Abstract
IL-4 promotes Th2 differentiation and provides immunity to helminth infections but is also associated with allergy and asthma. This suggests that precise adjustment of IL-4 responsiveness is needed to correctly balance immune responses. The IL-4Rα chain is an essential component of the IL-4 receptor and signals via STAT6. In this study, we show that infection with a helminth pathogen elicited broad upregulation of IL-4Rα on bystander CD4+ T cells in the draining lymph node, while simultaneously resulting in the loss of IL-4Rα expression on activated Th2 cells. IL-4Rα upregulation was restricted to the reactive lymph node, occurred within 4 d of infection, and was driven by an IL-4– and STAT6–dependent mechanism. Mice heterozygous for Stat6 exhibited reduced IL-4Rα upregulation and a correspondingly attenuated Th2 response. Indeed, the enhanced IL-4Rα upregulation in BALB/c mice, compared with that in C57BL6 mice, predicted their stronger Th2 response. The selective downregulation of IL-4Rα on highly activated Th cells was triggered by antigenic stimulation, was accompanied by loss of IL-7Rα, and rendered the cells unresponsive to IL-4. Together these data reveal a tightly controlled program of changing IL-4 responsiveness that characterizes the initiation, amplification, and restriction of a Th2 response in vivo.
Helminth parasites elicit Th2 responses in their hosts, defined by cytokines such as IL-4, IL-5, and IL-13 and high levels of IgE and IgG1 (1–3). IL-4 has a particular importance in orchestrating Th2 responses. Of the canonical Th2 cytokines, IL-4 alone can promote the priming of Th2 cells in vitro (4, 5), is critical for the Th2 cytokine response to helminth infection (6), and instructs B cells to class switch to IgG1 and IgE (7, 8). Effective Th2 polarization contributes to host survival during helminth infection (9) and is required for lasting immunity against reinfection (10). However, Th2 differentiation also is associated with allergy and asthma (11), suggesting that control of IL-4 responsiveness is key.
IL-4 signals through either the type I IL-4 receptor, which comprises IL-4Rα and the common γ (γc) chain, or the type II IL-4 receptor in which IL-4Rα is paired with IL-13Rα1 (12). In both cases, signal transduction is mediated by the phosphorylation of STAT6 (12–15). Expression of each of the IL-4 receptor subunits is restricted to specific cell populations: naive T lymphocytes possess only the type I receptor, whereas nonhematopoeitic cells predominantly use only the type II receptor, and monocytes and macrophages are unusual in their expression of both (12). The precise expression pattern of the IL-4 receptors determines the contrasting cytokine sensitivities of different cell types (16).
In vitro, IL-4 stimulation of naive CD4+ T cells results in rapid upregulation of surface IL-4Rα (17), a process that involves increased transcription of the Il4ra gene and de novo protein synthesis (18, 19). A recent study by Leonard and colleagues (20) reported that IL-4Rα expression is enhanced by IL-2 signaling, thereby facilitating Th2 differentiation in vitro. However, the in vivo significance of these observations remains unclear.
In this study, we use both in vitro cocultures of naive, responder, and bystander CD4+ T cells and infection with a murine helminth parasite to examine the regulation of IL-4Rα expression and IL-4 responsiveness during the development of the Th2 response. We show that IL-4Rα is upregulated on nonactivated, bystander CD4+ T cells by cytokine stimulation but is profoundly downregulated on highly activated Th cells in response to Ag encounter. Thus, changes in IL-4Rα expression and the different sensitivities to IL-4 that they confer define distinct stages of initiation, amplification, and termination during Th2 progression in vivo.
Materials and Methods
Mice
4get (C.129-Il4tm1Lky/J) (21) and KN2 (22) IL-4 reporter mice and IL-4−/− (KN2 homozygotes), IL-4Rα−/− (23), STAT6−/− (C.129S2-Stat6tm1Gru/J) (24), TCRα−/− (25), and Yeti IFN-γ reporter mice (26) were on a BALB/c CD90.2 or CD90.1 genetic background. 4get mice on a C57BL/6 CD90.2 background were backcrossed for >10 generations. TCR transgenic mice specific for the Salmonella typhimurium flagellin427–441 epitope (SM1) (27) were used on a C57BL/6 RAG2−/−, CD90.1+ background; TCR transgenic mice specific for I-Ad–restricted epitopes of OVA (DO11.10) (28) and influenza virus hemagglutinin (HNT) (29) were used on BALB/c CD90.2+ and BALB/c CD90.1+ backgrounds, respectively. Animals were kept under specific pathogen-free conditions in filter-top cages at the animal facility of the Trudeau Institute and were used at 8–12 wk old. All of the experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee.
Infections and immunizations
Mice were inoculated by gavage with 200 Heligmosomoides polygyrus infective third-stage larvae, as described (22). Two hundred H. polygyrus larvae were used for s.c. immunization. In Toxoplasma gondii infections, mice were given 10 cysts ME49 by gavage as described (30).
Flow cytometry and cell sorting
The following mAbs against mouse Ags were used as biotin, FITC, PE, allophycocyanin, Alexa Fluor 750, or Pacific Blue conjugates: CD4 (RM4–5), CD25 (7D4), CD44 (IM7), CD90.1 (CD90.1; OX-7), CD124 (IL-4Rα, M1), CD127 (IL-7Rα; A7R34), and CD132 (IL-2Rγc; 4G3). Additional reagents included anti-human CD2 (RPA-2.10), streptavidin-PE, and streptavidin-allophycocyanin. Dead cells were discriminated by the addition of propidium iodide (0.5 µg/ml; Sigma-Aldrich, St. Louis, MO) and excluded from the analyses. Surface staining with mAb was performed as described (22). Phosphorylation of STAT6 at tyrosine 641 (pY641) and total STAT6 were detected by intracellular staining with the Phosflow kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Samples were acquired on a FACSCalibur or Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star, Ashland, OR) software. Cell sorting was performed on a FACSVantage flow cytometer (BD Biosciences) equipped with DiVa electronics.
Cell cultures
For 24-h activation, naive (CD44lo) CD4+ T cells were sorted from pooled lymph node cells from either DO11.10 or HNT TCR transgenic mice and cocultured (5 × 105 cells per milliliter) with irradiated APCs (5 × 106 cells per milliliter), using either TCRαβ−/− splenocytes or IL-4−/− splenocytes depleted of CD4+ and CD8+ cells by MACS. Recombinant IL-4 (0.5 ng/ml), OVA323–339 peptide (2 µM), and neutralizing Abs against IL-2 (S4B6, 20 µg/ml) or IL-2Rα (PC61, 20 µg/ml) were included as indicated. For 8-d cultures, naive CD4+ T cells were likewise sorted from 4get mice and stimulated with plate-bound anti-CD3 (145-2C11, 10 µg/ml) and anti-CD28 (37.51, 5 µg/ml) in the presence of IL-2 alone (10 U/ml; “neutral” conditions); IL-2, IL-4, and anti-IFNγ (10 U/ml, 50 ng/ml, and 20 µg/ml, clone XMG1.2; “Th2”); or IL-2, IL-12, and anti–IL-4 (10 U/ml, 5 ng/ml, and 20 µg/ml, clone 11B11; “Th1”). Cells were recovered on day 3, rested in IL-2 alone, and restimulated with anti-CD3 on day 7. Flow cytometric analysis was performed 24 h later. To test the IL-4 responsiveness of CD4+ T cells, mesenteric lymph node (mesLN) cells from 14-d H. polygyrus infected 4get mice were first incubated with anti–IL-4 (11B11, 20µg/ml) for 1 h, washed extensively, and then cultured in the absence or presence of IL-4 (50 ng/ml) for 15 min. Subsequent flow cytometric analysis was gated on CD4+ T cells.
Quantitative RT-PCR
RNA was extracted from sorted cell populations using the RNAqueous-4PCR kit (Ambion, Austin, TX) and reverse transcribed with Superscript II RNase H (Invitrogen, Carlsbad, CA) with oligo(dT)18 priming. Quantitative real-time RT-PCR with specific primers and probes (22) was performed using an ABI Prism 7700 Sequence BioDetector according to the manufacturer’s instructions (TaqMan; PE Applied Biosystems, Foster City, CA). Additionally, we designed primers and probes to discriminate the alternative splice variants of the IL-4Rα mRNA (31, 32):
membrane IL-4R (mIL-4R) forward, 5′-CTACTATACGGCGCGTGTGAT-3′ (exon 7);
mIL-4R reverse, 5′-GCAGCTGGAAGTGGTTGTACCA-3′ (exon 9);
mIL-4R probe, 5′-TCCCAGATACTCACTGGCACCTGGA-3′;
soluble IL-4R (sIL-4R), forward 5′-CTACTATACGGCGCGTGTGAT-3′ (exon 7);
sIL-4R reverse, 5′-CCGTGAGCTCTCCTCACC-3′ (exon 8);
sIL-4R probe, 5′-TCCCAGATACTCACTGGCACCTGGA-3′.
