Abstract
The regulatory machinery that governs genetic and epigenetic control of gene expression for biological processes and cancer is organized in nuclear microenvironments. Strategic placement of transcription factors at target gene promoters in punctate microenvironments of interphase nuclei supports scaffolding of co-regulatory proteins and the convergence as well as integration of regulatory networks. The organization and localization of regulatory complexes within the nucleus can provide signatures that are linked to regulatory activity. Retention of transcription factors at gene loci in mitotic chromosomes contributes to epigenetic control of cell fate and lineage commitment, as well as to persistence of transformed and tumor phenotypes. Mechanistic understanding of the architectural assembly of regulatory machinery can serve as a basis for treating cancer with high specificity and minimal off-target effects.
Keywords: gene expression, nuclear structure, histone
I. INTRODUCTION
Genetic and epigenetic regulation of gene expression is operative in biological control of proliferation, growth, and phenotype. Both regulatory mechanisms synergistically contribute to compromised gene expression that is functionally linked to transformation and tumorigenesis. There is growing recognition that regulatory machinery is compartmentalized in subnuclear domains in which the components for combinatorial control are organized and assembled.1–12
Clinical relevance is emerging from well-documented modifications in the localization of regulatory machinery that govern transcription, replication, and repair in cancer cell nuclei that provide new dimensions to diagnosis and therapy.13,14 We review evidence that the architectural organization of genetic and epigenetic regulatory machinery is obligatory. We also emphasize strategies to mechanistically relate the focal organization of regulatory complexes with genetic and epigenetic parameters of control that are architecturally configured as integrated networks in the interphase nucleus (Fig. 1). In addition, we explore emerging indications that transcriptional machinery is retained at target gene loci of chromosomes during mitosis, epigenetically contributing to sustained competency for sustained expression of genes in progeny cells.
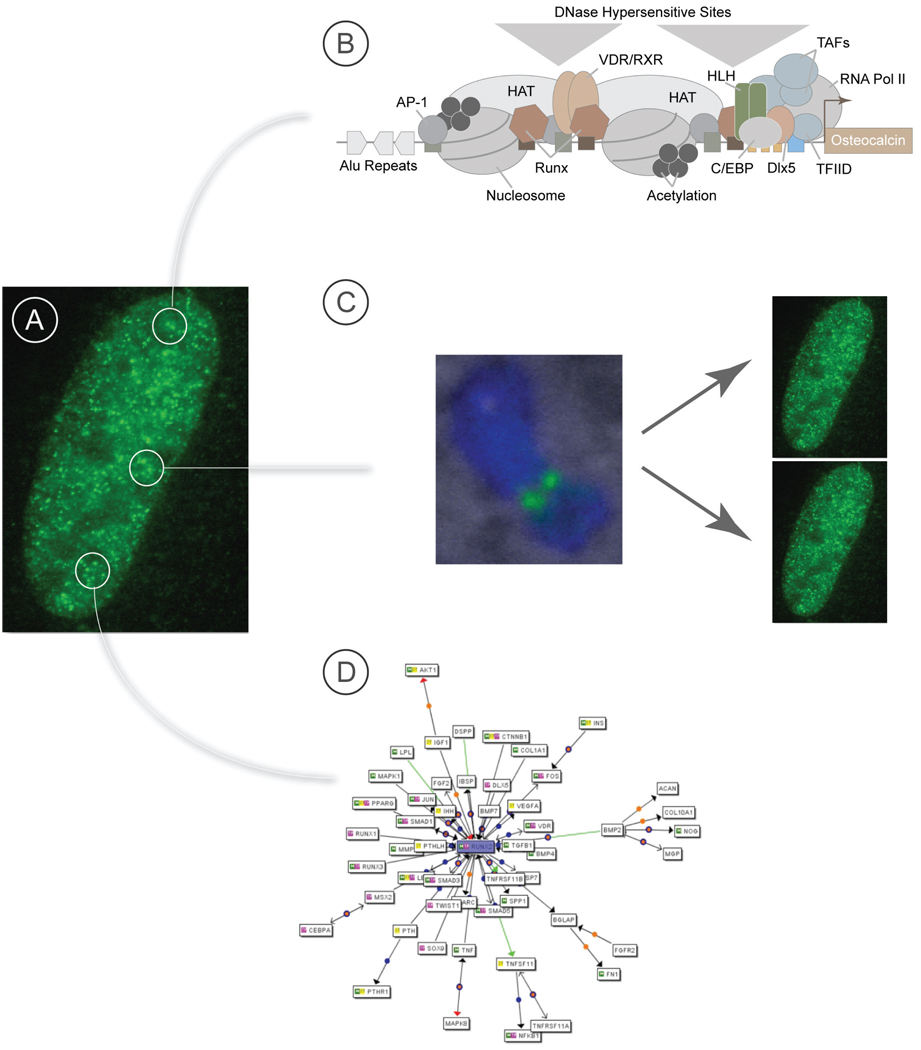
FIGURE 1.
