Abstract
The adenomatous polyposis coli (APC) tumor suppressor is an essential negative regulator in the evolutionarily conserved Wnt/Wingless (Wg) signal transduction pathway. During normal development, Wnt signaling is required not only to induce cell proliferation and cell fate specification, but also to induce apoptotic cell death. However in some malignant states triggered by APC loss, inappropriate activation of Wnt signaling promotes cell survival and inhibits cell death, indicating that the cellular response to APC loss and Wnt signaling is highly dependent on cell context. This chapter summarizes our current understanding of the role of APC and Wnt signaling in the regulation of apoptosis, based upon studies from fly and mouse in vivo models, as well as cultured carcinoma cells.
Keywords: APC, β-catenin, Wnt, Wg, Arm, Armadillo, TCF, signaling, apoptosis, caspase, Drosophila, retinal photoreceptors, neural crest, colon cancer, intestinal epithelial cells, crypt
Introduction
Mutation of both alleles of the human adenomatous polyposis coli (APC) tumor suppressor triggers the development of upper and lower gastrointestinal polyps and carcinoma, and less frequently, hepatocellular carcinoma and hepatoblastoma.1–7 In addition to developing a number of extra-intestinal manifestations, individuals with a germline mutation in one APC allele develop hundreds to thousands of neoplastic colorectal polyps as a consequence of somatic mutation in the other APC allele (reviewed in 8). Some of these polyps invariably progress to carcinoma, and without surgical intervention, the mean age at diagnosis of colorectal adenocarcinoma is 39 years.9 Biallelic inactivation of APC is also found in the earliest developmental stages of greater than 80% of sporadic colorectal carcinomas, which are the second and third leading cause of cancer-related death, respectively, in men and women living in the United States.10–12 Together, these observations identify APC as a primary gatekeeper of cell proliferation and survival in the colonic epithelium.13 (see also Kwong and Dove, this book)
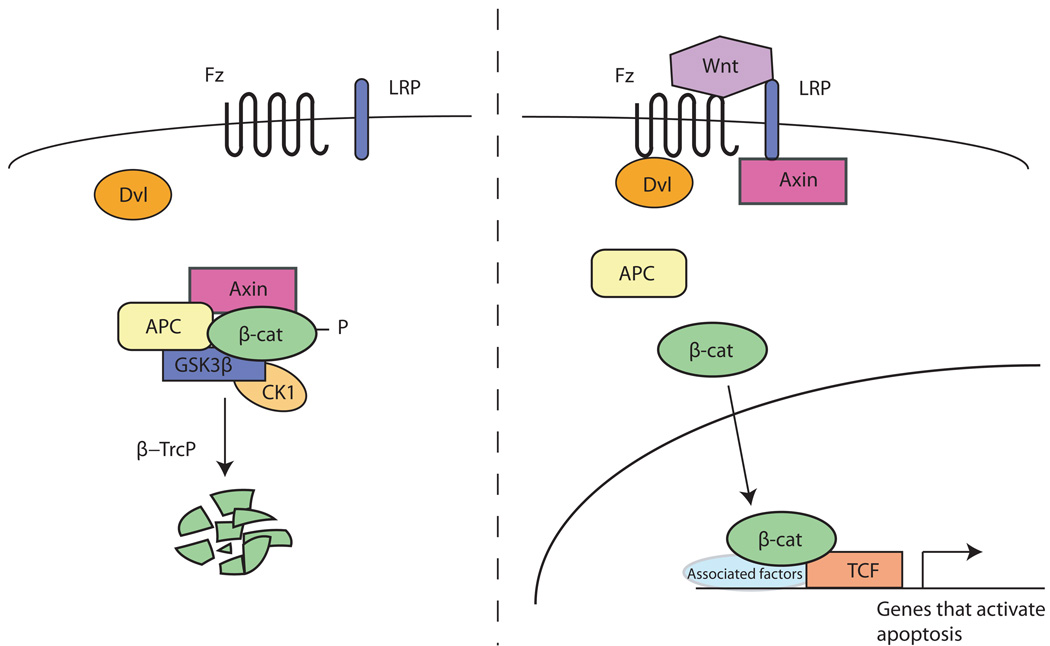
APC is an essential negative regulator in the evolutionarily conserved Wnt/Wingless (Wg) signal transduction pathway (Figure 1).14–16 In the absence of Wnt signaling, APC forms a “destruction complex” in the cytoplasm with Glycogen Synthase Kinase 3 (GSK3), Casein Kinase 1 (CK1), Axin, and β-catenin/Armadillo (Arm). GSK3 and CK1 sequentially phosphorylate specific serine and threonine residues in the amino-terminus of β-catenin/Arm, an event that depends on the scaffolding functions of Axin and APC. Phosphorylated β-catenin/Arm is targeted for ubiquitination by the E3 ubiquitin-ligase β-TrCP and subsequently degraded by the proteasome. Binding of the Wnt ligand to its co-receptors Frizzled and Low-density lipoprotein-receptor-related proteins 5/6 (LRP5/6), stimulates the dissociation of Axin from APC, and leads to the stabilization of cytoplasmic and nuclear β-catenin/Arm. Nuclear β-catenin/Arm binds to the transcription factor T-cell factor/lymphoid enhancement factor (TCF/LEF) and activates transcription of target genes in cooperation with Legless/Bcl-9 and Pygopus. β-catenin/Arm regulates the transcription of genes involved in a number of cellular events, including cell fate determination, cell proliferation, cell differentiation and apoptosis (see Cadigan, this book).