Control experiments established a >95% efficiency of serial 10-fold dilutions, and no amplification was detected within 40 cycles with mRNA isolated from IL-4Rα−/− lymphocytes. Cycle threshold values for GAPDH were routinely between 17 and 20 cycles.
Adoptive transfers
To assess the Ag specificity of IL-4Rα upregulation, 1 × 107 lymphocytes from pooled spleen and lymph nodes of naive SM1 × RAG2−/− × CD90.1+ TCR transgenic mice were transferred i.v. into naive or 14d H. polygyrus infected C57BL/6 CD90.2+ 4get mice, and recipient mesLNs were taken 18 h later. To assess the longevity of IL-4Rα upregulation, 2 × 107 mesLN cells from 4get CD90.1+ mice infected with H. polygyrus 14 d earlier were transferred i.v. into naive or 14d H. polygyrus infected 4get CD90.2+ recipients. Twenty-four hours later, mesLN cells were harvested from the recipient mice and analyzed by flow cytometry.
Mixed bone marrow chimeras
To compare wild-type (WT) and gene-deficient cells in the same animal, 4get CD90.1+ cells were lethally irradiated (950 rad) and reconstituted with a total of 1 × 107 donor bone marrow (BM) cells from WT 4get CD90.1+ mice mixed in equal parts with either STAT6−/− 4get CD90.2+ or IL-4−/− CD90.2+ BM cells.
To generate chimeras with a defined population of TCR transgenic cells, C57BL/6 4get CD90.2+ mice were lethally irradiated and reconstituted with 10% SM1 × RAG2−/− × CD90.1+-derived and 90% WT C57BL/6 4get CD90.2+ BM. Mice were allowed to reconstitute for 6–8 wk before infection.
Statistical analysis
The differences between two data sets were assessed using Student t test. Significant values are denoted by the following symbols: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Results
Opposite effects of cytokine and antigenic stimulation on IL-4Rα expression
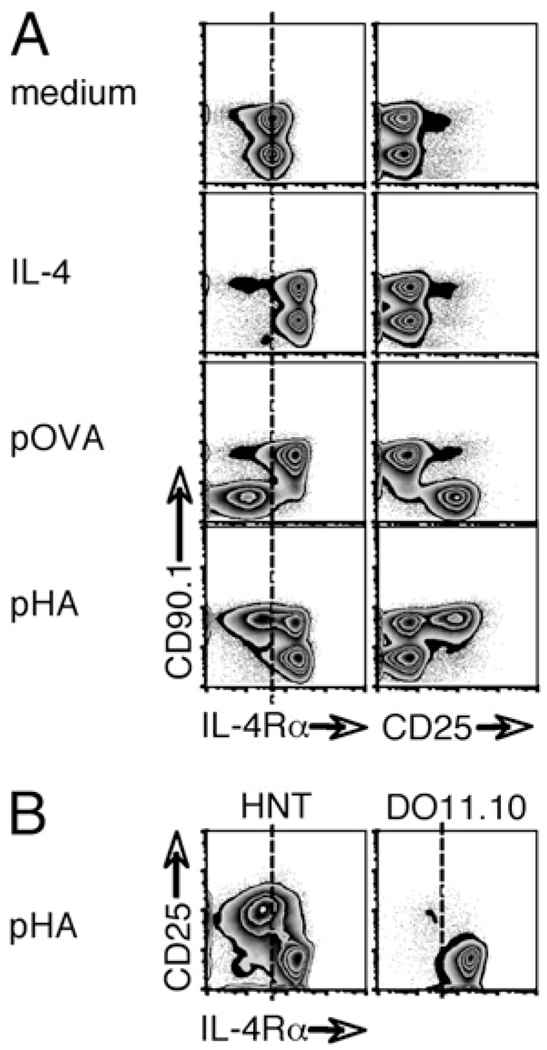
To investigate changes in the surface expression of the IL-4Rα chain during Th2 development, we first used a highly defined in vitro system. To mimic the presence of Ag-specific and nonspecific populations in a reactive lymph node, we sort purified naive (CD44lo) CD4+ T cells from DO11.10 and HNT TCR transgenic mice, which have distinct Ag specificities, and cocultured them in the presence of irradiated APCs (Fig. 1A). In the absence of stimulation, DO11.10 (CD90.1−) and HNT (CD90.1+) T cells expressed identical levels of IL-4Rα. As predicted (12, 17), the addition of IL-4 resulted in the upregulation of IL-4Rα on both populations within 24 h. When DO11.10 cells (CD90.1−) were selectively stimulated with OVA peptide, IL-4Rα expression was substantially reduced on the Ag-specific population but increased on the bystander HNT cells (CD90.1+) present in the same culture. Reciprocal results were obtained when the HNT population was selectively stimulated with the hemagglutinin peptide: Ag-responsive cells lost receptor expression, whereas cocultured and nonspecific cells instead upregulated IL-4Rα. Although only ~50% of the HNT donor CD4+ T cells reacted to the hemagglutinin peptide, as described (29), costaining confirmed that the loss of IL-4Rα was restricted to activated, CD25+ cells, whereas the nonactivated, CD25− cells upregulated IL-4Rα like the defined bystander DO11.10 cells (Fig. 1B). Of note, the increase in IL-4Rα expression on bystander cells elicited by either recombinant IL-4 or the activation of specific T cells occurred despite the absence of CD25, the high-affinity IL-2Rα chain, on these cells (Fig. 1). Moreover, neither the addition of a neutralizing anti–IL-2 nor a blocking IL-2RmAb caused any inhibition of the upregulation of IL-4Rα (data not shown), and activated DO11.10 cells downregulated IL-4Rα despite robust CD25 expression (Fig. 1A). IL-2 signals therefore appear to be dispensable for the induction of IL-4Rα on naive CD4+ T cells in vitro.
FIGURE 1.
IL-4Rα expression is oppositely influenced by cytokine-mediated and antigenic stimulation. A, CD4+ CD44lo cells sorted from naive DO11.10 animals (CD90.1−, specific for the OVA peptide 323–339 [pOVA]) were mixed 1:1 with equivalent cells from HNT mice (CD90.1+, specific for the hemagglutinin peptide 126–138 [pHA]) and cultured in the presence of irradiated APCs. Medium was supplemented with recombinant IL-4 or peptide Ag as indicated. Cells were recovered 24 h later and analyzed by flow cytometry for expression of IL-4Rα and CD25. B, Identical cocultures were established, and pHA-stimulated cells were analyzed for dual expression of IL-4Rα and CD25, gating on each transgenic population as shown. In both cases, data are representative of five independent experiments.
Together these data indicate that IL-4Rα expression on CD4+ T cells can be differentially regulated by antigenic stimulation and IL-4 signals and that the primary activation of naive CD4+ T cells by their cognate Ag is sufficient to alter receptor expression on bystander CD4+ T cells.
