Abstract
The heterotrimeric PDZ complex containing LIN-2, LIN-7 and LIN-10 is known to be involved in the organization of epithelial and neuronal junctions in Caenorhabditis elegans and mammals. We report here that mammalian LIN-7 PDZ proteins form a complex with cadherin and β-catenin in epithelia and neurons. The association of LIN-7 with cadherin and β-catenin is Ca2+ dependent and is mediated by the direct binding of LIN-7 to the C-terminal PDZ target sequence of β-catenin, as demonstrated by means of co-immunoprecipitation experiments and in vitro binding assays with the recombinant glutathione S-transferase:LIN-7A. The presence of β-catenin at the junction is required in order to relocate LIN-7 from the cytosol to cadherin-mediated adhesions, thus indicating that LIN-7 junctional recruitment is β-catenin dependent and that one functional role of the binding is to localize LIN-7. Moreover, when LIN-7 is present at the β-catenin-containing junctions, it determines the accumulation of binding partners, thus suggesting the mechanism by which β-catenin mediates the organization of the junctional domain.
Keywords: β-catenin/hippocampal neurons/LIN-7/MDCK cells/PDZ proteins
Introduction
In order to exert their physiological functions, cells must be able to generate and maintain specialized plasma membrane domains. Each of these domains is characterized by a unique molecular composition, which includes adhesion molecules, receptors, ion channels and their associated cytosolic signaling elements. The assembly of these components in a functional complex requires the presence of a multidomain protein, which acts as a molecular scaffold to organize the different elements in the appropriate region (Fanning and Anderson, 1999). Scaffolding proteins are often membrane-associated proteins containing one or more PDZ domains, which are protein–protein interaction modules that usually bind the C-terminal sequences of target proteins (Craven and Bredt, 1998). One scaffold complex, which is common to epithelia and neuronal cells and has been found to be involved in the organization of junctional domains, is the heterotrimeric complex consisting of the LIN-2, LIN-7 and LIN-10 PDZ proteins (Borg et al., 1998; Butz et al., 1998; Kaech et al., 1998).
In Caenorhabditis elegans, this complex ensures the localization of the tyrosine kinase receptor LET-23 at the lateral/junctional domain of vulval precursor cells, a localization necessary in order to maintain the signaling cascade leading to cell proliferation and the differentiation of vulval cells (Simske et al., 1996; Kaech et al., 1998).
The cloned mammalian orthologs of LIN-2, LIN-7 and LIN-10 are also known as CASK, Velis/MALS and Mint1/X11 (Hata et al., 1996; Borg et al., 1998; Butz et al., 1998; Cohen et al., 1998; Kaech et al., 1998; Irie et al., 1999; Jo et al., 1999). Both C.elegans and mammalian LIN-2 can bind LIN-10 and LIN-7 independently of the PDZ domains, which are free to recruit cell adhesion molecules, receptors and signaling proteins (Borg et al., 1998; Butz et al., 1998; Kaech et al., 1998). Important advances have been made in characterizing the molecular components of the complex, and it has been shown that adhesion molecules, such as neurexin and syndecan (Hata et al., 1996; Cohen et al., 1998; Hsueh et al., 1998), as well as transporters, receptors and ion channels, such as the epithelial γ-aminobutyric acid (GABA) transporter (BGT-1), the N-methyl-d-aspartate (NMDA) receptor and the N-type Ca2+ channel (Jo et al., 1999; Maximov et al., 1999; Perego et al., 1999), interact with the PDZ domains of mammalian LIN-2, LIN-7 and LIN-10. The heterotrimeric PDZ complex plays a role in regulating the localization of interacting proteins (Simske et al., 1996; Perego et al., 1999), but it is still uncertain how its components are targeted to the junctional surface.
The epithelial and neuronal junctional domains are composed primarily of Ca2+-dependent adhesion molecules called cadherins. These are transmembrane proteins whose extracellular domains promote cell–cell homotypic adhesion, whereas their cytoplasmic regions are linked to cytoplasmic proteins called catenins [α-catenin, β-catenin and γ-catenins (plakoglobins)] (Gumbiner, 1996). Catenins play a central role in cadherin function by mediating the connection of cadherin to the actin cytoskeleton, and thus regulating the strength of cell adhesion (Adams et al., 1996; Angres et al., 1996). Various integral proteins and signaling molecules are accumulated and organized at cadherin–catenin junctions (Daniel and Reynolds, 1997), and the multi-PDZ domain complex containing LIN-7 is an excellent candidate for the mediation of these processes, which lead to the establishment of structurally and functionally distinct plasma membrane domains. We explored the possibility that the direct binding of PDZ proteins to the structural components of the cadherin adhesion system may recruit multi-PDZ domain complexes at the junctions.
When examining the amino acid sequence of the proteins of the cadherin-mediated adhesion system (Table I), we noted the presence of a PDZ-like target sequence (-D-T-D-L) in the C-terminal end of β-catenin, which corresponds to the consensus for binding the type I PDZ motif of LIN-7 (Kaech et al., 1998; Jo et al., 1999). We demonstrate here that LIN-7 forms a complex with cadherin and β-catenin at epithelial and neuronal junctions by means of direct binding to the C-terminal domain of β-catenin, and provide data supporting the role of this interaction in the LIN-7 junctional recruitment.
Table I. Alignment of the cadherin and catenin C-terminal sequences.
| Organism | Name | C-terminal sequence | Accession No. |
|---|---|---|---|
| Human | E-cadherin | admygggedd | P12830 |
| Human | N-cadherin | ladmygggdd | P19022 |
| Human | VE-cadherin | gsdpreelly | P33151 |
| Human | α-catenin | lsefkamdsi | P35221 |
| Human | plakoglobin | pyptadhmla | P14923 |
| Human | p-120 1AC | kgttplmqki | O60714 |
| Human | δ-catenin | hypaspdswv | O00379 |
| Human | β-catenin | nqlawfdtdl | P35222 |
| Rat | β-catenin | nqlawfdtdl | Q9WU82 |
| Mouse | β-catenin | nqlawfdtdl | Q02248 |
| Chicken | β-catenin | nqlawfdtdl | O42486 |
| Xenopus laevis | β-catenin | nqlawfdtdl | P26233 |
| Zebrafish | β-catenin | nqlawfdtdl | Q90424 |
| Ciona intestinalis | β-catenin | vqqpwldtdl | BAA92185 |
| Sea urchin | β-catenin | tglaffdtdl | O61229 |
| Drosophila | arm | nlaawydtdc | P18824 |
| C.elegans | hmp-2 | pnhnwydtdl | O44326 |
| Hydra | β-catenin | vtqgwfdpdl | Q25100 |
| Saccharomyces cerevisiae | VAC8 | itqqilqflh | P39968 |
Results
The recruitment of LIN-7 to E-cadherin–β-catenin-based junctions
We analyzed the temporal and spatial distribution of LIN-7 during the formation of cell–cell contacts, as well as in fully polarized Madin–Darby canine kidney (MDCK) cells. The cell–cell adhesion of epithelial cells is based mainly on the E-cadherin–catenin system, and we therefore compared the localizations of LIN-7, E-cadherin and β-catenin by means of triple immunofluorescence experiments and confocal analysis. In order to follow the localization of LIN-7, we developed a polyclonal antibody raised against synthetic peptides designed in the LIN-7 PDZ domain (see Materials and methods), which recognized an ∼23 kDa band in MDCK cell lysates (see Figure 4C). It has been shown that MDCK cells express the LIN-7C and/or LIN-7Ba isoforms, which are both resolved as a band with an apparent molecular mass of ∼23 kDa by means of SDS–PAGE (Irie et al., 1999; Jo et al., 1999).
