Abstract
Objective
The TB/HIV in Rio (THRio) study was launched in September 2005 to assess the impact of integrated tuberculosis (TB) and HIV treatment strategies in 29 HIV clinics in Rio de Janeiro, Brazil.
Design
THRio is a cluster-randomized trial (CRT) to determine whether routine screening for and treatment of latent TB in HIV clinic patients with access to antiretroviral therapy will reduce TB incidence at the clinic level. THRio is part of the Consortium to Respond Effectively to AIDS/TB Epidemic that is implementing research studies to assess the impact of bold, new public health paradigms for controlling the AIDS/TB epidemic.
Methods
Twenty-nine public primary HIV clinics were randomly assigned a date to begin implementing TB screening procedures and provision of isoniazid preventive therapy (IPT) for TB/HIV coinfected patients. Final analysis of the CRT is expected in 2011.
Results
Starting at date of tuberculin skin test (TST)/IPT implementation at each clinic through August 2010, 1670 HIV-infected patients initiated IPT, of which 215 are still receiving treatment. Of the remaining 1455 patients, 1230 (85%) completed therapy and only 20 (1.2%) patients initiating IPT reported adverse reactions leading to discontinuation of therapy. IPT completion was higher among HIV-infected patients receiving HAART (87%) than those not yet receiving HAART (79%, P < 0.01). Times to TST and IPT have markedly decreased postintervention, but remain considerably long. The richness of the THRio database has resulted in several analyses of this expansive cohort of HIV-infected patients that are reviewed here.
Conclusions
The national implementation of TST and IPT for HIV-positive patients in Brazil has been invigorated partly due to THRio’s baseline results. Expanded use of IPT in HIV patients in Rio de Janeiro is achievable with high adherence and low adverse events, although this effort requires a package of activities including training, advocacy and reorganization of services.
Keywords: adherence, HIV, implementation, isoniazid preventive therapy, tuberculosis
Introduction
The overlap of latent tuberculosis (TB) infection (LTBI) and HIV infection has resulted in marked increases in TB incidence in countries with dual epidemics and TB has become one of the most common opportunistic infections and the leading cause of death in HIV-infected people in Africa, Asia and Latin America [1].
Potential medical and public health strategies to reduce the incidence of HIV-related TB in developing countries include the use of isoniazid (INH) preventive therapy (IPT) and provision of antiretroviral (ARV) therapy to patients with advanced HIV disease, as well as measures to prevent transmission of infection in health facilities and the community.
Available data demonstrate that ARVs dramatically reduce the risk of TB in HIV-infected patients, but rates of TB remain unacceptably high despite this intervention. Cohort studies in Italy, the United States, Brazil and South Africa found a remarkably consistent effect, with reduction in the relative rate of TB by 80% in patients who received triple combination ARVs compared with those not receiving ARVs, even after adjusting for CD4 cell count. In the South African and Brazilian studies, however, the incidence of TB in patients with advanced HIV disease who received ARVs was still 2–3 cases per 100 person years, a rate that vastly exceeds that seen in HIV-uninfected populations [2–5]. Therefore, although combination ARV should be considered an essential component of the response to the HIV and HIV/TB epidemics, it may not be sufficient to prevent progression from LTBI to active disease among HIV-infected populations.
IPT has been shown to reduce the incidence of TB in HIV-infected people with LTBI by 70–90% [6–10], although a recent meta-analysis reported a 33% reduction in TB incidence among eight clinical trials [11]. This report emphasized the strong effect among HIV-infected patients with a positive tuberculin skin test (TST) (64% reduction) compared with those with a negative or unknown TST (14% reduction in both). Finally, there was no protection conferred for mortality, although TST-positive patients had a 26% reduction that was borderline significant. In Brazil, individuals in an HIV-infected cohort with access to medical care, including ARVs, had a 62% lower risk of developing TB and a 76% lower risk of dying if they received TB preventive therapy [12].
The specific impact of the combination of ARV and IPT was uncertain, but together these strategies could potentially reduce TB risk to extremely low levels. Thus, the TB/HIV in Rio (THRio) study was launched in 2005 to assess the impact of integrated TB and HIV treatment strategies.
Brazil has the highest number of TB cases in Latin America, and ranks 19th in the world for estimated incident TB cases according to the WHO [13]. Rio de Janeiro is the second largest city in Brazil with the second highest TB incidence in the country; TB is the leading cause of AIDS-related death even in patients with access to ARV [14].
