SUMMARY
The progressive loss of muscle strength during aging is a common degenerative event of unclear pathogenesis. Although muscle functional decline precedes age-related changes in other tissues, its contribution to systemic aging is unknown. Here, we show that muscle aging is characterized in Drosophila by the progressive accumulation of protein aggregates that associate with impaired muscle function. The transcription factor FOXO and its target 4E-BP remove damaged proteins at least in part via the autophagy/lysosome system, while foxo mutants have dysfunctional proteostasis. Both FOXO and 4E-BP delay muscle functional decay and extend lifespan. Moreover, FOXO/4E-BP signaling in muscles decreases feeding behavior and the release of Insulin from producing cells, which in turn delays the age-related accumulation of protein aggregates in other tissues. These findings reveal an organism-wide regulation of proteostasis in response to muscle aging, and a key role of FOXO/4E-BP signaling in the coordination of organismal and tissue aging.
Keywords: Muscle aging, Proteostasis, FOXO, 4E-BP, Systemic regulation of aging, Feeding behavior
INTRODUCTION
Aging of multi-cellular organisms involves distinct pathogenic events that include higher mortality, the progressive loss of organ function, and susceptibility to degenerative diseases, some of which arise from protein misfolding and aggregation. Recent genetic studies in the mouse, the nematode Caenorhabditis elegans, and the fruitfly Drosophila melanogaster have expanded our understanding of the evolutionarily conserved signaling pathways regulating aging, with the identification of several mutants which have prolonged or shortened lifespan (Kenyon, 2005). Manipulation of longevity-regulating pathways in certain tissues is sufficient to extend life expectancy, indicating that some tissues have a predominant role in lifespan extension (Libina et al., 2003; Wang et al., 2005; Wolkow et al., 2000). For example, foxo overexpression in the Drosophila fat body extends lifespan, indicating a key role of this tissue in the regulation of longevity (Giannakou et al., 2004; Hwangbo et al., 2004). In addition, because most tissues undergo progressive deterioration during aging (Garigan et al., 2002), it is thought that organismal lifespan may be linked to tissue senescence. However, our understanding of the mechanisms regulating tissue aging and their interconnection to lifespan is limited. For example, analysis in Drosophila has revealed that prevention of age-dependent changes in cardiac performance does not alter lifespan (Wessells et al., 2004), raising the possibility that functional decline in distinct tissues may have different outcomes on the systemic regulation of aging.
The Insulin/IGF-1 signaling pathway has been implicated in the control of aging across evolution via its downstream signaling component FOXO (DAF-16 in C. elegans), a member of the fork-head box O transcription factor family (Salih and Brunet, 2008). FOXO regulates the expression of a series of target genes involved in metabolism, cell growth, cell proliferation, stress resistance and differentiation via direct binding to target gene promoter regions (Salih and Brunet, 2008). Mutations in foxo/daf-16 reduce lifespan and stress resistance in both C. elegans and flies, indicating a key role in organism aging (Junger et al., 2003; Salih and Brunet, 2008). In addition to regulating lifespan, FOXO has been reported to prevent the pathogenesis of some age-related diseases. For example, FOXO reduces the toxicity associated with aggregation-prone human mutant Alzheimer’s and Huntington’s disease proteins (proteotoxicity) in C. elegans and mice, suggesting that regulating protein homeostasis (proteostasis) during aging may have a direct effect on the pathogenesis of human neurodegenerative diseases (Cohen et al., 2006; Hsu et al., 2003; Morley et al., 2002). However, little is known on the protective mechanisms induced in response to FOXO signaling and whether they vary in different aging tissues and disease contexts.
Among the plethora of age-related pathological conditions, the gradual decay in muscle strength is one of the first hallmarks of aging in many organisms, including Drosophila, C. elegans, mice and, importantly, humans (Augustin and Partridge, 2009; Herndon et al., 2002; Nair, 2005; Zheng et al., 2005). However, despite its medical relevance, the mechanisms underlying muscle aging are incompletely understood. Functional changes in skeletal muscles temporally precede the manifestation of aging in other tissues (Herndon et al., 2002), and reduced muscle strength is associated with an increased risk in developing Alzheimer’s and Parkinson’s diseases (Boyle et al., 2009; Chen et al., 2005). However, although aging-related changes in skeletal muscles have been proposed to affect physiological processes in distal organs (Nair, 2005), whether muscle senescence modulates the pathogenesis of degenerative events in other tissues is unknown.
The fruit fly Drosophila is an excellent model to study muscle aging. The progressive decline in muscle strength and function observed in humans is recapitulated in this system (Rhodenizer et al., 2008), which is amenable to extensive genetic manipulation. By using this model organism, we have searched for the molecular mechanisms responsible for muscle aging and found that decreased protein quality control plays a role in the pathogenesis of age-related muscle weakness. Interestingly, increased activity of the transcription factor FOXO and its target Thor/4E-BP are sufficient to delay this process and preserve muscle function at least in part by promoting the basal activity of the autophagy/lysosome system, an intracellular protein degradation pathway which removes aggregates of damaged proteins (Rubinsztein, 2006).
Moreover, we report that FOXO/4E-BP signaling in muscles extends lifespan and regulates proteostasis organism-wide by regulating feeding behavior, release of Insulin from producing cells, and 4E-BP induction in non-muscle tissues. Thus, we propose a model by which FOXO/4E-BP signaling in muscles preserves systemic proteostasis by mimicking some of the protective effects of decreased nutrient intake.
RESULTS
Loss of Proteostasis during Muscle Aging is Prevented by FOXO
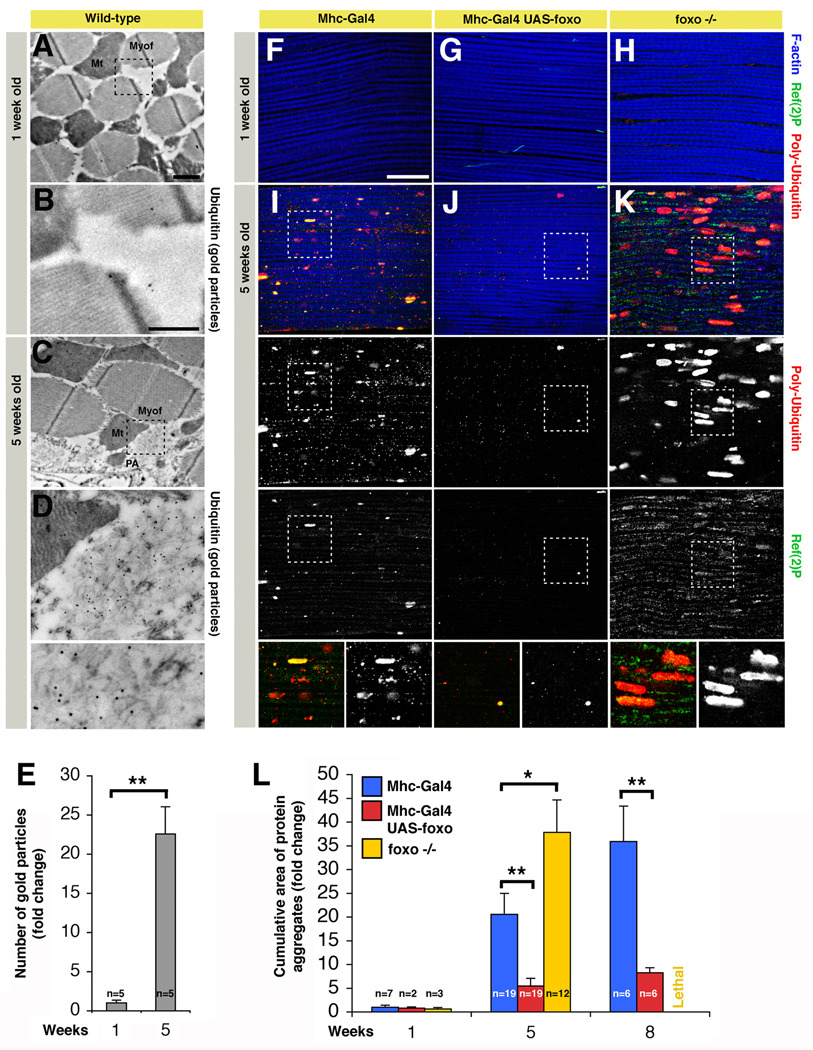
To detect cellular processes that are responsible for decreased muscle strength in aging flies, we monitored cellular changes in indirect flight muscles of wild-type flies by immunogold-electron microscopy (IEM). In older flies, we detected filamentous cytoplasmic structures that were instead absent in muscles from young flies (Figure 1A–D). Filamentous materials present in these structures stained with an anti-Ubiquitin antibody (Figure 1D), a marker for proteins that are poly-Ubiquitinated, suggesting that the cytoplasmic structures are aggregates of damaged proteins. Aggregates were variable in size and were detected in both resin-embedded sections (Figure 1) and cryosections (data not shown) of thoracic muscles of old but not young flies, in parallel with an increase in the overall number of gold particles (Figure 1E). To test the hypothesis that muscle function during aging may decrease due to defects in protein homeostasis, we better characterized the age-related deposition of protein aggregates by immunofluorescence. In agreement with the IEM analysis (Figure 1A–E), we observed that aging skeletal muscles progressively accumulate aggregates of poly-Ubiquitinated proteins (ranging up to several µm) that co-localize with p62/Ref(2)P, an inclusion body component (Figure 1F, I). The cumulative area of protein aggregates increases during aging (Figure 1L), suggesting that the progressive protein damage together with a decrease in the turnover of muscle proteins may result in the age-related decline of muscle strength.