Figure 1.
Schematic representation of the evolutionarily conserved Wnt/Wingless signal transduction pathway.
This chapter summarizes our current understanding of the role of APC and Wnt signaling in induction of apoptotic cell death. Activation of the Wnt signaling pathway has been shown to both positively and negatively regulate apoptosis.17–27 In concordance with its essential role as a negative regulator of Wnt signaling, APC activity impacts the induction of programmed cell death in a number of cell types. We describe examples from fly and mouse model systems in which APC is required to prevent apoptosis, and thereby promote cell survival, during normal development. Conversely, analysis of APC loss in cultured colonic carcinoma cells reveals that the resultant activation of β-catenin signaling promotes cell survival and inhibits cell death. Together, these studies indicate an essential role for APC and Wnt transduction in regulating the decision to undergo apoptotic death, and reveal the importance of cell context in determining the response to APC loss.
Overview of apoptotic cell death
Apoptotic cell death is an evolutionarily-conserved process that is critical for normal development and for homeostasis in the adult life of animals (reviewed in 28). During embryogenesis, apoptotic death is required for the patterning of many tissues, and for eliminating tissues that have outlived their usefulness. In adults, apoptosis serves to eliminate infected cells, cells having undergone DNA damage, and inappropriately proliferating cells. In addition, apoptotic death is also important in selecting the immune repertoire and for maintaining homeostasis in tissue size. Apoptosis is particularly important in maintaining homeostasis in regenerating tissues, such as the absorptive epithelium of the small intestine, which in mice, turns over entirely every three to five days (reviewed in 14). Maintenance of the normal crypt-villus intestinal structures requires continual production of new cells in the crypt compartment, as well as elimination of cells by apoptotic death at the tip of the villus (for more details see Kwong and Dove, this volume).
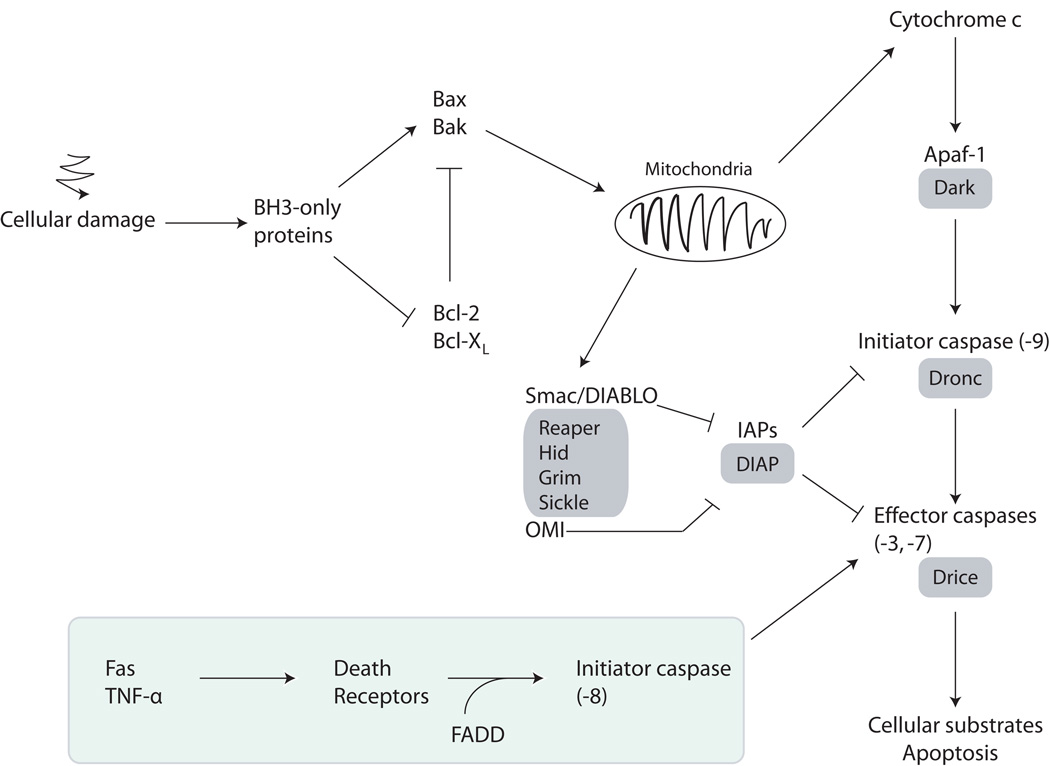
Apoptosis is mediated by a family of cysteine proteases known as caspases. Caspases are initially expressed as inactive pro-caspase precursors that must undergo proteolytic activation. Initiator caspases are activated in response to developmental signals and cellular stress, and cleave the precursor forms of effector caspases. Activated effector caspases in turn cleave a series of cellular substrates, such as Bid, RB and Poly(ADP-ribose) polymerase (reviewed in 29), resulting in apoptosis. Two pathways trigger caspase activation and apoptosis in mammalian cells: the intrinsic and extrinsic pathways (Figure 2; reviewed in refs. 30–32). The intrinsic apoptosis pathway involves the release of Cytochrome c from mitochondria and is regulated through the Bcl-2 family of proteins, while the extrinsic apoptosis pathway is initiated by the binding of death ligands, such as Fas and TNF-α, to cell surface death receptors.30, 31
Figure 2.
Simplified schematic representation of the apoptosis pathway in mammals and Drosophila. The Drosophila proteins are boxed in grey. The extrinsic pathway is boxed in green.
APC prevents neuronal apoptosis during retinal development in Drosophila
As the Wnt/Wg signaling pathway is evolutionarily conserved, genetic studies in Drosophila have been instrumental in identifying new components in the pathway, and for elucidating their in vivo function. While Wg signaling has well-established roles in cell proliferation and cell fate specification during normal development (see also Cadigan, this book), recent studies have revealed that Wg signaling is also important for developmentally regulated apoptotic cell death, and highlight the importance of β-catenin/Arm signaling in this process. Two well-established examples of Wg-induced apoptosis occur during the development of the fly retina. First, Wg signaling is required to refine the highly-ordered compound eye structure by eliminating excess cells that are present between each of approximately 750 unit ommatidia.33 Second, Wg signaling is also required to induce the death of all retinal neurons (photoreceptors) at the edge of the eye (Figure 3), a process believed to be important for eliminating defective neurons, and for sculpting of the retinal periphery.18,34 During eye development, Wg is secreted by epithelial cells that surround the retina, and spreads into the eye edge to activate β-catenin/Arm signaling. Transduction of high-level β-catenin/Arm signaling activates the transcription of the three major apoptosis effectors, reaper, hid, and grim (see Figure 2). Inactivation of Wg signaling in the retinal periphery results in an excessive accumulation of retinal neurons, and a disordered eye structure, revealing an essential role for Wg in developmentally regulated apoptotic death.18,34
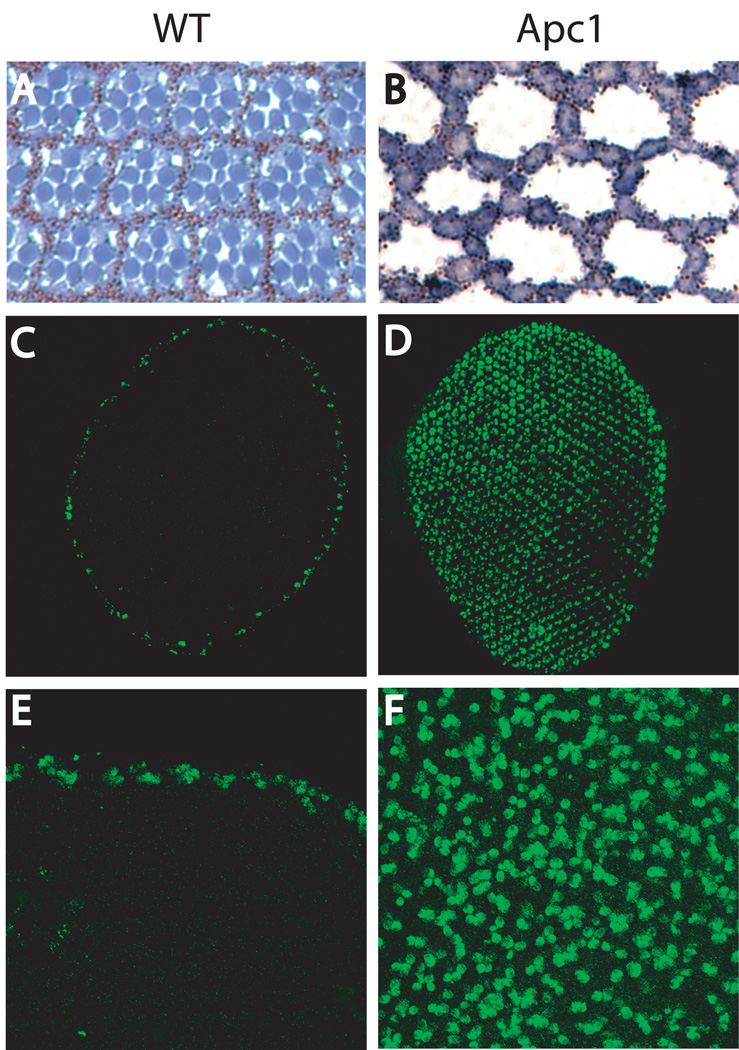
Figure 3.
Inactivation of Apc1 causes apoptosis of Drosophila retinal photoreceptors. (A, C, E) Wild type (WT) eye. (B, D, F) Apc1 mutant. Cross-section of the retina are presented in A and B. In WT (A), Photoreceptors (in blue) are surrounded by pigment cells. In the Apc1 mutant (B), photoreceptors are absent and only pigment cells remain. Activated Caspase-3 expression is presented in C–F. Whole retinas are shown in C and D and close-ups of the retina are shown in E and F.