Since 1997, Brazil has had a well known policy of universal and free access to combination ARV therapy to all patients who meet clinical criteria. The Brazilian policies for the provision of treatment to HIV-infected persons stand as a model for implementing ARV therapy in a relatively resource-poor setting. An analysis of the incidence of TB, AIDS and mortality in Rio de Janeiro found that AIDS mortality rates decreased dramatically after ARV therapy was introduced, but the proportion of patients with TB diagnosed within 1 year of the diagnosis of AIDS has remained stable at 15.2% since 1998 [15].
Brazilian national TB and HIV guidelines emphasize the need to conduct TSTs with purified protein derivative in all HIV-infected patients and to provide IPT to those who have LTBI [16,17], but uptake of these policies has been extremely limited.
In this article, we describe the implementation of and lessons learned from the THRio study of integrated TB/HIV care. THRio is a cluster randomized trial (CRT) to determine whether routine screening for and treatment of LTBI in patients receiving HIV care, including ARV drugs, in 29 HIV clinics in Rio de Janeiro, Brazil, will reduce TB incidence at the clinic level. THRio is part of the Consortium to Respond Effectively to AIDS/TB Epidemic (CREATE) that is implementing research studies to assess the impact of bold, new public health paradigms for controlling the AIDS/TB epidemic [18].
Methods
Study design
The THRio study design and methodology have been described elsewhere in detail [19]. The unit of analysis in THRio is the clinic. In brief, 29 public primary care clinics where ARV therapy was available to all HIV-infected patients who met the Brazilian national guidelines were randomly assigned a date to begin implementing TB screening procedures and IPT for TB/HIV coinfected patients, starting in September 2005. Kaplan–Meier curves were generated to compare preintervention and post-intervention time to TST and time to IPT. The results presented here are from analyses at different points in time during the THRio study. Relevant periods are provided for each analysis.
Study implementation
Community advisory board
A community advisory board (CAB) was established prior to the implementation of the study to have a group to advise the study team. The group has volunteer representatives from nongovernmental organizations (NGOs), people living with HIV/AIDS (PLWHA), healthcare workers (HCWs) and universities. The CAB meets monthly to look at issues related to the guarantee of human rights, ethical considerations and voluntary participation in the study. The group also contributed to the design and provision of educational materials and community education to PLWHA.
Training of healthcare workers
THRio implementation relies on the HCW that routinely provide care to HIV and TB patients in 29 public clinics in Rio de Janeiro city. Therefore, the primary activity of the implementation phase was the design and development of a comprehensive training package emphasizing recent scientific evidence and highlighting the importance of preventing TB in HIV-infected patients, including those with access to ARV. We conducted pilot trainings in a nonparticipating clinic to test the materials and gather feedback. Training was developed in three topical areas:
TB-HIV. TB and HIV doctors of two clinics at a time were trained together. Emphasis was placed on integration of the programs’ activities to provide patients with good-quality standard care, including screening for both diseases and IPT to control the burden of TB. Results of studies demonstrating the safety of IPT were communicated to these physician groups.
TST. Nurses involved in the application and reading of the test were trained to achieve a standard for the procedure.
Counseling. The main topics covered were pretest and posttest counseling for TST for HIV-infected patients, HIV counseling for TB patients and adherence counseling for the 6 months required to complete IPT.
Educational materials
Information and education materials (pamphlets and posters) were developed in consultation with the CAB members to support IPT implementation.
Field supporters
Six field supervisors were hired to support the development of the study in the 29 clinics. They were trained in TB–HIV and good clinical practices. Their primary role was to collect operational data on TST and IPT, so evaluation could be conducted in real time and provided to the study staff on a weekly basis, including reports of the number of patients examined and put on IPT. The supervisors also developed a system to remind the physicians of the dates when patients should be screened for LTBI. All the doctors regularly received a list of their patients with current TST status and indication for, or completion of, IPT.
Advocacy
The THRio study advocacy activities are connected with the broader activities of CREATE and involve engagement with key governmental and nongovernmental stakeholders. At the local level, a series of workshops has been conducted to inform and promote TB–HIV integration goals. These workshops included local HIV and TB networks, NGOs, faith-based organizations and activists. A video with an HIV activist and TB testimonies was recorded and used in several NGO events.
