Abstract
BACKGROUND
A high body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) is associated with increased mortality from cardiovascular disease and certain cancers, but the precise relationship between BMI and all-cause mortality remains uncertain.
METHODS
We used Cox regression to estimate hazard ratios and 95% confidence intervals for an association between BMI and all-cause mortality, adjusting for age, study, physical activity, alcohol consumption, education, and marital status in pooled data from 19 prospective studies encompassing 1.46 million white adults, 19 to 84 years of age (median, 58).
RESULTS
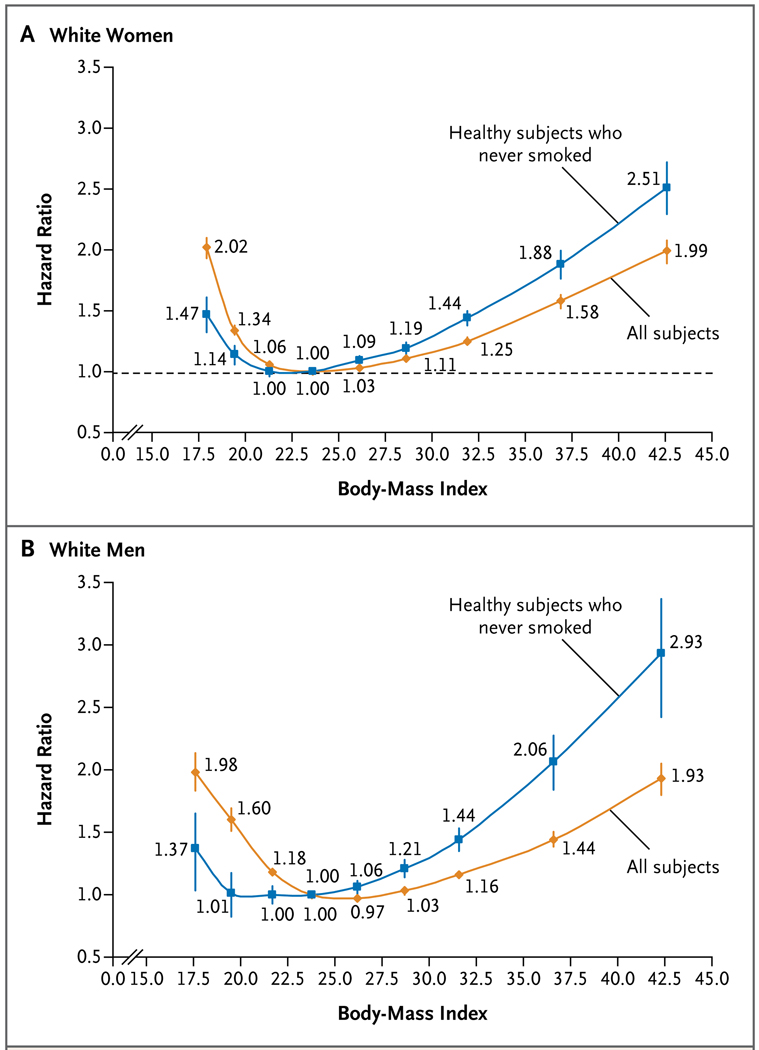
The median baseline BMI was 26.2. During a median follow-up period of 10 years (range, 5 to 28), 160,087 deaths were identified. Among healthy participants who never smoked, there was a J-shaped relationship between BMI and all-cause mortality. With a BMI of 22.5 to 24.9 as the reference category, hazard ratios among women were 1.47 (95 percent confidence interval [CI], 1.33 to 1.62) for a BMI of 15.0 to 18.4; 1.14 (95% CI, 1.07 to 1.22) for a BMI of 18.5 to 19.9; 1.00 (95% CI, 0.96 to 1.04) for a BMI of 20.0 to 22.4; 1.13 (95% CI, 1.09 to 1.17) for a BMI of 25.0 to 29.9; 1.44 (95% CI, 1.38 to 1.50) for a BMI of 30.0 to 34.9; 1.88 (95% CI, 1.77 to 2.00) for a BMI of 35.0 to 39.9; and 2.51 (95% CI, 2.30 to 2.73) for a BMI of 40.0 to 49.9. In general, the hazard ratios for the men were similar. Hazard ratios for a BMI below 20.0 were attenuated with longer-term follow-up.
CONCLUSIONS
In white adults, overweight and obesity (and possibly underweight) are associated with increased all-cause mortality. All-cause mortality is generally lowest with a BMI of 20.0 to 24.9.