Analysis of APC function in the fly eye confirms the critical role of Wg signaling in regulating apoptosis during retinal development. In Drosophila, as in humans, there are two APC homologs (Apc1 and Apc2), and genetic studies have provided evidence that the function of APC as a critical negative regulator of β-catenin signaling is conserved from flies to humans.17,35,36 Inactivation of Apc1 results in hyperactivation of β-catenin/Arm signaling in all retinal photoreceptors17,37, which induces both their apoptotic death, and a concomitant hypertrophy of the neighboring retinal pigment cells (Figure 3).17 Supporting the conclusion that this photoreceptor apoptosis results from ectopic β-catenin/Arm signaling, the apoptosis can be suppressed simply by reducing the gene dosage of Arm, or its transcriptional activator dTCF/Pangolin. Conversely, in otherwise wild-type flies, photoreceptor apoptosis can be induced by overexpression of Wingless or Arm.17,38,39 Thus, the apoptotic response of all photoreceptors upon Apc1 loss is similar to the response of peripheral photoreceptors to Wg signaling at the retinal edge during normal eye development.18,34 Furthermore, in both wild-type and in Apc1 mutant flies, elimination of the apoptosis effectors hid, reaper, and grim prevents photoreceptor apoptosis18; however, whether these three genes are direct targets of Wnt transduction remains to be elucidated. Together, these studies reveal a critical role for Wg transduction in promoting apoptosis, and for APC in promoting cell survival in retinal cells in which Wg is low or absent.
Unexpectedly, the fly retinal cell death phenotype induced by Apc1 loss parallels a retinal defect found in humans with germline mutations in APC, termed congenital hypertrophy of the retinal pigment epithelium.40 In these individuals, congenital retinal lesions are often bilateral and multifocal. In the rare instances in which these lesions have been sectioned at autopsy, they have been found to be composed of degenerated photoreceptors and hypertrophied pigment cells.41,42 Thus, reduction of APC activity in flies and humans results in retinal defects that are at least superficially similar. A mouse model for APC loss recapitulates these retinal lesions43, and provides a means to determine whether, as in the fly, the retinal neuronal degeneration induced by APC loss in mammals results from an apoptotic response to hyperactivation of β-catenin signaling.
