Abstract
Background
Respiratory tract viral infections result in asthma exacerbations. Toll-like receptor (TLR) 7 is a receptor for viral single-stranded RNA and is expressed at high levels in the lungs.
Objective
Because TLR7 polymorphisms are associated with asthma, we examined the effects of TLR7 agonists in guinea pig airways.
Methods
We induced bronchoconstriction in guinea pigs in vivo by means of electrical stimulation of the vagus nerve or intravenous administration of acetylcholine and measured the effect of a TLR7 agonist administered intravenously. We induced contraction of airway smooth muscle in segments of isolated guinea pig tracheas in vitro and measured the effect of TLR7 agonists, antagonists, and pharmacologic inhibitors of associated signaling pathways administered directly to the bath.
Results
TLR7 agonists acutely inhibited bronchoconstriction in vivo and relaxed contraction of airway smooth muscle in vitro within minutes of administration. Airway relaxation induced by the TLR7 agonist R837 (imiquimod) was partially blocked with a TLR7 antagonist and was also blocked by inhibitors of large-conductance, calcium-activated potassium channels; prostaglandin synthesis; and nitric oxide generation. Another TLR7 agonist, 21-mer single-stranded phosphorothioated polyuridylic acid (PolyUs), mediated relaxation that was completely blocked by a TLR7 antagonist.
Conclusions
These data demonstrate a novel protective mechanism to limit bronchoconstriction and maintain airflow during respiratory tract viral infections. The fast time frame is inconsistent with canonical TLR7 signaling. R837 mediates bronchodilation by means of TLR7-dependent and TLR7-independent mechanisms, whereas PolyUs does so through only the TLR7-dependent mechanism. TLR7-independent mechanisms involve prostaglandins and large-conductance, calcium-activated potassium channels, whereas TLR7-dependent mechanisms involve nitric oxide. TLR7 is an attractive therapeutic target for its ability to reverse bronchoconstriction within minutes.
Keywords: Toll-like receptor 7, asthma, virus, bronchodilator, large-conductance, calcium-activated potassium channel, prostaglandins, adenosine, nitric oxide, imiquimod, IRS661, guinea pig
Viruses have been detected in 80% of acute exacerbations of asthma in children and 50% of acute exacerbations in adults.1,2 These airway viruses induce inflammatory responses in lungs through multiple signaling pathways, including protein kinase R, retinoid inducible gene I (and related helicases), and multiple Toll-like receptors (TLRs).
TLRs respond to multiple viruses as an early and rapid defense. TLR signaling results in an immediate inflammatory response and induction of an adaptive immune response to clear viral infection.3 Most respiratory viruses have a single-stranded RNA (ssRNA) genome. On recognition of viral ssRNA4, TLR7 signals through the adaptor molecule MyD88, activating transcription factors that induce production of antiviral cytokines, such as interferons, and inflammatory cytokines, such as TNF-α.4 Although necessary to clear infection, the resulting inflammation might also impair airway function and participate in virus-induced asthma attacks. The highest TLR7 expression level is found in the lungs,5 most likely in multiple cell types, including inflammatory cells,6 epithelial cells,7 and human airway smooth muscle cells.8 Furthermore, expression in human smooth muscle cells is potentiated by IL-1β and TNF-α, cytokines commonly found in lungs in response to infection.8 TLR7 polymorphisms have been associated with asthma, although the functional effects of these polymorphisms are not known.9,10
To understand whether TLR7 directly affects respiratory physiology, we tested the effects of a synthetic TLR7 agonist, R837 (imiquimod), in guinea pigs in vivo. We were surprised to find that R837 rapidly and completely abolished bronchoconstriction. This was reproduced in isolated guinea pig tracheas in vitro. Here we demonstrate that R837-mediated bronchodilation is mediated by TLR7 at low doses but that off target (TLR7-independent) effects are seen at higher doses, which are also bronchodilating. In addition, we show that the mechanism of TLR7-dependent bronchodilation involves nitric oxide, whereas the TLR7-independent bronchodilation involves prostaglandins and large-conductance, calcium-activated potassium channels (BkCa). These might represent potent physiological mechanisms of bronchodilation in the presence of respiratory viral RNA by which airflow can be maintained during virus-induced inflammation and counteract virus-induced airway occlusion. TLR7 polymorphisms, if associated with loss of expression or function, might predispose to asthma by eliminating this compensatory bronchodilation during viral infections. The rapid bronchodilation observed within minutes makes TLR7 an attractive and novel target for the treatment of asthma.
METHODS
Animals
Specific pathogen-free female Hartley guinea pigs (300–350 g; Elm Hill Breeding Labs, Chelmsford, Mass) were shipped in filtered crates, kept in high-efficiency particulate-filtered air, and fed a normal diet.
Animals were handled in accordance with National Institutes of Health guidelines. Protocols were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University.
In vivo guinea pig bronchoconstriction
Bronchoconstriction was measured in vivo, as previously reported.11 Guinea pigs were anaesthetized with urethane (1.9 mg kg−1 administered intraperitoneally), chemically sympathectomized with guanethidine (5 mg kg−1 administered intravenously), and paralyzed with a constant infusion of succinylcholine (10 µg kg−1 min−1 administered intravenously). Animals were tracheostomized and mechanically ventilated (tidal volume, 2.5 mL; 100 breaths/min). Both vagus nerves were cut, and distal portions were attached to platinum electrodes submerged in liquid paraffin. Drugs were administered through cannulas in both jugular veins. Heart rate and blood pressure were measured by using a transducer connected to a cannula in one carotid artery. Pulmonary inflation pressure was measured by using a transducer connected to a side arm of the tracheal cannula. Bronchoconstriction was induced by means of electrical stimulation of the vagus nerves (10 V, 10 Hz, 0.2-ms pulse duration, 5 seconds on, and 60 seconds off) or administration of acetylcholine (2 µg kg−1). Each dose of R837 was administered cumulatively, and the next induced contraction was measured. One hundred percent maximum contraction is defined as the maximum contraction induced by the indicated bronchoconstricting stimulus after administration of vehicle and before the administration of bronchodilating drugs.