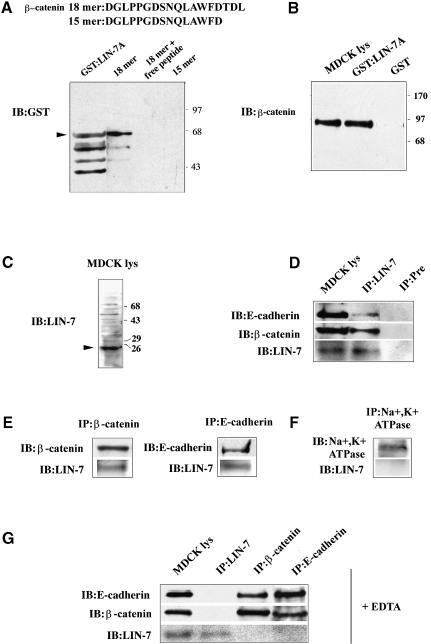
Fig. 4. LIN-7 forms a Ca2+-dependent complex with E-cadherin and β-catenin via its interaction with the β-catenin PDZ target motif. (A) Recombinant GST:LIN-7A was retained by the 18mer peptide containing the β-catenin PDZ target sequence, but not by the 15mer peptide. The sequences of the β-catenin 18mer and 15mer peptides immobilized on CNBr-activated Sepharose 4B are shown. A lysate from bacteria expressing the GST:LIN-7A fusion protein was incubated with the indicated immobilized peptides. Bound GST:LIN-7A was detected using a monoclonal antibody directed against GST (IB:GST). Purified GST:LIN-7A fusion protein was loaded in the gel as control (GST:LIN-7A). In addition to the band at the position expected for the fusion protein (arrowhead), lower molecular weight bands, presumably due to the proteolysis of the products, are visible. Molecular weight standards expressed in kilodaltons are indicated on the right. (B) β-catenin is retained specifically by the GST:LIN-7A but not by GST. MDCK cell lysates were incubated with immobilized GST or GST:LIN-7A fusion protein. The bound material was resolved by 10% SDS–PAGE and immunostained with monoclonal antibodies directed against β-catenin (IB:β-catenin). Ten percent of the total MDCK cell lysate (MDCK lys) used in the experiment was probed with the same antibody. Molecular weight standards expressed in kilodaltons are indicated on the right. (C) The LIN-7 antibody recognizes a band of the expected molecular weight in immunoblotting of MDCK cell lysate (arrowhead). A 20 µg aliquot of cell lysate was probed with the LIN-7 antibody (IB:LIN-7) raised against synthetic peptides designed in the LIN-7 PDZ domain (see Materials and methods). Molecular weight standards expressed in kilodaltons are indicated on the right. (D) Co-immunoprecipitation of E-cadherin and β-catenin with the LIN-7 antibody. (E) Co-immunoprecipitation of LIN-7 with the β-catenin or E-cadherin antibodies. (F) LIN-7 is not co-immunoprecipitated by the Na+,K+ ATPase antibody. (G) LIN-7 is not found in the complex with β-catenin and E-cadherin after pre-treatment with 5 mM EDTA. (D–G) The MDCK cell cultures were extracted in lysis buffer and immunoprecipitated with the indicated antibodies (IP:). The immunoprecipitates were loaded onto an 11% SDS–polyacrylamide gel followed by immunoblot analysis with the indicated antibodies (IB:). As controls, immunoprecipitation experiments were performed using a LIN-7 pre-immune serum (Pre) or the monoclonal antibody raised against Na+,K+ ATPase. When indicated, 10% of the cell lysates (MDCK lys) used in the immunoprecipitation experiments were probed with the indicated antibodies.
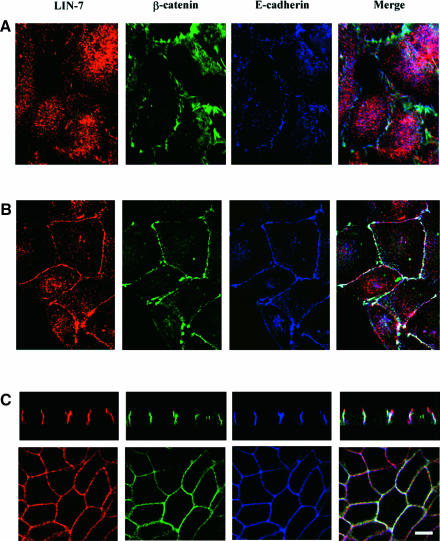
In order to follow the initial phases of junction formation, the MDCK cells were plated at high density for 2 h (early contacts) (Figure 1A). Under these conditions, LIN-7 staining was predominantly cytosolic (red), whereas E-cadherin (blue) and β-catenin (green) were already enriched in irregular structures along the cell–cell contacts (light blue in the merged image). It is interesting to note that co-localization of LIN-7, β-catenin and E-cadherin was only evident in areas with a continuous cell–cell contact (white in the merged image). Between 10 and 18 h after seeding in culture, the MDCK cells were not fully polarized but formed continous adhesions along the entire cell–cell contact surfaces in which LIN-7, β-catenin and E-cadherin co-localized (white in the merged image) (Figure 1B). In fully polarized MDCK cells (>4 days in culture, Figure 1C), LIN-7 co-localized with E-cadherin and β-catenin along the lateral junctional surface (vertical and horizontal sections), and LIN-7 staining was also observed at the apex of the lateral membrane (vertical section). This localization is similar to that of the tight junction-associated protein ZO-1 (data not shown), as previously documented by Irie et al. (1999). The different distribution of LIN-7 in cells with early irregular contacts versus cells with regular contacts cannot be explained by a higher expression of LIN-7 in the latter, because similar amounts of the PDZ protein were revealed by the immunoblotting of crude cell lysates (data not shown).
Fig. 1. The junctional localization of LIN-7 parallels the maturation of the junctions and the acquisition of cell polarity. MDCK cells were plated at high density onto poly-l-lysine-coated glass coverslips, cultured for different times and then fixed in 4% paraformaldehyde. LIN-7 distribution was compared with that of E-cadherin and β-catenin by means of confocal analyses of the triple immunofluorescence stainings. Rabbit anti-LIN-7 (red), mouse anti-β-catenin (green) and rat anti-E-cadherin (blue) antibodies were used. In the merged images, the white color indicates the co-localization of the three proteins, yellow the co-localization of LIN-7 with β-catenin, magenta the co-localization of LIN-7 with E-cadherin, and light blue the co-localization of β-catenin with E-cadherin. (A) In cells cultured for 2 h, E-cadherin appears as irregular and often punctate staining at the cell–cell boundary where it co-localizes with β-catenin (light blue in the merged image); predominantly, diffuse cytoplasmic staining for LIN-7 can be seen. (B) In cells cultured for 10–10–18 h, LIN-7 largely co-localizes with E-cadherin and β-catenin at the cell–cell contact regions. (C) In fully polarized MDCK cells, LIN-7 co-localizes with E-cadherin and β-catenin along the lateral plasma membrane domain, but LIN-7 labeling can also be seen in a more apical region (red in the merged image of the vertical section). Bar, 15 µm.
These data indicate that the accumulation of E-cadherin and β-catenin at the junctional surface precedes the accumulation of LIN-7. Moreover, the progressive recruitment of LIN-7 parallels the formation of regular cell–cell adhesions throughout the contact area.