Two thirds of the adult population in the United States and at least half the populations of many other developed countries are currently overweight or obese.1,2 Although it is well established that obese people — defined as having a body-mass index (BMI) (the weight in kilograms divided by the square of the height in meters) of 30.0 or more — have increased death rates from heart disease, stroke, and many specific cancers,3 the strength of the relationship between a high BMI and all-cause mortality remains uncertain, as does the optimal BMI with respect to mortality. Some studies suggest that being overweight (i.e., a BMI of 25.0 to 29.9) either is beneficial or has little effect on all-cause death rates,4,5 whereas others report a small increased risk.6–8 These inconsistencies could be due to confounding by tobacco use or disease-related weight loss, differences in age or duration of follow-up among study populations, use of different referent categories, or chance variation because of small numbers.9 Pooled analyses provide an opportunity to examine these issues in a large, diverse population with the use of a standard analytic approach across studies.
The Prospective Studies Collaboration recently assessed the association between BMI and mortality among 900,000 persons in studies that were primarily designed to evaluate risk factors for cardiovascular disease.3 On the basis of detailed analyses, particularly of cause-specific mortality, the investigators concluded that both overweight and obesity were associated with increased all-cause mortality and that the optimal BMI was 22.5 to 25.0. However, the main conclusions were based on analyses that included smokers and persons with preexisting cancer, possibly underestimating the association between BMI and all-cause mortality and overestimating optimal BMI.
We examined the relationship between BMI and all-cause mortality in a pooled analysis of 19 prospective studies, predominantly designed to study cancer, which included 1.46 million white (non-Hispanic) adults and 160,087 deaths. Three of the cohorts (in which 30,153 deaths occurred) were also included in the Prospective Studies Collaboration. 10–13 We systematically addressed the methodologic limitations described above by examining the extent to which the relationship between BMI and all-cause mortality varied with smoking status and prevalent disease. The very large sample and diverse population enabled us to evaluate variations according to age, sex, follow-up time, and physical activity. Our principal objectives were to assess the optimal BMI range and to provide stable estimates of the risks associated with being overweight, obese, and morbidly obese (BMI ≥40.0), with minimal confounding due to smoking or prevalent disease.
METHODS
INCLUSION CRITERIA
All prospective studies in the National Cancer Institute Cohort Consortium14 were eligible for inclusion if they satisfied the following criteria: the study had more than 5 years of follow-up, there were more than 1000 deaths among non-Hispanic white participants, and the baseline year was 1970 or later. Studies must also have ascertained height, weight, and smoking status at baseline. Most studies had information on preexisting conditions (particularly cancer other than nonmelanoma skin cancer or heart disease manifested as heart attack, arrhythmia, or angina), alcohol consumption, educational level, marital status, and level of physical activity. Key variables (height, weight, smoking status, and preexisting conditions) not available at baseline were later collected on questionnaires, so the baseline was redefined as the later date. We restricted the analyses to non-Hispanic whites (based on self-reported race or ethnic group) because the relationship between BMI and mortality may differ across racial and ethnic groups.15 Participants were also excluded if they were 85 years of age or older at baseline, had less than 1 year of follow-up, had missing information on height or weight, or had a BMI that was less than 15.0 or that was 50.0 or higher.
STUDY VARIABLES AND FOLLOW-UP
Variables were formatted to be consistently classified across studies into standard categories, including smoking status (never smoked, past smoker, or current smoker), number of years since the person stopped smoking (less than 10, 10 to 19, or 20 or more), alcohol consumption (in grams per day), overall level of physical activity (low, medium, or high), educational level (less than high-school graduate, high-school graduate, some college, college graduate, or postgraduate), and marital status (married, divorced, widowed, or single). Categories for missing data were included for all these variables.
Participants were followed from baseline to the date of death, end of follow-up, or loss to follow-up, whichever occurred first. The cause of death was ascertained from death certificates or medical records and was coded according to the International Classification of Diseases, Ninth Revision.
STATISTICAL ANALYSIS
Analyses involved the use of proportional-hazards models, with attained age as the underlying time variable and with stratification according to study, and were adjusted for alcohol intake, educational level, marital status, and physical activity. Analyses of BMI used the following predefined standard categories: 15.0 to 18.4, 18.5 to 19.9, 20.0 to 22.4, 22.5 to 24.9, 25.0 to 27.4, 27.5 to 29.9, 30.0 to 34.9, 35.0 to 39.9, and 40.0 to 49.9. We defined a BMI of 22.5 to 24.9 as the referent category on the basis of a preliminary analysis indicating that this was usually the range of BMI associated with the lowest mortality. Analyses were conducted for all subjects combined and for subgroups (e.g., subjects who had never smoked). All analyses were performed with the use of SAS statistical software, version 9.0 (SAS Institute).16 Because the relationship between BMI and all-cause mortality was nonlinear when evaluated across the whole BMI range (15.0 to 50.0), heterogeneity among cohorts was tested with the use of the Q statistic,17 with the BMI analyzed as a continuous variable in two categories of BMI: 15.0 to 24.9 and 25.0 to 49.9.