In vitro contraction of isolated guinea pig trachea
Contractions of isolated tracheas were measured in vitro, as previously reported.12 Rings (0.5 cm) of tracheas were suspended in a 5-mL organ bath (Radnoti Glass Technology, Inc, Monrovia, Calif) that contained Kreb’s solution bubbled with a 95% O2–5% CO2 gas mixture. The segments were supported by loops of silk through the tracheal lumen, with the lower thread tied to a hook at the bottom of the bath and the upper thread tied to a Grass FT03 isometric force transducer (Grass Instrument Co, Quincy, Mass). Except for studies involving isoproterenol, propranolol (1 µmol/L) was added to theKreb’s solution. Segments were equilibrated in the bath at 1 g of tension for 60 minutes and washed with Kreb’s solution every 15 minutes. Tracheal contractions were induced with methacholine (10 µmol/L), histamine (10 µmol/L), KCl (20 or 100 mmol/L), or electrical field stimulation (EFS; 100 V, 20 Hz, 0.2-ms pulse duration, 15 seconds on, and 150 seconds off). Tension recordings were made on a Powerlab/8SP (ADInstruments, Castle Hill, Australia). Except for EFS experiments, drugs were administered cumulatively to the bath after induction of sustained contractions. For EFS, drugs were administered between contractions because it induces transient contractions. One hundred percent maximum contraction is defined as the maximum contraction induced by the indicated contracting stimulus after administration of vehicle and before the administration of bronchodilating drugs. Values are normalized to a time control corresponding to each dose.
Treatments
TLR7 agonists (R837, R848, CL097, gardiquimod, 21-mer single-stranded phosphorothioated polyuridylic acid [PolyUs], and 21-mer single-stranded phosphorothioated polyadenylic acid [PolyAs]), the β2-adrenergic receptor agonist isoproterenol, and the adenosine receptor agonist 5′-(N-ethylcarboxamido) adenosine were added cumulatively in increasing concentrations to tissues precontracted as above. Before the agonist dose responses in vitro, some tracheal segments were treated for 1 hour with the TLR7 antagonist IRS661 (100 µmol/L) or for 15 minutes with the COX inhibitor indomethacin (1 µmol/L), the BkCa blocker paxilline (20 µmol/L), the general potassium channel blocker tetraethylammonium (TEA; 20 mmol/L), the nitric oxide synthase inhibitor N-methyl-L-arginine [L-NMMA] (100 µmol/L), the adenosine A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 2 µmol/L), or the adenosine A2A receptor antagonist SCH58261 (2 µmol/L).
Materials
TLR7 agonists R837, R848, CLO97, and gardiquimod were obtained from InvivoGen (San Diego, Calif). The previously described TLR7 antagonist IRS66113 and the 21-mer phosphorothioated ssRNA oligonucleotides PolyUs and PolyAs were custom synthesized by Invitrogen (Carlsbad, Calif). All other drugs were obtained from Sigma-Aldrich (St Louis, Mo).
Statistics
Two-way ANOVA with repeated measures and the Bonferroni multiple-comparison post-test was used to compare multiple means across groups. One-way ANOVA with repeated measures and the Bonferroni multiple-comparison post-test was used for the effect of a dose within a single group. Analysis was done with GraphPad Prism software (GraphPad Software, Inc, La Jolla, Calif). Significance is indicated at P values of less than .05, less than .01, and less than .001. All error bars represent SEMs.
RESULTS
A TLR7 agonist inhibits bronchoconstriction in guinea pigs in vivo
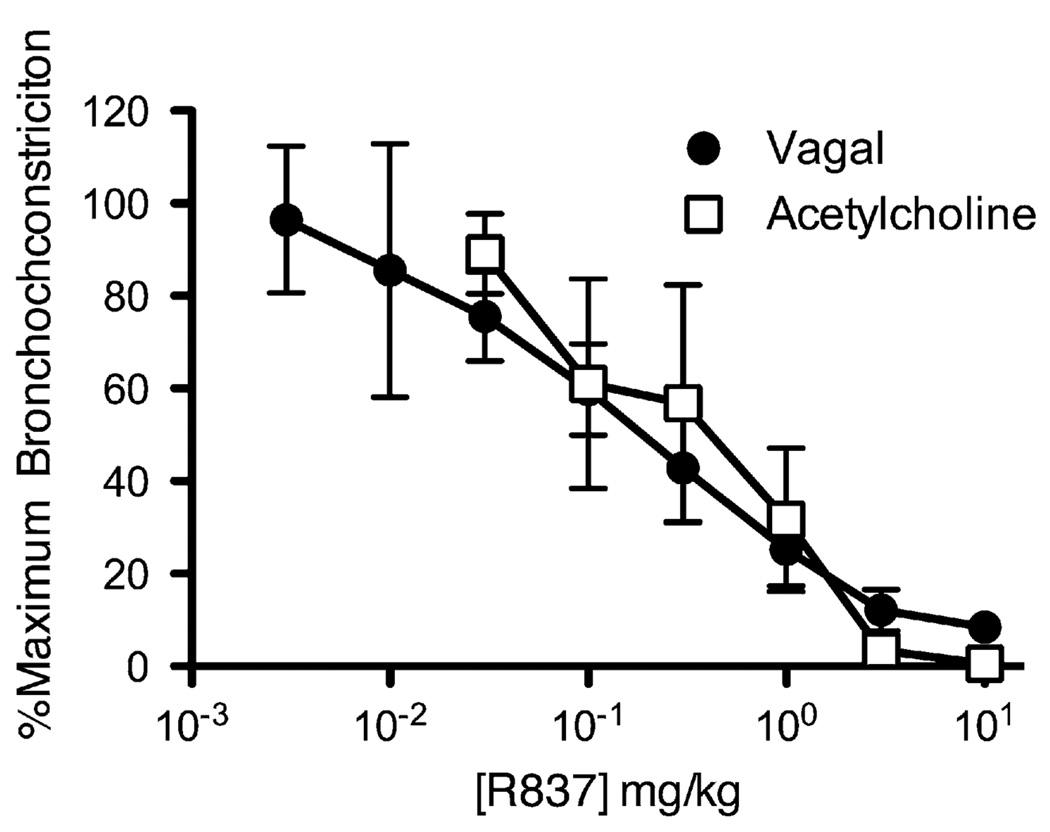
Electrical stimulation of the vagus nerves (10 V, 10 Hz, 0.2-ms pulse duration, 5 seconds on, and 60 seconds off) or intravenous administration of acetylcholine (2 µg kg−1) caused reproducible bronchoconstriction in anesthetized guinea pigs in vivo. R837 (0.003–10 mg kg−1) dose dependently inhibited subsequent bronchoconstriction induced by means of both vagal stimulation and intravenous acetylcholine (vagal stimulation inhibitory concentration of 50% [IC50] = 0.19 mg kg−1; acetylcholine IC50 = 0.49 mg kg−1; Fig 1). The effect occurred within 1 minute of administration of R837, and at higher doses, bronchoconstriction was completely inhibited. The effect of R837 was rapidly reversed, with recovery of bronchoconstriction within 20 minutes.
FIG 1.