LIN-7, E-cadherin and β-catenin are recruited progressively in a Triton X-100-insoluble fraction during junctional maturation
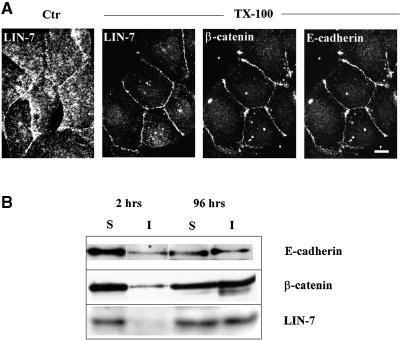
The maturation of cell–cell contacts leads to the reorganization of the actin cortical cytoskeleton, and the resistance of the E-cadherin–β-catenin complex to Triton X-100 extraction (Nathke et al., 1994; Adams et al., 1996). We investigated whether junctional LIN-7 was recruited in Triton X-100-insoluble complexes by means of immunofluorescence experiments in which the cells were extracted with Triton X-100 before paraformaldehyde fixation. Under these conditions, LIN-7 staining was restricted primarily to the cell–cell contact sites, where it co-localized with E-cadherin and β-catenin, and the intracellular staining was largely extracted by the treatment (Figure 2A). The distribution of LIN-7 in the Triton X-100-soluble and -insoluble fractions during junctional maturation was determined by means of a biochemical assay (Figure 2B). Cells at different stages of junctional development were extracted using 0.5% Triton X-100 for 20 min, and equal volumes of the soluble or insoluble fractions were loaded onto a 10% SDS–polyacrylamide gel and processed for immunoblotting. As in the case of E-cadherin and β-catenin, the amount of LIN-7 recovered in the Triton X-100-insoluble fraction increased during junctional maturation, reaching 50% in fully polarized cells. These data indicate that cytosolic LIN-7 is recruited progressively to the junctional domain, where it becomes stabilized in a Triton X-100-insoluble complex together with E-cadherin and β-catenin.
Fig. 2. LIN-7 is recruited progressively in the Triton X-100-insoluble fraction together with β-catenin and E-cadherin. (A) Immunofluorescence confocal analysis of LIN-7, β-catenin and E-cadherin staining after extraction with Triton X-100. Cells seeded in culture for 10–18 h were extracted (+ TX-100) or not (Ctr) with 0.5% Triton X-100 for 20 min before fixation with 4% paraformaldehyde. The distribution of LIN-7 in the control and treated cells is shown. On the Triton X-100-treated coverslips, the localization of LIN-7 is compared with that of β-catenin and E-cadherin. A horizontal section of triple immunofluorescence staining is shown. The detergent treatment largely extracts the cytosolic LIN-7, but the junctional staining is preserved. Bar, 15 µm. (B) Western blot analysis of the amount of LIN-7, β-catenin and E-cadherin in the Triton X-100-soluble (S) and -insoluble (I) fractions. High-density cultures of MDCK cells were extracted in Triton X-100 2 or 96 h after seeding in culture. Equivalent volumes of the Triton X-100-soluble or -insoluble fractions were separated by 10% SDS–PAGE and immunostained using the indicated antibodies (IB:). A concomitant association of these proteins with cytoskeletal elements during the junctional maturation is suggested by their similar reaction to Triton X-100 extraction.
The actin cytoskeleton is required in order to maintain the association of LIN-7 at the E-cadherin–β-catenin-based junctions
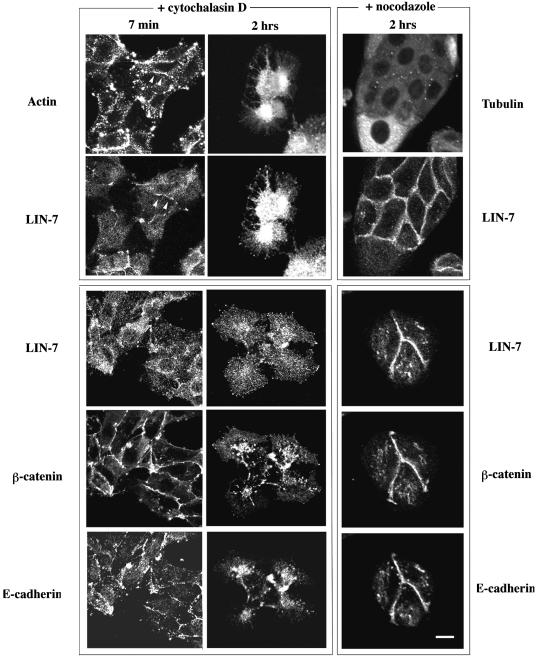
The results obtained with the Triton X-100 experiments suggest the presence of LIN-7, E-cadherin and β-catenin in a fraction containing the actin cytoskeleton. Since actin depolymerization causes the intracellular relocation of E-cadherin and β-catenin (Nathke et al., 1994), we examined the distribution of LIN-7 after treatment with 2 µM cytochalasin D (Figure 3, + cytochalasin D). E-cadherin, β-catenin and LIN-7 redistribution was observed after 2 h in the presence of the depolymerizing agent, but E-cadherin and β-catenin largely co-localized in the intracellular regions and LIN-7 staining was more diffuse. After short treatments with cytochalasin D (7 min), actin relocated to the cell interior, thus suggesting the partial disassembly of actin filaments, revealed as patchy intracellular and junctional staining (Figure 3, actin staining). LIN-7 redistributed mainly to the cytosol, but a minor fraction was maintained in the junctional regions containing an assembled actin cytoskeleton (continuous segments, arrowheads in the figure of double actin and LIN-7 staining). In contrast, short cytochalasin treatment had no effect on the localization of E-cadherin and β-catenin (LIN-7, β-catenin and E-cadherin triple staining). Athough staining with a tubulin antibody revealed the presence of microtubule cables running parallel to the contact area (data not shown), the treatment with 33 µM nocodazole that disassembled the microtubules (cytosolic staining, Figure 3, + nocodazole) apparently did not affect the junctional localization of the three proteins.
Fig. 3. The junctional localization of LIN-7 depends on a stably assembled actin cytoskeleton but not on the microtubule cytoskeleton. The localization of LIN-7, E-cadherin and β-catenin after disassembly of the actin (+ cytochalasin D) or the microtubule (+ nocodazole) cytoskeleton is shown. Low-density MDCK cells were treated with 2 µM cytochalasin D for 7 min or 2 h, or with 33 µM nocodazole for 2 h. The coverslips were cut into two parts: half was double stained with the LIN-7 antibody and phalloidin–FITC (actin) or with anti-tubulin antibodies (tubulin) in order to assess cytoskeleton depolymerization (upper panels); the other half was triple stained with LIN-7, β-catenin and E-cadherin antibodies (lower panels). Confocal analysis of the horizontal sections is shown. Partial depolymerization of the actin cytoskeleton (7 min) mainly affected the junctional localization of LIN-7, whereas the E-cadherin and β-catenin remained predominantly at the cell–cell contacts. The arrowheads indicate a contact region positive for LIN-7 staining in which the cytoskeleton is still assembled. Longer treatment (2 h) completely depolymerizes the actin cytoskeleton causing the cytosolic redistribution of the three proteins. Nocodazole treatment does not affect the junctional localization of the three proteins. Bar, 20 µm.
The association of LIN-7 with the E-cadherin– β-catenin-mediated adhesion system therefore requires the actin cytoskeleton.
The PDZ protein LIN-7 forms a complex with E-cadherin and β-catenin in MDCK cells
We next tested whether the association of LIN-7 with the cadherin-mediated junctional domain was due to its direct interaction with components of the adhesion system. When examining the C-termini of cadherins and catenins (Table I), we observed the presence of a PDZ-like target sequence (-D-T-D-L) at the C-terminal end of β-catenin. It is interesting to note that the C-terminal end of β-catenin containing the PDZ target motif is highly conserved throughout evolution with the exception of the yeast Saccharomyces cerevisiae, which does not have a LIN-7 ortholog (analysis of the yeast genome data bank by means of the FASTA 3 program at the EBI site) (Table I).