Regulation of IL-4Rα expression during helminth infection
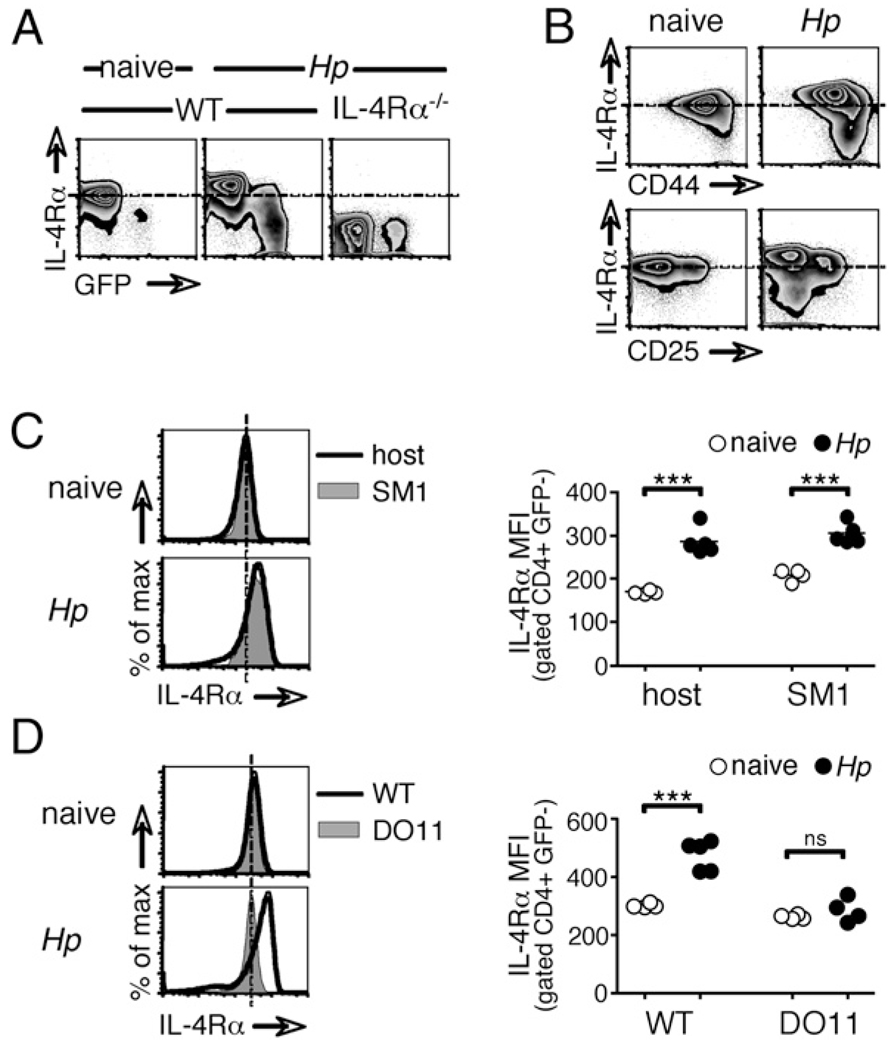
To assess whether a similar modulation of IL-4Rα also occurs during Th2 development in vivo, we infected WT and IL-4Rα−/− 4get IL-4 reporter mice with the enteric helminth parasite H. polygyrus and analyzed CD4+ T cells in the draining mesLNs 2 wk later. In 4get mice, GFP fluorescence marks IL-4 expression and indicates prior Ag encounter (21, 33). Consistent with our in vitro observations (Fig. 1), IL-4Rα expression was increased on the majority of CD4+ T cells in infected animals (Fig. 2A). These cells appeared to represent pathogen nonspecific bystanders, as denoted by their high frequency, their lack of GFP expression, and their predominantly CD44lo CD25− phenotype (Fig. 2B). A small subset of CD4+ T cells, with a GFP+ and CD44hi phenotype suggestive of Ag experience, instead displayed a profound downregulation of the IL-4Rα to a level equivalent to that of IL-4Rα−/− cells (Fig. 2A, 2B). Expression of the γc chain, which pairs with IL-4Rα to form the type I IL-4R, was not changed in response to infection (data not shown and Fig. 6A).
FIGURE 2.
Modulation of IL-4Rα expression upon infection with a helminth parasite. A, WT and IL-4Rα−/− 4get reporter mice, in which GFP fluorescence denotes IL-4 expression, were infected with H. polygyrus for 14 d. mesLN cells were analyzed by flow cytometry. Data shown are gated on CD4+ cells. B, WT mice were infected and analyzed as in A. C, CD4+ cells from SM1 TCR transgenic mice on a RAG−/− background, which are specific for an irrelevant Salmonella Ag, were transferred into congenically distinct, WT recipients 13 d after H. polygyrus infection. Twenty-four hours later, mesLN CD4+ cells of donor and recipient origins were analyzed for their expression of IL-4Rα by flow cytometry. D, WT BALB/c or DO11.10 TCR transgenic mice, in which T cells recognize only OVA were infected with H. polygyrus, and 14 d later, CD4+ cells from mesLNs were analyzed by flow cytometry. Data in all of the panels are representative of two or more independent experiments.
FIGURE 6.
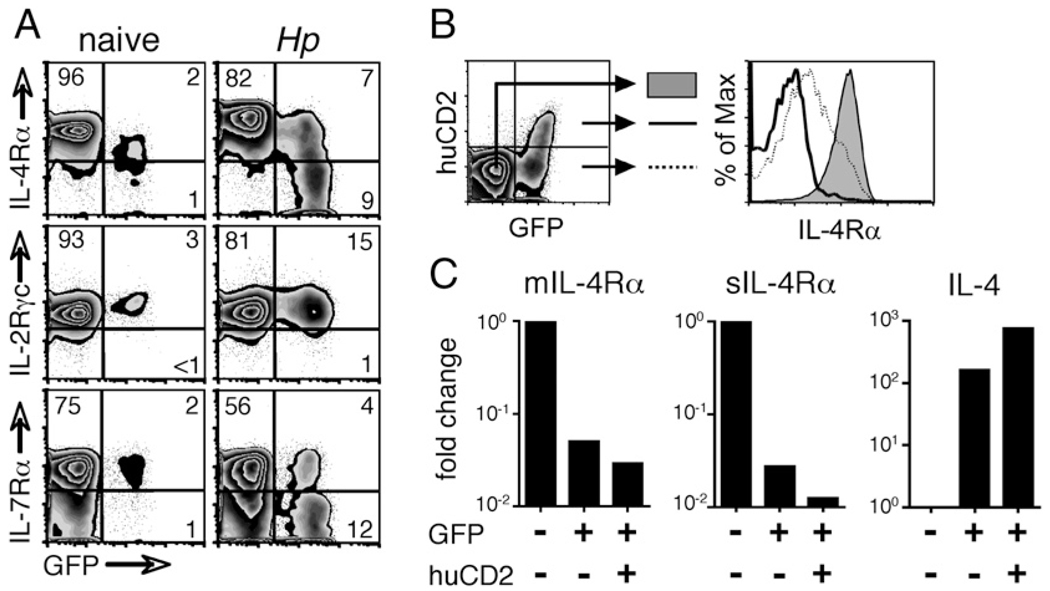
Highly activated Th2 cells lose IL-4Rα expression. A, WT 4get mice were infected with H. polygyrus, and 14 d later, CD4+ cells from mesLNs were analyzed for their expression of IL-4Rα, IL-2Rγc, and IL-7Rα. B, 4get/Kn2 dual-reporter mice were also infected, and on day 14, mesLN CD4+ cells were sorted into three populations as shown (GFP− huCD2−, filled histogram; GFP+ huCD2−, dashed line; GFP+ huCD2+, solid line). IL-4Rα expression on each population was measured by flow cytometry. C, Populations sorted as in B were lysed, their RNA was purified and reverse-transcribed, and their expression of the membrane-associated and soluble alternative splice variants of the IL-4Rα transcript and of IL-4 was assessed by quantitative PCR. Data are shown as a fold change in expression relative to that of GFP− huCD2− cells. In all of the panels, data are representative of at least two independent experiments.
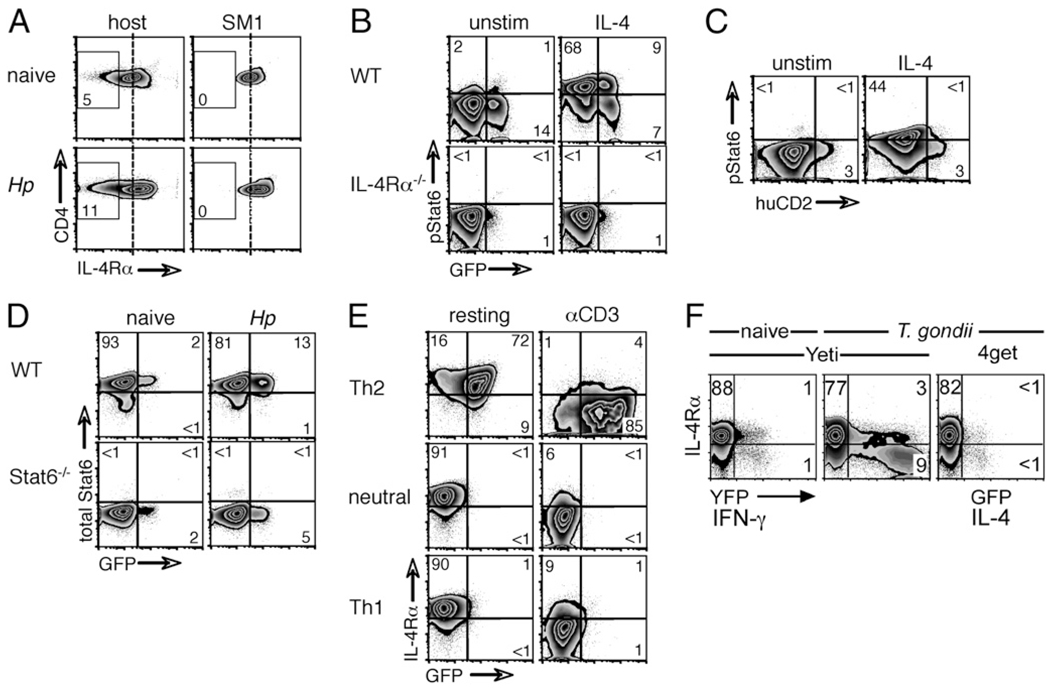
To formally confirm that the enhanced IL-4Rα expression seen in the bulk population of mesLN CD4+ T cells after H. polygyrus inoculation (Fig. 2A) was a feature of cells with TCR specificities irrelevant to the current infection, we transferred CD4+ T cells from SM1 TCR transgenic mice into naive or H. polygyrus infected C57BL/6 hosts and analyzed IL-4Rα expression 18 h later (Fig. 2C). SM1 cells recognize the flagellin427–441 epitope of S. typhimurium (27), a bacterium phylogenetically distant from the helminth parasite H. polygyrus. We used SM1mice on a RAG2−/− background to exclude the possibility of confounding endogenous TCR rearrangements. As shown in Fig. 2C, Salmonella-specific SM1 CD4+ T cells upregulated IL-4Rα expression within 18 h to a level equivalent to that of the endogenous cells of the infected host.
