Abstract
The average amount of cholesterol in the whole animal equals about 2100 mg/kg body weight, and 15% and 23% of this sterol in the mouse and human, respectively, is found in the central nervous system. There is no detectable uptake across the blood-brain barrier of cholesterol carried in lipoproteins in the plasma, even in the newborn. However, high rates of de novo cholesterol synthesis in the glia and neurons provide the sterol necessary for early brain development. Once a stable brain size is achieved in the adult, cholesterol synthesis continues, albeit at a much lower rate, and this synthesis is just balanced by the excretion of an equal amount of sterol, either as 24(S)-hydroxycholesterol or, presumably, as cholesterol itself.
Keywords: apolipoprotein E, ATP binding cassette transporters, blood-brain barrier, cerebellum, Niemann-Pick type C, oxysterols, 24(S)-hydroxycholesterol, 27-hydroxycholesterol
Introduction
Unesterified cholesterol (C) is an important component o\f many cellular membranes including, in particular, the plasma membrane (Lange, 1992) where it plays an essential role in determining the fluidity and electrical and permeability characteristics of this limiting structure in every cell. In the whole animal, the amount of C present averages about 2100 mg per kg body weight, regardless of the species, and this steady-state concentration remains essentially constant throughout the life of the animal. However, for reasons still not well understood, a fraction of this pool of C in the plasma membrane of cells is constantly replaced. In a small animal like the mouse, 7–9% of the whole body sterol pool is turned over each day, while in a larger animal with a lower metabolic rate like the human, only about 0.7% of this pool is replaced daily (Dietschy and Turley, 2004; Li et al., 2008). These observations indicate that mechanisms must be in place to constantly excrete or degrade the C molecule and, at the same time, to constantly supply an equivalent amount of new sterol to the cell plasma membranes. These two processes also must be so tightly regulated that the steady-state concentration of C in tissues, and in the whole animal, remains essentially constant.
The broad outlines of the processes that accomplish this regulated turnover of plasma membrane C in the whole animal are partially understood. Even though mammals can absorb sterol, there is no dietary requirement for C. Instead, virtually every cell in the body has invested in the elaborate machinery necessary to synthesize this molecule from acetyl-CoA (Dietschy et al., 1993). In addition, the cells of most tissues take up small amounts of C that are carried by various apolipoproteins in the surrounding interstitial fluid. This uptake involves processes such as receptor-mediated endocytosis, receptor-mediated selective uptake and bulk-phase endocytosis of the pericellular fluid. The relative importance of each of these processes varies from organ to organ.
Intestinal epithelial cells, for example, express a protein on the microvillus border known as Niemann-Pick type C like protein (NPC1L1) that selectively moves unesterified sterol from the intestinal lumen into the cytosolic compartment (Brown et al., 2007; Ge et al., 2008) where it is esterified (CE) and incorporated into nascent chylomicrons (CM). The low density lipoprotein receptor (LDLR) is expressed in the liver and many other tissues and is involved in cellular uptake through receptor-mediated endocytosis of C and CE carried in lipoproteins containing apoB100 or apoE (Brown and Goldstein, 1979; Innerarity and Mahley, 1978; Osono et al., 1995). In contrast, the transporter scavenger receptor class B type I (SR-BI), which is expressed principally in the liver and endocrine glands (Acton et al., 1996; Xie et al., 2006), selectively removes CE from HDL and transfers it directly into the cytosolic compartment of cells. Virtually all cells also take up sterol contained in lipoproteins through bulk-phase endocytosis of the pericellular fluid. It should be noted that C and CE taken up through bulk-phase endocytosis or by the action of LDLR pass through the late endosomal/lysosomal compartment of cells, whereas the sterol taken up by the activities of SR-BI and NPC1L1 bypasses this organelle and is delivered directly into the metabolically active pool in the cytosol (Liu et al., 2007; Xie et al., 2000b). As another sterol transporter, Niemann Pick type C1 (NPC1), acting in concert with NPC2, is involved in the movement of C from this late endosomal/lysosomal compartment to the cytosolic pool, mutational inactivation of this protein results in the accumulation of C in cells using LDLR or bulk-phase uptake of lipoproteins, whereas there is no accumulation of C in cells utilizing SR-BI or NPC1L1 for cellular sterol uptake (Li et al., 2008; Liu et al., 2007).