A TLR7 agonist, R837, inhibits bronchoconstriction in guinea pigs in vivo. Bronchoconstriction was induced by means of electrical stimulation of the vagus nerves (circles) or by means of intravenous administration of acetylcholine (squares), and the effect of cumulative increasing doses of R837 administered intravenously was measured (n = 9 for vagal stimulation: maximum, 93.0 ± 21.5 mm H2O; n = 4 for acetylcholine: maximum, 70.5 ± 32.2 mm H2O; P ≤ .001 for effect of dose).
A TLR7 agonist reverses contraction of isolated guinea pig trachea
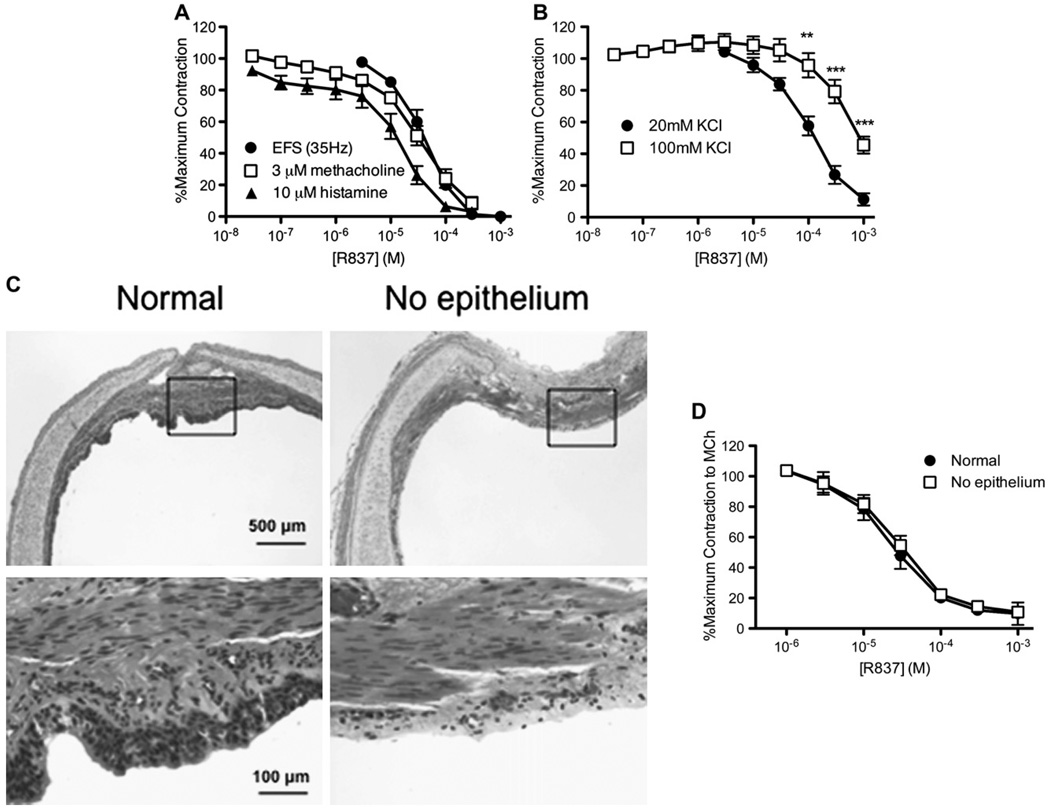
EFS of isolated guinea pig tracheas in organ baths (100 V, 20 Hz, 0.2-ms pulse duration, 15 seconds on, and 150 seconds off) caused reproducible contractions that were blocked by atropine, indicating that they were mediated through release of acetylcholine. R837 (3–1000 µmol/L) acutely reduced subsequent contractions induced by EFS (IC50 = 40 µmol/L; Fig 2, A), confirming in vivo inhibition of bronchoconstriction induced by electrical stimulation of the vagus nerves (Fig 1).
FIG 2.
A TLR7 agonist, R837, relaxes isolated guinea pig tracheas in vitro. A, Contraction of tracheal segments was induced by EFS (circles), methacholine (squares), and histamine (triangles), and the effect of cumulative increasing doses of R837 was measured (n = 3 for EFS: maximum, 0.53 ± 0.08 g; n = 6 for methacholine: maximum, 1.98 ± 0.31 g; n = 6 for histamine: maximum = 1.04 ± 0.13 g; P ≤ .001 for effect of dose). B, Contraction of tracheal segments was induced by KCl (circles, 20 mmol/L; squares, 100 mmol/L), and the effect of cumulative increasing doses of R837 was measured (n = 3 for 20 mmol/L KCl: maximum, 0.78 ± 0.23 g; n = 6 for 100 mmol/L KCl: maximum, 1.47 ± 0.30 g; P ≤ .001 for effect of dose; **P ≤ .01 and ***P ≤ .001 for 20 mmol/L vs 100 mmol/L KCl at indicated doses of R837). C, Magnification (×4 of hematoxylin and eosin–stained sections of tracheal segments with and without the epithelium, with a ×20 magnification of the region in the boxed area). The removal of epithelium can be viewed as the absence of dark gray nuclei lining the airway lumen. D, The effect of cumulative increasing doses of R837 on contraction was measured in intact tracheal segments (circles) or tracheal segments with the epithelium removed (squares; n = 2: maximum with epithelium, 1.48 ± 0.1 g; maximum without epithelium, 0.76 ± 0.28 g; P ≤ .001 for effect of dose). MCh, Methacholine.
Reproducible contractions were induced by methacholine (3 µmol/L) or histamine (10 µmol/L), both of which signal through G protein–coupled receptors, to determine whether R837 also relaxed contractions induced directly at airway smooth muscle. R837 relaxed established and sustained contractions induced by methacholine and histamine in a concentration-dependent manner (IC50 = 32 µmol/Lfor tissues contracted with methacholine and 7.2 µmol/L for tissues contracted with histamine; Fig 2, A).
To bypass surface receptors on smooth muscle, we used KCl (20 and 100 mmol/L) to directly depolarize the membrane and contract tracheal smooth muscle. R837 also relaxed KCl-induced contraction (Fig 2, B). The R837-mediated relaxation of contractions induced by 20 mmol/L KCl (IC50 = 113 µmol/L) was more potent and complete than the relaxation of contractions induced by 100 mmol/L KCl (IC50 = 779 µmol/L), suggesting a mechanism that might involve potassium channels.
As seen in vivo, relaxation of airway smooth muscle by R837 in vitro was reversible, and the full contractile response recovered within 15 minutes of washing R837 from the bath, demonstrating that relaxation of contracted airways is not due to toxic effects at airway smooth muscle.
Because we were able to replicate the in vivo bronchodilatory effect of R837 in vitro, all of the subsequent experiments were carried out in vitro.