Figure 1. FOXO Signaling in Skeletal Muscles Preserves Proteostasis during Aging.
(A–D) Electron micrographs of immunogold-labeled Drosophila skeletal muscles of wild-type flies at 1 (A–B) and 5 weeks of age (C–D). Protein aggregates (PA) are detected in the cytoplasm in proximity to mitochondria (Mt) and myofibrils (Myof) in old (C–D) but not young flies (A–B). Numerous gold particles (indicative of anti-Ubiquitin immunoreactivity) localize to filamentous structures at 5 weeks of age (C, D), while only few are present in muscles from young flies. Scale bars are 1 µm (A, C) and 500 nm (B, D). (E) The number of gold particles, indicative of Ubiquitin immunoreactivity, significantly increases in old age (sem is indicated with n; ** p<0.01).
(F–K) Immunostaining of indirect flight muscles from flies with (UAS-foxo/+;Mhc-Gal4/+) or without (Mhc-Gal4/+) foxo overexpression at 1 week (F, G) and 5 weeks of age (I, J), and foxo homozygous null (MhcGal4, foxo21/25) flies (H, K). Poly-Ubiquitin (red) and p62/Ref(2)P (green) immunoreactivities reveal an increased deposition of aggregates containing poly-Ubiquitin proteins during aging in muscles of control flies (F, I), and to a lesser extent in muscles overexpressing foxo (G, J). Conversely, muscles from foxo null animals display an accelerated deposition of protein aggregates (H, K), in comparison with controls (F, I). Note the significant increase in the cumulative area of protein aggregates (indicative of both aggregate size and number) in (K) versus (I), and (I) versus (J), indicating that the control of protein homeostasis is linked to FOXO activity in muscles (quantification in L, sem is indicated with n; * p<0.05; ** p<0.01). Representative poly-Ubiquitin and Ref(2)P immunoreactivities are shown in insets. Phalloidin staining (blue) outlines F-actin, which is a component of muscle myofibrils. Scale bar is 20 μm (F-K). See also Figures S1, S2, and S3.
To better characterize how protein quality control is linked with aging in muscles, we analyzed the deposition of protein aggregates in syngenic flies with foxo overexpression. Foxo overexpression results in its activation (Giannakou et al., 2004; Hwangbo et al., 2004) and was achieved specifically in muscles via the UAS-Gal4 system using the Mhc-Gal4 driver (Figure S1). Increased FOXO activity in muscles did not affect developmental growth and differentiation (as estimated by body weight and sarcomere assembly; Figure S2), and resulted in delayed accumulation of aggregates containing poly-Ubiquitinated proteins and Ref(2)P during aging (Figure 1G, J, compare with control muscles in Figure 1F, I). Next, we tested whether foxo null animals display accelerated muscle aging, and found an increased accumulation of protein aggregates (Figure 1H, K), indicating that FOXO is both necessary and sufficient to modulate muscle proteostasis (Figure 1L).
To further corroborate these findings, we overexpressed either wild-type or constitutive-active foxo transgenes using the Dmef2-Gal4 muscle driver in combination with the temperature sensitive tubulin-Gal80ts transgene to achieve adult-onset foxo overexpression in muscles (Figure S3). Transgene overexpression significantly preserved muscle proteostasis in both cases, while controls displayed increased accumulation of protein aggregates (Figure S3). Altogether, these results indicate that protein homeostasis depends on FOXO activity during muscle aging.
4E-BP Controls Proteostasis in Response to Pten/FOXO Activity
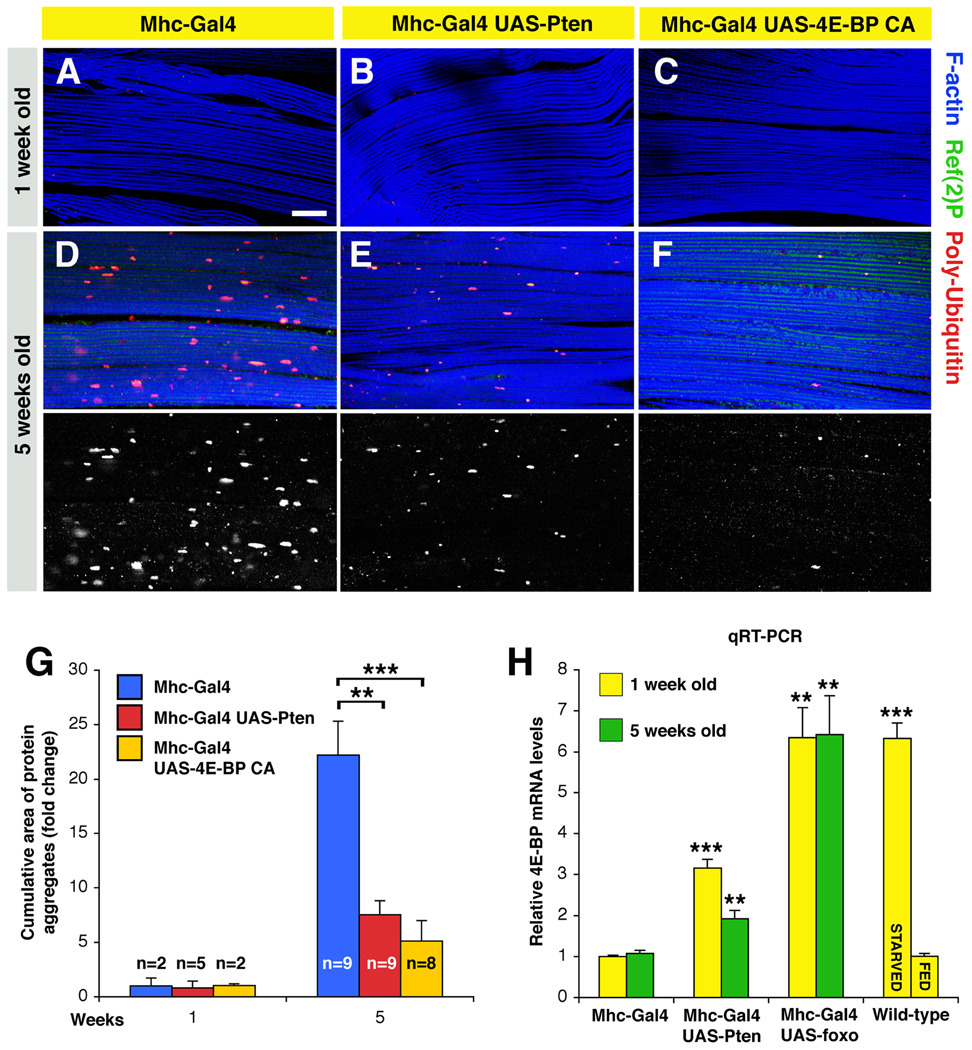
To dissect the stimuli that impinge on FOXO to control proteostasis, we tested whether Pten overexpression phenocopies FOXO activation. Consistent with its role in activating FOXO, we found that Pten decreased the accumulation of protein aggregates during aging (Figure 2B, E; see controls in Figure 2A, D).