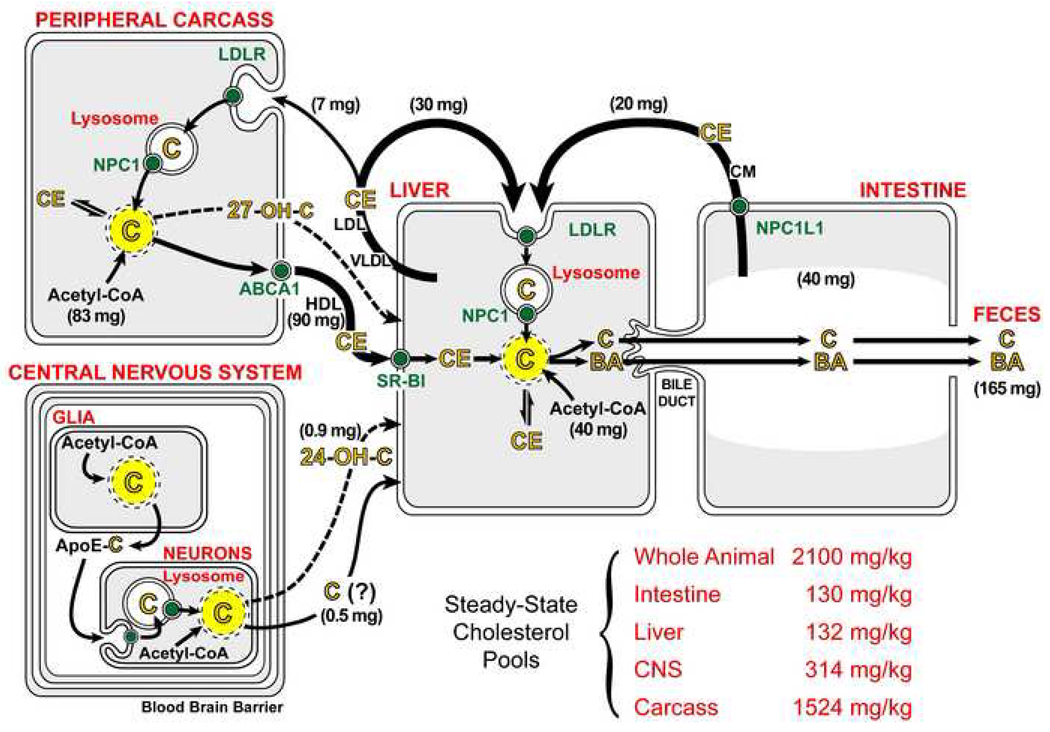
Cholesterol flux through tissues outside the central nervous system
In the whole animal, these biosynthetic and transport mechanisms act in concert to bring about an orderly and regulated flow of C across the plasma membrane of every cell while, at the same time, preventing the abnormal accumulation of sterol within any tissue. Although there are quantitative differences, the major steps in this turnover process are very similar in all mammalian species and are shown in diagrammatical form in Figure 1 for the mouse that is 7–8 weeks of age. Dietary C intake in such animals typically is about 40 mg/day/kg, of which about 50% is absorbed into the jejunal mucosa through the activity of NPC1L1 (Altmann et al., 2004; Repa et al., 2005). This absorbed sterol is esterified to CE and incorporated into the nascent CM along with large amounts of absorbed dietary triacylglycerol. During circulation through the plasma much of this triacylglycerol is removed from the particle in the periphery, following which the CM remnant is essentially totally cleared into the liver utilizing the interaction between the apoE present on this remnant and the LDLR (Ishibashi et al., 1994). After processing through the late endosomal/lysosomal compartment and being transported by NPC1 into the cytosolic compartment (Figure 1, yellow circle), this 20 mg of C joins another 40 mg of sterol synthesized locally in the liver during this same period of time.
Figure 1.
Flow of cholesterol through the major tissue compartments of the 7–8 week old mouse. The whole body is divided into four tissue compartments made up of the intestine, liver, central nervous system and remaining tissues of the peripheral carcass. The solid black arrows show the movement of cholesterol through the plasma carried in CM, VLDL/LDL and HDL. The numbers in parentheses adjacent to these arrows represent the milligrams of cholesterol moving through each of these pathways each day per kg of body weight (mg/day/kg). The dashed lines represent the flow of small amounts of various hydroxylated cholesterol molecules from the peripheral organs and CNS to the liver. The small green circles represent the specific cholesterol transporters NPC1L1, LDLR, SR-BI, NPC1 and ABCA1. Two separate intracellular pools of cholesterol are represented within each tissue compartment: one is isolated in the late endosomal/lysosomal compartment (white) while the other represents the metabolically active pool of unesterified cholesterol in the membranes of the cytosolic compartment (yellow). Movement of sterol from the lysosomal to the metabolically active pool requires functional NPC1. While not shown, a second protein, NPC2, acts in concert with NPC1 to promote C movement out of the lysosome. The numbers adjacent to acetyl-CoA represent the milligrams of cholesterol newly synthesized each day per kg of body weight (mg/day/kg). The size of the steady-state cholesterol pool in each of these tissue compartments is also listed. The abbreviations shown represent unesterified cholesterol (C), cholesteryl ester (CE), bile acid (BA) and hydroxylated cholesterol (OH-C). These flux rates are also available for larger animals and, in general, such rates decline in value as animal size increases (Dietschy et al., 1993).