Our in vitro data suggested that this heightened IL-4Rα expression on bystander cells could be triggered as a consequence of antigenic stimulation of naive CD4+ T cells. To test whether the activation of Ag-specific T cells is also required for increased IL-4R expression on CD4+ T cells in vivo, we infected intact DO11.10TCR transgenic mice and WT controls with H. polygyrus and analyzed IL-4Rα expression 2 wk later (Fig. 2D). In DO11.10 animals, all of the cells, including CD4+ T cells, are competent to make IL-4. However, their TCR specificity is restricted to the OVA323–339 epitope, and consequently, very little or no Ag-mediated activation of CD4+ cells can occur during H. polygyrus infection. Basal IL-4Rα expression was identical on CD4+ T cells from naive mice of both groups, but those of DO11.10 animals failed to increase IL-4Rα expression upon H. polygyrus infection. This suggests that antigenic stimulation of CD4+ T cells is required for IL-4R upregulation in vivo.
IL-4Rα upregulation is restricted to the draining lymph node
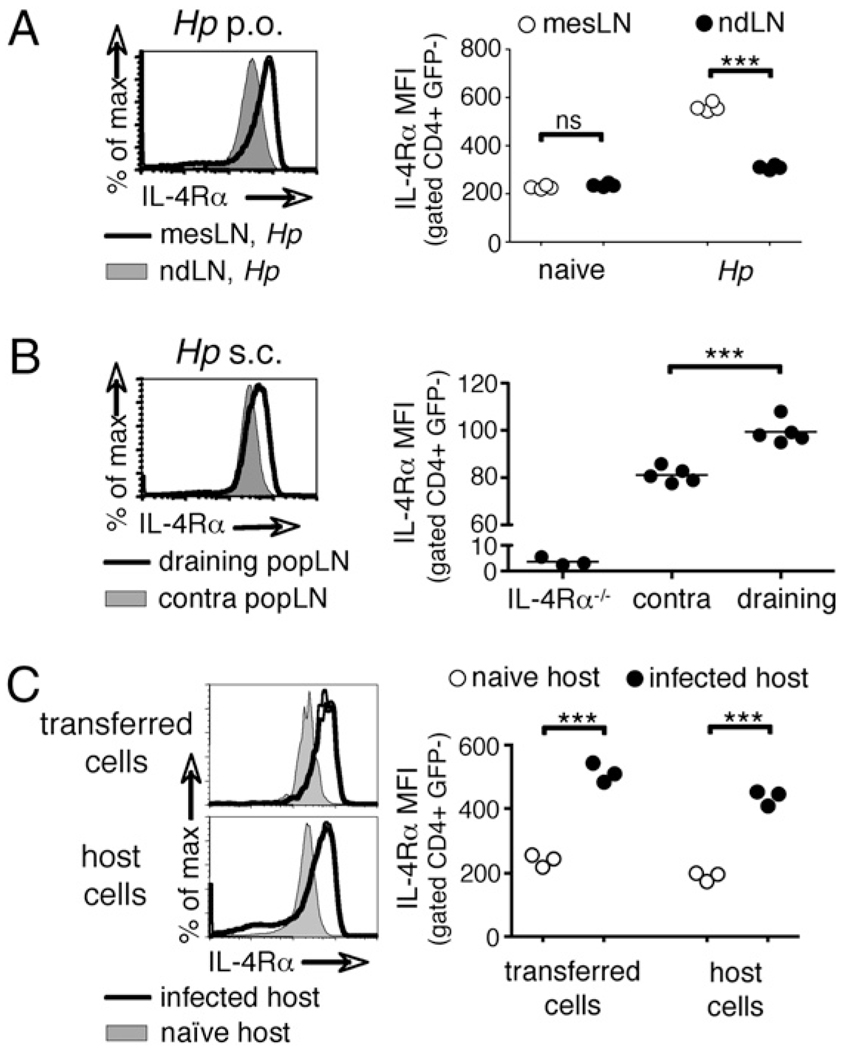
To determine whether the IL-4Rα upregulation in H. polygyrus infected animals is driven by local signals or represents a systemic event, CD4+ T cells in the draining mesLNs and nondraining lymph nodes were analyzed in WT mice that were infected orally with H. polygyrus 2 wk earlier. In naive mice, CD4+ cells from mesLNs and peripheral lymph nodes displayed equivalent basal levels of IL-4Rα. However, 2 wk after H. polygyrus infection, IL-4Rα expression was higher in the mesLNs than in nondraining lymph nodes (Fig. 3A). Similarly, the s.c. injection of live H. polygyrus larvae, which are known to elicit local Th2 responses in the absence of patient infection [(34) and data not shown], into one footpad triggered IL-4Rα upregulation in the draining popliteal lymph node but not in the contralateral popliteal lymph node, the mesLN, or any other nondraining lymph node (Fig. 3B and data not shown).
FIGURE 3.
Increased expression of IL-4Rα is determined by the local environment. A, IL-4Rα expression on CD4+ cells from mesLNs or pooled nondraining lymph nodes (cervical, axial, brachial, inguinal, and popliteal) was compared 14 d after oral H. polygyrus infection and (B) from draining and contralateral popliteal lymph nodes 7 d after s.c. immunization in the footpad with H. polygyrus larvae.C, CD4+ IL-4Rαhi cells were isolated from mesLNs 14 d after H. polygyrus infection and transferred into either 14 d infected or naive recipients. CD4+ cells in the recipient mesLNs were analyzed 24 h later by flow cytometry. Data are representative of three or more independent experiments.
The presence of IL-4Rαhi cells in only the draining lymph nodes suggested that IL-4Rα expression is increased in response to local stimuli. To test this, we transferred IL-4Rαhi CD4+ T cells from the mesLNs of 2-wk H. polygyrus infected mice into either naive or H. polygyrus infected congenic hosts. The IL-4Rα expression on donor cells adjusted within 24 h to a level equivalent to that of CD4+ host T cells in the local environment: transferred cells recovered from the mesLNs of naive hosts had downregulated IL-4Rα expression to basal levels, whereas those isolated from infected hosts maintained an IL-4Rαhi phenotype (Fig. 3C).
Increased IL-4Rα expression is dependent on IL-4 and direct STAT6 signaling
On the basis of our in vitro data (Fig. 1) and published observations (14, 17, 23), we hypothesized that the local signal driving the upregulation of IL-4Rα in vivo is IL-4. To examine this, we infected WT, IL-4−/−, and STAT6−/− mice with H. polygyrus and analyzed IL-4Rα expression 2 wk later. Indeed, both IL-4−/− and STAT6−/− animals failed to increase IL-4Rα expression on CD4+ cells upon infection (Fig. 4A).
FIGURE 4.