To determine whether R837 relaxes smooth muscle contraction by bronchodilators released from the epithelium,14 we removed the epithelium from tracheal segments with a cotton swab. Epithelial removal was confirmed by means of microscopic evaluation of tracheal segments used in the bath (Fig 2, C). Removal of airway epithelium had no effect on the ability of R837 to relax airway smooth muscle precontracted with methacholine (3 µmol/L) in vitro, indicating that R837 does not act through the epithelium to induce relaxation (Fig 2, D).
Other TLR7 agonists also relax airway smooth muscle in vitro
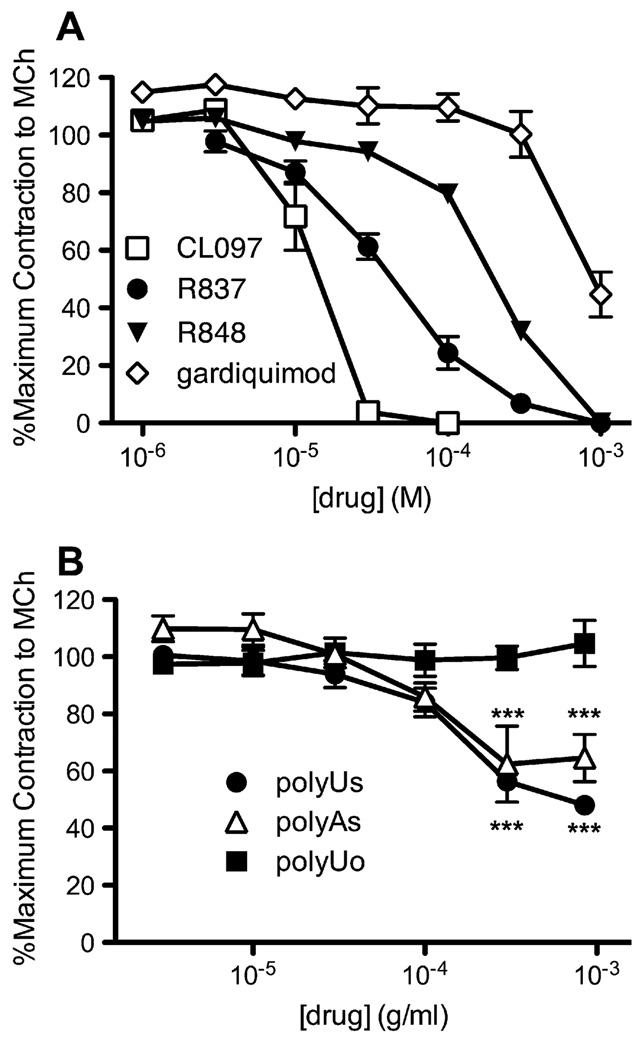
Other TLR7 agonists, CL097, R848, and gardiquimod, also relaxed isolated tracheal smooth muscle contracted with methacholine (3 µmol/L) in a concentration-dependent manner (Fig 3, A). Because R837 and these 3 other agonists are structurally related imidazoquinolines, we also tested a reported TLR7 ligand, PolyUs, of a different structural class.15 PolyUs partially relaxed contraction induced by methacholine (3 µmol/L) in a concentration-dependent manner (Fig 3, B). Mixed polyU oligonucleotides of various lengths on a phosphodiester backbone (Poly-Uo) did not induce relaxation, suggesting the relaxant effect is specific to TLR7 ligands. Another related compound, PolyAs, also partially relaxed methacholine-contracted tracheas (Fig 3, B).
FIG 3.
Other TLR7 agonists also relax isolated guinea pig tracheas in vitro. A, Contraction of tracheal segments was induced by methacholine, and the effect of cumulative increasing doses of R837 (circles), CL097 (squares), R848 (triangles), and gardiquimod (diamonds) was measured (R837, n = 8; R848, n = 4; gardiquimod, n = 3; CL097, n = 2; maximum, 1.64 ± 0.17 g; P ≤ .001 for effect of dose). B, Contraction of tracheal segments was induced by methacholine (3 µmol/L), and the effect of cumulative increasing doses of PolyUs (circles), mixed lengths of PolyU on a phosphodiester backbone (PolyUo; squares), and (PolyAs; triangles) was measured (n = 3; maximum, 1.27 ± 0.14 g; 300 µg · mL−1 and 1,000 µg mL−1 PolyUo vs PolyUs and PolyUo vs PolyAs, P ≤ .001; for effect of dose of PolyUs, P ≤ .001; PolyAs, P ≤ .01). MCh, Methacholine.
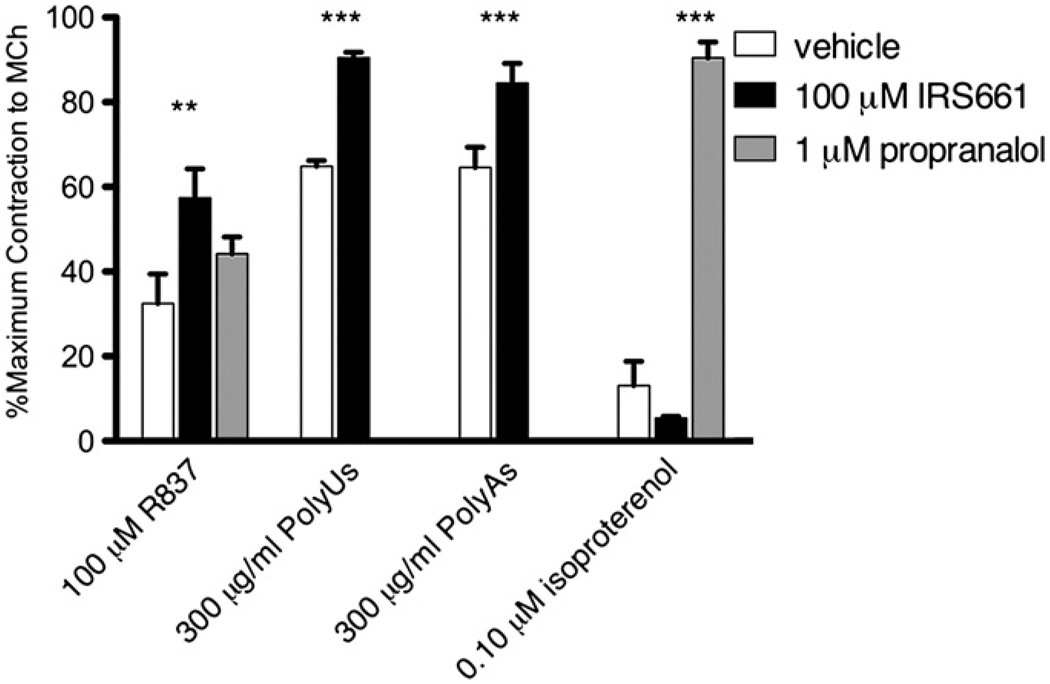
The TLR7 antagonist IRS661 partially blocks R837-mediated relaxation of airway smooth muscle and completely blocks PolyUs- and PolyAs-mediated relaxation
The TLR7 antagonist IRS661 (100 µmol/L) partially, although significantly, blocked smooth muscle relaxation by R837 (100 µmol/L) (Fig 4). IRS661 (100 µmol/L) was able to completely block the relaxation by PolyUs and PolyAs (300 µg · mL−1, Fig 4). The ability of IRS661 to block relaxation was specific to TLR agonists because it did not block relaxations induced by the β2-adrenergic receptor agonist isoproterenol (Fig 4, right bars). The β2-adrenergic receptor antagonist propranolol blocked relaxation by isoproterenol but did not block R837-mediated relaxation of airway smooth muscle (Fig 4), demonstrating that R837 is not mediating relaxation through β2-adrenergic receptors. Together, these data indicate that R837-induced relaxation is partially mediated by TLR7, whereas relaxation by PolyUs and PolyAs is completely mediated by TLR7.