Since all the studies except one18 ascertained height and weight by means of self-report, we conducted a sensitivity analysis to assess the potential effect of reporting error. We generated an adjusted BMI for each participant by regressing BMI as calculated from measured height and weight in participants in the U.S. National Health and Nutrition Evaluation Survey (NHANES)19 on the BMI as calculated from their self-reported height and weight, and results were compared with those based on the unadjusted BMI.
RESULTS
CHARACTERIATICS OF THE STUDY COHORTS
Descriptive statistics for the 19 cohorts7,10–13,18,20–39 and the combined population are provided in Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. Of the 1.46 million people in these studies, more than half (58%) were women. The median age at baseline was 58 years, and the median BMI was 26.2. Forty-seven percent of the study population reported at baseline that they had never smoked, and only 13% reported that they were currently smoking. A total of 160,087 deaths were reported during a 10-year median follow-up; 35,369 of these deaths were among subjects who were healthy at baseline (i.e., those who reported no history of cancer or heart disease) and had never smoked.
The prevalence of current smoking decreased with increasing BMI; smokers accounted for 25% of the study participants in the lowest BMI category (15.0 to 18.4) but for only 8% of those in the highest BMI category (40.0 to 49.9) (Table 2 in the Supplementary Appendix); in contrast, the prevalence of former smoking increased from 27% to 44% from the lowest to the highest BMI category. Preexisting cancer and emphysema were slightly more common in the low-BMI categories, whereas the prevalence of preexisting heart disease increased with increasing BMI. Physical inactivity and lack of a college degree were both associated with a higher BMI.
BMI AND ALL-CAUSE MORTALITY
The age-standardized rate of death from any cause was generally lowest among participants with a BMI of 22.5 to 24.9 (Table 3 in the Supplementary Appendix). As compared with this referent group, the hazard ratios increased with progressively higher and lower levels of BMI (Fig. 1, and Table 3 in the Supplementary Appendix). However, the shape of the relationship between BMI and the hazard ratio for death changed with the sequential exclusion of current and former smokers and participants who reported having cancer or heart disease at baseline. With each exclusion, the hazard ratios increased for a BMI of 25.0 or higher and decreased for a BMI of less than 22.5. Figure 1 shows the contrast between the pattern observed among healthy participants who never smoked and the pattern observed when all subjects were included in the analysis. Among both women (Fig. 1A) and men (Fig. 1B), the nadir of the curve flattened and expanded to the BMI range of 20.0 to 24.9 when the analysis was restricted to healthy participants who never smoked. For healthy women who never smoked, the estimated hazard ratios were 1.13 (95% confidence interval [CI], 1.09 to 1.17) for those who were overweight (BMI, 25.0 to 29.9), 1.44 (95% CI, 1.38 to 1.50) for those in obesity class I (BMI, 30.0 to 34.9), 1.88 (95% CI, 1.77 to 2.00) for those in obesity class II (BMI, 35.0 to 39.9), and 2.51 (95% CI, 2.30 to 2.73) for those in obesity class III (BMI, 40.0 to 49.9). Hazard ratios were broadly similar for men except they were higher for obesity classes II and III.
Figure 1. Estimated Hazard Ratios for Death from Any Cause According to Body-Mass Index for All Study Participants and for Healthy Subjects Who Never Smoked.
Hazard ratios and 95% confidence intervals are shown for white women (Panel A) and white men (Panel B). The hazard ratios were calculated with the use of age as the underlying time scale, were stratified by study, and were adjusted for alcohol intake (grams per day), educational level, marital status, and overall physical activity. Subjects were deemed healthy if they had no cancer or heart disease at baseline.
After we had excluded participants who smoked and those with cancer or heart disease, the further exclusion of those with emphysema or stroke did not materially alter the associations between BMI and the rate of death (Table 4 in the Supplementary Appendix). Adjustments for other potential confounders (alcohol consumption, physical activity, educational level, and marital status) slightly reduced the hazard-ratio estimates associated with a BMI of 25.0 or higher (Table 5 in the Supplementary Appendix). In the sensitivity analysis, the adjustment for reporting errors in height and weight with the use of NHANES data also slightly decreased the hazard ratios for a BMI of 25.0 or higher and slightly increased those for a BMI of less than 22.5 (Table 6 in the Supplementary Appendix).
Since the results of the following analyses were broadly similar for men and women, they were combined to increase statistical power. The analysis according to single units of BMI among healthy participants who never smoked confirmed a nadir for death rates at a BMI of 20.0 to 25.0 and showed an approximately linear relationship in the hazard ratios for the range of 25.0 to 40.0 (Fig. 1 in the Supplementary Appendix). When the BMI was analyzed as a continuous variable, the hazard ratio for each 5-unit increase was 1.31 (95% CI, 1.29 to 1.33) over the range of 25.0 to 49.9.