APC prevents the apoptotic death of mammalian cephalic and cardiac neural crest cells
Neural crest development in the mouse provides another interesting example of the role of APC in preventing apoptosis and promoting cell survival. Neural crest cells are a multipotent stem cell population that migrates from the dorsal neural tube to diverse positions throughout the body, differentiating into a variety of cell types.44 Derivatives of neural crest include bone and cartilage tissues, peripheral nerves, glia, smooth muscle cells, Schwann cells and melanocytes. The Wnt signaling pathway plays an important role in the early stages of neural crest development, such as neural crest induction and melanocyte formation.45–48 Hasegawa et al.49 analyzed the function of APC in neural crest cells by specifically inactivating Apc in the neural crest of mice, using the Cre-loxP recombination system. The mutant mice generated had markedly increased apoptosis, as revealed by TUNEL staining, in the ventral craniofacial mesenchyme, the branchial arch and the cardiac outflow tract, indicating that APC loss leads to the apoptosis of a subset of neural crest derivatives. As a result, these mice had severe craniofacial and cardiac defects and died shortly after birth. All bones derived from the cephalic neural crest were affected, and cardiac defects included ventricular septal defects and persistent truncus arteriosus. Remarkably, neural crest derivatives destined to become bone or cartilage undergo apoptosis, while those that differentiate as peripheral nerves, Schwann cells, or melanocytes survive, indicating that the apoptotic response of neural crest cells to Apc loss is context dependent. Increased β-catenin levels were observed in tissues containing TUNEL-positive cells, indicating that the apoptosis induced by Apc loss may result from increased β-catenin activity; however a direct link between the apoptosis and increased Wnt signaling awaits further investigation.
APC loss and activation of Wnt signaling results in both increased cell proliferation and increased apoptosis in mammalian intestinal epithelia
Analysis of the mammalian intestinal epithelium exposed to different levels of Wnt signaling revealed unexpected effects on proliferation and apoptosis. The mammalian intestinal epithelium is a self- renewing tissue organized into highly ordered structures composed of villi and crypts (see Kwong & Dove and Sansom, this volume). Mitotically active stem cells present at the base of each crypt migrate along the crypt-villus axis where they differentiate, carry out their specific role in the epithelium, and finally undergo apoptosis and are shed into the gut lumen. The Wnt signaling pathway is a key regulator of cell fate along the crypt-villus axis.14,50–52 Inactivation of Wnt signaling results in a complete loss of the crypt progenitor compartment, while hyperactivation of signaling results in ectopic expression of Wnt-target genes and hyperproliferation of cells in the crypt compartment. As a result of Wnt pathway activation, epithelial cells displayed a number of phenotypes associated with colorectal lesions. Migration of epithelial cells along the crypt-villus axis was abrogated, differentiation was arrested, and increased proliferation was observed. In addition, proliferation was no longer confined to the base of the crypt. Together, these studies reveal the critical role of Wnt signaling in regulating self-renewal of the intestinal epithelium.
Unexpectedly, hyperactivation of Wnt signaling in the intestinal epithelium results not only in increased cell proliferation, but also in increased apoptosis. Increased apoptosis was observed in three distinct mouse models that address the effects of increased Wnt signaling in the intestinal epithelium. First, directed expression of a constitutively activated β-catenin, similar to that found in some colonic carcinomas, resulted not only in increased cell proliferation in crypt epithelial cells, but also in increased apoptotic death.53 Second, conditional inactivation of APC in the mouse intestinal epithelium led to the accumulation and nuclear translocation of β-catenin, and increased Wnt signaling.54 This in vivo model for increased Wnt signaling resulted in qualitatively similar results: not only was cell proliferation increased, but apoptotic cell death was increased also. Third, expression of a β-catenin/Lef-1 fusion protein that enhances Wnt signaling in the intestinal epithelium of a chimeric mouse model also resulted in increased apoptosis.55 In these mice, increased apoptosis was restricted to only those intestinal epithelial cells that expressed the β-catenin/Lef-1 fusion protein, and unexpectedly was not associated with increased cell proliferation.
Based on these data, it has been proposed that different levels of β-catenin signaling result in qualitatively distinct cellular responses.55 In this model, high levels of β-catenin signaling in the intestinal epithelia induce apoptosis, while intermediate levels of signaling result in sustained cell proliferation. Conversely, complete loss of β-catenin signaling results in the absence of proliferation and differentiation. Evidence supporting dosage-dependent cellular responses to APC loss and β-catenin signaling has been documented in several models, including mouse embryonic stem cells.56,57 Whether high-level β-catenin signaling is important for an apoptotic response in intestinal epithelia, and whether there exists a direct link between β-catenin mediated target gene activation and the induction of apoptosis in these cells awaits further experimental analysis.