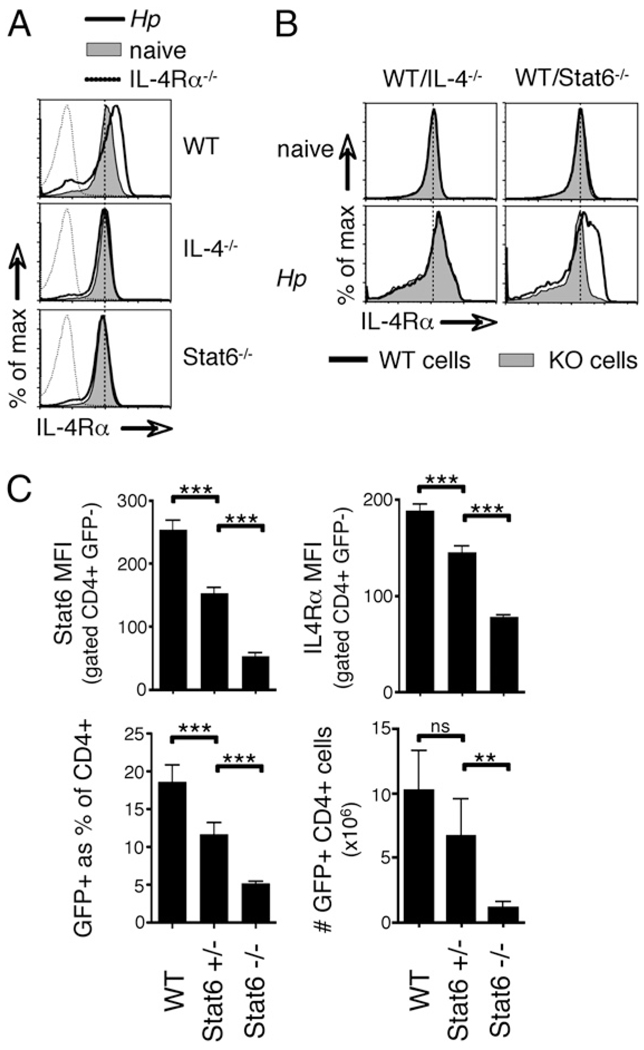
IL-4Rα upregulation in vivo is driven by IL-4 and STAT6 signaling. A, WT, STAT6−/−, and IL-4−/− mice were infected with H. polygyrus, and CD4+ cells from mesLNs were analyzed 14 d later by flow cytometry. Filled histograms indicate the staining of cells from naive mice of each genotype; solid line, H. polygyrus day 14; dashed line, cells from infected IL-4Rα−/− mice as a negative control. B, Mixed BM chimeras were generated by reconstituting CD90.1+ recipients with equal parts of CD90.1+ WT and either CD90.2+ IL-4−/− or CD90.2+ STAT6−/− BM. Reconstituted mice were infected with H. polygyrus and CD4+ cells from mesLNs analyzed 14 d later by flow cytometry. C, WT, STAT6+/−, and STAT6−/− 4get mice were infected with H. polygyrus and analyzed by flow cytometry 14 d later. The mean fluorescence of intracellular total Stat6 and surface IL-4Rα staining on bystander CD4+ GFP− cells from the mesLNs is shown, together with the total number of CD4+ GFP+ cells in the mesLNs. Error bars indicate the SD of five mice per group. Data in all of the panels are representative of two independent experiments.
To determine whether the upregulation of IL-4Rα expression in vivo requires direct IL-4 and STAT6 signaling, we generated mixed BM chimeras by reconstituting lethally irradiated, WT recipients (CD90.1) with BM from WT mice (CD90.1) mixed 1:1 with either CD90.2 IL-4−/− or CD90.2STAT6−/− BM. This approach allowed us to compare the gene-deficient populations with WT control cells in the same animal. As shown in Fig. 4B, WT and STAT6−/− or IL-4−/− cells in naive BM chimeras expressed identical levels of IL-4Rα. However, 2 wk after infection with H. polygyrus, STAT6−/− cells failed to upregulate the IL-4Rα, whereas IL-4−/− cells increased expression equivalently with their WT counterparts. These data show that CD4+ T cell-intrinsic STAT6 signals are required for IL-4Rα upregulation, whereas paracrine sources of IL-4 are sufficient.
Stat6 heterozygous animals have been reported to exhibit reduced IL-4 responsiveness compared with that of WT littermates (35). Because STAT6 is required for IL-4Rα upregulation in H. polygyrus infected mice, we reasoned that the phenotype observed in Stat6 heterozygous animals might be at least in part due to impaired IL-4Rα upregulation. As shown in Fig. 4C, in H. polygyrus infected Stat6+/− animals, the haplosufficiency translated directly into decreased STAT6 protein abundance and, importantly, into reduced upregulation of IL-4Rα surface expression on the bystander population of GFP− CD4+ cells in the mesLNs. Using Stat6+/− and Stat6−/− mice crossed onto the 4get IL-4 reporter background, we analyzed the magnitude of the Th2 response. Infection of Stat6 heterozygous animals with H. polygyrus elicited a frequency of GFP+ Th2 cells that was intermediate between those of WT and STAT6−/− controls (Fig. 4C). The total number of GFP+ cells followed the same trend but did not reach statistical significance (Fig. 4C). Together these data show that IL-4Rα expression is increased on CD4+ T cells during helminth infection by an IL-4- and STAT6-dependent mechanism. Paracrine sources of IL-4 are sufficient, but cell-intrinsic STAT6 signals are indispensable.
Early IL-4Rα expression predicts the size of the ensuing Th2 response
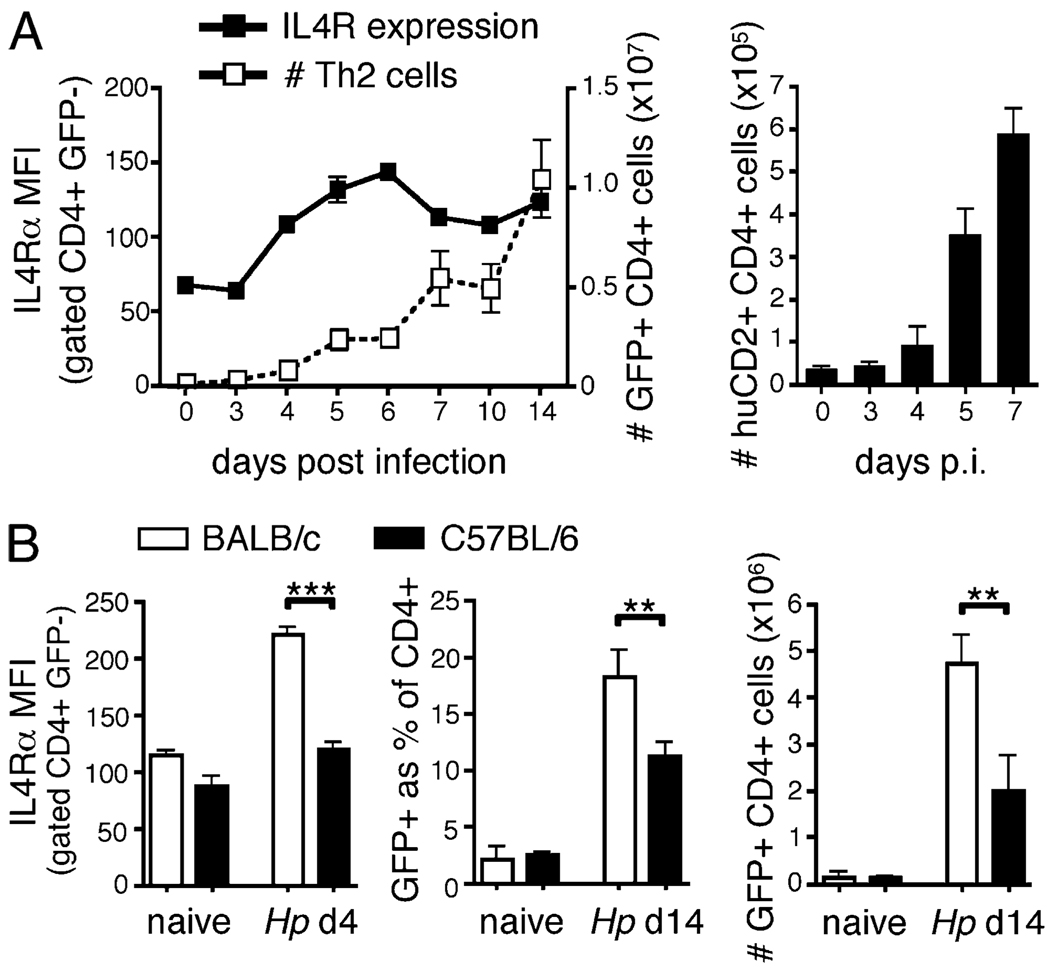
IL-4 drives both IL-4Rα upregulation and Th2 differentiation, which suggests that the two effects might comprise a positive feedback amplification loop. To investigate this, we performed kinetic studies in WT 4get IL-4 reporter mice to identify the temporal relation between IL-4Rα induction and Th2 differentiation during the onset of an H. polygyrus infection. Surface expression of IL-4Rα on bystander, GFP− CD4+ T cells increased markedly between 3 and 4 d after infection, a time point coincident with the earliest indication of Th2 development (Fig. 5A). Indeed, the first IL-4 production by T cells, revealed by huCD2 expression in 4get/KN2 IL-4 dual-reporter mice (22), also was detected at this time (Fig. 5A). Together with previous data (Fig. 4), this suggested that IL-4Rα upregulation occurs rapidly, initiated by early IL-4 expression.