An architectural perspective of genetic and epigenetic control in nuclear microenvironments. A schematic illustration of parameters to genetic and epigenetic control that are architecturally mediated. Panel (A) depicts a fluorescent micrograph showing the focal organization of RUNX regulatory machinery in nuclear microenvironments of the interphase nucleus. Using the RUNX transcription factors as a paradigm, the strategic localization of RUNX proteins at multiple sites of a target gene promoter are shown (B) where they strategically locate regulatory machinery that includes transcriptional co-activators and co-repressors, steroid hormone receptors, endpoints for signaling cascades and factors that contribute to epigenetic regulation by supporting histone modifications, chromatin remodeling and DNA methylation. Panel (C) illustrates the focal retention of RUNX transcription factors at target gene loci of chromosomes during mitosis, epigenetically conveying regulatory information for cell fate, lineage commitment and cell growth from parental to progeny cells. Panel (D) schematically depicts a RUNX regulatory network that supports the convergence and integration of regulatory signatures that contribute to RUNX-mediated biological control and RUNX control of aberrant gene expression in tumor cells.
II. AN ARCHITECTURAL PERSPECTIVE OF GENETIC AND EPIGENETIC REGULATION
A. Promoter Architecture
Combinatorial control of transcription involves the organization and assembly of regulatory complexes mediated by protein-DNA and protein-protein interactions at strategic sites of target gene promoters.15 The transcription factors bind to cognate regulatory sequences and provide scaffolds for recruitment and retention of co-regulatory proteins that include co-activators, co-repressors, steroid hormone receptors, and endpoints for signaling pathways. Transcription factor interactions with histone-modifying and chromatin-remodeling factors support the localization of epigenetic regulatory machinery for selectively influencing chromatin structure and nucleosome organization.16,17 Although the biochemistry of these components to epigenetic regulation has been characterized to a significant extent, there is a requirement to mechanistically define the placement of the components for epigenetic control that mediate physiologically responsive transcriptional activation and suppression. However, a mechanistic explanation for the fidelity of localization at promoter sites is minimally understood. The cross-talk between histone modifications, DNA methylation, and selective representation of histone subgroups to epigenetic control necessitates clarification. Further biochemical modifications, both independently and combinatorially with consideration of context, should not be dismissed.
The changes that occur during development and differentiation, differences that are cell type specific, and modifications that are observed in diseases that include cancer are conserved. There seems to be retention of architectural regulatory mediators of epigenetic control at genomic sites that require access to factors to determine the extent to which the genes are actively transcribed or suppressed. This architecturally based epigenetic control is exerted at two levels, which include both cause and effect. The first is placement of the factors that are required for architecturally configuring and remodeling genomes, whereas the second is the consequential remodeling of genomic DNA to accommodate demands for transcription.
Although it is evident that the orchestration of a complex cohort of regulatory factors is required for genetic and epigenetic control of gene loci, there is much to be learned about rate-limiting obligatory relationships that are proactive and responsive. As a strategy to probe mechanisms that render genes competent for expression or suppression in a physiologically responsive manner, the concept of signatures rather than single molecular determinants of control is becoming increasingly informative. With accruing insight into multiple levels of molecular organization contributing combinatorial control of gene expression, the informational contribution provided by architectural organization is becoming increasingly evident.18 The regulatory signatures that were once viewed as exclusive properties of nucleotide sequences in DNA have been extended to “codes” that are epigenetically based on histone modifications, 19,20 the selective use of histone subtypes, higher order genomic organization, and the configuration as well as localization of regulatory microenvironments within the cell nucleus.2,12
B. Nuclear Microenvironments
Regulatory machinery for biological control is not uniformly distributed throughout the nucleus. Rather, the nucleic acid and protein components of biological control are focally organized in specialized nuclear domains.12,21 These nuclear microenvironments are illustrated by two nucleoli where the regulatory machinery for ribosomal gene expression resides, chromosome territories where genes are localized in interphase nuclei, and sites of active transcription, processing of gene transcriptions, replication, and repair.