FIG 4.
The TLR7 antagonist IRS661 partially reverses R837-mediated relaxation and completely reverses PolyUs- and PolyAs-mediated relaxation but does not reverse β2-adrenergic receptor–mediated relaxation of isolated guinea pig tracheas in vitro. Tracheal segments were preincubated with vehicle (open bars), IRS661 (solid bars), or propranolol (gray bars). Contraction of tracheal segments was induced by methacholine, and the effect of R837, PolyUs/As, or isoproterenol was measured (n = 3; maximum, 1.49 ± 0.10 g; R837 ± IRS661, P ≤ .01; PolyUs/As ± IRS661, P ≤ .001; isoproterenol ± propranolol, P ≤ .001). MCh, Methacholine.
R837-mediated relaxation of airway smooth muscle involves prostaglandins, BkCa, and nitric oxide synthase
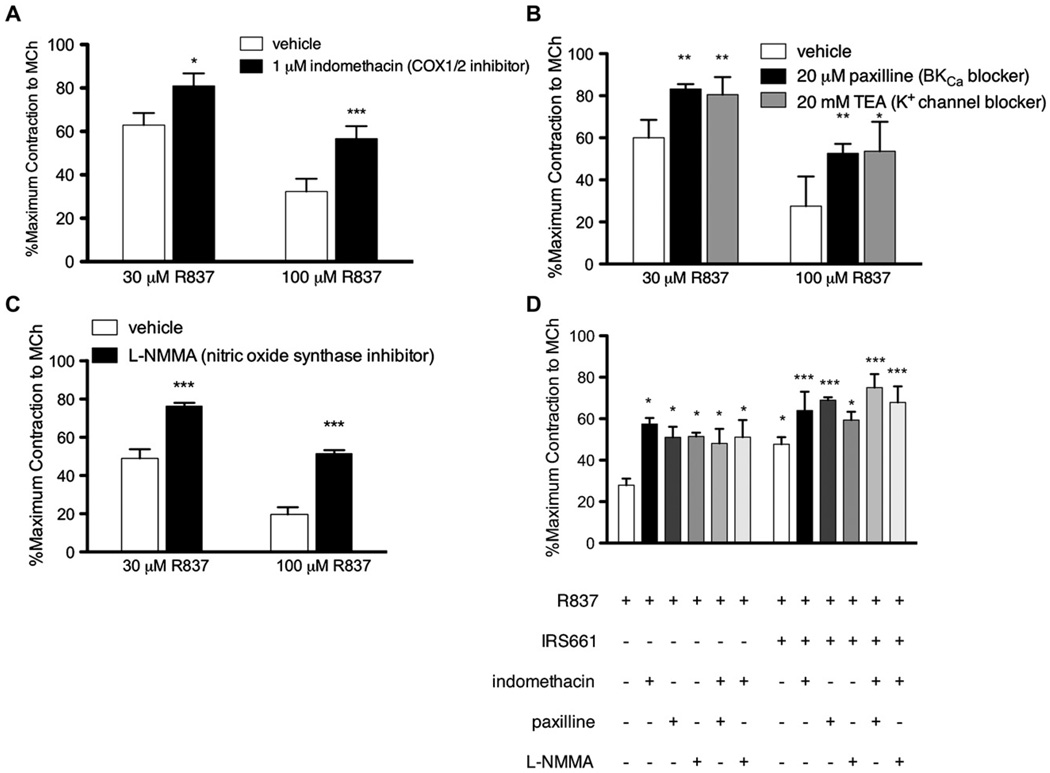
We investigated the involvement of rapid bronchodilatory pathways implicated in TLR signaling, including prostaglandins,16,17 BkCa,18 and nitric oxide.19,20 The COX inhibitor indomethacin (1 µmol/L) partially blocked R837-mediated relaxation of contractions induced by methacholine (3 µmol/L; Fig 5, A), indicating that prostaglandins are partially responsible for R837-mediated relaxation.
FIG 5.
The mechanism of R837-mediated relaxation of isolated guinea pig tracheas in vitro involves prostaglandins and BkCa. A, Tracheal segments were preincubated with vehicle or the COX inhibitor indomethacin, contraction was induced by methacholine, and the effect of R837 was measured (n = 3; maximum, 1.53 ± 0.18 g; 30 µmol/L R837, P ≤ .05; 100 µmol/L R837, P ≤ .001). B, Tracheal segments were preincubated with vehicle, paxilline, or TEA; contraction was induced by methacholine; and the effect of R837 was measured (n = 3; maximum, 1.22 ± 0.09 g; 30 µmol/L R837 vs paxilline, P ≤ .01; 30 µmol/L R837 vs TEA, P ≤ .01; 100 µmol/L R837 vs paxilline, P ≤ .01; 100 µmol/L R837 vs TEA, P ≤ .05). C, Tracheal segments were preincubated with vehicle or L-NMMA, contraction was induced with methacholine, and the effect of R837 was measured (n = 3; maximum, 2.14 ± 0.17 g; P ≤ .001). D, Tracheas were preincubated with vehicle control or IRS661 in combination with vehicle control, indomethacin, paxilline, L-NMMA, indomethacin and paxilline together, or indomethacin and L-NMMA together. Contraction was induced by methacholine, and the effect of R837 was measured (n = 3; maximum, 1.93 ± 0.15 g; *significantly different than R837 alone; ***significantly different than both R837 alone and R837 + IRS661). MCh, Methacholine.
Both TEA21 (a general potassium channel blocker, 20 mmol/L) and paxilline22 (a selective BkCa blocker, 20 µmol/L) partially and equivalently blocked relaxation induced by either 30 or 100 µmol/L R837 (Fig 5, B). This indicates that BkCa is involved in the relaxation because there is no additional blockade when all potassium channels are blocked by TEA.