Figure 2. 4E-BP Preserves Proteostasis in Response to Pten/FOXO Signaling.
(A–F) Immunostaining of muscles overexpressing Pten and constitutive active (CA) 4E-BP. In both cases, a decrease in the accumulation of Poly-Ubiquitin protein aggregates is observed at 5 weeks of age in comparison with age-matched controls, suggesting that these interventions can preserve proteostasis in aging muscles. Scale bar is 20 µm. Hsp70 overexpression has instead limited effects (Figure S4, Tables S1 and S2).
(G) A reduction in the cumulative area of protein aggregates is observed upon increased activity of either Pten or 4E-BP in comparison with controls (sem is indicated with n; ** p<0.01; *** p<0.001).
(H) Relative quantification of Thor/4E-BP mRNA levels from thoraces of syngenic flies at 1 and 5 weeks of age. A significant increase in 4E-BP expression is detected in response to fasting, and Pten and FOXO activity (**p<0.01; ***p<0.001; sem is indicated with n=4).
Next, we examined the responses induced by Pten/FOXO signaling. First, we examined whether FOXO activity delays protein damage by inducing chaperones that are key for protein quality control (Tower, 2009). In response to FOXO activity in muscles, we detected an increase in the mRNA levels of Hsp70 and its cofactors involved in protein folding (Hip, Hop, Hsp40 and Hsp90) but not in protein degradation (Chip and Chap; Figure S4 and Table S1). FOXO regulates directly the expression of Hsp70 and its co-factors as estimated with Luciferase transcriptional reporters based on the proximal promoter region of target genes (Figure S4 and Table S2). On this basis, we tested whether Hsp70 overexpression preserves proteostasis during aging but found little changes in the age-related accumulation of protein aggregates (Figure S4). Thus, we conclude that additional FOXO-dependent responses are involved.
Among the FOXO-target genes, Thor/4E-BP has a key role in delaying aging by regulating protein translation (Zid et al. 2009; Tain, 2009). However, the cellular mechanisms that are 4E-BP regulated are largely unknown. To test whether 4E-BP controls proteostasis during muscle aging, we overexpressed a constitutive active form of 4E-BP in muscles and observed limited accumulation of protein aggregates during aging (Figure 2C, F), in comparison with controls (Figure 2A, D). Altogether, increased activity of Pten or 4E-BP significantly decreases the cumulative area of protein aggregates (Fig. 2G).
In addition, a significant increase in 4E-BP mRNA levels is induced in muscles upon Pten, foxo overexpression, and fasting (Figure 2H). Altogether these findings suggest that 4E-BP is key to control proteostasis in response to Pten/FOXO signaling.
FOXO/4E-BP Signaling Regulates Proteostasis via the Autophagy/Lysosome System
While FOXO/4E-BP signaling mounts a stress resistance response that may decrease the extent of protein damage due to various stressors (Salih and Brunet, 2008; Tain et al., 2009), we wondered whether it regulates the removal of damaged proteins via macroautophagy. In this process, entire regions of the cytoplasm are sequestered in a double membrane vesicle (autophagosome) that subsequently fuses with a lysosome, where the autophagic cargo is degraded (Rubinsztein, 2006). Although the primary role of autophagy is to mount an adaptive response to nutrient deprivation, its basal activity is required for normal protein turnover (Hara et al., 2006). In agreement with this notion, suppression of basal autophagy leads to the accumulation of poly-Ubiquitin protein aggregates in a number of contexts (Korolchuk et al., 2009; Rubinsztein, 2006).
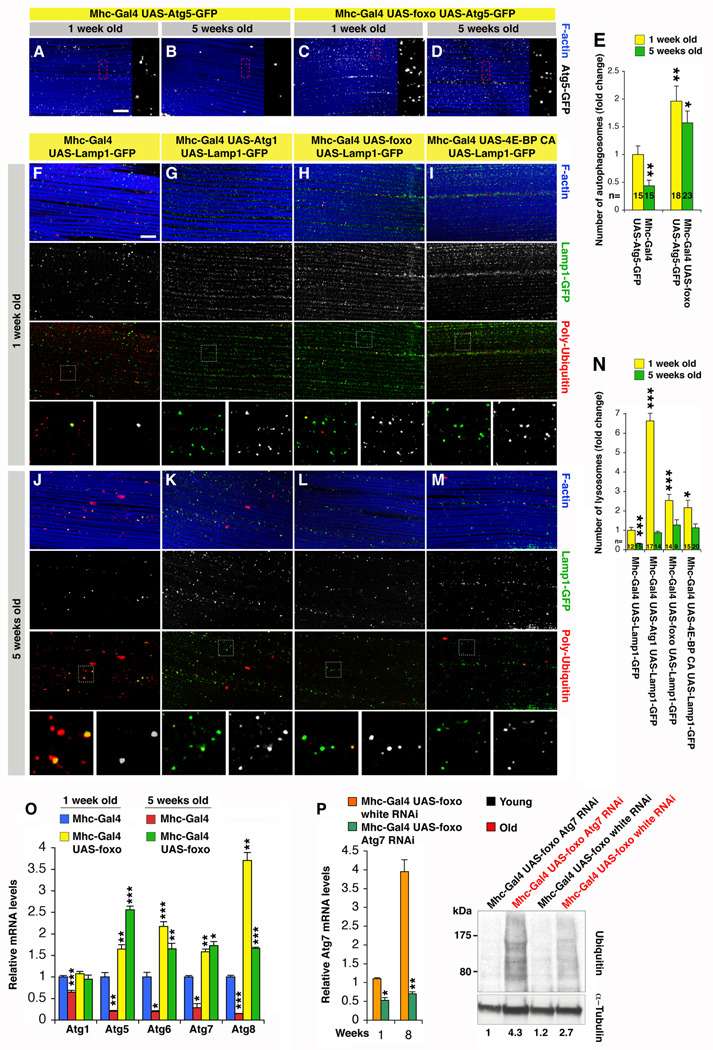
To test whether autophagy is regulated in response to FOXO signaling in muscles, we used a GFP-tagged version of the autophagosome marker Atg5 (Rusten et al., 2004). While the number of Atg5-GFP punctae decreases during aging in control muscles (Figure 3A, B), it is in part maintained in response to foxo overexpression (Figure 3C, D, and quantification in 3E). In addition, given the interconnection between the lysosome system and autophagy, we have monitored a GFP-tagged version of the lysosome marker Lamp1 (lysosome associated membrane protein 1) and detected an overall increase in the number of GFP punctae in response to overexpression of the autophagy inducer kinase Atg1, foxo, and 4E-BP CA in muscles at both 1 and 5 weeks of age (Figure 3G-I and K-M in comparison with controls in Figure 3F, J and quantification in 3N).
