Abstract
Coenzyme Q (ubiquinone or Q) functions in the respiratory electron transport chain and serves as a lipophilic antioxidant. In the budding yeast Saccharomyces cerevisiae, Q biosynthesis requires nine Coq proteins (Coq1–Coq9). Previous work suggests both an enzymatic activity and a structural role for the yeast Coq7 protein. To define the functional roles of yeast Coq7p we test whether Escherichia coli ubiF can functionally substitute for yeast COQ7. The ubiF gene encodes a flavin-dependent monooxygenase that shares no homology to the Coq7 protein and is required for the final monooxygenase step of Q biosynthesis in E. coli. The ubiF gene expressed at low copy restores growth of a coq7 point mutant (E194K) on medium containing a non-fermentable carbon source, but fails to rescue a coq7 null mutant. However, expression of ubiF from a multicopy vector restores growth and Q synthesis for both mutants, although with a higher efficiency in the point mutant. We attribute the more efficient rescue of the coq7 point mutant to higher steady state levels of the Coq3, Coq4, and Coq6 proteins and to the presence of demethoxyubiquinone, the substrate of UbiF. Coq7p co-migrates with the Coq3 and Coq4 polypeptides as a high molecular mass complex. Here we show that addition of Q to the growth media also stabilizes the Coq3 and Coq4 polypeptides in the coq7 null mutant. The data suggest that Coq7p, and the lipid quinones (demethoxyubiquinone and Q) function to stabilize other Coq polypeptides.
Coenzyme Q (ubiquinone or Q)3 is a prenylated benzoquinone lipid that is found in membranes throughout eukaryotic cells. The reversible redox chemistry of Q is responsible for its function in the respiratory electron transport chain of inner mitochondrial membranes, where it transports electrons from complexes I and II to complex III. Q is an acceptor of electrons from many cellular dehydrogenases involved in the oxidative metabolism of dihydroorotate, choline, fatty acyl-CoA, glycerolphosphate, sarcosine, and dimethylglycine (1). The hydroquinone or reduced form of Q (QH2) serves as a lipophilic antioxidant in cellular membranes and in lipoprotein particles (2). There is increasing interest in the use of Q as a nutritional supplement, and a recent study indicates that high doses of Q may help slow the progression of Parkinson disease (3).
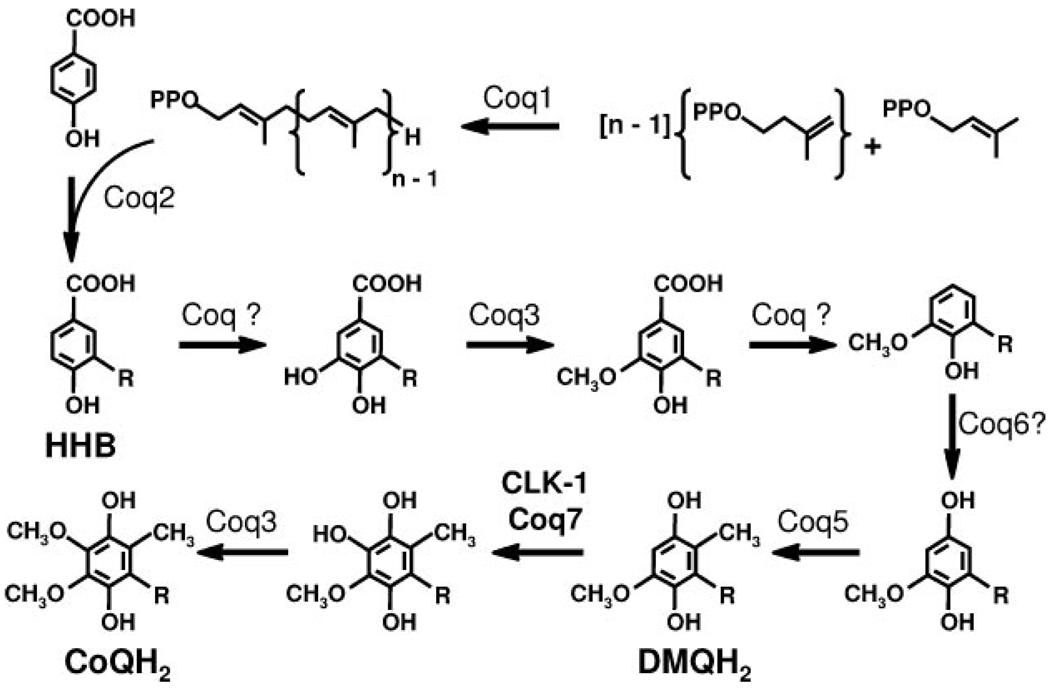
Cells commonly rely on de novo synthesis for their supply of Q. A proposed eukaryotic Q biosynthetic pathway is shown in Fig. 1. The number of the prenyl units in the hydrophobic tail varies among different organisms. In the budding yeast Saccharomyces cerevisiae, Q-deficient mutant strains have been categorized into nine complementation groups, coq1–coq9 (4, 5). The coq mutants are nuclear petite (ρ+) and non-respiring, and hence are unable to grow in media containing a non-fermentable carbon source such as ethanol or glycerol. The same early intermediate 3-hexaprenyl-4-hydroxybenzoic acid (HHB), the product of the Coq2 protein, accumulates in each of the coq null mutants (5–7). Several studies (8–11) have provided genetic evidence indicating that in S. cerevisiae, a complex of Coq polypeptides is required to convert 3-hexaprenyl-4-hydroxybenzoic acid to Q. The recent identification of a high molecular weight complex containing the Coq3, Coq4, and Coq6 polypeptides supports this model of a multienzyme Q biosynthetic complex (12).
FIGURE 1. Proposed eukaryotic Q biosynthetic pathway.
There are nine identified Coq proteins in S. cerevisiae ascribed the following functions: Coq1, hexaprenyl-diphosphate synthase; Coq2, 4-hydroxybenzoate:hexaprenyl-diphosphate transferase; Coq3, O-methyltransferase; Coq5, C-methyltransferase; Coq6, potential flavin-dependent monooxygenase; and Coq7/CLK-1, putative di-iron hydroxylase catalyzing the last hydroxylase step. The function of Coq4, Coq8, and Coq9 are unknown. The number of isoprene units in the tail of Q is designated by n; n = 6 in S. cerevisiae, 9 in C. elegans, and 10 in Homo sapiens.
Yeast Coq7/Cat5 was shown to be required for the hydroxylation of DMQ6 (13–15). Interestingly, the coq7-1 point mutant (G65D) accumulates DMQ6, whereas the coq7 null mutant produces only the earlier intermediate 3-hexaprenyl-4-hydroxybenzoic acid (15). Similarly, yeast mutants expressing Coq7p with a missense mutation (E194K) produced DMQ6, whereas DMQ6 was not detected in strains harboring a coq7-nonsense mutation (where the carboxyl-terminal half of the protein is missing) (16). These studies suggest that Coq7 is either involved in one or more monooxygenase steps or serves as an essential component of the putative multisubunit enzyme complex. Previous studies have offered indirect evidence indicating that yeast Coq7 functions as a hydroxylase responsible for the conversion of DMQ6 to Q6 at the penultimate step in the biosynthesis of Q. The yeast Coq7 protein, as well as its homologues in human and Caenorhabditis elegans (CLK-1), was shown to contain a conserved di-iron-binding motif found in alternative oxidase, the steroyl-CoA desaturase, and methane and toluene monooxygenases (17, 18) (Fig. 2). Coq7 homologues from Pseudomonas aeruginosa, Thiobacillus ferroxidans, and C. elegans were demonstrated to complement an Escherichia coli mutant with defects in ubiF (18, 19). E. coli lacks a homologue of COQ7/CLK-1 and instead the hydroxylation of DMQ8 in E. coli Q biosynthesis depends on UbiF, a flavin-dependent monooxygenase (20).
FIGURE 2. Identification of mutations in yeast COQ7.