The nitric oxide synthase inhibitor L-NMMA (100 µmol/L) partially blocked relaxation induced by either 30 or 100 µmol/L R837 (Fig 5, C), indicating that nitric oxide is involved in R837-mediated relaxation.
To test whether TLR7 receptors, prostaglandins, BkCa, and nitric oxide synthase are part of the same or parallel pathways of R837-mediated relaxation, we used combinations of inhibitors in vitro. Indomethacin, paxilline, L-NMMA, and IRS661 (TRL7 antagonist) each blocked R837-induced relaxation to the same extent. Blockade of R837-mediated relaxation by IRS661 was significantly greater in the presence of either indomethacin or paxilline compared with IRS661, indomethacin, or paxilline alone. However, blockade by IRS661 was not significantly greater in the presence of L-NMMA (Fig 5, D). There was no additive effect of indomethacin with paxilline (Fig 5, D). Together, these data indicate that R837-induced relaxation through 2 different pathways, one that is mediated through TLR7 and nitric oxide synthase, and a separate bronchodilator pathway that signals through prostaglandins and BkCa.
Adenosine antagonists do not relax airway smooth muscle, and a TLR7 antagonist does not block adenosine receptors in vitro
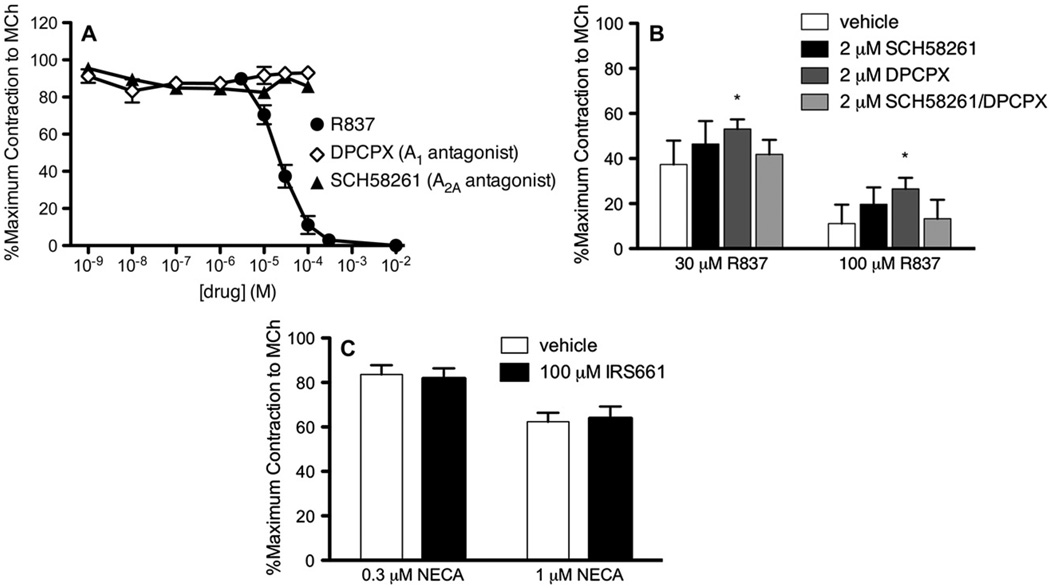
We tested whether relaxing effects of R837 are through adenosine receptors because it is a reported antagonist at A1 and A2A adenosine receptors.23 Neither the A1 receptor antagonist DPCPX nor the A2a receptor antagonist SCH58261 relaxed contractions induced by methacholine (Fig 6, A). Therefore R837 does not relax airway smooth muscle by acting as an antagonist at A1 or A2A receptors.
FIG 6.
Adenosine receptor antagonists do not induce relaxation and partially reverse the effect of R837-mediated relaxation of isolated guinea pig trachea in vitro. A, Contraction of tracheal segments was induced by methacholine, and the effect of cumulative increasing doses of R837 (circles), the adenosine A1 receptor antagonist DPCPX (diamonds), or the adenosine A2A receptor antagonist SCH58261 (triangles) was measured (n = 3; maximum, 1.29 ± 0.10 g; effect of dose of R837, P ≤ .001). B, Contraction of tracheal segments was induced by methacholine and incubated in the absence or presence of SCH58261, DPCPX, or SCH58261 and DPCPX together, and the effect of R837 was measured (n = 3; maximum, 1.3 ± 0.16 g; R837 vs 2 µmol/L DPCPX, P ≤ .05). C, Tracheal segments were preincubated with vehicle (open bars) or IRS661 (solid bars). Contraction of tracheal segments was induced by methacholine, and the effect of 5′-(N-ethylcarboxamido)adenosine (NECA) was measured (n = 2; maximum, 1.78 ± 0.30 g).
To address whether the adenosine antagonists bind and compete at the same receptor as R837 but fail to relax contraction, we measured the ability of R837 to relax methacholine-induced contractions when coincubated with adenosine receptor antagonists. When coincubated with DPCPX, R837-mediated relaxation was partially reversed (30 and 100 µmol/L; Fig 6, B). Because of the minimal reversal, it is unlikely that these receptors are mediating the majority of the R837-mediated relaxation. Moreover, the TLR7 antagonist IRS661 (100 µmol/L) did not reverse the relaxation of tracheal segments induced by 30 or 100 µmol/L 5′-(N-ethylcarboxamido)adenosine (a general adenosine receptor agonist; Fig 6, C), indicating that IRS661 is not blocking adenosine receptors and specifically reverses the relaxant effects of TLR7 agonists.
DISCUSSION
Because of the association of ssRNA respiratory viruses with asthma attacks,1,2,24 the relatively high expression of TLR7 in the lungs,5 and the association of TLR7 polymorphisms with asthma,9,10 we investigated the effects of acute TLR7 signaling on airway physiology. We were surprised to find that the TLR7 agonist R837 completely and potently abolished bronchoconstriction in vivo and trachealis muscle contraction in vitro, both effects occurring within minutes. This drug clearly has direct potent effects on airway smooth muscle, as seen by its ability to relax contractions induced by methacholine and histamine, although additional effects on airway nerves cannot be excluded. It is also possible that other cells, including leukocytes, which are known to express TLR7, might be contributing bronchodilating mediators.
The effect is not specific to G protein-coupled receptor mediated contractions because R837 relaxed contraction induced by chemical depolarization of the smooth muscle membrane with KCl. The mechanism of R837-induced relaxation is independent of epithelium and adenosine receptors.