Within the national governmental sector, the goal was to promote the scale-up of IPT and TB/HIV collaborative activities in Brazil. The THRio team has been promoting this agenda in major national meetings and serves on the board of consultants to both the AIDS and the TB national programs.
Data collection
The study uses an intensified clinic monitoring and surveillance system to evaluate the outcomes in this trial. To obtain data on all HIV-infected patients registered at the 29 HIV study clinics, medical record abstractions were initially conducted for all HIV-infected patients by a team of data abstractors who were trained to collect the information from the charts on a regular basis throughout the THRio study. The information collected and data management have been described elsewhere [20].
Results and lessons learned
Tuberculin skin test and isoniazid preventive therapy uptake
Starting at date of TST/IPT implementation at each clinic through October 2010, 1670 HIV-infected patients initiated IPT, of which 215 are still receiving treatment. Of the remaining 1455 patients, 1230 (85%) completed therapy.
Among all patients receiving IPT, only 20 patients experienced adverse events that led to discontinuation of therapy. The following 21 events were experienced among the 20 patients: gastrointestinal disturbances (6), mild-to-moderate hepatotoxicity (3), pruritus (6), seizures (2), headache (2) and arthralgia (2).
Prior to the THRio training intervention, IPT completion was 81% compared with 85% posttraining (P = 0.09), although 84% of all patients initiating IPT during the THRio study period started after the THRio training. Table 1 describes the eligible patients who started IPT following the THRio intervention (n = 1455), stratified by whether they completed IPT. Men (85%) and women (84%) had similar completion rates, and the median age among completers (42 years) was slightly higher than noncompleters (39 years, P = 0.01). Median CD4 cell count at IPT initiation was higher in completers (527 cells/µl) than noncompleters (491 cells/µl; P = 0.03).
Table 1.
Characteristics of HIV-infected patients who completed isoniazid preventive therapy vs. HIV-infected patients who did not complete isoniazid preventive therapy in the 29 TB/HIV in Rio clinics, post-HIV/tuberculosis training (n = 1455).
| Completed IPT (n = 1230) | Did not complete IPT (225) | P-value | |
|---|---|---|---|
| Male | 742 (85%) | 133 (15%) | 0.73 |
| Female | 488 (84%) | 92 (16%) | |
| Median age (IQR) | 42 (34, 49) | 39 (32, 47) | 0.01 |
| Median CD4 cell count (IQR) | 527 (368, 729) | 491 (306, 710) | <0.03 |
| Receiving HAART at time of IPT | 846 (87%) | 125 (13%) | <0.01 |
| Not receiving HAART at time of IPT | 384 (79%) | 100 (21%) | |
| Mean time on HAART (months) | 52.5 | 50.5 | 0.55 |
| Median (IQR) time on HAART (months) | 49 (25, 78) | 42 (18, 74) | 0.39 |
IPT, isoniazid preventive therapy; IQR, interquartile range.
Patients who started IPT after they had already been receiving HAART had higher completion rates (87%) compared with those who initiated IPT prior to HAART (79%, P < 0.001). Among patients on HAART when starting IPT, completers had been receiving HAART for a median of 49 months compared with 42 months for noncompleters (P = 0.39).