In order to verify whether β-catenin can interact with LIN-7, we tested the ability of a peptide consisting of the last 18 amino acids of β-catenin to bind a recombinant glutathione S-transferase (GST):LIN-7A fusion protein (Kaech et al., 1998) (Figure 4A). The peptide containing the PDZ target motif of β-catenin (18mer), but not the peptide from which the motif was removed (15mer), retained the mouse LIN-7A fusion protein. Binding was also prevented by an excess of free 18mer peptide in the incubation reaction. These data indicate that LIN-7 can interact with the C-terminal domain of β-catenin.
To investigate whether LIN-7 is capable of binding the native β-catenin endogenously expressed in MDCK cells, the mLIN-7A isoform fused to the GST protein and immobilized on Sepharose beads was incubated with an MDCK cell lysate. The bound molecules were revealed by means of SDS–PAGE and immunoblotting with specific antibodies. A consistent fraction of β-catenin (∼10%) was retained specifically by the GST:LIN-7A fusion protein (Figure 4B), but not by the immobilized GST.
In order to demonstrate the association between LIN-7 and β-catenin in vivo, we performed co-immunoprecipitation experiments. Antibodies raised against synthetic peptides designed in the LIN-7 PDZ domain were produced in rabbits, and recognized a major band of the expected mobility in western blots of MDCK cell lysates (Figure 4C). When confluent MDCK cell lysates were immunoprecipitated with this LIN-7 antiserum, β-catenin was co-immunoprecipitated. E-cadherin was also recovered in the immunocomplex, thus suggesting that LIN-7 is part of the E-cadherin–β-catenin adhesion system (Figure 4D). Control experiments using pre-immune serum confirmed the selectivity of the association. The cell extract was similarly subject to immunoprecipitation with anti-E-cadherin or β-catenin antibodies and, in both cases, LIN-7 was co-immunoprecipitated (Figure 4E). To exclude the possibility that the co-immunoprecipitation was due merely to the proximity of LIN-7 to E-cadherin and β-catenin and/or their common association with the actin cytoskeleton, we tested whether LIN-7 was co-immunoprecipitated with Na+,K+ ATPase, a basolateral/junctional protein known to interact with the cytoskeleton (McNeill et al., 1990). Confluent MDCK cell lysates were immunoprecipitated with specific antibodies raised against Na+,K+ ATPase, and no co-immunoprecipitation of LIN-7 was observed (Figure 4F).
Cadherins are Ca2+-dependent cell adhesion molecules, and so the chelation of extracellular calcium destabilizes the cell junctions and causes the internalization of the E-cadherin–β-catenin complex (Le et al., 1999). We investigated whether the association of LIN-7 with the E-cadherin–β-catenin complex was preserved in the absence of extracellular calcium. When MDCK cells were incubated with 5 mM EDTA for 30 min before cell lysis, the LIN-7 protein was immunoprecipitated, but neither β-catenin nor E-cadherin was found in the immunocomplex (Figure 4G); conversely, the β-catenin antibodies co-immunoprecipitated E-cadherin but not LIN-7, and the same result was obtained after immunoprecipitation with the E-cadherin antibodies. These data suggest that the recruitment of LIN-7 to the E-cadherin– β-catenin complex requires calcium-dependent cell–cell adhesions.
LIN-7 associates with the neuronal cadherin– β-catenin adhesion complex
Synaptic junctions in the central nervous system are morphologically similar to epithelial adherens junctions and have identical molecular components (Uchida et al., 1996; Shapiro and Colman, 1999). Given that brain LIN-7 isoforms have been identified and are reported to localize at synaptic junctions (Irie et al., 1999; Jo et al., 1999), we investigated whether LIN-7 can associate with the cadherin–catenin complex in brain.
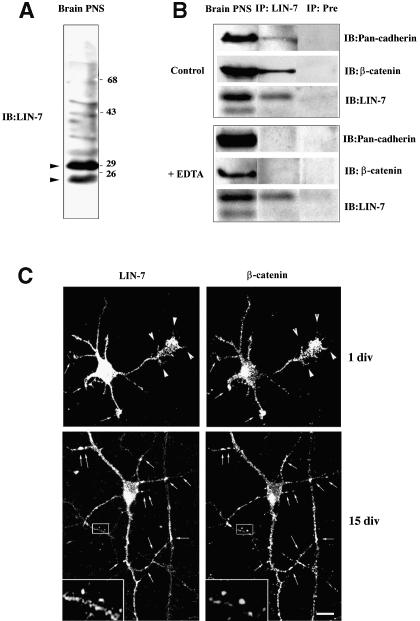
To this end, co-immunoprecipitation experiments were performed using the LIN-7 immune serum. In brain lysate, our LIN-7 antibody recognized 23 and 30 kDa proteins (Figure 5A, arrowheads), which correspond to the reported molecular masses of neuronal LIN-7 isoforms (Jo et al., 1999). In order to detect neuronal cadherins, we used a commercial monoclonal Pan-cadherin antibody that recognizes N-cadherins and which immunostained a single band of 120 kDa in the brain lysates. When the lysate was immunoprecipitated with the LIN-7 antibody, both neuronal cadherin and β-catenin were recovered in the same immunocomplex. The specificity of the interaction was confirmed by means of control experiments using a pre-immune serum. When EDTA was added to the lysis buffer, LIN-7 was immunoprecipitated but neither neuronal cadherin nor β-catenin was found in the immunocomplex (Figure 5B).
Fig. 5. LIN-7 associates with the cadherin–catenin adhesion system in neurons. (A) The LIN-7 antibody recognizes two main bands of the expected molecular weights in immunoblots of PNS brain fractions (arrowheads). A 20 µg aliquot of PNS was probed with the LIN-7 antibody (IB:LIN-7). Molecular weight standards expressed in kilodaltons are indicated on the right. (B) LIN-7 forms a Ca2+-dependent immunocomplex with β-catenin and cadherin in brain. PNS brain fractions were extracted with lysis buffer (Control) or lysis buffer containing 5 mM EDTA (+ EDTA). The brain extracts were immunoprecipitated with LIN-7 (IP:LIN-7) or pre-immune serum (IP:Pre). The immunoprecipitates were separated by SDS–PAGE, and immunoprobed with the LIN-7, the monoclonal β-catenin and the Pan-cadherin antibodies (IB:). Ten percent of the PNS extract (Brain PNS) was immunostained with the same antibodies. (C) LIN-7 co-localizes with β-catenin in cultured rat hippocampal neurons. One-day-old (1 div) or 15-day-old (15 div) hippocampal neurons were fixed and double stained with the LIN-7 and β-catenin antibodies. In the undifferentiated neurons (1 div), the arrows indicate the dendritic growth cones, and the arrowheads the filopodia emanating from the growing axon and the axonal growth cone. In the differentiated neurons (15 div), the arrows indicate sites reminiscent of synaptic labeling and positive for β-catenin and LIN-7. The boxed region is shown at higher magnification (3.5×) in the inset. Bar, 20 µm.
The cadherin–catenin adhesion complex has recently been implicated in the initial formation of synaptic junctions, as well as in the maturation and plasticity of synapses. We therefore analyzed the distribution of LIN-7 and β-catenin in hippocampal neurons cultured at early or late stages of neuron development and synapse formation (Figure 5C). One-day cultured neurons (1 div) had not formed synapses, and presented an immature axon that can be identified by its greater length and finer caliber, and several short processes that are young dendrites. At this stage, LIN-7 staining was diffuse in the somatodendritic and axonal compartments. A slight enrichment of both β-catenin and LIN-7 was observed in dendritic (arrows) and axonal growth cones and in the filopodia originating from the axonal growth cones (arrowheads), which are thought to initiate synaptogenic interactions (Cooper and Smith, 1992). After synaptogenesis (15 div), LIN-7 and β-catenin largely co-localized in punctate structures reminiscent of synaptic boutons (arrows, boxed region). Thus, in both neurons and polarized epithelial cells, LIN-7 forms a complex with cadherin and catenin that is enriched in junctional domains.