Figure 3. FOXO and 4E-BP Regulate Proteostasis at Least in Part via the Autophagy/ Lysosome System.
(A–E) Immunostaining of muscles expressing the marker of autophagosomes Atg5-GFP reveals a significant increase in their number (E) and maintenance at 1 and 5 weeks of age upon foxo overexpression (C, D), in comparison with controls (A, B). In (E), sem is indicated with n; *p<0.05 and **p<0.01.
(F–N) Immunostaining of muscles expressing the lysosomal marker Lamp1-GFP, and overexpressing either Atg1, foxo, and 4E-BP CA. Note an increase in the number of lysosomes (N) at both 1 (G–I) and 5 weeks of age (K–M), which inversely correlates with poly-Ubiquitin immunoreactivity, in comparison with control muscles (F, J). Scale bar is 10 µm (A–D and F–M). In (N), sem is indicated with n; *p<0.05 and ***p<0.001.
(O) Relative mRNA levels of autophagy genes from thoraces of 1 and 5 weeks old flies decrease during normal muscle aging while their expression increases and persists in response to FOXO. Sem is indicated with n=4; *p,0.05, **p<0.01 and ***p<0.001.
(P) RNAi treatment against Atg7 results in a ~50% knock-down of its mRNA levels in muscles and partially impairs FOXO-mediated proteostasis, as indicated by the increased detection of Ubiquitin-conjugated proteins in Triton X-100 insoluble fractions at 8 weeks (old, red) in comparison with mock treated (white RNAi) and young flies (1 week old, black). Normalized values based on α-Tubulin levels are indicated.
Closer inspection revealed that the abundance of Lamp1-GFP vesicles inversely correlates with the progressive deposition of poly-Ubiquitin protein aggregates, suggesting that FOXO/4E-BP signaling regulates proteostasis at least in part via the autophagy/lysosome system, To further test this hypothesis, we analyzed the age-related changes in autophagy gene expression, which have been previously used as a correlative measurement of autophagic activity (Gorski et al., 2003; Simonsen et al., 2008). Interestingly, the expression of several autophagy genes involved in autophagosome induction (Atg1), nucleation (Atg6), and elongation (Atg5, Atg7 and Atg8) progressively declines during aging in muscles (Figure 3O), suggesting that gene expression changes likely contribute to the accumulation of damaged proteins. Conversely, foxo overexpression increased the basal expression of several Atg genes at both young and old age, suggesting that their increased expression contributes to the beneficial effects of FOXO on proteostasis. To test this hypothesis, we knocked-down Atg7 levels in foxo overexpressing flies and analyzed the deposition of poly-Ubiquitin protein aggregates. Interestingly, RNAi treatment brought about a ~50% decrease in Atg7 mRNA levels and resulted in a partial increase in the buildup of insoluble ubiquitinated proteins at 8 weeks, in comparison with age-matched, mock treated flies (white RNAi), and 1 week old flies (Figure 3P).
Altogether, these findings suggest that FOXO/4E-BP signaling prevents the buildup in protein damage at least in part by promoting the basal activity of the autophagy/lysosome system.
Prevention of Muscle Aging by FOXO and 4E-BP Extends Lifespan
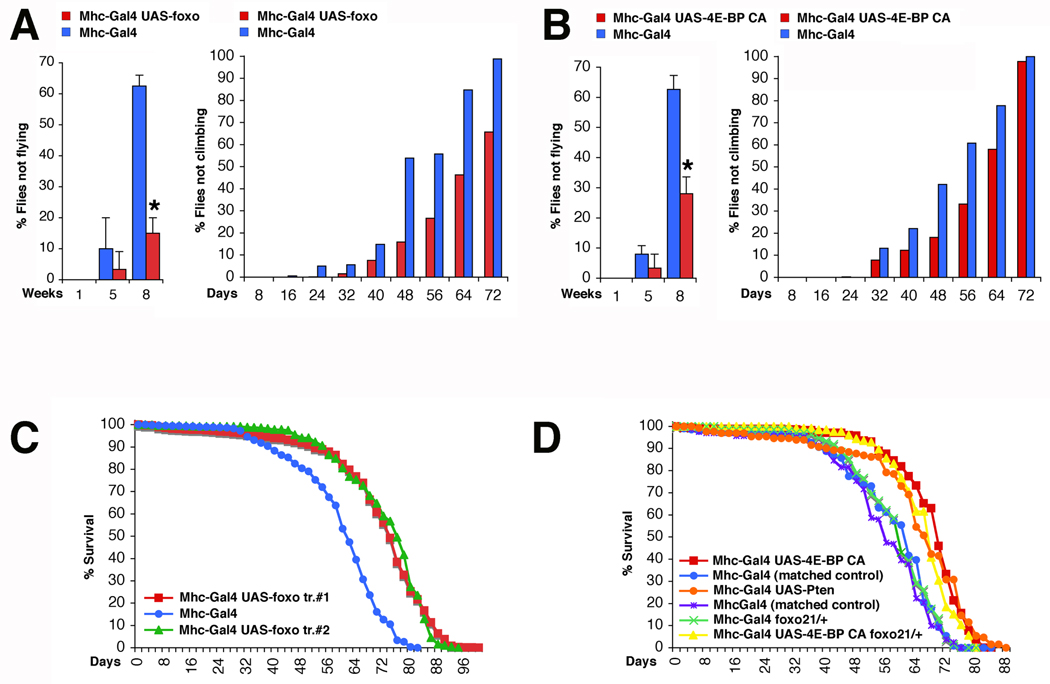
To evaluate whether preserving proteostasis can prevent functional alterations in aging muscles, we assessed muscle strength with negative geotaxis and flight assays (see Experimental Procedures). As shown in Figure 4A–B, muscle functionality gradually decreases in aging flies, resulting in impaired climbing and flight ability. Notably, foxo (Figure 4A) and 4E-BP activity (Figure 4B) significantly preserve muscle strength during aging. Thus, FOXO and 4E-BP prevent both the cellular degenerative events and the functional decay of aging muscles.
Figure 4. FOXO/4E-BP Signaling Preserves Muscle Function and Extends Lifespan.
(A) Muscle function gradually decreases during aging as indicated by an increase in the percentage of flies with climbing and flight defects. However, foxo preserves their function in comparison with controls (Flight ability: n[flies]= 10 (week 1 and 5) and 30 (week 8) with n[batch]= 3 (week 1 and 5) and 2 (week 8); sd is indicated and *p<0.05. Climbing ability: (n[Mhc-Gal4/+]=1264, n[Mhc-Gal4/UAS-foxo]=966, with n indicating the number of flies at day 1; p<0.001).
(B) Similar to FOXO, 4E-BP activity also results in decreased age-related flight and climbing deficits in comparison with controls (Flight ability: n[flies]≥10 (week 1 and 5) and 25 (week 8) with n[batch]≥3 (week 1 and 5) and 2 (week 8); sd is indicated and *p<0.05. Climbing ability: (n[Mhc-Gal4/+]=204, n[Mhc-Gal4/UAS-4E-BP CA]=403, p<0.001).
(C) Survival of flies during aging. Foxo overexpression in muscles significantly extends the median and maximum lifespan (Median and maximum lifespan: Mhc-Gal4/+=~61 and 82 days (n=1264); UAS-foxo tr.#1/+;Mhc-Gal4/+=~73 and 100 days (n=1184); Mhc-Gal4/UAS-foxo tr.#2=~76 and 94 days (n=966); p<0.001).
