incubated for 1 hour, the reaction was initiated by adding 10 nM biotin-Hsp90 peptide, measurements were taken at different time points. A different well was used for each time point to avoid photo-bleaching. Data are expressed as raw signal of count per second. Data are expressed as mean +/− SD from 3 experiments.
(B) Time for the competition assay to reach equilibration. His6-tagged TPR2A (100 nM), biotin-Hsp90 peptide (10 nM) and beads (10 µg/ml) were incubated for 1 hour before the addition of 10 µM free Hsp90 peptide. A different well was used for each time point to avoid photo-bleaching. Data are expressed as raw signal of count per second. Data are expressed as mean +/− SD from 3 experiments performed in triplicates.
The C-terminal peptides of Hsp70 and Hsp90 serve as test peptides to assess the specificity of the AlphaScreen™ competition assay. Competitive displacement measurements were performed using the free 20 mer Hsp90 and Hsp70 C-terminal peptides, with IC50s of 4.9 µM and 24 µM, respectively (Figure 4), which are consistent with the reported values11. Multiple independent measurements yielded an average IC50 of 4.4 µM with a standard deviation of 2.2 µM for the Hsp90-TPR2A interaction. A C-terminally amidated Hsp90 peptide, deficient in binding due to its inability to form a “two carboxylate clamp” with TPR2A11, did not compete with biotin-Hsp90 for TPR2A binding in this assay (data not shown). These results validate the use of this assay to identify small molecule compounds that disrupt the Hsp90-HOP interaction.
Figure 4. Competition of free non-biotinylated Hsp90, Hsp70 peptides with biotinylated Hsp90 peptide for the binding to TPR2A protein.
Biotinylated Hsp90 peptide (10 nM) and TPR2A protein (100 nM) and increasing concentrations of free Hsp90 (■) or Hsp70 (▲) peptides were incubated for one hour first, the reaction was initiated by addition of 10 µg/ml Donor and Acceptor beads. The plate was read after 1 hour incubation. The Y axis represents the percentage inhibition of the maximal Alphascreen signal in the absence of any inhibitor. Data are expressed as mean +/− SD from 3 experiments.
Optimization of the assay
We tested the effect of DMSO on the AlphaScreen™ signal, S/B ratio and IC50 for competition curves at concentrations up to 5%. DMSO decreased the signals in a concentration dependent manner, without affecting the IC50 values of the competition curves. The S/B ratios were 51, 42, 35 for 0%, 2.5%, 5% DMSO concentration respectively (Data not shown). The actual HTS was performed in the presence of 0.1% DMSO.
We also explored the possibility of lowering the beads concentration to lower the assay cost. The effect of different beads concentration (20, 10, 7.5, 5 µg/ml) on IC50, assay signal, S/B ratio were tested using the same peptide and protein concentration. The signal decreased with decreased beads concentration in a dose-dependent but not a linear way. The IC50 values of the competition curves at different beads concentration were the same within measurement error (data not shown). To ensure a minimum signal >20,000, we used a bead concentration of 10 µg/ml, with a maximum signal of over 100,000 and S/B ratio of 130.
Robustness of the AlphaScreen assay
To evaluate the suitability of the Hsp90 competition assay for HTS, we studied the intra-plate variability on two control populations: the positive controls (10 nM biotin-Hsp90, 100 nM His6-tagged TPR2A, 10 µg/ml Donor and Acceptor beads in the absence of any inhibitors) and the negative controls (10 nM biotin-Hsp90, 100 nM His6-tagged TPR2A, 10 µg/ml Donor and Acceptor beads with 30 µM free Hsp90 peptide). We used 48 wells in a 384-well plate to measure the positive controls and 48 different wells to measure the negative controls (Figure 5). The average signal for positive controls was 121,833 with a standard deviation of 8,118 (CV = 6.7%); the average signal for negative controls was 2,022 with a standard deviation of 442 (CV =21.9%). The S/B ratio obtained was 60. Inter-day variability was around 11%. The Z’ factor was 0.76 for the two day measurements, indicating the good quality of the assay. For day to day variance measurements, a replicate plate was read on a different day to avoid photo bleaching.
Figure 5. High throughput assay format evaluation using two control populations.
(A) Intra-plate variance using 48 positive controls (●) (10 nM biotin-Hsp90, 100 nM histag-TPR2A and 10 µg/ml beads), negative controls (◦) (10 nM biotin-Hsp90, 100 nM histag-TPR2A and 10 µg/ml beads in the presence of 30 µM free non-biotinylated Hsp90 peptide) in one 384-well plate. The lines represent the mean (solid) and 3 standard deviations (dashed) from the mean of the positive and negative controls. (B) Inter-day variation. Two plates with samples from the same preparation were incubated and read at two different days. Data were expressed as percent of maximum signals by normalizing against the average of positive controls in each plate. Data were plotted by row first, then by column.