Impact of the intervention on clinical practice
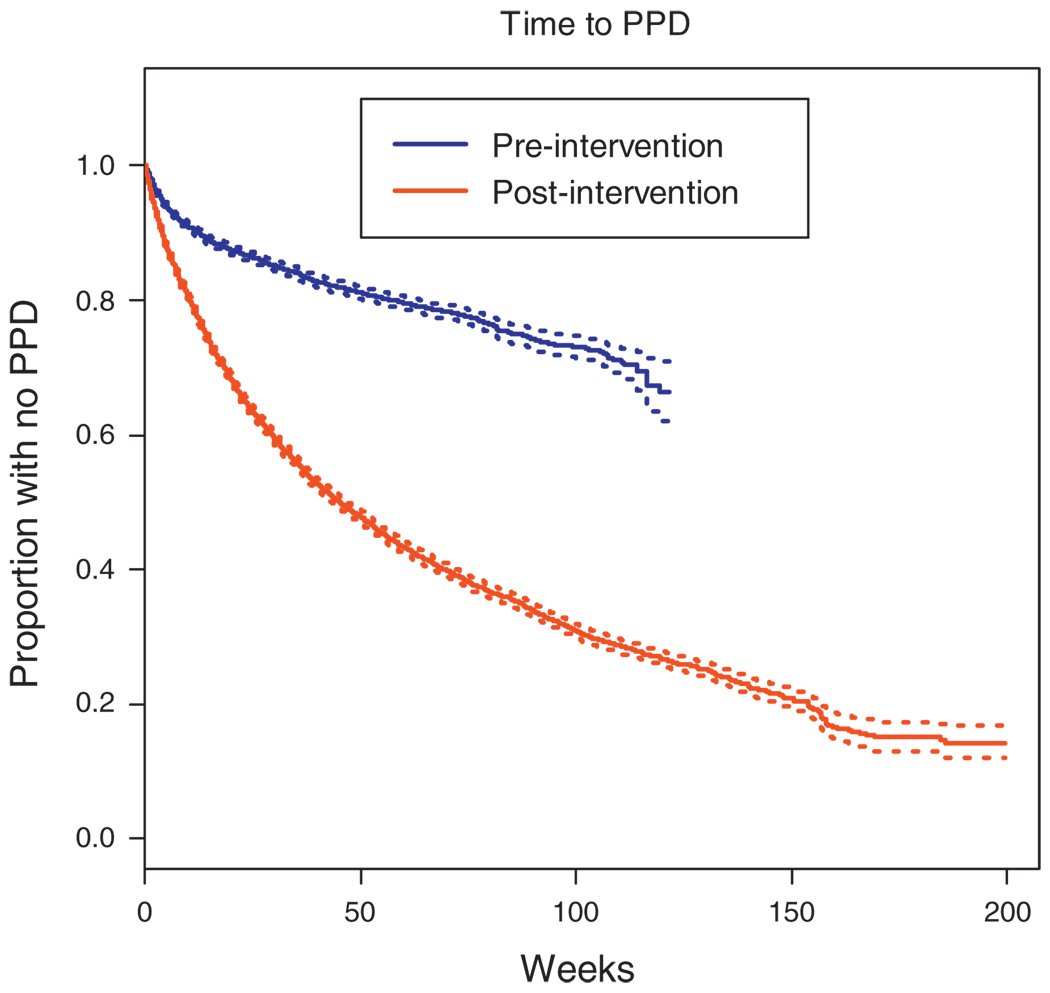
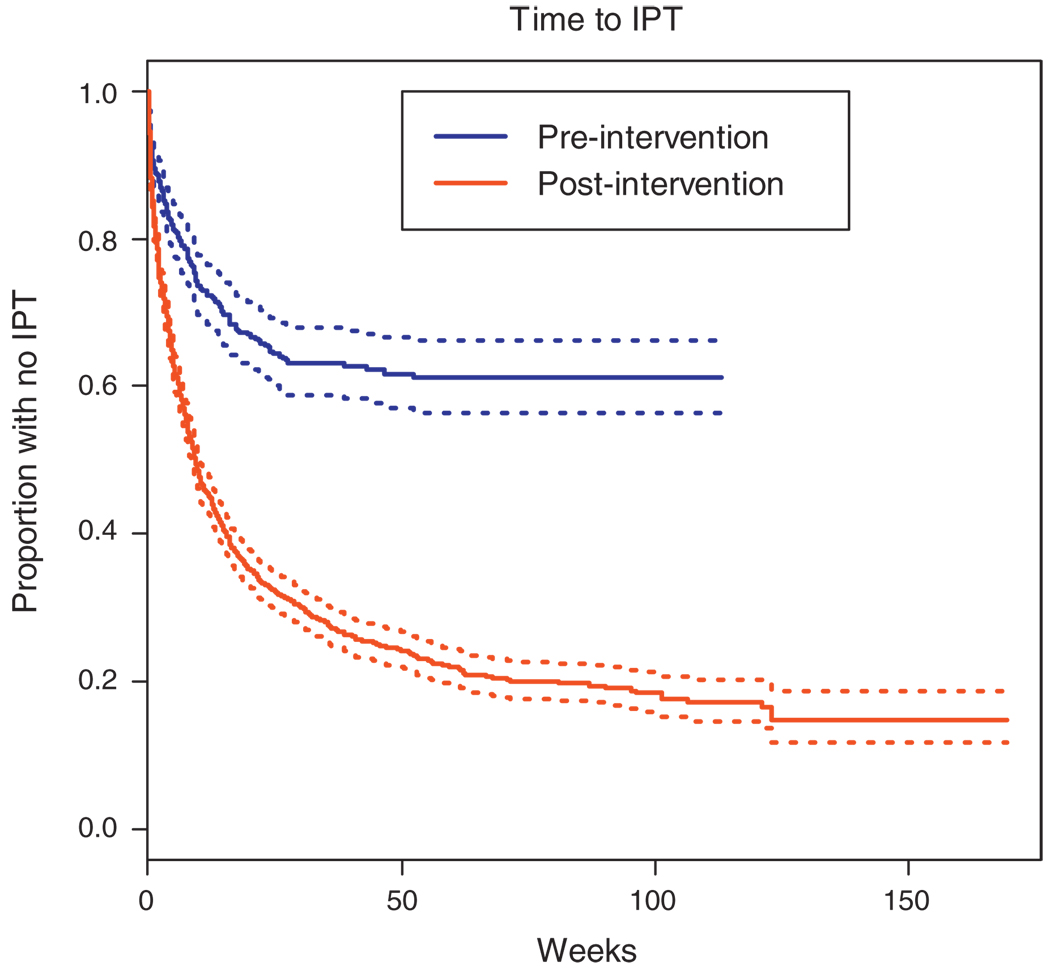
To investigate the impact of the intervention on clinical practice, we evaluated the time between each step of the process from the first clinic visit after HCW training until initiation of IPT. Time to TST and IPT are both markedly improved postintervention, however, time to both remains alarmingly long (Figs 1 and 2). Figure 1 presents the time to provision TST for clinic patients starting with the date of HCW training at each clinic, and compares this to the time to provision of TST for clinic patients prior to HCW training. The median time to TST postintervention was 44 weeks and 80% of patients had received a TST within 3 years following implementation of the intervention. In over 2 years since September 2005, less than 50% of patients in the preintervention clinics had received a TST. Figure 2 describes the time from a positive TST result to initiation of IPT in clinics where the HCW training has been conducted with the time to IPT initiation in clinics where the training had not yet been done. The median time to IPT for eligible patients was 8.9 weeks in the postintervention period, whereas approximately 60% of eligible patients in the preintervention period had yet to start IPT within 2 years following the intervention start.
Fig. 1. Time from first clinic visit to completion of tuberculin skin test prior to and following the intervention.
PPD, purified protein derivative.
Fig. 2. Time from positive tuberculin skin test to start of isoniazid preventive therapy for eligible patients prior to and following the intervention.
IPT, isoniazid preventive therapy.
Healthcare worker and the intervention: the qualitative study