FIGURE 5.
Early IL-4Rα upregulation predicts subsequent Th2 differentiation. A,WT 4get or 4get/Kn2 dual-reporter mice, in which GFP and huCD2 indicate IL-4 transcription and translation, respectively, were infected with H. polygyrus, and their mesLN CD4+ cells analyzed by flow cytometry at the indicated time points. B, 4get mice were bred on a BALB/c or C57BL/6 background, infected with H. polygyrus, and their mesLN cells analyzed 4 and 14 d later. In both panels, IL-4Rα expression is shown as the mean fluorescence of staining on CD4+ GFP− cells. Error bars indicate the SD of 3–5 mice per group, and data are representative of two independent experiments.
To explore whether the extent of early IL-4R upregulation influences the magnitude of the subsequent Th2 response, we compared 4get IL-4 reporter mice on BALB/c and C57BL/6 genetic backgrounds. BALB/c mice characteristically show sustained and enhanced Th2 development, relative to that of C57BL/6 animals (36). Although basal IL-4Rα expression was similar on mesLN CD4+ T cells from both strains, by 4 d after H. polygyrus infection, cells from BALB/c mice expressed markedly higher levels of IL-4Rα than those from C57BL/6 animals (Fig. 5B). This was consistent with an enhanced, earliest detectable IL-4 expression in BALB/c versus C57BL/6 mice (data not shown). Importantly, subsequent Th2 expansion was correspondingly stronger in BALB/c mice than that in C57BL/6 mice (Fig. 5B).
Loss of IL-4Rα expression occurs in highly activated Th2 cells
Although IL-4Rα expression was generally increased on CD4+ cells in the mesLNs of H. polygyrus infected animals, GFPhi cells downregulated IL-4Rα to levels equivalent to those of IL-4Rα−/− cells (Fig. 2). To analyze the loss of IL-4Rα expression in relation to Th2 differentiation, we infected 4get IL-4 reporter mice with H. polygyrus and examined mesLN cells 14 d later. Downregulation of IL-4R was specific for the IL-4Rα component of the type I IL-4R complex because expression of its partner subunit, the γc chain, was not altered (Fig. 6A). Interestingly, the IL-7Rα chain, which also depends on the γc chain for signal transduction, was also markedly downregulated in GFPhi cells (Fig. 6A).
We have previously shown that in 4get mice enhanced GFP fluorescence correlates with increased IL-4 transcript levels and the production of IL-4 protein in GFPhi cells (22). To test whether the loss of IL-4Rα expression was associated with IL-4 production in vivo, we analyzed CD4+ T cells in the mesLNs of H. polygyrus infected 4get/KN2 IL-4 dual-reporter mice (Fig. 6B). Indeed, although IL-4Rα expression on GFPlohuCD2− cells was reduced compared with that on the GFP− population, essentially all of the GFPhihuCD2+ cells had lost IL-4Rα expression [Fig. 6B and (22)].
The absence of surface IL-4Rα on IL-4–producing Th2 cells could indicate either globally reduced expression of the full-length mIL-4Rα mRNA or alternative splicing to sIL-4Rα RNA (31, 32). To distinguish these possibilities, CD4+ T cells with GFP−huCD2−, GFPlohuCD2−, and GFPhihuCD2+ phenotypes were sorted from the mesLNs of H. polygyrus infected 4get/KN2 mice and analyzed by quantitative PCR for the expression of the respective IL-4Rα mRNA species. The abundance of both IL-4Rα mRNA species was substantially decreased in all of the GFP+ cells as compared with that of the GFP− cells, and there was no apparent skewing toward the alternatively spliced sIL-4Rα mRNA in either GFP+ population (Fig. 6C). The sort purity was internally confirmed by the direct correlation between the abundance of IL-4 transcripts and the intensity of GFP fluorescence (Fig. 6C) (22).
IL-4Rα downregulation in vivo is induced by TCR engagement and renders the cell unresponsive to IL-4
The data above indicated that the loss of IL-4Rα expression in GFPhi CD4+ T cells is associated with highly polarized Th2 cells. From our in vitro data (Fig. 1) and because acute TCR activation results in increased IL-4 mRNA expression, the production of IL-4 (22), and a substantial downregulation of IL-7Rα expression (37), we reasoned that antigenic stimulation could be responsible for the IL-4Rα downregulation that occurs in vivo. To test this, we sought an experimental approach in which an identifiable population of CD4+ T cells is present throughout the course of a H. polygyrus infection but cannot receive TCR stimulation by H. polygyrus Ags. Because it is difficult to track adoptively transferred naive CD4+ T cells for extended periods of time, particularly in the presence of an ongoing, irrelevant immune response, we devised a BM chimeric approach. Lethally irradiated CD90.2 C57BL/6 4get mice were reconstituted with BM comprising 10% SM1 × RAG2−/− × CD90.1 (27) and 90% WT cells. The TCR transgenic BM provided an internal source of CD4+ T cells unable to recognize H. polygyrus derived Ags. Eight weeks after reconstitution, the CD4+ T cell compartment contained a stable population of 6–14% SM1 TCR transgenic cells that expressed IL-4Rα levels identical to those of the polyclonal nontransgenic population. As expected from Fig. 3, H. polygyrus infection caused increased IL-4Rα expression on both the polyclonal WT and the Salmonella-specific SM1 CD4+ T cells (Fig. 7A). However, unlike cells within the WT population, none of the SM1 cells displayed reduced IL-4Rα expression, despite their presence throughout the 2-wk course of infection. These data show that Ag nonspecific cells do not downregulate IL-4Rα expression despite their presence in the reactive lymph node, suggesting that TCR engagement is required for this process.
FIGURE 7.
IL-4Rα downregulation is triggered by Ag encounter and terminates IL-4R signaling. A, Mixed BM chimeras were generated by reconstituting WT CD90.2+ C57BL/6 recipients with a 90:10 mix of WT CD90.2+ and SM1 TCR transgenic CD90.1+ BM. Chimeras were infected with H. polygyrus, and 14 d later, CD4+ cells from mesLNs were analyzed for IL-4Rα expression by flow cytometry. Data shown are gated on CD4+ cells, and the numbers indicate the percentage of IL-4Rαlo cells within each population. B, WT and IL-4Rα−/− 4get mice were infected with H. polygyrus, and mesLN cells were isolated on day 14. Cells were first incubated with anti–IL-4 to remove endogenous STAT6 phosphorylation and then either incubated in medium alone or stimulated with IL-4 for 15 min. Resulting STAT6 phosphorylation was assessed by intracellular staining and flow cytometry. C, WT 4get/Kn2 dual-reporter mice were infected with H. polygyrus, and mesLN cells were harvested, stimulated, and stained as in B. D, WT and STAT6−/− 4get mice were infected with H. polygyrus, and mesLN cells were isolated on day 14. Total STAT6 expression was assessed by intracellular staining and flow cytometry. E, Naive CD4+ cells from 4get mice were activated with αCD3 + αCD28 in Th2, neutral, or Th1 conditions. Cells were washed, rested, and on day 7 either cultured in medium alone or restimulated with αCD3. IL-4Rα expression was measured 24 h later by flow cytometry. F, Yeti IFN-γ and 4get IL-4 reporter mice were infected with the Th1-inducing protozoan T. gondii, and 7 d later, CD4+ cells from mesLNs were analyzed for their expression of YFP or GFP and IL-4Rα. Data in all of the panels are representative of at least two independent experiments.
