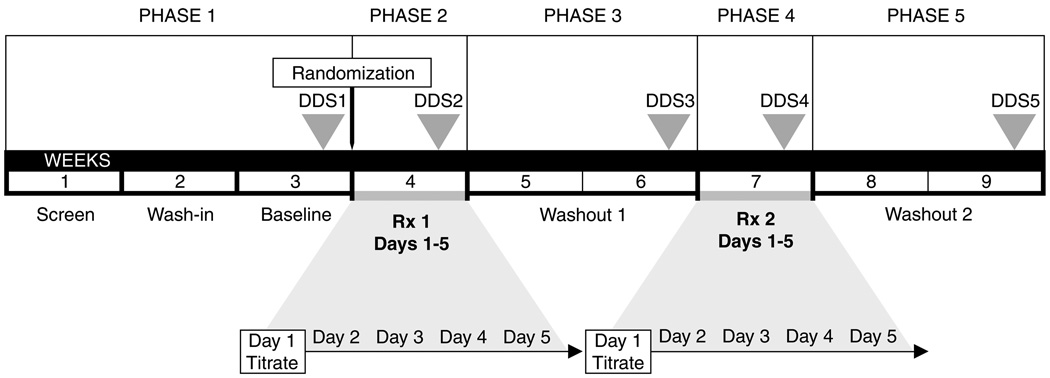
Figure 1.
Study Schema. After screening, eligible subjects were randomized to receive cannabis or placebo first (treatment week 1; Rx 1), followed by the alternative treatment (treatment week 2; Rx 2). The principal measure of pain, the Descriptor Differential Scale (DDS), was measured at five time points (DDS1–5; arrowheads). The primary outcome was the difference in DDS change from baseline (DDS1) to the end of each treatment (active or placebo) week (DDS2/4). Remaining DDS assessments (3, 5) were used in secondary analyses. During each day of the 5-day treatment week, subjects smoked cannabis or placebo cigarettes four times daily. On day 1 of each week, cannabis dose was titrated to efficacy and tolerability as described in the text. On the remaining days (2–4), subjects smoked the maximum tolerated dose achieved on day 1.