Amino acid sequence of Coq7 from S. cerevisiae (NCBI accession number NP_014768), C. elegans (AAG00035), H. sapiens (H. sap; NP_057222), Mus musculus (M. mus; AAH38681), Drosophila melanogaster (D. mel; NP_651967), Pseudomonas aeruginosa (P. aer; AAG04044), Rickettsia prowazekii (R. pro; CAA14656) were aligned with the Clustal method in the Megalign program of DNASTAR. Identical residues are shaded black. The previously proposed Coq7 amino acid sequence of S. cerevisiae (X82930) was modified by the Saccharomyces Genome Data base, which predicts that translation initiated at the second in-frame ATG codon of the original reported ORF, leading to exclusion of the first 39 amino acids. Consequently, the G104D and E233K mutations noted previously (15, 16) are now designated as G65D and E194K, respectively. Seven distinct mutations in the COQ7 ORF were identified via sequencing of amplified genomic DNA segments of nine mutant strains from the G64 (coq7) complementation group (4) and amino acid substitution mutations are designated by arrows: G185A (G62D) (coq7-2, N183 and E2–196), G194A (G65D) (coq7-1, NM101) (15), G215A (G72D) (coq7-3, N558), G580A (E194K) (coq7-4, C104), and C589T (H197K) (coq7-5, W202). Asterisks designate nonsense mutations: G276A (W92STOP) (coq7-6, C291 and E4-140) and G360A (W120STOP) (coq7-7, N49). The region predicted to be adjacent to the DMQ binding pocket is designated by a bracket; predicted ligands to the di-iron center (EXXH) are in gray.
The goal of this study was to define the multiple functional roles of yeast Coq7p. Here we characterize eight independent coq7 mutants and identify five new mutations. We demonstrate that many of these mutations affect residues predicted to function as ligands of the di-iron center, or affect invariant residues predicted to be adjacent to the DMQ6 substrate binding pocket. To compare the coq7 point mutant phenotypes with that of the wild-type and the coq7 null mutant, a stable integrant was generated where the E194K mutation is expressed from the endogenous COQ7 chromosomal locus. The phenotypes were then evaluated with respect to production of Q6, DMQ6, and rescue by expression of the E. coli ubiF monooxygenase. Because E. coli UbiF is unlikely to interact with the yeast Coq polypeptides, the interspecific complementation analyses with UbiF could help define the functional roles of the Coq7 polypeptide. Evidence is presented that Coq7p, its lipid substrate DMQ6, and Q6 itself, play important roles in stabilizing other Coq polypeptides, and that Coq7p is a member of the high molecular weight Coq biosynthetic complex.
EXPERIMENTAL PROCEDURES
Yeast Strains and Growth Media
The yeast strains used in this study are listed in Table 1. Growth media for yeast were prepared as described (21) and included SDC (0.18% yeast nitrogen base without amino acids, 2% dextrose, 0.14% NaH2PO4, 0.5% N2H8SO4, and complete amino acid supplements), SD-Leu (SDC, but amino acid supplement lacks leucine), YPD (1% yeast extract, 2% peptone, 2% dextrose), YPG (1% yeast extract, 2% peptone, 3% glycerol), YPGal (1% yeast extract, 2% peptone, 2% galactose, 0.1% dextrose), YPE (1% yeast extract, 2% peptone, 2% ethanol), and YPEG (1% yeast extract, 2% peptone, 3% glycerol, 2% ethanol). E. coli were grown in Luria-Bertani (LB) broth. When required, antibiotics were added to final concentrations of 100 µg/ml ampicillin, 50 µg/ml kanamycin, and 200 µg/ml G418. Solid media contained 2% Difco Bacto agar. Yeast and bacteria were grown at 30 and 37 °C, respectively.
TABLE 1. Genotypes and sources of S. cerevisiae strains.
| Strain | Genotype | Source |
|---|---|---|
| JM43 | MATα leu2-3,112 ura3-52 trp1-289 his4-580 | Ref. 41 |
| JM43Δcoq7-1 (JM43Δcoq7) | MATα leu2-3,112 ura3-52 trp1-289 his4-580 coq7Δ::LEU2 | Ref. 15 |
| E194KCoq7 | MATα leu2-3,112 ura3-52 trp1-289 his4-580 coq7 G580A (KAN) | This work |
| WtCoq7 | MATα leu2-3,112 ura3-52 trp1-289 his4-580 COQ7 (KAN) | This work |
| NM101 | MATa leu2-3,112 ura3-52 coq7-1 | Ref. 15 |
| W303-1A | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-52 | –a |
| N183 | MATα coq7-2 | –b |
| E2-196 | MATα coq7-2 | –b |
| N558 | MATα coq7-3 | –b |
| C104 | MATα coq7-4 | –b |
| W202 | MATα coq7-5 | –b |
| C291 | MATα coq7-6 | –b |
| E4-140 | MATα coq7-6 | –b |
| N49 | MATα coq7-7 | –b |
Dr. R. Rothstein, Department of Human Genetics, Columbia University, New York.
Dr. A. Tzagoloff, Department of Biological Services, Columbia University, New York.
Plasmid Construction
The pQMF plasmid is a low copy yeast shuttle vector containing E. coli ubiF with a 35 amino-terminal mitochondrial targeting sequence from yeast COQ3, expressed from the yeast CYC1 promoter. The ORF of ubiF was amplified via PCR from the genomic DNA of E. coli DH5α (Invitrogen), with primers pTUP1 (5′-TTGGCGCGCCTATGACAAATCAACCA-3′) and pTUP2 (5′-TTGGCGCGCCCTACAACCCTAACGC-3′). These primers contained an AscI restriction site (underlined) and 15 nucleotides corresponding to either the 5′ end or complementary to the 3′ end of the ORF. The resulting 1.176-kb product was digested with AscI and ligated into the MluI site of pQM (22) to form the vector pQMF. The pQM plasmid contains the yeast CYC1 promoter (23) and the first 105 nucleotides from the COQ3 ORF, encoding a proposed mitochondrial targeting sequence (24) in the low copy yeast CEN-based shuttle vector pRS316 (25). The multicopy yeast shuttle vector expressing E. coli ubiF, pCHF, was constructed by digestion of pQMF with BamHI and KpnI to release the intact CYC1 promoter-COQ3 mitoleader-ubiF ORF DNA fragment, and ligation of this fragment to the high copy number vector pRS426 (26).
Identification of coq7 Mutations via Genomic Sequencing
Genomic DNA from each of eight mutant strains from the coq7 (G64) complementation group (4) and its parental wild-type strain, D273-10B/A1, was isolated with the Wizard Genomic DNA kit (Promega). The coq7 locus was amplified from the genomic DNA with primers annealing 231 base pairs upstream and 177 base pairs downstream of the COQ7 ORF: pUPQ7PCR forward (5′-GAATGCAGTAATCCTTTGTACG-3′) and pUPQ7PCR reverse (5′-CCTGTCATATTGGTCATATAGCG-3′). Nucleotide sequencing of the DNA fragments was performed by the University of California at Los Angeles (UCLA) Sequencing Core with the two PCR primers and three internal primers: pQ7seqF2 (5′-TTTGTGGAAGGCAGGAGC-3′), pQ7seqR1 (5′-CATTGTAGTGCCCTCCGATT-3′), and pQ7seqF1 (5′-CACGTATACGGGAGAGATACATAG-3′).
Construction of the E194KCoq7 Integrant and Its Wild-type Control, WtCoq7
The loxP-Kan-loxP DNA fragment was PCR amplified from pUG6 (27) with the primers ploxPu (RI) Kan (5′-CCGGAATTCTGAAGCTTCGTACGCTGCAGG-3′) and ploxPdKanBamHI (5′-CGGGATCCTAACCTTAAGATGATAAATAGGAATTTTAACAATATCATCAAAAtagtggatctgatatcaccta-3′). The ploxPu (RI) Kan primer contained an EcoRI restriction site (underlined) and +16 to +38 of the pUG6 plasmid sequence; the ploxPdKanBamHI primer contained a BamHI restriction site (underlined), downstream region of the COQ7 ORF (+892 to +936 from the stop codon, bold), and +1615 to +1593 of the pUG6 plasmid sequence. The resulting 1.6-kb Kan-cassette was digested with EcoRI and BamHI and was then ligated to the EcoRI and BamHI cut pNMQ71 (15) or pe2519 (16) to generate pCoq7-Kan or pCoq7E194K-Kan, respectively. pCoq7-Kan contains a wild-type COQ7 ORF tagged at the 3′ terminus with the Kan/G418 resistance marker; pCoq7E194K-Kan contains G580A coq7 ORF also tagged at the 3′ terminus with the Kan/G418 resistance marker. The E194KCoq7 and WtCoq7 integrants were constructed by a one-step gene replacement/integration procedure (28). In brief, the JM43Δcoq7 strain was transformed with a 3.7-kb PvuII fragment obtained from either pCoq7-Kan or pCoq7E194K-Kan. To generate the WtCoq7 integrant, the previously disrupted COQ7 segment and downstream non-essential gene UBP2 (29) of the genome was replaced with the COQ7 (KAN) fragment via homologous recombination leading to loss of the LEU2 marker, gain of the Kan/G418 resistance marker, and restored ability to grow on YPG media. This strain was designated as WtCoq7 after verifying the replacement of the disrupted COQ7 locus by PCR analysis. G418R transformants obtained via transformation with the coq7 G580A (KAN) fragment failed to grow on SD-Leu and YPG media and were selected and designated as E194KCoq7 after verifying the replacement of the disrupted COQ7 locus by PCR analysis and sequencing.