The liver also assembles another cholesterol- and triacylglycerol-rich lipoprotein known as VLDL. This particle functions to move excessive amounts of triacylglycerol out of the liver to the sites of utilization and storage in the periphery. During this process, both VLDL remnants (not shown in Figure 1) and LDL are formed within the vascular space. The C and CE contained in this latter particle are cleared from the plasma predominantly by the liver (30 mg/day/kg) while smaller amounts (7 mg/day/kg) are taken up by many of the peripheral organs in the carcass. In the normal mouse, this uptake process predominantly utilizes the LDLR and receptor-mediated endocytosis (88%), while the remainder apparently is taken up through bulk-phase endocytosis (12%) (Osono et al., 1995). The CE contained in this LDL particle must then be hydrolyzed and processed through the late endosomal/lysosomal compartment in the cells of all of these organs before being moved by NPC1 into the metabolically active pool of C in the cytosol.
Within the different organs of the peripheral carcass, an additional 83 mg/day/kg of C is synthesized and added to the 7 mg/day/kg taken up in LDL. As the steady-state pool of C in these tissues is constant at about 1524 mg/kg, about 90 mg/kg of C must be removed each day. This is apparently accomplished by transfer of C from the outer leaflet of the plasma membranes of these cells to HDL circulating in the pericellular fluid. In some tissues, but not all, this transfer may be facilitated by another transport protein, ABCA1. As is also indicated in Figure 1, very small amounts of C may move out of some of these organs after first being hydroxylated in the 27 position to form 27-hydroxycholesterol (Björkhem et al., 1999; Lund et al., 1996). Thus, based on these values, 5.9% of the C pool present in all of these peripheral organs that make up 87% of the body mass is turned over each day. As will be discussed later, the CNS contributes only trivial amounts of C (< 1.5 mg/day/kg) to this turnover process.
The C taken up by HDL is rapidly esterified within the particle and the CE is then selectively transported into the cytosolic compartment of liver (and endocrine) cells utilizing SR-BI (Acton et al., 1996). After hydrolysis of this molecule by a neutral cholesteryl ester hydrolase (Shimada et al., 1994), the C enters the metabolically active pool of the cell (Xie et al., 2006). In species other than the mouse, there is a second mechanism that involves the transfer of CE from HDL to one of the apoB-containing lipoproteins by cholesterol ester transfer protein. Ultimately, these particles are taken up into the liver through intervention of the LDLR, the CE is hydrolyzed in the lysosome by an acidic cholesteryl ester hydrolase, and the C is then transferred to the metabolically active pool of sterol by NPC1. From these considerations it is clear that, in the mouse, the liver plays a central role in the flux of sterol through the body. While the steady-state pool of C in this organ is very small (132 mg/kg), the net flow of sterol through the liver from the intestine, peripheral organs and local synthesis (143 mg/day/kg) is such that 108% of this pool turns over every day.
The net excess of sterol reaching the liver, whether as cholesterol itself or as a hydroxylated derivative, ultimately must be excreted from the body since no cells have enzymes capable of degrading the sterol nucleus. While a small amount of C is lost from the body through the sloughing of skin and after conversion to steroid hormones, the great majority must be excreted in the feces. In the mouse, about half of this excess C is converted to bile acids (BA). This bile acid, along with a nearly equal amount of C (about 50 – 70 mg/day/kg each) is then transported across the canalicular membrane of the hepatocyte through the combined action of three proteins, ABCG5/8, ABCB4 and ABCB11, and, ultimately, excreted in the feces in either the acidic or neutral sterol fractions (Dietschy and Turley, 2002). In this manner, the animal maintains the whole body C pool constant at 2100 mg/kg while 165 mg (7.8% of the total pool) of dietary and newly synthesized sterol flows through the plasma membranes of cells each day.