The hazard-ratio estimates varied with the age at which the participant’s BMI was ascertained (Table 1). For a BMI of 25.0 or higher as compared with a BMI of 22.5 to 24.9, the hazard ratios were higher for participants whose height and weight were ascertained at 20 to 49 years of age than for those whose height and weight were ascertained after the age of 70 years (P = 0.005 for trend across categories of age). Annual excess mortality was higher for the older participants because their absolute death rates were higher.
Table 1.
Estimated Hazard Ratios for Death from Any Cause among Healthy Subjects Who Never Smoked, According to Body-Mass Index and Age at Baseline.*
| Variable | Body-Mass Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15.0–18.4 | 18.5–19.9 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0–39.9 | 40.0–49.9 | |
| 20–49 yr of age | |||||||||
| No. of deaths | 68 | 196 | 916 | 958 | 844 | 477 | 529 | 214 | 114 |
| Hazard ratio (95% CI) | 1.45 (1.13–1.85) | 0.95 (0.82–1.11) | 1.04 (0.95–1.14) | 1.00 | 1.30 (1.19–1.43) | 1.44 (1.29–1.61) | 1.79 (1.61–1.99) | 2.48 (2.14–2.88) | 3.70 (3.03–4.50) |
| Annual deaths per 1000 participants | 2.3 | 1.5 | 1.7 | 1.6 | 2.1 | 2.3 | 2.9 | 4.0 | 5.9 |
| Annual excess deaths per 1000 participants | 0.7 | −0.1 | 0.1 | — | 0.5 | 0.7 | 1.3 | 2.4 | 4.3 |
| 50–59 yr of age | |||||||||
| No. of deaths | 66 | 246 | 1151 | 1942 | 1879 | 1157 | 1378 | 491 | 257 |
| Hazard ratio (95% CI) | 1.15 (0.90–1.46) | 1.14 (1.00–1.30) | 0.96 (0.89–1.03) | 1.00 | 1.10 (1.03–1.17) | 1.22 (1.13–1.31) | 1.56 (1.46–1.68) | 2.06 (1.86–2.28) | 2.77 (2.42–3.16) |
| Annual deaths per 1000 participants | 4.5 | 4.4 | 3.7 | 3.9 | 4.3 | 4.8 | 6.1 | 8.0 | 10.8 |
| Annual excess deaths per 1000 participants | 0.6 | 0.5 | −0.2 | — | 0.4 | 0.9 | 2.2 | 4.1 | 6.9 |
| 60–69 yr of age | |||||||||
| No. of deaths | 218 | 456 | 2125 | 3803 | 4067 | 2640 | 2754 | 905 | 358 |
| Hazard ratio (95% CI) | 1.49 (1.30–1.71) | 1.15 (1.04–1.27) | 1.00 (0.95–1.06) | 1.00 | 1.03 (0.99–1.08) | 1.15 (1.09–1.21) | 1.34 (1.28–1.41) | 1.77 (1.64–1.91) | 2.27 (2.03–2.53) |
| Annual deaths per 1000 participants | 12.2 | 9.4 | 8.2 | 8.2 | 8.4 | 9.4 | 11.0 | 14.5 | 18.6 |
| Annual excess deaths per 1000 participants | 4.0 | 1.2 | 0.0 | — | 0.2 | 1.2 | 2.8 | 6.3 | 10.4 |
| 70–84 yr of age | |||||||||
| No. of deaths | 152 | 254 | 845 | 1282 | 1193 | 699 | 546 | 137 | 52 |
| Hazard ratio (95% CI) | 1.65 (1.39–1.95) | 1.32 (1.15–1.51) | 1.06 (0.97–1.16) | 1.00 | 1.04 (0.96–1.13) | 1.15 (1.04–1.26) | 1.24 (1.12–1.38) | 1.59 (1.33–1.90) | 1.91 (1.44–2.52) |
| Annual deaths per 1000 participants | 27.1 | 21.6 | 17.4 | 16.4 | 17.1 | 18.9 | 20.3 | 26.1 | 31.3 |
| Annual excess deaths per 1000 participants | 10.7 | 5.2 | 1.0 | — | 0.7 | 2.5 | 3.9 | 9.7 | 14.9 |
The age categories are for the age at the time that the body-mass index (BMI) (the weight in kilograms divided by the square of the height in meters) was calculated. Participants were considered healthy if they had no cancer or heart disease at baseline. Hazard ratios were calculated with age as the underlying time scale, stratified by study, and adjusted for sex, alcohol consumption (grams per day), educational level, marital status, and overall physical activity. The annual rates of death per 1000 participants are based on the crude death rates over a median follow-up of 10 years among the healthy study participants who never smoked. The annual rates of excess deaths per 1000 participants are compared with the death rate in the reference category of 22.5 to 24.9. The P values for trend across the categories of age were as follows: P = 0.002 for participants with a BMI below 25.0 and P = 0.005 for those with a BMI of 25.0 or more when estimated with the use of the model for continuous BMI.