Generation of Yeast Coq7 Antibodies
E. coli strain BL21-CodonPlus (DE3)-RIL competent cells (Stratagene) were transformed with a plasmid encoding a glutathione S-transferase-Coq7 fusion protein (13). The fusion protein was induced by 0.5 mm isopropyl-β-d-thiogalactoside (Fisher Biotech) at 30 °C for ~3 h. The insoluble fraction of the bacterial lysate was separated by preparative SDS-PAGE. The 55-kDa GST-Coq7 fusion protein was visualized by copper staining (30), and eluted from the gel by diffusion (31). The recovered protein was identified as the GST-Coq7 fusion by mass spectrometry analysis of tryptic fragments, and rabbit antisera was obtained by a standard immunization protocol (Animal Pharm Services).
Mitochondrial Isolation and Western Blot Analysis
Yeast strains were grown in YPGal media to an optical density (A600) of 3–5. Preparation of spheroplasts and cell lysate fractionation were performed as described (32). Crude mitochondria were isolated and further purified over a linear Nycodenz gradient as described previously (33). Protein concentrations were determined by the bicinchoninic acid assay (Pierce). Indicated amounts of protein from the Nycodenz-purified mitochondrial fractions were separated by electrophoresis on 12% acrylamide, 2.5 m urea, Tris glycine gels, and then transferred to Hybond ECL nitrocellulose (Amersham Biosciences). Subsequent immunoblot analyses and treatment of membranes were as described earlier (34). Primary antibodies to yeast mitochondrial polypeptides were used at the following concentrations: Coq1, 1:10,000; Coq3, 1:1000; Coq4, 1:2000; Coq5, 1:5000; Coq6, 1:500; Coq7, 1:1000; Cytb2, 1:5000; Cytc, 1:5000; Cytc1, 1:800; F1β-ATPase, 1:10000; and Rip1, 1:10000. Goat anti-rabbit and goat anti-mouse secondary antibodies conjugated to horseradish peroxidase (Calbiochem) were each used at a 1:10,000 dilution.
Lipid Extraction and Quantification of Quinones
Lipid extraction and analyses of quinone content by HPLC/ECD were as described (35), with the following modifications. Q4 was added to yeast cell pellets (1.6 pmol/mg wet weight) to provide an internal standard. Lipids were extracted with 9 ml of methanol and 6 ml of petroleum ether, and the first extraction was performed overnight at 4 °C on a shaking platform (200 rpm). Chromatographic separations were performed with a Beta basic 5-mm, 4.6 × 150-mm C18 column (Thermo) with MeOH:H2O (98:2), 10 mm lithium perchlorate as mobile phase. External standards were injected at the beginning and end of the HPLC/ECD analyses. Standards consisted of a stock solution containing DMQ3 (100 pmol/µl), Q4 (75 pmol/µl), and Q6, (75 pmol/µl). To generate a standard curve, 10 µl of the stock solution, or 10 µl of 2-, 10-, and 30-fold dilutions were injected. Quinones were quantified with an ESA Coulochem II electrochemical detector. A precolumn electrode was set to 650 mV, and the analytical cell electrodes were E1, −650 mV, and E2, 550 mV. Limits of detection for Q6 and DMQ3 were 0.5 and 0.75 pmol/injection, respectively. For each analysis a volume of lipid extract representing 50–75 mg wet weight cell pellet was injected. Yeast were cultured in YPGal to A600 = 4–6, or where stated, in YPG.
Detergent Extraction, Gel Filtration, and Methyltransferase Assays
Purified mitochondria were isolated from W303-1A, 2 mg were solubilized with digitonin (Biosynth International, Switzerland) and the 100,000 × g supernatant was subjected to gel filtration as described (12). Fractions were collected and analyzed for polypeptide content by Western blot analysis and for Coq3 O-methyltransferase activity as described (12).
Mitochondrial Solubilization and Blue Native (BN)-PAGE Analysis
Nycodenz-purified mitochondria were isolated from JM43, E194KCoq7, JM43Δcoq7, and JM43Δcoq7 grown in the presence of exogenous Q6. 300 µg of purified mitochondria was solubilized by digitonin as described previously (12), except that the final concentration of Pefabloc was 0.4 mm. The 100,000 × g supernatant was subjected to BN-PAGE (5–13.5%) in the first dimension and SDS-PAGE (10–13%) in the second dimension as described (12). Molecular mass calibration standards for native electrophoresis (Amersham Biosciences) were as follows: thyroglobulin, 669 kDa; ferritin, 440 kDa; catalase, 232 kDa; lactase dehydrogenase, 140 kDa; and bovine serum albumin, 67 kDa.
RESULTS
Sequence Analysis of Mutants from the G64/coq7 Complementation Group
Eight different mutants in the G64/coq7 complementation group (4) whose coq7 gene defects had not previously been characterized were studied: C104, C291, E2-196, E4-140, N49, N183, N558, and W202. These mutants were obtained via mutagenesis of the parental wild-type strain D273-10B/A1 (36, 37). To identify the mutation(s) in the COQ7 coding region of these mutants, DNA segments surrounding the ORF plus 231 bp of 5′ flanking and 177 bp of 3′ flanking sequences were amplified from the corresponding genomic DNA. Sequence analysis revealed that each mutant harbored a unique nucleotide substitution, resulting in the following amino acid changes: E194K in C104; W92STOP in C291 and E4-140; G62D in E2-196 and N183; W120STOP in N49; G72D in N558; and H197K in W202 (Fig. 2). The W92STOP and W120STOP nonsense mutations would result in truncated and inactive Coq7 proteins. The G62D and G72D mutations, like the previously characterized G65D mutation (15), affect invariant residues present in the region predicted to be adjacent to the DMQ binding pocket (18). The G65D mutation would be expected to interfere with the predicted function of the adjacent Glu-63 residue as an iron atom ligand. The E194K and H197K mutations affect other invariant residues predicted to function as ligands to the di-iron center, EXXH (18). In particular, the Glu-194 residue, corresponding to Glu-148 in C. elegans and Glu-178 in P. aeruginosa, was proposed to function as a bridge between the two iron atoms of the di-iron center, and thus is predicted to be an essential residue for the formation of a stable di-iron site (18). Our previous work showed that coq7 null mutants harboring the coq7E194K gene on either low or multicopy plasmids were respiratory defective, lacked Q6, and accumulated relatively large amounts of DMQ6 (16). Because this DMQ6 accumulation indicates the retention of the other Coq polypeptides, we chose to utilize this mutation for further analyses.
Stable Integration of the coq7E194K Mutation
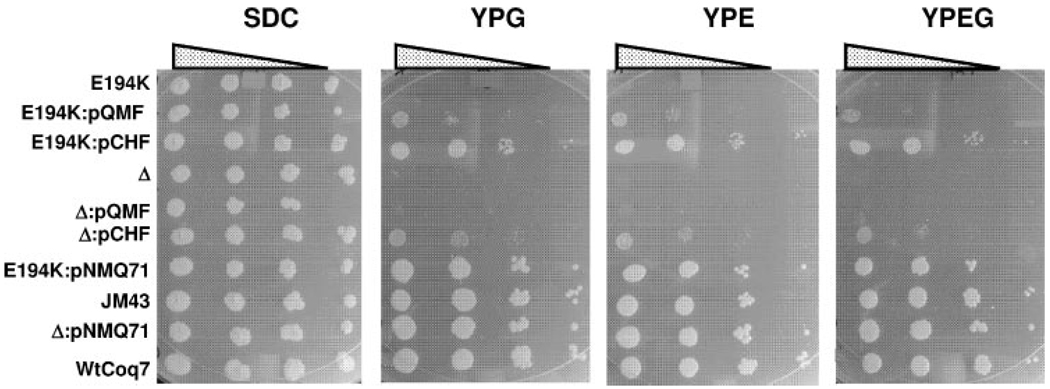
The coq7 mutants described above were generated by chemical mutagenesis and may contain other genetic defects. This was apparent in the strain C104 (coq7-2, E194K) as it was observed to grow slowly on SDC media. To compare the phenotype of the yeast strain harboring the coq7E194K mutation with that of the wild-type yeast, stable integrants containing either the coq7E194K mutation or the COQ7 wild-type gene were prepared as described under “Experimental Procedures.” The yeast strain E194KCoq7 contains one copy of the coq7E194K point mutation, integrated at the COQ7 locus. Similarly, the wild-type control integrant WtCoq7 was prepared. These strains are isogenic, except for the presence of either the integrated coq7E194K point mutation or the integrated wild-type COQ7 allele. E194KCoq7 and the coq7 null mutant fail to grow on media containing the non-fermentable carbon sources glycerol or ethanol, but grow well on media containing glucose (SDC, Fig. 3). As expected, both the JM43 parental strain, and the wild-type control integrant (WtCoq7) grow on nonfermentable carbon sources.