Regulation of these cholesterol homeostatic mechanisms
Obviously, because of this very large flux of C across the cells of the body and the likelihood that the input of dietary sterol may vary erratically, mechanisms must be in place to regulate sterol flux and, therefore, the amount of C in the plasma membrane of every cell. Recent studies have identified at least four different types of mechanisms that control the amount of C in the metabolically active pool. First, expansion of this pool of C always triggers esterification with a subsequent increase in the concentration of CE within the cytosol. This important reaction presumably converts the potentially toxic, amphipathic C molecule into the more inert, hydrophobic CE. Second, expansion of this C pool also triggers binding of the key enzyme in sterol biosynthesis, HMG-CoA reductase, to INSIG-1 and INSIG-2 which, in turn, initiates ubiquitination of this protein and its subsequent degradation. This leads to very rapid suppression of the rate of sterol synthesis within the cell (DeBose-Boyd, 2008; Goldstein et al., 2006). Third, an increase in the C content of the metabolically active pool also blocks the processing of SREBPs to their active, nuclear forms by the golgi. Since the nuclear forms of these transcription factors normally drive the expression of many of the enzymes required for cholesterogenesis, including HMG-CoA reductase, this leads to more prolonged suppression of the rate of cholesterol synthesis (Goldstein et al., 2006; Horton et al., 2002). Finally, expansion of this cellular pool of C activates a number of nuclear receptors such as LXR and FXR that control the synthesis of several sterol transporters or enzymes involved in the metabolism of the sterol molecule to bile acids (Repa and Mangelsdorf, 2000). Thus, in a tissue like the liver, the sudden influx of a large bolus of sterol is followed by a rapid increase in the concentration of CE, a rapid decline in the rate of C synthesis that is secondary to degradation of HMG-CoA reductase and, later, to a decrease in the mRNA level for this enzyme, and an increase in the expression of cholesterol 7α-hydroxylase and transporters such as ABCG5/8.
It should be emphasized that these events occur only when the bolus of sterol reaches the metabolically active pool of C in the cytosol and nucleus. In the presence of a mutation that inactivates NPC1, C or CE entering cells through either receptor-mediated or bulk-phase endocytosis becomes trapped in the late endosomal/lysosomal compartment of the cells and cannot reach the metabolically active pool. As a result, even though the C pool in these cells is massively expanded, CE virtually disappears, the rate of cholesterol synthesis is actually increased and there is no activation of the LXR target genes (Li et al., 2005; Xie et al., 1999).
Cholesterol pools in the central nervous system
Because of its unique anatomy and relative isolation from the rest of the body, many features of C metabolism in the central nervous system (CNS) differ significantly from those just described in these other organs. One of the most striking differences is seen in the distribution and size of the C pool in the cells of the brain and spinal cord. In the other organs, this pool of sterol is found mostly as C in the plasma membrane of cells or, in the case of the endocrine organs, as CE stored in the cytosol. With the evolution of larger organisms, the need arose to increase the velocity of electrical conduction along neurons, and this was accomplished largely through reducing the capacitance of these cells by increasing the thickness of the hydrophobic membranes surrounding each axon. The task of creating these membranes is carried out by oligodendrocytes that are able to synthesize vast sheets of plasma membrane that are wrapped around numerous adjacent neurons and dehydrated to form compact myelin (Dietschy and Turley, 2004). As these sheets of myelin are rich in C, both the concentration and pool size of sterol in the CNS is much higher than in most of the other organs in the body.
In the adult mouse, for example, the concentration of C in the brain averages about 15 mg/g wet weight whereas in most other tissues, it is in the range of 2 – 6 mg/g. The pool of C in the CNS of the mouse is 314 mg/kg (Figure 1) or 15% of the whole animal pool, whereas this organ accounts for only 1.7% of whole body mass. Because of the relatively larger brain in the human (2.1% of body mass), and the increased development of the neocortex, the mean concentration of C exceeds 20 mg/g and the pool equals 490 mg/kg (23% of the whole human pool). In other organs, most of this C is in the plasma membrane of cells whereas in the CNS, the great majority is in myelin. Of the total pool of C in the mouse CNS (314 mg/kg), about 245 mg/kg represents C in the membranes of compact myelin while only about 69 mg/kg is presumably in the plasma membranes of cellular elements. As neurons represent only about 10% of the cells in the brain, the pool of C in these nerve cells may be only about 7 mg/kg (0.3% of the body pool). It is likely that the rate of C turnover in the myelin and in the plasma membranes of the glia and neurons varies greatly.