Redistribution of LIN-7 to the junctional domain in a β-catenin-dependent manner and its functional consequence
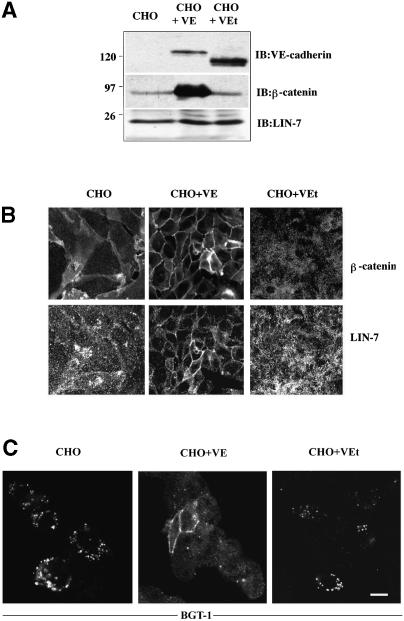
Having demonstrated the direct association of LIN-7 with β-catenin, we investigated whether the recruitment of LIN-7 at the junctional domains was dependent on β-catenin. To this end, we used a system introduced by Navarro et al. (1995), consisting of Chinese hamster ovary (CHO) cells (which normally do not express cadherins) stably expressing either wild-type VE-cadherin or a truncated form lacking the β-catenin-binding site. We compared the expression (Figure 6A) and localization (Figure 6B) of LIN-7, β-catenin and VE-cadherin in untransfected cells and in the transfected cell lines. Two intense bands of the apparent molecular mass corresponding to the expected size for the wild-type (130 kDa) and the truncated VE-cadherin (100 kDa) were detected by means of western blot analysis in the VE-cadherin transfectants. No detectable VE-cadherin expression or cadherin activity was found in the untransfected cells. Small amounts of β-catenin were detected in untransfected and truncated VE-cadherin transfectants. The higher expression of β-catenin in wild-type VE-cadherin-transfected cells is probably due to the stabilization of the β-catenin when integrated in the cadherin–catenin complex (Navarro et al., 1995). A LIN-7 isoform with a molecular mass identical to that found in MDCK cells was expressed equally in the three cell lines. Immunofluorescence showed that LIN-7 and β-catenin localized to the cytosol of CHO cells, but upon the expression of wild-type VE-cadherin, LIN-7 redistributed to the junctions where it co-localized with β-catenin. This localization was due to the presence of β-catenin at the junctions because, although forming adhesive junctions (Navarro et al., 1995), the cells expressing truncated VE-cadherin failed to recruit β-catenin at the cadherin-mediated adhesions and LIN-7 remained in the cytosol. The subcellular distribution of LIN-7 therefore seems to be regulated by its binding to β-catenin.
Fig. 6. The junctional localization of β-catenin mediates the redistribution of LIN-7. (A) Western blot analyses of LIN-7, β-catenin and VE-cadherin expression in untransfected CHO cells (CHO), and CHO cells transfected with wild-type (CHO + VE) or truncated VE-cadherin (CHO + VEt). A 20 µg aliquot of the total proteins from each cell line was loaded onto an 11% SDS–polyacrylamide gel and immunoprobed with the indicated antibodies (IB:). Bands corresponding to the wild-type (∼130 kDa) and truncated (∼100 kDa) VE-cadherin were revealed by specific staining. The CHO cells transfected with wild-type VE-cadherin express larger amounts of β-catenin. An equal level of LIN-7 expression was detected in the three cell lines. Molecular weight standards expressed in kilodaltons are indicated on the left. (B) Immunolocalization of β-catenin and LIN-7 in untransfected and transfected CHO cells. The cells were fixed in 4% paraformaldehyde and double stained with the β-catenin and LIN-7 antibodies. The presence of β-catenin at the junction determines the redistribution of LIN-7 from the cytosol to the junctions. (C) Immunolocalization of the epithelial GABA transporter BGT-1 in untransfected and transfected CHO cells. The cells were transiently transfected with the BGT-1 cDNA and, 48 h later, were fixed in 4% paraformaldehyde and processed for immunofluorescence using an antibody raised against the BGT-1 transporter (BGT-KLH antibody; Perego et al., 1997). Only CHO cells localizing LIN-7 at the junctions retain and accumulate the BGT-1 transporter at the cell surface. Bar: (B) 20 µm; (C) 15 µm.
We then analyzed the functional consequences of LIN-7 recruitment to the junctions. MDCK cells exposed to hypertonic conditions expressed the epithelial GABA transporter (BGT-1); we have shown previously that this transporter is a partner of LIN-7 and that their interaction leads to the plasma membrane BGT-1 retention. The prevention or disruption of LIN-7–BGT-1 binding determined the internalization of the transporter and its accumulation in an intracellular recycling compartment (Perego et al., 1999). Given that LIN-7 interacts with BGT-1 at the plasma membrane, we expected to find surface BGT-1 accumulation only in the CHO cells in which LIN-7 was localized at the junctions. The three CHO cell lines were transfected individually with BGT-1 cDNA and transporter distribution was analyzed by means of immunofluorescence staining. BGT-1 was retained at the junctional domain only in wild-type VE-cadherin transfectants, whereas it accumulated in the intracellular structures of cells in which LIN-7 remained cytosolic (untransfected CHO cells and cells transfected with truncated VE-cadherin) (Figure 6C). These results confirm that LIN-7 is recruited at the junctional domain only in cells expressing wild-type VE-cadherin and that, once there, it may play a role in organizing the domain through the retention of interacting proteins.
Discussion
Recruitment of LIN-7 to the junctional domain
In this study, we investigated the mechanisms by which a component of the trimeric complex is recruited to the junctional domain. We found that, during establishment of cell polarity, LIN-7 translocates from the cytosol to cadherin-mediated cell–cell contacts, where it accumulates progressively until it reaches a complete junctional localization in fully polarized MDCK cells. In particular, its recruitment appears to coincide with the maturation of the junctional domain because: (i) in MDCK cells, LIN-7 was only contained in organized contacts (in which E-cadherin appeared as a continuous line versus those in which cadherin staining was irregular; Figure 1); (ii) in neurons, there was diffuse LIN-7 and β-catenin staining in the immature axons and dendrites before synaptogenesis but, afterwards, LIN-7 largely co-distributed with β-catenin at synaptic sites (Figure 5); and (iii) the junctional but not the cytosolic LIN-7 was Triton X-100 insoluble, and the amount of LIN-7 found in the Triton X-100-insoluble fraction increased progressively with the time of culture, thus paralleling the Triton X-100 insolubility of E-cadherin and β-catenin (Figure 2).
In an attempt to clarify the molecular basis of the junctional recruitment of LIN-7, we carried out affinity chromatography and co-immunoprecipitation experiments. A complex containing E-cadherin, β-catenin and LIN-7 could be demonstrated in epithelia and neurons by means of co-immunoprecipitation. The association was calcium dependent and specific, because no co-immunoprecipitation was obtained using pre-immune sera. Furthermore, the association of LIN-7 with the cadherin–catenin adhesion system was not due to the physical proximity of the proteins or their interactions with the actin cytoskeleton because no co-immunoprecipitation of LIN-7 was observed with Na+,K+ ATPase, a protein that localizes in the basolateral/junctional domain and interacts with the actin cytoskeleton (McNeill et al., 1990). The association between LIN-7 and the E-cadherin–β-catenin complex was mediated by the PDZ target motif of β-catenin because recombinant GST:LIN-7A from a bacterial lysate was retained by a β-catenin synthetic peptide containing the PDZ target motif (but not by the peptide lacking the PDZ-binding site), and the binding was competed by an excess of free β-catenin peptide. Analysis of the expression of β-catenin and LIN-7 throughout evolution revealed a further link between them: the C-terminal end of β-catenin is conserved in organisms expressing LIN-7 but not in the yeast S.cerevisiae in which genomic LIN-7 has not been found (BLASTA analysis).