The increased hazard ratios for a BMI below 20.0 as compared with a BMI of 22.5 to 24.9 were reduced as the length of follow-up increased; at 15 or more years, the only hazard ratio that was elevated was for a BMI of 15.0 to 18.4 (P = 0.007 for trend across follow-up periods) (Table 2). The hazard ratios for all BMI categories below 20.0 were also lower for participants who reported higher levels of physical activity than for those who reported lower levels, although the trend was not significant (P = 0.14). The hazard ratio for a BMI of 15.0 to 18.4, as compared with 22.5 to 24.9, was 1.62 (95% CI, 1.38 to 1.91) versus 1.22 (95% CI, 1.02 to 1.46) for those reporting low versus high levels of activity (Table 7 in the Supplementary Appendix).
Table 2.
Estimated Hazard Ratios for Death from Any Cause among Healthy Subjects Who Never Smoked, According to Body-Mass Index and Follow-up Period.*
| Variable | Body-Mass Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15.0–18.4 | 18.5–19.9 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0–39.9 | 40.0–49.9 | |
| <5 yr of follow-up | |||||||||
| No. of deaths | 504 | 1152 | 5037 | 7985 | 7983 | 4973 | 5207 | 1747 | 781 |
| Hazard ratio (95% CI) | 1.73 (1.46–2.06) | 1.19 (1.03–1.36) | 1.04 (0.96–1.13) | 1.00 | 1.07 (1.00–1.15) | 1.19 (1.10–1.29) | 1.44 (1.33–1.56) | 1.67 (1.48–1.88) | 2.28 (1.93–2.69) |
| 5–9 yr of follow-up | |||||||||
| No. of deaths | 363 | 916 | 4089 | 6485 | 6400 | 3954 | 4126 | 1425 | 624 |
| Hazard ratio (95% CI) | 1.51 (1.31–1.75) | 1.23 (1.11–1.36) | 1.03 (0.97–1.09) | 1.00 | 1.07 (1.02–1.13) | 1.20 (1.13–1.27) | 1.42 (1.34–1.51) | 1.93 (1.78–2.10) | 2.39 (2.12–2.69) |
| 10–14 yr of follow-up | |||||||||
| No. of deaths | 166 | 493 | 2351 | 3609 | 3334 | 1958 | 2072 | 718 | 318 |
| Hazard ratio (95% CI) | 1.29 (1.03–1.61) | 1.18 (1.03–1.36) | 1.03 (0.95–1.12) | 1.00 | 1.04 (0.97–1.12) | 1.19 (1.10–1.29) | 1.47 (1.35–1.59) | 1.97 (1.75–2.21) | 2.65 (2.23–3.15) |
| ≥15 yr of follow-up | |||||||||
| No. of deaths | 86 | 267 | 1359 | 2052 | 1825 | 992 | 1042 | 379 | 176 |
| Hazard ratio (95% CI) | 1.21 (0.97–1.50) | 0.92 (0.81–1.04) | 0.92 (0.86–0.98) | 1.00 | 1.12 (1.06–1.20) | 1.19 (1.11–1.29) | 1.41 (1.31–1.52) | 2.04 (1.82–2.27) | 3.11 (2.66–3.63) |
Participants were considered healthy if they had no cancer or heart disease at baseline. Hazard ratios were calculated with age as the underlying time scale, stratified by study, and adjusted for sex, alcohol consumption (grams per day), educational level, marital status, and overall physical activity. The numbers are based on the crude death rate over a median follow-up of 10 years among the healthy study participants who had never smoked; these rates are compared with the death rate in the reference category of 22.5 to 24.9. The P values for trends across the categories of age at BMI ascertainment are P = 0.007 for participants with a BMI less than 25.0 and P = 0.04 for those with a BMI of 25.0 or more when estimated with the use of the model for continuous BMI.
HETEROGENEITY AMONG STUDIES
Significant heterogeneity in the association between BMI and all-cause mortality was observed across the cohorts (P<0.001 for women and men). For a BMI of less than 25.0, there was some qualitative heterogeneity between studies, whereas for a BMI of 25.0 or more, the heterogeneity was largely quantitative. Heterogeneity was reduced in analyses that were restricted to healthy participants who never smoked (P = 0.01 for women and P = 0.09 for men with a BMI of 25.0 or more, and P = 0.02 for women and P = 0.003 for men with a BMI of less than 25.0) (Fig. 2 in the Supplementary Appendix) and was further reduced when the first 5 years of follow-up were excluded (P = 0.18 for women and = 0.20 for men with a BMI of 25.0 or more, and = 0.05 for women and P = 0.02 for men with a BMI of less than 25.0). Even when we excluded each study in turn (Table 8 in the Supplementary Appendix), changes in hazard ratios were small.