Cholesterol acquisition by the central nervous system
In theory, this large pool of C could be acquired either by uptake of plasma lipoproteins across the blood-brain barrier or by de novo synthesis within the neurons and glia themselves. However, the endothelial cells forming the brain capillaries are uniquely different from those making up other capillary beds in the body. There are no fenestrations, as in hepatic sinusoidal capillaries, there is little or no trans-endothelial bulk-phase vesicular transport, and the junctions between cells are very tight with high electrical resistance. Thus, any net movement of lipoproteins between the plasma and the pericellular fluid in the brain would have to take place through some sort of protein-mediated transport. The observation that the transporters LDLR, SR-BI and ABCA1 are expressed in brain endothelial cells (Dehouck et al., 1997; Panzenboeck et al., 2002), raised the possibility that the CNS did acquire at least part of its C pool from the plasma. However, direct measurements of net C movement into the brain in vivo have failed to identify such transport. For example, in the fetal sheep model, LDL uptake cannot be detected in any region of the brain, even as early as 90 days before birth when the blood-brain barrier is just forming (Turley et al., 1996). The rate of lipoprotein clearance in all areas of the brain is < 2 µl/hr/g throughout fetal development, even as the content of C in the CNS increases nearly 100-fold, from 9 to 876 mg. Furthermore, no net LDL transport across the blood-brain barrier can be detected in the newborn or adult sheep even though the level of LDL in the plasma becomes very high during the suckling period (Cavender et al., 1995; Turley et al., 1998).
Similar negative experimental results also have been reported in the mouse utilizing a variety of knockout animals. If one of the apolipoproteins such as apoAI or apoE is important in delivering C into the brain, or if one of the transporters participates in net trans-endothelial lipoprotein movement, then knockout of one of these proteins would be expected to increase the rate of C synthesis and, possibly, lower the content of sterol in the CNS. When tested, however, this has proved not to be the case. Even though there are profound changes in C metabolism in the rest of the body, there are no alterations in brain C metabolism with deletion of function of ABCA1, SR-BI, LDLR, apoE or apoAI (Quan et al., 2003). Similar negative results have been reported in other species, including humans, using other techniques (summarized in (Dietschy and Turley, 2004)). Taken together, these many observations provide compelling evidence that there is no net contribution of C from lipoproteins in the plasma to the pool of sterol within the CNS. If there is such a contribution, it is very small and below the level of detection by current techniques.
It follows from these observations that the C required for brain development and myelination must come from local synthesis. In the mouse, there is little myelination of the CNS at birth so that the mean concentration of C equals only ~ 4 mg/g and the total pool is < 1 mg (Xie et al., 2000a). However, during the first three weeks of life, myelination proceeds rapidly and the mean concentration of C in the brain approaches 12–13 mg/g. During this time, therefore, the rate of sterol accretion by the CNS is about 250 µg of C per day (Quan et al., 2003). Importantly, during this same interval, the rate of C synthesis also equals about 250 µg/day. Thus, the rate of synthesis fully accounts for the rate of C accretion in the CNS. However, beyond three weeks of age, the rate of accretion drops rapidly to only 10–15 µg/day as mature brain size is achieved between 13 and 26 weeks of age. Over this same interval, there is also an abrupt decrease in the rate of C synthesis, although this decrease is not as great as seen with the accretion rate. As a result, in 13 to 26 week-old mice, the rate of synthesis is in the range of 25–35 µg/day, which is 2–3 times higher than the rate of accretion. Thus, beyond three weeks of age, more C is being synthesized in the CNS than is required for new cell development and myelination.
During the early period of development, the majority of this synthetic activity presumably takes place in the oligodendrocytes which must assemble large quantities of plasma membrane from which compact myelin is made. In general, the regional rate of C synthesis reflects the degree of myelination taking place in that same area. In the mouse, for example, the rate of synthesis is lowest in the cerebrum and highest in the spinal cord (Quan et al., 2003). The fact that in the developing brain there is a direct correlation between the rate of synthesis and the ultimate concentration of C found in the cerebrum (12 mg/g), cerebellum (14 mg/g), mid-brain (23 mg/g), brainstem (30 mg/g) and the spinal cord (38 mg/g) further suggests that this sterol is synthesized locally to meet the needs of each region, and is not transported from some other area of the CNS (Quan et al., 2003). An identical correlation between regional synthesis rates and regional C concentrations is also seen in the developing CNS of the neonatal lamb (Turley et al., 1998). It should be noted, however, that the timing of these bursts of C and myelin synthesis varies in different species. In the guinea pig and ungulate, C synthesis and myelin formation are very active in the fetal CNS during late intrauterine development so that the brain of the newborn is well developed and the animal is fully mobile at birth. In contrast, species like the mouse, hamster and human only develop high rates of C and myelin synthesis after birth and, so, are essentially helpless when born (Dietschy and Turley, 2004). What triggers this burst of C and myelin synthesis either in the developing fetus or in the newborn is currently poorly understood.