Having established the existence of a direct interaction between β-catenin and LIN-7, we investigated its functional significance. Using CHO cells transfected with a wild-type or a truncated VE-cadherin lacking the binding site for β-catenin, we found that the recruitment of LIN-7 depended on the presence of β-catenin at the junctions, thus suggesting that LIN-7–β-catenin binding may mediate the junctional localization of LIN-7.
However, LIN-7 was not recruited in early contacts, and its association with the cadherin–β-catenin contacts paralleled the maturation of the junctions. Junctional maturation and cell polarity development are accompanied by dephosphorylation of the components of the adhesion system (Daniel and Reynolds, 1997; Lampugnani et al., 1997), and many protein tyrosine phosphatases and signaling molecules have been found to accumulate in the mature junctions (Daniel and Reynolds, 1997). Our preliminary results indicate that tyrosine-phosphorylated β-catenin is not retained on the immobilized GST:LIN-7A fusion protein, thus suggesting that the association between these proteins is regulated by tyrosine phosphorylation. This may explain why the recruitment of LIN-7 parallels junctional maturation, and supports the hypothesis that β-catenin plays a role in LIN-7 localization. The association of LIN-7 with the target protein may therefore be regulated by post-translational modifications, as has been documented in the case of the PDZ protein discs large DLG, whose interaction with the synaptic complex is regulated by a cAMP-dependent kinase II-dependent phosphorylation during neuronal development and plasticity (Ho Koh et al., 1999).
The requirement for β-catenin in LIN-7 localization could also be explained by its role in organizing the actin cytoskeleton at the junctional domain. This would be in line with our finding that depolymerization of the actin cytoskeleton caused a rapid relocation of LIN-7 to the cytosol. Since CASK, but not LIN-7, contains binding sites for interactions with cytoskeletal proteins (Cohen et al., 1998), the association of LIN-7 with the cytoskeleton should be mediated by its binding to CASK. The results obtained by Straight et al. (2000) showing that the binding site for CASK mediates the basolateral localization of LIN-7 support this possibility.
The interaction with β-catenin (mediated by the PDZ domain) and the cytoskeleton (mediated by the binding site for CASK) may contribute to the localization of LIN-7 at cadherin-mediated junctions. Various lines of evidence indicate that the targeting of PDZ proteins to the appropriate plasma membrane region relies on both PDZ and non-PDZ domains, and that the PDZ domains binding to target proteins affect the efficiency and the specificity of the targeting (Lue et al., 1996; Wu et al., 1998; Firenstein et al., 1999).
Functional consequences of LIN-7 junctional recruitment
Our data indicate that LIN-7 is a component of the cadherin-mediated adhesion that is common to the pre- and post-synaptic compartments, as well as to epithelial junctions; but what are the functional consequences of LIN-7–β-catenin binding to the junctions? We have demonstrated previously that LIN-7 causes the selective accumulation of the interacting BGT-1 transporter in the basolateral junctional domain of MDCK cells by means of retentional mechanisms, and that the disruption of LIN-7–BGT-1 binding causes intracellular BGT-1 accumulation (Perego et al., 1999). By analogy, LIN-7 binding to β-catenin might control the amount of cytosolic β-catenin that is free to function as a coactivator of gene expression (Gumbiner, 1995). This aspect was not investigated in this study, but we have documented that, once recruited to the junctions, LIN-7 participates in the organization of this domain by localizing and accumulating binding partners. Using CHO cells, we found that only the LIN-7 recruited in the β-catenin-containing junctions is capable of retaining the epithelial GABA transporter at the surface domain, whereas the transporter accumulated in an intracellular compartment in the cells not containing LIN-7 at the junctions. This result implies that, as documented in the case of other PDZ proteins (Hata et al., 1996; Hsueh et al., 1998), the single PDZ-containing protein LIN-7 may interact with different partners (β-catenin and BGT-1) in the same cell type. It is not yet clear whether the same LIN-7 isoform forms a single complex containing both β-catenin and BGT-1, or multiple complexes containing either one or the other, nor whether the composition of these complexes is regulated during the development of cell polarity. Since several LIN-7 isoforms are expressed in epithelia and neurons, and LIN-7 can associate with CASK or other members of the membrane-associated guanylate kinase subfamily (PALs: proteins associated with LIN-7; Kamberov et al., 2000) via the non-PDZ domain, it is possible that LIN-7 forms multiple complexes characterized by a specific molecular composition. However, it is interesting to note that β-catenin also appears to be a binding partner of the PDZ protein MAGI 1, but whereas the interaction with MAGI 1 occurs mainly under conditions of Ca2+ depletion (Dobrosotskaya and James, 2000), we found that the interaction with LIN-7 was disrupted by Ca2+ depletion, thus suggesting that the composition of the complexes may be regulated during the acquisition of cell polarity.
LIN-7 as a component of the heterotrimeric complex may play a further role in coupling the cadherin–catenin system (via LIN-7) to the cell adhesion molecule neurexin or the matrix receptor syndecan (via CASK). Given that two different components of these adhesion systems (β-catenin and CASK) may both function as co-activators of gene expression (Gumbiner, 1995; Hsueh et al., 2000), the link between the various adhesion systems may be important for the integration and processing of information from the junction in order to generate intracellular signaling.
Materials and methods
Cell cultures and treatments
MDCK strain II cells were cultured as previously described (Perego et al., 1997). High-density cultures (5 × 105 cells/35 mm diameter dish) were seeded on poly-l-lysine-coated glass coverslips, or Transwell filter inserts (0.4 µm pores; Costar), and processed for immunofluorescence or biochemical studies at different times. At 2 h after seeding in culture, the cells presented sparse cell–cell contacts (early contacts); after 10–18 h in culture, they were characterized by continuous cell–cell contacts but still had a spread morphology; after >5 days, they had a fully polarized phenotype. Low-density cultures (5 × 104 cells/35 mm diameter dish) were used when indicated.
CHO cells, untransfected and transfected with the wild-type and truncated human VE-cadherin (Navarro et al., 1995), were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum. They were a kind gift from the laboratory of Dr Dejana. CHO cells were transiently transfected with the cDNA encoding BGT-1 using the calcium phosphate method (Pietrini et al., 1994).
The primary hippocampal neurons were a kind gift from the laboratory of Dr Matteoli, and were cultured as described by Goslin and Banker (1991). After 1 or 15 days in culture, the neurons were fixed and processed for immunoflurescence (Cameron et al., 1991).
Primary antibodies
The initial immunofluorescence experiments were performed using the previously described affinity-purified polyclonal antiserum raised against amino acids 86–207 of mLIN-7A (Perego et al., 1999). Similar results were obtained with the rabbit polyclonal anti-LIN 7 antibody used in this study, which was produced against synthetic peptides corresponding to amino acids 110–125 and 126–145 of mouse LIN-7A/Veli-2 PDZ domain (Borg et al., 1998; Kaech et al., 1998). Cysteines were added to the C-termini of the synthetic peptides to facilitate coupling to keyhole limpet hemocyanin, with the haptenization and injection of the rabbits being performed as previously described (Pietrini et al., 1994). The following monoclonal antibodies were also used: anti-β-catenin (Transduction Laboratories; Sigma), anti-Pan cadherin (Sigma), anti-tubulin (Boehringer Mannheim), anti-GST (Santa Cruz), anti-E-cadherin (Uvomorulin clone DECMA-1; Sigma) and anti-VE-cadherin (gift of Dr Dejana). Fluorescein isothiocyanate (FITC)-labeled phalloidin (Sigma) was used to detect filamentous actin (final concentration 125 ng/ml).