High-resolution strategies that incorporate antibodies to regulatory factors and in situ hybridization for detection of genes and transcripts, together with sophisticated microscopy, biochemistry and molecular approaches, have permitted the identification and functional characterization of regulatory domains within the interphase nucleus. These are dynamic, rather than static, subnuclear compartments that exhibit exchange and turnover of components to regulatory complexes in a physiologically responsive manner.
The identification of intranuclear trafficking signals in transcription factors that include RUNX/AML, AML/ETO, and glucocorticoid, estrogen, and androgen receptors provides examples of regulatory proteins that can begin to be understood in relation to mechanisms that are linked to obligatory nuclear localization. Aberrant or abortive proliferation, differentiation, and/or development in vivo point to obligatory relationships between nuclear organization and biological control. Together with regulatory signals for nuclear import, retention, and DNA binding, it is becoming apparent that multiple components of control are operative in the focal assembly of machinery for gene expression in nuclear microenvironments.18,22
Sophisticated imaging and algorithms that define localization of regulatory machinery, in relation to an extensive series of regulatory parameters that are context-dependent, provide a basis for configuring a signature to characterize a component of control that is retained and conveyed to progeny cells during mitosis, thereby contributing an architectural dimension to epigenetic control. 23,24 The architectural features of focally organized regulatory complexes are further illustrated by genomic and proteomic analyses that provide insight into the complex cohort of signals that converge at nuclear domains where regulatory networks support the integration of cues to initiate, sustain, or down-regulate biological processes as well as changes that occur in cancer.23–25
III. EPIGENETIC RETENTION OF REGULATORY MACHINERY DURING MITOSIS
The retention of transcription factors at target gene loci of mitotic chromosomes establishes a dimension to epigenetic control of cell fate and lineage commitment that complements DNA methylation and histone modifications.26 Osteogenic, myogenic, and adipogenic transcription factors have been shown to remain bound to promoter sequences during mitosis, supporting persistence of RNA polymerase-II-dependent and tissue-specific gene expression in progeny cells following cell division.27–30 Ribosomal genes also retain phenotypic transcription factors in nuclear organizing regions of chromosomes and in the interphase nucleoli, reflecting epigenetic control of RNA polymerase-I-dependent transcription to support cell growth and protein synthesis postmitotically.23,25 In addition to growing appreciation for epigenetic mechanisms, a potential obligatory relationship between cell cycle, growth, and phenotype is suggested.12
A fundamental question is the extent to which the cohort of co-regulatory proteins that are complexed with tissue-specific transcription factors are conveyed to progeny cells during mitosis. This architectural epigenetic component of control has implications for understanding the requirements to reinitiate transcription of tissue-specific genes following completion of mitosis to sustain the cellular phenotype. Recent results indicate that RUNX transcription factors associated with ribosomal genes during mitosis retain UBF,23 reflecting persistence of a principal RNA polymerase-I co-regulatory factor. Retention of TLE with RUNX during mitosis is another example of co-regulatory protein persistence.27,28 An architectural epigenetic perspective of mitotic control that accounts for the full complement of DNA-binding transcription factors and co-regulatory proteins that are retained at target gene promoters in mitotic cells can provide an indication of the extent to which progeny cells are poised to express or suppress genes that are consistent with requirements for specialized structure and function.2,12,31,32
There are additional examples of regulatory proteins that remain associated with target genes during mitosis epigenetically “bookmarking” genes33 for expression in progeny cells. Among these examples are the globin gene regulatory factor NF-E234,35 and HSF1,36 extending architecture-mediated epigenetic control beyond phenotypic transcription factors.37–42 However, as with other parameters of control, all transcription factors are not retained during cell division. The SP1-related regulatory proteins43 and HMG44 are two examples of factors that are genomically associated during interphase but not mitosis. Taken together, these observations provide a basis for a mechanism that can support commitment to lineages and/or specific phenotypes with a superimposed capability to modify parameters of control in a physiologically responsive manner.
In transformed and tumor cells, there is a similar requirement for retention of transcriptional competency to sustain a cancer phenotype. Retention of AML/ETO, transformation-fusion protein with RNA polymerase I and II target genes during mitosis epigenetically facilitates continued expression of genes that are conducive to transformation and/or tumor progression. It is realistic to anticipate that chromosomal retention of ALL45 may similarly contribute to epigenetic persistence of tumor-related transcription. The cohorts of regulatory and co-regulatory proteins that remain associated with target genes in tumor cells may provide signatures for diagnosis and prognosis and combinatorial blueprints for therapeutic targets.