Cholesterol excretion from the central nervous system
The observation that in the adult animal the rate of C synthesis exceeds the need for further sterol accretion in the CNS indicates that, as in the other organs (Figure 1), there is constant C turnover in the brain, and mechanisms must be in place, therefore, to excrete sterol across the blood-brain barrier. One mechanism was identified when it was recognized that the P450 enzyme, cholesterol 24-hydroxylase is uniquely expressed in the brain (Lund et al., 1999; Smith et al., 1972) and, in studies performed in humans, it was found that the CNS makes a net contribution of 24(S)-hydroxycholesterol to the plasma (Lütjohann et al., 1996). When the gene encoding this hydroxylase (CYP46A1) is inactivated in the mouse, there is essentially no change in C turnover in any organ of the body except the brain where synthesis is suppressed approximately 40% (Lund et al., 2003). Subsequent balance studies in these animals revealed that the rate of excretion of sterol from the CNS equals 1.4 mg/day/kg, and about 0.9 mg/day/kg of this is as 24(S)-hydroxycholesterol (Xie et al., 2003). The rate of excretion of this oxysterol from the human brain equals 0.09 mg/day/kg (Björkhem et al., 1998). Thus, the net flux of sterol out of the CNS is very small compared to the flux coming from all of the other organs of the peripheral carcass (Figure 1) and, unlike these other organs, the majority of this excreted sterol is as the oxysterol, 24(S)-hydroxycholesterol.
This low rate of excretion relative to the large pool of C in the CNS suggests that turnover is very slow and of the order of 0.4%/day. However, it is clear that turnover may vary among the different cell types. The cholesterol 24-hydroxylase has been localized to a small subset of large neurons in the cortex and cerebellum (Lund et al., 1999). If the excretion of 24(S)-hydroxycholesterol reflects only turnover in this small population of neurons (0.9 mg/day/kg), and if the pool of C in this subset of cells is of the order of 3 mg/kg, then the turnover of C in these nerve cells may be nearly 30%/day. If, on the other hand, the excretion of C through the second pathway (0.5 mg/day/kg) reflects turnover in the remainder of the CNS pool made up predominantly of glial plasma membranes and myelin, then turnover of this pool is extremely slow and equal to only 0.16%/day. These calculations suggest that C turnover in these large neurons may be 5-fold greater than turnover in all of the organs of the peripheral carcass (Figure 1).
While the majority of the C synthesized early during development of the CNS presumably occurs in oligodendrocytes, the sources for the sterol that drive this turnover in the adult brain are less clear. Cholesterol synthesis takes place in both glia and neurons in vitro, although in the case of nerve cells, this synthesis may occur predominantly in the cell body and proximal axon and not in the distal axon (Vance et al., 1994). Several lines of evidence suggest that both nerve growth and synapse formation require additional amounts of C made elsewhere in the CNS (Hayashi et al., 2004; Mauch et al., 2001). Astrocytes, in particular, synthesize C and apoE and, under the influence of ABCA1 and, possibly, ABCG1 (Karten et al., 2006; Wahrle et al., 2004), secrete these two molecules as a complex into the pericellular fluid. Importantly, this secretory process continues unaltered in the presence of a mutation that inactivates NPC1 (Karten et al., 2005). This apoE-C complex is then presumably taken up by neurons during growth or synapse remodeling, utilizing one of the LDLR family of transporters present on nerve cells (Hayashi et al., 2004). Thus, as shown in Figure 1, one pathway that may be active in C turnover in a subset of neurons involves the synthesis of C in astrocytes, secretion of this sterol along with apoE into the pericellular fluid, receptor-mediated uptake of this complex into nerves, processing of this C through the late endosomal/lysosomal pathway, 24-hydroxylation of this C, and, ultimately, the movement of this 24(S)-hydroxycholesterol across the blood-brain barrier into the plasma. In genetic conditions where NPC1 is not functioning, this C accumulates within neurons (Xie et al., 2000a). Although less well understood, a second pathway may also involve apoE-associated C movement from glia and, possibly, myelin directly to the blood-brain barrier for excretion from the CNS. In the presence of neurodegeneration and demyelination, the movement of C through the 24-hydroxylation pathway decreases while C excretion through the second pathway increases.