Immunocytochemistry
The cells were fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Immunostaining with primary antibodies was followed by incubation with rhodamine-conjugated anti-rabbit, Cy2-conjugated anti-rat (E-cadherin), FITC-conjugated anti-mouse or Cy5-conjugated anti-mouse (triple immunofluorescence) IgG from Jackson Immunoresearch (West Grove, PA). Where indicated, the cells were extracted in extraction buffer (50 mM NaCl, 10 mM PIPES pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose) at 4°C for 10 min before paraformaldehyde fixation.
The confocal images were obtained using a Bio-Rad MRC-1024 confocal microscope.
Triton X-100 extraction and western blot analysis
MDCK cells grown on Petri dishes were washed twice with Ca2+- and Mg2+-containing phosphate-buffered saline (PBS) and then incubated in extraction buffer [150 mM NaCl, 10 mM Tris–HCl pH 7.4, 1 mM MgCl2, 0.5% Triton X-100, 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and a cocktail of protease inhibitors] for 20 min on ice under gentle agitation. The extraction buffer was collected and centrifuged at 14 000 g for 5 min at 4°C, and the supernatant was defined as the Triton X-100-soluble fraction. After extraction, the cells were washed gently twice with Ca2+- and Mg2+-containing PBS and then extracted using the same volume of extraction buffer with 0.5% SDS for 20 min. The extract was collected and centrifuged at 14 000 g for 5 min at 4°C, and the supernatant was defined as the Triton X-100-insoluble fraction. The same volume of Triton X-100-soluble and -insoluble fractions was solubilized in solubilization buffer (Perego et al., 1999), loaded onto a 10% SDS–polyacrylamide gel and blotted onto nitrocellulose membranes at 120 mA overnight. In the experiments performed using CHO cells, the cells were solubilized in a solubilization buffer as described in Perego et al. (2000), and equal amounts were loaded onto an 11% SDS–polyacrylamide gel and immunoblotted. The blots were immunostained with [125I]protein A (Amersham) as the secondary reagent (Perego et al., 2000) or with anti-IgG or protein A conjugated to peroxidase (Supersignal West femto maximum sensitivity substrate; Pierce).
Disassembly of the cytoskeleton in MDCK cells
In order to disrupt the actin cytoskeleton, the cells were treated with 2 µM cytochalasin D (Sigma) in normal medium for 7 min or 2 h at 37°C (Nathke et al., 1996). To disrupt the microtubules, the cells were incubated in normal medium at 4°C for 30 min, and then the medium was supplemented with 33 µM nocodazole (Sigma) and the cells incubated at 4°C for a further 30 min. Finally, the cells were transferred at 37°C for 1 h in the continuous presence of nocodazole (Nathke et al., 1996).
Immunoprecipitation
MDCK cell lysate. The MDCK cells (2 × 107 cells) were washed twice with cold Ca2+- and Mg2+-containing PBS, scraped with a rubber policeman and centrifuged at 800 g for 10 min at 4°C. The pellet was resuspended in lysis buffer (100 mM NaCl, 25 mM Tris–HCl pH 7.4, 1 mM MgCl2, 1% Triton X-100, 0.1 mM PMSF and a cocktail of protease inhibitors) (Husken et al., 1994) with or without 5 mM EDTA, and incubated at 4°C for 30 min. In one set of immunoprecipitation experiments (Figure 4E and F), the cells were homogenized by means of repeated passages through a ball-bearing homogenizer. The post-nuclear supernatant (PNS) was centrifuged at 100 000 g for 1 h, and the pellet lysed as described above.
Brain lysate. Approximately 0.5 g of rat brain was homogenized in 4 vols (w/v) of buffer containing 320 mM sucrose and 4 mM HEPES–NaOH pH 7.3. The homogenate was centrifuged at 800 g for 10 min at 4°C in order to obtain a PNS, which was then incubated for 30 min at 4°C with an equal volume of 2× lysis buffer, with or without 10 mM EDTA.
After centrifugation at 14 000 g for 15 min at 4°C, the brain or MDCK cell lysates were incubated overnight at 4°C with anti-LIN-7 rabbit polyclonal serum or a pre-immune serum (1:100), monoclonal anti- β-catenin (5 µg), anti-E-cadherin (50 µg) and anti-Pan-cadherin (50 µg) antibodies; 20 µl of protein A–Sepharose were added, and the bead-bound immunocomplexes were recovered after 2 h, washed four times with lysis buffer, solubilized with loading buffer, separated by SDS–PAGE and analyzed by means of immunoblotting with the appropriate antibodies. The immunoprecipitation results shown in the biochemical figures came from the same gel. Each immunoprecipitate (IP) was loaded into a single lane that was cut at the level of the 43 kDa molecular weight standard. The upper part was stained sequentially with the β-catenin and cadherin antibodies, whereas the lower part was immunostained with the LIN-7 antibody. Within each set of bands, the exposure time for each antibody was the same.
Affinity chromatography assays
The expression of mLIN-7A fused to GST and inserted into the pGEX-1 vector (Kaech et al., 1998) was induced in Escherichia coli DH 5α. The fusion protein was purified on glutathione–Sepharose 4B (Pharmacia) following the manufacturer’s protocol.
Binding of recombinant GST:LIN-7A to immobilized peptides. Bacterial lysates containing ∼2 µg of GST:LIN-7A were incubated with ∼20 µg of peptides immobilized on CNBr-activated Sepharose 4B (Pharmacia). In competition experiments, 60 µg of free peptides were pre-incubated with the bacterial lysate for 2 h at room temperature before overnight incubation with the Sepharose-bound peptides. Four washes were carried out at 4°C using 1.5 ml of PBS containing 0.5% Triton X-100 (50% of the detergent concentration in the bacterial lysate). The proteins bound to the Sepharose beads were solubilized, loaded onto a 10% SDS–polyacrylamide gel and blotted. The bound GST:LIN-7A fusion protein was detected using a monoclonal antibody directed against GST (Santa Cruz).
Binding of MDCK cell lysate to immobilized GST or GST:LIN-7A. MDCK cells (5 × 106 cells) were solubilized in solubilization buffer [100 mM NaCl, 25 mM Tris–HCl pH 7.4, 1 mM MgCl2, 0.5% NP-40, 5 mM dithiothreitol (DTT), 0.1 mM PMSF and a cocktail of protease inhibitors] and incubated overnight with ∼2 µg of the GST:LIN-7A fusion protein or with GST immobilized on Sepharose beads. Four washes were carried out at 4°C using 1.5 ml of PBS containing 0.5% NP-40, and the proteins were eluted with SDS–PAGE loading buffer. The bound material was resolved by SDS–PAGE, transferred to a nitrocellulose membrane and immunostained using the monoclonal antibody directed against β-catenin.
Acknowledgments
Acknowledgements
We would like to thank Dr S.K.Kim for the GST:LIN-7A fusion protein, Dr E.Dejana for the CHO cells transfected with the wild-type and truncated human VE-cadherin, Dr M.Matteoli for the hippocampal neurons, Dr A.Flora for his assistance in data bank analysis, Drs F.Clementi and N.Borgese for their helpful suggestions and comments on the manuscript, and K.Smart for his help in preparing the text.