Other structurally related TLR7 agonists, R848, CL097, and gardiquimod, as well as the structurally unrelated TLR7 agonists PolyUs and PolyAs, also relaxed guinea pig tracheas in vitro. Unlike R837, these latter 2 compounds only partially relaxed contraction. A TLR7 antagonist, IRS661, partially reversed R837-mediated relaxation and completely reversed relaxation induced by PolyUs and PolyAs. Taking together that multiple TLR7 agonists are bronchodilators and an antagonist reverses some or all of this bronchodilation strongly suggests a role for TLR7 in mediating the bronchodilating effects. Thus PolyUs and PolyAs induce a partial relaxation of contracted tissue that is completely TLR7 dependent. In contrast, the rapid and potent relaxant effect of R837 is significantly greater than these 2 agonists but is only partially TLR7 dependent. This is an important distinction because many groups currently use imidazoquinolines, rather than PolyUs, to study TLR7 and might be seeing off-target effects with the R837-related imidazoquinolines. There are conflicting reports based on species and organ system as to whether existing TLR7 agonists also stimulate TLR8,25–30 leaving open the possibility that some bronchodilator effect is TLR8 mediated.
TLR7 agonist–mediated relaxation occurred in minutes in vivo and in vitro. The speed of this effect is important but is not consistent with typical TLR signaling through induction of gene expression. To address the cellular mechanisms involved, we tested pathways associated both with TLR7 signaling and rapid airway smooth muscle relaxation. The TLR7 agonist R848 can induce human neutrophils to produce lipid mediators of inflammation, including the bronchodilator prostaglandin E2.16 We have shown that part of the R837-mediated relaxant effect depends on prostaglandins because indomethacin, an inhibitor of prostaglandin synthesis, partially blocked the relaxant effect of R837. Prostaglandin E2 induction might be a mechanism for the TLR7-independent portion of the R837 response because treatment of tissues with both the TLR7 antagonist IRS661 and indomethacin shows an additive effect.
TLR2 and TLR4 ligands increase the opening probability of BkCa.18 BkCa opens in response to a combination of membrane depolarization and increased intracellular Ca2+, repolarizing smooth muscle membrane to limit contraction. We have shown that part of the R837-mediated relaxant effect depends on BkCa because it is partially blocked by paxilline. The effect of treatment with the TLR7 antagonist and paxilline together was additive and significantly greater than either paxilline or the TLR7 antagonist alone, suggesting that the TLR7-independent portion of bronchodilation also signals through BkCa.
Furthermore, the effect of treatment with both paxilline and indomethacin together was not greater than either inhibitor alone. Because prostaglandins can signal in a common pathway with BkCa,31,32 the lack of an additive effect suggests they are part of a common pathway.
TLR7 agonists can stimulate the production of nitric oxide in B cells,19 although this is through enhancement of inducible nitric oxide synthase expression in addition to nitric oxide release. We have shown that a portion of the relaxant effect of R837 is mediated by nitric oxide because it is partially blocked by the nitric oxide synthase inhibitor L-NMMA. The effect of treating with a TLR7 antagonist and L-NMMA together was not additive or significantly different from the blockade of the TLR7 antagonist or L-NMMA alone, suggesting that nitric oxide mediates the TLR7-dependent relaxant effect.
The combination of inhibitors reverses most but not all of the airway smooth muscle–relaxing effect of R837. Thus we cannot exclude additional pathways and mediators that might contribute to the potent bronchodilating effect of this compound.
Others have explored TLR7 as a therapeutic target for asthma33–37 but in a context of chronic protection from airway inflammation rather than rescue from an acute asthma attack. This is shown not by the inhibition of bronchoconstriction, as we show here, but by the inhibition of airway hyperreactivity and allergic inflammation characteristic of asthma. This is thought to be a consequence of canonical TLR7 signaling, resulting in increased expression of TH1 cytokines, such as IL-12 and IFN-γ,38,39 skewing the airway response away from a TH2 immune environment.40 Our data show there is also a fast bronchodilating action of these drugs that is too rapid in onset to occur through modulation of the immune environment by using changes in gene expression. In sum, this class of compounds might provide both chronic protection against airway hyperreactivity and rapid bronchodilation in the event of an exacerbation, indicating that they might be successful as both prophylactic and rescue medications. Furthermore, the data emphasize the need to test shorter incubation times when conducting studies with TLR7 ligands and to consider noncanonical TLR7 signaling, as well as off-target effects of these ligands.
The TLR7-dependent portion of this bronchodilation also suggests a mechanism to limit airway obstruction resulting from inflammation, edema, and sloughing of dead cells associated with clearance of respiratory tract viral infections. TLR7 polymorphisms are associated with asthma,9,10 and it is tempting to speculate that these might result in a defective bronchodilator response to respiratory tract viruses, leaving bronchoconstrictive effects of viral infection unopposed in asthma.
In summary, TLR7 agonists are potent bronchodilators in vivo and in vitro, a rapid effect inconsistent with the time frame of canonical TLR signaling. The rapid and potent bronchodilatory effects of TLR7 agonists, along with their longer-term effects of biasing the immune response away from TH2 and toward TH1, make these compounds attractive candidates for treatment of asthma.
Acknowledgments
Supported by the US National Institutes of Health (5T32AI007472, NIH HL61013 [D.B.J.], HL71795 [D.B.J.], AI75064 [D.B.J.], HL55543 [A.D.F.], and ES14601 [A.D.F.]), an Oregon Health and Science University Tartar Trust Research Fellowship (E.H.K.), and an Achievement Awards for College Scientists Foundation scholarship (E.H.K.).
Disclosure of potential conflict of interest: The authors have received research support from the National Institutes of Health. In addition, E. H. Kaufman has received support from the Oregon Health and Science University Tartar Trust and Achievement Awards for College Scientists Foundation.