(D) Lifespan of flies with increased Pten and 4E-BP activity in muscles is extended in comparison with matched controls (Median and maximum lifespan of 4E-BP: Mhc-Gal4/+=~63 and 78 days (n=204); Mhc-Gal4/UAS-4E-BP CA =~71 and 84 days (n=403); Pten: Mhc-Gal4/+=~55 and 76 days (n=162); Mhc-Gal4/UAS-Pten=~66 and 88 days (n=130); p<0.001). Similar increase in lifespan is brought about by 4E-BP CA overexpression in foxo21 heterozygous null flies. See also Figures S5 and S7.
Epidemiological studies in humans have associated muscle senescence with increased mortality (Nair, 2005), implying that muscle aging may have organism-wide consequences beyond muscle function. To ask whether prevention of muscle aging affects the organism lifespan, we manipulated the activity of components of the Akt pathway in muscles and scored for their effects on viability. As shown in Figure 4C–D, either Pten, foxo, or 4E-BP CA overexpression in muscles is sufficient to significantly extend longevity by increasing the median and maximum lifespan. 4E-BP increased lifespan also in foxo heterozygous null animals (Figure 4D), while Hsp70 overexpression on the other hand showed little effects (Figure S5). Altogether, these findings indicate that the extent of muscle aging is interconnected with the lifespan of the organism.
FOXO/4E-BP Signaling in Muscles Influences Feeding Behavior and the Release of Insulin from Producing Cells
Considering that both fasting and FOXO induce 4E-BP expression (Figure 2H), we wondered whether the systemic effect of FOXO signaling on lifespan extension can result, at least in part, from reduced food intake.
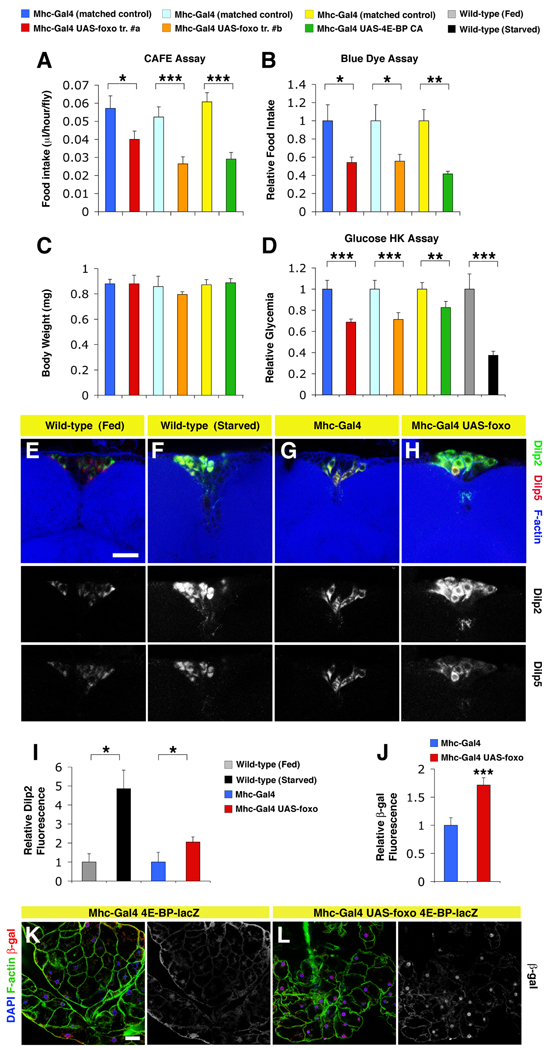
To test this hypothesis, we examined whether feeding behavior would be decreased in adults with FOXO and 4E-BP activation in muscles. We first monitored the amount of liquid food ingested using the CAFÉ assay (capillary feeding; (Ja et al., 2007)). Interestingly, feeding was decreased in response to FOXO/4E-BP signaling in muscles (Figure 5A). To substantiate this finding, we measured the ingestion of blue colored food (Xu et al., 2008). Also with this assay, we detected significant differences in food intake (Figure 5B), confirming that feeding behavior is affected. Next, to assess whether decreased feeding behavior arises from developmental defects, we measured the body weight of adult flies, which is a sensitive indicator of developmental feeding (Demontis and Perrimon, 2009), but found no significant differences (Figure 5C). Thus, the behavior of flies overexpressing foxo and 4E-BP CA in muscles most likely is not caused by developmental defects. To assess the metabolic status, we monitored the glucose concentration (glycemia) in the hemolymph. Similar to wild-type flies starved for 24 hours, we detected a significant decrease of glycemia in flies with FOXO and 4E-BP activation in muscles (Figure 5D). Altogether these findings suggest that FOXO and 4E-BP act as a metabolic brake in muscles that, by influencing feeding behavior, mimics at least in part the physiological changes that are associated with fasting.
Figure 5. FOXO Signaling in Muscles Partially Mimics Systemic Metabolic Changes Associated with Fasting by Modulating Feeding Behavior.
(A–C) Flies in which FOXO/4E-BP activity has been altered specifically in muscles consume less food than matched controls. Food consumption was determined via capillary feeding CAFÉ assay over 2 hours periods (A), and by monitoring the ingestion of blue colored food in 24 hours (B). Error bars represent sem with n[measurements]=44, 46, 52, 37, 103, and 61 in (A) and n=2 in (B), with * p<0.05; ** p<0.01; *** p<0.001. Decreased feeding does not result from developmental defects, as indicated by similar body weights of flies analyzed (C; error bars represent sd with n≥3).
(D) Relative glucose levels (glycemia) in the hemolymph of flies overexpressing either foxo or 4E-BP CA in muscles, and matched controls. Manipulation of FOXO/4E-BP signaling in muscles brings about a reduction of glycemia similar in part to that of wild-type flies starved for 24 hours, as estimated with the Glucose Hexokinase Assay (sem is indicated with n=5, and ** p<0.01; *** p<0.001).
(E–H) Immunostaining of Dilp-producing median neurosecretory cells in the brain of starved wild-type flies, flies overexpressing foxo in muscles, and controls. Increase in the immunoreactivity of Insulin-like peptides Dilp2 (green) is detected in producing cells in response to either starvation (F) or foxo overexpression in muscles (H), in comparison respectively with fed wild-type flies (E), and controls with no foxo overexpression in muscles (G). Smaller changes in Dilp5 levels are observed. Phalloidin staining (blue) detects F-actin (scale bar is 20 µm; images in E–H have the same magnification). (I) Quantification of the intensity of staining indicates that differences in Dilp2 fluorescence are significant (sd is indicated with n[measurements]=35, 69, 37, and 96 from n[brains]=2, 4, 3, and 4; *p<0.05). (J–L) Quantification and immunostaining of adipose tissue (peripheral fat body of the abdomen) from 2 weeks old flies. (J) Note a significant increase in nuclear β-galactosidase immunoreactivity (red) in the adipose tissue from flies with a nuclear 4E-BP-lacZ reporter and foxo overexpression in muscles (L), in comparison with controls (K). F-actin (green) and DAPI staining (indicative of nuclei, blue) are shown. Scale bar is 20 µm. In (J), sem is indicated with n=20 and *** p<0.001.
To gain mechanistic insights into the systemic regulation of aging by FOXO/4E-BP signaling in muscles, we next monitored the release of Insulin-like peptides (Dilps) from the Dilp-producing median neurosecretory cells in the brain, that have been previously shown to mediate the response of lifespan to nutrition in Drosophila (Broughton et al., 2010). We detected a significant accumulation of the Insulin-like peptide Dilp2 (and to a lesser extent Dilp5) in starved, wild-type flies in comparison with fed flies (Figure 5E–F). Increased immunoreactivity indicates decreased release of Dilps, and has been previously shown to occur in response to starvation (Geminard et al., 2009). Next, we tested whether similar changes would occur upon FOXO signaling in muscles and found a partial accumulation of Dilps (Figure 5G–I).