CAUSE OF DEATH
The pattern and magnitude of the hazard ratios varied according to the broad cause of death (Table 3). The hazard-ratio estimates associated with BMI of 25.0 or more were highest for death from cardiovascular conditions and were lowest for cancer. For a BMI of less than 22.5, the hazard ratios were highest for other causes of death and were not elevated for deaths due to cancer. Although absolute death rates varied according to sex, the hazard ratios were similar.
Table 3.
Estimated Hazard Ratios for Death from Specific Causes among Healthy Subjects Who Never Smoked, According to Body-Mass Index.*
| Cause of Death | Body-Mass Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15.0–18.4 | 18.5–19.9 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | 35.0–39.9 | 40–49.9 | |
| Cancer | |||||||||
| No. of deaths | 140 | 400 | 1933 | 3099 | 2948 | 1755 | 1851 | 520 | 207 |
| Hazard ratio (95% CI) | 1.12 (0.94–1.33) | 0.9 (0.87–1.07) | 0.95 (0.90–1.00) | 1.00 | 1.06 (1.00–1.11) | 1.13 (1.07–1.20) | 1.37 (1.29–1.45) | 1.55 (1.41–1.70) | 1.85 (1.60–2.13) |
| Cardiovascular disease | |||||||||
| No. of deaths | 96 | 186 | 752 | 1391 | 1671 | 1132 | 1307 | 465 | 216 |
| Hazard ratio (95% CI) | 1.47 (1.20–1.81) | 1.07 (0.92–1.25) | 0.89 (0.82–0.98) | 1.00 | 1.25 (1.16–1.34) | 1.52 (1.40–1.64) | 2.04 (1.89–2.21) | 3.05 (2.74–3.40) | 4.42 (3.82–5.11) |
| Other causes | |||||||||
| No. of deaths | 237 | 487 | 2040 | 2925 | 2756 | 1680 | 1678 | 642 | 312 |
| Hazard ratio (95% CI) | 1.84 (1.61–2.10) | 1.30 (1.18–1.43) | 1.11 (1.05–1.17) | 1.00 | 1.02 (0.97–1.07) | 1.13 (1.06–1.20) | 1.29 (1.22–1.38) | 2.00 (1.84–2.18) | 3.00 (2.66–3.37) |
Participants were considered healthy if they had no cancer or heart disease at baseline. Hazard ratios were calculated with age as the underlying time scale, stratified by study, and adjusted for sex, alcohol consumption (grams per day), educational level, marital status, and overall physical activity.
DISCUSSION
In this large, pooled analysis of prospective studies, both overweight and obesity (and possibly underweight) were associated with increased all-cause mortality in analyses restricted to participants who never smoked and did not have diagnosed cancer or heart disease. Thus, analyses of this subgroup should be minimally confounded by smoking or prevalent illness. The associations were strongest among participants whose BMI was ascertained before the age of 50 years. The lowest all-cause mortality was generally observed in the BMI range of 20.0 to 24.9. Longer follow-up attenuated the associations with lower BMI levels.
Our findings are broadly consistent with those of the Prospective Studies Collaboration, which showed an optimal BMI of 22.5 to 25.0 in analyses of all study participants3 and of 20.0 to 25.0 in analyses restricted to participants who never smoked.40 Results from two cohorts that were not included in either of the pooled analyses, Cancer Prevention Study II and the European Prospective Investigation into Cancer and Nutrition, also support an optimal BMI range of 20.0 to 24.9.6,8 In addition, the current study and these previous studies all showed that being overweight is associated with increased all-cause mortality.3,6–8 Among healthy persons who never smoked, our estimated hazard ratio per 5-unit increase in BMI was similar to the estimate in the Prospective Studies Collaboration — 1.31 (95% CI, 1.29 to 1.33) and 1.32 (95% CI, 1.29 to 1.36), respectively, for the BMI range of 25.0 to 49.9. In contrast, analyses of NHANES data and the Canadian National Health Survey, which included smokers and persons with preexisting diseases, showed that being overweight was not associated with increased all-cause mortality.4,5 These studies were smaller than our pooled study, with only about 11,000 deaths combined (7% of the total deaths in our study), so it is unlikely that their inclusion would have altered the main results of the current analysis. A recent study that used NHANES data to forecast the effects of overweight and obesity on life expectancy may also have underestimated these effects.41
Debate over the importance of overweight and obesity for all-cause mortality generally focuses on whether it is appropriate to exclude from analyses all smokers and persons with prevalent diseases. It is argued that smoking and preexisting illness contribute disproportionately to deaths that occur before average life expectancy, so the results of analyses that exclude them cannot be extrapolated to the general population. The counterargument is that smoking and preexisting conditions that cause weight loss are powerful confounders and analyses that include them lack validity — an attribute that is more important in etiologic studies than is generalizability. Stratification or exclusion rather than adjustment is necessary because smoking is so strongly related to obesity and mortality (Tables 2 and 9 in the Supplementary Appendix), making it difficult to avoid residual confounding by means of typical adjustments for smoking status and number of cigarettes smoked per day. Two aspects of our findings support our approach of focusing on healthy participants who never smoked. First, long-term follow-up strengthened rather than weakened the association between obesity and all-cause mortality, which is the expected result if preexisting illness confounds this association, especially early during follow-up. Second, the relationship between low BMI and all-cause mortality is stronger among former smokers who quit less than 20 years ago than among current smokers (Table 9 in the Supplementary Appendix). This result is probably a reflection of cessation of smoking because of illness.