Abbreviations used
- BkCa
Large-conductance, calcium-activated potassium channel
- DPCPX
1,3-Dipropyl-8-cyclopentylxanthine
- EFS
Electrical field stimulation
- IC50
Inhibitory concentration of 50%
- L-NMMA
N-methyl-L-arginine
- PolyAs
21-mer single-stranded phosphorothioated polyadenylic acid
- PolyUs
21-mer single-stranded phosphorothioated polyuridylic acid
- ssRNA
Single-stranded RNA
- TEA
Tetraethylammonium
- TLR
Toll-like receptor
REFERENCES
- 1.Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, et al. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 2.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 4.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 5.Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 6.Demedts IK, Bracke KR, Maes T, Joos GF, Brusselle GG. Different roles for human lung dendritic cell subsets in pulmonary immune defense mechanisms. Am J Respir Cell Mol Biol. 2006;35:387–393. doi: 10.1165/rcmb.2005-0382OC. [DOI] [PubMed] [Google Scholar]
- 7.Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, et al. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118:641–648. doi: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Moller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Borglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008;63:1064–1069. doi: 10.1136/thx.2007.094128. [DOI] [PubMed] [Google Scholar]
- 10.Roponen M, Yerkovich ST, Hollams E, Sly PD, Holt PG, Upham JW. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J. 2010;35:64–71. doi: 10.1183/09031936.00172008. [DOI] [PubMed] [Google Scholar]
- 11.Verhein KC, Jacoby DB, Fryer AD. IL-1 receptors mediate persistent, but not acute, airway hyperreactivity to ozone in guinea pigs. Am J Respir Cell Mol Biol. 2008;39:730–738. doi: 10.1165/rcmb.2008-0045OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray TC, Jacoby DB. Viral infection increases contractile but not secretory responses to substance P in ferret trachea. J Appl Physiol. 1992;72:608–611. doi: 10.1152/jappl.1992.72.2.608. [DOI] [PubMed] [Google Scholar]
- 13.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freed AN, Peters SP, Menkes HA. Airflow-induced bronchoconstriction: role of epithelium and eicosanoid mediators. J Appl Physiol. 1987;62:574–581. doi: 10.1152/jappl.1987.62.2.574. [DOI] [PubMed] [Google Scholar]
- 15.Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 16.Hattermann K, Picard S, Borgeat M, Leclerc P, Pouliot M, Borgeat P. The Toll-like receptor 7/8-ligand resiquimod (R-848) primes human neutrophils for leukotriene B4, prostaglandin E2 and platelet-activating factor biosynthesis. FASEB J. 2007;21:1575–1585. doi: 10.1096/fj.06-7457com. [DOI] [PubMed] [Google Scholar]
- 17.Balzary RW, Cocks TM. Lipopolysaccharide induces epithelium- and prostaglandin E(2)-dependent relaxation of mouse isolated trachea through activation of cyclooxygenase (COX)-1 and COX-2. J Pharmacol Exp Ther. 2006;317:806–812. doi: 10.1124/jpet.105.097634. [DOI] [PubMed] [Google Scholar]
- 18.Scheel O, Papavlassopoulos M, Blunck R, Gebert A, Hartung T, Zahringer U, et al. Cell activation by ligands of the toll-like receptor and interleukin-1 receptor family depends on the function of the large-conductance potassium channel MaxiK in human macrophages. Infect Immun. 2006;74:4354–4356. doi: 10.1128/IAI.01783-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammadi A, Billard C, Faussat AM, Kolb JP. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-kappaB activation. Nitric Oxide. 2008;19:138–145. doi: 10.1016/j.niox.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Tumurkhuu G, Koide N, Dagvadorj J, Noman AS, Khuda II, Naiki Y, et al. B1 cells produce nitric oxide in response to a series of toll-like receptor ligands. Cell Immunol. 2009;261:122–127. doi: 10.1016/j.cellimm.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima S. Analysis of K inactivation and TEA action in the supramedullary cells of puffer. J Gen Physiol. 1966;49:629–640. doi: 10.1085/jgp.49.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 23.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Lai K, Brownile R, Babiuk LA, Mutwiri GK. Porcine TLR8 and TLR7 are both activated by a selective TLR7 ligand, imiquimod. Mol Immunol. 2008;45:3238–3243. doi: 10.1016/j.molimm.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Spaner DE, Miller RL, Mena J, Grossman L, Sorrenti V, Shi Y. Regression of lymphomatous skin deposits in a chronic lymphocytic leukemia patient treated with the Toll-like receptor-7/8 agonist, imiquimod. Leuk Lymphoma. 2005;46:935–939. doi: 10.1080/10428190500054426. [DOI] [PubMed] [Google Scholar]
- 27.Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol. 2006;177:6584–6587. doi: 10.4049/jimmunol.177.10.6584. [DOI] [PubMed] [Google Scholar]
- 28.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 29.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 30.Lan T, Kandimalla ER, Yu D, Bhagat L, Li Y, Wang D, et al. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci U S A. 2007;104:13750–13751. doi: 10.1073/pnas.0706059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SW, Wang HZ, Zhao W, Ney P, Brink PR, Christ GJ. Prostaglandin E1 activates the large-conductance KCa channel in human corporal smooth muscle cells. Int J Impot Res. 1999;11:189–199. doi: 10.1038/sj.ijir.3900399. [DOI] [PubMed] [Google Scholar]
- 32.Yamaki F, Kaga M, Horinouchi T, Tanaka H, Koike K, Shigenobu K, et al. MaxiK channel-mediated relaxation of guinea-pig aorta following stimulation of IP receptor with beraprost via cyclic AMP-dependent and -independent mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:538–550. doi: 10.1007/s002100100485. [DOI] [PubMed] [Google Scholar]
- 33.Camateros P, Tamaoka M, Hassan M, Marino R, Moisan J, Marion D, et al. Chronic asthma-induced airway remodeling is prevented by toll-like receptor-7/8 ligand S28463. Am J Respir Crit Care Med. 2007;175:1241–1249. doi: 10.1164/rccm.200701-054OC. [DOI] [PubMed] [Google Scholar]
- 34.Moisan J, Camateros P, Thuraisingam T, Marion D, Koohsari H, Martin P, et al. TLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L987–L995. doi: 10.1152/ajplung.00440.2005. [DOI] [PubMed] [Google Scholar]
- 35.Camateros P, Kanagaratham C, Henri J, Sladek R, Hudson TJ, Radzioch D. Modulation of the allergic asthma transcriptome following resiquimod treatment. Physiol Genomics. 2009;38:303–318. doi: 10.1152/physiolgenomics.00057.2009. [DOI] [PubMed] [Google Scholar]
- 36.Stokes JR, Sorkness RL, Kaplan MR, Castleman WL, Tomai MA, Miller RL, et al. Attenuation of virus-induced airway dysfunction in rats treated with imiquimod. Eur Respir J. 1998;11:324–329. doi: 10.1183/09031936.98.11020324. [DOI] [PubMed] [Google Scholar]
- 37.Xirakia C, Koltsida O, Stavropoulos A, Thanassopoulou A, Aidinis V, Sideras P, et al. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am J Respir Crit Care Med. 2010;181:1207–1216. doi: 10.1164/rccm.200908-1255OC. [DOI] [PubMed] [Google Scholar]
- 38.Tomai MA, Gibson SJ, Imbertson LM, Miller RL, Myhre PE, Reiter MJ, et al. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res. 1995;28:253–264. doi: 10.1016/0166-3542(95)00054-p. [DOI] [PubMed] [Google Scholar]
- 39.Fogel M, Long JA, Thompson PJ, Upham JW. Dendritic cell maturation and IL-12 synthesis induced by the synthetic immune-response modifier S-28463. J Leukoc Biol. 2002;72:932–938. [PubMed] [Google Scholar]
- 40.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]