FIGURE 3. Expression of E. coli UbiF rescues the E194KCoq7 point mutant more effectively than the coq7 null mutant.
Each strain was grown overnight in selective media and serial dilutions were spotted on SDC, YPG, YPE, and YPEG plate media, at a final optical density 600 nm (A600) of 0.2, 0.02, 0.002, or 0.0002. Growth is depicted after 6 days at 30 °C. E194K, Δ, and WtCoq7, represent E194KCoq7, the coq7 null mutant (JM43Δcoq7-1) or the wild-type integrant, respectively. JM43 designates the parental wild-type strain. Yeast strains were transformed with pQMF, a low copy plasmid expressing E. coli UbiF; pCHF, a multicopy plasmid expressing E. coli UbiF; or pNMQ71, a low copy plasmid containing the yeast COQ7 gene.
Complementation of Yeast coq7 Null and E194KCoq7 Mutants by the E. coli ubiF Gene
E. coli ubiF encodes a predicted flavin-dependent monooxygenase, required for the hydroxylation of DMQ in E. coli Q biosynthesis (20). Although E. coli UbiF shares no homology with other prokaryotic or eukaryotic Coq7 homologs, expression of Coq7 homologs have been shown to rescue Q biosynthesis in an ubiF E. coli mutant (18, 19). To investigate whether the E. coli ubiF gene could functionally substitute for yeast COQ7, UbiF expression constructs in low copy (pQMF) and multicopy (pCHF) yeast shuttle plasmids were tested for the ability to restore growth of the coq7 null and E194KCoq7 yeast mutants on plate media containing a nonfermentable carbon source. As expected, all tested yeast strains grew well on SDC plate media (Fig. 3). Whereas expression of E. coli ubiF from a multicopy vector restored growth of both the E194KCoq7 point and the coq7 null mutants on medium containing a non-fermentable carbon source, the expression of ubiF from a low copy yeast shuttle vector rescued only E194KCoq7. The low copy ubiF-mediated rescue of E194KCoq7 but not the coq7 null mutant was also observed in growth assays performed in YPG liquid media (data not shown).
Restoration of Q6 Biosynthesis in coq7 Null and E194KCoq7 Mutants Harboring ubiF Expressing Constructs
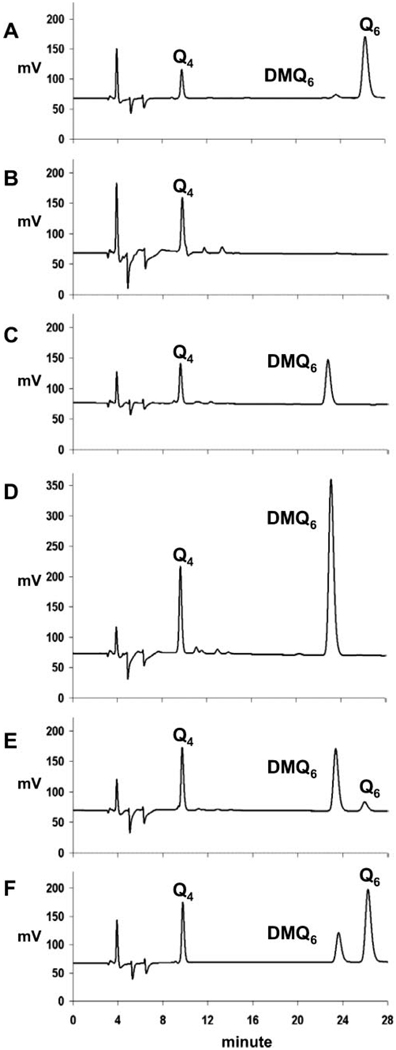
To examine the effects of E. coli UbiF protein expression on Q content, lipid extracts were prepared from yeast cultured in media containing galactose (YPGal). Galactose serves as a fermentable, nonrepressing carbon source and enables the comparison of respiratory competent and incompetent strains of yeast. The Q6 and DMQ6 content of WtCoq7, E194KCoq7, and coq7 null mutant yeast strains harboring the ubiF expression plasmids were determined by HPLC and ECD analysis of cell lipid extracts (Fig. 4 and Table 2). As expected, Q6 is the predominant quinone species in the WtCoq7 yeast strain, although a small amount of DMQ6 is detected (Fig. 4A). The null coq7 mutant failed to synthesize detectable amounts of either Q6 or DMQ6 (Fig. 4B). E194KCoq7 contained DMQ6, but Q6 was not detectable (Fig. 4C). A small amount of Q6 was detected in E194KCoq7:pCHF (harboring the multicopy ubiF expressing construct) (Fig. 4E). E194K:pNMQ71 (harboring wild-type COQ7) produced predominantly Q6 (Fig. 4F), although DMQ6 was present at higher quantities as compared with the WtCoq7 integrant control strain (Fig. 4A, Table 2).
FIGURE 4. Assays of Q6 and DMQ6 in coq7 null and E194KCoq7 mutants harboring low copy and multicopy plasmids expressing E. coli ubiF.
All strains were cultured in YPGal media. Lipid extracts of cell pellets were prepared and analyzed for quinone content by HPLC/ECD as described under “Experimental Procedures.” The elution positions of Q4, Q6, and DMQ6 are indicated: WtCoq7 (A), JM43Δcoq7 (B), E194KCoq7 (C), E194KCoq7:pQMF (D), E194KCoq7:pCHF (E), and E194KCoq7:pNMQ71 (F). The plasmid descriptions are as follows: pQMF, a low copy plasmid expressing E. coli UbiF; pCHF, a multicopy plasmid expressing E. coli UbiF; and pNMQ71, a low copy plasmid containing the yeast COQ7 gene.
TABLE 2. Amounts of Q6 and DMQ6 in Δcoq7, E194K, and WtCoq7 yeast strains in the presence and absence of E. coli ubiF.
Total lipid was extracted from whole yeast cultured in YPGal and quinones were quantified as described under “Experimental Procedures.” Results are expressed as the average of three injections ± S.D. Quinone content was expressed as pmol/mg wet weight yeast cell pellet.
| Strain | Q6 | DMQ6 |
|---|---|---|
| pmol/mg wet weight | ||
| JM43Δcoq7 | NDa | ND |
| JM43Δcoq7:pQMF | ND | 0.23 ± 0.02 |
| JM43Δcoq7:pCHF | ND | ND |
| E194KCoq7 | ND | 8.61 ± 0.15 |
| E194KCoq7:pQMF | ND | 13.7 ± 0.53 |
| E194KCoq7:pCHF | 0.75 ± 0.06 | 7.65 ± 0.35 |
| E194KCoq7:pNMQ71 | 12.7 ± 0.36 | 6.21 ± 0.19 |
| WtCoq7 | 27.1 ± 2.0 | 0.92 ± 0.05 |
ND, not detected.
The coq7 null mutant harboring multicopy ubiF and E194KCoq7 harboring low copy ubiF grew on media containing ethanol/glycerol (Fig. 3). Thus, the apparent absence of Q6 in these same strains when cultured on galactose was unexpected (Table 2 and Fig. 4D). To investigate the relationship between the ubiF rescue of growth in media containing glycerol and Q6 content, lipid extracts of E194KCoq7 and coq7 null mutants expressing low and multicopy ubiF were analyzed for Q6 content. As expected, Q6 was present in each of the yeast strains able to grow on YPG: E194KCoq7:pQMF (1.01 ± 0.07 pmol/mg wet weight), E194KCoq7:pCHF (1.89 ± 0.03 pmol/mg wet weight), and JM43Δcoq7-1:pCHF (2.39 ± 0.24 pmol/mg wet weight).