To better understand barriers HCWs experience in following the national policy, we conducted a qualitative analysis of motivations and practices concerning IPT. This study was designed to identify strengths and weaknesses of ongoing TB prevention activities at selected clinics during the implementation of the intervention. The aim was to understand the differences in performance among the units. We hoped that this would help identify training opportunities and ultimately improve IPT provision in HIV clinics [21,22].
The qualitative study was performed in four health clinics in the first year of THRio’s implementation. On the basis of a summary measure incorporating proportion of eligible patients receiving TST and IPT, two of the worst and two of the best performing clinics were chosen. Ten HIV and TB physicians were interviewed about their practices and focus groups were convened with six local health administrators and six nurses. Qualitative interviews queried the physicians and nurses regarding their motivation to work in the public health field, opinions of the Brazilian Public Health System (SUS), routine practices of the health unit, knowledge of the THRio study and personal capacity to promote improvements and improve the prevention of TB. The primary driving questions of the focus group of local health administrators was ‘What could they do to improve TB prevention?’, and for the nurse group ‘What could they do to improve TST and IPT adherence?’ The main findings were as follows:
The Brazilian SUS is an ideal vision that, in reality, is poor at providing proper healthcare for its citizens.
Although physicians at the public health units say they are too busy, tense and solitary, they evaluated their health units very positively.
Prior to implementation of THRio, physician knowledge about the national recommendations for TB prevention in HIV patients was limited.
There was a strong belief among physicians that the complexity of HIV treatment and HIV patients’ lifestyles adversely affected TB prevention in these patients.
Potential adverse effects of IPT were an important limitation to prescribing INH.
Fragmented responsibilities, lack of integration among TB and HIV services and lack of management tools were the primary problems at the local level.