Promotion of cell survival and negative regulation of apoptosis by Wnt signaling in carcinomas
Although activation of Wnt signaling can induce both proliferation and apoptosis during normal development, Wnt signaling is also thought to have the opposite role of promoting cell survival and increasing cell proliferation in cancer cells. For instance, Wnt expression is upregulated in a number of human cancers,58–64 and monoclonal antibodies or siRNA directed against Wnt-1 and Wnt-2 in cultured carcinoma cells leads to β-catenin downregulation and induction of apoptosis.22–25 When either melanoma, non-small cell lung carcinoma, breast carcinoma, mesothelioma, or sarcoma cells overexpressing Wnt-1 or Wnt-2 are injected in nude mice along with the Wnt-1 or Wnt-2 monoclonal antibody, respectively, tumor growth is inhibited and an increased number of apoptotic cells is observed as compared to mice injected with the Wnt-1 or Wnt-2 overexpressing cells alone.22, 24
Similarly, in several different types of cultured carcinoma cells, decreased β-catenin levels resulting from forced expression members of the destruction complex leads to induction of apoptosis. Hepatocellular and colorectal carcinoma cells, for instance, have high levels of nuclear β-catenin due to loss of function mutations in APC or Axin, or hyperactivating mutations in β-catenin. Expressing APC or Axin in these cells induces apoptosis.65 Similarly, expressing APC or a dominant negative TCF in a colon carcinoma cell line lacking endogenous APC increases caspase expression and activity and stimulates apoptosis.66,67 Together, these studies provide evidence that activation of Wnt signaling is important for promoting cell survival in some types of carcinoma cells, and again suggest that the cellular response to Wnt signaling is highly dependent upon cell context.
Recent studies have identified several candidate target genes that act to prevent apoptosis, and are regulated by Wnt signaling. Huang et al.68 analyzed the expression profile of HeLa cells in which β-catenin expression was downregulated by RNAi. The expression of a number of apoptosis-related genes, including MYBL2, BAG2, BAG3, PTEN, HIF1A, PDCD6IP and DAP3, is increased in these cells. The anti-apoptotic protein Bcl-XL has also been identified as a potential target of Wnt transduction.69 In thymocytes, whose survival depends on Wnt signaling, inactivation of TCF-1 leads to a decrease in Bcl-XL expression. These studies are among the first to delineate targets of Wnt transduction that are important for inhibition of apoptosis.
Conclusions
The Wnt signal transduction pathway has well-established roles in promoting cell proliferation and differentiation in both vertebrates and invertebrates. Recent studies have revealed that during normal development, Wnt signaling also has an essential role in promoting apoptosis. In some cells that are not normally destined to die, loss of APC activates ectopic Wnt signaling and results in excessive apoptotic death, revealing that APC is required to promote cell survival in different developmental contexts. Conversely, in some cultured carcinoma cells, Wnt signaling is critical for cell survival, and inhibition of apoptosis, and recent work has revealed a number of putative target genes that might directly regulate this Wnt signaling-induced response. Together, these studies in both in vivo models and cultured cell lines highlight the importance of cellular context in determining the apoptotic response to APC loss and Wnt transduction.
How do some cells respond to the loss of APC by proliferating, while others respond by dying? One current challenge in addressing this question is to determine the cell contexts in which elevated β-catenin signaling directly induces an apoptotic response to APC loss, and whether apoptosis results specifically from high-level of β-catenin signaling. The recent generation of numerous reagents that allow either conditional activation or conditional inhibition of Wnt transduction will be instrumental in addressing this issue. In addition, determining the molecular factors that establish the context of a cell, and thereby influence the cellular response to activated Wnt signaling, also awaits further analysis. Understanding how these effectors regulate the decision to induce proliferation, differentiation, or death is of fundamental importance in developing novel therapies that redirect APC mutant colonic tumor cells from a proliferation to a cell death program.
Acknowledgements
We thank Edward G. Hughes and Jason R. Baird for the photographs in Figure 3. Work in our laboratory is supported by the Norris Cotton Cancer Center, the Emerald Foundation, the Scholars Program of the General Motors Cancer Research Foundation, the American Cancer Society (IRG-82-003-21), the National Institutes of Health (RO1CA105038), and the Howard Hughes Medical Institute, through an award from the Biomedical Research Support Program for Medical Schools to Dartmouth Medical School (76200-560801).