Steady State Levels of Coq Polypeptides in coq7 Null and E194KCoq7 Mutants
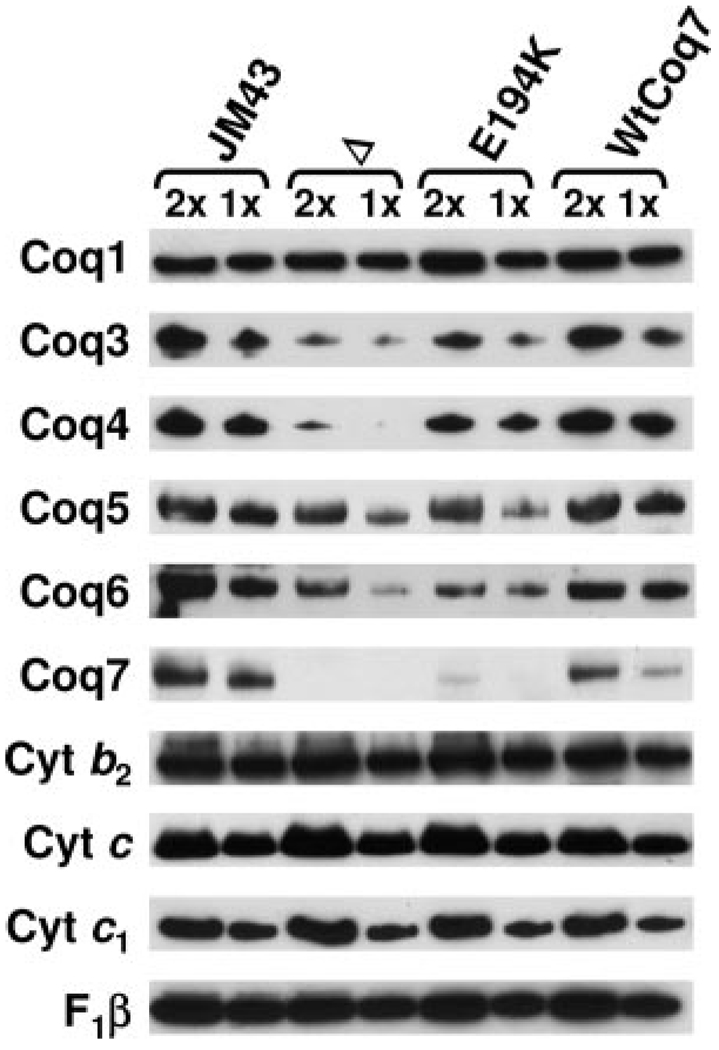
Previous studies indicated that steady state levels of the Coq3, Coq4 and Coq6 polypeptides were decreased in a coq7 null mutant (11). To determine whether E194KCoq7 mutant yeast might have a less severe phenotype relative to the coq7 null mutant, the steady state levels of the Coq1, Coq3, Coq4, Coq5, Coq6, and Coq7 polypeptides were determined in mitochondria isolated from JM43, coq7 null, E194KCoq7, and WtCoq7 yeast. For these studies we generated new antisera to Coq7. The Coq7 antibodies reacted with a polypeptide of about 23 kDa in wild-type samples that was absent from the null coq7 samples (Fig. 5). Based on the mobility in the SDS-PAGE, the apparent molecular mass of Coq7 is slightly smaller than the predicted precursor (26 kDa), and is consistent with the removal of a putative mitochondrial leader sequence. Although the Coq7 polypeptide is detected in mitochondria from E194KCoq7, levels are decreased relative to the wild-type Coq7p, suggesting that it is destabilized by the E194K mutation. The steady state levels of Coq3p and Coq4p were severely diminished in the coq7 null mutant, whereas Coq3p and Coq4p levels were higher in E194KCoq7. The steady state levels of Coq6p were decreased in both the coq7 null and E194KCoq7 mutants. Steady state levels of Coq1 and Coq5 proteins were not significantly affected, nor were mitochondrial constituents participating in respiratory complexes (cytochrome b2, cytochrome c, cytochrome c1, and F1β, the β subunit of F1-ATPase).
FIGURE 5. Steady state levels of Coq1, Coq3, Coq4, Coq5, Coq6, and Coq7 polypeptides in wild-type and coq7 mutants.
Purified mitochondria were isolated from yeast strains grown in YPGal media; parental wild type (JM43), JM43Δcoq7 (Δ), E194KCoq7 (E194K), and wild-type Coq7 integrant control (WtCoq7). Samples of mitochondria protein 20 (2×) or 10 µg (1×) were separated by SDS-PAGE and analyzed by Western blotting with antibodies against the Coq polypeptides (Coq1, Coq3, Coq4, Coq5, Coq6, and Coq7), and for other polypeptides of the mitochondrial respiratory chain including the cytochromes b2, c, and c1, and F1β (the F1-ATP synthase β subunit).
Steady State Levels of Coq3, Coq4, and Coq7 Polypeptides in coq7 Null and E194KCoq7 Mutants in the Presence and Absence of UbiF and Q6
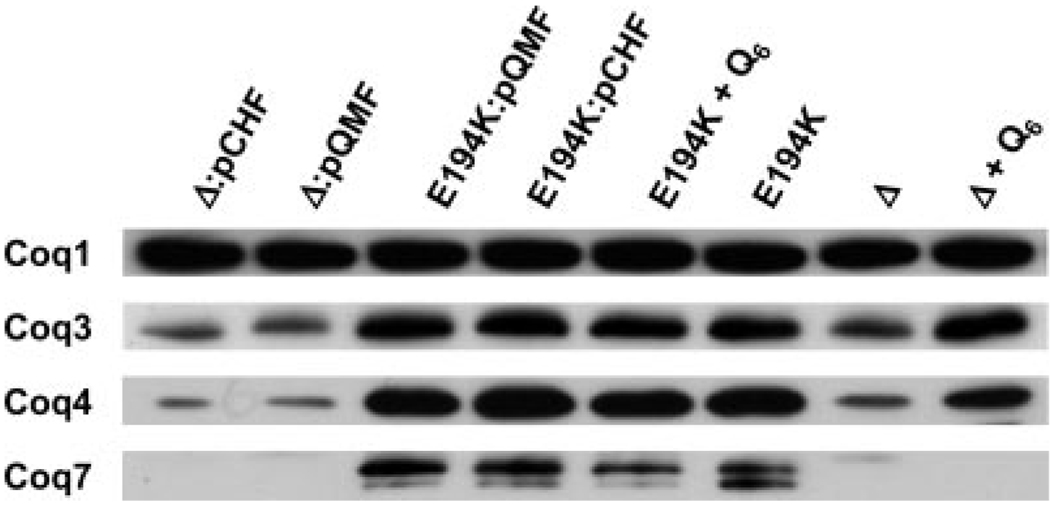
Expression of E. coli ubiF rescued E194KCoq7 more effectively than the coq7 null mutant. We determined the steady state levels of the Coq3, Coq4, and Coq7 polypeptides in the coq7 null mutant and E194KCoq7 in the presence or absence of E. coli ubiF expression. As expected, steady state levels of Coq3 and Coq4 proteins in E194KCoq7 were elevated relative to the coq7 null mutant. However, expression of E. coli ubiF in E194KCoq7 did not increase levels of the Coq3, Coq4, and Coq7 polypeptides (Fig. 6).
FIGURE 6. Steady state levels of Coq1, Coq3, Coq4, and Coq7 polypeptides in the presence and absence of UbiF or exogenous Q6.
Purified mitochondria were prepared from the following yeast strains grown in YPGal media: JM43Δcoq7:pCHF (Δ:pCHF), JM43Δcoq7:pQMF (Δ:pQMF), E194KCoq7:pQMF (E194K:pQMF), E194KCoq7:pCHF (E194:pCHF), E194KCoq7 with Q6 supplement (E194K + Q6), E194KCoq7 without Q6 supplement (E194K), JM43Δcoq7 (Δ), and JM43Δcoq7 with Q6 supplement (Δ + Q6). Samples (20 µg of protein) were separated by SDS-PAGE and analyzed by Western blotting with antibodies against Coq1, Coq3, Coq4, and Coq7 proteins. The plasmid descriptions are as follows: pQMF, a low copy plasmid expressing E. coli UbiF; and pCHF, a multicopy plasmid expressing E. coli UbiF.