IV. AN ARCHITECTURAL GENETIC AND EPIGENETIC LANDSCAPE
Further refinement is required of the rules that govern functional relationships between nuclear organization and fidelity of genetic and epigenetic regulation to support biological control and that are compromised in transformed and tumor cells. We are beginning to mechanistically understand the organization of regulatory machinery at target gene promoters and in functionally organized subnuclear domains. The mitotic retention of regulatory proteins with genes transcribed by RNA polymerase I and II indicate that transcription factor-mediated epigenetic control contributes to cell fate, lineage commitment, and cross-talk between control of cell growth and phenotype. An emerging concept is that transcriptional control requires organization and assembly of regulatory machinery in nuclear microenvironments where threshold levels of rate-limiting factors can support activation and suppression of genes and the required convergence and integration of regulatory networks that determine transcriptional responsiveness (Fig. 1).
An architectural underpinning for genetic and epigenetic control is supported by the genomic scaffolding of biochemical mediators for transcription replication, chromatin structure, nucleosome organization, and DNA methylation during interphase and mitosis. Architectural signatures that reflect specificity of localization for regulatory domains and perturbations that occur in tumor cells may represent targets for the detection and treatment of tumors.
Combining high-resolution microscopy, in situ gene analysis, and transcription regulatory complexes with characterization of the mitotic and interphase chromosomal proteome can be instructive. We can anticipate new dimensions to understanding regulatory mechanisms operative in control of gene expression that can support requirements for epigenetically retaining components of control with the superimposed capabilities for accommodating requirements for dynamic responsiveness to a broad spectrum of regulatory cues.
ACKNOWLEDGMENTS
The authors thank Patricia Jamieson for her assistance in the preparation of this article. This work was supported by National Institutes of Health grants P01 AR048818, P01 CA082834, and 5 P30 DK32520. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Cremer T, Kupper K, Dietzel S, Fakan S. Higher order chromatin architecture in the cell nucleus: on the way from structure to function. Biol Cell. 2004 Oct;96(8):555–567. doi: 10.1016/j.biolcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi SK, Young DW, Javed A, Pratap J, Montecino M, van Wijnen A, Lian JB, Stein JL, Stein GS. Nuclear microenvironments in biological control and cancer. Nat Rev Cancer. 2007 Jun;7(6):454–463. doi: 10.1038/nrc2149. [DOI] [PubMed] [Google Scholar]
- 3.Saltman LH, Javed A, Ribadeneyra J, Hussain S, Young DW, Osdoby P, Amcheslavsky A, van Wijnen AJ, Stein JL, Stein GS, Lian JB, Bar-Shavit Z. Organization of transcriptional regulatory machinery in osteoclast nuclei: compartmentalization of Runx1. J Cell Physiol. 2005 Apr 12;204(3):871–880. doi: 10.1002/jcp.20329. [DOI] [PubMed] [Google Scholar]
- 4.Vradii D, Doan DN, Wagner S, Nickerson JA, Lian JB, Stein JL, van Wijnen AJ, Imbalzano AN, Stein GS. Brg1, the ATPase subunit of SWI/SNF chromatin remodeling complex, is required for myeloid differentiation to granulocytes. J Cell Physiol. 2006;206:112–118. doi: 10.1002/jcp.20432. [DOI] [PubMed] [Google Scholar]
- 5.Zaidi SK, Javed A, Pratap J, Schroeder TM, Westendorf J, Lian JB, Lian JB, van Wijnen AJ, Stein GS, Stein JL. Alterations in intranuclear localization of Runx2 affect biological activity. J Cell Physiol. 2006 Sep 13;209(3):935–942. doi: 10.1002/jcp.20791. [DOI] [PubMed] [Google Scholar]
- 6.Hall LL, Byron M, Butler J, Becker KA, Nelson A, Amit M, Itskovitz-Eldor J, Stein J, Stein G, Ware C, Lawrence JB. X-inactivation reveals epigenetic anomalies in most hESC but identifies sublines that initiate as expected. J Cell Physiol. 2008;216:445–452. doi: 10.1002/jcp.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuardo M, Martinez M, Hidalgo K, Montecino M, Javed A, Lian JB, Stein GS, Stein JL, Gutiérrez SE. Altered chromatin modifications in AML1/RUNX1 breakpoint regions involved in (8;21) translocation. J Cell Physiol. 2009 Feb;218(2):343–349. doi: 10.1002/jcp.21599. [DOI] [PubMed] [Google Scholar]