References
- 1.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 2.Joslyn G, Carlson M, Thliveris A, et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 4.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 5.Gruner BA, DeNapoli TS, Andrews W, et al. Hepatocellular carcinoma in children associated with Gardner syndrome or familial adenomatous polyposis. J Pediatr Hematol Oncol. 1998;20(3):274–278. doi: 10.1097/00043426-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Attard TM, Cuffari C, Tajouri T, et al. Multicenter experience with upper gastrointestinal polyps in pediatric patients with familial adenomatous polyposis. Am J Gastroenterol. 2004;99(4):681–686. doi: 10.1111/j.1572-0241.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirschman BA, Pollock BH, Tomlinson GE. The spectrum of APC mutations in children with hepatoblastoma from familial adenomatous polyposis kindreds. J Pediatr. 2005;147(2):263–266. doi: 10.1016/j.jpeds.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Bronner MP. Gastrointestinal polyposis syndromes. Am J Med Genet A. 2003;122(4):335–341. doi: 10.1002/ajmg.a.20476. [DOI] [PubMed] [Google Scholar]
- 9.Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121(1):198–213. doi: 10.1053/gast.2001.25581. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi Y, Ando H, Nagase H, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci U S A. 1992;89(10):4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell SM, Petersen GM, Krush AJ, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993;329(27):1982–1987. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- 12.Smith KJ, Johnson KA, Bryan TM, et al. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993;90(7):2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 14.Clevers H. Wnt/beta-Catenin Signaling in Development and Disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20(11):1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 16.Stadeli R, Hoffmans R, Basler K. Transcription under the control of nuclear Arm/beta-catenin. Curr Biol. 2006;16(10):R378–R385. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed Y, Hayashi S, Levine A, et al. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93(7):1171–1182. doi: 10.1016/s0092-8674(00)81461-0. [DOI] [PubMed] [Google Scholar]
- 18.Lin HV, Rogulja A, Cadigan KM. Wingless eliminates ommatidia from the edge of the developing eye through activation of apoptosis. Development. 2004;131(10):2409–2418. doi: 10.1242/dev.01104. [DOI] [PubMed] [Google Scholar]
- 19.Olmeda D, Castel S, Vilaro S, et al. Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell. 2003;14(7):2844–2860. doi: 10.1091/mbc.E03-01-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Pang KM, Evans M, et al. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11(10):3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edlund S, Lee SY, Grimsby S, et al. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25(4):1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He B, You L, Uematsu K, et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6(1):7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You L, He B, Uematsu K, et al. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 2004;64(10):3474–3478. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- 24.You L, He B, Xu Z, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64(15):5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 25.You L, He B, Xu Z, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23(36):6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 26.You Z, Saims D, Chen S, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157(3):429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Guttridge DC, You Z, et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152(1):87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay BA, Huh JR, Guo M. The genetics of cell death: approaches, insights and opportunities in Drosophila. Nat Rev Genet. 2004;5(12):911–922. doi: 10.1038/nrg1491. [DOI] [PubMed] [Google Scholar]
- 29.Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007;14(1):66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- 30.Hersey P, Zhuang L, Zhang XD. Current strategies in overcoming resistance of cancer cells to apoptosis melanoma as a model. Int Rev Cytol. 2006;251:131–158. doi: 10.1016/S0074-7696(06)51004-6. [DOI] [PubMed] [Google Scholar]
- 31.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 32.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17(6):617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordero J, Jassim O, Bao S, et al. A role for wingless in an early pupal cell death event that contributes to patterning the Drosophila eye. Mech Dev. 2004;121(12):1523–1530. doi: 10.1016/j.mod.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Tomlinson A. Patterning the peripheral retina of the fly: decoding a gradient. Dev Cell. 2003;5(5):799–809. doi: 10.1016/s1534-5807(03)00326-5. [DOI] [PubMed] [Google Scholar]
- 35.McCartney BM, Dierick HA, Kirkpatrick C, et al. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146(6):1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Waltzer L, Bienz M. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat Cell Biol. 1999;1(3):144–151. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129(7):1751–1762. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- 38.Brunner E, Brunner D, Fu W, et al. The dominant mutation Glazed is a gain-of-function allele of wingless that, similar to loss of APC, interferes with normal eye development. Dev Biol. 1999;206(2):178–188. doi: 10.1006/dbio.1998.9136. [DOI] [PubMed] [Google Scholar]