HIV patients are resistant to TST because it takes too much time and they are concerned about costs.
The results of these studies strongly support the importance of training and education of health unit staff regarding HIV/TB coinfection, emphasizing current epidemiology, current data supporting IPT for HIV-infected patients and current Brazilian policies regarding treatment of active TB disease and LTBI. Moreover, the health unit staff emphasized the importance of educating the HIV patient population regarding TB, and better operationalization of TST and IPT in HIV clinics. New communication strategies for physicians and patients need to be developed and implemented
Strengthen country-wide implementation of isoniazid preventive therapy for HIV-positive patients
Preliminary results from the THRio study can significantly inform the implementation of more widespread use of IPT among HIV-infected people in Brazil and worldwide. The THRio team has participated with other organizations throughout Brazil in technical and normative meetings of the AIDS and TB national programs that set and define policies on national levels. The evidence gathered through THRio and the strong advocacy of various organizations contributed to the change the Brazilian Ministry of Health implemented in July 2009. The new guidelines suggest the systematic collection of TST status and the use of IPT for all HIV-infected patients entered in the national ARV prescription system. Considering the robust experience with IPT implementation in Rio de Janeiro, the THRio study team is developing a plan to work with other sites to implement similar programs.
Discussion
Brazil HIV-AIDS policies have been recognized as a model for developing countries. Even before the implementation of its policy to provide universal access to ARV therapy in 1997, the widespread use of cotrimoxazole preventive therapy (CPT) for pneumocystis pneumonia was successfully implemented early in the epidemic. As TB became the leading cause of death among HIV patients, one would expect that IPT would receive the same attention and prioritization as CPT from HIV programs and that doctors as well as patients would recognize that IPT is a crucial prevention measure in their package of care. However, although IPT has been recommended in Brazilian guidelines and TST and INH are available in most TB clinics, uptake remains very low and a revamped strategy is necessary to cope with HIV-related TB.
The THRio study is a CRT evaluating the impact of routine screening for and treatment of LTBI in patients receiving HIV care in Rio de Janeiro, Brazil. Despite evidence that IPT reduces TB incidence in HIV-infected patients and WHO policy recommendations, uptake of IPTf or PLWHA has been limited worldwide. Our baseline data revealed a 76% reduction in TB incidence among patients with a history of HAART and IPT, suggesting that THRio would have a strong impact on reducing TB incidence in this population [20]. THRio follow-up has recently been completed, and final results are pending. However, many lessons have been learned, both clinical and operational, that can be used for future implementation of IPT for PLWHA in Brazil and elsewhere.
The literature is replete with reports of poor adherence to IPT, with rates rarely topping 65% even in developed countries. However, since implementation of THRio, 1455 HIV-infected patients have been placed on IPT, and 85% have completed therapy, a completion rate that is extremely high, particularly in the developing world. This scale up of IPT was achieved through a focused training program for all HIV clinic staff designed for THRio that emphasized the clinical interactions of HIV and TB, the public health importance of detecting and treating active disease in HIV/AIDS patients and detecting and treating LTBI in this population. We also report high completion of therapy (81%) among patients initiating IPT prior to the THRio training, though far fewer were screened for and started on IPT. Fewer physicians prescribed IPT prior to THRio, and it is likely that they were highly motivated to ensure adherence among their patients. The high reported completion rates in THRio provide promise that initiation and adherence to IPT can be achieved on a large scale without the need for incentives.
THRio set out to provide IPT to all eligible HIV-infected patients regardless of current HAART status; thus, it was encouraging to find high completion rates among patients receiving HAART (87%) and those not yet receiving HAART (79%). We hypothesize that patients receiving HAART are accustomed to taking daily medication, thus, IPT is less likely perceived as burdensome and these patients are less likely to forget. Completion among non-HAART patients was also much higher than most worldwide estimates, thus, providing evidence that high IPT adherence can be achieved in all HIV-infected patients regardless of HAART status.