Assuming that decreased Dilps secretion may result in systemic FOXO activation, we monitored its activity using a nuclear 4E-BP-lacZ transcriptional reporter. By immunostaining adipose tissues with anti-β-galactosidase antibodies, we detected higher 4E-BP expression upon foxo activation in muscles, in comparison with controls (Figure 5J–L). Thus, FOXO signaling in muscles appears to systemically activate 4E-BP expression in other tissues by regulating food intake and Insulin release.
FOXO/4E-BP Signaling in Muscles Regulates Proteostasis in Other Aging Tissues
Our demonstration that FOXO/4E-BP signaling in muscles extends lifespan in Drosophila and induces a systemic fasting-like response, along with the observation that muscles undergo age-related structural and functional changes precociously in comparison to other tissues (Herndon et al., 2002; Zheng et al., 2005), raises the possibility that muscle senescence may influence the progression of age-related degenerative events in the entire organism.
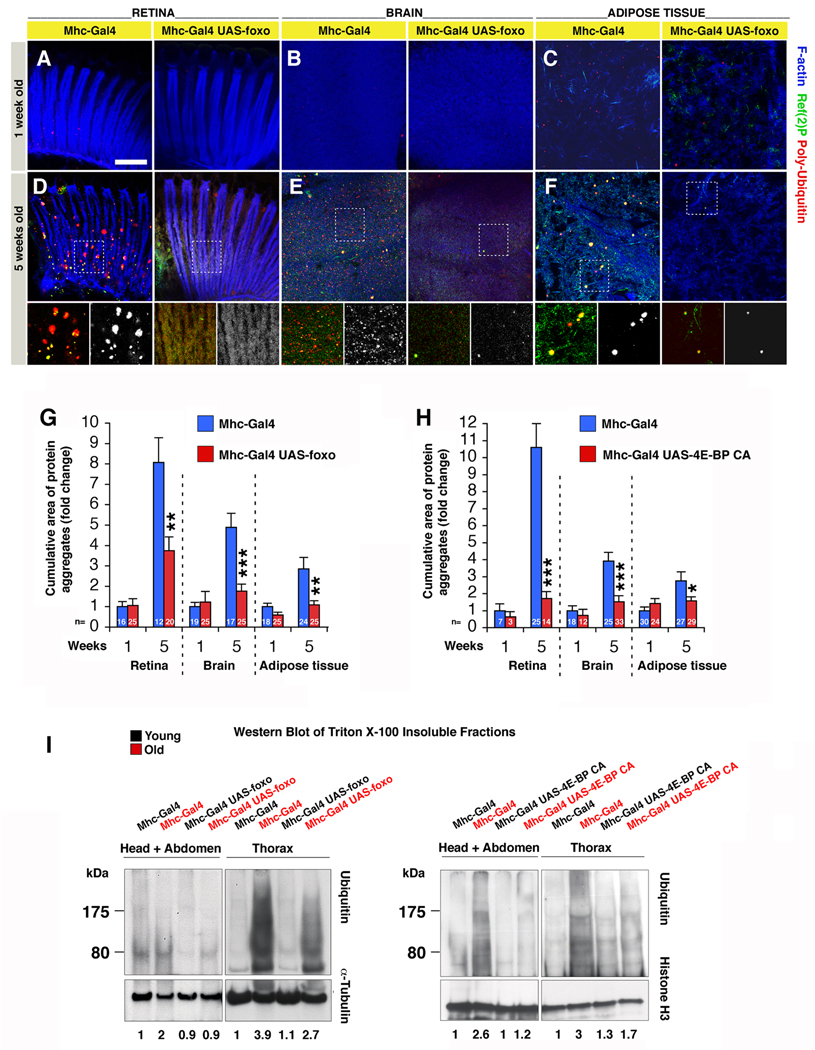
To test this hypothesis, we examined whether, in addition to lifespan extension, FOXO signaling in muscles can affect protein homeostasis in other tissues. As in the case of muscles (Figures 1 and 2), we found that Ref(2)P/poly-Ubiquitin aggregates progressively accumulate in aging retinas (Figure 6A, D), brains (Figure 6B, E), and adipose tissue (Figure 6C, F; peripheral fat body of the abdomen). However, foxo overexpression in muscle resulted in decreased accumulation of protein aggregates in other aging tissues (Figure 6D-F; quantification in Figure 6G). Similar changes were observed in response to 4EBP activity in muscles, in comparison with syngenic controls (Figure 6H). Importantly, this regulation is muscle non-autonomous as Mhc-Gal4 drives transgene expression only in muscles (and not in the retina, brain or adipose tissue; Figure S1). To further test the finding that FOXO/4E-BP signaling in muscles delays the systemic impairment of proteostasis in other tissues (Figure 6A–H), we analyzed by Western blot the Ubiquitin levels of Triton X-100 insoluble fractions, which include protein aggregates, from either thoraces (which mainly consist of foxo-overexpressing muscles) or heads and abdomens (which are enriched in non-muscle tissues and muscles with little foxo overexpression; Figure S1), at 1 and 8 weeks of age. In agreement with the increased deposition of protein aggregates observed during aging by immunofluorescence (Figures 1, 2 and 6A–F), Ubiquitin levels were dramatically increased in the Triton X-100 insoluble fractions from control thoraces, and head and abdominal extracts at 8 weeks of age, in comparison with 1 week of age (Figure 6I). However, Ubiquitin levels were only partially increased in old foxo-overexpressing flies in both thoracic, and head and abdominal extracts. No substantial differences were instead detected in the Triton X-100 soluble fractions (not shown). Similar results were obtained by 4E-BP CA but not Hsp70 overexpression in muscles (Figure 6I and S5), indicating that 4E-BP activity in muscles also confers systemic protection from the age-related decline in proteostasis. To test whether this effect is muscle-specific, we overexpressed foxo in the adipose tissue (abdominal fat body) with the S106GS-Gal4 driver, and analyzed the deposition of poly-Ubiquitinated proteins in Triton X-100 insoluble fractions from thoraces. Under these conditions, we seemingly detected no differences (Figure S6), suggesting that, although other tissues may be involved, muscles may play a key role in this regulation. Altogether, these observations suggest that FOXO and 4E-BP activity in muscles mitigates the loss of proteostasis non-autonomously by influencing feeding behavior, Insulin release from producing cells, and 4E-BP activity in other tissues.
Figure 6. Systemic Proteostasis is Remotely Controlled by FOXO/4E-BP Signaling in Muscles.
(A–F) Aggregates of poly-Ubiquitin proteins accumulate during aging in the retina (A, D), brain (B, E), and the adipose tissue (C, F) of control flies (Mhc-Gal4/+), but to a lesser extent in tissues from flies overexpressing foxo in muscles (UAS-foxo/+;Mhc-Gal4/+), as indicated by poly-Ubiquitin (red) and p62/Ref(2)P (green) stainings. Phalloidin staining (blue) outlines F-actin. Note that Mhc-Gal4 does not drive transgene expression in these tissues (Figure S1). Scale bar is 10 μm.
(G–H) The age-related increase in the cumulative area of protein aggregates is significantly less prominent in tissues from flies overexpressing foxo (G) or 4E-BP CA (H) in muscles in comparison with controls (sem is indicated with n; *p<0.05. **p<0.01, and ***p<0.001).