References
- Adams C.L., Nelson,W.J. and Smith,S.J. (1996) Quantitative analysis of cadherin–catenin–actin reorganization during development of cell–cell adhesion. J. Cell Biol., 135, 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angres B., Barth,A. and Nelson,W.J. (1996) Mechanism for transition from initial to stable cell–cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J. Cell Biol., 134, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J.P., Straight,S.W., Kaech,S.M., de Taddeo-Borg,M., Kroon,D.E., Karnak,D., Turner,R.S., Kim,S.K. and Margolis,B. (1998) Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem., 273, 31633–31636. [DOI] [PubMed] [Google Scholar]
- Butz S., Okamoto,M. and Sudhof,T.C. (1998) A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell, 94, 773–782. [DOI] [PubMed] [Google Scholar]
- Cameron P.L., Sudhof,T.C., Jahn,R. and De Camilli,P. (1991) Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J. Cell Biol., 115, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.R., Woods,D.F., Marfatia,S.M., Walther,Z., Chishti,A.H. and Anderson,J.M. (1998) Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J. Cell Biol., 142, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M.W. and Smith,S.J. (1992) A real-time analysis of growth cone–target cell interactions during the formation of stable contacts between hippocampal neurons in culture. J. Neurobiol., 23, 814–828. [DOI] [PubMed] [Google Scholar]
- Craven S.E. and Bredt,D.S. (1998) PDZ proteins organize synaptic signaling patways. Cell, 93, 495–498. [DOI] [PubMed] [Google Scholar]
- Daniel J.M. and Reynolds,A.B. (1997) Tyrosine phosphorylation and cadherin/catenin function. BioEssays, 19, 883–891. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya I.Y. and James,G.L. (2000) MAGI-1 interacts with β-catenin and is associated with cell–cell adhesion structures. Biochem. Biophys. Res. Commun., 270, 903–909. [DOI] [PubMed] [Google Scholar]
- Fanning A.S. and Anderson,J.M. (1999) Protein modules as organizers of membrane structure. Curr. Opin. Cell Biol., 11, 432–439. [DOI] [PubMed] [Google Scholar]
- Firenstein B.L., Brenman,J.E., Aoki,C., Sanchez-Peres,A.M., El-Husseini,A.E-D. and Bredt,D.S. (1999) Cypin: a cytosolic regulator of PSD-95 postsynaptic targeting. Neuron, 24, 659–672. [DOI] [PubMed] [Google Scholar]
- Goslin K. and Banker,G. (1991) Rat hippocampal neurons in low-density culture. In Banker,G. and Goslin, K (eds), Culturing Nerve Cells. MIT Press, Cambridge, MA, pp. 251–281. [Google Scholar]
- Gumbiner B.M. (1995) Signal transduction of β-catenin. Curr. Opin. Cell Biol., 7, 634–640. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell, 84, 343–357. [DOI] [PubMed] [Google Scholar]
- Hata Y., Butz,S. and Sudhof,T.C. (1996) CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J. Neurosci., 16, 2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Koh Y., Popova,E., Thomas,U., Griffith,L.C. and Budnik,V. (1999) Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell, 98, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh Y.-P., Yang,F.-C., Kharazia,V., Naisbitt,S., Cohen,A.R., Weinberg,R.J. and Sheng,M. (1998) Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J. Cell Biol., 142, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh Y.-P., Wang,T.-F., Yang,F.-C. and Sheng,M. (2000) Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature, 404, 298–302. [DOI] [PubMed] [Google Scholar]
- Husken J., Birchmeier,W. and Behrens,J. (1994) E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J. Cell Biol., 127, 2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M., Hata,Y., Deguchi,M., Ide,N., Hirao,K., Yao,I., Nishioka,H. and Takai,Y. (1999) Isolation and characterization of mammalian homologues of Caenorhabditis elegans lin-7: localization at cell–cell junctions. Oncogene, 18, 2811–2817. [DOI] [PubMed] [Google Scholar]
- Jo K., Derin,R., Li,M. and Bredt,D.S. (1999) Characterization of MALS/Velis-1, -2 and -3: a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J. Neurosci., 19, 4189–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S.M., Whitfield,C.W. and Kim,S.K. (1998) The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C.elegans EGF receptor LET-23 in vulval epithelial cells. Cell, 94, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberov E., Makarova,O., Roth,M., Liu,A., Karnak,D., Straight,S. and Margolis,B. (2000) Molecular cloning and characterization of Pals, proteins associated with mLin-7. J. Biol. Chem., 275, 11425–11431. [DOI] [PubMed] [Google Scholar]
- Lampugnani M.G., Corada,M., Andriopoulou,P., Esser,S., Risau,W. and Dejana,E. (1997) Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J. Cell Sci., 110, 2065–2077. [DOI] [PubMed] [Google Scholar]
- Le T.L., Yap,A.M. and Stow,J.L. (1999) Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol., 146, 219–230. [PMC free article] [PubMed] [Google Scholar]
- Lue R.A., Brandin,E., Chan,E. and Branton,D. (1996) Two independent domains of hDlg are suffucient for subcellular targeting: the PDZ1–2 conformational unit and an alternatively spliced domain. J. Cell Biol., 135, 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A., Sudhof,T.C. and Bezprozvanny,I. (1999) Association of neuronal calcium channels with modular adaptor proteins. J. Biol. Chem., 274, 24453–24456. [DOI] [PubMed] [Google Scholar]
- McNeill H.M., Ozawa,R., Kemler,R. and Nelson,W.J. (1990) Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell, 62, 309–316. [DOI] [PubMed] [Google Scholar]
- Nathke S., Hinck,L., Swedlow,J.R., Papkoff,J. and Nelson,W.J. (1994) Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J. Cell Biol., 125, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke S., Adams,C.L., Polakis,P., Sellin,J.H. and Nelson,W.J. (1996) The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol., 134, 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P., Caveda,L., Breviario,F., Mandoteanu,I., Lampugnani,M.G. and Dejana,E. (1995) Catenin-dependent and -independent functions of vascular endothelial cadherin. J. Biol. Chem., 270, 30965–30972. [DOI] [PubMed] [Google Scholar]
- Perego C., Bulbarelli,A., Longhi,R., Caimi,M.,Villa,A., Caplan,M.J. and Pietrini,G. (1997) Sorting of two polytopic proteins, the γ-aminobutyric acid and betaine transporters, in polarized epithelial cells. J. Biol. Chem., 272, 6584–6592. [DOI] [PubMed] [Google Scholar]
- Perego C., Vanoni,C., Villa,A., Longhi,R., Kaech,S.M., Frohli,E., Hajnal,A., Kim,S.K. and Pietrini,G. (1999) PDZ-mediated interactions retain the epithelial GABA transporter on the basolateral surface of polarized epithelial cells. EMBO J., 18, 2384–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C., Vanoni,C., Bossi,M., Massari,S., Basudev,H., Longhi,R.and Pietrini,G. (2000) The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J. Neurochem., 75, in press. [DOI] [PubMed] [Google Scholar]
- Pietrini G., Suh,Y.J., Edelmann,L., Rudnick,G. and Caplan,M.J. (1994) The axonal γ-aminobutyric acid transporter GAT-1 is sorted to the apical membranes of polarized epithelial cells. J. Biol. Chem., 269, 4668–4674. [PubMed] [Google Scholar]
- Shapiro L. and Colman,D.R. (1999) The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron, 23, 427–430. [DOI] [PubMed] [Google Scholar]
- Simske J.S., Kaech,S.M., Harp,S.A. and Kim,S.K. (1996) LET-23 receptor localization by the cell junction protein LIN-7 during C.elegans vulval induction. Cell, 85, 195–204. [DOI] [PubMed] [Google Scholar]
- Straight S.W., Karnak,D., Borg,J.P., Kamberov,E., Dare,H., Margolis,B. and Wade,J.B. (2000) mLin-7 is localized to the basolateral surface of renal epithelia via its NH2 terminus. Am. J. Physiol., 278, F464–F475. [DOI] [PubMed] [Google Scholar]
- Uchida N., Honjo,Y., Johnson,K.R., Wheelock,M.J. and Takeichi,M. (1996) The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol., 135, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Reuver,S.M., Kuhlendahl,S., Chung,W.J. and Garner,C.C. (1998) Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J. Cell Sci., 111, 2365–2376. [DOI] [PubMed] [Google Scholar]