The THRio study did have a significant impact in reducing delays in receiving TSTs and implementing IPT compared with the pre-THRio period, however, delays remained alarmingly long. The longest delays were observed between implementation of the THRio intervention and placement of TST for all clinic patients. It is important to note that the THRio study was designed to train HCWs about the importance of TB as an opportunistic infection among HIV-infected patients and the need to properly screen for LTBI and active TB among these patients. Thus, after training, we did not place study personnel in the units to administer TST and IPT. Rather, the clinic personnel conducted their day-to-day activities and our team of data abstractors collected data on TST and IPT from the medical records. Because we did not actively pursue clinic patients to come to the clinic specifically for a TST, much of the delay was caused by waiting for patients to come to the clinic for their next scheduled appointment. If a patient missed a visit, or if the physician did not ask for a TST, then the delay to TST for the patient could be as long as 6 months until the next scheduled visit. Moreover, TST is not routinely offered in all clinics on all days; thus, a patient who has a clinic visit on a day when TST is not scheduled will have increased delays.
Once an asymptomatic patient receives a positive TST, a chest radiograph result is required to rule out active TB. The protocol in most THRio clinics is the following. A chest radiograph prescription is written and the patient is responsible for scheduling the appointment and returning the result to the clinic physician. As many patients with LTBI do not feel ill, they may not feel the urgency to return quickly to the clinic with their results. Moreover, the same issue described above with missed clinic visits may significantly prolong time to IPT.
In Brazil, the prescription of IPT is recommended only after a diagnosis of LTBI using the TST and the exclusion of active TB. These steps take many weeks in most situations, thus, leading to prolonged delays in initiating IPT. Although we cannot identify the primary reason for delay for every individual HIV-infected patient, our experience shows the lack of coordination between the TB and HIV programs is a strong contributing factor. Efforts to integrate the HIV and TB programs are currently being explored on the basis of our findings. However, patients with a positive TST should be considered as high risk and a system to bring them to the clinics sooner is a necessity. We must reorganize services to make the procedures easier for patients, provide TST placement and readings on all clinic days, and ensure that both physicians and patients recognize the need for quickly taking the necessary steps towards initiating IPT.
Recently, the WHO has recommended a simple four symptom clinical screening algorithm for identifying TB suspects among HIV-infected patients in high burden settings [23]. This screening algorithm also identifies patients unlikely to have TB who are candidates for IPT, without relying on TST. Eliminating the need for TST could markedly reduce delays in initiating IPT observed in our study. Though this new algorithm has tremendous potential for wider prevision of IPT, it’s applicability to Brazil, where TST is still recommended, requires further evaluation. Data forthcoming from the THRio study should help address this important issue.
A further step is now being taken by the Brazilian Ministry of Health as TST and INH are now to be made available in all HIV clinics, and not only in TB clinics. Thus, HIV-infected patients will not need to travel to multiple clinics to initiate IPT. Our experience in scaling-up IPT in HIV patients in Rio has shown that in spite of being very knowledgeable about ARV therapy recommendations, HIV physicians lack knowledge about TB. Training and supervision were well received and contributed to increased use of IPT, but training alone was clearly not enough to guarantee that all patients in need would access IPT in an adequate timeframe.
A fear of many public health programs considering IPT for HIV-infected patients is the risk of adverse events. THRio reported adverse events in only 1.2% of all patients initiating IPT, and all were minor. A recent meta-analysis reported a 1.66-fold increased risk of adverse events leading to stoppage of treatment among HIV-infected patients receiving IPT compared with placebo [11]. Our results, among a patient population that is predominantly receiving HAART, suggest that adverse events are not a major concern. Of course, patients should continue to be monitored for adverse events while receiving IPT.
The goal of operational research such as THRio is to impact health outcomes and to help formulate policy recommendations for scale-up implementation of the studied intervention. CREATE has had a strong commitment to policy and advocacy from its implementation, which has resulted in fast track policy changes on the basis of THRio’s baseline results. The inclusion of TST and IPT on all ARV therapy prescription forms is a strong first step, along with the representation of these results in the Brazilian TB guidelines. On the basis of our preliminary results, we have made significant contributions to policy and advocacy activities for TB control among HIV-infected patients in Brazil.
Future directions
The final results for THRio, particularly the overall effectiveness of the intervention in reducing TB incidence, are still pending. However, we know that additional operational research is needed to better articulate and understand the barriers to implementing TST and IPT in Brazil, and to determine, in our setting, the utility of the new WHO screening recommendations. The national implementation of TST and IPT for HIV-positive patients has been invigorated partly due to THRio’s baseline results, and interventions to maximize provision of TST and IPT are currently being assessed by the THRio team in conjunction with the National Tuberculosis Program.