(I) Ubiquitin levels (indicative of protein aggregates) are detected in Triton X-100 insoluble fractions from thoraces, and head and abdominal tissues from flies overexpressing foxo in muscles or control flies at 1 (young, black) and 8 (old, red) weeks of age. Ubiquitin levels are increased in old flies in comparison with young flies in extracts from both muscles (thoraces) and non-muscle tissues (heads and abdomens). However, flies overexpressing foxo in muscles have reduced deposition of protein aggregates at 8 weeks of age in both muscles and non-muscle tissues. Similar results are obtained in response to increased 4E-BP activity in muscles (I), but not Hsp70 (Figure S5). Quantification of Ubiquitin-conjugated proteins normalized to α-Tubulin or Histone H3 levels is indicated. See also Figures S1, S5 and S6.
DISCUSSION
By using a number of behavioral, genetic and molecular assays, we have described a mechanism in the pathogenesis of muscle aging that is based on the loss of protein homeostasis (proteostasis) and the resulting decrease in muscle strength (Figure 7). Increased activity of Pten and the transcription factor FOXO is sufficient to delay this process, while foxo null animals experience accelerated loss of proteostasis during muscle aging. Pten and FOXO induce multiple protective responses, including the expression of folding chaperones, and the regulator of protein translation 4E-BP that has a pivotal role in preserving proteostasis. FOXO and 4E-BP preserve muscle function at least in part by sustaining the basal activity of the autophagy/lysosome system, which removes aggregates of damaged proteins. However, additional mechanisms may be involved. For example, the proteasome system may degrade damaged proteins and thus avoid their accumulation in aggregates (Rubinsztein, 2006). Thus, perturbation in proteasome assembly and subunit composition may contribute to muscle aging in response to FOXO activity. In addition, while overexpression of a single chaperone had limited effects, interventions to effectively limit the extent of protein damage are likely to delay the decay in proteostasis by decreasing the workload for the proteasome and autophagy systems (Tower, 2009).
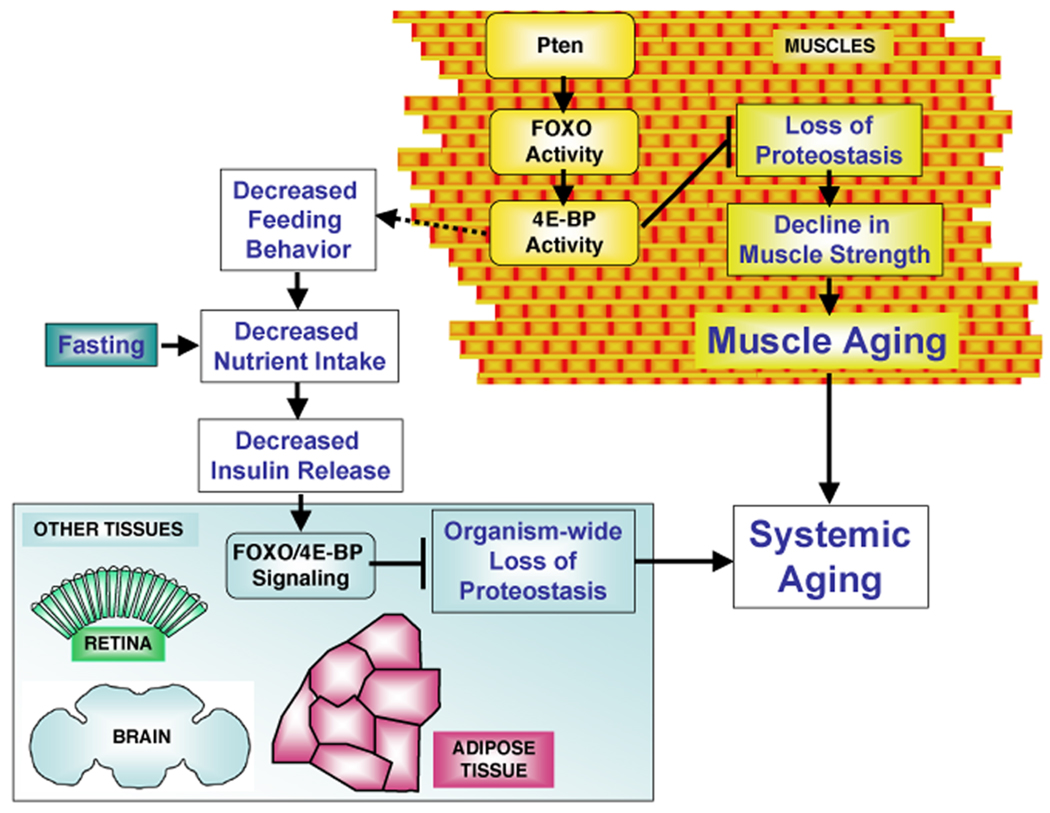
Figure 7. FOXO/4E-BP Signaling in Muscles Controls Proteostasis and Systemic Aging.
Muscle aging is characterized by protein damage and accumulation of cytoplasmic aggregates. Loss of protein homeostasis (proteostasis) associates with the progressive decrease in muscle strength and can affect the lifespan of the organism. Pten/FOXO signaling induces multiple targets, including several folding chaperones and the regulator of protein translation 4E-BP. FOXO/4E-BP activity regulates muscle proteostasis at least in part via the autophagy/lysosome pathway of protein degradation, preserves muscle function, and extends lifespan. In addition, FOXO/4E-BP signaling in muscles decreases feeding behavior that, similar to fasting, results in reduced Insulin release from producing cells. This in turn promotes FOXO and 4E-BP activity in other tissues, preserving proteostasis organism-wide and mitigating systemic aging.
By comparing the accumulation of poly-Ubiquitinated proteins in aggregates of aging muscles, retinas, brains, and adipose tissue, we have found that reduced protein homeostasis is a general feature of tissue aging that is particularly prominent in muscles (Figures 1, 6 and S6). The observation that muscle aging is characterized by loss of proteostasis further suggests some similarity between muscle aging and neurodegenerative diseases, many of which are characterized by the accumulation of protein aggregates (Rubinsztein, 2006).
Mechanical, thermal, and oxidative stressors occur during muscle contraction (Arndt et al., 2010) and therefore muscle proteins may be particularly susceptible to damage in comparison with other tissues. While our findings refer to the loss of proteostasis in the context of normal aging, it is likely that a better understanding of this process will likely help cure muscle pathologies associated with aging, as some of the underlying mechanisms of etiology may be shared. For example, most cases of inclusion body myositis (IBM) arise over the age of 50 years, defining aging as a major risk factor for the pathogenesis of this disease. Interestingly, muscle weakness in patients with IBM is characterized by the accumulation of protein aggregates (Needham and Mastaglia, 2008), which we have now described to occur in the context of regular muscle aging in Drosophila. Thus, FOXO may interfere with the pathogenesis of muscle degenerative diseases in addition to muscle aging. Studies in animal disease models of IBM will be needed to test this hypothesis.
There is an apparent contradiction between our findings and data describing the FOXO-dependent induction of muscle atrophy in mice (Bodine et al., 2001; Sandri et al., 2004), a serious form of age-related muscle degeneration that results in decreased muscle strength (Augustin and Partridge, 2009). The observation that different degrees of FOXO activation can promote stress resistance or rather cell death (Salih and Brunet, 2008) could explain why FOXO activity can be protective or rather detrimental during muscle aging. In particular, while physiologic FOXO activation can preserve protein homeostasis and muscle function, its excessive activation may lead to decreased muscle function due to hyper-activation of protein turnover pathways. Consistent with this view, the macroautophagy pathway has also been involved in both muscle atrophy (Mammucari et al., 2007; Zhao et al., 2007) as well as in the preservation of muscle sarcomere organization (Arndt et al., 2010; Masiero et al., 2009), highlighting the importance of fine tuning the degree of activation of stress resistance pathways to maintain muscle homeostasis. In addition, the output of FOXO activity may radically differ in growing versus pre-existing myofibers. In particular, our present study indicates that FOXO protects pre-existing myofibers against age-dependent changes in proteostasis, while it also blunts developmental muscle growth in flies (Demontis and Perrimon, 2009), as observed in mammals (Kamei et al., 2004). Thus, deleterious effects of FOXO activation as observed in mammalian muscles may result from the inhibition of growth of novel myofibers in post-natal development and adulthood, a process which is thought to be limited to development in Drosophila (Grefte et al., 2007).