To assess the functional consequence of the loss of IL-4Rα on activated Th2 cells, we tested their ability to phosphorylate STAT6 in response to IL-4 stimulation. Lymphocytes from mesLNs of H. polygyrus infected 4get or 4get × IL-4Rα−/− mice were cultured for 15 min in the absence or presence of IL-4, and CD4+ T cells were analyzed for the presence of phosphorylated STAT6 (Fig. 7B). In contrast to GFP− and GFPlo cells, the GFPhi cells, which show pronounced IL-4Rα downregulation (Fig. 6B), did not phosphorylate STAT6 in response to IL-4 stimulation; instead, they behaved equivalently to the IL-4Rα−/− cells that served as negative controls. These findings were corroborated in similarly infected and analyzed 4get/KN2 dual-reporter mice in which IL-4–producing GFPhi huCD2+ cells that have lost surface expression of IL-4Rα [Fig. 6B and (22)] failed to phosphorylate STAT6 upon IL-4 stimulation (Fig. 7C). Importantly, the absence of STAT6 phosphorylation in GFPhi cells was not due to the loss of STAT6 protein per se (Fig. 7D). These experiments illustrate that the selective downregulation of IL-4Rα on IL-4–producing GFPhi Th2 cells renders them unresponsive to IL-4.
To explore whether the loss of IL-4Rα and IL-4 responsiveness is limited to activated Th2 cells or is elicited by TCR signals regardless of the Th subset, we first polarized naive, 4get CD4+ cells in vitro. The cultures were rested and restimulated by TCR ligation. All of the cells, whether polarized under Th2, Th0, or Th1 conditions, displayed pronounced downregulation of IL-4Rα upon TCR stimulation (Fig. 7E). To confirm this observation in vivo, we analyzed IL-4Rα expression on highly polarized Th1 cells induced by acute infection with the protozoan parasite T. gondii. Using both Yeti IFN-γ reporter (26) and 4get IL-4 reporter mice, we assessed IL-4Rα expression on CD4+ T cells 7 d after T. gondii infection (Fig. 7F). Indeed, CD4+ T cells that expressed high levels of the yellow fluorescent protein (YFP) IFN-γ reporter selectively lost IL-4Rα expression. As expected, T. gondii did not induce any expression of IL-4, illustrated by the absence of GFP in infected 4get mice, and consequently, IL-4Rα expression was not increased on YFP− or GFP− bystander cells (Fig. 7F). These data suggest that TCR engagement triggers a reduction in IL-4Rα expression on CD4+ T cells irrespective of their Th polarization.
Discussion
IL-4 plays a key role in governing Th2 development (4–6, 8). IL-4 expression is tightly regulated (21, 22, 38, 39), but its impact also depends on the ability of target cells to bind the cytokine and transmit IL-4 signals. In this study, we demonstrate that the Th2 response to a murine helminth pathogen involves a series of precise adjustments in IL-4R expression. Infection triggers both a rapid upregulation of the IL-4Rα chain on the bystander majority of CD4+ T cells in the draining mesLN and a profound downregulation on the Ag-activated population. Increased IL-4Rα expression is elicited by IL-4, and its loss occurs in response to TCR engagement. We propose that the different effects of cytokine and Ag on IL-4R expression result in a series of changes in IL-4 sensitivity that guide the initiation, expansion, and restriction of Th2 development.
Many studies have examined the sources and kinetics of IL-4 production during infection (21, 22, 38, 40), but only recently has attention focused on the regulation of IL-4 responsiveness (16, 41, 42). There is precedence for the differential expression of cytokine receptors rather than cytokine production playing a decisive role in vivo. In acute lymphocytic choriomeningitis virus infection, the transition from an effector phenotype into a long-lasting memory CD8+ cell correlates with persistent IL-7Rα expression (43); cells lacking a functional receptor unit are eliminated during immune contraction (44). In a Th2 context, myeloid cells determine their response to IL-3, IL-5, and GM-CSF, which signal through a common receptor β-chain, by selective expression of the cytokine-specific α-subunits (45). Indeed, it has recently been proposed that the contrasting regulatory and effector functions of IL-4 and IL-13, respectively, are the consequence of differential receptor expression on specific target cells, conferring distinct patterns of cytokine sensitivity (16).
In this study, we show that a single cell type, the CD4+ T lymphocyte, modulates its expression of the IL-4 receptor during Th2 differentiation in vivo. STAT6 signaling mediates IL-4 function, and although IL-4 expression can be STAT6-independent (39), STAT6−/− cells, like IL-4Rα−/− cells, do not respond to IL-4 stimulation (12–15, 23, 46). Targeted deletions of both Il4ra and Stat6 have revealed gene dosage effects in heterozygous mice, indicating that a 2-fold alteration in IL-4Rα/STAT6 signaling has functional significance: heterozygous Il4ra animals display an intermediate disease phenotype in situations of Th2-mediated pulmonary and pancreatic inflammation compared with those of WT and IL-4Rα-deficient controls (47, 48). Similarly, the reduced IL-4R signaling capacity of Stat6 heterozygotes causes sufficiently impaired Th2 development such that, in contrast to WT littermates, these mice are as resistant to Leishmania major infection as STAT6−/− animals (35).
Our observation that IL-4 alone is sufficient for the upregulation of IL-4Rα is in contrast to a recent study that proposed a critical role for IL-2 signals in the same process in vitro (20). The discrepancy may reflect the different culture regimes employed in the two studies: we analyzed naive TCR transgenic CD4+ T cells within the first 24 h of peptide stimulation, whereas Liao et al. (20) examined cells that were preactivated with anti-CD3 and anti-CD28 for 2 d and then rested overnight prior to their exposure to IL-2. We and others have previously shown that naive CD4+ T cells undergo substantial cell division within the first 3 d of stimulation and are then compromised in the plasticity of their cytokine repertoire (49–52). Importantly, we extend our studies in vivo and show that in H. polygyrus infected mice, IL-4Rα upregulation on CD4+ T cells is strictly dependent on direct IL-4 signals and occurs independently of CD25 expression, the high-affinity IL-2R (Figs. 2, 4). In mixed BM chimeras, IL-4Rα induction is selectively abrogated in STAT6−/− CD4+ cells compared with that in WT equivalents in the same animal, despite the equal availability of IL-2 for both populations (Fig. 4). Nonetheless, IL-2 has been shown to support the Th2 response to helminth infections (53, 54), and its influence may occur later in Th2 development, stabilizing the open Il4 locus in previously activated cells (55) rather than enhancing IL-4Rα expression early during Th differentiation.
The ability of IL-4 alone to elicit IL-4Rα upregulation implies that the effect is not restricted to T cells of a particular Ag specificity. Indeed, with the exception of activated Th2 cells, all of the CD4+ T cells within the draining lymph node show an increase in IL-4Rα expression during H. polygyrus infection (Figs. 2, 3). Heightened IL-4Rα expression might be expected to predispose naive CD4+ T cells toward Th2 differentiation. Bottomly and colleagues (56, 57) have reported a similar effect, termed “collateral priming,” in which IL-4 promotes Th2 development by conditioning both dendritic cells and T cells to circumvent the need for TLR signals to activate the APCs. In the context of helminth infection, during which the parasite undergoes a series of developmental molts that likely result in a succession of distinct Ags, the ability of IL-4 to facilitate Th2 polarization in bystander cells could be beneficial, ensuring that Ag presentation occurring after the initiation of the Th2 response will also result in a supportive Th2 bias in newly activated T cells. Conversely, it may also have deleterious consequences: patients with allergies of a single specificity are more likely to become allergic to other, unrelated Ags than are healthy controls (58, 59).
The loss of IL-4Rα expression on acutely activated Th cells renders them unable to respond to IL-4 (Fig. 7). The absence of IL-4Rα on activated Th cells is unusual among lymphocytes, because the IL-4Rα chain is generally believed to be ubiquitously expressed on hematopoietic cells (12). IL-4 is a growth factor for T cells (60, 61), and consequently the downregulation of IL-4Rα may serve to limit the extent of a Th2 response by depriving activated effector cells from the survival signals provided by IL-4. Such a mechanism of immune regulation would mirror that of IL-7Rα, which is downregulated on activated Th cells and thus disconnects them from essential survival signals, curtailing their response (62). This hypothesis would predict that a failure to extinguish IL-4Rα expression would result in uncontrolled Th2 proliferation. Intriguingly, Tanaka and colleagues (41) recently demonstrated that Dock2−/− mice, which show impaired downregulation of IL-4Rα on CD4+ T cells, develop exaggerated Th2 responses and consequent allergic disease. The disease phenotype was prevented when Dock2−/− mice were either treated with anti-CD4 or bred to the IL-4Rα−/− background, which demonstrates a direct role for CD4+ T cells and IL-4Rα (41). In the context of a Th1 response, the same consequence of IL-4Rα downregulation on highly activated cells may not serve to curtail Th2 differentiation but instead to reinforce correct Th1 polarization.