- 39.Freeman M, Bienz M. EGF receptor/Rolled MAP kinase signalling protects cells against activated Armadillo in the Drosophila eye. EMBO Rep. 2001;2(2):157–162. doi: 10.1093/embo-reports/kve019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair NP, Trempe CL. Hypertrophy of the retinal pigment epithelium associated with Gardner's syndrome. Am J Ophthalmol. 1980;90(5):661–667. doi: 10.1016/s0002-9394(14)75133-5. [DOI] [PubMed] [Google Scholar]
- 41.Buettner H. Congenital hypertrophy of the retinal pigment epithelium. Am J Ophthalmol. 1975;79(2):177–189. doi: 10.1016/0002-9394(75)90069-0. [DOI] [PubMed] [Google Scholar]
- 42.Traboulsi EI, Murphy SF, de la Cruz ZC, et al. A clinicopathologic study of the eyes in familial adenomatous polyposis with extracolonic manifestations (Gardner's syndrome) Am J Ophthalmol. 1990;110(5):550–561. doi: 10.1016/s0002-9394(14)77880-8. [DOI] [PubMed] [Google Scholar]
- 43.Marcus DM, Rustgi AK, Defoe D, et al. Retinal pigment epithelium abnormalities in mice with adenomatous polyposis coli gene disruption. Arch Ophthalmol. 1997;115(5):645–650. doi: 10.1001/archopht.1997.01100150647013. [DOI] [PubMed] [Google Scholar]
- 44.Dorsky RI, Moon RT, Raible DW. Environmental signals and cell fate specification in premigratory neural crest. Bioessays. 2000;22(8):708–716. doi: 10.1002/1521-1878(200008)22:8<708::AID-BIES4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 45.Ikeya M, Lee SM, Johnson JE, et al. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389(6654):966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 46.Lee HY, Kleber M, Hari L, et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303(5660):1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297(5582):848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- 48.Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396(6709):370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa S, Sato T, Akazawa H, et al. Apoptosis in neural crest cells by functional loss of APC tumor suppressor gene. Proc Natl Acad Sci U S A. 2002;99(1):297–302. doi: 10.1073/pnas.012264999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111(2):241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 51.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111(2):251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 52.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 53.Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated beta-Catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998;141(3):765–777. doi: 10.1083/jcb.141.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18(12):1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong MH, Huelsken J, Birchmeier W, et al. Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef-1/beta-catenin signaling. J Biol Chem. 2002;277(18):15843–15850. doi: 10.1074/jbc.M200184200. [DOI] [PubMed] [Google Scholar]
- 56.Kielman MF, Rindapaa M, Gaspar C, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32(4):594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 57.Gaspar C, Fodde R. APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol. 2004;48(5–6):377–386. doi: 10.1387/ijdb.041807cg. [DOI] [PubMed] [Google Scholar]
- 58.Katoh M. Frequent up-regulation of WNT2 in primary gastric cancer and colorectal cancer. Int J Oncol. 2001;19(5):1003–1007. doi: 10.3892/ijo.19.5.1003. [DOI] [PubMed] [Google Scholar]
- 59.Katoh M. Expression and regulation of WNT1 in human cancer: up-regulation of WNT1 by beta-estradiol in MCF-7 cells. Int J Oncol. 2003;22(1):209–212. [PubMed] [Google Scholar]
- 60.Wong SC, Lo SF, Lee KC, et al. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196(2):145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki H, Watkins DN, Jair KW, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 62.Smith K, Bui TD, Poulsom R, et al. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer. 1999;81(3):496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dimitriadis A, Vincan E, Mohammed IM, et al. Expression of Wnt genes in human colon cancers. Cancer Lett. 2001;166(2):185–191. doi: 10.1016/s0304-3835(01)00428-1. [DOI] [PubMed] [Google Scholar]
- 64.Holcombe RF, Marsh JL, Waterman ML, et al. Expression of Wnt ligands and Frizzled receptors in colonic mucosa and in colon carcinoma. Mol Pathol. 2002;55(4):220–226. doi: 10.1136/mp.55.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satoh S, Daigo Y, Furukawa Y, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24(3):245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 66.Chen T, Yang I, Irby R, et al. Regulation of caspase expression and apoptosis by adenomatous polyposis coli. Cancer Res. 2003;63(15):4368–4374. [PubMed] [Google Scholar]
- 67.Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci U S A. 1996;93(15):7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang M, Wang Y, Sun D, et al. Identification of genes regulated by Wnt/beta-catenin pathway and involved in apoptosis via microarray analysis. BMC Cancer. 2006;6:221. doi: 10.1186/1471-2407-6-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ioannidis V, Beermann F, Clevers H, et al. The beta-catenin--TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2(8):691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]