In previous work we have observed an effect of exogenously added Q6 on steady state levels of Coq polypeptides in a coq1 null mutant (11).We postulated that the rescue of the coq7 null mutant by E. coli ubiF may be because of the conversion of a small amount of quinone intermediates to Q6, which in turn acted to stabilize yeast Coq polypeptides. Accordingly, we determined the steady state levels of Coq polypeptides in the presence or absence of exogenously added Q6 (Fig. 6). Levels of Coq3 and Coq4 polypeptides were significantly increased when the coq7 null mutant strain was cultured in media supplemented with Q6. In this case, the stabilization occurs despite the complete absence of the yeast Coq7 polypeptide, and suggests that Q6 itself may associate with and stabilize these polypeptides. However, addition of exogenous Q6 did not result in a similar degree of Coq7E194K stabilization.
Coq7p Elutes as a High Molecular Mass Complex with O-Methyltransferase Activity, Coq3p and Coq4p
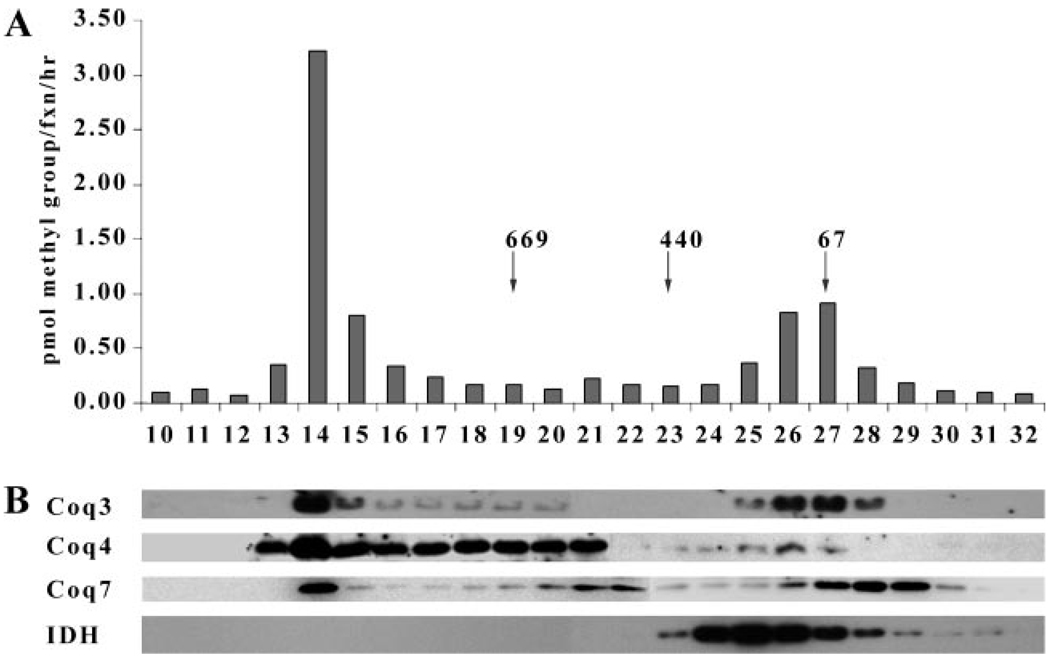
Gel filtration analyses were performed to determine the oligomeric state of Coq7p. Mitochondria were solubilized with digitonin and the 100,000 × g supernatant was applied to a Superose 6 column. This procedure had been employed previously to determine the native molecular mass of Coq3, Coq4, and Coq6, and had indicated that most of the O-methyltransferase activity and Coq3p eluted as a predominant high molecular mass complex >669 kDa (12). A second peak of O-methyltransferase activity and Coq3p was observed to elute with a lower molecular mass of 150 kDa. As shown in Fig. 7, Coq3p and O-methyltransferase activity co-eluted with the Coq7 and Coq4 polypeptides as a high molecular weight complex, suggesting that Coq7 co-migrates with polypeptide components of the high molecular mass Q biosynthetic complex.
FIGURE 7. Coq7 polypeptide co-elutes with Coq3 and Coq4 as a high molecular mass complex.
A, gel filtration analysis of Coq3 O-methyltransferase activity. Wild-type mitochondria (2 mg) were solubilized with digitonin (2:1, w/w, detergent/protein) and the 100,000 × g supernatant was subjected to gel filtration chromatography with calibration standards (denoted by arrows) as described under “Experimental Procedures.” Coq3 O-methyltransferase (pmol of methyl groups/fraction/h) is depicted. B, gel filtration analysis of Coq polypeptides. The Coq3, Coq4, Coq7, and isocitrate dehydrogenase (IDH) polypeptides were detected in eluate fractions by immunoblot analysis.
Coq7p Co-migrates as a High Molecular Mass Complex with Coq3p and Coq4p in the Presence of Q6/DMQ6
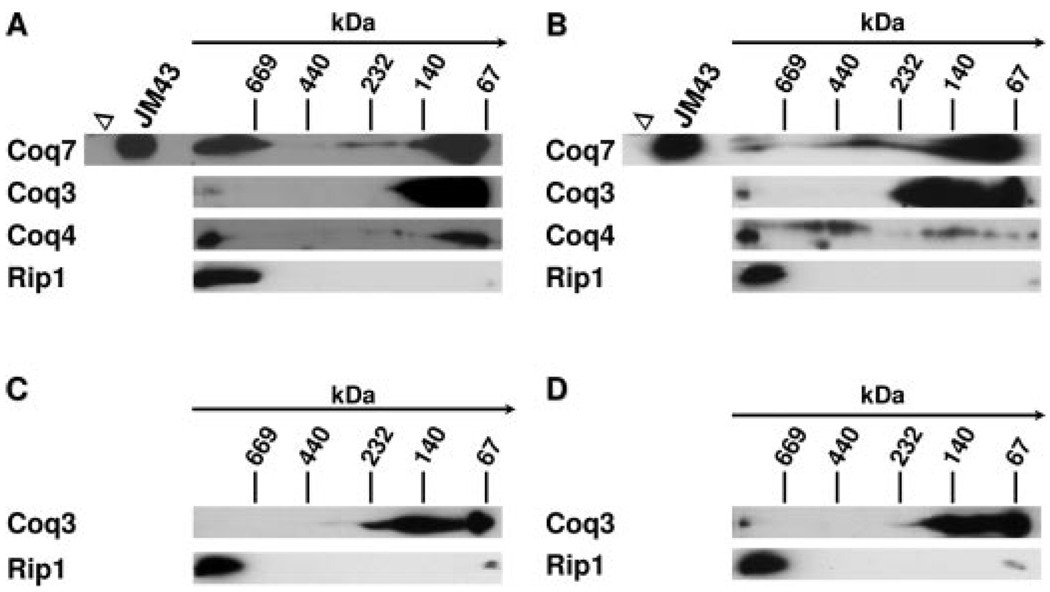
To better characterize the oligomeric complex containing Coq3, Coq4, and Coq7 polypeptides (indicated by above gel filtration data), two-dimensional BN-PAGE/SDS-PAGE analyses were performed on digitonin-solubilized mitochondria isolated from JM43 (wild-type), E194KCoq7, JM43Δcoq7, and JM43Δcoq7 grown in the presence of exogenous Q6 (Fig. 8). In both the wild-type (Fig. 8A) and the point coq7 mutant (Fig. 8B), Coq7 co-migrated with the Coq3 polypeptide in high molecular mass complexes (>669 kDa) as well as in lower molecular mass complexes. Migration of the high molecular mass oligomeric complexes containing Coq7 coincided with the Rip1 polypeptide, which is a component of the respiratory bc1 complex (38). The high molecular mass complex containing Coq3p was absent in the coq7 null mutant but was restored in the coq7 null mutant grown in the presence of exogenous Q6 (Fig. 8, C and D). The Rip1 containing high molecular mass complexes were readily detected in the coq7 null mutant in the absence and presence of exogenous Q6, suggesting that Coq7 is not required for formation of the bc1 complex.
FIGURE 8. Coq7 polypeptide co-migrates with Coq3 and Coq4 as a high molecular mass complex.