Our results show that it is possible to expand the use of IPT in HIV patients while achieving high IPT adherence and low toxicity. This effort requires a package of activities including training, advocacy and reorganization of services. Finally, THRio has clearly shown that treatment of LTBI would enormously benefit from new tools to speed diagnosis of LTBI and for diagnosing and ruling out active TB disease.
Acknowledgements
Support for this work was provided by the Bill and Melinda Gates Foundation grant for the Consortium to Respond Effectively to the AIDS-TB Epidemic (CREATE) and National Institutes of Health grants AI066994 and AI001637.
Footnotes
Conflicts of interest: None.
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 3.Jones JL, Hanson DL, Dworkin MS, DeCock KM Adult/Adolescent Spectrum of HIV Disease Group. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–1031. [PubMed] [Google Scholar]
- 4.Girardi E, Antonucci G, Vanacore P, Libanore M, Errante I, Matteeli A, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- 5.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 6.Whalen CC, Johnson JL, Okwera A, Horn DL, Huebner R, Mugyeni P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 7.Halsey NA, Coberly JS, Desormeaux J, Losikoff P, Atkinson J, Moulton LH, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351:786–792. doi: 10.1016/S0140-6736(97)06532-X. [DOI] [PubMed] [Google Scholar]
- 8.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 9.Mwinga A, Hosp M, Godfrey-Fausset P, Quigley M, Mwaba P, Mugala BN, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–2457. doi: 10.1097/00002030-199818000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Gordin FM, Matts JP, Miller C, Brown LS, Hafner R, John SL. A controlled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med. 1997;337:315–320. doi: 10.1056/NEJM199707313370505. [DOI] [PubMed] [Google Scholar]
- 11.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Pinho AM, Santoro-Lopes G, Harrison LH, Schechter M. Chemoprophylaxis for tuberculosis and survival of HIV-infected patients in Brazil. AIDS. 2001;15:2129–2135. doi: 10.1097/00002030-200111090-00008. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Geneva: WHO Press; 2009. Global tuberculosis control: a short update to the 2009 report. Report No. WHO/HTM/TB/2009.426. [Google Scholar]
- 14.Saraceni V, King BS, Cavalcante SC, Golub JE, Lauria LM, Moulton LH, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2008;12:769–772. [PMC free article] [PubMed] [Google Scholar]
- 15.Pacheco AG, Durovni B, Cavalcante SC, Lauria LM, Moore RD, Moulton LH, et al. AIDS-related tuberculosis in Rio de Janeiro, Brazil. PLoS ONE. 2008;3:e3132. doi: 10.1371/journal.pone.0003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelo Filho A, Kritski AL, Barreto AW, Lemos ACM, Netto AR, Gueimarares CA, et al. II Brazilian consensus on treatment of tuberculosis - Brazilian guidelines for tuberculosis 2004 [in Portuguese] J Bras Pneumol. 2004;30 Suppl 2:S62–S64. [Google Scholar]
- 17.Recommendations for antiretroviral therapy for adults infected with HIV: 2008/Ministry of Health, Secretary for Health Surveillance, National Program for DST and AIDS. 7th edition. Brasilia: Ministry of Health; 2008. [Google Scholar]
- 18.Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE) [accessed 17 June 2010]; www.tbhiv-create.org. [Google Scholar]
- 19.Moulton L, Golub JE, Durovni B, Cavalcante SC, Pacheco AG, Saraceni V, et al. Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials. 2007;4:190–199. doi: 10.1177/1740774507076937. [DOI] [PubMed] [Google Scholar]
- 20.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saraceni V, Durovni B, King B, Eldred L, Chaisson RE. Physician adherence to HIV and TB guidelines in Rio de Janeiro, Brazil. Programs and abstract of the 38th Union World Conference on Lung Health, International Union Against Tuberculosis and Lung Disease; 8–12 November 2007; Cape Town, South Africa. [abstract] [Google Scholar]
- 22.Vellozo V, Israel G, Saraceni V, Ferreira R, Durovni B, DeLuca A. THRio qualitative study. Programs and abstract of the 39th Union World Conference on Lung Health, International Union Against Tuberculosis and Lung Disease; 16–20 October 2008; Paris, France. [Google Scholar]
- 23.World Health Orgainzation. Geneva, Switzerland: WHO Press; 2010. Guidelines for intensified tuberculosis case finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. [Google Scholar]