An interesting observation of our study is that interventions that decrease muscle aging also extend the lifespan of the organism. In particular, our work raises the prospect that the extent of muscle aging may be a key determinant of systemic aging (Figure 7). Reduced muscle proteostasis may be detrimental per se for life expectancy, presumably due to the involvement of muscles in a number of key physiological functions. Consistent with this view, overexpression in muscles of aggregation-prone human Huntington’s disease proteins is sufficient to decrease lifespan (Figure S7). Moreover, FOXO signaling in muscles regulates proteostasis in other tissues, via inhibition of feeding behavior and decreased release of Insulin from producing cells, that in turn promote 4E-BP activity systemically. Thus, we propose that FOXO/4E-BP signaling in muscles regulates lifespan and remotely controls aging events in other tissues by bringing about some of the protection associated with decreased food intake.
In mammals, muscles produce a number of cytokines involved in the control of systemic metabolism (Nair, 2005; Pedersen and Febbraio, 2008). For example, Interleukin-6 (IL-6) is produced by muscles and has been proposed to control glucose homeostasis and feeding behavior through peripheral and brain mechanisms (Febbraio and Pedersen, 2002; Plata-Salaman, 1998). Thus, a muscle-based network of systemic aging as observed in flies may occur in humans.
This study supports the common belief that preserving muscle function is beneficial for overall aging (Boyle et al., 2009; Chen et al., 2005) and the notion that muscles are central tissues to coordinate organism-wide processes, including aging and metabolic homeostasis (Nair, 2005). Moreover, the observation that FOXO signaling in muscles influences aging events in other tissues suggests that the systemic regulation of aging relies on tissue-to-tissue communication (Russell and Kahn, 2007), which may provide the basis for interventions to extend healthy lifespan.
EXPERIMENTAL PROCEDURES
Drosophila Strains and Lifespan Analysis
Details on fly strains can be found in Supplemental Experimental Procedures. For longevity measurement, male flies were collected within 24 hours from eclosion and reared at standard density (20 flies per vial) on cornmeal/soy flour/yeast fly food at 25°C. Dead flies were counted every other day and food changed. For each genotype, at least two independent cohorts of flies, raised at different times from independent crosses, were analyzed. For starvation treatments, flies were kept in normal vials with 1.5% agar as a water source for the period of time indicated. For all experiments, Mhc-Gal4 females were mated with male transgenic and syngenic control flies and the resulting male offspring analyzed in parallel by comparing transgene expressing flies with matched controls flies having the same genetic background. For transgene expression with the Gal4-UAS system, flies were reared at 25°C.
Behavioral and Metabolic Assays
Flight ability was scored according to Park et al. (2006), and negative geotaxis assays were performed as previously described (Rhodenizer et al., 2008). In brief, flies were gently tapped to the bottom of a plastic vial, and the number of flies that could climb to the top of the vial after 20 seconds was scored. Quantification of the glucose concentration in the hemolymph, and capillary (CAFÉ) and blue colored food feeding assays were done as previously (Geminard et al., 2009; Xu et al., 2008) and are described in detail in Supplemental Experimental Procedures.
Immunostaining, Confocal and Electron Microscopy, and Image Analysis
For whole-mount immunostaining of fly tissues, indirect flight muscles, peripheral fat body of the abdomen, retinas, and brains were dissected from male flies and fixed for 30–40 minutes in PBS with 4% paraformaldehyde and 0.2% Triton X-100. After washing, samples were incubated over-night with appropriate primary and secondary antibodies. Image analysis was done with ImageJ and Photoshop. Immuno-gold electron microscopy was done similar to Nezis et al., (2008). See Supplemental Experimental Procedures for further information and list of antibodies used.
Quantitative Real-Time RT-PCR
qRT-PCR was done as previously described (Demontis and Perrimon, 2009). Total RNA was prepared from fly thoraces and qRT-PCR was performed with the QuantiTect SYBR Green PCR kit (Qiagen). Alpha-Tubulin 84B was used has normalization reference. Relative quantification of mRNA levels was calculated using the comparative CT method.
Statistical Analysis
Statistical analysis was performed with Excel (Microsoft) and p-values were calculated with Student’s t-tests and logrank tests.
Western Blot and Biochemical Analysis of Detergent Insoluble Fractions
Western blot and biochemical analysis of detergent insoluble fractions were done substantially as previously (Nezis et al., 2008). In brief, dissected flies were homogenized in ice-cold PBS with 1% Triton X-100 and protease inhibitors, and the resulting unsoluble pellet resuspended in RIPA buffer with 5% SDS and 8M urea. See Supplemental Experimental Procedures for a complete protocol.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Andreas Brech, Didier Contamine, Ernst Hafen, Pierre Leopold, Susan Lindquist, Ioannis Nezis, Amita Sehgal, Marc Tatar, Robert Tjian, John Tower, the DRSC/TRiP, and members of the Perrimon lab for fly stocks, reagents and advice. We thank Maria Ericsson for assistance with electron microscopy, Christians Villalta for embryo injection, and Chris Bakal, Rami Rahal, and Jonathan Zirin for critically reading the manuscript. This work was supported by the NIH (1P01CA120964-01A1) and a Pilot Project Grant from the Paul F. Glenn Labs for the Molecular Biology of Aging. FD is an Ellison Medical Foundation/AFAR postdoctoral fellow. NP is an investigator of the Howard Hughes Medical Institute.
Footnotes
SUPPLEMENTAL DATA
Supplemental Data include seven figures, two tables, Supplemental Experimental Procedures, and References.
REFERENCES
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Furst DO, Saftig P, Saint R, Fleischmann BK, et al. Chaperone-Assisted Selective Autophagy Is Essential for Muscle Maintenance. Curr Biol. 2010;20:143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Augustin H, Partridge L. Invertebrate Models of Age-Related Muscle Degeneration. Biochim Biophys Acta. 2009;1790:1084–1094. doi: 10.1016/j.bbagen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66:1339–1344. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, Partridge L. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9:336–346. doi: 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Demontis F, Perrimon N. Integration of Insulin receptor/Foxo signaling and dMyc activity during muscle growth regulates body size in Drosophila. Development. 2009;136:983–993. doi: 10.1242/dev.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. Faseb J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Gorski SM, Chittaranjan S, Pleasance ED, Freeman JD, Anderson CL, Varhol RJ, Coughlin SM, Zuyderduyn SD, Jones SJ, Marra MA. A SAGE approach to discovery of genes involved in autophagic cell death. Curr Biol. 2003;13:358–363. doi: 10.1016/s0960-9822(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Grefte S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Skeletal muscle development and regeneration. Stem Cells Dev. 2007;16:857–868. doi: 10.1089/scd.2007.0058. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- Needham M, Mastaglia FL. Sporadic inclusion body myositis: a continuing puzzle. Neuromuscul Disord. 2008;18:6–16. doi: 10.1016/j.nmd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, Brech A. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:1065–1071. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR. Cytokines and Feeding. News Physiol Sci. 1998;13:298–304. doi: 10.1152/physiologyonline.1998.13.6.298. [DOI] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol. 2008;43:739–748. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Lindmo K, Juhasz G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev Cell. 2004;7:179–192. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12:1129–1135. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. Hsps and aging. Trends Endocrinol Metab. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Zheng J, Edelman SW, Tharmarajah G, Walker DW, Pletcher SD, Seroude L. Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci U S A. 2005;102:12083–12088. doi: 10.1073/pnas.0503374102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.