Mitochondria were isolated from the following yeast strains: JM43 (A), E194KCoq7 (B), JM43Δcoq7 (C), and JM43Δcoq7 with Q6 supplement (D). Mitochondria (300 µg) were solubilized with digitonin and subjected to BN-PAGE (5–13.5%) in the first dimension and SDS-PAGE (10–13%) in the second dimension as described. The point of origin and size separation for the first BN dimension is designated by an arrow with the position of the molecular mass standards (in kDa) indicated. Aliquots of control mitochondria from JM43Δcoq7 (Δ) and wild type (JM43) were subjected only to the second dimension (SDS-PAGE) separation. The indicated that Coq polypeptides were detected via immunoblotting with specific antisera. A representative blot from each panel was stripped and re-probed with anti-Rip1.
DISCUSSION
S. cerevisiae Coq7 protein and its C. elegans homologue, CLK-1, have been shown to be required for the conversion of DMQ to 5-hydroxyubiquinone at the last monooxygenase step in Q biosynthesis (15, 39). In this work we identify five new coq7 mutations (Fig. 2) producing defects in Q biosynthesis. The missense mutations affect invariant and conserved residues that reside either in the region predicted to be adjacent to the DMQ6 binding site (G62D, G65D, and G72D) or that are proposed to ligand the di-iron center, EXXH (E194K and H197Y). Antibodies raised to a Coq7-GST fusion protein identify yeast Coq7 as a 23-kDa polypeptide, a size consistent with use of the methionine 39 amino acids internal to the ORF reported originally (15), and with processing of a predicted mitochondrial leader sequence. This modified full-length amino acid sequence of S. cerevisiae demonstrates a higher degree of homology among the Coq7 homologues, especially at the NH2 terminus (Fig. 2).
In this study a stable integrant yeast strain was prepared (E194KCoq7) that contains one copy of the coq7E194K point mutation integrated at the COQ7 genomic locus. This strain together with the coq7 null, the parental wild-type, and a control strain with the wild-type COQ7 gene integrated in exactly the same manner were examined for quinone content and steady state levels of Coq polypeptides. As observed previously, the coq7 null strain was found to lack Q6 and DMQ6, (15, 16), and steady state levels of the Coq3, Coq4, and Coq6 polypeptides were severely diminished (11). The E194KCoq7 point mutant also lacked Q6, however, DMQ6 was present and steady state levels of the Coq3 and Coq4 polypeptides were elevated relative to the coq7 null. These results suggest that the Coq7 polypeptide may interact with and stabilize the Coq3 and Coq4 polypeptides. In support of this idea we show that Coq7p co-elutes with O-methyltransferase activity, Coq3p, and Coq4p as a high molecular mass complex in digitonin extracts of mitochondria (Fig. 7). Further evidence that Coq3, Coq4, and Coq7 coexist in high molecular mass complex was provided by BN-PAGE analysis (Fig. 8). These data, together with previous studies suggest that Coq7p is a polypeptide component of a large Q-biosynthetic complex (12).
The Coq7E194K polypeptide was present at very low levels relative to wild-type Coq7p (Fig. 5), suggesting that the E194K mutation may cause instability. However, these low levels of the mutated Coq7p are sufficient to stabilize the Coq3 Coq4 polypeptides, and a partially defective Q-biosynthetic complex. Indeed, the high molecular mass complex containing Coq7, Coq3, and Coq4 polypeptides was detected in the point mutant by two-dimensional BN-PAGE, although there was less Coq7p in the high molecular mass region as compared with the wild-type control strain (Fig. 8, A and B). Moreover, because DMQ6 has been found to co-elute with the Coq3/Coq4/Coq6 Q-biosynthetic polypeptide complex (12), we favor the model that the quinone intermediate DMQ6 may also play a role in this stabilization. Thus, the presence of the Coq7E194K polypeptide is predicted to stabilize a small amount of Coq3p and Coq4p, in turn allowing production of DMQ6, which has additional stabilizing influences.
We performed complementation assays with E. coli ubiF, a predicted flavin-dependent monooxygenase required for hydroxylation of DMQ8 in E. coli (20). UbiF shares no homology to eukaryotic or prokaryotic Coq7/Clk-1 homologs (20). Expression of E. coli ubiF restored growth of the coq7 null and E194KCoq7 point mutants on media containing non-fermentable carbon sources, although the complementation was much more effective in the coq7 point mutant. The E194KCoq7 mutant was rescued by expression of E. coli ubiF from either low copy or multicopy constructs. However, the coq7 null mutant was rescued only when E. coli ubiF was provided in multicopy. It is possible that in the coq7 null mutant, a slow flux through the Q biosynthetic pathway occurs that is not absolutely dependent on the formation of a high molecular mass complex. We postulate that provision of E. coli ubiF at high copy allows for the conversion of DMQ6 to the hydroxylated intermediate, and hence to Q6 and thus supports respiration, allowing growth on non-fermentable carbon source. However, because the Q6 level in the UbiF-rescued coq7 null mutant would be much lower than exogenously added Q6, there is no increase in the steady state level of Coq3 and Coq4 polypeptides. UbiF provides a more efficient rescue of the coq7 point mutant because of the relatively high steady state levels of Coq3p, Coq4p, and DMQ6, the substrate of UbiF.
How much Q6 is present in the cells grown in the presence of exogenous Q6? Previous work has demonstrated that coq7 mutants of W303 genetic background are able to assimilate exogenously added Q6 and thus acquire the ability to grow on media containing non-fermentable carbon sources (40). In fact, the amount of exogenously added Q6 recovered in isolated mitochondria represents about 80% of the Q6 amount present in mitochondria of wild-type cells. Because JM43 and W303 share the same genetic background (both are derived from D273-10B) (41, 42), it is very likely that exogenous Q6 is similarly assimilated and transported to JM43 mitochondria. Indeed, the work presented here shows that supplementation of glycerol containing medium (YPG) with 2 µm Q6 rescues growth of null coq7 yeast.
We observed that the mutants able to grow on non-fermentable carbon sources did synthesize Q6. However, the content of Q6 is dramatically affected by the carbon source of the media. For example, although Q6 was not detected in some of the “rescued” mutants cultured on galactose, it was readily detected in these same mutants following culture on media containing glycerol. Culture of yeast on non-fermentable carbon source is known to increase the content of Q (43).
How does carbon source in the growth media affect the endogenous Q6 content? The most likely mechanism is via the release of catabolite repression and up-regulating the expression of the COQ genes so that more Q6 is produced thus satisfying the demand(s) of aerobic respiration. In fact, previous studies have demonstrated that mRNA levels of COQ3, COQ4, COQ5, and COQ7 genes were higher in yeasts grown in glycerol containing media than in cultures containing fermentable dextrose (9, 15, 24, 44). In addition, Jonassen et al. (13) has shown that Coq7 protein amount was significantly increased in non-fermentable (ethanol) growth conditions.
We tested whether Q6 itself may stabilize Coq3 and Coq4 polypeptides in the coq7 mutant strains. The results presented in Fig. 6 indicate that steady state levels of the Coq3 and Coq4 polypeptides are dramatically increased when the coq7 null mutant is cultured in media supplemented with Q6. This stabilizing influence of Q6 on Coq3p and Coq4p is mediated in the complete absence of the Coq7 polypeptide. These results are further confirmed by the two-dimensional BN-PAGE analyses performed on digitonin-solubilized mitochondria isolated from the coq7 null mutant, grown in either the absence or presence of exogenously added Q6 (Fig. 8). As shown in Fig. 8, C and D, the presence of the Coq3p at high molecular weight was detectable only when the coq7 null mutant was grown in the presence of exogenous Q6. Conversely, the high molecular mass complexes containing the Rip1 protein were readily detected in each of the panels (Fig. 8, A–D), indicating that the presence or absence of Coq7p does not affect the assembly of the super-respiratory complexes containing the bc1 complex. Taken together, the data suggest that Coq3 and Coq4 polypeptides are stabilized by the presence of either Q6 or DMQ6. Future studies are aimed at identifying the partner Coq proteins that interact with Coq7 protein and DMQ6, and developing an in vitro assay for the hydroxylase activity of Coq7 protein using farnesylated analogues of DMQ6.
Acknowledgments
We thank Dr. A. Tzagoloff (Columbia University) for the cytochrome c1 antibody and the original yeast coq mutant strains, Dr. M. P. Yaffe (UCSD) for the F1β-ATPase antibody, Drs. Horst and Schartz (University of Basel, Switzerland) for the cytochrome b2 antibody, Dr. B Trumpower for the monoclonal antisera to Rip1, and Dr. Entian (Universitat Frankfurt, Germany) for the plasmid expressing GST-Coq7 fusion protein. We are grateful to the following people at UCLA: Dr. C. M. Koehler for the antibody to cytochrome c, Dr. J. P. Whitelegge for performing mass spectrometry analysis of isolated GST-Coq7 fusion protein, and A. Lunceford for providing technical support for the HPLC/ECD system.