Regulation of C flux through the central nervous system
Current evidence suggests that the four major processes known to control the content of C in the metabolically active pool in the cells of other tissues also operate to control C metabolism in the cells of the CNS. These cells express abundant mRNA for ACAT2, SREBP, HMGR, and LDLR. In addition, in the mouse brain, there is also significant expression of the nuclear receptors LXRα, LXRβ, RXRα and RXRγ, but little or no PXR or FXR (Repa et al., 2007; Wang et al., 2002). Deleting the function of LXRα and LXRβ leads to diminished expression of a number of LXR target genes (Wang et al., 2002), while administration of an LXR agonist enhances the expression of genes controlling the synthesis of ABCA1, ABCG1 and apo D (Repa et al., 2007). Thus, although the magnitude of change in C flux across the cells of the CNS is probably small compared to the changes possible in other organs, these findings suggest that the response of the brain to diminution or expansion of the metabolically active pool of C in the cytosolic compartment of glia and neurons is the same as in the cells of other organs.
One line of evidence supporting this conclusion comes from studies in mice with an inactivating mutation of NPC1. In these animals, the C taken up through receptor-mediated endocytosis of the apoE-C complex becomes sequestered in the late endosomal/lysosomal compartment of glia and neurons. Treatment of these animals with cyclodextrin that overcomes this transport defect allows this C to flow into the metabolically active pool in the cytosol. This, in turn, leads to an increase in the CE concentration in the cytosol, suppression of sterol synthesis and activation of a number of LXR target genes (Liu et al., 2008). Thus, the same metabolic responses to the movement of C into the metabolically active pool are seen in the brain as are found in the other tissues of the body. A second line of evidence comes from mice treated with an LXR agonist. Such treatment leads to an increase in the mRNA levels for a number of target genes including ABCA1 and ABCG1, and to a significant increase in C, but not 24(S)-hydroxycholesterol, excretion from the CNS (Repa et al., 2007).
Concluding Remarks
Thus, recent studies indicate that the cells of the CNS synthesize the C required for brain growth and myelination of axons in the developing brain and for continued axon growth and synapse remodeling in the mature brain. These cells appear to express the same control mechanisms seen in cells of other tissues for maintaining the concentration and turnover of C in the metabolically active pool and plasma membrane. However, there are many other aspects of the metabolism of C in the CNS that remain to be elucidated, as well as the possible role of disordered sterol metabolism in the genesis of various syndromes of neurodegeneration.
ACKNOWLEDGMENTS
Much of the research presented in this review was supported by grant R01 HL009610 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Other support has come from the Moss Heart Fund, the Ara Parseghian Foundation and Dana's Angels Research Medical Trust.
REFERENCES
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Altmann SW, Davis HR, Jr, Zhu L-j, Yao X, Hoos LM, Tetzloff G, Iyer SPN, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- Björkhem I, Diczfalusy U, Lütjohann D. Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr. Opin. Lipidol. 1999;10:161–165. doi: 10.1097/00041433-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Björkhem I, Lütjohann D, Diczfalusy U, Ståhle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem. J. 2007;406:273–283. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. U.S.A. 1979;76:3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender CP, Turley SD, Dietschy JM. Sterol metabolism in fetal, newborn, and suckled lambs and their response to cholesterol after weaning. Am. J. Physiol. 1995;269:E331–E340. doi: 10.1152/ajpendo.1995.269.2.E331. [DOI] [PubMed] [Google Scholar]
- DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18:609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehouck B, Fenart L, Dehouck M-P, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J. Biol. Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- Ge L, Wang J, Qi W, Miao H-H, Cao J, Qu Y-X, Li B-L, Song B-L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 2004;279:14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity TL, Mahley RW. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978;17:1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in "knockout" mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten B, Campenot RB, Vance DE, Vance JE. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J. Biol. Chem. 2006;281:4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