- 8.Pande S, Ali SA, Dowdy C, Zaidi SK, Ito K, Ito Y, Montecino MA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Subnuclear targeting of the Runx3 tumor suppressor and its epigenetic association with mitotic chromosomes. J Cell Physiol. 2009 Mar;218(3):473–479. doi: 10.1002/jcp.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghule PN, Becker KA, Harper JW, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Cell cycle dependent phosphorylation and subnuclear organization of the histone gene regulator p220NPAT in human embryonic stem cells. J Cell Physiol. 2007;213(1):9–17. doi: 10.1002/jcp.21119. [DOI] [PubMed] [Google Scholar]
- 10.Stein GS, Lian JB, van Wijnen AJ, Stein JL, Javed A, Montecino M, Zaidi SK, Young D, Choi JY, Gutierrez S, Pockwinse S. Nuclear microenvironments support assembly and organization of the transcriptional regulatory machinery for cell proliferation and differentiation. J Cell Biochem. 2004 Feb 1;91(2):287–302. doi: 10.1002/jcb.10777. [DOI] [PubMed] [Google Scholar]
- 11.Stein GS, Davie JR, Knowlton JR, Zaidi SK. Nuclear microenvironments and cancer. J Cell Biochem. 2008 Aug 15;104(6):1949–1952. doi: 10.1002/jcb.21846. [DOI] [PubMed] [Google Scholar]
- 12.Zaidi SK, Young DW, Montecino M, Lian JB, van Wijnen AJ, Stein JL, Stein GS. Mitotic bookmarking of genes: a novel dimension to epigenetic control. Nat Rev Genet. 2010 Aug;11(8):583–589. doi: 10.1038/nrg2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lever E, Sheer D. The role of nuclear organization in cancer. J Pathol. 2010 Jan;220(2):114–125. doi: 10.1002/path.2651. [DOI] [PubMed] [Google Scholar]
- 14.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004 Sep;4(9):677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 15.Weake VM, Workman JL. Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet. 2010 Jun;11(6):426–437. doi: 10.1038/nrg2781. [DOI] [PubMed] [Google Scholar]
- 16.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 17.Cairns BR. The logic of chromatin architecture and remodelling at promoters. Nature. 2009 Sep 10;461(7261):193–198. doi: 10.1038/nature08450. [DOI] [PubMed] [Google Scholar]
- 18.Cook PR. A model for all genomes: the role of transcription factories. J Mol Biol. 2010 Jan 8;395(1):1–10. doi: 10.1016/j.jmb.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007 Dec;8(12):983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007 Nov;14(11):1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao R, Bodnar MS, Spector DL. Nuclear neighborhoods and gene expression. Curr Opin Genet Dev. 2009 Apr;19(2):172–179. doi: 10.1016/j.gde.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007 May 24;447(7143):413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 23.Young DW, Hassan MQ, Pratap J, Galindo M, Zaidi SK, Lee SH, Yang X, Xie R, Javed A, Underwood JM, Furcinitti P, Imbalzano AN, Penman S, Nickerson JA, Montecino MA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007;445(7126):442–446. doi: 10.1038/nature05473. [DOI] [PubMed] [Google Scholar]
- 24.Young DW, Zaidi SK, Furcinitti PS, Javed A, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Quantitative signature for architectural organization of regulatory factors using intranuclear informatics. J Cell Sci. 2004;117(Pt 21):4889–4896. doi: 10.1242/jcs.01229. [DOI] [PubMed] [Google Scholar]
- 25.Young DW, Hassan MQ, Yang X-Q, Galindo M, Javed A, Zaidi SK, Furcinitti P, Lapointe D, Montecino M, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic retention of gene expression patterns by the cell fate determining transcription factor Runx2. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3189–3194. doi: 10.1073/pnas.0611419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaidi SK, Young DW, Pockwinse SH, Javed A, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic partitioning and selective reorganization of tissue specific transcription factors in progeny cells. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14852–14857. doi: 10.1073/pnas.2533076100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali SA, Zaidi SK, Dacwag CS, Salma N, Young DW, Shakoori AR, Ali SA, Zaidi SK, Dacwag CS, Salma N, Young DW, Shakoori AR. Phenotypic transcription factors epigenetically mediate cell growth control. Proc Natl Acad Sci U S A. 2008 May 6;105(18):6632–6637. doi: 10.1073/pnas.0800970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali SA, Zaidi SK, Dobson JR, Shakoori AR, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Transcriptional corepressor TLE1 functions with Runx2 in epigenetic repression of ribosomal RNA genes. Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4165–4169. doi: 10.1073/pnas.1000620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakshi R, Zaidi SK, Pande S, Hassan MQ, Young DW, Montecino M, Lian JB, van Wijnen AJ, Stein JL, Stein GS. The leukemogenic t(8;21) fusion protein AML1-ETO controls ribosomal RNA genes and associates with nucleaolar organizing regions at mitotic chromosomes. J Cell Sci. 2008;21:3981–3990. doi: 10.1242/jcs.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakshi R, Hassan MQ, Pratap J, Lian JB, Montecino MA, van Wijnen AJ, Stein JL, Imbalzano AN, Stein GS. The human SWI/SNF complex associates with RUNX1 to control transcription of hematopoietic target genes. J Cell Physiol. 2010 Nov;225(2):569–576. doi: 10.1002/jcp.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou GL, Xin L, Liu DP, Liang CC. Remembering the cell fate during cellular differentiation. J Cell Biochem. 2005 Dec 1;96(5):962–970. doi: 10.1002/jcb.20572. [DOI] [PubMed] [Google Scholar]
- 32.Delcuve GP, Rastegar M, Davie JR. Epigenetic control. J Cell Physiol. 2009 May;219(2):243–250. doi: 10.1002/jcp.21678. [DOI] [PubMed] [Google Scholar]
- 33.John S, Workman JL. Bookmarking genes for activation in condensed mitotic chromosomes. BioEssays. 1998 Apr;20(4):275–279. doi: 10.1002/(SICI)1521-1878(199804)20:4<275::AID-BIES1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Martin DI, Orkin SH. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- 35.Whitelaw E, Tsai SF, Hogben P, Orkin SH. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarge KD, Park-Sarge OK. Mitotic bookmarking of formerly active genes: keeping epigenetic memories from fading. Cell Cycle. 2009 Mar 15;8(6):818–823. doi: 10.4161/cc.8.6.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009 Dec;20(23):4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delcuve GP, He S, Davie JR. Mitotic partitioning of transcription factors. J Cell Biochem. 2008 Sep 1;105(1):1–8. doi: 10.1002/jcb.21806. [DOI] [PubMed] [Google Scholar]
- 39.Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat Rev Mol Cell Biol. 2008 Jul;9(7):505–516. doi: 10.1038/nrm2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing H, Wilkerson DC, Mayhew CN, Lubert EJ, Skaggs HS, Goodson ML, Hong Y, Park-Sarge OK, Sarge KD. Mechanism of hsp70i gene bookmarking. Science. 2005 Jan 21;307(5708):421–423. doi: 10.1126/science.1106478. [DOI] [PubMed] [Google Scholar]
- 41.Yan J, Xu L, Crawford G, Wang Z, Burgess SM. The forkhead transcription factor FoxI1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol Cell Biol. 2006 Jan;26(1):155–168. doi: 10.1128/MCB.26.1.155-168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke LJ, Zhang R, Bartkuhn M, Tiwari VK, Tavoosidana G, Kurukuti S, Weth C, Leers J, Galjart N, Ohlsson R, Renkawitz R. CTCF binding and higher order chromatin structure of the H19 locus are maintained in mitotic chromatin. EMBO J. 2005 Sep 21;24(18):3291–3300. doi: 10.1038/sj.emboj.7600793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He S, Davie JR. Sp1 and Sp3 foci distribution throughout mitosis. J Cell Sci. 2006 Mar 15;119(Pt 6):1063–1070. doi: 10.1242/jcs.02829. [DOI] [PubMed] [Google Scholar]
- 44.Hock R, Scheer U, Bustin M. Chromosomal proteins HMG-14 and HMG-17 are released from mitotic chromosomes and imported into the nucleus by active transport. J Cell Biol. 1998 Dec 14;143(6):1427–1436. doi: 10.1083/jcb.143.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blobel GA, Kadauke S, Wang E, Lau AW, Zuber J, Chou MM, Vakoc CR. A reconfigured pattern of MLL occupancy within mitotic chromatin promotes rapid transcriptional reactivation following mitotic exit. Mol Cell. 2009;36(6):970–983. doi: 10.1016/j.molcel.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]