Footnotes
This work was supported by National Institutes of Health Grant GM45952.
The abbreviations used are: Q, coenzyme Q or ubiquinone; DMQ, demethoxy-Q, demethoxy-ubiquinone or 2-polyprenyl-6-methoxy-3-methyl-1,4-benzoquinone; ECD, electrochemical detection; HPLC, high performance liquid chromatography; ORF, open reading frame; BN, blue native.
REFERENCES
- 1.Lenaz G, De Santis A. In: Coenzyme Q. Lenaz G, editor. Chichester, UK: John Wiley & Sons; 1985. pp. 165–199. [Google Scholar]
- 2.Turunen M, Olsson J, Dallner G. Biochim. Biophys. Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M. Arch. Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 4.Tzagoloff A, Dieckmann CL. Microbiol. Rev. 1990;54:211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson A, Gin P, Marbois BN, Hsieh EJ, Wu M, Barros MH, Clarke CF, Tzagoloff A. J. Biol. Chem. 2005;280:31397–31404. doi: 10.1074/jbc.M503277200. [DOI] [PubMed] [Google Scholar]
- 6.Poon WW, Marbois BN, Faull KF, Clarke CF. Arch. Biochem. Biophys. 1995;320:305–314. doi: 10.1016/0003-9861(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 7.Poon WW, Do TQ, Marbois BN, Clarke CF. Mol. Aspects Med. 1997;18:s121–s127. doi: 10.1016/s0098-2997(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 8.Hsu AY, Do TQ, Lee PT, Clarke CF. Biochim. Biophys. Acta. 2000;1484:287–297. doi: 10.1016/s1388-1981(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 9.Belogrudov GI, Lee PT, Jonassen T, Hsu AY, Gin P, Clarke CF. Arch. Biochem. Biophys. 2001;392:48–58. doi: 10.1006/abbi.2001.2448. [DOI] [PubMed] [Google Scholar]
- 10.Baba SW, Belogrudov GI, Lee JC, Lee PT, Strahan J, Shepherd JN, Clarke CF. J. Biol. Chem. 2004;279:10052–10059. doi: 10.1074/jbc.M313712200. [DOI] [PubMed] [Google Scholar]
- 11.Gin P, Clarke CF. J. Biol. Chem. 2005;280:2676–2681. doi: 10.1074/jbc.M411527200. [DOI] [PubMed] [Google Scholar]
- 12.Marbois B, Gin P, Faull KF, Poon WW, Lee PT, Strahan J, Shepherd JN, Clarke CF. J. Biol. Chem. 2005;280:20231–20238. doi: 10.1074/jbc.M501315200. [DOI] [PubMed] [Google Scholar]
- 13.Jonassen T, Proft M, Randez-Gil F, Schultz JR, Marbois BN, Entian KD, Clarke CF. J. Biol. Chem. 1998;273:3351–3357. doi: 10.1074/jbc.273.6.3351. [DOI] [PubMed] [Google Scholar]
- 14.Proft M, Kotter P, Hedges D, Bojunga N, Entian KD. EMBO J. 1995;14:6116–6126. doi: 10.1002/j.1460-2075.1995.tb00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marbois BN, Clarke CF. J. Biol. Chem. 1996;271:2995–3004. doi: 10.1074/jbc.271.6.2995. [DOI] [PubMed] [Google Scholar]
- 16.Padilla S, Jonassen T, Jimenez-Hidalgo MA, Fernandez-Ayala DJ, Lopez-Lluch G, Marbois B, Navas P, Clarke CF, Santos-Ocana C. J. Biol. Chem. 2004;279:25995–26004. doi: 10.1074/jbc.M400001200. [DOI] [PubMed] [Google Scholar]
- 17.Berthold DA, Stenmark P. Annu. Rev. Plant Biol. 2003;54:497–517. doi: 10.1146/annurev.arplant.54.031902.134915. [DOI] [PubMed] [Google Scholar]
- 18.Stenmark P, Grunler J, Mattsson J, Sindelar PJ, Nordlund P, Berthold DA. J. Biol. Chem. 2001;276:33297–33300. doi: 10.1074/jbc.C100346200. [DOI] [PubMed] [Google Scholar]
- 19.Adachi A, Shinjyo N, Fujita D, Miyoshi H, Amino H, Watanabe Y, Kita K. FEBS Lett. 2003;543:174–178. doi: 10.1016/s0014-5793(03)00419-8. [DOI] [PubMed] [Google Scholar]
- 20.Kwon O, Kotsakis A, Meganathan R. FEMS Microbiol. Lett. 2000;186:157–161. doi: 10.1111/j.1574-6968.2000.tb09097.x. [DOI] [PubMed] [Google Scholar]
- 21.Burke D, Dawson D, Stearns T. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. 2000 Ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 22.Hsu AY, Poon WW, Shepherd JA, Myles DC, Clarke CF. Biochemistry. 1996;35:9797–9806. doi: 10.1021/bi9602932. [DOI] [PubMed] [Google Scholar]
- 23.Guarente L, Mason T. Cell. 1983;32:1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- 24.Clarke CF, Williams W, Teruya JH. J. Biol. Chem. 1991;266:16636–16644. [PubMed] [Google Scholar]
- 25.Sikorski RS, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Gene (Amst.) 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 27.Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothstein RJ. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 29.Baker RT, Tobias JW, Varshavsky A. J. Biol. Chem. 1992;267:23364–23375. [PubMed] [Google Scholar]
- 30.Garfin DE. Methods Enzymol. 1990;182:425–441. doi: 10.1016/0076-6879(90)82035-z. [DOI] [PubMed] [Google Scholar]
- 31.Harrington MG. Methods Enzymol. 1990;182:488–495. doi: 10.1016/0076-6879(90)82039-5. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe MP. Methods Enzymol. 1991;194:627–643. doi: 10.1016/0076-6879(91)94046-f. [DOI] [PubMed] [Google Scholar]
- 33.Glick PS, Pon LA. Methods Enzymol. 1995;260:213–223. doi: 10.1016/0076-6879(95)60139-2. [DOI] [PubMed] [Google Scholar]
- 34.Gin P, Hsu AY, Rothman SC, Jonassen T, Lee PT, Tzagoloff A, Clarke CF. J. Biol. Chem. 2003;278:25308–25316. doi: 10.1074/jbc.M303234200. [DOI] [PubMed] [Google Scholar]
- 35.Jonassen T, Clarke CF. J. Biol. Chem. 2000;275:12381–12387. doi: 10.1074/jbc.275.17.12381. [DOI] [PubMed] [Google Scholar]
- 36.Tzagoloff A, Akai A, Needleman RB. J. Biol. Chem. 1975;250:8228–8235. [PubMed] [Google Scholar]
- 37.Tzagoloff A, Akai A, Needleman RB, Zulch G. J. Biol. Chem. 1975;250:8236–8242. [PubMed] [Google Scholar]
- 38.Beckmann JD, Ljungdahl PO, Trumpower BL. J. Biol. Chem. 1989;264:3713–3722. [PubMed] [Google Scholar]
- 39.Miyadera H, Amino H, Hiraishi A, Taka H, Murayama K, Miyoshi H, Sakamoto K, Ishii N, Hekimi S, Kita K. J. Biol. Chem. 2001;276:7713–7716. doi: 10.1074/jbc.C000889200. [DOI] [PubMed] [Google Scholar]
- 40.Santos-Ocana C, Do TQ, Padilla S, Navas P, Clarke CF. J. Biol. Chem. 2002;277:10973–10981. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- 41.McEwen JE, Ko C, Kloeckner-Gruissem B, Poyton RO. J. Biol. Chem. 1986;261:11872–11879. [PubMed] [Google Scholar]
- 42.Tzagoloff A, Akai A, Needleman RB. J. Bacteriol. 1975;122:826–831. doi: 10.1128/jb.122.3.826-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon PA, Stewart PR. Biochim. Biophys. Acta. 1969;177:358–360. doi: 10.1016/0304-4165(69)90150-0. [DOI] [PubMed] [Google Scholar]
- 44.Hagerman RA, Trotter PJ, Willis RA. Free Radic. Res. 2002;36:485–490. doi: 10.1080/10715760290021360. [DOI] [PubMed] [Google Scholar]