High Throughput Screen of compound libraries
20,000 compounds were screened at single concentration of ~ 10 µM at Yale Chemical Genomics Screening Facility using the optimized assay in 384-well format. Using a selection criterion of >50% inhibition at ~ 10 µM compound, 60 primary “hits” were identified from this library.
76,174 compounds were screened at NCGC using the optimized assay in qHTS in 1536-well format. Each compound was titrated in 7 to 15 concentrations, generating concentration-response (CR) curves. This titration format, rather than single concentration screening with follow-up CR curve measurements, allowed us to more efficiently select the hits and quickly narrowing them down to a small group of active compounds for follow-up study 17. We included 0.1% BSA and 0.01% Tween-20 in the assay buffer to minimize promiscuous inhibition that could be caused by compounds aggregation18. We selected the active compounds based on their potencies instead of the percentage inhibition in the traditional single concentration screening. A selection criterion of IC50 values < 10 µM and maximal activity > 50% was used to identify 149 active compounds as the primary “hits” from this screen.
Identifying target-independent positives using the TrueHits screen or a counter screen
In compound screen process, each assay format may produce its assay specific false positives in addition to the screen operation-related target-independent positives such as lint and dispensing glitch. In this assay design, the Ni2+ chelate coated beads is used to capture the TPR2A-Histag protein which could be interfered by the metal chelators in the compound library. The singlet oxygen transferring between the Acceptor and Donor beads may also be interrupted by certain compounds in the compound collection. In addition, the binding of biotin-Hsp90 peptide and streptavidin coated beads can be blocked by the compounds such as the biotin analogs.
To identify target-independent positives, we applied the AlphaScreen™ TrueHits kit to screen the 60 “hit” compounds identified from the 20,000 compounds at single concentration of ~ 10 µM. Interfering compounds were identified as those with > 30% inhibition. No interfering compound was identified from the 60 “hits” from the 20,000 Maybridge compounds. We then measured the full competition curves for the 60 compounds. Target-independent positives typically exhibit non-sigmoidal, or even linear competition curves. 31 out of the 60 “hit” compounds displayed “normal” dose dependent and sigmoidal responses (See Supplementary Figure S1). All compounds have IC50 values on average similar to that of the free Hsp90 C-terminal peptide. Their Hillslopes were close to −1, suggesting there was no cooperativity in the competitive compounds binding to TPR2A and no aggregation of reagents in the assay 19.
From the NCGC screen, 149 “primary hits” were identified based on their <10 µM IC50 values (see PubChem AID=595). 41 compounds representative of different structural clusters were selected, reordered and retested. The binding of 39 out of these 41 compounds were confirmed. These 39 compounds were tested in the counter screen assay (see PubChem AID=632). 36 of these 39 were positive in the counter-screen assay with sigmoidal shape competition curves, thus were discarded as interfering compounds. Among these some had imidazole-like structures, implying that they were competing with the His6-tagged protein for binding to the Nickel chelated Acceptor beads. Many other mechanisms of non-specific inhibition are possible, such as being singlet oxygen or fluorescence quenchers.
The final three confirmed NCGC compounds had close activity in the competition assay, with IC50 values of 0.49, 0.37 and 0.36 µM respectively (Figure 6) likely due to their highly related structures. It is worth noting that even though this class of compounds were not active in the counter screen, they must be further characterized and confirmed using independent assays. We do not expect that the counter screen to be 100% effective in ruling out all target-independent positives, but at least they allow us to trim the “hit” list and remove compounds that are certainly not true positives.
Figure 6.
AlphaScreen™ detected competition curves (A) and counter screen (B) of 3 hit compounds from the 76,314 compounds NCGC library.
CONCLUSION
We have established the first high throughput screening assay to identify compounds that inhibit the Hsp90-HOP interaction using AlphaScreen™ technology. The assay can distinguish between ligands with different affinities for TPR2A, as with free Hsp90 and Hsp70 peptides. The average Z’ factor value was over 0.7, indicating that the assay can adapt well to high-throughput assay format. By screening this assay against a total of 96,174 compounds from different sources, we identified 209 primary “hits” that potentially interrupt the Hsp90-HOP interaction in vitro. After excluding target-independent positives using a TrueHits kit screen or a counter screen, followed by full concentration-response curve measurements, we have 34 “hit” compounds (overall 0.03% hit rate) with average IC50 values in the micromolar range. Further characterization of these “hits” using secondary in vitro and in vivo assays will be presented elsewhere 20 (Yi, F and Regan, L. in press).
This study represents the first effort to identify a novel class of Hsp90 inhibitors by interrupting its cochaperone interaction. The compounds identified using this assay have the potential to be used as molecular probes to study the role of cochaperones in Hsp90 dependent cellular function and signaling pathways. Although a major attraction of current Hsp90 inhibitors is their ability to simultaneously inhibit multiple signaling pathways, the pleiotropic effects resulting from inhibition of Hsp90 ATPase activity also make it very difficult to determine which specific Hsp90 client proteins or signaling pathways are the most critical for the anti-cancer activity of Hsp90 inhibitors in a particular tumor. Because the specificity of Hsp90 for different client proteins is to some extent determined by its cochaperones 21,22, it is reasonable to speculate that inhibitors that inhibit Hsp90 function by disrupting its specific cochaperone interactions might provide the attractive feature of specific inhibition of client proteins.