Two findings suggest that the association between a low BMI (less than 20.0) and increased mortality is probably, at least in part, an artifact of preexisting disease. First, the association between underweight and increased mortality was substantially weaker after 15 years of follow-up (hazard ratio, 1.21) than after 5 years of follow-up (hazard ratio, 1.73), which is consistent with greater confounding by other prevalent diseases (diseases that were undiagnosed or those we did not have data for) in the early years of follow-up. Second, the association was somewhat weaker among persons who were physically active (those who were lean and fit) than among persons who were inactive (those with illness-induced wasting). However, another factor that could attenuate the hazard ratios for underweight people with longer follow-up is weight gain over time. Therefore, we cannot rule out the possibility that being underweight is associated with increased mortality.
The strengths of our study include the very large and diverse study population, long-term follow-up with the majority of deaths occurring in the last decade, and the broad age range. This permitted statistically precise estimates of the relationship between BMI and mortality across a wide range of BMI categories even in analyses restricted to healthy participants who never smoked. In our study, there were more than five times as many deaths among participants in the highest obesity categories (BMI of 35.0 to 39.9 and 40.0 to 49.9) than in previous studies3,6,8 because severe obesity had become more common. Among non-Hispanic persons in the United States as a whole, an estimated 11% of men and 17% of women had a BMI of 35 or higher in 2008.
The principal limitation of our study is its reliance on height, weight, and preexisting conditions at a single point in time. As explained above, changes in these factors may contribute to the change in hazard ratios over time (Table 2), but without repeated measures of these factors, we cannot assess their relative contributions. Although BMI is not a perfect measure of adiposity, since it does not distinguish fat from lean body mass, height and weight are more easily measured or self-reported than other indexes of excess adiposity, such as waist circumference.42 Nevertheless, there will be errors in recall and self-reporting of height and weight. Prevalent diseases were also self-reported, and details varied across studies. Finally, an important limitation in terms of generalizability was the fact that the population was restricted to non-Hispanic whites.
We conclude that for non-Hispanic whites, both overweight and obesity are associated with increased all-cause mortality, and underweight may be as well. All-cause mortality is generally lowest within the BMI range of 20.0 to 24.9. The results of our analysis are most relevant to whites living in affluent countries; similar analyses are under way in other populations.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health (NIH) and the Division of Cancer Control and Population Sciences, National Cancer Institute (NCI), NIH. Details regarding funding for the individual studies are listed in the Supplementary Appendix.
We thank Deborah Winn and Scott Rogers (Division of Cancer Control and Population Sciences, NCI); Arti Varanasi, Michelle Brotzman, and Brenda Sun (Westat); Franklin Demuth, Roy Van Dusen, and Li Cheung (Information Management Services); and Sir David Cox (Nuffield College, Oxford).
APPENDIX
From the Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD (A.B.G., P.H., S.C.M., G.S.T., L.B.F., D.M.F., M.S.L., Y.P., A.S.); the Division of Epidemiology, College of Medicine, Mayo Clinic, Rochester, MN (J.R.C.); the Department of Nutrition, Harvard School of Public Health, (A.J.F., W.C.W.), Brigham and Women’s Hospital and Harvard Medical School (I-M.L.), and the Divisions of Preventive Medicine and Aging, Brigham and Women’s Hospital (H.D.S.) — all in Boston; the Department of Epidemiology and Surveillance Research, American Cancer Society, Atlanta (L.H., M.J.T.); the Centre for Molecular, Environmental, Genetic, and Analytic Epidemiology, University of Melbourne (R.J.M., D.R.E.), and the Cancer Epidemiology Center, Cancer Council Victoria (G.G.) — both in Melbourne, Australia; Cancer Research UK Genetic Epidemiology Unit, University of Cambridge, Cambridge, United Kingdom (R.J.M.); the Department of Epidemiology, School of Medicine, University of California, Irvine, Irvine (H.A.-C.), Loma Linda University School of Public Health, Loma Linda (W.L.B.), and City of Hope National Medical Center, Department of Population Sciences, Duarte (K.D.H.) — all in California; the Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore (S.L.C., J.H.-B.); the University of Minnesota School of Public Health, Minneapolis (A.R.F.); the Division of Nutritional Epidemiology, National Institute of Environmental Medicine (N.H., A.W.), and the Department of Medical Epidemiology and Biostatistics (E.W.), Karolinska Institutet, Stockholm; the Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC (J.A.H.); the Department of Environmental Medicine, New York University School of Medicine, New York (K.L.K., A.Z.-J.); the Department of Epidemiology, School of Public Health, University of Washington, and the Cancer Prevention Program, Fred Hutchinson Cancer Research Center — both in Seattle (G.P.); the Cancer Registry of Norway, Oslo, and the Department of Community Medicine, Tromso — both in Norway (E.W.); Samfundet Folkhalsan, Helsinki (E.W.); and Pacific Health Research Institute and Queen’s Medical Center, Honolulu (B.J.W.).