Another hypothesis, not exclusive to that above, is that the loss of IL-4Rα expression on acutely activated T cells enables them to provide effective help to B cells. In the lymph node, activated, IL-4–producing CD4+ T cells are restricted to B cell areas and display the phenotype of follicular Th helper cells (63–65). The dialogue between these cells and the B cells that they support is mediated in part by CD40–CD40L interaction, yet IL-4 signaling attenuates CD40L expression on activated CD4+ T cells (66). Downregulation of IL-4Rα could allow IL-4–producing Th cells to maintain CD40L expression, thus promoting the B cell response. Moreover, because IL-4 has been proposed to be a limiting factor for B cell maturation (63), the absence of IL-4Rα on IL-4–producing Th2 cells may also serve an altruistic purpose, preventing the consumption of IL-4 by follicular Th cells and thereby maximizing the local concentration available to B cells. A similar mechanism of regulating cytokine availability by modulating receptor expression on T cells has been suggested for IL-7 (67).
We have shown that, during helminth infection, IL-4 stimulation alone drives IL-4Rα upregulation on the bulk population of CD4+ T cells in the lymph node and Ag encounter causes its profound downregulation on highly activated Th cells. These changes in IL-4Rα expression, and the different sensitivities to IL-4 that they confer, characterize distinct stages of Th2 progression in vivo.
Acknowledgments
We thank Ron LaCourse and Brandon Sells for cell sorting, Irah King and Edward Pearce for ideas and discussion, and Andrew MacDonald and Stephen Jenkins for critical review of the manuscript.
This work was supported by funds from the Trudeau Institute and the National Institutes of Health Grants AI072296 and AI076479 (to M.M.).
Abbreviations used in this paper
- γc
common γ
- BM
bone marrow
- HNT, TCR
transgenic mice specific for influenza virus hemagglutinin
- mesLN
mesenteric lymph node
- mIL-4R
membrane IL-4R
- sIL-4R
soluble IL-4R
- SM1
TCR transgenic mice specific for the Salmonella typhimurium flagellin427–441 epitope
- YFP
yellow fluorescent protein
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 4.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 6.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 7.Snapper CM, Finkelman FD, Paul WE. Regulation of IgG1 and IgE production by interleukin 4. Immunol. Rev. 1988;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 8.Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 9.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-α-mediated cachexia and death during acute schistosomiasis. J. Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 10.Urban JF, Jr, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int. Arch. Allergy Immunol. 2004;135:173–186. doi: 10.1159/000080899. [DOI] [PubMed] [Google Scholar]
- 12.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 16.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, Paul WE. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Rα, IL-13Rα1, and γc regulates relative cytokine sensitivity. J. Exp. Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara J, Paul WE. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc. Natl. Acad. Sci. USA. 1988;85:8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dokter WH, Borger P, Hendriks D, van der Horst I, Halie MR, Vellenga E. Interleukin-4 (IL-4) receptor expression on human T cells is affected by different intracellular signaling pathways and by IL-4 at transcriptional and posttranscriptional level. Blood. 1992;80:2721–2728. [PubMed] [Google Scholar]
- 19.Renz H, Domenico J, Gelfand EW. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J. Immunol. 1991;146:3049–3055. [PubMed] [Google Scholar]
- 20.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat. Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 22.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohrs M, Ledermann B, Köhler G, Dorfmüller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor α-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 24.Kaplan MH, Wurster AL, Smiley ST, Grusby MJ. Stat6-dependent and -independent pathways for IL-4 production. J. Immunol. 1999;163:6536–6540. [PubMed] [Google Scholar]
- 25.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor αβ-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 26.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 28.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes invivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 29.Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 2003;197:801–806. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum H, Wolf M, Enssle K, Röllinghoff M, Gessner A. Two distinct stimulus-dependent pathways lead to production of soluble murine interleukin-4 receptor. J. Immunol. 1996;157:1846–1853. [PubMed] [Google Scholar]
- 32.Mosley B, Beckmann MP, March CJ, Idzerda RL, Gimpel SD, VandenBos T, Friend D, Alpert A, Anderson D, Jackson J, et al. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- 33.Mohrs K, Harris DP, Lund FE, Mohrs M. Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J. Immunol. 2005;175:5306–5313. doi: 10.4049/jimmunol.175.8.5306. [DOI] [PubMed] [Google Scholar]
- 34.Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A, Pesce J, VanNov J, Sharpe AH, Urban JF, Gause WC. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J. Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 35.Burgis S, Gessner A. Unexpected phenotype of STAT6 heterozygous mice implies distinct STAT6 dosage requirements for different IL-4 functions. Int. Arch. Allergy Immunol. 2007;143:263–268. doi: 10.1159/000100571. [DOI] [PubMed] [Google Scholar]
- 36.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 37.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7Rα-expressing cells. J. Exp. Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, et al. Basophils produce IL-4 and accumulate in tissues after infectionwithaTh2-inducingparasite. J.Exp.Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, Schopf L, Urban JF., Jr Stat6 regulation of in vivo IL-4 responses. J. Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 40.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. Int. Immunol. 1999;11:1811–1818. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y, Hamano S, Gotoh K, Murata Y, Kunisaki Y, Nishikimi A, Takii R, Kawaguchi M, Inayoshi A, Masuko S, et al. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-α subunit controlled by the Rac activator Dock2. Nat. Immunol. 2007;8:1067–1075. doi: 10.1038/ni1506. [DOI] [PubMed] [Google Scholar]
- 42.Maldonado RA, Soriano MA, Perdomo LC, Sigrist K, Irvine DJ, Decker T, Glimcher LH. Control of T helper cell differentiation through cytokine receptor inclusion in the immunological synapse. J. Exp. Med. 2009;206:877–892. doi: 10.1084/jem.20082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 44.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 45.Geijsen N, Koenderman L, Coffer PJ. Specificity in cytokine signal transduction: lessons learned from the IL-3/IL-5/GM-CSF receptor family. Cytokine Growth Factor Rev. 2001;12:19–25. doi: 10.1016/s1359-6101(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 46.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller U, Stenzel W, Köhler G, Polte T, Blessing M, Mann A, Piehler D, Brombacher F, Alber G. A gene-dosage effect for interleukin-4 receptor α-chain expression has an impact on Th2-mediated allergic inflammation during bronchopulmonary mycosis. J. Infect. Dis. 2008;198:1714–1721. doi: 10.1086/593068. [DOI] [PubMed] [Google Scholar]
- 48.Radu DL, Noben-Trauth N, Hu-Li J, Paul WE, Bona CA. A targeted mutation in the IL-4Rα gene protects mice against autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 2000;97:12700–12704. doi: 10.1073/pnas.230431397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohrs M, Lacy DA, Locksley RM. Stat signals release activated naive Th cells from an anergic checkpoint. J. Immunol. 2003;170:1870–1876. doi: 10.4049/jimmunol.170.4.1870. [DOI] [PubMed] [Google Scholar]
- 50.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 51.Richter A, Löhning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J. Exp. Med. 1999;190:1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z, Liu Q, Hamed H, Anthony RM, Foster A, Finkelman FD, Urban JF, Jr, Gause WC. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J. Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88. J. Immunol. 2004;172:4527–4534. doi: 10.4049/jimmunol.172.7.4527. [DOI] [PubMed] [Google Scholar]
- 57.Dittrich AM, Chen HC, Xu L, Ranney P, Connolly S, Yarovinsky TO, Bottomly HK. A new mechanism for inhalational priming: IL-4 bypasses innate immune signals. J. Immunol. 2008;181:7307–7315. doi: 10.4049/jimmunol.181.10.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Des Roches A, Paradis L, Menardo JL, Bouges S, Daurés JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J. Allergy Clin. Immunol. 1997;99:450–453. doi: 10.1016/s0091-6749(97)70069-1. [DOI] [PubMed] [Google Scholar]
- 59.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin. Exp. Allergy. 2001;31:1392–1397. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 60.Brown M, Hu-Li J, Paul WE. IL-4/Bcell stimulatory factor 1 stimulates T cell growth by an IL-2-independent mechanism. J. Immunol. 1988;141:504–511. [PubMed] [Google Scholar]
- 61.Fernandez-Botran R, Sanders VM, Oliver KG, Chen YW, Krammer PH, Uhr JW, Vitetta ES. Interleukin 4 mediates autocrine growth of helper T cells after antigenic stimulation. Proc. Natl. Acad. Sci. USA. 1986;83:9689–9693. doi: 10.1073/pnas.83.24.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee BO, Haynes L, Eaton SM, Swain SL, Randall TD. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J. Exp. Med. 2002;196:693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]