Supplementary Material
Flowchart tracking “hits” identification and characterization. Page 21
ACKNOWLEDGEMENT
We thank Dr. Paul Fletcher for his invaluable suggestions and help with the early assay development and screening at the Yale Chemical Genomics Screening Facility. This work was supported by NIH grant (5R01CA113677) and Breast Cancer Alliance (L.R.); Anna Fuller Postdoctoral Fellowship foundation, NIH Molecular Libraries Screening Centers Network (XO1-MH077625-01), Concept Award, Department of Defense, Breast Cancer Research Program (W91ZSQ7167N696) (F.Y.). Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Program of the National Human Genome Research Institute, National Institute of Health (P.Z., N.S., J.I., C.P.A., W.Z.).
References
- 1.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32(3):517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 2.Powers MP, Workman P. Inhibitors of the heat shock response: Biology and pharmacology. FEBS Lett. 2007;581(19):3758–3769. doi: 10.1016/j.febslet.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, Rosen N. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol. 2001;8(3):289–299. doi: 10.1016/s1074-5521(01)00015-1. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle A, Tomlin P, Norris C, Richards J, Cordwell M, Boxall K, Rowlands M, Jones K, Collins I, McDonald E, Workman P, Aherne W. A duplexed phenotypic screen for the simultaneous detection of inhibitors of the molecular chaperone heat shock protein 90 and modulators of cellular acetylation. Mol Cancer Ther. 2007;6(3):1112–1122. doi: 10.1158/1535-7163.MCT-06-0496. [DOI] [PubMed] [Google Scholar]
- 5.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. Phase I Trial of 17-Allylamino-17-Demethoxygeldanamycin in Patients With Advanced Cancer. J. of Clinical Oncology. 2005;23(6):1076–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 6.Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, Kelly W, DeLaCruz A, Curley T, Heller G, Larson S, Schwartz L, Egorin MJ, Rosen N, Scher HI. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13(6):1775–1782. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanathan RK, Egorin MJ, Eiseman JL, Ramalingam S, Friedland D, Agarwala SS, Ivy SP, Potter DM, Chatta G, Zuhowski EG, Stoller RG, Naret C, Guo J, Belani CP. Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers. Clin Cancer Res. 2007;13(6):1769–1774. doi: 10.1158/1078-0432.CCR-06-2233. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AJ. What is a cochaperone? Cell Stress Chaperones. 2003;8(2):105–107. doi: 10.1379/1466-1268(2003)008<0105:wiac>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;3:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Smith DF. Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem. 1998;273(52):35194–35200. doi: 10.1074/jbc.273.52.35194. [DOI] [PubMed] [Google Scholar]
- 11.Scheufler C, Brinker A, Bourenkov G, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: Critical elements of the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 12.Brinker A, Scheufler C, von der Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl FU. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp90-HOP-Hsp90 complexes. J. Biol. Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200. [DOI] [PubMed] [Google Scholar]
- 13.Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271(50):32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 14.Cortajarena AL, Yi F, Regan L. Designed TPR modules as novel anticancer agents. ACS Chem Biol. 2008;3(3):161–166. doi: 10.1021/cb700260z. [DOI] [PubMed] [Google Scholar]
- 15.Ullman EF, Kirakossian H, Singh S, Wu ZP, Irvin BR, Pease JS, Switchenko AC, Irvine JD, Dafforn A, Skold CN. Luminescent oxygen channeling immunoassay: measurement of particle binding kinetics by chemiluminescence. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5426–5430. doi: 10.1073/pnas.91.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner G, Illy C, Pedro L, Roby P, Bosse R. AlphaScreen kinase HTS platforms. Curr. Med. Chem. 2004;11:721–730. doi: 10.2174/0929867043455693. [DOI] [PubMed] [Google Scholar]
- 17.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng BY, Shoichet BK. A Detergent-Based Assay for the Detection of Promiscuous Inhibitors. Nature Protocols. 2002;1(2):550–553. doi: 10.1038/nprot.2006.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGovern SL, Helfand BT, Feng B, Shoichet BK. A specific mechanism of nonspecific inhibition. J Med Chem. 2003;46(20):4265–4272. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- 20.Yi F, Regan L. A novel class of Hsp90 inhibitors. ACS Chemical Biology. 2008 doi: 10.1021/cb800162x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roe SM, Ali MM, Meyer P, Vaughan CK, Panaretou B, Piper PW, Prodromou C, Pearl LH. The mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50cdc37. Cell. 116:87–98. doi: 10.1016/s0092-8674(03)01027-4. [DOI] [PubMed] [Google Scholar]
- 22.Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF. Functional specificity of cochaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. doi: 10.1080/10409230490892513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart tracking “hits” identification and characterization. Page 21