Footnotes
Dr. Sesso reports receiving consulting fees from Iovate Health Sciences USA. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.International Obesity Task Force. Global prevalence of adult obesity. http://www.iotf.org/database/documents/GlobalPrevalenceofAdultObesityOctober2009v2.pdf. [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 5.Orpana HM, Berthelot JM, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity (Silver Spring) 2010;18:214–218. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 8.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [Erratum, N Engl J Med 2010;362:2433.] [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. Estimating the number of deaths due to obesity: can the divergent findings be reconciled? J Womens Health (Larchmt) 2007;16:168–176. doi: 10.1089/jwh.2006.0080. [DOI] [PubMed] [Google Scholar]
- 10.Baik I, Ascherio A, Rimm EB, et al. Adiposity and mortality in men. Am J Epidemiol. 2000;152:264–271. doi: 10.1093/aje/152.3.264. [DOI] [PubMed] [Google Scholar]
- 11.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 13.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II — a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute Cohort Consortium home page. http://epi.grants.cancer.gov/Consortia/cohort.html.
- 15.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [Erratum, Lancet 2004;363:902.] [DOI] [PubMed] [Google Scholar]
- 16.SAS, version 9.0. Cary, NC: SAS Institute; 2009. [Google Scholar]
- 17.Takkouche B, Cadarso-Suárez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150:206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 18.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 19.National Health and Nutrition Examination Survey data. Hyattsville, MD: Department of Health and Human Services; 2006. [Google Scholar]
- 20.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 21.Beeson WL, Mills PK, Phillips RL, Andress M, Fraser GE. Chronic disease among Seventh-day Adventists, a low-risk group: rationale, methodology, and description of the population. Cancer. 1989;64:570–581. doi: 10.1002/1097-0142(19890801)64:3<570::aid-cncr2820640303>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore SC, Mayne ST, Graubard BI, et al. Past body mass index and risk of mortality among women. Int J Obes (Lond) 2008;32:730–739. doi: 10.1038/sj.ijo.0803801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schairer C, Byrne C, Keyl PM, Brinton LA, Sturgeon SR, Hoover RN. Menopausal estrogen and estrogen-progestin replacement therapy and risk of breast cancer (United States) Cancer Causes Control. 1994;5:491–500. doi: 10.1007/BF01831376. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–635. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 26.Comstock GW, Alberg AJ, Huang HY, et al. The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acid, carotenoids, alpha-tocopherol, selenium, and total peroxyl radical absorbing capacity. Cancer Epidemiol Biomarkers Prev. 1997;6:907–916. [PubMed] [Google Scholar]
- 27.Orsini N, Bellocco R, Bottai M, Pagano M, Michaelsson K, Wolk A. Combined effects of obesity and physical activity in predicting mortality among men. J Intern Med. 2008;264:442–451. doi: 10.1111/j.1365-2796.2008.01985.x. [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR, Kaye SA, Sellers TA, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269:483–487. [Erratum, JAMA 1993;269:1254.] [PubMed] [Google Scholar]
- 29.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 30.Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(Suppl):349S–355S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 31.Hayes RB, Sigurdson A, Moore L, et al. Methods for etiologic and early marker investigations in the PLCO trial. Mutat Res. 2005;592:147–154. doi: 10.1016/j.mrfmmm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [Erratum, N Engl Med 2009;360:1797.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296:1371–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- 34.Freedman DM, Ron E, Ballard-Bar-bash R, Doody MM, Linet MS. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30:822–829. doi: 10.1038/sj.ijo.0803193. [DOI] [PubMed] [Google Scholar]
- 35.White E, Patterson RE, Kristal AR, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004;159:83–93. doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 36.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 39.Weiderpass E, Braaten T, Magnusson C, et al. A prospective study of body size in different periods of life and risk of pre-menopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1121–1127. [PubMed] [Google Scholar]
- 40.Whitlock G, Lewington S, Sherliker P, Peto R. Body-mass index and mortality. Lancet. 2009;374:114. [Google Scholar]
- 41.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.