- Karten B, Hayashi H, Francis GA, Campenot RB, Vance DE, Vance JE. Generation and function of astroglial lipoproteins from Niemann-Pick type C1-deficient mice. Biochem. J. 2005;387:779–788. doi: 10.1042/BJ20041694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y. Tracking cell cholesterol with cholesterol oxidase. J. Lipid Res. 1992;33:315–321. [PubMed] [Google Scholar]
- Li H, Repa JJ, Valasek MA, Beltroy EP, Turley SD, German DC, Dietschy JM. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann-Pick type C disease. J. Neuropathol. Exp. Neurol. 2005;64:323–333. doi: 10.1093/jnen/64.4.323. [DOI] [PubMed] [Google Scholar]
- Li H, Turley SD, Liu B, Repa JJ, Dietschy JM. GM2/GD2 and GM3 gangliosides have no effect on cellular cholesterol pools or turnover in normal or NPC1 mice. J. Lipid Res. 2008;49:1816–1828. doi: 10.1194/jlr.M800180-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in Niemann-Pick type C disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. U.S.A. 2008 doi: 10.1073/pnas.0810895106. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Xie C, Richardson JA, Turley SD, Dietschy JM. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann-Pick C disease. J. Lipid Res. 2007;48:1710–1723. doi: 10.1194/jlr.M700125-JLR200. [DOI] [PubMed] [Google Scholar]
- Lund E, Andersson O, Zhang J, Babiker A, Ahlborg G, Diczfalusy U, Einarsson K, Sjövall J, Björkhem I. Importance of a novel oxidative mechanism for elimination of intracellular cholesterol in humans. Arterioscler. Thromb. Vasc. Biol. 1996;16:208–212. doi: 10.1161/01.atv.16.2.208. [DOI] [PubMed] [Google Scholar]
- Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- Lütjohann D, Breuer O, Ahlborg G, Nennesmo I, Sidén Å, Diczfalusy U, Björkhem I. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller E-C, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Osono Y, Woollett LA, Herz J, Dietschy JM. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J. Clin. Invest. 1995;95:1124–1132. doi: 10.1172/JCI117760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzenboeck U, Balazs Z, Sovic A, Hrzenjak A, Levak-Frank S, Wintersperger A, Malle E, Sattler W. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- Quan G, Xie C, Dietschy JM, Turley SD. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Dev. Brain Res. 2003;146:87–98. doi: 10.1016/j.devbrainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Li H, Frank-Cannon TC, Valasek MA, Turley SD, Tansey MG, Dietschy JM. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J. Neurosci. 2007;27:14470–14480. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J. Lipid Res. 2005;46:779–789. doi: 10.1194/jlr.M400475-JLR200. [DOI] [PubMed] [Google Scholar]
- Shimada A, Tamai T, Oida K, Takahashi S, Suzuki J, Nakai T, Miyabo S. Increase in neutral cholesteryl ester hydrolase activity produced by extralysosomal hydrolysis of high-density lipoprotein cholesteryl esters in rat hepatoma cells (H-35) Biochim. Biophys. Acta. 1994;1215:126–132. doi: 10.1016/0005-2760(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Smith LL, Ray DR, Moody JA, Wells JD, van Lier JE. 24-hydroxycholesterol levels in human brain. J. Neurochem. 1972;19:899–904. doi: 10.1111/j.1471-4159.1972.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Turley SD, Burns DK, Dietschy JM. Preferential utilization of newly synthesized cholesterol for brain growth in neonatal lambs. Am. J. Physiol. 1998;274:E1099–E1105. doi: 10.1152/ajpendo.1998.274.6.E1099. [DOI] [PubMed] [Google Scholar]
- Turley SD, Burns DK, Rosenfeld CR, Dietschy JM. Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J. Lipid Res. 1996;37:1953–1961. [PubMed] [Google Scholar]
- Vance JE, Pan D, Campenot RB, Bussière M, Vance DE. Evidence that the major membrane lipids, except cholesterol, are made in axons of cultured rat sympathetic neurons. J. Neurochem. 1994;62:329–337. doi: 10.1046/j.1471-4159.1994.62010329.x. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system apoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson J-Å. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Burns DK, Turley SD, Dietschy JM. Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J. Neuropathol. Exp. Neurol. 2000a;59:1106–1117. doi: 10.1093/jnen/59.12.1106. [DOI] [PubMed] [Google Scholar]
- Xie C, Lund EG, Turley SD, Russell DW, Dietschy JM. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- Xie C, Richardson JA, Turley SD, Dietschy JM. Cholesterol substrate pools and steroid hormone levels are normal in the face of mutational inactivation of NPC1 protein. J. Lipid Res. 2006;47:953–963. doi: 10.1194/jlr.M500534-JLR200. [DOI] [PubMed] [Google Scholar]
- Xie C, Turley SD, Dietschy JM. Centripetal cholesterol flow from the extrahepatic organs through the liver is normal in mice with mutated Niemann-Pick type C protein (NPC1) J. Lipid Res. 2000b;41:1278–1289. [PubMed] [Google Scholar]
- Xie C, Turley SD, Pentchev PG, Dietschy JM. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am. J. Physiol. 1999;276:E336–E344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]